Abstract
Retinopathy of prematurity (ROP) with early vessel loss (Phase I) followed by uncontrolled vessel growth (Phase II) causes visual impairment in premature infants. Although supplementation with omega-3 (n-3) docosahexaenoic acid (DHA) alone shows mixed results in preventing ROP, supplementation with both n-3 DHA and n-6 arachidonic acid (ARA) in early postnatal life reduces severe ROP by 50 % (Mega Donna Mega study). In the Mega Donna Mega study, 146 (72.6 %) of 201 included infants had at least one hyperglycemic episode during the first 14 days of life, which is a strong ROP risk factor. We therefore evaluated the protective effects and mechanisms of combined dietary n-3 DHA and n-6 ARA in a neonatal mouse model of hyperglycemia-induced suppression of retinal vascular development (Phase I ROP). At postnatal day (P) 10, retinal vessel growth was improved in pups from mothers on diets enriched with 1 % DHA + 2 % ARA vs. 3 % DHA. Lipid changes in pup plasma and RPE complex (retinal pigment epithelium with choroid and sclera) were in accordance with maternal diets’ DHA and ARA levels, indicating that milk lipids reflected maternal diets. Proteomic retinal analysis revealed increased abundances of proteins related to mitochondrial respiration and glucose metabolism with the combined diet. Inhibition of mitochondrial ATP synthase negated the protective effects of the combined diet. In conclusion, combined DHA+ARA oral maternal supplementation protects against hyperglycemia-induced retinopathy in mouse neonates (Phase I ROP model) through enhanced retinal metabolism, suggesting the potential of balanced lipid supplementation for ROP prevention.
Keywords: Postnatal hyperglycemia, Retinopathy of prematurity, DHA, ARA, Retinal vasculature
1. Introduction
Retinopathy of prematurity (ROP) is a potentially blinding eye disorder [1] characterized by two phases. In Phase I, physiological retinal vascular growth and neuroretinal development are suppressed [2]; in Phase II, pathological retinal neovascularization is triggered by both local hypoxia in vaso-obliterated retinal areas and oxygen-independent stimuli [3].
Metabolic factors modulate ROP development and progression [4,5]. In Phase I, perinatal hyperglycemia is common, and alterations in omega-3 (n-3) and omega-6 (n-6) long-chain polyunsaturated fatty acid (LCPUFA) levels, particularly n-3 docosahexaenoic acid (DHA) and n-6 arachidonic acid (ARA), occur, as the normal supply from the uterine environment is disrupted.
DHA and ARA, “conditionally essential” fatty acids, are crucial during fetal [6] and postnatal development [7], particularly in the retina [8]. DHA makes up ~ 50 % of total phospholipid fatty acids in retinal photoreceptor outer segments [9], while ARA is enriched in photoreceptors and retinal vessels [10]. After preterm birth, DHA and ARA levels fall dramatically due to disrupted in utero supply of these lipids. Reduced DHA and ARA blood levels persist for at least 4 weeks after birth [11,12] and are associated with increased ROP severity [13,14]. Clinical trials have explored the potential of nutritional/metabolic interventions in ROP [15]. Parenteral supplementation of n-3 LCPUFAs alone yields inconsistent results regarding ROP development and progression [16–22]. The DIAMOND (DHA Intake And Measurement Of Neural Development) study found that supplementation of DHA and ARA (1:2 ratio) in early life improved visual acuity at 12 months of age. Increased DHA supplementation, however, yielded no additional improvement of visual acuity [23]. The recent Mega Donna Mega study showed that enteral supplementation immediately after birth with n-3 DHA (50 mg/kg/d) and n-6 ARA (100 mg/kg/d) at a 1:2 ratio reduced severe ROP incidence by 50 % in extremely preterm infants (< 28 weeks of gestational age) [24], suggesting that the balance between DHA and ARA may be crucial in protecting against ROP. However, the underlying mechanisms are unknown.
Hyperglycemia in the early postnatal period after preterm birth, seen in 45 % of preterm infants with birth weight < 1000 g [25] and 80 % of preterm infants with birth weight < 750 g [26], is associated with increased ROP incidence and severity [27–32]. In this study, we examined postnatal hyperglycemic status in the Mega Donna Mega trial. We tested a balanced maternal rodent diet containing 1 % DHA + 2 % ARA vs. 3 % DHA in hyperglycemia-associated retinopathy in pups, modeling Phase I ROP [33–36]. We have previously shown that maternal diet composition is reflected in milk-fed pups’ retinas [37]. We assessed the impact of DHA+ARA vs. DHA maternal diet on pup retinal vascular development, fatty acid profile of retina, retinal pigment epithelium (RPE) complex, and plasma, as well as retinal proteome, to uncover protective mechanisms of combined nutritional n-3 and n-6 LCPUFAs as a potential strategy for prevention and treatment of Phase I ROP.
The primary aim of this study was to determine whether combined maternal DHA+ARA supplementation provides benefit over DHA alone in mouse models of ROP, as well as to investigate the underlying mechanisms. The 1:2 DHA:ARA ratio was chosen according to the Mega Donna Mega [24] and the DIAMOND [23] clinical trials. We hypothesized that maternal DHA+ARA vs. DHA may be protective in murine ROP models.
2. Materials and methods
2.1. Ethics statement
Mouse experiments followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication 8523, revised 2011) and the ARVO Statement for Use of Animals in Ophthalmic Vision and Research. The work was approved by Boston Children’s Hospital’s Institutional Animal Care and Use Committee (protocol 0001619).
The previously published Mega Donna Mega study, an open-label, randomized controlled trial (ClinicalTrials.gov Identifier: NCT03201588) aimed to evaluate if a combined enteral fatty acid supplementation with DHA and ARA could reduce severe ROP [13,24,38]. The Regional Ethics Review Board in Gothenburg (303–11 and T570–15) and the Swedish Ethical Review Authority (2020–02381) approved the trial protocol. All procedures followed the Helsinki Declaration of 1975 (revised 2008). The WHO Guiding Principles on Human Cell, Tissue and Organ Transplantation are not applicable. Parents or guardians provided written informed consent. We analyzed longitudinal glucose blood samples of infants included in the study born before 28 weeks of gestational age at three neonatal intensive care units in Sweden between 2016 and 2019.
2.2. Mouse model of hyperglycemia-associated retinopathy and quantification
For all animal experiments, C57BL/6J mice (#000664, Jackson Laboratory, Bar Harbor, ME, USA) were bred in-house with 12-hour light/dark cycle and ad libitum chow. Because hyperglycemia in the early postnatal period is a strong ROP risk factor, and as most infants in our clinical study had at least one incidence of hyperglycemia, we used the neonatal hyperglycemia-associated retinopathy mouse model to study lipid intervention in early ROP. The dietary ratio of DHA:ARA (1:2) used in mice was aligned with the Mega Donna Mega clinical trial [24]. To induce hyperglycemia-associated retinopathy (a Phase I ROP model with suppression of physiological retinal vascular growth), mouse pups were intraperitoneally injected with 50 μg/g body weight streptozotocin (STZ, S0130, Sigma-Aldrich, St. Louis, MO, USA; 50 μg/μL in PBS) daily from postnatal day (P) 1–P9 [33]. C57BL/6J moms were fed fully defined rodent feed (Research Diets, Inc., New Brunswick, NJ, USA) including either 3 % DHA or 1 % DHA + 2 % ARA (DHA+ARA) of the diets’ lipid content (10 % of the chow) from P1–P10 (Table 1). DHA and ARA oils were sourced from DSM (Heerlen, Netherlands). At P10, after using a lethal intraperitoneal injection of ketamine/xylazine, plasma, RPE complex (retinal pigment epithelium with choroid and sclera), and retinas of pups with body weight ≥ 3.8 g were collected.
