Abstract
Membrane homeostasis is maintained by exocytosis and endocytosis. The molecular mechanisms regulating the interplay between these two processes are not clear. Inositol hexakisphosphate (InsP6) is under metabolic control and serves as a signal in the pancreatic β cell stimulus-secretion coupling by increasing Ca2+-channel activity and insulin exocytosis. We now show that InsP6 also promotes dynamin I-mediated endocytosis in the pancreatic β cell. This effect of InsP6 depends on calcineurin-induced dephosphorylation and is accounted for by both activation of protein kinase C and inhibition of the phosphoinositide phosphatase synaptojanin and thereby formation of phosphatidylinositol 4,5-bisphosphate. In regulating both exocytosis and endocytosis, InsP6 thus may have an essential integral role in membrane trafficking.
Sequential exocytosis and endocytosis are basic features of secretory cells and ensure plasma membrane homeostasis. The molecular machinery regulating the interplay between exocytosis and endocytosis is poorly understood. Inositol hexakisphosphate (InsP6) is under metabolic control and serves as a signal in the pancreatic β cell stimulus-secretion coupling by increasing Ca2+-channel activity and insulin exocytosis (1, 2). These actions depend on inhibition of serine-threonine protein phosphatases and increased protein kinase C (PKC) activity (1, 2).
The process of endocytosis is complex and involves the interaction of several components similar to phosphoinositides, e.g., phosphatidylinositol 4,5-bisphosphate (PIP2), clathrin and the clathrin adaptors, the guanosine triphosphatase dynamin I, synaptojanin 1 and the amphiphysin dimer (3–5). Dynamin is a force-generating molecule responsible for membrane fission during endocytosis (6, 7). Dynamin is also a high-affinity substrate for calcineurin, and its guanosine triphosphatase activity is determined by the balance between PKC-mediated phosphorylation and calcineurin-dependent dephosphorylation (8, 9). Moreover, dynamin I serves as a switch for depolarization-evoked synaptic vesicle endocytosis (10).
Synaptojanin 1 is a phosphoinositide 5-phosphatase, and in mice deficient in this phosphatase there are increased neuronal levels of PIP2 (11). PIP2 has been demonstrated to play a role in the nucleation of clathrin coats and an actin-based cytoskeletal scaffold at endocytotic zones, and dephosphorylation of this phosphoinositide accompanies the release of newly formed vesicles from these interactions (5). The amphiphysin dimer binds both dynamin I and synaptojanin 1 (12). Whereas phosphorylation of dynamin I and synaptojanin 1 inhibits their binding to amphiphysin, phosphorylation of amphiphysin inhibits its binding to AP-2 and clathrin (12). Hence formation between multimeric complexes of various endocytic proteins is inhibited by phosphorylation.
Here we demonstrate that InsP6 plays an important role in the regulation of endocytosis in the β cell through modulation of intracellular levels of phosphoinositides and influence on protein phosphorylation.
Materials and Methods
Preparation and Culture of β Cells.
Mouse pancreatic β cells were isolated from NMRI-mice (Bomholtgard Breeding and Research Center, Ry, Denmark). The animals were stunned by a blow against the head and killed by cervical dislocation. The pancreas was removed quickly, and pancreatic islets were isolated by collagenase digestion. The islets were dispersed into single cells by shaking in Ca2+-free solution, and the resulting cell suspension was plated on Nunc Petri dishes. The cells were maintained for up to 3 days in RPMI 1640 medium supplemented with 10% FCS/100 μg/ml streptomycin/100 units/ml penicillin. HIT-T15 insulinoma cells were cultured as described (2).
Cell Capacitance Measurements.
Endocytosis was monitored in single tissue-cultured mouse β cells as decreases in cell membrane capacitance (13) by using the standard patch whole-cell configuration, an EPC-9 patch-clamp amplifier (HEKA Electronics, Lambrecht/Pfalz, Germany), and PULSE 8.01 software (HEKA Electronics). The holding potential was −70 mV. The extracellular medium consisted of 138 mM NaCl, 5.6 mM KCl, 1.2 mM MgCl2, 2.6 mM CaCl2, 5 mM Hepes (pH 7.40 with NaOH), and 5 mM d-glucose. All electrophysiological recordings were done at 33°C. The pipette solution contained 125 mM K-glutamate, 10 mM KCl, 10 mM NaCl, 1 mM MgCl2, 5 mM Hepes, 3 mM MgATP, 10 mM EGTA, and 0, 2, and 5 mM CaCl2. The resulting free Ca2+ concentrations of the buffers were 10, 54, and 220 nM. InsP6 was dissolved in water, and PIP2 was sonicated in water (stock concentration 0.5 mM; Sigma). All other agents were dissolved in DMSO (final concentration, 0.01–0.1%) and included in the medium as indicated in the test and figures.
