Abstract
Background
The No Specific Molecular Profile (NSMP) subtype accounts for ∼30%-40% of endometrial cancer (EC), comprising a heterogeneous group of EC.
Patients and methods
The primary outcome of this study was the prevalence of actionable genomic alterations in NSMP EC, classified according to the European Society for Medical Oncology (ESMO) Scale for Clinical Actionability of molecular Targets (ESCAT). Oncogenic and likely oncogenic alterations, pathways, and co-mutation patterns were reported. The analysis was stratified by risk group according to the European Society of Gynaecological Oncology (ESGO)–ESMO–European SocieTy for Radiotherapy and Oncology (ESTRO) guidelines. Patients with NSMP EC enrolled in the FPG500 comprehensive cancer genome profiling program (NCT06020625) were included.
Results
Two hundred and fifty-three patients with NSMP EC of any International Federation of Gynecology and Obstetrics (FIGO) stage were enrolled between 1 January 2022 and 31 December 2023. Median age was 62 years, and the most frequent histotype was endometrioid (97%). Ninety-five percent of patients were estrogen receptor positive. Two hundred and thirty-three patients (92%) had at least one ESCAT tier I-III alteration. The most frequent variants were in PTEN [88%, 95% confidence interval (CI) 84% to 92%], PIK3CA (42%, 95% CI 36% to 49%), FGFR2 (15%, 95% CI 11% to 20%), and AKT1 (6%, 95% CI 3% to 10%); 4% (95% CI 2% to 8%) of patients had an ESR1 variant, while KRAS G12C was found in 3% (95% CI 1% to 6%) of patients. The majority of PTEN variants were on the R130 hotspot. More frequent PIK3CA hotspot variants were H1047R (9%), E545D/K/Q/A (6%), and E542K (4%). In the overall population, PIK3CA with PIK3R1 (odds ratio [OR] = 0.07, P value = 4.25 × 10−14), KRAS with CTNNB1 (OR = 0.03, P value = 1.98 × 10−7), and AKT1 and PTEN (OR = 0.06, P value = 5.73 × 10−6) were mutually exclusive. Significant co-occurrence was found between PTEN and ARID1A (OR = 6.94, P value = 3.39 × 10−6) and PTEN with PIK3R1 (OR = 4.36, P value = 0.009). An alteration in the phosphatidylinositol-3 kinase (PI3K) pathway was found in 94% of patients in the overall population and in 98% of patients with an ESCAT tier I-III alteration.
Conclusion
Our findings highlight potentially actionable alterations in NSMP EC patients, supporting the exploration of tailored molecular-matched therapies according to risk groups.
Key words: No Specific Molecular Profile endometrial cancer, genomic description, ESCAT, target therapy
Highlights
-
•
Ninety-two percent of NSMP EC patients had at least one ESCAT tier I-III alteration.
-
•
The most frequent ESCAT tier III variants were PTEN (88%), PIK3CA (42%), FGFR2 (15%), and AKT1 (6%).
-
•
Co-occurrent genes were PTEN-ARID1A and PTEN-PIK3R1. PIK3CA-PIK3R1, KRAS-CTNNB1, and AKT1-PTEN were mutually exclusive.
Introduction
The advent of molecular profiling radically changed the classification systems and therapeutic algorithms for endometrial cancer (EC). In 2013, based on The Cancer Genome Atlas (TCGA) study, four distinct prognostic subgroups were identified: (i) ultramutated, characterized by extremely high mutation rates caused by a pathogenetic alteration in the exonuclease domain of DNA polymerase epsilon (POLE) gene (5%-15%); (ii) microsatellite instable, hypermutated due to defects in the DNA mismatch repair (MMR) system (25%-30%); (iii) copy number-low exhibiting a low mutation rate and minimal copy number alterations (30%-40%); (iv) copy number-high with extensive copy number alterations and a high frequency of TP53 mutations (5%-15%).1 POLE-mutated patients exhibited an excellent prognosis, with 5-year recurrence-free survival rates of 90%-95%, in sharp contrast to those with TP53-mutated tumors, facing poorer prognosis, with 5-year recurrence-free survival rates of 50%-60%.2, 3, 4
To facilitate the widespread adoption of molecular profiling, a pragmatic classifier, mainly based on immunochemistry (IHC) was developed and validated.5 Four prognostic subgroups, closely mirroring those identified by the TCGA, were established: (i) POLE-mutated evaluated through sequencing, (ii) p53-aberrant (p53abn) evaluated through IHC, (iii) mismatch repair-deficient evaluated through IHC, and (iv) patients without any of the aforementioned characteristics. The latter, which accounts for ∼30%-40%, is known as ‘No Specific Molecular Profile’ (NSMP).6 It represents a heterogeneous subgroup in terms of clinical, histological, and molecular features, with an intermediate prognosis. In this context, the research on molecular characteristics to further stratify NSMPs is an open field of research with multiple potential implications including treatment de-escalation approach in lower-risk patients and the introduction of additional target therapies in higher-risk patients. In the past years, several parameters, both clinicopathological and molecular, have been investigated trying to stratify the outcome including estrogen receptor expression that could potentially be able to define a good prognosis population within the NSMP group.5,7,8
NSMP patients in the advanced (stage III) and metastatic (stage IV) setting can currently benefit from the combination of poly (ADP-ribose) polymerase inhibitors (olaparib) and immunotherapy (dostarlimab) which has been recently approved regardless of histological and/or molecular characteristics.7, 8, 9 However, the lack of a predictive biomarker is likely to diminish the benefits of these drugs in this subgroup, while exacerbating clinical and financial burdens.
Hence, in this context the implementation of targetable alteration could enlarge treatment options, not only in the maintenance setting. In this article, we present a comprehensive genomic, pathological, and clinical characterization of an unselected series of prospectively clinically sequenced EC patients from a large referral center with the objective of delineating the presence of potentially targetable alterations. To better understand the clinical impact of our molecular findings, we decided to further categorize them according to the European Society for Medical Oncology (ESMO) Scale for Clinical Actionability of molecular Targets (ESCAT).10,11
Patients and methods
The primary outcome of this study was the prevalence of actionable genomic alterations classified according to the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT), within a prospectively sequenced cohort of NSMP EC patients. This was designed as a descriptive analysis without predefined hypothesis testing.
Case selection
In January 2022, our institution launched a comprehensive cancer genome profiling (CGP) program (FPG500; IRB approval 3837, NCT06020625) that encompasses 11 different cancer types, including EC.12 Eligible patients were ≥18 years of age and had newly diagnosed NSMP EC for which a CGP report was available. Exclusion criteria were represented by the presence of p53 alterations, MMR deficiency or POLE hotspot mutations, non-epithelial histotype (e.g. sarcoma), and failure of genomic sequencing. Sequencing data of the NSMP EC cohort were retrieved for the present study. Institutional review board approval was received (protocol U 00194/23, ID number: 3837) and before participation, all patients provided informed consent. The program was conducted according to the Declaration of Helsinki.
