Abstract
Despite clear evidence that vitamin C levels are depleted in the brains of Alzheimer's disease (AD) patients, dietary supplementation has consistently failed in clinical trials, suggesting a critical bottleneck not in systemic supply, but in its transport into brain cells. Here, we identify this bottleneck as a progressive downregulation of the ascorbate transporter, Slc23a2, also known as SVCT2, in microglia. Then we hypothesized that bypassing this cellular deficiency via targeted SVCT2 overexpression in microglia could either prevent the onset of pathology or rescue established functional deficits. Indeed, overexpressing SVCT2 in microglia before disease onset in 5xFAD mice triggered a profound redox reprogramming, resulting in a unique "hybrid" neuroprotective microglial phenotype that co-expressed both homeostatic and disease-associated markers. Functionally, this leads to decreased amyloid plaque burden and strengthens the synaptic bioenergetic capacity, which consequently prevents the development of synaptic and memory deficits. Strikingly, when employed after disease establishment, SVCT2 overexpression rescued synaptic plasticity and memory performance despite not affecting the existing amyloid burden. This rescue was driven by changes in the microglial secretory pathways. Collectively, these findings resolve a long-standing clinical paradox by establishing that neuroprotection depends not on systemic vitamin C intake but on the brain's cellular uptake machinery. This offers a mechanistic explanation for the failure of dietary supplementation in AD and identifies SVCT2 as a promising therapeutic target against the neurodegenerative process in AD.
Keywords: Ascorbate, Slc23a2, SVCT2, Neurodegenerative diseases, 5xFAD, LTP, RNA sequencing, Proteomics, APP/PS1
1. Background
Alzheimer's Disease (AD) is a progressive neurodegenerative disorder and the leading cause of dementia globally. Characterized by progressive memory loss and behavioral changes, AD manifests through diminished decision-making abilities, language difficulties, and personality alterations [1], with anxiety and depression frequently observed as comorbidities [2]. Histopathologically, AD is marked by reduced brain volume and the presence of amyloid plaques and neurofibrillary tangles. Central to AD's pathogenesis is oxidative stress, a precursor to disease hallmarks [3,4]. It is well documented that plasma antioxidants, including vitamin C, are depleted in individuals with mild cognitive impairment and AD patients [5,6]. Additionally, there is a significant decrease in ascorbate levels in the cerebrospinal fluid of AD patients compared to healthy subjects [5,7].
Ascorbate, the reduced form of vitamin C, possesses antioxidant properties and is present at high concentrations in the central nervous system (CNS), which is crucial for CNS development, functioning, and homeostasis [8]. High ascorbate concentrations in the brain result from the activity of the Sodium-dependent Vitamin C Transporter 2 (SVCT2), which is encoded by the Slc23a2 gene. This transporter is expressed in choroid plexus epithelial cells, facilitating ascorbate entry into the CNS [8]. After reaching the brain's interstitial space, ascorbate is taken up by SVCT2 in neurons, microglia, and oligodendrocytes [[9], [10], [11]]. The essential role of the ascorbate/SVCT2 system in the brain is illustrated by the severe phenotypes observed in SVCT2 knockout mice [12] and confirmed in a mouse model in which SVCT2 expression is decreased in the brain [13]. In both models, it was observed that drastically diminished ascorbate levels in the brain resulted in severe oxidative damage, brain hemorrhages, and death shortly after birth.
Despite the recognized importance of vitamin C in combating oxidative stress in AD, epidemiological studies have not consistently linked vitamin C supplementation with AD prevention or improvement [14,15]. This inconsistency may stem from variations in vitamin C bioavailability, ultimately determined by the transport mechanisms governed by SVCT2. In line with this, single-nucleus sequencing data from AD patients revealed decreased SVCT2 expression in microglia [16], suggesting compromised vitamin C uptake in these cells. This finding critically shows the necessity of re-evaluating the role of vitamin C in AD, focusing on the transport mechanisms that govern its bioavailability in the brain.
Genetic analyses of diagnosed AD patients highlight the significance of microglial genes as genetic risk factors for the disease [17], a finding corroborated by animal studies identifying genes modulating microglial responses to amyloid-beta (Aβ) plaques [18]. This evidence positions microglial homeostasis at the forefront of AD pathology research, underscoring the complex interplay between genetic predispositions and environmental triggers in disease progression. Notably, exposure of microglia to Aβ induces a distinct phenotype known as Disease-Associated Microglia (DAM) [19]. DAM is characterized by an increase in the expression of genes involved in lysosomal/phagocytic pathways and lipid metabolism, as well as a decrease in the expression of microglial homeostatic genes. This transcriptional signature is closely mirrored by the neurodegenerative microglia (MGnD) profile [20], and these states are now understood to represent a conserved microglial response observed across various neurodegenerative disease models [21].
Previous work from our lab has revealed a central role for SVCT2 and ascorbate uptake in maintaining microglial homeostasis [11]. Adult heterozygous SVCT2 mice exhibit significant microgliosis, indicating the critical nature of SVCT2-mediated ascorbate uptake for microglial function in the adult brain [11]. Moreover, acquiring a proinflammatory microglial state requires decreasing the abundance of SVCT2 on the cell surface [11]. Conversely, overexpression of SVCT2 can counteract microglial oxidative stress and proinflammatory activation [11], highlighting the therapeutic potential of modulating SVCT2 expression in microglia in neurological diseases with a neuroinflammatory component, such as AD.
Building on this groundwork, here we investigate whether SVCT2 affects microglial function in mouse models of AD-like pathology and its implications for disease progression. We demonstrate that overexpressing SVCT2 in microglia prior to the onset of AD-like pathology prevents the development of key disease hallmarks, such as memory impairment and decreased synaptic function. Notably, after disease onset, microglial SVCT2 overexpression restores synaptic plasticity and rescues memory impairments. Our work suggests that SVCT2 is a potential therapeutic target for AD, shifting the emphasis of antioxidant therapies from vitamin C intake and/or supplementation to cellular uptake and bioavailability.
2. Methods
2.1. Experimental models
Mice used in this work were bred at the i3S animal facility. Mice were housed under specific pathogen-free conditions in standard laboratory conditions with a 12-h light/dark cycle, maintained at 20 °C, 45–55 % humidity, and provided with access to water and food ad libitum. All procedures were conducted according to the European Union guidelines for animal welfare (European Union Council Directive 2010/63/EU) and Portuguese law (DL 113/2013). All procedures were considered in accordance with Russell and Burch's 3Rs principle. The Portuguese regulatory entity Direção Geral de Alimentação e Veterinária (DGAV) approved all experiments involving animal models (2022-02-18 003669). All efforts were made to minimize animal suffering, and humane endpoints were adopted to safeguard animal welfare.
5xFAD Mouse Model: The 5xFAD transgenic mouse model overexpresses five familial Alzheimer's disease (FAD) mutations in human APP and PS1 [APP: K670 N/M671L (Swedish) + I716V (Florida) + V717I (London); PS1: M146L + L286V] [22] Both transgenes are under the regulation of the Thy1 promoter. Founders were purchased from The Jackson Laboratory (MMRRC stock #34840). This mouse line was rederived and maintained in a C57BL/6J background. The breeding scheme implemented was informed by the observation that female 5xFAD subjects exhibit a more rapid disease progression [23]. As such, we selectively bred males heterozygous for the 5xFAD transgene with wild-type females. Progeny was genotyped using the following primers: Primer mutant (5′ to 3′): AAG CTA GCT GCA GTA ACG CCA TTT; Primer WT (5′ to 3′): ACC TGC ATG TGA ACC CAG TAT TCT ATC; Primer common (5′ to 3′): CTA CAG CCC CTC TCC AAG GTT TAT AG. This research employed only heterozygous 5xFAD female mice.
AßPPswe/PS1A246E Mouse Model: The AβPPswe/PS1A246E, hereafter, APP/PS1 mice, coexpress a chimeric mouse-human amyloid-β protein precursor (AβPP) bearing a human Aβ domain with mutations (K595 N and M596L) linked to Swedish familial AD pedigrees and human presenilin-1 A246E mutation, with both transgenes under the control of the mouse prion protein promoter [24]. APP/PS1 mice were genotyped by PCR using PSEN primers (5′ to 3′): AAT AGA GAA CGG CAG GAG CA (forward) and GCC ATG AGG GCA CTA ATC AT (reverse); APP primers (5′ to 3′): GAC TGA CCA CTC GAC CAG GTT CTG (forward) and CTT GTA AGT TGG ATT CTC ATA TCC G (reverse); WT primers (5′ to 3′): CCT CTT TGT GAC TAT GGT GAC TGA TGT CGG (forward) and GTG GAT AAC CCC TCC CCC AGC CTA GAC C (reverse). APP/PS1 mice were maintained in the SV129 background. This study exclusively utilized female APP/PS1 mice with heterozygous genetic profiles.
2.2. Experimental procedures
2.2.1. Behavioral tests
Procedures were conducted during the dark phase of the light/dark cycle and were performed blind to the genotypes. The tests were carried out in the following order: (1) Novel Object Recognition followed by an interval of 6 days, and (2) Morris Water Maze.
Novel Object Recognition (NOR): The NOR test is employed to evaluate recognition memory in mice, as previously described [[25], [26], [27]]. This test is divided into three parts. In the first phase, the habituation, mice were placed in a box (40 cm × 40 cm x 40 cm) and allowed to explore the apparatus for 10 min (Fig. 5B). After 24 h; mice were put in the same box in the presence of 2 identical objects equidistant from each other (blue squares; Fig. 5B). Free exploration in the presence of both objects was allowed for 10 min. Four hours later, one of the familiar objects was replaced by a new one that differed from the previous one in color, shape, and texture (a yellow triangle; Fig. 3, Fig. 5B). The mice were again positioned in the apparatus, and 3 min were allowed for assessment. Exploration was defined as follows: the mouse touched the object with its nose, or its nose was directed toward the object at a distance shorter than 2 cm. The analyses for the test day were performed using the Boris software [28] to extract the exploration time of the novel () and familiar () objects. After that, the percentage of recognition was calculated using the following ratio:
Fig. 5.
Early microglial SVCT2 overexpression prevents memory deficits and synaptic plasticity impairments in 5xFAD mice.
(A) Timeline of behavioral testing for 6-month-old mice that received AAV injections at 2 months of age. The Novel Object Recognition (NOR) test was followed by the Morris Water Maze (MWM).
(B) Assessment of recognition memory using the NOR test. The bar graph shows the percentage of time spent exploring the novel object. SVCT2 overexpression rescued the memory deficit observed in 5xFAD-Empty mice, restoring performance to wild-type (WT) levels. Data are presented as mean ± SEM. N = 11 (WT-Empty), N = 6 (5xFAD-Empty), N = 9 (5xFAD-SVCT2) mice. p < 0.05 (One-way ANOVA).
(C) Assessment of spatial memory using the MWM. Representative swim paths (left) and quantification of time spent in the target quadrant and total distance swam (right). 5xFAD-SVCT2 mice spent significantly more time in the target quadrant than 5xFAD-Empty mice. Data are presented as mean ± SEM. N = 9 (WT-Empty), N = 7 (5xFAD-Empty), N = 10 (5xFAD-SVCT2) mice. ∗p < 0.01 (One-way ANOVA).
(D) Time course of field excitatory postsynaptic potential (fEPSP) slope changes in hippocampal CA1 synapses following θ-burst stimulation. Representative fEPSP traces before (solid line) and 50–60 min after (dashed line) θ-burst stimulation are also shown. Scale bars: 5 ms (horizontal), 0.5 mV (vertical).
(E) Quantification of LTP magnitude. Data are mean ± SEM from n = 15 slices from N = 5 animals in each group. ∗p < 0.05 (One-way ANOVA).
(F) Quantification of post-tetanic potentiation (PTP) magnitude. Data are mean ± SEM from n = 15 slices from N = 5 animals in each group.
(G) Quantification of Paired-pulse facilitation (PPF) ratio. Data are mean ± SEM from n = 15 slices from N = 5 animals in each group.
(H–J) Input/output (I/O) curves showing fEPSP slope and presynaptic fiber volley (PSFV) amplitude versus stimulus intensity, and fEPSP slope versus PSFV amplitude. Data are mean ± SEM from n = 15 slices from N = 5 animals in each group. ∗p < 0.05 (One-way ANOVA for maximal slope).
Fig. 3.
SVCT2-driven redox modulation induces a hybrid homeostatic and disease-associated microglial state.
(A) Heatmap illustrating the upregulation of canonical Disease-Associated Microglia (DAM) marker genes in SVCT2-overexpressing microglia from 6-month-old 5xFAD mice, identified from RNA-seq data.
(B, C) Representative high-resolution confocal images from the hippocampus of 6-month-old 5xFAD mice, showing increased expression of the DAM markers Cd11c (B) and Lpl (C) in SVCT2-overexpressing microglia (Iba1, green; mCherry, red) compared to controls. Data are represented as mean ± SD. N = 3 mice per group. ∗∗∗p < 0.001 (Unpaired t-test). Scale bars = 20 μm.
(D) Gene Ontology (GO) enrichment analysis of biological processes associated with the upregulated DAM genes in SVCT2-overexpressing microglia.
(E) GO enrichment analysis of cellular components, suggesting that SVCT2 overexpression enhances microglial capacity for phagocytic vesicle formation, lysosomal activity, and endocytic trafficking.
(F) Heatmap illustrating the expression of homeostatic microglial genes that are typically downregulated in DAM states. SVCT2 overexpression partially restored the expression of several of these markers, indicating the emergence of a unique hybrid transcriptional signature.
Morris Water Maze (MWM): The MWM was used to evaluate spatial memory. The apparatus consisted of a circular pool (110 cm diameter) of fiberglass filled with water (21 ± 1 °C). This test is divided into three consecutive steps. Firstly, the cued training was conducted for 2 days with four trials per animal per day. A non-visible escape platform (7 × 8 cm) was submerged 1 cm below the water surface in the quadrant center. Mice were trained to find the platform with a visual clue. Animals were subjected to 4 swimming trials with different start and goal positions. The training phase lasts 5 days, with four trials per animal per day and a completion time of 1 min. The visual clue on top of the platform was removed, but others were placed across the room walls to identify the platform position. Only the mice are released from different positions in this stage, while the platform's location remains unaltered. Once again, if the mice could not reach the platform within 1 min, they were guided to it, and 10 s were given to the mice on top of the platform for each trial, allowing them to learn its location. The test day occurs 24 h after the last training. At this stage, the platform was removed, forcing the mice to recall where it was supposed to be. Each mouse was released from the same position (opposite quadrant) and allowed to freely swim around the apparatus for 30 s to search the platform's position. All parameters were automatically evaluated by SMART v3.0 software (Panlab, Barcelona, Spain).
2.2.2. Stereotaxic injection of Adeno-Associated Virus (AAV) into the hippocampus
The mice were initially sedated using a 3 % isoflurane anesthesia, followed by the subcutaneous administration of the analgesic buprenorphine at a dosage of 0.08 mg/kg to ensure adequate pain management throughout the procedure. Subsequently, each mouse was carefully positioned within a digitally controlled stereotaxic apparatus equipped with an integrated microinjection system (Stoelting Co.). To maintain the depth of anesthesia while minimizing respiratory depression, the isoflurane concentration was adjusted to 1 %. A rectal thermal probe linked to a rodent warming unit (Stoelting Co.) was employed to sustain the subject's body temperature at 37 °C. Precise hippocampal targeting was achieved by using the bregma as a landmark, with the following coordinates: Anteroposterior (AP) at −2.0 mm; Mediolateral (ML) at ±1.3 mm; and Dorsoventral (DV) at −2.2 mm.
Afterward, stereotaxic delivery of custom-engineered self-complementary AAV9 vectors was carried out. These vectors harbored a bicistronic transgene payload, encoding for SVCT2 and mCherry proteins (the "SVCT2 virus"). The control virus carries a similar construct containing only the mCherry reporter (the "empty virus"). The expression of these transgenes was driven by the CD68 promoter, chosen for its specificity to the target microglia within the hippocampal region [[29], [30], [31], [32], [33]].
The CD68 promoter sequence used was the following: CCTGCAGGGCCCACTAGTCATTTCTTACCTCCCCTTCCCTCTCCCACCTGCTACTGGGTGCATCTCTGCTCCCCCCTTCCCCAGCAGATGGTTACCTTTGGGCTGTTGCTTTCTTGTCACCATCTGAGTTCTCAGACGCTGGAAAGCCATGTTCTCGGCTCTGTGAATGACAATGCTGACTGGAGTGCTGCCCCTCTGTAAAGGGCTGGGTGTGGATGGTCACAAGCCCCTCACATGCCTCAGCCAAGAGGAAGTAGTACAGGGGTCAGCCCAGAGGTCCAGGGGAAAGGAGTGGAAACCGATTTCCCCACCAAGGGAGGGGCCTGTACCTCAGCTGTTCCCATAGCTACTTGCCACAACTGCCAAGCAAGTTTCGCTGAGTTTGACACATGGATCCCTGTGGATCAACTGCCCTAGGACTCCGTTTGCACCCATGTGACACTGTTGACTTTGCCCTGATGAAGCAGGGCCAACAGTCCCCTAACTTAATTACAAAAACTAATGACTAAGAGAGAGGTGGCTAGAGCTGAGGCCCCTGAGTCAGGCTGTGGGTGGGATCATCTCCAGTACAGGAAGTGAGACTTTCATTTCCTCCTTTCCAAGAGAGGGCTGAGGGAGCAGGGTTGAGCAACTGGTGCAGACAGCCTAGCTGGACTTTGGGTGAGGCGGTTCAGCC.
2.2.3. Tissue dissociation and microglial enrichment
Percoll Gradient: As outlined in previous studies, the method for separating cells employed a 24 % isotonic Percoll gradient [34]. In brief, mice were euthanized under deep anesthesia induced by pentobarbital (400 mg/kg) and subsequently perfused with ice-cold phosphate-buffered saline (PBS). The brains were carefully extracted and subjected to mechanical dissociation using a glass tissue grinder in Hank's Balanced Salt Solution (HBSS), supplemented with 15 mM HEPES and 0.6 % glucose. The resulting tissue homogenates were passed through 70 μm cell strainers that had been moistened to achieve single-cell suspensions. These suspensions were centrifuged at 300×g for 10 min at 4 °C. After discarding the supernatant, the cell pellets were resuspended in an isotonic solution of 24 % Percoll, over which PBS was gently layered. The samples were centrifuged at 800×g for 30 min at 4 °C to form gradients. After centrifugation, the cell pellets were again suspended in HBSS containing 15 mM HEPES, 0.6 % glucose, and 1 mM EDTA, followed by two washing steps. Cells were finally readied for analysis via flow cytometry.
