Abstract
Introduction
Selpercatinib’s consistent efficacy and manageable safety profile were observed in patients with RET fusion–positive NSCLC across geographies in single-arm studies (LIBRETTO-001 and LIBRETTO-321). Here, we report the efficacy and safety of the phase 3 study LIBRETTO-431 in patients from East Asia.
Methods
LIBRETTO-431 (NCT04194944) is a randomized, open-label phase 3 trial comparing first-line selpercatinib versus pemetrexed and platinum with or without pembrolizumab. Geography (East Asia versus non-East Asia) was a stratification factor. Efficacy end points including progression-free survival (PFS), objective response rate, and duration of response as assessed by means of blinded independent central review were evaluated in patients from East Asia. Pharmacokinetics were assessed in the selpercatinib group. Safety data were collected for all patients who received at least one dose of study treatment.
Results
Of the 261 patients enrolled, 142 (54.4%) were from East Asia, with 116 randomized to the intention-to-treat-pembrolizumab population (selpercatinib: n = 75, control: n = 41). With a median follow-up of 19.4 and 21.2 months in the selpercatinib and control groups respectively, the median PFS in patients from East Asia was not yet reached for selpercatinib (95% confidence interval (CI): 16.4–not evaluable) versus 11.1 months (95% CI: 7.0–16.8) for control (hazard ratio: 0.38 [95%CI: 0.22–0.68]; p = 0.0008). Safety and pharmacokinetics were consistent with those previously reported across the development program and adverse events were generally manageable with dose adjustments.
Conclusions
Consistent with results in the overall LIBRETTO-431 population, selpercatinib exhibited superior PFS compared with chemotherapy plus pembrolizumab in patients from East Asia with a manageable safety profile. These data further highlight the importance of early comprehensive genomic testing and the use of selpercatinib as a preferred first-line regimen in patients with RET fusion–positive NSCLC across geographies.
Keywords: Non–small cell lung cancer (NSCLC), RET fusion, Selpercatinib, East Asia
Introduction
Selpercatinib is a first-in-class, highly selective, potent RET inhibitor with central nervous system (CNS) activity, approved by health authorities in multiple geographies for the treatment of patients with RET-driven cancers.1, 2, 3, 4 On the basis of the marked clinical benefit observed in single-arm and randomized studies, treatment guidelines recommend selpercatinib for patients with RET fusion–positive NSCLC.5,6 The single-arm studies LIBRETTO-001 and LIBRETTO-321 initially characterized selpercatinib’s efficacy and safety, including in patients from East Asia with RET-driven cancers.7, 8, 9, 10 Previous reports from LIBRETTO-431 revealed first-line selpercatinib more than doubled progression-free survival (PFS) compared with chemotherapy with pembrolizumab and reported that selpercatinib effectively treats existing CNS metastases and may prevent or delay the formation of new CNS metastases.1,4 The overall safety profile of selpercatinib was consistent with previous studies in the development program and adverse events (AEs) were manageable with dose adjustments.1,7,10 Here, we report in more detail the efficacy and safety of selpercatinib in patients from East Asia with newly diagnosed RET fusion–positive NSCLC, most of the LIBRETTO-431 study population.
Materials and Methods
Patients and Study Design
The LIBRETTO-431 study methodology has been reported previously.1 Patients were randomly assigned to receive selpercatinib (160 mg twice daily) in continuous 21-day cycles or control, which consisted of pemetrexed (500 mg/m2 with vitamin supplementation) and investigator’s choice of platinum therapy (carboplatin area under curve 5, maximum dose of 750 mg, or cisplatin 75 mg/m2) with or without pembrolizumab 200 mg every 21 days. Randomized patients were stratified by geography (East Asia versus non-East Asia), presence of brain metastases, and physician’s decision to treat with or without pembrolizumab in the event of control arm assignment. After the completion of four cycles of control arm treatment without progressive disease, patients could continue to receive pemetrexed with or without pembrolizumab. Pembrolizumab was administered up to a maximum of 35 cycles. Because of differences in treatment administration, the trial was open-label to patients and investigators; however, the sponsor was kept blinded to aggregate data and response assessments and disease progression assessed by blinded independent central review (BICR) and by investigator per Response Evaluation Criteria in Solid Tumors version 1.1. The study was performed in accordance with the International Conference on Harmonization Guidelines on Good Clinical Practice and the Declaration of Helsinki. All patients provided informed written consent. Detailed study design and statistical analyses are reported in the Supplementary Appendix.