Table 1.
Composition of the rodent diets containing either 3 % DHA or 1 % DHA + 2 % ARA of the diets’ lipid content (10 % of the chow).
| Diet name | 1 % DHA + 2 % ARA | 3 % DHA | ||
|---|---|---|---|---|
|
| ||||
| Product # | D21032502-38 | D21032503S | ||
| Macronutrients | gm% | kcal% | gm% | kcal% |
| Protein | 21 | 20 | 21 | 20 |
| Carbohydrate | 59.9 | 58 | 59.9 | 58 |
| Fat | 10 | 22 | 10 | 22 |
| Total | 100 | 100 | ||
| kcal/gm | 4.1 | 4.1 | ||
| Ingredients | gm | kcal | gm | kcal |
| Casein | 200 | 800 | 200 | 800 |
| L-Cystine | 3 | 12 | 3 | 12 |
| Corn Starch | 337 | 1348 | 337 | 1348 |
| Maltodextrin 10 | 132 | 528 | 132 | 528 |
| Sucrose | 100 | 400 | 100 | 400 |
| Cellulose, BW200 | 50 | 0 | 50 | 0 |
| Soybean Oil | 0 | 0 | 0 | 0 |
| DHASCO (43.3 % DHA) | 2.3 | 21 | 6.8 | 61 |
| ARASCO (42.2 % ARA) | 4.6 | 41 | 0 | 0 |
| Safflower Oil, High Oleic | 90.1 | 811 | 90.3 | 813 |
| t-ButyIhydroquinone | 0.014 | 0 | 0.014 | 0 |
| Mineral Mix S10022G | 35 | 0 | 35 | 0 |
| Vitamin Mix V10037 | 10 | 40 | 10 | 40 |
| Choline Bitartrate | 2.5 | 0 | 2.5 | 0 |
| FD&C Blue Dye #1 | 0.04 | 0 | 0.04 | 0 |
| FD&C Yellow Dye #5 | 0.01 | 0 | 0.01 | 0 |
| Total | 966.564 | 4001 | 966.664 | 4002 |
| DHA (g/kg) | 1 | 3 | ||
| ARA (g/kg) | 2 | 0 | ||
DHA, docosahexaenoic acid; ARA, arachidonic acid.
For inhibition of mitochondrial ATP synthase, oligomycin A (0.25 μg/g body weight) (#11342, Cayman Chemical, Ann Arbor, MI, USA; 0.25 μg/μL in PBS with 30 % dimethylsulfoxide) [39] was intraperitoneally injected daily from P7–P9 during deep retinal vessel formation. A higher concentration of oligomycin A (0.5 μg/g body weight) in neonatal mice caused postnatal growth retardation, which affects retinal vascular development (data not shown).
After fixation of the whole eye in 4 % paraformaldehyde, retinas were dissected and permeabilized using 1 % Triton X-100 in PBS. Isolectin B4 (Alexa Fluor 594, I21413, Invitrogen™, Carlsbad, CA, USA) in 1 mM CaCl2 in PBS was used overnight to stain vessels. Four images (one per quadrant) were taken between the optic nerve head and the leading vessel edge in the deep and superficial vascular layer on a Zeiss Axio Observer Z1 microscope (200x magnification) [33]. The retinal vascular status (number of meshes and total vessel length per field) was assessed with the “Angiogenesis analyzer” ImageJ plugin. At least 3 litters per group were analyzed.
2.3. Mouse model of oxygen-induced retinopathy and quantification
To validate findings from the hyperglycemia-associated retinopathy model, maternal DHA+ARA vs. DHA diet from P1–P17 was applied in the oxygen-induced retinopathy model. C57BL/6 J pups and their nursing dams were exposed to 75 % oxygen from P7–P12 with hyperoxia leading to retinal vessel regression (vaso-obliteration, VO; modeling oxygen-induced Phase I ROP) and return to room air at P12 resulting in relative hypoxia and subsequent formation of pathological retinal neovascularization (NV; modeling hypoxia-induced Phase II ROP) [40]. At P17, both eyes of pups with body weight 5.0–7.5 g [41] were collected after intraperitoneal injection of ketamine/xylazine.
Retinas were dissected and stained as described above. Images were acquired at 50x magnification with a Zeiss fluorescent microscope. Quantification of VO and NV was performed using ImageJ [42], and the proportions of NV and VO to the total retinal area were compared between diet groups.
2.4. Analysis of postnatal hyperglycemia
In the Mega Donna Mega study, premature infants receiving combined DHA+ARA (1:2 ratio) within 3 days after birth until 40 weeks postmenstrual age vs. standard of care without oral lipid supplementation showed a 50 % reduction in severe ROP [24]. As hyperglycemia in the first weeks of life is associated with increased ROP incidence and severity [27–32], we analyzed maximum daily glucose values over the first 14 postnatal days of infants born before 28 weeks of gestational age. Of the 206 randomized infants, 201 (97.6 %) had available glucose measurements during the first 14 days. Included infants’ (n = 201) mean gestational age was 25.5 weeks (SD ± 1.4), and mean birth weight was 792.1 g (SD ± 195.1). Routine blood samples for glucose measurement were drawn according to local guidelines and clinical indications. All glucose levels were retrieved from the infants’ medical records until 14 postnatal days. When multiple glucose levels were available for an infant on the same day, the highest value during that day was used to define presence of hyperglycemia (> 10 mmol/L) [43].
2.5. Fatty acid profiling
P10 retinal samples of known weight from pups after STZ and maternal DHA+ARA vs. DHA diet (n = 18 samples/group; both retinas pooled) were disrupted with glass homogenizers. For total lipid extraction [44], the following were sequentially added: 1.75 mL 0.88 % potassium chloride, 2 mL methanol, 4 mL chloroform. Heptadecanoic acid (17:0) was used as an internal standard. Total lipids were extracted overnight and then methylated with methanolic boron trifluoride (100 °C, 1 h). Fatty acid methyl esters (FAMEs) were quantified in a gas chromatograph with a flame ionization detector (Varian, Bruker, Billerica, MA, USA). FAMEs were eluted on a DB-FFAP column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) by set program at initial oven temperature of 50 °C for 1 min, following with a ramp-up to 130 °C at a 30 °C/min rate, 175 °C at 10 °C/min, 230 °C at 5 °C/min, then held for 9.5 min, and a ramp-up to 240 °C at 5 °C/min for 11.13 min. Using peak comparison to the internal standard, 31 fatty acid species were quantified (8 species of saturated and 8 monounsaturated fatty acids, 8 species of n-6 and 7 species of n-3 PUFAs). At least 4 litters per group were analyzed.
2.6. Proteomics
Retinas from P10 pups after STZ and maternal DHA+ARA vs. DHA diet (n = 8 mice/group; both retinas pooled) were processed for label-free LC-MS/MS proteomics [39]. At least 2 litters per group were analyzed. An Orbitrap Fusion Lumos coupled to an Easy-nLC1000 HPLC pump (Thermo Fisher Scientific, Waltham, MA, USA) was used for mass spectrometry. Peptide spectra were analyzed with Proteome Discoverer (v2.5, Thermo Fisher Scientific). Included proteins had ≥ 2 unique peptides [39]. For each protein, logarithmic transformation, student’s t-test (p-value), and Benjamini-Hochberg method (FDR-adjusted p-value, called q-value) were performed (Qlucore Omics Explorer, v3.5, Qlucore, Lund, Sweden). Gene Ontology pathway enrichment (Biological Process, Molecular Function, Cellular Component) of significantly decreased or increased proteins (p < 0.05; |log2 fold change| > 0.2) was analyzed using EnrichR (retrieved 10/2024) [45–47]. Pathways with p < 0.05 (Fisher’s exact test) were considered significantly enriched.