Microglia Isolation Using MACS: To isolate microglia from the hippocampus, mice were anesthetized with pentobarbital (400 mg/kg) and perfused with ice-cold PBS. The hippocampi were dissected and then dissociated mechanically in ice-cold Dounce buffer, which contained 15 mM HEPES, 0.5 % glucose, 160U/mL DNase, and 20U/mL RNasin® Plus Ribonuclease Inhibitor, adjusted to pH 7.4. The resulting cell suspensions were centrifuged at 300 rcf for 10 min at 4°C. The cell pellets were resuspended in MACS buffer (0.5 % BSA and 1 mM EDTA in PBS, pH 7.4) and incubated with CD11b MicroBeads (Miltenyi Biotec) for 30 min to label the microglial cells. After removing excess beads through centrifugation, cells were washed twice and passed through LS columns (Miltenyi Biotec) mounted on a quadroMACS separator (Miltenyi Biotec) and pre-rinsed with MACS buffer. The columns were then washed, and the CD11b-positive cells were flushed, centrifuged, and set aside for flow cytometry analysis or further RNA isolation and sequencing processing.
Microglia isolated for RNA sequencing underwent a second round of purification by being passed through a new LS column to enhance purity further. After washing, the enriched CD11b-positive cells were lysed using RLT buffer plus (Qiagen) supplemented with 40 μM DTT. RNA was then isolated from these lysates using the Qiagen RNeasy kit, following the manufacturer's protocol. The Bioanalyzer 2100 RNA Pico chips assessed the RNA integrity according to the manufacturer's protocol.
2.2.4. Flow cytometry
Analysis of whole-brain microglia: Following the tissue dissociation procedure outlined above using a 24 % Percoll gradient, the resultant cell suspensions were quantified using the Countess™ Automated Cell Counter (Invitrogen). A total of 2.5 × 106 viable cells were allocated to each well of a U-bottom 96-well plate. The cells were first stained with Zombie Green™ viability dye (diluted 1:500 in PBS) for 30 min to identify dead cells. After Fc receptor blocking using CD16/CD32 antibodies (1:100 dilution), cells were labeled with CD45 (PE-conjugated, 1:200 dilution) and CD11b (PE/Cy7 conjugated, 1:200 dilution) markers. After staining, cells were fixed with 2 % paraformaldehyde (PFA) and permeabilized using eBioscience™ Permeabilization Buffer to allow intracellular staining. Intracellular SVCT2 was detected using a primary antibody (1:100 dilution) followed by incubation with Alexa Fluor™ 647-conjugated goat anti-rabbit IgG secondary antibody (1:500 dilution). After washing, samples were analyzed on an LSRFortessa flow cytometer (BD Biosciences). Compensation was automatically set using monolabel controls (cells stained with individual antibodies or dyes), and SVCT2 staining was validated using a fluorescence-minus-one (FMO) control. Data analysis was conducted using FlowJo X10 Software (TreeStar). SVCT2 staining in APP/PS1 mice was performed using the SVCT2 (G19) antibody from Santa Cruz, while the staining in 5xFAD was performed using the SVCT2 antibody from Abcam.
Analysis of hippocampal microglia: Microglia isolated from the hippocampus, as described in the previous section using the MACS system, were prepared in a similar manner. After plating and PBS washes, cells were stained with Zombie Violet™ (1:500 in PBS) for viability assessment. For oxidative stress measurements, cells were concomitantly incubated with CellRox™ green reagent (1:100). Following washing steps, Fc receptors were blocked (CD16/CD32, 1:100 dilution) before staining with CD11b-PE/Cy7. Following fixation and permeabilization (2 % PFA and eBioscience™ Permeabilization Buffer, respectively), cells were incubated with anti-SVCT2 and anti-mCherry antibodies (both 1:100). Secondary antibodies, Alexa Fluor™ 568 for mCherry and Alexa Fluor™ 647 for SVCT2 (both 1:500), were then applied. After a final wash, cells were analyzed using the LSRFortessa. Compensation and FMO controls were set up as described above, including specific FMO controls for SVCT2, mCherry, and CellRox™ green. Data analysis was performed using FlowJo X10 Software.
2.2.5. Ascorbate measurements using the OxiSelect™ ascorbic acid assay kit
Mice were euthanized under deep anesthesia induced by pentobarbital (400 mg/kg) and subsequently perfused with ice-cold phosphate-buffered saline (PBS). Hippocampi were dissected, and the microglia were isolated using the MACS System. Microglia from both hippocampi from each animal were resuspended in the kit assay buffer, sonicated (6 pulses of 1s at 60Hz), and centrifuged (18000g for 15 min at 4 °C). The supernatant was collected, and the ascorbate concentration was measured according to the manufacturer's instructions. Absorbance was measured at 540 nm using a multimode microplate reader (Synergy HT, BioTek, USA). Ascorbate concentrations were calculated using a standard curve generated with known concentrations.
2.2.6. Tissue preparation for immunofluorescence analysis
Mice were euthanized under deep anesthesia induced by pentobarbital (400 mg/kg) and subsequently perfused with ice-cold PBS to clear the blood. The brains were carefully excised and fixed in 4 % paraformaldehyde (PFA) for a full day to ensure thorough fixation. Following fixation, the brains were rinsed with PBS and then subjected to cryoprotection by immersing the tissue in successive solutions of 15% followed by 30% sucrose, each supplemented with 0.01% sodium azide to prevent microbial growth. For sectioning, the cryoprotected brains were embedded in an Optimal Cutting Temperature (OCT) compound to support the tissue structure. They were then rapidly frozen to preserve cellular integrity and sectioned at a thickness of 30 μm using a Leica CM3050S Cryostat, ensuring consistent slice thickness for uniform staining. The brain sections underwent a permeabilization step with 1 % Triton X-100 for 15 min to increase antibody accessibility, followed by a quenching step with 0.2 M NH4Cl for another 15 min to reduce autofluorescence. For 1 h, sections were incubated in a 1 % BSA solution within the permeabilization buffer to block non-specific binding. Primary antibodies targeting Iba-1, mCherry, and BAM-10 (diluted at 1:400, 1:400, and 1:100, respectively, in blocking solution) were applied to the sections, which were then incubated at 4 °C for 72 h to allow for thorough binding. Notably, sections incubated with BAM-10 antibody were previously incubated with 70 % formic acid for antigen retrieval. Following primary antibody incubation, sections were washed with PBS and incubated with fluorescently labeled secondary antibodies (Alexa Fluor™ 647, Alexa Fluor™ 488, and Alexa Fluor™ 568 conjugated antibodies, all at 1:1000 dilution) in blocking solution for 24 h at 4 °C. Following secondary antibody incubation, sections were washed and stained with DAPI (125 μg/mL) for 15 min to label nuclei, followed by a final PBS wash. The staining procedures were conducted with gentle orbital agitation to ensure even staining. After the final wash, brain sections were mounted onto glass slides. Excess PBS was carefully removed, and a 90 % glycerol was applied. After 1 h, the slides were sealed with nail polish to prevent drying and left to cure in a dark environment for at least 24 h before imaging.
2.2.7. Imaging acquisition
High-Throughput Imaging Analysis of Amyloid and Microglia: To elucidate the dynamic interplay between amyloid beta (Aβ) deposition (BAM-10 labeling) and microglia (Iba1 labeling) within the 5xFAD model hippocampus, our methodology leveraged the PhenoImager HT system for high-throughput imaging of tissue slides. This system's automation and rapid scanning capabilities were crucial, efficiently acquiring comprehensive whole-slide images within a 15 mm × 15 mm area. In the post-imaging phase, we employed QuPath software for the rigorous quantification and characterization of Aβ deposition and microglial distribution patterns.
Confocal imaging: High-resolution images of tissue sections from the dorsal hippocampus were captured using a Leica TCS SP5 confocal microscope. The imaging was conducted in 8-bit sequential mode at a scan speed of 400 Hz, utilizing the standard TCS mode to ensure optimal resolution and minimize signal noise. The pinhole was adjusted to 1 Airy unit across all imaging sessions to achieve consistent optical sectioning. Images were generated using various laser excitation combinations to illuminate the samples effectively. The resolution of the acquired images was set to either 512 x 512 or 1024 x 1024 pixels, depending on the detail required for the analysis. Hybrid Detector (HyD) technology was employed for its superior sensitivity and dynamic range, facilitating the capture of high-quality images. Comprehensive Z-stacks were collected for each tissue section to encompass the entire sample depth, ensuring a thorough volumetric analysis. To maintain consistency and allow accurate sample comparison, equivalent stereological regions were delineated and imaged across all tissue sections within a given slide. This approach ensured that data collection was systematic and representative of the entire tissue architecture.
2.2.8. Electrophysiology
Preparation of Acute Hippocampal Slices: Acute hippocampal slices were meticulously prepared as previously described [35,36]. 5xFAD mice and WT littermates were humanely euthanized using rapid cervical dislocation followed by decapitation. Brains were swiftly excised and submerged in ice-cold artificial cerebrospinal fluid (aCSF) containing (in mM): 124 NaCl, 3 KCl, 1.2 NaH2PO4, 25 NaHCO3, 2 CaCl2, 1 MgSO4, and 10 glucose, continuously oxygenated with a 95 % O2 and 5 % CO2 mixture. Hippocampi were carefully dissected and sectioned perpendicularly along their long axis into 400 μm-thick slices using a precision tissue chopper. These slices were then incubated in oxygenated aCSF at room temperature (22–25 °C) for at least 1 h to ensure functional and energetic recovery before electrophysiological assessments.
Extracellular fEPSP Recordings: Post-recovery, slices were positioned in a specialized recording chamber designed for submerged slices, with a constant superfusion of warm (32 °C) oxygenated aCSF at a 3 ml/min flow rate. Extracellular fEPSPs were recorded in the CA1 stratum radiatum using microelectrodes (4–8 MΩ) filled with aCSF. Stimuli were delivered to Schaffer collateral fibers via a bipolar concentric electrode (platinum/iridium, 25 μm diameter, <800 kΩ impedance), with each stimulus comprising a 0.1 ms pulse at 20-s intervals. Data acquisition was managed with an Axoclamp-2B amplifier and analyzed using WinLTP software [37]. The initial phase slope of averaged fEPSPs (from six consecutive responses) was quantified, setting the stimulus intensity to achieve a near 0.5 mV/ms slope, indicative of approximately 50 % maximal response. Input/output (I/O) relationships were delineated by incrementally increasing stimulus intensity (20 μA every 4 min, ranging from 60 to 340 μA), plotting fEPSP slopes against stimulus intensities. Maximal slope values were extrapolated from non-linear I/O curve fitting, utilizing an F-test for parameter differentiation. Paired-pulse facilitation (PPF) was evaluated by the slope ratio of two consecutive fEPSPs (fEPSP1/fEPSP0) with a 50 ms interstimulus gap. 6 paired responses were averaged for each PPF metric.
Long-term potentiation (LTP) was induced using a θ-burst stimulation pattern, which is more physiologically relevant to learning and memory processes [38]. This involved a single train of four bursts (200 ms apart), each containing four 100 Hz pulses. LTP magnitude was gauged as the percentage change in fEPSP slope (50–60 min post-induction) relative to pre-induction averages (10 min prior). Post-tetanic potentiation (PTP), reflecting transient transmitter release enhancements due to residual Ca2+ during high-frequency activity [39], was assessed by averaging fEPSP slopes in the initial 4 min following LTP induction [40]. Recording protocols were systematized to capture PPF data via one pathway stimulation, I/O metrics through an alternate pathway, and PTP and LTP measurements by reverting to the initial pathway. Baseline fEPSP stability was confirmed under standard stimulation conditions for over 10 min before any protocol adjustments. Each slice, representing two independent pathways, was utilized for one set of experiments per day.
2.2.9. Enzyme-linked immunosorbent assay (ELISA) for Aβ1-42 detection in soluble and insoluble fractions
Mice were euthanized under deep anesthesia induced by pentobarbital (400 mg/kg) and subsequently perfused with ice-cold PBS. Hippocampi were carefully dissected, immediately snap-frozen in dry ice, and stored at −80 °C until further processing. Upon thawing, tissues were homogenized in 200 μL of ice-cold homogenization buffer (50 mM Tris, 137 mM NaCl, 4 mM EDTA, 0.2 % Triton X-100, pH 7.4) supplemented with a protease inhibitor cocktail. The homogenates were centrifuged at 17000g for 15 min at 4 °C to separate soluble and insoluble fractions. The supernatant was collected as the soluble fraction. The pellet was then resuspended in 70 % formic acid, homogenized, and centrifuged at 17000g for 15 min at 4 °C. The resulting supernatant was neutralized with 1 M Tris, pH 11, to obtain the insoluble fraction. Both fractions were analyzed for amyloid beta 1–42 (Aβ1-42) levels using a specific ELISA kit (Invitrogen, Cat# KHB3441) following the manufacturer's instructions. Absorbance was measured at 450 nm using a multimode microplate reader (Synergy HT, BioTek, USA). Aβ1-42 concentrations were calculated using a standard curve generated with known concentrations.
2.2.10. Library preparation, RNA sequencing, and bioinformatics
Ion Torrent sequencing libraries were prepared according to the AmpliSeq Library prep kit protocol, as we did before [25,41]. Briefly, 1 ng of highly intact total RNA was reverse transcribed. The resulting cDNA was amplified for 16 cycles by adding PCR Master Mix and the AmpliSeq mouse transcriptome gene expression primer pool. Amplicons were digested with the proprietary FuPa enzyme, and then barcoded adapters were ligated onto the target amplicons. The library amplicons were bound to magnetic beads, and residual reaction components were washed off. Libraries were amplified, re-purified, and individually quantified using Agilent TapeStation High Sensitivity tape. Individual libraries were diluted to a 50 pM concentration and pooled equally. Emulsion PCR, templating, and 550 chip loading were performed with an Ion Chef Instrument (Thermo Scientific MA, USA). Sequencing was performed on an Ion S5XL™ sequencer (Thermo Scientific MA, USA) as we did before [41].
The S5 XL run data was processed using the Ion Torrent platform-specific pipeline software Torrent Suite v5.12 to generate sequence reads, trim adapter sequences, filter and remove poor signal reads, and split the reads according to the barcode. FASTQ and BAM files were generated using the Torrent Suite plugin FileExporter v5.12. Automated data analysis was done with Torrent Suite™ Software using the Ion AmpliSeq™ RNA plugin v.5.12 and target region AmpliSeq_Mouse_Transcriptome_V1_Designed as we did before [41].
Raw data was loaded into Transcriptome Analysis Console (4.0 Thermo Fisher Scientific, MA, EUA) and first filtered based on ANOVA eBayes using the Limma package and displayed as fold change. Significant changes had a fold change of < -1.5 and >1.5, p-value <0.05, and FDR <0.1. Functional enrichment analyses were performed using STRING [42]. Pathway enrichment was conducted using the REACTOME database with default settings. Enrichment scores for gene sets were calculated using an FDR cutoff of 0.05. Enriched pathways were manually recategorized to core transcriptomic modules and displayed as a network (constructed using Cytoscape).
2.2.11. Synaptosome preparation
Synaptosomes were acutely prepared with the Syn-PER™ Synaptic Protein Extraction Reagent (Thermo Fisher Scientific, Cat# 87793) as before [[25], [26], [43]]. Briefly, mice were euthanized under deep anesthesia induced by pentobarbital (400 mg/kg) and subsequently perfused with ice-cold PBS. Hippocampi were carefully dissected and homogenized in ice-cold Syn-PER™ Reagent. The homogenate was centrifuged at 1,200g for 10 min at 4 °C to remove debris. The supernatant was centrifuged at 15,000g for 20 min at 4 °C. The resulting synaptosome pellet was resuspended in Syn-PER™ Reagent for downstream applications. Synaptosomes used in proteomics and western blotting were prepared and maintained in the presence of protease and phosphatase inhibitors.
2.2.12. Mitochondrial membrane potential assessment
Synaptosomes were probed using the MitoPT® JC-1 Assay kit (Immunochemistry Technologies LLC, USA) to evaluate mitochondrial membrane potential, following the manufacturer's instructions. A positive control using the Carbonyl cyanide 3-chlorophenylhydrazone (CCCP), a proton gradient uncoupling agent, was employed to decrease the electrochemical potential of the inner mitochondrial membrane. Synaptosome washes were performed by centrifugation (12,000g; 10 min at 4 °C). Fluorescence dual emission was measured using excitation at 488 nm and emission at 527 nm and 590 nm, with a multi-detection microplate reader (CLARIOstar, BMG Labtech, DE). The emission ratio red/green was used to evaluate the mitochondrial membrane potential. All samples were normalized for protein content using the BCA method (Pierce BCA Protein Assay Kit).
2.2.13. ATP measurements
Following synaptosome preparation, 2uL of synaptosome solution was added to a luminometer 96-well plate. All procedures were done exactly as described by the manufacturer. The ATP amount was calculated using a standard curve with known concentrations. Luminescence was measured using a multi-detection microplate reader (CLARIOstar, BMG Labtech, DE). All samples were normalized by protein content using the BCA method (Pierce™ BCA protein assay kit).
2.2.14. Western blotting
Synaptosomes were sonicated (6 pulses of 1 s at 60 Hz) and centrifuged at 16,000×g (4 °C) for 10 min. The supernatants were collected, and the BCA method was used to determine the protein concentration. All samples were denatured with sample buffer (0.5 M Tris-HCl pH 6.8, 30 % glycerol, 10 % SDS, 0.6 M DTT, 0.02 % bromophenol blue) at 95 °C for 5 min and stored at −20 °C until use. Notably, the samples used to evaluate vGLUT-1 expression were not boiled. Samples were resolved in a 10 % SDS-PAGE gel, transferred to PVDF membranes, and incubated overnight with the following primary antibodies PSD-95 (1:2000); vGLUT-1 (1:1000) and GAPDH (1:100000). Membranes were washed in TBS-T buffer pH 7.6, incubated with peroxidase-conjugated secondary antibodies (1:10000), and developed using an ECL chemiluminescent reagent. Images were acquired in a ChemiDoc XRS System (Bio-Rad).
