Abstract
Aims
Several clinical factors, such as ageing and atrial dilatation, are associated with post-discharge atrial fibrillation (late-POAF) after cardiac surgery, but it is currently unknown if atrial histological characteristics are linked to late-POAF. Therefore, the aim of this study was to determine the association of atrial histological characteristics with POAF incidence and burden during 2.5 years of continuous rhythm monitoring after cardiac surgery.
Methods and results
Consecutive patients with and without AF history were prospectively included. Intraoperatively, biopsies were taken of left (LAA) and right atrial appendages (RAA), and all patients received an implantable loop recorder for 2.5 years. Biopsies were analysed for overall and endomysial fibrosis, cardiomyocyte diameter, capillary density and size, and fibroblast density. POAF incidence was defined as any AF episode during the follow-up, while POAF burden was the percentage of time in AF. A total of 133 patients were included (90 without and 43 with AF history). Late-POAF occurred in 53 patients (40.8%). While several histological traits were associated with POAF incidence in the unadjusted analyses, none of the histological traits were significantly associated with POAF incidence after adjustments for clinical confounders. Increased RAA endomysial fibrosis was the only histological trait significantly associated with increased overall (St. Beta = 0.32, 95% CI: 0.06–0.58, P = 0.017) and late-POAF burden (St. Beta = 0.43, 95% CI: 0.13–0.72, P = 0.006) after adjusting for age, sex, type of surgery, reduced LV function, RA volume, and AF history.
Conclusion
Increased RAA endomysial fibrosis appears to be associated with increased late-POAF persistence, reflected by increased burden, but not with incidental late-POAF recurrences.
Keywords: Cardiac surgery, Postoperative atrial fibrillation, Histology, Atrial fibrillation burden
Graphical Abstract
Graphical Abstract.
What’s new?
Atrial histological characteristics do not appear to be associated with incidental device-detected AF (re)occurrence after cardiac surgery, suggesting a greater role for trigger mechanisms rather than structural pro-arrhythmic remodelling in incidental AF.
Right atrial endomysial fibrosis appears to be a strong marker of atrial fibrillation burden after cardiac surgery in both patients with and without preoperative AF history, highlighting the right atrium’s important role in AF persistence.
Introduction
Postoperative atrial fibrillation (POAF) is the most frequent complication after cardiac surgery, occurring in both the early (early-POAF) and late postoperative phases (late-POAF). POAF is associated with an increased incidence of late adverse events, particularly late mortality and stroke.1–5 Recent studies have reported several preoperative clinical characteristics that are associated with an increased risk of developing both early- and late-POAF, such as older age, increased atrial volumes, and several blood biomarkers, all of which appear to be related to an advanced underlying atrial cardiomyopathy.1–4 Additionally, several studies performing histopathological analyses of atrial appendages have identified increased overall fibrosis to be associated with increased in-hospital early-POAF incidence.6–8 Furthermore, endomysial fibrosis, but not overall fibrosis, measured in both left and right atrial appendage biopsies (LAA and RAA) was identified to be strongly associated with persistent atrial fibrillation (AF), implying an important role in the pathophysiology of AF.9,10 Increased levels of endomysial fibrosis also seem to be associated with increased conduction disturbances determined by high-coverage high-density epicardial mapping.11
However, it is currently unknown how atrial histological characteristics, especially overall and endomysial atrial fibrosis, are related to the incidence and burden of late-POAF. Therefore, the main objective of the current study was to determine the association of atrial histological traits with POAF incidence and burden during 2.5 years of continuous rhythm monitoring after cardiac surgery, both in patients with and without a preoperative AF history.
Methods
Study population
After approval of the study protocol by the local ethical committee, consecutive patients were prospectively included in the RACE V—Tissue Bank Project at the Maastricht University Medical Centre (clinicaltrials.gov identifier NCT03124576). Eligible patients were ≥18 years of age, underwent their first cardiac surgical procedure, and had a life expectancy of >2.5 years. Patients with and without an AF history (paroxysmal or persistent) were included. Those with preoperative AF underwent various types of surgical ablation concomitant to their index cardiac surgical procedure. The specific type of surgical ablation was determined by a specialized heart team, comprising an arrhythmia-focused cardiac surgeon and an electrophysiologist. Objectives, designs, and study procedures of the RACE V Tissue Bank Project were published previously.12
Clinical patient assessment
Preoperative data on clinical history, comorbidity profile, clinical presentation, and preoperative medication were collected through chart review after informed consent was obtained. All patients underwent a transthoracic echocardiography (TTE) within the 6 months prior to cardiac surgery. After patients’ enrolment in the study, all baseline echocardiographic studies were reanalysed by a blinded imaging cardiologist who was part of our research team. Standard 12-lead electrocardiograms (ECG) were recorded preoperatively. All measurements were performed in MUSE™ (GE Medical Systems Information Technologies, Inc., Milwaukee, WI, USA). Biomarker analysis was performed by Roche Diagnostics (Basel, Switzerland). The rationale for selection of blood biomarkers assessed in the current study was described previously.3 Additionally, procedural and postoperative data were prospectively collected and analysed.
Histological analysis
Snap frozen tissue samples of the LA and RA appendage (LAA/RAA) were collected during cardiac surgery and analysed as previously described.9,13 In brief, all samples were cryosectioned (6 µm) and stained with wheat germ agglutinin [WGA (connective tissue)], mouse monoclonal anti-CD31 (capillary density), and Rabbi monoclonal anti-vimentin (fibroblast density). Next, slides were submerged in ice-cold acetone for 10 min to fixate antigens. Non-specific binding sites were blocked by application of a blocking solution (2 m/v% fraction V BSA and 0.3 M glycine) for 60 min. WGA conjugated to Alexa 594 [1/200, ThermoFisher (W11262), Netherlands] was applied for 120 min. Prolong Gold Antifade mounting medium [ThermoFisher (P10144), Netherlands] was used to mount coverslips. Multiple non-overlapping areas were imaged at 400× magnification using a Leica DM4B microscope with a Leica MC170 HD camera. Only areas with transversely cut myocytes were photographed. Automated analysis of structural tissue characteristics was performed using JavaCyte.13 The fraction of WGA-positive pixels was determined as a measure for total ECM content (overall fibrosis). Moreover, cardiomyocytes were segmented by detection of local fluorescence minima. Inter-cardiomyocyte distances between neighbouring cardiomyocytes were obtained as a measure for endomysial fibrosis. Additionally, the size of cardiomyocytes was quantified as the minimal Feret diameter for each object.13 Capillary density was calculated as the number of capillaries per myocyte while fibroblast density was defined as number of fibroblasts per myocyte.13
Postoperative AF detection
The extensive description of the continuous monitoring strategy was described previously.3 In short, a Reveal Linq™ (Medtronic Minneapolis, MN, USA) was inserted at the left parasternal area at the end of cardiac surgery. All patients received a remote monitoring system (CareLink®) before discharge for a period of 2.5 years. Because of the intrinsic set-up of the Medtronic algorithm, the ILR recorder detected all POAF episodes lasting at least 2 min.14 AF episodes were only included if they were captured on an ECG lead recorded by the ILR and subsequently could be confirmed by the investigators and the treating cardiologist.
Postoperative AF definitions
Any-POAF was defined as the time to the first POAF episode detected by the ILR during the follow-up period. Early-POAF was defined as any AF episode occurring within the first 30 postoperative days, and late-POAF as any episode occurring thereafter. The choice of the 30-day threshold was deliberate and based on previous analyses from the RACE V cohort, which demonstrated that POAF episodes occurring beyond the first postoperative month were significantly more predictive of late-POAF during long-term monitoring.15 This threshold likely reflects a transition from transient, surgery-induced arrhythmia to more sustained, substrate-driven AF.