Histopathological data
Histopathological data were obtained from pathology reports generated by the gynecologic pathology unit, which operates with a standardized diagnostic approach (https://www.mayocliniclabs.com/test-catalog/overview/35466). All cases underwent comprehensive surgical staging, which included sentinel lymph node mapping. The presence and extent of lymphovascular space invasion (LVSI) was scored as absent, focal, or substantial. Results of the immunohistochemistry (IHC) analysis of the p53 and DNA MMR proteins MLH1, MSH2, MSH6, and PMS2 were recorded. Patients included were classified as NSMP according to the ProMisE molecular classification, with POLE status assessed through next-generation sequencing (NGS) and MMR and p53 status assessed through IHC.13 Alterations in TP53 or MMR genes detected by NGS that were inconsistent with IHC findings were not considered for patients’ classification but were included in the analysis. Estrogen receptor (ER) expression was categorized with a cut-off of 10%, defining as positive those patients with ER ≥10%.14 Stage I-II patients were classified into four groups based on molecular and clinicopathological features, following the European Society of Gynaecological Oncology (ESGO)–ESMO–European SocieTy for Radiotherapy and Oncology (ESTRO) guidelines: low risk (LR), intermediate risk (IR), high-intermediate risk (HIR), and high risk (HR). Patients with locally advanced or metastatic tumors were analyzed separately.15
Data collection
The demographic and clinical details of the enrolled patients were gathered using a customized electronic case report form (eCRF) based on a thorough review of medical records. This eCRF was developed using RedCap (https://redcap-irccs.policlinicogemelli.it), a web-based platform compliant with the European Union’s data protection and management standards as stipulated in the General Data Protection Regulation-2016/679.
Statistical analysis and sample size
The sample size was determined through the consecutive enrollment of patients. Since the study was structured as a descriptive analysis, no formal power calculations were conducted. Descriptive statistics, including means and standard deviations for continuous variables and frequencies with percentages for categorical variables, were utilized to summarize the characteristics of the study population. The 95% confidence intervals (CIs) for proportions were calculated using the Clopper–Pearson method. Relapse-free survival (RFS) was defined as the time interval from diagnosis to the first radiological detection of disease recurrence, assessed based on RECIST 1.1 criteria, or death due to any cause. Progression-free survival (PFS) was defined as the time interval from the start of first-line systemic therapy to radiological disease progression or death from any cause. The median follow-up period was calculated using the reversed Kaplan–Meier method and was reported with 95% CI. The Cochran–Armitage test for trend was used to assess trends in gene mutation proportions across risk groups. Fisher’s test was applied to compare the proportion of mutated patients between the ER-positive and ER-negative groups. The false discovery rate was corrected using the Benjamini–Hochberg (BH) method. All statistical tests were two-sided, with a P value threshold of 0.05 and an adjusted P value of 0.10 set to determine statistical significance. Analyses were conducted using R (version 4.4.0) and Python (version 3.9.21).
Genomic preprocessing
Details on the genomic methodologies used in this study have been previously published.16,17 The TruSight Oncology 500 (TSO500) multigene panel (Illumina, San Diego, CA) was applied on samples containing >20% tumor cells, as determined by a review conducted by a gynecologic pathologist (GFZ). Raw sequencing data were processed using the Illumina software TSO500 v2.2 Local App and a custom analysis pipeline (https://github.com/lucianogiaco/lianne). Data were then sent to the Clinical Genomics Workspace software platform by Velsera (Boston, MA) for variant interpretation and reporting. Sequencing was carried out with a mean depth of >500×, with a minimum coverage of 100× for 90% of regions and 250× for hotspots. Variant call format files were converted to mutation annotation format and annotated using the Ensembl variant effect predictor18 and vcf2maf (https://github.com/mskcc/vcf2maf.git), and then further annotated with OncoKB.19 Data were filtered for non-synonymous, exonic variants with an allelic frequency <0.04% in gnomAD v2 and a variant allele frequency (VAF) ≥5%. Only mutations annotated as ‘Oncogenic’ or ‘Likely Oncogenic’ according to OncoKB were considered for the analysis. Copy number variation files were first annotated with OncoKB and then discretized into categories, including amplifications with five or more copies and homozygous deletion. Microsatellite instability (MSI) status was determined by analyzing 130 homopolymer repeat loci (10-20 base pairs) with a minimum coverage of 60 full-spanning reads. Tumor mutational burden (TMB) was considered high if ≥10 mutation/megabase (mut/Mb).
Genomic data analysis
ESCAT tiers were assigned to genetic variants detected according to Mosele et al.,11 with a comprehensive list of all considered variants available in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2025.105755.20 Genomic data were analyzed using the maftools package.21
Pathways were reported according to Sanchez-Vega et al.22 In the analysis of co-occurrence and mutual exclusivity, P values were calculated using pairwise Fisher’s exact test. Intersection size was calculated as the number of times that a specific combination of genes occurs, whereas set size was calculated as the number of times that a specific gene is altered.
Non-negative matrix factorization (NMF) was carried out using the Lee method on the Gene Ontology (GO)-smoothed mutation matrix. Genes in the top decile of variance were retained and then converted to a binary matrix. GO semantic similarity was computed using the Wang measure based on the Biological Process terms. Network smoothing was applied via random walk with a restart probability of 0.5. Analyses were conducted for factorization ranks 2-8 to identify the optimal number of latent factors.
Multiple correspondence analysis (MCA) was carried out on a binary matrix representing the mutation state (mutated or wild-type) of somatic variants across samples. The quality of representation (cos2) and the contribution of each mutation state to the dimensions were extracted, with cos2 values indicating the proportion of variance of a given mutation state, and vector length reflecting its discriminative power in the MCA space.
A co-mutation network was constructed from somatic variants by mapping each tumor sample to its set of mutated genes and calculating pairwise co-occurrences. Network topology was visualized using the Fruchterman–Reingold force-directed layout. Centrality measures (degree, betweenness, closeness, and eigenvector centrality) were computed. For the PIK3CA network, the four most frequent PIK3CA hotspots were considered, and the network graph was generated using the spring layout. The NetworkX (v2.8) package was used.
Results
Between 1 January 2022 and 31 December 2023, 3124 patients were enrolled in the institutional CGP program, including 695 EC. High-quality clinical and genomic data were available for 596 patients, 42% of whom (n = 253 patients) were classified as NSMP and included in the present analysis (Figure 1).
Figure 1.
STROBE diagram summarizing the NSMP patients included in the study. dMMR, mismatch repair-deficient; FPG, Fondazione Policlinico Gemelli; HT, high-throughput; NSMP, non-specific molecular profile; TSO, TruSight Oncology 500.
