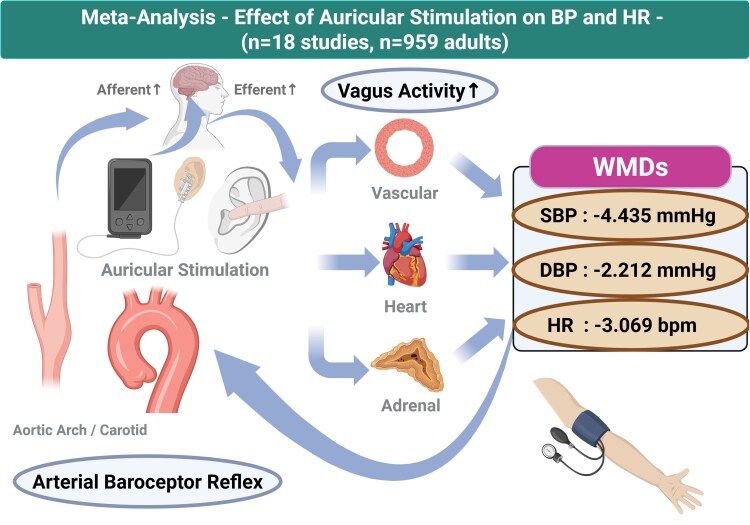
Abstract
Earlier clinical trials have investigated the efficacy of auricular stimulation for hypertension, but the overall evidence regarding the effect of non-invasive auricular stimulation on blood pressure (BP) reduction remains unclear. This systematic review summarizes the effects of non-invasive auricular stimulation on cardiovascular haemodynamics. We searched for studies published in English through PubMed, ICHUSHI, and Cochrane Central Library databases and reviewed randomized controlled trials involving adults. Data collection and analysis were performed on the relationships of non-invasive auricular electrical stimulation and acupressure with changes in haemodynamics. A meta-analysis was conducted on the effects of non-invasive auricular stimulation on systolic BP (SBP), diastolic BP (DBP), and heart rate (HR). In the primary analysis, effect sizes were extracted from 18 studies for a total analytic sample of n = 959. Non-invasive auricular stimulation significantly reduced in SBP [weighted mean difference (WMD) = −4.435 mmHg, 95% confidence interval (CI) (−7.037 to −1.832)], DBP [WMD = −2.212 mmHg, 95% CI (−3.734 to −0.690)], and HR [WMD = −3.069 beats/min, 95% CI (−5.389 to −0.749)]. Overall, heterogeneity in each analysis was high, which could be explained by the stimulation duration and baseline values of SBP, DBP, and HR. There were no serious adverse events across all 18 studies. Enhancing vagus nerve activity through non-invasive auricular stimulation leads to clinically safe reductions in BP and HR. Further studies are needed to clarify whether non-invasive auricular stimulation can be used as a viable treatment for hypertension.
Keywords: Auricular stimulation, Autonomic nervous, Blood pressure, Heart rate, Vagus nervous system
Graphical Abstract
Graphical Abstract.
Introduction
High blood pressure (BP) is the leading cause of death and disability worldwide. In the USA, hypertension accounted for more cardiovascular deaths than any other modifiable risk factor of cardiovascular disease. In the National Health and Nutrition Examination Survey, more than half of deaths from coronary heart disease and stroke occurred among individuals with hypertension.1 In the report of Global Cardiovascular Risk Consortium, the population attributable fraction of the 10-year incidence of cardiovascular disease related to systolic BP (SBP) was 29.3% in women and 21.6% in men. Elevated SBP was the largest population attributable cause of cardiovascular disease development.2 Although hypertension has traditionally been considered to affect primarily older people, the diagnosis of hypertension is increasing among younger and middle-aged adults.3
The main pathophysiological mechanism of essential hypertension is vasoconstriction caused by increased sympathetic tone. In hypertension, enhanced sympathetic outflow also increases peripheral vascular resistance which results in higher afterload for heart.4,5 Therefore, sympathovagal imbalance has been proposed to be closely associated with cardiovascular outcomes.6 From a therapeutic standpoint, sympathetic overactivity is a target for the treatment of hypertension. Previous studies have demonstrated that transcutaneous auricular stimulation enhances vagal nerve activity and parasympathetic signalling, while it significantly reduced sympathetic nervous system activity.7 Thus, auricular vagally mediated neuromodulation could be a viable prevention or treatment for hypertension.
There has been no comprehensive evidence that non-invasive auricular stimulation has an effect on haemodynamics as shown in meta-analysis to date. Invasive neuromodulation via vagus nervous system might have the possibility of stimulating sympathetic nervous system activity, making it difficult to find a direct relationship between stimulation and haemodynamic change. Given the safety and scalability of non-invasive auricular stimulation, it is worthwhile to evaluate the evidence for its effectiveness on SBP, diastolic BP (DBP), and heart rate (HR) lowering. In this systematic review and meta-analysis, the efficacy of non-invasive auricular stimulation vs. sham or no intervention for haemodynamic changes in BP and/or HR is summarized in adult populations with and without hypertension.
Methods
Data sources and searches
The method used to conduct this systematic review has been previously registered in PROSPERO, an international database of prospectively registered systematic reviews (CRD42024594032). This systematic review and meta-analysis were conducted according to the PRISMA statement (checklist in the Supplementary material). A systematic review and meta-analysis of the studies assessing the association between non-invasive auricular stimulation and BP-lowering effect were conducted for studies published up to November 2024.
Inclusion and exclusion criteria
The inclusion according to the PICO criteria consisted of (P) patients/participants: adults (age > 18 years old); (I) intervention: non-invasive auricular stimulation, which includes acupressure or low-level auricular electrical stimulation; (C) comparison: sham stimulation or no intervention; and (O) outcomes: BP and HR after stimulation between active and sham groups. This systematic review and meta-analysis include human randomized controlled trials (RCTs). RCTs of invasive auricular stimulation such as acupuncture and non-RCTs were excluded.
Types of outcome measures
The main outcome variables are differences in SBP, DBP, and HR between active and sham/control groups presented as mean and standard deviation. Effect size is calculated using mean difference between active and sham/control groups. The additional outcome variable is number of patients with adverse effects which we defined as including any hypotension, bradycardia, dizziness, nausea, vomiting, itching, headache, bruising, erythema, skin irritation, or/and pain. Serious adverse events were defined as adverse effects from auricular stimulation that might result in serious harm, neuronitis, skin carcinoma, stroke, coronary artery disease, disability, or/and even sudden death.
Data sources and search strategies
Systematic search was performed based on PubMed/MEDLINE, Cochrane Library, and Igaku Chuo Zasshi (ICHUSHI: database of Japanese journals launched by the Japan Medical Abstracts Society). The study language is restricted to English. We searched with the following term; ((‘blood pressure’ AND ‘acupressure’) OR (‘hypertension’ AND ‘acupressure’) OR (‘baroreflex’ AND ‘acupressure’)) OR ((‘blood pressure’ AND ‘transcutaneous tragus stimulation’) OR (‘blood pressure’ AND ‘noninvasive vagus nerve stimulation’) OR (‘blood pressure’ AND ‘transcutaneous tragus stimulation’) OR (‘blood pressure’ AND ‘noninvasive vagus nerve stimulation’) OR (‘blood pressure’ AND ‘low level tragus stimulation’) OR (‘blood pressure’ AND ‘auricular stimulation’)) OR ((‘hypertension’ AND ‘transcutaneous tragus stimulation’) OR (‘hypertension’ AND ‘noninvasive vagus nerve stimulation’) OR (‘hypertension’ AND ‘transcutaneous tragus stimulation’) OR (‘hypertension’ AND ‘noninvasive vagus nerve stimulation’) OR (‘hypertension’ AND ‘low level tragus stimulation’) OR (‘hypertension’ AND ‘auricular stimulation’)) OR ((‘baroreflex’ AND ‘acupressure’) OR (‘baroreflex’ AND ‘transcutaneous tragus stimulation’) OR (‘baroreflex’ AND ‘noninvasive vagus nerve stimulation’) OR (‘baroreflex’ AND ‘transcutaneous tragus stimulation’) OR (‘baroreflex’ AND ‘noninvasive vagus nerve stimulation’) OR (‘baroreflex’ AND ‘low level tragus stimulation’) OR (‘baroreflex’ AND ‘auricular stimulation’)).
