Abstract
Background:
Icosapent ethyl (IPE) has demonstrated efficacy and safety in reducing the risk of ischemic cardiovascular disease. This study aimed to systematically gather and synthesize existing cost-effectiveness analyses of IPE combined with statin therapy for cardiovascular risk reduction in primary and secondary prevention settings.
Methods:
Comprehensive electronic searches were conducted across PubMed/MEDLINE, Scopus, Web of Science Core Collection, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL), the NHS Economic Evaluation Database (NHS EED), and the Health Technology Assessment (HTA) database to identify relevant literature (up to May 2024). From an initial pool of 580 studies, 11 met the predefined inclusion criteria.
Results:
The findings demonstrated that IPE significantly decreased hospitalization and mortality rates compared to standard treatments. The study indicated that IPE provided greater quality-adjusted life years and life-years gained than statin therapy alone. However, IPE is more expensive than conventional medications, such as statins. For instance, the 1-year cost of IPE is $3768 in Australia and $3497 in the United States per patient. Additionally, the results revealed that the threshold for assessing the effectiveness of IPE ranged from $50,000 to $150,000 in the United States and AUD 50,000 ($39,000) in Australia.
Conclusion:
Based on the current study, IPE is cost-effective, with a higher probability of cost-effectiveness in patients undergoing secondary prevention than those in primary prevention.
Keywords: Cost-effectiveness, Icosapent Ethyl, Statins, Cardiovascular disease, Systematic review
Introduction
Despite significant advancements in science and pharmacology, cardiovascular disease (CVD) remains the leading cause of mortality and rising healthcare costs, posing a substantial epidemiological and societal burden.1, 2 Cardiometabolic, behavioral, environmental, and social risk factors are key contributors to CVD.1 In the United States, CVD is the cause of 1 in every 3 deaths in the United States and results in direct and indirect costs exceeding $300 billion annually, with projected annual costs surpassing $1 trillion by 2035 (3, 4). Even among patients receiving treatment for primary or secondary prevention of cardiovascular risk factors, the rates of cardiovascular events remain persistently high.5, 6
Adjunctive therapies proven to reduce CVD events when combined with statin therapy include the omega-3 fatty acid eicosapentaenoic acid (EPA) and ezetimibe.7–9 In the Japan EPA Lipid Intervention Study (JELIS), the risk of major coronary events decreased by 19% in the group receiving EPA plus statin therapy, significantly lower than in the group receiving statin therapy alone.3, 10, 11 According to the REDUCE-IT trial, treatment with IPE significantly reduced the risk of ischemic events, including cardiovascular death, myocardial infarction, and stroke, in patients with elevated triglyceride (TG) levels despite statin use across both primary and secondary prevention populations.9, 12 On 13 December 2019, the United States Food and Drug Administration (FDA) approved Vascepa (Icosapent Ethyl) as an adjunctive therapy to reduce the risk of ischemic CVD events in adults with elevated TG levels of 150 mg /dL or higher. Eligible patients must also have either established CVD or diabetes, along with 2 or more additional CVD risk factors. Patients are advised to maintain physical activity and a healthy diet.13
Given the demonstrated efficacy and safety of IPE in trials such as JELIS and REDUCE-IT, it is crucial to evaluate the long-term cost-effectiveness of this novel drug to optimize the allocation of limited healthcare resources. To our knowledge, no systematic review has yet assessed the lifetime economic impact of IPE for cardiovascular risk reduction. In this context, the present study aimed to systematically gather and synthesize available cost-effectiveness and cost-utility analyses of IPE combined with statin therapy, compared to statin therapy alone, for cardiovascular risk reduction in primary and secondary prevention settings. The analysis considers various healthcare systems and perspectives.
Methods
The current systematic review aimed to evaluate the cost-effectiveness of IPE in conjunction with statin therapy compared to statin therapy alone for reducing cardiovascular risk. The protocol for this study has been registered with the Code of Ethics IR.IUMS.REC.1399.701 from the Iran University of Medical Sciences. This study was conducted at the Hospital Management Research Center, Iran University of Medical Sciences, Tehran, Iran.
Study Identification Database search
To conduct this study, the following electronic scholarly databases were systematically searched: PubMed / MEDLINE, Scopus, Web of Science Core Collection, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL), the NHS Economic Evaluation Database (NHS EED), and the Health Technology Assessment (HTA) database. These databases were searched without restrictions on language, time frame, study design, or publication status. A specific and tailored search strategy was developed for each database. The strategy incorporated a combination of relevant keywords and medical subject headings (MeSH terms in the case of PubMed/MEDLINE) to identify pertinent literature, with the search updated to May 2024.
Search for other resources
The reference lists of included studies were further reviewed to identify additional related studies. The Google Scholar search engine was also utilized to ensure the search was as comprehensive as possible. All identified studies were imported into EndNote software (version X7; Thomson Reuters) for organization and management.
Study screening and selection
After compiling the articles and removing duplicates, their titles and abstracts were screened, and irrelevant articles were excluded. The full texts of the remaining articles were thoroughly reviewed based on predefined inclusion and exclusion criteria, and the reasons for exclusion were documented. All steps of the screening and selection process were conducted independently by 2 researchers. Any disagreements were resolved through discussion to reach a final consensus.
Inclusion and exclusion criteria
Inclusion criteria: Intervention and comparator:
Studies were included if they compared the use of IPE in combination with statin therapy against statin therapy alone for reducing cardiovascular risk in patients.
Types of outcomes:
Studies were eligible if they reported at least 1 of the following outcomes:
Mortality,
Hospitalization,
Incremental cost-effectiveness ratio (ICER),
Cost per quality-adjusted life year (cost per QALY),
Cost per life-year gained (cost per LYG),
Cost per unit of effectiveness (in natural units), and
Net monetary benefit (NMB).
