Abstract
Aneuploidy is ubiquitous in cancer, and its phenotypes are inevitably dominant and abnormal. In view of these facts we recently proposed that aneuploidy is sufficient for carcinogenesis generating cancer-specific aneusomies via a chain reaction of autocatalytic aneuploidizations. According to this hypothesis a carcinogen initiates carcinogenesis via a random aneuploidy. Aneuploidy then generates transformation stage-specific aneusomies and further random aneusomies autocatalytically, because it renders chromosome segregation and repair mechanisms error-prone. The hypothesis predicts that several specific aneusomies can cause the same cancers, because several chromosomes also cooperate in normal differentiation. Here we describe experiments on the Chinese hamster (CH) that confirm this hypothesis. (i) Random aneuploidy was detected before transformation in up to 90% of CH embryo cells treated with the carcinogen nitrosomethylurea (NMU). (ii) Several specific aneusomies were found in 70–100% of the aneuploid cells from colonies transformed with NMU in vitro and from tumors generated by NMU-transformed cells in syngeneic animals. Among the aneuploid in vitro transformed cells, 79% were trisomic for chromosome 3, and 59% were monosomic for chromosome 10, compared with 8% expected for random distribution of any aneusomy among the 12 CH chromosomes. Moreover, 52% shared both trisomy 3 and monosomy 10 compared with 0.6% expected for random distribution of any two aneusomies. Among the tumor cells, 65% were trisomic for chromosome 3, 51% were trisomic for chromosome 5, and 30% shared both trisomies. Aneuploid cells without these specific aneusomies may contain minor transformation-specific aneusomies or may be untransformed. (iii) Random aneusomies and structurally altered chromosomes increased with the generations of transformed cells to the point where their origins became unidentifiable in tumors. We conclude that specific aneusomies are necessary for carcinogenesis.
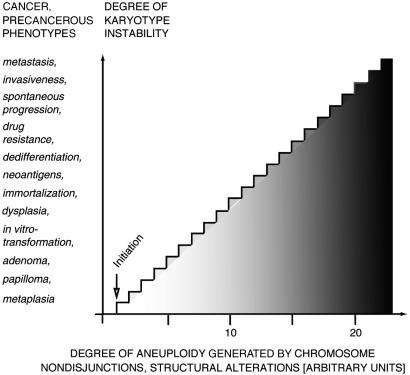
Aneuploidy is ubiquitous in cancer (1–4), and its phenotypes are inevitably dominant and abnormal because of abnormal dosages and expression of thousands of normal genes (5–11, 78). Down's syndrome, generated by trisomy of chromosome 21, is a classic example of an abnormal phenotype generated by abnormal expression of many normal genes (7). In view of these facts we have recently proposed that aneuploidy is sufficient for carcinogenesis generating cancer-specific aneusomies via a chain reaction of autocatalytic aneuploidizations (1–4). According to this hypothesis, carcinogens initiate carcinogenesis via a random aneuploidy. Aneuploidy then generates cancer-specific aneusomies and further random aneusomies autocatalytically, because it renders chromosome segregation and repair mechanisms error-prone (Fig. 1). The hypothesis predicts that several specific aneusomies can cause the same cancers and stages of carcinogenesis, because several chromosomes also cooperate in normal differentiation, just like several mutations of a gene may generate the same phenotype.
Figure 1.
The correlations between the degrees of aneuploidization and chromosomal instability and the generation of cancer-specific phenotypes according to the aneuploidy cancer hypothesis. The shading reflects the increasing numerical and structural alterations of chromosomes and the corresponding phenotypes that are generated by the chain reaction of autocatalytic aneuploidization.
Cancer-specific aneusomies are thought to encode the various stages of carcinogenesis such as morphological transformation, dedifferentiation or anaplasia, immortality, neoantigens, invasiveness, and metastasis (ref. 12; Fig. 1) by abnormal expressions of normal genes. Indeed, abnormal gene expression is “the sine qua non of malignant transformation” (13), and many hundreds of abnormally expressed genes have been identified recently in cancer cells (14–16). Likewise, specific aneusomies are thought to encode the phenotypes of irreversible precancerous stages of carcinogenesis such as metaplasias, dysplasias, adenomas, papillomas, and cellular transformations (refs. 1 and 12; Fig. 1).
Because the probability that random karyotype variation will generate a new karyotype that out-competes a normal diploid cell in its own habitat is very low, the average time for the autocatalytic evolution of a neoplastic karyotype will be long, analogous to the evolution of a new species. However, the process can be accelerated by supplemental carcinogens or by tumor promoters (17, 18).
In an effort to test the karyotype evolution predicted by the aneuploidy cancer hypothesis, we have analyzed here the karyotypes of three stages of experimental carcinogenesis with an inbred line of the Chinese hamster (CH): (i) as-yet-untransformed cells in which carcinogenesis had been “initiated” with the mutagenic carcinogen nitrosomethylurea (NMU; refs. 19 and 20) (ii); CH embryo (CHE) cells transformed in vitro with NMU to three-dimensional growth and neoplastic morphology; and (iii) tumor cells generated by inoculation of in vitro-transformed cells into syngeneic animals.
In contrast to the analysis of clinical human cancers (21), the experimental system used here is reproducible and controllable. It allows the simultaneous generation of multiple transformants with the same carcinogen in the same isogenic cells in vitro and of multiple tumors with in vitro transformed cells or with carcinogens in isogenic animals. This method should limit the number of transformation-specific aneusomies, if they exist, by eliminating variations caused by different carcinogens, genetic backgrounds, and latent periods from unknown initiation to manifestation of carcinogenesis, which all complicate the analysis of clinical cancers (21–23). Moreover, the experimental system used here allows us to determine exactly when after initiation of carcinogenesis with a carcinogen aneuploidy is first generated by analyzing preneoplastic aliquots of carcinogen-treated cells or animals (24).
If successful, this system should help to decide between our hypothesis, which postulates that carcinogenesis is initiated by aneuploidy and that specific aneusomies encode specific cancer phenotypes, and the competing hypothesis, which postulates that cancer is caused by transformation-specific gene mutations (3, 4, 25–30) and that aneuploidy may play an indirect role in carcinogenesis. For example, aneuploidy has been proposed to “select for gains of chromosomes containing activated oncogenes and for loss of chromosomes containing tumor-suppressor genes” (31) or to “accelerate the acquisition of growth promoting mutations” (23), possibly as a result of genes altered by chromosome rearrangements (32, 33).
In agreement with our hypothesis, we have found here random aneuploidy in carcinogen-treated cells before transformation, transformation stage-specific aneusomies in cells transformed in vitro as well as in tumors, and also random aneuploidy in all transformed cells.
Materials and Methods
Cells.
Male CHE cells were prepared and maintained as described (34, 35).
Cytogenetic Analysis.
The analysis of metaphase chromosomes followed published procedures (35–37).
