Abstract
Recent trends promote the replacement of animal-based products using plant sources. Such food alternatives can be achieved utilizing bigels, tailorable materials which combine plant protein-based hydrogel (HG) and oleogel (OG) phases into one gel system. The effect of salt addition, commonly used in the food industry, into plant-based bigel formulation based on pea protein hydrogel and candelilla wax oleogel is the main goal of this research while the effect of transglutaminase (TG) cross-linker concentration (0–20 % out of the protein mass) and OG/HG ratio (30/70-60/40) were also examined. Overall, bigel composition of 10 % TG, which was found vital for consistency, and 40/60 OG/HG ratio, which was found preferable with respect to mechanical strength and appearance, was used to thoroughly explore the effect of salt addition. The results show that NaCl significantly impacts the bigel structure and performance. More specifically, increase salt concentration transitioned the bigel structure from OG-in-HG type to an unorganized bi-continuous-like microstructure. These structural changes were correlated to protein screening effect of the salt ions which affected the bulk gelation and interfacial properties of the protein. The mechanical behavior demonstrated a transition from flaky and brittle texture to softer and spreadable texture with elevated salt concentrations. Moreover, all bigels thermal rheology showed an abrupt softening due to oleogel melting finalized by increasing moduli around protein denaturation point. It seems that TG concentration and OG/HG ratio have a significant impact on the bigel properties, while even a low concentration of NaCl can significantly alter the bigel performance and thus should be considered while developing a specific alternative product.
Keywords: Bigel, Pea protein, NaCl, Microstructure, Texture
Graphical abstract
Highlights
-
•
Plant-based bigels were formulated using pea protein hydrogel and wax oleogel.
-
•
TG enzyme is vital for pea protein-based bigel consistency.
-
•
NaCl charge screening effect on protein induced unorganized bigel microstructure.
-
•
NaCl reduced bigel mechanical properties and increased its shear resistance.
-
•
NaCl significantly affects the texture of plant protein-based bigel.
1. Introduction
In the last few years, there has been an increased awareness of the environmental, ethical, and health issues attributed to animal-based food such as meat and dairy. Aiming to advocate reduction or even eliminate animal sourced products, alternative food options are required. Recent studies focus on the development of foods based on plant proteins; however, plant proteins raise some challenges related to their poor functional properties compared to animal proteins, such as undesirable textures and organoleptic attributes (Xiao et al., 2023). Furthermore, some existing alternative products do not provide comparable nutritional values such as cheese analogues with low protein and high carbohydrate content. Moreover, the fat phase usually relies on the use of coconut or palm oils which suffer from some limitation related to their high saturation level (Mefleh et al., 2022). Therefore, current research aims to find solutions with improved nutritional profile and comparable texture, based on plant proteins.
Gels are semi-solid materials that attain tailorable textures due to their component diversity, preparation conditions, and internal interactions. Their structure relies on a major liquid phase entrapped within a three-dimensional network of components called gelling agents or gelators. Food gels include oil and water as a liquid phase creating oleogels and hydrogels, respectively, when their gelators are various hydrophobic and hydrophilic substances including lipids, proteins, and polysaccharides (Banerjee and Bhattacharya, 2012; Co and Marangoni, 2018). Bigels are unique hybrid systems which combine these two gels, i.e. oleogel and hydrogel, and have been studied in various fields such as cosmetics and pharmaceuticals. Their utilization in the food field can provide improved texture and nutritional profile (Shakeel et al., 2019; Silva et al., 2022). Bigels were studied previously using various oil and water gelation mechanisms. Oil gelation was examined using oleogelators such as glycerol monostearate (Samui et al., 2021), fatty acid mixtures (Zheng et al., 2023), ethyl cellulose (Xue et al., 2024), and waxes (Chen et al., 2023; Xie et al., 2023). For example, candelilla wax (CW), a plant-based low molecular weight oil gelator, has a high melting point (60 °C–73 °C) while creating platelet-like crystals in oil leading to its solidification (Blake et al., 2018). Water gelation agents previously used in bigels included polysaccharides such as carrageenan and locust bean gum (Martins et al., 2023) and sodium alginate (Martins et al., 2019), and animal proteins such as whey proteins (Hashemi et al., 2023) and gelatin (Samui et al., 2021). Although being an emerging trend at alternative food products, plant proteins function as sole hydrogelators in bigels has limited occurrence including pea protein (Lotfi Shirazi and Koocheki, 2025), potato protein (Gonçalves et al., 2024), chickpea and potato protein (Glusac et al., 2024), soy protein (Qiu et al., 2024), and canola protein (Moguiliansky et al., 2025).
Plant proteins can be found in cereals, nuts, seeds, algae, and legumes, differing in their functional properties such as solubility, emulsification, and gelation. Most plant proteins have large and compact structures and function as storage proteins, leading to lower functionalities than proteins from animal sources. Two major legume protein fractions are globulins (50–70 % out of the total seed protein) and albumins (∼20–25 % out of the total seed protein). Globulins are defined as salt soluble globular proteins, while albumins are water soluble (Day et al., 2022). Some plant proteins can aggregate and form a gel matrix due to heat treatment. During heating, proteins are first unfolded exposing their reactive sites and hydrophobic regions, then can interact with neighboring molecules leading to crosslinking and aggregation. This process is also termed heat-induced gelation due to the formation of a 3D network structure (Ma and Chen, 2023; Nicolai and Chassenieux, 2019). Pea (Pisum sativum L.) is considered as a proper source of plant proteins, due to a high nutritional value and low allergenicity. Pea globulins present better emulsifying and gelling features than albumins. There are three main globulin types divided into two fractions by their sedimentation coefficient. One fraction is the legumin section (11S) and other (7S) is composed of the proteins vicilin and covicilin. The final ratio between protein groups in the isolated protein can vary between suppliers due to the extraction method effecting their functional properties (Mession et al., 2015; Yang et al., 2021).
Chemical crosslinking of proteins is a common way for enhancing the formation of a network thus altering the hydrogel properties by creating protein structures with covalent interactions. Transglutaminase (TG) is an enzyme which catalyzes protein intra-molecular and inter-molecular crosslinking when acyl transfers from glutamine's residue to lysine's creating glutamyl lysine isopeptides. TG is a common cross-linker used in the food industry, having an optimal temperature range of 45–55 °C and an activity lost point above 70 °C, and can be obtained from animals, plants, and microorganisms (Vasić et al., 2023). TG was used to generate protein modification of various plant sources including pea protein (Vasić et al., 2023; Li et al., 2022; Moreno et al., 2020a). Its use in bigel systems was studied for pea protein (Lotfi Shirazi and Koocheki, 2025; Yang et al., 2024), chickpea and potato protein (Glusac et al., 2024), and canola protein (Moguiliansky et al., 2025).
Salts such as sodium chloride (NaCl) are commonly food additives used in the food industry as flavoring and preservatives by reducing water activity. For example, salt added during cheese manufacturing affects bacteria and enzyme activity, also causing physical changes in milk proteins, syneresis and water loss, affecting its texture (Man, 2007). Moreover, aqueous solution with ionic strength can alter plant proteins surface charge, which alters their conformation and heat-induced gelation affecting gel properties (Guo et al., 2023a; Lei et al., 2022). NaCl addition was reported to have dual effect on plant-protein-based hydrogel properties depending on its concentration, pH value, and protein nature. Some cases reported on salting-in effect of NaCl such as in the case of faba bean and pea globulins at pH 7 and 4.5, respectively, resulting with stronger gels (Johansson et al., 2023; Tanger et al., 2022). Others, such as walnut protein combined with κ-carrageenan to form a composite gel had salting-out effect, aggregation and protective effect from denaturation. This effect resulted in either stronger gels derived from aggregates being active filling particles or weaker protein network (Lei et al., 2022). Additionally, NaCl can affect the interfacial behavior of plant proteins in biphasic systems. It was previously established that such proteins can act as emulsifiers by creating a direct thick coating layer on the oil phase droplets or Pickering stabilizers. For example, previous studies with bigel systems used plant protein particles for interface stabilization, i.e. Pickering mechanism, and by that improved bigel properties (Guo et al., 2023b; Li et al., 2023). Such stabilization behavior can be affected by the presence of salt ions. For example, the screening effect of 50–100 mM NaCl induced soy globular proteins aggregation at the aqueous bulk and water-oil interface of emulsion gels, and by that impaired their emulsification and interface stability, while high concentration induced an opposite salting-in effect (Zhang et al., 2022). However, up to date the effect of salt on plant proteins in bigel systems is still unknown, although proteins may play a crucial role in gel stabilization in the bulk and water-oil interface.
In this research pea protein (PP)-based hydrogel was used to formulate a bigel matrix with CW oleogel while the effects of salt addition, oleogel:hydrogel (OG/HG) ratio, and TG addition were examined. First, the formulation was adjusted by analyzing the effect of TG concentration and OG/HG ratio, and then the addition of salt, NaCl, to the matrix was examined aiming to explore the effect of such additive on bigel performance. The bigel properties were examined by analyzing the effect of the above parameters on the mechanical, rheological, thermal, and structural properties. It was hypothesized that NaCl concentration can change bigel texture due to its effect on plant protein conformation and functionality.
2. Materials and methods
2.1. Materials
Refined canola oil was purchased from a local supermarket (private label Shufersal, Israel). CW was purchased from Plant Guru (USA). PP isolate Peazzaz® (Merit Functional Foods, Canada) was kindly donated by Hirshberg Brothers Chemicals (Israel). Transglutaminase Preparation ACTIVA® (TG) was purchased from Ajinomoto (USA) with an activity of 86–135 U/gr, as specified by the manufacturer. NaCl was purchased from Fisher Scientific (UK). Nile red and Nile blue A dyes and the reagents: Ammonium persulfate (APS), Sodium dodecyl sulfate (SDS), TEMED, Tris, β-mercaptoethanol, ethanol, acetic acid, methanol, Coomassie brilliant blue (R-250), HCl were purchased from Sigma-Aldrich (Israel). High range protein marker [PM2600] was purchased from Smobio (China).