Types of studies:
All types of complete economic evaluation studies were included in the systematic review, including
Cost-benefit analysis (CBA),
Cost-effectiveness analysis (CEA), and
Cost-utility analysis (CUA), whether model-based or trial-based.
Additionally, health technology assessment (HTA) studies were included if they incorporated economic evaluations.
Language restrictions:
Only studies with full text available in English were included.
Exclusion criteria:
Review studies, editorials, letters to the editor, conference/proceeding abstracts, and unpublished grey literature such as dissertations and theses were excluded.
Studies with incomplete evaluations, such as cost-minimalization analyses, cost-of-illness (CoI) studies, cost analyses, cost outcome descriptions, or cost descriptions, were excluded.
Studies without full text or with full text in a language other than English were excluded.
Animal studies were excluded.
Redundant studies with results published across multiple articles were excluded, with only the highest-quality publication retained after quality assessment.
Assessing the reporting quality of studies:
The reporting quality of economic evaluation studies was assessed using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) tool.14 The CHEERS tool consists of 24 questions organized into the following 6 sections:
Title and abstract;
Introduction;
Methods;
Results;
Discussion; and
Other.
Each question is evaluated using 1 of 4 assessment options as follows:
Yes (if the item is fully reported);
Partially reported;
No (not reported); or
Not Applicable.
Two researchers independently assessed the quality of the studies, and any discrepancies were resolved through discussion.
Data extraction and analysis (synthesis):
A data collection form was utilized to extract the relevant data. This form captured the key characteristics of the studies and outcome information, including author, year of publication, sample size (n), intervention, comparators, and primary and secondary outcomes.
Data extraction for each study was conducted independently by 2 researchers and verified by a third researcher. The cost-effectiveness information of the compared technologies was organized into tables and qualitatively synthesized. All currency values were converted to 2022 US dollars.
The present study was conducted and reported following the principles outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.15
Results
Out of 580 identified items, 40 articles underwent full-text review. Ultimately, 11 studies that met the criteria for complete economic evaluation and aligned with the research objectives, inclusion, and exclusion criteria were included in the current study (Figure 1). Tables 2 and 3 summarize the key assumptions and cost-effectiveness findings of each study. The extracted components include the author, country and year of the study, patient population (sample size), health outcomes, study perspective, time horizon, study question and intervention performed, mean age of patients in the clinical trial or estimated model, type of model, sensitivity analysis performed, discount rate, costs included in the study, mortality rate, hospitalization rate, LYGs, QALYs, annual and total costs, ICER, and cost-effectiveness threshold.
Fig. 1.
Process of the systematic literature search, according to the preferred reporting items for systematic review and meta-analyses
Table 2:
Characteristics of included studies in the review
| Study/ citation | country | Patient population | Health outcome | Perspective | Time horizon | Research question | Mean or Median age | subgroup | Type of model | Sensitive analysis | Discount rate |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ademi et.al, 2019 | Australia | The Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial | Mortality, Hospitalization, QALY, LYQs | Australian public healthcare system | 20-years | Icosapent + statins vs statins | 64 years | Primary vs secondary prevention | Markov model | Y | 5% |
| Gao et.al, 2019 | Australia | A cohort of Australian patients aged 45 years and over with established CVD | QALY, LYQs | Australian healthcare system | 25-years | Icosapent + statins vs statins | 64 years | - | Markov model | Y | 3% |
| ICER, 2019 | US | adults with established CVD being treated with optimal medical management and patients without known CVD but at high risk for cardiovascular events | QALY, LYQs | health care sector | lifetime time horizon | Icosapent vs statins | 64 years | - | A Markov cohort model | Y | 3% |
| Kodera, et.al 2018 | Japan | The Japan Eicosapentaenoic Acid Lipid Intervention Study | QALY, LYQs | Public healthcare funder | 30-years | Eicosapentaenoic + statins vs statins | 61 years | Primary vs secondary prevention | Markov model | Y | 2% |
| Philip et.al, 2016 | US | QALY | Third-party payer | 5-years | Eicosapentaenoic + statins vs statins | - | secondary prevention | decision analytic model | Y | 3% | |
| Weintraub et.al, 2020 | US | REDUCE-IT PATIENTS | QALY, ICER | Payer | in-trial | Icosapent vs standard care | 64 years | primary vs secondary prevention | - | Y | - |
| Michaeli et.al, 2023 | Germany | Dyslipidemia patients | QALY, LY, ICER | Germany’s healthcare system | 20 years | statin combinations with icosapent ethyl vs statin monotherapy | 63 years | primary vs secondary prevention | Markov cohort model | Y | 3 % |