Data extraction and assessment of risk of bias in included trials
Two researchers screened independently titles, abstracts, and full texts for eligibility. The information included the country in which the study was conducted, publication year, sample size, age, gender, intervention, comparison, outcomes, withdrawal/dropout, and adverse events using a standardized data extraction template. All information was extracted in duplicate by two reviewers and in the event of any inconsistencies was resolved by discussion with each other to form consensus. The risk of bias tool was used to evaluate the risk of bias for the primary outcome change in BP and/or HR. The tool includes the following seven domains of bias: (i) randomization process, (ii) deviations from the intended interventions, (iii) missing outcome data, (iv) measurement of the outcome, and (v) selection of the reported result.8 We rated each domain as well as the overall risk of bias as low, some concerns or high risk of bias.
Strategy for data synthesis
The SPSS 28 (IBM Corp., Armonk, NY) and EZR (Saitama Medical Center, Jichi Medical University), which is a graphical user interface for R (The R Foundation for Statistical Computing, version 2.13.0, Vienna, Austria), were used to conduct the meta-analysis. Continuous outcomes of differences in SBP, DBP, and HR after stimulation between active and sham groups are expressed as inverse-variance weighted mean differences (WMDs) with 95% confidence interval (CI). As all outcomes are measured on the same scale (e.g. mmHg or beats/min), the WMD effect sizes are reported in the original units. Cochran’s Q-test and I² index were used to assess the statistical heterogeneity between studies, with I² ≥ 50% indicating significant heterogeneity. When no significant heterogeneity was detected, the fixed or common effects model was applied. The random effects model was applied otherwise. P < 0.05 was considered statistically significant. Subgroup analyses and meta-regression analysis were attempted to address potential sources of heterogeneity. Stratified analyses were conducted on age [young/middle age (<65 years) or older adults (65 years or more)], auricular acupressure or electrical stimulation, populations with or without cardiovascular disease or high cardiovascular disease risk, populations with or without hypertension, populations with or without antihypertensive medications, stimulation duration (<1 h or 1 h to 1 week or more than 1 week), and short or chronic stimulation. Sensitivity analyses were performed using a leave-one-out (LOO) method for studies in which BP and HR were measured in specific conditions or among populations. Meta-regression was used incorporating factors of SBP, DBP, or HR at baseline conditions. To assess potential publication bias, funnel plots were used, and Egger tests were performed if the number of included studies was greater than 10.
In addition, among the studies of auricular electrical stimulation, stratified analyses were conducted on stimulation frequency (5, 20, or 25 Hz) and laterality (left, right, left or right side, or both sides). In the meta-regression, stimulation amplitude (mA) was used as an incorporating factor.
Results
Based on the search method, 324 studies were found, 44 duplicates were removed, and 31 records removed for other reasons. After 249 records screened for titles and abstracts, 213 records were excluded. After careful evaluation, from 44 articles evaluated at full text, 18 studies were finally included, as per the inclusion criteria.9–26 Those studies were analysed in the systematic review and meta-analysis (Tables 1 and 2 and Figure 1).
Table 1.
Non-invasive auricular electrical stimulation and changes in blood pressure and heart rate
| Study (author/year/design) | Subjects/age | Laterality/location of stimulation | Duration of stimulation | Frequency/amplitude | Changes in BP (mmHg) and HR (b.p.m.) before and after stimulations |
|---|---|---|---|---|---|
| Sellaro et al., 20159: sham controlled | The 24 healthy young: male 13%: mean 19 (18–22) years | Lt. ear lobe (control), Lt. tragus (active) | 8 min (3 times) | 25 Hz: 0.5 mA | In the sham stimulation, SBP/DBP/HR before and after stimulation were 119 ± 16/72 ± 10/82 ± 13 and 119 ± 14/73 ± 10/74 ± 11, respectively. In the active stimulation, SBP/DBP/HR before and after stimulation were 118 ± 15/70 ± 10/80 ± 14 and 116 ± 15/71 ± 8.8/80 ± 14, respectively. |
| Fischer et al., 201811: sham controlled | The 21 healthy young: male 14%: 20 ± 1 years | Lt. ear lobe (sham), Lt. concha (active) | 36 min | 25 Hz: 1.3 (active)/1.5 (sham) mA | In the sham stimulation, SBP/DBP/HR before and after stimulation were 112 ± 7.3/73 ± 7.8/75 ± 2.3 and 109 ± 7.3/73 ± 6.4/66 ± 2.8, respectively. In the active stimulation, SBP/DBP/HR before and after stimulation were 112 ± 12/72 ± 9.6/77 ± 13 and 108 ± 8.7/74 ± 6.4/66 ± 9.6, respectively. |
| Ventura-Bort et al., 201812: sham controlled | 21 healthy young males: male 14%: 20 ± 1 years | Lt. ear lobe (sham), Lt. concha (active) | 35 min | 25 Hz: 1.3 (active)/1.5 (sham) mA | In the sham stimulation, SBP/DBP/HR before and after stimulation were 112 ± 7.5/73 ± 8.2/75 ± 14 and 109 ± 7.4/73 ± 6.6/66 ± 9.3, respectively. In the active stimulation, SBP/DBP/HR before and after stimulation were 112 ± 13/72 ± 5.6/78 ± 13 and 108 ± 9.1/74 ± 6.9/66 ± 9.8, respectively. |
| Giraudier et al., 202013: sham controlled | 60 healthy young males: male 23%: 24 ± 5 years | Lt. ear lobe (sham), Lt. concha (active) | 23 min | 25 Hz 1.5 (active)/1.3 (sham) mA | In the sham stimulation, SBP/DBP/HR before and after stimulation were 116 ± 9.8/78 ± 7.1/73 ± 12 and 109 ± 12/74 ± 6.5/70 ± 7.9, respectively. In the active stimulation, SBP/DBP/HR before and after stimulation were 116 ± 12/77 ± 8.3/74 ± 14 and 108 ± 11/73 ± 7.6/71 ± 9.0, respectively. |
| Gauthey et al., 202014: crossover controlled | 28 healthy young males: male 100%: 27 ± 4 years | Rt. ear lobe (control), Rt. concha (active) | 10 min | 5 Hz, 20 Hz: NA (amplitude) | The SBP/DBP/HR before and after stimulation at control, at 5 Hz and at 20 Hz were 115 ± 11/59 ± 9/62 ± 8.4 and 115 ± 12/59 ± 9/61 ± 7.7, 116 ± 11/59 ± 9/61 ± 9.2 and 115 ± 10/59 ± 9/61 ± 8.7, and 115 ± 12/59 ± 9/62 ± 8.3 and 115 ± 10/59 ± 8/61 ± 8.3, respectively. |
