Abstract
Current kidney function assessment primarily relies on estimation of glomerular filtration rate (GFR), which fails to capture the full spectrum of kidney functions and may limit accurate disease classification and targeted treatments. To propose innovative strategies to comprehensively assess kidney functions, aiming to improve our capacity to uncover pathophysiologic mechanisms and delineate novel disease subgroups, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) convened a workshop titled “Re-imagining Kidney Function Assessment.” This workshop brought together experts to discuss current limitations and potential advancements in kidney function evaluation. Key themes emerged, including the following: (i) the need for standardized protocols for measured GFR using exogenous filtration markers, (ii) the potential of kidney functional reserve (KFR) and stress tests to reveal subclinical kidney dysfunction, (iii) the importance of assessing tubular secretion alongside glomerular filtration, (iv) the value of glomerular permselectivity measurements in predicting disease progression; and (v) the promise of integrating molecular profiling with functional assessments for precision medicine in nephrology. The workshop highlighted the critical need for a more comprehensive approach to kidney function assessment. Integrating diverse kidney function measures into tailored, individual-level assessments could lead to more accurate disease classification, targeted interventions, ability to track response to therapies, and improved patient outcomes. Future research should focus on developing and validating these novel assessment strategies to advance precision medicine in nephrology.
Introduction and Objectives of the NIDDK Workshop on “Re-imagining Kidney Function Assessment”
Limitations of Current Kidney Function Assessment
Kidney function assessment is a critical aspect of diagnosing, managing, and treating kidney diseases. The kidneys play a vital role in maintaining overall health and homeostasis, extending far beyond the commonly assessed GFR or proteinuria as markers of kidney damage. These remarkable organs are responsible for a wide range of essential functions, including filtration and excretion of waste products and toxins; as well as regulation of extracellular fluid volume, blood pH, ionic composition, osmolality, and blood pressure. They also serve important endocrine functions, producing hormones such as erythropoietin, renin, calcitriol, and klotho, and contribute to glucose metabolism. Impairment in any of these domains can result in clinically significant complications that are not captured by assessing GFR or proteinuria alone. For example, declines in erythropoietin or calcitriol production can contribute to anemia and mineral and bone disorders, respectively. Moreover, current approaches do not account for the kidneys’ intrinsic compensatory capacity, in which 1 kidney can preserve overall function for extended periods even when the other kidney is injured or loses function. This compensation can delay the detection of early or localized pathology, potentially underestimating the true burden of kidney disease. In selected cases, kidney biopsy provides complementary structural information that can help clarify these limitations. Although it is an invasive procedure, biopsy offers direct visualization of glomerular, tubular, and interstitial compartments and can uncover abnormalities not evident through functional markers alone. Discrepancies are not uncommon; for example, some individuals may have significant histologic fibrosis despite preserved GFR, reflecting compensatory hyperfiltration by unaffected nephrons. These observations reinforce the need to integrate structural and functional assessments to better capture the full spectrum of kidney dysfunction.
A more comprehensive assessment of kidney functions is needed to open new avenues for discovery in research and clinical care. This includes the development of novel biomarkers and imaging modalities, alongside refinement and standardization of existing methodologies. In this context, standardization refers to the establishment of consensus protocols for measurement techniques, reference ranges, and reporting criteria to ensure consistency, reproducibility, and comparability across studies and clinical settings. This will enable comparative analyses and generate more reliable data that might yield novel insights into kidney disease pathogenesis, with applications to differential diagnosis, monitoring, treatment, and subclassification of diseases to promote individualized therapies.
Case Examples Illustrating Shortcomings of Current Kidney Function Assessments
In contrast to nephrology, other medical specialties have embraced comprehensive functional assessments to guide clinical decision-making and advance patient care. For example, cardiology routinely uses stress tests, echocardiograms, cardiac magnetic resonance imaging (MRI), and biomarkers such as B-type natriuretic peptide and cardiac troponin to evaluate cardiac function and direct therapy. Similarly, hepatology employs liver function tests to assess liver health by examining inflammation, synthetic capacity, and bile secretion, whereas pulmonology uses measurements of oxygen and carbon dioxide exchange alongside evaluations of parenchymal integrity. These multifunctional approaches have facilitated the identification of disease subgroups and the development of targeted treatment strategies.
To illustrate the shortcomings of our current approach, consider a 45-year-old male with no significant medical or family history, a body mass index of 28 kg/m2, and a blood pressure of 132/85 mm Hg, who underwent routine laboratory testing, which revealed a serum creatinine concentration of 1.2 mg/dl. Subsequent evaluations showed an estimated GFR (eGFR) of 75 ml/min per 1.73 m2 and a urine albumin-to-creatinine ratio (uACR) of 70 mg/g. Current kidney function assessments are unable to clearly determine whether these findings reflect intrinsic kidney disease, previous or ongoing kidney injury, or are attributable to other factors. Even with directly measured GFR, early kidney disease may go undetected because of compensatory hyperfiltration by noninjured glomeruli. In addition, although the uACR is diagnostically informative at very low (< 30 mg/g) or high (> 300 mg/g) values, it is less conclusive within the intermediate range of 30 to 300 mg/g. Similarly, a 45-year-old male with well-controlled hypertension and a 10-year history of type 2 diabetes presents with the same kidney function metrics: an eGFR of 75 ml/min per 1.73 m2 and a uACR of 70 mg/g. These cases underscore several limitations of eGFR and uACR, including the diagnostic challenges in determining whether the patient has diabetic kidney disease or an alternative kidney disease etiology. Furthermore, eGFR and uACR provide limited information for disease subclassification or identifying the specific nephron compartments affected. These measures cannot differentiate between glomerular and tubulointerstitial injury, and they do not assess functional impairments such as disturbances in permselectivity, tubular reabsorption, erythropoiesis control, renin-angiotensin-aldosterone system (RAAS) regulation, or calcium/phosphorus/calcitriol and klotho regulation.
These examples underscore the limitations of current kidney function assessments, which often lack the sensitivity to detect early kidney injury and provide an incomplete characterization of kidney dysfunction. The reliance on eGFR and uACR as primary biomarkers presents diagnostic challenges, particularly in cases where their diagnostic interpretation remains uncertain, making it difficult to determine the presence and etiology of kidney disease. This uncertainty complicates the ability to differentiate between distinct forms of kidney injury and to ascertain which kidney compartment is primarily affected. Addressing these challenges necessitates the development of more advanced diagnostic methodologies capable of providing a comprehensive evaluation of kidney function. These approaches should encompass assessments of key physiological processes, including acid-base and electrolyte regulation, volume homeostasis under physiological stress, responsiveness to renoprotective therapies, and intrinsic kidney reserve capacity. The integration of these functional assessments with molecular profiling and genetic testing has the potential to refine disease classification by elucidating distinct pathophysiological and causal mechanisms across the spectrum of kidney disease, ultimately facilitating the advancement of clinical application and targeted therapeutic strategies.
In sum, the kidneys play a pivotal role in maintaining health and homeostasis. However, the totality of these life-sustaining functions are not captured through GFR, and the extent of the damage is not assessed through uACR. The NIDDK workshop on “Re-imagining Kidney Function Assessment,” led by Afshin Parsa et al.,1 (recorded webinar is openly available at: Reimagining Kidney Function Assessment Workshop - 2024 - NIDDK) aimed to address the challenge of modernizing and expanding the assessment of kidney function. In the following sections, we delve into specific aspects of kidney function assessment that were discussed during the workshop, including measuring GFR, assessing tubular functions, assessing KFR, evaluating the kidney’s inherent physiologic response to stressors, and characterizing glomerular permselectivity. By exploring these facets of kidney function in detail, we aimed to provide a more comprehensive understanding of the challenges and opportunities in advancing kidney function assessment and ultimately improving patient care.
Measuring GFR in Clinical and Research Settings
Evaluation of the GFR in patients is generally considered the best single overall assessment of kidney health, even though it does not capture all critical renal functions.2 In clinical practice, the GFR is estimated using endogenous biomarkers.3,4 There are clinical situations in which the GFR should be measured (mGFR) instead of estimated to provide a more accurate assessment of the patient’s GFR.5 The GFR can be measured by administering exogenous filtration markers and following their clearance either through urinary clearance or plasma disappearance protocols.6, 7, 8 Several of the historical filtration markers employed to perform mGFR studies, such as inulin and nonradioactive iothalamate, are currently unavailable for purchase. GLOFIL-125, a radioactive iothalamate, is still available for purchase and has a documented clinical protocol.9 However, most institutions prefer to use nonradioactive exogenous markers to reduce radioactive waste and limit patient exposure to radioactive compounds. This leaves iohexol as one of the most widely available and researched exogenous filtration markers currently employed for research and clinical mGFR studies.10 Plasma disappearance protocols for mGFR involve an i.v. infusion of the filtration marker and carry the benefit of only sampling a patient’s plasma11 either through a single blood collection sampling or multiple blood collections.12,13 Single time point for plasma iohexol clearance appears to be sufficient to estimate GFR in individuals with normal GFR; however, it has decreased accuracy in patient populations with lower levels of GFR. Some studies have shown that multiple time points, including a 24-hour sample, are required for accurate measurement of GFR in chronic kidney disease (CKD) stage 4 or 5.14, 15, 16, 17 Laboratory analysis of iohexol specimens typically employs the use of high-performance liquid chromatography or liquid chromatography tandem mass spectrometry.18 Analytical accuracy and precision of iohexol measurement at performance laboratories are essential for an accurate GFR measurement.19 There is an external proficiency program that laboratories can subscribe to in order to assess accuracy, called EQUALIS, which assists in keeping laboratory measurement procedures on calibration.20
A recent international consensus from the European Kidney Function Consortium has provided practical, evidence-based guidance for the implementation of iohexol plasma clearance protocols.21 Importantly, this consensus highlights the need for methodological flexibility within a standardized framework, acknowledging that institutional differences in staffing, sample collection logistics, and analytical capacity may require tailored approaches. The objective is not to mandate a single uniform procedure, but to ensure that varied protocols can produce consistent and reliable results across clinical and research settings. However, significant barriers persist; for example, in the United States there is no current procedural terminology code or established reimbursement model for nonradioactive mGFR testing. In the absence of a defined payment structure, broad clinical adoption remains limited, regardless of feasibility or clinical value. To support more reliable capture of mGFR, coordinated efforts are needed to strengthen protocol alignment while addressing policy and reimbursement challenges. Expanding the clinical utility of GFR measurement will require not only technical innovation, such as rapid mGFR approaches,22 but also the creation of accessible and sustainable implementation pathways.
