Cancer is a disease traditionally associated with aging, yet recent trends show a troubling rise in cancers occurring at younger ages, often referred to as ‘early-onset’ cancers.1 Nevertheless, these malignancies remain comparatively understudied, despite their unique challenges and the significant burden they impose. Traditionally, ‘early onset’ referred to patients diagnosed in their adulthood before the age of 50 years, although this definition may be overly simplistic. With nearly 1 million cancers occurring among young adults worldwide, early-onset cancers now represent the leading cause of mortality in this age group in regions with a middle-to-high socio-demographic index—surpassing cardiovascular diseases. People younger than 50 years were the only age group to experience a sustained increase in cancer incidence from 1995 through 2021.2 Between 1990 and 2019, the global incidence of early-onset cancer in this age group rose by 79.1%, while mortality from these cancers increased by 27.7%.3
The incidence of various cancers across multiple organ systems has been rising among adults under 50 years in many regions worldwide, including digestive cancers (colorectum, esophagus, extrahepatic bile duct, gall-bladder, stomach, pancreas, and liver), gynecological cancers (breast and endometrium), urogenital cancers (kidney and prostate), as well as head and neck and thyroid cancers.4 Recent epidemiological data also suggest an increasing trend in the incidence of lung cancer among never smokers, with ∼12.5% of cases observed in this demographic occurring among young adults.5 Concurrently, sex disparities in cancer incidence among young adults have become increasingly pronounced, reflecting distinct evolving trends over time.2 Historically, women under 50 years have carried a heavier cancer burden than men in the same age group, and this disparity has widened considerably over time. In 2021, the incidence rate among young women was 141.1 per 100 000—82% higher than in young men (77.4 per 100 000) compared with a 51% difference reported in 2002. Overall, cancer incidence in young women rose by ∼20%, driven primarily by surges in breast and thyroid cancers, which together now account for nearly half of all cancers in this age group.2 In contrast, between 2002 and 2021, the overall cancer incidence in young men declined slightly, mostly due to falling rates of melanoma, non-Hodgkin lymphoma, and prostate cancer, despite increases in the four most common cancers in this group—colorectal, testicular, kidney, and leukemia.
The rapid increase in the incidence of cancer is among patients under 40 years (Figure 1), making this group particularly relevant for studying the determinants of early-onset cancers.6 The age range of 20-40 years is biologically distinct, with individuals having passed puberty, but not yet experienced the effects of hormonal decline, immune response deterioration, or organ dysfunction associated with chronic health conditions.7,8 This phase of life is also marked by significant physical, emotional, and psychosocial milestones. Moreover, individuals in this age group generally have a large portion of their expected life span ahead of them, contribute substantially to the economy, and play critical roles in their families and communities. Many young adults prioritize academic, professional, or social commitments over their health, often perceiving themselves as invincible and dismissing early symptoms, which can lead to delays in both diagnosis and seeking medical care. Additionally, few evidence-based screening guidelines specifically target young adults—cervical cancer screening begins at age 21 years, and certain high-risk syndromes may warrant earlier engagement, but most standard protocols exclude this demographic.9 Despite many advances in oncology, outcomes for malignancies in these younger patients remain poorer compared with similar diseases in older populations.1,10 This disparity arises from multiple factors, including distinct tumor biology, delayed diagnoses, and challenges in tailoring treatment for young adults. Finally, adherence to treatment regimens, follow-up plans, and clinical trial enrollment is often lower in this age group.11
Figure 1.
Age-standardized cancer incidence rate per 100 000. Temporal trends in incidence of cancer among males (A) and females (B) according to age groups in the most populous countries (using available data within GLOBOCAN21) of each continent.
