Abstract
Thermogenetics uses temperature-sensitive proteins to regulate cellular functions via temperature changes. Compared to optogenetics, which utilizes visible light and is limited by light penetration, thermogenetics offers a practical alternative by enabling deeper and more accessible control of cellular processes via heat. Herein, we report the development of a thermogenetically controlled programmed cell death system that enables heat-activated human caspase 8 (CASP8) using temperature-responsive elastin-like polypeptides (ELPs). The core functionality of this system relies on the reversible phase transition behavior of ELPs, which transition from a soluble state to a coacervate state in response to thermal stimuli. By exploiting this temperature-dependent behavior, we fused ELP[V60] with the catalytic domain of CASP8 to construct the ELP[V60]-CASP8 fusion. Upon heating at temperatures above 35 °C, the ELP[V60] in the fusion protein underwent coacervation, increasing the local concentration of CASP8 to facilitate dimerization-induced activation and promote cell death in HEK293T cells. We observed a correlation between the heating temperature and the duration required to induce cytotoxicity at higher temperatures, requiring shorter heating times. Additionally, we developed a CASP8 indicator to monitor CASP8 activation and demonstrated its functionality in HEK293T cells. We further used optical heating with a 1470 nm laser combined with fluorescence lifetime-based thermometry to achieve localized activation of CASP8 in target single cells with precise and controlled temperature increments.
Keywords: thermogenetics, elastin-like polypeptides, caspase 8, programmed cell death, optical heating, FLIM


The ability to control the activity of target proteins in live cells enables the manipulation of cellular processes. Several approaches have been developed to achieve precise protein regulation. Chemogenetics uses chemical agents such as rapamycin to induce protein–protein interactions between FK506-binding protein (FKBP) and the FKBP-rapamycin-binding domain. However, achieving precise control at a single-cell resolution with high spatial and temporal precision remains challenging. Moreover, chemical reagents are often difficult to wash out quickly, hindering rapidly reversed inactivation of the system. Optogenetics, which uses light-sensitive proteins, offers a solution by enabling high spatial and temporal control of ion channels or target proteins through visible light irradiation. Various optogenetic tools have been developed to control the activity of ion channels, such as halorhodopsins and channelrhodopsins, as well as intracellular proteins, using photosensitive proteins, such as the light-oxygen-voltage (LOV) domain, Cryptochrome 2, and Vivid. Although optogenetics provides versatile tools for biological studies, the requirement for visible light irradiation has some drawbacks, as light has poor penetration into deep tissues and may unintentionally activate endogenous photoreceptors. However, thermogenetics employs temperature-sensitive proteins to modulate cellular functions by changing temperature. Unlike optogenetics, which requires visible light irradiation, thermogenetics can leverage localized temperature control using techniques such as infrared (IR), focused ultrasound, ultrahigh frequency, or radio-wave irradiation, , enabling the manipulation of deep tissues where light penetration is restricted. This feature renders thermogenetics a more practical tool for certain experiments and offers an alternative to optogenetics.
The development of thermogenetic tools has predominantly focused on the regulation of ion channels, particularly for controlling neuronal activities (see reviews and ). Transient receptor potential (TRP) channels are among the most extensively studied thermogenetic systems that permit cation influx upon heat activation, leading to membrane depolarization and subsequent neuronal activation. Bath et al. have investigated fly strains expressing intrinsic Drosophila dTRPA1 and developed an IR stimulation system called FlyMAD to precisely target and activate the dTRPA1 thermosensitive channel. By applying localized IR-mediated heating using FlyMAD, they successfully manipulated neurons associated with mating behavior in freely moving flies. Ermakova et al. have used the TRPA1 channel from snakes as a thermogenetic stimulator and demonstrated its functionality in cultured mammalian neurons and zebrafish. In addition to TRP channels, the Drosophila taste receptor family member Gr28bD has been identified as a potential thermosensitive channel for thermogenetic applications. Recently, Chee et al. have developed a thermogenetic tool using temperature-sensitive Tlpa protein, which forms a coiled-coil domain at a temperature below 30 °C for regulating the function of split-T7 RNA polymerase. Benman et al. have developed thermogenetic systems to modulate the mammalian cell fate using an engineered BcLOV4 protein that underwent oligomerization and translocation to the plasma membrane when the temperature was lowered. Both systems rely on a temperature decrease for activation, which can be achieved using a stage-top incubator on a microscope or a thermoPlate device. However, these approaches face significant challenges, including slow response times (on the minute scale) and the inability to achieve localized cooling, which limits the rapid activation of target single cells. Additionally, laboratory procedures such as washing and exchanging the medium at room temperature may lead to unintended activation. Compared with cooling, heating technologies for local and rapid temperature elevation are well-developed. However, no thermogenetic tools have been established for the heat-controlled regulation of cellular protein function. Expanding the versatility of heat-activated thermogenetic tools requires the discovery and engineering of temperature-responsive biomolecules. These biomolecules should exhibit rapid and reversible responses to temperature increases, enabling precise control of cellular activity without causing irreversible damage or thermal stress.
Owing to their unique thermal responsiveness, elastin-like polypeptides (ELPs) are candidates for the development of thermogenetic tools. ELPs are composed of repeating pentapeptides (VPGXG)n, where X is a guest amino acid residue except proline, and n represents the number of repeats. ELPs exhibit a lower critical solution temperature in aqueous solutions: they remain in a soluble state below the phase transition temperature (T t) and undergo a reversible self-assembly process to form coacervates at elevated temperatures above T t. The T t of ELPs can be fine-tuned by modifying the hydrophobicity of the guest residue X or altering the number of repeats n. More hydrophobic residues or a higher number of repeats result in a lower T t, while less hydrophobic residues or fewer repeats increase T t. These properties make the ELPs versatile scaffolds for creating temperature-responsive systems that can be activated by heating.
In this study, we harnessed the thermally responsive properties of ELPs to develop a thermogenetic tool to control the activation of human caspase 8 (CASP8), a key initiator caspase in the extrinsic apoptotic pathway. CASP8 is produced as an inactive zymogen and is activated through dimerization and autocleavage, leading to the initiation of programmed cell death. By fusing CASP8 with ELP, we achieved temperature-dependent control of its activation as demonstrated using apoptosis detection and cytotoxicity assays. Additionally, we developed a CASP8 indicator to monitor CASP8 activation and validated its functionality in HEK293T cells. Furthermore, we employed optical heating with an infrared laser (1470 nm) and demonstrated the highly localized spatial and temporal regulation of apoptosis in target single cells.
Results
Design of Heat-Activated Human CASP8
We exploited the thermal responsiveness of ELP to control human CASP8 activation. CASP8 is a member of the cysteine protease family and, like other caspases, is synthesized as an inactive zymogen (pro-caspase 8) in the cytosol. Activation of CASP8 occurs via proteolytic cleavage, either through autoactivation after recruitment to an oligomeric complex or through trans-cleavage by other caspases. Once activated, pro-caspase 8 undergoes dimerization and autocleavage into large (∼20 kDa) and small (∼12 kDa) subunits, forming the α2β2 heterotetrameric structure, which is known as the active form. CASP8 is initially cleaved at one of two aspartic acid residues (Asp-374 or Asp-384) between its large and small subunits, followed by cleavage at one of three sites (Asp-210, Asp-216, or Asp-223) between the large subunit and the prodomain (Figure A). Active CASP8 cleaves and activates downstream executioner caspases such as caspase 3, ultimately leading to apoptosis and cell death. To design heat-activated CASP8, we fused ELP with the catalytic domain of CASP8 (Figure B). At temperatures below the phase transition temperature (T < T t), ELP remains in a soluble state, preventing CASP8 activation. When the temperature rises above the phase transition temperature (T > T t), ELP undergoes coacervation, bringing CASP8 molecules into close proximity. This may promote CASP8 activation through increased local concentration within condensates, potentially facilitating dimerization-triggered autocleavage of CASP8. Activated CASP8 is cleaved from ELP coacervates and then initiates programmed cell death (Figure C).