Difference in mGFR Versus eGFR Versus True GFR
True GFR is a physiological property which cannot be measured directly. It is assessed using measured urinary or plasma clearance of exogenous filtration markers (mGFR) and estimated (eGFR) from serum concentrations of endogenous filtration markers.23 mGFR differs from true GFR because of errors in clearance methods, nonideal filtration marker properties, errors in filtration marker assays, and biological variability (Figure 1).6 Thus, observed differences in mGFR from one day to another may be due to true biological variation in GFR, including day-to-day variation, or to errors secondary to analytical errors in the assay or the clearance procedure.24 Causes of biological variation include factors that influence the determinants of GFR, such as changes in renal blood flow, intraglomerular pressure, filtration coefficient, hydraulic pressure, and oncotic pressure. These determinants may be affected by extracellular fluid volume, blood pressure, exercise, obesity, and dietary protein intake. Furthermore, many of the kidney physiological functions display circadian rhythms, which contribute to variation within a day.25, 26, 27, 28, 29, 30, 31 Evidence for diurnal variation in GFR in healthy individuals in highly controlled environments with 33% daily variability has been demonstrated, with less consistent evidence for diurnal variation in patients with CKD.32
Figure 1.
Factors influencing true, measured, and estimated glomerular filtration rate.
In addition, eGFR differs from mGFR and true GFR due to non-GFR determinants of filtration markers including, generation, tubular reabsorption and secretion, extrarenal elimination, and measurement errors in filtration marker assays.33,34 Consequently, discrepancies between eGFR and true GFR may arise from inherent inaccuracies related to these eGFR-associated factors or from variability and limitations in mGFR measurements themselves.35 Further, eGFR estimated using serum creatinine versus using cystatin C can give discordant values, prompting the question of which estimate is to be trusted and whether 1 or both are influenced by the non-GFR determinants specific to each marker (Table 1).36, 37, 38 These differences in mGFR, true GFR, and the distinction between immediate and average GFR are often underappreciated factors that limit the development of more reliable eGFR equations. These factors may in part explain findings from the Chronic Renal Insufficiency Cohort study, which found that mGFR may not necessarily provide a better prediction of outcomes in individuals with CKD.39 A further challenge lies in the application of eGFR equations across diverse populations. Many commonly used equations were developed in cohorts with limited racial, ethnic, age, or clinical heterogeneity, and may perform suboptimally in individuals with differing body composition, comorbid conditions, or socioeconomic backgrounds.40 These limitations underscore the need for broader external validation, the use of more inclusive reference populations, and the development of approaches that reduce reliance on any single biomarker.
Table 1.
Effects of non-GFR determinants on creatinine and cystatin C as endogenous filtration markers
| Factor | Creatinine | Mechanism of change | Cystatin C | Mechanism of Change |
|---|---|---|---|---|
| Increasing age | ↓ | Decreased muscle mass | ↓ | Decreased total body cell mass |
| Female sex | ↓ | Less muscle mass compared to males | ↓ | Lower cell mass compared to males |
| African American ethnicity | ↑ | Higher average muscle mass compared to other ethnicities | – | – |
| Inflammation | – | – | ↑ | Increased cystatin C production |
| Hyperthyroidism | – | – | ↑ | Increased cystatin C production |
| Hypothyroidism | – | – | ↓ | Decreased cystatin C production |
| Smoking | – | – | ↑ | Increased cystatin C production due to inflammation |
| Vegetarian diet | ↓ | Reduced creatinine production | – | – |
| Consumption of meat or creatinine supplements | ↑ | Increased creatinine production (in some cases may show no change due to transient GFR increase) | – | – |
| Body builders or individuals with high muscle mass | ↑ | Increased creatinine production or from high protein intake | – | – |
| Low muscle mass (e.g., limb amputation) | ↓ | Decreased creatinine production | – | – |
| Malnutrition or muscle wasting due to chronic illness | ↓ | Decreased creatinine production or reduced protein intake | ↑ | Increased cystatin C production due to inflammation |
| Obesity | – | – | ↑ | Increased cystatin C production due to higher fat mass |
| Trimethoprim, cimetidine, fibric acid derivatives (except gemfibrozil) | ↑ | Inhibit tubular secretion of creatinine | No data | No data |
| Ketoacids and some cephalosporins | ↑ | Interfere with creatinine measurement by alkaline picrate assay | No data | No data |
↑, increase; ↓, decrease; -, no change; GFR, glomerular filtration rate.
The ultimate goal is an accurate, precise, and reliable assessment of GFR that is easy and inexpensive with fast turnaround. Ongoing studies are evaluating panels of endogenous filtration markers that may attenuate the impact of non-GFR determinants of any single marker.41 Ideally, such markers are able to detect short-term changes in GFR, such as when assessing individuals with acute kidney injury (AKI), or can capture average GFR over several days. Realistic and immediate goals for mGFR include clear indicators, when to use; standardized protocols with reliable data on accuracy, and how accuracy varies by individual characteristics, ensure stable supply chain for the filtration markers, and payment systems to encourage more widespread use.
The Dual Mechanisms of Kidney Clearance: Glomerular Filtration and Tubular Secretion
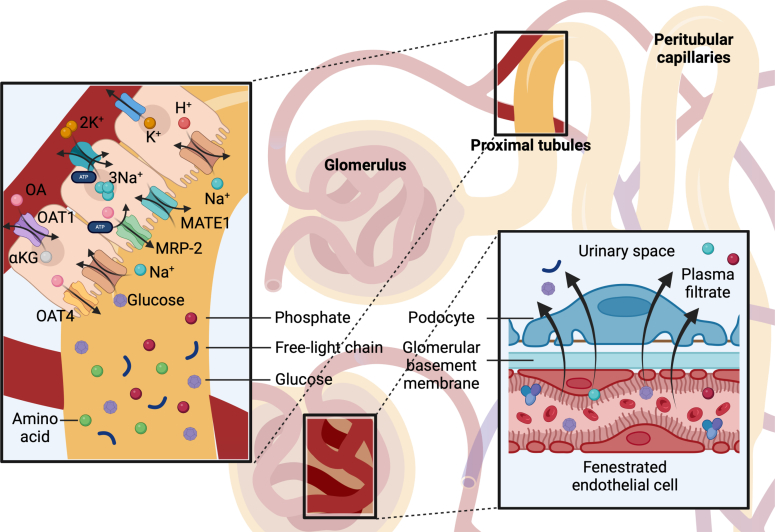
The human kidneys clear endogenous solutes and medications from the circulation by 2 mechanisms: glomerular filtration and tubular secretion. Filtration is the primary mechanism for eliminating freely circulating solutes that can readily traverse the glomerular basement membrane (GBM). Although only approximately 20% of renal plasma flow is filtered through the glomeruli, this process is highly effective because of the substantial volume of plasma delivered to the kidneys. Humans accomplish this task by routing approximately 20% of the total cardiac output to the kidneys. For an average-sized person with intravascular plasma volume of 5 L and a “normal” GFR of 120 ml/min, the entirety of the plasma volume is filtered through the kidneys every 42 minutes.
In contrast, the remaining approximately 80% of renal plasma flow that is not filtered at the glomerulus is directed to the proximal tubules, where specific transporters actively secrete retained solutes and drugs into the urine (Figure 2).42 Tubular secretion is a highly efficient mechanism for solute clearance and represents the primary route of elimination for protein-bound substances that are minimally filtered due to size and charge specificity of the GBM. Many candidate uremic solutes are known substrates of secretory transporters, for example indoxyl sulfate, and indoleacetic acid.43,44 Tubular secretion is also the primary kidney mechanism for eliminating hundreds of commonly prescribed drugs, including antibiotics, diuretics, antidiabetes medications, antiviral agents, and chemotherapies.45 While secretion accounts for a smaller overall fraction of total solute clearance than filtration, it is essential for the removal of specific compounds not efficiently handled by glomerular filtration, especially in settings of reduced GFR.
Figure 2.
The dual mechanisms of kidney clearance. The kidneys clear endogenous solutes and medications via two main mechanisms: glomerular filtration, which removes freely circulating solutes that can cross the glomerular basement membrane, and tubular secretion, which actively transports solutes and drugs into the urine. Only about 20% of renal plasma flow is filtered, necessitating a high plasma delivery—roughly 20% of the cardiac output—to achieve effective clearance. The remaining 80% of renal plasma flow reaches the proximal tubules, where specialized transporters efficiently eliminate protein-bound and other solutes poorly filtered at the glomerulus. This robust secretion pathway is essential for excreting toxic substances and is the primary route for eliminating many commonly prescribed drugs (e.g., antibiotics, diuretics, antiviral agents, and chemotherapies).
