Abstract
Pancreatic ductal adenocarcinoma (PDAC) remains highly resistant to therapy, surviving despite hypoxia, oxidative stress, and nutrient deprivation. Redox effector factor-1 (Ref-1) regulates several oncogenic transcription factors (TFs) and is controlled by peroxiredoxins (PRDX). We investigated how Ref-1 inhibition by APX2014, combined with PRDX expression, affects pancreatic cancer cells from multiple patient lines. Silencing or CRISPR/Cas9 knockout of PRDX1—but not PRDX2-6—sensitized PDAC cell lines to APX2014 both in vitro and in vivo without affecting Ref-1's DNA repair function of apurinic/apyrimidinic endonuclease. The combination of PRDX1 loss and APX2014 treatment increased apoptosis and decreased TF activity (NF-κB, HIF-1α) and their downstream targets, TNFAIP2, Survivin, and CA9. A 3D co-culture with PRDX1-null tumor spheroids and cancer-associated fibroblasts (CAFs) showed that (i) Ref-1 inhibition impaired PRDX1-null tumor growth, and (ii) PRDX1 loss reduced CAF viability, highlighting redox crosstalk within the tumor microenvironment (TME). In a PDAC xenograft model, PRDX1-knockout tumors treated with APX2014 had smaller volumes, lighter weights, lower Ref-1, and decreased Ki-67, with improved animal survival. Patient microarrays indicated increased Ref-1 and PRDX1 levels versus normal tissue, emphasizing their clinical relevance. Overall, these data identify PRDX1 as a key factor for PDAC's susceptibility to Ref-1 blockade, suggesting dual targeting could disrupt TME redox signaling, limit tumor progression, and improve APX therapy. These findings support dual targeting of Ref-1 and PRDX1 as a promising therapeutic approach in PDAC and other redox-driven cancers.
Keywords: Pancreatic cancer, Redox factor-1, Peroxiredoxin, Tumor microenvironment, HIF-1α, NF-κB
Graphical abstract
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is highly heterogeneous, genetically unstable, and intrinsically resistant to therapy. Redox effector factor-1 (Ref-1) is highly elevated in PDAC and coordinates several oncogenic transcription factors (TFs) such as HIF-1α, NF-κB, STAT3, and AP-1, thereby driving multiple cancer pathways [[1], [2], [3], [4], [5], [6]]. Ref-1 serves another significant role beyond its redox regulation of TFs; it also acts as a DNA repair endonuclease, with both functions entirely separable. Since Ref-1's redox and DNA-repair domains are distinct, APX inhibitors selectively inhibit only the redox activity [[7], [8], [9]] while sparing the repair function [2,[10], [11], [12], [13]]. Studies utilizing HSQC NMR experiments have previously mapped the binding pocket for Ref-1 specific redox inhibitors, APX3330 and APX2009 [14,15], and the latest APX2014 binding data are presented here.
The TME of PDAC is a complex and dynamic network of cell types that exhibit plasticity and contribute to the desmoplastic stroma characteristic of the disease [16]. This stroma not only drives drug resistance but also acts as a barrier to infiltration of immune cells and delivery of chemotherapy [17]. Comprised largely of tightly packed CAFs, the stroma produces extracellular matrix (ECM) and elevates interstitial fluid pressure (IFP), which can induce oxidative stress [18,19]. CAFs are well known to secrete growth factors and cytokines that promote epithelial-mesenchymal transition (EMT), remodel the stromal ECM, promote metastasis, and contribute to therapeutic resistance [20,21]. Oxidative stress also plays a key role in the crosstalk between tumors and CAFs yet the contribution of specific redox proteins such as Ref-1 and PRDX1 are still under investigation. H2O2 levels in the TME can drive CAF activation and signaling, influencing both resident fibroblasts and tumor cells. Signaling through TGFβ pathways can contribute to this by downregulating redox signaling protein, glutathione peroxidase 1 (GPX1) [19]. For the studies presented here, we utilize co-culture models of PDAC to recapitulate these interactions and to further define the role of Ref-1 and PRDX1 in modulating tumor-stroma crosstalk. The fibrotic phenotype accompanying PDAC that contributes to the resistance of the disease and precludes chemotherapeutic agents from penetrating the tumor provides further rationale for the use of this 3D co-culture system.
The activity of Ref-1 is further modified by the PRDX1/Ref-1/TRX1 cycle [[22], [23], [24]]. Peroxiredoxins (PRDXs) are a family of six thiol-dependent peroxidases that maintain cellular redox homeostasis by reducing reactive oxygen species (ROS) [25,26]. Among these, PRDX1 stands out due to its frequent overexpression in various cancers, including PDAC, and its wider role in tumor progression, metastasis, and therapy resistance [25,[27], [28], [29], [30], [31], [32]]. Unlike other PRDX family members—which are often restricted to specific organelles (e.g., PRDX3 in mitochondria, PRDX4 in the endoplasmic reticulum, PRDX6 in cytosol/lysosomes)—PRDX1 is localized in both the nucleus and cytoplasm, enabling it to directly influence redox-sensitive transcription factors such as NF-κB, HIF-1α, and STAT3. Specifically, PRDX1 has been associated with the transformation of fibroblasts into cancer-associated fibroblasts (CAFs), further aiding stromal remodeling and tumor microenvironment (TME) adaptation [25]. Notably, prior studies have shown a direct redox interaction between PRDX1, thioredoxin (TRX1), and Ref-1, creating a unique redox relay essential for keeping Ref-1 in its active reduced state, a function not significantly reported for other PRDX family members [22]. This distinctive interaction positions PRDX1 as a critical node regulating the sensitivity of PDAC cells and CAFs to Ref-1 targeted therapies, making it an appealing and specific therapeutic target compared to other PRDX isoforms.
To investigate how PRDX modulates Ref-1 inhibitor sensitivity, we used siRNA knockdown and CRISPR knockout to target PRDX1, while also exploring the knockdown of PRDX2-6. The knockdown of PRDX1 (PRDX1KD) significantly increased cell death in pancreatic cancer cells, Pa03C and Panc-1, as well as in CAF19, after treatment with three Ref-1 inhibitors—APX3330, APX2009, and APX2014. However, the knockdown of PRDX2-6 did not enhance the killing caused by APX2014. Focusing on PRDX1, we created PRDX1-knockout (PRDX1KO) clones in Pa03C, treated them with APX2014, and evaluated tumor behavior in 3D co-cultures and mouse xenografts. The loss of PRDX1 significantly increased the tumor-spheroid response to APX2014, while CAF proliferation was reduced even without the drug. Under hypoxia, PRDX1KO cells showed suppressed NF-κB and HIF-1α-driven targets, confirming effective transcription factor inhibition. In vivo, mice with PRDX1KO tumors treated with APX2014 were significantly smaller and had longer survival than controls. Additionally, Ki-67 and Ref-1 immunostaining levels decreased, indicating reduced tumor cell proliferation. Tumor microarrays showed elevated levels of Ref-1 and PRDX1 in PDAC than in normal adjacent pancreas, emphasizing their clinical importance. These results identify PRDX1 as the main peroxiredoxin regulating PDAC dependence on Ref-1 redox signaling. Combining PRDX1 depletion with APX-mediated Ref-1 inhibition disrupts tumor-stromal redox balance, improves anti-tumor effects, and supports a dual targeting approach of Ref-1 and PRDX1 as a new therapeutic strategy in PDAC.
2. Materials and methods
2.1. In vitro cell culture
Pancreatic cancer cells, Pa03C, along with cancer-associated fibroblasts, CAF19, were generously provided by Dr. Anirban Maitra at The Johns Hopkins University, while Panc-1 was sourced from the In Vivo Therapeutics Core at IUSCCC. The cells were maintained in DMEM culture media (Gibco ThermoFisher, Waltham, MA), supplemented with FBS (Bio-techne, Minneapolis, MN), at 37 °C and 5 % CO2. All cell lines were authenticated through short tandem repeat (STR) analysis (IDEXX BioResearch, Columbia, MO) and confirmed to be free of mycoplasma.
Pa03C PRDX1KO cells were generated using CRISPR/Cas9 genome editing (Synthego Corporation, Redwood City, CA, USA). Briefly, the Pa03C cell line was confirmed to be free of mycoplasma and Synthego created a pool of PRDX1KO cells via CRISPR/Cas9. The guide delivery and editing efficiency (Indel % and pooled KO-score) achieved an efficiency and positivity of 87 %, respectively. Clones were produced using an in-house single-clone strategy that involved a dilution series of cell populations across 96-well plates to generate colonies derived from single cells. These colonies were later expanded in 12-well tissue culture plates and transferred to T25 tissue culture flasks. Some clones exhibited cell balling (not shown), and the addition of 1 mM sodium pyruvate to the media resolved this issue. Further expansion did not require the pyruvate supplement, which was subsequently discontinued. Finally, clones were screened by Western blotting to confirm PRDX1 KO using 100 μg lysate. The guide RNA sequence and scoring success, as well as the clone screening, are provided in Supplemental Fig. S2.
2.2. Ref-1 inhibitor treatment
To analyze cell viability and proliferation after drug treatment, tumor cells were seeded at 2.5 × 103 cells per well in 96-well tissue culture plates (ThermoFisher, Waltham, MA), as we have previously performed [7,33,34]. Cells were allowed to attach overnight followed by treatment with Ref-1 inhibitor at optimized concentrations in DMEM containing 5 % FBS. The cells were treated for 24–48 h with optimized drug dosages of Ref-1 inhibitors APX3330, APX2009, APX2014, or the control drug RN7-58 (Apexian Pharmaceuticals, Indianapolis, IN). The fluorescent cell viability reagent, alamarBlue™ (Invitrogen, Eugene, USA), was added 5 h before the treatment ended at a final concentration of 10 %. Fluorescence was measured at 544/590 nm using the Synergy H1 plate reader (BioTek, Winooski, VT). Relative fluorescence units (RFU) were averaged from three wells, and background values consisting of drug media only were subtracted. To calculate the final percent viability, RFU was normalized to cells exposed to media only. Experiments were repeated in triplicate or more.
2.3. siRNA transfections
siRNA knockdown studies were conducted as in previous studies with various genes [26]. Tumor and CAF19 cells were seeded at 3 × 105 cells per well in a 6-well plate (Corning, New York, NY) and allowed to attach overnight. Cells were then transfected with an optimized amount (5–30 nM) of PRDX1-6 siRNA or Universal/Scrambled control (SCR) (OriGene Technologies, Rockville, MD) (Supplemental Table S1) using RNAiMax (Invitrogen, Carlsbad, CA) in Opti-MEM™ (0.3–0.4 % of total volume) (Gibco ThermoFisher, Waltham, MA) and incubated for 24 h. Based on the optimal time point for achieving at least 70 % target protein knockdown efficiency (48–96 h; confirmed by Western analysis), transfected cells were collected and reseeded at 2.5 × 103 cells per well in 96-well plates for 48 h. Increasing doses of Ref-1 inhibitor(s) were added, and final viability testing was performed. The remaining transfected cells were seeded at 5 × 105 cells per 6-well for collection on the day of treatment to confirm knockdown. At least three independent transfections were conducted.