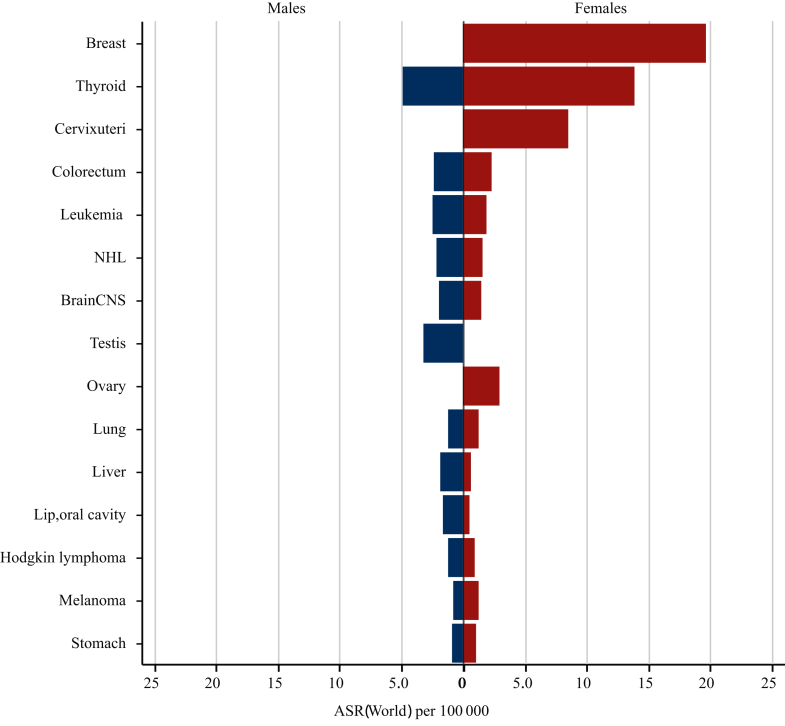
In 2022, GLOBOCAN reported 1 248 230 new cancer cases and 351 110 cancer deaths among young adults aged 20-40 years worldwide (Figure 2).12 Global epidemiological data indicate that early-onset cancers have an age-standardized incidence rate of 50.3 cases per 100 000 person-years, with mortality rates reaching 14.2 deaths per 100 000 person-years. By 2050, early-onset cancers are projected to show a significant upward trajectory, with models predicting a parallel rise in both incidence and mortality. Specifically, incidence rates may increase by 12.8% compared with current levels, while mortality rates could rise by 12.9%. Among young adults, age-standardized rates (ASRs) of incidence and mortality were 50.3 and 14.2 per 100 000 person-years, respectively. Among men, the most commonly diagnosed cancers were thyroid (14.4%), testis (9.0%), and colorectal (6.9%) neoplasms, and leading causes of death included liver (12.1%) and central nervous system (9.2%) cancers. Among women, breast cancer accounts for the highest incidence (30.0%), followed by thyroid cancer (20.4%) and cervical cancer (12.9%). Regarding mortality in young adult women, breast cancer remains the leading cause (24.6%), followed by cervical cancer (16.6%).
Figure 2.
Estimated number of new cancer cases among young adults for the 15 most common anatomical sites, worldwide in 2022.21
NHL, non-Hodgkin lymphoma.
In this 20- to 40-year-old population, the overall incidence trend for ‘all cancers combined’ conceals important variations across tumor sites.12 For instance, in France, the ASR of incidence increased from 77.3 to 94 per 100 000 among women and 53.7 to 65.3 per 100 000 among men between 1998 and 2017—a 21.6% rise in both sexes, corresponding to +1.1% per year. Notably, many cancers demonstrated increased ASR, including gastric, colorectal, gall-bladder, pancreas, melanoma, thyroid, breast, uterus, prostate, and testis, whereas incidence of certain other cancers