| Michaeli et.al, 2022 | UK | Dyslipidaemia patients | QALY, LY, ICER | UK’s National Health Service | 20-year time horizon (lifetime) | statin combinations with icosapent ethyl vs statin monotherapy | 63 years | Icosapent ethyl in primary vs secondary prevention: Age < 65 years ≥ 65 years Baseline triglyceride ≥ 200 mg/dL and HDL-C ≤ 35 mg/dL No Yes Baseline LDL-C ≥ 100 mg/dL No Yes Baseline high-sensitivity CRP ≤ 2 mg/L > 2 mg/LL |
Markov model | Y | 3.5% (±1.5%) |
| Lachaine et.al, 2023 | Canada | Statin-treated patients with elevated triglycerides | QALY, ICER | Canadian healthcare payer perspective | 20 years | Icosapent ethyl vs Placebo | Median starting age: Range in REDUCE-IT trial | ------------ | Markov model | Y | 1.5% |
| Weintraub et.al, 2022 | US | Hypertriglyceridemia and known cardiovascular disease risk factor who were treated or diabetes and at least 1 other with statins. | QALY, LY, ICER | US health care sector perspective | lifetime | Icosapent ethyl vs Standard care | 64 years | age (≥65 vs <65 years), sex, trial recruit-ment cohort (primary vs secondary prevention), baseline diabetes status, baseline serum triglyc-eride level (≥200 vs <200 mg/dL and ≥150 vs <150 mg/dL), and baseline low-density lipoprotein cholesterol level (≥70 vs <70 mg/dL). | Markov model | Y | 3 % |
| Weintraub et .al, 2024 | US | Statin-stabilized patients were eligible with fasting triglycerides ≥135 and <500 mg/dL and LDL-C> 40 and ≤100 mg/dL | QALY, LY, ICER | US health sector perspective | Lifetime | Icosapent ethyl vs Standard care | aged 65 to 84 years | age (≥65 versus <65 years), sex, primary versus secondary prevention, baseline diabetes, baseline serum triglycerides (≥200 versus <200 mg/dL, and ≥150 versus <150 mg/dL), and baseline LDL-C (≥70 versus <70 mg/dL). | Markov model | Y | 3 % |
Table 3:
Summary results of included economic evaluation studies
| Study/citation | Mortality | Hospitalization | QALYs | LYQs | Annual cost | Total Cost | ICER | Threshold | Result |
|---|---|---|---|---|---|---|---|---|---|
| Ademi et.al, 2019 | Icosapent + statin= 736.5 in 1000 individuals statin= 794.3 Difference=−57.8 |
Non-fatal MI/non-fatal Stroke: Icosapent + statin=877 statin= 1,147.8 Difference= −270.8 Serious Bleeding: Icosapent + statin= 220.6 statin= 208.2 Difference=12.4 Coronary Revascularization: Icosapent + statin= 772.4 statin= 1,068 Difference= −295.8 Hospitalization for AF: Icosapent + statin= 437.7 statin= 300.9 Difference= 136.8 |
Icosapent + statin =7.82 statin =7.53 Difference=0.28 |
Icosapent + statin=10.11 statin=9.78 Difference=0.33 |
Icosapent + statin= $1637 statin= $173 |
Icosapent + statin= $89,333 statin=$76,311 |
Cost per QALY gained (overall)= AUD $45,039 Cost per QALY gained (primary prevention) = $96,136 Cost per QALY gained (secondary prevention) = $35,935 Cost per YoLS (overall)= $38,480 Cost per YoLS (primary prevention) = $113,916 Cost per YoLS (secondary prevention) = $29,250 |
AUD50,000 | Compared with statin alone, Icosapent ethyl in combination with statin therapy is likely to be cost-effective in the prevention of cardiovascular disease, especially in the secondary preventive setting. |
| Gao et.al, 2019 | - | - | Icosapent = 10.57 Placebo= 10.28 Difference= 0.29 |
Icosapent = 12.78 Placebo= 12.47 Difference=0.31 |
AUD3768 per patient | Icosapent = $83,258 Placebo= $66,453 Difference= 16,805 |
Cost per QALY = $59,036 Cost per LYQs = $54,358 |
AUD50,000 | Icosapent is not a cost-effective from an Australian healthcare system perspective. The government may consider subsidising this medication given the clinical need but at a discounted acquisition cost. |
| ICER, 2019 | - | - | Icosapent =10.19 Statins=9.69 Difference=0.5 |
Icosapent =10.21 Statins=9.69 Difference=0.52 |
Net Price per Year Icosapent =$1,625 | Icosapent: Total costs=$40,000 Intervention Costs=$15,000 Non-Intervention Costs=$25,000 Statins: Total costs=$31,000 Intervention Costs=$800 Non-Intervention Costs=$30,000 Difference=$9,000 |
$18,000 per QALY gained, $17,000 per LYQs and $53,000 per MACE avoided | $50,000, $100,000, and $150,000 per QALY | Results suggest that the use of icosapent ethyl (in patients receiving statins) provide clinical benefit in terms of gains in quality-adjusted survival and overall survival compared to optimal medical management alone in the adult,established CVD cohort, and adults without known CVD but at high risk for cardiovascular events. |
| Kodera, et.al 2018 | primary prevention: Eicosapentaenoic + statin=18.8 statin=18.7 Difference=0.1 secondary prevention: Eicosapentaenoic + statin=18.1 statin=17.9 Difference=0.2 |
primary prevention: Eicosapentaenoic + statin=21.2 statin=21.1 Difference=0.1 secondary prevention: Eicosapentaenoic +statin=20.8 statin=20.6 Difference=0.2 |
A dose of 1,800mg costs ¥210.8 in Japan | primary prevention: Eicosapentaenoic + statin= ¥3,987,474 statin= ¥2,517,209 Difference= ¥1,470,265 secondary prevention: Eicosapentaenoic + statin= ¥6,551,407 statin= ¥5,281,864 Difference= ¥ 1,269,543 |
primary prevention: Cost per QALY = ¥29,567,364 Cost per LYQs = ¥32,198,787 secondary prevention: Cost per QALY = ¥5,450,831 Cost per LYQs = ¥5,410,598 |
¥5 million per QALY | Eicosapentaenoic +statin combination therapy showed acceptable cost-effectiveness for secondary prevention, but not primary prevention, of CVD in patients with hypercholesterolemia in Japan. | ||