2.2.15. High-throughput proteomics, data acquisition, and quantification
100 μg of synaptosome proteins from each sample were processed for proteomic analysis following the solid-phase-enhanced sample-preparation protocol [44]. Enzymatic digestion was performed with trypsin/LysC (2 μg) overnight at 37 °C at 1000 rpm. The resulting peptide concentration was measured by fluorescence.
NanoLC-MS/MS performed protein identification and quantification as before [25,43]. This equipment comprises an Ultimate 3000 liquid chromatography system coupled to a Q-Exactive Hybrid Quadrupole-Orbitrap mass spectrometer (Thermo Scientific). 500 ng of peptides of each sample were loaded onto a trapping cartridge in a mobile phase of 2 % ACN, 0.1 % FA at 10 μL per minute. After 3 min of loading, the trap column was switched in-line to a 50 cm × 75 μm inner diameter EASY-Spray column at 250 nL per minute. Separation was achieved by mixing A (0.1 % FA) and B (80 % ACN, 0.1 % FA) with the following gradient: 5 min (2.5 % B to 10 % B), 120 min (10 % B to 30 % B), 20 min (30 % B to 50 % B), 5 min (50 % B to 99 % B), and 10 min (hold 99 % B). Afterward, the column was equilibrated with 2.5 % B for 17 min. Data acquisition was controlled by Xcalibur 4.0 and Tune 2.11 software (Thermo Scientific).
The mass spectrometer was operated in the data-dependent (DD) positive acquisition mode, alternating between a full scan (m/z 380–1580) and subsequent HCD MS/MS of the 10 most intense peaks from a full scan (normalized collision energy of 27 %). The ESI spray voltage was 1.9 kV. The global settings were: use lock masses best (m/z 445.12003), lock mass injection Full MS, and chrom. peak width (FWHM) of 15 s. The full scan settings were as follows: 70k resolution (m/z 200), AGC target 3 x 106, maximum injection time 120 ms; DD settings: minimum AGC target 8 x 103, intensity threshold 7.3 x 104, charge exclusion: unassigned, 1, 8, >8, peptide match preferred, exclude isotopes on, and dynamic exclusion 45 s. The MS2 settings were as follows: micro scans 1, resolution 35k (m/z 200), AGC target 2 x 105, maximum injection time 110 ms, isolation window 2.0 m/z, isolation offset 0.0 m/z, dynamic first mass, and spectrum data type profile.
The raw data was processed using the Proteome Discoverer 2.5.0.400 software (Thermo Scientific) and searched against the UniProt database for the reviewed Mus musculus Proteome (2021_03 with 17,077 entries). A common protein contaminant list from MaxQuant was also considered in the analysis. The Sequest HT search engine was used to identify tryptic peptides. The ion mass tolerance was 10 ppm for precursor ions and 0.02Da for fragment ions. The maximum allowed missing cleavage sites was set to 2. Cysteine carbamidomethylation was defined as a constant modification. Methionine oxidation, deamidation of glutamine and asparagine, peptide terminus glutamine to pyroglutamate, and protein N-terminus acetylation, Met-loss, and Met-loss + acetyl were defined as variable modifications. Peptide confidence was set to high. The processing node Percolator was enabled with the following settings: maximum delta Cn 0.05; decoy database search target false discovery rate 1 %; validation based on q-value. Protein label-free quantitation was performed with the Minora feature detector node at the processing step. Precursor ions quantification was conducted at the consensus step with the following parameters: unique plus razor peptides were considered, precursor abundance was based on intensity, and normalization was based on total peptide amount.
For the determination of DE between groups, the following filters were used: (1) only master proteins detected with high/medium confidence FDR; (2) a protein/phosphoprotein must be detected in more than 50 % of samples in each experimental group (except for proteins that were depleted entirely in one of the experimental groups); (3) the p-value adjusted using Benjamini–Hochberg correction for the FDR was set to ≤ 0.05; (4) at least 50 % of samples with protein-related peptides sequenced by MS/MS.
2.2.16. Proteomics network analyses
For constructing granular networks, DE proteins retrieved from the LFQ experience were uploaded to STRING [42] within Cytoscape as before [25]. Enrichment analyses (FDR cutoff <0.05, with the Benjamini-Hochberg multiple test adjustment) were conducted using REACTOME and GO as functional databases. Network construction and topography were carried out following manual pathway annotation and clustering in Cytoscape.
2.2.17. Primary cultures of cortical microglia
Primary microglial cell cultures were prepared as previously described [11,45]. In brief, rat pups (2-day-old) were sacrificed, and their cerebral cortices were dissected in HBSS, pH 7.2, and digested with 0.07 % trypsin plus 50 μL (w/v) DNAse for 15 min. Next, cells were gently dissociated using a glass pipette in DMEM F12 GlutaMAX™-I (Thermo Fisher) supplemented with 10 % FBS and 1 % Pen/Strep. Cells were plated in poly-d-lysine-coated T-flasks (75 cm2) at 1.5x106 cells per cm2. Cultures were kept at 37 °C and 95 % air/5 % CO2 in a humidified incubator. Culture media was changed every 3–4 days up to 20 days. Culture flasks were subjected to orbital shaking at 200 rpm for 2 h to detach microglia. Next, the culture supernatant was collected and centrifuged at 453g for 5 min at room temperature. The supernatant was discarded, and the microglia pellet was resuspended in culture medium. Ultimately, cells were seeded in poly-d-lysine-coated 12-well culture plates at 2.5x105 cells/cm2 with Dulbecco's Modified Eagle Medium (DMEM) F12 + GlutaMAX™-I (Thermo Fisher) supplemented with 1 % FBS, 1 % Pen/Strep, and 1 ng/ml mCSF.
For oxidative stress measurements and immunocytochemistry, cultures were infected with Lenti-Empty or Lenti-SVCT2 viruses, as before [11], and allowed to recover for 5 days. Then, cultures were loaded with CellRox™ Deep Red reagent for 30 min before incubation with saline (control) or Aβ1-42 oligomers (1 μg/ml) for 3 h. Cultures were then fixed with 2 % PFA, and fluorescence was measured using a fluorescence microscope as before [11,26,27,45]. Aβ1-42 oligomers were prepared as before [26,27]. Cells were also used to validate SVCT2 overexpression induced by the Lenti-SVCT2 virus. For that, cells were transduced and kept in culture for 5 days. After that, the cells were fixed with 1 % PFA and processed for immunocytochemistry exactly as before [11]. Image acquisition and quantification were performed exactly as before [11].
2.2.18. Quantification and statistical analysis
Experimenters were blinded to genotypes and housing conditions. Data were tested for Gaussian distribution using the D'Agostino-Pearson omnibus test. All tests considered mice as experimental units with a significance level of P < 0.05.
Descriptive statistics, including mean, median, standard deviation, and interquartile range, were provided for each dataset and detailed within the Figure Legends accompanying the results. All statistical analyses and the creation of graphical representations of the data were conducted using GraphPad Prism (version 9.0.2 for macOS) and Python, ensuring rigorous and accurate data analysis and visualization.
The assembly of figure panels for publication was performed using Adobe Illustrator 2020 (version 24.3). Schematic diagrams were created using BioRender.
The source identifiers related to this manuscript can be found in the Supplementary Table 5.
3. Results
3.1. Decreased SVCT2 expression and ascorbate content in 5xFAD microglia
AD is characterized by progressive memory loss and cognitive decline, in which pathological changes in microglia that drive inflammation, oxidative stress, and neurodegeneration are thought to play a significant role. We previously found that decreasing the abundance of SVCT2 on the microglial cell surface reduces ascorbate uptake, triggering oxidative stress and the secretion of proinflammatory molecules [11]. More recently, Prater and colleagues have reported a decrease in SVCT2 mRNA expression in microglia from AD patients [16]. This raises the hypothesis that the SVCT2 expression in microglia might become compromised in age-related neurodegenerative processes, such as AD.
Using flow cytometry, we evaluated the SVCT2 expression in microglia from 5xFAD mice (a pre-clinical mouse model for studying AD [22]) at different time points (2, 4, and 6 months of age - Fig. 1A). Our results revealed a distinct age-dependent decline in SVCT2 expression in the 5xFAD microglia compared to age-matched WT controls. As expected, this decrease in SVCT2 expression was associated with a significant decrease in intracellular ascorbate content in 5xFAD microglia at 6 months of age (Fig. 1B).
Fig. 1.
Age-dependent decrease in microglial SVCT2 expression in 5xFAD mice and validation of AAV-mediated SVCT2 overexpression.
(A) Flow cytometry analysis of SVCT2 expression in microglia from wild-type (WT) and 5xFAD mice at 2, 4, and 6 months of age. The gating strategy shows debris exclusion (FSC-A vs. SSC-A), singlet selection, live cell gating (Zombie GreenTM negative), and identification of microglia (CD11b+ CD45Dim). Representative histograms and summary quantification of SVCT2 median fluorescence intensity (MFI) show a progressive decline in 5xFAD microglia compared to age-matched WT controls. Data are represented as mean ± SEM. N = 3 (WT, 2mo), N = 4 (WT, 4mo), N = 5 (WT, 6mo), N = 4 (5xFAD, 2mo), N = 5 (5xFAD, 4mo), N = 5 (5xFAD, 6mo) mice per group. ∗p < 0.05 (Two-way ANOVA).
(B) Quantification of intracellular ascorbate levels in microglia isolated from the hippocampus of 6-month-old 5xFAD mice, showing a significant decrease in ascorbate content compared to WT mice. Data are represented as mean ± SEM. N = 3 mice per group. ∗p < 0.05 (Unpaired t-test).
(C) Schematic representation of the experimental design for the early intervention study. 5xFAD mice were injected at 2 months of age with AAV9 vectors driving mCherry (Empty) or SVCT2-IRES-mCherry (SVCT2) expression under the microglial-specific CD68 promoter in both hippocampi. A battery of analyses was performed at 6 months of age to assess the impact of the intervention.
(D) Representative confocal images from the hippocampus of 6-month-old 5xFAD mice injected with AAV9-Empty or AAV9-SVCT2. Images show successful transduction of microglia (Iba1, green) with the viral vectors, as indicated by mCherry fluorescence (red). Scale bar = 20 μm
(E) Flow cytometry gating strategy and quantification confirming SVCT2 overexpression in transduced (mCherry+) microglia isolated from the hippocampus of 6-month-old 5xFAD mice. The bar graph shows a significant increase in SVCT2 MFI in the 5xFAD-SVCT2 group compared to the 5xFAD-Empty group. Data are represented as mean ± SEM. N = 5 (5xFAD-Empty) and N = 7 (5xFAD-SVCT2) mice. ∗p < 0.05 (Unpaired t-test).
(F) Quantification of intracellular ascorbate levels in isolated microglia from 6-month-old 5xFAD-Empty and 5xFAD-SVCT2 mice hippocampi, confirming that SVCT2 overexpression leads to increased ascorbate intracellular content. Data are represented as mean ± SEM. N = 3 mice per group. ∗p < 0.05 (Unpaired t-test).
3.2. SVCT2 overexpression reconfigures the redox landscape of 5xFAD microglia
To counteract the decrease in SVCT2 expression in 5xFAD microglia, we employed a self-complementary AAV9-CD68 vector system, recognized for its selective targeting of myeloid cells [[29], [30], [31], [32], [33]]. We stereotactically delivered either SVCT2-IRES-mCherry (SVCT2) or mCherry-only (Empty) constructs to the hippocampi of 2-month-old 5xFAD mice, a time point preceding overt pathology [22] (Fig. 1C). By six months—a stage of robust AD-like pathology [22]—we thoroughly confirmed selective microglial transduction and SVCT2 overexpression (Fig. 1D and E and Suppl. Fig. 1A–D). Moreover, ascorbate measurements confirmed elevated intracellular ascorbate levels in SVCT2-overexpressing microglia, thereby validating the functional impact of SVCT2 overexpression (Fig. 1F).
Following the validation of SVCT2 overexpression in 5xFAD microglia, we performed RNA sequencing (RNA-seq) on isolated hippocampal microglia (Fig. 2A). Transcriptomic analysis revealed widespread reprogramming of gene expression (Fig. 2A, Suppl. Fig. 2, and Suppl. Table 1) with 328 upregulated and 193 downregulated transcripts.
Fig. 2.
SVCT2 overexpression reconfigures the microglial redox landscape in 5xFAD mice.
(A) Schematic workflow of the RNA sequencing (RNA-seq) analysis performed on microglia isolated by Magnetic-Activated Cell Sorting (MACS) from the hippocampi of 6-month-old 5xFAD-SVCT2 and 5xFAD-Empty mice. Volcano plot visualizing differentially expressed genes (DEGs) between microglia from 5xFAD-SVCT2 and 5xFAD-Empty mice. Upregulated genes are shown in red and downregulated genes in blue. The x-axis represents the log2 fold change, and the y-axis represents the -log10 (p-value), with significance thresholds indicated by dashed lines. Scatter plot showing the per-group correlation between Cd68 and Slc23a2 (SVCT2) transcipt levels in microglia. Each point represents an individual animal from 5xFAD-Empty or 5xFAD-SVCT2 groups. The lines depict the linear regression, with the Pearson correlation coefficient (r) shown for each respective group.
(B) The redox signature of 5xFAD microglia overexpressing SVCT2. The heatmaps show the z-scores of selected transcripts related to redox homeostasis, including glutathione (GSH) metabolism, NADH metabolism, and NF-kB signaling.
(C) Functional validation of the antioxidant effect of SVCT2 in vitro. Representative fluorescence images and quantification of the ROS probe CellRox Deep Red (grey) in primary rat microglial cultures (DIV 5–8) infected with lentiviral vectors for Empty or SVCT2 overexpression (GFP, green). Cultures were challenged with Aβ1-42 oligomers (1 μM for 3 h) or control saline. Data represent N = 4 independent cultures per group. ∗∗∗∗p < 0.0001 (Two-way ANOVA). Scale bar = 50 μm.
(D) In vivo validation showing decreased ROS levels (via CellRox Green) in hippocampal microglia from 6-month-old 5xFAD-SVCT2 mice compared to 5xFAD-Empty controls. Data are represented as mean ± SEM. N = 3 (5xFAD-Empty) and N = 4 (5xFAD-SVCT2) mice. ∗p < 0.05 (Unpaired t-test).
Because the Cd68 promoter drives Slc23a2 (SVCT2) overexpression, we then examined the relationship between their transcript levels (Fig. 2A – scatter plot). In 5xFAD-Empty mice, we observed a strong negative correlation, indicating that in the early disease stage, the most activated microglia (highest Cd68) have the lowest endogenous Slc23a2 (SVCT2) levels. Of note, SVCT2 overexpression reversed this trend, resulting in a strong positive correlation (Pearson r = 0.74).
Furthermore, this SVCT2-driven transcriptional change caused no overt alteration in the expression levels of SVCT1 (Slc23a1) in microglia (Suppl. Table 1). Gene ontology (GO) enrichment highlighted two major components controlled by SVCT2 overexpression in microglia: a core redox homeostasis module and a stress response program (Fig. 2B).
The redox homeostasis core comprised pathways essential for glutathione metabolism, NADH metabolism, thioredoxin activity, NF-kappaB signaling, and FoxO-mediated stress responses, indicating that SVCT2-overexpressing microglia adopt a transcriptional state optimized for oxidative stress resilience (Fig. 2B). Upregulated genes included, Psme2b which enhances proteasomal degradation of oxidized proteins, and Dock2, a key regulator of actin remodeling and immune responses (Fig. 2B). Plasma membrane-localized transcripts such as Hvcn1, a proton channel involved in pH regulation, and Rab32, a regulator of mitochondrial fitness and vesicle trafficking, were also elevated, suggesting adaptations in metabolic and vesicular processes. Nuclear transcriptional regulators, such as Irf4 and Nfya, which are known to modulate oxidative stress and immune homeostasis, were also enriched, underscoring SVCT2's role in coordinating a multi-tiered response to oxidative stress (Fig. 2B). In contrast, downregulated genes largely consisted of metabolic and cytoskeletal regulators typically associated with high-energy ion transport, receptor signaling, and inflammation, reinforcing a functional shift favoring oxidative stress resilience over excessive immune activation (Fig. 2B).
To validate the redox-associated transcriptomic changes at a functional level, we examined whether SVCT2 overexpression could alleviate oxidative stress in microglia. In primary microglial cultures transduced with the SVCT2 construct [11](validation in Suppl. Fig. 1E), we observed a significant decrease in Aβ1-42–induced ROS production compared to microglia transduced with the Empty virus (Fig. 2C). This antioxidant effect was also observed in vivo, as hippocampal microglia from 5xFAD-SVCT2 mice displayed decreased levels of ROS compared to hippocampal microglia from 5xFAD-Empty mice (Fig. 2D). These data suggest that SVCT2 overexpression enhances the oxidative stress resilience of microglia, consistent with the transcriptional shift in redox reprogramming.
3.3. SVCT2-driven redox rewiring modulates microglial response in 5xFAD mice
Given the extensive transcriptomic reprogramming observed, we further leveraged our RNA-seq data to characterize whether SVCT2-induced redox reprogramming in microglia also influences the microglial response commonly reported in Alzheimer's pathology [21,46,47]. Thus, using the RNA-seq-derived transcriptomic profiles of hippocampal microglia from 5xFAD-Empty and 5xFAD-SVCT2 mice, we specifically examined established gene markers associated with disease-associated microglia (DAM). This analysis identified a pronounced upregulation of canonical DAM markers (Fig. 3A), including Cd11c and Lpl (Fig. 3B and C), in SVCT2-overexpressing microglia. Enriched biological processes highlighted by these differentially expressed DAM genes notably included antigen processing and presentation, macrophage activation, and negative regulation of neuroinflammation (Fig. 3D). Moreover, cellular component enrichment suggested an increased microglial capacity for phagocytic vesicle formation, lysosomal activity, and endocytic trafficking (Fig. 3E)—key mechanisms influencing microglial-amyloid interactions.