Clinicopathological data
Detailed clinicopathological characteristics are provided in Table 1. Most patients were classified as LR (n = 121, 48%) or IR (n = 60, 24%). All LR patients had stage IA, well-differentiated endometrioid tumors, with 93% lacking LVSI and 98% showing ER positivity. In the IR group, 90% were presented with stage IB, low-grade endometrioid tumors, characterized by high ER positivity (98%) and no LVSI in 78% of cases. A smaller portion of the cohort was classified as HIR (n = 40, 16%) or HR (n = 27, 11%). Among HIR patients, all had an endometrioid histotype, predominantly low grade (60%), with stage II disease in 53% and ER positivity in 95%. However, LVSI was present in 21 patients (53%). In the HR subgroup, most had ER positivity (78%) and low-grade endometrioid neoplasms (67%) at stage IIIC1 (63%), with a lower LVSI positivity rate (37%). In the advanced setting, most of the patients had ER-positive high-grade endometrioid tumors (40%) diagnosed at stage IVB (60%). In the overall population, the median membrane expression level of the ER was 60% (interquartile range 30%-80%). Overall, 27% of NSMP patients received adjuvant treatment. Specifically, 32 patients (13%) were treated with platinum-based chemotherapy (76%). In 29 patients, chemotherapy was associated with radiotherapy, in 65 (26%) with external radiotherapy, while in 107 (42%) with brachytherapy. Considering risk classes, 1 patient in the LR (1%), 9 patients in the IR (15%), and 24 patients (60%) in the HIR groups were treated with radiotherapy. The combination of chemotherapy and radiotherapy was the treatment choice in HR patients (89%) and in the advanced setting (60%). Targeted therapy was not administered as patients were treated according to the ESMO–ESGO guidelines, and no approved or accessible conditions (such as trial eligibility or expanded access program availability) allowed for its use.
Table 1.
Clinical and pathological characteristics
| Characteristics | All cases |
NSMP |
|---|---|---|
| N = 596 | N = 253 | |
| Mean age at diagnosis, years | 62 (27-89) | 62 (27-89) |
| BMI, kg/m2 | 28.6 (16.5-64.4) | 30.1 (17.7-64.4) |
| Immunohistochemistry | ||
| ER | ||
| Negative (<10%) | 96/595 (16.1) | 14/253 (5.5) |
| Positive (≥10%) | 499/595 (83.9) | 239/253 (94.5) |
| PR | ||
| Negative | 96 (16.1) | 17 (6.7) |
| 1+ | 63 (10.6) | 22 (8.7) |
| 2+ | 173 (29.0) | 63 (24.9) |
| 3+ | 264 (44.3) | 151 (59.7) |
| p53 | ||
| Wild-type | 498/594 (83.8) | 252/252 (100.0) |
| Aberrant | 96/594 (16.2) | 0/252 (0.0) |
| Mismatch repair (MMR) | ||
| Proficient | 353/573 (61.6) | 234/234 (100.0) |
| Deficient | 220/573 (38.4) | 0/234 (0.0) |
| POLE | ||
| Wild-type | 535 (89.8) | 253 (100.0) |
| Mutated | 61 (10.2) | 0 (0.0) |
| Prognostic risk class | ||
| Low | 237/579 (40.9) | 121/253 (47.8) |
| Intermediate | 104/579 (18.0) | 60/253 (23.7) |
| Intermediate-high | 104/579 (18.0) | 40/253 (15.8) |
| High | 106/579 (18.3) | 27/253 (10.7) |
| Advanced metastatic | 28/579 (4.8) | 5/253 (2.0) |
| Histotype | ||
| Endometrioid G1-2 | 372 (62.4) | 217 (85.8) |
| Endometrioid G3 | 117 (19.6) | 28 (11.1) |
| Serous | 46 (7.7) | 1 (0.4) |
| Clear cell | 9 (1.5) | 1 (0.4) |
| Mixed | 34 (5.7) | 4 (1.6) |
| Dedifferentiated | 6 (1.0) | 1 (0.4) |
| Carcinosarcoma | 12 (2.0) | 1 (0.4) |
| Grading | ||
| 1 | 57 (9.6) | 42 (16.6) |
| 2 | 315 (52.9) | 175 (69.2) |
| 3 | 224 (37.6) | 36 (14.2) |
| 2023 FIGO pathological stage | ||
| IA1 | 32 (5.4) | 19 (7.5) |
| IA2 | 150 (25.2) | 101 (39.9) |
| IA3 | 2 (0.3) | 1 (0.4) |
| IAmPOLEmut | 57 (9.6) | 0 (0.0) |
| IB | 74 (12.4) | 51 (20.2) |
| IIA | 25 (4.2) | 14 (5.5) |
| IIB | 25 (4.2) | 11 (4.3) |
| IIC | 82 (13.8) | 28 (11.1) |
| IICp53abn | 60 (10.1) | 2 (0.8) |
| IIIA1 | 11 (1.8) | 3 (1.2) |
| IIIA2 | 2 (0.3) | 0 (0.0) |
| IIIB1 | 5 (0.8) | 1 (0.4) |
| IIIB2 | 2 (0.3) | 1 (0.4) |
| IIIC1i | 21 (3.5) | 16 (6.3) |
| IIIC1ii | 20 (3.4) | 3 (1.2) |
| IIIC2ii | 3 (0.5) | 0 (0.0) |
| IVA | 2 (0.3) | 0 (0.0) |
| IVB | 16 (2.7) | 1 (0.4) |
| IVC | 7 (1.2) | 1 (0.4) |
| LVSI | ||
| Not availablea | 14 (2.3) | 3 (1.2) |
| Negative | 369 (61.9) | 183 (72.3) |
| Focal | 87 (14.6) | 34 (13.4) |
| Substantial | 126 (21.1) | 33 (13.0) |
| Recurrence or progression during treatment | 31/596 (5.2) | 5/253 (2.0) |
| Progression | 10/31 (32.3) | 0/5 (0.0) |
| Recurrence | 21/31 (67.7) | 5/5 (100.0) |
| Cancer-specific death | 14/596 (2.3) | 2/253 (0.8) |
Results are given as n/N (%) and as median (min-max) as appropriate. For categorical variables, denominators could differ due to missing data.
BMI, body mass index; ER, estrogen receptor; FIGO, International Federation of Gynecology and Obstetrics; LVSI, lymphovascular space invasion; NSMP, No Specific Molecular Profile; POLE, DNA polymerase epsilon; PR, progesterone receptor.
In 12 cases only biopsy was carried out.
At data cut-off (1 June 2025), four patients died of cancer disease (2%), with six patients (2%) experiencing a RFS or a PFS event. Of note, one of the patients was categorized as LR. The median follow-up duration was 25.5 months (95% CI 23.5-26.5 months) (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2025.105755).