| Philip et.al, 2016 | - | - | Eicosapentaenoic+statin=3.627 statin=3.575 Difference=0.052 |
- | Eicosapentaenoic +Statin= $3,497 Statin= $994 Difference=$2503 |
Eicosapentaenoic +Statin= $29,377 Statin= $30,587 Difference=$−1210 |
- | - | Combining Eicosapentaenoic with statin therapy for secondary prevention of cardiovascular disease in the United States may be a cost-saving. |
| Weintraub et.al, 2020 | - | - | - | - | - | $4.16 a day | primary prevention=$36,118/QALY | $50,000, $100,000, and $150,000 per QALY | In the United States, icosapent ethyl was shown to be dominant overall, cost-effective in primary prevention, and dominant in secondary prevention |
| Michaeli et.al, 2023 | Primary prevention CVD death: 3.9 Non-CVD death: 41.7 |
Primary prevention 4.6 |
Primary prevention Incremental QALYs: 0.81 |
Primary prevention Incremental LYs: 0.97 |
Icosapent ethyl: €2,400 | Primary prevention €14,732 | Primary prevention ICER (costs/LY): 15,130 ICER (costs/QALY): 18,133 |
€20,000 | For primary cardiovascular prevention, a combination therapy of icosapent ethyl plus statin is a cost-effective use of resources compared to statin monotherapy. |
| Secondary prevention CVD death: 3.8 Non-CVD death: 48.8 |
Secondary prevention 4.3 |
Secondary prevention Incremental QALYs: 0.99 |
Secondary prevention Incremental LYs: 1.34 |
Statins: €131.62 | Secondary prevention €14,333 |
Secondary prevention ICER (costs/LY): 10,695 ICER (costs/QALY): 14,485 |
For secondary prevention, icosapent ethyl increases atient benefit at different economic costs. | ||
| Michaeli et.al, 2022 | Primary prevention CVD death: 3.9 Non-CVD death: 41.7 |
Primary prevention 4.6 |
Primary prevention Incremental QALYs: 0.79 |
Primary prevention Incremental LYs: 0.9 |
Icosapent ethyl + statin: £2064 | --------------- | Primary prevention ICER (costs/LY): 17,121 ICER (costs/QALY): 19,485 |
£17,000 per QALY | Icosapent ethyl is cost effective for primary and secondary cardiovascular prevention at an annual price of £2064 in the UK |
| Secondary prevention CVD death: 3.8 Non-CVD death: 48.8 |
Secondary prevention 4.3 |
Secondary prevention Incremental QALYs: 0.98 |
Secondary prevention Incremental LYs: 1.25 |
Secondary prevention ICER (costs/LY): 10,409 ICER (costs/QALY): 13,285 |
|||||
| Lachaine et.al, 2023 | -------------- | ------------------ | Icosapent ethyl: 9.88 (0.52) Placebo: 9.58 (0.49) |
---------------- | ----------------- | Icosapent ethyl: $54.864 ($4483) Placebo: $42.341 ($4777) |
$42,797 ($15,884) | $50,000/QALY | Icosapent ethyl could be a cost-effective strategy for treating these patients in Canada. |
| Weintraub et.al, 2022 | • Death from any cause, nonfatal MI, or nonfatal stroke Icosapent ethyl: Trial:13.4 Model:13.6 Standard care: Trial: 16.9 Model:17.5 |
• During the trial period New heart failure Icosapent ethyl: 176 (4.30) Standard care: 167 (4.08) Atrial fibrillation/flutter Icosapent ethyl: 144 (3.52) Standard care: 105 (2.57) |
In trial analysis Icosapent ethyl: SSR: 3.34 WAC: 3.34 Standard care: SSR: 3.27 WAC:3.27 |
In trial analysis Icosapent ethyl: SSR: 4.31 WAC: 4.31 Standard care: SSR: 4.25 WAC: 4.25 |
Icosapent ethyl: SSR: $1518 WAC: $3387 |
In trial analysis Icosapent ethyl: LY and QALY SSR: $18786 WAC: $24544 Standard care: SSR: $17273 WAC: $17273 |
In trial analysis SSR: $26,328 per LY WAC: $126,524 per LY SSR: $22311 Per QALY WAC: $107218 per QALY |
$50,000 | Both in-trial and over the lifetime, Icosapent ethyl offers better cardiovascular out-comes than standard care in REDUCE-IT participants at common willingness-to-pay thresholds. |
| • Death from any cause Icosapent ethyl: Trial:6.7 Model: 6.9 Standard care: Trial: 7.6 Model:7.8 |
Ventricular tachycardia/fibrillation Icosapent ethyl: 35 (0.86) Standard care: 40 (0.98) Peripheral arterial disease Icosapent ethyl: 199 (4.87) Standard care: 206 (5.04) Unstable angina Icosapent ethyl: 132 (3.23) Standard care: 200 (4.89) • Over the lifetime New heart failure Icosapent ethyl: 513 (6.84) Standard care: 486 (6.48) Atrial fibrillation/flutter Icosapent ethyl: 428 (5.71) Standard care: 374 (4.99) Ventricular tachycardia/fibrillation Icosapent ethyl: 74 (0.99) Standard care: 76 (1.01) Peripheral arterial disease Icosapent ethyl: 475 (6.33) Standard care: 502 (6.69) Unstable angina Icosapent ethyl: 647 (8.63) Standard care: 982 (13.09) |
Lifetime model Icosapent ethyl: SSR: 10.59 WAC:10.59 Standard care: SSR: 10.35 WAC:10.35 |
Lifetime model Icosapent ethyl: SSR: 14.08 WAC:14.08 Standard care: SSR: 13.94 WAC:13.94 |
Not reported for standard care | Lifetime model Icosapent ethyl: LY and QALY SSR: $195276 WAC: $202830 Standard care: SSR: $197064 WAC: $197064 |
Lifetime model SSR: Dominant WAC: $36042 per LY SSR: Dominant WAC: $23866 per QALY al. |
|||
| Weintraub et.al, 2024 | •Death from any cause, nonfatal MI, or nonfatal stroke Icosapent ethyl: Trial: 14.3 Model: 14.7 Standard care: Trial:19.3 Model:19.5 • Death from any cause Icosapent ethyl: Trial: 7.2 Model: 7.4 Standard care: Trial:9.8 Model:9.9 |