Raw data are available on the ProteomeXchange Consortium via the PRIDE [48] repository (identifier PXD061307, https://doi.org/10.6019/PXD061307).
2.7. Statistics
Infant glucose values were displayed graphically as a longitudinal curve using locally estimated scatterplot smoothing (LOESS). For infant characteristics and neonatal morbidities, Fisher’s exact test was used for dichotomous variables, Mantel-Haenszel Chi-square trend test was performed for categorical ordered variables, and Mann-Whitney U test was applied for continuous variables.
Mouse data are presented as mean ± SEM. For non-omics data, normality (quantile-quantile plot) and variance (F-test) were tested, followed by parametric (unpaired t-test or Welch’s t-test) or non-parametric (Mann-Whitney U test) analysis (Prism v9.0, GraphPad Software, La Jolla, CA, USA). Statistical significance was considered at p < 0.05. Heatmaps were created using https://software.broadinstitute.org/morpheus/.
3. Results
3.1. In hyperglycemia-associated retinopathy in neonatal mice (modeling Phase I ROP), maternal DHA+ARA vs. DHA diet promoted physiological vessel growth
In the hyperglycemia-associated retinopathy model, the impact of maternal DHA+ARA vs. DHA diet was examined (Fig. 1A). The diets had no effect on pup body weight and blood glucose levels (Fig. 1B, C). Oral milk from mothers on DHA+ARA vs. DHA maternal diet resulted in increased density of the deep (Fig. 1D, E) and superficial (Fig. 1F, G) retinal vasculature in pups at P10, indicating that combined DHA+ARA vs. DHA lipid diet promoted physiological retinal vessel growth in mice with neonatal hyperglycemia-associated retinopathy.
Fig. 1.
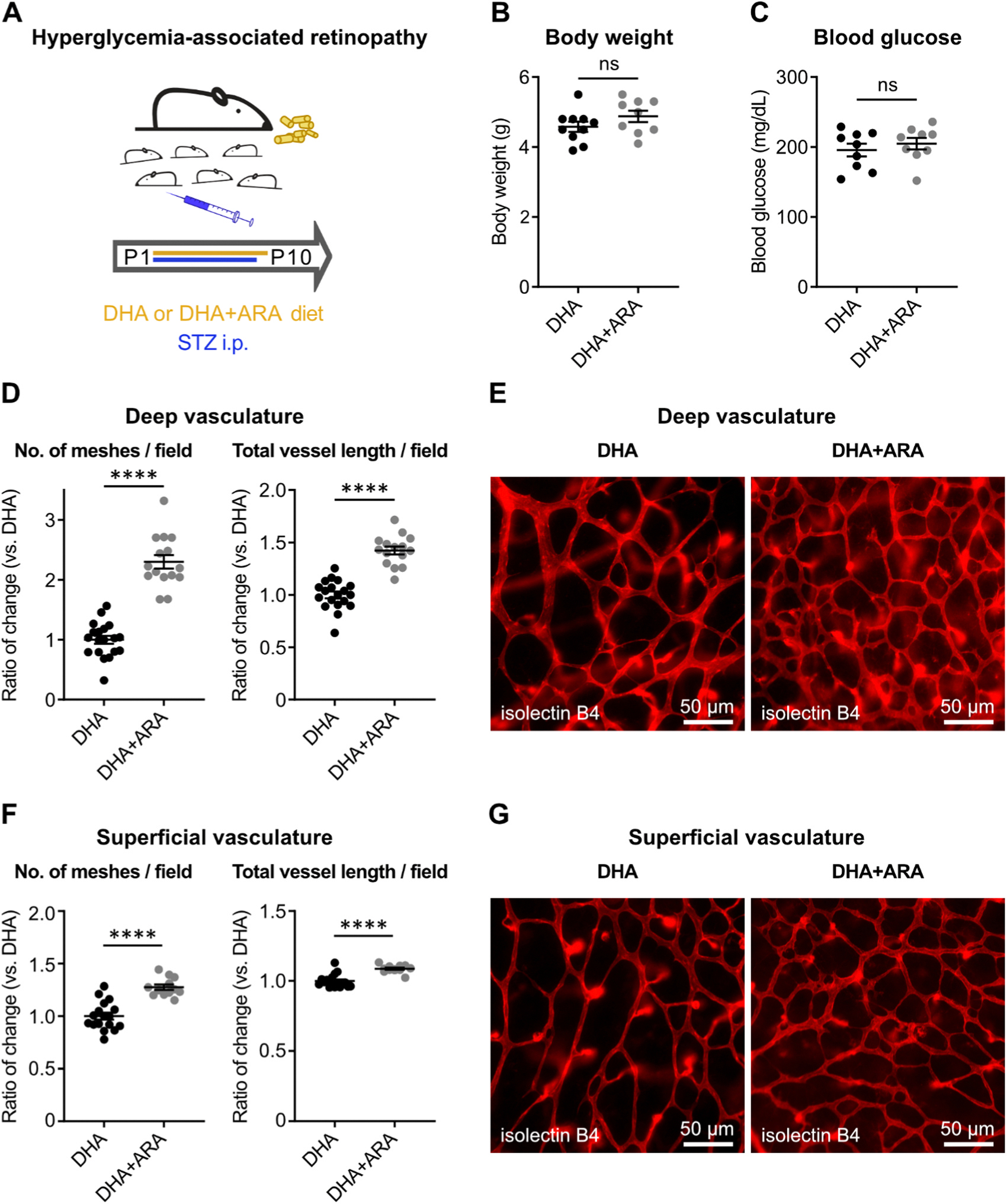
DHA+ARA diet vs. DHA promoted physiological retinal vascularization in hyperglycemia-associated retinopathy in neonatal mice. (A) Schematic of mouse model. To induce hyperglycemia-associated retinopathy, mouse pups were injected daily with streptozotocin (STZ, 50 μg/g body weight intraperitoneally) in PBS from postnatal day (P) 1–P9. Nursing moms were fed a diet supplemented with docosahexaenoic acid alone (DHA) or DHA plus arachidonic acid (DHA+ARA) from P1–P10. (B) P10 mouse pup body weight and (C) blood glucose levels were measured. DHA pups, n = 9–10; DHA+ARA pups, n = 9; unpaired t-test; ns, not significant. (D–G) Retinal vessel analysis of STZ-induced pups at P10. (D, F) Number of vascular meshes (left) and total vessel length (right) per field in deep (D) and superficial (F) retinal vasculature were quantified. DHA pup retinas, n = 17–19; DHA+ARA pup retinas, n = 12–15; unpaired t-test; **** p < 0.0001. (E, G) Representative images of deep (E) and superficial (G) retinal vasculature. Retinal vessels were visualized with isolectin B4 (red). Magnification, 200x; scale, 50 μm.
3.2. In oxygen-induced retinopathy in neonatal mice, maternal DHA+ARA vs. DHA diet promoted physiological vessel growth
To validate findings in hyperglycemia-associated retinopathy, the maternal diets were tested in the oxygen-induced retinopathy mouse model (Supplemental Fig. 1A, B). Body weight was unchanged by the diets (data not shown). Maternal DHA+ARA vs. DHA diet resulted in a reduction of vaso-obliteration, suggesting improved physiological retinal vascularization (Supplemental Fig. 1C, D), while pathological neovascularization was unaffected (Supplemental Fig. 1E, F). These data confirm the protective effect of maternal DHA+ARA vs. DHA diet on physiological retinal vessel growth in two mouse models of Phase I ROP.