Dynamin Antisense Nucleotides.
Two dynamin I antisense oligonucleotides designated DAS1A (5′-GGTTGCCCATGGTTGCGGCGGGCCC-3′) and DAS1B (5′-GGCGGGTTGCGATCCCGGCAGCCGG-3′) were used (14). Control sense oligonucleotides (sense 1A and 1B) were synthesized as the reversed complements of the antisense. Dynamin II antisense oligonucleotides (DAS2A and DAS2B) were designed from the same nucleotide spans as those for dynamin I: DAS2A (5′-CCGCGGATCCCCCGACTACGACTCG-3′) and DAS2B (5′-GGTTGCCCATGGTGCCCGCGTCGCC-3′). Oligonucleotides (TAG Copenhagen, Denmark) were added (25 μM) to the tissue-culture medium 24 h before use.
Dynamin I Western Blot.
HIT T15 cells were homogenized in a buffer containing 50 mM Tris⋅HCl, 2 mM EDTA, 250 mM sucrose, 0.5 mM phenylmethylsulfonyl fluoride, and protease inhibitor mixture (Roche, Gipf-Oberfrick, Switzerland). Protein content was determined (15), and proteins were analyzed by 7.5% acrylamide SDS/PAGE with the Laemmli buffer system (16). Enhanced chemiluminescence was used for Western blot detection. Dynamin I mAb as well as PC12 cell lysate were obtained from Transduction Laboratories (Lexington, KY).
FM1-43 Fluorescence Measurements.
Membrane internalization in HIT T15 cells was recorded with a Leica TCS NT laser-scanning confocal microscope by using FM1-43 as the indicator essentially as described (17). Before the experiments, the cells were loaded with 40 μM FM1-43 on ice for 15 min, washed, and subsequently permeabilized (5 pulses of a 3-kV/cm electric field at 4°C) in 140 mM K-glutamate/5 mM NaCl/1 mM MgCl2/10 mM EGTA/25 mM Hepes/0.025% (wt/vol) albumin (pH 7.0 with KOH). After permeabilization, cells were incubated (15 min at 37°C) in buffer with 2 mM Mg-ATP/2 mM creatine phosphate/10 units/ml creatine phosphokinase, pCa 7.5 or 5.0. Incubation was stopped by cooling the samples on ice. Cells were resuspended in buffer without ATP, and fluorescence was recorded. InsP6 was present during the incubation step with ATP, whereas deltamethrin was present during dye-labeling and incubation steps.
Calcineurin Activity Assay.
The QuantiZyme assay system (Biomol, Plymouth Meeting, PA) was used to determine calcineurin activity, with RII phosphopeptide from cAMP-dependent protein kinase as substrate. Assay medium (50 μl total volume) containing 50 mM Tris⋅HCl (pH 7.5), 100 mM NaCl, 6 mM MgCl2, 0.5 mM DTT, 0.02% Nonidet P-40, 0.5 mM CaCl2, 0.25 μM calmodulin, 0.15 mM RII phosphopeptide, 0.4 units/μl recombinant human calcineurin, and 20 μM InsP6 was incubated for 40 min at 30°C. The free phosphate released was detected by a colorimetric assay (absorbance at 620 nm) using the Malachite green assay.
Phosphoinositide Measurements.
For measurements of phosphoinositide concentrations, HIT T15 cells were cultured for 2 days in RPMI 1640 medium supplemented with 5 μCi/ml (1 Ci = 37 GBq) myo-[3H]inositol. Cells then were permeabilized with electric field, washed, and incubated in permeabilization buffer containing ATP and an ATP-regenerating system for 15 min at 37°C. After incubation, lipids were extracted (18) and separated by thin layer chromatography (TLC) on silica gel 60 glass plates (Merck; ref. 19). Separated phosphoinositides were labeled with iodine vapor, regions corresponding to phosphatidylinositol, phosphatidylinositol 4-monophosphate (PIP), and PIP2 were scraped and transferred to a scintillation vial, and radioactivity was counted.