declined. Analysis of the so-called theoretical ‘tobacco-associated’ malignancies reveals notable trends in the 20- to 40-year-old population, although these cancers can be partly attributed to other causes. When examining the ASR per 100 000 for these cancers—including lip, oral cavity, and pharynx; esophagus; pancreas; larynx; lung; and bladder—significant sex-specific variations emerged. In France, between 1998 and 2017, females had an overall ASR increase from 3.3 to 4.4 (+1.7% per year), with head and neck cancers specifically rising from 0.9 to 1.6 (+4% per year). Conversely, males experienced a decline in the incidence of these cancers, from 7.0 to 4.8 (−1.6% per year). However, pancreatic cancer incidence among men exhibited a concerning upward trend, increasing nearly five-fold from 0.12 to 0.56 (+19.3% per year). In the United States, between 1978 and 2017, women showed an overall decrease in alcohol- and tobacco-related cancers from 4.7 to 3.7 (−0.5% per year), driven mostly by a reduction in lung cancer from 2.3 to 0.87 (−1.6% per year). Nevertheless, pancreatic cancer rose from 0.32 to 0.49 (+1.4% per year), and head and neck cancers increased from 1.5 to 1.8 (+5.1% per year). Among men in the United States, the burden of these cancers declined from 6.0 to 4.5 (−0.6% per year), with lung cancer dropping substantially from 2.2 to 0.97 (−1.4% per year), while pancreatic cancer continued to rise, moving from 0.40 to 0.71 (+2.0% per year).12
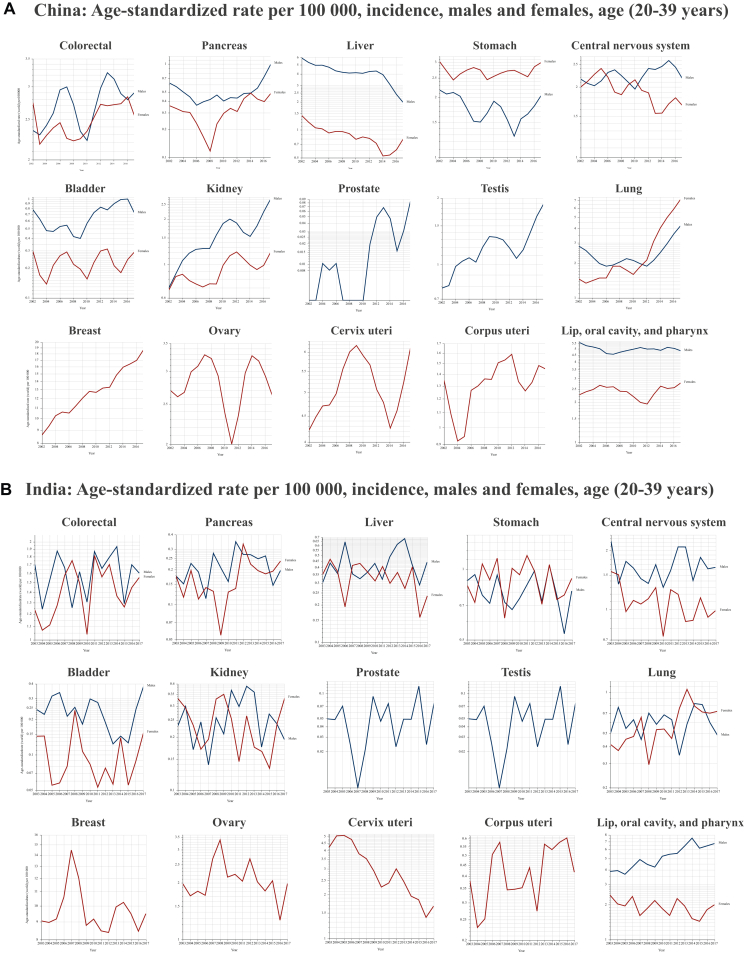
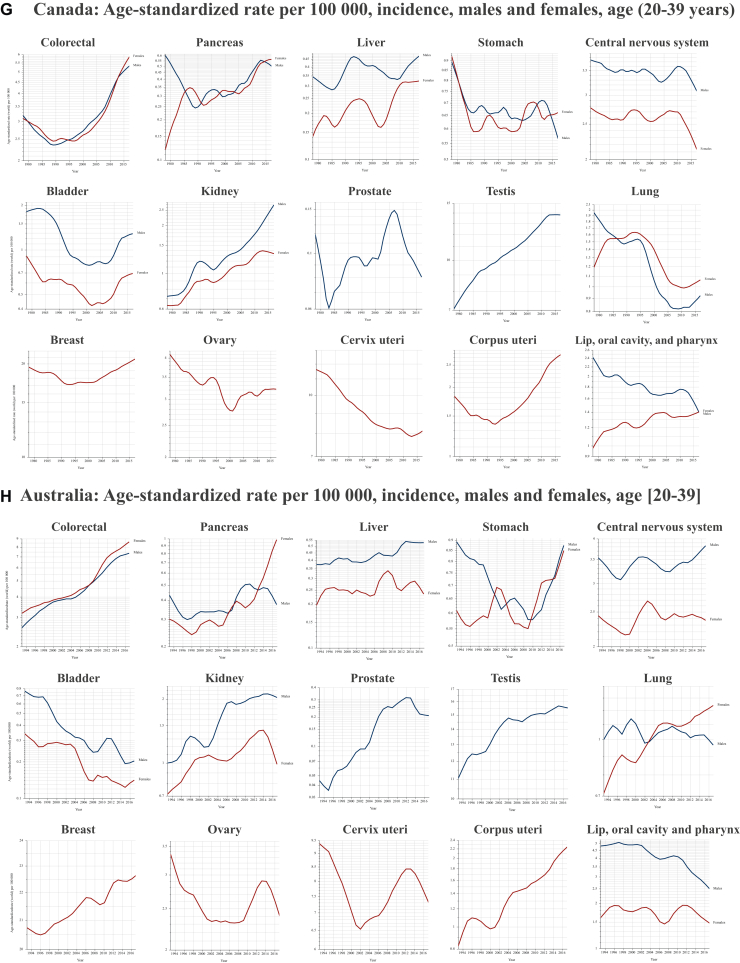
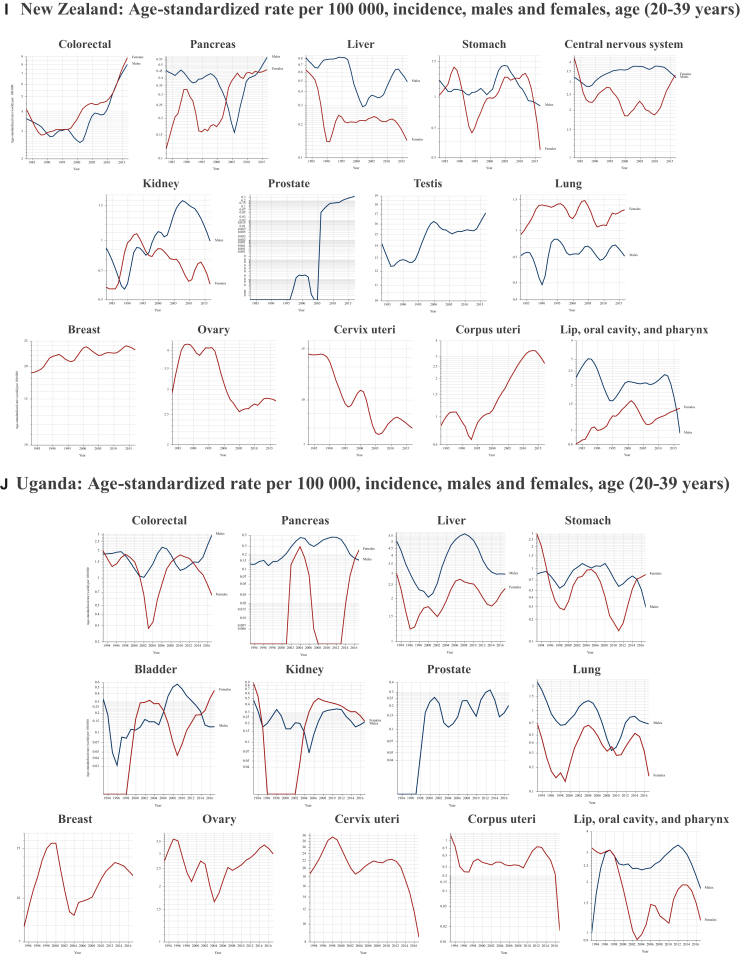
Several critical research questions arise from the pressing public health issue of increasing early-onset cancers. The most urgent query concerns their etiology.13 Currently, there is no single validated hypothesis to explain the dramatic increase in cancer incidence among young adults. The varied temporal trends among cancer type, sexes, and geographic regions suggest that changes in testing availability, lifestyle, and