Abstract
Loss of integrity of the capillary network is directly associated with the development of kidney fibrosis resulting in chronic kidney disease. Here, we characterized long non-coding RNAs (lncRNAs) in endothelial cells (ECs) during the development of kidney fibrosis. Using a murine EC lineage-tracing model, we observed expression of the conserved lncRNA metastasis-associated lung adenocarcinoma transcript 1 (Malat1) to be elevated in ECs upon kidney injury; either by ischemia-reperfusion injury or by unilateral ureteral obstruction (UUO). In addition, we found elevated MALAT1 expression in the kidney and circulation of patients with fibrotic kidney diseases. Pharmacological intervention of Malat1 initiated protection against fibrosis in the UUO model, illustrated by a marked decline in collagen deposition and a concomitant decrease in interstitial alpha-smooth muscle actin (α-SMA)-positive cells in the kidney. This protective effect was further highlighted by an increase in capillary density and reduced endothelial-to-mesenchymal transition. Mechanistically, transcriptomic analyses of kidney ECs upon Malat1 knockdown demonstrated increased EC-matrix-receptor interaction. Furthermore, we show that silencing of MALAT1 results in increased barrier function and angiogenic response, less vascular leakage, and decreased focal adhesions. Finally, integration with in silico analyses and RNA immunoprecipitation confirmed binding of MALAT1 to SUZ12, a member of the PRC2 complex, suggesting a transcriptional regulatory role for MALAT1. Collectively, our findings classify the lncRNA MALAT1 as an important regulator of EC function and kidney health. As such, targeting MALAT1 may provide novel strategies to reduce kidney fibrosis.
Keywords: MT: Non-coding RNAs, long non-coding RNAs, vascular integrity, kidney fibrosis, endothelial cell function, MALAT1, chronic kidney disease, antisense oligonucleotides, RNA-therapeutics
Graphical abstract

Loss of integrity of the capillary network is directly associated with kidney fibrosis and chronic kidney disease. Bijkerk and colleagues show that pharmacological intervention of the lncRNA Malat1 protected against fibrosis in experimental kidney injury and preserved vascular integrity and may serve as potential treatment for chronic kidney disease.
Introduction
Chronic kidney disease (CKD) has a worldwide prevalence of >10%.1 Besides its high morbidity, CKD is a leading cause of premature cardiovascular disease.2 It is estimated that by 2040, CKD will become the 5th leading cause of death, due to the aging population and increased prevalence of non-communicable diseases such as diabetes and hypertension.3 Irrespective of the etiology, the common pathway in the pathology of CKD involves glomerular sclerosis, tubulointerstitial fibrosis associated with inflammation, myofibroblast proliferation, extracellular matrix accumulation, and tubular atrophy.4 A central feature of CKD is the progressive loss of the peritubular capillary network, a process that is referred to as rarefaction. Microvascular rarefaction is directly correlated with the severity of fibrosis,5 and the extent of rarefaction has been found to predict the degree of interstitial damage as well as changes in the glomerular filtration rate in CKD patients.6 Likewise, emerging evidence indicates that the renal microvascular endothelium of the outer medullary peritubular network is the primary site of injury in kidney allograft nephropathy7 and that regression of the peritubular network is directly related to a decline in glomerular filtration, interstitial fibrosis, and the severity of chronic allograft nephropathy.8 These findings suggest an early, rate-limiting role for integrity of the peritubular capillary network in the pathogenesis of kidney fibrosis. Therefore, finding therapeutic strategies to stabilize the microvasculature in patients at risk for progressive renal failure may provide novel treatment options.
Emerging evidence suggests that long non-coding RNAs (lncRNAs) are critical regulators of gene regulatory networks in diverse biological processes, including kidney and endothelial cell (EC) (dys)function.9,10 lncRNAs are defined as non-coding RNA transcripts longer than 200 nucleotides that exert structural and regulatory functions through their interaction with proteins, DNAs, or RNAs in the nucleus or cytoplasm.11 lncRNAs have been demonstrated to be involved in gene regulation through a variety of mechanisms, such as epigenetic and transcriptional regulation through their association with chromatin-modifying complexes, regulation of mRNA processing and splicing, and acting as competitive inhibitors of endogenous RNAs (e.g., microRNAs).11 An example of an lncRNA-directed function in kidney fibrosis can be found in lncRNA lnc-TSI that was demonstrated to inhibit renal fibrogenesis by negatively regulating the transforming growth factor β (TGF-β)/Smad3 pathway.12 Additionally, the lncRNAs RNA imprinted and accumulated in the nucleus and myocardial infarction-associated transcript were shown to mediate myofibroblast formation in kidney fibrosis.13 lncRNAs are also essential to EC function. For example, lncRNA activated by shear stress in endothelium regulates shear stress sensing and endothelial barrier function through its association with and stabilization of junction proteins, e.g., Platelet endothelial cell adhesion molecule-1 (PECAM-1 and CD31).14
In the current study, we aimed to identify lncRNAs that are dysregulated in ECs during kidney fibrosis and to determine whether the modulation of lncRNA expression levels can augment the development of kidney fibrosis. To that end, we used a Cdh5-creER;tdTomato mouse model to genetically label ECs and isolated them through fluorescence-activated cell sorting (FACS) from healthy kidneys and injured kidneys that were exposed to unilateral ureteral obstruction or ischemia-reperfusion injury (IRI). Following lncRNA profiling of the sorted cells, we found a signature of differentially expressed lncRNAs in injured cells and demonstrated that lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is part of a rate-limiting post-transcriptional network involved in vascular integrity that can potentially be targeted to counteract kidney fibrosis.
Results
Kidney fibrosis is associated with loss of vascular integrity and endothelial-to-mesenchymal transition
To allow fate tracing of ECs upon kidney injury, we used a genetic mouse model expressing tamoxifen-inducible Cre driven by the Cdh5 (VE-Cadherin) promoter (Cdh5-Cre-ERT2), as previously described,15 and crossed these to Rosa-TdTomatofl/fl reporter mice, in which Cre-mediated excision resulted in Tomato expression specific to ECs. Next, we applied the unilateral ureteral obstruction (UUO) model to induce severe kidney fibrosis. In addition, we included the unilateral IRI model as an acute kidney injury model that associates with microvascular injury and develops kidney fibrosis in the longer term. At 10 and 2 days post-intervention, respectively, studies were terminated (Figure 1A). In the UUO model, upon kidney fibrosis, we observed less organized EC-derived Tomato signal (Figure 1B). Notably, several tomato-positive cells appeared elongated and enlarged, suggesting that ECs possibly underwent endothelial-to-mesenchymal transition (EndMT). We co-stained the Tomato signal with mesenchymal marker alpha-smooth muscle actin (α-SMA) and indeed found several double-positive ECs, confirming that a fraction of ECs underwent EndMT (Figure S1A). We subsequently sorted the Tomato-positive cells using FACS (Figure S1B) and via RT-qPCR confirmed EndMT: acquirement of mesenchymal markers α-SMA (Acta2) and Col-1α1 in ECs derived from UUO kidneys compared to ECs derived from healthy kidneys, while EC-markers VE-cadherin (Cdh5) and Pecam-1/Cd31 were downregulated, suggesting loss of EC phenotype (Figures S1C and S1D). Interestingly, in the IRI model, we observed a trend toward increased levels of mesenchymal marker Col-1α1 in ECs already after 2 days, potentially indicative of an early initiation of the pro-fibrotic phenotype.
Figure 1.
Loss of vascular integrity during kidney fibrosis and differential lncRNA expression in endothelial cells
(A) Schematic overview of study setup. (B) Representative images of tdTomato-positive cells (red) in healthy contralateral (CLK), IRI, and UUO kidneys. (C) Volcano plots visualizing differential lncRNA expression between indicated conditions. The blue and red dots correspond to lncRNAs with p < 0.05 and −1<logFC>1 that are down- or up-regulated, respectively. Gray dots indicate non-significantly changed lncRNAs (n = 3 per condition). (D) Hierarchical clustering shows a distinguishable lncRNA expression pattern in VE-cadherin-derived cells in IRI and UUO compared to healthy CLK kidneys (n = 3 per condition). (E) Venn diagram indicating number of differentially expressed lncRNAs in IRI and UUO. (F) Scatterplot and heatmap of lncRNAs that are significantly differentially expressed in both models. The blue and red dots correspond to lncRNAs with p < 0.05 and −1<logFC>1 that are down- or up-regulated, respectively. Gray dots indicate non-significantly changed lncRNAs.
LncRNAs are differentially expressed in ECs during kidney injury
To implicate lncRNAs involved in the injury response of ECs in fibrotic kidney disease, we profiled lncRNAs in FACS-sorted Cdh5-derivative ECs isolated from injured and contralateral control kidneys (CLK). These efforts provided an EC-specific lncRNA signature within the in vivo kidney injury setting. Differential expression analysis and hierarchical clustering of lncRNA expression revealed clear differential lncRNA expression profile in ECs in diseased kidneys compared to healthy kidneys for both models (Figures 1C and 1D). After IRI, we found 417 lncRNAs to be significantly altered in ECs, of which 280 were decreased and 137 increased. Upon UUO, 587 lncRNAs (322 down and 265 up) were dysregulated in ECs (−1 < logFC > 1, p < 0.05; Figure 1E; Tables S1 and S2). Simultaneously, mRNAs were profiled, and subsequent differential expression analysis, hierarchical clustering, and pathway analyses revealed a clear differential mRNA expression profile in ECs in diseased kidneys compared to healthy kidneys (Figures S2A–S2D; Tables S3 and S4). Gene Ontology (GO) biological process analysis of genes differentially expressed in ECs of both IRI and UUO models when compared to CLK indicates “Actin Cytoskeleton Organization” and “Cell-Matrix Adhesion” to be affected (Figure S2E).