Tumorigenicity of Cells.
Between 4 and 6 million CH cells, the equivalent of 0.5–1 confluent 10-cm Petri dish, were dissociated with trypsin, resuspended in ≈0.3 ml of PBS, and injected into in the upper hind leg of 6–9-month-old syngeneic, male CHs (34). Tumors of 1–1.5 cm were explanted at the time of diagnosis, minced with scalpels, dissociated into single cells with trypsin, and then cultured until a confluent Petri dish was obtained for the analysis of metaphases as described (24).
Results
NMU Is Highly Aneuploidogenic.
To test the prediction of the aneuploidy hypothesis that carcinogens are aneuploidogenic, we investigated whether the “directly mutagenic” carcinogen NMU causes aneuploidy under the conditions in which it is carcinogenic (20, 38). Following protocols used for tumorigenicity by others (39–42), ≈2 × 106 CHE cells were incubated in a 10-cm culture dish at 37°C with 0.5 μg (5 nmol) of NMU (Sigma) dissolved in 4 ml of PBS for 2 h, because in that condition the half-life of NMU is 1 h (Sigma lists half-life in their catalog). The NMU-treated cells reached confluence in 2–4 days in complete DMEM. Because it is known that the same amount of carcinogen is more effective if delivered at multiple low doses than at one high dose (17), these treatments were repeated six times. This dosage of NMU had no detectable toxicity on CHE cells. The half-lethal dose of NMU is over 100 μg in our condition (data not shown). Before each NMU treatment, confluent cultures were diluted 4-fold. After the six NMU treatments, the cells had become more refractile than untreated controls but were not growing three-dimensionally like neoplastic cells (35). At this time the karyotypes were analyzed and reported in accord with the International System for Cytogenetic Nomenclature (ISCN; refs. 21 and 37).
As shown in Table 1, 11 of 20 (55%) of the six-times NMU-treated cells had become aneuploid via losses of chromosomes 2, 5, 7, 9, 10, X, and Y or via gains of chromosomes 1, 2, 3, 7, and 9. A reciprocal chromosome translocation, t(1;6), observed in four of these cells is not relevant here, because it does not alter the normal gene balance of the cell. The remaining nine (45%) cells had normal balances of autosomal chromosomes, although seven had lost the Y chromosome; but because the loss of the Y chromosome generates the normal female chromosome balance, the chromosome balance of these cells is not a source of abnormal phenotypes. In normal females, one of two X chromosomes is inactivated naturally to match the gene dosis with that of males (43)
Table 1.
Karyotypes of untreated, NMU-treated, and DMBA-treated CH cells
| Treatment | Karyotypes* |
|---|---|
| NMU, 6× 0.5 μg | Aneuploid; 20,−X,−Y[1]; 20,XY,−2,−5[1], 21,X,−Y,−7,+9[1]; 21,XY,t(1;6)(q;q),+3,−5,−9[2]; 22,X,−Y,+2[1]; 22,XY,+3,−9[1]; 22,XY,+7,−10[1]; 22,X,−Y,+1[1]; 22,XY,t(1;6)(q;q),+3,−9[2]; Normal chromosome balance; 22,XY[2]; 21,X,−Y[7] |
| NMU, 3× 1 μg | Aneuploid; 18,XY,+3,−5,−6,−7,−9,−10[1]; 21,XY,+3,−9,−10[1]; 22,XY,+3,−10[3]; 24,XY,+1,+3[1]; 23,XY,+3[1]; 44,XXYY,+3,+3,−10,−10[1]; Normal chromosome balance; 22,XY[2] |
| NMU, 3× 10 μg | Aneuploid; 21,X,−Y+3,−10[1]; 22,XY,+3,−10[1]; 22,X,−Y,+3[2]; 23,XY,+3[14]; Normal chromosome balance; 22,XY[1]; 21,X,−Y[1] |
| NMU, 3× 100 μg | Aneuploid; 22,XY,+4,−10[1]; 22,X,−Y,+2[1]; 22,X,−Y,+4[2]; 23,XY,+3[1]; 24,XY,Yp−,+3[3]; 24,XY,Yp−,+4[3]; 24,XY,+4,+8[1]; Normal chromosome balance; 22,XY[3] |
| 12 generations in vitro | Aneuploid; 23,XY,+X[5]; 22,XY,+X,−10[1]; Normal chromosome balance; 22,XY[3]; 21,X,−Y[2]; 21,X,−Y,der(9)t(Y;9)(q;q)[9] |
| DMBA, 3× 2.5 μg | Aneuploid; 20,XY,2p−,−9,−10[1]; 20,XY,Yp+,−7,−9[1]; 20,XY,Yp+,+4,−5,−8,−10[1]; 21,XY,Yp+,+3,−7,−9[1]; 22,XY,4q+[1]; 22,XY,Yp+[6]; 22,XY,Yp+,+3,−10[1]; 23,XY,Yp+,+3[1]; 23,XY,Yp+,+4[1]; 23,XY,Yp+,+7[1]; 23,XY,Yp+,+9[1]; 23,XY,+2,+4,−10[1]; 29,XY,Yp+,+1,+2,+3,+4,+5,+6,+8[1]; 41,XXYY,Yp+,Yp+,−7,−8,−9[1]; 46,XXYY,Yp+,Yp+,+10,+10[1] |
The CH has 11 chromosome pairs. According to the International System for Cytogenetic Nomenclature (21, 37), the numbers in square brackets represent cells with identical karyotypes. +, trisomic chromosome; −, monosomic chromosome; p and q, short and long arms of chromosomes relative to the centromere, respectively; p or q +/−, additions to or deletions from these chromosome arms, respectively; der, derivative generated by a translocation.
To confirm the surprisingly efficient aneuploidization by NMU in view of its notoriety for “causing tumors by direct mutagenesis” (20, 38), three independent sets of 2 million CHE cells were analyzed for aneuploidy after only three treatments with 1, 10, and 100 μg of NMU. As can be seen in Table 1, between 80 and 90% of the cells were rendered aneuploid by these NMU treatments generating either gains or losses of all 10 autosomal and both sex chromosomes. Thus NMU is highly aneuploidogenic in conditions used for carcinogenesis.
By contrast, only 1 of 20 (5%) untreated control cells had lost an autosomal chromosome, i.e., chromosome 10, after 6 passages or 12 generations in culture (Table 1). However, six of the 20 cells (30%) had gained a second X chromosome, confirming earlier evidence that the karyotypes of CHE cells are relatively unstable in vitro (ref. 44; Table 1). In addition, 2 of the 20 cells had lost the Y chromosome and 9 had lost all but a q fragment of the Y chromosome, which was added to the end of chromosome 9 to form a monocentric hybrid chromosome, der(9)t(Y;9)(q;q) (Table 1). Again the loss of Y does not generate an abnormal gene balance (see above). During their six passages in culture these cells retained contact inhibition of growth and the morphology that are typical of primary CHE cells in vitro.