| Sinkovec et al., 202315: sham controlled | The 50 healthy young: male 52%: mean 26 (20–39) years | Rt. tragus-no-stimulation (control), Rt. tragus stimulation (active) | 20 min | 20 Hz: 90 μA, 130 μA | In the control, SBP/DBP/HR was 113 ± 12/71 ± 8.9/65 ± 7.8, respectively. In the active stimulation at 90 μA and at 130 μA, SBP/DBP/HR were 113 ± 11/71 ± 8.1/65 ± 8.1 and 113 ± 13/72 ± 9.5/65 ± 8.4, respectively. |
| Hatik et al., 202316: sham controlled | The 90 healthy young: male 50%: 18–35 years | Bi. concha/tragus-no-stimulation (sham, n = 30) Lt. concha/tragus stimulation (active, n = 30), Bi. concha/tragus stimulation (active, n = 30) |
20 min (4 days) | 20 Hz: NA (amplitude) | In the group with bilateral sham stimulation, the values in SBP/DBP/HR at control and after stimulation were 164 ± 5.5/94 ± 9.2/134 ± 11 and 153 ± 5.8/90 ± 9.2/116 ± 11, respectively. In the active group with left tragus stimulation, the values in SBP/DBP/HR at control and after stimulation were 163 ± 6.1/92 ± 7.2/133 ± 8.2 and 142 ± 6.2/86 ± 6.7/109 ± 9.0, respectively. In the active group with bilateral tragus stimulation, the values in SBP/DBP/HR at control and after stimulation were 160 ± 6.4/91 ± 8.9/129 ± 7.8 and 130 ± 6.1/82 ± 8.6/102 ± 6.1, respectively. |
| de Moraes et al., 202317: parallel-group controlled | 30 patients with metabolic syndrome: male 33%: 43 ± 9 (active, n = 20)/46 ± 10 (control, n = 10) years | Lt. concha no-stimulation (control) Lt. concha stimulation (active) |
30 min once a week (8 weeks) | 25 Hz: 0.1–5.0 mA | In the control group, the values in SBP/DBP/HR before and after stimulation (8 weeks) were 135 ± 21/80 ± 10/74 ± 10 and 133 ± 21/83 ± 10/76 ± 10, respectively. In the active group, the values in SBP/DBP/HR before and after stimulation (8 weeks) were 137 ± 21/81 ± 10/72 ± 7 and 121 ± 11/77 ± 8/68 ± 8, respectively. |
| Percin et al., 202418: sham controlled | The 76 healthy young: male 53%: mean 25–26 years | Bi. tragus no-stimulation (sham, n = 19), Bi. tragus stimulation (active, n = 19), Bi. behind-ear no-stimulation (sham, n = 19), Bi. behind-ear stimulation (active, n = 19) | 20 min | 25 Hz: 0.13–50 mA | In the sham group at tragus, the values in SBP/DBP/HR before and after stimulation were 125 ± 15/88 ± 10/93 ± 15 and 123 ± 13/87 ± 12/95 ± 9.5, respectively. In the active group at tragus, the values in SBP/DBP/HR before and after stimulation were 136 ± 12/93 ± 14/90 ± 14 and 119 ± 16/82 ± 14/82 ± 5.3, respectively. In the sham group at behind-ear, the values in SBP/DBP/HR before and after stimulation were 119 ± 15/86 ± 8.5/93 ± 10 and 118 ± 16/88 ± 10/92 ± 5.9, respectively. In the active group at behind-ear, the values in SBP/DBP/HR before and after stimulation were 120 ± 15/86 ± 8.4/91 ± 13 and 107 ± 16/77 ± 10/88 ± 6.1, respectively. |
| Mbikyo et al., 202419: sham controlled | 40 patients with Grade 1 hypertension: male 58%: 29 ± 5.4 (sham, n = 19)/31 ± 7.1 (active, n = 21) years | Tragus (Lt. or Rt. not specified) | 60 min. a day, 5 days a week (12 weeks) | 20 Hz: 1 mA | In the sham group, the values in SBP/DBP/HR before and those after stimulation (12 weeks) were 143 ± 8.6/92 ± 6.1/71 ± 6.3 and 137 ± 8.0/88 ± 5.3/72 ± 5.9, respectively. In the active group, the values in SBP/DBP/HR before and those after stimulation (12 weeks) were 143 ± 8.2/90 ± 5.8/70 ± 8.7 and 129 ± 7.1/81 ± 6.0/69 ± 9.0, respectively. |
| Gentileet al., 202420: sham controlled | 16 patients with chronic heart failure with ejection fraction < 50%: male 63%: mean 65 ± 8 years | Lt. tragus stimulation (n = 16), Rt. tragus stimulation (n = 16) | 10 min | 20 Hz: Lt. median 23 mA, Rt. median 23 mA | The SBP/DBP/HR at baseline and after Lt. and Rt. tragus stimulation were 106 ± 13/60 ± 11/76 ± 11, 107 ± 11/62 ± 10/78 ± 10, and 108 ± 13/61 ± 10/77 ± 10, respectively. |
BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate.
Table 2.
Non-invasive auricular acupressure and changes in blood pressure and heart rate
| Study (author/year/design) | Subjects/age | Laterality/location of stimulation | Duration of stimulation | Changes in BP (mmHg) and HR (b.p.m.) before and after stimulations |
|---|---|---|---|---|
| Barker et al., 200621: sham controlled | The 38 elderly with hip fracture: male 13.2%: 86 ± 4.8 (control, n = 20) vs. 87 ± 4.0 (active, n = 18) years | 1 acupoint without affect haemodynamics (control) 3 selected acupoints (active) |
1 min | In the sham stimulation group, the values in SBP/DBP/HR before and after procedure were 125 ± 7.4/78 ± 7.7/92 ± 12 and 126 ± 20/81 ± 13/90 ± 8.0, respectively. In the active stimulation group, the mean changes in SBP/DBP/HR before and after procedure were 125 ± 12/74 ± 9.4/95 ± 8.3 and 120 ± 19/73 ± 13/73 ± 9.4, respectively. |
| Yeh et al., 201522: randomized, controlled | 123 hypertensives: male 48.8%: 61 ± 7.7 (control, n = 60) vs. 60 ± 7.4 (active, n = 63) years | Routine care (control) 6 selected acupoints (active) |
2 min (3 times/day for 10 weeks) | In the control group, the values in SBP/DBP/HR before and after procedure were 125 ± 14/78 ± 4.2/69 ± 12 and 122 ± 14/77 ± 3.6/70 ± 13, respectively. In the active acupressure group, the values in SBP/DBP/HR before and after procedure were 127 ± 14/78 ± 5.3/64 ± 11 and 124 ± 13/76 ± 5.2/70 ± 11, respectively. |
| Kuo et al., 201623: randomized, controlled | 76 females with postpartum: male 0%: 33 ± 4.3 (control, n = 37) vs. 33 ± 3.6 (active, n = 39) years | Routine care (control) 1 selected acupoints (active) |
3 min (2 times/day for 4 days) | In the control group, the values in SBP/DBP/HR before and after procedure were 113 ± 16/72 ± 9.1/84 ± 12 and 119 ± 13/79 ± 9.6/86 ± 9.1, respectively. In the active acupressure group, the values in SBP/DBP/HR before and after procedure were 108 ± 16/70 ± 9.5/84 ± 12 and 113 ± 16/76 ± 12/77 ± 9.0, respectively. |
| Park et al., 202324: sham controlled | The 46 hypertensives: male 39.1%: 84 ± 5.7 (control, n = 22) vs. 82 ± 5.6 (active, n = 24) | 5 selected acupoints not related with haemodynamics (control) 5 selected acupoints related with haemodynamics (active) |
N/A (5 times/day for 8 weeks) |
In the control group, the values in SBP/DBP/HR before and after procedure were 139 ± 3.2/79 ± 1.4/72 ± 2.4 and 133 ± 2.7/73 ± 2.2/73 ± 2.0, respectively. In the active acupressure group, the values in SBP/DBP/HR before and after procedure were 146 ± 3.1/82 ± 1.3/74 ± 2.3 and 128 ± 2.6/77 ± 2.1/73 ± 1.9, respectively. |
| Kim and Park, 202325: placebo controlled | The 46 hypertensives: male 0%: 70 ± 4.8 (control, n = 23) vs. 71 ± 5.3 (active, n = 23) | No acupressure (control) 5 selected acupoints (active) |