environmental exposures are fundamental factors (Figure 3). Screening does not appear as the primary driver of rising cancer rates in young adults, as most screening protocols do not target this demographic, apart from cervical cancer. Even in the case of hereditary cancer predisposition syndromes, cancer screening alone fails to fully account for the observed escalation.14 Moreover, in young adults, ‘screening’ tests often function more as diagnostic tools than true prevention measures. Increased accessibility worldwide to such diagnostics may partially explain rising incidence rates, but is still insufficient to explain the broader trend. Particularly concerning is the marked rise in early-onset malignancies of organs where screening is limited, non-existent, or does not cover this age group, such as colorectal and pancreatic cancers; here the increase likely reflects a genuine epidemiological shift rather than an artifact of better detection. Evolving exposure patterns to risk factors during pre-life, early life, and young adulthood—from the mid-20th century onward—may have contributed significantly to the rise in early-onset cancer.13,15 Notable associations include obesity and metabolic syndrome, early-life antibiotic use (and its impact on the microbiome), greater exposure to carcinogens and environmental pollutants, endocrine disruptors from various sources, and lifestyle changes (e.g. diet, physical activity, and sleep patterns).3 The interaction between these compounds and (poly)genetic or epigenetic susceptibilities adds another layer of complexity.6,16 Additionally, recent findings indicate that ‘accelerated aging’ is more frequent among individuals born in or after 1965, relative to those born between 1950 and 1954, and that each standard deviation increase in this metrics correlated with a 42% greater risk of early-onset lung cancer, a 22% greater risk of early-onset gastrointestinal malignancies, and a 36% greater risk of early-onset uterine cancer.17
Figure 3.