3.3. Hyperglycemia incidence in the first two weeks after preterm birth in the Mega Donna Mega study
In the Mega Donna Mega study, the median number of glucose blood samples per infant was 27 (IQR 17–47). The mean glucose level across all days was 7.9 mmol/L (SD ± 2.9), and, considering the maximum glucose levels, the mean was 9.5 mmol/L (SD ± 5.4). Of the 2298 samples, 770 (33.5 %) met the criteria for hyperglycemia (> 10 mmol/L), and 146 infants (72.6 %) had at least one hyperglycemic sample (Supplemental Fig. 2). Infants with hyperglycemia were more immature at birth and more often affected by neonatal morbidities, including ROP (Table 2).
Table 2.
Characteristics and neonatal morbidities of infants with and without hyperglycemia.
| Variables | No hyperglycemia n = 55 | Hyperglycemia n = 146 | p-value |
|---|---|---|---|
|
| |||
| Birth characteristics | |||
| Gestational age (weeks) | 26.6 (0.9) | 25.1 (1.4) | < 0.0001 |
| 26.7 (23.6; 27.9) | 25.1 (22.3; 27.9) | ||
| (26.1; 27.3) | (24.1; 26.1) | ||
| Birth weight (grams) | 948.5 (179.4) | 733.2 (166.5) | < 0.0001 |
| 940 (580; 1330) | 707 (420; 1345) | ||
| (815; 1120) | (610; 829) | ||
| Birth weight SDS (Fenton) | 0.38 (0.73) | −0.04 (0.82) | < 0.0001 |
| 0.45 (−1.61; 1.81) | 0.06 (−2.29; 2.34) | ||
| (−0.04; 0.89) | (−0.51; 0.41) | ||
| Sex, boys | 29 (52.7 %) | 86 (58.9 %) | 0.52 |
| Morbidities | |||
| ROP stage | 0.0012 | ||
| No ROP | 35 (63.6 %) | 60 (41.1 %) | |
| Mild ROP (stages 1 and 2) | 14 (25.5 %) | 41 (28.1 %) | |
| Severe ROP (stage 3 and treated) | 6 (10.9 %) | 45 (30.8 %) | |
| ROP treatment | 2 (3.7 %) | 34 (27.4 %) | 0.0002 |
| BPD (Jensen 1, 2, 3) | 19 (35.2 %) | 79 (64.8 %) | 0.0005 |
| NEC | 2 (3.6 %) | 19 (13.0 %) | 0.07 |
| Severe IVH (grades 3 and 4) | 3 (5.5 %) | 21 (14.4 %) | 0.09 |
| Neonatal course first month of life | |||
| Mechanical ventilation (days) | 3.7 (7.4) | 14.3 (10.2) | < 0.0001 |
| 0 (0; 28) | 15 (0; 28) | ||
| (0; 4) | (4; 25) | ||
| CPAP (days) | 16.9 (9.7) | 12.0 (9.8) | < 0.0001 |
| 19 (0; 28) | 11 (0; 28) | ||
| (7; 27) | (3; 20) | ||
| Sepsis | 4 (7.3 %) | 24 (16.4 %) | 0.11 |
| CRP, max (mg/L) | 10.1 (19.9) | 26.8 (42.6) | < 0.0001 |
| 3.3 (0.3; 129.0) | 13.0 (0.3; 300.0) | ||
| (0.9; 11.0) | (4.0; 29.0) | ||
| Hb, min (g/L) | 107.4 (15.6) | 97.6 (14.8) | < 0.0001 |
| 107.0 (56.0; 154.0) | 100.0 (38.0; 151.0) | ||
| (101.0; 113.0) | (95.0; 104.0) | ||
| Platelet count, min (109/L) | 205.2 (79.1) | 132.0 (76.8) | < 0.0001 |
| 194 (28; 400) | 130 (8; 391) | ||
| (148; 259) | (72; 174) | ||
| Glucose, max (mmol/L) | 8.5 (2.0) | 20.2 (10.1) | < 0.0001 |
| 9 (4; 15) | 17 (10; 59) | ||
| (7; 10) | (14; 23) | ||
Infants with at least one blood glucose measurement > 10 mmol/L (n = 146) and infants without any episode of hyperglycemia (n = 55) during the first 14 postnatal days. Data are presented as mean (standard deviation), median (minimum, maximum), and (IQR), or number (percentage). For dichotomous variables Fisher’s exact test was used. For categorical ordered variables Mantel- Haenszel Chi-square trend test was performed. For continuous variables Mann- Whitney U test was applied. BPD, bronchopulmonary dysplasia; CPAP, continuous positive airway pressure; CRP, C-reactive protein; Hb, hemoglobin; IVH, intraventricular hemorrhage; NEC, necrotizing enterocolitis; ROP, retinopathy of prematurity.
3.4. Maternal diet composition was reflected in the pup lipid profile of plasma and RPE complex
Given the frequency of hyperglycemia and its association with ROP in premature infants in the Mega Donna Mega trial, we further focused on the hyperglycemia-associated retinopathy mouse model. At P10 in plasma and RPE complex, DHA levels were significantly increased in DHA diet group pups, while ARA was higher in DHA+ARA diet group pups (Fig. 2A, B), suggesting that fatty acids in the maternal diet can reach the pups via maternal milk and can incorporate into the pups’ ocular tissue. However, in the retina, DHA and ARA were comparable between the diet groups, and higher maternal dietary DHA (3 % vs. 1 %) did not further increase retinal DHA in pups (Fig. 2C), suggesting a tight regulation of local retinal lipid composition [49]. Other retinal (C14:0, C18:2n-6) and circulating (C14:0, C18:0, C20:5 n-3, C22:4n-6) fatty acids were changed by diets.
Fig. 2.

Maternal diet composition was reflected in the pup lipid profile of plasma and RPE complex. Targeted fatty acid analysis of neonatal mice with hyperglycemia-associated retinopathy. P10 (A) plasma, (B) RPE complex (retinal pigment epithelium with choroid and sclera), and (C) retina were isolated from mouse pups. Nursing moms were fed a diet supplemented with DHA or DHA+ARA from P1–P10. DHA pups, n = 18 (2 retinas pooled); DHA+ARA pups, n = 18 (2 retinas pooled); multiple unpaired t-test; * p < 0.05. Only comparisons with statistically significant changes are labeled. Fatty acids on the x-axis are denoted as Cx: yn-z, with x, number of carbons; y, number of double bonds; z, position of the first double bond from the omega (methyl) end.
3.5. The maternal DHA+ARA vs. DHA diet increased retinal proteins related to mitochondrial respiration
We assessed the global proteome of P10 STZ retinas isolated from DHA+ARA vs. DHA maternal diet pups to uncover mechanisms involved in the combined diets’ protective effect on retinal vessels. In total, 4203 proteins with ≥ 2 unique peptides were detected. Each diet group displayed a distinct proteome profile. DHA+ARA vs. DHA retinas showed 198 proteins with increased and 176 proteins with decreased abundance passing p < 0.05 (q < 0.37) and |log2 fold change| > 0.2 (Fig. 3A, B). Gene ontology pathways related to mitochondrial respiration, fatty acid and glucose metabolism were significantly (p < 0.05) enriched (Fig. 3C) with increased protein abundance of mitochondrial complex I subunits (NDUFS8, NDUFB6, NDUFS3) and of glucose transporter 1 (SLC2A1) (Fig. 3D). Pathways with decreased protein abundance in the DHA+ARA vs. DHA group were related to autophagy, pexophagy, mitochondrial apoptosis, lysosomes, endosomes, and negative regulation of growth factor signaling (data not shown).
Fig. 3.