POAF burden was defined as the percentage of time spent in AF during a specific period of rhythm monitoring. Additionally, the duration of the longest individual POAF episode was analysed as a surrogate of AF burden, as previously described.15,16 These parameters were calculated for three postoperative phases: early (first 30 days), late (beyond 30 days), and overall (entire follow-up period).
Statistical analysis
Continuous variables were reported as means with standard deviations (SDs) or medians with interquartile ranges (IQRs), depending on distribution assessed via the Shapiro–Wilk test. Categorical variables were summarized as counts and percentages. Group differences were analysed using ANOVA, Kruskal–Wallis, or χ2 tests, as appropriate. To improve group sizes, patients with paroxysmal and persistent AF were combined into one group (preoperative AF history). Non-normally distributed variables were log-transformed prior to regression analyses. Concordance between LAA and RAA histological features was assessed using Spearman’s rho in patients with paired samples. Associations between clinical, imaging, and histological variables were also evaluated with Spearman’s rho and presented in a correlation matrix.
Cox proportional hazards models were used to identify histological predictors of POAF incidence. Proportional hazards assumptions were tested using Schoenfeld residuals. Multivariable Cox models were adjusted for age, sex, AF history, surgery type, atrial volumes, and reduced LV function (LVEF < 50% on preoperative TTE), based on variables identified in unadjusted analyses. Separate subgroup analyses were conducted in patients without preoperative AF, including both unadjusted and adjusted models.
Linear regression was used to assess associations between POAF burden, clinical variables, and histological features. POAF burden was log-transformed to ensure linearity. Variables with significant univariable associations were entered into multivariable models adjusted for the same clinical confounders listed above. Subgroup analyses were again performed for patients without preoperative AF.
Adjustment variables were selected a priori based on literature and clinical relevance.9 Multivariable results were presented in forest plots using standardized beta coefficients to allow direct comparison of effect sizes. A P-value of <0.05 was considered statistically significant. No correction for multiple testing was applied due to the exploratory nature of the study. All analyses were performed in R (version 4.2.2) and SPSS (v26.0, IBM Corp., Armonk, NY).
Results
Patient and procedural characteristics
A total of 133 consecutive patients were prospectively included. Median follow-up was 988 days (IQR: 790–1062), with 98 patients (73.7%) completing 2.5 years of continuous rhythm monitoring (Figure 1). During follow-up, 14 patients died, 14 had ILR explantation, and 7 were lost to follow-up. Most patients were male (102/133, 76.7%) and had no preoperative AF history (90/133, 67.7%) (Table 1). Patients with AF history were older and had larger atrial volumes and higher blood biomarker levels. Coronary artery bypass grafting (CABG) was performed in 89 patients (66.9%) (Table 2).
Figure 1.
Study inclusion diagram. AF, atrial fibrillation; FU, follow-up; ILR, implantable loop recorder; LAA, left atrial appendage; RAA, right atrial appendage. Created in BioRender. Kawczynski, M. (2025) https://BioRender.com/25ue4ow.
Table 1.
Baseline patient characteristics
| Variable | Overall cohort (n = 133) | No AF history (n = 90) | AF history (n = 43) | P-value |
|---|---|---|---|---|
| Patient characteristics | ||||
| No preoperative AF history (%) | 90 (67.7) | 90 (100) | 0 (0) | NA |
| Preoperative paroxysmal AF (%) | 25 (18.8) | 0 (0) | 25 (58.1) | NA |
| Preoperative persistent AF (%) | 18 (13.5) | 0 (0) | 18 (41.9) | NA |
| Age, years (IQR) | 68 (62–72) | 67 (60–72) | 70 (67–73) | 0.031 |
| Male sex (%) | 102 (76.7) | 74 (82.2) | 28 (65.1) | 0.029 |
| BSA, m2 (IQR) | 1.97 (1.87–2.12) | 1.97 (1.90–2.11) | 1.98 (1.84–2.17) | 0.709 |
| BMI, kg/m2 (IQR) | 27.1 (24.8–29.7) | 27.3 (24.8–29.7) | 27.1 (24.8–29.5) | 0.551 |
| NYHA classification (IQR) | 2 (1–3) | 2 (1–2) | 2 (2–3) | 0.010 |
| Hypertension (%) | 122 (91.7) | 84 (93.3) | 38 (88.4) | 0.331 |
| Dyslipidaemia (%) | 113 (84.9) | 79 (87.8) | 34 (79.1) | 0.189 |
| Diabetes mellitus (%) | 27 (20.3) | 19 (21.1) | 8 (18.6) | 0.737 |
| COPD (%) | 16 (12.0) | 12 (13.3) | 4 (9.3) | 0.504 |
| Pulmonary hypertension (%) | 16 (12.0) | 7 (7.8) | 9 (20.9) | 0.029 |
| Peripheral artery disease (%) | 24 (18.0) | 19 (21.1) | 5 (11.6) | 0.183 |
| Creatinine, μmol/L (IQR) | 71.2 (60.8–83.1) | 73.4 (63.3–85.4) | 66.7 (56.5–78.6) | 0.044 |
| Sleep apnoea (%) | 9 (6.8) | 7 (7.8) | 2 (4.7) | 0.502 |
| Smoking history (%) | 86 (64.7) | 59 (65.6) | 27 (62.8) | 0.755 |
| Alcohol use (%) | 78 (58.6) | 52 (57.8) | 26 (60.5) | 0.768 |
| HASBLED-score (IQR) | 2 (2–3) | 2 (1–2) | 2 (2–3) | 0.060 |
| CHA₂DS₂-VASc-score (IQR) | 3 (3–4) | 3 (3–4) | 3 (3–4) | 0.209 |
| Preoperative medication | ||||
| Beta-blocker (%) | 98 (73.7) | 64 (71.1) | 34 (79.1) | 0.330 |
| ACE-inhibitor (%) | 53 (39.9) | 37 (41.1) | 16 (37.2) | 0.667 |
| ARB (%) | 31 (23.3) | 23 (25.6) | 8 (18.6) | 0.375 |
| Diuretics (%) | 37 (27.8) | 19 (21.1) | 18 (41.9) | 0.012 |
| Statins (%) | 99 (74.4) | 73 (81.1) | 26 (60.5) | 0.011 |
| Preoperative transthoracic echocardiography | ||||
| LVEF, % (IQR) | 55 (50–60) | 55 (50–60) | 56 (51–61) | 0.642 |