2.4. 3D Co-culture assays
3D co-culture assays were conducted as previously described using 96-well ultra-low attachment plates (Corning, New York, NY) [35]. After spheroid formation for 4 days, spheroids were treated with different concentrations of APX2014 on Days 4, 8, and 12 with 3D culture media (DMEM, 5 % FBS, and 3 % Matrigel Growth Factor Reduced) (Corning, New York, NY). PRDX1KO cells were stably transduced with CAG-RFP lentivirus (Cellomics Technology, LLC, Cat No. PLV-10071-50) with an MOI of 10 and CAF19 was obtained from Dr. Anirban Maitra at The Johns Hopkins University and were stably transduced with EGFP as previously described [59]. CAF19 cells were transfected with PRDX1 siRNA and were included in the 3D co-culture assay at a ratio of 1:4 (tumor:CAF). A baseline fluorescence reading was taken on Day 4 prior to drug treatment and subsequently on Days 8, 12, and 14 before treatment was administered. Fluorescence intensity was monitored using the Thermo Scientific™ ArrayScan™ High-content Imaging System (ThermoFisher, Waltham, MA, Chemical Genomics Core, IUSM). Fold change was calculated by normalizing to media control intensity on Day 14 to assess the drug treatment effect and KD/KO effect on spheroid growth over time.
2.5. Western blotting analysis
Protein expression and knockdown efficiency were quantified by Western analysis, as described previously [34]. Briefly, cell pellets were lysed in either 1 % SDS or RIPA extraction buffer supplemented with protease inhibitors (Santa Cruz Biotechnology, Dallas, TX). The cells were then sonicated, clarified, and the protein was quantified using a detergent-compatible protein assay (Bio-Rad Laboratories, Hercules, CA). Denatured samples were loaded onto stain-free TGX SDS-PAGE (Bio-Rad Laboratories, Hercules, CA) for electrophoresis. Proteins were transferred onto either nitrocellulose or PVDF membranes (Bio-Rad Laboratories, Hercules, CA) using the Trans-blot® Turbo™ (Bio-Rad Laboratories, Hercules, CA). Membranes were blocked in 5 % Milk TBS-T and incubated overnight on a rocker at slow speed with PRDX1-5 (Proteintech Group, Rosemont, IL) or PRDX2, PRDX6 (Santa Cruz, Dallas, TX) (Supplemental Table S1), followed by 1 h with either a horseradish peroxidase-conjugated secondary antibody (Bio-Rad Laboratories, Hercules, CA) or IRDye (LI-COR®, Lincoln, NE). Finally, the membranes were imaged on ChemiDoc™ (Bio-Rad Laboratories, Hercules, CA) using either Pierce™ ECL substrate (ThermoFisher, Waltham, MA) or 680RD-800CW filters, respectively. Band intensities were quantified using Image Lab software (Bio-Rad Laboratories, Hercules, CA) and normalized to both total protein and loading controls, Vinculin or Actin (EMD Millipore, Burlington, MA).
2.6. Real-time quantitative PCR (qRT-PCR)
qRT-PCR was conducted as in previous studies [33,36]. Cells were harvested, and RNA was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA) following the manufacturer's protocol. RNA concentrations were measured using a NanoDrop spectrophotometer (ThermoFisher, Waltham, MA). For cDNA synthesis, 1 μg of RNA was reverse-transcribed in a 20 μL reaction mix with the qScript cDNA synthesis kit (Quantabio, Beverly, MA). qRT-PCR was conducted in a 96-well plate with a total reaction volume of 20 μL per well using SYBR Green PCR Master Mix (Applied Biosystems, Waltham, MA) on a CFX96 real-time PCR detection system (Bio-Rad Laboratories, Hercules, CA). The primers for target genes CA9, Survivin, TNFα‐induced protein 2 (TNFAIP2), and housekeeping gene RPL13A were synthesized (ThermoFisher, Waltham, MA), and their sequences are listed in Supplemental Table S2 qRT-PCR cycling conditions were as follows: initial denaturation at 95 °C for 1 min, followed by 40 cycles of 15 s at 95 °C and 1 min at 60 °C. Relative mRNA expression levels were calculated using the 2−ΔΔCT method, normalizing to the expression of the RPL13A gene.
2.7. Apoptosis assay
The APC-Annexin V/PI Apoptosis Detection kit was utilized for the apoptosis assay (ThermoFisher, Cat#: 88-8007-74). Briefly, Pa03C Cas9 Control and PRDX1KO 1A2 were seeded at 3 × 105 cells per 12-well and allowed to attach overnight. Cells were then treated with either DMSO vehicle control (EMD Millipore, Burlington, MA) or APX2014 for 24–48 h. Following treatment, cells were washed with PBS, counted, and stained in the dark with 10 μL of Annexin V-APC in 100 μL of 1x binding buffer for 30 min. Cells were thoroughly washed with binding buffer and finally resuspended in 200 μL of binding buffer. Subsequently, 5 μL of Propidium iodide was added for necrosis staining. All samples were assessed for apoptosis (Attune NxT) in the IUSCCC flow cytometry core.
2.8. Luciferase reporter assay
To assess transcription factor transactivation, we utilized stably transduced Pa03C cells expressing HIF-1α or NFκB luciferase reporters (TR412VA-P & TR426VA-P), along with a negative control (TR411VA-P) from System Biosciences, Palo Alto, CA, as previously described [7]. These cells were then transfected under optimized conditions for KD of PRDX1 or a scrambled control, incubated for 24 h, and subsequently seeded at 2.5 × 103 cells per well in either a 96-well white tissue culture Costar® plate (Corning, Kennebunk, ME) for luciferase assay or a clear 96-well plate for AlamarBlue™ cytotoxicity testing (ThermoFisher, Waltham, MA). PRDX1KD was confirmed through Western analysis on the day of treatment. Treatment consisted of APX2014 at 5, 7.5, and 10 μM for 24 h. Reporter activity following treatment was first normalized to cells under normoxia for HIF-1α and to cells without treatment (media only) for NF-κB and then normalized to the fluorescent values to ensure that only live cells were evaluated for HIF-1α or NF-κB activity.
2.9. NMR studies of Ref-1 interactions with APX2014
Protein expression and purification. For NMR studies, we expressed 15N -Ref-1 (39–318/C138A) as a His-SUMO fusion in BL21/Rosetta E. coli. We introduced 15N using 15N -ammonium chloride (Cambridge Isotopes) as the sole nitrogen source in 2 L minimal media cultures [37,38]. Cell cultures were induced with 0.2 mM IPTG at an OD of 0.6 and grown for 4 h at 37 °C. They were then pelleted and stored at −80 °C before purification. Lysis buffer containing 50 mM sodium phosphate, 0.3 M sodium chloride, and 20 mM imidazole at pH 7.8 was used to resuspend the cells prior to lysis with a microfluidizer M110L. The cell lysate was centrifuged at 35,000 rpm for 30 min. Following ultracentrifugation, the supernatant was filtered and applied to two 0.5 ml Ni-NTA (Qiagen, Inc.) columns (one column per liter of growth), run at 4 °C. After loading the supernatant, the columns were washed with 10 column volumes of buffer. On-column cleavage to remove the His-SUMO tag occurred via incubation with Ulp1 protease (10 μg/column) in 8 ml per column overnight. Isotopically labeled Ref-1 was collected as the flowthrough the next day. 15N-Ref-1 was then subjected to SP-sepaharose ion-exchange chrormatographic purification. The protein was eluted using a salt gradient of 0–1 M NaCl in 50 mM MES pH 6.0. Peak fractions were combined and assessed using SDS-PAGE and QToF intact mass spectrometric analysis to confirm purity and mass.
NMR spectroscopy. NMR spectroscopy was performed in the same manner as previously published [39]. 15N-Ref-1 at a concentration of 80 μM was dialyzed against 20 mM sodium phosphate (pH 6.5) and 0.1 M sodium chloride in a 90 %/10 % H2O/D2O mix, then placed in an NMR tube. NMR spectra, specifically 2D 1H/15 N heteronuclear single-quantum coherence (HSQC) spectra, were acquired at 25 °C on a 600 MHz Bruker AVANCE spectrometer equipped with a 5-mm triple-resonance cryoprobe (1H, 13C, and 15 N) along with a z-axis pulsed gradient. NMR data were processed using TOPSPIN and analyzed with CCPN NMR. The inhibitor APX2014 was dissolved in acetonitrile-D3. For the inhibitor experiments, acetonitrile-D3 concentrations were capped at 2 % (v/v). Chemical shift perturbations (CSP) (Δδ) were calculated using eq. (1), which reflects the total weighted change in 1H and 15 N chemical shift for a given peak in the 2D spectra: Δδ = [(ΔδH)2 + (0.1ΔδN)2]1/2.
Molecular Docking. A structural model of APX2014 bound to Ref-1 was obtained using rigid-receptor docking with the AutoDock 4.2 and AutoDock Vina programs [[40], [41], [42]]. Prior to docking, the MolProbity webserver [43,44] and PROPKA [45] tools were used to analyze a crystal structure of Ref-1 (PDBID: 4QHD) [46] to check for residue flips and determine appropriate protonation states for titratable residues at pH = 7.0, respectively. APX2014 was constructed using UCSF Chimera [47]. The AutoDock Tools program was used to format receptor and ligand coordinate files and generate parameters for docking [40,42,48]. Docking grids were created with AutoGrid [40,42,48]. To account for the many degrees of freedom that could be sampled in APX2014, docking was carried out with an exhaustiveness setting of 50 or 100 for AutoDock 4.2 and AutoDock Vina, respectively. All other settings used the default options. The lowest energy consensus structures for APX2014 bound to Ref-1 were determined by manual inspection of the top 10 rank-ordered poses obtained from both programs, including ligand clustering commonly performed for AutoDock 4.2 results in AutoDock Tools. Figures were generated using PyMOL [40,41,49].
2.10. IL-8 cytokine quantification by ELISA
Culture supernatant was used for IL-8 screening via the Human IL-8 ELISA kit (ThermoFisher, Waltham, MA) according to the manufacturer's instructions. Briefly, Pa03C Cas9 Control and PRDX1KO 1A2, 1C3 were seeded at 3 × 105 cells per 6-well and allowed to attach overnight. Cells were then treated with either DMSO vehicle control (EMD Millipore, Burlington, MA) or 10 μM APX2014 for 24 h. The following day, culture supernatant was collected and stored at −80 °C until processed. Final readings were taken at an absorbance of 450 nm using a Synergy H1 microplate reader (BioTek, Winooski, VT). All samples were run in duplicates and repeated at least three times in independent experiments. Results can be found in Supplemental Fig. S3.
2.11. In vivo xenograft study
In vivo studies in mice were conducted under Protocol 21165 approved by the Institutional Animal Care and Use Committee of Indiana University. Age-matched (4–6-week-old) NSG (NOD.Cg-Prkdc scid Il2rg tm1Wjl/SzJ (NOD/SCIDγ(−/−))) mice were purchased from the IUSCCC Preclinical Modeling and Therapeutics Core and maintained in pathogen-free conditions. Following a one-week acclimatization period, mice were subcutaneously implanted with 2 × 106 Cas9 control cells or PRDX1KO (clones 1A2 and 1C3) cells on the right flank. One week post-implantation, mice were randomized based on their tumor volumes (∼120 mm3). They were then dosed once a day with either vehicle (PKT (Propylene glycol: Kolliphor HS15: Tween 80)) or 50 mg/kg APX2014 by intratumoral injection, administered with a frequency of 5 days of treatment followed by 2 days of rest. Tumors were measured with calipers twice a week, and body weight was recorded once a week. When the tumor size reached a limit of 2000 mm3, the mice were sacrificed, and the tumors were collected for further processing.