DHA+ARA vs. DHA diet increased mitochondrial proteins in P10 retinas from mouse pups induced with hyperglycemia-associated retinopathy. Nursing moms were fed a diet supplemented with DHA or DHA+ARA from P1–P10. DHA pups, n = 8 (2 retinas pooled); DHA+ARA pups, n = 8 (2 retinas pooled). At P10, mouse retinas were isolated for proteomic analysis. (A) Principal component analysis of all detected proteins (≥ 2 unique peptides) in retinas isolated from mouse pups in DHA+ARA vs. DHA diet group. (B) Volcano plot of proteins with significantly (p < 0.05; q < 0.37; log2 fold change (log2FC) > 0.2 or < −0.2) increased (orange) or decreased (blue) abundance in retinas isolated from pups in DHA+ARA vs. DHA diet group. (C) Selected enriched (p < 0.05) gene ontology pathways associated with energy and lipid metabolism (biological process, BP; cellular component, CC; molecular function, MF) of proteins with significantly increased abundance (p < 0.05; log2FC > 0.2) in retinas isolated from pups in DHA+ARA vs. DHA diet group. Pathways related to oxidative phosphorylation are written in bold. Protein count refers to the number of distinct proteins with significantly increased abundance in the respective gene ontology cluster. Protein ratio describes the proportion of proteins with significantly increased abundance to all listed proteins in a cluster. (D) Heatmap depicting the altered proteins in the selected enriched pathways shown in (C).
3.6. Pharmacological inhibition of mitochondrial ATP production negated the maternal DHA+ARA vs. DHA diet promotion of retinal vessel growth
Next, we tested whether mitochondrial respiration contributed to the combined diet promotion of retinal vessel growth. In hyperglycemia-associated Phase I retinopathy, pups with maternal DHA+ARA or DHA diet received intraperitoneal injection of oligomycin A, inhibiting mitochondrial ATP synthase (Fig. 4A). No differences in body weight and blood glucose levels between maternal diet groups were observed (Fig. 4B, C). After pharmacological suppression of mitochondrial ATP energy production, no differences between DHA+ARA and DHA maternal diet groups in deep (Fig. 4D, E) and superficial (Fig. 4F, G) retinal vasculature at P10 were observed. These findings suggested that mitochondrial energy production was a key component in the combined DHA+ARA diet promotion of retinal vessel growth in neonatal hyperglycemia-associated retinopathy.
Fig. 4.

Pharmacological inhibition of mitochondrial ATP production negated the promotion of physiological retinal vascularization by dietary DHA+ARA. (A) Schematic of intervention. Mouse pups induced with hyperglycemia-associated retinopathy were treated with mitochondrial ATP synthase inhibitor oligomycin A (25 μg/g body weight intraperitoneally) in 30 % dimethylsulfoxide in PBS from P7–P9. Nursing moms were fed DHA or DHA+ARA diet from P1–P10. (B) Body weight and (C) blood glucose levels of P10 oligomycin A-treated pups with maternal DHA or DHA+ARA diet were measured. DHA pups, n = 5–6; DHA+ARA pups, n = 8; unpaired t-test; ns, not significant. (D–G) Retinal vessel analysis of STZ-induced pups at P10. (D, F) Number of vascular meshes (left) and total vessel length (right) per field in deep (D) and superficial (F) retinal vasculature were quantified. DHA pup retinas, n = 12; DHA+ARA pup retinas, n = 16; unpaired t-test or Mann-Whitney U test; ns, not significant. (E, G) Representative images of deep (E) and superficial (G) retinal vasculature. Retinal vessels were visualized with isolectin B4 (red). Magnification, 200x; scale, 50 μm.
4. Discussion
The LCPUFA supplementation approach preventing ROP development and progression should be refined. Our study demonstrates that combined dietary supplementation with DHA+ARA compared to DHA alone protects against hyperglycemia-associated retinopathy in neonatal mice (mimicking Phase I ROP associated with hyperglycemia) by enhancing retinal metabolism. Our findings highlight the importance of maintaining a balance between n-3 and n-6 LCPUFAs in promoting retinal vascular development during the critical early postnatal period.
While clinical trials of DHA supplementation have yielded inconsistent results regarding suppression of ROP [16–22], DHA vs. ARA supplementation is protective in a mouse model of neovascular ROP (oxygen-induced retinopathy) [37,50–52] by promoting physiological vascularization in Phase I and inhibiting pathological neovascularization in Phase II. Currently, the focus has shifted towards synergistic/complementary effects of oral DHA+ARA. Two clinical trials (Mega Donna Mega and DIAMOND) demonstrated that oral supplementation (starting close to birth) in Phase I ROP with DHA+ARA (1:2 ratio) improved retinal health in very preterm infants [23,24], but the underlying mechanisms are unclear. Our current work shows that combined DHA+ARA vs. DHA oral supplementation better promotes physiological vascular development in a Phase I ROP model of hyperglycemia-associated vascular development suppression. The mouse study results align with clinical data, providing the foundation for further mechanistic investigations.
This study focused on the hyperglycemia-associated retinopathy mouse model of Phase I ROP based on findings from the Mega Donna Mega trial showing that premature infants at risk for ROP exhibit a mean blood glucose level of 7.9 mmol/L. In contrast, published studies report that postnatal mean blood glucose levels in full-term infants range from 2.3 to 4.6 mmol/L at various timepoints [53–55]. In our clinical data, infants who experienced hyperglycemia during the early postnatal period were more likely to be of lower gestational age and birth weight and to present with comorbidities such as bronchopulmonary dysplasia. Infants with hyperglycemia were also more likely to receive mechanical ventilation or continuous positive airway pressure and to present with elevated CRP and reduced hemoglobin and platelet count. As some of the mentioned observations are known risk factors for ROP [56,57], it is likely that hyperglycemia cooccurs with other risk factors and that distinct risk factors modulate one another. This highlights the multifactorial nature of ROP and underscores the importance of employing different models of ROP focusing on distinct underlying factors, such as oxygen exposure or hyperglycemia, to further enhance our understanding of the disease’s multifaceted pathogenesis. To verify our findings, we therefore also carried out maternal diet intervention with DHA+ARA vs. DHA in the OIR mouse model, confirming the protective effect of maternal DHA+ARA diet on physiological vascularization in systems modeling Phase I ROP.
Photoreceptor metabolism and metabolic needs dictate retinal vascular development [33,58]. Our findings suggest that a combination of DHA+ARA vs. DHA alone promotes physiological retinal vascularization by improving retinal metabolism as indicated by proteomics data and confirmed by loss of the protective effect after inhibition of mitochondrial energy production. Of note, maternal DHA+ARA vs. DHA diet did not affect pup blood glucose levels, suggesting that maternal DHA+ARA supplementation does not prevent hyperglycemia, a factor leading to retinopathy, but instead protects the retina from the harmful effects of hyperglycemia. This effect could be mediated through enhanced retinal metabolism and potentially improved local glucose utilization, as indicated by the enrichment of the Gene Ontology pathway, glucose import, in the proteome. Additionally, maternal DHA+ARA diet might alter cell membrane compositions and thereby improve membrane fluidity and mitochondrial function [59–61]. The beneficial effects of DHA+ARA are not likely to be mediated through mitochondrial β-oxidation of these lipids for energy production. DHA and ARA can be oxidized by some cells such as murine astrocytes but to a much lower extent and at slower rates than other lipids, e.g., palmitic or oleic acid [62]. However, DHA+ARA might influence mitochondrial β-oxidation by modulating the supply of other saturated and unsaturated fatty acids. For example, we found significantly altered circulating (C14:0, C18:0, C20:5n-3, and C22:4n-6) and retinal (C14:0, C18:2n-6) fatty acids in DHA+ARA vs. DHA diet groups. Further studies are required to identify preferred lipids for retinal energy production and to discover whether mitochondrial β-oxidation of other lipids mediates DHA+ARA protection against neonatal retinopathy.