| LAVImax, mL/m2 (IQR) | 36.4 (28.4–49.0) | 33.7 (26.8–42.4) | 42.9 (35.6–64.6) | <0.001 |
| LAVImin, mL/m2 (IQR) | 17.5 (11.5–28.6) | 13.8 (9.3–20.1) | 29.3 (18.8–46.5) | <0.001 |
| LATEF, % (SD) | 49.9 ± 16.1 | 56.2 ± 12.6 | 36.9 ± 14.8 | <0.001 |
| RAVImax, mL/m2 (IQR) | 27.1 (21.0–37.2) | 24.7 (18.6–34.0) | 33.8 (25.9–47.0) | <0.001 |
| RAVImin, mL/m2 (IQR) | 13.4 (9.6–19.3) | 11.7 (8.6–16.0) | 17.4 (13.6–29.9) | <0.001 |
| RATEF, % (SD) | 46.9 ± 14.7 | 49.9 ± 12.4 | 40.7 ± 17.1 | <0.001 |
| LVM, grams (SD) | 210.4 ± 68.2 | 203.9 ± 72.2 | 224.9 ± 56.5 | 0.105 |
| LVMI, grams/m2 (SD) | 105.4 ± 32.9 | 101.6 ± 34.1 | 113.7 ± 28.9 | 0.052 |
| E-wave, cm/s (IQR) | 76 (63–98) | 73 (63–86) | 94 (63–130) | 0.008 |
| A-wave, cm/s (IQR) | 76 (65–94) | 80 (68–99) | 69 (53–80) | 0.013 |
| Preoperative 12-lead electrocardiography | ||||
| PR-interval, ms (IQR) | 165 (150–184) | 163 (148–183) | 176 (160–199) | 0.032 |
| P-wave duration, ms (IQR) | 116 (105–128) | 112 (104–124) | 128 (116–136) | 0.002 |
| QRS duration, ms (IQR) | 96 (86–104) | 96 (84–104) | 98 (86–108) | 0.199 |
| QT-interval, ms (SD) | 401.6 ± 38.8 | 406.2 ± 32.9 | 392.0 ± 47.8 | 0.086 |
| QTc-interval, ms (IQR) | 424 (410–447) | 421 (408–443) | 434 (412–462) | 0.070 |
| Preoperative computed tomography | ||||
| Epicardial adipose tissue (SD) | 124.4 ± 48.8 | 121.1 ± 45.4 | 133.2 ± 57.0 | 0.289 |
| Pericardial adipose tissue (SD) | 207.9 ± 85.3 | 205.9 ± 82.9 | 213.4 ± 92.7 | 0.711 |
| Preoperative biomarkers | ||||
| ANG2, ng/mL (IQR) | 2.1 (1.6–2.9) | 1.9 (1.5–2.4) | 2.9 (2.0–5.0) | <0.001 |
| BMP10, ng/mL (IQR) | 1.8 (1.6–2.1) | 1.7 (1.6–1.9) | 2.2 (1.8–2.7) | <0.001 |
| DKK3, ng/mL (IQR) | 55.6 (48.5–64.6) | 53.6 (47.7–62.9) | 59.6 (52.6–66.9) | 0.025 |
| ESM1, pg/mL (IQR) | 1887.9 (1566.6–2409.3) | 1774.9 (1545.3–2070.3) | 2451.4 (1773.5–3065.6) | <0.001 |
| FGF23, ng/mL (IQR) | 154.2 (124.3–206.2) | 139.9 (111.7–165.9) | 182.7 (154.6–385.3) | <0.001 |
| IGFBP7, ng/mL (IQR) | 102.8 (89.4–116.5) | 99.6 (87.7–111.9) | 112.5 (99.8–126.6) | 0.001 |
| NT-proBNP, pg/mL (IQR) | 1444.1 (549.7–2867.2) | 1036.2 (481.6–2076.0) | 2887.7 (1598.9–4315.5) | <0.001 |
| GDF15, pg/mL (IQR) | 1504.5 (1019.7–2251.5) | 1436.0 (981.1–2081.8) | 1681.0 (1175.0–2573.0) | 0.130 |
| IL6, pg/mL (IQR) | 3.4 (2.0–6.4) | 3.2 (2.2–5.6) | 4.8 (1.8–7.7) | 0.459 |
| Pro-BNP2, pg/mL (IQR) | 349.1 (120.0–935.1) | 206.3 (92.4–571.1) | 840.8 (304.7–1484.0) | <0.001 |
| Troponin T, pg/mL (IQR) | 14.8 (10.4–22.4) | 14.4 (10.2–20.4) | 16.9 (10.6–28.4) | 0.283 |
Bold values imply statistical significance at an alpha threshold of 0.05.
ACE, angiotensin converting enzyme; ANG-2, angiopoietin 2; ARB, angiotensin receptor blocker; BMI, body mass index; BMP-10, bone morphogenic protein 10; BSA, body surface area; COPD, chronic obstructive pulmonary disease; DKK-3, dickkopf 3; ESM-1, endothelial cell-specific molecule 1; FGF-23, fibroblast growth factor 23; GDF-15, growth differentiation factor 15; GFR, glomerular filtration rate; IGFBP-7, insulin-like growth factor-binding protein 7; IL-6, interleukin 6; LATEF, left atrial total ejection fraction; LAVI, left atrial volume indexed for body surface area; LVEF, left ventricle ejection fraction; LVMI, left ventricular mass indexed for body surface area; ng, nanogram; NT-proBNP, N-terminal brain natriuretic peptide; NYHA, New York Hear Association; PAF, paroxysmal atrial fibrillation; pg, picogram; pro-BNP2, pro brain natriuretic peptide 2; RATEF, right atrial total ejection fraction; RAVI, right atrial volume indexed for body surface area; SD, standard deviation; SR, sinus rhythm.
Table 2.
Procedural and postoperative characteristics
| Variable | Overall cohort (n = 133) | No AF history (n = 90) | AF history (n = 43) | P-value |
|---|---|---|---|---|
| Surgical data | ||||
| Type of operation (%) | 133 (100) | 90 (100) | 43 (100) | <0.001 |
| CABG (%) | 89 (66.9) | 72 (80.0) | 17 (39.5) | |
| AVR (%) | 18 (13.5) | 11 (12.2) | 7 (16.3) | |
| MV surgery (%) | 22 (16.5) | 6 (6.7) | 16 (37.2) | |
| Aortic surgery (%) | 4 (3.1) | 1 (1.1) | 3 (7.0) | |
| Rhythm surgery (%) | 36 (100) | NA | 36 (100) | NA |
| PVI (%) | 11 (30.6) | NA | 11 (30.6) | |
| Left-sided Maze (%) | 21 (58.3) | NA | 21 (58.3) | |
| Full Cox Maze IV (%) | 4 (11.1) | NA | 4 (11.1) | |
| CPB-time, minutes (IQR) | 97 (70–134) | 85 (63–111) | 145 (96–175) | <0.001 |
| X-time, minutes (IQR) | 65 (45–87) | 57 (42–76) | 97 (52–127) | <0.001 |
| Postoperative data | ||||
| Reoperation for bleeding (%) | 1 (0.8) | 1 (1.1) | 0 (0.0) | 0.488 |
| Reoperation for tamponade (%) | 4 (3.0) | 2 (2.2) | 2 (4.7) | 0.443 |
| ICU admission, days (IQR) | 1 (1–2) | 1 (1–1) | 1 (1–3) | 0.100 |
| Hospitalization in days (IQR) | 6 (5–8) | 6 (5–7) | 8 (6–11) | <0.001 |
| Rhythm control during admission (%) | 17 (12.8) | 6 (6.7) | 11 (25.6) | 0.002 |
| Rhythm control at discharge (%) | 10 (7.5) | 4 (4.4) | 6 (14.0) | 0.052 |
| (N)OAC at discharge (%) | 65 (48.9) | 22 (24.4) | 43 (100) | <0.001 |
| Diuretics at discharge (%) | 55 (41.4) | 27 (30.0) | 28 (65.1) | <0.001 |
| Beta blockers at discharge (%) | 118 (88.7) | 84 (93.3) | 34 (79.1) | 0.015 |
Bold values imply statistical significance at an alpha threshold of 0.05.
AF, atrial fibrillation; AVR, aortic valve replacement; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; ICU, intensive care unit; IQR, interquartile range; MV, mitral valve; OAC, oral anticoagulation; PVI, pulmonary vein isolation; SD, standard deviation; X, aortic cross clamp.