N/A (8 weeks) | In the control group, the values in SBP/DBP/HR before and after procedure were 151 ± 8.3/83 ± 5.8/77 ± 4.0 and 150 ± 9.3/86 ± 8.1/77 ± 3.8, respectively. In the active stimulation, the values in SBP/DBP/HR before and after procedure were 147 ± 6.8/83 ± 5.6/79 ± 6.8 and 141 ± 5.3/79 ± 4.5/74 ± 5.6, respectively. |
| Sajadi et al., 202326: sham controlled | 94 males undergoing non-emergency coronary angiography: male 100%: 52 ± 12 (control, n = 47) vs. 55 ± 12 (active, n = 47) years | 4 selected acupoints not-related with anxiety and endocrine (control) 4 selected acupoints related with anxiety and endocrine (active) |
Each point for 1 min (60 min before angiography) | In the sham group, the values in SBP/DBP/HR before and after procedure were 134 ± 1.3/90 ± 2.0/81 ± 1.9 and 133 ± 1.2/90 ± 1.9/81 ± 1.7, respectively. In the active acupressure group, the values in SBP/DBP/HR before and after procedure were 135 ± 1.2/89 ± 1.6/85 ± 1.3 and 132 ± 1.1/88 ± 1.6/81 ± 1.2, respectively. |
| Keshtkar et al., 202410: randomized controlled | 80 females with gestational hypertension: male 0%: 28 ± 5.0 (control, n = 40) vs. 27 ± 4.9 (active, n = 40) years | Routine care (control) Selected acupoints (active) |
6 times a day for at least 1 min (twice a week for 1 month) | In the control group, the values in SBP/DBP before and after procedure were 142 ± 4.6/88 ± 6.2/and 136 ± 5.0/84 ± 4.9, respectively. In the active acupressure group, the values in SBP/DBP before and after procedure were 143 ± 4.4/88 ± 6.2 and 131 ± 4.0/80 ± 1.2, respectively. |
BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Figure 1.
Flow diagram of included studies.
Study characteristics
Among all studies, one each study was published from Austria,21 Belgium,14 Brasil,17 China,19 Italy,20 Netherlands,9 and Slovenia15; two studies from Iran,10,26 South Korea,24,25 Taiwan,22,23 and Turkey16,18 and three studies from Germany (Tables 1 and 2).11,13
The percentage of males was ranged from 0% to 100%.9–26 The four studies were conducted on people aged 65 years or older.20,21,24,25
There were 11 studies that utilized electric auricular stimulation,9,20 while seven studies were based on auricular acupressure.10,21–26
There were eight studies conducted on the healthy population,9,11–16,18 five studies were conducted on persons with hypertension,10,19,22,24,25 and one study each was conducted on chronic heart failure (HF),20 coronary heart disease,26 metabolic syndrome,17 hip fracture,21 and postpartum period.23
Five studies included subjects using antihypertensive medications.10,20,22,24,25 The rates of antihypertensive medication use in each study were approximately 5.3%,10 78.3%,25 94%,20 and 100%,22,24 respectively.
The 17 studies specified information on SBP, DBP, and HR,9,11–26 and one study described only SBP and DBP (Tables 1 and 2).10 A total of 18 studies were included with a total of 959 participants, 674 interventions vs. 285 controls. And, among these, 220 received both active and sham stimulations.
Risk of bias
All included studies were evaluated for risk of bias using the Cochrane Risk of Bias tool (ROB2.0). None of ROB2.0 raters were authors on the included RCTs. One auricular electrical stimulation study13 and one auricular acupressure study10 were assessed as being at overall high risk of bias. Fifteen studies were evaluated to be at some concerns for the bias.9,12,14–21,23–26 One study with acupressure was assessed to be at low risk of bias.22 This indicates that while most studies had some concerns regarding bias. The complete risk of bias assessment is found in Figure 2A and B.
Figure 2.
(A) Risk of bias assessment for each study included in the meta-analysis. (B) Risk of bias graph across all included studies.
Results of meta-analysis
Non-invasive auricular stimulation and changes in systolic blood pressure, diastolic blood pressure, and heart rate
Eighteen studies analysed the relationship between non-invasive auricular stimulation and changes in SBP and DBP, and 17 studies analysed HR. Compared to the control methods, non-invasive auricular stimulation had a significant influence on reduction in SBP [WMD = −4.435, 95% CI (−7.037 to −1.832) mmHg, test for overall effect, Z = −3.340, P = 0.0008; I2 = 93.1%] (Figure 3A). Similar results were found for reduction in DBP [WMD = −2.212, 95% CI (−3.734 to −0.690) mmHg, test for overall effect, Z = −2.848, P = 0.004; I2 = 84.2%] (Figure 3B) and in HR [WMD = −3.069, 95% CI (−5.389 to −0.749) beats/min, test for overall effect, Z = −2.593, P = 0.0095; I2 = 93.0%) (Figure 3C). Overall, heterogeneity of each analysis was very high with I2 ≥ 50%. The overall magnitude of effect of non-invasive auricular stimulation was greater on SBP compared with DBP.
Figure 3.
Forest plots of the outcomes for the association of non-invasive auricular stimulation with changes in systolic blood pressure, diastolic blood pressure, and heart rate. Effect size reported for changes in systolic blood pressure (A), diastolic blood pressure (B), and heart rate (C). *Active stimulation vs. control procedure. #Active stimulation vs. control status. Listed with ‘a’ to ‘i’ when there were multiple stimulation sites or methods. ‘a’ indicates pulse frequency of 5 Hz; ‘b’, pulse frequency of 20 Hz; ‘c’. stimulation amplitude of 90 μA; ‘d’, stimulation amplitude of 130 μA; ‘e’, left side stimulation; ‘f’, bilateral stimulation; ‘g’, tragus stimulation; ‘h’, behind-ear stimulation; ‘i’, right side stimulation.
Meta-regression and subgroup analyses
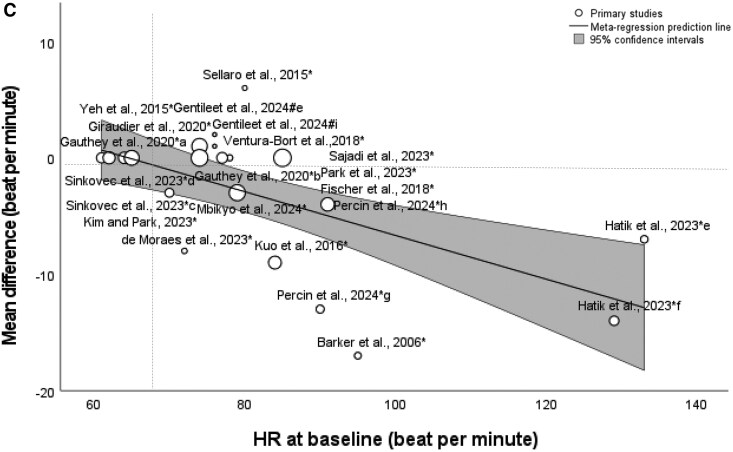
In the meta-regression analysis, SBP at baseline condition significantly moderated the relationship between non-invasive auricular stimulation and reduction in SBP [coefficient, −0.254 (95% CI, −0.356 to −0.152), P < 0.0001] (Figure 4A). Similar results were found for DBP at baseline condition [coefficient, −0.192 (95% CI, −0.321 to −0.062), P = 0.004] (Figure 4B) and for HR at baseline condition [coefficient, −0.188 (95% CI, −0.279 to −0.097), P < 0.0001] (Figure 4C). In the meta-regression among the studies of auricular electrical stimulation, stimulation amplitude (mA) significantly moderated the relationship between non-invasive auricular electrical stimulation and reduction in HR [coefficient, −0.202 (95% CI, −0.378 to −0.0259), P = 0.0246] (see Supplementary material online, Data Supplement; Figure S1).