Genomic landscape
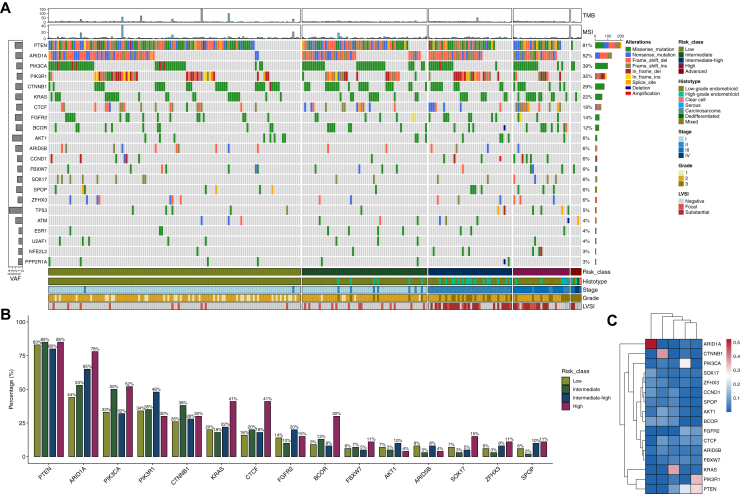
Molecular findings from the overall NSMP cohort, including oncogenic/likely oncogenic variants, are shown in Figure 2A. Two hundred and fifty-one patients (99%) had at least one oncogenic/likely oncogenic variant. The most frequently altered genes were: PTEN (n = 206, 81%, 95% CI 76% to 86%), ARID1A (n = 132, 52%, 95% CI 46% to 58%), PIK3CA (n = 99, 39%, 95% CI 33% to 45%), PIK3R1 (n = 89, 35%, 95% CI 29% to 41%), and CTNNB1 (n = 73, 29%, 95% CI 23% to 35%). A KRAS variant was found in 22% (95% CI 17% to 28%, n = 56) of patients, mainly in the G12 hotspot (n = 45, 80% of KRAS-mutated patients). TP53 alterations were found in 5% (95% CI 2% to 8%, n = 12) of the entire population.
Figure 2.
Genomic characteristics in the overall cohort stratified by risk groups. (A) Oncoplot displaying variants in the most frequently mutated genes and clinical features. Bar chart on the left represents the mean VAF. When a gene had multiple mutations, variants were prioritized in the following order: frameshift deletions or frameshift insertions, nonsense mutations, splice sites, in-frame deletions or in-frame insertions, missense mutations. (B) Bar plot illustrating the percentage of patients harboring mutations in the most frequently mutated genes, stratified by risk classification. (C) Heatmap of the GO-smoothed mutation matrix. Rows represent genes and columns represent NMF-derived modules, with hierarchical clustering. The color scale indicates the intensity of the smoothed mutation signal for each gene–module pair, with blue reflecting low and red indicating high mutation signal. GO, Gene Ontology; LVSI, lymphovascular space invasion; MSI, microsatellite instability; NMF, non-negative matrix factorization; TMB, tumor mutational burden; VAF, variant allele frequency.
PTEN and ARID1A mutations were the more prevalent alterations regardless of the risk subgroup (83% and 44% in LR, 85% and 53% in IR, 80% and 65% in HIR, and 85% and 78% in HR, respectively; Figure 2B). A statistically significant trend in mutation frequency across risk groups was observed for ARID1A (BH-adjusted P value = 0.005, Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2025.105755). Additionally, a numerical increase in mutation frequency in the high-risk group was noted for BCOR, CTCF, and KRAS (respectively, 30%, 41%, and 41%). These trends did not retain statistical significance after multiple testing correction. ER-negative patients (n = 14, 6%), compared with ER-positive patients (n = 239, 94%), more frequently exhibited alterations in BCOR (29% versus 11%, BH-adjusted P value 0.008), KRAS (43% versus 21%, adjusted P value = 0.005), and TP53 (14% versus 4%, adjusted P value = 0.048). FBXW7 showed a non-significant difference (14% versus 6%, adjusted P value = 0.15). Conversely, PTEN (85% versus 43%, adjusted P value = 1 × 10−8) and CTNNB1 (30% versus 7%, adjusted P value = 0.0002) alterations were more frequently found in ER-positive patients (Supplementary Figure S2A and B, available at https://doi.org/10.1016/j.esmoop.2025.105755). The median TMB in the overall cohort was 8.0 mut/Mb, while the median MSI value was 2.9% (Supplementary Figure S3A and B, available at https://doi.org/10.1016/j.esmoop.2025.105755).
Mutually exclusive genes were PIK3CA with PIK3R1 (odds ratio [OR] = 0.07, adjusted P value = 4.25 × 10−14), KRAS with CTNNB1 (OR = 0.03, adjusted P value = 1.98 × 10−7) and FGFR2 (OR = 0.09, adjusted P value = 0.03), and AKT1 with PTEN (OR = 0.06, adjusted P value = 5.73 × 10−6). Significant co-occurrence was found between PTEN and ARID1A (OR = 6.94, adjusted P value = 3.39 × 10−6) and PTEN with PIK3R1 (OR = 4.36, adjusted P value = 0.009) (Supplementary Figure S4, available at https://doi.org/10.1016/j.esmoop.2025.105755). Co-occurrence between PTEN and PIK3CA (P value = 0.03) did not retain statistical significance after multiple testing correction. With respect to risk subgroups, BCOR and PIK3CA (adjusted P value = 0.095) in the IR group, as well as CTNNB1 and KRAS in both the IR (adjusted P value = 0.094) and LR groups (P = 0.009), were identified as mutually exclusive genes. PTEN co-occurrence with PIK3CA and ARID1A and PIK3CA and PIK3R1 co-exclusivity were consistent across all class risks (Supplementary Figure S5A-D, available at https://doi.org/10.1016/j.esmoop.2025.105755).
After GO-guided network smoothing and NMF-based clustering, we observed optimal clustering stability around rank 5 (Supplementary Figure S6, available at https://doi.org/10.1016/j.esmoop.2025.105755). In the mutation matrix (Figure 2C), distinct gene modules emerged, including PTEN–PIK3R1, PIK3CA, KRAS, CTNNB1, and ARID1A. These modules suggest the presence of at least four underlying biological processes driving tumor heterogeneity: phosphatidylinositol-3 kinase (PI3K) signaling, mitogen-activated protein kinase cascade activation, WNT/β-catenin signaling, and SWI/SNF-mediated chromatin deregulation.