Histological characteristics
Overall, RAA biopsies were obtained in 109 patients (82.0%) and LAA biopsies in 96 patients (72.2%), with paired samples available in most cases (Figure 1). In patients with paired samples, concordance between RAA and LAA histological findings was moderate for fibroblast density (ρ = 0.55, P < 0.001), endomysial fibrosis (ρ = 0.39, P < 0.001), capillary size (ρ = 0.38, P = 0.005), and cardiomyocyte diameter (ρ = 0.25, P = 0.031) (see Supplementary material online, Figure S1). Correlations were weaker and not statistically significant for total ECM content (ρ = 0.21, P = 0.064) and capillary density (ρ = 0.24, P = 0.077) (see Supplementary material online, Figure S1).
Compared to patients without preoperative AF, those with AF history had significantly larger LAA cardiomyocyte diameters (P < 0.001), increased LAA endomysial fibrosis (P < 0.001), increased LAA capillary (P = 0.015) and fibroblast density (P = 0.004), as well as increased RAA endomysial fibrosis (P = 0.007) and fibroblast density (P = 0.025) (Table 3). No significant differences were observed in total ECM content between groups.
Table 3.
Histological characteristics
| Variable | Overall cohort (n = 133) | No AF history (n = 90) | AF history (n = 43) | P-value |
|---|---|---|---|---|
| Left atrial characteristics | ||||
| Myocyte diameter, in μm (IQR) | 11.3 (10.5–12.2) | 10.9 (10.1–11.6) | 12.0 (11.3–12.7) | <0.001 |
| Endomysial fibrosis, in μm (IQR) | 5.1 (4.6–5.9) | 4.8 (4.5–5.4) | 5.6 (5.2–6.7) | <0.001 |
| Extracellular matrix content, in % (IQR) | 23.2 (18.9–27.2) | 24.3 (19.9–27.7) | 22.3 (18.4–25.1) | 0.161 |
| Capillary density, ratio (IQR) | 0.26 (0.20–0.36) | 0.24 (0.19–0.30) | 0.30 (0.24–0.46) | 0.015 |
| Capillary size, in μm (IQR) | 4.3 (3.8–4.7) | 4.4 (4.0–4.7) | 4.2 (3.6–4.8) | 0.522 |
| Fibroblast density, ratio (IQR) | 0.51 (0.42–0.63) | 0.46 (0.40–0.59) | 0.61 (0.48–0.70) | 0.004 |
| Right atrial characteristics | ||||
| Myocyte diameter, in μm (IQR) | 11.1 (10.5–11.8) | 11.1 (10.5–11.7) | 11.4 (10.6–12.3) | 0.202 |
| Endomysial fibrosis, in μm (IQR) | 6.3 (5.7–6.8) | 6.1 (5.6–6.6) | 6.4 (6.2–7.1) | 0.007 |
| Extracellular matrix content, in % (IQR) | 23.6 (20.3–26.1) | 23.5 (20.2–25.9) | 24.1 (21.1–27.3) | 0.569 |
| Capillary density, ratio (IQR) | 0.30 (0.23–0.36) | 0.30 (0.23–0.35) | 0.30 (0.25–0.44) | 0.742 |
| Capillary size, in μm (IQR) | 3.8 (3.4–4.3) | 3.8 (3.4–4.2) | 3.8 (3.4–4.7) | 0.598 |
| Fibroblast density, ratio (IQR) | 0.53 (0.44–0.66) | 0.51 (0.42–0.60) | 0.62 (0.50–0.82) | 0.025 |
Bold values imply statistical significance at an alpha threshold of 0.05.
AF, atrial fibrillation; SD, standard deviation; μm, micrometre.
Fibrosis, clinical characteristics, and imaging features
LAA endomysial fibrosis was significantly positively associated with age (ρ = 0.21, P = 0.038), LA volume (LAVImax: ρ = 0.43, P < 0.001; LAVImin: ρ = 0.47, P < 0.001), LV mass (LVM: ρ = 0.24, P = 0.021; LVMI: ρ = 0.28, P = 0.009), RA volume (RAVImax: ρ = 0.31, P = 0.001; RAVImin: ρ = 0.33, P = 0.001), and significantly negatively associated with LA total emptying fraction (LATEF: ρ = -−0.41, P < 0.001) (see Supplementary material online, Figure S2). RAA endomysial fibrosis was significantly positively associated with LV mass (LVM: ρ = 0.26, P = 0.006; LVMI: ρ = 0.28, P = 0.003), and with prolonged QRS duration (ρ = 0.24, P = 0.012) (see Supplementary material online, Figure S2).
Histological traits and POAF incidence
Any-POAF incidence
In total, 81 patients (60.9%) experienced at least one POAF episode during follow-up (Table 4). In unadjusted analyses, only RAA endomysial fibrosis (HR = 1.09, 95% CI: 1.02–1.16, P = 0.015) and LAA capillary density (HR = 7.51, 95% CI: 1.01–55.8, P = 0.049) were significantly associated with any-POAF incidence (see Supplementary material online, Table S1). In combined models including both RAA and LAA histological characteristics, no significant associations were observed (see Supplementary material online, Table S2). After adjusting for age, sex, AF history, surgery type, atrial volumes, and reduced LVF, none of the histological traits remained significantly associated with any-POAF incidence.
Table 4.
Incidence and occurrence patterns of postoperative AF
| Variable | Overall cohort (n = 133) | No AF history (n = 90) | AF history (n = 43) | P-value |
|---|---|---|---|---|
| Postoperative AF incidence and patterns | ||||
| Any-POAF (%) | 81 (60.9) | 43 (47.8) | 38 (88.4) | <0.001 |
| Overall-POAF burden, % (IQR) | 0.163 (0.045–1.204) | 0.132 (0.006–0.628) | 0.403 (0.119–2.219) | 0.017 |
| AF episodes per patient (IQR) | 19 (4–52) | 8 (3–29) | 42 (10–72) | 0.014 |
| AF episodes ventricular rate (IQR) | 94 (73–111) | 97 (79–118) | 92 (70–109) | <0.001 |
| AF episodes duration, minutes (IQR) | 14 (4–140) | 8 (2–78) | 20 (4–182) | <0.001 |
| Longest AF episode duration, minutes (IQR) | 877 (202–3080) | 756 (38–1084) | 2104 (326–5840) | 0.022 |
| AF episodes duration (%) | 3607 (100) | 965 (100) | 2642 (100) | <0.001 |
| <10 min | 1662 (46.1) | 517 (53.6) | 1145 (43.3) | |
| 10 min to 6 h | 1302 (36.1) | 350 (36.2) | 952 (36.0) | |
| >6 h | 643 (17.8) | 98 (10.2) | 545 (20.7) | |
| Early postoperative AF incidence and patterns | ||||
| Early-POAF first 30 days (%) | 73 (56.2) | 40 (44.9) | 33 (80.5) | 0.001 |
| Early-POAF burden first 30 days, % (IQR) | 2.819 (0.909–6.590) | 2.519 (0.342–7.736) | 3.055 (1.333–5.337) | 0.660 |
| AF episodes per patient (IQR) | 6 (2–22) | 4 (2–14) | 10 (3–23) | 0.305 |
| AF episodes ventricular rate (IQR) | 103 (90–120) | 98 (82–121) | 105 (95–120) | 0.004 |
| AF episodes duration, minutes (IQR) | 17 (4–120) | 14 (2–95) | 20 (4–132) | 0.116 |
| Longest AF episode duration, minutes (IQR) | 648 (254–988) | 479 (126–920) | 769 (326–990) | 0.495 |
| AF episodes duration (%) | 730 (100) | 311 (100) | 419 (100) | 0.245 |
| <10 min | 309 (42.3) | 141 (45.3) | 168 (40.1) | |
| 10 min to 6 h | 307 (42.1) | 128 (41.2) | 179 (42.7) | |
| >6 h | 114 (15.7) | 42 (13.5) | 72 (17.2) | |
| Late postoperative AF incidence and patterns | ||||
| Late-POAF after first 30 days (%) | 53 (40.8) | 27 (30.3) | 26 (63.4) | <0.001 |
| Late-POAF burden after first 30 days, % (IQR) | 0.048 (0.005–0.659) | 0.014 (0.002–0.390) | 0.138 (0.008–0.908) | 0.146 |
| AF episodes per patient (IQR) | 17 (3–48) | 4 (2–25) | 23 (16–80) | 0.006 |
| AF episodes ventricular rate (IQR) | 91 (71–109) | 94 (78–118) | 88 (68–107) | <0.001 |
| AF episodes duration, minutes (IQR) | 14 (4–152) | 6 (2–74) | 18 (4–200) | <0.001 |
| Longest AF episode duration, minutes (IQR) | 166 (22–1005) | 122 (21–942) | 349 (22–1328) | 0.373 |
| AF episodes duration (%) | 2877 (100) | 654 (100) | 2223 (100) | <0.001 |
| <10 min | 1353 (47.0) | 376 (57.5) | 977 (43.9) | |
| 10 min to 6 h | 995 (34.6) | 222 (33.9) | 773 (34.8) | |
| >6 h | 529 (18.4) | 56 (8.6) | 473 (21.3) | |
Bold values imply statistical significance at an alpha threshold of 0.05.