Balancing LCPUFAs has gained attention in studies of ROP [16–22] and other complications of preterm birth, including bronchopulmonary dysplasia [63], as well as other retinal disorders, such as diabetic retinopathy, age-related macular degeneration [64], type 3 Stargardt dystrophy [65], and dry eye syndrome [66]. Interestingly, our data revealed distinct diet-dependent changes in fatty acid profiles across different tissues, suggesting that plasma DHA and ARA levels are reflected in the RPE complex, while being tightly regulated in the retina [49]. Higher dietary DHA (3 % vs. 1 %) did not further increase retinal DHA levels. Notably, supplementation of DHA without n-6 ARA supplementation reduces serum ARA levels in infants [19] and animals [67,68], and low postnatal serum ARA levels are associated with increased ROP incidence [14]. Supplementation of DHA+ARA at a 1:2 ratio leads to increased DHA and ARA serum levels in infants [23,24], offering a promising approach for ROP prevention. These findings suggest a complex interplay between dietary LCPUFAs and endogenous fatty acid metabolism.
Our study has limitations that could be addressed in future research. First, while our mouse model of hyperglycemia-associated retinopathy provides valuable insights into mechanisms of neonatal hyperglycemic retinal disease, it does not fully recapitulate the complexity of human ROP. Second, the long-term effects of combined DHA+ARA supplementation on retinal function and structure beyond the neonatal period remain to be elucidated. Third, the optimal ratio of DHA to ARA for retinal protection requires further investigation, as it may depend on the specific developmental stage and pathological condition. Finally, the fatty acid profiling targeted lipids of interest and did not capture potential changes in other fatty acid species, particularly very long-chain fatty acids. A limitation to the generalizability of the animal study is that it did not consider gender/sex.
In conclusion, our findings provide evidence for the protective effects of combined DHA+ARA supplementation against hyperglycemia-associated retinopathy in neonatal mice through increased mitochondrial metabolism. This study offers mechanistic insights into the clinical observations of reduced ROP severity with oral DHA+ARA supplementation and highlights the importance of balanced LCPUFA intake for optimal retinal development, which may also have a beneficial effect on other retinal disorders.
Supplementary Material
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.phrs.2025.107877.
Acknowledgements
LEHS is supported by NIH EY017017, EY03090401, BCH IDDRC (1U54HD090255), Massachusetts Lions Eye Foundation 74543. AH is supported by The Swedish Research Council (DNR# #2020–01092), Government grants under the ALF agreement ALFGBG-717971, and The Wallenberg Clinical Scholars. ZF is supported by NIH EY032492, EY017017, BCH Ophthalmology Foundation 85010, Massachusetts Lions Eye Research Fund 77821, and Knights Templar Eye Foundation 71212. MA is supported by NIH R01HL126901, R01HL149302, and Kowa Company Ltd, Nagoya, Japan.
Footnotes
Declaration of Competing Interest
The authors have declared no conflict of interest.
CRediT authorship contribution statement
Masanori Aikawa: Writing – review & editing, Resources, Funding acquisition. Chuck T. Chen: Writing – review & editing, Investigation, Formal analysis, Methodology. Sasha A. Singh: Writing – review & editing, Methodology. Chaomei Wang: Writing – review & editing, Investigation, Formal analysis. Hitomi Yagi: Writing – review & editing, Investigation, Formal analysis, Data curation. Katherine Neilsen: Writing – review & editing, Investigation, Formal analysis. Myriam Boeck: Writing – original draft, Visualization, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Mathew Yu: Writing – review & editing, Investigation, Formal analysis. Aldina Pivodic: Writing – review & editing, Investigation, Formal analysis. Jeff Lee: Writing – review & editing, Investigation, Formal analysis. Pia Lundgren: Writing – review & editing, Investigation, Formal analysis. Zhongjie Fu: Writing – review & editing, Resources, Methodology, Funding acquisition, Conceptualization. Deokho Lee: Writing – review & editing, Investigation, Formal analysis. Richard P. Bazinet: Writing – review & editing, Resources. Taku Kasai: Writing – review & editing, Investigation, Formal analysis, Data curation. Lois EH Smith: Writing – review & editing, Supervision, Resources, Methodology, Funding acquisition, Conceptualization. Victoria Hirst: Writing – review & editing, Investigation, Formal analysis. Ann Hellström: Writing – review & editing, Resources, Methodology, Funding acquisition, Conceptualization. Shen Nian: Writing – review & editing, Investigation, Formal analysis. Yan Zeng: Writing – review & editing, Investigation, Formal analysis. Andrew McCutcheon: Writing – review & editing, Investigation, Formal analysis. Anders K. Nilsson: Writing – review & editing, Investigation, Formal analysis.
Declaration of Generative AI and AI-assisted technologies in the writing process
During the preparation of this work, the authors used ChatGPT and Perplexity AI to refine the manuscript language. After using these tools, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Data availability
Data will be made available on request.
References
- [1].Garcia H, Villasis-Keever MA, Zavala-Vargas G, Bravo-Ortiz JC, Perez-Mendez A, Escamilla-Nunez A, Global prevalence and severity of retinopathy of prematurity over the last four decades (1985–2021): a systematic review and meta-analysis, Arch. Med Res 55 (2) (2024) 102967. [DOI] [PubMed] [Google Scholar]
- [2].Dai C, Xiao J, Wang C, Li W, Su G, Neurovascular abnormalities in retinopathy of prematurity and emerging therapies, J. Mol. Med. 100 (6) (2022) 817–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].de Las Rivas Ramirez N, Luque Aranda G, Rius Diaz F, Perez Frias FJ, Sanchez Tamayo T, Risk factors associated with Retinopathy of Prematurity development and progression, Sci. Rep. 12 (1) (2022) 21977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tomita Y, Usui-Ouchi A, Nilsson AK, Yang J, Ko M, Hellstrom A, et al. , Metabolism in Retinopathy of Prematurity, Life (Basel) 11 (11) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fu Z, Nilsson AK, Hellstrom A, Smith LEH, Retinopathy of prematurity: metabolic risk factors, Elife 11 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Crawford MA, Sinclair AJ, Hall B, Ogundipe E, Wang Y, Bitsanis D, et al. , The imperative of arachidonic acid in early human development, Prog. Lipid Res. 91 (2023) 101222. [DOI] [PubMed] [Google Scholar]
- [7].Carlson SE, Colombo J, Docosahexaenoic acid and arachidonic acid nutrition in early development, Adv. Pediatr. 63 (1) (2016) 453–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shindou H, Koso H, Sasaki J, Nakanishi H, Sagara H, Nakagawa KM, et al. , Docosahexaenoic acid preserves visual function by maintaining correct disc morphology in retinal photoreceptor cells, J. Biol. Chem. 292 (29) (2017) 12054–12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Stinson AM, Wiegand RD, Anderson RE, Fatty acid and molecular species compositions of phospholipids and diacylglycerols from rat retinal membranes, Exp. Eye Res. 52 (2) (1991) 213–218. [DOI] [PubMed] [Google Scholar]