2.12. Immunohistochemistry
Following deparaffinization and rehydration, 5 μm thick tumor tissue sections underwent antigen retrieval in 0.1 M EDTA (pH 8.0) using a pressure cooker and were treated with 3 % hydrogen peroxide for 10 min to block endogenous peroxidase activity. Samples were incubated with primary antibodies Ref-1 (Novus, cat#: NB100-116) and PRDX1 (Proteintech, cat# 66820-1), diluted in TBST, either overnight at 4 °C or for 45 min at room temperature. Incubation with appropriate secondary antibodies was carried out for 1 h at room temperature. Immunoreactivity was detected using VECTASTAIN DAB for 10 min, and the reaction was stopped by rinsing the slides in distilled water. Counterstaining was performed with modified Mayer's hematoxylin (Vector), and the sections were dehydrated, cleared, and sealed with a coverslip. Immunohistochemical staining for Ki67 was conducted using the Agilent Dako Autostainer Link 48 system. Following antigen retrieval, as previously described, endogenous peroxidase activity was blocked by incubating the sections with the Envision FLEX Peroxidase-Blocking Solution (Agilent Dako, SM801) for 5 min at room temperature. Sections were then incubated with primary antibody (Ki67, Abcam, cat# ab205718, 1:100) and secondary antibody (Anti-Rabbit HRP, Abcam, cat# ab16667, 1:800) in TBST for 30 min each at room temperature. For visualization, the EnVision FLEX High pH Link visualization system (Agilent Dako, K8000) was utilized according to the manufacturer's protocol. Briefly, slides were incubated with EnVision FLEX/HRP detection reagent for 20 min, followed by visualization with 3,3′-diaminobenzidine (DAB) chromogen solution for 10 min. Sections were counterstained with Mayer's hematoxylin (Agilent Dako, SM806) for 5 min, followed by bluing in 0.1 % lithium carbonate solution for 1 min. Stained sections were dehydrated, cleared, and mounted as described above. Images were acquired at 20x magnification using an Aperio ScanScope CS digital slide scanner. Quantitative immunohistochemical analysis was performed using Indica Labs HALO image analysis software Cytonuclear v2.05 (Ref-1, Ki67) or Area Quantification v2.4.9 (PRDX1) modules. The total percentage of positive tissue (PRDX1) or cells (Ref-1, Ki67) was used for statistical analyses, which were conducted using GraphPad Prism software as described below [50,51].
2.13. Human Tissue Microarray (TMA)
A Tissue Microarray (TMA) was developed from human specimens collected at the Indiana University School of Medicine, IU Simon Comprehensive Cancer Center, in compliance with the guidelines of Indiana University School of Medicine. Patients' consent was obtained under institutional biobanking collection protocols IUSCC-0678 Total Cancer Care (IRB: #1807389306), IUCRO-0280IUSCC Tissue Bank (IRB: #1106005767), or IUCRO-0454 (IRB: #1312105608) for the use of biological specimens in cancer research. The TMA included 174 patients, with 127 patients serving as normal controls, and 167 patients providing a total of 319 tumor slides. (Note: some patients contributed more than one tumor slide, which explains why the total number of slides exceeds the number of patients) [52].
2.14. APE1 endonuclease activity assay
Pa03C PRDX1KO cell line 1A2 and the Cas9 control cell line were tested for apurinic/apyrimidinic (AP) endonuclease activity in an enzymatic assay previously described [53] to decipher if lacking PRDX1 affected efficiency at repairing an abasic (AP) site. Briefly, the AP target comprised annealed oligonucleotides containing tetrahydrofuran and the fluorescent 6-FAM label ((5′) 6-FAM-GAA-TCC-6CC-ATA-CGT-ATT-ATA-TCC-AAT-TCC) along with a Dabcyl quencher on the complementary strand ((3′) Dabcyl-GGA-ATT-GGA-TAT-AAT-ACG-TAT-GGT-GGA-TTC) (Eurogentec Ltd. Belgium). Cleavage of the oligo by the APE1 enzyme resulted in the release of 6-FAM from the quencher, with the amount of fluorescence proportional to APE1's repair efficiency. Cell lysate representing three cell passages was serially diluted 1:2 in a 4-point spread from 100 μg/mL to 12.5 μg/mL. The fluorescence was read kinetically at 1-min intervals for 5 min using the BioTek Synergy H1Ⓡ (BioTek, Winooski, VT). The rate of the reaction was used to determine the change in APE1 repair activity in 1A2 compared to Cas9. Additionally, a cytotoxicity assay was performed with 2000 cells/well (96-well) of Pa03C Cas9 and 1A2 and treated with an APE1 repair inhibitor, APE1 Inhibitor III (Sigma-Aldrich, St. Louis, MO) for 48 h (3 separate replications). Results can be found in Supplemental Fig. S4.
2.15. Statistics
The experiments were repeated in triplicate or more, and the obtained data were shown as ‘Mean + Standard Error or Standard Deviation’ normalized to the control. One-way and two-way ANOVA, as well as Student's t-test, were calculated using GraphPad Prism Version 10. Survival analysis was conducted with Cox regression. Changes in tumor volume over time were assessed using a linear mixed effects model, with mouse IDs treated as a random effect. The implementation was conducted in R version 4.1. When multiple statistical tests were conducted, Bonferroni correction was applied.
3. Results
3.1. PRDX1 knockdown increases PDAC sensitivity to Ref-1 inhibitors
A prior report indicated that PRDX1 interacts with Ref-1 and impacts its redox activity [22]. To investigate this relationship in PDAC, we silenced PRDX1 (>70 % reduction) with siRNA in Pa03C and Panc-1 tumor cells, as well as in CAF19 cancer-associated fibroblasts (Fig. 1A, B, and C). After 48 h, the cells were treated with Ref-1 redox inhibitors APX3330, APX2009, or APX2014, along with the inactive analog RN7-58 (Fig. 1D–G). In all three cell lines, PRDX1 knockdown (PRDX1KD) led to a significant, dose-dependent loss of viability with each active inhibitor compared to scrambled controls, while RN7-58 showed no effect. These results suggest that PRDX1 critically influences PDAC cell survival in the context of Ref-1 blockade and that reducing PRDX1 expression significantly increases the efficacy of Ref-1 inhibitors (see Supplemental Fig. S1).
Fig. 1.
Reduced PRDX1 expression in PDAC significantly enhanced cell sensitivity to Ref-1 Inhibitors. Peroxiredoxin-1 (PRDX1) expression was lowered by siRNA and confirmed by Western blot (A) in PDAC cell lines Pa03C and Panc-1, as well as in the PDAC cancer-associated fibroblast CAF19. The reduced PRDX1 expression (PRDX1KD) versus Scrambled control (SCR) increased cytotoxicity (alamarBlue) caused by several Ref-1 Inhibitors APX3330 (B), APX2009 (C), and APX2014 (D) after 48 h of treatment, while no effect was observed with the 1,4-naphthoquinone negative control, RN7-58 (E); IC50 indicated for each condition (Mean ± SEM). Two-way ANOVA statistical analysis, Mean ± SEM, n = 3 or greater: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001.
3.2. APX2014 binds to a small pocket in Ref-1
Chemical shift perturbations (CSPs) calculated from 1H–15 N HSQC spectra collected for a 1, 2, 4, 6, 10, and 12-fold molar excess of APX2014 over Ref-1 were used to assess molecular interactions. Shown in Fig. 2 are the CSPs for a 10-fold molar excess mapped onto the structure of Ref-1. Residues Ser135, Arg136, Gln137, Asp163, Tyr264, Phe266, Ile300, and Leu305 (Fig. 2A and B) exhibited CSPs with a threshold above 0.02, indicating a significant difference. Among these, residues 62, 135, 136, 137, and 163 define a small pocket on Ref-1, located on the opposite face of the protein from the DNA-binding site (Fig. 2C). The remaining residues with CSPs above 0.02 include 264, 266, 300, and 305, all of which, except for 305, are buried residues. This analysis suggests that APX2014 binds directly to Ref-1. APX3330 was previously reported to bind to the endonuclease active site pocket and, more recently, to the small distant pocket [54]. Thus, the interactions of APX2014 with Ref-1 are consistent with those of other known Ref-1 inhibitors [15,54]. Neither APX3330 nor APX2014 inhibits the endonuclease activity of Ref-1. This finding suggests that APX2014 mediates its effects through binding to the distant small pocket (Fig. 2D).
Fig. 2.
Interaction of APX2014 results in chemical shift perturbations (CSPs) in APE1/Ref-1. (A) 2D15N-HSQC spectrum with the superimposition of the free protein spectrum (black) and spectra with APX2014 concentration (10-fold molar excess) in red. Each 1H/15N peak corresponds to one backbone or side chain amide. Specific chemical shift perturbations are shown in small boxes. (B) CSPs vs residue number reveal interactions with APE1. Interacting residues with CSPs higher than 0.02 include Ser135, Arg136, Gln137, Asp163, Tyr264, Phe266, Ile300, and Leu305. (C) These interacting residues are shown as ball-and-stick renderings (N, blue, O, red, and C green in a cartoon rendering of APE1 (4QHD). In (D), a semi-transparent surface of APE1 is shown in light gray, cartoon rendering in gray, and interacting residues ball-and-stick renderings (colors as indicated in (C) with the view rotated approximately 90°). Surface residues appear in color on the surface. Residues 62, 135, 136, 137, and 163 define a small pocket on the opposite face of APE1 as the endonuclease active site. (E) A close-up view of the small pocket (same orientation as in (D) is shown with three poses obtained by molecular docking with Vina Autodock. “Ring out” poses are shown with C, magenta or yellow, (F) and (G), respectively, “ring in” with C in cyan (H). Increased sensitivity to Ref-1 inhibition by loss of PRDX1 is unique among the PRDX family. Pa03C pancreatic cancer cell line was transfected with siRNA specific to PRDX1, PRDX2, PRDX3, PRDX4, PRDX5, or PRDX6. Western blot analysis confirmed at least 70 % expression reduction in (I) PRDX1 (PRDX1KD), (J) PRDX2 (PRDX2KD), (K) PRDX3 (PRDX3KD), (L) PRDX4 (PRDX4KD), (M) PRDX5 (PRDX5KD), and (N) PRDX6 (PRDX6KD). Vinculin or Actin served as the loading controls. Cells were then treated with Ref-1 inhibitor APX2014 for 48 h (O), and cytotoxicity was normalized to cells containing media only, and viability was compared. Two-way ANOVA statistical analysis, Mean ± SEM, n = 3 or greater: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
Molecular docking of APX2014 to the small pocket of Ref-1 was performed with AutoDock 4.2 and AutoDock Vina, which use different conformational search algorithms and scoring functions to determine and energetically score ligand poses within a protein binding site [[1], [2], [3]]. Three consensus poses were identified in Autodock Vina (similar results were obtained using Autodock), which support the WaterLOGSY characterization of APX2014 binding [55]. These poses (collectively shown in Fig. 2E), two with the naphthyl “ring out” (Fig. 2F and G) and one with the “ring in” (Fig. 2H) are predicted to have similar binding affinities, within 0.2 kcal/mol of each other (which is within the predictive accuracy of rigid receptor docking) and either may be equally likely to occur. When the 1,4-naphthoquinone core of APX2014 binds into the pocket, it makes favorable hydrophobic interactions with Phe162, Phe165, Leu62, and Ile91. The rest of APX2014 protrudes outward from the pocket and can interact with water or several residues surrounding the pocket due to the greater flexibility of this part of the molecule. In the “ring-out” conformation, the n-propyl group buries into the pocket and sits adjacent to Ile91 and Leu62, forming favorable hydrophobic interactions. Consequently, the 1,4-naphthoquinone core sticks out into solution and can accept a hydrogen bond from Ser164 in one of two possible poses (Fig. 2G). The amide moiety in APX2014 is also spatially near Gln137 and may form a hydrogen bond upon side chain or ligand rotation.