Results
Patients
Of the 261 patients enrolled, 142 patients (54.4%) were enrolled at sites across East Asia (Supplementary Fig. 1). The intention-to-treat pembrolizumab population included 116 patients from East Asia randomly assigned to selpercatinib (n = 75) or pembrolizumab with chemotherapy (n = 41). Baseline demographic characteristics of patients from East Asia in this population were generally well-balanced. Similar to the overall study population, most patients were female, younger than 65 years of age, and never-smokers. In most patients (53%), RET fusions were identified by next-generation sequencing, with the most common being KIF5B (36%) and CCDC6 (9.5%). In addition, RET fusions in 47% of patients were identified by polymerase chain reaction, which does not specify RET fusion partners. The most common RET fusions identified by next-generation sequencing were KIF5B (36%) and CCDC6 (9.5%).
The selpercatinib arm had a higher proportion of patients from the People's Republic of China (73% versus 66%) and Japan (11% versus 5%) compared with the control arm, whereas the control arm had a higher proportion of patients from Korea (17% versus 11%) compared with the selpercatinib arm.
Baseline clinical and demographic characteristics were similarly balanced among patients from East Asia in the intention-to-treat population (Supplementary Table 1).
Efficacy
With a median follow-up time of approximately 19.4 and 21.2 months in the selpercatinib and control groups respectively, the median time on treatment was 16.6 (± 7.8) months in the selpercatinib group and 9.7 (±7.9) months in the control group. The median PFS assessed by BICR for patients from East Asia in the intent-to-treat pembrolizumab population was not yet reached (95% confidence interval [CI]: 16.4–not estimable) with selpercatinib and 11.1 months (95% CI: 7.0–16.8) with control treatment (hazard ratio [HR]: 0.38 [95% CI: 0.22–0.68]; p = 0.0008) (Fig. 1). At 12 months, the rate of PFS was 72.8% in the selpercatinib group versus 41.7% in the control group. The objective response rate assessed by BICR was higher in the selpercatinib group (86.7% [95% CI: 76.8–93.4]) than in the control group (61.0% [95% CI: 44.5–75.8]). The median duration of response (DOR) was not yet reached (95% CI: 15.4–not estimable) in the selpercatinib group, as compared with 11.5 months (95% CI: 9.6–not estimable) in the control group. At 12 months, the DOR rate was 76.9% (95% CI: 63.4–85.9) in the selpercatinib group versus 46.9% (95% CI: 24.3–66.6) in the control group (Table 1). Similar results were observed in the intention-to-treat population from East Asia across all end points assessed by BICR (Supplementary Table 2, Supplementary Fig. 2).
Figure 1.
Progression-free survival assessed by blinded independent central review in the intention-to-treat pembrolizumab population. Kaplan-Meier estimate of progression-free survival assessed by blinded independent central review in the patients from East Asia in the intention-to-treat pembrolizumab population. CI, confidence interval; HR, hazard ratio.
Table 1.
Summary of Efficacy End Points Assessed in the Intention-to-Treat Pembrolizumab Population by BICR in Patients From East Asia
| End point | Intention-to-treat Pembrolizumab Population |
|
|---|---|---|
| Selpercatinib |
Control |
|
| (n = 75) | (n = 41) | |
| Progression-free survival - mo (95% CI) | ||
| Median progression-free survival | NE (16.4–NE) | 11.1 (7.0–16.8) |
| Median duration of follow-up | 19.4 (14.1–19.7) | 21.2 (11.1–24.7) |
| Objective response rate - % (95% CI) | 86.7 (76.8–93.4) | 61.0 (44.5–75.8) |
| Best overall response - no. (%) | ||
| Complete response | 5 (6.7) | 5 (12.2) |
| Partial response | 60 (80.0) | 20 (48.8) |
| Stable disease | 9 (12.0) | 11 (26.8) |
| Progressive disease | 1 (1.3) | 3 (7.3) |
| Not evaluable | 0 | 2 (4.9) |
| Median Time to Response – mo (range) | 1.5 (1.2–6.8) | 1.6 (1.3–11.3) |
| Duration of response | ||
| patients with a response and censored data - no. (%) | 65 (86.7) | 25 (61.0) |
| Median duration of response - mo (95% CI) | NE (15.4–NE) | 11.5 (9.6–NE) |
| 12-mo duration of response rate - % (95% CI) | 76.9 (63.4–85.9) | 46.9 (24.3–66.6) |
BICR, blinded independent central review; CI, confidence interval; n, no. of people in the subgroup; NE, not evaluable.