1.
Design of heat-activated human CASP8. (A) Schematic representation of the domain structure of human CASP8 (adapted with permission under a Creative Commons CC-BY License from ref . Copyright 2010 Elsevier). CASP8 consists of a prodomain containing two death effector domains (DEDs) and a catalytic domain composed of large and small subunits. Upon activation, CASP8 is first cleaved at one of two aspartic acid residues (Asp-374 or Asp-384) between the large and small subunits, followed by cleavage at one of three sites (Asp-210, Asp-216, or Asp-223) between the large subunit and the prodomain. The cleaved aspartic acid sites are denoted in bold and underlined. (B) Design of ELP-CASP8 fusion. The catalytic domain of CASP8 is fused with a thermal-responsive ELP. (C) Working mechanism of heat-activated human CASP8. When the temperature (T) exceeds the phase transition temperature (T t), ELP undergoes coacervation, bringing CASP8 molecules into close proximity. The resulting increase in local concentration of CASP8 may promote dimerization-triggered autocleavage, leading to the initiation of programmed cell death. Illustration from NIH BIOART Source (https://bioart.niaid.nih.gov/bioart/71).
Construction and Characterization of Heat-Activated Human CASP8
We designed our thermogenetic system to be activated at a temperature just a few degrees Celsius (°C) above body temperature (37 °C), allowing the system to be triggered by mild heating. The T t of an ELP can be adjusted by modifying either its composition or the length of its penta-repeating unit. However, fusing ELP with other proteins alters the T t compared with that of the unfused ELP, which has been known as the “fusion ΔT t effect” (fusion ΔT t = T t (ELP fusion protein) – T t (free ELP)). This effect arises from changes in conformational stability and the hydration shell surrounding the ELP when fused with other proteins. To construct heat-activated human CASP8, we selected ELP[V60], as a previous study demonstrated that ELP[V60] fused with a green fluorescent protein (GFP) exhibited an intracellular T t of 40 °C in HEK293 cells. Additionally, since the molecular weight of GFP (∼27 kDa) is comparable to that of the catalytic domain of CASP8 (∼32 kDa), we anticipated that the ELP[V60]-CASP8 fusion would exhibit a similar T t of approximately 40 °C. We then constructed an ELP[V60]-CASP8 fusion protein (Figure A). As a control, we introduced a C360A mutation to inactivate the enzymatic function of CASP8 (details of the amino acid and DNA sequences of ELP[V60]-CASP8 and ELP[V60]-CASP8_C360A are provided in Figures S1A,B and S2.
2.
Construction and characterization of heat-activated human CASP8. (A) Gene design. ELP[V60] was fused with a CASP8 molecule. For control, we introduced a mutation of C360A of CASP8 to inactivate the catalytic function of CASP8. (B) SDS-PAGE of ELP[V60]-CASP8_C360A (molecular weight, M w = 60.7 kDa including 6xHis-tag). (C) Temperature-dependent turbidity of ELP[V60]-CASP8_C360A. A plot of optical density (OD) at 350 nm against temperature in the PBS buffer. (D) Temperature-dependent hydrodynamic radius (R h) of ELP[V60]-CASP8_C360A in PBS buffer. The concentration was 10 μM for (C,D). Data are mean ± standard deviation (SD; n = 3).
We expressed ELP[V60]-CASP8 and ELP[V60]-CASP8_C360A inEscherichia coli (E. coli) to produce recombinant proteins. However, we observed that E. coli expressing ELP[V60]-CASP8 began to die after expression induction, suggesting that CASP8 was activated, leading to the death of the host cells. The observed phenomenon likely results from the responsiveness of ELP not only to heat but also to other factors, such as high protein concentration and molecular crowding within the E. coli environment, which could have induced the coacervation of ELP and promoted CASP8 activation. In contrast, the control ELP[V60]-CASP8_C360A was successfully purified to the correct size (Figure B), indicating that the mutation prevented unwanted activation during expression. These results support the hypothesis that CASP8 in the ELP[V60]-CASP8 fusion is activated during protein expression in E. coli, leading to host cell death.
We characterized the temperature response of the heat-activated CASP8 system. Because the ELP[V60]-CASP8_C360A control contained only a single mutation, its transition temperature was expected to be similar to that of ELP[V60]-CASP8. We examined the temperature-dependent turbidity of the ELP[V60]-CASP8_C360A control (Figure C), and the results showed that it underwent a phase transition from 35 to 55 °C, with a T t of 43.6 °C in phosphate-buffered saline (PBS) solution. To mimic the molecular crowding environment in mammalian cells, we used a PBS solution containing 14% w/w Ficoll PM70 and observed a similar response with a slight decrease in T t to 41.8 °C (Figure S3). Temperature-dependent hydrodynamic radius (R h) measurements showed a phase transition from 35 to 55 °C, with a T t of 48.7 °C (Figure D). Additionally, we evaluated the concentration-dependent turbidity of ELP[V60]-CASP8_C360A (Figure S4). Optical density at 350 nm increased with protein concentrations. At 1 and 5 μM, the T t values were 43.8 °C and 43.4 °C, respectively, which are comparable to that at 10 μM (Figure C). However, at 20 μM, the phase transition shifted to a lower range of 30–50 °C, with a T t of 39.8 °C. These results suggest that the activation temperature of the system is influenced by the expression levels and can be tuned within a temperature range from 30 to 55 °C, allowing precise control of CASP8 activation through mild temperature shifts.
Development of a CASP8 Indicator
To monitor the activation of CASP8, we developed a CASP8 indicator. The indicator was designed by fusing the red fluorescent protein mCherry with a CASP8 substrate (IETD) and a triple nucleus export signal (3xNES) (Figures A and S1C for details of the amino acid sequence). When expressed in cells, CASP8 indicator is localized in the cytoplasm. Upon activation of CASP8, the active enzyme cleaves mCherry from the 3xNES, allowing mCherry to translocate into the nucleus (Figure B). We examined the functionality of CASP8 indicator in HEK293T cells. To track apoptosis, we used Annexin XII conjugated to a polarity-sensitive green fluorescent dye that fluoresces only when bound to the outer membrane of apoptotic cells. In untreated HEK293T cells, we observed a red fluorescence signal in the cytoplasm without green fluorescence in the Annexin XII channel (Figure C), indicating the absence of CASP8 activation or healthy cell conditions. To induce apoptosis, we treated the cells with the apoptosis-inducing drug staurosporine. After 5 h of treatment, we observed an increase in red fluorescence in the nucleus (Figure D) and green fluorescence on the membrane of the Annexin XII channel (Figure C), indicating CASP8 activation and cell apoptosis. Our results demonstrated that the CASP8 indicator functions effectively in HEK293T cells.
3.
Development of CASP8 indicator. (A) Gene design. The red fluorescent protein mCherry is fused with a CASP8 substrate (IETD) and a triple nucleus export signal (3xNES). (B) Schematic illustration of the mechanism. In the absence of CASP8 activation, mCherry resides in the cytoplasm. Upon CASP8 cleavage at the IET/D substrate, mCherry is released and translocated into the nucleus, enabling detection of CASP8 activity. (C) Fluorescence images of mCherry and Annexin XII of HEK293T cells expressing the CASP8 indicator. (D) Relative fluorescence intensity in the nucleus (F/F 0) of four cells exhibiting nucleus translocation of mCherry following treatment with the apoptosis-inducing drug staurosporine (2 μM) at 30 min. Color arrows indicate CASP8-activating cells. Scale bar, 20 μm. Illustrations were created with BioRender.com.