Another set of 2 million CHE cells was treated three times with 2.5 μg of the carcinogen 7,12-dimethylbenz[a]anthracene (DMBA) to serve as a standard of aneuploidization by carcinogens (24, 35). The three treatments with DMBA had rendered 100% of the CHE cells aneuploid (Table 1). Thus per molecule of carcinogen, the nonmutagenic DMBA (2.5 μg = 10 nmol) is an even better aneuploidogen than the mutagenic NMU (1, 10, and 100 μg correspond to 10, 100, and 1,000 nmol).
Specific Aneusomies in NMU-Transformed Cells.
To test the prediction of our hypothesis that neoplastic transformation depends on specific aneusomies, we have isolated transformed colonies arising from NMU-treated cells and then analyzed their karyotypes. For this purpose, NMU-treated cells were incubated under conditions that allow formation of three-dimensional colonies by transformed cells, whereas normal cells remain contact-inhibited. Contact inhibition of normal cells was achieved by transferring the cells that had been treated six times with NMU from one confluent 10-cm dish to three dishes and incubating them without further dissociation. Under these conditions, transformed cells grow into three-dimensional foci as described (35, 45). Approximately 4 weeks later, over 90 three-dimensional foci (Fig. 2) and ≈450 less pronounced foci appeared in the three culture dishes. Five foci were picked and grown into confluent cultures on 10-cm dishes for analysis of their karyotypes.
Figure 2.
Two three-dimensional foci, F1 and F2 (Table 2), of CHE cells transformed with NMU in vitro. The cultures were magnified at ×125 with a phase-contrast microscope. (Left) Note that the peak of the focus F1 appears black because the cell layers were too thick for the light to penetrate.
It can be seen in Table 2 that the five foci, numbered F1–F5, were between 75 and 100% aneuploid. Based on previous studies, the low percentages of diploid cells found in four of the five foci are likely to reflect nontransformed cells from the original culture, because the focal cells were analyzed without prior clonal selection for the transformed phenotype (35, 45). It is shown in Table 3 that 79% (52/66) of all aneuploid cells of F1–F5 were trisomic for chromosome 3, and 59% (39/66) were monosomic for chromosome 10. By contrast, random distribution predicts only 8% for a given aneusomy among the 12 CH chromosomes. Remarkably, 52% (34/66) of the aneuploid cells were both trisomic for chromosome 3 and monosomic for chromosome 10 compared with 0.6% predicted for random distribution of any two aneusomies among the 12 CH chromosomes.
Table 2.
Karyotypes of five foci of CHE cells transformed with NMU in vitro
| Focus | Karyotypes* |
|---|---|
| F1 | Aneuploid; 20,XY,t(1;6)(q;q),+3,−7,−8,−10[1]; 22,XY,t(1;6)(q;q),+3,−10[13] |
| F2 | Aneuploid; 22,XY,+3,−8[1]; 23,XY,+3[3]; 24,XY,+3,+10[1]; 46,XXYY,+3,+7[1]; Diploid; 22,XY[2] |
| F3 | Aneuploid; 21,XY,t(1;6)(q;q),−10[1]; 22,XY,t(1;6)(q;q),+3,−10[14]; 23,XY,t(1;6)(q;q),+3,+5,−10[1]; 23,XY,+3[3]; Diploid; 22,XY[1]; 22,XY,t(1;6)(q;q)[1] |
| F4 | Aneuploid; 20,XY,−8,−10[1]; 20,XY,t(1;6)(q;q),−9,−10[1]; 21,XY,−1[1]; 21,XY,−6[1]; 21,XY,−10[2]; 21,Y,−X[1]; 22,XY,t(1;6)(q;q),+3,−10[2]; 23,XY,+3[1]; 23,XY,+7[3]; 23,XY,t(1;6)(q;q),+3,+4,−10[1]; Diploid; 22,XY,t(1;6)(q;q)[1]; 22,XY[5] |
| F5 | Aneuploid; 22,XY,−6,+r[2]; 22,XY,t(1;6)(q;q),+3,−10[2]; 23,XY,+3[7]; 24,XY,+X,+5[1]; 24,XY,+3,+10[1]; Diploid; 22,XY[2] |
Specific aneusomies are marked in bold.
Table 3.
Percentage of aneuploid cells with specific aneusomies from five foci, generated with NMU in vitro, and from 10 tumors, generated with NMU-transformed cells
| Cells | Trisomies
|
Monosomies
|
Double Aneusomies
|
|||
|---|---|---|---|---|---|---|
| 3+ | 4+ | 5+ | 10− | 3+/10− | 3+/5+ | |
| NMU foci | 79 | 59 | 52 | |||
| Tumors | 65 | 21 | 51 | 30 | ||
Thus under our conditions trisomy 3, monosomy 10, and the combination of both seem to be transformation-specific aneusomies. Indeed, the coincidence that F1 was the first and fastest growing three-dimensional focus to appear in NMU-treated cells (Fig. 2), the first of the five foci to be tumorigenic (below, Table 4), and that all of its cells contained both trisomy 3 and monosomy 10 (Table 2) suggests that trisomy 3 and monosomy 10 may have complementary transforming functions.
Table 4.
Latent periods from inoculation of NMU-transformed CHE cells to tumorigenesis in syngeneic CHs
| Weeks | 7 | 8 | 9 | 10 | 12 | 15 | 21 |
|---|---|---|---|---|---|---|---|
| Tumors | T1-1a | T3-1a | T3-2 | T5 | T4-1 | T4-2 | T2 |
| T1-1b | T3-1b | ||||||
| T1-2 |
Only 14% (9/66) of the aneuploid cells of the five foci that were analyzed did not contain either trisomy 3 or monosomy 10. However, one of these cells, from focus F5, contained an alternate transformation-specific aneusomy, i.e., trisomy 5, that has been identified here in CH tumors (see below) and in other neoplastic CH cells described by others (see Discussion). Another one of the nine cells contained an unidentifiable ring chromosome, r, that may or may not contain elements of chromosomes 3 (and 5). Based on our hypothesis the focus-derived aneuploid cells without major specific aneusomies may contain either minor transformation-specific aneusomies or may be aneuploid, but untransformed cells.
It may be argued that the specific aneusomies of the five foci are not transformation-specific, because they already occur in some NMU-treated CHE cells from which the transformed foci were isolated. For example, 59% (29/49) of the 49 aneuploid NMU-treated cells described in Table 1 had trisomy 3, 20% (10/49) had monosomy 10, and 16% (8/49) even had the double aneusomy, trisomy 3 plus monosomy 10 (Table 1). However, this argument fails to consider that (i) survival of aneuploidy already reflects a selection of nonrandom karyotypes, and (ii) the aneuploid precursor cells of the NMU foci are likely to include already transformed cells that have remained undetected, because they could not develop into foci as long as the cultures were dissociated for NMU treatments at weekly intervals. It indeed was observed that undissociated aliquots of CHE cells treated three, four, and five times with NMU did develop foci on further incubation, although fewer than the cells treated six times, which generated the foci that were analyzed. Thus the existence of these aneusomies in NMU-treated cells not cultured for focus formation indeed may signal the existence of transformed cells.
