Abstract
Intestinal diseases, particularly inflammatory bowel disease (IBD) and colorectal cancer (CRC), are increasingly prevalent and difficult to manage due to complex pathology, poor drug targeting, and systemic toxicity. Albumin nanoparticles (ANPs) have emerged as a promising drug delivery platform, offering biocompatibility, controlled degradation, and targeting potential. This review summarizes advances in ANP-based therapies for intestinal diseases, briefly outlining key fabrication approaches and focusing on targeting strategies that exploit pathological features such as leaky vasculature, acidic pH, and oxidative stress. ANPs can also be modified for active targeting via receptor-mediated mechanisms. Preclinical studies demonstrate that drug-loaded ANPs enhance local drug accumulation, suppress inflammation, and improve therapeutic efficacy in IBD and CRC models. In addition, the review addresses potential toxicity concerns related to crosslinkers and drug-albumin interactions. Despite ongoing challenges in oral bioavailability, mucus penetration, and scale-up, ANPs represent a promising avenue for precision treatment of intestinal diseases.
Keywords: Albumin nanoparticles, Intestinal diseases, Targeted drug delivery, Inflammatory bowel disease, Colorectal cancer, Nanoparticle toxicity, Precision medicine
Graphical abstract
Highlights
-
•
Various fabrication strategies enable tunable size, stability, and drug encapsulation of ANPs.
-
•
ANPs achieve targeted and controlled drug release by leveraging pathological features of intestinal diseases.
-
•
Preclinical studies confirm that ANPs improve drug retention and therapeutic efficacy in intestinal diseases.
-
•
Toxicity assessments reveal that ANP safety depends on crosslinkers, drug compatibility, and in vivo behavior.
1. Introduction
The intestine, a vital digestive organ, not only facilitates the absorption of nutrients and water but also protects systemic health by blocking the entry of pathogens and harmful substances. Recent studies have identified high-saturated-fat diets (Mana et al., 2021) and worsening air pollution (Vignal et al., 2021) as major risk factors contributing to the rising global incidence of intestinal diseases. Epidemiological data reveal that gastrointestinal diseases claim approximately 8 million lives worldwide each year (Abuhayi et al., 2024). Furthermore, according to the World Health Organization's Global Cancer Observatory (GLOBOCAN), gastrointestinal malignancies, including colorectal, gastric, and esophageal cancers, accounted for 17.1 % of new cancer diagnoses globally in 2022, with a disproportionately high mortality rate of 20.7 % (Bray et al., 2024). Etiologically, intestinal diseases are classified as inflammatory, functional, infectious, or structural. Among these, inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), and colorectal cancer (CRC) are the most prevalent clinical entities (Meng et al., 2020). These conditions impair nutrient absorption and often lead to debilitating complications such as chronic pain and anemia, thereby profoundly compromising patients' quality of life.
Current treatment strategies for intestinal diseases primarily rely on conventional pharmacotherapies, including immunosuppressants, miRNA-based therapeutics, antibiotics, and interferons, and surgical intervention when necessary (Liu et al., 2021b). However, these approaches frequently suffer from poor target specificity, systemic side effects, and short-lived efficacy (Tian et al., 2025), underscoring the urgent need for more effective and targeted therapies. Since their conceptualization in the 1950s, nanoparticle-based drug delivery systems have evolved into sophisticated platforms characterized by physicochemical stability, low cytotoxicity, precise tissue targeting, gastric acid resistance, and modifiable surfaces—traits particularly advantageous for gastrointestinal applications. Based on their origin, nanocarriers are broadly divided into synthetic (e.g., polymers, inorganic particles, carbon-based materials) and natural (e.g., proteins, liposomes) categories. Among natural carriers, protein-based nanoparticles are especially attractive due to their intrinsic biocompatibility, low immunogenicity, and predictable degradation profiles.
Albumin nanoparticles (ANPs), derived from endogenous protein sources, have gained considerable attention in nanodrug delivery. Their well-defined amino acid sequence and high lysine content enable electrostatic adsorption of both positively (e.g., ganciclovir) and negatively charged (e.g., oligonucleotide) molecules without additional carriers (Irache et al., 2005; Weber et al., 2000). Compared to synthetic carriers such as PLGA, ANPs have lower immunogenicity due to their biological origin (Mehta et al., 2023; Sivadasan et al., 2021). Notably, ANPs exploit FcRn receptor-mediated transcytosis to bypass lysosomal degradation and prolong drug retention at inflammatory sites (Lamichhane and Lee, 2020; Schmidt et al., 2017). Their targeting efficacy can be further enhanced through surface modification strategies, thereby increasing drug accumulation at lesion sites while minimizing off-target toxicity (Bhushan et al., 2017). Despite these advantages, limitations such as poor aqueous solubility, low oral bioavailability, and potential non-specific distribution remain barriers to clinical translation (Younis et al., 2022). Addressing these issues calls for hybrid engineering approaches, such as enzyme-resistant coatings and conjugation with mucosal repair peptides, to bridge the gap between localized therapeutic action and broader mucosal healing.
Several prior reviews have discussed albumin-based nanocarriers for various indications, particularly in oncology and IBD. For example, Kratz (2008) outlined three major albumin-based drug delivery strategies and their clinical relevance in tumor targeting Other reviews have focused on the use of ANPs in controlled release and site-specific cancer therapy (Elzoghby et al., 2012; Qu et al., 2024). However, these are predominantly cancer-oriented and lack depth regarding colorectal-specific pathology. Similarly, reviews on oral nanodelivery systems in IBD often cover diverse nanocarriers without focusing specifically on albumin-based platforms (Liu et al., 2021b). While some mention the advantages of ANPs in treating IBD (Hua et al., 2015), few offer in-depth discussions linking albumin nanoparticle design to distinct intestinal disease mechanisms.
In light of this gap, there is a clear need for a comprehensive review focused exclusively on the application of ANPs in intestinal diseases. Compared with previous reviews that primarily focus on cancer applications or general nanocarrier strategies, our work highlights several distinctive aspects of ANPs in the treatment of intestinal diseases. Specifically, this review is the first to comprehensively correlate ANP design strategies, such as size-dependent delivery, microenvironment responsiveness, and receptor targeting, with the pathological features of IBD, irritable IBS, and CRC. In addition, we emphasize the translational relevance of ANPs by incorporating toxicity analyses and clinical considerations, which are often overlooked in earlier literature. These unique perspectives contribute to the novelty and practical value of this review.
Therefore, we hypothesize that ANPs, through their tunable surface properties, biocompatibility, and responsiveness to pathological cues (e.g., pH, ROS, enzymes), represent a uniquely suited nanoplatform for targeted drug delivery in various intestinal diseases. This review aims to systematically evaluate their mechanisms, therapeutic potentials, safety profiles, and translational challenges to establish a comprehensive foundation for future research and clinical translation.
2. Literature search and selection
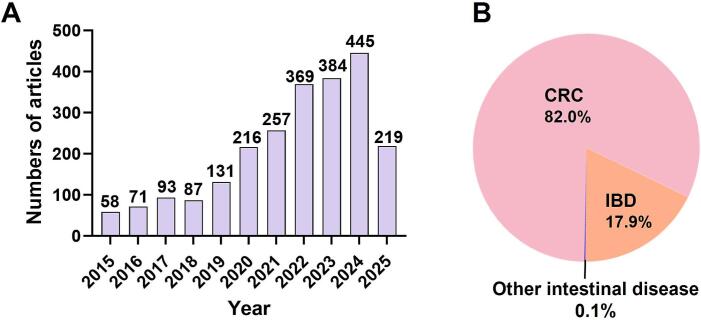
To ensure a rigorous and up-to-date foundation for this review, we conducted a systematic literature search focusing on recent advances in ANPs for intestinal disease therapy. As depicted in Fig. 1A, the annual publication trend indicates steadily increasing research interest in ANP-based drug delivery systems over the past decade. Moreover, Fig. 1B illustrates the disease-specific distribution of ANP-related studies, highlighting CRC as the most extensively investigated condition (1911 articles, ∼82 %), followed by IBD (416 articles, 17.9 %), with only 3 articles (0.1 %) addressing the other intestinal diseases. By systematically summarizing these findings, this review aims to enhance the current understanding of ANP applications in intestinal disease therapy, serve as a valuable reference for researchers, and support the clinical translation of ANP-based therapeutic strategies.
Fig. 1.
(A) Annual publication trends related to ANP-based nanoformulations from January 2015 to May 2025. (B) Proportional distribution of ANP research across different categories of intestinal diseases.
3. Types of albumin
The most frequently used albumins include human serum albumin (HSA), bovine serum albumin (BSA), ovalbumin (OVA) derived from egg whites, α-lactalbumin extracted from milk, and storage albumins sourced from plant seeds, such as legumes, amaranth, and soybeans (Visentini et al., 2023). Among these, OVA, BSA, and HSA are predominantly utilized for the fabrication of ANPs, especially for drug loading aimed at treating intestinal diseases.
3.1. Ovalbumin
OVA, accounting for approximately 54–60 % of egg white proteins, is a 45 kDa globular protein consisting of 386 amino acids (Xiong et al., 2024). Its structure features four free thiol groups and one disulfide bond, and is primarily composed of α-helices and β-sheets (Guha et al., 2019). The hydrophobic core of OVA serves as a binding site, particularly suitable for lipophilic molecules and small-molecule therapeutic agents. With an isoelectric point (pI) of approximately 4.8, OVA maintains a net negative charge at physiological pH, enabling electrostatic interactions with positively charged inflammatory mediators (Vesković et al., 2022). Furthermore, OVA exhibits pH- and temperature-sensitive characteristics, facilitating targeted drug delivery within the intestinal environment and modulating inflammatory responses (Wongsasulak et al., 2010). Despite its demonstrated therapeutic potential for intestinal conditions, clinical application of OVA-based ANPs remains relatively limited, suggesting a significant opportunity for further exploration.
3.2. Bovine serum albumin
Compared to OVA, BSA is more extensively employed in drug delivery due to its superior biocompatibility, biodegradability, and reduced immunogenicity (Elzoghby et al., 2012; Spada et al., 2021). BSA is a single-chain protein composed of 583 amino acids, with a molecular weight of approximately 66.5 kDa (Koh et al., 2019). Structurally, BSA comprises three homologous domains (I–III), each further subdivided into two subdomains (A and B) (Szymaszek et al., 2022). The primary drug-binding sites reside within domains IIA and IIIA, effectively accommodating fatty acids, pharmaceuticals, and metal ions. At physiological pH, BSA carries a net negative surface charge due to its low isoelectric point (∼4.7), which facilitates the electrostatic adsorption of positively charged inflammatory factors (Kubiak-Ossowska et al., 2019). Due to its high stability, minimal toxicity, and osmotic regulatory properties, BSA-based ANPs enhance intestinal drug retention, protect the mucosal barrier, and thus offer significant therapeutic potential in managing various intestinal diseases (Duan et al., 2022b; Yu et al., 2022).
3.3. Human serum albumin
HSA, an abundant protein found in human plasma, is often selected over BSA to minimize potential immunogenic responses in clinical applications. HSA consists of 585 amino acids with a molecular weight of approximately 66.5 kDa. Its three-dimensional structure includes three highly conserved domains (I–III), each containing six α-helices (Tao et al., 2021). The primary ligand-binding sites are located in domains IIA and IIIA, capable of binding both endogenous molecules (e.g., bilirubin and fatty acids) and exogenous therapeutic drugs (Zsila et al., 2011). Similar to BSA, HSA carries a net negative charge at physiological pH, with an isoelectric point typically ranging between 4.7 and 5.2, facilitating effective neutralization of positively charged inflammatory agents in the gastrointestinal environment (Kubiak-Ossowska et al., 2019). Additionally, the degradation products of HSA provide amino acids as nutritional substrates for peripheral tissues. Given its favorable characteristics, including preferential uptake in inflamed or tumor tissues, ready availability, biodegradability, and minimal toxicity, HSA-based ANPs are increasingly recognized as optimal candidates for intestinal drug delivery applications (Fasano et al., 2005; Kratz, 2008).
To facilitate the comparison among different albumin sources, we summarized their key characteristics in Table 1, including molecular weight, isoelectric point, drug-binding domains, advantages and disadvantages as nanoparticle carriers, and therapeutic efficacy. These parameters are essential for selecting suitable albumin types for specific disease applications and guiding future formulation optimizations.
Table 1.
Comparative properties and therapeutic efficacy of different types of albumin.
| Albumin type | Molecular weight | Isoelectric point | Drug-binding domains | Advantages | Disadvantages | Therapeutic efficacy | References |
|---|---|---|---|---|---|---|---|
| OVA | 45 kDa | ∼4.8 | Hydrophobic core | Responsive to pH and temperature; High loading of lipophilic drugs | Potential allergenicity; Limited stability | Targeted anti-inflammatory modulation | (Vesković et al., 2022; Wongsasulak et al., 2010; Xiong et al., 2024) |
| BSA | 66.5 kDa | ∼4.7 | Domains IIA & IIIA | Good mucosal adhesion; Low production cost | Immunogenicity risks; Batch-to-batch variability | Enhanced mucosal protection | (Duan et al., 2022b; Kubiak-Ossowska et al., 2019; Koh et al., 2019; Szymaszek et al., 2022; Yu et al., 2022) |
| HSA | 66.5 kDa | 4.7–5.2 | Domains IIA & IIIA | High affinity to human tissues; Minimal immunogenicity | High production costs; Endogenous competition risks | Preferential uptake in inflamed or CRC tissues | (Fasano et al., 2005; Kratz, 2008; Kubiak-Ossowska et al., 2019; Tao et al., 2021; Zsila et al., 2011) |
4. Preparation of ANPs
ANPs are promising drug delivery carriers due to their biocompatibility, low immunogenicity, and biodegradability. Optimizing ANP preparation techniques is essential for enhancing drug-loading and targeted delivery efficiency. Current fabrication methods can be broadly categorized as crosslinking-dependent or crosslinking-free. Crosslinking-dependent approaches, including desolvation (Langer et al., 2003), pH-induced coagulation (Merodio et al., 2001), and thermal gelation (Hassanin and Elzoghby, 2020), typically use chemical (e.g., glutaraldehyde) or physical crosslinking to stabilize nanoparticles. These methods provide high stability and controlled particle sizes, but may compromise biocompatibility and induce protein denaturation. Crosslinking-free techniques, such as emulsification (Szczech and Szczepanowicz, 2020), Nab™ technology (Fu et al., 2009), spray drying (Heng et al., 2011), self-assembly (Asghar et al., 2014), and microfluidics (Minetti et al., 2022), form nanoparticles through hydrophobic interactions, solvent evaporation, or hydrodynamic forces. They preserve albumin's native structure and bioactivity, making them ideal for sensitive drugs. However, they may exhibit reduced long-term stability, necessitating further formulation optimization. Table 2 summarizes the mechanisms, advantages, and disadvantages of these ANP preparation methods.