The development of accurate measurements of tubular secretory clearance could expand the assessment of kidney functions beyond prevailing measurements of GFR and albuminuria. Filtration and secretion occur via distinct physiologic mechanisms: glomerular filtration is primarily a passive process dependent on hemodynamics and effective surface area, whereas secretion requires the coordinated actions of basolateral and apical transporters coupled with cellular energy, suggesting potential contrasts across individuals and disease processes. The loss of tubular secretory clearance relative to GFR may suggest the presence of disorders that preferentially involve the tubules and interstitium, including ischemic and toxic causes of AKI. Measures of secretory clearance may provide new opportunities for the early detection of kidney disease, in which GFR is often preserved because of some degree of compensatory hyperfiltration. The central role of tubular secretory clearance in kidney drug elimination suggests further potential for improving drug dosing strategies, which are currently based exclusively on estimates of eGFR.46
Despite its central physiological importance as an essential intrinsic kidney function, tubular secretory clearance is rarely assessed in clinical or research settings. Conceptually, secretory clearance can be estimated based on the kidney clearance of endogenous or exogenous markers that have high specificity for tubular solute transporters, for example, p-aminohippurate. Kestenbaum et al. have developed an assay to measure a set of endogenous substrates of tubular organic anion transporters. They have used the timed kidney clearances of these markers to demonstrate the following: that (i) lower estimated tubular secretory clearance is associated with the progression of CKD, cognitive decline, and mortality after adjustment for GFR and albuminuria; and (ii) measures of tubular secretory clearance predict kidney drug elimination.47, 48, 49 Further interdisciplinary research across clinical, translational, and laboratory medicine is needed to improve the estimation of this vital intrinsic kidney function and understand its clinical implications.
Renal Stress Tests - Using the Kidney’s Inherent Physiologic Response to Assess Function
Using the kidney’s inherent physiologic response to a known stressor or stimuli may provide clues into previous or ongoing acute or chronic injuries. The renal literature has several examples of how tracking kidney response may help in the prognostication or diagnosis of certain disease states.50 Although this section highlights selected examples, it is important to note that a wide array of physiologic stress tests can also reveal important insights into kidney function.
A physiologic stress test in AKI, the furosemide stress test, assesses renal tubular integrity and prognosticates outcomes in patients with hypervolemic and euvolemic stage 1 and 2 AKI.51,52 Furosemide is nearly completely bound to plasma albumin and negligibly filtered. Its actions within the kidneys require active secretion via an intact proximal tubule (organic anion transporters) and subsequent interaction with the functional thick ascending limb of the loop of Henle, and functional distal nephron to have its maximal effect.53,54 In 2013, Chawla and colleagues showed that in early AKI, the inability to increase urine output > 200 ml within 2 hours of the i.v. administration of 1.0 to 1.5 mg/kg furosemide predicts progression to stage 3 AKI across a variety of clinical settings.51 Since then, the furosemide stress test has been validated across a variety of cohorts, prospective and retrospective, and across different settings of AKI.55, 56, 57 More recently, the furosemide stress test has been combined with other tools (biochemical biomarkers and AKI risk scores) to provide a multimodal approach to assessing tubular function and AKI risk.58,59
In addition to assessing the response to furosemide in the setting of AKI, others have investigated how furosemide affects kidney oxygen consumption as a method to study CKD progression. Quantifying oxygen availability using blood oxygen level–dependent MRI before and after i.v. furosemide administration, has permitted the estimation of oxygen consumption, a test termed furosemide suppressible oxygen consumption. Furosemide suppressible oxygen consumption has differentiated ischemic kidneys (from renal artery stenosis) from those with tubular injury.60,61
In the setting of CKD, there is a predictable physiologic response to RAAS antagonism. Initiating any RAAS inhibitor, irrespective of class, leads to a decrease in intraglomerular pressure through reductions in efferent arteriolar resistance. This attenuates intraglomerular pressure and lowers GFR.62 In a post hoc analysis of the SOLVD trial, which investigated the role of enalapril on survival in patients with impaired left ventricular function and established heart failure, Testani et al.63 examined outcomes stratified by RAAS inhibitor use and whether participants experienced a decline in eGFR. Those patients who developed a 20% decrease in GFR within 14 days of starting enalapril, had the best long-term survival; and not surprisingly, those who had a similar decrease in GFR while getting placebo (essentially AKI) had the worst prognosis.63 Therefore, in the setting of congestive heart failure, those patients who displayed intact glomerular hemodynamic function were the ones to experience the best outcomes. However, not all studies have confirmed these findings when studied in larger and more heterogeneous populations.64 Future investigations should help determine the role and impact of the changes in glomerular hemodynamic across various patient populations.
Similar data exist around starting spironolactone in patients with proteinuria and CKD; those with an immediate reduction in GFR developed the largest decrease in their chronic proteinuria.65 However, studies examining the initiation of RAAS inhibitors across the general population (i.e., not specific to congestive heart failure or CKD) have not shown the same benefits, with some studies documenting that across the general population, the greater the increase in serum creatinine, the greater the risk of adverse outcomes.64 In the near future, we would anticipate applying this same stress test to other medications to treat CKD. Do patients who start a sodium-glucose cotransporter 2 inhibitor or newer nonsteroidal mineralocorticoid receptor antagonists (e.g., finerenone) do better if they have the anticipated increase in serum creatinine or decrease in eGFR from drug initiation? Some of the existing data support this notion; however, future studies should investigate whether those patients who do not exhibit the expected changes from these medications still derive a benefit compared with those who do not receive the medication.66
In the setting of a dietary acid load, the kidney will normally produce ammonia and other buffers (e.g., sulfates and phosphates) to bind the excess hydrogen ions. However, in the setting of CKD, these compensatory mechanisms are impaired, leading to the net retention of hydrogen ions, reduced urinary citrate excretion, and the development of metabolic acidosis.67 Several cohorts have demonstrated that patients who are not able to buffer a dietary acid load in the setting of kidney disease are more likely to experience CKD progression and the development of end-stage disease.68 In the future, protocolized acid loads combined with timed urine collections may be developed to help identify those patients at higher risk for CKD progression who may benefit from therapeutic interventions to prevent the development of metabolic acidosis and ESKD. Other physiological measures such as water restriction, water loading, and sodium MRI to assess urine concentrating ability, bicarbonate administration to evaluate acid base regulatory capacity, and measurement of the tubular maximum for specific solutes may also provide insight into tubular function and kidney health.69, 70, 71, 72, 73
Measuring KFR
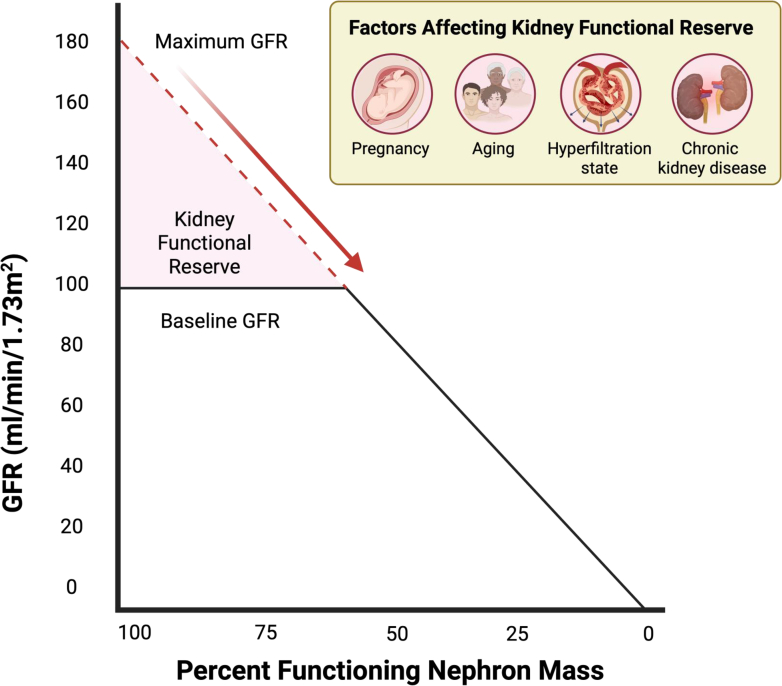
Consistently adapting to physiological and pathological stimuli, glomerular filtration is a dynamic rather than static process. For example, black bears exhibit a reduction in GFR by 70% during hibernation.74 Although humans display less pronounced fluctuations in GFR, there is still notable variability. Human GFR can adjust in response to dietary intake, hydration status, and medications, underscoring the kidney's adaptive role in maintaining homeostasis. The increase in serum creatinine as a marker of kidney dysfunction often becomes clinically evident only when there is a significant loss of kidney mass, which at times in certain circumstances, may not become evident until ≥ 50% of the nephrons are compromised.75,76 This threshold highlights the concept of KFR, which measures the capacity of the kidneys to augment GFR in response to increased demands or stress (Figure 3). Importantly, the assessment of KFR provides insight not only into subclinical nephron loss but also into the interpretation of elevated GFR, particularly in hyperfiltration states. Hyperfiltration may occur through an increase in single-nephron GFR across structurally intact kidneys or as a compensatory response in kidneys with reduced nephron number. Although this response may be initially physiologic, such as during pregnancy or after unilateral nephrectomy, persistent hyperfiltration is often maladaptive and has been implicated in the progression of kidney injury, particularly in conditions such as obesity and diabetes. In individuals with elevated baseline GFR, a blunted or absent increase in GFR following a standardized stress test suggests that the kidneys are already functioning near their maximal capacity, indicating a loss of reserve. Impaired KFR has been documented in diverse clinical contexts, including aging,77 pregnancy,78 hyperfiltration states,79 and CKD80 (Figure 3).