Long Latent Periods from Transformation in Vitro to Tumorigenicity.
Next we investigated whether stage-specific aneusomies set apart cells transformed in vitro from those of tumors induced by in vitro transformed cells. Numerous experimentalists have observed that transformation of cells in vitro is not necessarily sufficient for transformation in vivo (refs. 13 and 46; Fig. 1). Therefore, typically millions of in vitro transformed cells are injected into animals to improve the odds for the missing “steps” from transformation in vitro to tumorigenesis to occur in one of these cells (40, 47–49).
In view of this, we inoculated 4–6 million cells from each of the five NMU-induced foci into pairs of 6–9-month-old syngeneic CHs to induce tumors (ref. 34; Materials and Methods). Seven to twenty-one weeks after inoculation of the in vitro transformed cells palpable tumors of 1–1.5 cm appeared in one or both of two inoculated hamsters (Table 4). By contrast, normal, diploid CHE cells are not tumorigenic in this system (35). The tumors were numbered T1–T5, to designate their origin from the foci F1–F5, and with a second number to designate their origin from individual animals, i.e., T1-1 and T1-2 (Tables 4 and 5). In two of the animals, two distinct tumors appeared that were labeled a and b. No tumors appeared in one of two animals inoculated with cells of F2 and F5 during a 27-week period of observation (Table 4).
Table 5.
The karyotypes of CH tumors induced with NMU-transformed CHE cells
| Tumor | Karyotypes* |
|---|---|
| T1-1a | Aneuploid; 23,XY,+3[1]; 23,XY,+4[1]; 24,XY,+3,+6[1]; 24,XY,+3,+10[1]; 24,XY,+6,+10[1]; 24,XY,+3,+mar[1]; 24,XY,+4,+mar[1]; 25,XY,+3,+4,+mar[2]; 25,XY,+3,+6,+mar[1] |
| T1-1b | Aneuploid; 23,XY,+3[24]; 23,XY,+4[1]; 24,XY,+3,+4[1]; 24,XY,+3,+6[3]; 25,XY,+3,+4,+9[1] |
| T1-2 | Aneuploid; 23,XY,+3,−4,+10[1]; 23,XY,+3,−7,+9[1]; 24,XY,+5,−7,+9,+mar[1]; 24,Y,−X,+3,+10,+mar[1]; 24,XY,+10,+mar[1]; 25,XY,+9,+10,+mar[1]; 25,XY,+3,+9,+mar[1]; 26,XY,+9,+9,+10,+mar[1]; 26,XY,+3,+5,+9,+mar[2]; 26,Y,−X,+3,+5,+9,+10,+mar[1]; 27,XY,+3,+5,+7,+9,+10[1]; 27,XY,+3,+4,+5,+6,−7,+10,+mar[1]; 27,XY,+3,+5,+9,+10,+mar[1]; 28,XY,+3,+5,+9,+9,+10,+mar[1] |
| T2-1 | Aneuploid; 22,XY,3q+,5q+[16]; 22,XY,5q+[1]; 23,XY,3q+,5q+,+6[1]; 24,XY,+3,+10[1]; Diploid; 22,XY[1] |
| T3-1 | Aneuploid; 23,XY,Xp−,+4[1]; 24,XY,Xp−,+3,+5[6]; 24,XY,Xp−,+4,+5[5]; 24,XY,+3,+5[1]; 24,XY,+4,+5[1]; 25,XY,Xp−,+4,+5,+10[1] |
| T3-2a | Aneuploid; 21,Y,−X,+4,−7[1]; 23,XY,+3[1]; 23,XY,+4[6]; 23,XY,+4,7q+[1]; 24,XY,+3,+4[1]; 24,XY,+4,+5[2]; 24,XY,+4,+5,7q+[1]; 24,XY,+3,+5[6]; 24,XY,+3,+5,7q+[1]; 24,XY,+3,+10[1]; 25,XY,+3,+4,7q+,+10[5]; Diploid; 22,XY[2] |
| T3-2b | Aneuploid; 23,XY,+3[8]; 24,XY,+3,+8[8]; 24,XY,+3,+5[2]; 25,XY,+3,+8,+mar[1]; 26,XY,+3,+4,+4,+5[1] |
| T4-1 | Aneuploid; 24,XY,+X,+5q−[18]; 24,XY,+5q−,+10[2]; 24,XY,+X,+5q−,+6,−9[1]; 25,XY,+X,+5q−,+10[2]; 24,XY,+5q−,+mar[2]; 25,XY,+X,+5q−,+mar(10)[3]; Diploid; 22,XY[1] |
| T4-2 | Aneuploid; 20,Y,Xq−,−8,−9[1]; 21,XY,7q−,mar(8),−9[1]; 22,XY,7q−,mar(8)[1]; 22,X,−Y,+3,+4,−7[1]; 22,Y,−X,2p−,−2,−3,−5,−7,−10,+6mar[1]; 22,XY,7q−,mar(8)[1]; 23,XY,2p−,7q−,mar(8),+9[1]; 23-majority structurally altered,XY,+4,mar(8)[1]; 23,XY,7q−,mar(8),+9[2]; 23,XY,+3,+4,−6,mar(8)[1]; 23,XY,+4,7q−,mar(8)[1]; 24-majority altered,XY[1]; 24,XY,+4,+4,mar(8)[1]; 24,XY,+3,+3,mar(8)[1]; 24,XY,+3,+4,mar(8)[1]; 25,XY,+3,+4,+5,mar(8)[1]; 25,XY,+X,+3,mar(8),+10[1]; 32,XY,+Y,+1,+2,+3,+4,+5,+7,mar(8),+10,+2mar[1]; 42,XXYY,−2,−4,7q−x2[1]; 43−majority altered,+5, [1]; 46−majority altered,XXYY,+5,+10[1]; 50,XXY,−Y,+3,+4,+5,+9,+3mar[1]; 53,XXYY,+4,+9,+10,+6mar[1] |
| T5-1 | Aneuploid; 23,XY,+3[1]; 25,XY,+3,+5,+9[17]; 25,XY,+5,+7,+9[1]; 27,XY,+3,+3,+5,+8,+9[1] |
Specific aneusomies are marked in bold.
Because the generation time of a CH cell is ≈14 h (34), a single tumorigenic cell could have generated in 7 weeks, the shortest of the observed latent periods, ≈284 or 2 × 1025 tumor cells, which is the equivalent of over 1014 hamsters. Thus the lengths and the relatively wide range of these latent periods, i.e., 7–21 weeks, indicate that tumorigenic cells probably were generated de novo from in vitro transformed cells via several time-dependent steps.