Table 2.
Mechanism, advantages, and disadvantages of various ANP preparation methods.
| Methods | Mechanism | Advantages | Disadvantages | Reference |
|---|---|---|---|---|
| Desolvation | Adding a desolvating agent to reduce the solubility of albumin, causing it to precipitate and form nanoparticles | Offers high drug loading, controllable particle size, and well-established protocols for precise nanoparticle engineering | Requires organic solvents and toxic crosslinkers, posing biocompatibility concerns | (Jahanban-Esfahlan et al., 2016; Langer et al., 2003; Lei et al., 2021) |
| Emulsification | Utilizing oil-water interfacial tension to encapsulate oil droplets with albumin in the aqueous phase, forming nanoparticles after solidification | Provides formulation flexibility and is particularly suitable for lipophilic drugs with customizable systems | Involves potentially toxic surfactants and complex purification steps | (Bilati et al., 2005; Qu et al., 2024; Verma et al., 2020; Zhou et al., 2013) |
| Nab™ technology | High-pressure homogenization binds hydrophobic drugs with albumin to form stable nanoparticles | Clinically validated, crosslinker-free, and scalable for hydrophobic drug delivery | Relies on energy-intensive processes like high-pressure homogenization with limited drug applicability | (Kim et al., 2017; Min et al., 2015) |
| pH coagulation | Adjusting the pH near albumin's isoelectric point to induce aggregation and solidification into nanoparticles | Simple and cost-effective method using pH adjustment for particle formation | Difficult to control particle size distribution and potential protein denaturation | (Merodio et al., 2001; Qu et al., 2024) |
| Thermal gelation | Heating induces albumin denaturation and cross-linking, forming thermally stable nanogel particles | Simple, solvent-free process suitable for rapid production of basic nanoparticles | Exhibits poor drug compatibility and inconsistent particle size control with limited applications | (Monahan et al., 1995) |
| Spray drying | Rapid solvent evaporation via spray drying solidifies albumin into nano-sized dry powder particles | Continuous process enabling high-yield production of dry powder formulations | High equipment costs and potential thermal degradation of sensitive drugs | (Fatima et al., 2023; Meng et al., 2022) |
| Self-assembly | Exploiting albumin's amphiphilicity to spontaneously form micellar or nanoparticle structures | Enables mild, solvent-free preparation of multifunctional hybrid nanoparticles | Suffers from lower drug loading efficiency and potential stability issues requiring optimization | (Abolhassani and Shojaosadati, 2019; Safavi et al., 2017; Thao et al., 2016a; Xu et al., 2011) |
| Microfluidics | Precise fluid mixing via microfluidic technology enables controlled and uniform albumin nanoparticle preparation | Exceptional control over particle characteristics with excellent reproducibility | Complex setup requirements and limited scalability for industrial production | (Minetti et al., 2022; Zhang et al., 2023b) |
5. Mechanisms of action of drug-loaded ANPs in the treatment of intestinal diseases
Although traditional drug therapies can alleviate symptoms of intestinal diseases such as IBD and CRC to some extent, their clinical application faces significant limitations due to poor drug stability, low bioavailability, and considerable systemic toxicity. In recent years, ANP-based drug delivery systems have emerged as a research focus for intestinal disease treatment owing to their exceptional biocompatibility, targeting capability, and drug stabilization properties. Accumulating evidence demonstrates that drug-loaded ANPs can leverage unique pathological features of inflammatory regions and tumor microenvironments to achieve efficient drug delivery, thereby markedly enhancing therapeutic efficacy while minimizing adverse effects.
5.1. Key pathological features and therapeutic challenges of intestinal diseases
Key pathological features of intestinal diseases include chronic inflammation, impaired barrier function, gut flora imbalance (dysbiosis), and, in the case of intestinal cancers, an abnormal tumor microenvironment.
-
•
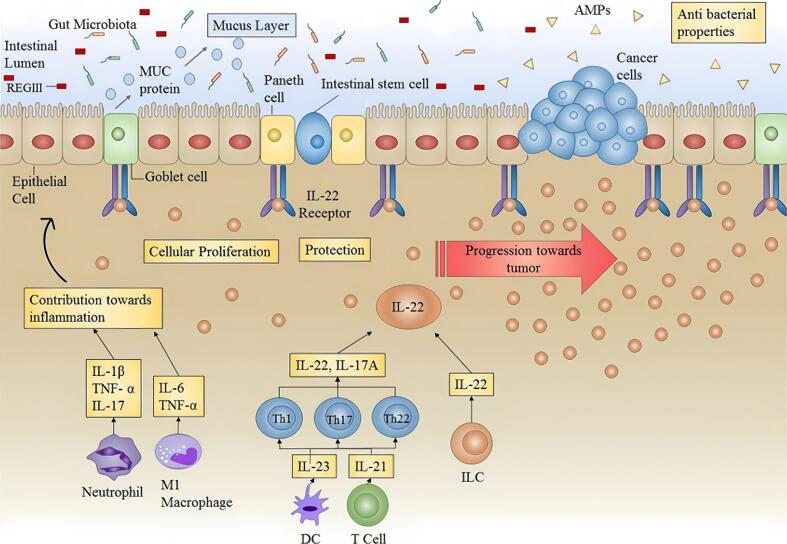
Inflammatory response: Patients with IBD (and to some extent IBS) often exhibit persistent inflammation. Clinical studies have shown decreased levels of anti-inflammatory cytokines and increased levels of pro-inflammatory cytokines in these patients, resulting in an imbalance between the two (Bashashati et al., 2017; Macsharry et al., 2008; Schmulson et al., 2012). Inflammation can disrupt intestinal tight junctions and cellular integrity, leading to massive immune cell infiltration. This significantly increases the permeability of inflamed colonic tissue and can result in dysplastic changes and eventual progression to small bowel cancer or CRC (Fig. 2) (Porter et al., 2021).
-
•
Impaired barrier function: Normally, the intestinal barrier effectively blocks the invasion of harmful substances. However, when various internal or external factors disrupt the intestinal barrier, intestinal permeability increases, triggering pathological changes such as bacterial translocation and endotoxin absorption (Chelakkot et al., 2018). Moreover, loss of barrier integrity may cause abnormal activation of the local immune response, creating a vicious cycle that exacerbates inflammation. This loss of barrier integrity not only undermines the structural integrity of the intestine but also provides a potential window for targeted drug delivery.
-
•
Abnormal tumor microenvironment: In malignant tumors such as CRC, the tumor microenvironment is often characterized by poor perfusion, hypoxia, and immunosuppression. These features impede the penetration of traditional chemotherapeutics into tumor tissue and readily induce drug resistance and recurrence (Bejarano et al., 2021). Furthermore, the accumulation of high levels of growth factors, inflammatory mediators, and stromal components in the tumor microenvironment constitutes an additional therapeutic barrier, enabling tumor cells to resist treatment. Thus, targeted strategies to modulate or exploit the properties of the tumor microenvironment have become important for improving therapeutic efficacy.
Fig. 2.
Role of IL-22 in the inflammatory environment. Prolonged or uncontrolled expression of IL-22 (as in colitis) can promote tumor progression (Arshad et al., 2020). IL-22, interleukin-22; AMPs, antimicrobial peptides; MUC, mucins. Copyright 2020, adapted with permission from Arshad et al. under the Creative Commons Attribution-Non Commercial license.
Traditional treatments (e.g., aminosalicylic acid, corticosteroids, immunosuppressants, surgery) can effectively relieve symptoms, but long-term use is often associated with drug resistance, reduced immune function, and multi-organ damage (Ouyang et al., 2023). Furthermore, although biologics have been introduced in clinical practice, some patients do not respond adequately, and surgical or radiotherapy treatments are highly invasive with substantial risks of complications and side effects (Kajiwara and Ueno, 2024). Therefore, there is an urgent need for therapeutic strategies that increase drug concentrations at the lesion site while reducing systemic toxicity.
5.2. Targeted delivery mechanisms of drug-loaded ANPs
Drug-loaded ANPs can harness the unique physiological and pathological characteristics of disease sites to achieve precise drug delivery and controlled release, thereby overcoming the limitations of traditional drug therapy. Their mechanisms of action mainly encompass two broad strategies: passive targeting by leveraging pathological cues (e.g., size-dependent, pH-dependent, ROS-responsive, enzyme-dependent, or microenvironment-responsive) and active targeting via specific ligand–receptor interactions.
5.2.1. Size-dependent targeting
Inflammatory and tumor tissues often exhibit impaired mucosal barriers and reduced epithelial integrity, resulting in an enhanced permeability and retention (EPR) effect that promotes passive nanoparticle accumulation (Barua and Mitragotri, 2014; Matsumura and Maeda, 1986). In many tumors, vascular pore cutoffs range from 380 to 780 nm, allowing liposomes or viral vectors of 100–300 nm to preferentially localize at these sites via specific vascular channels (Hobbs et al., 1998). At the same time, reducing particle size to the nanoscale (10–200 nm) facilitates diffusion through the mucus mesh (Murgia et al., 2018). ANPs measuring 50–200 nm not only evade gastrointestinal clearance but also accumulate effectively in the permeable microenvironment of inflamed regions, such as the colon. Lamprecht et al. (2001) demonstrated this size-dependent targeting in a TNBS-induced colitis model: fluorescent particles of ∼100 nm showed the highest retention at sites of inflammation compared to larger or smaller counterparts.
5.2.2. pH-dependent targeting
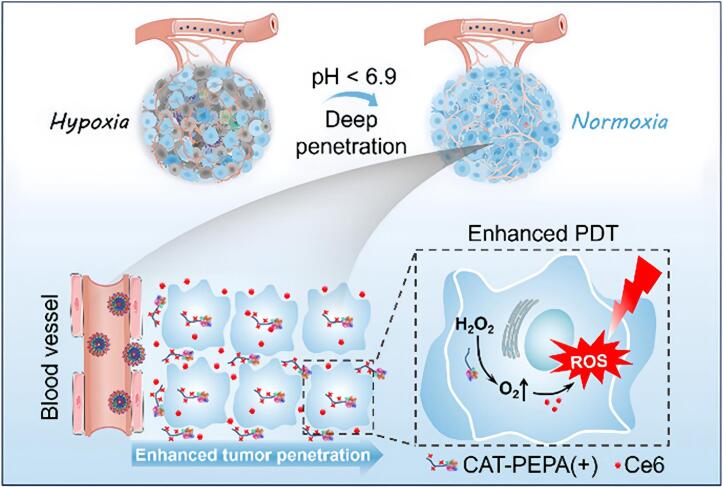
pH differences along the gastrointestinal tract (e.g., gastric pH ∼2; ileal pH 6.8–8.0; colonic pH 5.3–7.5) provide a basis for designing pH-sensitive nanomedicines (Zeeshan et al., 2019). A local acidic environment is a common pathological feature of IBD and intestinal tumors. To exploit this, pH-responsive ANPs have been developed as an effective controlled-release strategy. Notably, albumin undergoes significant conformational changes with pH (transitions at pH 2.7, 4.3, 8.0, and 10.0). For example, at pH 7.4, albumin adopts a normal heart-like structure (N isoform), while at pH 3.5 it unfolds into a partially expanded cigar-like shape (F isoform) (Baler et al., 2014). When ANPs reach an acidic disease site, these conditions induce structural changes or nanoparticle dissociation, thereby triggering drug release. Huang et al. (2024) prepared ultra pH-sensitive HSA nanomedicines loaded with the photosensitizer Ce6 (HSA/CAT-PEPA@Ce6) via self-assembly (Fig. 3). These nanoparticles rapidly disassembled in the acidic tumor environment and, aided by positively charged monomers (through electrostatic adsorption), further penetrated tumor cells. The nanoparticles released a catalase (CAT) payload to generate oxygen, which in turn produced more reactive oxygen species upon light irradiation, achieving synergistic killing of tumor cells. Additionally, a pH- and redox-responsive albumin hydrogel demonstrated controlled drug release at physiological pH (7.4), but underwent rapid degradation and payload release in a reducing environment (e.g., 100 mM glutathione), highlighting its potential for targeted therapy in glutathione-rich cancer microenvironments (Raja et al., 2015). In IBS-D (diarrhea-predominant) patients, a decrease in colonic pH may similarly trigger drug release, thereby helping modulate intestinal motility and secretion.
Fig. 3.
Schematic diagram illustrating enhanced tumor penetration and alleviation of tumor hypoxia by HSA/CAT-PEPA@Ce6 for improved photodynamic therapy (Huang et al., 2024). Copyright 2024, adapted with permission from Huang et al. under the Creative Commons Attribution-Non Commercial License.
5.2.3. ROS-responsive targeting
Reactive oxygen species (ROS) in the gut arise both physiologically—from mitochondrial electron leakage and NADPH oxidase activity—and pathologically—from neutrophil respiratory bursts, dysbiosis, and ischemia–reperfusion injury (Aviello and Knaus, 2017). Excess ROS exacerbate IBD by inducing DNA damage, degrading tight-junction proteins, and triggering inflammatory cascades (Sies and Jones, 2020). Indeed, mucosal ROS levels in UC can be 10- to 100-fold higher than normal, making ROS an attractive biomarker for targeted therapy (Sedghi et al., 1993).
Albumin-based nanomedicines can be designed to release drugs in oxidative stress microenvironments using ROS-responsive components, such as polymers containing disulfide bonds or nitrophenyl ether groups (Chen et al., 2017; Jacob et al., 2020). Moreover, ANPs can exert antioxidant effects by scavenging free radicals and thereby alleviating inflammation. For example, GBP@HA nanoparticles scavenged over 60 % of ROS (e.g., PTIO•) and achieved radical scavenging rates of ∼70 % for certain reactive nitrogen species (e.g., DPPH•) and 98 % for ABTS• radicals at concentrations of 50–500 μg/mL (Zhao et al., 2024).