Figure 3.
Kidney functional reserve (KFR) and factors influencing its decline. KFR represents the difference between the baseline GFR and the maximum GFR. Under conditions where the nephron mass is functioning at 100%, the maximum GFR may increase to as much as 180 ml/min per 1.73 m2. Factors such as aging,77 pregnancy,78 baseline glomerular hyperfiltration states,79 and CKD,80 can lead to a attenuated in kidney functional reserve. CKD, chronic kidney disease; GFR, glomerular filtration rate.
One common method to measure KFR involves administering a protein load, typically in the form of a meat meal or amino acid infusion and observing the subsequent increase in GFR. Several mechanisms have been proposed to explain the changes in GFR that occur in response to a protein load. Notably, the resetting of tubuloglomerular feedback has been demonstrated in animal models. For instance, studies using lithium clearance methods in dogs have shown that an i.v. infusion of amino acids can enhance proximal tubular transport through the sodium amino acid cotransport system.81 This leads to reduced sodium chloride delivery to the distal tubule, effectively deactivating tubuloglomerular feedback and resulting in an increase in GFR. Furthermore, these physiological responses are often accompanied by changes in renal blood flow, as consistently observed in animal studies following protein ingestion. In addition, the release of various humoral factors during protein consumption is believed to play a role in the observed increase in GFR.82
Recently, the potential of KFR to predict outcomes such as AKI or CKD progression has been explored. In a study of adult patients with preserved baseline GFR undergoing elective cardiac surgery, preoperative KFR was documented to be significantly lower in those who developed AKI postoperatively than in those who did not.83 Specifically, an KFR value < 15 ml/min per 1.73 m2 was associated with an 11.8-fold increased likelihood of developing AKI. The quantification of KFR before high-risk kidney events, such as cardiopulmonary bypass, nephrotoxin exposure, or kidney transplantation, is emerging as a promising tool in the early detection of subclinical kidney disease. Assessing KFR may enable clinicians to identify patients who might benefit from preemptive therapeutic interventions, potentially preventing the onset of AKI as demonstrated in recent clinical study.84 In addition, KFR assessment could be valuable for monitoring kidney recovery following AKI.
Glomerular Filtration and the Pathogenesis of Albuminuria
Despite decades of research on the glomerular filtration barrier, a biophysical model to explain how the kidney filter allows extensive fluid filtration while restricting the passage of macromolecules was lacking until relatively recently.85 The glomerular filtration barrier consists of the following 3 layers: (i) a specialized and fenestrated endothelium that lines the luminal side of the capillary wall; (ii) an extracellular matrix–based GBM that contains type IV collagen, laminin-521, and nidogen, as well as sulfated proteoglycans; and (iii) podocytes that cover the outer surface of the GBM. Podocytes, which are terminally differentiated cells with a limited capacity for self-renewal and regeneration, closely envelope the glomerular capillaries through extensions (foot processes) that interdigitate with those of adjacent podocytes. They are connected by a membrane-like cell junction that allows the formation of an extended filtration slit and serves as a hub for podocyte signaling.86 The remarkable luminal pressure in the capillaries required for fluid filtration exerts physical forces on the capillary wall that are counteracted by the GBM and podocytes.87 Interdigitating podocyte foot processes serve as buttresses against the physical forces of hydrostatic pressure in the glomerular capillaries, compressing the gel-like structure of the GBM. With these adaptive biophysical properties, the GBM acts as a permselective filter and restricts the permeability to macromolecules transported by diffusion and bulk flow.85 When podocytes are injured, they take on a more simplified architecture, and the slit-diaphragm length is much reduced, resulting in a reduction in the filtration slit area and a decline in the GFR of water and small solutes. Concomitantly, the buttressing force of podocytes is lost and the compressive force of the GBM is reduced, which increases the permeability to albumin and explains early changes that cause albuminuria.85 New evidence suggests that at more advanced stages in disease development, shear forces exerted by extensive fluid filtration over a shortened filtration slit may then cause podocytes to detach.88 Loss of podocytes then allows free passage of macromolecules of all sizes in denuded areas. To best withstand shear forces, podocytes can orient the filtration slit in a preferred direction along the axis of the capillary. This orientation is lost in podocyte disease, further predisposing to podocyte loss and progressive kidney disease.89,90 Taken together, these underscore the importance of preserving podocyte integrity and slit diaphragm orientation, highlighting the need for comprehensive assessments of glomerular permselectivity and structural integrity to improve early detection and management of kidney diseases.
Glomerular Permselectivity and Loss of Kidney Function
Proteinuria is a canonical indicator of glomerular injury and a predictor of loss of kidney function. The selective permeability of the glomerulus to proteins (permselectivity) can be quantitatively characterized based on the kidney's handling of (exogenous) test macromolecules (e.g., dextrans) covering a range of molecular sizes. So-called dextran sieving curves have been used for decades to characterize changes in glomerular permselectivity in human kidney disease. These curves may be modeled in terms of a small number of parameters, including the shunt magnitude, ω0, the fraction of the total glomerular filtrate that passes through parts of the filtration barrier with essentially no restriction to the passage of large molecules.91 A recent long-term (median: 17.7 years) follow-up study of a population of American Indians with type 2 diabetes and a high risk of developing diabetic kidney disease, showed that the shunt magnitude measured at baseline, when proteinuria was modest and kidney function essentially normal, was a strong predictor of eventual loss of kidney function.92 The hazard ratio (adjusted for potential confounding factors) was 1.55 for each SD increment in ω0. Although dextran sieving could represent a powerful risk assessment tool, it is invasive, time-consuming, and not currently feasible for routine clinical use.
An alternative approach, more practical in clinical setting, is the selectivity index, which was initially explored in the 1960s.93 This test was based on the glomerular handling of a small number of endogenous proteins covering a range of molecular sizes, in some ways analogous to dextran sieving. The slope (angle) of the linear regression line to the (log-log) plot of urine/serum protein ratio against molecular size strongly predicted, among other things, responsiveness of the individual patient to steroid therapy. It was suggested at the workshop that the selectivity index might be a reasonable surrogate (inverse) predictor of ω0. It was also proposed that a physical interpretation of the permselective shunt may reflect initially transient detachment of podocyte foot processes from the filtration surface. Of note in this regard is that in an earlier study,94 ω0 did not exceed the normal range until the uACR was nearly in the nephrotic range, suggesting that different mechanisms are responsible for lower-grade and nephrotic-range albuminuria, with the latter reflecting podocyte “stress” or loss. This may explain why in focal segmental glomerular sclerosis, a treatment response lowering urine protein-to-creatinine ratio to < 1.5 g/g, approximately the point at which ω0 started to increase in diabetes, was essentially as protective against eventual kidney failure as achieving complete remission,95 possibly reflecting protection from podocyte loss. Standardization, broader use, and further study of the selectivity index (as a predictor of ω0) has the potential to identify early pathogenesis and clinically meaningful subtypes of proteinuria, as well as refine the assessment of treatment response.
Functional Imaging Techniques
Advances in functional imaging have provided valuable tools that enhance our understanding of kidney physiology and pathology, complementing traditional measures of kidney function. A summary of the functional imaging modalities commonly used for kidney assessment is presented in Table 2. MRI with specialized sequences offers critical insights into key processes affecting kidney health, including perfusion, oxygenation, and fibrosis. Blood oxygen level–dependent MRI noninvasively assesses kidney tissue oxygenation by quantifying the transverse relaxation rate (R2∗), which reflects local deoxyhemoglobin levels.96 Changes in tissue oxygenation indicate imbalances between oxygen supply and demand, serving as early markers of kidney injury and functional impairment. Diffusion-weighted imaging MRI evaluates water molecule movement within the renal parenchyma, providing surrogate markers such as the apparent diffusion coefficient that reflect microstructural alterations such as fibrosis and inflammation linked to kidney damage and disease progression.97 Magnetic resonance relaxometry, including T1 mapping, measures tissue longitudinal relaxation time (T1), which correlates with fibrosis and edema, offering a sensitive indicator of tissue remodeling and injury.98 Arterial spin labeling MRI quantifies renal blood flow without contrast agents, capturing a fundamental aspect of kidney function as perfusion supports filtration and tubular processes.99 However, MRI techniques are limited by variability in platforms, protocols, and image analysis.
Table 2.
Summary of functional imaging modalities used in kidney assessment
| Imaging modality | Principle | Measurement | Interpretation of parameters |
|---|---|---|---|
| BOLD MRI | Measures paramagnetic effects of deoxyhemoglobin to assess tissue oxygenation | Transverse relaxation rate (R2∗) |
|
| Diffusion-weighted imaging (DWI) MRI | Measures Brownian motion of water molecules reflecting tissue microstructure | Apparent diffusion coefficient (ADC) |
|
| T1 Mapping (magnetic resonance relaxometry) | Quantifies longitudinal relaxation time to evaluate tissue composition and fibrosis | Longitudinal relaxation time (T1) |
|
| Arterial spin labeling (ASL) MRI | Uses magnetically labeled blood water as an endogenous tracer to quantify perfusion | Renal blood flow |
|
| Positron emission tomography (PET) | Uses radioactive tracers to measure renal blood flow, oxygen consumption, and metabolism | Tracer uptake rate, renal metabolic rate |
|
| Contrast-enhanced ultrasound (CEUS) | Uses microbubble contrast agents to visualize real-time blood flow in microvasculature | Contrast intensity, time-intensity curves | Decreased contrast intensity or altered time-intensity patterns reflect reduced perfusion |
↑, increase; ↓, decrease; BOLD, blood oxygen level–dependent; MRI, magnetic resonance imaging.
