Abstract
Informed, voluntary, ongoing consent is a central tenet of ethical research. However, consent processes are prone to exclusionary practices and inaccessibility. Consent materials are often too long and complex to foster understanding and ensure that people make truly informed decisions to participate in research. While this complexity is problematic for all people, these challenges are compounded for autistic people and people with intellectual disability. Consent materials and procedures rarely incorporate accommodations for processing and communication differences common in autism and intellectual disability. Failure to provide such accommodations ultimately threatens the conduct of ethical research. We describe lessons learned across multiple major U.S. research institutions that improved informed consent materials and procedures, with the goal of fostering responsible inclusion in research for autistic people and people with intellectual disability. We used these alternative materials and procedures in multiple research projects with samples of autistic people and people with intellectual disability. Each contributing team partnered with university human research participant protections personnel, accessibility experts, community members, and researchers to develop rigorous procedures for improving the readability and accessibility of informed consent materials. We present guidelines for designing consent materials and procedures and assert that participatory methods are vital to the success of ongoing accessibility initiatives. Adoption of understandable consent materials and accessible consent procedures can cultivate more equitable, respectful, and inclusive human research practices. Future work should expand on this work to design inclusive practices for populations with additional considerations.
Keywords: autism, intellectual disability, informed consent, research ethics, human research participant protections, participatory research
Introduction
The National Institutes of Health (NIH) designated people with disabilities as a health disparities population in 2023, highlighting the importance of research to advance our understanding of disparate health outcomes for people with disabilities.1 Autistic people and people with intellectual disability may have differences in cognition, sensory processing, and communication. Researchers must consider these differences in the conduct of ethical human subject research.2 Recent research highlights that people with disabilities may experience social marginalization and systemic exclusion in all areas of life,3–5 and this extends to the way research is framed and conducted.6–8 Widespread research processes and institutional structures, such as the consent process, are prone to inaccessibility and obstacles to meaningful participation.6,7,9 Historically, this has led to science that fails to include marginalized populations or includes them without ensuring informed consent, threatening the conduct of ethical research.6,7,9,10
Researchers are bound by ethical codes (e.g., Declaration of Helsinki and Belmont Report) that protect participants’ rights and ensure safety.11 Informed consent, the ongoing process where prospective participants are provided with information about a research study to make an informed and voluntary decision about joining it, is a central ethical requirement for research that some countries have codified into federal policies or laws. For example, in the United States, researchers must follow The Common Rule.10,12 This process protects the ethical principle of autonomy, a person’s right to make their own decision and free choice.10 In order for consent to be considered legal, ethical, and effective, researchers are required by federal law to disclose all information necessary for the individual to make an informed decision. Required information includes stating the purpose of the research as well as identifying the study team, foreseeable risks, benefits to research participants, how confidential information will be stored and whether it will be shared, and that participation is voluntary. Failure to thoroughly provide this information is considered a failure to respect a person’s autonomy.11
Central to these regulations is that the consent process must be presented to prospective participants in an understandable manner so that the decision about whether to participate is truly informed and voluntary.12 In the United States, federal regulations of research and institutional Human Research Protection Programs (HRPP) advise that consent materials (e.g., consent document, authorization form to access Protective Health Information [PHI]) should be readable at the 6–8th grade level.12,13 Yet, in practice, many institutions require long, complex consent materials that prioritize thoroughness and protection from legal action over clarity and readability. Prior work has shown that a concerning number of consent materials require reading comprehension at a post-baccalaureate level and beyond, far exceeding the 6–8th grade level guidelines.14 Such materials use medical and/or scientific jargon, which can impede prospective participants’ ability to fully understand study information.10 In our experience, most templated consent materials are filled with complex, convoluted language, including legal statements and medical terminology, often written by the institution. Such forms fail to meet even basic accessibility requirements and may lead individuals to consent without fully understanding. Furthermore, the length of consent materials has increased drastically over the last three decades to an average of 19 pages in clinical research.10 The length and complexity of such forms may prevent a substantial proportion of people from being adequately informed or participating in research at all, and we would argue that it may itself be a breach of ethics.10
These challenges are a known problem across all research and especially among marginalized groups (e.g., people from non-English-speaking communities), which have produced positive accommodations such as expectation for translation of study material and utilizing staff who speak native languages to ensure understanding of consent information. However, the complexity of consent materials is particularly problematic in research with autistic people and people with intellectual disability. Imprecise and unnecessarily complex language is likely to be misinterpreted or misunderstood by autistic people and people with intellectual disability due to processing and communication differences.15 Furthermore, the inaccessibility of the informed consent process for autistic people and people with intellectual disability has received less attention and advocacy, resulting in the perpetuation of accessibility and comprehension barriers.