5.2.4. Enzyme-dependent targeting
In inflamed or pathological intestinal sites, specific enzymes are often overexpressed and can act as “switches” for targeted responses. ANPs can be engineered to be sensitive to such enzymes (e.g., matrix metalloproteinases MMP-2/9 (Xiong and Gao, 2017) or esterases (Wan et al., 2023) by incorporating enzyme-cleavable bonds or linkages, thereby achieving precise drug release upon enzymatic cleavage. For example, Xiong and Gao (2017) developed an HSA nanoparticle coupled with an MMP-specific substrate peptide, exploiting the high MMP-2/9 expression in the tumor microenvironment to trigger the release of chemotherapeutic drugs.
In the context of IBD, myeloperoxidase (MPO) released by activated neutrophils is markedly elevated in inflamed regions. Taking advantage of MPO's affinity for HSA, Tiruppathi et al. conjugated 5-aminosalicylic acid (5-ASA) to HSA nanoparticles. These MPO-responsive NPs accumulated selectively at inflamed sites and exhibited anti-inflammatory efficacy over 1000-fold greater than free 5-ASA (Tiruppathi et al., 2004a). By pairing the passive EPR effect with enzyme-triggered release, this strategy significantly enhances local drug concentration and treatment precision. More broadly, designing ANPs to respond to overexpressed enzymes in intestinal inflammation or tumors offers a powerful means to improve therapeutic index and minimize off-target exposure.
5.2.5. Microenvironment-responsive targeting
The tumor microenvironment (TME) in intestinal cancers is defined by hypoxia, chronic inflammation, immunosuppression, acidic pH, and elevated reducing agents—all of which can be harnessed for stimulus-responsive ANP delivery (Thakkar et al., 2020). Under hypoxia, HIF-1α activation increases gp60 receptor expression by 2–3-fold, enhancing endothelial transcytosis of albumin (Semenza, 2012). The mildly acidic milieu (pH ≈ 6.8) induces conformational shifts in albumin that strengthen its binding to SPARC in the tumor stroma (Sleep, 2015). High intracellular glutathione levels (2–10 mM) then cleave disulfide bonds within ANPs, triggering controlled drug release (Kratz, 2008). Furthermore, recombinant HSA can exploit native endocytic pathways to accumulate in tumor cells.
By integrating pH-, redox-, and enzyme-responsive elements into nanoparticle design, payloads are liberated specifically within the TME. For example, Wang et al. (2021) formulated atovaquone-loaded HSA nanoparticles (∼164.5 nm, ∼90 % encapsulation efficiency, ∼10 % drug loading) that alleviated tumor hypoxia and potentiated anti–PD-1 therapy. These HSA-ATO NPs exhibited robust tumor accumulation in a colorectal cancer patient-derived xenograft model, demonstrating the power of microenvironment-responsive targeting.
5.2.6. Active targeting via ligand-receptor interactions
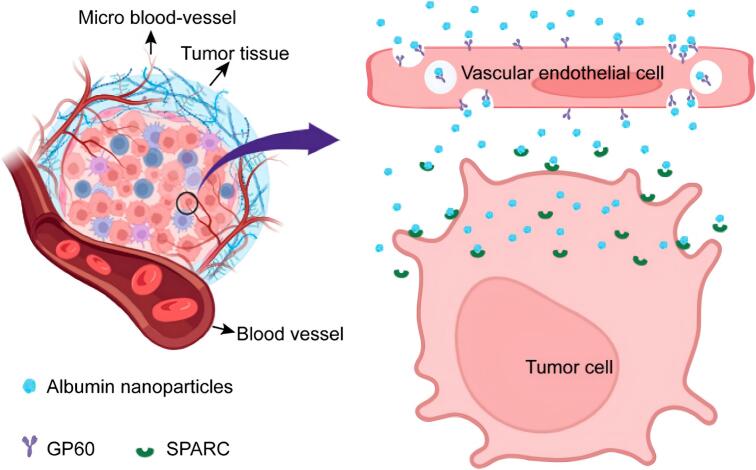
ANPs can be actively targeted via surface modifications. For inflammatory conditions, one approach is to use albumin or attached ligands that have a high affinity for inflammation-associated molecules (e.g., myeloperoxidase released by neutrophils) or for receptors overexpressed on colonic epithelial cells and activated macrophages (e.g., CD44, mannose receptor, CD98, PepT1, F4/80). Among these, CD44 and the mannose receptor are the most extensively studied targets (Table 3), while others have been explored less frequently. Such ligand-directed modifications promote receptor-mediated endocytosis and enhance drug accumulation and cellular uptake at sites of inflammation (Hirata et al., 2010; Hua et al., 2015; Hua, 2014; Kumar et al., 2022; Tiruppathi et al., 2004b; Wang et al., 2014; Zheng et al., 2022). By binding to specific receptors on inflamed cells, ligand-decorated ANPs deliver drugs directly to the target area and achieve improved therapeutic effects. Receptor-mediated active targeting systems thus represent a promising therapeutic strategy for IBD (see Table 3). In tumors, cancer cells express albumin-binding receptors such as gp60 (Fig. 4). ANPs bind to gp60 on endothelial cells; this receptor is associated with caveolin-1 and leads to caveolae-mediated endocytosis. This process allows ANPs to transcytose across the endothelium and subsequently bind to SPARC (Secreted Protein Acidic and Rich in Cysteine), an albumin-binding protein overexpressed in many cancers. Through this gp60/SPARC pathway, ANPs achieve enhanced accumulation in tumor cells.
Table 3.
Representative receptor-targeted drug delivery systems for the treatment of IBD.
| Receptor | Ligand | Carrier | Loading cargo | Delivery route | Characterization | Principal finding | Diseases | Reference |
|---|---|---|---|---|---|---|---|---|
| CD44 | HA | GBP@HA NPs | EGCG | Oral | S: 441.93 ± 17.27 nm Z: −22.63 ± 3.62 mV PDI: 0.181 |
Regulating the Keap1-Nrf2 and NF-κB signaling pathways, as well as promoting Caspase-1 dependent pyroptosis | Inflammation | (Zhao et al., 2024) |
| HA | TA/CUR-NPs | CUR | Oral | S: 220 nm Z: −28.8 mV |
Prolonged colonic adhesion and increased uptake in Caco-2 cells | Inflammation | (Luo et al., 2020) | |
| HA | BSA·SH-Mag NPs | Mag | Oral | S: 403 ± 4 nm Z: −23.6 mV |
HA conjugated via an amide reaction to target CD44+ receptors | Inflammation | (Li et al., 2024) | |
| HA | BSA-KPV/PLGA/PVA-chitosan | KPV | Oral | S: 272.3 nm Z: −5.3 mV PDI: 0.20 ± 0.06 |
HA-modified nanoparticles were selectively absorbed by Colon-26 cells and RAW264.7 macrophages | Cancer | (Xiao et al., 2017) | |
| HA | BSA-Ce6@MPN | EGCG | Injection | S: 219.62 ± 9.30 nm Z: −11.87 ± 0.31 mV |
HA can specifically bind to the CD44 receptor overexpressed on the surface of CT26 cells | Cancer | (Liang et al., 2022) | |
| Mannose receptor | Mannose | Man-BSA@Rb1 NPs | Rb1 | Injection | S: 106 nm Z: −20.0 mV |
Inhibiting NF-κB and MAPK signaling pathways in LPS-induced Raw264.7 cells | Inflammation | (Fu et al., 2022) |
| Mannose | DOX@MAN-BSA | DOX | Injection | S: 150 nm Z: −15 mV |
Internalized by colon tumor cells and M2 tumor-associated macrophages via mannose receptor-mediated endocytosis | Cancer | (Zeng et al., 2022) | |
| Mannose | eHSA | CA | Injection | S: 108.8 nm Z: −25.3 mV |
Inhibiting Wnt signaling for CRC therapy | Cancer | (Yang et al., 2024) |
Abbreviations: HA, hyaluronic acid; EGCG, Epigallo-catechin 3-gallate; S: Sizes; Z: Zeta-potentials; PDI: Polydispersity indexes; NF-κB, nuclear factor-κB; CUR, Curcumin; Mag, Magnolol; LPS, lipopolysaccharide; Man, Mannose; KPV: Lysine-proline-valine; PLGA, Poly(lactic-co-glycolic acid); PVA, Polyvinyl Alcohol; MAPK, mitogen-activated protein kinase; DOX, doxorubicin; CA, carnosic acid.
Fig. 4.
Schematic diagram of ANPs targeting tumor cells via the gp60 and SPARC pathways (Ji et al., 2024). Copyright 2024, adapted with permission from Ji et al. under the Creative Commons Attribution-Non Commercial License.
6. Advances in preclinical studies of drug-loaded ANPs for intestinal diseases
ANPs have emerged as a promising platform for the targeted treatment of intestinal diseases, owing to their favorable biocompatibility, stability, and ability to enhance site-specific drug delivery. Recent preclinical studies have explored ANP-based delivery systems in several prevalent intestinal conditions, demonstrating improved drug stability, targeted release, and therapeutic efficacy in disease models. In this chapter, we provide an overview of these advances, focusing on three common intestinal diseases: IBD, CRC, and other intestinal diseases. We discuss how drug-loaded ANPs can address the treatment challenges in each of these conditions, highlight representative preclinical findings, and outline the therapeutic potential of ANP-mediated strategies in intestinal disease management.
6.1. Inflammatory bowel disease
IBD, which includes Crohn's disease and ulcerative colitis, affects approximately 7 million individuals worldwide (Maddineni et al., 2024). It is characterized by chronic, relapsing inflammation of the intestinal mucosa, leading to epithelial damage, ulceration, and fibrotic remodeling (McDowell et al., 2025). Conventional treatments such as aminosalicylates, corticosteroids, immunosuppressants, and biologics, particularly anti-TNF agents, are often limited by poor targeting and systemic side effects (Cai et al., 2021; Peyrin-Biroulet et al., 2021).
Drug administration routes for IBD include oral, intravenous, and rectal delivery, each with unique pharmacokinetic profiles (Fig. 5). Oral delivery, while convenient, is hindered by the extreme pH variation in the gastrointestinal tract (pH 1.0–8.3), compromising drug stability (Zhang et al., 2017). To overcome this, researchers have developed ANP systems with pH-sensitive coatings (e.g., Eudragit® FS30D) or azo-reductase-responsive linkages for colon-specific release. Moreover, Zhang et al. (2023a) engineered a multistage albumin nanomedicine depots (MANDs) system that exhibited a high internalization rate (up to 99 %) in Caco-2 cells, confirming enhanced cellular uptake (Fig. 6A–C). In a DSS-induced colitis mouse model, MANDs significantly improved anti-inflammatory outcomes, as indicated by colon length, spleen weight, and colonic injury scores (Fig. 6D–F). Histological analyses further confirmed low organ toxicity (Fig. 6G). Similarly, He et al. (2023) developed an oral polyphenol armor ANP that synergistically inhibited intestinal inflammation in DSS-induced colitis mice via targeted TNF-α siRNA delivery and ROS scavenging. In vitro, these ANPs enhanced macrophage uptake and eliminated intracellular ROS, while in vivo they modulated gut microbiota composition and alleviated inflammation through gut-brain axis interactions. Intravenous delivery, while offering improved bioavailability, often leads to off-target hepatic accumulation and rapid renal clearance, especially for nanoparticles smaller than 10 nm (Liu et al., 2021a). Gou et al. (2018) demonstrated that intravenously delivered EGCG-loaded nanoparticles (EGCG-NPs) achieved a 1.4-fold higher accumulation in inflamed tissues compared to free EGCG, highlighting improved targeting efficiency and minimal organ toxicity. Rectal delivery facilitates localized drug administration with limited systemic exposure. For instance, curcumin-loaded albumin microspheres released 90 % of their drug payload at colonic pH 7.2 compared to only 15 % under acidic conditions, significantly alleviating symptoms in a DSS-induced colitis model (Beloqui et al., 2014).
Fig. 5.
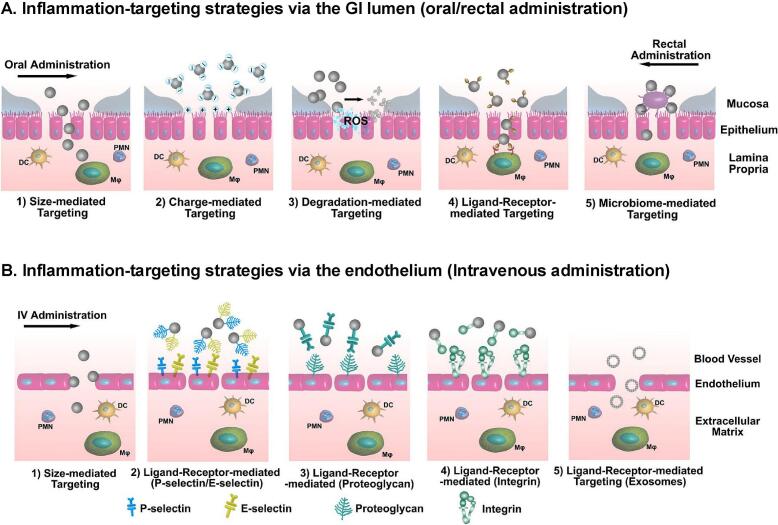
Delivery strategies and targeting mechanisms of NPs for intestinal inflammation in IBD (Zhang et al., 2017). (A) After oral or rectal administration, NPs reach the inflamed colonic mucosa via the epithelial route, mediated by particle size, surface charge, degradation rate, ligand–receptor interactions, and modulation of the gut microbiome. (B) After intravenous administration, NPs target the inflamed colon via the endothelial route, exploiting size-dependent distribution and ligand–receptor interactions, including selective homing of exosomes. DC, dendritic cells; MΦ, macrophages; PMN, polymorphonuclear leukocytes; ROS, reactive oxygen species. Copyright 2017, adapted with permission from Zhang et al. under the Creative Commons Attribution-Non Commercial License.
Fig. 6.
Oral administration of MANDs for the treatment of IBD (Zhang et al., 2023a). (A) Bright-field microscopic images of MANDs. (B) Caco-2 cells incubated with FITC-labeled MTX@HSA NPs for 1, 2, 4, and 8 h. (C) Flow cytometry analysis of Caco-2 cells incubated with FITC-labeled MTX@HSA NPs for the indicated times. (D and E) Colon length (D) and Spleen wight (E) were measured and analyzed (n = 6); (F) Colonic damage scores assessed by H&E staining (n = 6); (G) Representative H&E-stained colon sections from each group. Copyright 2023, adapted with permission from Zhang et al. under the Creative Commons Attribution-Non Commercial License.
