Abstract
Refractory celiac disease is a rare complex autoimmune-mediated disorder, which has multiple complications, among the best known is enteropathy-associated T-cell lymphoma, other rare complications are central nervous system demyelinating disorder and cavitary mesenteric lymph node syndrome. In our case report, we present a 43-year-old female with a known history of refractory celiac disease who developed a progressive demyelinating disorder of unknown origin and elevated liver enzymes that warranted further investigation. This led to the diagnosis of the cavitary mesenteric lymph node syndrome and celiac disease associated with demyelinating disorder that are both very rare presentations of refractory celiac disease. Treatment options for this case presented a challenge, firstly due to the lack of literature but also to continued complications.
Keywords: CMLNS, Autoimmune Enteropathy, Malabsorption, Gluten-Related Neurological Disorder
Introduction
Refractory celiac disease is defined as persistent villous atrophy with crypt hyperplasia, divided into 2 types: type I (normal intraepithelial lymphocytes) and type II (aberrant intraepithelial lymphocytes).1 Complications such as enteropathy-associated T-cell lymphoma are more common, but complications such as central nervous system (CNS) demyelinating disorders and cavitating mesenteric lymph node syndrome (CMLNS) are very rare and present in patients with refractory celiac disease.2 The pathology of CNS demyelinating disease is rarely described and is hypothesized to be linked to circulating neoplastic T cells; complications are often fatal.3,4 Findings show massive loss of Purkinje cells and local immune responses in patients with neurological symptoms and celiac disease.4 CMLNS is a rare manifestation of refractory celiac disease; fewer than 40 cases are reported. This condition is often associated with refractory celiac disease, enteropathy-associated T-cell lymphoma, and hyposplenism.5 In CMLNS, lymph node biopsy is crucial to exclude malignancy like lymphoma.6 Treatment for CMLNS without malignancy aims to control celiac disease; steroids may improve symptoms but are not beneficial long-term.6 Similarly, treatment options for CNS demyelinating disorder in celiac disease are scarce. We report a patient with refractory celiac disease who developed CNS demyelinating disorder and CMLNS without malignancy.
Case Report
A 43-year-old female with a past medical history of refractory celiac disease type 2 not on treatment for celiac disease except a gluten-free diet got lost to follow-up for 2 years (human leukocyte antigen-DQ (HLA-DQ) 2+, tissue transglutaminase (TG) negative and positive biopsy for celiac disease). Recent past medical history included 2 months of unknown multifocal demyelinating CNS disease being treated with rituximab. The patient presented with several concerning symptoms, including nausea, generalized weakness, and presyncope. The physical examination revealed icteric sclera, jaundice, and right upper quadrant tenderness, along with known generalized lower extremity weakness.
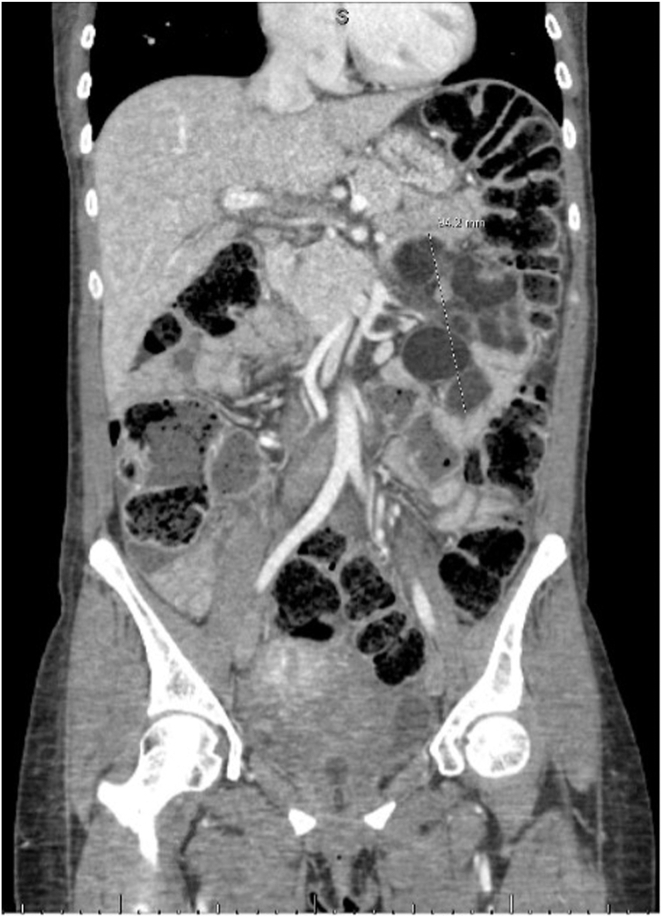
Labs showed elevated bilirubin (6.6 mg/dL), aspartate aminotransferase (1151 U/L), alanine aminotransferase (1447 U/L), and alkaline phosphatase (635 U/L), indicating liver dysfunction results seen in Table 1. Computed tomography scan showed enlarged mesenteric lymph nodes and splenic atrophy, consistent with cavitary mesenteric lymph node syndrome seen in Figure 1. Additionally, there was abnormal enhancement of the liver, along with inferior vena cava and hepatic vein distention, suggesting possible congestive hepatopathy. A magnetic resonance cholangiopancreatography was performed to rule out bile duct obstruction, and it showed findings compatible with nonspecific hepatocellular dysfunction, such as hepatitis or congestive hepatopathy. No biliary obstruction was noted, but there were markedly enlarged left mid-abdominal fat density masses, presumed to be lymph nodes with fluid levels, most suggestive of cavitary mesenteric lymph node syndrome.