Heat Activation of CASP8 in HEK293T Cells
We performed heat-activated CASP8 system in HEK293T cells. To track the expression of ELP[V60]-CASP8, we coexpressed a blue fluorescent marker, mTagBFP2, along with a self-cleavage peptide, P2A (Figure A). This design allowed us to monitor the expression of ELP[V60]-CASP8 systems without disrupting their phase transition behavior. Additionally, the mTagBFP2 fluorescence signal was used to estimate the intracellular concentration of ELP[V60]-CASP8 and its mutant to be 3.3 ± 2.3 μM and 4.2 ± 4.3 μM, respectively (Figure S5). Protein expression was further confirmed by Western blot analysis (Figure S6). To determine the phase transition temperature in cells, we incubated HEK293T cells expressing mTagBFP2-ELP[V60]-CASP8 or mTagBFP2-ELP[V60]-CASP8_C360A mutant at 30, 35, 37, 39, or 41 °C for 24 h. We tracked apoptosis using Annexin XII and captured 5 × 5 montage fluorescence images of the mTagBFP2 and Annexin XII channels for merging (Figure B). We plotted the fluorescence ratio of Annexin XII/mTagBFP2 against temperature (Figure C) and found that the cells expressing mTagBFP2-ELP[V60]-CASP8 began apoptosis at a temperature above 35 °C, with the effect increasing at higher temperatures. In contrast, cells expressing the mutant exhibited negligible apoptosis under similar conditions. To validate the apoptosis data, we performed a lactate dehydrogenase cytotoxicity assay (see the Materials and Methods section for details). At 30 °C, the cytotoxicity of cells expressing mTagBFP2-ELP[V60]-CASP8 was not significantly different compared with that of cells expressing the mutant (Figure D). However, at temperatures above 35 °C, the cytotoxicity significantly increased with stronger effects at higher temperatures. This supports the apoptosis results shown in Figure C, confirming that the heat-responsive CASP8 system effectively induced cell death at elevated temperatures, whereas the mutant remained inactive. The temperature responsiveness of ELP[V60]-CASP8, as observed through apoptosis and cytotoxicity assays, was consistent with the phase transition temperature determined in vitro (Figures C,D, S3, and S4). Additionally, cytotoxicity was found to correlate with the incubation time with longer exposure, leading to increased cell death (Figure E). It should also be noted that inducing significant cell death required different durations depending on the temperature: 24 h at 35 °C, 18 h at 37 °C, and only 12 h at 39 °C compared with 30 °C (Figure E). Cells expressing the mutant exhibited some cytotoxicity at 41 °C after 18 h (Figure F), which was likely attributable to heat stress. Notably, when HEK293T cells were transfected with mTagBFP2-CASP8 and cultured at 30 °C, we observed significant apoptosis and cytotoxicity (Figure S7), which may be attributed to CASP8 overexpression leading to autoactivation. This result highlights the functional role of the ELP[V60] domain in the ELP[V60]-CASP8 fusion, which prevents CASP8 dimerization and activation at temperatures below the phase transition threshold.
4.
Heat-activated human CASP8 in HEK293T cells. (A) Gene design. A fluorescent marker, mTagBFP2, along with a self-cleavage peptide, P2A, is fused upstream the ELP[V60]-CASP8 (left) or ELP[V60]-CASP8_C360A mutant (right). (B) Fluorescence images of HEK293T cells expressing mTagBFP2-ELP[V60]-CASP8 or mTagBFP2-ELP[V60]-CASP8_C360A mutant with Annexin XII staining at different temperatures for 24 h. The blue color represents mTagBFP2 and the green color represents Annexin XII signals. (C) A graph of the fluorescence ratio of Annexin XII/mTagBFP2 against temperature after 24 h of incubation. (D) Cytotoxicity assay of HEK293T cells expressing mTagBFP2-ELP[V60]-CASP8 or mTagBFP2-ELP[V60]-CASP8_C360A mutant at different temperatures for 24 h. Cell cytotoxicity at different times of HEK293T cells expressing (E) mTagBFP2-ELP[V60]-CASP8 or (F) mTagBFP2-ELP[V60]-CASP8_C360A mutant. ns indicates no significant difference, while * (p < 0.05), ** (p < 0.01), and *** (p < 0.001) denote statistically significant differences, as determined using a two-tailed Student’s t-test. Data show mean ± SD (n = 5). Bright field (BF) and fluorescence images of HEK293T cells coexpressing the CASP8 indicator and (G) mTagBFP2-ELP[V60]-CASP8 or (H) mTagBFP2-ELP[V60]-CASP8_C360A mutant. Time-lapse imaging of HEK293T cells coexpressing the CASP8 indicator and (I) mTagBFP2-ELP[V60]-CASP8 or (K) mTagBFP2-ELP[V60]-CASP8_C360A mutant at 30 and 39 °C. Arrows indicate CASP8-activating cells. Scale bar, 200 μm for panel (B) and 50 μm for panels (G,H,I,K).
We visualized the heat-activated human CASP8 in real time. We cotransfected HEK293T cells with the CASP8 indicator and mTagBFP2-ELP[V60]-CASP8 or mTagBFP2-ELP[V60]-CASP8_C360A mutant and confirmed their coexpression (Figure G,H). To prevent premature activation of the ELP[V60]-CASP8 system, we cultured the cells at 30 °C after transfection. Additionally, to avoid phototoxicity caused by UV excitation, we did not capture mTagBFP2 images during time-lapse imaging. To activate the system, we heated the medium to 39 °C using a stage-top incubator. As shown in Figure I, at 30 °C, the CASP8 indicator remained in the cytoplasm, and no green fluorescence signal from Annexin XII was detected, indicating that the CASP8 was not activated, and the cells were healthy. Upon heating the medium to 39 °C for over 4 h, we observed cell apoptosis, indicated by the Annexin XII fluorescence signal on the cell membrane, as well as the translocation of the CASP8 indicator into the nucleus (Figure I and see Movie S1 for details). This result demonstrated that CASP8 was activated by heating, leading to apoptosis and cell death. For cells expressing the mutant, we observed negligible CASP8 activation or cell apoptosis upon heating to 39 °C (Figure K and Movie S2). Similar results were also observed in HeLa cells, confirming the broader applicability of heat-activated human CASP8 system (, Movies S3 and S4).
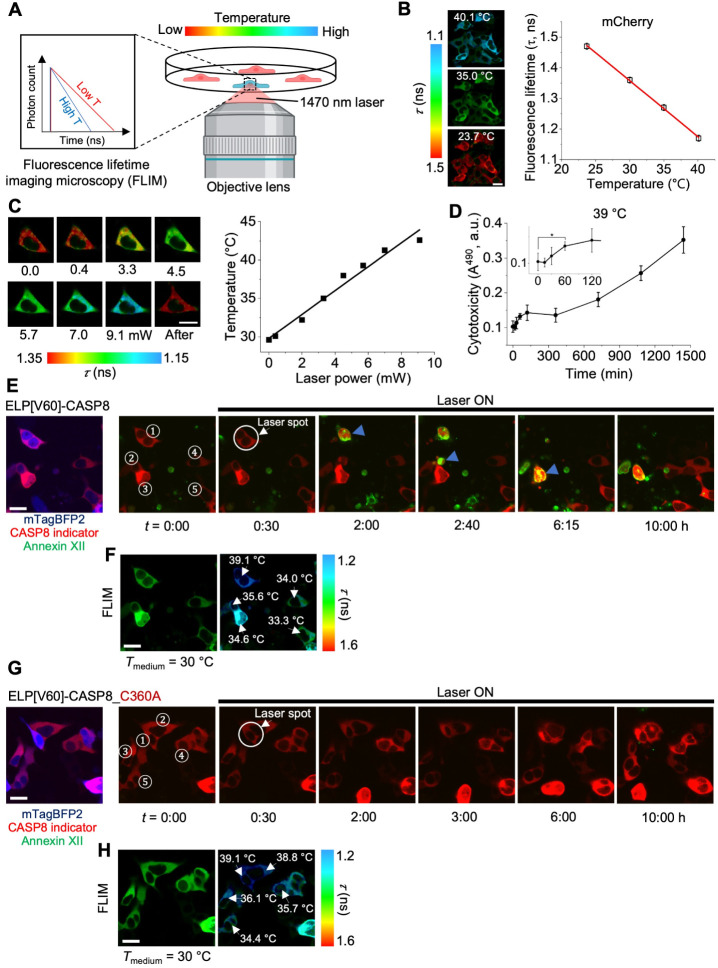
Local Heat-Activated CASP8 at Single Cells Using Optical Heating
We performed local heat activation of CASP8 in single-target cells using optical heating. To achieve precise local heating, a single cell was illuminated with a 1470 nm laser (Figure A), a well-established method in the IR-LEGO system. To quantify the temperature increase accurately, we used fluorescence lifetime imaging microscopy (FLIM) to measure the fluorescence lifetime of mCherry using the CASP8 indicator. The fluorescence lifetime of mCherry was calibrated against the temperature of the medium (Figure B) and subsequently used to determine the temperature increment within the cells upon local heating. This approach enabled the highly localized and controlled heat activation of CASP8 at the single-cell level. We demonstrated local heating by varying the 1470 nm laser power, and the temperature in the cells was quantified using FLIM-based thermometry. We observed a positive correlation between the temperature increment and the 1470 nm laser power (Figure C). We also investigated the heating duration required to induce CASP8 activation by incubating HEK293T cells expressing mTagBFP2-ELP[V60]-CASP8 at 39 °C for varying durations and measuring cytotoxicity (see Methods for details). Significant cytotoxicity was observed after 60 min of incubation at 39 °C compared with cells incubated at 30 °C (t = 0) (Figure D).