Comparisons were performed based on individual patient median values for AF characteristics.
AF, atrial fibrillation; IQR, interquartile range; POAF, postoperative atrial fibrillation.
Subgroup analysis in patients without preoperative AF history showed no significant associations between histological traits and any-POAF incidence (see Supplementary material online, Table S3).
Early-POAF incidence
Early-POAF occurred in 73 patients (Table 4). No significant associations between histological characteristics and early-POAF incidence were observed in the overall cohort (see Supplementary material online, Table S4). Similarly, combined models including both RAA and LAA histological characteristics showed no significant associations (see Supplementary material online, Table S5). Subgroup analysis in patients without preoperative AF history also revealed no significant associations between histological traits and early-POAF incidence (see Supplementary material online, Table S6).
Late-POAF incidence
Late-POAF was identified in 53 patients. In unadjusted analyses, increased LAA cardiomyocyte diameter (HR = 1.40, 95% CI: 1.15–1.71, P < 0.001), LAA fibroblast density (HR = 4.52, 95% CI: 1.02–20.1, P = 0.047), RAA fibroblast density (HR = 3.98, 95% CI: 1.23–12.9, P = 0.021), and RAA endomysial fibrosis (HR = 1.09, 95% CI: 1.02–1.17, P = 0.017) were significantly associated with increased late-POAF incidence (see Supplementary material online, Table S7).
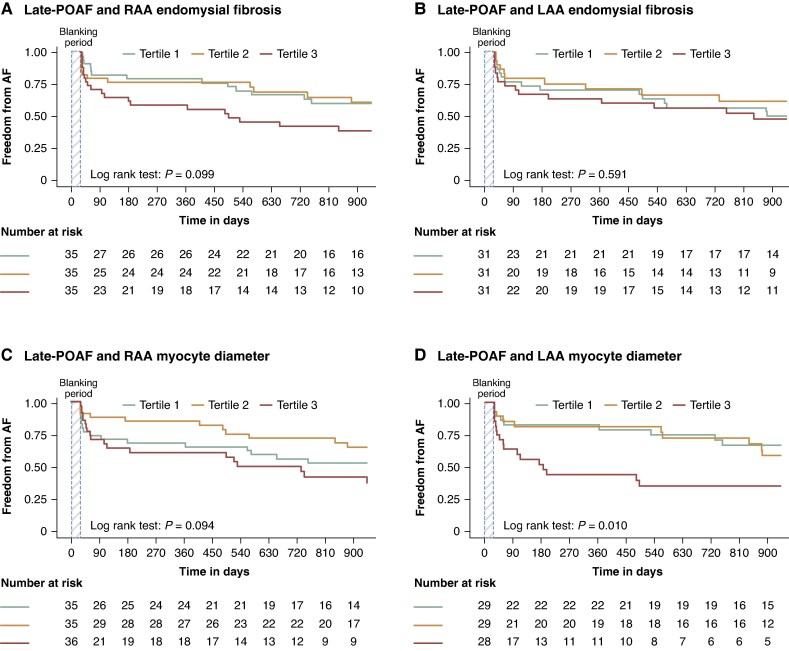
Tertile analysis showed no significant association between late-POAF and RAA endomysial fibrosis, LAA endomysial fibrosis, or RAA myocyte diameter (Figure 2A–C). However, LAA myocyte diameter remained significantly associated with late-POAF, particularly in the highest tertile (13.0 ± 1.8 µm; P = 0.010) (Figure 2D). This association remained significant in a combined model including both RAA and LAA myocyte diameter (HR = 1.37, 95% CI: 1.02–1.85, P = 0.039; Supplementary material online, Table S8). There were no significant associations between fibroblast density tertiles (RAA or LAA) and late-POAF incidence (P = 0.157 and P = 0.096, respectively). None of the histological characteristics remained significantly associated with late-POAF after adjusting for age, sex, AF history, surgery type, atrial volumes, and reduced LVF.
Figure 2.
Kaplan–Meier curves for POAF incidence and histological traits. (A) Kaplan–Meier curves for RAA endomysial fibrosis divided by tertiles and late-POAF incidence. (B) Kaplan–Meier curves for LAA endomysial fibrosis divided by tertiles and late-POAF incidence. (C) Kaplan–Meier curves for RAA cardiomyocyte diameter divided by tertiles and late-POAF incidence. (D) Kaplan–Meier curves for LAA cardiomyocyte diameter divided by tertiles and late-POAF incidence. AF, atrial fibrillation; LAA, left atrial appendage; POAF, postoperative atrial fibrillation; RAA, right atrial appendage.
In subgroup analysis of patients without preoperative AF history, both RAA capillary density and fibroblast density were associated with increased late-POAF incidence (see Supplementary material online, Table S9). However, after adjustment for age, sex, surgery type, atrial volumes, and reduced LVF, none of the histological traits remained significantly associated with late-POAF in this subgroup.
Histological traits and POAF burden
Overall-POAF burden
LAA endomysial fibrosis (St. Beta: 0.36, P = 0.016), RAA endomysial fibrosis (St. Beta: 0.42, P = 0.004), and RAA cardiomyocyte diameter (St. Beta: 0.36, P = 0.016) were significantly associated with higher overall-POAF burden (see Supplementary material online, Table S10). After adjustment for age, sex, AF history, surgery type, RAVI, and reduced LVF, only RAA endomysial fibrosis remained significantly associated (St. Beta: 0.32, P = 0.012; Figures 3A and 4A; Supplementary material online, Table S11). In subgroup analyses of patients without preoperative AF history, both RAA cardiomyocyte diameter (St. Beta = 0.26, P = 0.044) and RAA endomysial fibrosis (St. Beta = 0.33, P = 0.006) remained significantly associated with overall-POAF burden after adjustment for clinical confounders (see Supplementary material online, Table S12). Individual histological differences by preoperative AF history in patients with paired RAA and LAA samples are illustrated in Figure 5.
Figure 3.
Adjusted analysis for POAF burden and histological traits. (A) Adjusted association between RAA endomysial fibrosis and overall-POAF burden. (B) Adjusted association between RAA endomysial fibrosis and early-POAF burden. (C) Adjusted association between RAA endomysial fibrosis and late-POAF burden. (D) Adjusted association between RAA endomysial fibrosis and longest late-POAF episode duration. AF, atrial fibrillation; CABG, coronary artery bypass grafting; POAF, postoperative atrial fibrillation; RAA, right atrial appendage.