Figure 4.
Bubble plots of meta-regression result for blood pressure and heart rate at baseline as covariate. The size of the bubble reflects the weight of the study. Line represents fitted meta-regression line for the associations of systolic blood pressure (A), diastolic blood pressure (B), and heart rate (C) at baseline with weighted mean differences.
Subgroup analyses by age (young/middle age or older) (see Supplementary material online, Figure S1), auricular acupressure or electrical stimulation (see Supplementary material online, Figure S2), populations with or without cardiovascular disease or high cardiovascular disease risk (see Supplementary material online, Figure S3), populations with or without hypertension (see Supplementary material online, Figure S4), populations with or without antihypertensive medications (see Supplementary material online, Figure S5), stimulation duration (<1 h, 1 h to 1 week, or more than 1 week) (see Supplementary material online, Figure S6), short or chronic period stimulation (see Supplementary material online, Figure S7) are reported in the Supplementary material online, Data Supplement. The analysis for subgroup differences regarding cardiovascular disease or its risk was significant for the relationship between non-invasive auricular stimulation and reduction in HR (P = 0.003). The analysis for subgroup differences regarding stimulation duration was significant for the relationship between non-invasive auricular stimulation and reduction in SBP (P = 0.001), DBP (P = 0.037), and HR (P = 0.001) (see Supplementary material online, Data Supplement). The relationship between non-invasive auricular stimulation and reduction in DBP and HR was not significant in the elderly but was significant in the young/middle age. In the hypertensive group, the relationship between non-invasive auricular stimulation and reduction in DBP and HR was not significant, while that in SBP was significant with an effect size like the overall analysis. In the populations with antihypertensive medication, the relationship between non-invasive auricular stimulation and reduction in SBP, DBP, and HR was not significant. The relationship between non-invasive auricular stimulation and reduction in DBP was not significant in the group with 1-week stimulation (P = 0.09), and the relationship between non-invasive auricular stimulation and reduction in HR was not significant in the groups with 1 min∼1 h (P = 0.25) and with 1-week stimulation (P = 0.098) (see Supplementary material online, Data Supplement). The LOO sensitivity analyses for the study by Fischer et al.,11 Ventura-Bort et al.,12 Hatik et al.,16 or Barker et al.21 did not reveal any significant change in effect size or heterogeneity for the association between non-invasive auricular stimulation and reductions in SBP, DBP, or HR (see Supplementary material online, Data Supplement).
In stratified analysis among the studies of auricular electrical stimulation, there were significant differences regarding the stimulation laterality in the relationship between auricular electrical stimulation and reduction in SBP (P = 0.004), DBP (P < 0.0001), and HR (P = 0.02) (see Supplementary material online, Figure S9), while there were no significant differences regarding the stimulation frequency (see Supplementary material online, Data Supplement; Figure S8).
Publication bias
The funnel plot was drawn based on studies that included the outcome of SBP, DBP, and HR, and the funnel plots of SBP and HR were asymmetrical with visual inspection (see Supplementary material online, Figure S11). The Egger’s test showed significant results for SBP (P < 0.0001), DBP (P < 0.001), and HR (P = 0.002) suggesting significant publication bias.
Adverse effects
None of the 18 studies reported any serious adverse effects. And there were no reports of symptomatic hypotension. Among the three studies that described side effects,11–13 one reported that subjective ratings of ear irritation were significantly higher in the active group than in the sham group,12 while the other two studies found no significant differences in subjective symptoms of side effects between the two groups using a side effects checklist11,13 (see Supplementary material online, Table S1A and B).
Discussion
This systematic review and meta-analysis demonstrated that non-invasive auricular stimulation is associated with reduced SBP, DBP, and HR in adults. Findings were generally consistent across types of auricular stimulation and settings for comparison. There were no serious adverse events across all the 18 studies and minimal evidence for other adverse effects. The findings in this meta-analysis are derived from RCTs that were generally free from excessive serious risk of bias. However, there was evidence of substantial heterogeneity, which could be explained by stimulation duration or the overall high variability in baseline SBP, DBP, and HR values.
In this meta-analysis, compared to sham stimulation, the differences in SBP and DBP in the active stimulation were −4.44 and −2.21 mmHg, respectively. An earlier meta-analysis conducted by the Blood Pressure Lowering Treatment Trialists’ Collaboration has shown that the SBP- and DBP-lowering effects of antihypertensive treatment were associated with a significant reduction in the incidence of stroke, coronary artery disease, and HF at levels of 2–5 and 1–2 mmHg, respectively.27 In this respect, the degree of BP-lowering caused by non-invasive auricular stimulation is also considered to be beneficial from a clinical perspective.
Non-invasive auricular stimulation and systolic blood pressure
In this meta-analysis, non-invasive auricular stimulation was significantly associated with reduced SBP in adults. Specifically, non-invasive auricular stimulation showed the strongest SBP reduction in populations with hypertension.
Low-level electrical auricular stimulation28,29 and auricular acupressure25 are non-invasive treatments with few side effects that activate the auricular branch of the vagus nerve in tragus which decreases sympathetic nervous system activity.7,11 Baroreflex plays a pivotal role in BP regulation, and impaired baroreflex sensitivity (BRS) could have resulted in increased BP instability. Vagus nerve stimulation is associated with an increase in BRS,30 and electrical vagus nerve stimulation is effective in reducing BP that is reflective of decreased sympathetic nervous system activity. Low-level intermittent vagus nerve stimulation improves the set point for BP regulation modulated by BRS which links in BP reduction.31 In addition, increased sympathetic nervous system activity promotes vascular remodelling and increases vascular resistance.4 Thus, electrical auricular stimulation or acupressure might diminish the heightened responsiveness of the vessel wall for vasoconstriction.
Because most cardiovascular diseases or those risks are associated with autonomic dysregulation characterized by decreased BRS gain, increased sympathetic nervous system activity, and withdrawal of parasympathetic nervous system activity,29 non-invasive auricular stimulation might be associated with SBP reduction via recovery of sympathovagal balance. In this analysis, non-invasive auricular stimulation showed the strongest SBP reduction in the hypertensive populations. Thus, non-invasive auricular stimulation could serve as an adjunctive treatment of hypertension.
Non-invasive auricular stimulation and diastolic blood pressure
Concerning DBP, the clinical implications for BP level in autonomic neuromodulation is slightly different from those in SBP. While non-invasive auricular stimulation is significantly reduced DBP in total, the stratified analysis revealed that the relationship between non-invasive auricular stimulation and reduction in DBP was not significant in older adults.