Table 1.
Liver Enzymes Descending in Chronological Order
| Alkaline phosphatase (U/L) | ALT (U/L) | AST (U/L) | Total bilirubin (mg/dL) | INR |
|---|---|---|---|---|
| 381 | 2557 | 1792 | 1.0 | 1.3 |
| 684 | 613 | 390 | 9.3 | 1.4 |
| 342 | 330 | 253 | 13.1 | 1.2 |
| 248 | 231 | 164 | 13.8 | 1.2 |
| 512 | 102 | 57 | 2.8 | 1.3 |
| 393 | 96 | 74 | 0.9 | 1.1 |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; INR, international normalized ratio.
Figure 1.
CT of the abdomen coronal view with cavitary mesenteric lymph nodes. CT, computed tomography.
Specific laboratory tests were ordered due to the transaminitis, including antinuclear antibody, anti-smooth muscle antibody, immunoglobulin G, hepatitis C antibody, hepatitis C virus viral load, hepatitis B antibody, hepatitis B core immunoglobulin M, hepatitis B core total antibody, iron panel, thiopurine methyltransferase enzyme level, and F-actin antibody. All these tests returned negative or normal results. Due to the continued increase in transaminitis, a liver biopsy and a biopsy of a paragastric-mesenteric lymph node were warranted to further investigate the underlying cause of the patient's condition.
The liver biopsy showed fragmented hepatocytes and mild lymphocytic infiltrate in the portal tracts with rare plasma cells. Eosinophils were virtually absent. Bile ducts had intraepithelial lymphocytosis and focal injury, with no acute cholestasis, florid duct lesions, granulomas, or steatosis. Trichrome stain indicated no significant fibrosis (stage 0). Immunohistochemical stains revealed predominantly small CD3+ T lymphocytes coexpressing CD8, with rare CD4+ T cells. The findings suggested panlobular hepatitis with frequent apoptotic hepatocytes, mild lymphocytic portal inflammation, and bile duct injury, indicative of autoimmune hepatitis/cholangitis (including drug-induced). There was no evidence of lymphoproliferative disorder involvement. Paragasrtic lymph node biopsy showed no evidence of malignancy.
As transaminitis worsened, mentation and neurological deficits also deteriorated. A repeat brain magnetic resonance image showed T2/flair intense lesions in the right basal ganglia, left periventricular white matter, brainstem, and cerebellum, as seen in Figure 2. Cerebral spinal fluid analysis was nonspecific. Brain biopsy of the right cerebellar lesion revealed multifocal necrotic white matter lesions with severe axonal injury and no inflammatory infiltrates. Flow cytometry showed T cells with an inverted CD4 to CD8 ratio, a nonspecific finding seen in both reactive and neoplastic conditions.
Figure 2.
MRI image: Cerebellum demyelinating lesions. MRI, magnetic resonance image.
A trial of intravenous immunoglobulin was attempted but discontinued after 3 sessions due to bleeding at the biopsy site. The patient was started on high-dose steroids to treat refractory celiac, autoimmune hepatitis, and demyelinating disorder, showing slight improvement after 14 days of IV methylprednisolone. After physical therapy, the patient was discharged home with close follow-up with a celiac disease specialist and continued prednisone, remaining stable.
Discussion
Refractory celiac disease is complex to treat and has a high risk for malignancy. Our patient had refractory celiac disease with two rare complications: CMLNS and demyelinating disorder, both associated with the autoimmune nature of celiac disease. Literature describes several cases of refractory celiac with CMLNS and CNS demyelinating disorder separately. This case is significant as it links refractory celiac disease to CMLNS and a demyelinating disorder without lymphoma.
Refractory celiac disease is seen in patients who fail to respond to a strict gluten-free diet or have persistent symptoms, with an increased risk of malignancies or complications.7 Our patient was diagnosed with biopsy- and antibody-proven refractory celiac disease 2 years prior. The patient had been on a gluten-free diet without relief. Biopsy of the mesenteric lymph node showed no malignancy, ruling out lymphoma.