5.
Local heat activation of human CASP8 at single cells. (A) Schematic illustration of the optical heating using 1470 nm laser combined with FLIM-based thermometry for precise temperature control and measurement in single cells. (B) Calibration of fluorescence lifetime of mCherry in the CASP8 indicator as a function of medium temperature. The temperature sensitivity of mCherry was determined to be 0.018 ns/°C, which is based on the slope of the linear fitting curve. (C) Optical heating of single HEK293T cells expressing the CASP8 indicator. The cell temperature was calculated using the calibration curve shown in panel (B) and plotted as a function of the 1470 nm laser power. (D) Cytotoxicity of HEK293T cells expressing mTagBFP2-ELP[V60]-CASP8 after incubation at 39 °C for various durations. The inset shows an enlarged view from 0 to 120 min. The * denotes statistically significant differences (p < 0.05), as determined using two-tailed Student’s t-test. Data show mean ± SD (n = 5). (E) Optical heating of HEK293T cells coexpressing mTagBFP2-ELP[V60]-CASP8 and the CASP8 indicator, with corresponding FLIM images shown in (F). Blue arrows indicate CASP8-activating cells. (G) Optical heating of HEK293T cells coexpressing mTagBFP2-ELP[V60]-CASP8_C360A mutant and the CASP8 indicator, with its FLIM images shown in (H). Cells were maintained at 30 °C under 5% CO2. Color bars indicate fluorescence lifetime (τ). Scale bars, 20 μm. Illustrations were created with BioRender.com.
We next demonstrated localized heat activation at the single-cell level. We cotransfected HEK293T cells with mTagBFP2-ELP[V60]-CASP8 and the CASP8 indicator, and confirmed successful coexpression (Figure E). We locally heated up the target cells while quantifying the temperature via FLIM-based thermometry (Figure F). The 1470 nm laser was adjusted to reach a target temperature of 39.1 °C in cell 1, while neighboring cells 2, 3, 4, and 5 exhibited temperatures of 35.6, 34.6, 34.0, and 33.3 °C, respectively. After a 30 min preincubation, the 1470 nm laser was turned on, and time-lapse imaging was carried out for 10 h. We observed that cell 1 showed CASP8 activation and apoptosis within 2 h, as indicated via the CASP8 indicator and Annexin XII signal. Notably, cells 2 and 3, which were exposed to slightly lower temperatures, underwent delayed CASP8 activation and apoptosis at 2:40 h and 6:15 h, respectively. In contrast, cells 4 and 5 remained viable throughout the 10 h period (Figure E and Movie S5). Additionally, we carried out the control experiment using mTagBFP2-ELP[V60]-CASP8_C360A mutant (Figure G). We observed no cell death under similar heating condition (Figure H and Movie S6), confirming that activation was specific to the functional CASP8 domain. Our results demonstrated the temperature sensitivity and spatial precision of the ELP[V60]-CASP8 system for regulating programmed cell death.
Discussion
We exploited the unique phase transition behavior of ELPs to develop a thermogenetic tool for the activation of human CASP8 through temperature elevation. By fusing ELP[V60] with CASP8, we harnessed the reversible phase transition of ELPs, which shifted from a soluble to a coacervate state at specific temperatures to induce CASP8 dimerization and activation. Our results demonstrated that the ELP[V60]-CASP8 system effectively induced apoptosis in a temperature-dependent manner, as demonstrated using apoptosis detection (Figure C) and cytotoxicity assays (Figure D). Moreover, the system exhibited time-dependent activation with prolonged heating, resulting in increased cytotoxicity (Figures E and D). Control experiments using a CASP8-inactivating mutant showed negligible apoptosis and cytotoxicity under similar heating conditions (Figure C,D,F), confirming the specificity of heat-activated human CASP8 in the ELP[V60]-CASP8 system. We successfully achieved the localized activation of the CASP8 system in targeted single cells using optical heating (Figure E,F). Our results highlight the potential of the ELP[V60]-CASP8 system as a powerful tool for precise induction of temperature-controlled apoptosis.
The ELP[V60]-CASP8 system effectively functions at a working temperature starting from 35 °C, with apoptosis and cytotoxicity occurring at the physiological temperature of 37 °C. However, the activation temperature of the ELP-CASP8 system can be adjusted by modifying the length or composition of the ELPs. More hydrophobic guest residues (X) or a greater number of repeats (n) in the repeating pentapeptide sequence (VPGXG)n result in a lower T t, whereas less hydrophobic residues or fewer repeats lead to an increase in T t. For example, shortening the length of the ELPs from ELP[V60] to ELP[V48] raised the intracellular T t in fusion with GFP from 40 °C for GFP-ELP[V60] to 45 °C for GFP-ELP[V48]. Conversely, increasing the length to ELP[V96] lowered the intracellular T t of GFP-ELP[V96] to 24 °C.23 This tunability enables control of the working temperature of the system for various applications across different species with varying physiological temperatures. T t of ELPs can be influenced by fusion with other proteins. To monitor the expression of ELP[V60]-CASP8, we incorporated mTagBFP2 along with a P2A self-cleaving peptide (Figure A) to ensure that the fluorescent marker did not interfere with the phase transition behavior of the ELP-CASP8 system. Additionally, factors such as self-concentration (Figure S4), molecular crowding, and ionic strength can also affect the T t of ELPs. Therefore, careful optimization of experimental conditions and appropriate control experiments are essential to ensure the reliable performance of ELP-based thermogenetic tools.
ELPs have been utilized to develop tools for controlling various cellular functions, including clustering of the epidermal growth factor receptor (EGFR) to modulate signaling pathway, and regulation of endocytosis by fusing ELPs with proteins such as dynamin, clathrin, or caveolin. Our ELP[V60]-CASP8 system expands the utility of ELP-triggered assembly by enabling direct control of enzymatic activity of CASP8. This represents a functional application of thermally responsive phase behavior for inducing programmed cell death.
The use of optical heating provides significant advantages over conventional heating methods, such as stage-top incubators. When cells were heated to ∼39 °C, optical heating (Figure E) enabled selective activation of CASP8 in a single target cell with approximately half the duration required by stage-top incubation (Figure I), demonstrating the superior temporal precision and spatial selectivity of the optical approach.