It is important to note that consent should not be reduced to a document. Rather, it is an ongoing process involving a dialogue and interaction, shaped by context and policies that can contribute to the systematic exclusion or inclusion of marginalized people. For example, in-person or telephone consent processes that do not allow for alternative forms of communication risk the exclusion of people who are nonspeaking or who rely on forms of communication other than speech, even if they can comprehend written documentation. Similarly, failure to accommodate sensory processing differences can have unintended consequences of making consent procedures highly disabling.16 While a few mechanisms for exception exist (such as waivers of documentation of consent when written consent creates a significant barrier to participation or electronic consent), they may be inconsistently used. These issues of accessibility are important to address across all research, but they are particularly important in research with autistic and intellectual disability populations. Inaccessible consent materials and protocols ultimately compromise freedom of choice and introduce barriers to responsible inclusion.
Improving consent materials and protocols requires partnership with all relevant constituents. HRPP offices do not require professionals to have expertise in the language and cognitive needs of disability populations. This often results in a mismatch between standard required language from institutional HRPP offices and cognitive needs of the research participants. Modifying institution-standard language to suit the needs of people with varying language and cognitive needs can yield delays in project approval for researchers who are already managing tight timelines. These challenges create barriers and disincentives to establishing accessible and inclusive consent processes.
Our consortium of researchers, community leaders, and HRPP professionals developed guidelines for more accessible consent materials (i.e., consent and authorization forms) and procedures, with an emphasis on fostering understanding for autistic research participants and people with intellectual disability. While we formed our consortium with a focus on autism research, we derived our guidelines from research projects at multiple major U.S. institutions using participatory research processes with autistic people with and without intellectual disability and with nonautistic people with intellectual disability. Furthermore, we have used the materials we created across other disability populations (e.g., broader samples with disabilities that affect cognition), and we hope our guidelines may be useful to making consent procedures more accessible in general. Our collective efforts produced a comprehensive set of inclusive consent language and sample consent materials (see Supplementary Data S1), which we hope will support widespread improvements of informed consent processes.
Methods
Participatory research offers ethical and scientific advantages to conduct more inclusive, meaningful, and impactful research. As such, we used participatory approaches in all research studies, partnering with autistic adults, HRPP professionals, and research team members to design informed consent materials and protocols. Each project team implemented participatory approaches in slightly different ways, influenced by the needs of community partners and research populations as well as institutional policies.
Institutional and project context
Pittsburgh Adult Autism Research Community Collaborative at University of Pittsburgh.
Pittsburgh Adult Autism Research Community Collaborative (PAARCC) is a community-based participatory research (CBPR) group comprising eight community partners (age 18–65), including autistic adults, autistic parents of autistic children, and community leaders of autistic organizations. Autistic PAARCC members’ age of autism diagnosis ranges from 2 to 64 years, and members include people from the LGBTQIA+ community, people of color, and people with co-occurring conditions. All PAARCC members are able to communicate using speech, although two members prefer to communicate through text. PAARCC members meet monthly with autism researchers using a CBPR approach. In this project, PAARCC members, two autism researchers, and a university HRPP analyst revised required consent language for their institution and consent and assent materials for a qualitative study.
Academic Autism Spectrum Partnership in Research and Education projects at Portland State University and Oregon Health and Science University.
Academic Autism Spectrum Partnership in Research and Education (AASPIRE) is a multi-decade community–academic partnership that conducts CBPR with the autistic community.1,2 AASPIRE is led by two neurodivergent codirectors (one of whom is autistic), a 10-member Community Council, and a Steering Committee comprising the principal investigators (PIs), community project leads, and project coordinators of each active project. Distinct AASPIRE research projects are led by a PI and a community co-PI or community project lead and include 6–10 community partners. Autistic community partners bring diverse perspectives, including people with and without intellectual disability, speaking and nonspeaking people, people with a variety of support needs, and people with multiple intersectional marginalized identities related to race, ethnicity, gender, and/or sexual orientation. Some projects have also included parents of autistic adults with high support needs, clinicians, and other constituents. Projects that address intellectual disability populations have included nonautistic community partners with other disabilities (e.g., people with intellectual disability). Academic and community team members work together as equal partners throughout all phases of the research.