2.2. PP isolate characterization
CHNS (i.e. carbon, hydrogen, nitrogen, and sulfur) elemental analysis was performed for protein content determination of the isolate using a Flash 2000 Organic Elemental Analyzer (Thermo Scientific, USA), based on a previous study method (Moguiliansky et al., 2025). Essentially, the nitrogen fraction was measured for duplicated powder samples, and the final protein content was calculated using a conversion factor of 5.4 that was recently used for pea protein (Emkani et al., 2023) and derived from previously calculated nitrogen to protein ratio of pea amino acid composition (Mariotti et al., 2008).
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) was used to evaluate the different PP fractions using 12 % separating gel and 4 % stacking gel (Moguiliansky et al., 2025). The protein powder was diluted and treated in reducing conditions, achieving a concentration of 20 μg/30 μl that was loaded on the gel in duplicate.
2.3. Sample preparation
Bigels were fabricated using a direct hot emulsification method based on combination of oil and water phases. First, PP was gently stirred in distilled water for 1 h at room temperature, to create a 15 %wt. suspension. For formulations in which NaCl addition was examined, the salt was mixed with the suspension as well. Then, oil and wax (6 %wt. out of the oil phase) were stirred while heating up to ∼75 °C until a full dissolution of CW was achieved. At the same time, the aqueous phase was mixed and heated to ∼70 °C. After heating, oil phase was gradually poured into the protein suspension, and the mixture was then subjected to high sheer mixing by an Omni General Laboratory Homogenizer (GLH 850). The homogenization occurred in two cycles of 1 min at 20,000 rpm, as TG was added to the second homogenization step to minimize its exposure to high shear and temperature. The obtained mixture, ∼24 g of a white dense paste, was transferred into a 60 mm aluminum weighing dish, covered with aluminum foil and stored at 4 °C for 2 days. Before analysis, the gels were allowed to equilibrate at room temperature.
The examined bigels were prepared using various TG concentrations (5, 10, 15, and 20 % out of the protein mass) and OG/HG ratios (30/70, 40/60, 50/50, and 60/40). These parameters were examined to reach the best formulation based on favorable consistency. This formulation was further examined by analyzing the salt effect where NaCl concentrations of 0, 75, 150 and 225 mg/100 g bigel were tested. As a control, hydrogel and oleogel samples were prepared separately and characterized. Oleogel sample was prepared in the same way described prior to homogenization and was transferred to a 20 ml syringe to mold a cylindrical shape, while preserving the same heating conditions. Hydrogel was prepared by mixing and heating (∼70 °C) 24 g of the protein suspension with and without NaCl, then cooling to ∼55 °C for 2 min while mixing, adding TG, followed by stirring at 800 rpm for 2 min and transferring the paste to aluminum dish.
2.4. Protein suspension analysis
The effect of NaCl on the protein behavior in solution was examined using particle size analysis and ζ-potential. Particle size distribution was measured by Static Light Scattering (SLS) using Mastersizer 3000 (Malvern Instruments Ltd, Dr. Golik, Israel). Refractive Index values of 1.33 and 1.45 were used for water and protein, respectively. Analyzed suspensions of 15 %wt. PP were prepared in triplicates with the same NaCl content added to water phase of 40/60 bigels: 0 mg/60 g, 75 mg/60 g, 150 mg/60 g, and 225 mg/60 g. Protein suspensions were added to the measurement cell, containing 125 ml of Milli-Q water and mixed at 1200 rpm, until the laser obscuration was stabilized on 4–9 %. Size distribution data was reported as the mean volume-weighted particle diameter d(4,3) = Σnidi4/Σnidi3, when ni is the number of particles having di diameter. The analysis was done in triplicates, when each measurement represented the average of five runs. The ζ-potential of the same protein suspensions was determined using a Zetasizer Ultra, coupled with capillary electrophoresis (Malvern Instruments, Dr. Golik, Israel). Samples were diluted in Milli-Q water at a ratio of 1:100 (v/v) before 700 μl were inserted into a DTS1070 capillary cell and tested at 25 °C.
2.5. Microstructural analysis
The effect of different OG/HG ratios, TG concentrations, and NaCl contents on bigel microstructure was explored using an inverted confocal laser scanning microscope (LSM 700, Zeiss). Bigel morphology and type (i.e., oleogel-in-hydrogel, hydrogel-in-oleogel or bi-continuous) were determined by a visual assessment of the distribution of the different phases (i.e., hydrogel and oleogel). The divided phases were stained during heating by fluorescent dyes – 0.1 w/v% in ethanol solution, Nile red for oleogel and Nile blue A for hydrogel, in a ratio of 0.05 v/w. The emission between 555 and 639 nm was detected. Image analyzes and brightness contrast display modification were done using a Zeiss Zen 3.7.97, blue edition (Carl Zeiss Microscopy GmbH, Germany).
2.6. Mechanical properties analysis
The mechanical properties representing the gel strength, were evaluated using a TA1 texture analyzer (Lloyd Instruments LTD, AMETEK, Israel) equipped with 50 N load cell. The Texture profile analysis testing procedure was operated with two compression cycles between two flat plates (11.5 cm diameter), 3 s waiting between cycles, and 50 mm/min head speed, to compress a 16 mm circular sample up to 50 % of its height. Hardness (N), cohesiveness, and springiness, defined as the maximum force required for the first compression, the ratio of the second to the first peak area under the force-time curve, and the ratio of the sample height at the start of the second to the first compression, respectively, were calculated. Six to nine replicates of each formulation were tested. The samples were visualized before and after the compression test while a small portion of each NaCl added bigel was smeared by a spatula to assess its spreadability and the result was also analyzed visually.
2.7. Viscoelastic properties analysis
The rheological behavior of systems with different NaCl concentrations was assessed using MCR 302 rheometer (Anton Paar, Prime Lab Scientific, Israel). A 25 mm diameter sandblasted parallel plates geometry (PP25S) at 1 mm gap was selected for the procedure. The viscoelastic behavior of the salt added hydrogel and bigel was examined through measuring the storage and loss modulus (G′ and G″, respectively) with respect to frequency and temperature. Modulus-frequency dependency was tested at a temperature of 25 °C at the range of 0.01–100 rad s−1 in a linear viscoelastic region (LVR), which was determined priorly. The measured data was fitted to a power-law model:
| (1) |
where G′0 is the modulus value at the frequency of 1 rad s−1, ω is the angular frequency, and n′ is the exponent parameter expressing the dependency between G′ and ω (Moreno et al., 2020b). The effect of temperature on the viscoelastic behavior was tested during a temperature ramp between 20 and 100 °C in heating rate of 10 °C/min. Three replicates of each formulation were examined.
2.8. Thermal analysis
Thermal gravimetric analysis (TGA) was performed using TGA 5500 (TA Instruments, BARGAL, Israel) to estimate the bigel composition and thermal stability at different NaCl concentrations. Approximately 10–20 mg of sample was placed in a platinum pan heated from 20 to 700 °C at a 20 °C∙min−1 rate under nitrogen flow (25 ml min−1). Samples were tested in triplicates.
2.9. Statistical analysis
Results were statistically examined using GraphPad Prism 7.03 (GraphPad Software Inc., USA) and JMP® Pro 15 (SAS Institute Inc.) softwares. ANOVA comparable analysis via Brown-Forsythe test with Tukey's multiple comparison was conducted between samples with similar group variance. Additionally, the GamesHowell test with Welch's correction was used for samples with a significantly different group variance. The results are presented at a significant level of 5 % and shown as a mean ± standard deviation.
3. Results and discussion
3.1. The effect of transglutaminase addition
The gelation of PP using TG was previously suggested and explored (Djoullah et al., 2018), however, the effect of TG on PP cross-linking in bigel system was not examined to date. Therefore, the effect of TG addition on bigel was examined using 40/60 OG/HG ratio, no NaCl added, and 0, 5, 10, 15, 20 %wt. TG (out of the protein mass). The effect of TG was examined to optimize its concentration as a stable bigel was not formed without TG addition as can be seen in Fig. S1.
Based on the visual appearance of bigels their texture and consistency were significantly influenced by TG content, Fig. S1. Overall, the gels were white with a semi-solid appearance. Moreover, the images clearly show the important role of TG in the bigel formulation, where the sample without TG had a viscous white paste texture (Figs. S1 and A0), therefore, it could not be analyzed using TPA (as described below). The important role of TG in gel formation was previously shown for canola protein-based bigels, where sample without TG at 40/60 OG/HG ratio was liquid (Moguiliansky et al., 2025). Thus, the addition of TG led to the formation of a white stable gel with defined shape and structure (Fig. S1, A1-4).
The visual appearance was followed by microscopic confocal analysis where the oil phase (red) and water phase (blue) were visually observed, Fig. 1. The observed morphology demonstrated dispersed oil phase droplets (in red) inside a continuous water phase composed of protein aggregates (in blue), i.e. oleogel-in-hydrogel type structure, in all TG concentrations. The black voids that appeared at the combined images can be related to air pockets formed during homogenization. Overall, a clear change in the gel density can be observed with TG concentration. More specifically, the oil droplets density increased, and the content of air pockets decreased leading to overall higher sample density when TG concentration increased. Such microstructural changes can explain the changes seen visually, i.e. increase in consistency and stability. Previous studies showed similar appearance for fermentation-induced PP isolate emulsion gels with 0 and 0.05 %w/w TG (Masiá et al., 2023) and chickpea-potato protein bigels with 0 and 100 U/g TG (Glusac et al., 2024), where for both examples, TG addition created a visible denser hydrogel assembly. Consequently, it seems that bigel microstructure changes with TG concentration forming a denser protein network, therefore, can explain the differences seen in the overall gel physical properties.
Fig. 1.
Confocal images of bigels produced with 6 %wt. CW in the oil phase, 15 %wt. PP in the water phase, and 40/60 OG/HG ratio using different TG concentrations (out of the protein mass) at × 20 magnification.