Specific Aneusomies in Tumor Cells Induced by Cells Transformed in Vitro.
To determine whether the lag between inoculation of in vitro transformed cells and tumorigenesis reflects the evolution of tumor-specific aneusomies via chromosome assortments, the karyotypes of the tumors were analyzed.
As shown in Table 5, among the 211 cells of the 10 tumors that were analyzed, 207 were aneuploid. Almost all aneuploid cells had gained and some had lost intact chromosomes. In addition, 9 of 10 tumors also contained structurally altered chromosomes, which are termed “marker chromosomes” when they are clonal (21). These structurally altered chromosomes included those that differed from a known progenitor either by specific deletions and additions, e.g., 5q− and 5q+, or by more complex alterations in which only the major progenitor could be identified, e.g., mar(10) which indicates that it was derived from chromosome 10, and finally those termed mar, the origin of which could not be determined by the techniques available to us now (21, 37).
To calculate aneusomic specificity, only intact and structurally altered aneusomic chromosomes with an origin that could be identified were counted (Table 3). In these calculations it was assumed that complete and altered derivatives of aneusomic chromosomes are selected for common transformation-specific segments. For example, an altered aneusomic chromosome 5, e.g., 5q−, was counted like an intact chromosome 5 in Table 3. But structurally altered chromosomes with unidentified origins were omitted from the calculation, leaving open the possibility that there actually is more aneusomic specificity in these tumors than our calculations reveal.
It can be seen in Table 3 that 65% (134/207) of all 207 aneuploid tumor cells analyzed were trisomic for chromosome 3, and 51% (105/207) were trisomic for chromosome 5, compared with 8% for random distributions of a given aneusomy among the 12 CH chromosomes. Moreover, 30% (62/207) of the tumor cells, occurring in 7 of 10 tumors analyzed, i.e., T1-2, T2-1, T3-1, T3-2a, T3-2b, T4-2, and T5-1, were trisomic for both chromosomes 3 and 5, compared with 0.6% based on random distribution. Thus under our conditions trisomies 3 and 5 and the combination of both are highly tumor-specific.
Chromosome 4 achieved the next highest chromosomal aneuploidy index, being trisomic in 21% (43/207) of all aneuploid tumor cells (Table 3). The aneusomic indices of all other chromosomes of the tumor cells were 18% and lower (Table 5). Aneusomies of chromosomes 1 or 2 were found only in tumor T4-2.
On a per tumor basis, 70–100% of the aneuploid cells of 9 of the 10 tumors were trisomic for chromosomes 3, 5, or both but only 42% (10/24) of the cells of tumor T4-2. However, because 21 of the 24 aneuploid cells of T4-2 contained between 1 and 6 (!) structurally altered chromosomes of partially or completely unknown origin, the real percentage of cells with segmental aneusomies of chromosomes 3 and 5 probably is higher than 42%. As suggested above, the aneuploid tumor cells without major specific aneusomies may contain minor transformation-specific aneusomies or may be aneuploid, but untransformed cells.
A comparison shows that the two stages of transformation, in vitro transformation versus tumorigenic transformation, both share high indices for trisomy 3 but differ in other specific aneusomies and in combinations thereof (Tables 2, 3, and 5). For example, monosomy 10 and combinations of monosomy 10 and trisomy 3 were specific for in vitro transformed cells, whereas trisomy 5 and combinations of trisomy 5 and 3 were specific for tumors. In addition, the tumors differ from the in vitro transformed cells in numerous structurally altered chromosomes that may include as-yet-undetected aneusomies. Thus, different stages of transformation display common and distinct aneusomies.
Discussion
Transformation-Specific Aneusomies.
Our experiments confirm three predictions of the aneuploidy cancer hypothesis. (i) The mutagenic carcinogen NMU proved to be an efficient aneuploidogen, rendering up to 90% cells aneuploid before transformation. According to our hypothesis NMU achieves aneuploidization by rendering spindle proteins and chromosomes nonfunctional by alkylation (1, 2) rather than by mutation of genes (see below). (ii) Several transformation stage-specific aneusomies were found in most CH cells transformed with NMU in vitro and in most cells of tumors generated with in vitro transformed cells in animals. (iii) Random aneusomies were found in all transformed cells and increased with the generations of transformed cells in vitro and tumor cells in vivo.
Because specific aneusomies correlate with specific stages of transformation and aneusomy is inevitably dominant and abnormal, e.g., Down's syndrome (see above), we conclude that specific aneusomies are necessary and possibly sufficient (see below) for neoplastic transformation. Distinct, specific aneusomies thus can explain the distinct metabolic, morphological, and clinical properties of tumors of the same kind and with the same somatic mutations and of different cells of the same tumors (1, 12). The choice between alternative transformation-specific aneusomies, which were observed here, probably is determined by the likelihood with which an abnormal chromosome assortment arises that upsets the balance of those chromosomes that encode the normal differentiated state of the progenitor from which the transformed cell is derived. Once the cell is liberated from differentiation-specific growth control by specific aneusomies, the stage is set for accelerated karyotype evolution (Fig. 1).
This conclusion is supported by cancer-specific and cancer stage-specific aneusomies observed by others both in human cancers and in experimental cancers of animals including the CH (see below). By contrast, there is no consistent evidence that mutations of known oncogenes and tumor suppressor genes are transformation stage-specific, and there is no evidence that these mutations are dominant in transfected diploid cells and in transgenic animals or animals with null mutations (1, 2, 43, 50, 51).
Literature Supports Transformation-Specific Aneusomies.
Surprisingly, in view of the now-prevailing focus on mutations of oncogenes and tumor-suppressor genes, transformation-specific aneusomies were found over 30 years ago in experimentally transformed animals and animal cells in culture, including CH. The most particular case was made by Sachs et al. (52, 53), who have distinguished “E” chromosomes for expression and “S” chromosomes for suppression of neoplastic transformation. Indeed, 11 studies on karyotypes of spontaneously, chemically, or simian virus 40 virus-transformed CH cells have identified one or both of the two most prominent transformation-specific aneusomies that we have identified here, i.e., trisomies 3 and 5, regardless of the carcinogen or strain of the animal used (47, 48, 53–61). Three of these studies also reported prominent trisomies of chromosomes 8 (61), 9 (55), and 10 (48). Thus the literature confirms and extends our evidence for several, transformation-specific aneusomies in neoplastic CH cells.