In each AASPIRE project, academic and community partners collaboratively created consent and authorization documents and procedures. We started from either Oregon Health and Science University (OHSU) or Portland State University (PSU) templates. The PI and staff of each study adapted the templates to create draft materials and then worked collaboratively with community partners, over one or more meetings, to further adapt materials to increase accessibility and reduce participation barriers. One of the AASPIRE codirectors had in-depth discussions, as needed, with the OHSU Institutional Review Board (IRB) Chair and PSU HRPP director on the nuances of each project, including how to make the consent and authorization process as accessible as possible. Using these approaches has facilitated subsequent IRB approvals.
AASPIRE suicide prevention project at the University of Utah.
Established in 2022, the AASPIRE Suicide Prevention Project (SPP) team is housed at the PI’s institution—the University of Utah—and works in collaboration with AASPIRE. The AASPIRE SPP team comprises of seven autistic community partners with a passion for community advocacy, clinicians, and research staff (PI and research assistants). Team members have a diverse range of backgrounds including representation from a wide range of ages, the nonspeaking community, the LGBTQIA+ community, people of color, and people with co-occurring conditions; the team collaborates virtually, and members live across multiple regions of the United States. The team meets 1–2 times per month to collaborate on research, has created consent materials for qualitative interviews, and is currently developing an education and community empowerment program for suicide prevention.
Consortium guideline formation
Each research team selected projects that (1) used participatory methods with autistic people and (2) led to substantial changes in university template consent materials and/or protocols. These changes occurred iteratively over time across multiple projects. All of the studies discussed in the article were approved by the IRB of their respective institutions (i.e., PSU, OHSU, University of Pittsburgh, and University of Utah). The IRB at Vanderbilt University and San Diego State University waived authorization to the PSU IRB for the Outcomes Project.
Then, the consortium collaboratively synthesized asynchronously and synchronously the various approaches to develop the practical guidelines presented herein. In Supplementary Data S1, we present sample autistic-inclusive required language and consent documents that were approved by HRPPs.
Guidelines for Fostering Understanding in Autism Research
Guidelines for designing consent materials
Box 1 provides an overview of the guidelines for consent materials.
Box 1. Guidelines for Designing Consent Materials.
Involve people with lived experience on the research team.
Seek out collaborators with expertise in human research participant protections.
Start with checklists instead of templates.
Improve written language and formatting.
Use participant-centered language.
Use precise phrasing.
Structure information in a logical order.
Integrate visuals.
Simplify and integrate authorization language, as applicable.
A. Involve people with lived experience on the research team.
All projects contributing to this consortium used participatory methods.17 In this context, people with lived experience refers to autistic people, including both formally and self-diagnosed individuals or people with intellectual disability. People with lived experience have necessary expertise to design consent materials and procedures to maximize understanding. Academic researchers and HRPP professionals have necessary knowledge of institutional systems and research ethics to ensure the necessary components are included and to act on community partners’ recommendations. For example, the Pitt team iteratively worked in collaboration to improve consent materials—where PAARCC members shared points of confusion and recommendations to revise required language and the academic researchers and HRPP analyst worked to revise and advocate for change based on these recommendations at the institutional level.
B. Seek out collaborators with expertise in human research participant protections.
Success across the consortium projects was equally fostered by working closely and intentionally with our respective institutions’ HRPP (e.g., IRB analysts). Readability revisions can be so dramatically different from institutional template language that they may appear not to meet federal and/or institutional requirements. Thus, partnering with HRPP professionals directly to collaboratively revise consent language in a way that meets the needs of autistic people and satisfies requirements is essential.
C. Start with consent checklists over consent templates.
Most university HRPP utilize consent templates with boilerplate paragraphs of required text acceptable to the university. However, the language is not widely accessible, and researchers aiming to edit templates for their autistic study participants may face barriers. Recognizing these and other limitations of templates, in 2018, the University of Utah switched to a checklist approach to better meet the needs of a wide range of research projects (this change is further described on their website).18 The AASPIRE SPP team found that starting with a checklist allowed the team to collaboratively write the consent document using clear language and structure. Community partners were able to freely write text that helped clarify the study and process without concern about adherence to a template or the need to make “workaround” edits to existing text. Likely as a result, AASPIRE SPP’s study went smoothly through the IRB review process. The team felt that the checklist approach made it easier to ensure their materials met all the IRB’s requirements while also maximizing the readability and understandability of the consent document. Using checklists rather than templates may help to mitigate the challenges faced at other universities.