PIP2 Phosphatase Activity.
For measurements of a total PIP2 phosphatase activity, HIT T15 cells were lysed in 25 mM Tris⋅HCl/4 mM 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS)/0.5 mM phenylmethylsulfonyl fluoride and protease inhibitor mixture (Roche), pH 7.4. The lysate was incubated on ice for 30 min and cleared by high-speed centrifugation (100,000 × g for 1 h). [inositol-3H]PIP2 (0.05 μCi per sample; Dupont/NEN) supplemented with 10 μg per sample of crude brain phosphoinositides (Sigma) was dried under a stream of nitrogen and resuspended in assay buffer containing 50 mM Hepes-NaOH, 4 mM CHAPS, 0.1 mM NaCl, and 0.5 mM EGTA (pH 7.4). HIT T15 cell lysate (10 μg of protein) was incubated in a final volume of 50 μl of this assay buffer for 10 min at 37°C. Lipids were extracted, separated by TLC, and counted by the scintillation assay.
Synaptojanin Expression and Purification.
For expression of FLAG-tagged synaptojanin 1 and measurements of synaptojanin activity in immunoprecipitate, HIT T15 cells were transfected with FLAG-tagged synaptojanin 1 (p145) cDNA using the Lipofectamine 2000 transfection system (Life Technologies, Paisley, Scotland). The cDNA of FLAG-tagged human synaptojanin 1, kindly provided by P. De Camilli (Yale University, New Haven, CT), was inserted into pcDNA3 (Invitrogen). Ninety-six hours after transfection, cells were lysed in a buffer containing 50 mM Tris⋅HCl, 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 30 mM NaF, 30 mM Na4P2O7, and 4 mM CHAPS (pH 7.4). Lysate was centrifuged at 10,000 × g for 5 min, and supernatant was collected. One milliliter of supernatant containing 0.6 mg of protein was incubated overnight with 25 μl of anti-FLAG affinity gel (Sigma). After incubation, gel was washed once with the incubation buffer and three times with the PIP2 phosphatase assay buffer. The synaptojanin 1 phosphatase assay was run with the immunoprecipitate analogously to the PIP2 phosphatase assay with the cell lysate.
Statistical Analysis.
Data are presented as mean values ± SE for the indicated number of experiments, and statistical significance was evaluated using the Student's t test for unpaired observations.
Results and Discussion
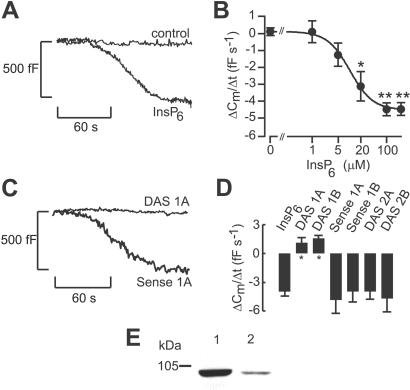
In β cells, InsP6 (100 μM) stimulated a time-dependent decrease in cell capacitance (reflecting endocytosis) at low cytoplasmic Ca2+ levels (54 nM; Fig. 1A). After an initial delay (37 ± 4 s), the cell capacitance decreased and reached a new steady state within 2 min. On average, stimulation of endocytosis by InsP6 amounted to −4.3 ± 1.5 fF/s (n = 10) when measured 30–90 s after establishment of the whole-cell configuration. No effect was observed in the absence of Ca2+ (10 nM) (control, 0.1 ± 0.5 fF/s, n = 5; 100 μM InsP6, 0.5 ± 0.6 fF/s, n = 6). At elevated [Ca2+]i (220 nM), exocytosis was stimulated even under control conditions, and here InsP6 (100 μM) reduced the increase in cell capacitance by 42% (from 4.8 ± 1.2 fF/s, n = 8, to 2.8 ± 0.8 fF/s, n = 7), reflecting the simultaneous operation of exocytosis and endocytosis. The ability of InsP6 to stimulate endocytosis depended on the dose (EC50 = 10.2 μM, as shown in Fig. 1B). Fig. 1 C and D illustrates that the stimulatory effects of InsP6 on endocytosis were mediated by activation of dynamin I as decreases in cell capacitance were abolished in cells treated with dynamin I but not dynamin II antisense oligonucleotides. The expression of dynamin I in the β cell was shown by Western blotting with a specific antibody for dynamin I (Fig. 1E). Dynamin 2 was also detected (data not shown).