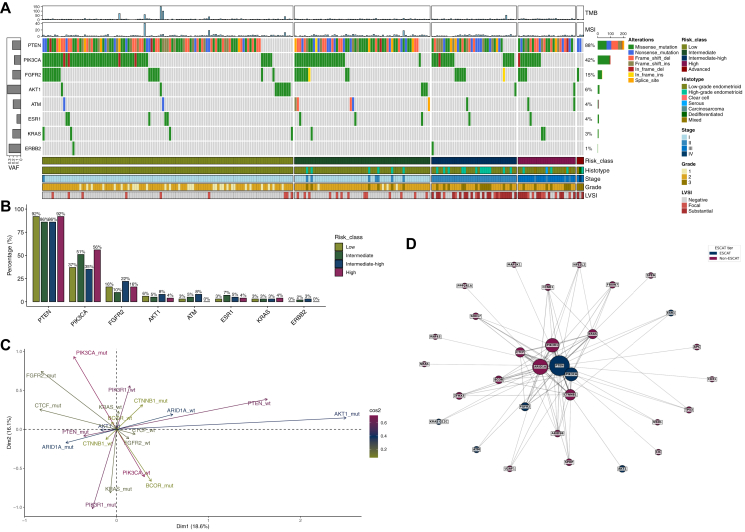
Genomic landscape under the light of ESCAT framework
Out of 253 patients, 233 (92%, 95% CI 88% to 95%) were found to have at least one ESCAT tier I-III alteration. No ESCAT tier IA-B alterations were found. The more frequent variants were in PTEN (n = 206, 88%, 95% CI 84% to 92%), PIK3CA (n = 99, 42%, 95% CI 36% to 49%), FGFR2 (n = 35, 15%, 95% CI 11% to 20%), and AKT1 (n = 14, 6%, 95% CI 3% to 10%) (Figure 3A); 4% (95% CI 2% to 8%, n = 10) of patients had an ESR1 variant. PTEN, PIK3CA, and FGFR2 were the most frequently altered genes regardless of risk class (Figure 3B). In the advanced/metastatic setting, PIK3CA accounted for 40% of alterations, followed by ATM (20%) and PTEN (20%).
Figure 3.
Genomic characteristics according to ESCAT classification. (A) Oncoplot displaying variants in the most frequently mutated genes and clinical features. Bar chart on the left represents the mean VAF. When a gene had multiple mutations, variants were prioritized in the following order: frameshift deletions or frameshift insertions, nonsense mutations, splice sites, in-frame deletions or in-frame insertions, missense mutations. (B) Bar plot illustrating the percentage of patients harboring mutations in the most frequently mutated genes, stratified by risk classification. (C) Multiple correspondence analysis of somatic mutation patterns. The color of the labels and arrows reflects the quality of representation (cos2) of each mutation state (wild-type versus mutated) on the MCA dimensions, while the length of the arrow from the origin indicates the contribution to tumor discrimination along these dimensions, with longer vectors representing mutation states that explain greater variance. (D) Co-occurrence network of ESCAT and non-ESCAT genes, mutated in at least five patients. Edge thickness represents co-mutation frequency, while node size corresponds to mutation frequency. ESCAT and non-ESCAT genes are color coded. Layout: Fruchterman–Reingold (k = 3). ESCAT, European Society for Medical Oncology Scale for Clinical Actionability of molecular Targets; LVSI, lymphovascular space invasion; MCA, multiple correspondence analysis; MSI, microsatellite instability; mut, mutated; TMB, tumor mutational burden; VAF, variant allele frequency; wt, wild-type.
We next focused on the interplay between ESCAT genes and non-ESCAT genes. In MCA of mutation patterns, PTEN, PIK3CA, PIK3R1, and AKT1 were the principal contributors to sample separation in the first two dimensions (34.7% of total variance) (Figure 3C and Supplementary Figure S7, available at https://doi.org/10.1016/j.esmoop.2025.105755). Tumors harboring PTEN and PIK3CA localized to distinct regions of the MCA space, while the PTEN wild-type and AKT1-mutated tumors showed a tendency to cluster together. In the co-mutation network (Figure 3D), we observed distinct connectivity patterns: the main ESCAT hubs were PTEN [eigenvector centrality (EvC): 0.563] and PIK3CA (EvC: 0.356), while the main non-ESCAT hubs were ARID1A (EvC: 0.484), PIK3R1 (EvC: 0.33), KRAS nonG12C (EvC: 0.176), and CTNNB1 (EvC: 0.260). On the other hand, ESCAT genes such as ESR1, AKT1, ATM, and KRAS G12C were peripheral nodes, with lower connectivity and centrality scores (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2025.105755).
Analysis of ESCAT variants
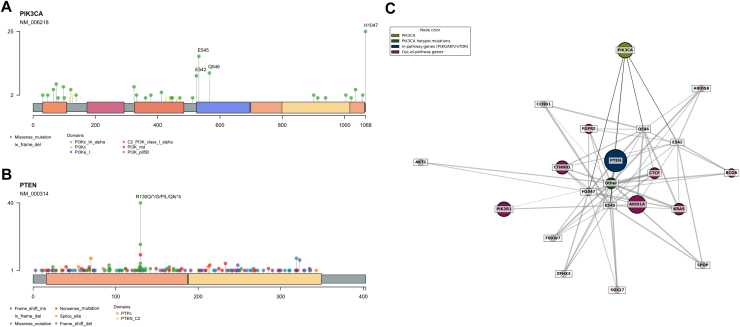
The majority of PTEN variants were on the R130 residue: R130G (n = 40, 17%), R130Q (n = 17, 7%), and R130∗ (n = 10, 4.3%) (Figure 4A). The most frequently altered PIK3CA hotspots were H1047R (n = 21, 9%), E545D/K/Q/A (n = 13, 6%), and E542K (n = 9, 4%) (Figure 4B). The KRAS G12 was the most frequently altered (n = 19, 8%), and a KRAS G12C mutation was found in 3% (95% CI 1% to 6%, n = 7) of patients (Supplementary Figure S8A, available at https://doi.org/10.1016/j.esmoop.2025.105755). The most frequent FGFR2 hotspot was S252W (n = 16, 7%), almost all (n = 15, 6%) AKT1 mutations were E17K, and ESR1 hotspots were S463P (n = 4, 2%) and L536P (n = 3, 1%) (Supplementary Figure S8B-D, available at https://doi.org/10.1016/j.esmoop.2025.105755).
Figure 4.
ESCAT gene hotspots and PIK3CA co-mutation network. (A, B) Lollipop plots for PTEN (A) and PIK3CA (B). The x-axis represents the aminoacidic position, while the y-axis indicates the frequency of each variant. The most frequent hotspot mutations are highlighted. (C) Co-occurrence network of PIK3CA mutations. Nodes represent genes, with PIK3CA and its hotspot mutations (Q546, H1047, E545, E542) highlighted. Genes within the PI3K/AKT/mTOR pathway (in-pathway) and genes from other pathways (out-of-pathway) are color coded. Edge thickness represents co-mutation frequency, while node size corresponds to mutation frequency. Layout: spring. ESCAT, European Society for Medical Oncology Scale for Clinical Actionability of molecular Targets; mTOR, mammalian target of rapamycin.