As reported previously, overall survival data in the intention-to-treat population were not yet mature at the interim analysis. The HR for death for patients from East Asia in the intention-to-treat pembrolizumab and overall intention-to-treat populations were 0.81 (95% CI: 0.34–1.94) and 0.81 (95% CI: 0.37–1.79), respectively (Supplementary Fig. 3). With a median follow–up of 22 months, 77% of patients in the intention-to-treat pembrolizumab and 75% of patients in the overall intention-to-treat populations were still alive.
Safety and Pharmacokinetics
Of the 142 randomized patients from East Asia, 140 patients received the assigned study treatment and were included in the safety population (91 of which received selpercatinib), pharmacokinetic data were available from 72 patients in the selpercatinib arm on 160 mg twice daily. At C1D8, the geometric mean trough plasma concentration of selpercatinib was 3070 ng/mL and trough concentrations remained consistent in subsequent cycles (up to C6D1) (Supplementary Fig. 4).
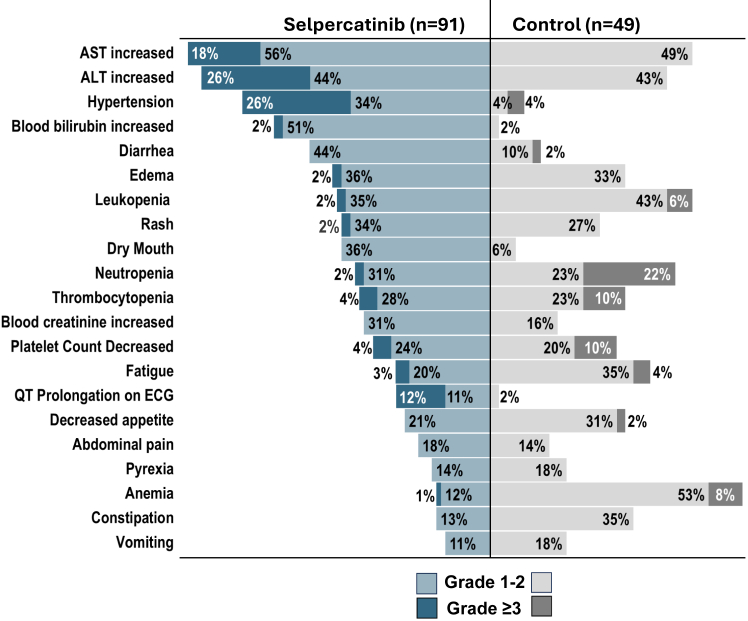
AEs that occurred at a higher incidence with selpercatinib than with control treatment (by ≥10%) included increased aspartate aminotransferase levels, increased alanine aminotransferase levels, hypertension, prolonged QTc interval, increased bilirubin levels, diarrhea, and increased blood creatinine levels (Fig. 2). AEs that occurred at a higher incidence with control treatment than with selpercatinib (by ≥10%) included anemia, constipation, fatigue, decreased appetite, leukopenia, and neutropenia (Fig. 2). As previously reported, patients from East Asia had a higher incidence of grade 3 or higher AEs and treatment discontinuations because of AEs than was reported in the overall study population, though most AEs were manageable with dose adjustments. Overall, the incidence of grade 3 or higher AEs was greater with selpercatinib than with control treatment (87% versus 65%). A total of 11 patients (12%) in the selpercatinib group and one patient (2%) in the control group discontinued study treatment because of AEs. Dose adjustments occurred in 77% of the patients who received selpercatinib, as compared with 51% of those who received control treatment (Supplementary Table 3).
Figure 2.
Adverse events among patients from East Asia in the safety population. The tornado plot includes AEs that occurred during treatment in at least 15% of the patients in either group. The terms used to describe the AEs are adapted from or composites of the Medical Dictionary for Regulatory Activities, Version 25.0, preferred terms. AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ECG, electrocardiogram; n, no. of patients in the subgroup.