We leveraged FLIM-based thermometry to precisely quantify the temperature increase in cells upon irradiation with a 1470 nm laser. Compared with fluorescence intensity-based thermometry, FLIM-based thermometry offers several advantages, as it is independent of factors such as expression levels, excitation intensity, photobleaching, focus drift, and cell shape. , When the cells were irradiated with the 1470 nm laser, we observed drastic changes in cell morphology. Despite these changes, we could precisely quantify the temperature using FLIM (Figure C), demonstrating its superiority and robustness in cell imaging applications. Using mCherry in the CASP8 indicator, we achieved a temperature sensitivity of 0.018 ns/°C. This temperature sensitivity could be further improved by replacing mCherry with other fluorescent proteins that exhibit higher temperature sensitivity, such as Sirius or tdTomato, enabling more precise temperature measurements. Our results emphasized the advantages of FLIM-based thermometry for experiments requiring accurate and reliable temperature measurements under dynamic cellular conditions.
The activation of human CASP8 can be controlled using different strategies. A chemogenetic approach has been developed by fusing CASP8 with FKBP. Upon introduction of the AP20187 drug, FKBP-CASP8 underwent dimerization, leading to CASP8 activation. Although the FKBP-CASP8 system has been successfully demonstrated in HeLa cells, achieving spatial control of activation in target cells poses a significant challenge. However, the use of photocaged rapamycin would introduce a solution for the local control of CASP8 activation through light-mediated release of the chemical inducer. In contrast, the optogenetic approach enables direct control of CASP8 activation upon light irradiation. By fusing CASP8 with the photosensitive protein Cryptochrome-2, which undergoes homo-oligomerization upon exposure to blue light, light-activatable human CASP8 systems were developed. Despite these advantages, optogenetics often has poor depth penetration owing to light scattering, which necessitates high light intensities for tissue or in vivo specimens, leading to phototoxicity and unintended activation of endogenous light-sensitive systems, particularly by visible light in the blue region. Although efforts have been made to develop red and near-infrared optogenetics, thermogenetic approaches offer alternatives to overcome these limitations. By using temperature-responsive ELPs as a motif to drive CASP8 activation, the ELP-CASP8 system offers precise control of CASP8 activation through mild temperature increases using optical heating methods such as IR-mediated heat production.
Deep tissue heating technologies are essential for therapeutic applications. Although IR-based optical heating offers high spatial and temporal precision, its effectiveness in deep tissues is limited by light scattering, posing a challenge for clinical translation. To overcome this limitation, the ELP-CASP8 system can be integrated with advanced heating technologies such as focused ultrasound and magnetic hyperthermia. Focused ultrasound has been used to activate chimeric antigen receptor (CAR) T cells in vivo, demonstrating its potential for precise thermal modulation in therapeutic applications. Similarly, magnetic hyperthermia, which is currently under clinical investigation, uses magnetic nanoparticles (e.g., iron oxide) to generate heat under an alternating magnetic field, thereby inducing cell death. However, targeting nanoparticles to specific subcellular compartments remains a significant challenge. In contrast, the genetically encoded ELP-CASP8 system can be readily localized to specific organelles by fusing it with targeting peptides or proteins, offering a distinct advantage for intracellular control. A combination of focused ultrasound or magnetic hyperthermia with the ELP-CASP8 system could present a promising strategy for therapeutic applications by integrating deep tissue heating with genetically programmable apoptotic control.
Deep tissue penetration can also be achieved using magnetogenetics, an emerging technique that employs external magnetic fields to modulate cellular functions. One approach, known as magneto-thermal-genetics, uses magnetic nanoparticles to generate localized heat under an alternating magnetic field, thereby activating thermally responsive ion channels such as TRPV1. , Another method, referred to magneto-mechanical-genetics, uses torque-generating magnetic nanoparticle (m-Torquer) to apply mechanical forces that stimulate mechanosensitive ion channels, such as Piezo1. , While magnetogenetics has been successfully demonstrated in mammalian cells and in vivo, its applications have largely been limited to the modulation of ion channels and membrane receptors. However, a recent report on the magnetic field responsiveness of fluorescent protein mScarlet3 suggests a promising direction for extending magnetogenetic strategies toward direct control of intracellular protein function.
Expanding the newly developed field of thermogenetics requires significant efforts, as it involves both the development of new thermosensitive biomolecules and heating/cooling technologies. TRP channels have been extensively used for the thermogenetic control of neural system regulation via IR irradiation. , In addition to ion channel regulation, temperature-sensitive proteins such as Tlpa , and BcLOV4 have been used to modulate cellular protein functions by lowering temperature. If CASP8 was fused with Tlpa or BcLOV4, it could potentially allow for temperature-dependent control of CASP8 activation, where CASP8 remains active at lower temperatures and is inhibited when the temperature increases. This inverse thermogenetic control may provide a new approach to regulating apoptosis. Although heating technologies for thermogenetics are well-established, implementing precise cooling strategies to achieve controlled temperature reduction remains a challenge. Recent advancements in laser-cooling systems , offer promising directions for refining cooling-based thermogenetic control in biological applications. Additionally, integrating thermogenetics with existing tools, such as magnetogenetics, optogenetics, and chemogenetics, could lead to multimodal control systems, expanding the versatility and precision of temperature-mediated protein regulation across various biological applications.
Conclusion
We successfully developed ELP[V60]-CASP8 system as a thermogenetic tool for temperature-controlled program cell death. Our results demonstrated that the ELP[V60]-CASP8 system effectively induced apoptosis in a temperature- and time-dependent manner. This system provides a versatile platform for expanding the thermogenetic toolkit beyond the regulation of apoptosis. By replacing CASP8 with other biomolecules, additional thermogenetic tools can be developed to modulate various cellular functions, such as enzyme activity, protein–protein interactions, and gene expression. This approach opens new avenues for the temperature-mediated control of biological processes, offering broad applications in biotechnology and biomedical research.
Materials and Methods
Gene Construction
We purchased plasmids encoding ELP[V60] containing the sequence (VPGVG)60 (plasmid #67013), human caspase 8 (plasmid #11817), mCherry (plasmid #124429), and mTagBFP2 (plasmid #55327) from Addgene. For the caspase 8 indicator, we used overlapping PCR to amplify the gene encoding mCherry fused with the caspase 8 substrate sequence IETD and a triple nuclear export signal (3xNES, LALKLAGLDIGS) at the C-terminus. The resulting PCR product, mCherry-IETD-3xNES, was inserted into the pcDNA3.1(−) vector (Invitrogen) for mammalian expression. We amplified the gene encoding ELP[V60] using BamHI and EcoRI, and human CASP8 using EcoRI and HindIII, then fused and cloned these fragments into the pRSETA vector (Invitrogen) for bacterial expression. For mammalian cell expression, we amplified ELP[V60]-CASP8 and fused it upstream with the fluorescent marker mTagBFP2 using a P2A self-cleaving peptide. This construct was cloned into the pcDNA3.1(−) vector. As a control, we introduced a C360A mutation in CASP8 to inactivate its catalytic function. All gene constructions were transformed into DH5α E. coli competent cells and cultured in Luria–Bertani (LB) broth medium containing 100 μg/mL carbenicillin (C1389, Sigma-Aldrich) for 10–12 h at 37 °C, followed by plasmid purification.
Protein Purification
We transformed E. coli strain JM109 (DE3) (P9801, Promega) with pRSETA plasmids encoding ELP[V60]-CASP8 or ELP[V60]-CASP8_C360A. The bacteria were cultured at 20 °C in 200 mL of LB medium supplemented with 100 μg/mL ampicillin (19769-64, NACALAI TESQUE), with gentle shaking at 120 rpm. After 3 days of incubation, the bacteria were harvested, and protein purification was carried out according to the procedure described previously.
Turbidity Tests
We measured turbidity using a V-650 UV–vis Spectrophotometer (JASCO). A protein solution at a concentration of 10 μM in PBS buffer or PBS supplemented with 14% w/w Ficoll PM70 (F2878, Sigma-Aldrich) was placed into a quartz cuvette with a path length of 1 cm. Optical density (OD) at 350 nm was recorded as a measure of turbidity across a temperature range from 25 to 60 °C, with measurements taken at 5 °C intervals. The phase transition temperature (T t ) was identified as the temperature at which the largest turbidity gradient occurred.