Among differentially expressed lncRNAs, we found 17 lncRNAs that were differentially expressed in both models (Figure 1F). Based on consistent upregulation in both injury models, as well as known conservation between mice and humans and previously reported increased circulating levels in patients with diabetic kidney disease,16 we hypothesized that lncRNA Malat1 may play an important role in ECs in kidney fibrosis and was selected to study further. Additionally, transcription factor motif enrichment analysis in the promoter regions of the differentially expressed lncRNAs identified among others HMGA1 to be enriched and to potentially bind the promoter region of MALAT1 (Figures S3A–S3C). Interestingly, HMGA1 has been linked to regulate EC function via controlling endothelial plasticity and angiogenesis.17,18,19 We applied chromatin immunoprecipitation (ChIP) for HMGA1 and confirmed binding to the Malat1 promoter by this transcription factor (Figure S3D), supporting direct regulation.
MALAT1 is increased in human fibrotic kidney disease
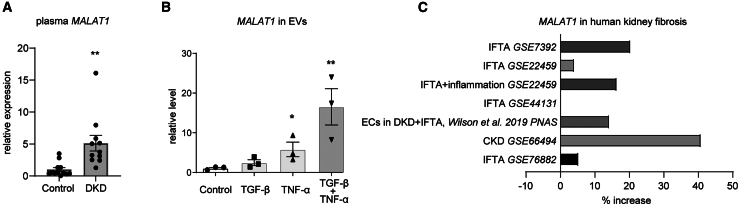
To demonstrate relevance for humans, we aimed to determine whether MALAT1 dysregulation is consistent in human vascular and fibrotic kidney disease. We previously demonstrated that circulating MALAT1 levels were increased in patients with diabetic kidney disease.16 We reassessed circulating MALAT1 levels in the plasma of these patients and confirmed increased MALAT1 levels when compared to controls (Figure 2A). Since circulating lncRNA levels are often carried in extracellular vesicles (EVs), we hypothesized that activated ECs may secrete increased levels of MALAT1 in EVs. Indeed, when human umbilical vein endothelial cells (HUVECs) were stimulated with TGF-β and/or tumor necrosis factor alpha (TNF-α), we observed increased MALAT1 levels in EC-secreted EVs (Figure 2B). Finally, we consulted online available datasets that contained transcriptomics data from human kidneys from patients with fibrotic kidney disease (GSE7392, GSE22459, GSE22459, GSE44131, GSE66494, and GSE76882) and found an upregulation of MALAT1 in the majority of datasets (Figure 2C), which is consistent with our mouse studies. Importantly, in a single-cell RNA sequencing (RNA-seq) dataset that contained data from ECs in kidneys from patients with diabetes with or without kidney fibrosis,20 we observed that MALAT1 is increased in ECs when kidney fibrosis is present (Figure 2C).
Figure 2.
Increased kidney and circulating MALAT1 levels in fibrotic kidney disease
(A) Quantitative reverse-transcription PCR (RT-qPCR) analysis of MALAT1 levels in plasma of patients with diabetic kidney disease (DKD, n = 11) and healthy controls (n = 12); student’s t test. (B) RT-qPCR analysis of MALAT1 in extracellular vesicles from HUVECs stimulated with TGF-β and/or TNF-α or vehicle control (n = 3); one-way ANOVA. (C) MALAT1 expression levels in fibrotic kidney tissue compared to controls from different datasets. IFTA, interstitial fibrosis and tubular atrophy; CKD, chronic kidney disease. ∗p < 0.05 and ∗∗p < 0.01.
In vivo knockdown of Malat1 reduces kidney fibrosis and preserves vascular integrity
Given the increase of Malat1 in ECs in UUO and IRI, we hypothesized that knockdown of Malat1 may protect against disturbed microvascular integrity and the subsequent development of kidney fibrosis. To investigate this, we applied the UUO model in the Cdh5-creER;tdTomato mice while inhibiting Malat1 expression using intraperitoneal injection of Malat1-targeting GapmeRs. Silencing of Malat1 in the kidneys was confirmed by in situ hybridization (ISH) for Malat1 within the kidney tissue (Figures 3A–3C and S4), as well as by RT-qPCR (Figure S5). Interestingly, ISH indicates that, upon UUO, Malat1 is elevated in the kidney cortex, while gapMalat1-mediated knockdown also occurs mainly in the kidney cortex. To assess whether Malat1 knockdown resulted in decreased kidney fibrosis, we next used a Sirius Red staining to determine collagen deposition (Figure 3D). Indeed, we observed a strong decrease in kidney fibrosis upon inhibition of Malat1, amounting to a ∼50% reduction in collagen deposition (Figure 3E). Furthermore, we observed a marked reduction in the number of α-SMA-positive myofibroblasts in kidneys from the gapMalat1 mice, compared to kidneys of gapC-treated mice, 10 days after UUO (Figures 3F and 3G). We confirmed this decrease in kidney α-SMA expression upon Malat1 inhibition using western blot (Figures 3H, 3I, and S6). Given the observed role of Malat1 in regulating EC function, we then determined the impact of Malat1 knockdown on renal vascular integrity after UUO. Indeed, we observed that the density of MECA32+ peritubular capillaries in the gapMalat1-treated mice was markedly higher than that in the control kidneys (Figures 3J and 3K). Similarly, we observed higher levels of tdTomato+ VE-cadherin(-derived) cells upon Malat1 knockdown (Figures 3L and 3M). Given that our lineage trace model allows fate tracing of the ECs, we next assessed whether EndMT was also affected by silencing Malat1. Indeed, we found decreased numbers of α-SMA-tdTomato double-positive cells, indicating a decrease in EndMT (Figure S7). Taken together, these data further confirm that Malat1 plays an important function in vivo in regulating vascular integrity and thereby in mediating kidney fibrosis.
Figure 3.
In vivo Malat1 knockdown inhibits kidney fibrosis and preserves vascular integrity
(A) Quantification of in situ hybridization for Malat1 in the kidney upon Malat1-targeting GapmeR treatment (gapMalat1), compared to control GapmeR (gapC)-treated mice, n = 3; one-way ANOVA. (B and C) Representative whole-mount images (B) and zoomed-in images (C) of in situ hybridization for Malat1. (D and E) Representative images of Sirius Red staining (D) and corresponding quantification (E). (F and G) Representative images of α-SMA staining (F) and corresponding quantification (G). (H and I) Representative western blots for α-SMA (H) and corresponding quantification (I), normalized for glyceraldehyde 3-phosphate dehydrogenase (GAPDH). (J and K) Representative images of endothelial cell marker MECA32 (J) and corresponding quantification (K). (L and M) Representative images of endogenous Tomato label (L) and corresponding quantification (M). For (D)–(M), n = 5 (gapC) and n = 8 (gapMalat1); student’s t test. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. CLK, healthy contralateral kidney; UUO, fibrotic kidney from unilateral ureteral obstruction model; gapC, control GapmeR; gapMalat1, Malat1 GapmeR.
Knockdown of MALAT1 affects cell-cell and cell-matrix interaction in ECs
To understand how depletion of MALAT1 preserves vascular integrity and decreases fibrosis and myofibroblast formation, we isolated RNA from FACS-sorted VE-cadherin;tdTomato+ cells in the UUO model (10 days) after silencing Malat1 and performed transcriptomic analysis by RNA-seq. Unsupervised hierarchical clustering of genes differentially expressed in Malat1-sufficient and -deficient ECs revealed that Malat1 significantly reprogrammed transcriptional responses in response to UUO induction (Figure 4A, −1 < logFC > 1, p < 0.05; Table S5). In addition, using RT-qPCR, we confirmed knockdown of Malat1 in the sorted VE-cadherin-tdTomato+ cells (Figure 4B). Gene set enrichment analysis of differentially expressed genes identified that “ECM-Receptor Interaction” is activated, while metabolic and mitochondrial pathways are suppressed (Figure 4C). In addition, analysis of enriched cellular components indicates that “Focal Adhesion” and “Cell-Substrate Junction” are affected (Figure 4D). These pathway analyses were confirmed by ingenuity pathway analysis (IPA) that also indicated increased “Focal Adhesion Kinase (FAK) signaling,” important for cell-matrix interactions, and impaired metabolic pathways, mainly reduced “Oxidative Phosphorylation” and increased “Mitochondrial Dysfunction” (Figure S8). As such, these data pose two possible mechanisms (cell-matrix/cell-cell interactions and metabolic/mitochondrial function) via which Malat1 affects EC function and kidney fibrosis.
Figure 4.
Knockdown of MALAT1 affects cell-cell and cell-matrix interaction
(A) Volcano plots visualizing differential gene expression in ECs in vivo upon GapmeR-mediated Malat1 knockdown. The blue and red dots correspond to mRNAs with p < 0.05 and −1<logFC>1 that are down- or up-regulated, respectively. Gray dots indicate non-significantly changed mRNAs, n = 3. (B) Malat1 levels as determined by quantitative reverse-transcription PCR (RT-qPCR) on FACS-sorted mouse kidney ECs; n = 3; student’s t test. (C and D) Gene set enrichment analyses (C) and cellular component analysis on differentially expressed genes from (A). (E) Heatmap showing relative expression of genes involved in ECM-receptor interaction. (F and G) Representative images of VE-cadherin staining (green) and F-actin (red) (zoomed images in G) on HUVECs treated with gapMALAT1 or gapC, in the presence of TGF-β. Examples of gaps (loss cell-cell contacts) are shown by white arrows, and focal adhesion junctions by yellow arrows. (H–K) Quantification of total VE-cadherin staining (H), number of gaps (loss of cell-cell contacts) (I), area of gaps (J), and focal adhesion junctions (K); n = 4; student’s t test. ∗p < 0.05 and ∗∗p < 0.01.