Despite the pronounced DAM-like gene expression, SVCT2 overexpression concurrently restored the expression of several homeostatic microglial markers (e.g., Cx3cr1, Csf1r, Serinc3), which are usually suppressed in classical DAM states (Fig. 3F). These findings show that SVCT2-driven redox regulation supports a unique transcriptional state in microglia, combining DAM-associated changes with preservation of the homeostatic signature.
3.4. SVCT2 overexpression in 5xFAD microglia decreases amyloid pathology
We also directly assessed the impact of SVCT2 overexpression on microglia-amyloid interactions within the 5xFAD hippocampus. Firstly, we asked if transduced microglia, identifiable by the mCherry reporter, interact with Aβ plaques. High-resolution confocal imaging confirmed that Iba1-positive microglia co-expressing mCherry were indeed clustered around BAM-10-positive amyloid deposits (Suppl. Fig. 3). Moreover, quantitative analysis revealed a clear decrease of hippocampal amyloid-beta deposition in SVCT2-overexpressing mice, indicated by a significantly smaller amyloid (BAM-10-positive) area compared to control mice (Fig. 4A). Furthermore, SVCT2 overexpression in microglia induced a decrease in amyloid plaque size and, critically, enhanced microglial coverage of amyloid deposits (Fig. 4B). A regression analysis further solidified these findings, demonstrating a stronger correlation between plaque size and microglial encapsulation in 5xFAD-SVCT2 mice compared to controls (Fig. 4C). This improved microglial coverage was significant for medium and large plaques (Fig. 4D).
Fig. 4.
Early SVCT2 overexpression in microglia decreases amyloid pathology in 5xFAD mice.
(A) Representative immunofluorescence images of the hippocampus and quantification of amyloid plaque burden in 6-month-old 5xFAD mice. Amyloid plaques are labeled with BAM-10 (red) and nuclei with DAPI (blue). SVCT2 overexpression significantly decreased the total Aβ load (% area) compared to the Empty control group. Data are expressed as mean ± SD from N = 4 mice per group. ∗p < 0.05 (Unpaired t-test). Scale bar = 200 μm
(B) High-magnification images and quantification of plaque size and microglial coverage. Amyloid plaques (BAM-10, red) are shown with associated microglia (Iba1, green). SVCT2 overexpression resulted in significantly smaller plaque sizes and enhanced microglial plaque coverage. Data shown are mean ± SD. N = 67 plaques from 4 mice (5xFAD-Empty) and 102 plaques from 4 mice (5xFAD-SVCT2). ∗p < 0.05; ∗∗∗∗p < 0.0001 (Unpaired t-test). Scale bar = 10 μm
(C) Scatter plot with regression analysis showing a stronger positive correlation between amyloid plaque size and microglial plaque coverage in the 5xFAD-SVCT2 mice compared to 5xFAD-Empty controls. Each point represents an individual plaque.
(D) Quantification of microglial plaque coverage stratified by plaque size (Small, Medium, Large). Data points represent individual plaques. ∗∗p < 0.01, ∗∗∗p < 0.001 (Two-way ANOVA).
To strengthen the relevance of our findings, we also evaluated SVCT2 expression and amyloid burden in an additional, well-established AD transgenic mouse model, the APP/PS1 mice [24]. In line with observations from 5xFAD mice, microglia from APP/PS1 mice also displayed diminished SVCT2 expression compared to wild-type littermates (Suppl. Fig. 4A), stating that decreased SVCT2 expression in microglia is a common feature to both AD mouse models.
Consistent with the data obtained in the 5xFAD model, microglial-specific SVCT2 overexpression in APP/PS1 mice (validation in Suppl. Fig. 4B) resulted in a significant decrease in amyloid burden (Suppl. Fig. 4C and D), highlighting the reproducibility and broader applicability of SVCT2-driven microglial modulation across multiple amyloid-depositing paradigms. We also observed an increase in excitatory synaptic density in the hippocampus of APP/PS1 mice overexpressing SVCT2 in microglia compared to APP/PS1 mice injected with the control virus (Suppl. Fig. 4E). This result suggests that SVCT2 overexpression in microglia influences the hippocampal synapse landscape, potentially affecting synaptic function and memory in Alzheimer's mouse models.
3.5. Overexpressing SVCT2 in microglia before disease onset prevents memory deficits and synaptic plasticity impairments in the 5xFAD mice
Following the validation of SVCT2 overexpression as a regulator of redox homeostasis in 5xFAD microglia, we next investigated the effects of this targeted microglial approach on memory performance and synaptic plasticity in 5xFAD mice. Memory functions were evaluated with the Novel Object Recognition (NOR) test to assess recognition memory and the Morris Water Maze (MWM) to evaluate spatial memory (Fig. 5A). In the NOR test, 5xFAD mice injected with the control vector (5xFAD - Empty) exhibited a marked decrease in novel object preference, indicating a recognition memory impairment. Notably, 5xFAD mice overexpressing SVCT2 (5xFAD - SVCT2) demonstrated a significant increase in novel object interaction comparable to WT mice inject with the empty virus (Fig. 5B). Spatial memory was then assessed using the Morris water maze (MWM), a well-established assay for hippocampal-dependent memory (Fig. 5C). Path tracking analysis revealed that 5xFAD mice overexpressing SVCT2 showed efficient search patterns and spent a more significant percentage of time in the target quadrant, indicative of successful spatial learning and memory retention. In contrast, 5xFAD mice injected with the control vector exhibited less targeted search patterns and a reduced time spent in the target quadrant, revealing spatial memory impairment (Fig. 5C). Of note, memory performance in the MWM was comparable between WT – Empty and 5xFAD – SVCT2 groups (Fig. 5C).
Synaptic dysfunction is a hallmark of cognitive decline in AD. Hence, we analyzed the impact of microglial SVCT2 overexpression on synaptic plasticity. We evaluated LTP at CA3-CA1 hippocampal synapses in 5xFAD mice overexpressing SVCT2 in microglia or 5xFAD mice injected with the empty virus. Consistent with our hypothesis, θ-burst stimulation of Schaffer collateral pathways led to an initial surge in the field excitatory postsynaptic potential (fEPSP) slope, which subsequently decreased to a plateau markedly above pre-stimulation baseline values (Fig. 5D). Notably, the LTP magnitude, expressed as the percentage change in fEPSP slope pre- and post-50-60 min of θ-burst stimulation, was significantly enhanced in the SVCT2-overexpressing group compared to 5xFAD mice injected with empty virus, signifying pronounced facilitation of synaptic strength (Fig. 5E). As expected, 5xFAD injected with the empty virus displayed a significantly decreased LTP magnitude compared to WT mice injected with the same virus (Fig. 5D and E). This effect was specific to LTP, as post-tetanic potentiation (PTP) magnitudes remained unaltered between groups (Fig. 5F), underscoring the specificity of microglial SVCT2's role in modulating long-term forms of synaptic plasticity.
Paired-pulse facilitation (PPF) assay, designed to probe presynaptic alterations, indicated no significant differences in glutamate release probabilities or quantal content between groups (Fig. 5G), indicating a specific impact of SVCT2 overexpression in microglia on post-synaptic long-term plasticity.
Further investigation into basal synaptic transmission via input/output (I/O) curves recorded from Schaffer collateral-commissural fibers showed that synaptic responses to afferent stimulation were significantly increased in SVCT2-overexpressing 5xFAD microglia compared to controls. Specifically, the maximum fEPSP slope, a critical parameter indicating synaptic strength, was higher in hippocampal slices from SVCT2-overexpressing 5xFAD microglia than in slices from control 5xFAD microglia (Fig. 5H). Importantly, the PSFV amplitude elicited with the strongest stimulus did not appreciably differ between the groups (Fig. 5I), confirming that the increased synaptic responses observed in SVCT2-overexpressing microglia were not due to changes in presynaptic fibers firing. We plotted the fEPSP slope values against the presynaptic fiber volley (PSFV) amplitude to further dissect this observation. The resulting I–O curve (Fig. 5J) showed an appreciable increase in the fEPSP slope in hippocampal slices from SVCT2-overexpressing 5xFAD microglia compared to control 5xFAD microglia. This enhancement in fEPSP slope occurred despite similar presynaptic fiber volley (PSFV) amplitudes between the groups (Fig. 5J), suggesting that the observed increase in synaptic responses was not due to changes in presynaptic input strength but instead to an increase in postsynaptic efficiency.
Taken together, our electrophysiological data showed that the overexpression of SVCT2 in 5xFAD microglia enhances both LTP and basal synaptic transmission, likely through mechanisms that increase postsynaptic efficiency without altering presynaptic input strength.
3.6. Overexpressing SVCT2 in 5xFAD microglia before disease onset remodels the synaptic proteome critical to mitochondrial energetics
To elucidate the synaptic underpinnings associated with SVCT2 overexpression in microglia in 5xFAD mice, we performed an in-depth high-throughput LC-MS/MS analysis in synaptosomes (Fig. 6A, Suppl. Fig. 2 and Suppl. Fig. 5). The results revealed a substantial reconfiguration of the synaptic proteome landscape (Suppl. Table 2). Differential expression analysis indicated that 85 proteins were significantly upregulated and 52 were downregulated in the synaptosomes from the 5xFAD-SVCT2 group compared to the 5xFAD-Empty cohort (Fig. 6B). Subcellular localization analyses underscored a mitochondrial enrichment of altered proteins, suggesting the influence of microglial SVCT2 overexpression on synaptic mitochondrial proteomic composition (Fig. 6C). Gene Ontology (GO) and pathway enrichment analyses revealed significant proteomic shifts, specifically highlighting an increase in the mitochondrial electron transport chain (ETC) and its related components. Notably, the analysis revealed an upregulation in critical subunits of the mitochondrial respiratory chain Complex IV, such as Cox5a, Cox7a2, and Cox7c, suggesting a fortified mitochondrial respiratory function. This enhancement was complemented by an increase in the ATP synthase component Atp5e, indicating a potential increase in ATP production capacity—a fundamental aspect for synaptic efficiency and adaptability (Suppl. Table 2; Fig. 6D).
Fig. 6.
Early SVCT2 overexpression remodels the synaptic mitochondrial proteome.
(A) Schematic diagram of the proteomic workflow, where synaptosomes were isolated from the hippocampi of 6-month-old 5xFAD-Empty and 5xFAD-SVCT2 mice for LC-MS/MS-based label-free quantitative proteomics.
(B) Volcano plot showing differentially expressed proteins in synaptosomes from 5xFAD-SVCT2 versus 5xFAD-Empty mice. The analysis identified 85 upregulated (red) and 52 downregulated (blue) proteins. Data were derived from N = 4 (5xFAD-Empty) and N = 5 (5xFAD-SVCT2) mice.
(C, D) Gene Ontology (GO) enrichment analysis of the differentially expressed proteins. Bubble plots show enrichment for subcellular localizations, highlighting the mitochondrial membrane and matrix (C), and for biological pathways, highlighting the TCA cycle and mitochondrial electron transport chain (D).
(E, F) Protein-protein interaction (PPI) networks constructed using STRING and visualized in Cytoscape. Networks show upregulated proteins involved in the mitochondrial matrix, inner membrane, and envelope (E), and key metabolic pathways including the TCA cycle and ATP synthesis (F). Node color indicates log2 fold change (red = upregulated, blue = downregulated).
(G) Functional validation of mitochondrial membrane potential (ΔΨm) using the JC-1 assay in isolated synaptosomes. The bar graph shows a significant increase in the red/green fluorescence ratio in the 5xFAD-SVCT2 group compared to controls ∗∗p < 0.01 (Unpaired t-test).
(H) Functional validation of ATP levels in synaptosomes. The bar graph shows significantly higher ATP concentrations in the 5xFAD-SVCT2 group compared to controls. ∗p < 0.05 (Unpaired t-test).
Dashed lines in the graphs indicate the average measurements taken from a matched WT-Empty mouse.
Protein-protein interaction (PPI) analysis provided detailed insights into mitochondrial protein dynamics, with alterations indicating an enhancement of mitochondrial functioning in response to SVCT2 overexpressing microglia (Fig. 6E and F). The elevation in the expression of Uqcrq and Uqcrh, components of the mitochondrial respiratory chain complex III, and Sdhd, a part of Complex II, mirrors a potential enhancement in the ETC's efficiency facilitated by microglia overexpressing SVCT2 (Fig. 6E). The presence of proteins such as Ndufa2 and Ndufb8 from Complex I underscores a broad-based support for mitochondrial electron transport enhancement.
Within the mitochondrial matrix, the overexpression of SVCT2 in microglia markedly enhances the expression of pivotal proteins, thereby optimizing bioenergetic processes. The elevated levels of Hspa1l and Hspe1, chaperone proteins, enhance protein folding and stress response mechanisms, which are crucial for preserving mitochondrial functionality across various physiological states. The upregulation of Mrpl28, an integral component of the mitochondrial ribosome, indicates a heightened synthesis of mitochondrial proteins, which are crucial for maintaining the continuity of the electron transport chain (ETC) and ATP production. Furthermore, Grpel2's role as a mitochondrial chaperone, involved in protein import and organizing the mitochondrial network, suggests an improved capacity for protein import and mitochondrial structural integrity. This is pivotal for the efficient operation of the mitochondria. Me3, which facilitates the malate/aspartate shuttle, is instrumental in efficiently transferring reducing equivalents into the mitochondria, enhancing NADH utilization for ATP synthesis. The observed increase in Suclg1, a crucial enzyme in the succinate-CoA ligase complex of the TCA cycle, highlights an enriched ability to synthesize ATP from TCA cycle intermediates. This enhancement underscores the critical function of the mitochondrial matrix in energy metabolism, pointing to a significant microglial overexpression of SVCT2-mediated boost in synaptic mitochondrial ATP production (Fig. 6E).
Further analysis of the interaction between pyruvate metabolism, the TCA cycle, the ETC, and ATP production highlights a group of proteins crucial to the energy pathways in synaptic mitochondria of 5xFAD mice, especially increased by SVCT2 overexpression in microglia (Fig. 6F). The enhanced expression of Idh2 and Sdhd, crucial for the TCA cycle, marks a robust increase in NADH production. This uptick in NADH feeds into the ETC and likely amplifies synaptic ATP synthesis efficiency, further reflecting an optimized bioenergetic state [48]. The upregulation of Complex III components, Uqcrq and Uqcrh, alongside Cycs—a critical player in the cytochrome c oxidase complex—illustrates a fortified electron transport mechanism [49]. This enhancement ensures a seamless electron flow through the ETC, which is pivotal for sustaining high rates of oxidative phosphorylation and ATP generation. The involvement of Complex IV subunits, Cox5a, Cox7a2, and Cox7c, further solidifies the link between the TCA cycle and the ETC, facilitating efficient energy substrate conversion and bolstering mitochondrial respiratory function [49]. Lastly, the increased levels of Atp5e and mt-Atp6, which are essential for the final stage of oxidative phosphorylation, further indicate enhanced ATP production capacity [49].
Consistent with these proteomic indicators of enhanced mitochondrial bioenergetics, we next sought to validate these changes functionally. To directly probe synaptic mitochondrial function, we first quantified the mitochondrial membrane potential (ΔΨm) using the JC-1 assay in freshly isolated hippocampal synaptosomes. Because JC-1 preferentially accumulates in polarized mitochondria, an increased red/green fluorescence ratio indicates a more negative ΔΨm and thus a greater proton-motive force available for oxidative phosphorylation. Synaptosomes from 5xFAD-SVCT2 displayed a significant elevation in JC-1 signal compared to controls (Fig. 6G).
Given that ΔΨm is the primary electrochemical driver for ATP synthesis via the F1F0-ATP synthase, we hypothesized that this observed hyperpolarization would translate into increased ATP levels. Using an ATP measurement kit, ATP concentrations were determined in matched synaptosomal lysates. Indeed, SVCT2-overexpressing preparations contained significantly higher ATP levels than those expressing empty controls (Fig. 6H).
The increased expression of these mitochondrial proteins, along with the functional validation of higher mitochondrial membrane potential and ATP levels, indicates a highly efficient and robust bioenergetic environment in the synaptic terminals of microglial SVCT2-overexpressing 5xFAD mice hippocampus.
3.7. Overexpressing SVCT2 in microglia after AD-like pathology establishment induced a secretome-related transcriptional signature in 5xFAD microglia
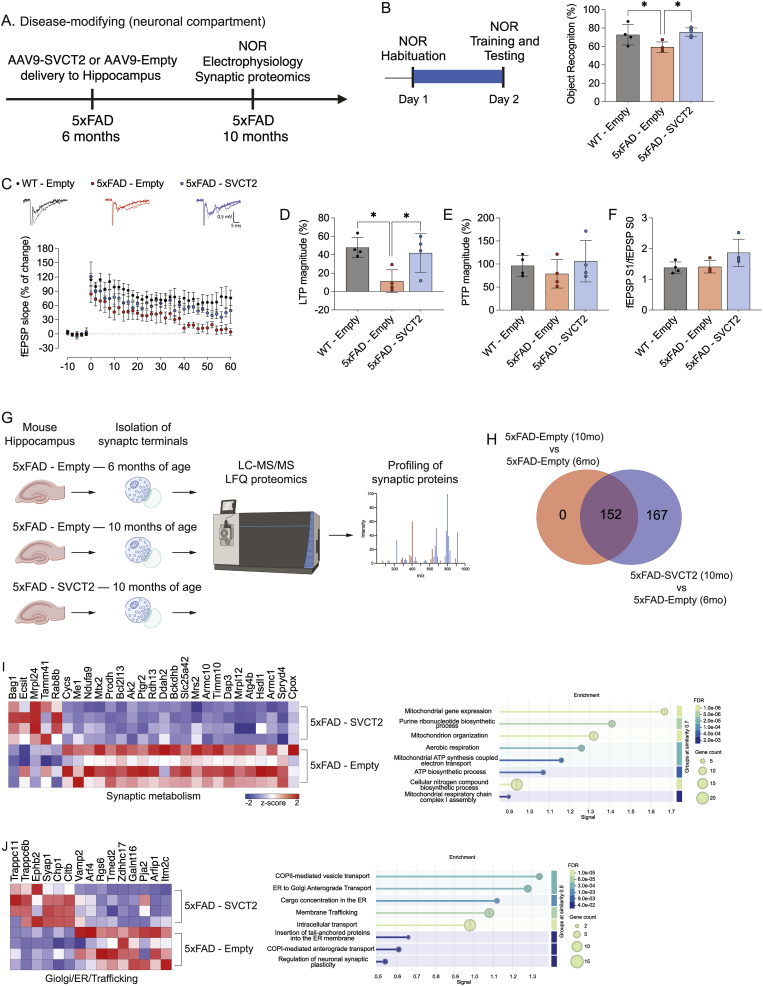
After demonstrating that SVCT2 overexpression in microglia before disease onset can prevent the synaptic and memory impairments associated with AD-like pathology, we aimed to determine whether enhancing microglial ascorbate uptake would modify the progression of AD-like pathology in 5xFAD mice. To investigate this, we targeted microglia by injecting AAV9-CD68:SVCT2 (SVCT2) or AAV9-CD68:Empty (Empty) vectors into the hippocampi of 6-month-old 5xFAD mice (a period in which amyloid pathology, synaptic dysfunction, and memory deficits are present) and conducted analyses four months later, at 10 months of age (Fig. 7A). This approach aimed to determine if microglial SVCT2 overexpression works as a disease-modifying intervention in 5xFAD mice.