- [10].Kulkarni P, Cai J, Hurst HE, Lipids and nitric oxide in porcine retinal and choroidal blood vessels, J. Ocul. Pharmacol. Ther. 18 (3) (2002) 265–275. [DOI] [PubMed] [Google Scholar]
- [11].Martin CR, Dasilva DA, Cluette-Brown JE, Dimonda C, Hamill A, Bhutta AQ, et al. , Decreased postnatal docosahexaenoic and arachidonic acid blood levels in premature infants are associated with neonatal morbidities, J. Pediatr. 159 (5) (2011) 743–749, e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Agostoni C, Marangoni F, Stival G, Gatelli I, Pinto F, Rise P, et al. , Whole blood fatty acid composition differs in term versus mildly preterm infants: small versus matched appropriate for gestational age, Pediatr. Res. 64 (3) (2008) 298–302. [DOI] [PubMed] [Google Scholar]
- [13].Hellstrom A, Pivodic A, Granse L, Lundgren P, Sjobom U, Nilsson AK, et al. , Association of docosahexaenoic acid and arachidonic acid serum levels with retinopathy of prematurity in preterm infants, JAMA Netw. Open 4 (10) (2021) e2128771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lofqvist CA, Najm S, Hellgren G, Engstrom E, Savman K, Nilsson AK, et al. , Association of retinopathy of prematurity with low levels of arachidonic acid: a secondary analysis of a randomized clinical trial, JAMA Ophthalmol. 136 (3) (2018) 271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hellstrom A, Kermorvant-Duchemin E, Johnson M, Saenz de Pipaon M, Smith LE, Hard AL, et al. , Nutritional interventions to prevent retinopathy of prematurity, Pediatr. Res. 96 (4) (2024) 905–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pawlik D, Lauterbach R, Walczak M, Hurkala J, Sherman MP, Fish-oil fat emulsion supplementation reduces the risk of retinopathy in very low birth weight infants: a prospective, randomized study, JPEN J. Parent. Enter. Nutr. 38 (6) (2014) 711–716. [DOI] [PubMed] [Google Scholar]
- [17].Hill NS, Cormack BE, Little BS, Bloomfield FH, Growth and clinical outcome in very low-birth-weight infants after the introduction of a multicomponent intravenous lipid emulsion, JPEN J. Parent. Enter. Nutr. 44 (7) (2020) 1318–1327. [DOI] [PubMed] [Google Scholar]
- [18].Beken S, Dilli D, Fettah ND, Kabatas EU, Zenciroglu A, Okumus N, The influence of fish-oil lipid emulsions on retinopathy of prematurity in very low birth weight infants: a randomized controlled trial, Early Hum. Dev. 90 (1) (2014) 27–31. [DOI] [PubMed] [Google Scholar]
- [19].Najm S, Lofqvist C, Hellgren G, Engstrom E, Lundgren P, Hard AL, et al. , Effects of a lipid emulsion containing fish oil on polyunsaturated fatty acid profiles, growth and morbidities in extremely premature infants: a randomized controlled trial, Clin. Nutr. ESPEN 20 (2017) 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Vayalthrikkovil S, Bashir RA, Rabi Y, Amin H, Spence JM, Robertson HL, et al. , Parenteral fish-oil lipid emulsions in the prevention of severe retinopathy of prematurity: a systematic review and meta-analysis, Am. J. Perinatol. 34 (7) (2017) 705–715. [DOI] [PubMed] [Google Scholar]
- [21].Kapoor V, Malviya MN, Soll R, Lipid emulsions for parenterally fed preterm infants, Cochrane Database Syst. Rev. 6 (6) (2019) CD013163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bernabe-Garcia M, Villegas-Silva R, Villavicencio-Torres A, Calder PC, Rodriguez-Cruz M, Maldonado-Hernandez J, et al. , Enteral docosahexaenoic acid and retinopathy of prematurity: a randomized clinical trial, JPEN J. Parent. Enter. Nutr. 43 (7) (2019) 874–882. [DOI] [PubMed] [Google Scholar]
- [23].Birch EE, Carlson SE, Hoffman DR, Fitzgerald-Gustafson KM, Fu VL, Drover JR, et al. , The DIAMOND (DHA Intake And Measurement Of Neural Development) Study: a double-masked, randomized controlled clinical trial of the maturation of infant visual acuity as a function of the dietary level of docosahexaenoic acid, Am. J. Clin. Nutr. 91 (4) (2010) 848–859. [DOI] [PubMed] [Google Scholar]
- [24].Hellstrom A, Nilsson AK, Wackernagel D, Pivodic A, Vanpee M, Sjobom U, et al. , Effect of enteral lipid supplement on severe retinopathy of prematurity: a randomized clinical trial, JAMA Pediatr. 175 (4) (2021) 359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Binder ND, Raschko PK, Benda GI, Reynolds JW, Insulin infusion with parenteral nutrition in extremely low birth weight infants with hyperglycemia, J. Pediatr. 114 (2) (1989) 273–280. [DOI] [PubMed] [Google Scholar]
- [26].Beardsall K, Vanhaesebrouck S, Ogilvy-Stuart AL, Vanhole C, Palmer CR, Ong K, et al. , Prevalence and determinants of hyperglycemia in very low birth weight infants: cohort analyses of the NIRTURE study, J. Pediatr. 157 (5) (2010) 715–719, e1–3. [DOI] [PubMed] [Google Scholar]
- [27].Chavez-Valdez R, McGowan J, Cannon E, Lehmann CU, Contribution of early glycemic status in the development of severe retinopathy of prematurity in a cohort of ELBW infants, J. Perinatol. 31 (12) (2011) 749–756. [DOI] [PubMed] [Google Scholar]
- [28].Kaempf JW, Kaempf AJ, Wu Y, Stawarz M, Niemeyer J, Grunkemeier G, Hyperglycemia, insulin and slower growth velocity may increase the risk of retinopathy of prematurity, J. Perinatol. 31 (4) (2011) 251–257. [DOI] [PubMed] [Google Scholar]
- [29].Mohamed S, Murray JC, Dagle JM, Colaizy T, Hyperglycemia as a risk factor for the development of retinopathy of prematurity, BMC Pediatr. 13 (2013) 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ahmadpour-Kacho M, Motlagh AJ, Rasoulinejad SA, Jahangir T, Bijani A, Pasha YZ, Correlation between hyperglycemia and retinopathy of prematurity, Pediatr. Int. 56 (5) (2014) 726–730. [DOI] [PubMed] [Google Scholar]
- [31].Mohsen L, Abou-Alam M, El-Dib M, Labib M, Elsada M, Aly H, A prospective study on hyperglycemia and retinopathy of prematurity, J. Perinatol. 34 (6) (2014) 453–457. [DOI] [PubMed] [Google Scholar]
- [32].Almeida AC, Silva GA, Santini G, Brizido M, Correia M, Coelho C, et al. , Correlation between hyperglycemia and glycated albumin with retinopathy of prematurity, Sci. Rep. 11 (1) (2021) 22321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fu Z, Lofqvist CA, Liegl R, Wang Z, Sun Y, Gong Y, et al. , Photoreceptor glucose metabolism determines normal retinal vascular growth, EMBO Mol. Med. 10 (1) (2018) 76–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fu Z, Lundgren P, Pivodic A, Yagi H, Harman JC, Yang J, et al. , FGF21 via mitochondrial lipid oxidation promotes physiological vascularization in a mouse model of Phase I ROP, Angiogenesis 26 (3) (2023) 409–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Harman JC, Pivodic A, Nilsson AK, Boeck M, Yagi H, Neilsen K, et al. , Postnatal hyperglycemia alters amino acid profile in retinas (model of Phase I ROP), iScience 26 (10) (2023) 108021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fu Z, Yan W, Chen CT, Nilsson AK, Bull E, Allen W, et al. , Omega-3/Omega-6 long-chain fatty acid imbalance in phase I retinopathy of prematurity, Nutrients 14 (7) (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, et al. , Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis, Nat. Med. 13 (7) (2007) 868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sjobom U, Andersson MX, Pivodic A, Lund AM, Vanpee M, Hansen-Pupp I, et al. , Modification of serum fatty acids in preterm infants by parenteral lipids and enteral docosahexaenoic acid/arachidonic acid: a secondary analysis of the Mega Donna Mega trial, Clin. Nutr. 42 (6) (2023) 962–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yagi H, Boeck M, Petrishka-Lozenska M, Lundgren P, Kasai T, Cagnone G, et al. , Timed topical dexamethasone eye drops improve mitochondrial function to prevent severe retinopathy of prematurity, Angiogenesis 27 (4) (2024) 903–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Smith LE, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, et al. , Oxygen-induced retinopathy in the mouse, Invest Ophthalmol. Vis. Sci. 35 (1) (1994) 101–111. [PubMed] [Google Scholar]