• During the trial period New heart failure Icosapent ethyl: 86 (5.6%) Standard care: 91 (5.7%) Atrial fibrillation/flutter Icosapent ethyl: 64 (4.1%) Standard care: 66 (4.1%) Ventricular tachycardia/fibrillation Icosapent ethyl: 17 (1.1%) Standard care: 20 (1.3%) Peripheral arterial disease Icosapent ethyl: 93 (6.0%) Standard care: 115 (7.2%) Unstable angina Icosapent ethyl: 49 (3.2%) Standard care: 94 (5.9%) • Over the lifetime New heart failure Icosapent ethyl: 428 (5.71%) Standard care: 374 (4.99%) Atrial fibrillation/flutter Icosapent ethyl: 74 (0.99%) Standard care: 76 (1.01%) Ventricular tachycardia/fibrillation Icosapent ethyl: 475 (6.33%) Standard care: 502 (6.69%) Peripheral arterial disease Icosapent ethyl: 647 (8.63%) Standard care: 982 (13.09%) Unstable angina Icosapent ethyl: 85 (1.13%) Standard care: 87 (1.16%) |
In trial analysis Icosapent ethyl: Net cost: 3.28 WAC: 3.28 Standard care: Net cost: 3.13 WAC:3.13 Lifetime model Icosapent ethyl: Net cost:10.36 WAC:10.36 Standard care: Net cost: 9.83 WAC: 9.83 |
In trial analysis Icosapent ethyl: Net cost: 4.23 WAC: 4.23 Standard care: Net cost: 4.10 WAC: 4.10 Lifetime model Icosapent ethyl: Net cost: 13.68 WAC:13.68 Standard care: Net cost: 13.27 WAC:13.27 |
----------------- | In trial analysis Icosapent ethyl: LY Net cost: $33806 WAC: $41904 Standard care: Net cost: $35386 WAC: $35386 QALY Net cost: $29420 WAC: $36364 Standard care: Net cost: $30947 WAC: $30947 Lifetime model LY Icosapent ethyl: Net cost: $216243 WAC: $221403 Standard care: Net cost: $219212 WAC: $219212 QALY Icosapent ethyl: Net cost: $216243 WAC: $221403 Standard care: Net cost: $219212 WAC: $219212 |
In trial analysis LY Net cost: Dominant WAC: $48674 QALY Net cost: Dominant WAC: $36208 Lifetime model LY Net cost: Dominant WAC: $12385 QALY Net cost: Dominant WAC: $9582 |
$50,000 | The REDUCE-IT USA cost effectiveness analysis has shown that IPE provides excellent value, even being cost saving (dominant) both in trial over the lifetime as well as in most sensitivity analyses and subgroups, and even within the conservative US WTP threshold of $50 000 per QALY gained, both in primary and secondary prevention. |
Abbreviations: wholesale acquisition cost(WAC)
Table 1:
Annex A - Search strategy for each database
| Database | Date conducted | Search strategy |
|---|---|---|
| PubMed | May 4, 2024 | “Icosapent ethyl” [tiab] OR vascepa[tiab] OR amr101[tiab] OR amr-101[tiab] OR “eicosapentaenoic acid ethyl ester”[Supplementary Concept] OR “ethyl eicosapentaenoate”[tiab] OR “ethyl icosapentaenoate”[tiab] OR “ethyl eicosapentaenoic acid”[tiab] OR ethyl-EPA[tiab] OR “icosapent ethyl”[tiab] OR “ethyl eicosapentaenoic acid”[tiab] OR Epadel[tiab] OR icosapent[tiab] |
| Web of Science | May 4, 2024 | TS=(“Icosapent ethyl” OR vascepa OR amr101 OR amr-101 OR “eicosapentaenoic acid ethyl ester” OR “ethyl eicosapentaenoate”) |
| NHS Economic Evaluation Database (NHS EED) and the health technology assessment | ((icosapent ethyl):TI OR (vascepa ):TI OR (amr101):TI) and ((Systematic review:ZDT and Bibliographic:ZPS) OR (Systematic review:ZDT and Abstract:ZPS) OR (Cochrane review:ZDT) OR (Cochrane related review record:ZDT) OR (Economic evaluation:ZDT and Bibliographic:ZPS) OR (Economic evaluation:ZDT and Abstract:ZPS) OR Project record:ZDT OR Full publication record:ZDT) IN DARE, NHSEED | |
| Embase | May 4, 2024 | ‘icosapentaenoic acid ethyl ester’/exp OR ‘icosapentaenoic acid ethyl ester’ OR vascepa:ti,ab,kw OR amr101:ti,ab,kw OR amr-101:ti,ab,kw |
| Scopus | May 4, 2024 | TITLE-ABS-KEY (“’icosapentaenoic acid ethyl ester” OR vascepa OR amr101 OR amr-101 OR “Icosapent ethyl”) |
Of the 11 studies included in the final analysis, 5 were conducted in the United States,3, 7, 16–18 2 in Australia,9, 19 1 in Germany,20 1 in the United Kingdom,21 1 in Canada,22 and 1 in Japan.23 Most studies were published between 2019 and 2024, following the approval of IPE by the FDA on 13 December 2019, after randomized controlled trials demonstrated a significant reduction in the risk of ischemic events. The most commonly reported health outcomes, in addition to mortality and hospitalization rates, were QALYs, LYGs, and ICER indices. The perspectives of the studies primarily included health systems and payers.
The study time horizon was determined based on the average patient age of 60 years, with most studies adopting a 20-year or lifetime horizon, except for the study by Philip et al. The mean age of patients across the studies was 64 years, except for the study by Kodera et al.7 Studies utilized either Markov models or decision-tree methods for their design. All included studies incorporated sensitivity analysis to assess the impact of variable changes on the results. The discount rate applied in these studies ranged from 2% to 5%, consistent with the standards for economic evaluations in developed countries.
Table 2 presents the key results extracted from studies evaluating the cost-effectiveness of IPE combined with statins versus statin therapy alone. The study by Ademi et al19 demonstrated that, over a 20-year time horizon, the mortality rate per 1000 simulated patients was 736.5 in the IPE plus statin group compared to 794.3 in the statin-only group, indicating a reduction of 58 deaths per 1000 individuals. Additionally, IPE was associated with a significant decrease in hospitalizations. The same study reported that hospitalizations for nonfatal myocardial infarction or nonfatal stroke were 877 cases per 1000 in the IPE plus statin group, compared to 1,147.8 cases per 1000 in the statin-only group, reflecting a reduction of 270 hospitalizations.