This can be explained by the association of more advanced structural arterial stiffness, not amenable to changes in sympathetic tone. Low DBP causes reduction in myocardial perfusion,32 which is related to increasing the risk of cardiovascular events in the elderly.32 Moreover, increased aortic stiffness reduced cerebral blood flow in the elderly.33 Isolated systolic hypertension is a common phenotype in the elderly, and there has been a dilemma about the way how to treat those patients with low DBP.34 Because non-invasive auricular stimulation did not significantly reduce DBP in the elderly, non-invasive auricular stimulation has the potential to reduce specifically SBP while maintaining target organ haemodynamics in the elderly. This meta-analysis suggests that non-invasive auricular stimulation could safely provide a BP-lowering effect on isolated systolic hypertension.
Non-invasive auricular stimulation and heart rate
In this meta-analysis, non-invasive auricular stimulation was significantly associated with reduced HR in the adults, and there were no reports of symptomatic bradycardia. On the other hand, in the stratified analyses, non-invasive auricular stimulation did not significantly reduce HR in older adults or patients with hypertension.
Heart rate is a key predictor of adverse events in the cardiovascular diseases such as HF and stroke.35 On the contrary, cardiovascular benefits are associated with HR control.36 Numerous physiological studies have reported that the postganglionic vagus nerve acts on muscarinic receptors to regulate the heart via acetylcholine which leads to reduce HR.37 Although there were concerns based on experimental investigations with direct stimulation of cervical vagus nerves in dogs that demonstrated the pronounced effect on bradycardia,38 this systematic review showed no symptomatic bradycardia in non-invasive auricular stimulation, suggesting that auricular stimulation may reduce HR without serious side effects such as bradycardia in adults. However, it should be emphasized that none of the included RCTs utilizing auricular stimulation were performed on patients with severe atrioventricular block or sick sinus syndrome.
The relationship between non-invasive auricular stimulation and reduction in HR was not significant in older adults or patients with hypertension. Hypertension in the elderly is characterized as reduced elastic arterial compliance in which the impact of sympathetic overdrive is limited.29 The earlier meta-analysis found that renal denervation, another kind of neuromodulation, effectively reduced HR only in young/middle-aged persons with hypertension.39 Because HR is a close indicator of the autonomic nervous involvement, auricular stimulation might be also more effective for reducing HR in the young/middle-aged population with hypertension than in older adults.
Meta-regression analysis in systolic blood pressure, diastolic blood pressure, and heart rate
In the meta-regression analysis, higher baseline SBP, DBP, and HR correlated with a greater effect of non-invasive auricular stimulation on reduction in those parameters. While this might indicate the suitability of the therapy for patients with hypertension, an effect for the regression to an optimum value is also possible.
Reassuringly, this meta-regression analysis suggests that stimulation does not have excessive lowering effects even if baseline SBP, DBP, and HR are below approximately 110∼115 mmHg, 65∼70 mmHg, and 65∼70 b.p.m., respectively. The findings suggest that auricular stimulation could be used safely even in patients with lower BP or a tendency to bradycardia.
Increased baseline BP and HR are associated with more pronounced activation of the renin-angiotensin-aldosterone system, increased sympathetic nervous system activity, and higher norepinephrine level,40,41 and those resulted in greater reduction in BP and HR after renal denervation.39–41 This allows for a similar interpretation to the results of the current analysis regarding neuromodulation of non-invasive auricular stimulation. Although the precise mechanisms particularly in auricular acupressure are not fully understood, non-invasive auricular stimulation might safely reduce higher BP and HR at baseline.
Limitation and perspectives
The inclusion of RCTs with different populations, interventions, and outcome measures might increase heterogeneity of results. In particular, compared to low-level electrical auricular stimulation, the exact mechanisms of auricular acupressure remain unclear. There is currently a lack of precision as to whether both methods of non-invasive auricular stimulation share the same physiological mechanisms. In this subgroup analysis, only electrical stimulation contributed to a significant reduction in both SBP and DBP. Compared with auricular acupressure, electrical stimulation might be specifically useful in lowering SBP and DBP. Acute transcutaneous tragus stimulation improved cardiac vagal baroreflex gain but did not significantly alter BP or HR in patients with chronic HF with left ventricular ejection fraction < 50%, of whom 88% were taking β-blockers.20 In this systematic review, the studies included contained very little information on classes of antihypertensives, making it difficult to analyse whether patients received β-blockers. In this subgroup analysis, no significant decrease in SBP, DBP, or HR was observed in the group with antihypertensive medication. Auricular stimulation might be useful for mild hypertension before starting oral antihypertensive treatment. However, there were a few studies20,22,24,25 that included populations taking antihypertensives, and there is little information on the type, dose, and duration of antihypertensive medication, so the results must be interpreted with great caution. On the other hand, non-invasive auricular stimulation specifically reduced SBP in the elderly. Although details were unknown, it is possible that many of the elderly were taking antihypertensives. Further research is needed to determine which types of hypertension (e.g., mild, isolated systolic or resistant hypertension) would benefit from non-invasive auricular stimulation. The degree of SBP reduction was greater in the group with stimulation for longer than 1 h, and that of DBP was not significant in the group with stimulation for longer than 1 week. From the viewpoint of lowering SBP, long-term stimulation might be more useful in isolated systolic hypertension. For the treatment of hypertension, it might be important to consider which class of antihypertensive medication is appropriate in combination with auricular stimulation. Furthermore, as additional therapy for patients with poor tolerance to antihypertensives, auricular stimulation could play a pivotal role.
This analysis could not provide detailed and precise results for the setting and laterality of auricular electrical stimulation due to the limited number of studies. In this stratified and meta-regression analysis, stimulation amplitude and laterality might be the causes of heterogeneity in the relationship between non-invasive auricular electrical stimulation and changes in haemodynamic parameters. The degree of reduction in SBP, DBP, or HR might be particularly high with higher amplitude or with bilateral stimulation, but the number of studies analysed is small. Further research, including larger sample sizes and more refined methodologies, is needed. Because the right vagal nerve sends efferent projections to the heart,42 there might be a difference in the effect on haemodynamics between the right and left sides of auricular stimulation. To date, there have been few RCTs in which the effect of auricular stimulation on haemodynamics was investigated regarding to laterality in the stimulation site. Thus, further investigation is needed to resolve the issue of laterality effect on haemodynamics regarding non-invasive auricular stimulation. Stratified analysis based on stimulation sites is also necessary, but some studies involved simultaneous stimulation of the tragus and concha and, in most of acupressure studies, stimulation of multiple sites on the auricle, making it difficult to strictly classify sites.
This review is also limited by the low number of participants in the original studies, likely explained by the relative experimental nature of non-invasive auricular stimulation. Despite 18 eligible RCTs, there were fewer than 1000 persons receiving the intervention vs. control which limits the generalizability of these findings to the general population. The analyses were also marked by considerable statistical heterogeneity. Some of the studies included in this meta-analysis were conducted for purposes other than lowering BP but including these studies could increase generalizability and reduce selection bias. Particularly, this meta-analysis included one study16 on stimulation after exercise, in which measurements were taken under conditions of severe fluctuations in haemodynamics. Recent reports have shown that low-level electrical auricular stimulation improved exercise capacity,43 and in this respect, the inclusion of the study by Hatik et al.16 is also reasonable. Furthermore, LOO sensitivity analyses did not reveal any significant change in effect size or heterogeneity. In this meta-analysis, the Egger’s test showed significant results for SBP, DBP, and HR. Publication bias is a well-known problem when conducting meta-analyses and increases the risk of overestimating the effects found.44 It should be noted that the results suggest a possible publication bias showing a larger positive effect size for the primary outcomes. Other limitations of meta-analyses in general are that the analyses may compound and accentuate the methodological limitations and biases in the original studies.