Before investigating this, we explored the effect of Malat1 knockdown on macrophages and proximal tubular epithelial cells, as these cells could also be affected by the Malat1 GapmeRs. By using mouse bone marrow-derived macrophages (BMDMs) and phorbol 12-myristate 13-acetate (PMA)-stimulated human THP-1 cells to induce macrophage phenotype, we found Malat1 knockdown to induce a modest decrease in inflammatory phenotype, as evidenced by gapMALAT1-mediated lower Il6 and TNF-α expression upon lipopolysaccharide (LPS) stimulation in PMA-differentiated THP-1 cells (Figure S9A). We next assessed the “pro-fibrotic” phenotype and found no statistically significant alterations, although a trend toward increased expression levels of pro-fibrotic markers was observed in both BMDM and THP-1 cells upon knockdown (Figures S9B and S9C). Then, we assessed kidney macrophage content by F4/80 staining and observed lower number of macrophages in fibrotic kidneys upon Malat1 knockdown (Figures S9D and S9E). Next, we analyzed the effect of MALAT1 knockdown on proximal tubular epithelial cells (PTEC) using HK-2 cells. While TGF-β induced a “mesenchymal” pro-fibrotic and pro-inflammatory phenotype, knockdown of MALAT1 did not affect this phenotype (Figure S10).
To further study the above identified affected pathways in ECs upon MALAT1 knockdown, we started out with testing the effect of MALAT1 knockdown in HUVECs on mitochondrial function in vitro using Seahorse respirometry. Under basal conditions, MALAT1 knockdown increased oxygen consumption in ECs (Figure S11A), corresponding to increased basal respiration, maximal respiration, and proton leak (Figure S11B). Upon TGF-β treatment, simulating the in vivo-activated state, gapMALAT1-treated HUVECs displayed decreased oxygen consumption corresponding to a trend toward decreased basal respiration, maximal respiration, and proton leak (Figures S11A and S11B). While the latter is in line with the RNA-seq data showing decreased mitochondrial function and oxidative phosphorylation, this presumably negative effect may not solely explain the beneficial effects of Malat1 knockdown in vivo. Therefore, we further investigated the role of MALAT1 in cell-cell and cell-matrix interactions and FAK signaling, as identified under Figures 4C and 4D. When visualizing relative gene expression from genes involved in the ECM-receptor interaction pathway, it is apparent that decreased Malat1 levels result in higher levels of many of these genes (Figure 4E). Following this notion, we next examined cell-cell and cell-matrix interaction by staining HUVECs for VE-cadherin and F-actin to visualize the actin fibers. While we did not find differences in absolute VE-cadherin staining, a clear decrease in cell-cell contacts was visible upon measuring the number of gaps between cells and gap area (Figures 4F–4J). More importantly, when analyzing focal adhesion junctions (FAJs), a measure of EC activation and junction remodeling,21 we found a reduction in these FAJs upon MALAT1 knockdown (Figure 4K), indeed implicating a more stable cell-matrix interaction, which is in line with Figure 3 demonstrating that MALAT1 knockdown preserves vascular integrity.
MALAT1 potentially acts in trans and in cis
We next sought to gain more insight into the possible underlying molecular mechanisms of MALAT1 in EC function. First, we performed fluorescence in situ hybridizationfor MALAT1 on HUVECs and confirmed nuclear localization (Figure S12). As it was previously indicated that MALAT1 plays a cis-regulatory role in gene transcription,22 we performed virtual chromosome conformation capture followed by high-throughput sequencing (Hi-C) analysis to identify coregulatory genes and potential in cis mechanisms driven by MALAT1 in its genomic locus.23 MALAT1 appears to have a strong association with nearby genes, potentially FAUP4 and NEAT1 (Figures 5A and 5B). Increased Neat1 expression upon Malat1 knockdown was confirmed in our RNA-seq dataset (Figure 5C). These data suggest a relation between Malat1 and the genes in its topological associated domain, indicating a potential cis-regulatory effect of Malat1 depletion in kidney ECs. Next, we investigated the potential in trans functional roles of MALAT1. We identified “RNA Binding” to be the top enriched molecular function in our RNA-seq dataset (Figure 5D) and found a strong overlap with genes involved in RNA binding by chromatin-associated proteins (Figure 5E).24,25 These data prompted us to analyze the presence of potential RNA-binding domains in MALAT1, and we found a strong enrichment of the PF00076 RNA recognition motif (Figure 5F). Using CatRAPID, we subsequently found numerous RNA-binding proteins that are predicted to directly interact with MALAT1 and Malat1 (Figure 5G). Most strikingly, we found polycomb protein SUZ12 to be the top hit, while ChIP-X Enrichment Analysis on the differentially expressed genes from our RNA-seq indeed identified SUZ12 and enhancer of zeste homolog 2 that together with SUZ12 forms the polycomb repressive complex 2 (PRC2), to be the strongest associated transcription factors (Figure 5H). These findings prompted us to investigate direct binding of MALAT1 to SUZ12. To that end, we assessed whether MALAT1 associated with SUZ12 by performing RNA immunoprecipitation. Indeed, qPCR of SUZ12 immunoprecipitates showed enrichment of MALAT1 when compared to control immunoglobulin G (IgG) immunoprecipitates (Figure 5I). This interaction of MALAT1 has been previously confirmed in other cell types26,27 and suggests that the nuclear MALAT1 may be involved in ECs in in trans chromatin binding and transcriptional regulation via SUZ12 and the PRC2.
Figure 5.
Virtual Hi-C analysis indicates possible coregulation with NEAT1, while MALAT1 directly binds SUZ12
(A and B) Virtual Hi-C analysis in HUVECs (A) and mouse C2C12 (B) within the 300 kb region of MALAT1 or Malat1, respectively. (C) Expression of Malat1 neighboring genes in sorted mouse kidney endothelial cells after gapMalat1 treatment or gapC, n = 3. (D) Gene set enrichment analysis for enriched molecular functions based on differentially expressed genes in sorted endothelial cells from mouse kidneys upon Malat1 knockdown, compared to control. (E) “Rummagene” analysis compared differentially expressed genes with other datasets. In parentheses the specific subset of data is indicated that overlaps. (F) RNA-binding domain prediction analysis of both human and mouse MALAT1. (G) CatRAPID analysis of both human and mouse MALAT1. (H) ChEA analysis on differentially expressed genes in sorted mouse kidney endothelial cells after gapMalat1 treatment or gapC. (I) SUZ12 RNA immunoprecipitation in HUVECs demonstrates direct binding of MALAT1 to SUZ12; n = 3; student’s t test. ∗p < 0.05.
In vitro knockdown of MALAT1 inhibits EC activation
Given our findings that MALAT1 inhibition preserves vascular integrity and affects cell-cell/cell-matrix contacts, we next set out to determine whether MALAT1 knockdown indeed impacts EC and vascular function. First, as we observed EndMT to occur during kidney fibrosis, we used our in vitro mouse EC model for EndMT28 to investigate the role of MALAT1 and showed that TGF-β induces Malat1 expression (Figure S13A). However, Malat1 knockdown did not prevent the TGF-β induced elongation of the cells associated with EndMT (Figures S13B and S13C), nor the increase in mesenchymal marker α-SMA (Figures S13D and S13E). Malat1 silencing decreased secreted pro-collagen1α1 levels (Figure S13F), while α-SMA and col1α1 gene expression were not affected (Figure S13G). These data suggest that reducing cellular Malat1 levels may not affect the cellular transition but could influence pro-fibrotic properties. Next, we knocked down MALAT1 in HUVECs using GapmeR antisense oligonucleotides (gapMALAT1) or control GapmeRs (gapC) and performed a series of functionality assays. First, we assessed the angiogenic response of HUVECs treated with gapMALAT1 in the Organoplate microfluidic system-based angiogenesis assay and found that silencing of MALAT1 resulted in less angiogenesis (Figure 6A) as shown by reduced sprout length and area (Figures 6B and 6C). Then, the endothelial barrier function was assessed by trans-endothelial electrical resistance measurements of HUVECs. Silencing of MALAT1 expression increased the capacity of HUVECs to form a tight barrier (Figure 6D). In line with the data provided in Figure 4, application of mathematical modeling (provided by the ECIS software) indicated that the increased barrier function upon knockdown of MALAT1 was driven by significantly enhanced cell-cell contacts as well as a trend toward more efficient cell-matrix contacts (Figure 6E). Since a tight barrier prevents vascular leakage, we leveraged the Organoplate microfluidic system29 to test the effect of MALAT1 depletion on the ability of HUVECs to form a leak tight 3D capillary-like vessel. Perfusion of these capillary-like vessels with fluorescently labeled albumin allows measurement of its potential leakage through the vessel (Figure 6F). We observed that MALAT1 knockdown in HUVECs leads to decreased vascular leakage when compared to gapCTRL (Figures 6G and 6H). Collectively, MALAT1 emerges to be an important regulator in driving EC integrity/barrier function and activation.
Figure 6.