For QALY and LYQ indices, studies report higher values for EPA plus statins versus statins. For instance, the QALY index in the ICER study was 10.19 and 9.69 for the EPA and statin groups, respectively. Results show that EPA is more expensive than conventional drugs such as statins. For example, the 1-year cost of an EPA in Australia is $ 3768 per patient, and the cost in the United States is $ 3497. The results also show that the threshold for evaluating the effectiveness of EPA varies from $ 50,000 to $ 150,000 in the United States, AUD 50,000 ($ 39,000) in Australia, and ¥5 million per QALY ($ 46,000) in Japan.
Discussion
This systematic review aimed to assess the economic implications of employing IPE for reducing the risk of CVD in primary and secondary prevention. The findings demonstrated that studies evaluating the cost-effectiveness of this novel pharmacological approach encompassed a diverse array of healthcare systems, perspectives, models, costs, and thresholds.
This research focused on analyzing several key aspects related to the cost-effectiveness of IPE compared to standard drugs:
Efficacy indices, including mortality rates, hospitalization rates, QALYs, and LYGs, in the IPE group versus standard drugs;
Annual and total costs associated with IPE versus standard drugs; and
The cost-effectiveness of IPE compared to standard drugs across various countries with differing cost-effectiveness thresholds.
Evaluation of efficacy indices
The findings of this study indicate that IPE was associated with reduced hospitalization and mortality rates compared to standard drugs. Ademi et al19 demonstrated a reduction in mortality of 58 individuals per 1000 patients in the long term with IPE use, aligning with the results of the REDUCE-IT US clinical trial, which used IPE to reduce cardiovascular mortality.
This study’s findings reveal that IPE demonstrates higher QALYs and LYGs efficacy indices than statins. As displayed in Table 4, IPE yielded an average of 10.2 QALYs, while statins resulted in 9.95 QALYs, suggesting a superior quality of life for patients in the IPE group. The observed increase in QALYs may be attributed to the reduced mortality and hospitalization rates among patients receiving IPE.
Table 4:
CHEERS checklist
| Section/item | Item No | Recommendation | Ademi et.al 2019 | Gao et.al, 2019 | ICER, 2019 | Kodera, et.al 2018 | Philip et.al, 2016 | Weintraub et.al, 2020 | Michaeli et.al, 2023 | Michaeli et.al, 2022 | Lachaine et.al, 2023 | Weintraub et.al, 2022 | Weintraub et.al, 2024 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title and abstract | |||||||||||||
| Title | 1 | Identify the study as an economic evaluation or use more specific terms such as “cost-effectiveness analysis”, and describe the interventions compared. | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Abstract | 2 | Provide a structured summary of objectives, perspective, setting, methods (including study design and inputs), results (including base case and uncertainty analyses), and conclusions. | Y | Y | Y | Y | Y | Y | Y | Y | ✘ | Y | ✘ |
| Introduction | |||||||||||||
| Background and objectives | 3 | Provide an explicit statement of the broader context for the study. | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Present the study question and its relevance for health policy or practice decisions. | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | ||
| Methods | |||||||||||||
| Target population and subgroups | 4 | Describe characteristics of the base case population and subgroups analysed, including why they were chosen. | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Setting and location | 5 | State relevant aspects of the system(s) in which the decision(s) need(s) to be made. | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Study perspective | 6 | Describe the perspective of the study and relate this to the costs being evaluated. | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Comparators | 7 | Describe the interventions or strategies being compared and state why they were chosen. | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Time horizon | 8 | State the time horizon(s) over which costs and consequences are being evaluated and say why appropriate. | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Discount rate | 9 | Report the choice of discount rate(s) used for costs and outcomes and say why appropriate. | Y | Y | Y | Y | Y | - | Y | Y | Y | Y | Y |
| Choice of health outcomes | 10 | Describe what outcomes were used as the measure(s) of benefit in the evaluation and their relevance for the type of analysis performed. | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Measurement of effectiveness | 11a | Single study-based estimates: Describe fully the design features of the single effectiveness study and why the single study was a sufficient source of clinical effectiveness data. | Y | Y | Y | Y | Y | - | Y | Y | Y | Y | Y |
| 11b | Synthesis-based estimates: Describe fully the methods used for identification of included studies and synthesis of clinical effectiveness data. | Y | Y | Y | Y | Y | - | Y | Y | Y | Y | Y | |
| Measurement and valuation of preference-based outcomes | 12 | If applicable, describe the population and methods used to elicit preferences for outcomes. | Y | Y | Y | - | Y | - | Y | Y | ✘ | Y | ✘ |
| Estimating resources and costs | 13a | Single study-based economic evaluation: Describe approaches used to estimate resource use associated with the alternative interventions. Describe primary or secondary research methods for valuing each resource item in terms of its unit cost. Describe any adjustments made to approximate to opportunity costs. | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 13b | Model-based economic evaluation: Describe approaches and data sources used to estimate resource use associated with model health states. Describe primary or secondary research methods for valuing each resource item in terms of its unit cost. Describe any adjustments made to approximate to opportunity costs. | Y | - | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Currency, price date, and conversion | 14 | Report the dates of the estimated resource quantities and unit costs. Describe methods for adjusting estimated unit costs to the year of reported costs if necessary. Describe methods for converting costs into a common currency base and the exchange rate. | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Choice of model | 15 | Describe and give reasons for the specific type of decision-analytical model used. Providing a figure to show model structure is strongly recommended. | Y | Y | Y | - | - | - | Y | Y | Y | Y | Y |