Despite these advantages, challenges remain. Targeting efficiency varies between pH-sensitive and enzyme-responsive systems (Zhang et al., 2023a), and the acute DSS colitis model used in many studies does not fully replicate the chronic, fibrotic features of human IBD (Chassaing et al., 2014). Future research should prioritize humanized animal models and explore combination therapies with biologics (such as anti-TNF antibodies). Additionally, scalable manufacturing and batch consistency are critical for translating ANP-based IBD treatments into clinical use.
6.2. Colorectal cancer
CRC, a malignancy arising from colonic epithelial cells, ranks as the third most common cancer and the second leading cause of cancer-related deaths globally. Chronic inflammation, such as that seen in IBD, significantly increases CRC risk (Lucafò et al., 2021). In 2020 alone, CRC caused approximately 1.9 million new cases and 0.9 million deaths worldwide (Xi and Xu, 2021). Clinical symptoms typically appear in advanced stages and may include hematochezia, obstruction, and systemic manifestations (Duan et al., 2022a). Traditional chemotherapy drugs, including doxorubicin, suffer from limited tumor specificity and serious adverse effects such as cardiotoxicity and liver damage (Injac and Strukelj, 2008; Kim et al., 2017; Oleaga et al., 2016; Rivankar, 2014).
ANPs offer a solution through enhanced drug stability and tumor targeting. Albumin carriers such as HSA and BSA can evade clearance by the reticuloendothelial system and be functionalized for active targeting. A landmark example is Abraxane, an FDA-approved albumin-bound paclitaxel formulation (Ma and Mumper, 2013). Simultaneously, Wang et al. (2025) found that the combination of A4-Based ANPs with anti-CTLA4 antibodies can effectively reduce lung metastasis in colon cancer models by inhibiting endothelial microtubules. Nevertheless, achieving sufficient intratumoral drug accumulation remains a major challenge, particularly in tumors with poor vascular permeability, such as melanoma, pancreatic cancer, and CRC. In a CRC mouse model, Kinoshita et al. (2017) demonstrated that SNO-HSA dimers significantly enhanced the tumor targeting of nab-paclitaxel by strengthening the enhanced permeability and retention (EPR) effect, leading to a 2.5-fold increase in intratumoral PTX concentration and reduced systemic toxicity. This highlights the potential of ANPs for improving nanodrug delivery in poorly perfused tumors.
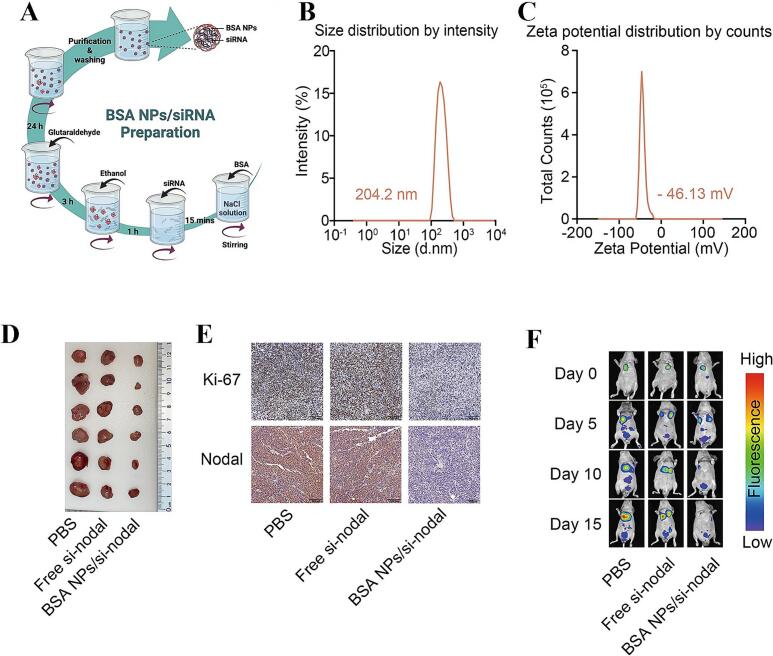
Recent advances include sirolimus-loaded ANPs (ABI-009, Aadi Bioscience) and BSA/si-Nodal nanocomplexes, which have shown potent antitumor activity in CRC models, including inhibition of metastasis (Wu et al., 2023). Figs. 7A–C present the preparation process, size distribution (204.2 nm), and zeta potential (−46.13 mV) of the BSA-NP/siRNA complexes, highlighting their well-defined physicochemical characteristics. In vivo tumor imaging (Fig. 7D), tumor volume analysis (Fig. 7E), and fluorescence imaging of lung metastases (Fig. 7F) further confirmed that the BSA-NP/si-Nodal nanocomplexes effectively suppressed tumor progression while minimizing systemic toxicity. Moreover, photothermal therapeutic strategies such as HSA-IR825 nanoparticles, developed by Chen et al. (2014), integrate imaging and targeted therapy to enhance treatment precision for CRC. Likewise, Liang et al. (2022) reported that hyaluronic acid (HA)-EGCG-modified metal–phenolic network (MPN) nanoreactors, targeting CD44 receptors, induced ferroptosis and photodynamic synergy, achieving a 12-fold reduction in tumor volume in CT26-bearing mice compared with controls, with minimal off-target toxicity.
Fig. 7.
(A) Schematic representation of the preparation of BSA-NP/si-Nodal nanocomplexes. (B and C) Hydrodynamic size (B) and zeta potential (C) distributions of BSA-NP/si-Nodal complexes measured by dynamic light scattering. (D and E) Representative images of mouse tumors (D) and IHC-stained tissue sections (E). (F) Fluorescence images of nude mice with lung metastases and quantitative analysis of fluorescence intensity (Wu et al., 2023). Copyright 2023, adapted with permission from Wu et al. under the Creative Commons Attribution-Non Commercial License.
These findings underscore the strong translational potential of ANPs in malignant intestinal diseases. Compared to IBD therapy, where ANPs primarily facilitate colon-specific drug delivery and reduce systemic side effects (Zhang et al., 2023a; Zhang et al., 2017), CRC-focused applications aim to improve tumor penetration and overcome chemoresistance. However, challenges such as tumor heterogeneity and limited intratumoral diffusion persist (Ma and Mumper, 2013; Wu et al., 2023). To address these issues, multifunctional ANPs with pH-, enzyme-, and redox-responsiveness are being developed to improve tumor accumulation and therapeutic precision (Kuang et al., 2023). Future progress will require not only technological refinements but also clinical trials targeting refractory IBD and metastatic CRC, alongside the standardization of large-scale production processes, to enable successful clinical translation of ANP-based therapies.
6.3. Other intestinal diseases
ANP-based delivery systems offer excellent biocompatibility, intestinal targeting, and drug-loading versatility for various intestinal therapy. ANPs have shown promise in treating conditions such as IBS, infectious enteropathies, and intestinal barrier dysfunction. By binding to intestinal epithelial receptors (such as the neonatal Fc receptor, FcRn, or SPARC) and by enhancing drug penetration of the intestinal mucosal barrier, ANPs can efficiently concentrate therapeutic agents at lesion sites, thereby improving drug absorption. Supporting this approach, Xie et al. (2020) demonstrated that graphdiyne-modified BSA nanoparticles (GDY-BSA NPs) provided significant radioprotective effects in a radiation-induced diarrhea model. Under whole-body X-ray irradiation, when the survival rate of mice in the untreated group dropped to 30 %, the GDY-BSA NP-treated group maintained a 90 % survival rate. These nanoparticles markedly reduced the severity of diarrhea, prevented weight loss, and mitigated histopathological damage in intestinal tissues, highlighting their strong potential as gastrointestinal radioprotectants.
Although ANP platforms have not yet reached clinical application for these conditions, the preclinical evidence supports their potential for future translation. However, mechanistic studies remain limited. Research into infectious enteropathies, for instance, has primarily focused on antibiotic-loaded ANPs, with relatively little exploration of antiviral or antiparasitic formulations (Hussain et al., 2018; Pisani et al., 2024).
Currently, the exploration of ANPs in these diseases is still in its early stages—reflecting high potential but low maturity. The relatively modest market size for these conditions may dampen industry enthusiasm for investment, underscoring the need for policy incentives and academic–industry collaboration. In the years ahead, research should prioritize the development of combination-loaded ANPs tailored to diverse intestinal pathologies, along with detailed mechanistic studies and long-term efficacy assessments.
7. Toxictity analysis of ANPs
Assessing nanodrug toxicity primarily involves evaluating cell viability, organ-specific toxicity, and biological survival. ANPs, as natural nanocarriers, exhibit inherently low cytotoxicity and can effectively mitigate off-target effects when loaded with chemotherapeutics such as methotrexate or paclitaxel. Notably, ANPs also enhance drug cytotoxicity against multidrug-resistant tumor cells. Gad et al. (2018) demonstrated that docetaxel-loaded HSA nanoparticles showed superior cytotoxic effects against multidrug-resistant NCI/ADR-RES cells compared to free docetaxel within 4 h, highlighting their intracellular sustained-release capability (Gad et al., 2018).
ANPs preferentially target inflamed or tumor tissues, enhancing local drug accumulation and reducing systemic toxicity. Histopathological evaluations are valuable indicators of organ toxicity. For example, Kushwah et al. (2018) reported minimal organ toxicity through biodistribution studies using coumarin-6-loaded AA-GEM-BSA nanoparticles (Kushwah et al., 2018). Furthermore, ANPs significantly extend survival in disease models; experiments by Taheri et al. (2012) demonstrated that biotin-modified HSA nanoparticles prolonged survival to 47.5 ± 0.43 days in tumor-bearing mice, significantly exceeding the 30 ± 0.96 days observed with free methotrexate (Taheri et al., 2012).
Despite these promising attributes, ANPs' nanoscale dimensions may pose unique toxicological challenges (Fischer and Chan, 2007). For instance, residual glutaraldehyde from crosslinking processes can compromise cellular viability (Meng et al., 2022). Consequently, alternative, lower-toxicity crosslinkers (e.g., tannic acid, ascorbic acid, citric acid, sorbitol, and glucose) have been explored to mitigate these concerns, although further refinement is necessary (Amighi et al., 2020; Weiner et al., 2011). Additionally, albumin, although inherently nontoxic, may amplify drug-related toxicity under certain conditions. In a rat model of aspirin poisoning, for instance, combining albumin with lactated Ringer's solution exacerbated toxicity (Harb et al., 2024). Moreover, interactions between albumin and blood proteins may reduce nanoparticle delivery efficiency to tumors (Saha et al., 2019). Thus, ANP safety profiles depend significantly on nanoparticle preparation methods, drug-albumin compatibility, and in vivo interactions. Future research should prioritize developing safer crosslinking strategies and more precise targeting approaches to further enhance the clinical safety and therapeutic efficacy of ANP-based nanomedicines.
8. Clinical potential of drug-loaded ANPs in intestinal disease therapy
By virtue of its unique structure and abundant surface functional groups, albumin can interact specifically with a broad range of molecules. This property provides an ideal basis for numerous modifications, making albumin a preferred nanocarrier system. Albumin-based nanocry stallization approaches have been widely applied in diagnostics and personalized medicine, and several albumin formulations have been successfully marketed (Table 4). Abraxane, the world's first albumin-bound paclitaxel formulation, has achieved promising clinical outcomes by reducing PTX toxicity, increasing drug loading (up to 49 %), and minimizing allergic reactions. Clinical trials have demonstrated that Abraxane is highly effective in cancer patients with severe hypersensitivity reactions and does not induce immune tolerance; however, its efficacy in treating CRC remains unsatisfactory (Fader and Rose, 2009).
Table 4.
Albumin drug delivery system for the treatment of intestinal diseases.