Positron emission tomography employs targeted radiotracers to measure renal perfusion, oxygen consumption, metabolism, inflammation and fibrosis, with parameters such as tracer uptake rate and renal metabolic rate, offering detailed mechanistic insights into kidney physiology and pathology100,101; however, its clinical use is restricted by ionizing radiation exposure, high cost, and limited accessibility. When MRI or positron emission tomography are contraindicated or unavailable, contrast-enhanced ultrasound (CEUS) provides real-time evaluation of renal microvascular perfusion by analyzing contrast intensity and time-intensity curves, serving as a practical bedside option.102 However, its use may be limited by operator dependency and reduced image quality in patients with high body mass index or challenging anatomy. Collectively, these modalities expand the scope of kidney assessment by providing detailed physiological and structural information that supports earlier diagnosis, improved risk stratification, and more precise monitoring of disease progression and therapeutic response. When integrated with clinical parameters, functional imaging can enhance decision-making and help avoid unnecessary or nonbeneficial procedures, particularly in patients with advanced or irreversible kidney damage. Further research and standardization are essential to optimize their clinical implementation.
Urine Biomarkers for Kidney Function Assessment
Urine biomarkers provide a noninvasive and sensitive means to evaluate kidney health by detecting specific physiological changes and injury patterns within the nephron. The stablished and emerging urine biomarkers relevant to kidney function assessment are summarized in Table 3. Biomarkers of glomerular integrity include urinary transferrin, immunoglobulin G, α2-macroglobulin, and ferritin.103 Markers of tubular injury and reabsorption defects comprise beta-2-microglobulin, retinol-binding protein, N-acetyl-β-D-glucosaminidase, alpha-1-microglobulin, and urinary cystatin C.104,105 Although these markers offer valuable insights, their clinical interpretation requires consideration of the inherent trade-offs between sensitivity and specificity. In AKI, early tubular damage can be identified by biomarkers such as neutrophil gelatinase–associated lipocalin, kidney injury molecule-1, interleukin-18, and the US Food and Drug Administration–approved TIMP-2/IGFBP7 assay.106 For CKD, biomarkers, including trefoil factor 3, monocyte chemoattractant protein-1, chitinase-3-like protein-1, dickkopf-related protein 3, uromodulin, and procollagen type III N-terminal propeptide, reflecting inflammation and fibrotic remodeling reveal ongoing disease progression.107 Collectively, these clinically established and investigational urine biomarkers complement traditional filtration markers and proteinuria, expanding the scope of kidney function assessment. Despite ongoing challenges in validation and standardization, integrating multimarker panels with advanced analytic approaches holds promise for advancing precision nephrology and enabling individualized patient care.
Table 3.
Summary of established and emerging urine biomarkers
| Biomarker | Associated condition | Primary implications/ clinical association | Physiological basis/ mechanism | Key limitations |
|---|---|---|---|---|
| Urinary transferrin (TRF) | Glomerular injury or dysfunction | Early glomerular charge barrier damage |
|
Requires further clinical validation beyond early DKD studies |
| Urine IgG | Glomerular injury or dysfunction | Severe GBM damage |
|
|
| Urine α2-macroglobulin (A2M) | Glomerular injury or dysfunction | Diabetic nephropathy progression |
|
Requires further validation for routine clinical use |
| Ferritin | Glomerular and tubular injury or dysfunction |
|
|
|
| Beta-2-microglobulin (B2M) | Tubular injury or dysfunction | Proximal tubular damage/ dysfunction |
|
|
| Retinol-binding protein (RBP) | Tubular injury or dysfunction | Proximal tubular injury or dysfunction (early marker) |
|
Suboptimal specificity (affected by proteinuria, chronic tubulopathies) |
| N-acetyl-β-D-glucosaminidase (NAG) | Tubular injury or dysfunction | Active renal tubular injury |
|
|
| Alpha-1-microglobulin (A1MG) | Tubular injury or dysfunction | Tubular damage or reabsorptive defects (drug-induced kidney damage) |
|
Suboptimal specificity (affected by proteinuria, chronic tubulopathies) |
| Urinary cystatin C | Tubular injury or dysfunction | Tubular reabsorption defects (acute tubular damage) |
|
Suboptimal specificity (affected by proteinuria, chronic tubulopathies) |
| Neutrophil gelatinase-associated lipocalin (NGAL) | AKI | Early tubular injury, AKI prediction/severity | Earliest and most robustly induced gene/protein in kidney following ischemic/nephrotoxic injury |
|
| Kidney injury molecule-1 (KIM-1) | AKI | Specific proximal tubule injury |
|
Influenced by CKD, and urinary tract infection |
| Interleukin-18 (IL-18) | AKI | Diagnosing ATN, AKI duration/mortality |
|
|
| Tissue inhibitor of metalloprotease-2 (TIMP-2) and insulin-like growth factor binding protein 7 (IGFBP7) | AKI | AKI risk assessment (critically ill patients) |
|
Nonkidney-specific elevations possible |
| Na+/H+ exchanger isoform 3 (NHE3) | AKI | Differentiating AKI types |
|
Limited studies |
| Liver-type fatty acid-binding protein (L-FABP) | AKI & CKD |
|
Primarily expressed in kidney’s proximal tubular epithelial cells | Still under investigation |
| Trefoil factor 3 (TFF3) | CKD Progression |
|
|
Still under investigation |
| Monocyte chemoattractant protein-1 (MCP-1) | CKD Progression |
|
|
Needs further validation |
| Chitinase-3-like protein-1 (YKL-40) | CKD Progression |
|
Glycoprotein involved in inflammation and tissue remodeling | Association with eGFR decline lost after albuminuria adjustment |
| Dickkopf-related protein 3 (DKK-3) | CKD Progression |
|
|
Needs further validation |
| Uromodulin (UMOD) / Tamm-Horsfall protein | CKD Progression |
|
|
Needs further validation |
| Procollagen type III N-terminal propeptide (PIIINP) | CKD Progression | Renal fibrosis progression |
|
Needs further validation |
AKI, acute kidney injury; CKD, chronic kidney disease; DKD, diabetic kidney disease; ECM, extracellular matrix; eGFR, estimated glomerular filtration rate; GBM, glomerular basement membrane; MW, molecular weight.
Conclusion
The NIDDK workshop on “Re-imagining Kidney Function Assessment” highlighted the pressing need to transcend current evaluation methods, such as GFR and proteinuria, which inadequately capture the multifaceted nature of kidney physiology and pathology. Key research questions identified by the workshop are summarized in Figure 4. The workshop emphasized returning to fundamental physiological principles, advocating for capturing diverse measures of kidney function, and standardizing protocols, such as those for measured GFR using exogenous filtration markers like iohexol, to mitigate variability and enhance reliability. In addition, the development of KFR and stress tests was identified as pivotal for unveiling subclinical dysfunction and predicting clinical outcomes. When integrated with tubular secretion assessments, these advancements promise a more comprehensive and nuanced understanding of kidney function.
Figure 4.
Key research questions. CKD, chronic kidney disease; eGFR, estimated GFR; GFR, glomerular filtration rate.
The workshop further emphasized the potential of glomerular permselectivity and molecular profiling as cornerstones of precision nephrology. These approaches enable more refined disease classification, the elucidation of distinct pathophysiological pathways, and the development of targeted therapeutic strategies. Although these approaches hold considerable promise for advancing early detection, prognostication, identification of disease subgroups, and personalized treatment, several challenges remain. Validation through large-scale, multicenter studies, cost-effectiveness analyses, and specialized training will be critical to their successful implementation. Standardized clinical protocols are imperative to ensure these methods can be seamlessly integrated into clinical practice.
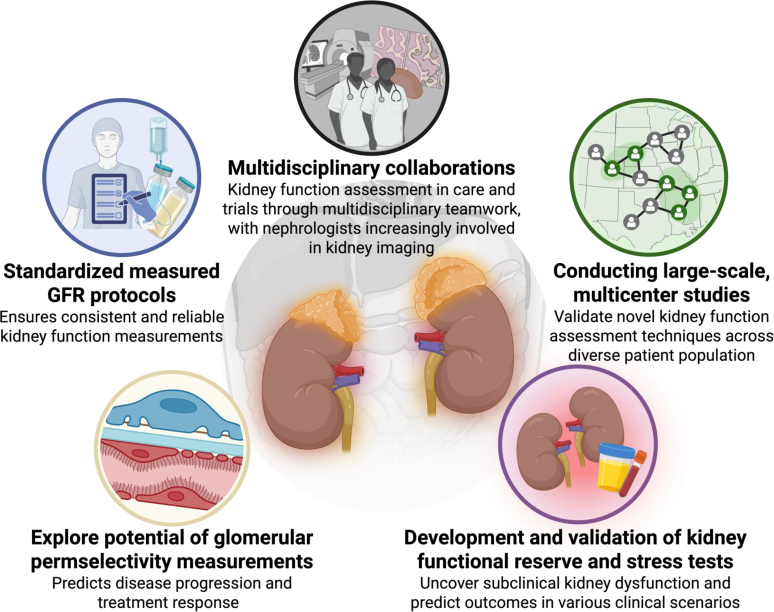
Achieving this integration will require robust interdisciplinary collaboration involving nephrology, radiology, laboratory medicine, and related specialties (these key innovations and strategies are summarized in Figure 5). By embracing these strategies, the nephrology community is well-positioned to redefine kidney functional assessment and advance the field through more precise, patient-centered approaches. Moving beyond traditional paradigms to a more comprehensive, multifaceted framework for kidney function evaluation will help reclassify kidney health and disease states, fostering innovations that ultimately improve the lives of individuals affected by kidney disease.