3.3. Increased sensitivity to Ref-1 inhibition in PRDX1KD PDAC demonstrates a unique dynamic within the PRDX family
While we show that PRDX1 knockdown enhances the effects of APX2014-mediated Ref-1 inhibition, we also examined whether lower expression of other peroxiredoxins similarly affects cell viability in this context. Pa03C was transfected with optimized siRNA specific to PRDX2, PRDX3, PRDX4, PRDX5, and PRDX6, achieving a knockdown expression of 70 % or higher, or a scrambled control (Fig. 2I–N; Supplemental Table S1), followed by a 48-h treatment with APX2014. Although PRDX3KD showed significance at the highest APX2014 dosage, only PRDX1KD exhibited a significant reduction in cell viability across all drug combinations with APX2014 (Fig. 2O).
3.4. Enhanced sensitivity to Ref-1 inhibition is confirmed in CRISPR/Cas9-derived PRDX1KO clones
PRDX1 was successfully knocked out of Pa03C cells (PRDX1KO) using CRISPR/Cas9 gene editing (Synthego Corporation, Redwood City, CA, USA) (Supplemental Fig. S2A–C). Subsequent single clones were isolated and screened for PRDX1 expression. The absence of PRDX1 was confirmed by Western blot in clones 1A2, 1C3, and 1B3, while clone 1A5 retained PRDX1 expression (Supplemental Fig. S2D). A significant increase in sensitivity to Ref-1 inhibition, as measured by cytotoxicity (alamarBlue™), was confirmed in clones 1A2, 1B3, and 1C3 compared to the Cas9 control, but was not observed in the PRDX1-positive clone 1A5 (Supplemental Fig. S2E and F), further emphasizing the importance of PRDX1 expression in PCC cellular response to Ref-1 inhibition.
3.5. Combining Ref-1 inhibition with decreased PRDX1 expression leads to increased cell death through apoptosis
To further evaluate the mode of cell death in PRDX1KO clones following Ref-1 inhibition, we used an apoptosis assay using Annexin V/PI staining on APX2014-treated PRDX1KO clone 1A2 and Cas9 control cells at 24 and 48 h (Fig. 3). Annexin V/PI staining demonstrated a significant increase in the percentage of cells undergoing late apoptosis in PRDX1KO cells (11.2 %) compared to the Cas9 control cells (3.8 %) when treated with 10 μM APX2014 at 24 h (Fig. 3A and B). Further increases in late apoptosis were observed after 48 h in PRDX1KO cells (52.6 %) compared to the Cas9 control (12.4 %) (Fig. 3A–C). Moreover, cell death in the 1A2 clone was dose- and time-dependent, with a 10 μM concentration of APX2014 leading to significantly higher cell death (52.6 %) compared to 5 μM (14.0 %). These results confirm that the loss of PRDX1 sensitizes cells to APX2014, resulting in cell death via late apoptosis/necrosis rather than just a reduction in cell proliferation.
Fig. 3.
The flow cytometry-based apoptosis assay demonstrates increased cell death due to late apoptosis in PRDX1KO clone 1A2 compared to Cas9 control cells following treatment with APX2014. (A) Cell death was evaluated in PRDX1KO clone 1A2 (PRDX1KO 1A2) and Cas9 control cells (Cas9 Control). (B) After 24 h of treatment with 10 μM APX2014, PRDX1KO 1A2 cells exhibited a significant increase in late apoptosis (11.15 %) compared to Cas9 Control (3.83 %). (C) At 48 h, late apoptosis was markedly higher in PRDX1KO 1A2 (52.60 %) compared to Cas9 Control (12.42 %). The level of apoptosis was dose-dependent, with 10 μM APX2014 inducing significantly higher cell death (52.60 %) in PRDX1KO 1A2 compared to 5 μM APX2014 (13.97 %).
3.6. PRDX1 knockdown upregulates oncogenic transcription factor NF-κB and downregulates NF-κB and HIF-1α in combination with Ref-1 inhibition
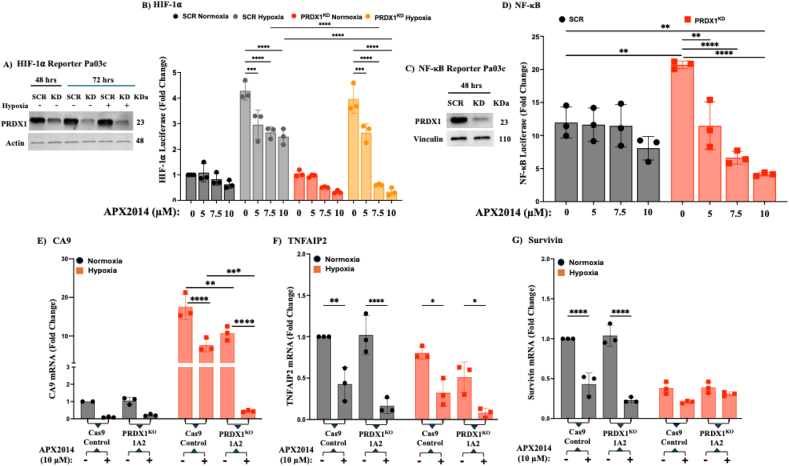
PDAC is recognized as a highly hypoxic tumor. Under conditions of oxidative and/or hypoxic stress, Ref-1-regulated oncogenic TFs such as STAT3, HIF-1α, and NF-κB control tumor progression and survival. Both HIF-1α and NF-κB have been shown to be highly active in PDAC cells as well as in stromal cells within the tumor microenvironment (TME) [33]. To compare the activation or inhibition of TFs regulated by Ref-1 and how PRDX1 may coregulate their expression, luciferase reporter assays and qPCR were performed to analyze downstream targets. Briefly, stably transduced PDAC Pa03C cell lines expressing HIF-1α and NF-κB-driven luciferase were transfected with PRDX1 siRNA to decrease PRDX1 expression (PRDX1KD). Western analysis confirmed knockdown efficiencies of over 70 % (Fig. 4A and C). Hypoxic conditions (1 % O2) were used to stimulate HIF-1α in both scrambled control and PRDX1KD cells to assess the effect of Ref-1 inhibition (APX2014: 5, 7.5, and 10 μM) on HIF-1α activity in the presence of PRDX1 and reduced levels. While no significant differences in HIF-1α transcriptional activity were observed in normoxia since basal HIF-1α activity was very low, as expected, under hypoxia, the substantial increases in HIF-1α activity (∼5-fold) in both the scrambled and knockdown conditions were significantly reduced under APX2014 treatment, while an even greater decrease was observed in the PRDX1KD cells (Fig. 4B).
Fig. 4.
Downregulation of transcription factors HIF1⍺ and NF-κB activity by Ref-1 inhibition in PDAC was more significant in the PRDX1KD condition than in the Scrambled control. Pa03C cells stably expressing the HIF-1⍺ Luciferase reporter were transfected with siRNA PRDX1 (PRDX1KD) or a scrambled control (SCR) for 72 h, and the results were confirmed by Western blot (A). PRDX1KD and SCR cells were then exposed for 24 h to either normoxic or hypoxic conditions (1 % O2). After this, both cell viability (measured with alamarBlue) and HIF-1⍺ activity were assessed following a 24-h treatment with APX2014 (10 μM) or DMSO as a vehicle control (B). Separately, Pa03C cells stably expressing the NF-κB Luciferase reporter were transfected with siRNA PRDX1 (PRDX1KD) or a scrambled control (SCR) for 48 h and confirmed by Western blot (C). Both cell viability and NF-κB activity were measured after 24 h of treatment with APX2014 (10 μM) or DMSO (D). Transcription factor data are presented as Mean ± SEM, with n ≥ 3, normalized to the vehicle, analyzed via two-way ANOVA (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001). Findings indicate that the inflammatory regulation and response of HIF-1⍺ and NF-κB pathways to Ref-1 inhibition in PDAC were more significantly downregulated in PRDX1 knockout clone 1A2 (PRDX1KO 1A2) than Cas9 Control cells (Cas9 Control); but not the STAT3 pathway. The Ref-1-regulated inflammatory response pathways—HIF-1⍺, NF-κB, and STAT3—represented by the gene expressions of CA9 (E), TNFAIP2 (F), and Survivin (G), were analyzed by qPCR in PRDX1KO 1A2 and Cas9 Control treated with the Ref-1 inhibitor APX2014, under both normoxic and hypoxic (1 % O2) conditions. Relative mRNA expression levels were calculated using the 2−ΔΔCT method and normalized to the RPL13A housekeeping gene; data were analyzed via two-way ANOVA (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001).
We also examined the transcriptional activity of NF-κB in PRDX1KD and scrambled control cells after treatment with APX2014. Interestingly, knocking down PRDX1 led to an unexpectedly higher basal NF-κB activity compared to the scrambled control (Fig. 4D). However, while treatment with APX2014 showed only a trending reduction in NF-κB activity in the scrambled control, inhibition of Ref-1 combined with PRDX1 reduction resulted in a clear dose-dependent decrease in NF-κB activity following APX2014 treatment, despite the higher baseline levels. These findings strongly indicate that PRDX1 and Ref-1 have a distinct relationship in regulating HIF-1⍺ and NF-κB activity in PDAC and may help explain the increased sensitivity observed, as these critical TFs are no longer able to activate pro-survival genes.
3.7. PRDX1 and Ref-1 influence the activation of gene transcription regulated by Ref-1 target transcription factors HIF-1α, NF-κB, and STAT3
To further examine the impact of Ref-1 and PRDX1 on the downstream targets of TFs HIF-1α, NF-κB, and STAT3, we measured the mRNA levels of Carbonic Anhydrase 9 (CA9), TNFAIP2, and Survivin in Pa03C Control Cas9 and PRDX1KO clone 1A2 (Fig. 4E–G). We assessed the expression of these target genes under both normoxic and hypoxic conditions (1 % O2). As shown by the luciferase reporter assay, HIF-1α activity was suppressed with Ref-1 inhibition and PRDX1 reduction in combination. We found that CA9 expression decreased both with the complete loss of PRDX1 and more significantly when combined with Ref-1 inhibition, the latter showing a surprising 10-fold reduction or more compared to both the Cas9 control and untreated 1A2 cells under hypoxia. While Ref-1 inhibition alone lowers CA9 expression by impairing HIF-1α activation, the absence of PRDX1 likely increases oxidative stress, making cells more susceptible to the effects of Ref-1 inhibition. This leads to a more pronounced suppression of CA9 expression in PRDX1KO cells treated with the Ref-1 inhibitor compared to other conditions. Additionally, we examined the TNFAIP2 gene, a target of TNF-α downstream of NF-kB, which was previously identified as a marker of Ref-1 inhibition [26]. While NF-kB luciferase activity showed little effect with APX2014 and a greater effect in PRDX1KD, TNFAIP2 expression under normoxia was significantly reduced with Ref-1 inhibition, with an even greater reduction observed in 1A2 (Fig. 4F). Under hypoxia conditions, the sensitivity was noticeably lower for either Ref-1 inhibition, PRDX1 loss, or their combination.