Knockdown of MALAT1 in ECs increases barrier function and reduces angiogenesis
(A–C) Representative images (A) of vascular endothelial growth factor (VEGF)-, basic fibroblast growth factor (bFGF)-, and sphingosine-1-phosphate (S1P)-induced angiogenesis upon knockdown of MALAT1 (gapMALAT1) or control (gapC) and corresponding quantification of average sprout length (B) and total area (C); n = 8; student’s t test. (D and E) Trans-endothelial electrical resistance of ECs after treatment with gapMALAT1 or gapC over time (D), attributable to cell-matrix contacts (alpha) and cell-cell contacts (Rb) (E); n = 4; student’s t test. (F) Schematic representation of the leakage assay, with HUVEC-based 3D capillary-like vessels in the upper perfusion channel, separated from a collagen gel in the lower channel with a phaseguide. Leak tight vessels have limited leakage of fluorescently labeled albumin, while increased permeability of the vessels results in increased fluorescent signal in the gel channel. (G and H) Analysis of leakage assay after knockdown of MALAT1 (gapMALAT1) or control (gapC) in HUVECs presented in (F), n = 5. Representative photographs (G) and quantification of the permeability of capillary-like vessels (H). ∗p < 0.05 and ∗∗p < 0.01.
Discussion
In this study, we demonstrate that lncRNA MALAT1 is strongly increased in ECs during the fibrotic response upon kidney injury. Knockdown of Malat1 inhibited EC activation and resulted in preserved vascular integrity in the kidney and decreased kidney fibrosis Together, our findings support a facilitatory role for MALAT1 negatively impacting vascular integrity in reverberating kidney fibrosis.
Cell-matrix and cell-cell interactions are vital to ECs to maintain stable barrier function and integrity of the blood vessels.30 We found that knockdown of MALAT1, using antisense oligonucleotides in vitro, increased barrier function, decreased vascular leakage, preserved cell-cell contacts, and decreased the formation of FAJs, all features of activation and remodeling of ECs.21 In addition, we observed decreased angiogenesis, which corroborates with previous reports showing that depletion of MALAT1 inhibits the angiogenic response of ECs,31,32 as well as other studies demonstrating a pathogenic role of MALAT1 in EC dysfunction.33,34 Given the increase in MALAT1 levels upon kidney injury both in humans and mice, these data clearly point toward a role for MALAT1 in activation of ECs and loss of vascular integrity that may be averted by using GapmeRs in vivo to inhibit MALAT1. Indeed, we show in the UUO model that this approach results in silencing of MALAT1 followed by preserved kidney vascular integrity and suppression of kidney fibrosis.
To gain more insight into MALAT1’s in cis and in trans molecular mechanisms, we performed in silico analyses on our in vivo transcriptomics data and identified a potential regulatory role for MALAT1 via SUZ12, a member of the repressive complex PRC2, which is important in chromatin regulation and transcriptional repression.35 Supporting this notion, it has indeed been previously demonstrated that MALAT1 can directly bind SUZ1226 and is important in transcriptional regulation via chromatin interactions.27,36,37 Interestingly, it was also described that co-analysis of data from capture hybridization analysis of RNA targets (CHART) and ChIP followed by sequencing showed that MALAT1 occupies the same sites as PRC2 throughout the mouse and human genomes.38 Importantly, we validated this direct physical interaction of SUZ12 and MALAT1 in HUVECs using RNA immunoprecipitation. Moreover, PRC2 has been found to decrease integrin expression in ECs and is important in maintenance of vascular health,39,40 while a strong link has been found between VE-cadherin expression and PRC2/SUZ12-mediated regulation of an EC-transcriptional program involved in vascular stability.41 As such, a picture emerges that MALAT1 is involved in chromatin regulation and transcription via binding SUZ12 leading to repression of a transcriptional program involved in maintaining EC function, possibly in conjunction with its neighboring lncRNA NEAT1,27,42 that our data suggest are co-regulated.
While many studies also describe a role for MALAT1 as competing endogenous RNA for microRNA, including in the setting of kidney fibrosis,42,43 our data suggest that MALAT1 functions as a transcriptional regulator. The subcellular localization of MALAT1 that we, and others,27,44 confirmed to be prominent in the nucleus, suggests that MALAT1 functions in the nucleus and has a less significant role in sponging (cytoplasmic) microRNAs. In addition, since MALAT1 localizes to the nucleus, we used a GapmeR-based strategy of targeting MALAT1, as GapmeRs are designed and shown to reach the nucleus, while short hairpin RNAs mediate RNA degradation primarily in the cytoplasm. In light of these differences in targeting, this may result in different mechanisms of action yet providing protection against kidney fibrosis.43
While we find a clear effect of MALAT1 knockdown on EC function, it cannot be excluded that the protective effect that is observed by MALAT1 silencing in vivo is also mediated through other cell types. For example, MALAT1 regulates differential activation of macrophages45 and mediates podocyte injury46 that may contribute to the development of kidney fibrosis. Indeed, we found that MALAT1 knockdown results in a less inflammatory and potentially pro-fibrotic phenotype, in line with previous findings.45 This combined anti-inflammatory, pro-fibrotic macrophage phenotype as a result of Malat1 knockdown was previously shown to induce fibrosis in the setting of lung fibrosis45 and would therefore not explain our protective findings in kidney fibrosis, making it likely that the observed lower number of renal macrophages is the consequence of less injury. Yet, a protective effect based on the less inflammatory phenotype cannot be excluded. In addition, MALAT1 is also highly expressed in tubular epithelial cells, where we, upon GapmeR treatment, also observe knockdown using ISH. However, we found no effect of MALAT1 knockdown on the pro-fibrotic phenotype of HK-2 cells, in line with previous findings that MALAT1 silencing in HK-2 kidney epithelial cells did not result in any functional alterations but mainly altered EC function,47 suggesting that tubular epithelial cells are not implicated in mediating the protective effects of Malat1 knockdown but hinting at at least a partial regulatory function for Malat1 in ECs. Surprisingly, genetic Malat1 deletion in this study did not result in any antifibrotic effect in the renal IRI model.47 This may be explained by the fact that, besides being a different injury model, full knockout of a lncRNA may yield different results than pharmacological knockdown; i.e., it has been demonstrated that knockout of Malat1 has no major phenotypes in mice,22,48 however, it does affect several physiological and pathophysiological processes in adult mice. Moreover, compensatory mechanisms may be in play as lncRNAs often function as rheostats, suggesting pharmacological targeting of Malat1 may be more relevant in the setting of kidney fibrosis. Yet, it should be noted that it cannot be excluded that GapmeR-mediated knockdown can result in off-target effects.
Next to increased levels of MALAT1 in mouse kidneys, we observed upregulation of (endothelial) MALAT1 in human fibrotic kidney disease. Furthermore, we validated that circulating levels of MALAT1 are increased in human fibrotic kidney disease, which is in line with previous reports.16,47,49 Interestingly, since most circulating lncRNAs are carried by EVs, it is tempting to speculate that ECs may be responsible for increased secretion of EVs containing MALAT1, especially as it has been demonstrated that, upon kidney injury, more activated EC-derived EVs appear in the circulation.50,51 Moreover, high glucose levels increase total HUVEC-derived EVs.52 Following this, our in vitro studies indicate that activated ECs secrete more EV-carried MALAT1, suggesting that this may indeed be the reason for the higher circulating MALAT1 levels as observed in patients with kidney disease.
Taken together, our studies identify MALAT1 as an important regulator of EC function potentially driving kidney fibrosis. Silencing MALAT1 reduced fibrosis by preserving vascular integrity, and therapies aimed at inhibiting MALAT1 in the vasculature may serve as potential treatment for CKD.
Materials and methods
Animals
All animal experiments were approved by the animal welfare committee of the veterinary authorities of the Leiden University Medical Center. Standard chow diet and drinking water were provided ad libitum. Eight-week-old male B6.Cdh5-Cre-ERT2;tdTomato mice (Cdh5-creER;tdTomato) were used, in which tamoxifen-inducible Cre-mediated excision results in endothelial Tomato expression. Mice received intraperitoneal 2 mg/0.2 mL tamoxifen for 5 consecutive days. The UUO model was performed through a left flank incision, followed by identification of the ureter and double ligation thereof close to the lower pole of the kidney with two separate silk ties. After 10 days, the mice were killed. For the Malat1 GapmeR experiment, locked nucleic acid (LNA) control GapmeR or LNA Malat1 GapmeR (20 mg/kg; Exiqon) were injected intraperitoneally twice: 2 days before and 2 days after UUO surgery. The unilateral IRI model was performed via an abdominal incision, after which the renal artery and vein were identified and, using surgical clamps (S&T, Neuhausen, Switzerland), unilaterally clamped for 45 min. The contralateral kidneys were used as controls. After 2 days, the mice were killed. Kidneys were removed, and RNA and protein were isolated as described in the following.
lncRNA and mRNA profiling
Profiling of lncRNAs and mRNAs was performed by Arraystar Inc. according to protocol using the Agilent Array platform. Sample preparation and microarray hybridization were based on standard protocols of the manufacturer with minor modifications. In brief, mRNA was purified from total RNA after rRNA removal (mRNAONLY Eukaryotic mRNA Isolation Kit, Epicentre). Each sample was then amplified and transcribed into fluorescent cRNA along the full length of transcripts without bias using a mix of oligo(dT) and random primers (Arraystar Flash RNA Labeling Kit, Arraystar). Labeled cRNAs were then hybridized on the “Mouse lncRNA Array v.3.0” (8 K × 60 K, containing 35.923 lncRNAs and 24.881 coding transcripts, Arraystar). After washing the slides, the arrays were scanned on Agilent Scanner G2505C. Agilent Feature Extraction software (version 11.0.1.1) was used for analysis of acquired array images. GeneSpring GX v.12.1 software package (Agilent Technologies) was used to perform quantile normalization and data processing. Following quantile normalization of raw data, lncRNAs and mRNA with at least 6 out of 12 samples having flags in present or marginal (“All Targets Value”) were selected for further data analysis. Statistically significant differentially expressed lncRNAs and mRNAs between the two groups were identified through volcano plot filtering.