| Assumptions | 16 | Describe all structural or other assumptions underpinning the decision-analytical model. | Y | Y | Y | N | Y | - | Y | Y | Y | Y | ✘ |
| Analytical methods | 17 | Describe all analytical methods supporting the evaluation. This could include methods for dealing with skewed, missing, or censored data; extrapolation methods; methods for pooling data; approaches to validate or make adjustments (such as half cycle corrections) to a model; and methods for handling population heterogeneity and uncertainty. | - | N | Y | Y | Y | Y | ✘ | ✘ | Y | Y | ✘ |
| Study parameters | 18 | Report the values, ranges, references, and, if used, probability distributions for all parameters. Report reasons or sources for distributions used to represent uncertainty where appropriate. Providing a table to show the input values is strongly recommended. | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Incremental costs and outcomes | 19 | For each intervention, report mean values for the main categories of estimated costs and outcomes of interest, as well as mean differences between the comparator groups. If applicable, report incremental cost-effectiveness ratios. | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Characterising uncertainty | 20a | Single study-based economic evaluation: Describe the effects of sampling uncertainty for the estimated incremental cost and incremental effectiveness parameters, together with the impact of methodological assumptions (such as discount rate, study perspective). | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y |
| 20b | Model-based economic evaluation: Describe the effects on the results of uncertainty for all input parameters, and uncertainty related to the structure of the model and assumptions. | Y | Y | Y | Y | Y | - | Y | Y | Y | Y | Y | |
| Characterising heterogeneity | 21 | If applicable, report differences in costs, outcomes, or cost-effectiveness that can be explained by variations between subgroups of patients with different baseline characteristics or other observed variability in effects that are not reducible by more information. | N | N | Y | N | - | - | ✘ | ✘ | ✘ | ✘ | ✘ |
| Study findings, limitations, generalisability, and current knowledge | 22 | Summarise key study findings and describe how they support the conclusions reached. Discuss limitations and the generalisability of the findings and how the findings fit with current knowledge. | Y | Y | Y | Y | Y | - | Y | Y | Y | Y | Y |
| Source of funding | 23 | Describe how the study was funded and the role of the funder in the identification, design, conduct, and reporting of the analysis. Describe other non-monetary sources of support. | Y | Y | Y | Y | Y | - | Y | Y | Y | Y | Y |
| Conflicts of interest | 24 | Describe any potential for conflict of interest of study contributors in accordance with journal policy. In the absence of a journal policy, we recommend authors comply with International Committee of Medical Journal Editors recommendations. | Y | Y | Y | Y | Y | - | Y | Y | Y | Y | Y |
Similarly, IPE achieved an LYGs index of 13.57, which is 0.31 higher than that of statins, indicating a potential increase in life expectancy for patients in the IPE group. The higher LYGs value observed in the IPE group may be explained by the decreased mortality rate among these patients.
Annual and total cost of IPE versus statins in different countries
The annual cost for IPE was found to be highest in the United States and lowest in Japan. This study revealed that the total costs for IPE, in all studies except Philip et al,7 were higher than those of standard drugs, such as statins. The highest total cost for IPE was reported in Australia at $73,164, while the lowest was observed in the United States at $31,774.
The higher total costs associated with IPE may be attributed to the elevated annual costs of this medication in various countries. Nonetheless, when comparing the total costs between IPE and statins, the difference diminished, potentially due to the higher readmission rates and subsequent complications experienced by patients in the statin group.
Cost-effectiveness of IPE in selected countries with different thresholds
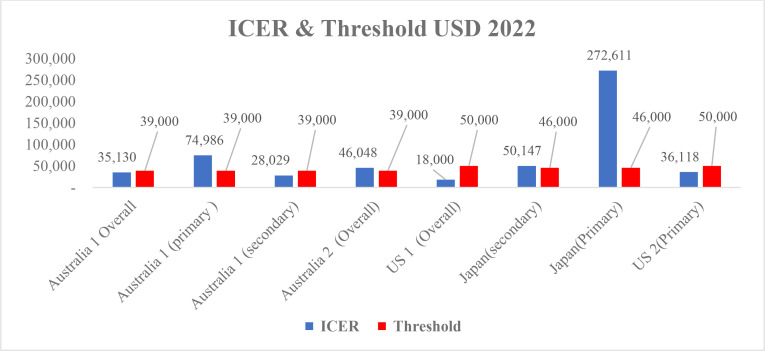
The cost-effectiveness of IPE was found to differ between primary and secondary prevention patient groups, as demonstrated by a study conducted in Australia. In the primary prevention group, the cost-effectiveness threshold was lower than the ICER index ($39,000 vs $75,000), as shown in Figure 2. Conversely, in the secondary prevention group, the ICER index was lower than the willingness-to-pay (WTP) threshold, indicating the cost-effectiveness of IPE in this population ($28,000 vs $39,000).
Fig. 2.
Cost-effectiveness Ratio and Threshold in selected countries
Studies in the United States have reported that IPE is a cost-effective alternative to statins within the United States healthcare system, in primary and secondary prevention. Similarly, a study in Japan demonstrated that the cost-effectiveness of IPE was higher in the secondary prevention group than in the primary prevention group. In the secondary prevention group, the ICER and threshold indices were relatively close ($46,000 and $50,000, respectively), while the ICER index for the primary prevention group was considerably higher than the threshold ($46,000 vs $272,000). A comparison of results across different countries revealed that the highest and lowest ICER indicators for primary prevention were found in Japan and the United States, respectively.
Limitation
The present study identified 11 economic evaluation studies examining the cost-effectiveness of IPE, indicating a need for further research to establish more conclusive results across diverse countries and healthcare systems. It is essential to consider long-term outcomes and potential complications associated with IPE to comprehensively evaluate its cost-effectiveness and potential impact on patients’ quality of life.