Fig. 7.
Late-stage SVCT2 overexpression remodels the microglial secretory pathway without altering amyloid burden.
(A) Schematic of the disease-modifying experimental design. AAV9-SVCT2 or AAV9-Empty vectors were delivered to the hippocampus of 6-month-old 5xFAD mice, with analysis of amyloid pathology and RNAseq performed at 10 months of age.
(B) Quantitative immunofluorescence analysis of Aβ load, plaque size, and microglia plaque coverage in 10-month-old 5xFAD mice. No significant differences were observed between the 5xFAD-SVCT2 and 5xFAD-Empty groups. Data are from N = 4–5 mice per group. ns = non-significant (Unpaired t-test).
(C) Venn diagram from RNAseq analysis comparing DE genes between 10mo 5xFAD-Empty, 6mo5xFAD-Empty, and 10mo 5xFAD-SVCT2 mice. The analysis identified 1093 genes specifically modified by late-stage SVCT2 overexpression.
(D) Venn diagram and table showing minimal overlap between the 1085 genes uniquely regulated by SVCT2 (from panel C, excluding shared genes with aging) and a set of 462 classical DAM genes. The low Jaccard index (0.0051) and significant Fisher's exact test (p < 0.0001) indicate that the SVCT2-driven transcriptional program is distinct from the classical DAM signature.
(E) Heatmaps showing expression changes (z-score) of DE genes related to the microglial secretory machinery. Genes associated with the endoplasmic reticulum, Golgi, endosome, and exocytosis clusters are shown, indicating a significant reconfiguration of the secretory pathway in response to late-stage SVCT2 overexpression.
Firstly, we evaluated amyloid pathology in the hippocampi of 5xFAD mice. In the hippocampi of 5xFAD-SVCT2 mice, the BAM-10-stained area did not show a significant decrease (Fig. 7B). Additionally, there was no noticeable reduction in amyloid plaque size or increase in microglial clustering around amyloid plaques in the SVCT2 overexpressing group (Fig. 7B). These results suggest that, after disease onset, overexpressing SVCT2 in microglia does not improve amyloid pathology in the 5xFAD hippocampus.
We then profiled MACS-isolated hippocampal microglia from 5xFAD mice using RNA-seq (Suppl. Fig. 2 and Suppl. Table 3). Our analysis included three different groups: microglia from 5xFAD mice injected with either an empty vector or an SVCT2 vector at six months and evaluated at ten months of age, and microglia from 5xFAD mice injected with an empty vector at two months and assessed at six months of age. Using these three groups, we established two datasets: the first one comparing 5xFAD-Empty at 10 months of age with 5xFAD-Empty at 6 months of age (to assess regular disease progression) and another one comparing 5xFAD-SVCT2 at 10 months of age with 5xFAD-Empty at 6 months of age (to evaluate the impact of SVCT2 overexpression in disease progression) (Fig. 7C).
Our analyses, utilizing set theory and Venn diagrams, identified 1093 genes specifically modified by SVCT2 overexpression in hippocampal 5xFAD microglia and 766 genes which the modification were impaired by SVCT2 overexpression (Fig. 7C). These DE genes represent almost 25 % of the total transcriptomic changes in 5xFAD microglia after AD-like pathology manifestation (Fig. 7C). This suggests a strategic redirection of the transcriptional landscape in diseased 5xFAD microglia.
To further characterize the nature of the SVCT2-induced transcriptional shift, we compared the SVCT2-associated gene signature (1869 genes – 766 + 1093) with the canonical DAM profile (Fig. 7D). Quantitative analysis revealed that only 20 transcripts were shared between the SVCT2-regulated and DAM gene sets out of a total of 2309 unique genes (1859 + 450). This yielded a Jaccard index of 0.0051, indicative of minimal overlap (<0.5 %). Fisher's exact test further confirmed that this overlap was significantly lower than expected by chance (p < 0.0001), demonstrating a statistically robust lack of association between the two gene sets. These findings strongly suggest that SVCT2 overexpression did not impact the DAM signature when injected after disease establishment. This molecular divergence is consistent with our histopathological observations showing that SVCT2 overexpression did not mitigate amyloid burden when delivered after pathology onset, implying that at later disease stages, SVCT2 modulates microglia via mechanisms that are independent of classical DAM activation.
We next investigated which cellular pathways were most significantly affected by SVCT2 overexpression following disease establishment. Gene ontology and pathway enrichment analyses revealed a significant reorganization of genes involved in the microglial secretory machinery, particularly those associated with the endoplasmic reticulum, Golgi apparatus, endosomal system, and exocytosis (Fig. 7E).
The endoplasmic reticulum (ER) underwent extensive transcriptional remodeling. Differentially expressed transcripts included genes associated with protein processing (e.g., Pdia6, Uggt2, Os9), response to ER stress (e.g., Bok, Tmco1, Arv1, Pdia6, and Atf6), lipid metabolism (e.g., Osbpl6, Arv1), and calcium homeostasis (e.g., Tmco1 and C2Cd2). Notably, Uggt2 and Pdia6 are key components of the ER protein quality control system, while Os9 promotes the degradation of misfolded glycoproteins, collectively supporting ER homeostasis. Additional changes in C2cd2l, Arv1, and lipid-handling genes, along with the upregulation of ER stress regulators such as Atf6, Tmco1, and Nrros, suggest a broader adaptive response of the ER to SVCT2 overexpression.
Similarly, the Golgi apparatus displayed altered gene expression across its cisternae and networks. Transcriptional changes in Arfgef2, St3gal1, Yipf6, Golph3, and Fut4 reflect structural and functional remodeling of the Golgi apparatus in response to SVCT2 overexpression. These genes regulate vesicular formation, terminal glycosylation, and membrane dynamics. These adaptations suggest enhanced Golgi efficiency and vesicle trafficking, potentially supporting an altered pattern of secretion.
The endosomal-lysosomal system was also significantly affected. Transcripts involved in early endosome function (Tmem165, Egfr, Vps16) and late endosome/lysosomal pathways (Pikfyve, Lamtor4) were upregulated, implying enhanced vesicle maturation, degradation capacity, and possibly restored mTOR signaling. Changes in Slc9a9, Rab22a, and Clcn5 further suggest altered recycling endosome activity, impacting vesicle acidification, pH regulation, and intracellular trafficking.
Components of the endosomal–lysosomal system were also differentially expressed, including genes regulating vesicular acidification (Slc9a9, Clcn5), receptor recycling (Rab22a, Egfr), endosome–lysosome fusion (Vps16, Pikfyve), and nutrient sensing (Lamtor4). Tmem165, a Golgi/endosomal ion transporter required for glycosylation, was also affected, pointing to integration between secretory and degradative pathways.
These intracellular changes ultimately converged on the exocytic pathway, resulting in increased expression of genes critical for vesicle docking, fusion, and secretion. Notable among these were Syt9 (a synaptotagmin involved in calcium-triggered exocytosis), Stxbp5 (a SNARE complex regulator), and Kif1a (a kinesin motor protein for vesicle transport).
In summary, although SVCT2 overexpression after pathology onset does not seem to reduce the amyloid burden or activate DAM-like programs, it triggers a significant reorganization of the microglial secretory machinery.
3.8. After disease establishment, overexpressing SVCT2 in 5xFAD microglia rescues memory deficits and synaptic plasticity impairments
Given the critical role of microglial secreted factors in modulating synaptic plasticity and memory [50,51], we hypothesized that enhancing SVCT2 expression in microglia could ameliorate memory deficits and synaptic dysfunction, even after their manifestation in the 5xFAD mice. To test this hypothesis, we again targeted microglia by injecting AAV9-CD68 or control AAV9-CD68 vectors into the hippocampi of 6-month-old 5xFAD mice (a period in which LTP impairments and memory deficits were already established – Fig. 5). We then assessed memory performance using the NOR test and synaptic plasticity through electrophysiological recordings at ten months of age 5xFAD mice (Fig. 8A). Our results revealed that 5xFAD mice with microglial SVCT2 overexpression (5xFAD-SVCT2) exhibited significantly improved memory performance compared to the 5xFAD-Empty group (Fig. 8B). This indicates that SVCT2 overexpression in microglia not only mitigates but also rescues memory deficits even after the manifestation of AD-like pathology.
Fig. 8.
SVCT2 overexpression after disease establishment rescues memory deficits and synaptic plasticity impairments in 5xFAD mice.
(A) Schematic representation of the disease-modifying experimental design. 6-month-old 5xFAD mice received intra-hippocampal delivery of AAV9-SVCT2 or AAV9-Empty vectors, with memory, electrophysiology, and synaptic proteomics analyses conducted at 10 months of age.
(B) Quantification of the Novel Object Recognition (NOR) test showing that late SVCT2 overexpression rescues recognition memory deficits in 10-month-old 5xFAD mice compared to controls. Data are from N = 4–5 mice per group. ∗p < 0.05 (Unpaired t-test).
(C) Time course of field excitatory postsynaptic potential (fEPSP) slopes recorded from CA1 synapses, showing the induction of LTP following θ-burst stimulation. Representative fEPSP traces before (solid line) and 50–60 min after (dashed line) θ-burst stimulation are also shown. Scale bars: 5 ms (horizontal), 0.5 mV (vertical).
(D) Bar graph quantifying the LTP magnitude from (C). Data are from n = 12 slices from N = 4 mice per group. ∗p < 0.05 (One-way ANOVA).
(E) Comparison of post-tetanic potentiation (PTP) magnitudes. Data are from n = 12 slices from N = 4 mice per group.
(F) Paired-pulse facilitation (PPF) ratios were comparable between groups. Data are from n = 12 slices from N = 4 mice per group.
(G) Schematic of the proteomics workflow comparing synaptosomes from 6- and 10-month-old 5xFAD-Empty mice and 10-month-old 5xFAD-SVCT2 mice.
(H) Venn diagram illustrating the set-theoretical analysis that identified 167 synaptic proteins uniquely altered by SVCT2 overexpression in aged 5xFAD mice, independent of aging-related changes.
(I, J) Heatmap representations of differentially expressed proteins involved in synaptic metabolism and Golgi/ER/Synaptic trafficking, showing distinct profiles between 5xFAD-SVCT2 and 5xFAD-Empty groups at 10 months of age. Gene Ontology (GO) enrichment analysis of these clusters, depicted in the associated bubble plots, is also shown.
We then examined how overexpressing microglial SVCT2 affects synaptic plasticity in this disease-modifying context. We measured LTP at CA3-CA1 hippocampal synapses in 10-month-old 5xFAD mice that overexpressed SVCT2 in microglia compared to those injected with the control virus. As expected, θ-burst stimulation of Schaffer collateral pathways caused an initial increase in the fEPSP slope, which then stabilized at a plateau above the pre-stimulation baseline, indicating successful LTP induction (Fig. 8C). Unsurprisingly, WT mice injected with the control virus showed a significantly higher LTP magnitude than 5xFAD mice injected with the control virus (Fig. 8C). Importantly, the LTP magnitude was significantly higher in the 5xFAD SVCT2-injected group compared to the 5xFAD-Empty control group, demonstrating a substantial enhancement of synaptic plasticity (Fig. 8D). PTP magnitudes did not differ significantly between groups (Fig. 8E), indicating that the effect of microglial SVCT2 is more related to long-term synaptic changes than short-term synaptic changes. Furthermore, PPF assays indicated that presynaptic function related to quantal glutamate release probabilities was consistent across groups (Fig. 8F), suggesting that SVCT2 overexpression in microglia primarily affects postsynaptic mechanisms involved in long-term plasticity rather than presynaptic release probabilities. The significant improvement in memory (assessed by the NOR test) and the enhanced LTP underscore the potential of microglial SVCT2 overexpression as a disease-modifying strategy in the 5xFAD mice.
3.9. Proteomic profiling maps the disease-modifying alterations in the 5xFAD synapses following microglial SVCT2 overexpression
To understand the molecular basis of the synaptic improvements observed following microglial SVCT2 overexpression, we conducted quantitative high-throughput proteomic profiling of hippocampal synaptosomes isolated from 5xFAD mice (Fig. 8G and Suppl. Fig. 2). This analysis enabled the discrimination of protein expression changes throughout the disease course in 5xFAD hippocampus, offering crucial insight into how SVCT2 overexpression in microglia alters the synaptic trajectory in the context of AD-like pathology.
To identify the proteomic changes specifically caused by SVCT2 overexpression, we employed a similar set-theory approach described in Fig. 7C, comparing the proteomic datasets using Venn diagrams (Fig. 8H). This method revealed a subset of 167 synaptic proteins uniquely affected by microglial SVCT2 overexpression in 5xFAD mice (Fig. 8H and Suppl. Table 4). Similar to the prevention approach (Fig. 6), SVCT2 overexpression in microglia within this disease-modifying strategy also enhances synaptic metabolism networks, as shown by changes in protein associated with mitochondrial respiratory chain complex I assembly and ATP biosynthetic process (Fig. 8I).
SVCT2 overexpression in microglia also affected synaptic protein networks related to the Golgi apparatus, ER-to-Golgi vesicle trafficking, and plasticity (Fig. 8J). Key components of the presynaptic secretory apparatus, including Vamp2, Tmed2, and Arf4, all decreased following SVCT2 overexpression (Fig. 8J). Given that hyper-excitability in the 5xFAD model may drive a compensatory upregulation of presynaptic proteins [52], their reduction suggests a normalization of excessive presynaptic turnover once the local environment is buffered by SVCT2-overexpressing microglia. Concurrently, the postsynaptic scaffold may be strengthened by an enhancement in Ephb2 (Fig. 8J). EphB2 is a key modulator of NMDAR-dependent plasticity and is essential for the proper synaptic localization and function of NMDA receptors [53]. Given that EphB2 overexpression is known to rescue impaired NMDAR trafficking and cognitive dysfunction in AD models [54], this microglia SVCT2-driven upregulation suggests a direct molecular bridge to the rescued LTP magnitude we observed.
4. Discussion
Alzheimer's pathology is complex and involves microglial dysfunction, ongoing neuroinflammation, and disrupted redox balance. An important yet often overlooked factor in AD progression is the impaired ascorbate homeostasis within microglia. Recently, Prater and colleagues observed a decrease in Slc23a2 levels in microglia from post-mortem AD patients [16]. Using the 5xFAD and APP/PS1 mouse models, we confirmed and expanded these findings by showing an age-dependent decrease in microglial SVCT2 protein levels and intracellular ascorbate content. This demonstrates that reduced SVCT2 expression and subsequent ascorbate deficiency in microglia are mechanistically linked to AD development. This decline was directly related to worsening Aβ pathology and the emergence of cognitive deficits, suggesting microglial ascorbate deficiency may be a key factor driving disease progression.
Overexpressing SVCT2 in the microglia of 5xFAD mice induced a marked increase in intracellular ascorbate levels, triggering a profound redox reprogramming within these cells. This intervention extended beyond merely enhancing microglia's intrinsic antioxidant capacity; it recalibrated their redox profile. Ascorbate itself can modulate gene expression via epigenetic mechanisms, serving as a cofactor for TET enzymes and histone demethylases [8,[55], [56], [57]]. Ascorbate can also modulate the activity of transcription factors, such as CREB and NF-κβ [58,59].
Although classical NF-κβ proinflammatory targets, such as Tnf, Il1β, Il6, Nos2, and Ccl2 remained unchanged in our model, we found increased expression of some NF-κβ-related genes, including Card6, Rhoh, Casp1, Il1a, Tnip2, and Trim25. This transcriptional profile may reflect a compensatory or regulatory adaptation rather than classical NF-κβ activation. Indeed, Card6, Rhoh, and Tnip2 are well-known inhibitors of NF-κβ signaling [[60], [61], [62]]. Moreover, ascorbate-induced low-ROS conditions may favor the formation of p50:p50 and p50:c-Rel dimers over classically pro-inflammatory p65-rich species. These alternative NF-κβ complexes are known to preferentially drive anti-apoptotic, antioxidant, and lysosomal gene programs [[63], [64], [65]]. Furthermore, the concurrent stabilization of Nrf2 by ascorbate [[66], [67], [68]] likely creates a synergistic interplay, where Nrf2 and specific NF-κβ complexes co-regulate genes involved in redox homeostasis and detoxification [69], contributing to the observed redox recalibration.
This redox recalibration sculpted a unique and neuroprotective microglial activation state—a "hybrid" phenotype. These "hybrid" microglia co-express DAM-associated markers, indicating their active engagement with Aβ and key homeostatic factors. The "hybrid" microglial state is further supported by ascorbate's role as a cofactor for TET and JmjC dioxygenases, which may maintain the epigenetic accessibility of homeostatic loci, even as DAM-related enhancers simultaneously gain activating marks. This suggests that NF-κβ functions on an epigenetically permissive landscape, where both homeostatic and DAM-response genes remain accessible, producing the mixed yet highly neuroprotective "hybrid" signature we observed. Indeed, this unique "hybrid" microglial state correlates with significantly improved interaction with Aβ plaques, resulting in a substantial reduction in amyloid burden in 5xFAD mice—a neuroprotective effect further validated in the APP/PS1 mouse model.