Figure 1.
InsP6-induced β cell endocytosis and the role of dynamin. (A) Decrease in cell capacitance (reflecting endocytosis) elicited by intracellular infusion of InsP6 (100 μM) at 54 nM [Ca2+]i in single mouse β cells. No change in cell capacitance was observed under control conditions. Cell capacitance is displayed against time after establishment of the whole-cell configuration. The membrane potential was clamped at −70 mV throughout the experiments to prevent Ca2+ influx through the voltage-dependent Ca2+ channels. (B) Concentration dependency of stimulation of endocytosis by InsP6. The cytoplasmic Ca2+ concentration was clamped at 54 nM. Stimulation of endocytosis is expressed as the average rate of decrease in cell capacitance 30–90 s after break-in into the cells. The curve represents a least-squares fit of the data points to the equation dCm/dt = A + B(1/{1 + ([IP6]/EC50)n}). This equation takes into account the fact that cell capacitance does not equal zero in the absence of InsP6. A is the change in cell capacitance in the absence of InsP6, and B is the maximal rate of InsP6-evoked decrease in cell capacitance. EC50 is the concentration of InsP6 giving half-maximal endocytosis, and n is the cooperativity factor. (C) Changes in cell capacitance in mouse β cells pretreated with dynamin I antisense oligonucleotides (DAS 1A, 25 μM for 24 h) or with the reverse complement (sense oligonucleotide 1A). (D) Histogram depicting the average rates of decrease in cell capacitance in the presence of InsP6 (100 μM) from cells treated with dynamin I antisense oligonucleotides (DAS 1A and DAS 1B) with the complementary sense oligonucleotides (sense 1A and sense 1B) or dynamin II antisense oligonucleotides (DAS 2A and DAS 2B). The data are mean values ± SE of 4–7 individual experiments. *, P < 0.05. (E) Dynamin I is expressed in HIT T15 cells. PC12 (10 μg, lane 1) and HIT T15 (10 μg, lane 2) cell lysates were probed with mAb directed against dynamin I.
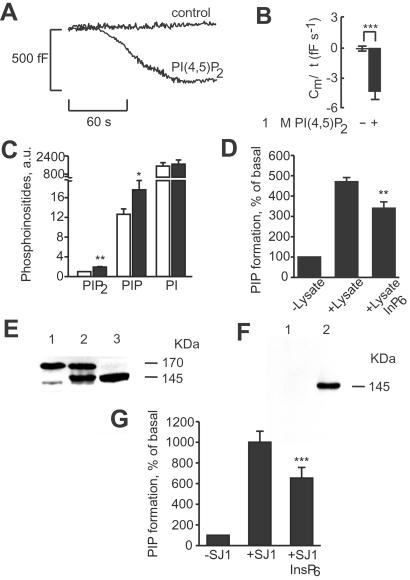
The effects of InsP6 on endocytosis were reproduced by 1 μM PIP2 (Fig. 2 A and B). Measurements of phosphoinositide concentrations in permeabilized HIT T15 cells revealed that InsP6 increased concentration of PIP2. The increase in PIP2 concentration was achieved by inhibition of phosphoinositide phosphatase activity with InsP6 (Fig. 2 C and D). Moreover, synaptojanin 1 was present in insulin-secreting cells, and the activity of this phosphoinositide 5-phosphatase was inhibited by InsP6 (Fig. 2 E–G). These data suggest that InsP6 promotes the formation of PIP2 by inhibiting synaptojanin 1, which leads to stimulation of endocytosis.
Figure 2.