D. Improve written language and formatting.
Informed consent templates commonly require a high literacy proficiency and utilize high information density. This occurs despite regulations requiring consent materials to be “in language understandable to the subject”12 and recommendations that consent documents be readable at a 6–8th grade level.10,13 Differences in neurocognitive processes common in autism and intellectual disability (e.g., processing, concrete thinking)19,20 can further exacerbate challenges with the dense and complex text commonly used in consent materials. As such, consent materials should maximize clarity and understanding while also considering the specific needs of the study population.13,21
Written Language:
Use shorter words: Identify words with three or more syllables and replace with shorter words.
Simplify sentences: Remove long sentences with multiple ideas. Revise so that each sentence conveys one single thought.
Limit parentheticals: Avoid excessive use of parentheticals to provide examples or define terms. Remove parentheticals and revise to complete sentences.
Limit or define complex terms: Identify complex terms (e.g., scientific, medical, or legal terms). Remove the complex words wherever possible. However, if it is important for participants to understand and use a complex term, maintain the term and provide a clear explanation or an example.
Limit or define scientific terms, such as “controlled trial,” “randomized,” “feasibility,” “prospective observational,” and so on. Replace or define such terms using descriptive language to simplify the concept, such as “by chance” for “randomized.”
Use active voice: Revise all sentences to use active voice instead of passive voice.
Note that improving readability should not be at the expense of limiting access to information. While it is often helpful to remove or simplify definitions or research terms, this should not result in an oversimplification of the information. We recommend that researchers use a variety of tools and methods to improve the readability of informed consent materials, including working directly with members from the autistic and intellectual disability communities.
Readability tools.
Readability tools can offer a quick test to evaluate readability; however, relying only on readability risks loss of precision and clarity. Such tools also do not consider font, formatting structure, or the use of visual aids. They should only be used as one method and marker for improving consent materials. The Flesch–Kincaid grade level formula and Flesch Reading Ease formula are the most used tools for assessing readability in health information-related materials.22 The Flesch–Kincaid Grade Level and Flesch Reading Ease formulas are available in Microsoft Word’s editing functions. The Flesch–Kincaid Grade Level formula assesses the approximate reading grade level of a text. The Flesch Reading Ease formula uses the average number of words per sentence and syllables per word to calculate a total readability score (0–100), where scores of ≥60 generally indicate acceptable readability.
Tools to reduce complexity of phrasing.
The Autistic Self Advocacy Network’s “One Idea Per Line: A Guide to Making Easy Read Resources”23 includes information about writing, formatting, and translating materials into easy read format. This guide is freely available online and answers common questions about creating easy read materials. In addition, the Upgoer Five and Upgoer Six Text Editor24 categorizes words according to how common they are, which can be helpful in differentiating between common and uncommon multisyllabic words. Although not originally intended as a tool for creating plain language materials, some professional plain language translators now use it in their work.23,25
Tools to remove scientific jargon.
The Centers for Disease Control and Prevention’s Every Day Words for Public Health Communication tool26 provides alternatives to scientific jargon and technical language, including both direct synonyms and example plain language translations of sentences. In addition, the Multi-Regional Clinical Trials Center of Brigham and Women’s Hospital and Harvard have a Clinical Research Glossary tool27 that provides plain language definitions of clinical research words that may be a useful tool when designing consent materials.
Font and Formatting:
Make font readable: Change text size to 14-point or larger and use clear fonts without serifs (e.g., Arial, Calibri, and Tahoma).
Visually emphasize key information: Use boldface to strategically emphasize and separate key information. Organize sections with clear headings.
Shorten line lengths: Set wider margins.
Increase white space: Increase line spacing to 1.5 or 2, use bulleted lists, and break up large blocks of text into smaller paragraphs.
Incorporate bullets: Use bulleted or numbered lists for sentences with three or more items.
See Table 1 for examples.