Cell culture
Primary HUVECs were isolated from human umbilical cords as previously described53 and cultured on 1% gelatin-coated surfaces in endothelial cell growth medium 2 (C-39216, PromoCell, Germany) supplemented with antibiotics. Where indicated, HUVECs were treated with 10 ng/mL TGF-β1 and/or 10 ng/mL TNF-α (Sigma). Mouse embryonic endothelial cells (MEECs), as previously described,28 were cultured on 1% (w/v) gelatin coating in Dulbecco’s modified Eagle’s medium (Gibco/Invitrogen, Breda, the Netherlands) supplemented with 10% fetal calf serum and 2 mM L-glutamine (Invitrogen). MEECs were treated for 48 h with 2 ng/mL TGF-β3 (Peprotech, London, UK). The human PTEC cell line (HK-2 cells) was grown in serum-free DMEM/HAM-F12 medium (Bio-Whittaker, Walkersville, MD) supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin (Invitrogen, Breda, The Netherlands), 1× insulin-transferrin-selenium, 40 ng/mL triiodothyronine, 10 ng/mL epidermal growth factor, 36 ng/mL hydrocortisone, triiodothyronine (40 ng/mL), epidermal growth factor (10 ng/mL), and hydrocortisone (36 ng/mL) (all from Sigma, Zwijndrecht, The Netherlands). HK-2 cells were stimulated with 10 ng/mL TGF-β1 (PeproTech). BMDMs were prepared through flushing bone marrow from tibiae and femora of 6- to 8-week-old mice. Cells were differentiated into macrophages in DMEM medium supplemented with 10% FBS, 1% P/S, and 15% L929-conditioned media for 7 days. THP-1 cells were obtained from ATCC (TIB202) and cultured in 10% FBS-supplemented RPMI1640 medium with 0.01 μg/mL L-glutamine, penicillin, and streptomycin, and 0.05 mM 2-mercaptoethanol was added. To differentiate THP-1 cells from THP-1 macrophages, 100 nM PMA (Sigma-Aldrich) was used. After 3 days, the PMA-containing medium was replaced by normal growth medium, and the cells were used for experiments where 100 ng/mL of LPS was used. Cells were transfected at 60%–75% confluence with 50–100 nM LNA GapmeR (Exiqon, Vedbaek, Denmark) targeting MALAT1/Malat1 or control GapmeR using Lipofectamine 3000 (Life Technologies, Carlsbad, CA) according to the manufacturer’s protocol.
FACS
Kidneys were extracted from three individual mice per injury model and mechanically dissociated and filtered through 100 and 40 μm filters. The obtained cell suspension was then sorted on the FACSAria II (BD Biosciences, Franklin Lakes, NJ, United States).
Cell debris was gated out using an FSC-A/SSC-A plot (gate 1), doublets were gated out using an SSC-W/SSC-H plot (gate 2) and an FSC-W/FSC-H plot (gate 3), and these three gates were combined. Next, from the combined exclusion gate, cells that were positively identified having a fluorescent tomato signal were collected.
RNA isolation and RT-qPCR analysis
Total RNA was isolated using Trizol reagent (Invitrogen) combined with the RNeasy Micro Kit (QIAGEN, The Netherlands) and reverse transcribed using iScript (Bio-Rad) according to the manufacturers protocol. RT-qPCR of target genes was performed using SYBR Green Master Mix (Applied Biosystems). Used primer sequences of target genes were mouse α-SMA (sense) CGTGGCTATTCCTTCGTGAC; mouse α-SMA (antisense): GCGTTCGTAGCTCTTCTCC; mouse Col1α1 (sense): TGACTGGAAGAGCGGAGAGT; mouse Col1α1 (antisense): GTTCGGGCTGATGTACCAGT; mouse β-actin (sense): AGGTCATCACTATTGGCAACGA; mouse β-actin (antisense): CCAAGAAGGAAGGCTGGAAAA; human IL6 (sense):
ACTCACCTCTTCAGAACGAATTG; human IL6 (antisense): CCATCTTTGGAAGGTTCAGGTTG; human TNF-α (sense):
TTCTGCCTGCTGCACTTTGG; human TNF-α (antisense):
TGATGGCAGAGAGGAGGTTG; human OPN (sense): CCACATGGCTAAACCCTGACC; human OPN (antisense):
CATGGCTTTCGTTGGACTTACTTG; human MMP12 (sense):
GGAATCCTAGCCCATGCTTTT; human MMP12 (antisense):
CATTACGGCCTTTGGATCACT; human TIMP2 (sense): TCCTCTTGATAGGGTTGCCA; human TIMP2 (antisense): CGTTTTGCAATGCAGATGTA; human S100A4 (sense): GATGAGCAACTTGGACAGCAA; human S100A4 (antisense):
CTGGGCTGCTTATCTGGGAAG; human CTGF (sense): CAGCATGGACGTTCGTCTG; human CTGF (antisense): AACCACGGTTTGGTCCTTGG; human CDH1 (sense):
CGAGAGCTACACGTTCACGG; human CDH1 (antisense): GGGTGTCGAGGGAAAAATAGG; human GAPDH (sense): GTCGGTGTGAACGGATTTG; human GAPDH (antisense): TCCCATTCTCAGCCTTGAC. For MALAT1/Malat1, mouse and human Taqman assays were used (Thermo Fisher Scientific, Waltham, MA). Gene expression levels were normalized to β-actin/GAPDH and quantified using the delta delta Ct method.
GapmeRs
The LNA GapmeRs for MALAT1 (human MALAT1: transcript variant ENST00000619449, mouse Malat1: transcript variant ESMUST00000173314) were obtained from Exiqon (Vedbaek, Denmark). Negative control A GapmeR was used as negative control.
Western blot
Western blot was performed on lysates harvested in lysis buffer (50 mM Tris-HCl pH7.5, 150 mM NaCl, 1% SDS, 0.5% deoxycholate, and 0.5% Triton X-100) with the addition of protease inhibitors (cOmplete protease inhibitor cocktail, Roche, Basel, Switzerland). BCA Protein Assay Kit (Pierce) was used to determine total protein content, and 5–20 μg. of total protein was applied on Any-kD Mini-PROTEAN TGX Precast SDS page gels (Bio-Rad). Gels were transferred to nitrocellulose membranes using the Trans-Blot Turbo Transfer System (Bio-Rad) and blocked with 5% non-fat milk powder in PBS with 0.01% of Tween (PBST). Primary antibodies against the following proteins were used: α-SMA (R&D, Minneapolis, MN, USA) and GAPDH (Cell Signaling Technology, Leiden, The Netherlands). Membranes were incubated with these primary antibodies overnight at 4°C, followed by incubation with appropriate secondary HRP-labeled antibodies for 1 h at room temperature. Upon PBST washing, membranes were incubated with SuperSignal West Dura Chemiluminescent Substrate (Thermo Fisher Scientific). Quantification of the protein bands was performed using ImageJ software and normalized to GAPDH.
RNA immunoprecipitation
Human SUZ12 was immunoprecipitated from HUVECs. All immunoprecipitations performed using the MagnaRIP RNA-Binding Protein Immunoprecipitation Kit (EMD Millipore, Burlington, MA) according to the manufacturers’ instructions. Briefly, an antibody targeting human SUZ12 (Cell Signaling, ab12073) or an isotype-matched control antibody (Sigma, 12–370) were bound to magnetic beads and incubated with lysed cells at 4°C for 24 h. Beads were isolated and cleaved from the bound proteins by proteinase K, and coprecipitated RNA was purified. qPCR analysis of total RNA was performed to detect enrichment of MALAT1 and control genes in the protein-of-interest precipitated fraction, which was determined as percentage of 1% input control.
Immunohistochemistry
Kidneys were fixed in 4% PFA for 1 h at 4°C, cryopreserved in 20% sucrose, and frozen in liquid nitrogen. Five micrometer sections were stained for α-SMA, MECA32, and F4/80 using a mouse fluorescein isothiocyanate-conjugated antibody against α-SMA (Sigma) or a specific antibody against MECA32 (Becton Dickinson, Franklin Lakes, New Jersey, USA) or F4/80 (Abcam, Cambridge, UK) followed by the corresponding Alexa or NovaRed-secondary antibodies (Invitrogen). For Picro Sirus Red (PSR), kidneys were sagittally sectioned and fixed in 10% neutral buffered formalin, processed, and embedded in paraffin wax. After sectioning, sections were dehydrated, xylene-cleared and mounted, and imaged using 3DHISTECH Pannoramic MIDI Scanner (Sysmex). PSR staining for collagen deposition was performed by 1 h incubation of the sections in 0.1% Sirius Red solution, followed by 2 rinses in 1% acidic acid. Stainings were quantified over the entire kidney section using HistoQuant software (3DHISTECH, Hungary) or ImageJ software (NIH, Bethesda, MD, USA). Staining of HUVECs for VE-cadherin and F-actin was performed by seeding HUVECs on an 8-well ibidi plate and fixation with 4% paraformaldehyde (Added Pharma, 1642810) and permeabilization with Triton X-100 (Merck, 11869). 5% BSA was used to block non-specific antigens. HUVECs were incubated with mouse anti-human CD144 (VE-cadherin) primary antibody (BD Pharmingen, 555661) and then secondary IgG goat anti-mouse Alexa 488 (Invitrogen, A32723), 1:200 Phalloidin-TRITC (Sigma, P1951), and 1:2,000 Hoechst (Thermo Fisher, H3569). Images were taken using a high-content confocal microscope (Molecular Devices, ImageXpress Micro Confocal) for determining FAJs: the cell boundary was identified by VE-cadherin staining, and the length of the boundary was measured using the length measurement tool in ImageJ. The cell-cell junction pattern is determined by the relative orientation of F-actin and membrane. The F-actin on FAJ was perpendicular to the cell membrane, while the F-actin bundle is parallel to the cell membrane on mature junctions.21 The FAJ length of the selected cell was measured, and the ratio of the FAJ length to the total length was calculated to obtain the FAJ ratio of each cell.