Our previous studies have utilized Survivin as a marker of STAT3 activity and observed that Ref-1 inhibition results in the downregulation of gene expression. As expected, when Cas9 control cells were treated with APX2014, the levels of Survivin mRNA significantly decreased under normoxic conditions (Fig. 4G). However, the lack of PRDX1 expression did not significantly enhance the reduction of Survivin levels in 1A2, nor in combination with APX2014 treatment. Surprisingly, under hypoxic conditions, Survivin levels dropped in both Cas9 control and 1A2 cells. While the decrease in Survivin expression upon Ref-1 inhibition under normoxia is likely due to disrupted STAT3 activation and changes in redox homeostasis, the regulation of STAT3/Survivin, especially under hypoxia, appears more complex in PDAC and less dependent on PRDX1 and Ref-1 signaling, as is also the case with TNFAIP2 expression. Together, these analyses demonstrate that both Ref-1 and PRDX1 have unique and co-regulatory roles in activating specific oncogenic transcription factors and significantly influence signaling pathways of transcription activity and their effectors.
3.8. Disruption of PRDX1 and Ref-1 redox activity reveals complex IL-8 regulation in PDAC cells
Numerous studies have demonstrated that PRDX1, Ref-1, and NF-κB regulate IL-8, which can be influenced by various factors. In one study, knocking down PRDX1 significantly increased IL-8 production and depended on NF-κB activation, which could be affected by Ref-1 knockdown [22]. Building on these findings, we aimed to determine whether such a relationship exists in PDAC and whether PRDX1KO cell lines treated with our specific Ref-1 redox inhibitor, APX2014, would prevent any increase in basal IL-8 levels by specifically inhibiting Ref-1 redox activity. Our results confirmed that loss of PRDX1 alone raised IL-8 basal levels in the PRDX1KO cells (1A2 clone, fourfold) and nearly doubled the levels in the 1C3 clone (Supplemental Fig. S3: A). Differences in basal IL-8 levels between 1A2 and 1C3 also suggested a clonality effect. Unexpectedly, IL-8 was also induced in Cas9 when treated with APX2014, and it did not significantly decrease IL-8 levels in the PRDX1KO clones, implying that other stress factors and signaling pathways beyond classical NF-κB and IL-8 stimulation, as well as regulation by Ref-1 redox activity, may be involved.
3.9. Effects on 3D co-culture growth after treatment with APX2014 in tumors and CAFs with and without PRDX1
To increase the complexity of our PDAC model by including CAFs and further exploring the Ref-1/PRDX1 interaction, we examined 3D spheroids made of either Pa03C tumor cells alone or co-cultured with CAFs. We used siRNA to knock down PRDX1 in the CAFs and compared tumor growth with CAF19SCR or CAF19PRDX1−KD (Fig. 5). We confirmed that PRDX1 was knocked down by Western blotting (Supplemental Fig. S5A) and comparisons were made to PRDX1KO in Pa03C tumor cells (Supplemental Fig. S5B). Ref-1 levels were also measured in the cells with reduced or knocked out PRDX1; there was a significant reduction in Ref-1 protein levels in the PRDX1KO tumor cells, but PRDX1 siRNA did not affect Ref-1 levels in the CAF19PRDX1−KD cells (Supplemental Fig. S5C). Spheroids derived from PRDX1KO Pa03C cells or Cas9 controls were treated with the Ref-1 inhibitor APX2014 on Days 4, 8, and 12. Similar to monolayers, spheroids from PRDX1KO cells were significantly more sensitive than controls (Fig. 5A; 2.5–10 μM), with representative images shown on the right. In co-cultures, the tumor spheroids’ response to APX2014 was partly protected by the presence of CAFs; yet PRDX1KO clones 1A2 and 1C3 remained highly susceptible to APX2014-induced killing (Fig. 5B, Cas9: black/gray, 1A2: red, 1C3: blue, top panel). APX2014 significantly reduces CAF viability at the highest dose (10 μM) when tumor cells expressing PRDX1 are present (Fig. 5B, bottom panel). Interestingly, treatment with APX2014 no longer affects the viability of CAFs in co-culture with PRDX1KO tumor cells (Fig. 5B, bottom panel). Next, we combined PRDX1KO tumors with CAFs transfected with siPRDX1 (PRDX1KD). Tumor killing improved modestly at 2.5 μM APX2014 (Fig. 5C, top panel, ∗p < 0.05), but CAF death was significant: across 2.5–10 μM, viability decreased earlier and more sharply than with PRDX1-positive counterparts (Fig. 5C, bottom panel; multiple p < 0.05–0.01 comparisons). For detailed statistical analysis and comparisons between the various co-culture groups, see Supplemental Table S3: 3D Co-culture Cytotoxicity assays: Pa03C (Cas9, PRDX1KO), CAF19.
Fig. 5.
PRDX1 expression affects 3D culture growth differently in tumors versus CAFs after Ref-1 inhibition. (A) Tumor spheroid growth over time of Pa03C Cas9 control and PRDX1KO clones (RFP+) treated with Ref-1 inhibitor, APX2014 (2.5, 5, 10 μM on Days 4, 8, and 12). (B) Quantification and representative images of 3D co-culture consisting of PRDX1KO tumor cells (RFP+) co-cultured with CAF19 expressing PRDX1 (GFP+) after treatment with APX2014. (C) Quantification and representative images of 3D co-culture with PRDX1KO tumor cells co-cultured with CAF19 cells transfected with siPRDX1 after treatment with APX2014. Total fluorescence intensity (INT) was measured using the ArrayScan™ High-content Imaging System on Days 4, 8, 12, and 14 post-plating. Fold change indicates normalized INT compared to media control on Day 14. (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, compared to Scrambled (Western) or DMSO control (3D). For PRDX1 and Ref-1 expression level statistics and comparisons, see Supplemental Fig. S5. Additional statistics and comparisons are available in Supplemental Table S3: 3D Co-culture Cytotoxicity assays: Pa03C (Cas9, PRDX1KO), CAF19).
Collectively, PRDX1 levels influence APX2014 response in both tumors and CAFs. Dual targeting of Ref-1 and PRDX1 could improve pancreatic cancer cell elimination, and the unexpected CAF dependence on tumor PRDX1 deserves further investigation into redox-mediated tumor–stroma interactions.
3.10. PRDX1 loss amplifies the effects of Ref-1 inhibition on PDAC tumor growth
We evaluated the growth of PRDX1KO tumors following Ref-1 inhibition using a subcutaneous PDAC model (Fig. 6A). Once tumor volume averaged around 120 mm3, mice were randomized to receive daily intratumoral injections of either vehicle or APX2014 (50 mg/kg; 5 days on/2 off). Tumors from both 1A2 and 1C3 clones lacking PRDX1 showed significantly smaller volumes (∗∗p < 0.001) and lower tumor weights (∗p < 0.01; ∗∗∗p < 0.0001) when treated with APX2014 compared to controls (Fig. 6B and C). Treatment with APX2014 in Cas9 tumors tended to reduce tumor sizes and weights (p = 0.06, Fig. 6B). Additionally, APX2014 significantly extended survival in all three groups (Fig. 6D) and did not exhibit in vivo toxicity associated with its analogs. These findings emphasize that targeting both PRDX1 and Ref-1 could improve tumor cell death, providing a promising approach for cancers urgently needing new targets and treatments. At the end of the study, tumors were collected and analyzed for various pharmacodynamic markers. PRDX1KO was confirmed via immunohistochemistry and quantified using the HALO platform (Fig. 6E). IHC analysis of Ref-1 levels showed consistent expression, with significant reductions only in APX2014-treated PRDX1KO 1A2 tumors (∗∗p < 0.001). Lastly, Ki-67 staining significantly decreased in APX2014-treated PRDX1KO 1A2 tumors (∗p < 0.01), indicating reduced proliferation. In summary, deleting PRDX1 renders PDAC tumors more sensitive to Ref-1 inhibition, thereby enhancing the anti-tumor effects of APX2014 both in vivo and in vitro.
Fig. 6.
Impact of PRDX1KO on PDAC tumor growth in response to Ref-1 inhibitor treatment.
(A) Tumor growth over time shows that PRDX1KO clones (1A2 and 1C3) treated with APX2014 were significantly smaller (∗p < 0.001) compared to their respective vehicle controls as compared to Cas9 control group (p = 0.06). (B) Tumor weights were significantly reduced in PRDX1KO clones (1A2, ∗p < 0.01 and 1C3, ∗∗∗p < 0.0001) treated with APX2014, compared to Cas9 control tumors, indicating enhanced anti-tumor effects of combined PRDX1KO and Ref-1 inhibition. (D) Survival studies demonstrated that mice treated with APX2014 exhibited significantly prolonged survival compared to vehicle-only treated mice. These results support the efficacy and safety of the 50 mg/kg APX2014 dose for in vivo studies. (E) Shows images of tumors that were stained with PRDX1, Ref-1, and Ki67 in all treatment groups for Cas9 and the PRDX1KO clones (1A2 and 1C3), which were then quantified, and (F) shows the quantification of positive regions for these stains. Scale bar is 1 mm.
3.11. Tumor microarray analysis reveals the roles of Ref-1 and PRDX1 in PDAC tumor progression and their potential as therapeutic targets
To further explore the clinical importance of Ref-1 and PRDX1, we stained a tissue microarray (TMA) containing PDAC patient-derived tumors and adjacent normal tissues for Ref-1 and PRDX1 expression (Fig. 7A). The primary aim was to assess the levels of these key biomarkers involved in tumor progression, cell proliferation, and redox regulation, providing insights into the potential of targeting Ref-1 as a therapy in human tumors with different levels of Ref-1 and PRDX1. These biomarkers are essential for understanding the molecular pathways in PDAC and could help develop targeted treatments to slow down metastasis. Immunohistochemical staining of the TMA from patient samples revealed significantly higher expression of both Ref-1 and PRDX1 (∗∗∗∗p < 0.00001) in tumors compared to normal adjacent tissues (Fig. 7B). Due to the importance of CAFs in PDAC and our findings that knocking PRDX1 down in the CAFs led to a reduction in CAF survival, we also looked at publicly available PDAC single cell RNA-seq data to determine whether different subtypes of cells from the TME including CAFs express PRDX1 [56]. Differential expression was carried out using the FindMarkers function in Seurat package and shows that PRDX1 is widely expressed in cells within the PDAC TME (Fig. S7A). However, within two CAF subtypes, the levels of PRDX1 are similar between tumor-promoting and tumor-restraining CAFs (Fig. S7B) [57].The increased expression of PRDX1 and Ref-1 in PDAC tumors and expression within the TME suggests they may play a role in promoting tumor development and resistance to therapy, further highlighting their potential as therapeutic targets.