Considering all variants, an alteration in the PI3K pathway was found in 236 (94%) patients, followed by RTK-RAS (n = 111, 44%) and WNT (n = 84, 33%) (Supplementary Figure S9A, available at https://doi.org/10.1016/j.esmoop.2025.105755). Focusing on ESCAT tiers I-III, an alteration in at least one of the PI3K pathway genes was found in 229 (98%) patients (Supplementary Figure S9B, available at https://doi.org/10.1016/j.esmoop.2025.105755).
In the PIK3CA network analysis (Figure 4C), PIK3CA hotspots exhibited possible distinct co-mutation patterns. Among genes within the PI3K/AKT/mTOR pathway, PTEN co-mutation rates ranged from 79% to 92%, according to PIK3CA hotspot. In contrast, AKT1 co-mutation frequency was generally low, occurring primarily with the H1047 hotspot (4%). Among out-of-pathway genes, 71% of patients with a variant in the E545 hotspot also had an ARID1A mutation, compared with 55% of those with an H1047 hotspot. Similarly, CTNNB1 was co-altered in 50% of patients with the H1047 hotspot variant, compared with 36% in the E545 hotspot cases. CTCF was co-altered with Q546 in 56% of patients, whereas the percentage was <30% for other PIK3CA hotspots (Supplementary Figure S10, available at https://doi.org/10.1016/j.esmoop.2025.105755).
Discussion
This study provides a descriptive analysis of actionable mutations based on the ESCAT framework in a large single-center cohort of prospectively sequenced NSMP EC patients. Consistently with literature data, most NSMP patients in this cohort were LR, ER-positive, and had PTEN alterations (81% of the entire NSMP population and 88% among those with at least one ESCAT variant).23 Other frequent ESCAT tier III alterations primarily involved the PIK3CA/AKT/mTOR pathway including PIK3CA (39% overall) and AKT1 (6% overall). Notably, the ER-negative population showed differences in the rate of TP53, BCOR, and FBXW7 alterations, suggesting a more aggressive phenotype in accordance with already published data.24, 25, 26
PTEN mutations are highly prevalent in endometrioid EC, associated with early-stage, low-grade disease and hence a good prognosis.27 In 2022, the Memorial Sloan Kettering research group analyzed 472 NSMP patients (endometrioid and non-endometrioid), identifying three genomic clusters: (1) PTEN and PIK3R1 mutations; (2) PTEN and PIK3CA mutations; (3) chromosome 1q high-level gain without PTEN mutation.28 Cluster 3 primarily consisted of patients with a poor prognosis, characterized by predominantly non-endometrioid tumors, advanced-stage disease, negative or weak ER expression, and low differentiation (grade 3). Conversely, cluster 1 patients were associated with a good prognosis, with the most frequently mutated genes being PTEN (64%), ARID1A (45%), PIK3CA (42.5%), CTNNB1 (36%), PIK3R1 (32%), and KRAS (29%).
In 2023, Jamieson et al. published a series of 1110 NSMPs characterized through a small NGS panel including CTNNB1, KRAS, and PI3KCA.5 The study found that patients with hormone receptor-negative IHC expression (<1%), L1CAM IHC overexpression, and PIK3CA alteration were associated with a shorter disease-specific survival. Notably, ∼84% of NSMP patients were classified as very low risk (low-grade, ER-positive tumors), with a 5-year disease-specific mortality rate of only 1.6%, supporting the potential for treatment de-escalation and a more conservative surgical approach.
From a therapeutic standpoint, our results suggest that NSMP patients have two potential treatment options: hormonal therapy (HT) and PI3K/AKT/mTOR pathway inhibitors. HT use is currently limited to young patients with FIGO stage I disease seeking fertility preservation, medically unfit older patients, and as palliative treatment for advanced or recurrent EC. In the setting of advanced and recurrent EC, recent reviews and meta-analyses confirmed a response to progestin treatment of 30% in unselected patients even though only low-quality data were available.29, 30, 31 The lack of validation for EC-specific cut-off values for ER/progesterone receptor (PR) positivity by IHC, which are instead adopted from breast cancer, limits the widespread clinical use of ER/PR information.14 The efficacy of adjuvant HT in NSMP advanced EC patients is currently under investigation in a randomized trial (ClinicalTrials.gov identifier: NCT05255653). However, incorporating tests that provide deeper and dynamic insights into ER/PR pathway activity is strongly recommended to optimize patients’ selection and identify potential good responders. Moreover, the loss of PR at recurrence has been frequently reported, making relevant the acquisition of a subsequent tissue evaluation when HT is considered.32,33 Few data are available concerning the impact of activating mutations in ESR1 in this context. In 2019, Gaillard et al. published data from a large cohort of molecular-profiled gynecological cancer: ESR1 alterations accounted for 2% in the overall EC cohort and for 4.4% in the endometrioid histotype. Clinical findings were available only for two patients, previously exposed to HT and who benefitted from selective ER modulators and selective ER degrader therapies.34
Regarding PI3K/AKT/mTOR pathway inhibitors, our data suggest that nearly half of NSMP patients could potentially benefit from targeted inhibition of this pathway. However, considering the significant co-occurrence found between PTEN and PIK3CA and ARID1A regardless of the risk classes, the strength of actionability of these alterations need to be further investigated and validated. While data remain limited, it is important to acknowledge that co-alterations involving PIK3CA and PTEN or AKT1 (particularly E17K) could restore signaling axis function, potentially diminishing the efficacy of PIK3CA-targeted therapies.35,36 Such compensatory mechanisms might lead to primary or adaptive resistance, underscoring the need for combination treatment strategies. Furthermore, our findings suggest how different PIK3CA hotspots exhibit distinct co-mutation profiles, suggesting that not all PIK3CA-altered tumors may respond similarly to pathway inhibition.
Available data from a pre-treated EC cohort (47% of the total population) receiving the PIK3CA inhibitor alpelisib showed the highest overall response rate (35% versus 28% in the overall population).37 H1047R and E545K alterations were the most common mutations. No correlation with loss of PTEN and a controversial correlation with its mutation was reported. The EMBER trial (NCT04188548) is a phase I trial evaluating imlunestrant, a next-generation oral selective ER, as monotherapy and in combination with anticancer therapies, including everolimus (mTORC1 inhibitor), for patients with ER-positive advanced/metastatic EC progressed after platinum-containing chemotherapy. Within a phase I/II trial, CPI-0209, an EZH2 inhibitor, is under investigation in patients with advanced solid tumors, including pre-treated EC (NCT04104776); NGS detection of ARID1A alteration is mandatory. Future clinical trials should preliminarily explore specific co-mutation patterns to tailor therapeutic approaches while avoiding clinical and financial toxicities.