Conclusions
Non-invasive auricular stimulation was associated with reduced SBP, DBP, and HR in adults. Findings were generally consistent across types of auricular stimulation and settings for comparison. In the stratified analysis, non-invasive auricular stimulation had the strongest effect on SBP reduction in persons with hypertension, while the reduction in DBP was not significant in older adults. Collectively, the findings suggest that non-invasive auricular stimulation could serve as a safe adjunctive treatment of hypertension. Future large-sample, multicentre, well-designed clinical trials will warrant the exact effects of non-invasive auricular stimulation on cardiovascular regulation.
Supplementary Material
Contributor Information
Michiaki Nagai, Cardiovascular Section, Department of Medicine, University of Oklahoma, Health Science Center, Andrews Academic Tower, Suite 5400, Oklahoma City, OK 73104, USA; Department of Medicine, Hiroshima Asa Medical Association Hospital, 2-1-38, Kabeminami, Aaskita-ku, Hiroshima 7310223, Japan.
Karl-Philipp Rommel, Department of Cardiology, University Medical Center Mainz and German Center for Cardiovascular Research, Langenbeckstrasse 1, 55131 Mainz, Germany.
Yukiko Nakano, Department of Cardiovascular Medicine, Graduate School of Biomedical and Health Sciences, Hiroshima University, 1-2-3 Kasumi, Minami-Ku, Hiroshima 7348551, Japan.
Phillip J Tully, Faculty of Medicine and Health, School of Psychology, Deakin University, 221 Burwood Highway, Burwood, VIC 3125, Australia; Faculty of Medicine and Health, Centre for Men’s Health and Wellbeing, School of Medicine, University of Adelaide, Level 6 AHMS Bldg, The University of Adelaide, Adelaide, SA 5005, Australia.
Isabel J Sible, Department of Neurology, University of California San Francisco, 675 Nelson Rising Lane, Suite 190, San Francisco, CA 94158, USA.
Sunny Po, Cardiovascular Section, Department of Medicine, University of Oklahoma, Health Science Center, Andrews Academic Tower, Suite 5400, Oklahoma City, OK 73104, USA.
Tarun W Dasari, Cardiovascular Section, Department of Medicine, University of Oklahoma, Health Science Center, Andrews Academic Tower, Suite 5400, Oklahoma City, OK 73104, USA.
Lead author biography

Michiaki Nagai, MD, PhD, has completed his graduation from the Jichi Medical University School of Medicine and has been involved in cardiovascular medicine. He has been investigating the fields for target hypertensive organ damages including the relationships among hypertension, blood pressure variability, brain atrophy, cognitive impairment, and central autonomic nervous system including the insular cortex. He is engaged in internal medicine and cardiology at Hiroshima Asa Medical Association Hospital and serves as a volunteer faculty at the University of Oklahoma Health Science Center.
Data availability
The data sets used in the current analysis are available from the corresponding authors upon reasonable request.
Supplementary material
Supplementary material is available at European Heart Journal Open online.
Author contributions
Michiaki Nagai (Conceptualization [equal], Data curation [equal], Writing—original draft, Writing—review & editing [equal], Formal Analysis [equal], Methodology [equal], Software), Karl-Philipp Rommel (Writing—review & editing [equal], Methodology [equal]), Yukiko Nakano (Writing—review & editing [equal], Methodology [equal]), Phillip J. Tully (Writing—review & editing [equal], Formal Analysis [equal], Validation, Resources), Isabel J. Sible (Writing—review & editing [equal], Methodology [equal],), Sunny Po (Conceptualization [equal], Writing—review & editing [equal], Methodology [equal], Project administration), Tarun W. Dasari (Conceptualization [equal], Data curation [equal], Writing—review & editing [equal], Visualization)
Funding
There was no research funding regarding this study.
References
- 1. Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, Ezzati M. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med 2009;6:e1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Global effect of modifiable risk factors on cardiovascular disease and mortality . The Global Cardiovascular Risk Consortium. N Engl J Med 2023;389:1273–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol 2018;71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 4. Dinenno FA, Jones PP, Seals DR, Tanaka H. Age-associated arterial wall thickening is related to elevations in sympathetic activity in healthy humans. Am J Physiol Heart Circ Physiol 2000;278:H1205–H1210. [DOI] [PubMed] [Google Scholar]
- 5. Mazzone SB, Lim LH, Wagner EM, Mori N, Canning BJ. Sympathetic nerve-dependent regulation of mucosal vascular tone modifies airway smooth muscle reactivity. J Appl Physiol 1985;2010:1292–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nagai M, Ewbank H, Nakano Y, Scherlag BJ, Po SS, Dasari TW. Heart rate variability and heart failure with reduced ejection fraction: a systematic review of literature. Curr Cardiol Rev 2025;21:78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clancy JA, Mary DA, Witte KK, Greenwood JP, Deuchars SA, Deuchars J. Non-invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity. Brain Stimul 2014;7:871–877. [DOI] [PubMed] [Google Scholar]
- 8. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng H-Y, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. Rob 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 9. Sellaro R, Steenbergen L, Verkuil B, van IJzendoorn MH, Colzato LS. Transcutaneous vagus nerve stimulation (tVNS) does not increase prosocial behavior in cyberball. Front Psychol 2015;6:499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Keshtkar L, Ranjkesh F, Habibi M, Rashvand F. Effects of auriculotherapy on gestational hypertension: randomized controlled trial study. Iran J Nurs Midwifery Res 2024;29:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fischer R, Ventura-Bort C, Hamm A, Weymar M. Transcutaneous vagus nerve stimulation (tVNS) enhances conflict-triggered adjustment of cognitive control. Cogn Affect Behav Neurosci 2018;18:680–693. [DOI] [PubMed] [Google Scholar]
- 12. Ventura-Bort C, Wirkner J, Genheimer H, Wendt J, Hamm AO, Weymar M. Effects of transcutaneous vagus nerve stimulation (tVNS) on the P300 and alpha-amylase level: a pilot study. Front Hum Neurosci 2018;12:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giraudier M, Ventura-Bort C, Weymar M. Transcutaneous vagus nerve stimulation (tVNS) improves high-confidence recognition memory but not emotional word processing. Front Psychol 2020;11:1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gauthey A, Morra S, van de Borne P, Deriaz D, Maes N, le Polain de Waroux JB. Sympathetic effect of auricular transcutaneous vagus nerve stimulation on healthy subjects: a crossover controlled clinical trial comparing vagally mediated and active control stimulation using microneurography. Front Physiol 2020;11:599896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Šinkovec M, Trobec R, Kamenski T, Jerman N, Meglič B. Hemodynamic responses to low-level transcutaneous auricular nerve stimulation in young volunteers. IBRO Neurosci Rep 2023;14:154–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hatik SH, Asrlan M, Demirbilek Ö, Özden AV. The effect of transcutaneous auricular vagus nerve stimulation on cycling ergometry and recovery in healthy young individuals. Brain Behav 2023;13:e3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Moraes TL, Costa FO, Cabral DG, Fernandes DM, Sangaleti