Conclusion
The findings of this systematic review indicate that IPE effectively reduces cardiovascular risks, leading to decreased mortality and hospitalization rates, as well as increased life expectancy and quality of life in both primary and secondary prevention patients. The results further suggest that IPE is cost-effective, with a higher probability of cost-effectiveness observed in the secondary prevention group than in the primary prevention group.
Acknowledgements
We would like to extend our gratitude to everyone who contributed to the writing of this article.
Footnotes
Availability of Data and Materials
The data sets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Funding
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Notes:
This paper should be cited as: Pourasghari H, Azari S, Omidi N, Arabloo J, Rajaie S, Rezaei MA, et al. Cost-Effectiveness of Icosapent Ethyl for Ischemic Cardiovascular Events. J Teh Univ Heart Ctr 2024;19(S1):40-55.
References
- 1.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. 2020;76(25):2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ollendorf D, McQueen R, Fazioli KJIfC, Review E. Additive therapies for cardiovascular disease: effectiveness and value. 2019. [DOI] [PMC free article] [PubMed]
- 4.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke Statistics-2019 update a report from the American Heart Association. Circulation. 2019. [DOI] [PubMed] [Google Scholar]
- 5.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia.N Engl J Med. 2019;380(1):11–22. [DOI] [PubMed] [Google Scholar]
- 6.Virani SS, Akeroyd JM, Ramsey DJ, Chan WJ, Frazier L, Nasir K, et al. Comparative effectiveness of outpatient cardiovascular disease and diabetes care delivery between advanced practice providers and physician providers in primary care: implications for care under the Affordable Care Act.Am Heart J. 2016;181:74–82. [DOI] [PubMed] [Google Scholar]
- 7.Philip S, Chowdhury S, Nelson JR, Benjamin Everett P, Hulme-Lowe CK, Schmier JKJJome. A novel cost-effectiveness model of prescription eicosapentaenoic acid extrapolated to secondary prevention of cardiovascular diseases in the United States.J Med Econ. 2016;19(10):1003–10. [DOI] [PubMed] [Google Scholar]
- 8.Matsuzaki M, Yokoyama M, Saito Y, Origasa H, Ishikawa Y, Oikawa S, et al. Incremental effects of eicosapentaenoic acid on cardiovascular events in statin-treated patients with coronary artery disease secondary prevention analysis from JELIS.Circ J. 2009;73(7):1283–90. [DOI] [PubMed] [Google Scholar]
- 9.Gao L, Moodie M, Li S-C. The cost-effectiveness of omega-3 polyunsaturated fatty acids–The Australian healthcare perspective. European journal of internal medicine. 2019;67:70–6. [DOI] [PubMed] [Google Scholar]
- 10.Bhatt DL, Steg PG, Brinton EA, Jacobson TA, Miller M, Tardif JC, et al. Rationale and design of REDUCE-IT: reduction of cardiovascular events with icosapent ethyl–intervention trial.Clin Cardiol. 2017;40(3):138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis.Lancet. 2007;369(9567):1090–8. [DOI] [PubMed] [Google Scholar]
- 12.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. New England Journal of Medicine. 2019;380(1):11–22. [DOI] [PubMed] [Google Scholar]
- 13.Fares H, Lavie CJ, DiNicolantonio JJ, O’Keefe JH, Milani RVJT., management cr. Icosapent ethyl for the treatment of severe hypertriglyceridemia.Ther Clin Risk Manag. 2014;10:485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated health economic evaluation reporting standards (CHEERS)—explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force.Value Health. 2013;16(2):231–50. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG.medicine PGJP. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med.2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weintraub WS, Bhatt D, Zhang Z, Zhang C, Sarahfaye D, Boden WE, et al. Cost-effectiveness of icosapent ethyl in US REDUCE-IT patients. 2020;75(11_Supplement_1):1914-. [Google Scholar]
- 17.Weintraub WS, Bhatt DL, Zhang Z, Dolman S, Boden WE, Bress AP, et al. Cost-effectiveness of icosapent ethyl for high-risk patients with hypertriglyceridemia despite statin treatment. JAMA Network Open. 2022;5(2):e2148172–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weintraub WS, Bhatt DL, Zhang Z, Dolman S, Boden WE, Bress AP, et al. Cost-Effectiveness of Icosapent Ethyl in REDUCE-IT USA: Results From Patients Randomized in the United States. Journal of the American Heart Association. 2024;13(1):e032413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ademi Z, Ofori-Asenso R, Zomer E, Owen A, Liew D. The cost-effectiveness of icosapent ethyl in combination with statin therapy compared with statin alone for cardiovascular risk reduction. Eur J Prev Cardiol. 2021:28(8):897–904. [DOI] [PubMed] [Google Scholar]
- 20.Michaeli DT, Michaeli JC, Boch T, Michaeli T. Cost-effectiveness of lipid-lowering therapies for cardiovascular prevention in Germany. Cardiovascular drugs and therapy. 2023;37(4):683–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michaeli DT, Michaeli JC, Boch T, Michaeli T. Cost-effectiveness of icosapent ethyl, evolocumab, alirocumab, ezetimibe, or fenofibrate in combination with statins compared to statin monotherapy. Clinical Drug Investigation. 2022;42(8):643–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lachaine J, Charron J-N, Gregoire JC, Hegele RA, Leiter LA. Cost-Effectiveness of icosapent ethyl (IPE) for the reduction of the risk of ischemic cardiovascular events in Canada. Clinicoecon Outcomes Res. 2023;15:295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kodera S, Morita H, Kiyosue A, Ando J, Komuro IJCJ. Cost-Effectiveness of Statin Plus Eicosapentaenoic Acid Combination Therapy for Cardiovascular Disease Prevention in Japanese Patients With Hypercholesterolemia—An Analysis Based on the Japan Eicosapentaenoic Acid Lipid Intervention Study (JELIS)—.Circ J. 2018;82(4):1076–82. [DOI] [PubMed] [Google Scholar]