- [41].Stahl A, Chen J, Sapieha P, Seaward MR, Krah NM, Dennison RJ, et al. , Postnatal weight gain modifies severity and functional outcome of oxygen-induced proliferative retinopathy, Am. J. Pathol. 177 (6) (2010) 2715–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Connor KM, Krah NM, Dennison RJ, Aderman CM, Chen J, Guerin KI, et al. , Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis, Nat. Protoc. 4 (11) (2009) 1565–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mesotten D, Joosten K, van Kempen A, Verbruggen S, nutrition EEECwgopp. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: carbohydrates, Clin. Nutr. 37 (6 Pt B) (2018) 2337–2343. [DOI] [PubMed] [Google Scholar]
- [44].Folch J, Lees M, Sloane Stanley GH, A simple method for the isolation and purification of total lipides from animal tissues, J. Biol. Chem. 226 (1) (1957) 497–509. [PubMed] [Google Scholar]
- [45].Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, et al. , Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool, BMC Bioinforma. 14 (2013) 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, et al. , Enrichr: a comprehensive gene set enrichment analysis web server 2016 update, Nucleic Acids Res. 44 (W1) (2016) W90–W97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Xie Z, Bailey A, Kuleshov MV, Clarke DJB, Evangelista JE, Jenkins SL, et al. , Gene set knowledge discovery with enrichr, Curr. Protoc. 1 (3) (2021) e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Perez-Riverol Y, Bai J, Bandla C, Garcia-Seisdedos D, Hewapathirana S, Kamatchinathan S, et al. , The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences, Nucleic Acids Res. 50 (D1) (2022) D543–D552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Longoni B, Demontis GC, Polyunsaturated Lipids in the Light-Exposed and Prooxidant Retinal Environment, Antioxidants 12 (3) (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Stahl A, Sapieha P, Connor KM, Sangiovanni JP, Chen J, Aderman CM, et al. , Short communication: PPAR gamma mediates a direct antiangiogenic effect of omega 3-PUFAs in proliferative retinopathy, Circ. Res. 107 (4) (2010) 495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sapieha P, Stahl A, Chen J, Seaward MR, Willett KL, Krah NM, et al. , 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of omega-3 polyunsaturated fatty acids, Sci. Transl. Med. 3 (69) (2011), 69–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Fu Z, Lofqvist CA, Shao Z, Sun Y, Joyal JS, Hurst CG, et al. , Dietary omega-3 polyunsaturated fatty acids decrease retinal neovascularization by adipose-endoplasmic reticulum stress reduction to increase adiponectin, Am. J. Clin. Nutr. 101 (4) (2015) 879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Guemes M, Rahman SA, Hussain K, What is a normal blood glucose? Arch. Dis. Child 101 (6) (2016) 569–574. [DOI] [PubMed] [Google Scholar]
- [54].Harris DL, Weston PJ, Gamble GD, Harding JE, Glucose profiles in healthy term infants in the first 5 days: the glucose in well babies (GLOW) study, J. Pediatr. 223 (2020) 34–41, e4. [DOI] [PubMed] [Google Scholar]
- [55].Hoseth E, Joergensen A, Ebbesen F, Moeller M, Blood glucose levels in a population of healthy, breast fed, term infants of appropriate size for gestational age, Arch. Dis. Child Fetal Neonatal Ed. 83 (2) (2000) F117–F119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kim SJ, Port AD, Swan R, Campbell JP, Chan RVP, Chiang MF, Retinopathy of prematurity: a review of risk factors and their clinical significance, Surv. Ophthalmol. 63 (5) (2018) 618–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Cakir B, Liegl R, Hellgren G, Lundgren P, Sun Y, Klevebro S, et al. , Thrombocytopenia is associated with severe retinopathy of prematurity, JCI Insight 3 (19) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Joyal JS, Sun Y, Gantner ML, Shao Z, Evans LP, Saba N, et al. , Retinal lipid and glucose metabolism dictates angiogenesis through the lipid sensor Ffar1, Nat. Med. 22 (4) (2016) 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Fukaya T, Gondaira T, Kashiyae Y, Kotani S, Ishikura Y, Fujikawa S, et al. , Arachidonic acid preserves hippocampal neuron membrane fluidity in senescent rats, Neurobiol. Aging 28 (8) (2007) 1179–1186. [DOI] [PubMed] [Google Scholar]
- [60].Khairallah RJ, Kim J, O’Shea KM, O’Connell KA, Brown BH, Galvao T, et al. , Improved mitochondrial function with diet-induced increase in either docosahexaenoic acid or arachidonic acid in membrane phospholipids, PLoS One 7 (3) (2012) e34402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Khairallah RJ, Sparagna GC, Khanna N, O’Shea KM, Hecker PA, Kristian T, et al. , Dietary supplementation with docosahexaenoic acid, but not eicosapentaenoic acid, dramatically alters cardiac mitochondrial phospholipid fatty acid composition and prevents permeability transition, Biochim. Biophys. Acta 1797 (8) (2010) 1555–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Corales LG, Inada H, Owada Y, Osumi N, Fatty acid preference for beta-oxidation in mitochondria of murine cultured astrocytes, Genes Cells 29 (9) (2024) 757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wackernagel D, Nilsson AK, Sjobom U, Hellstrom A, Klevebro S, Hansen-Pupp I, Enteral supplementation with arachidonic and docosahexaenoic acid and pulmonary outcome in extremely preterm infants, Prostaglandins Leukot. Ess. Fat. Acids 201 (2024) 102613. [DOI] [PubMed] [Google Scholar]
- [64].Gong Y, Fu Z, Liegl R, Chen J, Hellstrom A, Smith LE, omega-3 and omega-6 long-chain PUFAs and their enzymatic metabolites in neovascular eye diseases, Am. J. Clin. Nutr. 106 (1) (2017) 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Hubbard AF, Askew EW, Singh N, Leppert M, Bernstein PS, Association of adipose and red blood cell lipids with severity of dominant Stargardt macular dystrophy (STGD3) secondary to an ELOVL4 mutation, Arch. Ophthalmol. 124 (2) (2006) 257–263. [DOI] [PubMed] [Google Scholar]
- [66].Miljanovic B, Trivedi KA, Dana MR, Gilbard JP, Buring JE, Schaumberg DA, Relation between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in women, Am. J. Clin. Nutr. 82 (4) (2005) 887–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Hahn KE, Dahms I, Butt CM, Salem N Jr., Grimshaw V, Bailey E, et al. , Impact of arachidonic and docosahexaenoic acid supplementation on neural and immune development in the young pig, Front. Nutr. 7 (2020) 592364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Hsieh AT, Anthony JC, Diersen-Schade DA, Rumsey SC, Lawrence P, Li C, et al. , The influence of moderate and high dietary long chain polyunsaturated fatty acids (LCPUFA) on baboon neonate tissue fatty acids, Pediatr. Res. 61 (5 Pt 1) (2007) 537–545. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.