However, these early quests for specific aneusomies causing cancer by upsetting the normal chromosome balance, “balance theory” (62), were abandoned just because several, rather than unique, transformation-specific aneusomies were found in the CH and other animals. For example, Deaven et al. (56) remained uncertain about “the specific chromosome change hypothesis,” because different “nonrandom” karyotypes were found in CH tumors in their lab and in others. Likewise, others remained undecided or unconfirmed, because no unique transformation-specific aneusomies were found (47, 48, 54, 59, 61). As a result some began to interpret transformation-specific aneuploidies as evidence for oncogenes (58, 60, 62). Even Sachs et al. (63) dropped their E and S chromosomes in favor of a “mutation [that] can occur in one of a small number of the same or different genes.”
The literature of human cancers provides recent evidence for cancer- and cancer stage-specific aneusomies (21, 64) despite a “plethora” of unspecific aneuploidies (21). For example, in addition to minor transformation-specific aneusomies, 54% of the cells of a group of breast cancers had gained part of chromosome 1 (65, 66), 66% of the cells of a group of skin cancers had trisomy 18 (67), 69% of the cells of a group of early ovarian cancers had trisomy 12 (68), and 55% of the cells of early adenomas of the colon had lost the short arm of one chromosome 5 (23). Moreover, certain transformation-specific aneusomies are even shared by different human cancers. For example, 68% and more of the cells of large samples of small cell lung cancers, head and neck carcinomas, and cervical cancers have gained the long arm and lost the short arm of chromosome 3 (69–74). Furthermore, precedents for cancer stage-specific aneusomies, which seem analogous to the transformation stage-specific aneusomies described here, have been observed in ovarian (68), head and neck (74), colon and cervical (64), and bladder cancer (22, 75).
However, the role of these transformation-specific aneusomies in human carcinogenesis is debated still, because (i) unique, transformation-specific aneusomies are not detected in all tumors of a kind and in all cells of the same tumors, (ii) the specific aneusomies are considered to be indicators of amplified oncogenes or lost tumor-suppressor genes (22, 31, 64, 67, 75) or indicators of karyotype instability that can “accelerate the acquisition of growth promoting mutations” (23), although there are no direct functional assays for any of these mutations, and (iii) the large number of genes whose expression is changed by aneusomies is hard to reconcile with the 3–10 gene mutations currently thought to cause cancer (3, 25–30).
By contrast the aneuploidy hypothesis provides a unifying explanation of why nonidentical, specific aneusomies could cause the same cancers and transform the same cells, why inevitably dominant aneusomies are more plausible causes of cancer phenotypes than gene mutations, and why the expression of many hundreds of genes is altered in cancer cells (see above). In addition, the aneuploidy hypothesis offers an explanation for the random aneuploidy and structurally altered chromosomes that increase with the generations of a cancer cell and can obscure cancer-specific aneusomies (21–23). The many structurally altered chromosomes of 1 of the 10 CH tumors described here make a case in point (Table 5).
Role of Mutation in NMU Carcinogenesis?
Because NMU is a mutagen, it may be argued that, besides specific aneusomies, unrecognized NMU-induced gene mutations are necessary for neoplastic transformation. However, because between 3 and 10 gene mutations are thought to be necessary for neoplastic transformation (see above) and chemical mutagens such as NMU only mutate a given gene in 1 of 106 cells even at half-lethal concentration (76, 77), the odds for transformation would be too low to be detected in our system. Only 1 in 1018 cells would become transformed if three dominant mutations had to be generated, and only 1 in 1036 if three recessive mutations had to be generated. Instead we have observed over 90 foci of transformed cells in ≈2 × 106 cells exposed to only 3% of the half-lethal dose of NMU (6 times to 1/200th of the half-lethal dose). Moreover, tumorigenesis occurred only 7–21 weeks and many cell generations (see above) after inoculation of in vitro transformed CHE cells in the total absence of NMU.
Thus, after its short half-life of 1 h, NMU must depend on either spontaneous mutagenesis or a nonmutagenic mechanism for carcinogenesis to occur many mitoses later, as for example the chain reaction of aneuploidization proposed here. Further work is needed to determine whether mutagenesis may play an indirect role in NMU carcinogenesis, as for example mutation of genes that control chromosome segregation (1).
Acknowledgments
We thank Dan Koshland, Jr. (University of California, Berkeley, CA) for encouragement, Susanne Brendel (III Medizinische Klinik Mannheim, Mannheim, Germany) for assistance with all experiments, and Björn Lemmer and Klaus Witte (Institut für Pharmakologie und Toxikologie, University of Mannheim, Mannheim, Germany) for offering their animal facilities. We are indebted to George Miklos (Human Genetic Signatures and GenetixXpress, Sydney, Australia), Iver Petersen (Institute of Pathology, Medical School Charite, Humboldt University, Berlin, Germany), Henry Pitot (McArdle Laboratory, University of Wisconsin, Madison, WI), and David Rasnick (University of California, Berkeley, CA) for critical reviews of the manuscript. The Abraham J. and Phyllis Katz Foundation (New York), Robert Leppo (philanthropist, San Francisco), an American foundation that prefers to be anonymous, other private sources, and the Forschungsfonds der Fakultaet for Klinische Medizin Mannheim are gratefully acknowledged for support. P.D. is the recipient of a guest professorship from the Mildred Scheel Stiftung of the Deutsche Krebshilfe at the III Medizinische Klinik (University of Heidelberg, Mannheim, Germany).