Figure 5.
Key innovations and strategies for advancing kidney function assessment. GFR, glomerular filtration rate.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors would like to acknowledge additional speakers and moderators for the National Institute of Diabetes and Digestive and Kidney Diseases sponsored “Re-imagining Kidney Function Assessment Workshop”. The authors acknowledge the following participants of the workshop: Afshin Parsa, MD, MPH; Kevin Bennett, PhD; Adam Bush, PhD; Gary Friedman, MD; Stuart Goldstein, MD; Chi-yuan Hsu, MD, MS; Lilach Lerman, MD, PhD; Valerie Luyck, MD, PhD, MSc; Denise Marciano, MD; Tim Meyer, MD; Ragnar Palsson, MD; Andrew Rule, MD, MS; Robert Star, MD; Zhen Jane Wang, MD; Mengxiao Yu, PhD; Yuanyuan Zhang, PhD; and Jie Zheng, PhD.
References
- 1.Afshin Parsa D.G., Gossett D., Mendley S., et al. Reimagining Kidney Function Assessment Workshop. https://www.niddk.nih.gov/news/meetings-workshops/2024/reimagining-kidney-function-assessment-workshop
- 2.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013:1–150. [Google Scholar]
- 3.Stevens L.A., Coresh J., Greene T., Levey A.S. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 4.Levey A.S., Inker L.A., Coresh J. GFR estimation: from physiology to public health. Am J Kidney Dis Off J Natl Kidney Found. 2014;63:820–834. doi: 10.1053/j.ajkd.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebert N., Bevc S., Bökenkamp A., et al. Assessment of kidney function: clinical indications for measured GFR. Clin Kidney J. 2021;14:1861–1870. doi: 10.1093/ckj/sfab042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soveri I., Berg U.B., Björk J., et al. Measuring GFR: a systematic review. Am J Kidney Dis. 2014;64:411–424. doi: 10.1053/j.ajkd.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Seegmiller J.C., Eckfeldt J.H., Lieske J.C. Challenges in measuring glomerular filtration rate: a clinical laboratory perspective. Adv Chronic Kidney Dis. 2018;25:84–92. doi: 10.1053/j.ackd.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Speeckaert M.M., Seegmiller J., Glorieux G., et al. Measured glomerular filtration rate: the query for a workable golden standard technique. J Pers Med. 2021;11:949. doi: 10.3390/jpm11100949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iso-Tex Diagnostics, I Glofil-125 Package insert. https://www.isotexdiagnostics.com/_files/ugd/37c9d8_092248e8160f4cb0932a7108afe8cada.pdf
- 10.Krutzen E., Back S.E., Nilsson-Ehle I., Nilsson-Ehle P. Plasma clearance of a new contrast agent, iohexol: a method for the assessment of glomerular filtration rate. J Lab Clin Med. 1984;104:955–961. [PubMed] [Google Scholar]
- 11.Brochner-Mortensen J. A simple method for the determination of glomerular filtration rate. Scand J Clin Lab Investig. 1972;30:271–274. doi: 10.3109/00365517209084290. [DOI] [PubMed] [Google Scholar]
- 12.Jacobsson L. A method for the calculation of renal clearance based on a single plasma sample. Clin Physiol (Oxford, England) 1983;3:297–305. doi: 10.1111/j.1475-097x.1983.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 13.Gaspari F., Guerini E., Perico N., Mosconi L., Ruggenenti P., Remuzzi G. Glomerular filtration rate determined from a single plasma sample after intravenous iohexol injection: is it reliable? J Am Soc Nephrol. 1996;7:2689–2693. doi: 10.1681/ASN.V7122689. [DOI] [PubMed] [Google Scholar]
- 14.Frennby B., Sterner G., Almén T., Hagstam K.E., Hultberg B., Jacobsson L. The use of iohexol clearance to determine GFR in patients with severe chronic renal failure--a comparison between different clearance techniques. Clin Nephrol. 1995;43:35–46. [PubMed] [Google Scholar]
- 15.Ebert N., Loesment A., Martus P., et al. Iohexol plasma clearance measurement in older adults with chronic kidney disease-sampling time matters. Nephrol Dial Transplant. 2015;30:1307–1314. doi: 10.1093/ndt/gfv116. [DOI] [PubMed] [Google Scholar]
- 16.Seegmiller J.C., Ebert N. Measuring glomerular filtration rate with iohexol plasma disappearance: blood collection duration is essential for accurate glomerular filtration rate determinations. Kidney Int. 2020;97:616. doi: 10.1016/j.kint.2019.11.034. [DOI] [PubMed] [Google Scholar]
- 17.Ognissanti D., Andresen Bergström M., Theodorsson E., Larsson A., Nordin G., Hammarsten O. Estimating analytical errors of glomerular filtration rate measurement. Clin Chem. 2022;68:1211–1218. doi: 10.1093/clinchem/hvac098. [DOI] [PubMed] [Google Scholar]
- 18.Schmit D.J., Carroll L.J., Eckfeldt J.H., Seegmiller J.C. Verification of separate measurement procedures where analytical determinations influence the clinical interpretation of GFR: iohexol quantitation by HPLC and LC-MS/MS. Clin Biochem. 2019;67:16–23. doi: 10.1016/j.clinbiochem.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz G.J., Wang H., Erway B., et al. Multicenter laboratory comparison of iohexol measurement. J Appl Lab Med. 2018;2:711–724. doi: 10.1373/jalm.2017.024240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Equalis. http://www.equalis.se/en/products-and-services/external-quality-assessment-eqa/eqa-schemes/g-l/iohexol-024/
- 21.Ebert N., Schaeffner E., Seegmiller J.C., et al. Iohexol plasma clearance measurement protocol standardization for adults: a consensus paper of the European Kidney Function Consortium. Kidney Int. 2024;106:583–596. doi: 10.1016/j.kint.2024.06.029. [DOI] [PubMed] [Google Scholar]
- 22.Rizk D.V., Meier D., Sandoval R.M., et al. A novel method for rapid bedside measurement of GFR. J Am Soc Nephrol. 2018;29:1609–1613. doi: 10.1681/asn.2018020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levey A.S., Coresh J., Tighiouart H., Greene T., Inker L.A. Measured and estimated glomerular filtration rate: current status and future directions. Nat Rev Nephrol. 2020;16:51–64. doi: 10.1038/s41581-019-0191-y. [DOI] [PubMed] [Google Scholar]
- 24.Rowe C., Sitch A.J., Barratt J., et al. Biological variation of measured and estimated glomerular filtration rate in patients with chronic kidney disease. Kidney Int. 2019;96:429–435. doi: 10.1016/j.kint.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 25.Firsov D., Bonny O. Circadian rhythms and the kidney. Nat Rev Nephrol. 2018;14:626–635. doi: 10.1038/s41581-018-0048-9. [DOI] [PubMed] [Google Scholar]
- 26.Mills J.N., Stanbury S.W. Persistent 24-hour renal excretory rhythm on a 12-hour cycle of activity. J Physiol. 1952;117:22–37. doi: 10.1113/jphysiol.1952.sp004730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costello H.M., Johnston J.G., Juffre A., Crislip G.R., Gumz M.L. Circadian clocks of the kidney: function, mechanism, and regulation. Physiol Rev. 2022;102:1669–1701. doi: 10.1152/physrev.00045.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore-Ede M., Fm S., Fuller C. Harvard University Press; 1982. The Clocks That Time Us: Physiology of the Circadian Timing System. [Google Scholar]
- 29.Hansen H.P., Tauber-Lassen E., Jensen B.R., Parving H.H. Effect of dietary protein restriction on prognosis in patients with diabetic nephropathy. Kidney Int. 2002;62:220–228. doi: 10.1046/j.1523-1755.2002.00421.x. [DOI] [PubMed] [Google Scholar]
- 30.Eckerbom P., Hansell P., Cox E., et al. Circadian variation in renal blood flow and kidney function in healthy volunteers monitored with noninvasive magnetic resonance imaging. Am J Physiol Ren Physiol. 2020;319:F966–F978. doi: 10.1152/ajprenal.00311.2020. [DOI] [PubMed] [Google Scholar]
- 31.Voogel A.J., Koopman M.G., Hart A.A., van Montfrans G.A., Arisz L. Circadian rhythms in systemic hemodynamics and renal function in healthy subjects and patients with nephrotic syndrome. Kidney Int. 2001;59:1873–1880. doi: 10.1046/j.1523-1755.2001.0590051873.x. [DOI] [PubMed] [Google Scholar]
- 32.Koopman M.G., Koomen G.C., Krediet R.T., De Moor E.A., Hoek F.J., Arisz L. Circadian rhythm of glomerular filtration rate in normal individuals. Clin Sci (Lond) 1989;77:105–111. doi: 10.1042/cs0770105. [DOI] [PubMed] [Google Scholar]
- 33.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shafi T., Zhu X., Lirette S.T., et al. Quantifying individual-level inaccuracy in glomerular filtration rate estimation: a cross-sectional study. Ann Intern Med. 2022;175:1073–1082. doi: 10.7326/M22-0610. [DOI] [PubMed] [Google Scholar]