Figure 4.
Boxplots depicting the association between RAA endomysial fibrosis and POAF burden. (A) Boxplot depicting the association between RAA endomysial fibrosis and overall-POAF burden, (B) boxplot depicting the association between RAA endomysial fibrosis and early-POAF burden, (C) boxplot depicting the association between RAA endomysial fibrosis and late-POAF burden. EMF, endomysial fibrosis; POAF, postoperative atrial fibrillation; RAA, right atrial appendage; μm, micrometre.
Figure 5.
Individual examples of paired right and left atrial appendage histological analyses in patients with and without preoperative AF history. For the group without preoperative AF history, three study subjects were selected as examples: one with no POAF, one with a low overall-POAF burden (0.004% time in AF, 10 POAF episodes, longest episode lasting 16 min), and one with a high overall-POAF burden (1.35% time in AF, 416 POAF episodes, longest episode lasting 980 min). For the group with preoperative AF history, patients with paroxysmal AF (2.78% time in AF, 203 POAF episodes, longest episode lasting 1856 min) and persistent AF (92.85% time in AF, 768 POAF episodes, longest episode lasting 21 794 min) were presented as illustrative examples. All images are presented at 400 times magnification. AF, atrial fibrillation; POAF, postoperative atrial fibrillation; WGA, wheat germ agglutinin. Created in BioRender. Kawczynski, M. (2025) https://BioRender.com/75vf53d.
For the longest POAF episode, only LAA endomysial fibrosis was significant in unadjusted analysis for overall-POAF burden (St. Beta: 0.36, P = 0.024; Supplementary material online, Table S13), but not after adjustment for clinical confounders (see Supplementary material online, Table S14).
Early-POAF burden
RAA endomysial fibrosis was the only histological feature significantly associated with early-POAF burden in both unadjusted (St. Beta: 0.28, P = 0.048) and adjusted analyses (St. Beta: 0.30, P = 0.037; Figures 3B and 4B; Supplementary material online, Tables S10 and S11). In subgroup analyses limited to patients without preoperative AF history, both RAA cardiomyocyte diameter (St. Beta = 0.25, P = 0.025) and RAA endomysial fibrosis (St. Beta = 0.29, P = 0.007) remained significantly associated with early-POAF burden after adjustment for clinical confounders (see Supplementary material online, Table S12).
No histological features were significantly associated with the longest early-POAF episode (see Supplementary material online, Tables S13 and S14).
Late-POAF burden
In unadjusted analysis, RAA cardiomyocyte diameter (St. Beta = 0.43, P = 0.014) and RAA endomysial fibrosis (St. Beta = 0.47, P = 0.007) were significantly associated with higher late-POAF burden (see Supplementary material online, Table S10). After adjustment for clinical confounders, only RAA endomysial fibrosis remained significant (St. Beta = 0.43, P = 0.006; Figures 3C and 4C; Supplementary material online, Table S11). Also, in subgroup analyses of patients without preoperative AF history, the association between RAA endomysial fibrosis and late-POAF burden was significant after adjustment for relevant confounders (St. Beta = 0.32, P = 0.008) (see Supplementary material online, Table S12).
For the longest late-POAF episode, RAA endomysial fibrosis was significantly associated in both unadjusted (St. Beta = 0.38, P = 0.032) and adjusted analyses (St. Beta = 0.41, P = 0.012; Figure 3D; Supplementary material online, Tables S13 and S14).
Discussion
This study is the first to examine associations between atrial histology and device-detected POAF during long-term monitoring after cardiac surgery. It is also among the few that allowed extensive adjustment for clinical confounders. We found that elevated RAA endomysial fibrosis, rather than overall fibrosis, was independently associated with increased POAF burden in both early and late postoperative phases. Although several histological features were linked to POAF incidence in unadjusted analyses, none remained significant after adjustment. Additionally, patients with a preoperative AF history had significantly higher levels of RAA and LAA endomysial fibrosis, larger LAA cardiomyocyte diameters, and increased fibroblast density in both atria compared to patients without AF history. These findings highlight the potential relevance of right atrial remodelling in the pathogenesis of POAF and suggest that RAA fibrosis may serve as a marker of advanced atrial substrate, in patients undergoing cardiac surgery.
Postoperative AF incidence
While previous reports indicated that increased levels of overall atrial fibrosis were associated with in-hospital POAF in patients undergoing various types of cardiac surgery,6–8 we did not observe a significant association between both overall and endomysial atrial fibrosis and POAF incidence in the current study. Previous studies have identified an association between increased levels of atrial fibrosis and older age, consistently reported as an important predictor of POAF.6,17 Notably, atrial fibrosis also seems to be associated with prolonged P-wave duration, supporting the hypothesis that age-related atrial changes contribute to conduction slowing and inhomogeneities in AF patients.6 In accordance, increased levels of endomysial fibrosis were linked to increased conduction disturbances determined by high-coverage high-density epicardial mapping in human AF in a previous study.11 Fibrotic remodelling is proposed to interfere with cell-to-cell electrical coupling between atrial muscle fibres, leading to electrophysiological instability in atrial tissue and promoting re-entry arrhythmias.6,18 While fibrosis plays a major role in atrial arrhythmogenic remodelling and consequent progression of AF, other trigger mechanisms, such as atrial premature beats, catecholamines, electrolyte disturbances, and inflammatory reactions may also influence POAF incidence and sustainability, particularly in the early postoperative phase.11 In the late postoperative phase, lifestyle-specific triggers such as alcohol consumption, caffeine intake, and rigorous exercise might promote POAF initiation, leading to relatively short-lasting and self-terminating episodes, as observed in our study, which may be less dependent on presence of atrial fibrosis.19,20 Therefore, while atrial fibrosis contributes to re-entry mechanisms and AF sustainability, other trigger mechanisms might be responsible for POAF initiation, especially in short-lasting device-detected POAF, potentially explaining the lack of association between atrial fibrosis and POAF incidence in the current study.
Considering the other histological characteristics assessed in the current study, our results are largely consistent with previous findings. For instance, it has been reported that there is no significant association between RAA cardiomyocyte hypertrophy and the incidence of early-POAF,7,21 with only one study describing a significant association.22 In a prior study that categorized patients undergoing cardiac surgery by histological phenotype, hypertrophic atrial cardiomyopathy (AtCM) was not associated with a history of AF, in contrast to fibrotic AtCM, which showed a strong association with both AF history and heart failure.9 These findings suggest that fibrotic remodelling is more critical for sustaining AF than myocyte hypertrophy. Regarding the association of fibroblast and capillary density with the incidence of early-POAF, a previous study found a significant unadjusted association for increased fibroblast density but not for capillary density in LAA biopsies.23 In the current study, we did not observe a significant association between either of these histological traits and the incidence of early-POAF. However, we found that the association between increased fibroblast density, both in the RAA and LAA, and late-POAF incidence was significant in the unadjusted analysis. Nevertheless, this association diminished after adjusting for relevant clinical confounders.