Abbreviations
- CH
Chinese hamster
- NMU
nitrosomethylurea
- CHE
CH embryo
- DMBA
7,12-dimethylbenz[a]anthracene
References
- 1.Duesberg P, Rasnick D. Cell Motil Cytoskeleton. 2000;47:81–107. doi: 10.1002/1097-0169(200010)47:2<81::AID-CM1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 2.Li R, Sonik A, Stindl R, Rasnick D, Duesberg P. Proc Natl Acad Sci USA. 2000;97:3236–3241. doi: 10.1073/pnas.040529797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Webb T. J Natl Cancer Inst. 2001;93:92–94. doi: 10.1093/jnci/93.2.92. [DOI] [PubMed] [Google Scholar]
- 4.Gibbs W W. Sci Am. 2001;285(2):30–32. doi: 10.1038/scientificamerican0801-52. [DOI] [PubMed] [Google Scholar]
- 5.Lindsley D L, Sandler L, Baker B S, Carpenter A T C, Denell R E, Hall J C, Jacobs P A, Gabor Miklos G L, Davis B K, Gethmann R C, et al. Genetics. 1972;71:157–184. doi: 10.1093/genetics/71.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandler L, Hecht F. Am J Hum Genet. 1973;25:332–339. [PMC free article] [PubMed] [Google Scholar]
- 7.Epstein C. The Consequences of Chromosome Imbalance: Principles, Mechanisms, and Models. Cambridge, U.K.: Cambridge Univ. Press; 1986. [Google Scholar]
- 8.German J, Crippa L P, Bloom D. Chromosoma. 1974;48:361–366. doi: 10.1007/BF00290993. [DOI] [PubMed] [Google Scholar]
- 9.Kajii T, Kawai T, Takumi T, Misu H, Mabuchi O, Takahashi Y, Tachino M, Nihei F, Ikeuchi T. Am J Med Genet. 1998;78:245–249. doi: 10.1002/(sici)1096-8628(19980707)78:3<245::aid-ajmg7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 10.Hughes T R, Roberts C J, Dai H, Jones A R, Meyer M R, Slade D, Burchard J, Dow S, Ward T R, Kidd M J, et al. Nat Genet. 2000;25:333–337. doi: 10.1038/77116. [DOI] [PubMed] [Google Scholar]
- 11.Liu P, Zhang H, McLellan A, Vogel H, Bradley A. Genetics. 1998;150:1155–1168. doi: 10.1093/genetics/150.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foulds L. Neoplastic Development. New York: Academic; 1975. [Google Scholar]
- 13.Ruddon R W. Cancer Biology. New York: Oxford Univ. Press; 1981. [Google Scholar]
- 14.Zhang L, Zhou W, Velculescu V E, Kern S E, Hruban R H, Hamilton S R, Vogelstein B, Kinzler K W. Science. 1997;276:1268–1272. doi: 10.1126/science.276.5316.1268. [DOI] [PubMed] [Google Scholar]
- 15.Wang K, Gan L, Jeffery E, Gayle M, Gown A M, Skelly M, Nelson P S, Ng W V, Schummer M, Hood L, Mulligan J. Gene. 1999;229:101–108. doi: 10.1016/s0378-1119(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 16.Petersen S, Heckert C, Rudolf J, Schluns K, Tchernitsa O I, Schafer R, Dietel M, Petersen I. Int J Cancer. 2000;86:512–517. doi: 10.1002/(sici)1097-0215(20000515)86:4<512::aid-ijc11>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 17.Iversen O H. Carcinogenesis. 1991;12:493–502. doi: 10.1093/carcin/12.3.493. [DOI] [PubMed] [Google Scholar]
- 18.Parry J M, Parry E M, Barrett J C. Nature (London) 1981;294:263–265. doi: 10.1038/294263a0. [DOI] [PubMed] [Google Scholar]
- 19.Cairns J. Cancer: Science and Society. San Francisco: Freeman; 1978. [Google Scholar]
- 20.Lawley P D. Adv Cancer Res. 1994;65:17–111. doi: 10.1016/s0065-230x(08)60065-2. [DOI] [PubMed] [Google Scholar]
- 21.Heim S, Mitelman F. Cancer Cytogenetics. New York: Wiley–Liss; 1995. [Google Scholar]
- 22.Hoglund M, Sall T, Heim S, Mitelman F, Mandahl N, Fadl-Elmula I. Cancer Res. 2001;61:8241–8246. [PubMed] [Google Scholar]
- 23.Shih I M, Zhou W, Goodman S N, Lengauer C, Kinzler K W, Vogelstein B. Cancer Res. 2001;61:818–822. [PubMed] [Google Scholar]
- 24.Duesberg P, Li R, Rasnick D, Rausch C, Willer A, Kraemer A, Yerganian G, Hehlmann R. Cancer Genet Cytogenet. 2000;119:83–93. doi: 10.1016/s0165-4608(99)00236-8. [DOI] [PubMed] [Google Scholar]
- 25.Lodish H, Berk A, Zipursky S L, Matsudaira P, Baltimore D, Darnell J. Molecular Cell Biology. New York: Freeman; 1999. [Google Scholar]
- 26.Lewin B. Genes V. Oxford: Oxford Univ. Press; 1994. [Google Scholar]
- 27.Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson J D. Molecular Biology of the Cell. New York: Garland; 1994. [Google Scholar]
- 28.Vogelstein B, Kinzler K W. Trends Genet. 1993;9:138–141. doi: 10.1016/0168-9525(93)90209-z. [DOI] [PubMed] [Google Scholar]
- 29.Vogelstein B, Kinzler K W. The Genetic Basis of Human Cancer. New York: McGraw–Hill; 1998. [Google Scholar]
- 30.Renan M J. Mol Carcinog. 1993;7:139–146. doi: 10.1002/mc.2940070303. [DOI] [PubMed] [Google Scholar]
- 31.Lengauer C, Kinzler K W, Vogelstein B. Nature (London) 1997;386:623–627. doi: 10.1038/386623a0. [DOI] [PubMed] [Google Scholar]
- 32.Lengauer C. Proc Natl Acad Sci USA. 2001;98:12331–12333. doi: 10.1073/pnas.231485898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitelman F, Mertens F, Johansson B. Nat Genet Suppl. 1997;15:S417–S474. doi: 10.1038/ng0497supp-417. [DOI] [PubMed] [Google Scholar]
- 34.Yerganian G, Leonard M. Science. 1961;133:1600–1601. doi: 10.1126/science.133.3464.1600. [DOI] [PubMed] [Google Scholar]
- 35.Li R, Yerganian G, Duesberg P, Kraemer A, Willer A, Rausch C, Hehlmann R. Proc Natl Acad Sci USA. 1997;94:14506–14511. doi: 10.1073/pnas.94.26.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fonatsch C, Schaadt M, Kirchner H, Diehl V. Int J Cancer. 1980;26:749–756. doi: 10.1002/ijc.2910260608. [DOI] [PubMed] [Google Scholar]
- 37.Mitelman F. ISCN: An International System for Human Cytogenetic Nomenclature. Basel: Karger; 1995. [Google Scholar]
- 38.Zarbl H, Sukumar S, Arthur A V, Martin-Zanca D, Barbacid M. Nature (London) 1985;315:382–385. doi: 10.1038/315382a0. [DOI] [PubMed] [Google Scholar]
- 39.Richards J, Nandi S. J Natl Cancer Inst. 1978;61:773–777. [PubMed] [Google Scholar]