Bigel mechanical properties, which are important for sensory evaluation of food, were estimated using TPA analysis, Fig. 2. Gel hardness can be related to the gel strength, derived from the maximum force obtained during the first compression (Szczesniak, 2002). The results show that bigel hardness increased as the TG content increased, up to a maximum value at 10 % TG out of the protein mass (21.40 ± 1.55 N), above which, hardness reduction was observed. This trend can be attributed to hydrogel network strengthening due to TG mediated crosslinking, which first produced stiffer hydrogels, but resulted with lower gel hardness at high concentration. Bigel strength decrease could be due to syneresis (i.e. water extraction from gel matrix) and hydrogel weakening derived from excessive crosslinking as water droplets were visible on the outer surface of the bigels with 15 and 20 % TG (Fig. S1 A3-4). Hydrogel strength decrease due to excessive TG crosslinking and syneresis was previously reported by Ruzengwe et al. (2020) for bambara groundnut protein hydrogel. In addition, such hardness decrease could also result from strong protein crosslinked network which hinder the interfacial connection with the oleogel phase. Previous study on soy protein emulsion gels demonstrated lower mechanical properties (fracture stress and strain) due to extensive TG crosslinking which was explained by the formation of a coarse protein network that impair the system hydrophobic interactions (Luo et al., 2019).
Fig. 2.
The effect of TG concentration (out of the protein mass) on the hardness (light grey), cohesiveness (white), and springiness (dark grey) of bigel prepared with 15 %wt. PP in the water phase, 6 %wt. CW in the oil phase, and 40/60 OG/HG ratio. Different letters represent significantly different values (p < 0.05). N.D. refers to samples that cannot be analyzed.
Gel cohesiveness represents its relative ability to withstand deformation before breaking, and is derived from the ratio of the deformation resistance of the second compression to that of the first compression (Szczesniak, 2002; Mei Wee et al., 2018). The cohesiveness values, Fig. 2, remained steady at 5 and 10 % TG out of the protein mass, followed by a decrease as enzyme concentration further increased. This behavior is aligned with the hardness value and can be related to weaker bigel consistency derived from weaker interactions between the two phases due to stronger protein-protein interactions compared to their interactions with the oleogel at the interface. The springiness parameter describes the degree of recovery after deformation, indicating its plastic or elastic behavior (Szczesniak, 2002; Mei Wee et al., 2018). The springiness values, Fig. 2, were similar at 5 and 10 % TG out of the protein mass, followed by a slight decrease at 15 and 20 %. This indicates that at 5 and 10 % enzyme out of protein mass, bigels were better able to recover to their original height after compression, while excessive crosslinking led to more plastic behavior where the material deformed permanently, consistent with the obtained reduction in gel strength. Previous study showed increased hardness, cohesiveness, and springiness in bigels composed of carboxymethyl cellulose and PP hydrogel and rice bran wax oleogel as TG content increased from 0 to 0.4 %. This effect was attributed to the hydrogel network strengthening with the absence of excessive crosslinking (Yang et al., 2024).
The hardness, cohesiveness, and springiness results are consistent with the visual analysis presented in Fig. S1, showing stable and undeformed samples before and after compression at TG concentration below 10 %wt. (A1-2 and B1-2, respectively), followed by solid appearance before and ruptured and smeared samples after compression at higher TG concentration (A3-4 and B3-4, respectively). In fact, deep cracks were observed at 15 % TG out of the protein mass (Fig. S1 B3) while at 20 % the sample was completely deformed and spread (Fig. S1 B4). This suggests that TG gives elastic properties and resilience to the bigel up to 10 % out of the protein mass, while higher TG concentration produces brittle material. Thus, excessive amounts of TG should be handled with caution to achieve specific mechanical behavior.
Overall, the hardest and most consistent bigel was obtained while using 10 % TG out of the protein mass, thus this concentration was used to optimize the water-oil ratio for pea protein-based bigels.
3.2. The effect of oleogel:hydrogel ratio
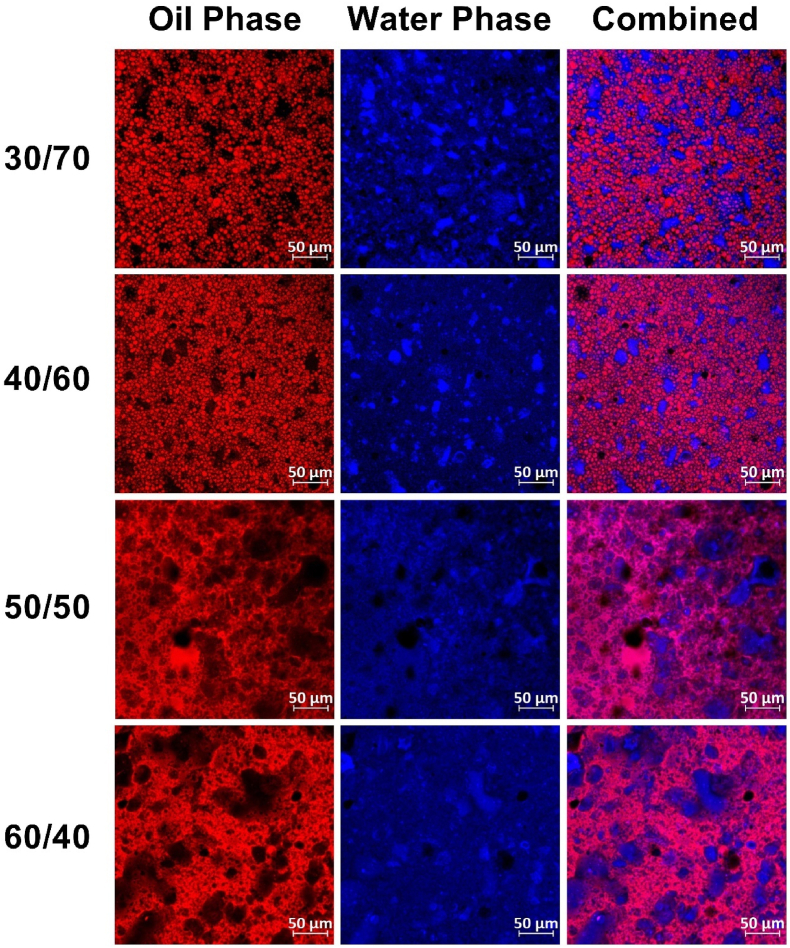
Previous studies have shown that OG/HG ratio can significantly impact the bigel behavior through changes in the biphasic gel morphology, i.e. W/O, O/W or bi-continuous, directly affecting the gel properties, performance, and functionality (Chen et al., 2023; Martins et al., 2019; Li et al., 2023). Therefore, the effect of bigel OG/HG ratio was examined for samples using higher water phase content (30/70 and 40/60 OG/HG), equal amount of both phases (50/50 OG/HG), and higher oil phase content (60/40 OG/HG), using 15 %wt. PP and 10 % TG out of the protein mass in the water phase, no added salt, and 6 %wt. CW in the oil phase. The OG/HG ratio changes the water/oil content, which directly affects the sample nutritional composition and overall texture. As such changes can significantly affect the biphasic system morphology and type, i.e. phase distribution, confocal microscopy imaging was performed at various OG/HG ratios, Fig. 3.
Fig. 3.
Confocal images of bigels produced with 6 %wt. CW in the oil phase, 15 %wt. PP and 10 % TG (out of the protein mass) in the water phase and various OG/HG ratios at × 20 magnification.
Gels with 30/70 and 40/60 OG/HG ratios produced an oleogel-in-hydrogel type bigel, characterized with oil droplets dispersed in a continuous water phase. Conversely, 50/50 and 60/40 OG/HG samples exhibited a bi-continuous morphology, characterized with water and oil phases integrated equally in one another. It seems that the high viscosity of the water phase, caused by the protein and TG content prevented the formation of water droplets within a continuous oil phase, as would be expected when oil content is higher than water content. Similar transition to a bi-continuous organization due to oil phase increase was observed previously for bigels based on fatty acids mixture oleogel and Xanthan gum hydrogel (Zheng et al., 2023), beeswax oleogel and tapioca hydrogel with an addition of zein nanoparticles (Li et al., 2023), and stearyl alcohol oleogel and agar hydrogel (Kodela et al., 2017).
Bigels at various OG/HG ratios were also visually examined with respect to native hydrogel and oleogel samples as a control before and after compression, Fig. S2. The images show that the bigel color changed from white glossy at low OG ratio (Fig. S2 A2-3 and B2-3) into a yellowish color at higher OG ratio (Fig. S2 A4-5 and B4-5). This transition seems to be correlated with the transition from oleogel-in-hydrogel type emulsion to bi-continuous type structure. Such color change could be related to the exposure of the oleogel phase at the bi-continuous organization, which is in accordance with its native yellowish color (Fig. S2 A6 and B6). In addition, 30/70 and 40/60 bigels had an elastic behavior revealed by the stable structure and integrity after compression (Fig. S2 B2-3), which is consistent with the stiff properties of the native hydrogel (Fig. S2 B1). In contrast, 50/50 and 60/40 OG/HG bigels showed a spreadable margarine-like behavior after compression (Fig. S2 B4-5), which can be related to the oleogel dominance having a viscoelastic behavior characterized with high deformability during compression (Fig. S2 B6).
The visual appearance results were verified using quantitative analysis by measuring the hardness, cohesiveness, and springiness during compression test, Fig. 4. The results show a gradual increase in hardness with OG concentration up to 40/60 OG/HG ratio, above which a notable deterioration in bigel hardness was detected. This change in mechanical properties can be directly correlated to the low OG content below 40/60 OG/HG ratio and the change in emulsion type seen in the confocal analysis at high OG/HG ratios, Fig. 3, and it is also consistent with the visual appearance seen in Fig. S2. Moreover, the results demonstrated lower hardness for the native oleogel sample (100/0 OG/HG) compared to the native hydrogel (0/100 OG/HG). It seems that in oleogel-in-hydrogel type system, the oleogel droplets act as active fillers contributing to an increase in hardness while increasing its fraction (shifting from 30/70 to 40/60 OG/HG). While for bigel with high oleogel content having a bi-continuous type organization, significant reduction in hardness is observed due to the exposure of the softer oleogel phase at this morphology. A similar trend of increased firmness due to oleogel fraction increase (10–60 %wt.) was observed in bigels produced with monoglyceride-beeswax oleogel and high acyl gellan gum hydrogel characterized with oleogel-in-hydrogel microstructure (Zhu et al., 2021). However, a shift in bigel compression force was also observed by Kodela et al. (2017) working with agar based hydrogel and stearyl alcohol based oleogel. In this case, an increase in compression force value was seen when the system was an oleogel-in-hydrogel type followed by a decrease while turning into a bi-continuous type system.