Fig. 7.
Levels of PRDX1 and Ref-1 in tumorigenic and normal adjacent PDAC patient pancreas. 174 patients and 127 patients contributed normal adjacent pancreas tissue in addition to tumor samples. Some patients contributed more tissue to the TMA, totaling 319 samples from only 167 patients. (A) Visual representation of IHC staining differences between PRDX1 and Ref-1 in tumor and normal tissue. In both panels, there is a direct comparison between each tumor image; the samples for PRDX1 are from the same patient as those for Ref-1. In the large panel on the left, two patients were selected as representatives, showing the differences in expression of PRDX1 and Ref-1 in tumor and normal pancreas. (B) Analysis of PRDX1 and Ref-1 in the patient TMAs of normal and tumor tissue. A two-way ANOVA was performed, presenting Mean ± SEM, n = 3 or greater: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001.
4. Discussion
Pancreatic ductal adenocarcinoma (PDAC) is marked by a highly oxidative and hypoxic tumor microenvironment (TME), which drives aggressive tumor growth, therapy resistance, and immune evasion. A key element linking redox stress to transcriptional regulation in PDAC is the multifunctional protein APE1/Ref-1. Ref-1 has two main roles: it is crucial for base excision DNA repair and also acts as a redox regulator of transcription factors (TFs) by keeping reactive cysteine residues in their reduced, DNA-binding state [9,58,59]. Ref-1 is essential for fully activating TFs that promote PDAC progression and therapy resistance, including HIF-1α, NF-κB, STAT3, AP-1, and p53 [9,58,59]. By coordinating redox-dependent transcriptional programs, Ref-1 helps tumor cells and stromal components survive oxidative and genotoxic stresses typical in pancreatic tumors. Given this critical role, small-molecule inhibitors have emerged as promising therapeutic agents that selectively block the redox function of Ref-1, such as APX3330, APX2009, and APX2014. APX3330 is currently in clinical trials, while APX2014 represents a second-generation analog with increased potency and redox-targeting specificity, as demonstrated [14,55]. However, despite these clinical advances, inhibiting Ref-1 alone has not consistently yielded strong responses in PDAC, suggesting that upstream redox regulators may influence Ref-1 activity and drug sensitivity.
Peroxiredoxins (PRDXs) are thiol peroxidases that neutralize reactive oxygen species (ROS) and help maintain redox homeostasis. Among the six members of the PRDX family, PRDX1 is the most abundant and widely expressed in mammalian cells. It has been linked to various cellular functions, including proliferation, inflammation, and survival, and is often overexpressed in cancers like PDAC [25,[29], [30], [31], [32]]. Previous studies have shown that PRDX1 interacts with Ref-1, with no evidence of other PRDXs interacting with Ref-1(22). We also reported a relationship involving NF-kB (RelA), PRDX1, and Ref-1 in a previous study, demonstrating that PRDX1 knockdown in RelA-deficient mouse PDAC and RelA add-back led to increased sensitivity to cell death in both conditions, with a more pronounced effect in the latter [24]. This study also proposed an additional mechanism, beyond ROS and NF-kB, involved in the increased sensitivity to Ref-1 inhibition, suggesting that direct PRDX1 regulation of Ref-1 may be a possible pathway. This led us to hypothesize that PRDX1 might also control Ref-1's redox activity in PDAC and that targeting both proteins could produce synergistic therapeutic effects.
To investigate this hypothesis, we first knocked down PRDX1 in two PDAC cell lines, Pa03C and Panc-1, and tested the effects of three different Ref-1 redox inhibitors. All inhibitors, APX3330, APX2009, and APX2014, significantly increased cytotoxicity in PRDX1 knockdown (PRDX1KD) cells compared to scrambled controls, demonstrating a clear functional link between PRDX1 and Ref-1 in supporting PDAC cell survival. These results were confirmed in cancer-associated fibroblasts (CAFs), where PRDX1KD also increased sensitivity to Ref-1 inhibition, suggesting a previously unrecognized dependence of stromal cells on PRDX1-Ref-1 signaling. To extend these findings, we generated CRISPR/Cas9-engineered PRDX1KO clones from Pa03C cells and validated them alongside Cas9 controls. In both monolayer and 3D spheroid models, PRDX1KO clones showed increased sensitivity to APX2014, further confirming that loss of PRDX1 makes PDAC cells more sensitive to Ref-1 inhibition. Importantly, Ref-1 endonuclease function remained intact in PRDX1KO cells, indicating that the observed synergy is driven by specific impairment of redox signaling rather than DNA repair.
Loss of PRDX1 increased the sensitivity of both tumor cells and CAFs to Ref-1 inhibition in our 3D co-culture system, highlighting a broader role for PRDX1 in supporting stromal and tumor survival. PRDX1KD CAFs were affected by APX2014 at doses that did not impact PRDX1-normal counterparts, while PRDX1KO tumor spheroids remained highly sensitive to the drug even within the protective tumor microenvironment (TME), suggesting that PRDX1 represents a common redox “addiction” in both malignant and stromal areas. These results indicate that PRDX1 supports survival in both cell types and that dual disruption of Ref-1 and PRDX1 weakens this supportive system. Notably, lowering PRDX1 in CAFs alone impaired their viability more than PRDX1 knockout in tumor cells, implying that tumor cells can partially compensate for increased oxidative stress, whereas CAFs remain highly dependent on PRDX1.
The 3D co-culture system also models the fibrotic, chemoresistant phenotype characteristic of PDAC, providing a physiologically relevant context to evaluate therapeutic responses. When PRDX1KO tumor cells were treated with APX2014, CAFs were unexpectedly protected from drug-induced cytotoxicity. While the mechanisms are not fully understood, two possibilities are proposed: (i) dual PRDX1/Ref-1 blockade may trigger compensatory NF-κB–mediated cytokine secretion from tumor cells, stimulating CAF growth (Fig. 4D and E), and (ii) loss of PRDX1 in tumor cells may impair detoxification of H2O2, increasing oxidative stress in the TME and promoting CAF activation. These possible mechanisms, along with other tumor–CAF secretome interactions influenced by PRDX1 and Ref-1, will be explored in future studies. Overall, the data support a tumor-intrinsic dependence on PRDX1 while revealing an important stromal component to the therapeutic vulnerability.
Because APX2014 is a naphthoquinone known to cycle through ROS-generating intermediates, we questioned whether the increased cytotoxicity in PRDX1KO cells was partly due to oxidative stress. We confirmed that APX2014 treatment caused necrotic, late apoptotic cell death in PRDX1KO cells. Knockdown of PRDX2-6 did not mimic the loss of PRDX1 in cytotoxicity assays, and endonuclease-competent Ref-1 remained intact, indicating that nonspecific ROS accumulation or global DNA repair deficiency are not the leading causes of cell death. The unique synergy, therefore, depends on Trx1 reducing PRDX1, which then regulates cellular redox homeostasis and supports Ref-1 reduction. This suggests a specific, non-redundant role for PRDX1 in controlling Ref-1 activity rather than a general imbalance of ROS. PRDX1KO increased basal NF-κB activity and IL-8 expression in PDAC cells, consistent with previous findings. However, treatment with APX2014 unexpectedly elevated IL-8 levels in Cas9 cells, while only slightly decreasing them in PRDX1KO clones. This indicates that APX2014 influences IL-8 via other redox-sensitive pathways interacting with NF-κB signaling. The differences between Cas9 control and PRDX1 knockout cells may also reflect their different responses to oxidative stress when PRDX1 is absent.
We also confirmed that PRDX1KO cells treated with APX2014 shift from apoptotic to necrotic cell death, further supporting a ROS-dependent mechanism. However, our data suggest that PRDX1's regulation of Ref-1 redox signaling, rather than overall oxidative imbalance, is the main factor driving increased drug sensitivity. This is further supported by our observation that PRDX1KO cells co-cultured with PRDX1-intact CAFs remained sensitive to Ref-1 inhibition, despite the potential for ROS scavenging by the microenvironment.
The combined inhibition of PRDX1 and Ref-1 showed effects that depended on the context on redox-sensitive gene programs relevant to PDAC biology. HIF-1α activity was significantly reduced in PRDX1 knockout cells when Ref-1 was inhibited via APX2014, especially under hypoxic conditions. This was supported by decreased CA9 levels, indicating impaired HIF-1–dependent adaptation to hypoxia. TNFAIP2, a gene involved in redox and inflammation-related tumor growth, was strongly downregulated under combined treatment in both normal and low-oxygen settings, pointing to the therapeutic potential of targeting both. Conversely, NF-κB activity was more influenced by the presence of PRDX1, which increased baseline NF-κB activity. Ref-1 inhibition had little additional effect, although combined treatment led to a clear decrease in NF-κB activity despite the baseline increase. Notably, Survivin (BIRC5) expression was mainly controlled by Ref-1; its levels decreased after APX2014 treatment in both control and PRDX1 knockout contexts, whereas loss of PRDX1 alone had little effect. Furthermore, hypoxia did not induce Survivin expression in Cas9 or PRDX1KO lines, suggesting a disconnect between hypoxic signaling and Survivin regulation in this model. These findings highlight the distinct roles of PRDX1 and Ref-1 in controlling transcription factor activity and redox-dependent gene expression. The use of siRNA-mediated knockdown in some experiments may have led to incomplete protein depletion, which could account for the weaker phenotypes observed in specific assays and underscores the need for validation using CRISPR-based models or protein quantification in future studies.
Our in vivo experiments confirmed the translational potential of this method. Mice implanted with PRDX1KO PDAC cells and treated with APX2014 exhibited significantly reduced tumors in size and weight compared to controls. These tumors also had lower Ref-1 levels and reduced proliferation, as indicated by Ki-67 staining. Overall, these results support that PRDX1 loss enhances the anti-tumor effects of Ref-1 inhibition in PDAC. Although intratumoral delivery maximized local drug exposure, the pharmacokinetic properties of oral APX3330/APX2014 suggest clinical feasibility. APX3330, the clinical equivalent of APX2014, has demonstrated oral bioavailability and a good safety profile in human Phase I/II studies, supporting systemic administration. Future preclinical studies will assess the effectiveness of oral APX2014 in orthotopic or metastatic PDAC models to replicate clinical dosing and distribution better. These findings will be crucial in addressing pharmacokinetic challenges and guiding dosing strategies for upcoming trials involving biomarker-enriched patient groups.
To enhance clinical translation, our findings support the potential to stratify PDAC patients based on intratumoral expression of PRDX1 and Ref-1. Tissue microarray (TMA) analysis showed that both proteins are significantly overexpressed in PDAC tumors compared to adjacent normal tissues. These expression patterns suggest that patients with high levels of PRDX1 and Ref-1 may respond particularly well to Ref-1-targeted redox inhibition strategies. As a potential biomarker approach, patients showing more than a 2-fold increase in PRDX1 and/or Ref-1 staining intensity relative to nearby non-tumor tissue could be considered for enrollment in future APX-based trials. Additional validation across independent PDAC cohorts and correlation with clinical outcomes will be essential for refining these thresholds and establishing biomarker-driven inclusion criteria. Additionally, an unbiased RNA-seq approach could offer a more detailed view of transcriptional changes following PRDX1 loss and Ref-1 inhibition, potentially identifying new pathways and resistance mechanisms. Although beyond the scope of the current study, our existing data already show consistent disruption of key transcriptional hubs, including HIF-1α and NF-κB, indicating broad downstream effects. Future transcriptomic analyses will build on these findings and might uncover additional regulatory networks affected by the PRDX1–Ref-1 axis.