Postoperative AF burden
Most studies on POAF have focused on its incidence during the early or late postoperative phases, while data on actual AF burden (time spent in AF) remain limited.2,3 Yet, assessing AF burden can provide meaningful insights into arrhythmia severity and help evaluate treatment strategies aimed at burden reduction.24 Although the relationship between AF burden and stroke risk remains uncertain, its measurement offers valuable information on AF susceptibility and persistence. The latter is particularly important, as it is influenced by episode frequency and duration.15 Clinically, patients with persistent AF are recognized to have a more advanced AF burden compared to those with paroxysmal AF, which on the histological level is reflected by increased levels of endomysial fibrosis and conduction disturbances.9,10
In the current study, we found that endomysial fibrosis, particularly in the RAA, was strongly associated with increased POAF burden, both in the early and late postoperative phases, independently of clinical confounders and atrial size. Furthermore, the duration of the longest POAF episode, a recognized surrogate for AF burden, also showed a significant association with RAA endomysial fibrosis.15 This observation raises the possibility that RAA fibrosis, rather than being a coincidental finding, may reflect an advanced stage of atrial remodelling relevant to AF persistence. While the majority of mechanistic literature has focused on LA remodelling as the primary driver of AF progression, the role of the right atrium (RA), particularly in relation to AF burden, remains less well defined. Based on our findings we hypothesize that RA involvement may emerge as a downstream consequence of advanced LA remodelling, which is not indefinite. Once a threshold of structural remodelling is reached in the LA, further haemodynamic stress and fibrotic remodelling may increasingly affect the RA. Supporting this, we previously identified bone morphogenetic protein 10 (BMP10), a RA-specific biomarker, as a robust predictor of both atrial fibrosis and late-POAF, with strong associations across both atria.4 In addition, prior studies consistently identified RAVI as the strongest predictor of late-POAF burden and recurrence. Together, these findings suggest that RA remodelling may serve as a clinically relevant marker of disease chronicity in patients undergoing cardiac surgery. Of note, the association between RAA endomysial fibrosis and POAF burden remained significant even after adjustment for RAVI, suggesting that mechanisms beyond atrial dilation may contribute. For example, increased endomysial fibrosis has been strongly linked to local conduction disturbances, particularly due to endo-epicardial dissociation. This may, at least in part, explain the volume-adjusted effect of RAA endomysial fibrosis on POAF burden.
Nevertheless, it should be noted that, despite the lack of a significant association between LAA endomysial fibrosis and POAF burden in the current study, the role of the posterior LA wall in AF pathophysiology remains undisputed, and LAA biopsies may not fully capture the overall severity of the LA substrate. Additionally, the relationship between the clinical classification of AtCM, based on mechanical and electrophysiological criteria, and histological findings from atrial biopsies warrants further investigation.25 Future studies should explore the association between AtCM severity and endomysial fibrosis in LAA samples to better understand this relationship.
Limitations
The study possesses several limitations that warrant consideration. First, as this is a single-centre study, the external generalizability of the findings needs to be demonstrated in future studies, despite the high level of internal validity resulting from consistent methodology and histological analysis. Secondly, there was a notably high rate of extraction of the ILR, attributable to both treatment and prevention of postoperative wound infections. In cases of suspected postoperative wound infection, ILRs were removed to mitigate potential study-associated risks for the participants. Thirdly, patients included in the study had likely different underlying mechanisms of atrial remodelling, reflected by varying indications for cardiac surgery, and diversities in surgical techniques among surgeons may have influenced the outcomes. Fourthly, the limited storage capacity of the ILR may have resulted in missed episodes of POAF. To address this, detailed instructions were provided to patients regarding night-time placement of the CareLink system and the frequency of manual transmissions. The investigators monitored transmission compliance on a weekly basis and reached out to patients who failed to transmit as instructed. Fifthly, it might be questionable whether the atrial appendage biopsies represent the structural status of the entire atrium, and, therefore, the results of the current study might be influenced by this uncertainty.
Conclusion
Increased endomysial fibrosis in RAA biopsies was associated with higher POAF burden, but not with POAF incidence, independent of relevant clinical confounders in a cohort of patients undergoing cardiac surgery with or without concomitant surgical AF ablation. These findings suggest that endomysial fibrosis, particularly in the RAA, exerts a strong pro-arrhythmic effect that may not be reflected in isolated AF recurrences, but rather in the persistence and duration of AF episodes (burden). This highlights that AF recurrences alone may not adequately capture the underlying pro-arrhythmic substrate in patients with POAF.
Supplementary Material
Contributor Information
Michal J Kawczynski, Department of Cardiothoracic Surgery, Heart and Vascular Centre Maastricht University Medical Centre, Maastricht, The Netherlands; Department of Physiology, Maastricht University, Universiteitssingel 50, 6229 ER Maastricht, The Netherlands; Cardiovascular Research Institute Maastricht (CARIM), Universiteitssingel 50, 6229 ER Maastricht, The Netherlands.
Martijn D Gilbers, Department of Cardiothoracic Surgery, Heart and Vascular Centre Maastricht University Medical Centre, Maastricht, The Netherlands; Department of Cardiothoracic Surgery, Medisch Spectrum Twente, Enschede, The Netherlands.
Joris Winters, Department of Physiology, Maastricht University, Universiteitssingel 50, 6229 ER Maastricht, The Netherlands; Cardiovascular Research Institute Maastricht (CARIM), Universiteitssingel 50, 6229 ER Maastricht, The Netherlands.
Bart Maesen, Department of Cardiothoracic Surgery, Heart and Vascular Centre Maastricht University Medical Centre, Maastricht, The Netherlands; Cardiovascular Research Institute Maastricht (CARIM), Universiteitssingel 50, 6229 ER Maastricht, The Netherlands.
Aaron Isaacs, Department of Physiology, Maastricht University, Universiteitssingel 50, 6229 ER Maastricht, The Netherlands; Cardiovascular Research Institute Maastricht (CARIM), Universiteitssingel 50, 6229 ER Maastricht, The Netherlands; Maastricht Centre for Systems Biology (MaCSBio), Maastricht University, Maastricht, The Netherlands.
Dominik Linz, Cardiovascular Research Institute Maastricht (CARIM), Universiteitssingel 50, 6229 ER Maastricht, The Netherlands; Department of Cardiology, Heart and Vascular Centre Maastricht University Medical Centre, P. Debyelaan 25, 6229 HX Maastricht, The Netherlands; Department of Biomedical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Michiel Rienstra, Department of Cardiology, University of Groningen, University Medical Centre Groningen, Groningen, The Netherlands.
Isabelle van Gelder, Department of Cardiology, University of Groningen, University Medical Centre Groningen, Groningen, The Netherlands.
Jos G Maessen, Department of Cardiothoracic Surgery, Heart and Vascular Centre Maastricht University Medical Centre, Maastricht, The Netherlands; Cardiovascular Research Institute Maastricht (CARIM), Universiteitssingel 50, 6229 ER Maastricht, The Netherlands.
Elham Bidar, Department of Cardiothoracic Surgery, Heart and Vascular Centre Maastricht University Medical Centre, Maastricht, The Netherlands; Cardiovascular Research Institute Maastricht (CARIM), Universiteitssingel 50, 6229 ER Maastricht, The Netherlands.
Ulrich Schotten, Department of Physiology, Maastricht University, Universiteitssingel 50, 6229 ER Maastricht, The Netherlands; Cardiovascular Research Institute Maastricht (CARIM), Universiteitssingel 50, 6229 ER Maastricht, The Netherlands; Department of Cardiology, Heart and Vascular Centre Maastricht University Medical Centre, P. Debyelaan 25, 6229 HX Maastricht, The Netherlands.
Supplementary material
Supplementary material is available at Europace online.