Age-standardized cancer rate per 100 000. (A-J) Temporal trends in early-onset cancer incidence among young adults in the most populous countries (with available data within GLOBOCAN21) of each continent.
The rising incidence of early-onset cancers represents an urgent scientific challenge demanding comprehensive investigation. The definition of early-onset cancers lacks formal biological justification and requires criteria beyond conventional age thresholds. Although the commonly used cut-off of 50 years aligns with established screening guidelines and coincides with significant biological transitions—particularly in women—a lower threshold may better speak for the distinct biological characteristics and the profound psychosocial implications of a cancer diagnosis at younger ages. For instance, prostate cancer at 45 years of age is far more unusual than breast cancer at the same age. Moreover, younger patients face unique challenges, including fertility preservation, career undermining, and profound alterations in life trajectory. The optimal definition ultimately depends on its purpose: standardization for epidemiological studies versus clinical decision making that both biological and psychosocial factors may nuance. In this editorial and our research program POWER (Precision Oncology for the Wellness, trEatment, and Research) for Young Adults, we focus on individuals under age 40 years, given the concerning surge in incidence among this demographic and the potential for better elucidating distinct risk factors and biological mechanisms in early-onset cancers.18 Understanding the underlying causes is crucial for targeted prevention strategies and more effective early detection. In recognition of this need, major research institutions worldwide have elevated early-onset cancer to a high-priority status.19,20 Notably, the United States has designated early-onset cancer as a research priority, and the National Institutes of Health/Cancer Research UK consortium on Cancer Grand Challenges identified it as a significant challenge, prompting substantial investment and international collaboration. This prioritization reflects both the complexity of the issue and its growing public health impact, necessitating coordinated global efforts that integrate molecular biology, epidemiology, environmental science, and clinical medicine.
Acknowledgements
The figures were generated from GLOBOCAN.21
Funding
None declared.
Disclosure
FA: research funding: AstraZeneca (Inst), Novartis (Inst), Pfizer (Inst), Eli Lilly (Inst), Roche (Inst), Daiichi (Inst); travel, accommodations, expenses: Novartis, Roche, GlaxoSmithKline, AstraZeneca. ER: research support (institutional): Gilead, MSD, Menarini; honoraria: Eli Lilly, Seagen, Novartis, AstraZeneca, Daiichi Sankyo, MSD; travel, accommodations, expenses: Pfizer, Roche, Eli Lilly, Gilead, Novartis, Menarini, Mundipharma. AB: consulting or advisory role, travel, accommodations, expenses: Mercks, Viatris, Ipsen, Servier. CS: principal investigator: AbbVie, Astellas, BMS, GSK, Boehringer Ingelheim, MSD; invited speaker: Roche, Pierre Fabre, Servier, MSD, Jazz Pharmaceutical; personal: Pierre Fabre, Servier, Merck. SD: consulting or advisory role: AstraZeneca; research funding: AstraZeneca (Inst), Pfizer (Inst), Roche/Genentech (Inst), Puma (Inst), Eli Lilly (Inst), Novartis (Inst), Sanofi (Inst); travel, accommodations, expenses: Pfizer, AstraZeneca, Roche. FB: institutional financial interests: AbbVie, ACEA, Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Eisai, Eli Lilly Oncology, F. Hoffmann–La Roche Ltd, Genentech, Ipsen, Ignyta, Innate Pharma, Loxo, Novartis, Medimmune, Merck, MSD, Pierre Fabre, Pfizer, Sanofi-Aventis, Summit Therapeutics, Takeda; non-financial interests: principal investigator for AstraZeneca, BMS, Innate Pharma, Merck, Mirati, Pierre Fabre, F. Hoffmann-La Roche, Ltd, sponsored trials (or ISR). BA-D has declared no conflicts of interest.