Dynamic Light Scattering (DLS)
We conducted DLS characterization using a Zetasizer Nano-ZSP (Malvern). A protein solution with a concentration of 10 μM in PBS buffer was introduced into a PMMA cuvette (DTS0012). We collected DLS data at a scattering angle of 173° over a temperature range of 25 to 60 °C with 5 °C intervals. Each temperature point was equilibrated for 480 s prior to measurement. The duration of each measurement was set to automatic mode. We performed the DLS measurements three times, and the results are reported as the mean ± standard deviation (SD).
Cell Culture and Transfection
We cultured HEK293T cells in Dulbecco’s Modified Eagle Medium (DMEM, 11965092, Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (FBS; 173012, Sigma-Aldrich) and 1% penicillin-streptomycin (PS, 09367–34, NACALAI TESQUE) at 37 °C in a 5% CO2 incubator. Cells were seeded onto glass bottom dishes and allowed to grow until reaching 60–70% confluence. For transfection, we prepared a mixture of 2.0 μg of plasmids and 5.0 μg of polyethylenimine MAX (PEI, 24765–1, Polysciences) in 200 μL of Opti-MEM reduced serum medium (31985062, Thermo Fisher Scientific), and allowed it to incubate for 20 min at room temperature. This mixture was then added to 1 mL of the culture medium containing the HEK293T cells and incubated at 30 °C in a 5% CO2 incubator. After 6 h post-transfection, the cells were washed with PBS, and the medium was exchanged with fresh DMEM. The cells were further incubated for 24–48 h at 30 °C in a 5% CO2 incubator, after which the medium was exchanged to DMEM/F12 (11039–021, Thermo Fisher Scientific) without phenol red prior to microscopy observation.
Cell Imaging
We conducted cell imaging experiments using an FV1200 confocal microscope (Olympus) equipped with an oil immersion objective lens (PLAPON 60×, NA = 1.42, Olympus) and a stage-top incubator (STXG-WSKMX, Tokai Heat) with 5% CO2 supplied. For apoptosis detection, we utilized an Annexin XII-based polarity-sensitive probe (Kinetic Apoptosis Kit, ab129817, Abcam) supplemented with 2 mM CaCl2 in DMEM/F12 medium. For imaging mTagBFP2, we utilized a 405 nm laser for excitation and captured the fluorescence emission through BA430–455 filter. For the Annexin XII channel, excitation was performed using a 488 nm laser and emissions were collected through BA490–540 filter. For the mCherry channel, we used a 559 nm laser for excitation and collected fluorescence through BA575–675 filter. The dichroic mirror was DM405/473/559/635 nm. Images were captured with a scanning speed of 1.109 s/frame at a resolution of 512 × 512 pixels.
Cytotoxicity Assay
We seeded approximate 8.5 × 10 HEK293T cells in 100 μL of DMEM supplemented with 10% FBS and 1% P/S in a CellView cell culture slide (543978, Greiner Bio-One). The following day, the cells were transfected with 0.5 μg of plasmids and 1.25 μg of PEI. After 6 h post-transfection, the cells were washed with PBS and the medium was replaced with DMEM/F12 supplemented with 10% FBS and 1% P/S. The cells were incubated at 30 °C for 1 day to allow protein expression. Subsequently, the cells were exposed to 30, 35, 37, 39, or 41 °C in a 5% CO2 incubator for 12, 18, and 24 h. At each time point, 10 μL of the medium was collected, and cytotoxicity was assessed using the Cytotoxicity LDH Assay Kit-WST (CK12, DOJINDO). Absorbance was measured at 490 nm using a plate reader (SpectraMax ABS Plus, Molecular Devices)
To investigate the time dependence of CASP8 activation, HEK293T cells expressing mTagBFP2-ELP[V60]-CASP8 were incubated at 39 °C for various durations. After the heating period, the cells were returned to 30 °C and maintained in a 5% CO2 incubator for an additional 24 h. Cytotoxicity was then measured using the LDH assay. As a control for t = 0, cells were kept at 30 °C without heating.
Optical Heating and Fluorescence Lifetime Imaging Microscopy (FLIM)
In the FV1200 confocal microscope, we installed an IR 1470 nm laser (MDL-MD-1470–2W, Changchun New Industries Optoelectronics Tech, China) to locally heat target cells. The dichroic mirror was 690/808/855/1030 nm. The 1470 nm laser power at the sample position through a 60× objective lens was measured using a photo power meter (Vega, Ophir Photonics) coupled with a 10A-V1.1-SH sensor (Ophir Photonics).
FLIM measurements were also performed on the same FV1200 confocal microscope, which was equipped with a rapidFLIMHiRes and a MultiHarp 150 Time-Correlated Single Photon Counting (TCSPC) unit from PicoQuant. We excited mCherry with a 560 nm pulsed laser (PicoQuant) and collected fluorescence emission through a 600/50 nm bandpass filter (Semrock). Fluorescence lifetime values were calculated using SymPhoTime 64 software (PicoQuant). The fluorescence lifetime decay histograms were fitted with a two-exponential function, with the arrival time fixed within the 0–8 ns range starting from the pulsed laser.
Supplementary Material
Acknowledgments
We thank Assoc. Prof. Takeshi Shimi and Dr. Yohei Kono (WPI-NanoLSI, Kanazawa University, Japan) for their assistance with the Western blot experiments, Prof. Hang Thu Ta (Griffith University, Australia) for her suggestions, Ms. Nahoko Shimizu (WPI-NanoLSI, Kanazawa University, Japan) for helping with protein purification, and Ms. Loan Thi Ngoc Nguyen (WPI-NanoLSI, Kanazawa University, Japan) for SDS-PAGE.
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsnano.5c07332.
Heat activation of HEK293T cells expressing mTagBFP2-ELP[V60]-CASP8 (AVI)
Heat activation of HEK293T cells expressing the mutant (AVI)
Heat activation of HeLa cells expressing mTagBFP2-ELP[V60]-CASP8 (AVI)
Heat activation of HeLa cells expressing the mutant (AVI)
Optical heating of HEK293T cells expressing the mTagBFP2-ELP[V60]-CASP8 system (AVI)
Optical heating of HEK293T cells expressing the mutant (AVI)
The amino acid sequence of ELP[V60]-CASP8, ELP[V60]-CASP8_C360A, and the CASP8 indicator (Figure S1); DNA map and sequence of mTagBFP2-ELP[V60]-CASP8 (Figure S2); temperature-dependent turbidity of ELP[V60]-CASP8_C360A in PBS + 14% w/w Ficoll PM70 (Figure S3); temperature-dependent turbidity of ELP[V60]-CASP8_C360A in PBS at different concentrations (Figure S4); estimation of protein concentration of ELP[V60]-CASP8 and its mutant expressed in HEK293T cells (Figure S5); Western blotting of HEK293T cells expressing ELP[V60]-CASP8 and its mutant (Figure S6); expression of CASP8 in HEK293T cells (Figure S7); heat-activated human CASP8 in HeLa cells (Figure S8) (PDF)
C.Q.V. and S.A. conceived and coordinated the project. C.Q.V. designed and conducted the experiments. C.Q.V. wrote the original manuscript. C.Q.V. and S.A. reviewed and edited the manuscript. All authors have read and agreed to the final version of the manuscript.
This work was supported by a grant-in-aid from the Ministry of Education, Culture, Sports, Science and Technology, Japan (22K20529 to C.Q.V.), the WPI-NanoLSI Transdisciplinary Research Promotion Grant, Kanazawa University to C.Q.V. in the fiscal years 2022–2023 and 2023–2024, the JST FOREST Program (JPMJFR201E to S.A.), and the World Premier International Research Center Initiative (WPI), MEXT.