| Name of disease | Loaded drugs | Delivery system | Characterization | Preparation method | Mode of administration | Design strategies | Therapeutic outcomes | Bibliography |
|---|---|---|---|---|---|---|---|---|
| IBD | 5-ASA | 5-ASA-HAS NPs | S: 190 nm Z: −11.8 mV PDI: 0.35 |
Desolvation | Oral administration | Active-targeting | Repair damaged intestinal mucosa, alleviate symptoms | (Iwao et al., 2018) |
| BUD, Vanco, GM-CSF | HEP-HSA NPs | S: 250–350 nm Z: −38 ∼ −42 mV PDI < 0.02 |
Desolvation | Rectal administration | Electrostatic interaction | Inhibit microvascular thromboses and Reduce proinflammatory cytokines/signaling molecules | (Zhang et al., 2020) | |
| CUR | TA/CUR-NPs | S: 220.4 ± 4.3 nm Z: −28.80 ± 0.40 mV PDI: 0.102 ± 0.018 |
Improved desolvation | Oral administration | pH-dependent and Enzyme-dependent | Suppress the expression levels of pro-inflammatory cytokines | (Luo et al., 2020) | |
| EGCG | EGCG-NPs | S: 202.9 nm Z: −13.2 mV PDI: 0.101 |
Self-assembling | Intravenous injection | pH-dependent and GSH-dependent | Enhanced EPR effect | (Gou et al., 2018) | |
| KPV | BSA-KPV/PLGA/PVA-chitosan | S: 272.3 nm Z: −5.3 mV PDI: 0.20 ± 0.06 |
Improved desolvation | Oral administration | Active-targeting | Prevent mucosa damage and downregulate TNF-α, alleviate colitis symptoms | (Xiao et al., 2017) | |
| MTX | MTX@HSA NPs/MANDs | S: 30 ± 3.2 nm Z: 37 mV |
Microfluidics | Oral administration | pH-dependent | Evades stomach acid, inhibit the synthesis of DNA and RNA and suppress the proliferation of inflammatory cells | (Zhang et al., 2023a) | |
| Se | Se@Albumin NPs | S: 96 ± 7.5 nm Z: −37.3 mV |
Self-assembly | Intravenous injection | Active-targeting | Maintain intestinal flora balance, alleviate colitis symptoms | (Deng et al., 2021) | |
| Tof | Tof@BSA-Chs-CP | S: 308.5 ± 4.8 nm Z: −22.4 ± 0.7 mV PDI: 0.065 ± 0.033 |
Desolvation | Oral administration | Active-targeting | Modulating the expression of pro-inflammatory cytokines and immune regulatory factors, alleviate colitis symptoms | (Wu et al., 2025) | |
| Tof | – | S: 250.6 ± 42.1 nm | Self-assembly | Oral administration | ROS-responsive and Active-targeting |
Remodulate immune microenvironment, alleviate colitis symptoms | (Li et al., 2023) | |
| CRC | Cetuximab | HSA-cetuximab nanoparticles | S: 241.5 ± 14.7 nm PDI: 0.068 ± 0.063 |
Desolvation | – | Active-targeting | Enhanced EPR effect | (Löw et al., 2011) |
| DOX | MSNP-BR-BSA | S: 210 nm Z: −12.2 mV |
Self-assembly | – | Enzyme-dependent | Inhibits tumor cell growth, defense against colon cancer | (Srivastava et al., 2017) | |
| DOX | DOX-HSA | S: 112 nm | Desolvation | Intravenous injection | Active-targeting | Inhibits tumor cell growth and enhanced EPR effect | (Kimura et al., 2019) | |
| DOX, TRAIL | TRAIL/Dox HSA NPs | S: 60–120 nm Z: −35 ∼ −22 mV |
Nab™ technology | Intravenous injection | Apoptosis synergy | Apoptotic synergy | (Thao et al., 2016b) | |
| DTX | Apt-NPs-DTX | S: 62 nm Z: −31.2 mV |
self-assembly | Intravenous injection | Active-targeting | Bind with nucleolin | (Yu et al., 2020) | |
| EGCG | BSA-Ce6@MPN | S:219.62 ± 9.30 nm Z:-11.87 ± 0.31 mV |
Self-assembly | Intravenous injection | Active targeting and ROS-responsive | Generating ROS to induce apoptosis and tissue damage | (Liang et al., 2022) | |
| FITC | FITC-HSA | S: 146.0 ± 2.1 nm PDI: 0.1 ± 0.01 |
Desolvation | Oral administration | Active-targeting | Enhanced EPR effect | (Hashem et al., 2018) | |
| 5-Fu | BLDH/5FU-ABX | S: 168.3 ± 2.0 nm Z: −14.0 mV PDI: 0.181 |
Self-assembly | Intravenous injection | pH-dependent and Charged-dependent | Exhibited synergetic inhibition of tumor proliferation and induction of cell apoptosis | (Li et al., 2021) | |
| 5-FU | 5-FU-rHSA-PEG-NPs | S: 65.7 ± 7.2 nm Z: 25.8 ± 3.5 mV PDI < 0.05 |
Covalent coupling method | Intravenous injection | Active-targeting | Inhibits tumor cell growth and enhanced EPR effect | (Sharma et al., 2017) | |
| OXA | Nps-BSA-OXA | S: 163.2 ± 6.3 nm Z: −27 mV PDI < 0.3 |
Desolvation | Intravenous injection | Active-targeting | Interfere with replication of DNA and transcription leading to cell death | (Wilson et al., 2024) | |
| PTX | SNO-HSA Dimer | S: 20 nm | Nab™ technology | Intravenous injection | ERF and Active-targeting | Enhance EPR and improve tumor microenvironment | (Kinoshita et al., 2017) |
Abbreviations: S: Sizes; Z: Zeta-potentials; PDI: Polydispersity indexes; 5-ASA: 5-Aminosalicylic acid; BUD: budesonide; GM-CSF, granulocyte macrophage colony-stimulating factor; HEP, Heparin; CUR, Curcumin; TA, Tannic Acid; KPV: Lysine-proline-valine; PLGA, Poly(lactic-co-glycolic acid); PVA, Polyvinyl Alcohol; Vano: vancomycin; TNF-α: tumor necrosis factor-α; MTX, methotrexate; MANDs, Multistage albumin nanomedicine depots; Tof: Tofacitinib; ERP effect: permeability and retention effect; DOX: doxorubicin; MSNP: mesoporous silica nanoparticles; BR: Bilirubin; TRAIL, TNF-α-related apoptosis-inducing ligands; NPs: nanoparticles; DTX: docetaxel; Apt: aptamer; EGCG, Epigallo-catechin 3-gallate; Ce6: Chlorin e6; MPN: metal-phenolic network; GSH: glutathione; ROS: reactive oxygen species; FITC: fluorescein isothiocyanate; 5-FU, 5-Fluorouracil; BLDH: albumin-stabilized layered double hydroxide; ABX: Abraxane; PEG: Polyethylene glycol; OXA, oxaliplatin; PTX: paclitaxel; SNO: S-nitrosated;.
To further improve therapeutic efficacy against CRC, Kinoshita et al. prepared an SNO-HSA dimer intended to augment the tumor's enhanced permeability and retention (EPR) effect, thereby increasing drug accumulation and efficacy at the tumor site (Kinoshita et al., 2017). In addition, Li et al. encapsulated a Janus kinase (JAK) inhibitor in reactive oxygen species (ROS)-responsive HSA nanoparticles and modified them with an anti-MAdCAM-1 antibody. This design significantly enhanced targeting to inflamed intestinal regions (Li et al., 2023). Both in vivo and ex vivo experiments confirmed that this nanomedicine produced favorable therapeutic effects in a DSS-induced acute colitis mouse model. It is noteworthy that current ANP-based therapies for IBS are relatively limited. This scarcity likely arises because most research on ANPs as drug carriers has focused on oncology, and their application in digestive diseases like IBS remains in an exploratory stage.
9. Challenges and future perspectives of drug-loaded ANPs for intestinal disease therapy
ANPs have emerged as highly promising drug carriers due to their excellent biocompatibility, intrinsic stability, and easily modifiable surface functional groups, which facilitate precise targeted delivery (Chen et al., 2014; Yoo et al., 2019). Nevertheless, the clinical application of ANPs in treating intestinal diseases still faces multiple significant challenges. Specifically, the harsh gastrointestinal environment, characterized by extreme pH variations, digestive enzymes, and complex microbiota interactions, can potentially compromise the structural integrity and stability of ANPs (Jahanban-Esfahlan et al., 2016). Furthermore, comprehensive validation of long-term safety, including potential chronic toxicity and metabolic fate, remains insufficiently explored.
We believe that a crucial step toward clinical translation involves addressing standardization and scalability challenges. Current ANP production methods, such as Nab™ technology, typically require specialized equipment, stringent process conditions, and high operational costs, limiting large-scale and cost-effective manufacturing (Min et al., 2015). Rigorous quality control and reproducibility across batches also remain significant hurdles. Additionally, most marketed ANP-based therapeutics are administered through injection, with few oral formulations available, thereby reducing patient compliance. The reliance on HSA derived from blood donations further raises ethical concerns and potential shortages in raw material supplies (Cho et al., 2024).
Moving forward, we advocate for the strategic integration of ANPs with advanced therapeutic modalities (e.g., biologics or corticosteroids) to enhance clinical efficacy and personalization. For example, combining ANPs with immune checkpoint inhibitors holds considerable promise in augmenting therapeutic outcomes within complex tumor microenvironments (Zhang et al., 2024). Additionally, incorporating real-time imaging techniques, such as fluorescence or magnetic resonance imaging, into ANP systems could facilitate precise monitoring and dynamic evaluation of drug distribution and therapeutic effectiveness in vivo (Duan et al., 2023). Stimuli-responsive materials capable of controlled and site-specific drug release at pathological lesions represent another critical advancement area that deserves further exploration (Wei et al., 2017).
From our perspective, achieving successful translation from bench to bedside will require concerted interdisciplinary collaboration aimed at refining material design, reducing manufacturing costs, and streamlining global regulatory frameworks. It remains essential to develop comprehensive toxicological assessments, particularly addressing the long-term biodistribution, biodegradation, and biocompatibility of ANPs (Bas et al., 2021). Moreover, addressing formulation challenges for oral delivery would significantly enhance patient acceptance and market penetration.
In conclusion, ANP-based drug delivery systems exhibit considerable potential in overcoming limitations associated with conventional therapies for intestinal diseases, such as IBD, CRC, and IBS. This review has highlighted the unique strengths of ANPs, especially their robust biocompatibility, stability, and versatile surface modifiability, and summarized various preparation methods, targeted delivery mechanisms, controlled release strategies, and significant preclinical and clinical advances. Despite landmark successes such as Abraxane, persistent challenges, including nanoparticle stability in harsh gastrointestinal conditions, targeted delivery efficiency, long-term safety validation, and scalable production processes, still need to be comprehensively addressed. We strongly recommend that future research efforts prioritize nanoparticle optimization for enhanced mucus penetration and site-specific targeting, extensive long-term safety evaluations, and refinement of production technologies to reduce costs. With sustained innovation and collaborative interdisciplinary research, next-generation ANPs are poised to offer more precise, effective, and patient-friendly therapies, significantly advancing the treatment landscape of intestinal diseases.
Funding
This work was supported by the Academician Foundation Program of Chongqing (cstb2023yszx-jcx0003), the Venture and Innovation Support Program for Chongqing Overseas Returnees (cx2024003), the Fundamental Research Funds for the Central Universities (SWU-KF25005), the Chongqing Natural Science Foundation (CSTB2023NSCQ-MSX0506), the Opening fund of State Key Laboratory of Resource Insects (SKLRI-ORP202502), the Chongqing GraduateStudent Research Innovation Project (CYS25197) and the Southwest University Municipal Training Program of Innovation and Entrepreneurship for Undergraduates (X202410635267).
CRediT authorship contribution statement
Xianrui Lin: Writing – original draft. Lin Wang: Methodology. Zhihao Lin: Methodology. Zhenlin Yang: Investigation. Ziheng Zhao: Formal analysis. Yuanyuan Wen: Conceptualization. Rui Xi: Methodology. Dingpei Long: Writing – review & editing, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare no conflict of interest in this work.
Data availability
Data will be made available on request.
References
- Abolhassani H., Shojaosadati S.A. A comparative and systematic approach to desolvation and self-assembly methods for synthesis of piperine-loaded human serum albumin nanoparticles. Colloids Surf. 2019;B 184 doi: 10.1016/j.colsurfb.2019.110534. [DOI] [PubMed] [Google Scholar]
- Abuhayi B.M., Bezabh Y.A., Ayalew A.M., Lakew M.A. Classification of gastrointestinal diseases using hybrid recurrent vision transformers with wavelet transform. Adv. Multimed. 2024;2024 doi: 10.1155/2024/8334358. [DOI] [Google Scholar]
- Amighi F., Emam-Djomeh Z., Labbafi-Mazraeh-Shahi M. Effect of different cross-linking agents on the preparation of bovine serum albumin nanoparticles. J. Iran. Chem. Soc. 2020;17:1223–1235. doi: 10.1007/s13738-019-01850-9. [DOI] [Google Scholar]
- Arshad T., Mansur F., Pálek R., Manzoor S., Liska V. A double edged sword role of interleukin-22 in wound healing and tissue regeneration. Front. Immunol. 2020;11:2148. doi: 10.3389/fimmu.2020.02148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar S., Salmani J.M.M., Hassan W., Xie Y., Meng F., Su Z., Sun M., Xiao Y., Ping Q. A facile approach for crosslinker free nano self assembly of protein for anti-tumor drug delivery: Factors’ optimization, characterization and in vitro evaluation. Eur. J. Pharm. Sci. 2014;63:53–62. doi: 10.1016/j.ejps.2014.06.022. [DOI] [PubMed] [Google Scholar]