References
- 1.Trama A., Botta L., Stiller C., et al. Survival of European adolescents and young adults diagnosed with cancer in 2010–2014. Eur J Cancer. 2024;202 doi: 10.1016/j.ejca.2024.113558. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Kratzer T.B., Giaquinto A.N., Sung H., Jemal A. Cancer statistics, 2025. CA Cancer J Clin. 2025;75(1):10–45. doi: 10.3322/caac.21871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao J., Xu L., Sun J., et al. Global trends in incidence, death, burden and risk factors of early-onset cancer from 1990 to 2019. BMJ Oncol. 2023;2(1) doi: 10.1136/bmjonc-2023-000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ugai T., Sasamoto N., Lee H.-Y., et al. Is early-onset cancer an emerging global epidemic? Current evidence and future implications. Nat Rev Clin Oncol. 2022;19(10):656–673. doi: 10.1038/s41571-022-00672-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LoPiccolo J., Gusev A., Christiani D.C., Jänne P.A. Lung cancer in patients who have never smoked — an emerging disease. Nat Rev Clin Oncol. 2024;21(2):121–146. doi: 10.1038/s41571-023-00844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mauri G., Patelli G., Sartore-Bianchi A., et al. Early-onset cancers: biological bases and clinical implications. Cell Rep Med. 2024;5(9) doi: 10.1016/j.xcrm.2024.101737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adolescent and Young Adult Oncology Progress Review Group Closing the Gap: Research and Care Imperatives for Adolescents and Young Adults with Cancer. 2006. https://www.cancer.gov/types/aya/research/ayao-august-2006.pdf Available at.
- 8.Tricoli J.V., Blair D.G., Anders C.K., et al. Biological and clinical characteristics of adolescent and young adult cancers: acute lymphoblastic leukemia, colorectal cancer, breast cancer, melanoma and sarcoma. Cancer. 2016;122(7):1017–1028. doi: 10.1002/cncr.29871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network (NCCN) version 2.2025. https://www.nccn.org/professionals/physician_gls/pdf/aya.pdf Available at.
- 10.Trama A., Botta L., Foschi R., et al. Survival of European adolescents and young adults diagnosed with cancer in 2000-07: population-based data from EUROCARE-5. Lancet Oncol. 2016;17(7):896–906. doi: 10.1016/S1470-2045(16)00162-5. [DOI] [PubMed] [Google Scholar]
- 11.Carr E., Rosengarten L. Teenagers and young adults with cancer: an exploration of factors contributing to treatment adherence. J Pediatr Oncol Nurs. 2021;38(3):190–204. doi: 10.1177/1043454221992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Global Cancer Observatory. https://gco.iarc.fr/ Available at.
- 13.Ogino S., Ugai T. The global epidemic of early-onset cancer: nature, nurture, or both? Ann Oncol. 2024;35(12):1071–1073. doi: 10.1016/j.annonc.2024.08.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garber J.E., Offit K. Hereditary cancer predisposition syndromes. J Clin Oncol. 2005;23(2):276–292. doi: 10.1200/JCO.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 15.Tateo V., Thompson Z.J., Gilbert S.M., et al. Epidemiology and risk factors for testicular cancer: a systematic review. Eur Urol. 2025;87:427–441. doi: 10.1016/j.eururo.2024.10.023. [DOI] [PubMed] [Google Scholar]
- 16.Argentieri M.A., Amin N., Nevado-Holgado A.J., et al. Integrating the environmental and genetic architectures of aging and mortality. Nat Med. 2025;31:1016–1025. doi: 10.1038/s41591-024-03483-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian R., Tica S., Hong D., et al. Paper presented at the American Association for Cancer Research Annual Meeting; San Diego, USA: April 7, 2024. 846/19 - Rising accelerated aging in recent generations associated with elevated risk of early-onset cancers. [Google Scholar]
- 18.Un cancer à 30 ans: Soutenez Gustave Roussy. https://guerirlecancer.gustaveroussy.fr/journees-mondiales/ Available at.
- 19.Danielson L., Prime P., Larter R. Mind the gap: why we need to consider teenage and young adult cancers across the research pipeline. Trends Cancer. 2024;10(9):774–776. doi: 10.1016/j.trecan.2024.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Ben-Aharon I., Smyth E., Wagner A.D. Dedicated centres and multinational platforms to improve patient care and address early-onset cancers. Nat Rev Cancer. 2025;25(3):145–146. doi: 10.1038/s41568-024-00777-5. [DOI] [PubMed] [Google Scholar]
- 21.GLOBOCAN. https://gco.iarc.fr/overtime/ Available at.