Fig. 4.
The effect of OG/HG ratio on the hardness (light grey), cohesiveness (white), and springiness (dark grey) of bigels prepared with 15 %wt. PP and 10 % TG (out of the protein mass) in the water phase and 6 %wt. CW in the oil phase. Different letters represent significantly different values (p < 0.05).
The OG/HG ratio effect on the microstructure can be correlated with cohesive behavior. More specifically, oleogel-in-hydrogel type bigels (30/70 and 40/60 OG/HG) were more cohesive than bi-continuous bigels (50/50 and 60/40 OG/HG), which had similar value as the native oleogel (100/0 OG/HG). Such behavior could be derived from the oleogel arrangement as dispersed droplets, leading to larger interfacial area than the bi-continuous, which enhance the interfacial interactions (Jurado et al., 2007). The hydrogel sample (0/100 OG/HG) had the highest cohesiveness compared to the other gels measured. This behavior could be related to the dispersed oleogel particles in the hybrid systems hindering the strong protein matrix formed by the covalent cross-links. A study working with whey protein nanofibers/sodium alginate hydrogel and emulsion template whey protein isolate/xanthan gum oleogel showed that bigel with 50/50 OG/HG ratio and bi-continuous morphology had lower cohesiveness compared with both the native hydrogel and oleogel-in-hydrogel type bigels with lower oleogel content (Jiang et al., 2024). Other study focused on TG-induced egg-soybean protein isolate emulsion gels demonstrated lower cohesiveness while adding 20 % (w/w) soybean oil compared with the native water based gel (Zhang et al., 2020).
Bigel springiness, Fig. 4, was the highest (close to 0.9) for the native hydrogel sample (0/100 OG/HG) and for the samples having oleogel-in-hydrogel morphology (30/70 and 40/60 OG/HG). In contrast, the springiness of bigels with bi-continuous structure (50/50 and 60/40 OG/HG) had significantly lower values along with the native oleogel sample (0/100 OG/HG). Bigel shift from elastic to plastic behavior along with the morphological changes can be explained by the native hydrogel and oleogel observed springiness and their organization affecting differently the combined matrix as was mentioned above. Jiang et al. (2024) demonstrated lower springiness for bi-continuous bigel having 50/50 OG/HG ratio compared with the native hydrogel and other oleogel-in-hydrogel bigels with lower oleogel fraction.
Based on these results, bigel consisting of 40/60 OG/HG ratio demonstrated the highest hardness and cohesiveness values thus was chosen to be further examined for the salt addition effect.
3.3. The effect of NaCl addition
3.3.1. The effect of salt addition on protein suspension
Salt addition is expected to affect the structural and physical behavior of proteins in water, therefore, initial evaluation of the protein content and the effect of salt on the protein's particle size and zeta-potential in water was performed.
First, CHNS elemental analysis was conducted to determine the protein content in the PP isolate (supplementary information), a value that can vary due to extraction conditions (Yang et al., 2021). The results revealed that the PP isolate contained ∼73 %wt. protein, derived from ∼13.5 %wt. detected nitrogen atoms (Table S1) multiplied by a nitrogen to protein conversion factor of 5.4 suitable for PP (Emkani et al., 2023; Mariotti et al., 2008). Previous analyses of commercial PP isolates reported lower protein content of ∼67 %wt. and ∼69 %wt. that could be due to different extraction conditions as well as the use of different conversion factors of 5.36 and 5.45, respectively (Moreno et al., 2020b; Moll et al., 2023), derived from variation between past calculations (Mariotti et al., 2008). Therefore, it can be concluded that 40/60 OG/HG bigel consists of 15 %wt. protein isolate in the water phase has a total protein weight fraction of 6.6 %wt.
Additionally, SDS-PAGE gel analysis was done to verify the PP protein composition, with respect to its various polypeptides (supplementary information and Fig. S3). Overall, the sample gel pattern exhibited a smeared coloring with notable bands implying on the aggregated and less soluble nature of PP. Pea legumin (11S) globular hexamer subunits – acidic (α) associated with ∼40 kDa band and basic (β) divided to ∼24 kDa and ∼17 kDa polypeptides, resulted from a cleavage of disulfide bonds. The band at ∼50 kDa could be related to un-cleaved vicilin globulin (7S) subunits, also having fragments of ∼20 kDa, ∼25 kDa and ∼35 kDa, product of disconnecting hydrophobic interactions. The band at ∼70 kDa can be attributed to convicilin globulin units (7S as well) that originally organized as trimers, sometimes in combinations with vicilin (Mession et al., 2015; Lam et al., 2018). Lastly, the band at ∼100 kDa could be attributed to pea lipoxygenases, a small fraction from total protein (∼3 %, as reported for protein isolate). Pea albumins, being less dominant than globulins, were not seen distinctively in this gel. As reported in literature, bands associated with albumins were previously reported at the range of 18–22 kDa or ∼30 kDa (Mession et al., 2015; Moll et al., 2023; Lam et al., 2018). Given the results above, it can be inferred that the gelation properties of PP, in current research, can be mainly associated with globulin behavior.
PP dispersions were prepared using 15 %wt. protein and increasing concentration of NaCl at room temperature corresponding with the water phase of 40/60 OG/HG ratio bigel samples (NaCl mg to 100 g bigel translated to NaCl mg to 60 g suspension). The pH value of all protein dispersions was kept constant at ∼7.4 (as used for bigel preparation).
Particle size and distribution can significantly affect protein gelling properties, interfacial behavior, and Pickering interface stabilization in bi-phasic systems (Janssen et al., 2024; Wu and Ma, 2016). The particle size and distribution curves of protein dispersions with different NaCl concentrations, Fig. 5 A, show one dominant particle size population around 100 μm and a minor particle size population around 10 μm. It can be assumed that the larger size population comprises protein aggregates while the smaller particle population can be attributed to disassociation of these aggregates. Similar size distribution range was observed previously for PP isolate particles in water at 25 °C and pH 6.8 with main size population around 100 μm (McCarthy et al., 2016). Interestingly, no significant difference between the samples at different salt concentrations was observed with respect to particle size distribution, Fig. 5 A and mean diameter, Table S2. Previous study showed that NaCl addition of 50 mM to PP concentrate solutions at pH 6.25 caused an increased average particle size from 1-2 μm to 4–5 μm and an addition of a shoulder at 20–30 μm (Kornet et al., 2022). At the current study, it seems that the large particle size and the large ratio between the protein content and the salt concentration led to a smaller impact on the measured particles size and distributions.
Fig. 5.
SLS particle size distribution (A) and ζ-potential (B) of 15 %wt. PP suspensions with various NaCl concentrations. Different letters represent significantly different values (p < 0.05).
The zeta potential of the protein particles in solution, determined by their electric potential and surface charge, is a value related to colloid stability of dispersions, which can affect their heat-induced gelation and surface activity. As a rule of thumb, system stability decreases along with its ζ-potential absolute value decrease (Bhattacharjee, 2016; Karaca et al., 2011; Schmitt et al., 2021). The measured zeta potential of protein suspensions, Fig. 5 B, decreased in absolute value from ∼ −38 mV for sample with no salt to ∼ −33 mV for NaCl added suspensions. This decrease is commonly correlated with increasing ionic strength of dispersions, when Na+ ions screen the negative charges on the protein surface affecting its electrostatic interactions (Guo et al., 2023a). Previous study reported similar behavior for PP suspensions with a gradual potential decrease with addition of NaCl between 0.03 and 0.6 M from −42 to −26 mV (Xu et al., 2023). Another study examining the surface charge of lentil, soy and pea protein isolates found that a larger absolute surface charge improved protein emulsifying properties (Karaca et al., 2011). Thus, it can be concluded that the dispersed protein particles were less negatively charged, meaning had lower electrostatic repulsion forces, which can impair their stability, affect network formation, and interfacial activity. However, additional aggregation of the protein particles due to NaCl addition was not noticed since there was no significant difference in particle size and distribution.
3.3.2. The effect of salt addition on bigel behavior
Bigels were prepared with 15 %wt. PP and 10 % TG (out of the protein mass) in the water phase, 6 %wt. CW in the oil phase, 40/60 OG/HG ratio, and various NaCl concentrations (0–225 mg/100 g) while their structure, appearance, and textural behavior were tested using various techniques. The salt concentrations were chosen with respect to the limit amount of salt required for labelling food as high in sodium according to the reform of the Israeli ministry of health (Anon, 2017). More specifically, the concentration range chosen was below this limit.