- Aviello G., Knaus U.G. ROS in gastrointestinal inflammation: rescue or Sabotage? Br. J. Pharmacol. 2017;174:1704–1718. doi: 10.1111/bph.13428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baler K., Martin O.A., Carignano M.A., Ameer G.A., Vila J.A., Szleifer I. Electrostatic unfolding and interactions of albumin driven by pH changes: a molecular dynamics study. J. Phys. Chem. B. 2014;118:921–930. doi: 10.1021/jp409936v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barua S., Mitragotri S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: a review of current status and future prospects. Nano Today. 2014;9:223–243. doi: 10.1016/j.nantod.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bas A., Burns N., Gulotta A., Junker J., Drasler B., Lehner R., Aicher L., Constant S., Petri-Fink A., Rothen-Rutishauser B. Understanding the development, standardization, and validation process of alternative in vitro test methods for regulatory approval from a researcher perspective. Small. 2021;17 doi: 10.1002/smll.202006027. [DOI] [PubMed] [Google Scholar]
- Bashashati M., Moradi M., Sarosiek I. Interleukin-6 in irritable bowel syndrome: a systematic review and meta analysis of IL-6 (-G174C) and circulating IL-6 levels. J. Interferon Cytokine Res. 2017;99:132–138. doi: 10.1016/j.cyto.2017.08.017. [DOI] [PubMed] [Google Scholar]
- Bejarano L., Jordāo M.J.C., Joyce J.A. Therapeutic Targeting of the Tumor Microenvironment. Cancer Discov. 2021;11:933–959. doi: 10.1158/2159-8290.Cd-20-1808. [DOI] [PubMed] [Google Scholar]
- Beloqui A., Coco R., Memvanga P.B., Ucakar B., des Rieux A., Préat V. pH-sensitive nanoparticles for colonic delivery of curcumin in inflammatory bowel disease. Int. J. Pharm. 2014;473:203–212. doi: 10.1016/j.ijpharm.2014.07.009. [DOI] [PubMed] [Google Scholar]
- Bhushan B., Khanadeev V., Khlebtsov B., Khlebtsov N., Gopinath P. Impact of albumin based approaches in nanomedicine: Imaging, targeting and drug delivery. Adv. Colloid Interface Sci. 2017;246:13–39. doi: 10.1016/j.cis.2017.06.012. [DOI] [PubMed] [Google Scholar]
- Bilati U., Allémann E., Doelker E. Strategic approaches for overcoming peptide and protein instability within biodegradable nano- and microparticles. Eur. J. Pharm. Biopharm. 2005;59:375–388. doi: 10.1016/j.ejpb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Bray F., Laversanne M., Sung H., Ferlay J., Siegel R.L., Soerjomataram I., Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024;74:229–263. doi: 10.3322/caac.21834. [DOI] [PubMed] [Google Scholar]
- Cai Z., Wang S., Li J. Treatment of inflammatory bowel disease: a comprehensive review. Front. Med. (Lausanne) 2021;8 doi: 10.3389/fmed.2021.765474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassaing B., Aitken J.D., Malleshappa M., Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014;104:152511–152514. doi: 10.1002/0471142735.im1525s104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelakkot C., Ghim J., Ryu S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018;50 doi: 10.1038/s12276-018-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., You Q., Hu L., Gao J., Meng Q., Liu W., Wu X., Xu Q. The antioxidant procyanidin reduces reactive oxygen species signaling in macrophages and ameliorates experimental colitis in mice. Front. Immunol. 2017;8:1910. doi: 10.3389/fimmu.2017.01910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Wang C., Zhan Z.X., He W.W., Cheng Z.P., Li Y.Y., Liu Z. Near-infrared dye bound albumin with separated imaging and therapy wavelength channels for imaging-guided photothermal therapy. Biomaterials. 2014;35:8206–8214. doi: 10.1016/j.biomaterials.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Cho Y.J., Kim H., Lim S.I. Preserved structure and function of human serum albumin self-folded in the oxidative cytoplasm of Escherichia coli. J. Biotechnol. 2024;390:62–70. doi: 10.1016/j.jbiotec.2024.05.005. [DOI] [PubMed] [Google Scholar]
- Deng L., Zeng H., Hu X., Xiao M., He D., Zhang Y., Jin Y., Hu Y., Zhu Y., Gong L., Wang Z., Xiang L., Zhu R., Zhang Y., Cheng Y., Chen X., Zhang S., Peng Y., Cao K. Se@Albumin nanoparticles ameliorate intestinal mucositis caused by cisplatin via gut microbiota-targeted regulation. Nanoscale. 2021;13:11250–11261. doi: 10.1039/d0nr07981b. [DOI] [PubMed] [Google Scholar]
- Duan B., Zhao Y., Bai J., Wang J., Duan X., Luo X., Zhang R., Pu Y., Kou M., Lei J., Yang S. In: Gastrointestinal Cancers. Morgado-Diaz J.A., editor. Exon Publications; Brisbane (AU): 2022. Colorectal Cancer: An Overview. [PubMed] [Google Scholar]
- Duan Q., Shi J., Zhou L., Zhang B., Wang X., Sang S. pH-responsive and sustained release drug delivery system of BSA coated CDs-DOX. J. Mol. Struct. 2022;1248 doi: 10.1016/j.molstruc.2021.131358. [DOI] [Google Scholar]
- Duan Q.-J., Zhao Z.-Y., Zhang Y.-J., Fu L., Yuan Y.-Y., Du J.-Z., Wang J. Activatable fluorescent probes for real-time imaging-guided tumor therapy. Adv. Drug Del. Rev. 2023;196 doi: 10.1016/j.addr.2023.114793. [DOI] [PubMed] [Google Scholar]
- Elzoghby A.O., Samy W.M., Elgindy N.A. Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release. 2012;157:168–182. doi: 10.1016/j.jconrel.2011.07.031. [DOI] [PubMed] [Google Scholar]
- Fader A.N., Rose P.G. Abraxane for the treatment of gynecologic cancer patients with severe hypersensitivity reactions to paclitaxel. Int. J. Gynecol. Cancer. 2009;19:1281–1283. doi: 10.1111/IGC.0b013e3181a38e2f. [DOI] [PubMed] [Google Scholar]
- Fasano M., Curry S., Terreno E., Galliano M., Fanali G., Narciso P., Notari S., Ascenzi P. The extraordinary ligand binding properties of human serum albumin. IUBMB Life. 2005;57:787–796. doi: 10.1080/15216540500404093. [DOI] [PubMed] [Google Scholar]
- Fatima M., Sheikh A., Almalki W.H., Talegaonkar S., Dubey S.K., Amin M.C.I.M., Sahebkar A., Kesharwani P. Recent advancement on albumin nanoparticles in treating lung carcinoma. J. Drug Target. 2023;31:486–499. doi: 10.1080/1061186x.2023.2205609. [DOI] [PubMed] [Google Scholar]
- Fischer H.C., Chan W.C.W. Nanotoxicity: the growing need for study. Curr. Opin. Biotechnol. 2007;18:565–571. doi: 10.1016/j.copbio.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Fu Q., Sun J., Zhang W.P., Sui X.F., Yan Z.T., He Z.G. Nanoparticle Albumin-Bound (NAB) technology is a promising method for anti-cancer drug delivery. Recent Pat. Anti-Canc. 2009;4:262–272. doi: 10.2174/157489209789206869. [DOI] [PubMed] [Google Scholar]
- Fu Z., Wang X., Lu X., Yang Y., Zhao L., Zhou L., Wang K., Fu H. Mannose-decorated ginsenoside Rb1 albumin nanoparticles for targeted anti-inflammatory therapy. Front. Bioeng. Biotechnol. 2022:10–2022. doi: 10.3389/fbioe.2022.962380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad S.F., Park J., Park J.E., Fetih G.N., Tous S.S., Lee W., Yeo Y. Enhancing docetaxel delivery to multidrug-resistant cancer cells with albumin-coated nanocrystals. Mol. Pharm. 2018;15:871–881. doi: 10.1021/acs.molpharmaceut.7b00783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou S., Chen Q., Liu Y., Zeng L., Song H., Xu Z., Kang Y., Li C., Xiao B. Green fabrication of ovalbumin nanoparticles as natural polyphenol carriers for ulcerative colitis therapy. ACS Sustain. Chem. Eng. 2018;6:12658–12667. doi: 10.1021/acssuschemeng.8b01613. [DOI] [Google Scholar]
- Guha S., Majumder K., Mine Y. In: Encyclopedia of Food Chemistry. Melton L., Shahidi F., Varelis P., editors. Academic Press; Oxford: 2019. Egg Proteins; pp. 74–84. [Google Scholar]
- Harb I., Medhat E., Samir M., Abdel Fattah S., Badawy H.A.A., Gamal S.M., Ateyya H. Assessment of IV albumin and ringer lactate on the acute oral toxicity of acetylsalicylic acid in albino rats. Future J. Pharm. Sci. 2024;10:139. doi: 10.1186/s43094-024-00714-1. [DOI] [Google Scholar]
- Hashem L., Swedrowska M., Vllasaliu D. Intestinal uptake and transport of albumin nanoparticles: potential for oral delivery. Nanomedicine (Lond.) 2018;13:1255–1265. doi: 10.2217/nnm-2018-0029. [DOI] [PubMed] [Google Scholar]
- Hassanin I., Elzoghby A. Albumin-based nanoparticles: a promising strategy to overcome cancer drug resistance. Cancer Drug Resist. 2020;3:930–946. doi: 10.20517/cdr.2020.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H., Qin Q., Xu F., Chen Y., Rao S., Wang C., Jiang X., Lu X., Xie C. Oral polyphenol-armored nanomedicine for targeted modulation of gut microbiota-brain interactions in colitis. Sci. Adv. 2023;9 doi: 10.1126/sciadv.adf3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng D., Lee S.H., Ng W.K., Tan R.B. The nano spray dryer B-90. Expert Opin. Drug Deliv. 2011;8:965–972. doi: 10.1517/17425247.2011.588206. [DOI] [PubMed] [Google Scholar]
- Hirata K., Maruyama T., Watanabe H., Maeda H., Nakajou K., Iwao Y., Ishima Y., Katsumi H., Hashida M., Otagiri M. Genetically engineered mannosylated-human serum albumin as a versatile carrier for liver-selective therapeutics. J. Control. Release. 2010;145:9–16. doi: 10.1016/j.jconrel.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Hobbs S.K., Monsky W.L., Yuan F., Roberts W.G., Griffith L., Torchilin V.P., Jain R.K. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc. Natl. Acad. Sci. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua S., Marks E., Schneider J.J., Keely S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: Selective targeting to diseased versus healthy tissue. Nanomed.-Nanotechnol. 2015;11:1117–1132. doi: 10.1016/j.nano.2015.02.018. [DOI] [PubMed] [Google Scholar]
- Hua S.S. Orally administered liposomal formulations for colon targeted drug delivery. Front. Pharmacol. 2014;5 doi: 10.3389/fphar.2014.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Zhang L., Sun J., Sun Y., Gong L., Ge S., Zheng Y., Gao W., Wei X. Hypoxia reversion by low-immunogenic ultra-acid-sensitive comicelles of protein–polymer conjugates sensitizes tumors to photodynamic therapy. J. Am. Chem. Soc. 2024;146:7543–7554. doi: 10.1021/jacs.3c13501. [DOI] [PubMed] [Google Scholar]
- Hussain S., Joo J., Kang J., Kim B., Braun G.B., She Z.-G., Kim D., Mann A.P., Mölder T., Teesalu T., Carnazza S., Guglielmino S., Sailor M.J., Ruoslahti E. Antibiotic-loaded nanoparticles targeted to the site of infection enhance antibacterial efficacy. Nat. Biomed. Eng. 2018;2:95–103. doi: 10.1038/s41551-017-0187-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Injac R., Strukelj B. Recent advances in protection against doxorubicin-induced toxicity. Technol. Cancer Res. Treat. 2008;7:497–516. doi: 10.1177/153303460800700611. [DOI] [PubMed] [Google Scholar]
- Irache J.M., Merodio M., Arnedo A., Camapanero M.A., Mirshahi M., Espuelas S. Albumin nanoparticles for the intravitreal delivery of anticytomegaloviral drugs. Mini-Rev. Med. Chem. 2005;5:293–305. doi: 10.2174/1389557053175335. [DOI] [PubMed] [Google Scholar]
- Iwao Y., Tomiguchi I., Domura A., Mantaira Y., Minami A., Suzuki T., Ikawa T., Kimura S., Itai S. Inflamed site-specific drug delivery system based on the interaction of human serum albumin nanoparticles with myeloperoxidase in a murine model of experimental colitis. Eur. J. Pharm. Biopharm. 2018;125:141–147. doi: 10.1016/j.ejpb.2018.01.016. [DOI] [PubMed] [Google Scholar]
- Jacob E.M., Borah A., Pillai S.C., Kumar D.S. Inflammatory Bowel Disease: the Emergence of New Trends in Lifestyle and Nanomedicine as the Modern Tool for Pharmacotherapy. Nanomaterials (Basel, Switzerland) 2020;10 doi: 10.3390/nano10122460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanban-Esfahlan A., Dastmalchi S., Davaran S. A simple improved desolvation method for the rapid preparation of albumin nanoparticles. Int. J. Biol. Macromol. 2016;91:703–709. doi: 10.1016/j.ijbiomac.2016.05.032. [DOI] [PubMed] [Google Scholar]
- Ji Q., Zhu H., Qin Y., Zhang R., Wang L., Zhang E., Zhou X., Meng R. GP60 and SPARC as albumin receptors: key targeted sites for the delivery of antitumor drugs. Front. Pharmacol. 2024;15 doi: 10.3389/fphar.2024.1329636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiwara Y., Ueno H. Essential updates 2022-2023: Surgical and adjuvant therapies for locally advanced colorectal cancer. Ann. Gastroent. Surg. 2024;8:977–986. doi: 10.1002/ags3.12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.H., Kim K.J., Kim J.H., Kwak J.H., Song H., Cho J.Y., Hwang D.Y., Kim K.S., Jung Y.S. Comparision of doxorubicin-induced cardiotoxicity in the ICR mice of different sources. Lab. Anim. Res. 2017;33:165–170. doi: 10.5625/lar.2017.33.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K., Yamasaki K., Nishi K., Taguchi K., Otagiri M. Investigation of anti-tumor effect of doxorubicin-loaded human serum albumin nanoparticles prepared by a desolvation technique. Cancer Chemother. Pharmacol. 2019;83:1113–1120. doi: 10.1007/s00280-019-03832-3. [DOI] [PubMed] [Google Scholar]
- Kinoshita R., Ishima Y., Chuang V.T.G., Nakamura H., Fang J., Watanabe H., Shimizu T., Okuhira K., Ishida T., Maeda H., Otagiri M., Maruyama T. Improved anticancer effects of albumin-bound paclitaxel nanoparticle via augmentation of EPR effect and albumin-protein interactions using-nitrosated human serum albumin dimer. Biomaterials. 2017;140:162–169. doi: 10.1016/j.biomaterials.2017.06.021. [DOI] [PubMed] [Google Scholar]
- Koh B.-B., Lee E.-J., Ramachandraiah K., Hong G.-P. Characterization of bovine serum albumin hydrolysates prepared by subcritical water processing. Food Chem. 2019;278:203–207. doi: 10.1016/j.foodchem.2018.11.069. [DOI] [PubMed] [Google Scholar]