CT, Dalboni MA, Motta e Motta J, de Souza LA, Montano N, Irigoyen MC, Brines M, Tracey KJ, Pavlov VA, Consolim Colombo FM. Brief periods of transcutaneous auricular vagus nerve stimulation improve autonomic balance and alter circulating monocytes and endothelial cells in patients with metabolic syndrome: a pilot study. Bioelectron Med 2023;9:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Percin A, Ozden AV, Yenisehir S, Pehlivanoglu BE, Yılmaz RC. The effect of in-ear and behind-ear transcutaneous auricular vagus nerve stimulation on autonomic function: a randomized, single-blind, sham-controlled study. J Clin Med 2024;13:4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mbikyo MB, Wang A, Ma Q, Miao L, Cui N, Yang Y, Fu H, Sun Y, Li Z. Low-level tragus stimulation attenuates blood pressure in young individuals with hypertension: results from a small-scale single-blind controlled randomized clinical trial. J Am Heart Assoc 2024;13:e032269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gentile F, Giannoni A, Navari A, Degl'Innocenti E, Emdin M, Passino C. Acute right-sided transcutaneous vagus nerve stimulation improves cardio-vagal baroreflex gain in patients with chronic heart failure. Clin Auton Res 2025;35:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barker R, Kober A, Hoerauf K, Latzke D, Adel S, Kain ZN, Wang SM. Out-of-hospital auricular acupressure in elder patients with hip fracture: a randomized double-blinded trial. Acad Emerg Med 2006;13:19–23. [DOI] [PubMed] [Google Scholar]
- 22. Yeh ML, Chang YC, Huang YY, Lee TY. A randomized controlled trial of auricular acupressure in heart rate variability and quality of life for hypertension. Complement Ther Med 2015;23:200–209. [DOI] [PubMed] [Google Scholar]
- 23. Kuo SY, Tsai SH, Chen SL, Tzeng YL. Auricular acupressure relieves anxiety and fatigue, and reduces cortisol levels in post-caesarean section women: a single-blind, randomised controlled study. Int J Nurs Stud 2016;53:17–26. [DOI] [PubMed] [Google Scholar]
- 24. Park S, Park H, Bang YY. The effects of auricular acupressure on physiological index, depression, anxiety, and stress for elders with hypertension. Holist Nurs Pract 2023;37:24–33. [DOI] [PubMed] [Google Scholar]
- 25. Kim B, Park H. The effects of auricular acupressure on blood pressure, stress, and sleep in elders with essential hypertension: a randomized single-blind sham-controlled trial. Eur J Cardiovasc Nurs 2023;22:610–619. [DOI] [PubMed] [Google Scholar]
- 26. Sajadi SA, Rahimi V, Farsi Z, Fournier A. The effect of auriculotherapy on anxiety and physiological parameters of male coronary angiography patients: a single-blind randomized clinical trial. J Perianesth Nurs 2023;38:102–107. [DOI] [PubMed] [Google Scholar]
- 27. Turnbull F; Blood Pressure Lowering Treatment Trialists’ Collaboration . Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet 2003;362:1527–1535. [DOI] [PubMed] [Google Scholar]
- 28. Nagai M, Dote K, Kato M, Sasaki S, Oda N, Förster CY. Afterload reduction after non-invasive vagus nerve stimulation in acute heart failure. Front Hum Neurosci 2023;17:1149449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nagai M, Rommel KP, Po SS, Dasari TW. Autonomic neuromodulation for cardiomyopathy associated with metabolic syndrome—prevention of precursors for heart failure with preserved ejection fraction. Hypertens Res 2024;47:3318–3329. [DOI] [PubMed] [Google Scholar]
- 30. Plachta DT, Gierthmuehlen M, Cota O, Espinosa N, Boeser F, Herrera TC, Stieglitz T, Zentner J. Blood pressure control with selective vagal nerve stimulation and minimal side effects. J Neural Eng 2014;11:036011. [DOI] [PubMed] [Google Scholar]
- 31. Schultz J, Annoni EM, Tolkacheva EG. Modified sequence method to assess baroreflex sensitivity in rats. Annu Int Conf IEEE Eng Med Biol Soc 2018;2018:2764–2767. [DOI] [PubMed] [Google Scholar]
- 32. Messerli FH, Mancia G, Conti CR, Hewkin AC, Kupfer S, Champion A, Kolloch R, Benetos A, Pepine CJ. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med 2006;144:884–893. [DOI] [PubMed] [Google Scholar]
- 33. Jefferson AL, Cambronero FE, Liu D, Moore EE, Neal JE, Terry JG, Nair S, Pechman KR, Rane S, Davis LT, Gifford KA, Hohman TJ, Bell SP, Wang TJ, Beckman JA, Carr JJ. Higher aortic stiffness is related to lower cerebral blood flow and preserved cerebrovascular reactivity in older adults. Circulation 2018;138:1951–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koracevic G, Stojanovic M, Kostic T, Lovic D, Tomasevic M, Jankovic-Tomasevic R. Unsolved problem: (isolated) systolic hypertension with diastolic blood pressure below the safety margin. Med Princ Pract 2020;29:301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nikolovska Vukadinović A, Vukadinović D, Borer J, Cowie M, Komajda M, Lainscak M, Swedberg K, Böhm M. Heart rate and its reduction in chronic heart failure and beyond. Eur J Heart Fail 2017;19:1230–1241. [DOI] [PubMed] [Google Scholar]
- 36. Böhm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L; SHIFT Investigators . Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomized placebo-controlled trial. Lancet 2010;376:886–894. [DOI] [PubMed] [Google Scholar]
- 37. Brack KE, Winter J, Ng GA. Mechanisms underlying the autonomic modulation of ventricular fibrillation initiation–tentative prophylactic properties of vagus nerve stimulation on malignant arrhythmias in heart failure. Heart Fail Rev 2013;18:389–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ardell JL, Randall WC. Selective vagal innervation of sinoatrial and atrioventricular nodes in canine heart. Am J Physiol 1986;251:H764–H773. [DOI] [PubMed] [Google Scholar]
- 39. Li L, Xiong Y, Hu Z, Yao Y. Effect of renal denervation for the management of heart rate in patients with hypertension: a systematic review and meta-analysis. Front Cardiovasc Med 2022;8:810321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vuignier Y, Grouzmann E, Muller O, Vakilzadeh N, Faouzi M, Maillard MP, Qanadli SD, Burnier M, Wuerzner G. Blood pressure and renal responses to orthostatic stress before and after radiofrequency renal denervation in patients with resistant hypertension. Front Cardiovasc Med 2018;5:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mahfoud F, Townsend RR, Kandzari DE, Kario K, Schmieder RE, Tsioufis K, Pocock S, David S, Patel K, Rao A, Walton A, Bloom JE, Weber T, Suppan M, Lauder L, Cohen SA, McKenna P, Fahy M, Böhm M, Weber MA. Changes in plasma renin activity after renal artery sympathetic denervation. J Am Coll Cardiol 2021;77:2909–2919. [DOI] [PubMed] [Google Scholar]
- 42. Nemeroff CB, Mayberg HS, Krahl SE, McNamara J, Frazer A, Henry TR, George MS, Charney DS, Brannan SK. VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology 2006;31:1345–1355. [DOI] [PubMed] [Google Scholar]
- 43. Ackland GL, Patel ABU, Miller S, Gutierrez Del Arroyo A, Thirugnanasambanthar J, Ravindran JI, Schroth J, Boot J, Caton L, Mein CA, Abbott TEF, Gourine AV. Non-invasive vagus nerve stimulation and exercise capacity in healthy volunteers: a randomized trial. Eur Heart J 2025;46:1634–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sterne J, Egger M, Moher D. Addressing reporting biases. In: Higgins JPT and Green S, eds. Cochrane Handbook for Systematic Reviews of Intervention. the Cochrane Collaboration. Stroebe M, St; 2008. Vol. 5.0.1, p. Chapter 10.46. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets used in the current analysis are available from the corresponding authors upon reasonable request.