The role of PIP2 in InsP6-induced β cell endocytosis. (A) Endocytosis elicited by inclusion of 1 μM PIP2 in the pipette solution with 54 nM [Ca2+]i. (B) Histogram showing average decreases in cell capacitance. The data are mean values ± SE for six (control) and five experiments (PIP2). ***, P < 0 001. (C) Effect of 50 μM InsP6 on phosphoinositide levels in permeabilized HIT T15 cells (n = 4). White bars depict phosphoinositide levels in the absence of InsP6, and black bars depict phosphoinositide levels in the presence of InsP6. *, P < 0.05, and **, P < 0.01, paired t test vs. concentration of corresponding phosphoinositide in the absence of InsP6. a.u., arbitrary units. (D) Inhibition of total PIP2 phosphatase activity with 50 μM InsP6 in HIT T15 cell lysate (n = 4). Phosphatase activity was monitored as formation of PIP from PIP2 without the addition of cell lysate and in the presence of cell lysate without or with the addition of InsP6. **, P < 0.01, paired t test vs. PIP formation in the absence of InsP6. (E) Western blot analysis with anti-synaptojanin 1 polyclonal Ab. Lane 1, HIT T15 cells; lane 2, HIT T15 cells overexpressing FLAG-tagged synaptojanin 1 isoform (p145); lane 3, mouse brain. (F) Western blot analysis with anti-FLAG mAb of anti-FLAG affinity gel precipitates in HIT T15 cell lysate (lane 1) and HIT T15 cell lysate expressing a 145-kDa isoform of FLAG-tagged synaptojanin 1 (lane 2). (G) Inhibition of synaptojanin 1 activity with 50 μM InsP6 in synaptojanin 1 immunoprecipitates (n = 4). Synaptojanin activity was monitored as formation of PIP from PIP2 in anti-FLAG affinity gel precipitates of lysates from either HIT T15 cells without treatment (−SJ1) or HIT T15 cells expressing FLAG-tagged synaptojanin 1 (+SJ1) without or with the addition of InsP6. ***, P < 0.001, paired t test vs. PIP formation in the absence of InsP6.
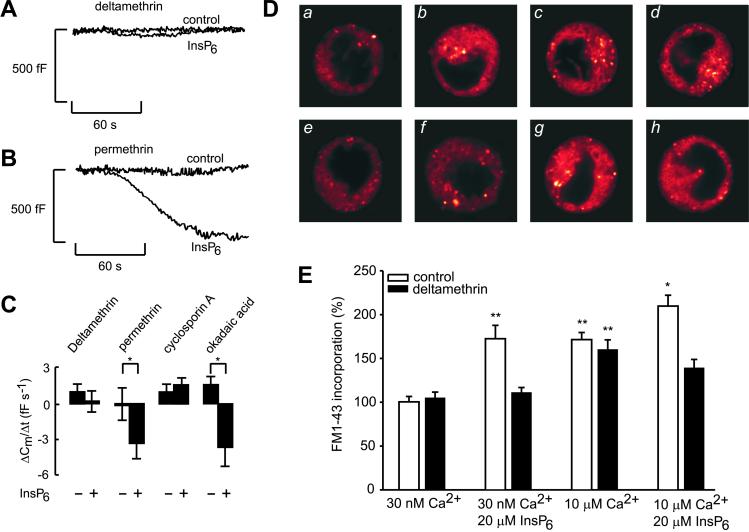
To explore the possibility that InsP6-mediated endocytosis depended on calcineurin (protein phosphatase 2B)-induced dephosphorylation, protein phosphatase 2B inhibitors were included in the cytoplasmic solution dialyzing the cell. Deltamethrin, an inhibitor of calcineurin, abolished the stimulatory effect of InsP6 on endocytosis (Fig. 3A), whereas the inactive analogue, permethrin, was ineffective (Fig. 3B). Cyclosporin A likewise removed the stimulatory action of InsP6 on endocytosis (Fig. 3C). By contrast, okadaic acid (an inhibitor of PP1, PP2A, and PP3 phosphatases) failed to counteract the stimulatory effect of InsP6 on endocytosis (Fig. 3C).
Figure 3.
The role of dephosphorylation in InsP6-induced β cell endocytosis. Cell capacitance was measured in mouse β cells pretreated with deltamethrin (20 nM for >1 h, A) or permethrin (20 nM for >1 h, B) in the absence and presence of 100 μM InsP6 in the recording pipette containing 54 nM [Ca2+]i. (C) Histogram depicting average data ± SE of 5–7 experiments in the absence (−) and presence (+) of 100 μM InsP6 in mouse β cells pretreated with deltamethrin, permethrin, cyclosporin A (1 μM for >15 min), or okadaic acid (0.1 μM for >10 min). *, P < 0.05. (D) Visualization of endocytosis by FM1-43. Cells were incubated with a permeabilization buffer containing 30 nM Ca2+ (a), 30 nM Ca2+ and 20 μM InsP6 (b), 10 μM Ca2+ (c), 10 μM and 20 μM InsP6 (d), 30 nM Ca2+ and 20 nM deltamethrin (e), 30 nM Ca2+ with 20 μM InsP6 and 20 nM deltamethrin (f), 10 μM Ca2+ and 20 nM deltamethrin (g), and 10 μM Ca2+ with 20 μM InsP6 and 20 nM deltamethrin (h). The images are representative of 24 (a–d) or 12 (e–h) cells from eight (a–d) or four (e–h) independent cell preparations. (E) Histogram showing average FM1-43 fluorescence (means ± SE) for the experiments shown in D. One hundred percent is equal to dye incorporation at 30 nM Ca2+. *, P < 0.05 vs. dye incorporated at 10 μM Ca2+; **, P < 0.001 vs. dye incorporated at 30 nM Ca2+.