- 35.Kwong Y.T., Stevens L.A., Selvin E., et al. Imprecision of urinary Iothalamate clearance as a gold-standard measure of GFR decreases the diagnostic accuracy of kidney function estimating equations. Am J Kidney Dis. 2010;56:39–49. doi: 10.1053/j.ajkd.2010.02.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fino N.F., Adingwupu O.M., Coresh J., et al. Evaluation of novel candidate filtration markers from a global metabolomic discovery for glomerular filtration rate estimation. Kidney Int. 2024;105:582–592. doi: 10.1016/j.kint.2023.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tio M.C., Zhu X., Lirette S., et al. External validation of a novel multimarker GFR estimating equation. Kidney360. 2023;360:1680–1689. doi: 10.34067/kid.0000000000000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adingwupu O.M., Barbosa E.R., Palevsky P.M., Vassalotti J.A., Levey A.S., Inker L.A. Cystatin C as a GFR estimation marker in acute and chronic illness: a systematic review. Kidney Med. 2023;5 doi: 10.1016/j.xkme.2023.100727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ku E., Xie D., Shlipak M., et al. Change in measured GFR versus eGFR and CKD outcomes. J Am Soc Nephrol. 2016;27:2196–2204. doi: 10.1681/asn.2015040341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inker L.A., Titan S. Measurement and estimation of GFR for use in clinical practice: core curriculum 2021. Am J Kidney Dis. 2021;78:736–749. doi: 10.1053/j.ajkd.2021.04.016. [DOI] [PubMed] [Google Scholar]
- 41.Inker L.A., Levey A.S., Coresh J. Estimated glomerular filtration rate from a panel of filtration markers-hope for increased accuracy beyond measured glomerular filtration rate? Adv Chronic Kidney Dis. 2018;25:67–75. doi: 10.1053/j.ackd.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Koepsell H., Endou H. The SLC22 drug transporter family. Pflugers Arch. 2004;447:666–676. doi: 10.1007/s00424-003-1089-9. [DOI] [PubMed] [Google Scholar]
- 43.Duranton F., Cohen G., De Smet R., et al. Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol. 2012;23:1258–1270. doi: 10.1681/asn.2011121175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wikoff W.R., Nagle M.A., Kouznetsova V.L., Tsigelny I.F., Nigam S.K. Untargeted metabolomics identifies enterobiome metabolites and putative uremic toxins as substrates of organic anion transporter 1 (Oat1) J Proteome Res. 2011;10:2842–2851. doi: 10.1021/pr200093w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morrissey K.M., Stocker S.L., Wittwer M.B., Xu L., Giacomini K.M. Renal transporters in drug development. Annu Rev Pharmacol Toxicol. 2013;53:503–529. doi: 10.1146/annurev-pharmtox-011112-140317. [DOI] [PubMed] [Google Scholar]
- 46.Granda M.L., Huang W., Yeung C.K., Isoherranen N., Kestenbaum B. Predicting complex kidney drug handling using a physiologically-based pharmacokinetic model informed by biomarker-estimated secretory clearance and blood flow. Clin Transl Sci. 2024;17 doi: 10.1111/cts.13678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Y., Zelnick L.R., Hoofnagle A.N., et al. Prediction of kidney drug clearance: a comparison of tubular secretory clearance and glomerular filtration rate. J Am Soc Nephrol. 2021;32:459–468. doi: 10.1681/ASN.2020060833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Y., Zelnick L.R., Wang K., et al. Kidney clearance of secretory solutes is associated with progression of CKD: the CRIC study. J Am Soc Nephrol. 2020;31:817–827. doi: 10.1681/ASN.2019080811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lidgard B., Bansal N., Zelnick L.R., et al. Association of proximal tubular secretory clearance with long-term decline in cognitive function. J Am Soc Nephrol. 2022;33:1391–1401. doi: 10.1681/ASN.2021111435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thurau K., Boylan J.W. Acute renal success. The unexpected logic of oliguria in acute renal failure. Am J Med. 1976;61:308–315. doi: 10.1016/0002-9343(76)90365-x. [DOI] [PubMed] [Google Scholar]
- 51.Chawla L.S., Davison D.L., Brasha-Mitchell E., et al. Development and standardization of a furosemide stress test to predict the severity of acute kidney injury. Crit Care (London, England) 2013;17 doi: 10.1186/cc13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rewa O.G., Bagshaw S.M., Wang X., et al. The furosemide stress test for prediction of worsening acute kidney injury in critically ill patients: a multicenter, prospective, observational study. J Crit Care. 2019;52:109–114. doi: 10.1016/j.jcrc.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nigam S.K., Wu W., Bush K.T., Hoenig M.P., Blantz R.C., Bhatnagar V. Handling of drugs, metabolites, and uremic toxins by kidney proximal tubule drug transporters. Clin J Am Soc Nephrol CJASN. 2015;10:2039–2049. doi: 10.2215/cjn.02440314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koyner J.L., Chawla L.S. Use of stress tests in evaluating kidney disease. Curr Opin Nephrol Hypertens. 2017;26:31–35. doi: 10.1097/mnh.0000000000000292. [DOI] [PubMed] [Google Scholar]
- 55.Matsuura R., Komaru Y., Miyamoto Y., et al. Response to different furosemide doses predicts AKI progression in ICU patients with elevated plasma NGAL levels. Ann Intensive Care. 2018;8:8. doi: 10.1186/s13613-018-0355-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McMahon B.A., Koyner J.L., Novick T., et al. The prognostic value of the furosemide stress test in predicting delayed graft function following deceased donor kidney transplantation. Biomark Biochem Indic Expo Resp Susceptibility Chem. 2018;23:61–69. doi: 10.1080/1354750x.2017.1387934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gist K.M., Penk J., Wald E.L., et al. Urine quantification following furosemide for severe acute kidney injury prediction in critically ill children. J Pediatr Intensive Care. 2023;12:289–295. doi: 10.1055/s-0041-1732447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meersch M., Weiss R., Gerss J., et al. Predicting the development of renal replacement therapy indications by combining the furosemide stress test and chemokine (C-C motif) ligand 14 in a cohort of postsurgical patients. Crit Care Med. 2023;51:1033–1042. doi: 10.1097/ccm.0000000000005849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goldstein S.L., Krallman K.A., Roy J.P., et al. Real-time acute kidney injury risk stratification-biomarker directed fluid management improves outcomes in critically ill children and young adults. Kidney Int Rep. 2023;8:2690–2700. doi: 10.1016/j.ekir.2023.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li L.P., Milani B., Pruijm M., et al. Renal BOLD MRI in patients with chronic kidney disease: comparison of the semi-automated twelve layer concentric objects (TLCO) and manual ROI methods. Magma. 2020;33:113–120. doi: 10.1007/s10334-019-00808-5. [DOI] [PubMed] [Google Scholar]
- 61.Gomez S.I., Warner L., Haas J.A., et al. Increased hypoxia and reduced renal tubular response to furosemide detected by BOLD magnetic resonance imaging in swine renovascular hypertension. Am J Physiol Ren Physiol. 2009;297:F981–F986. doi: 10.1152/ajprenal.90757.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abuelo J.G. Normotensive ischemic acute renal failure. N Engl J Med. 2007;357:797–805. doi: 10.1056/NEJMra064398. [DOI] [PubMed] [Google Scholar]
- 63.Testani J.M., Kimmel S.E., Dries D.L., Coca S.G. Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circ Heart Fail. 2011;4:685–691. doi: 10.1161/circheartfailure.111.963256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schmidt M., Mansfield K.E., Bhaskaran K., et al. Serum creatinine elevation after renin-angiotensin system blockade and long term cardiorenal risks: cohort study. BMJ. 2017;356 doi: 10.1136/bmj.j791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morales E., Millet V.G., Rojas-Rivera J., et al. Renoprotective effects of mineralocorticoid receptor blockers in patients with proteinuric kidney diseases. Nephrol Dial Transplant. 2013;28:405–412. doi: 10.1093/ndt/gfs429. [DOI] [PubMed] [Google Scholar]
- 66.Bakris G.L., Agarwal R., Anker S.D., et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383:2219–2229. doi: 10.1056/NEJMoa2025845. [DOI] [PubMed] [Google Scholar]
- 67.Goraya N., Simoni J., Sager L.N., Mamun A., Madias N.E., Wesson D.E. Urine citrate excretion identifies changes in acid retention as eGFR declines in patients with chronic kidney disease. Am J Physiol Ren Physiol. 2019;317:F502–F511. doi: 10.1152/ajprenal.00044.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mofrad M.D., Daneshzad E., Azadbakht L. Dietary acid load, kidney function and risk of chronic kidney disease: a systematic review and meta-analysis of observational studies. Int J Vitam Nutr Res. 2021;91:343–355. doi: 10.1024/0300-9831/a000584. [DOI] [PubMed] [Google Scholar]
- 69.López M., Moreno G., Lugo G., Marcano G. Dietary acid load in children with chronic kidney disease. Eur J Clin Nutr. 2020;74(suppl 1):57–62. doi: 10.1038/s41430-020-0687-3. [DOI] [PubMed] [Google Scholar]
- 70.Goraya N., Simoni J., Sager L.N., Madias N.E., Wesson D.E. Urine citrate excretion as a marker of acid retention in patients with chronic kidney disease without overt metabolic acidosis. Kidney Int. 2019;95:1190–1196. doi: 10.1016/j.kint.2018.11.033. [DOI] [PubMed] [Google Scholar]