The neuroprotective efficacy of this early SVCT2 intervention translated directly into the preservation of synaptic integrity, the prevention of LTP impairment, and a robust blockade of memory deficits in the 5xFAD mice. Mechanistically, these benefits were rooted in the remodeling of the synaptic mitochondrial proteome. Our data indicate that early SVCT2-mediated microglial support fostered an environment conducive to synaptic mitochondrial resilience. This resulted in enhanced synaptic mitochondrial bioenergetic capacity, evidenced by increased ATP production. Such bolstering of neuronal energy supply is essential for maintaining neuronal resilience and synaptic function against an ongoing neurodegenerative process [[70], [71], [72], [73]].
In contrast to the outcomes of early intervention, overexpressing SVCT2 in microglia after significant AD-like pathology was already established yielded a negligible impact on the existing, dense amyloid burden. This observation highlights the stage-dependent nature of microglial therapeutic responses, suggesting that late-stage plaques may be less amenable to microglial engagement due to their advanced maturation, or that ascorbate-replete microglia at this advanced stage prioritize alternative, non-amyloid driven protective functions. However, despite this lack of decreased amyloid pathology, late-stage SVCT2 intervention still significantly rescued synaptic plasticity, resulting in a robust improvement of memory performance. This dissociation between plaque reduction and cognitive improvement suggests alternative mechanisms elicited by late-stage ascorbate enhancement in microglia.
Transcriptomic analysis of microglia from these late-stage interventions revealed a distinct mechanistic signature underlying the cognitive rescue. Instead of driving the "hybrid" phenotype or overtly enhancing engulfment pathways, late-stage SVCT2 overexpression appeared to rewire microglial secretory functions. This involved alterations in pathways associated with the ER, Golgi apparatus, endosomal system, and exocytotic machinery. Given ascorbate's crucial role as an enzyme cofactor for prolyl and lysyl hydroxylases, which are vital for proper protein folding and maturation in the ER, enhanced intracellular ascorbate likely optimizes ER proteostasis, mitigates ER stress (a common feature in aged or diseased microglia [74]), and improves the fidelity and efficiency of protein synthesis and secretion. This suggests that at more advanced disease stages, SVCT2-mediated ascorbate enhancement shifts microglial therapeutic action primarily towards modulating their secretome. Thus, this intervention likely reshapes the neuronal microenvironment to support synaptic health and cognitive processes , independent of large-scale amyloid plaque removal. This mode of action, where microglia exert neuroprotection through paracrine signaling rather than directly tackling the amyloid burden, resonates with findings in other therapeutic contexts that demonstrate cognitive benefits without substantial clearance of aggregated proteins [[75], [76], [77], [78], [79]].
Further supporting this mechanism, proteomic profiling of synaptosomes from these late-stage interventions revealed a coordinated and beneficial remodeling of the synaptic proteome. This included changes in proteins crucial to postsynaptic signaling cascades and plasticity (e.g., components and regulators of glutamate receptor signaling complexes, such as AMPAR and NMDAR subunits or their trafficking proteins). These molecular alterations at the synapse indicate that late microglial ascorbate repletion promotes synaptic resilience and enhanced function by remodeling the postsynaptic molecular machinery.
Our findings provide a mechanistic explanation for the limited effectiveness of dietary vitamin C interventions in AD clinical trials. The core issue is not simply a deficiency of vitamin C in the diet, but rather a complex, multi-stage failure of biological transport that hinders this antioxidant from reaching the brain cells that require it the most. The initial bottleneck occurs at the level of intestinal absorption, mediated by SVCT1. This transport system reaches its maximum capacity at moderate dietary intakes, typically around 200–400 mg per day [80]. As a result, plasma vitamin C concentrations plateau, making oral megadoses (over 1 g per day) ineffective in significantly elevating CNS ascorbate levels beyond their natural saturation points [[81], [82], [83]].
This limitation at the gut is further compounded within the brain itself by a disease-induced failure of cellular ascorbate uptake. Our results revealed that SVCT2 expression and ascorbate levels are significantly decreased in diseased microglia. This downregulation of the transporter creates a paradoxical state of cellular ascorbate starvation, even when systemic plasma levels of vitamin C are ostensibly adequate.
Our data also provide an explanation for the consistent clinical observation that individuals with AD have markedly reduced vitamin C concentrations in their CSF and brain cortex, often 30 to 70 percent lower than those of healthy controls, despite maintaining normal dietary intake [14]. The tissue-specific deficiency results not from poor nutrition but as a direct consequence of the disease pathology, which includes both impaired uptake and increased oxidative consumption. The clinical significance of this is illustrated by findings that plasma ascorbate levels decrease in direct proportion to the severity of cognitive impairment. For instance, individuals with dementia are nearly three times more likely to have suboptimal plasma vitamin C concentrations than cognitively healthy controls, despite comparable dietary intakes [84]. Meta-analyses and systematic reviews further support that plasma and brain vitamin C levels are consistently lower in people with AD, which is potentially associated with faster disease progression [[85], [86], [87]].
This SVCT2-centric perspective also reconciles a long-standing discrepancy between observational studies, which often associate higher vitamin C levels with better cognitive function, and vitamin C supplementation clinical trials, which have shown inconsistent and mostly disappointing results. The protective links observed in epidemiological data likely occur during a pre-symptomatic phase, when SVCT2 expression and function are still intact, allowing for effective cellular uptake. However, in later stages of the disease, when the underlying AD pathology has reduced SVCT2 on the plasma membrane, thus decreasing vitamin C uptake, clinical trials are likely to fail, making increased systemic vitamin C from supplements largely ineffective.
Therefore, our results reframe cellular vitamin C deficiency in AD not only as a nutritional issue but also as a complex outcome of a disease-driven breakdown in cellular uptake and transport. This view also suggests that the ability of vulnerable brain cells to import and utilize ascorbate via SVCT2, rather than systemic availability alone, is the actual bottleneck and the key to future therapeutic intervention.
Although our findings provide compelling evidence for the therapeutic potential of targeting microglial SVCT2 in AD, our study has several caveats. Firstly, our investigations were conducted using transgenic mouse models of AD (5xFAD and APP/PS1). Although these models replicate key aspects of human AD pathology, such as amyloid deposition and cognitive deficits, they do not encompass the full spectrum of the human disease, including the significant heterogeneity and common co-morbidities observed in patients. Secondly, while the use of AAV9-CD68 vectors for microglia-specific overexpression is effective, there are translational considerations. The enduring safety, potential immunogenic responses, and optimal methods for administering AAV-based treatments in the human brain necessitate more in-depth investigation. Thirdly, although our transcriptomic and proteomic analyses offered valuable insights into the molecular changes caused by SVCT2 overexpression, a more granular understanding of the specific redox-sensitive signaling pathways and the exact composition of the altered microglial secretome is necessary. Ultimately, the long-term efficacy of SVCT2 modulation, especially in the context of late-stage interventions, requires more thorough research. Although we observed a robust improvement in cognitive function, it is crucial to assess how long these benefits last and if they can stop or even reverse the progression of neurodegenerative diseases over extended periods.
In conclusion, targeted enhancement of ascorbate uptake through microglial SVCT2 overexpression demonstrated significant preclinical potential as a therapeutic strategy for mitigating and modifying AD-like pathology. Early SVCT2 intervention induced substantial redox reprogramming, creating a hybrid microglial state that harmonized DAM-like responses with homeostatic functions, resulting in a reduced amyloid burden, improved synaptic mitochondrial health, and prevention of cognitive decline. In contrast, late-stage SVCT2 intervention, although not reducing existing amyloid pathology, effectively rescued synaptic plasticity and mitigated memory deficits by modulating the microglial secretome and positively impacting the postsynaptic proteome. Overall, our findings emphasize the crucial role of cellular ascorbate uptake mechanisms in the context of AD pathogenesis and highlight SVCT2 as a novel, druggable target for neurodegeneration.
5. Conclusions
Our findings demonstrate that AAV9-mediated overexpression of SVCT2 in microglia effectively delays the onset and modifies the progression of AD-like pathology in mouse models. By enhancing ascorbate uptake, SVCT2 overexpression improves microglial function, reduces amyloid burden when applied before disease onset, and restores synaptic plasticity and memory performance even after disease establishment. These results highlight the therapeutic potential of targeting SVCT2-mediated ascorbate uptake in microglia as a novel antioxidant strategy for neurodegeneration.
CRediT authorship contribution statement
Camila C. Portugal: Writing – review & editing, Writing – original draft, Project administration, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Evelyn C.S. Santos: Methodology, Formal analysis. Ana Monteiro-Pacheco: Methodology, Formal analysis. Sara Costa-Pinto: Methodology, Formal analysis. Tiago O. Almeida: Visualization, Methodology, Data curation. Joana Tedim-Moreira: Investigation, Formal analysis, Data curation. Dora Gavin: Methodology. Teresa Canedo: Validation, Methodology. Fabiana Oliveira: Visualization, Methodology. Isabel Cardoso: Resources. Teresa Summavielle: Resources, Formal analysis, Data curation. Sandra H. Vaz: Writing – original draft, Resources, Investigation, Formal analysis. Renato Socodato: Writing – review & editing, Writing – original draft, Validation, Funding acquisition, Conceptualization. João B. Relvas: Writing – review & editing, Writing – original draft, Resources, Conceptualization.
Ethics approval and consent to participate
All animal experiments were conducted in accordance with the European Union Directive 2010/63/EU and were approved by the Ethics Committee for Animal Experimentation of the University of Porto and the Portuguese National Authority for Animal Health (Direção-Geral de Alimentação e Veterinária), under the license reference number 2022-02-18 003669.
Consent for publication
Not applicable.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This work was funded by Portuguese funds through FCT - Fundação para a Ciência e a Tecnologia under framework of the project 2024.05324.RESTART and EXPL/MED-NEU/0588/2021. FCT through Fundos do Orçamento de Estado also supported the work realized at CCUL and GIMM by Sandra H. Vaz and Sara Costa-Pinto under the project PTDC/BTM-SAL/32147/2017. 2021 International Society for Neurochemistry (ISN) Career Development Grant funded the work realized at CCUL and GIMM by Sandra H. Vaz. H2020-WIDESPREAD-05-2017-Twinning (EpiEpinet) under grant agreement No. 952455 funded the work realized at CCUL and GIMM by Sandra H. Vaz and Sara Costa-Pinto. This work was funded (in part) by Programa Operacional Regional do Norte and co-funded by the European Regional Development Fund under the project "The Porto Comprehensive Cancer Center" with the reference NORTE-01-0145-FEDER-072678 - Consórcio PORTO.CCC – Porto.Comprehensive Cancer Center.
Camila C. Portugal held employment contracts financed by national funds through FCT - in the context of the program-contract described in paragraphs 4, 5, and 6 of art. 23 of Law no. 57/2016, of August 29, as amended by Law no. 57/2017 of July 2019 - DL 57/2016/CP1355/CT0025.Renato Socodato held an employment contract funded by FCT (2023.06380.CEECIND) Sara Costa-Pinto, Tiago O. Almeida, and Joana Tedim-Moreira hold Ph.D. fellowships funded by FCT (SFRH/BD/147277/2019, SFRH/BD/147981/2019 and UI/BD/151552/2021, respectively).
Declaration of competing interest
All the authors declare no conflict of interest related to this manuscript.
Acknowledgments
The authors acknowledge the support of the i3S Scientific Platform, HEMS - Histology and Electron Microscopy Service and ALM - Advanced Light Microscopy, both members of the national infrastructure PPBI - Portuguese Platform of Bioimaging (PPBI–POCI-01-0145-FEDER-022122). We Also acknowledge the support of the i3S Proteomics platform, a member of the Portuguese Mass Spectrometry Network, integrated into the National Roadmap of Research Infrastructures of Strategic Relevance (ROTEIRO/0028/2013; LISBOA-01-0145-FEDER-022125). The authors also acknowledge the support of the Genomics, Animal Facility, and Translational Cytometry i3S Scientific Platform. The authors also thank the Rodent Facility of Instituto de Medicina Molecular – João Lobo Antunes (IMM) for supporting this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2025.103851.
Contributor Information
Camila C. Portugal, Email: camila.portugal@i3s.up.pt.
Renato Socodato, Email: renato.socodato@ibmc.up.pt.
João B. Relvas, Email: jrelvas@i3s.up.pt.
Abbreviation List:
5xFAD – Five Familial Alzheimer's Disease Mutations (mouse model); Aβ – Amyloid Beta; Aβ1–42 – Amyloid Beta 1–42; AβPP – Amyloid Beta Precursor Protein; AβPPswe – Amyloid Beta Precursor Protein Swedish Mutation; AAV – Adeno-Associated Virus; ACN – Acetonitrile; AD – Alzheimer's Disease; AGC – Automatic Gain Control; APP – Amyloid Precursor Protein; APP/PS1 – Amyloid Precursor Protein/Presenilin 1 (mouse model); APV – Aminophosphonovaleric Acid; aCSF – Artificial Cerebrospinal Fluid; BAM-10 – Beta Amyloid Monoclonal Antibody 10; BCA – Bicinchoninic Acid; BSA – Bovine Serum Albumin; Bok – BCL2-Related Ovarian Killer Protein; CA1 – Cornu Ammonis 1 (hippocampal region); CD11b – Cluster of Differentiation 11b; CD16/CD32 – Cluster of Differentiation 16/32 (Fc Receptors); CD68 – Cluster of Differentiation 68; CNS – Central Nervous System; COX – Cytochrome c Oxidase; CyCS – Cytochrome c, Somatic; DAPI – 4′,6-Diamidino-2-Phenylindole; DD – Data-Dependent; DNA – Deoxyribonucleic Acid; DTT – Dithiothreitol; EDTA – Ethylenediaminetetraacetic Acid; EGFR – Epidermal Growth Factor Receptor; ELISA – Enzyme-Linked Immunosorbent Assay; ER – Endoplasmic Reticulum; ESI – Electrospray Ionization; ETC – Electron Transport Chain; FA – Formic Acid; FAD – Familial Alzheimer's Disease; fEPSP – Field Excitatory Postsynaptic Potential; FDR – False Discovery Rate; FoxO – Forkhead Box O; GM-CSF – Granulocyte-Macrophage Colony-Stimulating Factor; GO – Gene Ontology; GrpE – Gro-P Like Protein E; HBSS – Hank's Balanced Salt Solution; HCD – Higher-energy Collisional Dissociation; HEPES – (4-(2-Hydroxyethyl)-1-Piperazineethanesulfonic Acid); HIF-1 – Hypoxia-Inducible Factor 1; HPLC – High-Performance Liquid Chromatography; HSPA1L – Heat Shock Protein Family A Member 1 Like; HSPe1 – 10 kDa Heat Shock Protein, Mitochondrial; Iba1 – Ionized Calcium Binding Adaptor Molecule 1; IFN-γ – Interferon Gamma; IL-1β – Interleukin 1 Beta; IL-6 – Interleukin 6; I/O – Input/Output; IRES – Internal Ribosome Entry Site; LAMTOR4 – Late Endosomal/Lysosomal Adaptor, MAPK and MTOR Activator 4; LC-MS/MS – Liquid Chromatography–Tandem Mass Spectrometry; LFQ – Label-Free Quantification; LPS – Lipopolysaccharide; LTP – Long-Term Potentiation; MACS – Magnetic-Activated Cell Sorting; MAPK – Mitogen-Activated Protein Kinase; mCherry – Monomeric Cherry Fluorescent Protein; ME3 – Malic Enzyme 3; MRPL28 – Mitochondrial Ribosomal Protein L28; MWM – Morris Water Maze; NADH – Nicotinamide Adenine Dinucleotide (reduced form); NF-κB – Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells; NOX2 – NADPH Oxidase 2; NOR – Novel Object Recognition; NQO1 – NAD(P)H Quinone Dehydrogenase 1; Nrf2 – Nuclear Factor Erythroid 2–Related Factor 2; OCT – Optimal Cutting Temperature; Osbpl6 – Oxysterol Binding Protein-Like 6; PBS – Phosphate-Buffered Saline; PCR – Polymerase Chain Reaction; PDIA6 – Protein Disulfide Isomerase Family A Member 6; PFA – Paraformaldehyde; PI3Kγ – Phosphoinositide 3-Kinase Gamma; PPF – Paired-Pulse Facilitation; PS1 – Presenilin 1; PSEN1 – Presenilin 1 Gene; PSFV – Presynaptic Fiber Volley; PTP – Post-Tetanic Potentiation; qRT-PCR – Quantitative Reverse Transcription Polymerase Chain Reaction; Rab32 – Member of RAS Oncogene Family; RNAseq – RNA Sequencing; ROS – Reactive Oxygen Species; RT – Reverse Transcriptase; Sdhd – Succinate Dehydrogenase Complex Subunit D; Slc37a2 – Solute Carrier Family 37 Member 2; SVCT2 – Sodium-Dependent Vitamin C Transporter 2; SyT9 – Synaptotagmin 9; TCA – Tricarboxylic Acid (cycle); TBS – Tris-Buffered Saline; Tmco1 – Transmembrane and Coiled-Coil Domains 1; TNF-α – Tumor Necrosis Factor Alpha; UGGT2 – UDP-Glucose Glycoprotein Glucosyltransferase 2; UQCRH – Ubiquinol-Cytochrome c Reductase Hinge Protein; UQCRQ – Ubiquinol-Cytochrome c Reductase Complex III Subunit VII; VPS16 – Vacuolar Protein Sorting 16 Homolog; WT – Wild-Type; ΔΨm – Mitochondrial Membrane Potential.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Validation of AAV9-CD68 vector specificity and SVCT2 overexpression.
(A) Representative confocal images from the hippocampus of 6-month-old 5xFAD mice injected with AAV9-CD68 vectors expressing mCherry (red). The images show co-localization of mCherry with the microglial marker Iba1 (green), but not with the astrocyte marker GFAP (white) or the neuronal marker NeuN (white), confirming the microglia-specific transduction of the vector.
(B) Quantification of the percentage of mCherry-positive cells that are also positive for Iba1, NeuN, or GFAP. The analysis confirms that the vast majority of transduced cells are Iba1-positive microglia.