- 40.Richards J, Nandi S. Proc Natl Acad Sci USA. 1978;75:3836–3840. doi: 10.1073/pnas.75.8.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guzman R C, Osborn R C, Bartley J C, Imagawa W, Asch B B, Nandi S. Cancer Res. 1987;47:275–280. [PubMed] [Google Scholar]
- 42.Rhim J S, Jin S, Jung M, Thraves P J, Kuettel M R, Webber M M, Hukku B. Cancer Res. 1997;57:576–580. [PubMed] [Google Scholar]
- 43.Harris H. The Cells of the Body; A History of Somatic Cell Genetics. Plainview, NY: Cold Spring Harbor Lab. Press; 1995. [Google Scholar]
- 44.Cram L S, Bartholdi M F, Ray F A, Travis G L, Kraemer P M. Cancer Res. 1983;43:4828–4837. [PubMed] [Google Scholar]
- 45.Duesberg P, Rausch C, Rasnick D, Hehlmann R. Proc Natl Acad Sci USA. 1998;95:13692–13697. doi: 10.1073/pnas.95.23.13692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pitot H C. Fundamentals of Oncology. New York: Dekker; 1986. [Google Scholar]
- 47.Trewyn R W, Kerr S J, Lehman J M. J Natl Cancer Inst. 1979;62:633–637. doi: 10.1093/jnci/62.3.633. [DOI] [PubMed] [Google Scholar]
- 48.Connell J R. Br J Cancer. 1984;50:167–177. doi: 10.1038/bjc.1984.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kraemer P, Ray A, Bartholdi M F, Cram S L. Cancer Genet Cytogenet. 1987;27:273–287. doi: 10.1016/0165-4608(87)90010-0. [DOI] [PubMed] [Google Scholar]
- 50.Strauss B S. Cancer Res. 1992;52:249–253. [PubMed] [Google Scholar]
- 51.Boland C R, Ricciardello L. Proc Natl Acad Sci USA. 1999;96:14675–14677. doi: 10.1073/pnas.96.26.14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hitotsumachi S, Rabinowitz Z, Sachs L. Nature (London) 1971;231:511–514. doi: 10.1038/231511a0. [DOI] [PubMed] [Google Scholar]
- 53.Bloch-Shtacher N, Sachs L. J Cell Physiol. 1977;93:205–212. doi: 10.1002/jcp.1040930206. [DOI] [PubMed] [Google Scholar]
- 54.Kirkland D J, Venitt S. Br J Cancer. 1976;34:145–152. doi: 10.1038/bjc.1976.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Connell J R, Ockey C H. Int J Cancer. 1977;20:768–779. doi: 10.1002/ijc.2910200517. [DOI] [PubMed] [Google Scholar]
- 56.Deaven L L, Cram S L, Wells R S, Kraemer P M. In: Genes, Chromosomes, and Neoplasia. Arrighi F, Rao P, Stubblefield E, editors. New York: Raven; 1981. pp. 419–449. [Google Scholar]
- 57.Swindell J A, Ockey C H. Cancer Genet Cytogenet. 1983;8:23–36. doi: 10.1016/0165-4608(83)90102-4. [DOI] [PubMed] [Google Scholar]
- 58.Gadi I K, Harrison J J, Sager R. Somatic Cell Mol Genet. 1984;10:521–529. doi: 10.1007/BF01534856. [DOI] [PubMed] [Google Scholar]
- 59.Ray F A, Bartholdi M F, Kraemer P M, Cram S L. Cancer Genet Cytogenet. 1986;21:35–51. doi: 10.1016/0165-4608(86)90199-8. [DOI] [PubMed] [Google Scholar]
- 60.Shimizu T, Kato M V, Nikaido O, Suzuki F. Jpn J Cancer Res. 1995;86:546–554. doi: 10.1111/j.1349-7006.1995.tb02433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cram L S, Bartholdi M F, Ray F A, Travis G L, Kraemer P M. Cancer Res. 1983;43:4828–4837. [PubMed] [Google Scholar]
- 62.Oshimura M, Hesterberg T W, Barrett J C. Cancer Genet Cytogenet. 1986;22:225–237. doi: 10.1016/0165-4608(86)90159-7. [DOI] [PubMed] [Google Scholar]
- 63.Huberman E, Mager R, Sachs L. Nature (London) 1976;264:360–361. doi: 10.1038/264360a0. [DOI] [PubMed] [Google Scholar]
- 64.Ried T, Heselmeyer-Haddad K, Blegen H, Schrock E, Auer G. Genes Chromosomes Cancer. 1999;25:195–204. doi: 10.1002/(sici)1098-2264(199907)25:3<195::aid-gcc1>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 65.Persson K, Pandis N, Mertens F, Borg A, Baldetorp B, Killander D, Isola J. Genes Chromosomes Cancer. 1999;25:115–122. [PubMed] [Google Scholar]
- 66.Mertens F, Johansson B, Hoeglund M, Mitelman F. Cancer Res. 1997;57:2765–2780. [PubMed] [Google Scholar]
- 67.Jin Y, Mertens F, Persson B, Warloe T, Gullestad H P, Salemark L, Jin C, Jonsson N, Risberg B, Mandahl, et al. Cancer Genet Cytogenet. 1998;103:35–42. doi: 10.1016/s0165-4608(97)00356-7. [DOI] [PubMed] [Google Scholar]
- 68.Thompson F H, Liu Y, Emerson J, Weinstein R, Makar R, Trent J M, Taetle R, Alberts D S. Genes Chromosomes Cancer. 1994;10:262–266. doi: 10.1002/gcc.2870100407. [DOI] [PubMed] [Google Scholar]
- 69.Whang-Peng J, Kao-Shan C S, Lee E C, Bunn P A, Carney D N, Gazdar A F, Minna J D. Science. 1982;215:181–182. doi: 10.1126/science.6274023. [DOI] [PubMed] [Google Scholar]
- 70.Petersen I, Langreck H, Wolf G, Schwendel A, Psille R, Vogt P, Reichel M B, Ried T, Dietel M. Br J Cancer. 1997;75:79–86. doi: 10.1038/bjc.1997.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bockmuhl U, Schwendel A, Dietel M, Petersen I. Cancer Res. 1996;56:5325–5329. [PubMed] [Google Scholar]
- 72.Hidalgo A, Schewe C, Petersen S, Salcedo M, Gariglio P, Schluns K, Dietel M, Petersen I. Eur J Cancer. 2000;36:542–548. doi: 10.1016/s0959-8049(99)00323-8. [DOI] [PubMed] [Google Scholar]
- 73.Heselmeyer K, Macville M, Schrock E, Blegen H, Hellstrom A C, Shah K, Auer G, Ried T. Genes Chromosomes Cancer. 1997;19:233–240. [PubMed] [Google Scholar]
- 74.Jin C, Jin Y, Wennerberg J, Akervall J, Dictor M, Mertens F. Cancer Genet Cytogenet. 2002;132:85–96. doi: 10.1016/s0165-4608(01)00535-0. [DOI] [PubMed] [Google Scholar]
- 75.Richter J, Jiang F, Gorog J P, Sartorius G, Egenter C, Gasser T C, Moch H, Mihatsch M J, Sauter G. Cancer Res. 1997;57:2860–2864. [PubMed] [Google Scholar]
- 76.Bradley M O, Bhuyan B, Francis M C, Langenbach R, Peterson A, Huberman E. Mutat Res. 1981;87:81–142. doi: 10.1016/0165-1110(81)90029-4. [DOI] [PubMed] [Google Scholar]
- 77.Koeberle B, Roescheisen C, Helbig R, Speit G. Mutat Res. 1993;301:65–71. doi: 10.1016/0165-7992(93)90058-4. [DOI] [PubMed] [Google Scholar]
- 78.Friel J P, editor. Dorland's Illustrated Medical Dictionary. Philadelphia: Saunders; 1985. [Google Scholar]