Discussion
In LIBRETTO-431, selpercatinib more than doubled PFS (median PFS; selpercatinib: 24.8 mo, control: 11.2 mo) compared with chemotherapy with pembrolizumab, and delayed CNS progression in patients without CNS metastases in first-line patients with RET fusion–positive NSCLC.1,4 This report focuses on the efficacy and safety profile of selpercatinib in the 142 randomized patients from East Asia, comprising most of the study population. In patients from East Asia of the intention-to-treat-pembrolizumab population, PFS improved with selpercatinib compared with control (not reached versus 11.1 mo; HR: 0.38 [95% CI: 0.22–0.68]; p = 0.0008). Treatment with selpercatinib led to a higher objective response rate (87% versus 61%) and more durable responses (12-mo DOR rate: 77% versus 47%) in patients from East Asia compared with control. These results are consistent with those observed in the overall LIBRETTO-431 population.
With the development of more potent therapies for oncogene-addicted cancers, it is important to ensure the effectiveness and safety of new treatments in different geographies, as differences have been noted with other targeted agents.11 Pharmacokinetic results revealed that selpercatinib plasma concentrations were consistent with those observed in the global phase 1/2 LIBRETTO-001 study, further validating its stability and bioavailability across different populations. Although the incidence of grade 3 or higher AEs was higher with selpercatinib compared with control treatment, they were generally manageable with dose adjustments. Consistent with previous results from the phase 2 LIBRETTO-321 study, the most common grade 3 or higher AEs observed in the selpercatinib arm were increased alanine aminotransferase, increased aspartate aminotransferase, hypertension, thrombocytopenia, prolonged QTc, and edema. Similar to the overall study population, hematologic toxicities were among the most common AEs observed in patients from East Asia on control treatment. Taken together, selpercatinib continues to exhibit a favorable risk-benefit profile in patients with NSCLC from East Asia.
LIBRETTO-431 enrollment was generally well-balanced by geography (East Asia versus non–East Asia), though there were minor differences in enrollment by country (People’s Republic of China, Japan, Korea) in the intention-to-treat pembrolizumab and overall intention-to-treat populations. These differences may be attributed to geographic differences in physicians’ preferred choice of chemotherapy with or without pembrolizumab.
Advancements in available therapies for oncogene-driven NSCLC have reinforced the importance of comprehensive genomic testing at the time of diagnosis and the use of targeted treatments in the first-line setting. Taken together, these findings further support the use of first-line selpercatinib in patients with RET fusion–positive NSCLC across geographies.
CRediT Authorship Contribution Statement
Koichi Goto: Conceptualization, Investigation, Writing - review & editing.
Herbert H. Loong: Conceptualization, Investigation, Writing - review & editing.
Caicun Zhou: Conceptualization, Investigation, Writing - review & editing.
Silvia Novello: Investigation, Writing - review & editing.
Kazumi Nishino: Investigation, Writing - review & editing.
Dae Ho Lee: Investigation, Writing - review & editing.
Se-Hoon Lee: Investigation, Writing - review & editing.
James Chih-Dan Liu: Validation, Formal analysis, Methodology, Writing - review & editing.
Justin Williams: Writing - Original draft, review & editing, Visualization.
Minji Kim Uh: Conceptualization, Writing - review & editing.
Hongmei Han: Validation, Formal analysis, Writing - review & editing, Visualization.
Tarun Puri: Conceptualization, Writing - review & editing.
Aimee Bence Lin: Conceptualization, Methodology, Writing - review & editing.
Ying Cheng: Conceptualization, Investigation, Writing - review & editing.