Regarding the drug APX2014 specificity for Ref-1, our previous NMR and docking studies identified a distinct small surface-accessible pocket on Ref-1 that includes the residues Ser135, Arg136, Gln137, and Asp163, located on the opposite face from the endonuclease active site. NMR chemical-shift perturbations and docking identified APX2014 as part of a separate surface pocket bordered by Ser135–Gln137 and Asp163, which is distant from the DNA-repair active site (Fig. 2). Previous studies indicate that the redox activity of Ref-1 relies on maintaining Cys65 in a reduced state, often through interaction with thioredoxin (Trx1) [53,60]. The residues identified here are proposed to form a small pocket that, when bound by APX2014, may impair redox function by preventing Ref-1 from partially exposing Cys65, thus blocking its participation in redox reactions. Indeed, PRDX1KO cells showed exaggerated inhibition of NF-κB and HIF-1α reporters, as well as the downstream genes Survivin, CA9, and TNFAIP2 when treated with APX2014. These data show that PRDX1 can be modified to influence the response to Ref-1 inhibitors, and that dual-redox blockade might overcome both innate and TME-induced resistance.
Pharmacological inhibition of PRDX1, whether through covalent agents or genetic silencing, could (i) lower the necessary dose of Ref-1 inhibitors for effectiveness, (ii) counteract CAF protection, and (iii) decrease hypoxia-driven, TF-mediated survival pathways. Although PRDX1 loss increases ROS, it does not cause the same effects as other PRDX isoforms. Therefore, identifying patients with high levels of PRDX1 and targeting both Ref-1 and PRDX1 has the potential to enhance the efficacy of existing standard of care therapies in PDAC. Multi-drug regimens of either FOLFIRINOX or Gemcitabine/Abraxane are currently used to treat pancreatic cancer [61]. Additional preclinical work will be needed to determine an optimized regimen for the addition of Ref-1/PRDX1 inhibition to standard of care. Future in vivo studies will investigate how tumors respond to FOLFIRINOX or Gemcitabine/Abraxane with pre- or post-treatment with Ref-1/PRDX1 targeting. Sequencing of these chemotherapy regimens will be important. Administering Ref-1/PRDX1 inhibitors after standard of care (SOC) could potentially limit metastasis and recurrence as redox signaling pathways contribute to resistance and adaptation to stress. Conversely, targeting redox signaling to dramatically reduce tumor burden before the addition of SOC could allow for more complete tumor eradication. Although our in vitro studies used 1 % hypoxia to simulate in vivo conditions, orthotopic models and the 3D co-culture assays will continue to improve the translational relevance of this redox signaling axis in conjunction with SOC. Our ultimate goal is to leverage this redox signaling axis with SOC regimens to improve patient prognosis.
5. Conclusion
Our study reveals a crucial and previously unrecognized functional connection between PRDX1 and Ref-1 in PDAC. PRDX1 supports Ref-1's redox activity in both tumor and stromal cells, promoting transcriptional programs that favor tumor growth, survival, and therapy resistance. The absence of PRDX1 increases the sensitivity of PDAC cells to Ref-1 redox inhibition, disrupts key transcription factor pathways, including NF-κB and HIF-1α, and impairs protective stromal interactions. These findings strongly support dual targeting of Ref-1 and PRDX1 as a promising new therapeutic strategy for PDAC and potentially other redox-dependent cancers. Future research should focus on developing PRDX1 inhibitors, identifying predictive biomarkers for patient selection, determining if patients with higher PRDX1 levels would be better targets for dual inhibitor combinations, and testing combination therapies in clinical trials. Additionally, broader multi-omic profiling could help determine if redox failure intersects with DNA damage signaling, immunogenic cell death, or metabolic changes. Metabolic alterations have been observed in PDAC tumors treated with Ref-1 inhibitors [34,59].
CRediT authorship contribution statement
Sonia Kiran: Writing – review & editing, Methodology, Investigation, Formal analysis, Data curation. Randall S. Wireman: Writing – review & editing, Writing – original draft, Visualization, Validation, Project administration, Methodology, Investigation, Formal analysis, Data curation. Jacqueline Peil: Writing – review & editing, Methodology, Investigation, Data curation. Dana K. Mitchell: Methodology, Investigation, Data curation. Elizabeth Sierra Potchanant: Methodology, Investigation, Formal analysis. Ratan Rai: Validation, Methodology, Investigation, Formal analysis, Data curation. Jonah Z. Vilseck: Writing – review & editing, Validation, Methodology, Investigation, Formal analysis, Data curation. Sha Cao: Writing – review & editing, Validation, Methodology, Investigation. Sanya Haiaty: Methodology, Investigation, Formal analysis, Data curation. Millie M. Georgiadis: Writing – review & editing, Writing – original draft, Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Melissa L. Fishel: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Project administration, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Mark R. Kelley: Writing – review & editing, Writing – original draft, Validation, Supervision, Resources, Project administration, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
Mark R. Kelley licensed APX3330 to Apexian Pharmaceuticals LLC through the Indiana University Research and Technology Corporation. Second-generation compounds are also licensed to Apexian Pharmaceuticals. Apexian has licensed APX3330 to Ocuphire Parma now named Opus Genetics, Inc. Neither Apexian Pharmaceuticals nor Ocuphire Pharma/Opus Genetics had control or oversight of the studies, interpretation, or presentation of the data in this manuscript. Other authors do not have any conflict of interests.
Acknowledgements
This work was supported by grants from the National Institute of Health, National Cancer Institute R01CA282478 and R01CA254110 (M.R.K and M.L.F.), HT94252410689 (M.R.K. and M.L.F.) and U01 Pancreatic Ductal Adenocarcinoma (PDAC) Stromal Reprogramming Consortium (PSRC) (U01CA274304) (M.L.F.). M.R.K and M.L.F. were additionally supported by the Riley Children's Foundation and the IU Simon Comprehensive Cancer Center, P30CA082709. We also thank Dr. Silpa Gampala and Eyram Kpenu for their help in creating the graphical abstract.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2025.103848.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Smigiel J.M., Parameswaran N., Jackson M.W. Targeting pancreatic cancer cell plasticity: the latest in therapeutics. Cancers (Basel) 2018;10(1) doi: 10.3390/cancers10010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Georgiadis M.M., Luo M., Gaur R.K., Delaplane S., Li X., Kelley M.R. Evolution of the redox function in mammalian apurinic/apyrimidinic endonuclease. Mutat. Res. 2008;643(1–2):54–63. doi: 10.1016/j.mrfmmm.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fishel M.L., He Y., Reed A.M., Chin-Sinex H., Hutchins G.D., Mendonca M.S., et al. Knockdown of the DNA repair and redox signaling protein Ape1/Ref-1 blocks ovarian cancer cell and tumor growth. DNA Repair (Amst) 2008;7(2):177–186. doi: 10.1016/j.dnarep.2007.09.008. doi S1568-7864(07)00330-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fishel M.L., Kelley M.R. The DNA base excision repair protein Ape1/Ref-1 as a therapeutic and chemopreventive target. Mol. Aspect. Med. 2007;28(3–4):375–395. doi: 10.1016/j.mam.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Tell G., Damante G., Caldwell D., Kelley M.R. The intracellular localization of APE1/Ref-1: more than a passive phenomenon? Antioxidants Redox Signal. 2005;7(3–4):367–384. doi: 10.1089/ars.2005.7.367. [DOI] [PubMed] [Google Scholar]
- 6.Evans A.R., Limp-Foster M., Kelley M.R. Going APE over ref-1. Mutat. Res. 2000;461(2):83–108. doi: 10.1016/s0921-8777(00)00046-x. [DOI] [PubMed] [Google Scholar]
- 7.Gampala S., Moon H-r, Wireman R., Peil J., Kiran S., Mitchell D.K., et al. New Ref-1/APE1 targeted inhibitors demonstrating improved potency for clinical applications in multiple cancer types. Pharmacol. Res. 2024;201 doi: 10.1016/j.phrs.2024.107092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fishel M.L., Jiang Y., Rajeshkumar N.V., Scandura G., Sinn A.L., He Y., et al. Impact of APE1/Ref-1 redox inhibition on pancreatic tumor growth. Mol. Cancer Therapeut. 2011;10(9):1698–1708. doi: 10.1158/1535-7163.MCT-11-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caston R.A., Gampala S., Armstrong L., Messmann R.A., Fishel M.L., Kelley M.R. The multifunctional APE1 DNA repair–redox signaling protein as a drug target in human disease. Drug Discov. Today. 2021;26(1):218–228. doi: 10.1016/j.drudis.2020.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo M., Delaplane S., Jiang A., Reed A., He Y., Fishel M., et al. Role of the multifunctional DNA repair and redox signaling protein Ape1/Ref-1 in cancer and endothelial cells: small-molecule inhibition of the redox function of Ape1. Antioxidants Redox Signal. 2008;10(11):1853–1867. doi: 10.1089/ars.2008.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelley M.R., Fishel M.L. DNA repair proteins as molecular targets for cancer therapeutics. Anti Cancer Agents Med. Chem. 2008;8(4):417–425. doi: 10.2174/187152008784220294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bapat A., Fishel M., Kelley M.R. Going ape as an approach to cancer therapeutics. Antioxidants Redox Signal. 2009;11:651–668. doi: 10.1089/ars.2008.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zou G.M., Luo M.H., Reed A., Kelley M.R., Yoder M.C. Ape1 regulates hematopoietic differentiation of embryonic stem cells through its redox functional domain. Blood. 2007;109(5):1917–1922. doi: 10.1182/blood-2006-08-044172. [DOI] [PubMed] [Google Scholar]
- 14.Rai R., Dawodu O.I., Johnson S.M., Vilseck J.Z., Kelley M.R., Ziarek J.J., et al. 2023. Chemically Induced Partial Unfolding of the Multifunctional Apurinic/apyrimidinic Endonuclease 1. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muniyandi A., Kpenu E.K., Hartman G., Kelley M.R., Corson T.W. Ref-1 inhibitor APX2009 regulates hypoxia signaling in murine subretinal neovascularization and human retinal endothelial cells. Investig. Ophthalmol. Vis. Sci. 2024;65(7):2165. [Google Scholar]