We used the fluorescence membrane dye FM1-43 with quantitative fluorescence microscopy to separate the molecular events involved in endocytosis from those of exocytosis. Fig. 3 D and E shows that InsP6 stimulated FM1-43 incorporation in permeabilized HIT T15 cells incubated in either low (30 nM, a, b, e, and f) or high (10 μM, c, d, g, and h) Ca2+-containing medium. Deltamethrin (20 nM, images f and h) blocked InsP6 but not Ca2+-induced endocytosis (images e and g). The involvement of calcineurin is not the result of a direct action of InsP6, because phosphatase activity was not affected by this inositol polyphosphate. Calcineurin activity amounted to 0.38 nmol of phosphate/h under basal conditions (n = 4) and 0.40 nmol of phosphate/h in the presence of 20 μM InsP6 (n = 4). Similar negative results were observed in homogenates of β cells at 100 μM InsP6.
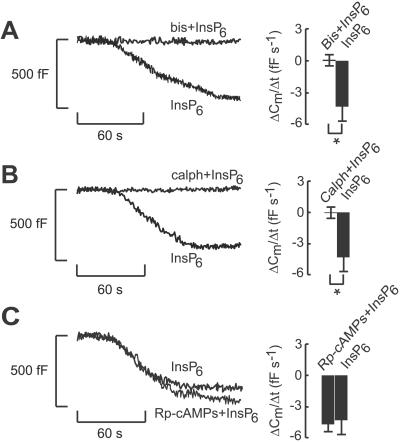
The stimulatory effect of InsP6 on endocytosis was abolished by the PKC inhibitors bisindolylmaleimide (Fig. 4A) or calphostin C (Fig. 4B). These observations argue that the stimulatory action of InsP6 depends on the activity of PKC. By contrast, activation of protein kinase A clearly is not required for the stimulatory effect, because InsP6 remained equally effective in the presence of 100 μM Rp-cAMPS, an inhibitor of protein kinase A (−5.06 ± 0.73 fF/s, n = 5 vs. 0.1 ± 0.5 fF/s, n = 5 under control conditions; Fig. 4C).
Figure 4.
The role of phosphorylation in InsP6-induced β cell endocytosis. Inhibition of InsP6-evoked decreases in cell capacitance in mouse β cells pretreated with bisindolylmaleimide (bis; 2.0 μM for 10 min, A) or calphostin C (calph; 1.5 μM for 15 min, B). (C) Rp-cAMPS (adenosine-3′,5′-cyclic monophosphothioate; 100 μM for >15 min), an inhibitor of protein kinase A, did not affect InsP6-induced endocytosis. The inhibitors also were included in the pipette solution dialyzing the cells. [Ca2+]i in the pipette-filling solution was 54 nM. The histograms depict average data ± SE for five and seven experiments, respectively.
We now show that InsP6 promotes endocytosis in the pancreatic β cell and that this process is mediated by dynamin I. Subsequent to glucose stimulation, cytoplasmic free-Ca2+ concentration rises, which leads to calcineurin-induced dephosphorylation, allowing dynamin to assemble and form multimeric protein complexes at the site of endocytosis (8, 9). Simultaneously, InsP6 concentration increases (1), resulting in activation of PKC, which may promote phosphorylation-induced dissociation of the various proteins involved in endocytosis (12). This scenario is compatible with a model in which proteins involved in endocytosis undergo constitutive phosphorylation and assemble in a dephosphorylated form (12). Because the phosphatidylinositol phosphatase synaptojanin can adjust the inositol lipid composition of membranes and thereby regulate dynamin recruitment (20), it is of interest to note that activity of this lipid phosphatase is inhibited by InsP6 (Fig. 2; ref. 21), resulting in accumulation of PIP2. Hence InsP6 promotes endocytosis by interacting at several distinct regulatory sites. The fact that InsP6 also promotes exocytosis (2) may suggest that this inositol polyphosphate has an important integral role in membrane trafficking in secretory cells by being part of the molecular mechanisms linking exocytosis to endocytosis.