Disclosure
Dr. Goto reports receiving grants (to institution) from Amgen Inc., AstraZeneca, AbbVie, AnHeart Therapeutics Inc., Bayer Yakuhin, Ltd., Boehringer-Ing elheim, Bristol-Myers Squibb, Blueprint Medicines Corporation., Chugai Pharmaceutical Co. Ltd., Craif Inc., Daiichi Sankyo Company. Limited; Eisai Co. Ltd., Eli Lilly and Company, Guardant Health Asia, Middle East and Africa Inc., Haihe Biopharma Co. Ltd., Ignyta Inc., Janssen Pharmaceutical, Kyowa Kirin Co. Ltd., Life Technologies Japan Ltd., Loxo Oncology, Inc., Lunit Inc., Medical and Biological Laboratories Co. Ltd., Merck Biopharma Co. Ltd., Merus Us Inc., Novartis Pharma, Ono Pharmaceutical Co. Ltd., Pfizer Research and Development, Precision Medicine Asia Co. Ltd., Riken Genesis Co. Ltd., Sumitomo Pharma Co. Ltd., Spectrum Pharmaceuticals Inc., Sysmex Corporation, Taiho Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., Turning Point Therapeutics Inc.; honoraria from Amgen Inc., Amoy Diagnostics Co. Ltd., AstraZeneca, Bayer, Bristol-Myers Squibb, Chugai Pharmaceutical Co. Ltd., Daiichi Sankyo Company Ltd., Eisai Co. Ltd., Eli Lilly, Guardant Health Japan Corp., iTeos Therapeutics Inc., Janssen Pharmaceutical, ThermoFisher Scientific, Merck Biopharma Co. Ltd., Nippon Kayaku Co. Ltd., Novartis Pharma, Ono Pharmaceutical Co. Ltd., Pharma Mar, Riken Genesis Co. Ltd., Taiho Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd.; and had an advisory board role for Amgen Inc., AstraZeneca, Bayer Healthcare Pharmaceuticals Inc., Bristol-Myers Squibb, Daiichi Sankyo Company, Ltd., Eli Lilly and Company, GlaxoSmithKline, Haihe Biopharma Co. Ltd., Janssen Pharmaceuticals, and Syneos Health Clinical. Dr. Loong reports receiving grants (to institution) from Merck Sharp & Dohme, Mundipharma, and Novartis; had consultancy roles in Boehringer-Ingelheim, Celgene, Eli Lilly and Company, Illumina, Novartis, Merck Sereno, Takeda, and George Clinical; received honoraria from AbbVie, Bayer, Eisai, Eli Lilly and Company, Guardant Health, and Novartis; and received travel support from Bayer, Boehringer-Ingelheim, Merck Sharp & Dohme, Novartis, and Pfizer. Dr. Zhou reports having consultancy role in QiLu Pharmaceutical and TopAlliance Biosciences Inc.; and received honoraria from Eli Lilly and Company, Roche, Merck Sharp & Dohme, QiLu Pharmaceutical, Hengrui Pharmaceutical, Innovent Biologics, Alice, CStone Pharmaceuticals, LUYE Pharma, and TopAlliance Biosciences Inc. Dr. Nishino reports receiving grants (to institution) from Ono Pharmaceutical Co. Ltd., Taiho Pharmaceutical Co. Ltd., Abbvie, Daiichi Sankyo Company Ltd., Amgen, Eisai Co. Ltd., Sanofi K.K., Janssen Pharmaceutical K.K., Novartis Pharmaceuticals, Pfizer, Merck, Biopharma Co. Ltd., and Takeda Pharmaceutical Co. Ltd.; received honoraria from AstraZeneca, Nippon Boehringer-Ingelheim, Life Technologies Japan Ltd., Merck Sharp & Dohme, Novartis Pharmaceuticals, Pfizer, Merck Biopharma Co. Ltd., Janssen Pharmaceutical K.K., Varian Medical Systems Inc., Nippon Kayaku, Ono Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., Amgen, Daiichi Sankyo Company Ltd., and Sun Pharma Japan Ltd. Dr. D.H. Lee reports having a consultancy role in St. Cube and Abion; and received honoraria from AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly and Company, Merck Sharp & Dohme, Janssen. Novartis, Ono, Pfizer, Roche, and Takeda. Dr. S-H. Lee reports receiving grants (to institution) from AstraZeneca, Daiichi Sankyo, Lunit, and Merck Sharp & Dohme; and had consultancy roles in Amgen, AstraZeneca/MedImmune, Bristol-Myers Squibb, Merck Sharp & Dohme, Roche, and Yuhan. Dr. Yang reports receiving grants (to institution) from AstraZeneca; had consultancy role in Boehringer, Ingelheim, Takeda, Pfizer, Amgen, AstraZeneca, Roche, Daiichi Sankyo, Merck Sharp & Dohme, Eli Lilly and Company, Janssen Oncology, and AbbVie; and received travel support from AstraZeneca, Jassen Oncology, Dizal, Takeda, and ArriVent Biopharma. Drs. Liu, Uh, Lin, Puri, and Ms. Han report employment with Eli Lilly and Company. Dr. Cheng declares no conflicts of interest.