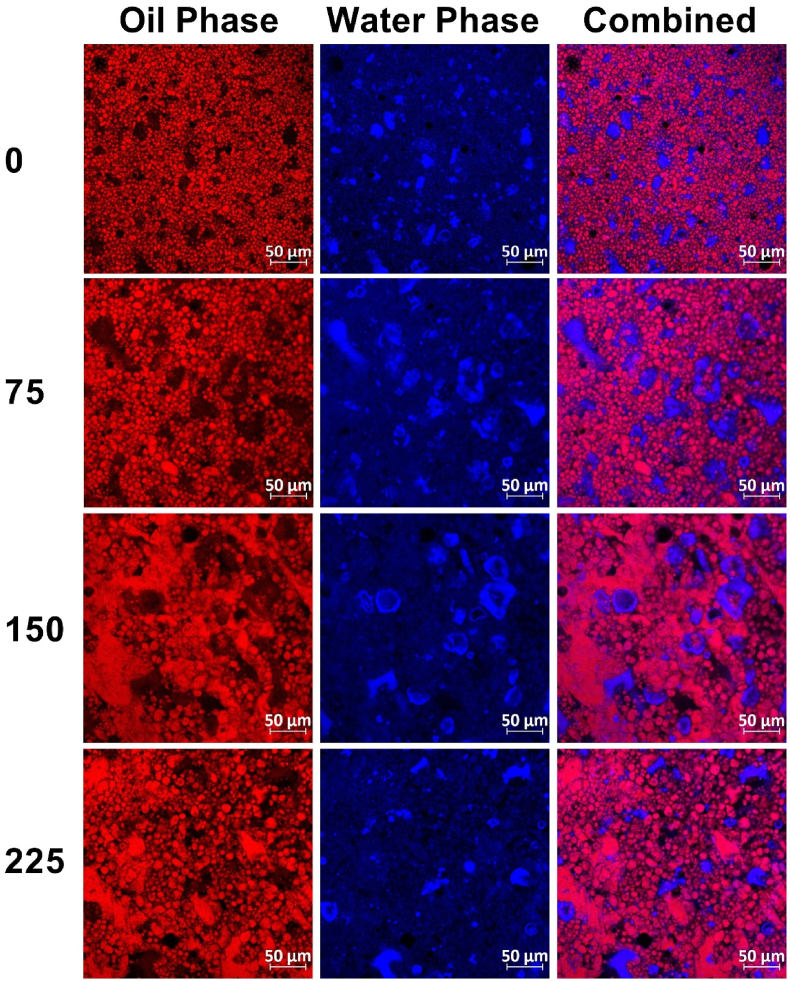
The effect of salt on the bigel structure was initially evaluated using confocal imaging and visual analysis while quantitative characterization can be seen below. Generally, confocal imaging revealed the formation of an oleogel-in-hydrogel type bigel for all salt concentrations. However, the morphology of the phases significantly changed due to salt addition, Fig. 6. More specifically, the oil phase (red) exhibited a droplet size increase from zero to 75 mg/100 g salt, followed by droplet coalescence and flocculation forming large clusters at higher salt concentrations of 150 and 225 mg/100 g. On the other hand, the hydrogel phase appearance (blue) exhibited a significant change upon salt addition characterized by clustering of the protein which appeared as large clusters of blue. The combined images show the overall effect of NaCl on both phases, turning an organized oleogel-in-hydrogel system into large oleogel droplets, forming unorganized bi-continuous-like structure.
Fig. 6.
Confocal images of bigels prepared using 15 %wt. PP and 10 % TG (out of the protein mass) in the water phase, 6 %wt. CW in the oil phase, and 40/60 OG/HG ratio with various NaCl concentrations (NaCl mg to 100 g bigel) at × 20 magnification.
Plant protein in bi-phasic systems such as bigels can act as interface stabilizers while coating the oil droplets as well as bulk stabilizers by formation a gel network. Flocculation of oil phase droplets in oil-in-water systems can be attributed to reduced repulsive forces including electrostatic and steric interaction between protein particles arranged at the oil droplet interface, due to a certain level of charge screening as was shown for NaCl concentration of 150–250 mM in Perilla seed protein-stabilized emulsions (Liu et al., 2018). Oil droplets flocculation was also found for emulsions stabilized with PP in the presence of NaCl at concentration of 20 and 100 mM at pH 7, which was attributed to decreased pea globulins solubility and protein re-organization at the oil-in-water interface (Chang et al., 2023). Such microscopic changes seen in the micrographs could result from the NaCl charge screening which influenced the PP particle zeta-potential seen in the results shown above, thus affecting heat-induced hydrogelation in the bulk and interfacial behavior of the protein at the water-oil interface affecting the oleogel droplet behavior. Hence, it seems that NaCl affected the protein behavior, interfering with the interface stabilization which directly affect oil droplets size and distribution leading to a less ordered microstructure.
Bigels appearance, Fig. 7, was analyzed before and after TPA compression while the ability to spread the sample was also visualized. Overall, the visual analysis revealed that the bigel properties were significantly affected by the salt addition. Based on the images, the control sample (with no NaCl) retained its shape after compression (Fig. 7 B1) suggesting elastic behavior. However, upon spreading the sample was completely disassembled forming a flaky texture (Fig. 7 C1) implying on a brittle texture. As NaCl concentration increased, a transition from flaky brittle to soft spreadable texture occurred. This transition is characterized by a solid appearance after compression and a crumbly texture after spreading the sample without salt (Fig. 7 B1 and C1, respectively), followed by deformed flaky appearance after compression and improved spreadability at 75 mg/100 g NaCl concentration (Fig. 7 B2 and C2, respectively). Furthermore, the smoothest texture and highest spreadability was obtained at 150 and 225 mg/100 g NaCl concentrations (Fig. 7 B3-4 and C3-4). This behavior can be correlated to the bigel microstructure seen above (Fig. 6) which demonstrates the formation of oleogel droplet clusters which turned the system into a bi-continuous-like morphology as a result of NaCl addition. The exposure of the oleogel phase at high NaCl concentration could contribute to bigel spreadability and smoothness which is attributed to oleogel phase.
Fig. 7.
Appearance of bigels with 15 %wt. PP and 10 % TG (out of the protein mass) in the water phase, 6 %wt. CW in the oil phase, 40/60 OG/HG ratio, and various NaCl concentrations. Photos A1-A4, B1-B4 and C1-C4 show bigels before and after compression by TA and after spreading, respectively. Sample 1 - no NaCl added, Sample 2–75 mg/100 g NaCl, sample 3–150 mg/100 g NaCl, and sample 4–225 mg/100 g NaCl.
The mechanical properties of the bigel and the corresponding hydrogel samples with various NaCl concentrations are presented in Fig. 8A and B, respectively. The salt addition caused a drastic decrease in hardness for both bigel (Fig. 8 A) and hydrogel (Fig. 8 B) samples. These results are inline with the bigels appearance after compression and spreading seen in Fig. 7. Therefore, it can be concluded that NaCl addition results in weaker hydrogel network which led to weaker bigel, since the hydrogel is the continuous phase. Hydrogel network weakening was consistent with the surface net charge reduction (Fig. 5 B) creating lower repulsion forces between protein particles and weaker electrostatic interactions with the water, which can reduce hydrogel water holding capacity resulting in lower textural stability (Moreira et al., 2023; Zheng et al., 2019a). In addition, the oleogel particle clustering and the formation of bi-continuous-like morphology due to salt concentration increase, seen in the confocal imaging (Fig. 6), could also contribute to the bigel weakening when larger oleogel droplets reduce the interfacial area leading to weaker internal interactions. Previous study with bigel stabilized with gelatin hydrogel and GMS oleogel concluded that smaller droplets lead to stronger bigel matrix as opposed to larger oil droplets which formulated softer gel (Golodnizky and Davidovich-Pinhas, 2020).
Fig. 8.
The effect of NaCl concentration on the hardness (light grey), cohesiveness (white), and springiness (dark grey) of bigels (A) and their corresponding hydrogels (B) for samples prepared with 15 %wt. PP and 10 % TG (out of the protein mass) in the water phase, 6 %wt. CW in the oil phase, and 40/60 OG/HG ratio. Different letters represent significantly different values (p < 0.05), and N.D. refers to samples that cannot be analyzed.
Salt addition effect on cohesiveness was also analyzed where significantly higher values were observed for bigel and hydrogel without added salt compared to the salt added samples, Fig. 8A and B, respectively. This could be related to the hindering effect of salt on the protein matrix leading to a less cohesive hydrogel network also seen in the bigel system. Moreover, salt addition also seems to take part in the interface instability, as discussed above, thus could weaken the internal attraction between the phases. This effect seems to change the phase distribution seen in the structural analysis (Fig. 6), also leading to reduced surface area to volume ratio, which can hinder physical interactions between phases, thus reducing cohesiveness. Previous syudy showed that addition of 0.3 M NaCl reduced the cohesiveness of egg yolk/κ-carrageenan dispersion and emulsion along with reduced dispersions zeta potential, but in this case it was coupled with increased hardness and reduced emulsion oil droplets flocculation degree (Li et al., 2020). This may be due to the relatively high NaCl concentration and the different system properties causing a more homogeneous microstructure and brittle behavior, as opposed to the current results.
The addition of NaCl resulted in a significant springiness decrease of both bigel and hydrogel. This correlates with the higher deformability seen after compression at the salt added bigels compared with the sample with no added salt, Fig. 7 B1-B4. The decrease in springiness could be attributed to the weaker gel network described before attaining lower resistance. Previous study showed that addition of 0.005 and 0.015 M of Na+ ions into soy protein isolate gel led to lower springiness of the heat-induced gels (Zheng et al., 2019b).
The mechanical behavior was further analyzed by measuring the effect of NaCl on the viscoelastic characteristics of bigel and the corresponding hydrogel with respect to frequency and temperature, demonstrating its deformation behavior at various conditions. The effect of shear measured by the frequency dependent module exhibited characteristic behavior of soft gels with frequency response, Fig. 9 A, B and Table 1. In order to quantify this tendency a power-law model (Eq. (1)) was fitted to the relation between the storage modulus (G′) and frequency. The n′ parameter can distinguish between two main gel types: weak physical gels with a positive dependency (n′ > 0) and strong chemical cross-linked gels with a zero-slop regarded as frequency independent (Golodnizky and Davidovich-Pinhas, 2020).
Fig. 9.
Typical G′ vs. frequency curves of bigels (A) and corresponding hydrogels (B) and typical G′ vs. temperature curves of bigels (C) and corresponding hydrogels (D) for bigels prepared with 15 %wt. PP and 10 % TG (out of the protein mass) in the water phase, 6 %wt. CW in the oil phase, and 40/60 OG/HG ratio at various NaCl concentrations.
Table 1.
Fit parameters obtained from Eq. (1) for the frequency sweep curves of bigels and their corresponding hydrogels prepared with 15 %wt. PP and 10 % TG out of the protein mass in the water phase, 6 %wt. CW in the oil phase, and 40/60 OG/HG ratio at various NaCl concentrations.
| NaCl [mg] | Bigel |
Hydrogel |
||
|---|---|---|---|---|
| G′0 [Pa] | n′ | G'0 [Pa] | n′ | |
| 0 | 25,317 ± 4971 a | 0.069 ± 0.024 a | 4484 ± 483 a | 0.114 ± 0.034 a |
| 75 | 34,127 ± 4876 ab | 0.067 ± 0.009 a | 4747 ± 380 a | 0.097 ± 0.010 a |
| 150 | 38,684 ± 1844 bc | 0.070 ± 0.010 a | 7066 ± 745 ab | 0.086 ± 0.004 a |
| 225 | 47,950 ± 2460 c | 0.063 ± 0.002 a | 9664 ± 1647 c | 0.084 ± 0.006 a |
∗Values at the same column followed by different lowercase letters are significantly different for the specific parameter (p < 0.05).