Acknowledgments
This study was supported by the Swedish Medical Research Council, the Royal Swedish Academy of Sciences, the Swedish Diabetes Association, Novo Nordisk Foundation, Juvenile Diabetes Foundation International, and Funds of Karolinska Institutet.
Abbreviations
- InsP6
inositol hexakisphosphate
- PKC
protein kinase C
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PIP
phosphatidylinositol 4-monophosphate
References
- 1.Larsson O, Barker C, Sjöholm Å, Carlquist H, Michell R H, Bertorello A, Nilsson T, Honkanen R E, Mayr G W, Zwiller J, Berggren P-O. Science. 1997;278:471–474. doi: 10.1126/science.278.5337.471. [DOI] [PubMed] [Google Scholar]
- 2.Efanov A, Zaitsev S V, Berggren P-O. Proc Natl Acad Sci USA. 1997;94:4435–4439. doi: 10.1073/pnas.94.9.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heuser J E, Reese T S. J Cell Biol. 1973;57:315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cremona O, De Camilli P. Curr Opin Neurobiol. 1997;7:323–330. doi: 10.1016/s0959-4388(97)80059-1. [DOI] [PubMed] [Google Scholar]
- 5.Cremona O, De Camilli P. J Cell Sci. 2001;114:1041–1052. doi: 10.1242/jcs.114.6.1041. [DOI] [PubMed] [Google Scholar]
- 6.Sweitzer S M, Hinshaw J E. Cell. 1998;93:1021–1029. doi: 10.1016/s0092-8674(00)81207-6. [DOI] [PubMed] [Google Scholar]
- 7.Schmid S L, McNiven M A, De Camilli P. Curr Opin Cell Biol. 1998;10:504–512. doi: 10.1016/s0955-0674(98)80066-5. [DOI] [PubMed] [Google Scholar]
- 8.Robinson P J, Sontag J-M, Liu J-P, Fykse E M, Slaughter C, McMahon H, Südhof T C. Nature (London) 1993;365:163–166. doi: 10.1038/365163a0. [DOI] [PubMed] [Google Scholar]
- 9.Robinson P J, Liu J-P, Powell K A, Fykse E M, Südhof T C. Trends Neurosci. 1994;17:348–353. doi: 10.1016/0166-2236(94)90179-1. [DOI] [PubMed] [Google Scholar]
- 10.Liu J-P, Sim A T R, Robinson P J. Science. 1994;265:970–973. doi: 10.1126/science.8052858. [DOI] [PubMed] [Google Scholar]
- 11.Cremona O, Di Paolo G, Wenk M R, Lüthi A, Kim W T, Takei K, Daniell L, Nemoto Y, Shears S B, Flavell R A, McCormick D A, De Camilli P. Cell. 1999;99:179–188. doi: 10.1016/s0092-8674(00)81649-9. [DOI] [PubMed] [Google Scholar]
- 12.Slepnev V I, Ochoa G-C, Butler M H, Grabs D, De Camilli P. Science. 1998;281:821–824. doi: 10.1126/science.281.5378.821. [DOI] [PubMed] [Google Scholar]
- 13.Eliasson L, Proks P, Ammala C, Ashcroft F M, Bokvist K, Renstrom E, Rorsman P, Smith P A. J Physiol (London) 1996;493:755–767. doi: 10.1113/jphysiol.1996.sp021420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torre E, McNiven M A, Urrutia R. J Biol Chem. 1994;269:32411–32417. [PubMed] [Google Scholar]
- 15.Bradford MM. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli UK. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Vida T A, Emr S M. J Cell Biol. 1995;128:779–792. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bligh E G, Dyer W J. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 19.Jolles J, Zwiers H, Dekar H, Wirtz W A, Gispen W H. Biochem J. 1981;194:283–291. doi: 10.1042/bj1940283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marsh M, McMahon H T. Science. 1999;285:215–220. doi: 10.1126/science.285.5425.215. [DOI] [PubMed] [Google Scholar]
- 21.Höer A, Oberdisse E. Biochem J. 1991;278:219–224. doi: 10.1042/bj2780219. [DOI] [PMC free article] [PubMed] [Google Scholar]