Acknowledgments
This study (NCT04194944) was supported by Eli Lilly and Company. The authors thank the investigators and site personnel for their participation in the study, and the clinical trial participants and their families and caregivers, without whom this work would not be possible. The authors also thank Justin Williams, an employee of Eli Lilly and Company, for his insights, guidance, and writing support of the manuscript. We also thank Deborah Rajakumar, a former employee of Eli Lilly and Company, for her careful analysis and insights on the safety data.
Data Sharing Statement
Eli Lilly and Company provides access to all individual data collected during the trial, after anonymization, with the exception of pharmacokinetic, genomic, or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date for data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
Footnotes
Cite this article as: Goto K, Loong H, Zhou C, et al. First-line selpercatinib or chemotherapy and pembrolizumab in patients from East Asia with RET fusion–positive NSCLC: a LIBRETTO-431 subgroup analysis. JTO Clin Res Rep 2025;6:100868.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2025.100868.
Supplementary Data
References
- 1.Zhou C., Solomon B., Loong H.H., et al. First-line selpercatinib or chemotherapy and pembrolizumab in RET fusion-positive NSCLC. N Engl J Med. 2023;389:1839–1850. doi: 10.1056/NEJMoa2309457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wirth L.J., Brose M.S., Elisei R., et al. LIBRETTO-531: a phase III study of selpercatinib in multikinase inhibitor-naive RET-mutant medullary thyroid cancer. Future Oncol. 2022;18:3143–3150. doi: 10.2217/fon-2022-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subbiah V., Wolf J., Konda B., et al. Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): a phase 1/2, open-label, basket trial. Lancet Oncol. 2022;23:1261–1273. doi: 10.1016/S1470-2045(22)00541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pérol M., Solomon B.J., Goto K., et al. CNS protective effect of selpercatinib in first-line RET fusion-positive advanced non-small cell lung cancer. J Clin Oncol. 2024;42:2500–2505. doi: 10.1200/JCO.24.00724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hendriks L.E., Kerr K.M., Menis J., et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:339–357. doi: 10.1016/j.annonc.2022.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Jaiyesimi I.A., Leighl N.B., Ismaila N., et al. Therapy for stage IV non-small cell lung cancer with driver alterations: ASCO living guideline, version 2023.3. J Clin Oncol. 2024;42:e1–e22. doi: 10.1200/JCO.23.02744. [DOI] [PubMed] [Google Scholar]
- 7.Drilon A., Subbiah V., Gautschi O., et al. Selpercatinib in patients with RET fusion-positive non-small-cell lung cancer: updated safety and efficacy from the registrational LIBRETTO-001 Phase I/II trial. J Clin Oncol. 2023;41:385–394. doi: 10.1200/JCO.22.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng X., Ji Q., Sun Y., et al. Efficacy and safety of selpercatinib in Chinese patients with advanced RET-altered thyroid cancers: results from the phase II LIBRETTO-321 study. Ther Adv Med Oncol. 2022;14 doi: 10.1177/17588359221119318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng Y., Huang D., Zhou J., et al. Intracranial activity of selpercatinib in Chinese patients with advanced RET fusion-positive non-small-cell lung cancer in the Phase II LIBRETTO-321 trial. JCO Precis Oncol. 2023;7 doi: 10.1200/PO.22.00708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu S., Cheng Y., Huang D., et al. Efficacy and safety of selpercatinib in Chinese patients with advanced RET fusion-positive non-small-cell lung cancer: a phase II clinical trial (LIBRETTO-321) Ther Adv Med Oncol. 2022;14 doi: 10.1177/17588359221105020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho B.C., Chewaskulyong B., Lee K.H., et al. Osimertinib versus standard of care EGFR TKI as first-line treatment in patients with EGFRm advanced NSCLC: FLAURA Asian subset. J Thorac Oncol. 2019;14:99–106. doi: 10.1016/j.jtho.2018.09.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.