(C) Quantification of SVCT2 mRNA levels in sorted mCherry-positive microglia from the hippocampi of 6-month-old 5xFAD-SVCT2 and 5xFAD-Empty mice, showing a significant increase in SVCT2 transcripts in the treatment group. Data are shown as mean ± SEM
(D) Flow cytometry data quantifying the median fluorescence intensity (MFI) of SVCT2 protein in transduced (mCherry+) microglia from 6-month-old 5xFAD mice, confirming higher protein expression in the 5xFAD-SVCT2 group compared to controls.
(E) Validation of lentivirus-mediated SVCT2 overexpression in primary rat microglial cultures (DIV 5). Representative immunofluorescence images show SVCT2 (red) and the viral reporter GFP (green). Nuclei are stained with DAPI (blue). The bar graph quantifies SVCT2 MFI in GFP-positive cells, confirming successful overexpression. Data represent N = 3 independent cultures. ∗∗∗∗p < 0.0001 (Unpaired t-test).
Principal Component Analysis (PCA) of transcriptomic and proteomic datasets.
Principal Component Analysis (PCA) mapping of transcriptomic (RNAseq) and proteomic data from hippocampal samples. The plots show clustering between the 5xFAD-SVCT2 and 5xFAD-Empty experimental groups for both the early intervention (6 months of age) and late intervention (10 months of age) time points.
Transduced microglia cluster around amyloid deposits in the 5xFAD hippocampus. Representative confocal micrographs from 6-month-old 5xFAD mice injected at 2 months with AAV9-CD68:Empty-mCherry (top row) or AAV9-CD68:SVCT2-IRES-mCherry (bottom row) show single channels for mCherry (transduced cells), Iba1 (microglia), and BAM-10 (Aβ deposits), plus the corresponding merge. Images illustrate that mCherry+/Iba1+ microglia are positioned adjacent to and encircle BAM-10+ deposits in both groups. Scale bar, 20 μm.
SVCT2 overexpression ameliorates pathology in the APP/PS1 mouse model.
(A) Flow cytometry analysis showing reduced SVCT2 expression (% SVCT2+ microglia) in microglia from 4-month-old APP/PS1 mice compared to age-matched wild-type (WT) controls.
(B) Schematic of the experimental design. 4-month-old APP/PS1 mice received intra-hippocampal injections of AAV9-SVCT2 or AAV9-Empty, with analyses performed at 9 months of age.
(C) Representative immunofluorescence images and quantification showing a significant reduction in amyloid plaque burden (BAM-10 staining, red) in the hippocampus of 9-month-old APP/PS1-SVCT2 mice compared to controls.
(D) ELISA quantification of soluble and insoluble Aβ1-42 levels in hippocampal lysates from 9-month-old APP/PS1 mice.
(E) Representative images and quantification of synaptic puncta (vGlut1, green; PSD95, red) in the hippocampus of 9-month-old APP/PS1 mice. SVCT2 overexpression significantly increased the density of co-localized synaptic puncta. For all panels, data are represented as mean ± SEM from N = 4–5 mice per group. ∗p < 0.05 (Unpaired t-test).
Quality control of synaptosome preparations.
Western blot analysis validating the enrichment of synaptosomal fractions isolated from the hippocampi of 6- and 10-month-old mice. Blots were probed for the presynaptic vesicular glutamate transporter 1 (vGlut1) and the postsynaptic density protein 95 (PSD95). Both vGlut1 and PSD95 are highly enriched in the synaptosome fraction compared to the cytosolic fraction.
Data availability
Data is available from the corresponding author upon reasonable request.
References
- 1.Rabinovici G.D. Late-onset alzheimer disease. Continuum. 2019;25(1):14–33. doi: 10.1212/con.0000000000000700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teri L., Ferretti L.E., Gibbons L.E., Logsdon R.G., McCurry S.M., Kukull W.A., McCormick W.C., Bowen J.D., Larson E.B. Anxiety in Alzheimer's Disease: prevalence and comorbidity. J. Gerontol.: Series A. 1999;54(7):M348–M352. doi: 10.1093/gerona/54.7.M348. [DOI] [PubMed] [Google Scholar]
- 3.Butterfield D.A., Reed T., Newman S.F., Sultana R. Roles of amyloid beta-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer's disease and mild cognitive impairment. Free Radic. Biol. Med. 2007;43(5):658–677. doi: 10.1016/j.freeradbiomed.2007.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nunomura A., Perry G., Aliev G., Hirai K., Takeda A., Balraj E.K., Jones P.K., Ghanbari H., Wataya T., Shimohama S., Chiba S., Atwood C.S., Petersen R.B., Smith M.A. Oxidative damage is the earliest event in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2001;60(8):759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 5.Chang Y.T., Chang W.N., Tsai N.W., Huang C.C., Kung C.T., Su Y.J., Lin W.C., Cheng B.C., Su C.M., Chiang Y.F., Lu C.H. The roles of biomarkers of oxidative stress and antioxidant in Alzheimer's disease: a systematic review. BioMed Res. Int. 2014 doi: 10.1155/2014/182303. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rinaldi P., Polidori M.C., Metastasio A., Mariani E., Mattioli P., Cherubini A., Catani M., Cecchetti R., Senin U., Mecocci P. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer's disease. Neurobiol. Aging. 2003;24(7):915–919. doi: 10.1016/S0197-4580(03)00031-9. [DOI] [PubMed] [Google Scholar]
- 7.Schippling S., Kontush A., Arlt S., Buhmann C., Stürenburg H.J., Mann U., Müller-Thomsen T., Beisiegel U. Increased lipoprotein oxidation in Alzheimer's disease. Free Radic. Biol. Med. 2000;28(3):351–360. doi: 10.1016/s0891-5849(99)00247-6. [DOI] [PubMed] [Google Scholar]
- 8.Portugal C.C. Ascorbate and its transporter SVCT2: the dynamic duo's integrated roles in CNS neurobiology and pathophysiology. Free Radic. Biol. Med. 2024;212:448–462. doi: 10.1016/j.freeradbiomed.2023.12.040. [DOI] [PubMed] [Google Scholar]
- 9.Guo Y.E., Suo N., Cui X., Yuan Q., Xie X. Vitamin C promotes oligodendrocytes generation and remyelination. Glia. 2018;66(7):1302–1316. doi: 10.1002/glia.23306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mun G.H., Kim M.J., Lee J.H., Kim H.J., Chung Y.H., Chung Y.B., Kang J.S., Hwang Y.I., Oh S.H., Kim J.G. Immunohistochemical study of the distribution of sodium-dependent vitamin C transporters in adult rat brain. J. Neurosci. Res. 2006;83(5):919–928. doi: 10.1002/jnr.20751. [DOI] [PubMed] [Google Scholar]
- 11.Portugal C.C., Socodato R., Canedo T., Silva C.M., Martins T., Coreixas V.S., Loiola E.C., Gess B., Rohr D., Santiago A.R., Young P., Minshall R.D., Paes-de-Carvalho R., Ambrosio A.F., Relvas J.B. Caveolin-1-mediated internalization of the vitamin C transporter SVCT2 in microglia triggers an inflammatory phenotype. Sci. Signal. 2017;10(472) doi: 10.1126/scisignal.aal2005. [DOI] [PubMed] [Google Scholar]
- 12.Sotiriou S., Gispert S., Cheng J., Wang Y., Chen A., Hoogstraten-Miller S., Miller G.F., Kwon O., Levine M., Guttentag S.H., Nussbaum R.L. Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nat. Med. 2002;8(5):514–517. doi: 10.1038/nm0502-514. [DOI] [PubMed] [Google Scholar]
- 13.Cao B., Xia Y., Cai Z., Wang Z., Tang C., Song Y. Construction of a brain-specific SLC23A2 gene knockout Mice model. Neuroscience. 2023;524:137–148. doi: 10.1016/j.neuroscience.2023.05.023. [DOI] [PubMed] [Google Scholar]
- 14.Bowman G.L. Ascorbic acid, cognitive function, and Alzheimer's disease: a current review and future direction. Biofactors. 2012;38(2):114–122. doi: 10.1002/biof.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monacelli F., Acquarone E., Giannotti C., Borghi R., Nencioni A. Vitamin C, aging and Alzheimer's Disease. Nutrients. 2017;9(7) doi: 10.3390/nu9070670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prater K.E., Green K.J., Mamde S., Sun W., Cochoit A., Smith C.L., Chiou K.L., Heath L., Rose S.E., Wiley J., Keene C.D., Kwon R.Y., Snyder-Mackler N., Blue E.E., Logsdon B., Young J.E., Shojaie A., Garden G.A., Jayadev S. Human microglia show unique transcriptional changes in Alzheimer's disease. Nat Aging. 2023;3(7):894–907. doi: 10.1038/s43587-023-00424-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Efthymiou A.G., Goate A.M. Late onset Alzheimer's disease genetics implicates microglial pathways in disease risk. Mol. Neurodegener. 2017;12(1) doi: 10.1186/s13024-017-0184-x. 43-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sala Frigerio C., Wolfs L., Fattorelli N., Thrupp N., Voytyuk I., Schmidt I., Mancuso R., Chen W.T., Woodbury M.E., Srivastava G., Moller T., Hudry E., Das S., Saido T., Karran E., Hyman B., Perry V.H., Fiers M., De Strooper B. The Major risk factors for Alzheimer's Disease: age, sex, and genes modulate the microglia response to abeta plaques. Cell Rep. 2019;27(4):1293–1306.e1296. doi: 10.1016/j.celrep.2019.03.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keren-Shaul H., Spinrad A., Weiner A., Matcovitch-Natan O., Dvir-Szternfeld R., Ulland T.K., David E., Baruch K., Lara-Astaiso D., Toth B., Itzkovitz S., Colonna M., Schwartz M., Amit I. A unique microglia type associated with restricting development of Alzheimer's Disease. Cell. 2017;169(7):1276–1290.e1217. doi: 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Krasemann S., Madore C., Cialic R., Baufeld C., Calcagno N., El Fatimy R., Beckers L., O'Loughlin E., Xu Y., Fanek Z., Greco D.J., Smith S.T., Tweet G., Humulock Z., Zrzavy T., Conde-Sanroman P., Gacias M., Weng Z., Chen H.…Butovsky O. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity. 2017;47(3):566–581 e569. doi: 10.1016/j.immuni.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sobue A., Komine O., Hara Y., Endo F., Mizoguchi H., Watanabe S., Murayama S., Saito T., Saido T.C., Sahara N., Higuchi M., Ogi T., Yamanaka K. Microglial gene signature reveals loss of homeostatic microglia associated with neurodegeneration of Alzheimer's disease. Acta Neuropathologica Communications. 2021;9(1):1. doi: 10.1186/s40478-020-01099-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oakley H., Cole S.L., Logan S., Maus E., Shao P., Craft J., Guillozet-Bongaarts A., Ohno M., Disterhoft J., Van Eldik L., Berry R., Vassar R. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J. Neurosci. 2006;26(40):10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forner S., Kawauchi S., Balderrama-Gutierrez G., Kramár E.A., Matheos D.P., Phan J., Javonillo D.I., Tran K.M., Hingco E., da Cunha C., Rezaie N., Alcantara J.A., Baglietto-Vargas D., Jansen C., Neumann J., Wood M.A., MacGregor G.R., Mortazavi A., Tenner A.J.…Green K.N. Systematic phenotyping and characterization of the 5xFAD mouse model of Alzheimer's disease. Sci. Data. 2021;8(1):270. doi: 10.1038/s41597-021-01054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borchelt D.R., Ratovitski T., van Lare J., Lee M.K., Gonzales V., Jenkins N.A., Copeland N.G., Price D.L., Sisodia S.S. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant Presenilin 1 and amyloid precursor proteins. Neuron. 1997;19(4):939–945. doi: 10.1016/S0896-6273(00)80974-5. [DOI] [PubMed] [Google Scholar]
- 25.Socodato R., Almeida T.O., Portugal C.C., Santos E.C.S., Tedim-Moreira J., Galvão-Ferreira J., Canedo T., Baptista F.I., Magalhães A., Ambrósio A.F., Brakebusch C., Rubinstein B., Moreira I.S., Summavielle T., Pinto I.M., Relvas J.B. Microglial Rac1 is essential for experience-dependent brain plasticity and cognitive performance. Cell Rep. 2023;42(12) doi: 10.1016/j.celrep.2023.113447. [DOI] [PubMed] [Google Scholar]
- 26.Socodato R., Henriques J.F., Portugal C.C., Almeida T.O., Tedim-Moreira J., Alves R.L., Canedo T., Silva C., Magalhaes A., Summavielle T., Relvas J.B. Daily alcohol intake triggers aberrant synaptic pruning leading to synapse loss and anxiety-like behavior. Sci. Signal. 2020;13(650) doi: 10.1126/scisignal.aba5754. [DOI] [PubMed] [Google Scholar]
- 27.Socodato R., Portugal C.C., Canedo T., Rodrigues A., Almeida T.O., Henriques J.F., Vaz S.H., Magalhaes J., Silva C.M., Baptista F.I., Alves R.L., Coelho-Santos V., Silva A.P., Paes-de-Carvalho R., Magalhaes A., Brakebusch C., Sebastiao A.M., Summavielle T., Ambrosio A.F., Relvas J.B. Microglia dysfunction caused by the loss of rhoa disrupts neuronal physiology and leads to neurodegeneration. Cell Rep. 2020;31(12) doi: 10.1016/j.celrep.2020.107796. [DOI] [PubMed] [Google Scholar]
- 28.Friard O., Gamba M. BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 2016;7(11):1325–1330. doi: 10.1111/2041-210X.12584. [DOI] [Google Scholar]
- 29.Aschauer D.F., Kreuz S., Rumpel S. Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0076310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grace P.M., Strand K.A., Galer E.L., Urban D.J., Wang X., Baratta M.V., Fabisiak T.J., Anderson N.D., Cheng K., Greene L.I., Berkelhammer D., Zhang Y., Ellis A.L., Yin H.H., Campeau S., Rice K.C., Roth B.L., Maier S.F., Watkins L.R. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc. Natl. Acad. Sci. U. S. A. 2016;113(24):E3441–E3450. doi: 10.1073/pnas.1602070113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grace P.M., Wang X., Strand K.A., Baratta M.V., Zhang Y., Galer E.L., Yin H., Maier S.F., Watkins L.R. DREADDed microglia in pain: implications for spinal inflammatory signaling in male rats. Exp. Neurol. 2018;304:125–131. doi: 10.1016/j.expneurol.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Carroll S.J., Cook W.H., Young D. AAV targeting of glial cell types in the central and peripheral nervous System and relevance to human gene therapy. Front. Mol. Neurosci. 2021;13 doi: 10.3389/fnmol.2020.618020. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosario A.M., Cruz P.E., Ceballos-Diaz C., Strickland M.R., Siemienski Z., Pardo M., Schob K.-L., Li A., Aslanidi G.V., Srivastava A., Golde T.E., Chakrabarty P. Microglia-specific targeting by novel capsid-modified AAV6 vectors. Molecular Therapy Methods & Clinical Development. 2016;3 doi: 10.1038/mtm.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galatro T.F., Vainchtein I.D., Brouwer N., Boddeke E., Eggen B.J.L. Isolation of microglia and immune infiltrates from mouse and primate central nervous system. Methods Mol. Biol. 2017;1559:333–342. doi: 10.1007/978-1-4939-6786-5_23. [DOI] [PubMed] [Google Scholar]
- 35.Abreu D.S., Gomes J.I., Ribeiro F.F., Diógenes M.J., Sebastião A.M., Vaz S.H. Astrocytes control hippocampal synaptic plasticity through the vesicular-dependent release of D-serine. Front. Cell. Neurosci. 2023;17 doi: 10.3389/fncel.2023.1282841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rei N., Rombo D.M., Ferreira M.F., Baqi Y., Müller C.E., Ribeiro J.A., Sebastião A.M., Vaz S.H. Hippocampal synaptic dysfunction in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis: reversal by adenosine A(2A)R blockade. Neuropharmacology. 2020;171 doi: 10.1016/j.neuropharm.2020.108106. [DOI] [PubMed] [Google Scholar]
- 37.Anderson W.W., Collingridge G.L. The LTP program: a data acquisition program for on-line analysis of long-term potentiation and other synaptic events. J. Neurosci. Methods. 2001;108(1):71–83. doi: 10.1016/s0165-0270(01)00374-0. [DOI] [PubMed] [Google Scholar]
- 38.Albensi B.C., Oliver D.R., Toupin J., Odero G. Electrical stimulation protocols for hippocampal synaptic plasticity and neuronal hyper-excitability: are they effective or relevant? Exp. Neurol. 2007;204(1):1–13. doi: 10.1016/j.expneurol.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 39.Korogod N., Lou X., Schneggenburger R. Posttetanic potentiation critically depends on an enhanced Ca(2+) sensitivity of vesicle fusion mediated by presynaptic PKC. Proc. Natl. Acad. Sci. U. S. A. 2007;104(40):15923–15928. doi: 10.1073/pnas.0704603104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Habets R.L., Borst J.G. Dynamics of the readily releasable pool during post-tetanic potentiation in the rat calyx of held synapse. J. Physiol. 2007;581(Pt 2):467–478. doi: 10.1113/jphysiol.2006.127365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Canedo T., Portugal C.C., Socodato R., Almeida T.O., Terceiro A.F., Bravo J., Silva A.I., Magalhães J.D., Guerra-Gomes S., Oliveira J.F., Sousa N., Magalhães A., Relvas J.B., Summavielle T. Astrocyte-derived TNF and glutamate critically modulate microglia activation by methamphetamine. Neuropsychopharmacology. 2021 doi: 10.1038/s41386-021-01139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szklarczyk D., Gable A.L., Lyon D., Junge A., Wyder S., Huerta-Cepas J., Simonovic M., Doncheva N.T., Morris J.H., Bork P., Jensen L.J., Mering C.v. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Costa-Pinto S., Gonçalves-Ribeiro J., Tedim-Moreira J., Socodato R., Relvas J.B., Sebastião A.M., Vaz S.H. Communication defects with astroglia contribute to early impairments in the motor cortex plasticity of SOD1G93A mice. Neurobiol. Dis. 2024 doi: 10.1016/j.nbd.2024.106435. [DOI] [PubMed] [Google Scholar]