- Kratz F. Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles. J. Control. Release. 2008;132:171–183. doi: 10.1016/j.jconrel.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Kuang Y., Chen S.-X., Chen H. Responsive nanoplatforms: Versatile design strategies for efficient cancer theranostics. Mater. Des. 2023;232 doi: 10.1016/j.matdes.2023.112076. [DOI] [Google Scholar]
- Kubiak-Ossowska K., Jachimska B., Al Qaraghuli M., Mulheran P.A. Protein interactions with negatively charged inorganic surfaces. Curr. Opin. Colloid Interface Sci. 2019;41:104–117. doi: 10.1016/j.cocis.2019.02.001. [DOI] [Google Scholar]
- Kumar S., Singh P., Kumar A. Targeted therapy of irritable bowel syndrome with anti-inflammatory cytokines. J. Clin. Gastroenterol. 2022;15:1–10. doi: 10.1007/s12328-021-01555-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushwah V., Katiyar S.S., Dora C.P., Kumar Agrawal A., Lamprou D.A., Gupta R.C., Jain S. Co-delivery of docetaxel and gemcitabine by anacardic acid modified self-assembled albumin nanoparticles for effective breast cancer management. Acta Biomater. 2018;73:424–436. doi: 10.1016/j.actbio.2018.03.057. [DOI] [PubMed] [Google Scholar]
- Lamichhane S., Lee S. Albumin nanoscience: homing nanotechnology enabling targeted drug delivery and therapy. Arch. Pharm. Res. 2020;43:118–133. doi: 10.1007/s12272-020-01204-7. [DOI] [PubMed] [Google Scholar]
- Lamprecht A., Schäfer U., Lehr C.M. Size-dependent bioadhesion of micro- and nanoparticulate carriers to the inflamed colonic mucosa. Pharm. Res. 2001;18:788–793. doi: 10.1023/A:1011032328064. [DOI] [PubMed] [Google Scholar]
- Langer K., Balthasar S., Vogel V., Dinauer N., von Briesen H., Schubert D. Optimization of the preparation process for human serum albumin (HSA) nanoparticles. Int. J. Pharm. 2003;257:169–180. doi: 10.1016/S0378-5173(03)00134-0. [DOI] [PubMed] [Google Scholar]
- Lei C., Liu X.R., Chen Q.B., Li Y., Zhou J.L., Zhou L.Y., Zou T. Hyaluronic acid and albumin based nanoparticles for drug delivery. J. Control. Release. 2021;331:416–433. doi: 10.1016/j.jconrel.2021.01.033. [DOI] [PubMed] [Google Scholar]
- Li B., Liu X.Y., Long Q., Zhuang X.D., Gao Y.F., Ali B., Chen H.T., Zhang D.Y., Wang X.Y., Guo W.S. Inflammation responsive tofacitinib loaded albumin nanomedicine for targeted synergistic therapy in ulcerative colitis. Nano Res. 2023;16:9873–9884. doi: 10.1007/s12274-023-5743-6. [DOI] [Google Scholar]
- Li L., Qian Y., Sun L., Han F.Y., Zhang R., Wang P.-Y., Xu Z.P. Albumin-stabilized layered double hydroxide nanoparticles synergized combination chemotherapy for colorectal cancer treatment. Nanomed. Nanotechnol. Biol. Med. 2021;34 doi: 10.1016/j.nano.2021.102369. [DOI] [PubMed] [Google Scholar]
- Li Y., Chen T., Chen L., Wu D., Hu J. Construction of hyaluronic acid-functionalized magnolol nanoparticles for ulcerative colitis treatment. Int. J. Biol. Macromol. 2024;268 doi: 10.1016/j.ijbiomac.2024.131920. [DOI] [PubMed] [Google Scholar]
- Liang X., Mu M., Chen B., Chuan D., Zhao N., Fan R., Tang X., Chen H., Han B., Guo G. BSA-assisted synthesis of nanoreactors with dual pH and glutathione responses for ferroptosis and photodynamic synergistic therapy of colorectal cancer. Mater. Today Adv. 2022;16 doi: 10.1016/j.mtadv.2022.100308. [DOI] [Google Scholar]
- Liu P., Gao C.F., Chen H.G., Vong C.T., Wu X., Tang X.D., Wang S.P., Wang Y.T. Receptor-mediated targeted drug delivery systems for treatment of inflammatory bowel disease: Opportunities and emerging strategies. Acta Pharm. Sin. B. 2021;11:2798–2818. doi: 10.1016/j.apsb.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.J., Dong Z.R., Liu K.H., Lu Y., Wu W., Qi J.P., Chen Z.J. Targeting strategies of oral nano-delivery systems for treating inflammatory bowel disease. Int. J. Pharm. 2021;600 doi: 10.1016/j.ijpharm.2021.120461. [DOI] [PubMed] [Google Scholar]
- Löw K., Wacker M., Wagner S., Langer K., von Briesen H. Targeted human serum albumin nanoparticles for specific uptake in EGFR-Expressing colon carcinoma cells. Nanomed. Nanotechnol. Biol. Med. 2011;7:454–463. doi: 10.1016/j.nano.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Lucafò M., Curci D., Franzin M., Decorti G., Stocco G. Inflammatory bowel disease and risk of colorectal cancer: an overview from pathophysiology to pharmacological prevention. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.772101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo R., Lin M., Zhang C., Shi J., Zhang S., Chen Q., Hu Y., Zhang M., Zhang J., Gao F. Genipin-crosslinked human serum albumin coating using a tannic acid layer for enhanced oral administration of curcumin in the treatment of ulcerative colitis. Food Chem. 2020;330 doi: 10.1016/j.foodchem.2020.127241. [DOI] [PubMed] [Google Scholar]
- Ma P., Mumper R.J. Paclitaxel nano-delivery systems: a comprehensive review. J. Nanomed. Nanotechnol. 2013;4 doi: 10.4172/2157-7439.1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macsharry J., O'Mahony L., Fanning A., Bairead E., Sherlock G., Tiesman J., Fulmer A., Kiely B., Dinan T., Shanahan F., Quigley E. Mucosal cytokine imbalance in irritable bowel syndrome. Scand. J. Gastroentero. 2008;43:1467–1476. doi: 10.1080/00365520802276127. [DOI] [PubMed] [Google Scholar]
- Maddineni G., Choday S., Morales A., Aakash F., Kajal D., Rehman O., Karpinska-Leydier K., Salami A., Begosh-Mayne D., El-Din M., Brahambhatt B. Shifts in Ibd Incidence, Mortality, and Burden: a Comprehensive Analysis of Us and Global Trends (1990-2019) Gastroenterology. 2024;166:S54. [Google Scholar]
- Mana M.D., Hussey A.M., Tzouanas C.N., Imada S., Barrera Millan Y., Bahceci D., Saiz D.R., Webb A.T., Lewis C.A., Carmeliet P., Mihaylova M.M., Shalek A.K., Yilmaz Ö.H. High-fat diet-activated fatty acid oxidation mediates intestinal stemness and tumorigenicity. Cell Rep. 2021;35 doi: 10.1016/j.celrep.2021.109212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura Y., Maeda H. A new concept for macromolecular therapeutics in cancer-chemotherapy - mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- McDowell C., Farooq U., Haseeb M. StatPearls Publishing; StatPearls: 2025. Inflammatory Bowel Disease. [PubMed] [Google Scholar]
- Mehta M., Bui T.A., Yang X., Aksoy Y., Goldys E.M., Deng W. Lipid-based nanoparticles for drug/gene delivery: an overview of the production techniques and difficulties encountered in their industrial development. ACS Materials Au. 2023;3:600–619. doi: 10.1021/acsmaterialsau.3c00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng R., Zhu H.M., Wang Z.W., Hao S.L., Wang B.C. Preparation of drug-loaded albumin nanoparticles and its application in cancer therapy. J. Nanomater. 2022;2022 doi: 10.1155/2022/3052175. [DOI] [Google Scholar]
- Meng X., Zhang G., Cao H., Yu D., Fang X., de Vos W.M., Wu H. Gut dysbacteriosis and intestinal disease: mechanism and treatment. J. Appl. Microbiol. 2020;129:787–805. doi: 10.1111/jam.14661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merodio M., Arnedo A., Renedo M.J., Irache J.M. Ganciclovir-loaded albumin nanoparticles: characterization and in vitro release properties. Eur. J. Pharm. Sci. 2001;12:251–259. doi: 10.1016/S0928-0987(00)00169-X. [DOI] [PubMed] [Google Scholar]
- Min S.Y., Byeon H.J., Lee C., Seo J., Lee E.S., Shin B.S., Choi H.G., Lee K.C., Youn Y.S. Facile one-pot formulation of TRAIL-embedded paclitaxel-bound albumin nanoparticles for the treatment of pancreatic cancer. Int. J. Pharm. 2015;494:506–515. doi: 10.1016/j.ijpharm.2015.08.055. [DOI] [PubMed] [Google Scholar]
- Minetti F., Mengatto L.N., Olivares M.L., Berli C.L.A. Generation of curcumin-loaded albumin nanoparticles by using off-the-shelf microfluidics driven by gravity. Food Res. Int. 2022;162 doi: 10.1016/j.foodres.2022.111984. [DOI] [PubMed] [Google Scholar]
- Monahan F.J., German J.B., Kinsella J.E. Effect of Ph and temperature on protein unfolding and thiol-disulfide interchange reactions during heat-induced gelation of whey proteins. J. Agric. Food Chem. 1995;43:46–52. doi: 10.1021/jf00049a010. [DOI] [Google Scholar]
- Murgia X., Loretz B., Hartwig O., Hittinger M., Lehr C.M. The role of mucus on drug transport and its potential to affect therapeutic outcomes. Adv. Drug Deliv. Rev. 2018;124:82–97. doi: 10.1016/j.addr.2017.10.009. [DOI] [PubMed] [Google Scholar]
- Oleaga C., Bernabini C., Smith A.S.T., Srinivasan B., Jackson M., McLamb W., Platt V., Bridges R., Cai Y.Q., Santhanam N., Berry B., Najjar S., Akanda N., Guo X.F., Martin C., Ekman G., Esch M.B., Langer J., Ouedraogo G., Cotovio J., Breton L., Shuler M.L., Hickman J.J. Multi-Organ toxicity demonstration in a functional human system composed of four organs. Sci. Rep. 2016;6 doi: 10.1038/srep20030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Y.L., Zhao J.L., Wang S.G. Multifunctional hydrogels based on chitosan, hyaluronic acid and other biological macromolecules for the treatment of inflammatory bowel disease: a review. Int. J. Biol. Macromol. 2023;227:505–523. doi: 10.1016/j.ijbiomac.2022.12.032. [DOI] [PubMed] [Google Scholar]
- Peyrin-Biroulet L., Sandborn W.J., Panaccione R., Domènech E., Pouillon L., Siegmund B., Danese S., Ghosh S. Tumour necrosis factor inhibitors in inflammatory bowel disease: the story continues. Therap. Adv. Gastroenterol. 2021;14 doi: 10.1177/17562848211059954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani S., Tufail S., Rosalia M., Dorati R., Genta I., Chiesa E., Conti B. Antibiotic-loaded nano-sized delivery systems: an insight into gentamicin and vancomycin. J. Funct. Biomater. 2024;15 doi: 10.3390/jfb15070194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R.J., Arends M.J., Churchhouse A.M.D., Din S. Inflammatory bowel disease-associated colorectal cancer: translational risks from mechanisms to medicines. J. Crohns Colitis. 2021;15:2131–2141. doi: 10.1093/ecco-jcc/jjab102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu N., Song K., Ji Y.T., Liu M.X., Chen L.J., Lee R.J., Teng L.S. Albumin nanoparticle-based drug delivery systems. Int. J. Nanomed. 2024;19:6945–6980. doi: 10.2147/Ijn.S467876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja S.T.K., Thiruselvi T., Mandal A.B., Gnanamani A. pH and redox sensitive albumin hydrogel: a self-derived biomaterial. Sci. Rep. 2015;5 doi: 10.1038/srep15977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivankar S. An overview of doxorubicin formulations in cancer therapy. J. Cancer Res. Ther. 2014;10:853–858. doi: 10.4103/0973-1482.139267. [DOI] [PubMed] [Google Scholar]
- Safavi M.S., Shojaosadati S.A., Yang H.G., Kim Y., Park E.J., Lee K.C., Na D.H. Reducing agent-free synthesis of curcumin-loaded albumin nanoparticles by self-assembly at room temperature. Int. J. Pharm. 2017;529:303–309. doi: 10.1016/j.ijpharm.2017.06.087. [DOI] [PubMed] [Google Scholar]
- Saha A., Pradhan N., Chatterjee S., Singh R.K., Trivedi V., Bhattacharyya A., Manna D. Fatty-amine-conjugated cationic bovine serum albumin nanoparticles for target-specific hydrophobic drug delivery. ACS Appl. Nano Mater. 2019;2:3671–3683. doi: 10.1021/acsanm.9b00607. [DOI] [Google Scholar]
- Schmidt E.G.W., Hvam M.L., Antunes F., Cameron J., Viuff D., Andersen B., Kristensen N.N., Howard K.A. Direct demonstration of a neonatal Fc receptor (FcRn)-driven endosomal sorting pathway for cellular recycling of albumin. J. Biol. Chem. 2017;292:13312–13322. doi: 10.1074/jbc.M117.794248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmulson M., Pulido-London D., Rodriguez O., Morales-Rochlin N., Martinez-García R., Gutierrez-Ruiz M.C., López-Alvarenga J.C., Robles-Díaz G., Gutiérrez-Reyes G. Lower serum IL-10 is an independent predictor of IBS among volunteers in Mexico. Am. J. Gastroenterol. 2012;107:747–753. doi: 10.1038/ajg.2011.484. [DOI] [PubMed] [Google Scholar]
- Sedghi S., Fields J.Z., Klamut M., Urban G., Durkin M., Winship D., Fretland D., Olyaee M., Keshavarzian A. Increased production of luminol enhanced chemiluminescence by the inflamed colonic mucosa in patients with ulcerative colitis. Gut. 1993;34:1191–1197. doi: 10.1136/gut.34.9.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G.L. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Kaur A., Jain U.K., Chandra R., Madan J. Stealth recombinant human serum albumin nanoparticles conjugating 5-fluorouracil augmented drug delivery and cytotoxicity in human colon cancer. HT-29 cells. Colloid. Surface. B. 2017;155:200–208. doi: 10.1016/j.colsurfb.2017.04.020. [DOI] [PubMed] [Google Scholar]
- Sies H., Jones D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nature Rev. Mol. Cell Biol. 2020;21:363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- Sivadasan D., Sultan M.H., Madkhali O., Almoshari Y., Thangavel N. Polymeric lipid hybrid nanoparticles (PLNs) as emerging drug delivery platform-a comprehensive review of their properties, preparation methods, and therapeutic applications. Pharmaceutics. 2021:13. doi: 10.3390/pharmaceutics13081291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleep D. Albumin and its application in drug delivery. Expert. Opin. Drug. Deliv. 2015;12:793–812. doi: 10.1517/17425247.2015.993313. [DOI] [PubMed] [Google Scholar]
- Spada A., Emami J., Tuszynski J.A., Lavasanifar A. The uniqueness of albumin as a carrier in nanodrug delivery. Mol. Pharm. 2021;18:1862–1894. doi: 10.1021/acs.molpharmaceut.1c00046. [DOI] [PubMed] [Google Scholar]