Vascular leakage and angiogenesis assay
HUVECs were used to culture 3D capillary-like vessels on the Organoplate microfluidic system (Mimetas, 9603-400B) and used to assess leakage/permeability based on the method described by van Duinen et al.29 After 7 days, the capillary-like vessels were used to assess permeability by adding Alexa 555-labeled albumin (75 μg/mL, Invitrogen, A34786) to the perfusion channel followed by assessment of leakage of albumin to the gel channel over a time period of 30 min using the ImageXpress confocal microscope (Molecular Devices). Quantification of vascular leakage was performed using ImageJ as previously described.29 For the angiogenesis assay, the same platform with the 3D capillary-like vessels on the Organoplate microfluidic system was used and performed as described previously.54 In short, angiogenic sprouts were stimulated with VEGF + bFGF + S1P for 4 days, where the last 48 h were in the presence of MALAT1 and control GapmeRs. Angiogenic factors were used in the following concentrations: 50 ng/mL for VEGF, 50 ng/mL for bFGF, and 500 nM for S1P. Sprouting was visualized with phalloidin staining (Sigma-Aldrich) and imaged using a high-content confocal microscope (Molecular Devices, ImageXpress Micro Confocal). Total sprouting area and average sprouting length were quantified using ImageJ by manually determining the distance between the microvessel and the (tip) cell sprouting furthest into the gel.
Endothelial barrier function
Using the electric cell-substrate impedance sensing system (ECIS Zθ, Applied Biophysics) and ECIS plates (96W20idf PET, Applied Biophysics), endothelial barrier function was assessed by measuring trans-endothelial electrical resistance, as previously described.53 Multiple frequency/time (MFT) mode was used to assess the barrier, and results are expressed as relative resistance at a frequency of 4,000 Hz. Using impedance data, the ECIS software was used for further mathematical modeling to calculate the cell morphological parameters of cell-cell (Rb) and cell-matrix (α) contacts.
ELISA
The mouse Pro-Collagen I alpha 1 ELISA (Abcam) was performed on cell lysates according to the instructions of the manufacturer.
Mitochondrial respiration
Oxygen consumption rate and corresponding analyses of basal respiration, maximal respiration, and proton leak were measured using the Seahorse XF96 analyzer and its associated Wave software (Agilent Technologies).
ISH
Chromogenic in situ detection was performed on formalin-fixed paraffin-embedded mouse kidney tissue sections using the RNAscope ISH technology (Advanced Cell Diagnostics, Bio-Techne, Minneapolis, MN). 5-μm sections were used and deparaffinized followed by boiling with RNAscope Target Retrieval Reagent for 15 min at 99°C and subsequent protease digestion for 30 min at 40°C. Hybridization was performed at 40°C for 2 h with RNAscope Probe - Mm-Malat1 (313391, Advanced Cell Diagnostics). RNAscope Negative Control Probe_dapB (310043) and RNAscope Probe - Mm-Ppib (313911) were used as negative and positive controls, respectively. RNAscope 2.5 HD Reagent Kit (Brown) (322310) was used to visualize the bound probes.
RNA-seq and pathway analysis
RNA-seq on FACS-sorted tdTomato-positive cells from mouse kidneys after the mice were treated with MALAT1 or control GapmeR was performed by Novogene (Cambridge, UK). In short, sequencing libraries were generated using NEBNext Ultra RNA Library Prep Kit for Illumina (NEB, USA) following manufacturer’s recommendations. mRNA was purified from total RNA using poly-T oligo-attached magnetic beads. Fragmentation was carried out using divalent cations under elevated temperature in NEBNext First Strand Synthesis Reaction Buffer (5×). First strand cDNA was synthesized using random hexamer primer and M-MuLV Reverse Transcriptase (RNase H). Second strand cDNA synthesis was subsequently performed using DNA polymerase I and RNase H. Remaining overhangs were converted into blunt ends via exonuclease/polymerase activities. After adenylation of 3′ ends of DNA fragments, NEBNext adaptor with hairpin loop structure was ligated to prepare for hybridization. In order to select cDNA fragments of preferentially 150–200 bp in length, the library fragments were purified with AMPure XP system (Beckman Coulter, Beverly, USA). Then 3 μL USER Enzyme (NEB, USA) was used with size-selected, adaptor-ligated cDNA at 37°C for 15 min followed by 5 min at 95°C before PCR. Then PCR was performed with Phusion high-fidelity DNA polymerase, universal PCR primers, and index (X) primer. At last, PCR products were purified (AMPure XP system) and library quality was assessed on the Agilent Bioanalyzer 2100 system. Sequencing reads were aligned to the mouse genome (GRCm39 M33) using STAR (v.2.7.7a). Mapped reads were quantified for genomic features with featureCounts. For differential expression analysis, a quasi-likelihood negative binomial generalized log-linear model was applied using the edgeR package (v.3.18) in R (v.4.4.0). Read counts were normalized using the trimmed mean of M values method. Genes were considered differentially expressed if the contrast between conditions reached statistical significance, defined by a false discovery rate-adjusted p value of less than 0.05. All statistical analyses were performed in R. Normalized data were used for gene set and GO enrichment analysis. Additional (pathway) analyses, where indicated, were performed using Enrichr,55 IPA software, and Rummagene.24 Morpheus tool (Broad institute, https://software.broadinstitute.org/morpheus/) was used to visualize gene expression in a max projection heatmap.56
Transcription factor motif enrichment, CatRAPID, and Hi-C
Discovery of enriched transcription factor-binding site motifs within lncRNA promoter regions (defined as 2,000 bp upstream of transcription start site) was performed by the analysis of motif enrichment tool,57 using the JASPAR general database. The catRAPID algorithm58 was used to determine RNA-binding domains and RNA-binding proteins in Malat1 and MALAT1. We visualized the 3-dimensional (3D) architecture within the genetic locus of MALAT1 and Malat1 using high-throughput chromosome conformation capture (Hi-C)59 (data from the 3D genome browser23) in HUVECs60 and C2C12.61
ChIP
MEECs were used to perform ChIP on HMGA1 with 10 μg anti-HMGA1a/HMGA1b antibody-ChIP grade (ab4078, Abcam) or negative control IgG using the EZ-Magna ChIP A/G chromatin immunoprecipitation kit (Merck-Millipore) according to the instructions of the manufacturer. Malat1 DNA was determined using qPCR. Data were normalized to IgG-negative controls. PCR primers that were used to detect Malat1 promoter region on the immunoprecipitated DNA were as follows: mmu-Malat1-promoter-bus/ree-fw AGCTTTAATCCAGCACTTGTGTAAG; mmu-Malat1-promoter-bus/ree-rev GGAGGTCCAGTGTTAGACCATT; mmu-Malat1-promoter-jaspar-fw GACGGGTTCCGCGGTC; mmu-Malat1-promoter-jaspar-rev CCAGGTCTATCTCATCGCTTCC; mmu-Malat1-promoter-manabe-fw GAAACATCTGAAAAACTTGGGGCT; mmu-Malat1-promoter-manabe-rev GGCCTCTTGGACCTTGCTAATA.
Statistical analyses
Results are expressed as mean ± standard error of the mean (SEM), unless otherwise indicated. Statistical analyses were performed using student’s t test or one-way ANOVA, and specific tests used have been indicated in the figure legends. p < 0.05 was considered statistically significant. For IPA, bias corrected Z scores were determined with a Z score higher than 2 or lower than −2 being considered statistically significant.
Data availability
The authors declare that the main data supporting the findings of this study are available within the article and its supplemental information files, extra data are available from the corresponding author upon request.
Acknowledgments
R.B. was supported by grants from the Dutch Kidney Foundation (14OIP13 and 20OK015) and EFSD/Novo Nordisk Foundation Future Leaders Awards Program (NNF23SA0087433). R.B. and A.J.v.Z. were further supported by a grant from the European Foundation for the Study of Diabetes (EFSD). C.v.S. is supported by a grant from the American Heart Association (23SCEFIA1153739).
Author contributions
Conceptualization, R.B., A.J.v.Z., and C.v.S.; methodology, R.B., Q.Z., L.A.K.v.d.P., M.G., D.P., W.G.R., H.K., A.M.v.O.-R., A.L., J.A.d.K., R.C.S., and L.M.H.; investigation, R.B., Q.Z., W.S., A.K., J.M.G.J.D., J.A.d.K., and L.A.K.v.d.P.; writing – original draft, R.B. and Q.Z.; writing – review and editing, R.B., C.v.S., A.J.v.Z., and J.I.R.; funding acquisition, R.B. and A.J.v.Z.; supervision, R.B., A.J.v.Z., and C.v.S.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omtn.2025.102689.