- 16.Cheng P.S.W., Zaccaria M., Biffi G. Functional heterogeneity of fibroblasts in primary tumors and metastases. Trends Cancer. 2024 doi: 10.1016/j.trecan.2024.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Vaish U., Jain T., Are A.C., Dudeja V. Cancer-associated fibroblasts in pancreatic ductal adenocarcinoma: an update on heterogeneity and therapeutic targeting. Int. J. Mol. Sci. 2021;22(24):13408. doi: 10.3390/ijms222413408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DuFort C.C., DelGiorno K.E., Hingorani S.R. Mounting pressure in the microenvironment: fluids, solids, and cells in pancreatic ductal adenocarcinoma. Gastroenterology. 2016;150(7):1545–1557. e2. doi: 10.1053/j.gastro.2016.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan J.S., Tan M.J., Sng M.K., Teo Z., Phua T., Choo C.C., et al. Cancer-associated fibroblasts enact field cancerization by promoting extratumoral oxidative stress. Cell Death Dis. 2017;8(1) doi: 10.1038/cddis.2016.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bulle A., Lim K.-H. Beyond just a tight fortress: contribution of stroma to epithelial-mesenchymal transition in pancreatic cancer. Signal Transduct. Targeted Ther. 2020;5(1):249. doi: 10.1038/s41392-020-00341-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosein A.N., Brekken R.A., Maitra A. Pancreatic cancer stroma: an update on therapeutic targeting strategies. Nat. Rev. Gastroenterol. Hepatol. 2020;17(8):487–505. doi: 10.1038/s41575-020-0300-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nassour H., Wang Z., Saad A., Papaluca A., Brosseau N., Affar E.B., et al. Peroxiredoxin 1 interacts with and blocks the redox factor APE1 from activating interleukin-8 expression. Sci. Rep. 2016;6 doi: 10.1038/srep29389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ando K., Hirao S., Kabe Y., Ogura Y., Sato I., Yamaguchi Y., et al. A new APE1/Ref-1-dependent pathway leading to reduction of NF-{kappa}B and AP-1, and activation of their DNA-binding activity. Nucleic Acids Res. 2008;36(13):4327–4336. doi: 10.1093/nar/gkn416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mijit M., Wireman R., Armstrong L., Gampala S., Hassan Z., Schneeweis C., et al. RelA is an essential target for enhancing cellular responses to the DNA Repair/Ref-1 redox signaling protein and restoring perturbated cellular redox homeostasis in mouse PDAC cells. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.826617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guan X., Ruan Y., Che X., Feng W. Dual role of PRDX1 in redox-regulation and tumorigenesis: past and future. Free Radic. Biol. Med. 2024;210:120–129. doi: 10.1016/j.freeradbiomed.2023.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Shah F., Goossens E., Atallah N.M., Grimard M., Kelley M.R., Fishel M.L. APE1/Ref-1 knockdown in pancreatic ductal adenocarcinoma - characterizing gene expression changes and identifying novel pathways using single-cell RNA sequencing. Mol. Oncol. 2017;11(12):1711–1732. doi: 10.1002/1878-0261.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim Y., Jang H.H. Role of cytosolic 2-Cys Prx1 and Prx2 in Redox signaling. Antioxidants (Basel, Switzerland) 2019;8(6) doi: 10.3390/antiox8060169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park M.H., Jo M., Kim Y.R., Lee C.-K., Hong J.T. Roles of peroxiredoxins in cancer, neurodegenerative diseases and inflammatory diseases. Pharmacol. Therapeut. 2016;163:1–23. doi: 10.1016/j.pharmthera.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bolduc J., Koruza K., Luo T., Pueyo J.M., Vo T.N., Ezeriņa D., et al. Peroxiredoxins wear many hats: factors that fashion their peroxide sensing personalities. Redox Biol. 2021;42 doi: 10.1016/j.redox.2021.101959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicolussi A., D'Inzeo S., Capalbo C., Giannini G., Coppa A. The role of peroxiredoxins in cancer. Mol. Clin. Oncol. 2017;6(2):139–153. doi: 10.3892/mco.2017.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y., Wang P., Hu W., Chen D. New insights into the roles of peroxiredoxins in cancer. Biomed. Pharmacother. 2023;164 doi: 10.1016/j.biopha.2023.114896. [DOI] [PubMed] [Google Scholar]
- 32.Cai C.Y., Zhai L.L., Wu Y., Tang Z.G. Expression and clinical value of peroxiredoxin-1 in patients with pancreatic cancer. Eur. J. Surg. Oncol. 2015;41(2):228–235. doi: 10.1016/j.ejso.2014.11.037. [DOI] [PubMed] [Google Scholar]
- 33.Caston R.A., Shah F., Starcher C.L., Wireman R., Babb O., Grimard M., et al. Combined inhibition of Ref-1 and STAT3 leads to synergistic tumour inhibition in multiple cancers using 3D and in vivo tumour co-culture models. J. Cell Mol. Med. 2021;25(2):784–800. doi: 10.1111/jcmm.16132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mijit M., Kpenu E., Chowdhury N.N., Gampala S., Wireman R., Liu S., et al. In vitro and in vivo evidence demonstrating chronic absence of Ref-1 Cysteine 65 impacts Ref-1 folding configuration, redox signaling, proliferation and metastasis in pancreatic cancer. Redox Biol. 2024;69 doi: 10.1016/j.redox.2023.102977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Logsdon D.P., Shah F., Carta F., Supuran C.T., Kamocka M., Jacobsen M.H., et al. Blocking HIF signaling via novel inhibitors of CA9 and APE1/Ref-1 dramatically affects pancreatic cancer cell survival. Sci. Rep. 2018;8(1):13759–13773. doi: 10.1038/s41598-018-32034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gampala S., Shah F., Zhang C., Rhodes S.D., Babb O., Grimard M., et al. Exploring transcriptional regulators Ref-1 and STAT3 as therapeutic targets in malignant peripheral nerve sheath tumours. Br. J. Cancer. 2021;124(9):1566–1580. doi: 10.1038/s41416-021-01270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jansson M., Li Y.C., Jendeberg L., Anderson S., Montelione G.T., Nilsson B. High-level production of uniformly (1)(5)N- and (1)(3)C-enriched fusion proteins in Escherichia coli. J. Biomol. NMR. 1996;7(2):131–141. doi: 10.1007/BF00203823. [DOI] [PubMed] [Google Scholar]
- 38.Snyder D.A., Chen Y., Denissova N.G., Acton T., Aramini J.M., Ciano M., et al. Comparisons of NMR spectral quality and success in crystallization demonstrate that NMR and X-ray crystallography are complementary methods for small protein structure determination. J. Am. Chem. Soc. 2005;127(47):16505–16511. doi: 10.1021/ja053564h. [DOI] [PubMed] [Google Scholar]
- 39.Muniyandi A., Hartman G.D., Sishtla K., Rai R., Gomes C., Day K., et al. Ref-1 is overexpressed in neovascular eye disease and targetable with a novel inhibitor. Angiogenesis. 2025;28(1):11. doi: 10.1007/s10456-024-09966-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J. Comput. Chem. 2009;30(16):2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trott O., Olson A.J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010;31(2):455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forli S., Huey R., Pique M.E., Sanner M.F., Goodsell D.S., Olson A.J. Computational protein-ligand docking and virtual drug screening with the AutoDock suite. Nat. Protoc. 2016;11(5):905–919. doi: 10.1038/nprot.2016.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davis I.W., Leaver-Fay A., Chen V.B., Block J.N., Kapral G.J., Wang X., et al. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 2007;35(suppl_2):W375–W383. doi: 10.1093/nar/gkm216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen V.B., Arendall W.B., 3rd, Headd J.J., Keedy D.A., Immormino R.M., Kapral G.J., et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010;66(Pt 1):12–21. doi: 10.1107/s0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olsson M.H., Søndergaard C.R., Rostkowski M., Jensen J.H. PROPKA3: consistent treatment of internal and surface residues in empirical pKa predictions. J. Chem. Theor. Comput. 2011;7(2):525–537. doi: 10.1021/ct100578z. [DOI] [PubMed] [Google Scholar]
- 46.He H., Chen Q., Georgiadis M.M. High-resolution crystal structures reveal plasticity in the metal binding site of apurinic/apyrimidinic endonuclease I. Biochemistry. 2014;53(41):6520–6529. doi: 10.1021/bi500676p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., et al. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 48.MGLTools: Autodocktools. 1.5.7. Molecular Graphics Laboratory: the Scripps Research Institute1999-2011.
- 49.The Pymol Molecular Graphics System. 2.5.2. Schrodinger LLC: New York, New York.
- 50.Mitchell D.K., Burgess B., White E.E., Smith A.E., Sierra Potchanant E.A., Mang H., et al. Spatial gene-expression profiling unveils immuno-oncogenic programs of NF1-Associated peripheral nerve sheath tumor progression. Clin. Cancer Res. 2024;30(5):1038–1053. doi: 10.1158/1078-0432.Ccr-23-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flint A.C., Mitchell D.K., Angus S.P., Smith A.E., Bessler W., Jiang L., et al. Combined CDK4/6 and ERK1/2 inhibition enhances antitumor activity in NF1-Associated plexiform neurofibroma. Clin. Cancer Res. 2023;29(17):3438–3456. doi: 10.1158/1078-0432.Ccr-22-2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chowdhury N.N., Yang Y., Dutta A., Luo M., Wei Z., Abrahams S.R., et al. Plasminogen deficiency suppresses pancreatic ductal adenocarcinoma disease progression. Mol. Oncol. 2024;18(1):113–135. doi: 10.1002/1878-0261.13552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mijiti Maihemuti S.G., Randall Wireman, Millie Georgiadis, Fishel Melissa, Kelley Mark. Cysteine 65 in Ref-1 is essential for Ref-1 redox function and transcriptional regulation in humanPDAC cells. Soc. Redox Biol. Med. 2022 Orlando, Florida. [Google Scholar]
- 54.Rai R., Dawodu O.I., Meng J., Johnson S.M., Vilseck J.Z., Kelley M.R., et al. Chemically induced partial unfolding of the multifunctional apurinic/apyrimidinic endonuclease 1. Protein Sci. 2025;34(6) doi: 10.1002/pro.70148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gampala S., Moon H.R., Wireman R., Peil J., Kiran S., Mitchell D.K., et al. New Ref-1/APE1 targeted inhibitors demonstrating improved potency for clinical applications in multiple cancer types. Pharmacol. Res. 2024;201 doi: 10.1016/j.phrs.2024.107092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elyada E., Bolisetty M., Laise P., Flynn W.F., Courtois E.T., Burkhart R.A., et al. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 2019;9(8):1102–1123. doi: 10.1158/2159-8290.Cd-19-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peng X.L., Kharitonova E.V., Xu Y., Kearney J.F., Luan C., Chan P.S., et al. Determination of permissive and restraining cancer-associated fibroblast (DeCAF) subtypes. bioRxiv. 2024 doi: 10.1101/2024.05.14.594197. [DOI] [Google Scholar]
- 58.Kpenu E.K., Kelley M.R. Combating PDAC drug resistance: the role of Ref-1 inhibitors in accelerating progress in pancreatic cancer research. J. Cell. Signal. 2024;5(4):208–216. doi: 10.33696/signaling.5.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gampala S., Shah F., Lu X., Moon H.R., Babb O., Umesh Ganesh N., et al. Ref-1 redox activity alters cancer cell metabolism in pancreatic cancer: exploiting this novel finding as a potential target. J. Exp. Clin. Cancer Res. 2021;40(1):251. doi: 10.1186/s13046-021-02046-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hirota K., Matsui M., Yodoi J. AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1. Proc Natl Acad Sci USA. 1997;94(8):3633–3638. doi: 10.1073/pnas.94.8.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Di Costanzo F., Di Costanzo F., Antonuzzo L., Mazza E., Giommoni E. Optimizing first-line chemotherapy in metastatic pancreatic cancer: efficacy of FOLFIRINOX versus Nab-Paclitaxel plus gemcitabine. Cancers (Basel) 2023;15(2) doi: 10.3390/cancers15020416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.