The authors declare no competing financial interest.
References
- Oberst A., Pop C., Tremblay A. G., Blais V., Denault J.-B., Salvesen G. S., Green D. R.. Inducible Dimerization and Inducible Cleavage Reveal a Requirement for Both Processes in Caspase-8 Activation*. J. Biol. Chem. 2010;285(22):16632–16642. doi: 10.1074/jbc.M109.095083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V., Thompson K. R., Deisseroth K.. eNpHR: a Natronomonas halorhodopsin enhanced for optogenetic applications. Brain Cell Biol. 2008;36(1–4):129–139. doi: 10.1007/s11068-008-9027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G., Szellas T., Huhn W., Kateriya S., Adeishvili N., Berthold P., Ollig D., Hegemann P., Bamberg E.. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Int. Acad. Sci. 2003;100(24):13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntas G., Hallett R. A., Zimmerman S. P., Williams T., Yumerefendi H., Bear J. E., Kuhlman B.. Engineering an improved light-induced dimer (iLID) for controlling the localization and activity of signaling proteins. Proc. Natl. Acad. Sci. U. S. A. 2015;112(1):112–117. doi: 10.1073/pnas.1417910112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shkarina K., Hasel de Carvalho E., Santos J. C., Ramos S., Leptin M., Broz P.. Optogenetic activators of apoptosis, necroptosis, and pyroptosis. J. Cell Biol. 2022;221(6):e202109038. doi: 10.1083/jcb.202109038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano F., Suzuki H., Furuya A., Sato M.. Engineered pairs of distinct photoswitches for optogenetic control of cellular proteins. Nat. Commun. 2015;6(1):6256. doi: 10.1038/ncomms7256. [DOI] [PubMed] [Google Scholar]
- Ratnayake K., Payton J. L., Lakmal O. H., Karunarathne A.. Blue light excited retinal intercepts cellular signaling. Sci. Rep. 2018;8(1):10207. doi: 10.1038/s41598-018-28254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermakova Y. G., Roshchin M. V., Lanin A. A., Balaban P. M., Zheltikov A. M., Belousov V. V., Nikitin E. S.. Thermogenetics as a New Direction in Controlling the Activity of Neural Networks. Neurosci. Behav. Physiol. 2020;50(8):1018–1023. doi: 10.1007/s11055-020-01001-1. [DOI] [Google Scholar]
- Chen Z., Li J., Zheng Y.. Heat-Mediated Optical Manipulation. Chem. Rev. 2022;122(3):3122–3179. doi: 10.1021/acs.chemrev.1c00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein J. G., Garrity P. A., Boyden E. S.. Optogenetics and thermogenetics: technologies for controlling the activity of targeted cells within intact neural circuits. Curr. Opin. Neurobiol. 2012;22(1):61–71. doi: 10.1016/j.conb.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath D. E., Stowers J. R., Hörmann D., Poehlmann A., Dickson B. J., Straw A. D.. FlyMAD: rapid thermogenetic control of neuronal activity in freely walking Drosophila. Nat. Methods. 2014;11(7):756–762. doi: 10.1038/nmeth.2973. [DOI] [PubMed] [Google Scholar]
- Ermakova Y. G., Lanin A. A., Fedotov I. V., Roshchin M., Kelmanson I. V., Kulik D., Bogdanova Y. A., Shokhina A. G., Bilan D. S., Staroverov D. B.. et al. Thermogenetic neurostimulation with single-cell resolution. Nat. Commun. 2017;8:15362. doi: 10.1038/ncomms15362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A., Salari A., Berigan B. R., Miguel K. C., Amirshenava M., Robinson A., Zars B. C., Lin J. L., Milescu L. S., Milescu M.. et al. The Drosophila Gr28bD product is a non-specific cation channel that can be used as a novel thermogenetic tool. Sci. Rep. 2018;8(1):901. doi: 10.1038/s41598-017-19065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee W. K. D., Yeoh J. W., Dao V. L., Poh C. L.. Highly Reversible Tunable Thermal-Repressible Split-T7 RNA Polymerases (Thermal-T7RNAPs) for Dynamic Gene Regulation. ACS Synth. Biol. 2022;11(2):921–937. doi: 10.1021/acssynbio.1c00545. [DOI] [PubMed] [Google Scholar]
- Benman W., Huang Z., Iyengar P., Wilde D., Mumford T. R., Bugaj L. J.. A temperature-inducible protein module for control of mammalian cell fate. Nat. Methods. 2025;22:539–549. doi: 10.1038/s41592-024-02572-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varanko A. K., Su J. C., Chilkoti A.. Elastin-Like Polypeptides for Biomedical Applications. Annu. Rev. Biomed. Eng. 2020;22(1):343–369. doi: 10.1146/annurev-bioeng-092419-061127. [DOI] [PubMed] [Google Scholar]
- MacEwan S. R., Chilkoti A.. Elastin-like polypeptides: biomedical applications of tunable biopolymers. Biopolymers. 2010;94(1):60–77. doi: 10.1002/bip.21327. [DOI] [PubMed] [Google Scholar]
- Kumar S.. Caspase function in programmed cell death. Cell Death Differ. 2007;14(1):32–43. doi: 10.1038/sj.cdd.4402060. [DOI] [PubMed] [Google Scholar]
- Kruidering M., Evan G. I.. Caspase-8 in apoptosis: the beginning of “the end”? IUBMB Life. 2000;50(2):85–90. doi: 10.1080/713803693. [DOI] [PubMed] [Google Scholar]
- Chang D. W., Xing Z., Capacio V. L., Peter M. E., Yang X.. Interdimer processing mechanism of procaspase-8 activation. EMBO J. 2003;22(16):4132–4142. doi: 10.1093/emboj/cdg414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen T., Hassouneh W., Trabbic-Carlson K., Chilkoti A.. Predicting Transition Temperatures of Elastin-Like Polypeptide Fusion Proteins. Biomacromolecules. 2013;14(5):1514–1519. doi: 10.1021/bm400167h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabbic-Carlson K., Meyer D. E., Liu L., Piervincenzi R., Nath N., LaBean T., Chilkoti A.. Effect of protein fusion on the transition temperature of an environmentally responsive elastin-like polypeptide: a role for surface hydrophobicity? Protein Eng., Des. Sel. 2004;17(1):57–66. doi: 10.1093/protein/gzh006. [DOI] [PubMed] [Google Scholar]
- Pastuszka M. K., Janib S. M., Weitzhandler I., Okamoto C. T., Hamm-Alvarez S., MacKay J. A.. A Tunable and Reversible Platform for the Intracellular Formation of Genetically Engineered Protein Microdomains. Biomacromolecules. 2012;13(11):3439–3444. doi: 10.1021/bm301090x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu C. Q., Fukushima S.-I., Wazawa T., Nagai T.. A highly-sensitive genetically encoded temperature indicator exploiting a temperature-responsive elastin-like polypeptide. Sci. Rep. 2021;11(1):16519. doi: 10.1038/s41598-021-96049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Li J., Enterina J. R., Shen Y., Zhang I., Tewson P. H., Mo G. C. H., Zhang J., Quinn A. M., Hughes T. E., Maysinger D., Alford S. C., Zhang Y., Campbell R. E.. Ratiometric biosensors based on dimerization-dependent fluorescent protein exchange. Nat. Methods. 2015;12(3):195–198. doi: 10.1038/nmeth.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong B., Liu M., Bai C., Ruan Y., Wang Y., Qiu L., Hong Y., Wang X., Li L., Li B.. Caspase-8 Induces Lysosome-Associated Cell Death in Cancer Cells. Mol. Ther. 2020;28(4):1078–1091. doi: 10.1016/j.ymthe.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei Y., Suzuki M., Watanabe K., Fujimori K., Kawasaki T., Deguchi T., Yoneda Y., Todo T., Takagi S., Funatsu T., Yuba S.. Infrared laser–mediated gene induction in targeted single cells in vivo. Nat. Methods. 2009;6:79. doi: 10.1038/nmeth.1278. [DOI] [PubMed] [Google Scholar]