The typical curves demonstrated frequency dependent behavior for bigels (Fig. 9 A) and hydrogels (Fig. 9 B), characterized with a positive slope and G′>G″ suggesting a solid-like behavior (data not shown). The curves were fitted to a power-law model (Eq. (1)) while the fitted values are summarized in Table 1. Surprisingly, there was a slight increase in the storage modulus for both bigels and hydrogels while increasing NaCl concentration as demonstrated by the G′0 values (Table 1), even though there was a decrease in hardness and cohesiveness (Fig. 8A and B). It can be inferred that the salt effect on protein, as was shown by the zeta potential decrease (Fig. 5 B), altered the hydrogel and bigel structure and caused a reduction in gel strength at large deformation when compressed by the TA but had stronger shear resistance resulting in higher G′ at small deformation in the LVR while sheared in a rheometer. Such deviation could be correlated to mechanical testing technique used while the first rely on uniaxial compression while the latter on shear forces. Increased G′ values were also observed for heat-induced walnut protein-κ-carrageenan gels, PP cold set hydrogels, and soy protein cold set emulsion gels while increasing NaCl concentration (Lei et al., 2022; Xu et al., 2023; Yu et al., 2022).
All NaCl added bigels exhibited n′ values close to zero with no significant difference between concentrations implying on the formation of consistent gels typical for chemically crosslinked gels. Similar n′ values were also obtained for all corresponding hydrogels indicating a similar frequency dependence behavior (Table 1). It seemed that the current ionic strength changed the protein network arrangement but did not alter TG stabilization mechanism which is responsible for the protein network stabilization. Previous study showed that the composition of NaCl and TG improved the strength of heat-induced arachin and basil seed gum composite gels thus salt did not interfere TG activity (Yang et al., 2023).
Bigel storage module was also monitored with respect to temperature to evaluate the thermal properties of the gels. The results revealed a softening profile (Fig. 9 C) characterized by a reduction in G′ values with temperature as previously seen for bigel based on CW oleogel and Xanthan gum hydrogel (Vershkov and Davidovich-Pinhas, 2023). The temperature dependent viscoelastic behavior exhibited similar behavior for all bigels regardless of NaCl content. The curves were characterized by a moderate decrease in G′ value with temperature up to ∼50 °C, above which an abrupt reduction in G′ was seen followed by a slight increase above 70 °C. These trends can be related to a slight bigel softening up to ∼50 °C followed by oleogel phase melting driven by CW melting as reported for CW oleogel (Vershkov and Davidovich-Pinhas, 2023), and ending with protein thermal aggregation and rearrangement also seen as abrupt G′ increase in the hydrogel temperature sweep curve (Fig. 9 D). Storage moduli increase at high temperatures was previously seen in PP gels with TG crosslinking and PP suspension with 0.3 M NaCl, which was related to structure development around pea globulins denaturation temperature at 85 °C and above 80 °C, respectively (Shand et al., 2008; Sun and Arntfield, 2010). Overall, an increase in slope and higher G′ values were observed for bigels (Fig. 9 C) and hydrogels (Fig. 9 D) with higher NaCl concentration, between 70 and 100 °C. This trend can be related to salt effect on protein denaturation and thermal rearrangement derived from salt ion screening effect which hinders the electrostatic repulsion between protein chains. Globular protein thermal denaturation involved exposure of its hydrophobic regions that enables bond formation leading to aggregation (Ma and Chen, 2023), which could be correlated with the current G′ increase. Protein surface charge reduction (Fig. 5 B) due to NaCl addition could favor hydrophobic interactions during the unfolding of former protein particles at high temperatures leading to an increase in bigel shear resistance around PP denaturation point.
Thermal analysis of salt added bigels was also performed by TGA, Fig. 10. This analysis can emphasize the material thermal stability and composition, derived from recorded decomposition events of substances as a mass reduction during heating. The derivative thermograms (DTGs) demonstrate the thermal events as peaks in specific temperatures. Similar DTGs curves were obtained for all NaCl concentrations showing four distinct mass loss stages. The first two peaks up to ∼100 °C can be attributed to moisture loss in two fractions. The first one owing to free water evaporation and the second to bounded water inside of hydrocolloid network (Crockett et al., 2011). It can be noticed that as NaCl concentration increased, the free moisture peak became relatively larger compared to the bound water peak. This trend could imply on a higher free water content at high salt concentration, which can be related to ion screening, and is consistent with gels weakness (Glusac et al., 2024). The third mass loss at ∼300 °C which can be seen as a shoulder of the larger peak at ∼415 °C could be related to protein thermal decomposition correlating with previous study on the thermal behavior of PP isolate having main derivative peak at 310 °C (Ricci et al., 2018). The last thermal event at ∼415 °C can be related to the oleogel phase as was previously received for oleogel made of 6 %wt. CW in canola oil (Moguiliansky et al., 2025). This analysis strengthens our conclusion related to the salt screening effect seen with NaCl addition, which reduced the interaction of water with the protein leading to weaker cohesive forces as seen in the TPA analysis which may contribute to the general weakening of the bigel.
Fig. 10.
Thermogravimetry (solid line) and derivative thermogravimetry (dashed line) curves of bigels prepared with 15 %wt. PP and 10 % TG (out of the protein mass) in the water phase, 6 %wt. CW in the oil phase, and 40/60 OG/HG ratio at various NaCl concentrations.
4. Conclusions
This study aimed to explore the formulation of bigel using PP hydrogel and CW oleogel and the effect of salt on this formulation. First, the effect of chemical crosslinking of PP by TG was examined searching for the most adequate enzyme concentration. TG was found to be a vital component for self-standing gel formulation, when 10 % TG out of the protein mass resulted in good gel physical properties avoiding excessive enzyme usage. Second, the effect of OG/HG mass ratios was examined where a significantly different microscopic organization was detected with respect to OG/HG ratio affecting the gel mechanical properties and appearance. It was found that the ratio of 40/60 OG/HG produced a consistent and the strongest gels thus, combination of 10 % TG (out of the protein mass) and 40/60 OG/HG ratio were chosen for bigel formulation for further examination of NaCl addition effect.
NaCl addition at a relatively small amount produced a significant effect on PP via reducing surface charge and colloidal stability, affecting protein assembly and interactions in hydrogel and bigel systems. In general, bigel microstructure became less organized as salt concentration increased, as observed by droplet size increase and formation of large heterogeneous oleogel clusters, which eventually led to transition from oleogel-in-hydrogel type organization to bi-continuous-like morphology. These changes resulted in reduction in bigel hardness, cohesiveness, and springiness, suggesting a softer protein network affected by salt screening. However, NaCl added bigels also showed a higher shear resistance demonstrated by G′0 increase as a result of NaCl addition. Such unique mechanical behavior showed a transition from flaky and brittle texture to softer and spreadable texture with elevated salt concentrations. In addition, similar thermal dependednt rheology behavior was seen for all bigels characterized by moderate and then abrupt softening due to oleogel melting and finalized by increasing moduli around protein denaturation point.
Overall, the use of bigels prepared with PP and TG in the water phase and CW in the oil phase has the potential to be used for alternative market while using various NaCl concentrations. It seems that the TG concentration, OG/HG ratio, and NaCl have a significant impact on the bigel properties, thus should be considered while developing a specific alternative product.
Credit author statement
N. Friedman: Methodology, Data curation, Validation, Formal analysis, Visualization, Writing- Original draft preparation. S. Moguiliansky: Methodology, Data curation, Validation, Formal analysis M. Davidovich-Pinhas: Conceptualization, Funding acquisition, Methodology, Supervision, Writing- Reviewing and Editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The research was funded by the Ministry of Science and Technology, the Ministry of Agriculture and Food Security, and the Good Food Institute (Israel). We acknowledge the Russell-Berrie Nanotechnology Institute (RBNI) at the Technion for supporting this research. We thank Dr. Ashkar-Abu-Uksa from the laboratory of Food Materials Engineering (Technion) for her help with the particle size and zeta potential analyses.