Cerebellar demyelinating disease in celiac patients has been linked to HLA-DQ2, suggesting a genetic predisposition, though few cases are reported.3 Six percent of celiac patients have neurological involvement, with HLA-DQ2 detected in 70%. Pathogenesis is hypothesized to be caused by anti-TG antibodies targeting TG2 and TG6.8 This antibody-mediated disorder may explain the biopsy results and improvement with steroids.
CMLNS, her second complication, was initially described in radiological images. CMLNS is often linked to neoplastic disease, celiac disease, and hyposplenism. We opted for a mesenteric lymph node biopsy to rule out malignancy. Histopathology shows a pseudocystic atrophic lymph node with a central cavity of chylous fluid, surrounded by fibrous material, with no infection or malignancy.7 Pathology is hypothesized to result from excessive antigenic exposure due to damaged intestinal mucosa in celiac disease, leading to depletion of lymphoid tissue in nodes and spleen.7,9
Cases like this are rare, making treatment decisions difficult. With an affected liver due to autoimmune disease or multiple drugs, treatment options are limited to short-term steroids.
CMLNS treatment aims to control refractory celiac disease to avoid progression. Celiac disease with demyelinating disorder has not responded to a gluten-free diet or immunosuppressive medication, except short-term corticosteroids.10 Survivors of this complication should continue long-term screenings for malignancies.
Conclusion
Celiac disease is a common disease throughout the world, but having refractory celiac is less common. Refractory celiac disease has well-known complications but the ones we expose here are very rare and still not well described in literature. This makes it even harder to get appropriate diagnosis and treatment in time for said conditions.
Footnotes
Conflicts of Interest: The authors disclose no conflicts.
Funding: The authors report no funding.
Ethical Statement: This manuscript describes a single patient case and does not constitute systematic research; therefore, institutional review board (IRB) or ethics committee approval was not required in accordance with local and international guidelines. Written informed consent was obtained from the patient for publication of the case details and any accompanying images. All identifying information has been omitted to protect patient confidentiality.
Reporting Guidelines: CARE.
References
- 1.Patey-Mariaud De Serre N., Cellier C., Jabri B., et al. Distinction between coeliac disease and refractory sprue: a simple immunohistochemical method. Histopathology. 2000;37(1):70–77. doi: 10.1046/j.1365-2559.2000.00926.x. [DOI] [PubMed] [Google Scholar]
- 2.Tye-Din J.A. Review article: follow-up of coeliac disease. Aliment Pharmacol Ther. 2022;56(Suppl 1):S49–S63. doi: 10.1111/apt.16847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keller C.E., Gamboa E.T., Hays A.P., et al. Fatal CNS vasculopathy in a patient with refractory celiac disease and lymph node cavitation. Virchows Arch. 2006;448:209–213. doi: 10.1007/s00428-005-0060-x. [DOI] [PubMed] [Google Scholar]
- 4.Rouvroye M.D., Bontkes H.J., Bol J.G.J.M., et al. Cerebellar presence of immune cells in patients with neuro-coeliac disease. Acta Neuropathol Commun. 2023;11(1):51. doi: 10.1186/s40478-023-01538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwock J., Hyjek E.M., Torlakovic E.E., et al. Enteropathy-associated intestinal T-cell lymphoma in cavitating mesenteric lymph node syndrome: fine-needle aspiration contributes to the diagnosis. Diagn Cytopathol. 2015;43(2):125–130. doi: 10.1002/dc.23144. [DOI] [PubMed] [Google Scholar]
- 6.Sanson E., Gassler N., Trautwein C., et al. Cavitating mesenteric lymph node syndrome: a rare complication of refractory celiac disease. Z Gastroenterol. 2010;48(9):1133–1137. doi: 10.1055/s-0028-1109948. [DOI] [PubMed] [Google Scholar]
- 7.McBride O.M., Skipworth R.J., Leitch D., et al. Cavitating mesenteric lymph node syndrome in association with coeliac disease and enteropathy associated T-cell lymphoma: a case report and review of the literature. Case Rep Med. 2010;2010 doi: 10.1155/2010/478269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitoma H., Manto M., Hampe C.S. Immune-mediated cerebellar ataxias: practical guidelines and therapeutic challenges. Curr Neuropharmacol. 2019;17(1):33–58. doi: 10.2174/1570159X16666180917105033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman H.J. Mesenteric lymph node cavitation syndrome. World J Gastroenterol. 2010;16(24):2991–2993. doi: 10.3748/wjg.v16.i24.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pengiran Tengah D.S., Wills A.J., Holmes G.K. Neurological complications of coeliac disease. Postgrad Med J. 2002;78:393–398. doi: 10.1136/pmj.78.921.393. [DOI] [PMC free article] [PubMed] [Google Scholar]