- Li Z., Tyrpak D. R., Park M., Okamoto C. T., MacKay J. A.. A new temperature-dependent strategy to modulate the epidermal growth factor receptor. Biomaterials. 2018;183:319–330. doi: 10.1016/j.biomaterials.2018.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila H., Truong A., Tyrpak D., Park S.-J., Lei S., Li Y., Okamoto C., Hamm-Alvarez S., MacKay J. A.. Intracellular Dynamin Elastin-like Polypeptides Assemble into Rodlike, Spherical, and Reticular Dynasomes. Biomacromolecules. 2022;23(1):265–275. doi: 10.1021/acs.biomac.1c01251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastuszka M. K., Okamoto C. T., Hamm-Alvarez S. F., MacKay J. A.. Flipping the Switch on Clathrin-Mediated Endocytosis using Thermally Responsive Protein Microdomains. Adv. Funct. Mater. 2014;24(34):5340–5347. doi: 10.1002/adfm.201400715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrpak D. R., Wang Y., Avila H., Guo H., Fu R., Truong A. T., Park M., Okamoto C. T., Hamm-Alvarez S. F., MacKay J. A.. Caveolin Elastin-Like Polypeptide Fusions Mediate Temperature-Dependent Assembly of Caveolar Microdomains. ACS Biomater. Sci. Eng. 2020;6(1):198–204. doi: 10.1021/acsbiomaterials.9b01331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T., Liu X., Chang Y.-T., Arai S.. Applicability and Limitations of Fluorescence Intensity-Based Thermometry Using a Palette of Organelle Thermometers. Chemosensors. 2023;11(7):375. doi: 10.3390/chemosensors11070375. [DOI] [Google Scholar]
- Vu C. Q., Arai S.. Quantitative Imaging of Genetically Encoded Fluorescence Lifetime Biosensors. Biosensors. 2023;13(10):939. doi: 10.3390/bios13100939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M., Arai Y., Kotera I., Okabe K., Kamei Y., Nagai T.. Genetically encoded ratiometric fluorescent thermometer with wide range and rapid response. PLoS One. 2017;12(2):e0172344. doi: 10.1371/journal.pone.0172344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K., Wazawa T., Sakamoto J., Vu C. Q., Nakano M., Kamei Y., Nagai T.. Intracellular Heat Transfer and Thermal Property Revealed by Kilohertz Temperature Imaging with a Genetically Encoded Nanothermometer. Nano Lett. 2022;22(14):5698–5707. doi: 10.1021/acs.nanolett.2c00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda N., Ueno T., Pohlmeyer C., Nagano T., Inoue T.. A Photocleavable Rapamycin Conjugate for Spatiotemporal Control of Small GTPase Activity. J. Am. Chem. Soc. 2011;133(1):12–14. doi: 10.1021/ja108258d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwasaki Y., Suzuki K., Yu G., Yamamoto S., Otabe T., Kakihara Y., Nishiwaki M., Miyake K., Fushimi K., Bekdash R.. et al. A red light–responsive photoswitch for deep tissue optogenetics. Nat. Biotechnol. 2022;40:1672–1679. doi: 10.1038/s41587-022-01351-w. [DOI] [PubMed] [Google Scholar]
- Chen S., Weitemier A. Z., Zeng X., He L., Wang X., Tao Y., Huang A. J. Y., Hashimotodani Y., Kano M., Iwasaki H., Parajuli L. K., Okabe S., Teh D. B. L., All A. H., Tsutsui-Kimura I., Tanaka K. F., Liu X., McHugh T. J.. Near-infrared deep brain stimulation via upconversion nanoparticle–mediated optogenetics. Science. 2018;359(6376):679–684. doi: 10.1126/science.aaq1144. [DOI] [PubMed] [Google Scholar]
- Wu Y., Liu Y., Huang Z., Wang X., Jin Z., Li J., Limsakul P., Zhu L., Allen M., Pan Y., Bussell R., Jacobson A., Liu T., Chien S., Wang Y.. Control of the activity of CAR-T cells within tumours via focused ultrasound. Nat. Biomed. Eng. 2021;5(11):1336–1347. doi: 10.1038/s41551-021-00779-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavilán H., Avugadda S. K., Fernández-Cabada T., Soni N., Cassani M., Mai B. T., Chantrell R., Pellegrino T.. Magnetic nanoparticles and clusters for magnetic hyperthermia: optimizing their heat performance and developing combinatorial therapies to tackle cancer. Chem. Soc. Rev. 2021;50(20):11614–11667. doi: 10.1039/D1CS00427A. [DOI] [PubMed] [Google Scholar]
- Huang H., Delikanli S., Zeng H., Ferkey D. M., Pralle A.. Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. Nat. Nanotechnol. 2010;5(8):602–606. doi: 10.1038/nnano.2010.125. [DOI] [PubMed] [Google Scholar]
- Stanley S. A., Kelly L., Latcha K. N., Schmidt S. F., Yu X., Nectow A. R., Sauer J., Dyke J. P., Dordick J. S., Friedman J. M.. Bidirectional electromagnetic control of the hypothalamus regulates feeding and metabolism. Nature. 2016;531(7596):647–650. doi: 10.1038/nature17183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin W., Lee Y., Lim J., Lee Y., Lah J. D., Lee S., Lee J.-U., Yu R., Lee P. H., Lee J.-H., Kwak M., Cheon J.. Nanoscale Magneto-mechanical-genetics of Deep Brain Neurons Reversing Motor Deficits in Parkinsonian Mice. Nano Lett. 2024;24(1):270–278. doi: 10.1021/acs.nanolett.3c03899. [DOI] [PubMed] [Google Scholar]
- Choi S.-H., Shin J., Park C., Lee J.-U., Lee J., Ambo Y., Shin W., Yu R., Kim J.-Y., Lah J. D., Shin D., Kim G., Noh K., Koh W., Lee C. J., Lee J.-H., Kwak M., Cheon J.. In vivo magnetogenetics for cell-type-specific targeting and modulation of brain circuits. Nat. Nanotechnol. 2024;19(9):1333–1343. doi: 10.1038/s41565-024-01694-2. [DOI] [PubMed] [Google Scholar]
- Xiang K. M., Lampson H., Hayward R. F., York A. G., Ingaramo M., Cohen A. E.. Mechanism of Giant Magnetic Field Effect in a Red Fluorescent Protein. J. Am. Chem. Soc. 2025;147(21):18088–18099. doi: 10.1021/jacs.5c03997. [DOI] [PubMed] [Google Scholar]
- Chee W. K. D., Yeoh J. W., Dao V. L., Poh C. L.. Thermogenetics: Applications come of age. Biotechnol. Adv. 2022;55:107907. doi: 10.1016/j.biotechadv.2022.107907. [DOI] [PubMed] [Google Scholar]
- Roder P. B., Smith B. E., Zhou X., Crane M. J., Pauzauskie P. J.. Laser refrigeration of hydrothermal nanocrystals in physiological media. Proc. Int. Acad. Sci. 2015;112(49):15024–15029. doi: 10.1073/pnas.1510418112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Chen Z., Liu Y., Kollipara P. S., Feng Y., Zhang Z., Zheng Y.. Opto-refrigerative tweezers. Sci. Adv. 2021;7(26):eabh1101. doi: 10.1126/sciadv.abh1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai, S. ; Itoh, H. ; Vu, C. Q. ; Nakayama, M. ; Oshima, M. ; Morita, A. ; Okamoto, K. ; Okuda, S. ; Teranishi, A. ; Osawa, M. ; et al. qMaLioffG: A single green fluorescent protein FLIM indicator enabling quantitative imaging of endogenous ATP. bioRxiv. 2023, 555275. 10.1101/2023.08.29.555275. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.