- 71.Crews D.C., Banerjee T., Wesson D.E., et al. Race/ethnicity, dietary acid load, and risk of end-stage renal disease among US adults with chronic kidney disease. Am J Nephrol. 2018;47:174–181. doi: 10.1159/000487715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Azim A., Murray J., Beddhu S., Raphael K.L. Urinary sulfate, kidney failure, and death in CKD: the African American study of kidney disease and hypertension. Kidney360. 2022;360:1183–1190. doi: 10.34067/kid.0000322022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Akbari A., Lemoine S., Salerno F., et al. Functional sodium MRI helps to measure corticomedullary sodium content in normal and diseased human kidneys. Radiology. 2022;303:384–389. doi: 10.1148/radiol.211238. [DOI] [PubMed] [Google Scholar]
- 74.Stenvinkel P., Jani A.H., Johnson R.J. Hibernating bears (Ursidae): metabolic magicians of definite interest for the nephrologist. Kidney Int. 2013;83:207–212. doi: 10.1038/ki.2012.396. [DOI] [PubMed] [Google Scholar]
- 75.Kellum J.A., Romagnani P., Ashuntantang G., Ronco C., Zarbock A., Anders H.J. Acute kidney injury. Nat Rev Dis Primers. 2021;7:52. doi: 10.1038/s41572-021-00284-z. [DOI] [PubMed] [Google Scholar]
- 76.Brenner B.M., Meyer T.W., Hostetter T.H. Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med. 1982;307:652–659. doi: 10.1056/nejm198209093071104. [DOI] [PubMed] [Google Scholar]
- 77.Musso C.G., Reynaldi J., Martinez B., Pierángelo A., Vilas M., Algranati L. Renal reserve in the oldest old. Int Urol Nephrol. 2011;43:253–256. doi: 10.1007/s11255-010-9769-9. [DOI] [PubMed] [Google Scholar]
- 78.Ronco C., Brendolan A., Bragantini L., et al. Renal functional reserve in pregnancy. Nephrol Dial Transplant. 1988;3:157–161. [PubMed] [Google Scholar]
- 79.Dedov I.I., Mukhin N.A., Shestakova M.V., et al. Renal functional reserve in diabetic patients without clinical nephropathy: comparisons with renal morphology. Diabet Med. 1991;8(Spec No):S43–S47. doi: 10.1111/j.1464-5491.1991.tb02155.x. [DOI] [PubMed] [Google Scholar]
- 80.Sharma A., Mucino M.J., Ronco C. Renal functional reserve and renal recovery after acute kidney injury. Nephron Clin Pract. 2014;127:94–100. doi: 10.1159/000363721. [DOI] [PubMed] [Google Scholar]
- 81.Woods L.L., Mizelle H.L., Montani J.P., Hall J.E. Mechanisms controlling renal hemodynamics and electrolyte excretion during amino acids. Am J Physiol. 1986;251:F303–F312. doi: 10.1152/ajprenal.1986.251.2.F303. [DOI] [PubMed] [Google Scholar]
- 82.Kleinman K.S., Glassock R.J. Glomerular filtration rate fails to increase following protein ingestion in hypothalamo-hypophyseal-deficient adults. Preliminary observations. Am J Nephrol. 1986;6:169–174. doi: 10.1159/000167107. [DOI] [PubMed] [Google Scholar]
- 83.Husain-Syed F., Ferrari F., Sharma A., et al. Preoperative renal functional reserve predicts risk of acute kidney injury after cardiac operation. Ann Thorac Surg. 2018;105:1094–1101. doi: 10.1016/j.athoracsur.2017.12.034. [DOI] [PubMed] [Google Scholar]
- 84.Landoni G., Monaco F., Ti L.K., et al. A randomized trial of intravenous amino acids for kidney protection. N Engl J Med. 2024;391:687–698. doi: 10.1056/NEJMoa2403769. [DOI] [PubMed] [Google Scholar]
- 85.Benzing T., Salant D. Insights into glomerular filtration and albuminuria. N Engl J Med. 2021;384:1437–1446. doi: 10.1056/NEJMra1808786. [DOI] [PubMed] [Google Scholar]
- 86.Benzing T. Signaling at the slit diaphragm. J Am Soc Nephrol. 2004;15:1382–1391. doi: 10.1097/01.asn.0000130167.30769.55. [DOI] [PubMed] [Google Scholar]
- 87.Butt L., Unnersjö-Jess D., Höhne M., et al. A molecular mechanism explaining albuminuria in kidney disease. Nat Metab. 2020;2:461–474. doi: 10.1038/s42255-020-0204-y. [DOI] [PubMed] [Google Scholar]
- 88.Butt L., Unnersjö-Jess D., Höhne M., Schermer B., Edwards A., Benzing T. A mathematical estimation of the physical forces driving podocyte detachment. Kidney Int. 2021;100:1054–1062. doi: 10.1016/j.kint.2021.06.040. [DOI] [PubMed] [Google Scholar]
- 89.Unnersjo-Jess D., Butt L., Höhne M., et al. Deep learning-based segmentation and quantification of podocyte foot process morphology suggests differential patterns of foot process effacement across kidney pathologies. Kidney Int. 2023;103:1120–1130. doi: 10.1016/j.kint.2023.03.013. [DOI] [PubMed] [Google Scholar]
- 90.Unnersjo-Jess D., Ramdedovic A., Butt L., et al. Advanced optical imaging reveals preferred spatial orientation of podocyte processes along the axis of glomerular capillaries. Kidney Int. 2023;104:1164–1169. doi: 10.1016/j.kint.2023.08.024. [DOI] [PubMed] [Google Scholar]
- 91.Deen W.M., Bridges C.R., Brenner B.M., Myers B.D. Heteroporous model of glomerular size selectivity: application to normal and nephrotic humans. Am J Physiol. 1985;249:F374–F389. doi: 10.1152/ajprenal.1985.249.3.F374. [DOI] [PubMed] [Google Scholar]
- 92.Saulnier P.J., Looker H.C., Layton A., Lemley K.V., Nelson R.G., Bjornstad P. Loss of glomerular permselectivity in type 2 diabetes associates with progression to kidney failure. Diabetes. 2023;72:1682–1691. doi: 10.2337/db23-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Joachim G.R., Cameron J.S., Schwartz M., Becker E.L. Selectivity of protein excretion in patients with the nephrotic syndrome. J Clin Invest. 1964;43:2332–2346. doi: 10.1172/JCI105107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lemley K.V., Blouch K., Abdullah I., et al. Glomerular permselectivity at the onset of nephropathy in type 2 diabetes mellitus. J Am Soc Nephrol. 2000;11:2095–2105. doi: 10.1681/ASN.V11112095. [DOI] [PubMed] [Google Scholar]
- 95.Troost J.P., Trachtman H., Nachman P.H., et al. An outcomes-based definition of proteinuria remission in focal segmental glomerulosclerosis. Clin J Am Soc Nephrol CJASN. 2018;13:414–421. doi: 10.2215/CJN.04780517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pruijm M., Mendichovszky I.A., Liss P., et al. Renal blood oxygenation level-dependent magnetic resonance imaging to measure renal tissue oxygenation: a statement paper and systematic review. Nephrol Dial Transplant. 2018;33(suppl 2):ii22–ii28. doi: 10.1093/ndt/gfy243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Caroli A., Schneider M., Friedli I., et al. Diffusion-weighted magnetic resonance imaging to assess diffuse renal pathology: a systematic review and statement paper. Nephrol Dial Transplant. 2018;33(suppl 2):ii29–ii40. doi: 10.1093/ndt/gfy163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tournebize C., Schleef M., De Mul A., et al. Multiparametric MRI: can we assess renal function differently? Clin Kidney J. 2024;18 doi: 10.1093/ckj/sfae365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Odudu A., Nery F., Harteveld A.A., et al. Arterial spin labelling MRI to measure renal perfusion: a systematic review and statement paper. Nephrol Dial Transplant. 2018;33(suppl 2):ii15–ii21. doi: 10.1093/ndt/gfy180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bjornstad P., Richard G., Choi Y.J., et al. Kidney energetics and cyst burden in autosomal dominant polycystic kidney disease: A pilot study. Am J Kidney Dis. 2024;84:286–297. doi: 10.1053/j.ajkd.2024.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Choi Y.J., Richard G., Zhang G., et al. Attenuated kidney oxidative metabolism in young adults with type 1 diabetes. J Clin Invest. 2024;134 doi: 10.1172/jci183984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cokkinos D.D., Antypa E.G., Skilakaki M., Kriketou D., Tavernaraki E., Piperopoulos P.N. Contrast enhanced ultrasound of the kidneys: what is it capable of? BioMed Res Int. 2013;2013 doi: 10.1155/2013/595873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lu Z., Ni W., Wu Y., et al. Application of biomarkers in the diagnosis of kidney disease. Front Med (Lausanne) 2025;12 doi: 10.3389/fmed.2025.1560222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen T.K., Coca S.G., Thiessen-Philbrook H.R., et al. Urinary biomarkers of tubular health and risk for kidney function decline or mortality in diabetes. Am J Nephrol. 2022;53:775–785. doi: 10.1159/000528918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Younes-Ibrahim M.S., Younes-Ibrahim M. Biomarkers and kidney diseases: a brief narrative review. J Lab Precis Med. 2022;7 doi: 10.21037/jlpm-22-1. 20-20. [DOI] [Google Scholar]
- 106.Ostermann M., Zarbock A., Goldstein S., et al. Recommendations on acute kidney injury biomarkers from the acute disease quality initiative consensus conference: a consensus statement. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.19209. [DOI] [PubMed] [Google Scholar]
- 107.Canki E., Kho E., Hoenderop J.G.J. Urinary biomarkers in kidney disease. Clin Chim Acta. 2024;555 doi: 10.1016/j.cca.2024.117798. [DOI] [PubMed] [Google Scholar]