- Srivastava P., Hira S.K., Srivastava D.N., Gupta U., Sen P., Singh R.A., Manna P.P. Protease-responsive targeted delivery of doxorubicin from bilirubin-BSA-capped mesoporous silica nanoparticles against colon cancer. ACS Biomater. Sci. Eng. 2017;3:3376–3385. doi: 10.1021/acsbiomaterials.7b00635. [DOI] [PubMed] [Google Scholar]
- Szczech M., Szczepanowicz K. Polymeric core-shell nanoparticles prepared by spontaneous emulsification solvent evaporation and functionalized by the layer-by-layer method. Nanomaterials-Basel. 2020;10 doi: 10.3390/nano10030496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymaszek P., Fiedor P., Chachaj-Brekiesz A., Tyszka-Czochara M., Świergosz T., Ortyl J. Molecular interactions of bovine serum albumin (BSA) with pyridine derivatives as candidates for non-covalent protein probes: aspectroscopic investigation. J. Mol. Liq. 2022;347 doi: 10.1016/j.molliq.2021.118262. [DOI] [Google Scholar]
- Taheri A., Dinarvand R., Ahadi F., Khorramizadeh M.R., Atyabi F. The in vivo antitumor activity of LHRH targeted methotrexate–human serum albumin nanoparticles in 4T1 tumor-bearing Balb/c mice. Int. J. Pharm. 2012;431:183–189. doi: 10.1016/j.ijpharm.2012.04.033. [DOI] [PubMed] [Google Scholar]
- Tao H.-Y., Wang R.-Q., Sheng W.-J., Zhen Y.-S. The development of human serum albumin-based drugs and relevant fusion proteins for cancer therapy. Int. J. Biol. Macromol. 2021;187:24–34. doi: 10.1016/j.ijbiomac.2021.07.080. [DOI] [PubMed] [Google Scholar]
- Thakkar S., Sharma D., Kalia K., Tekade R.K. Tumor microenvironment targeted nanotherapeutics for cancer therapy and diagnosis: A review. Acta Biomater. 2020;101:43–68. doi: 10.1016/j.actbio.2019.09.009. [DOI] [PubMed] [Google Scholar]
- Thao L.Q., Byeon H.J., Lee C., Lee S., Lee E.S., Choi H.G., Park E.S., Youn Y.S. Pharmaceutical potential of tacrolimus-loaded albumin nanoparticles having targetability to rheumatoid arthritis tissues. Int. J. Pharm. 2016;497:268–276. doi: 10.1016/j.ijpharm.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Thao L.Q., Byeon H.J., Lee C., Lee S., Lee E.S., Choi Y.W., Choi H.G., Park E.S., Lee K.C., Youn Y.S. Doxorubicin-bound albumin nanoparticles containing a TRAIL protein for targeted treatment of colon cancer. Pharm. Res. 2016;33:615–626. doi: 10.1007/s11095-015-1814-z. [DOI] [PubMed] [Google Scholar]
- Tian X.T., Zhan J.P., Qiao C., Ge J.L., Li D.H. Rising of natural therapies: Potential and challenges of traditional Chinese medicine in the management of gastrointestinal diseases. World J. Gastroenterol. 2025;31 doi: 10.3748/wjg.v31.i9.103145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiruppathi C., Naqvi T., Wu Y., Vogel S.M., Minshall R.D., Malik A.B. Albumin mediates the transcytosis of myeloperoxidase by means of caveolae in endothelial cells. Proc. Natl. Acad. Sci. USA. 2004;101:7699–7704. doi: 10.1073/pnas.0401712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiruppathi C., Naqvi T., Wu Y.B., Vogel S.M., Minshall R.D., Malik A.B. Albumin mediates the transcytosis of myeloperoxidase by means of caveolae in endothelial cells. Proc. Natl. Acad. Sci. USA. 2004;101:7699–7704. doi: 10.1073/pnas.0401712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma M.L., Dhanya B.S., Sukriti Rani V., Thakur M., Jeslin J., Kushwaha R. Carbohydrate and protein based biopolymeric nanoparticles: Current status and biotechnological applications. Int. J. Biol. Macromol. 2020;154:390–412. doi: 10.1016/j.ijbiomac.2020.03.105. [DOI] [PubMed] [Google Scholar]
- Vesković A., Bajuk-Bogdanović D., Arion V.B., Popović Bijelić A. Spectroscopic characterization of the binding and release of hydrophilic, hydrophobic and amphiphilic molecules from ovalbumin supramolecular hydrogels. Gels (Basel, Switzerland) 2022;9 doi: 10.3390/gels9010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignal C., Guilloteau E., Gower-Rousseau C., Body-Malapel M. Review article: Epidemiological and animal evidence for the role of air pollution in intestinal diseases. Sci. Total Environ. 2021;757 doi: 10.1016/j.scitotenv.2020.143718. [DOI] [PubMed] [Google Scholar]
- Visentini F.F., Perez A.A., Santiago L.G. Bioactive compounds: Application of albumin nanocarriers as delivery systems. Crit. Rev. Food Sci. Nutr. 2023;63:7238–7268. doi: 10.1080/10408398.2022.2045471. [DOI] [PubMed] [Google Scholar]
- Wan Y.X., Xie Z.Z., Wang M.J., Liu Y.L., Zheng M.B., Xu D., Du C. Esterase-triggered rapid release of succinic anhydride conjugated curcumin co-prodrug for osteosarcoma therapy. Eur. Polym. J. 2023;198 doi: 10.1016/j.eurpolymj.2023.112382. [DOI] [Google Scholar]
- Wang S.M., Zhou X.R., Zeng Z.K., Sui M.J., Chen L.H., Feng C., Huang C., Yang Q., Ji M.J., Hou P. Atovaquone-HSA nano-drugs enhance the efficacy of PD-1 blockade immunotherapy by alleviating hypoxic tumor microenvironment. J. Nanobiotechnol. 2021;19 doi: 10.1186/s12951-021-01034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Liu Y., Wang L., Chen Z., Ye Q., Chen K., Zhao C., Yang M., Xia W., Zhang M., Qiao J., Zhou G., Wu J. Combretastatin A4-based albumin nanoparticles remodeling the tumor immune microenvironment to enhance T cell immunotherapy in colon cancer. ACS Nano. 2025;19:23746–23759. doi: 10.1021/acsnano.5c03638. [DOI] [PubMed] [Google Scholar]
- Wang Z.J., Li J., Cho J., Malik A.B. Prevention of vascular inflammation by nanoparticle targeting of adherent neutrophils. Nat. Nanotechnol. 2014;9:204–210. doi: 10.1038/Nnano.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C., Coester C., Kreuter J., Langer K. Desolvation process and surface characterisation of protein nanoparticles. Int. J. Pharm. 2000;194:91–102. doi: 10.1016/S0378-5173(99)00370-1. [DOI] [PubMed] [Google Scholar]
- Wei M., Gao Y., Li X., Serpe M.J. Stimuli-responsive polymers and their applications. Polymer Chem. 2017;8:127–143. doi: 10.1039/c6py01585a. [DOI] [Google Scholar]
- Weiner J., Widman S., Golek Z., Tranquilli M., Elefteriades J.A. Role of bovine serum albumin-glutaraldehyde glue in the formation of anastomatic pseudoaneurysms. J. Card. Surg. 2011;26:76–81. doi: 10.1111/j.1540-8191.2010.01162.x. [DOI] [PubMed] [Google Scholar]
- Wilson B., Geetha K.M., Divekar K., Jenita J.L., Premakumari K.B., Sagar G. Albumin nanoparticle enhances oxaliplatin concentration in the colorectal region to treat colon cancer with increased efficacy. BioNanoScience. 2024;15:46. doi: 10.1007/s12668-024-01651-2. [DOI] [Google Scholar]
- Wongsasulak S., Patapeejumruswong M., Weiss J., Supaphol P., Yoovidhya T. Electrospinning of food-grade nanofibers from cellulose acetate and egg albumen blends. J. Food Eng. 2010;98:370–376. doi: 10.1016/j.jfoodeng.2010.01.014. [DOI] [Google Scholar]
- Wu C., Bi C., Kim G.-S., Yang Z., Li S., Dai T., Wu X., Tan J., He N., Li S. Oral colon-targeted responsive chitosan/pectin-based nanoparticles propels the application of tofacitinib in colitis therapy. Sci. Rep. 2025;15 doi: 10.1038/s41598-024-84322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T.Q., Wan J., Qu X., Xia K., Wang F.T., Zhang Z.C., Yang M.Q., Wu X.C., Gao R.Y., Yuan X.Q., Fang L., Chen C.Q., Yin L. Nodal promotes colorectal cancer survival and metastasis through regulating SCD1-mediated ferroptosis resistance. Cell Death Dis. 2023;14 doi: 10.1038/s41419-023-05756-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Y., Xu P.F. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021;14 doi: 10.1016/j.tranon.2021.101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B., Xu Z.G., Viennois E., Zhang Y.C., Zhang Z., Zhang M.Z., Han M.K., Kang Y.J., Merlin D. Orally targeted delivery of tripeptide KPV via hyaluronic acid-functionalized nanoparticles efficiently alleviates ulcerative colitis. Mol. Ther. 2017;25:1628–1640. doi: 10.1016/j.ymthe.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J.N., Wang C.Y., Wang N., Zhu S., Mei L.Q., Zhang X., Yong Y., Li L.L., Chen C.Y., Huang C.S., Gu Z.J., Li Y.L., Zhao Y.L. Graphdiyne nanoradioprotector with efficient free radical scavenging ability for mitigating radiation-induced gastrointestinal tract damage. Biomaterials. 2020;244 doi: 10.1016/j.biomaterials.2020.119940. [DOI] [PubMed] [Google Scholar]
- Xiong J.Y., Gao H.L. Matrix metalloproteases-responsive nanomaterials for tumor targeting diagnosis and treatment. J. Microencaps. 2017;34:440–453. doi: 10.1080/02652048.2017.1343873. [DOI] [PubMed] [Google Scholar]
- Xiong Z., Weng T., Wang J., Xiao S., Suo H., Li D. Regulatory mechanisms of phosphorylation in ovalbumin self-assembly for aggregates formation: Insights into size, structure, and morphology. Food Hydrocolloids. 2024;157 doi: 10.1016/j.foodhyd.2024.110431. [DOI] [Google Scholar]
- Xu R., Fisher M., Juliano R.L. Targeted albumin-based nanoparticles for delivery of amphipathic drugs. Bioconj. Chem. 2011;22:870–878. doi: 10.1021/bc1002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Li Z., Li Y., He W., Yan J. Transforming albumin into a trojan horse of immunotherapy-resistant colorectal cancer with a high microsatellite instability. ACS Nano. 2024;18:19332–19344. doi: 10.1021/acsnano.4c05893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo J., Park C., Yi G., Lee D., Koo H. Active targeting strategies using biological ligands for nanoparticle drug delivery systems. Cancers. 2019;11 doi: 10.3390/cancers11050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis M.A., Tawfeek H.M., Abdellatif A.A.H., Abdel-Aleem J.A., Harashima H. Clinical translation of nanomedicines: Challenges, opportunities, and keys. Adv. Drug Del. Rev. 2022;181 doi: 10.1016/j.addr.2021.114083. [DOI] [PubMed] [Google Scholar]
- Yu L., Yao L., Yang K., Fei W., Chen Q., Qin L., Liu S., Cao M., Liu Q., Qin B. cRGD-modified core–shell mesoporous silica@BSA nanoparticles for drug delivery. Polym. Bull. 2022;79:10555–10571. doi: 10.1007/s00289-021-03999-x. [DOI] [Google Scholar]
- Yu Z., Li X.D., Duan J.H., Yang X.D. Targeted treatment of colon cancer with aptamer-guided albumin nanoparticles loaded with docetaxel. Int. J. Nanomed. 2020;15:6737–6748. doi: 10.2147/Ijn.S267177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeshan M., Ali H., Khan S., Khan S.A., Weigmann B. Advances in orally-delivered pH-sensitive nanocarrier systems; an optimistic approach for the treatment of inflammatory bowel disease. Int. J. Pharm. 2019;558:201–214. doi: 10.1016/j.ijpharm.2018.12.074. [DOI] [PubMed] [Google Scholar]
- Zeng J., Sun Y., Sun S., Jiang M., Zhang D., Li W., Liu Z., Shang H., Guan X., Zhang W. Leveraging nanodrug delivery system for simultaneously targeting tumor cells and M2 tumor-associated macrophages for efficient colon cancer therapy. ACS Appl. Mater. Inter. 2022;14:50475–50484. doi: 10.1021/acsami.2c11534. [DOI] [PubMed] [Google Scholar]
- Zhang D.Y., Zhao J.J., Zhang Y.J., Jiang H.F., Liu D. Revisiting immune checkpoint inhibitors: new strategies to enhance efficacy and reduce toxicity. Front. Immunol. 2024;15 doi: 10.3389/fimmu.2024.1490129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F.K., Du Y.W., Zheng J.C., Cai Z.W., Ding T., Zhuang P.Z., Yang D., Liao F.L., Zhang Y., Yang W., Xiao Y.F., He W.L., Cui W.G., Guo W.S. Oral administration of multistage albumin nanomedicine depots (MANDs) for targeted efficient alleviation of chronic inflammatory diseases. Adv. Funct. Mater. 2023;33 doi: 10.1002/adfm.202211644. [DOI] [Google Scholar]
- Zhang H., Yang J., Sun R.Z., Han S.R., Yang Z.G., Teng L.S. Microfluidics for nano-drug delivery systems: From fundamentals to industrialization. Acta. Pharm. Sin. B. 2023;13:3277–3299. doi: 10.1016/j.apsb.2023.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.F., Langer R., Traverso G. Nanoparticulate drug delivery systems targeting inflammation for treatment of inflammatory bowel disease. Nano Today. 2017;16:82–96. doi: 10.1016/j.nantod.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.F., Cho W.J., Jin A.T., Kok L.Y., Shi Y.H., Heller D.E., Lee Y.A.L., Zhou Y.X., Xie X., Korzenik J.R., Lennerz J.K., Traverso G. Heparin-coated albumin nanoparticles for drug combination in targeting inflamed intestine. Adv. Healthc. Mater. 2020;9 doi: 10.1002/adhm.202000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Zhang Y., Wang P., Liu K., Zheng Y., Wen J., Wang K., Wen X. Layer by layer self-assembled hyaluronic acid nanoarmor for the treatment of ulcerative colitis. J. Nanobiotechnol. 2024;22 doi: 10.1186/s12951-024-02933-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Yu X., Wang C.L., Liu Y., Jia M., Lei F.T., Tian J., Li C.H. Targeted co-delivery biomimetic nanoparticles reverse macrophage polarization for enhanced rheumatoid arthritis therapy. Drug Deliv. 2022;29:1025–1037. doi: 10.1080/10717544.2022.2057616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Zhang X.M., Li M., Wu W.Q., Sun X., Zhang L., Gong T. Novel lipid hybrid albumin nanoparticle greatly lowered toxicity of pirarubicin. Mol. Pharm. 2013;10:3832–3841. doi: 10.1021/mp400303w. [DOI] [PubMed] [Google Scholar]
- Zsila F., Bikadi Z., Malik D., Hari P., Pechan I., Berces A., Hazai E. Evaluation of drug–human serum albumin binding interactions with support vector machine aided online automated docking. Bioinformatics. 2011;27:1806–1813. doi: 10.1093/bioinformatics/btr284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.