- 44.Osório H., Silva C., Ferreira M., Gullo I., Máximo V., Barros R., Mendonça F., Oliveira C., Carneiro F. Proteomics analysis of gastric cancer patients with diabetes mellitus. J. Clin. Med. 2021;10(3) doi: 10.3390/jcm10030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Socodato R., Portugal C.C., Canedo T., Domith I., Oliveira N.A., Paes-de-Carvalho R., Relvas J.B., Cossenza M. c-Src deactivation by the polyphenol 3-O-caffeoylquinic acid abrogates reactive oxygen species-mediated glutamate release from microglia and neuronal excitotoxicity. Free Radic. Biol. Med. 2015;79:45–55. doi: 10.1016/j.freeradbiomed.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 46.Deczkowska A., Keren-Shaul H., Weiner A., Colonna M., Schwartz M., Amit I. Disease-associated microglia: a universal immune sensor of neurodegeneration. Cell. 2018;173(5):1073–1081. doi: 10.1016/j.cell.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Rangaraju S., Dammer E.B., Raza S.A., Rathakrishnan P., Xiao H., Gao T., Duong D.M., Pennington M.W., Lah J.J., Seyfried N.T., Levey A.I. Identification and therapeutic modulation of a pro-inflammatory subset of disease-associated-microglia in Alzheimer's disease. Mol. Neurodegener. 2018;13(1):24. doi: 10.1186/s13024-018-0254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martínez-Reyes I., Chandel N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020;11(1):102. doi: 10.1038/s41467-019-13668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suomalainen A., Nunnari J. Mitochondria at the crossroads of health and disease. Cell. 2024;187(11):2601–2627. doi: 10.1016/j.cell.2024.04.037. [DOI] [PubMed] [Google Scholar]
- 50.Cornell J., Salinas S., Huang H.Y., Zhou M. Microglia regulation of synaptic plasticity and learning and memory. Neural Regen Res. 2022;17(4):705–716. doi: 10.4103/1673-5374.322423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parkhurst C.N., Yang G., Ninan I., Savas J.N., Yates J.R., 3rd, Lafaille J.J., Hempstead B.L., Littman D.R., Gan W.B. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155(7):1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barendrecht S., Schreurs A., Geissler S., Sabanov V., Ilse V., Rieckmann V., Eichentopf R., Künemund A., Hietel B., Wussow S., Hoffmann K., Körber-Ferl K., Pandey R., Carter G., Demuth H., Holzer M., Roßner S., Schilling S., Preuss C.…Cynis H. A novel human tau knock-in mouse model reveals interaction of Abeta and human tau under progressing cerebral amyloidosis in 5xFAD mice. Alzheimers Res. Ther. 2023;15 doi: 10.1186/s13195-022-01144-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nolt M., Lin Y., Hruska M., Murphy J., Sheffler-Colins S., Kayser M., Passer J., Bennett M., Zukin R., Dalva M. EphB controls NMDA receptor function and synaptic targeting in a subunit-specific manner. J. Neurosci. 2011;31:5353–5364. doi: 10.1523/JNEUROSCI.0282-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu R., Wei P., Jin L., Zheng T., Chen W.-Y., Liu X.-Y., Shi X., Hao J.-R., Sun N., Gao C. Overexpression of EphB2 in hippocampus rescues impaired NMDA receptors trafficking and cognitive dysfunction in Alzheimer model. Cell Death Dis. 2017;8 doi: 10.1038/cddis.2017.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maity J., Majumder S., Pal R., Saha B., Mukhopadhyay P.K. Ascorbic acid modulates immune responses through jumonji‐c domain containing histone demethylases and Ten eleven translocation (TET) methylcytosine dioxygenase. Bioessays. 2023;45(11) doi: 10.1002/bies.202300035. [DOI] [PubMed] [Google Scholar]
- 56.Minor E.A., Court B.L., Young J.I., Wang G. Ascorbate induces ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-Hydroxymethylcytosine. J. Biol. Chem. 2013;288(19):13669–13674. doi: 10.1074/jbc.C113.464800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yin R., Mao S.Q., Zhao B., Chong Z., Yang Y., Zhao C., Zhang D., Huang H., Gao J., Li Z., Jiao Y., Li C., Liu S., Wu D., Gu W., Yang Y.G., Xu G.L., Wang H. Ascorbic acid enhances Tet-mediated 5-methylcytosine oxidation and promotes DNA demethylation in mammals. J. Am. Chem. Soc. 2013;135(28):10396–10403. doi: 10.1021/ja4028346. [DOI] [PubMed] [Google Scholar]
- 58.Cárcamo J.M., Pedraza A., Bórquez-Ojeda O., Golde D.W. Vitamin C suppresses TNF alpha-induced NF kappa B activation by inhibiting I kappa B alpha phosphorylation. Biochemistry. 2002;41(43):12995–13002. doi: 10.1021/bi0263210. [DOI] [PubMed] [Google Scholar]
- 59.Domith I., Socodato R., Portugal C.C., Munis A.F., Duarte-Silva A.T., Paes-de-Carvalho R. Vitamin C modulates glutamate transport and NMDA receptor function in the retina. J. Neurochem. 2018;144(4):408–420. doi: 10.1111/jnc.14260. [DOI] [PubMed] [Google Scholar]
- 60.Li X., Bu X., Lu B., Avraham H., Flavell R.A., Lim B. The hematopoiesis-specific GTP-binding protein RhoH is GTPase deficient and modulates activities of other Rho GTPases by an inhibitory function. Mol. Cell Biol. 2002;22(4):1158–1171. doi: 10.1128/mcb.22.4.1158-1171.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qian X., Wang Y., Li X., Li Y., Li L. TNFAIP3 interacting protein 2 relieves lipopolysaccharide (LPS)-induced inflammatory injury in endometritis by inhibiting NF-kappaB activation. Immun. Inflamm. Dis. 2023;11(10) doi: 10.1002/iid3.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stehlik C., Hayashi H., Pio F., Godzik A., Reed J.C. CARD6 is a modulator of NF-kappa B activation by Nod1- and Cardiak-mediated pathways. J. Biol. Chem. 2003;278(34):31941–31949. doi: 10.1074/jbc.M300009200. [DOI] [PubMed] [Google Scholar]
- 63.Baldwin A. Regulation of cell death and autophagy by IKK and NF‐κB: critical mechanisms in immune function and cancer. Immunol. Rev. 2012;246 doi: 10.1111/j.1600-065X.2012.01095.x. [DOI] [PubMed] [Google Scholar]
- 64.Ghosh S., May M., Kopp E. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998;16:225–260. doi: 10.1146/ANNUREV.IMMUNOL.16.1.225. [DOI] [PubMed] [Google Scholar]
- 65.Rius-Pérez S., Pérez S., Martí-Andrés P., Monsalve M., Sastre J. NF-κB signaling complexes in acute inflammation. Antioxidants Redox Signal. 2019 doi: 10.1089/ars.2019.7975. [DOI] [PubMed] [Google Scholar]
- 66.Chen S.-J., Hseu Y., Gowrisankar Y., Chung Y., Zhang Y.-Z., Way T.-D., Yang H.-L. The anti-melanogenic effects of 3-O-ethyl ascorbic acid via Nrf2-mediated α-MSH inhibition in UVA-Irradiated keratinocytes and autophagy induction in melanocytes. Free Radical Biol. Med. 2021 doi: 10.1016/j.freeradbiomed.2021.07.030. [DOI] [PubMed] [Google Scholar]
- 67.Katsuyama Y., Tsuboi T., Taira N., Yoshioka M., Masaki H. 3-O-Laurylglyceryl ascorbate activates the intracellular antioxidant system through the contribution of PPAR-γ and Nrf2. J. Dermatol. Sci. 2016;82(3):189–196. doi: 10.1016/j.jdermsci.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 68.Ni Y., Wu G., Cai J., Zhang R., Zheng Y., Liu J., Yang X.-H., Yang X., Shen Y., Lai J., Ye X.-M., Mo S. Tubule-mitophagic secretion of SerpinG1 reprograms macrophages to instruct anti-septic acute kidney injury efficacy of high-dose ascorbate mediated by NRF2 transactivation. Int. J. Biol. Sci. 2022;18:5168–5184. doi: 10.7150/ijbs.74430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wardyn J., Ponsford A., Sanderson C. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015;43:621–626. doi: 10.1042/BST20150014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Minjarez B., Calderón-González K.G., Rustarazo M.L.V., Herrera-Aguirre M.E., Labra-Barrios M.L., Rincon-Limas D.E., Del Pino M.M.S., Mena R., Luna-Arias J.P. Identification of proteins that are differentially expressed in brains with alzheimer's disease using iTRAQ labeling and tandem mass spectrometry. J. Proteonomics. 2016;139:103–121. doi: 10.1016/j.jprot.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 71.Nolfi-Donegan D., Braganza A., Shiva S. Mitochondrial electron transport chain: oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020;37 doi: 10.1016/j.redox.2020.101674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tai Y.H., Engels D., Locatelli G., Emmanouilidis I., Fecher C., Theodorou D., Müller S.A., Licht-Mayer S., Kreutzfeldt M., Wagner I., de Mello N.P., Gkotzamani S.N., Trovò L., Kendirli A., Aljović A., Breckwoldt M.O., Naumann R., Bareyre F.M., Perocchi F.…Misgeld T. Targeting the TCA cycle can ameliorate widespread axonal energy deficiency in neuroinflammatory lesions. Nat. Metab. 2023;5(8):1364–1381. doi: 10.1038/s42255-023-00838-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Turner N.L., Macrina T., Bae J.A., Yang R., Wilson A.M., Schneider-Mizell C., Lee K., Lu R., Wu J., Bodor A.L., Bleckert A.A., Brittain D., Froudarakis E., Dorkenwald S., Collman F., Kemnitz N., Ih D., Silversmith W.M., Zung J.…Seung H.S. Reconstruction of neocortex: organelles, compartments, cells, circuits, and activity. Cell. 2022;185(6):1082–1100.e1024. doi: 10.1016/j.cell.2022.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Uddin M.E., Yu W., Lim L. Exploring ER stress response in cellular aging and neuroinflammation in Alzheimer's disease. Ageing Res. Rev. 2021;70 doi: 10.1016/j.arr.2021.101417. [DOI] [PubMed] [Google Scholar]
- 75.Leinenga G., To X.V., Bodea L.G., Yousef J., Richter-Stretton G., Palliyaguru T., Chicoteau A., Dagley L., Nasrallah F., Götz J. Scanning ultrasound-mediated memory and functional improvements do not require amyloid-β reduction. Mol. Psychiatr. 2024 doi: 10.1038/s41380-024-02509-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lv Z., Chen L., Chen P., Peng H., Rong Y., Hong W., Zhou Q., Li N., Li B., Paolicelli R.C., Zhan Y. Clearance of β-amyloid and synapses by the optogenetic depolarization of microglia is complement selective. Neuron. 2024;112(5):740–754. doi: 10.1016/j.neuron.2023.12.003. e747. [DOI] [PubMed] [Google Scholar]
- 77.Nagahara A.H., Merrill D.A., Coppola G., Tsukada S., Schroeder B.E., Shaked G.M., Wang L., Blesch A., Kim A., Conner J.M., Rockenstein E., Chao M.V., Koo E.H., Geschwind D., Masliah E., Chiba A.A., Tuszynski M.H. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat. Med. 2009;15(3):331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paolicelli R.C., Jawaid A., Henstridge C.M., Valeri A., Merlini M., Robinson J.L., Lee E.B., Rose J., Appel S., Lee V.M., Trojanowski J.Q., Spires-Jones T., Schulz P.E., Rajendran L. TDP-43 depletion in Microglia promotes amyloid clearance but also induces synapse loss. Neuron. 2017;95(2):297–308.e296. doi: 10.1016/j.neuron.2017.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shi Q., Chowdhury S., Ma R., Le K.X., Hong S., Caldarone B.J., Stevens B., Lemere C.A. Complement C3 deficiency protects against neurodegeneration in aged plaque-rich APP/PS1 mice. Sci. Transl. Med. 2017;9(392) doi: 10.1126/scitranslmed.aaf6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wilson J. Regulation of vitamin C transport. Annu. Rev. Nutr. 2005;25:105–125. doi: 10.1146/ANNUREV.NUTR.25.050304.092647. [DOI] [PubMed] [Google Scholar]
- 81.Blanchard J., Tozer T., Rowland M. Pharmacokinetic perspectives on megadoses of ascorbic acid. Am. J. Clin. Nutr. 1997;66(5):1165–1171. doi: 10.1093/AJCN/66.5.1165. [DOI] [PubMed] [Google Scholar]
- 82.Levine M., Padayatty S., Espey M. Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv. Nutr. 2011;2(2):78–88. doi: 10.3945/an.110.000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Padayatty S., Sun H., Wang Y., Riordan H., Hewitt S., Katz A., Wesley R., Levine M. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann. Intern. Med. 2004;140:533–537. doi: 10.7326/0003-4819-140-7-200404060-00010. [DOI] [PubMed] [Google Scholar]
- 84.Charlton K., Rabinowitz T., Geffen L., Dhansay M. Lowered plasma vitamin C, but not vitamin E, concentrations in dementia patients. J. Nutr. Health Aging. 2004;8(2):99–107. https://consensus.app/papers/lowered-plasma-vitamin-c-but-not-vitamin-e-concentrations-charlton-rabinowitz/6a08f2747492558883cea2351346299f/ [PubMed] [Google Scholar]
- 85.De Wilde M., Vellas B., Girault E., Yavuz A., Sijben J. Lower brain and blood nutrient status in Alzheimer's disease: results from meta-analyses. Alzheimer's Dement. : Transl. Res. Clin. Intervention. 2017;3:416–431. doi: 10.1016/j.trci.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hamid M., Mansoor S., Amber S., Zahid S.B. A quantitative meta-analysis of vitamin C in the pathophysiology of Alzheimer's disease. Front. Aging Neurosci. 2022;14 doi: 10.3389/fnagi.2022.970263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Travica N., Ried K., Sali A., Scholey A., Hudson I., Pipingas A. Vitamin C status and cognitive function: a systematic review. Nutrients. 2017;9 doi: 10.3390/nu9090960. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Validation of AAV9-CD68 vector specificity and SVCT2 overexpression.
(A) Representative confocal images from the hippocampus of 6-month-old 5xFAD mice injected with AAV9-CD68 vectors expressing mCherry (red). The images show co-localization of mCherry with the microglial marker Iba1 (green), but not with the astrocyte marker GFAP (white) or the neuronal marker NeuN (white), confirming the microglia-specific transduction of the vector.
(B) Quantification of the percentage of mCherry-positive cells that are also positive for Iba1, NeuN, or GFAP. The analysis confirms that the vast majority of transduced cells are Iba1-positive microglia.
(C) Quantification of SVCT2 mRNA levels in sorted mCherry-positive microglia from the hippocampi of 6-month-old 5xFAD-SVCT2 and 5xFAD-Empty mice, showing a significant increase in SVCT2 transcripts in the treatment group. Data are shown as mean ± SEM
(D) Flow cytometry data quantifying the median fluorescence intensity (MFI) of SVCT2 protein in transduced (mCherry+) microglia from 6-month-old 5xFAD mice, confirming higher protein expression in the 5xFAD-SVCT2 group compared to controls.
(E) Validation of lentivirus-mediated SVCT2 overexpression in primary rat microglial cultures (DIV 5). Representative immunofluorescence images show SVCT2 (red) and the viral reporter GFP (green). Nuclei are stained with DAPI (blue). The bar graph quantifies SVCT2 MFI in GFP-positive cells, confirming successful overexpression. Data represent N = 3 independent cultures. ∗∗∗∗p < 0.0001 (Unpaired t-test).
Principal Component Analysis (PCA) of transcriptomic and proteomic datasets.
Principal Component Analysis (PCA) mapping of transcriptomic (RNAseq) and proteomic data from hippocampal samples. The plots show clustering between the 5xFAD-SVCT2 and 5xFAD-Empty experimental groups for both the early intervention (6 months of age) and late intervention (10 months of age) time points.
Transduced microglia cluster around amyloid deposits in the 5xFAD hippocampus. Representative confocal micrographs from 6-month-old 5xFAD mice injected at 2 months with AAV9-CD68:Empty-mCherry (top row) or AAV9-CD68:SVCT2-IRES-mCherry (bottom row) show single channels for mCherry (transduced cells), Iba1 (microglia), and BAM-10 (Aβ deposits), plus the corresponding merge. Images illustrate that mCherry+/Iba1+ microglia are positioned adjacent to and encircle BAM-10+ deposits in both groups. Scale bar, 20 μm.
SVCT2 overexpression ameliorates pathology in the APP/PS1 mouse model.
(A) Flow cytometry analysis showing reduced SVCT2 expression (% SVCT2+ microglia) in microglia from 4-month-old APP/PS1 mice compared to age-matched wild-type (WT) controls.
(B) Schematic of the experimental design. 4-month-old APP/PS1 mice received intra-hippocampal injections of AAV9-SVCT2 or AAV9-Empty, with analyses performed at 9 months of age.
(C) Representative immunofluorescence images and quantification showing a significant reduction in amyloid plaque burden (BAM-10 staining, red) in the hippocampus of 9-month-old APP/PS1-SVCT2 mice compared to controls.
(D) ELISA quantification of soluble and insoluble Aβ1-42 levels in hippocampal lysates from 9-month-old APP/PS1 mice.
(E) Representative images and quantification of synaptic puncta (vGlut1, green; PSD95, red) in the hippocampus of 9-month-old APP/PS1 mice. SVCT2 overexpression significantly increased the density of co-localized synaptic puncta. For all panels, data are represented as mean ± SEM from N = 4–5 mice per group. ∗p < 0.05 (Unpaired t-test).
Quality control of synaptosome preparations.
Western blot analysis validating the enrichment of synaptosomal fractions isolated from the hippocampi of 6- and 10-month-old mice. Blots were probed for the presynaptic vesicular glutamate transporter 1 (vGlut1) and the postsynaptic density protein 95 (PSD95). Both vGlut1 and PSD95 are highly enriched in the synaptosome fraction compared to the cytosolic fraction.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Data is available from the corresponding author upon reasonable request.