Handling Editor: Professor A.G. Marangoni
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2025.101185.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Anon . 2017. The Protection of Public Health (Food) (Nutritional Labeling) Regulations, 5778-2017. [Google Scholar]
- Banerjee S., Bhattacharya S. Food gels: gelling process and new applications. Crit. Rev. Food Sci. Nutr. 2012;52:334–346. doi: 10.1080/10408398.2010.500234. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee S. DLS and zeta potential - what they are and what they are not? J. Contr. Release. 2016;235:337–351. doi: 10.1016/j.jconrel.2016.06.017. [DOI] [PubMed] [Google Scholar]
- Blake A.I., Toro-Vazquez J.F., Hwang H.-S. AOCS Press; 2018. Wax Oleogels. [DOI] [Google Scholar]
- Chang L., Lan Y., Chen B., Rao J. Interfacial, and emulsifying properties nexus of green pea protein fractions: impact of pH and salt. Food Hydrocoll. 2023;140 doi: 10.1016/j.foodhyd.2023.108652. [DOI] [Google Scholar]
- Chen Z., Bian F., Cao X., Shi Z., Meng Z. Novel bigels constructed from oleogels and hydrogels with contrary thermal characteristics: phase inversion and 3D printing applications. Food Hydrocoll. 2023;134 doi: 10.1016/j.foodhyd.2022.108063. [DOI] [Google Scholar]
- Co E.D., Marangoni A.G. AOCS Press; 2018. Oleogels. [DOI] [Google Scholar]
- Crockett R., Ie P., Vodovotz Y. How do xanthan and hydroxypropyl methylcellulose individually affect the physicochemical properties in a model gluten-free dough? J. Food Sci. 2011;76:E274–E282. doi: 10.1111/j.1750-3841.2011.02088.x. [DOI] [PubMed] [Google Scholar]
- Day L., Cakebread J.A., Loveday S.M. Food proteins from animals and plants: differences in the nutritional and functional properties. Trends Food Sci. Technol. 2022;119:428–442. doi: 10.1016/j.tifs.2021.12.020. [DOI] [Google Scholar]
- Djoullah A., Husson F., Saurel R. Gelation behaviors of denaturated pea albumin and globulin fractions during transglutaminase treatment. Food Hydrocoll. 2018;77:636–645. doi: 10.1016/j.foodhyd.2017.11.005. [DOI] [Google Scholar]
- Emkani M., Moundanga S., Oliete B., Saurel R. Protein composition and nutritional aspects of pea protein fractions obtained by a modified isoelectric precipitation method using fermentation. Front. Nutr. 2023;10 doi: 10.3389/fnut.2023.1284413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glusac J., Moguiliansky S., Fishman A., Davidovich-Pinhas M. Plant-based bigel based on chickpea-potato protein hydrogel and glycerol monostearate oleogel. Food Struct. 2024;41 doi: 10.1016/j.foostr.2024.100378. [DOI] [Google Scholar]
- Golodnizky D., Davidovich-Pinhas M. The effect of the hlb value of sucrose ester on physiochemical properties of bigel systems. Foods. 2020;9:1857. doi: 10.3390/foods9121857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves R.F.S., Zhou H., Vicente A.A., Pinheiro A.C., McClements D.J. Plant-based bigels for delivery of bioactive compounds: influence of hydrogel:oleogel ratio and protein concentration on their physicochemical properties. Food Hydrocoll. 2024;150 doi: 10.1016/j.foodhyd.2023.109721. [DOI] [Google Scholar]
- Guo R., Liu L., Huang Y., Lv M., Zhu Y., Wang Z., Zhu X., Sun B. Effect of Na+ and Ca2+ on the texture, structure and microstructure of composite protein gel of mung bean protein and wheat gluten. Food Res. Int. 2023;172 doi: 10.1016/j.foodres.2023.113124. [DOI] [PubMed] [Google Scholar]
- Guo J., Gu X., Du L., Meng Z. Spirulina platensis protein nanoparticle-based bigels: dual stabilization, phase inversion, and 3D printing. Food Hydrocoll. 2023;135 doi: 10.1016/j.foodhyd.2022.108160. [DOI] [Google Scholar]
- Hashemi B., Varidi M., Jafari S.M. Fabrication and characterization of novel whey protein-based bigels as structured materials with high-mechanical properties. Food Hydrocoll. 2023;145 doi: 10.1016/j.foodhyd.2023.109082. [DOI] [Google Scholar]
- Janssen S.W.P.M., Pouvreau L., de Vries R.J. Commercial plant protein isolates: the effect of insoluble particles on gelation properties. Food Hydrocoll. 2024;154 doi: 10.1016/j.foodhyd.2024.110049. [DOI] [Google Scholar]
- Jiang L., Wang Q., Rao Z., Lei X., Zhao J., Lei L., Ming J. Formulation and characterization of bigels utilizing whey protein and polysaccharides: potential applications as cream analogues. Food Hydrocoll. 2024;152 doi: 10.1016/j.foodhyd.2024.109884. [DOI] [Google Scholar]
- Johansson M., Karkehabadi S., Johansson D.P., Langton M. Gelation behaviour and gel properties of the 7S and 11S globulin protein fractions from faba bean (Vicia faba var. minor) at different NaCl concentrations. Food Hydrocoll. 2023;142 doi: 10.1016/j.foodhyd.2023.108789. [DOI] [Google Scholar]
- Jurado E., Bravo V., Camacho F., Vicaria J.M., Fernández-Arteaga A. Estimation of the distribution of droplet size, interfacial area and volume in emulsions, colloids and surf. A: physicochem. Eng. Aspects. 2007;295:91–98. doi: 10.1016/j.colsurfa.2006.08.037. [DOI] [Google Scholar]
- Karaca A.C., Low N., Nickerson M. Emulsifying properties of chickpea, faba bean, lentil and pea proteins produced by isoelectric precipitation and salt extraction. Food Res. Int. 2011;44:2742–2750. doi: 10.1016/j.foodres.2011.06.012. [DOI] [Google Scholar]
- Kodela S.P., Pandey P.M., Nayak S.K., Uvanesh K., Anis A., Pal K. Novel agar–stearyl alcohol oleogel-based bigels as structured delivery vehicles. Int. J. Polym. Mater. Polym. Biomater. 2017;66:669–678. doi: 10.1080/00914037.2016.1252362. [DOI] [Google Scholar]
- Kornet R., Roozalipour S.L., Venema P., van der Goot A.J., Meinders M.B.J., van der Linden E. Coacervation in pea protein solutions: the effect of pH, salt, and fractionation processing steps. Food Hydrocoll. 2022;125 doi: 10.1016/j.foodhyd.2021.107379. [DOI] [Google Scholar]
- Lam A.C.Y., Can Karaca A., Tyler R.T., Nickerson M.T. Pea protein isolates: structure, extraction, and functionality. Food Rev. Int. 2018;34:126–147. doi: 10.1080/87559129.2016.1242135. [DOI] [Google Scholar]
- Lei Y., Ouyang H., Peng W., Yu X., Jin L., Li S. Effect of NaCl on the rheological, structural, and gelling properties of walnut protein Isolate-κ-Carrageenan composite gels. Gels. 2022;8:259. doi: 10.3390/gels8050259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Xu L., Su Y., Chang C., Yang Y., Gu L. Flocculation behavior and gel properties of egg yolk/κ-carrageenan composite aqueous and emulsion systems: effect of NaCl. Food Res. Int. 2020;132 doi: 10.1016/J.FOODRES.2020.108990. 108990–108990. [DOI] [PubMed] [Google Scholar]
- Li M., He X., Zhao R., Shi Q., Nian Y., Hu B. Hydrogels as promising carriers for the delivery of food bioactive ingredients. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.1006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Han J., Xiao Y., Guo R., Liu X., Zhang H., Bi Y., Xu X. Fabrication and characterization of novel food-grade bigels based on interfacial and bulk stabilization. Foods. 2023;12:2546. doi: 10.3390/foods12132546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Chen Q., Li G., Zhu Z., Yi J., Li C., Chen X., Wang Y. Properties and stability of perilla seed protein-stabilized oil-in-water emulsions: influence of protein concentration, pH, NaCl concentration and thermal treatment. Molecules. 2018;23:1533. doi: 10.3390/molecules23071533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotfi Shirazi S., Koocheki A. Investigating the rheological properties and structure of grass pea protein isolate- sesame oil bigels for effective fat substitution in low-fat cream formulation. Food Res. Int. 2025;217 doi: 10.1016/j.foodres.2025.116820. [DOI] [PubMed] [Google Scholar]
- Luo K., Liu S., Miao S., Adhikari B., Wang X., Chen J. Effects of transglutaminase pre-crosslinking on salt-induced gelation of soy protein isolate emulsion. J. Food Eng. 2019;263:280–287. doi: 10.1016/j.jfoodeng.2019.07.008. [DOI] [Google Scholar]
- Ma Y., Chen F. Plant protein heat-induced gels: formation mechanisms and regulatory strategies. Coatings. 2023;13:1–20. doi: 10.3390/coatings13111899. [DOI] [Google Scholar]
- Man C.M.D. 2007. Technological Functions of Salt in Food Products, Reducing Salt in Foods: Practical Strategies; pp. 157–173. [DOI] [Google Scholar]
- Mariotti F., Tomé D., Mirand P.P. Converting nitrogen into protein—beyond 6.25 and jones' factors. Crit. Rev. Food Sci. Nutr. 2008;48:177–184. doi: 10.1080/10408390701279749. [DOI] [PubMed] [Google Scholar]
- Martins A.J., Silva P., Maciel F., Pastrana L.M., Cunha R.L., Cerqueira M.A., Vicente A.A. Hybrid gels: influence of oleogel/hydrogel ratio on rheological and textural properties. Food Res. Int. 2019;116:1298–1305. doi: 10.1016/j.foodres.2018.10.019. [DOI] [PubMed] [Google Scholar]
- Martins A.J., Guimarães A., Fuciños P., Sousa P., Venâncio A., Pastrana L.M., Cerqueira M.A. Food-grade bigels: evaluation of hydrogel:oleogel ratio and gelator concentration on their physicochemical properties. Food Hydrocoll. 2023;143 doi: 10.1016/j.foodhyd.2023.108893. [DOI] [Google Scholar]
- Masiá C., Ong L., Logan A., Stockmann R., Gambetta J., Jensen P.E., Rahimi Yazdi S., Gras S. Enhancing the textural and rheological properties of fermentation-induced pea protein emulsion gels with transglutaminase. Soft Matter. 2023;20:133–143. doi: 10.1039/d3sm01001e. [DOI] [PubMed] [Google Scholar]
- McCarthy N.A., Kennedy D., Hogan S.A., Kelly P.M., Thapa K., Murphy K.M., Fenelon M.A. Emulsification properties of pea protein isolate using homogenization, microfluidization and ultrasonication. Food Res. Int. 2016;89:415–421. doi: 10.1016/j.foodres.2016.07.024. [DOI] [PubMed] [Google Scholar]