Supplemental information
References
- 1.GBD Chronic Kidney Disease Collaboration Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levey A.S., Coresh J. Chronic kidney disease. Lancet. 2012;379:165–180. doi: 10.1016/S0140-6736(11)60178-5. [DOI] [PubMed] [Google Scholar]
- 3.Kalantar-Zadeh K., Jafar T.H., Nitsch D., Neuen B.L., Perkovic V. Chronic kidney disease. Lancet. 2021;398:786–802. doi: 10.1016/S0140-6736(21)00519-5. [DOI] [PubMed] [Google Scholar]
- 4.Long D.A., Norman J.T., Fine L.G. Restoring the renal microvasculature to treat chronic kidney disease. Nat. Rev. Nephrol. 2012;8:244–250. doi: 10.1038/nrneph.2011.219. [DOI] [PubMed] [Google Scholar]
- 5.Choi Y.J., Chakraborty S., Nguyen V., Nguyen C., Kim B.K., Shim S.I., Suki W.N., Truong L.D. Peritubular capillary loss is associated with chronic tubulointerstitial injury in human kidney: altered expression of vascular endothelial growth factor. Hum. Pathol. 2000;31:1491–1497. doi: 10.1053/hupa.2000.20373. [DOI] [PubMed] [Google Scholar]
- 6.Seron D., Alexopoulos E., Raftery M.J., Hartley B., Cameron J.S. Number of interstitial capillary cross-sections assessed by monoclonal antibodies: relation to interstitial damage. Nephrol. Dial. Transplant. 1990;5:889–893. doi: 10.1093/ndt/5.10.889. [DOI] [PubMed] [Google Scholar]
- 7.Rabelink T.J., Wijewickrama D.C., de Koning E.J. Peritubular endothelium: the Achilles heel of the kidney? Kidney Int. 2007;72:926–930. doi: 10.1038/sj.ki.5002414. [DOI] [PubMed] [Google Scholar]
- 8.Ishii Y., Sawada T., Kubota K., Fuchinoue S., Teraoka S., Shimizu A. Injury and progressive loss of peritubular capillaries in the development of chronic allograft nephropathy. Kidney Int. 2005;67:321–332. doi: 10.1111/j.1523-1755.2005.00085.x. [DOI] [PubMed] [Google Scholar]
- 9.Lorenzen J.M., Thum T. Long noncoding RNAs in kidney and cardiovascular diseases. Nat. Rev. Nephrol. 2016;12:360–373. doi: 10.1038/nrneph.2016.51. [DOI] [PubMed] [Google Scholar]
- 10.Bink D.I., Pauli J., Maegdefessel L., Boon R.A. Endothelial microRNAs and long noncoding RNAs in cardiovascular ageing. Atherosclerosis. 2023;374:99–106. doi: 10.1016/j.atherosclerosis.2023.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Mattick J.S., Amaral P.P., Carninci P., Carpenter S., Chang H.Y., Chen L.L., Chen R., Dean C., Dinger M.E., Fitzgerald K.A., et al. Long non-coding RNAs: definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023;24:430–447. doi: 10.1038/s41580-022-00566-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang P., Luo M.L., Song E., Zhou Z., Ma T., Wang J., Jia N., Wang G., Nie S., Liu Y., et al. Long noncoding RNA lnc-TSI inhibits renal fibrogenesis by negatively regulating the TGF-beta/Smad3 pathway. Sci. Transl. Med. 2018;10:eaat2039. doi: 10.1126/scitranslmed.aat2039. [DOI] [PubMed] [Google Scholar]
- 13.Bijkerk R., Au Y.W., Stam W., Duijs J.M.G.J., Koudijs A., Lievers E., Rabelink T.J., van Zonneveld A.J. Long Non-coding RNAs Rian and Miat Mediate Myofibroblast Formation in Kidney Fibrosis. Front. Pharmacol. 2019;10:215. doi: 10.3389/fphar.2019.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanicek L., Lozano-Vidal N., Bink D.I., Hooglugt A., Yao W., Wittig I., van Rijssel J., van Buul J.D., van Bergen A., Klems A., et al. Long non-coding RNA LASSIE regulates shear stress sensing and endothelial barrier function. Commun. Biol. 2020;3:265. doi: 10.1038/s42003-020-0987-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y., Nakayama M., Pitulescu M.E., Schmidt T.S., Bochenek M.L., Sakakibara A., Adams S., Davy A., Deutsch U., Lüthi U., et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465:483–486. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- 16.Groeneweg K.E., Au Y.W., Duijs J.M.G.J., Florijn B.W., van Kooten C., de Fijter J.W., Reinders M.E.J., van Zonneveld A.J., Bijkerk R. Diabetic nephropathy alters circulating long noncoding RNA levels that normalize following simultaneous pancreas-kidney transplantation. Am. J. Transplant. 2020;20:3451–3461. doi: 10.1111/ajt.15961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hopper R.K., Moonen J.R.A.J., Diebold I., Cao A., Rhodes C.J., Tojais N.F., Hennigs J.K., Gu M., Wang L., Rabinovitch M. Pulmonary Arterial Hypertension, Reduced BMPR2 Promotes Endothelial-to-Mesenchymal Transition via HMGA1 and Its Target Slug. Circulation. 2016;133:1783–1794. doi: 10.1161/CIRCULATIONAHA.115.020617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi C., Bin L., Yang Y., Yang Y., Li J., Zhou Q., Wen Y., Zeng C., Zheng L., Zhang Q., et al. Glipizide suppresses prostate cancer progression in the TRAMP model by inhibiting angiogenesis. Sci. Rep. 2016;6 doi: 10.1038/srep27819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camos S., Gubern C., Sobrado M., Rodriguez R., Romera V.G., Moro M.A., Lizasoain I., Serena J., Mallolas J., Castellanos M. The high-mobility group I-Y transcription factor is involved in cerebral ischemia and modulates the expression of angiogenic proteins. Neuroscience. 2014;269:112–130. doi: 10.1016/j.neuroscience.2014.03.036. [DOI] [PubMed] [Google Scholar]
- 20.Wilson P.C., Wu H., Kirita Y., Uchimura K., Ledru N., Rennke H.G., Welling P.A., Waikar S.S., Humphreys B.D. The single-cell transcriptomic landscape of early human diabetic nephropathy. Proc. Natl. Acad. Sci. USA. 2019;116:19619–19625. doi: 10.1073/pnas.1908706116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huveneers S., Oldenburg J., Spanjaard E., van der Krogt G., Grigoriev I., Akhmanova A., Rehmann H., de Rooij J. Vinculin associates with endothelial VE-cadherin junctions to control force-dependent remodeling. J. Cell Biol. 2012;196:641–652. doi: 10.1083/jcb.201108120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang B., Arun G., Mao Y.S., Lazar Z., Hung G., Bhattacharjee G., Xiao X., Booth C.J., Wu J., Zhang C., Spector D.L. The lncRNA Malat1 is dispensable for mouse development but its transcription plays a cis-regulatory role in the adult. Cell Rep. 2012;2:111–123. doi: 10.1016/j.celrep.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y., Song F., Zhang B., Zhang L., Xu J., Kuang D., Li D., Choudhary M.N.K., Li Y., Hu M., et al. The 3D Genome Browser: a web-based browser for visualizing 3D genome organization and long-range chromatin interactions. Genome Biol. 2018;19:151. doi: 10.1186/s13059-018-1519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clarke D.J.B., Marino G.B., Deng E.Z., Xie Z., Evangelista J.E., Ma'ayan A., Rummagene A. massive mining of gene sets from supporting materials of biomedical research publications. Commun. Biol. 2024;7:482. doi: 10.1038/s42003-024-06177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.G Hendrickson D., Kelley D.R., Tenen D., Bernstein B., Rinn J.L. Widespread RNA binding by chromatin-associated proteins. Genome Biol. 2016;17:28. doi: 10.1186/s13059-016-0878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan Y., Shen B., Tan M., Mu X., Qin Y., Zhang F., Liu Y. TGF-beta-induced upregulation of malat1 promotes bladder cancer metastasis by associating with suz12. Clin. Cancer Res. 2014;20:1531–1541. doi: 10.1158/1078-0432.CCR-13-1455. [DOI] [PubMed] [Google Scholar]
- 27.West J.A., Davis C.P., Sunwoo H., Simon M.D., Sadreyev R.I., Wang P.I., Tolstorukov M.Y., Kingston R.E. The long noncoding RNAs NEAT1 and MALAT1 bind active chromatin sites. Mol. Cell. 2014;55:791–802. doi: 10.1016/j.molcel.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bijkerk R., de Bruin R.G., van Solingen C., Duijs J.M.G.J., Kobayashi K., van der Veer E.P., ten Dijke P., Rabelink T.J., Goumans M.J., van Zonneveld A.J. MicroRNA-155 functions as a negative regulator of RhoA signaling in TGF-beta-induced endothelial to mesenchymal transition. MicroRNA. 2012;1:2–10. doi: 10.2174/2211536611201010002. [DOI] [PubMed] [Google Scholar]
- 29.van Duinen V., van den Heuvel A., Trietsch S.J., Lanz H.L., van Gils J.M., van Zonneveld A.J., Vulto P., Hankemeier T. 96 perfusable blood vessels to study vascular permeability in vitro. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-14716-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goligorsky M.S. Permissive role of vascular endothelium in fibrosis: focus on the kidney. Am. J. Physiol. Cell Physiol. 2024;326:C712–C723. doi: 10.1152/ajpcell.00526.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michalik K.M., You X., Manavski Y., Doddaballapur A., Zörnig M., Braun T., John D., Ponomareva Y., Chen W., Uchida S., et al. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ. Res. 2014;114:1389–1397. doi: 10.1161/CIRCRESAHA.114.303265. [DOI] [PubMed] [Google Scholar]