Furthermore, there is growing interest in combining AKT1 and PI3K inhibitors with HT due to the interplay between hormone signaling and these intracellular pathways.38 The combination of the PIK3CA inhibitor alpelisib and letrozole is under evaluation within the phase II randomized ENGOT-EN19/NSGO-CTU/ALPACA clinical trial. The study enrolls patients with PIK3CA-mutated, grade 1-2 endometrioid adenocarcinoma relapsed after first-line systemic therapy. Patients are randomized to receive letrozole alone (arm A) or letrozole plus alpelisib (arm B). Primary endpoint is investigator-assessed PFS. Within the ongoing phase II GOG-3069 trial (NCT05154487), ER-positive (>1%) PIK3CA-mutated endometrioid EC patients who underwent no more than three lines of prior chemotherapy are treated with alpelisib and fulvestrant (an ER agonist). The primary endpoint is objective response rate (ORR). An overlapping population has been investigated within another phase II trial including patients with hormone receptor-positive endometrial, breast, and ovarian cancers (>10%) harboring alterations that activate the PI3K pathway (presence of one or more PI3K and/or PTEN alterations in the tumor tissue; genetic alterations including PIK3CA gain-of-function mutations, PIK3R1 loss-of-function mutations, PTEN loss-of-function mutations, and PTEN deletions). Patients underwent oral therapy with the combination of fulvestrant and copanlisib, a PI3K-α and PI3K-δ inhibitor. Primary endpoints were safety, ORR, PFS, and overall survival. A 2023 systematic review and meta-analysis found no significant prognostic impact of PIK3CA mutations in EC, particularly in low-grade cases. However, mutations in exon 9 were linked to reduced survival and are highly concurrent with KRAS mutations, while mutations in exon 20 did not show a significant impact on survival.39
Several limitations should be acknowledged. This was a monocentric study with a relatively small patient cohort. Additionally, the absence of clinical data on targeted treatment responses to support the generated hypotheses, along with the short follow-up period, limits the ability to establish prognostic correlations with genomic findings. Longer follow-up will be crucial to robustly evaluate the survival impact of specific molecular features.
Conclusions
Our findings confirm the high molecular heterogeneity of NSMP EC patients, revealing that the vast majority harbor at least one potentially actionable alteration based on the ESCAT framework. Although the precise biological role of specific mutations and co-alterations remains to be clarified, these results support the exploration of tailored, molecular-matched therapies according to risk groups.
Acknowledgements
Ministry of Health—Ricerca Corrente 2025 MoH—Rc2025. We express our profound gratitude to Prof. Giovanni Scambia for his teachings and inspiration.
Funding
None declared.
Disclosure
CN declares travel support from MSD, Illumina, Menarini, and AZ; and honoraria from Veeva, GSK, MSD, AZ, Altems, Illumina, and Guardant Health. GS reports research support from MSD and honoraria from Clovis Oncology; and consultant for Tesaro and Johnson & Johnson. FF reports research supports for MSD, GSK, SYSMEX, and STRYKER; honoraria from SYSMEX, MSD, GSK, and STRYKER; and consultant for Clovis Oncology, GSK, MSD, and PharmaMar. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Levine D.A. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McConechy M.K., Talhouk A., Leung S., et al. Endometrial carcinomas with POLE exonuclease domain mutations have a favorable prognosis. Clini Cancer Res. 2016;22:2865–2873. doi: 10.1158/1078-0432.CCR-15-2233. [DOI] [PubMed] [Google Scholar]
- 3.León-Castillo A., de Boer S.M., Powell M.E., et al. Molecular classification of the PORTEC-3 trial for high-risk endometrial cancer: impact on prognosis and benefit from adjuvant therapy. J Clin Oncol. 2020;38:3388–3397. doi: 10.1200/JCO.20.00549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jamieson A., Thompson E.F., Huvila J., Gilks C.B., McAlpine J.N. p53abn Endometrial cancer: understanding the most aggressive endometrial cancers in the era of molecular classification. Int J Gynecol Cancer. 2021;31:907–913. doi: 10.1136/ijgc-2020-002256. [DOI] [PubMed] [Google Scholar]
- 5.Jamieson A., Huvila J., Chiu D., et al. Grade and estrogen receptor expression identify a subset of no specific molecular profile endometrial carcinomas at a very low risk of disease-specific death. Mod Pathol. 2023;36 doi: 10.1016/j.modpat.2022.100085. [DOI] [PubMed] [Google Scholar]
- 6.McAlpine J., Leon-Castillo A., Bosse T. The rise of a novel classification system for endometrial carcinoma; integration of molecular subclasses. J Pathol. 2018;244:538–549. doi: 10.1002/path.5034. [DOI] [PubMed] [Google Scholar]
- 7.Mirza M.R., Chase D.M., Slomovitz B.M., et al. Dostarlimab for primary advanced or recurrent endometrial cancer. N Engl J Med. 2023;388:2145–2158. doi: 10.1056/NEJMoa2216334. [DOI] [PubMed] [Google Scholar]
- 8.Eskander R.N., Sill M.W., Beffa L., et al. Pembrolizumab plus chemotherapy in advanced endometrial cancer. N Engl J Med. 2023;388:2159–2170. doi: 10.1056/NEJMoa2302312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fader A.N., Roque D.M., Siegel E., et al. Randomized phase II trial of carboplatin–paclitaxel compared with carboplatin–paclitaxel–trastuzumab in advanced (stage III–IV) or recurrent uterine serous carcinomas that overexpress Her2/Neu ( NCT01367002): updated overall survival analysis. Clin Cancer Res. 2020;26:3928–3935. doi: 10.1158/1078-0432.CCR-20-0953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mateo J., Chakravarty D., Dienstmann R., et al. A framework to rank genomic alterations as targets for cancer precision medicine: the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT) Ann Oncol. 2018;29:1895–1902. doi: 10.1093/annonc/mdy263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosele M.F., Westphalen C.B., Stenzinger A., et al. Recommendations for the use of next-generation sequencing (NGS) for patients with advanced cancer in 2024: a report from the ESMO Precision Medicine Working Group. Ann Oncol. 2024;35:588–606. doi: 10.1016/j.annonc.2024.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Nero C., Duranti S., Giacomini F., et al. Integrating a comprehensive cancer genome profiling into clinical practice: a blueprint in an Italian referral center. J Pers Med. 2022;12:1746. doi: 10.3390/jpm12101746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kommoss S., McConechy M.K., Kommoss F., et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann Oncol. 2018;29:1180–1188. doi: 10.1093/annonc/mdy058. [DOI] [PubMed] [Google Scholar]