- Mefleh M., Pasqualone A., Caponio F., De Angelis D., Natrella G., Summo C., Faccia M. Spreadable plant-based cheese analogue with dry-fractioned pea protein and inulin–olive oil emulsion-filled gel. J. Sci. Food Agric. 2022;102:5478–5487. doi: 10.1002/jsfa.11902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Wee M.S., Ting Goh A., Stieger M., Forde C.G. Correlation of instrumental texture properties from textural profile analysis (TPA) with eating behaviours and macronutrient composition for a wide range of solid foods. Food Funct. 2018;9:5301–5312. doi: 10.1039/C8FO00791H. [DOI] [PubMed] [Google Scholar]
- Mession J.L., Chihi M.L., Sok N., Saurel R. Effect of globular pea proteins fractionation on their heat-induced aggregation and acid cold-set gelation. Food Hydrocoll. 2015;46:233–243. doi: 10.1016/j.foodhyd.2014.11.025. [DOI] [Google Scholar]
- Moguiliansky S., Friedman N., Davidovich-Pinhas M. The effect of transglutaminase on the structure and texture of plant-protein based bigel. Food Hydrocoll. 2025;162 doi: 10.1016/j.foodhyd.2024.110981. [DOI] [Google Scholar]
- Moll P., Salminen H., Seitz O., Schmitt C., Weiss J. Characterization of soluble and insoluble fractions obtained from a commercial pea protein isolate. J. Dispersion Sci. Technol. 2023;44:2417–2428. doi: 10.1080/01932691.2022.2093214. [DOI] [Google Scholar]
- Moreira C., Machado L., Silva M., Nunes R., Pereira R.N., Rocha C.M.R., Geada P., Teixeira J.A. In: Sustainable Food Science - a Comprehensive Approach. Ferranti P., editor. Elsevier; Oxford: 2023. 3.14 - algal proteins; pp. 173–194. [DOI] [Google Scholar]
- Moreno H.M., Tovar C.A., Domínguez-timón F., Cano-báez J., Díaz M.T., Pedrosa M.M., Borderías A.J. Gelation of commercial pea protein isolate : effect of microbial transglutaminase and thermal processing. Food Sci. Technol. 2020;2061:800–809. doi: 10.1590/fst.19519. [DOI] [Google Scholar]
- Moreno H.M., Domínguez-Timón F., Díaz M.T., Pedrosa M.M., Borderías A.J., Tovar C.A. Evaluation of gels made with different commercial pea protein isolate: rheological, structural and functional properties. Food Hydrocoll. 2020;99 doi: 10.1016/j.foodhyd.2019.105375. [DOI] [Google Scholar]
- Nicolai T., Chassenieux C. Heat-induced gelation of plant globulins. Curr. Opin. Food Sci. 2019;27:18–22. doi: 10.1016/j.cofs.2019.04.005. [DOI] [Google Scholar]
- Qiu R., Qiu G., Zhao P., Awais M., Fan B., Huang Y., Tong L., Wang L., Liu L., Wang F. Regulation of rheological properties of soy protein isolate-beeswax based bigel inks for high-precision 3D printing. Food Hydrocoll. 2024;153 doi: 10.1016/j.foodhyd.2024.110052. [DOI] [Google Scholar]
- Ricci L., Umiltà E., Righetti M.C., Messina T., Zurlini C., Montanari A., Bronco S., Bertoldo M. On the thermal behavior of protein isolated from different legumes investigated by DSC and TGA. J. Sci. Food Agric. 2018;98:5368–5377. doi: 10.1002/jsfa.9078. [DOI] [PubMed] [Google Scholar]
- Ruzengwe F.M., Amonsou E.O., Kudanga T. Transglutaminase-mediated crosslinking of Bambara groundnut protein hydrogels: implications on rheological, textural and microstructural properties. Food Res. Int. 2020;137 doi: 10.1016/j.foodres.2020.109734. 109734–109734. [DOI] [PubMed] [Google Scholar]
- Samui T., Goldenisky D., Rosen-Kligvasser J., Davidovich-Pinhas M. The development and characterization of novel in-situ bigel formulation. Food Hydrocoll. 2021;113 doi: 10.1016/j.foodhyd.2020.106416. [DOI] [Google Scholar]
- Schmitt C., Bovetto L., Buczkowski J., De Oliveira Reis G., Pibarot P., Amagliani L., Dombrowski J. Plant proteins and their colloidal state. Curr. Opin. Colloid Interface Sci. 2021;56 doi: 10.1016/j.cocis.2021.101510. [DOI] [Google Scholar]
- Shakeel A., Farooq U., Iqbal T., Yasin S., Lupi F.R., Gabriele D. Key characteristics and modelling of bigels systems: a review. Mater. Sci. Eng. C. 2019;97:932–953. doi: 10.1016/j.msec.2018.12.075. [DOI] [PubMed] [Google Scholar]
- Shand P.J., Ya H., Pietrasik Z., Wanasundara P.K.J.P.D. Transglutaminase treatment of pea proteins: effect on physicochemical and rheological properties of heat-induced protein gels. Food Chem. 2008;107:692–699. doi: 10.1016/j.foodchem.2007.08.095. [DOI] [Google Scholar]
- Silva P.M., Cerqueira M.A., Martins A.J., Fasolin L.H., Cunha R.L., Vicente A.A. Oleogels and bigels as alternatives to saturated fats: a review on their application by the food industry. JAOCS, J. Am. Oil Chem. Soc. 2022;99:911–923. doi: 10.1002/aocs.12637. [DOI] [Google Scholar]
- Sun X.D., Arntfield S.D. Gelation properties of salt-extracted pea protein induced by heat treatment. Food Res. Int. 2010;43:509–515. doi: 10.1016/j.foodres.2009.09.039. [DOI] [Google Scholar]
- Szczesniak A.S. Texture is a sensory property. Food Qual. Prefer. 2002;13:215–225. doi: 10.1016/S0950-3293(01)00039-8. [DOI] [Google Scholar]
- Tanger C., Müller M., Andlinger D., Kulozik U. Influence of pH and ionic strength on the thermal gelation behaviour of pea protein. Food Hydrocoll. 2022;123 doi: 10.1016/j.foodhyd.2021.106903. 106903–106903. [DOI] [Google Scholar]
- Vasić K., Knez Ž., Leitgeb M. Transglutaminase in foods and biotechnology. Int. J. Mol. Sci. 2023;24 doi: 10.3390/ijms241512402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vershkov B., Davidovich-Pinhas M. The effect of preparation temperature and composition on bigel performance as fat replacers. Food Funct. 2023;14:3838–3848. doi: 10.1039/d3fo00002h. [DOI] [PubMed] [Google Scholar]
- Wu J., Ma G.-H. Recent studies of pickering emulsions: particles make the difference. Small. 2016;12:4633–4648. doi: 10.1002/smll.201600877. [DOI] [PubMed] [Google Scholar]
- Xiao X., Zou P.R., Hu F., Zhu W., Wei Z.J. Updates on plant-based protein products as an alternative to animal protein: technology, properties, and their health benefits. Molecules. 2023;28:4016. doi: 10.3390/molecules28104016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie D., Hu H., Huang Q., Lu X. Development and characterization of food-grade bigel system for 3D printing applications: role of oleogel/hydrogel ratios and emulsifiers. Food Hydrocoll. 2023;139 doi: 10.1016/j.foodhyd.2023.108565. [DOI] [Google Scholar]
- Xu G., Kang J., You W., Li R., Zheng H., Lv L., Zhang Q. Pea protein isolates affected by ultrasound and NaCl used for dysphagia's texture-modified food: rheological, gel, and structural properties. Food Hydrocoll. 2023;139 doi: 10.1016/j.foodhyd.2023.108566. [DOI] [Google Scholar]
- Xue Y., Zhong J., Liu X., Xiang D., Qin X. Improved physicochemical properties of bigels produced with ethyl cellulose-based oleogel and moderately deacetylated konjac glucomannan hydrogel. Food Chem. 2024;459 doi: 10.1016/j.foodchem.2024.140429. [DOI] [PubMed] [Google Scholar]
- Yang J., Zamani S., Liang L., Chen L. Extraction methods significantly impact pea protein composition, structure and gelling properties. Food Hydrocoll. 2021;117 doi: 10.1016/j.foodhyd.2021.106678. [DOI] [Google Scholar]
- Yang Q., Wang Y.R., Du Y.N., Chen H.Q. Heat-induced arachin and basil seed gum composite gels improved by NaCl and microbial transglutaminase: gelling properties and structure. Food Hydrocoll. 2023;135 doi: 10.1016/j.foodhyd.2022.108200. [DOI] [Google Scholar]
- Yang Y., Xu L., Zhang Q., Wang Y., Jiao A., Jin Z. Development and characterisation of a novel bigel based on pea protein hydrogel and rice bran wax oleogel: enhancement of rheological properties and freeze-thaw stability. Int. J. Biol. Macromol. 2024;282 doi: 10.1016/j.ijbiomac.2024.136606. [DOI] [PubMed] [Google Scholar]
- Yu J., Wang Y., Li D., jun Wang L. Freeze-thaw stability and rheological properties of soy protein isolate emulsion gels induced by NaCl. Food Hydrocoll. 2022;123 doi: 10.1016/j.foodhyd.2021.107113. [DOI] [Google Scholar]
- Zhang M., Yang Y., Acevedo N.C. Effect of oil content and composition on the gelling properties of Egg-SPI proteins stabilized emulsion gels. Food Biophys. 2020;15:473–481. doi: 10.1007/s11483-020-09646-8. [DOI] [Google Scholar]
- Zhang L., Lin W.F., Zhang Y., Tang C.H. New insights into the NaCl impact on emulsifying properties of globular proteins. Food Hydrocoll. 2022;124 doi: 10.1016/j.foodhyd.2021.107342. [DOI] [Google Scholar]
- Zheng H., Beamer S.K., Matak K.E., Jaczynski J. Effect of κ-carrageenan on gelation and gel characteristics of antarctic krill (Euphausia superba) protein isolated with isoelectric solubilization/precipitation. Food Chem. 2019;278:644–652. doi: 10.1016/j.foodchem.2018.11.080. [DOI] [PubMed] [Google Scholar]
- Zheng L., Teng F., Wang N., Zhang X.-N., Regenstein J.M., Liu J.-S., Li Y., Wang Z.-J. Addition of salt ions before spraying improves Heat- and cold-induced gel properties of soy protein isolate (SPI) Appl. Sci. 2019;9:1076. doi: 10.3390/app9061076. [DOI] [Google Scholar]
- Zheng R., Chen Y., Wang Y., Rogers M.A., Cao Y., Lan Y. Microstructure and physical properties of novel bigel-based foamed emulsions. Food Hydrocoll. 2023;134 doi: 10.1016/j.foodhyd.2022.108097. [DOI] [Google Scholar]
- Zhu Q., Gao J., Han L., Han K., Wei W., Wu T., Li J., Zhang M. Development and characterization of novel bigels based on monoglyceride-beeswax oleogel and high acyl gellan gum hydrogel for lycopene delivery. Food Chem. 2021;365 doi: 10.1016/j.foodchem.2021.130419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.