- 32.Wang C., Qu Y., Suo R., Zhu Y. Long non-coding RNA MALAT1 regulates angiogenesis following oxygen-glucose deprivation/reoxygenation. J. Cell Mol. Med. 2019;23:2970–2983. doi: 10.1111/jcmm.14204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J.Y., Yao J., Li X.M., Song Y.C., Wang X.Q., Li Y.J., Yan B., Jiang Q. Pathogenic role of lncRNA-MALAT1 in endothelial cell dysfunction in diabetes mellitus. Cell Death Dis. 2014;5 doi: 10.1038/cddis.2014.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu S.X., Zheng F., Xie K.L., Xie M.R., Jiang L.J., Cai Y. Exercise Reduces Insulin Resistance in Type 2 Diabetes Mellitus via Mediating the lncRNA MALAT1/MicroRNA-382-3p/Resistin Axis. Mol. Ther. Nucleic Acids. 2019;18:34–44. doi: 10.1016/j.omtn.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao R.W., Wang Y., Chen L.L. Cellular functions of long noncoding RNAs. Nat. Cell Biol. 2019;21:542–551. doi: 10.1038/s41556-019-0311-8. [DOI] [PubMed] [Google Scholar]
- 36.Lino Cardenas C.L., Kessinger C.W., Cheng Y., MacDonald C., MacGillivray T., Ghoshhajra B., Huleihel L., Nuri S., Yeri A.S., Jaffer F.A., et al. An HDAC9-MALAT1-BRG1 complex mediates smooth muscle dysfunction in thoracic aortic aneurysm. Nat. Commun. 2018;9:1009. doi: 10.1038/s41467-018-03394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yin Y., Lu J.Y., Zhang X., Shao W., Xu Y., Li P., Hong Y., Cui L., Shan G., Tian B., et al. U1 snRNP regulates chromatin retention of noncoding RNAs. Nature. 2020;580:147–150. doi: 10.1038/s41586-020-2105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aguilar R., Rosenberg M., Levy V., Lee J.T. An evolving landscape of PRC2-RNA interactions in chromatin regulation. Nat. Rev. Mol. Cell Biol. 2025;26:631–642. doi: 10.1038/s41580-025-00850-3. [DOI] [PubMed] [Google Scholar]
- 39.Dunn-Davies H., Dudnakova T., Baker A.H., Mitić T. Epigenetic control of vascular endothelial function revealed by multi-omics. Eur. Heart J. 2024;45:642–644. doi: 10.1093/eurheartj/ehad820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dunn-Davies H., Dudnakova T., Nogara A., Rodor J., Thomas A.C., Parish E., Gautier P., Meynert A., Ulitsky I., Madeddu P., et al. Control of endothelial cell function and arteriogenesis by MEG3:EZH2 epigenetic regulation of integrin expression. Mol. Ther. Nucleic Acids. 2024;35:102173. doi: 10.1016/j.omtn.2024.102173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morini M.F., Giampietro C., Corada M., Pisati F., Lavarone E., Cunha S.I., Conze L.L., O'Reilly N., Joshi D., Kjaer S., et al. VE-Cadherin-Mediated Epigenetic Regulation of Endothelial Gene Expression. Circ. Res. 2018;122:231–245. doi: 10.1161/CIRCRESAHA.117.312392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu P., Zhang B., Chen Z., He Y., Du Y., Liu Y., Chen X. A-induced lncRNA MALAT1 aggravates renal fibrogenesis in obstructive nephropathy through the miR-145/FAK pathway. Aging. 2020;12:5280–5299. doi: 10.18632/aging.102950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xia W., Chen X., Zhu Z., Chen H., Li B., Wang K., Huang L., Liu Z., Chen Z. Knockdown of lncRNA MALAT1 attenuates renal interstitial fibrosis through miR-124-3p/ITGB1 axis. Sci. Rep. 2023;13 doi: 10.1038/s41598-023-45188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tripathi V., Ellis J.D., Shen Z., Song D.Y., Pan Q., Watt A.T., Freier S.M., Bennett C.F., Sharma A., Bubulya P.A., et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol. Cell. 2010;39:925–938. doi: 10.1016/j.molcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cui H., Banerjee S., Guo S., Xie N., Ge J., Jiang D., Zörnig M., Thannickal V.J., Liu G. Long noncoding RNA Malat1 regulates differential activation of macrophages and response to lung injury. JCI Insight. 2019;4 doi: 10.1172/jci.insight.124522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu M., Wang R., Li X., Fan M., Lin J., Zhen J., Chen L., Lv Z. LncRNA MALAT1 is dysregulated in diabetic nephropathy and involved in high glucose-induced podocyte injury via its interplay with beta-catenin. J. Cell Mol. Med. 2017;21:2732–2747. doi: 10.1111/jcmm.13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kolling M., Genschel C., Kaucsar T., Hubner A., Rong S., Schmitt R., Sorensen-Zender I., Haddad G., Kistler A., Seeger H., et al. Hypoxia-induced long non-coding RNA Malat1 is dispensable for renal ischemia/reperfusion-injury. Sci. Rep. 2018;8:3438. doi: 10.1038/s41598-018-21720-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eißmann M., Gutschner T., Hämmerle M., Günther S., Caudron-Herger M., Groß M., Schirmacher P., Rippe K., Braun T., Zörnig M., Diederichs S. Loss of the abundant nuclear non-coding RNA MALAT1 is compatible with life and development. RNA Biol. 2012;9:1076–1087. doi: 10.4161/rna.21089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fawzy M.S., Abu AlSel B.T., Al Ageeli E., Al-Qahtani S.A., Abdel-Daim M.M., Toraih E.A. Long non-coding RNA MALAT1 and microRNA-499a expression profiles in diabetic ESRD patients undergoing dialysis: a preliminary cross-sectional analysis. Arch. Physiol. Biochem. 2020;126:172–182. doi: 10.1080/13813455.2018.1499119. [DOI] [PubMed] [Google Scholar]
- 50.Uil M., Hau C.M., Ahdi M., Mills J.D., Kers J., Saleem M.A., Florquin S., Gerdes V.E.A., Nieuwland R., Roelofs J.J.T.H. Cellular origin and microRNA profiles of circulating extracellular vesicles in different stages of diabetic nephropathy. Clin. Kidney J. 2021;14:358–365. doi: 10.1093/ckj/sfz145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodrigues K.F., Pietrani N.T., Fernandes A.P., Bosco A.A., de Sousa M.C.R., de Fátima Oliveira Silva I., Silveira J.N., Campos F.M.F., Gomes K.B. Circulating microparticles levels are increased in patients with diabetic kidney disease: A case-control research. Clin. Chim. Acta. 2018;479:48–55. doi: 10.1016/j.cca.2017.12.048. [DOI] [PubMed] [Google Scholar]
- 52.Burger D., Turner M., Xiao F., Munkonda M.N., Akbari S., Burns K.D. High glucose increases the formation and pro-oxidative activity of endothelial microparticles. Diabetologia. 2017;60:1791–1800. doi: 10.1007/s00125-017-4331-2. [DOI] [PubMed] [Google Scholar]
- 53.Vreeken D., Bruikman C.S., Stam W., Cox S.M.L., Nagy Z., Zhang H., Postma R.J., van Zonneveld A.J., Hovingh G.K., van Gils J.M. Downregulation of Endothelial Plexin A4 Under Inflammatory Conditions Impairs Vascular Integrity. Front. Cardiovasc. Med. 2021;8 doi: 10.3389/fcvm.2021.633609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Duinen V., Stam W., Borgdorff V., Reijerkerk A., Orlova V., Vulto P., Hankemeier T., van Zonneveld A.J. Standardized and Scalable Assay to Study Perfused 3D Angiogenic Sprouting of iPSC-derived Endothelial Cells In Vitro. J. Vis. Exp. 2019 doi: 10.3791/59678. [DOI] [PubMed] [Google Scholar]
- 55.Xie Z., Bailey A., Kuleshov M.V., Clarke D.J.B., Evangelista J.E., Jenkins S.L., Lachmann A., Wojciechowicz M.L., Kropiwnicki E., Jagodnik K.M., et al. Gene Set Knowledge Discovery with Enrichr. Curr. Protoc. 2021;1:e90. doi: 10.1002/cpz1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morpheus. https://software.broadinstitute.org/morpheus
- 57.McLeay R.C., Bailey T.L. Motif Enrichment Analysis: a unified framework and an evaluation on ChIP data. BMC Bioinf. 2010;11:165. doi: 10.1186/1471-2105-11-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Armaos A., Colantoni A., Proietti G., Rupert J., Tartaglia G.G. catRAPID omics v2.0: going deeper and wider in the prediction of protein-RNA interactions. Nucleic Acids Res. 2021;49:W72–W79. doi: 10.1093/nar/gkab393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lieberman-Aiden E., van Berkum N.L., Williams L., Imakaev M., Ragoczy T., Telling A., Amit I., Lajoie B.R., Sabo P.J., Dorschner M.O., et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326:289–293. doi: 10.1126/science.1181369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rao S.S.P., Huntley M.H., Durand N.C., Stamenova E.K., Bochkov I.D., Robinson J.T., Sanborn A.L., Machol I., Omer A.D., Lander E.S., Aiden E.L. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159:1665–1680. doi: 10.1016/j.cell.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barutcu A.R., Maass P.G., Lewandowski J.P., Weiner C.L., Rinn J.L. A TAD boundary is preserved upon deletion of the CTCF-rich Firre locus. Nat. Commun. 2018;9:1444. doi: 10.1038/s41467-018-03614-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the main data supporting the findings of this study are available within the article and its supplemental information files, extra data are available from the corresponding author upon request.