- 14.Perrone E., Capasso I., De Felice F., et al. Back to the future: the impact of oestrogen receptor profile in the era of molecular endometrial cancer classification. Eur J Cancer. 2023;186:98–112. doi: 10.1016/j.ejca.2023.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Concin N., Matias-Guiu X., Vergote I., et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer. 2021;31:12–39. doi: 10.1136/ijgc-2020-002230. [DOI] [PubMed] [Google Scholar]
- 16.Nero C., Trozzi R., Persiani F., et al. POLE mutations in endometrial carcinoma: clinical and genomic landscape from a large prospective single-center cohort. Cancer. 2025;131 doi: 10.1002/cncr.35731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vitale A., Mastrantoni L., Russo J., et al. Impact of comprehensive genome profiling on the management of advanced non-small cell lung cancer: preliminary results from the lung cancer cohort of the FPG500 program. JCO Precis Oncol. 2024;8 doi: 10.1200/PO.24.00297. [DOI] [PubMed] [Google Scholar]
- 18.McLaren W., Gil L., Hunt S.E., et al. The Ensembl Variant Effect Predictor. Genome Biol. 2016;17:122. doi: 10.1186/s13059-016-0974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chakravarty D., Gao J., Phillips S., et al. OncoKB: a precision oncology knowledge base. JCO Precis Oncol. 2017;1:1–16. doi: 10.1200/PO.17.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camarda F., Mastrantoni L., Parrillo C., et al. Actionable mutations in early-stage ovarian cancer according to the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT): a descriptive analysis on a large prospective cohort. ESMO Open. 2025;10 doi: 10.1016/j.esmoop.2024.104090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayakonda A., Lin D.-C., Assenov Y., Plass C., Koeffler H.P. Maftools: efficient and comprehensive analysis of somatic variants in cancer. Genome Res. 2018;28:1747–1756. doi: 10.1101/gr.239244.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchez-Vega F., Mina M., Armenia J., et al. Oncogenic signaling pathways in The Cancer Genome Atlas. Cell. 2018;173:321–337.e10. doi: 10.1016/j.cell.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Djordjevic B., Hennessy B.T., Li J., et al. Clinical assessment of PTEN loss in endometrial carcinoma: immunohistochemistry outperforms gene sequencing. Mod Pathol. 2012;25:699–708. doi: 10.1038/modpathol.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urick M.E., Yu E., Bell D.W. High-risk endometrial cancer proteomic profiling reveals that FBXW7 mutation alters L1CAM and TGM2 protein levels. Cancer. 2021;127:2905–2915. doi: 10.1002/cncr.33567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiang S., Lee C.-H., Stewart C.J.R., et al. BCOR is a robust diagnostic immunohistochemical marker of genetically diverse high-grade endometrial stromal sarcoma, including tumors exhibiting variant morphology. Mod Pathol. 2017;30:1251–1261. doi: 10.1038/modpathol.2017.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schultheis A.M., Martelotto L.G., De Filippo M.R., et al. TP53 mutational spectrum in endometrioid and serous endometrial cancers. Int J Gynecol Pathol. 2016;35:289–300. doi: 10.1097/PGP.0000000000000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mutter G.L., Baak J.P.A., Crum C.P., Richart R.M., Ferenczy A., Faquin W.C. Endometrial precancer diagnosis by histopathology, clonal analysis, and computerized morphometry. J Pathol. 2000;190:462–469. doi: 10.1002/(SICI)1096-9896(200003)190:4<462::AID-PATH590>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 28.Momeni-Boroujeni A., Nguyen B., Vanderbilt C.M., et al. Genomic landscape of endometrial carcinomas of no specific molecular profile. Mod Pathol. 2022;35:1269–1278. doi: 10.1038/s41379-022-01066-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Weelden W.J., Birkendahl P.B., Lalisang R.I., et al. The effect of progestin therapy in advanced and recurrent endometrial cancer: a systematic review and meta-analysis. BJOG. 2023;130:143–152. doi: 10.1111/1471-0528.17331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jerzak K.J., Duska L., MacKay H.J. Endocrine therapy in endometrial cancer: an old dog with new tricks. Gynecol Oncol. 2019;153:175–183. doi: 10.1016/j.ygyno.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 31.Ethier J.-L., Desautels D.N., Amir E., MacKay H. Is hormonal therapy effective in advanced endometrial cancer? A systematic review and meta-analysis. Gynecol Oncol. 2017;147:158–166. doi: 10.1016/j.ygyno.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Asaka S., Liu Y., Yu Z.-C., et al. ARID1A regulates progesterone receptor expression in early endometrial endometrioid carcinoma pathogenesis. Mod Pathol. 2023;36 doi: 10.1016/j.modpat.2022.100045. [DOI] [PubMed] [Google Scholar]
- 33.Tangen I.L., Werner H.M.J., Berg A., et al. Loss of progesterone receptor links to high proliferation and increases from primary to metastatic endometrial cancer lesions. Eur J Cancer. 2014;50:3003–3010. doi: 10.1016/j.ejca.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Gaillard S.L., Andreano K.J., Gay L.M., et al. Constitutively active ESR1 mutations in gynecologic malignancies and clinical response to estrogen-receptor directed therapies. Gynecol Oncol. 2019;154:199–206. doi: 10.1016/j.ygyno.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Varkaris A., Fece de la Cruz F., Martin E.E., et al. Allosteric PI3Kα inhibition overcomes on-target resistance to orthosteric inhibitors mediated by secondary PIK3CA mutations. Cancer Discov. 2024;14:227–239. doi: 10.1158/2159-8290.CD-23-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beaver J.A., Gustin J.P., Yi K.H., et al. PIK3CA and AKT1 mutations have distinct effects on sensitivity to targeted pathway inhibitors in an isogenic luminal breast cancer model system. Clin Cancer Res. 2013;19:5413–5422. doi: 10.1158/1078-0432.CCR-13-0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Passarelli A., Carbone V., Pignata S., et al. Alpelisib for PIK3CA-mutated advanced gynecological cancers: first clues of clinical activity. Gynecol Oncol. 2024;183:61–67. doi: 10.1016/j.ygyno.2024.02.029. [DOI] [PubMed] [Google Scholar]
- 38.Momeni-Boroujeni A., Yousefi E., Balakrishnan R., et al. Molecular-based immunohistochemical algorithm for uterine leiomyosarcoma diagnosis. Mod Pathol. 2023;36 doi: 10.1016/j.modpat.2022.100084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bredin H.K., Krakstad C., Hoivik E.A. PIK3CA mutations and their impact on survival outcomes of patients with endometrial cancer: a systematic review and meta-analysis. PLoS One. 2023;18 doi: 10.1371/journal.pone.0283203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.