Funding
This work was supported by Hartstichting (CVON2014-09, RACE V Reappraisal of Atrial Fibrillation: Interaction between hyperCoagulability, Electrical remodeling, and Vascular Destabilisation in the Progression of AF, and grant number 01-002-2022-0118, EmbRACE: Electro-Molecular Basis and the theRapeutic management of Atrial Cardiomyopathy, fibrillation and associated outcomEs), the European Union (ITN Network Personalize AF: Personalized Therapies for Atrial Fibrillation: a translational network, grant number 860974; CATCH ME: Characterizing Atrial fibrillation by Translating its Causes into Health Modifiers in the Elderly, grant number 633196; MAESTRIA: Machine Learning Artificial Intelligence Early Detection Stroke Atrial Fibrillation, grant number 965286).
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Bidar E, Zeemering S, Gilbers M, Isaacs A, Verheule S, Zink MD et al. Clinical and electrophysiological predictors of device-detected new-onset atrial fibrillation during 3 years after cardiac surgery. Europace 2021;23:1922–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abdelmoneim SS, Rosenberg E, Meykler M, Patel B, Reddy B, Ho J et al. The incidence and natural progression of new-onset postoperative atrial fibrillation. JACC Clin Electrophysiol 2021;7:1134–44. [DOI] [PubMed] [Google Scholar]
- 3. Gilbers MD, Kawczynski MJ, Bidar E, Maesen B, Isaacs A, Winters J et al. Clinical predictors of device-detected atrial fibrillation during 2.5 years after cardiac surgery: prospective RACE V cohort. JACC Clin Electrophysiol 2024;10:941–55. [DOI] [PubMed] [Google Scholar]
- 4. Winters J, Kawczynski MJ, Gilbers MD, Isaacs A, Zeemering S, Bidar E et al. Circulating BMP10 levels associate with late postoperative atrial fibrillation and left atrial endomysial fibrosis. JACC Clin Electrophysiol 2024;10:1326–40. [DOI] [PubMed] [Google Scholar]
- 5. Lin MH, Kamel H, Singer DE, Wu YL, Lee M, Ovbiagele B. Perioperative/postoperative atrial fibrillation and risk of subsequent stroke and/or mortality. Stroke 2019;50:1364–71. [DOI] [PubMed] [Google Scholar]
- 6. Goette A, Juenemann G, Peters B, Klein HU, Roessner A, Huth C et al. Determinants and consequences of atrial fibrosis in patients undergoing open heart surgery. Cardiovasc Res 2002;54:390–6. [DOI] [PubMed] [Google Scholar]
- 7. Mariscalco G, Engström KG, Ferrarese S, Cozzi G, Bruno VD, Sessa F et al. Relationship between atrial histopathology and atrial fibrillation after coronary bypass surgery. J Thorac Cardiovasc Surg 2006;131:1364–72. [DOI] [PubMed] [Google Scholar]
- 8. Swartz MF, Fink GW, Lutz CJ, Taffet SM, Berenfeld O, Vikstrom KL et al. Left versus right atrial difference in dominant frequency, K+ channel transcripts, and fibrosis in patients developing atrial fibrillation after cardiac surgery. Heart Rhythm 2009;6:1415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Winters J, Isaacs A, Zeemering S, Kawczynski M, Maesen B, Maessen J et al. Heart failure, female sex, and atrial fibrillation are the main drivers of human atrial cardiomyopathy: results from the CATCH ME consortium. J Am Heart Assoc 2023;12:e031220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maesen B, Verheule S, Zeemering S, La Meir M, Nijs J, Lumeij S et al. Endomysial fibrosis, rather than overall connective tissue content, is the main determinant of conduction disturbances in human atrial fibrillation. Europace 2022;24:1015–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maesen B, Nijs J, Maessen J, Allessie M, Schotten U. Post-operative atrial fibrillation: a maze of mechanisms. Europace 2012;14:159–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gilbers MD, Bidar E, Maesen B, Zeemering S, Isaacs A, Crijns H et al. Reappraisal of Atrial fibrillation: interaction between hyperCoagulability, Electrical remodelling and Vascular destabilisation in the progression of AF (RACE V) Tissue Bank Project: study design. Neth Heart J 2021;29:280–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Winters J, von Braunmuhl ME, Zeemering S, Gilbers M, Brink TT, Scaf B et al. JavaCyte, a novel open-source tool for automated quantification of key hallmarks of cardiac structural remodeling. Sci Rep 2020;10:20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pürerfellner H, Sanders P, Sarkar S, Reisfeld E, Reiland J, Koehler J et al. Adapting detection sensitivity based on evidence of irregular sinus arrhythmia to improve atrial fibrillation detection in insertable cardiac monitors. Europace 2018;20:f321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gilbers MD, Kawczynski MJ, Bidar E, Maesen B, Isaacs A, Winters J et al. Determinants and impact of postoperative atrial fibrillation burden during 2.5 years of continuous rhythm monitoring after cardiac surgery: results from the RACE V prospective cohort study. Heart Rhythm 2025;22:647–60. [DOI] [PubMed] [Google Scholar]
- 16. De With RR, Erküner Ö, Rienstra M, Nguyen BO, Körver FWJ, Linz D et al. Temporal patterns and short-term progression of paroxysmal atrial fibrillation: data from RACE V. Europace 2020;22:1162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dobrev D, Aguilar M, Heijman J, Guichard JB, Nattel S. Postoperative atrial fibrillation: mechanisms, manifestations and management. Nat Rev Cardiol 2019;16:417–36. [DOI] [PubMed] [Google Scholar]
- 18. Cosgrave J, Foley JB, Flavin R, O'Briain DS, Fitzpatrick E, Bennett K et al. Preoperative atrial histological changes are not associated with postoperative atrial fibrillation. Cardiovasc Pathol 2006;15:213–7. [DOI] [PubMed] [Google Scholar]
- 19. Groh CA, Faulkner M, Getabecha S, Taffe V, Nah G, Sigona K et al. Patient-reported triggers of paroxysmal atrial fibrillation. Heart Rhythm 2019;16:996–1002. [DOI] [PubMed] [Google Scholar]
- 20. Marcus GM, Modrow MF, Schmid CH, Sigona K, Nah G, Yang J et al. Individualized studies of triggers of paroxysmal atrial fibrillation: the I-STOP-AFib randomized clinical trial. JAMA Cardiol 2022;7:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nakai T, Chandy J, Nakai K, Bellows WH, Flachsbart K, Lee RJ et al. Histologic assessment of right atrial appendage myocardium in patients with atrial fibrillation after coronary artery bypass graft surgery. Cardiology 2007;108:90–6. [DOI] [PubMed] [Google Scholar]
- 22. Tinica G, Mocanu V, Zugun-Eloae F, Butcovan D. Clinical and histological predictive risk factors of atrial fibrillation in patients undergoing open-heart surgery. Exp Ther Med 2015;10:2299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van den Berg NWE, Neefs J, Kawasaki M, Nariswari FA, Wesselink R, Fabrizi B et al. Extracellular matrix remodeling precedes atrial fibrillation: results of the PREDICT-AF trial. Heart Rhythm 2021;18:2115–25. [DOI] [PubMed] [Google Scholar]
- 24. Chen LY, Chung MK, Allen LA, Ezekowitz M, Furie KL, McCabe P et al. Atrial fibrillation burden: moving beyond atrial fibrillation as a binary entity: a scientific statement from the American Heart Association. Circulation 2018;137:e623–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goette A, Corradi D, Dobrev D, Aguinaga L, Cabrera JA, Chugh SS et al. Atrial cardiomyopathy revisited-evolution of a concept: a clinical consensus statement of the European Heart Rhythm Association (EHRA) of the ESC, the Heart Rhythm Society (HRS), the Asian Pacific Heart Rhythm Society (APHRS), and the Latin American Heart Rhythm Society (LAHRS). Europace 2024;26:euae204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.