Table 1:
Illustrative examples of recommendations to enhance readability and accessibility
| RECOMMENDATION | CONVENTIONAL LANGUAGE | ACCESSIBLE LANGUAGE |
|---|---|---|
| Use shorter words | Your participation in this research study is entirely voluntary. | It is your choice to be in this study. |
| Limit complex terms | Voluntary Questionnaire |
Your choice Survey |
| Define technical terms when it is important for participants to know them | Confidential We may have to release information to others |
Confidential (private or secret) We may have to release (give) information to others |
| Simplify sentences | The above information has been explained to me and all of my current questions have been answered. I understand that I am encouraged to ask questions, voice concerns or complaints about any aspect of this research study during the course of this study, and that such future questions, concerns or complaints will be answered by a qualified individual or by the investigator(s) listed on the first page of this consent document at the telephone number(s) given. |
I understand what is involved in this study. The research team answered all my questions. I understand I can ask questions about the study. I understand I should tell the person in charge of the study or another person on the research team if I have problems with the study. The Principal Investigator’s name, number, and email is on the front of this form. |
| Limit parentheticals | When available, the study investigators and research staff will use your child/dependent’s medical information (e.g., scores from IQ tests, self or parent interview diagnostic assessments or language tests). | You might have been in our studies before. If so, we may use your past data in this study. That could include test scores, interviews, or surveys you already did. |
| Remove or simplify definitions | We will ask you to undergo electroencephalogram (EEG) testing, which is a way that we measure activity in your brain. | You will wear a cap with small metal discs that measures activity in your brain. This is called EEG testing, which is short for Electroencephalogram. |
| Use active voice | Questionnaires about your medical history, education, and daily life will be completed. | You will complete surveys about your medical history, education, and daily life. |
E. Use participant-centered language.
Using culturally appropriate, correct, and preferred language that reflects the value of the individual2,28 is vital to showing respect and building trust with potential research participants. Appropriate terminology across different disability communities is ever evolving, which requires ongoing efforts to recognize and replace outdated and/or offensive terms. This process is facilitated by the inclusion of people with lived experience on research teams.
Understand community preferences when deciding between the use of identity-first (“autistic person”) and person-first language (“person with autism”)
For example, “autistic person” instead of “person with autism” is preferred by many autistic self-advocates. In other communities, person-first language, such as “person with intellectual disability,” is preferred over identity-first, such as “intellectually disabled person.” Preferences are often highly specific to respective research populations.
Remove patronizing language
For example, avoid “talking down” to or using words that infantilize autistic people and people with intellectual disability. Researchers should avoid reference to a person’s “mental age” rather than their chronological age, assuming a participant needs a parent involved, and using childish pictures in the consent materials. Instead, consent materials should presume competence and ask about accommodations, such as the option to include another adult (e.g., caregiver, friend, support person). Autistic partners suggested the following phrasing as an example: “the interview can be just you and the researcher. Or, you can have another adult (e.g., caregiver, friend, support person) there too. It is your choice.”
Avoid using medicalized terms to refer to neuro-differences
For example, “co-morbid,” “symptoms,” “severity,” and crude functioning labels. Instead, use specific phrasing to describe the challenges addressed in this project. For example, “this research includes autistic people who also experience suicidal thoughts.”
Remove deficit-based language
Use neutral or strength-based language when possible. For example, avoid “vulnerable,” “at risk for autism,” and “restrictive interest.” Instead, researchers may use “people with an autistic parent or sibling” or “knowledgeable about a topic.”
Use second person pronouns
Second person pronouns (e.g. “you” and “your”) are preferred and easier to understand instead of “the participant.” For example, “We will ask you to” is more personal and direct than stating “we will ask the participant” within the consent materials.
F. Use precise phrasing.
Imprecise language, figures of speech, and vague terms can be confusing to autistic people and people with intellectual disability.15 Previous work suggested that imprecise and confusing phrasing can cause autistic people to agree to being in a study without a full understanding.29 As such, we recommend several steps to achieve precise and concrete phrasing in the consent process. Table 2 provides examples of modifying materials to have precise and concrete phrasing.
Table 2:
Illustrative examples of recommendations to revise conventional consent language
| Use precise and concrete phrasing | ||
|---|---|---|
| Figures of speech | “You will asked questions about feeling blue or down in the dumps”? | You will answer questions about depression and negative emotions. |
| Provide examples | Any information about you that we already collected will still be used in the study. | We will still use any information that you gave us while in this study. For example, if you come for the first visit and then stop being in the study. We would still use the information you gave us at the first visit. |
| Avoid vague phrasing | We will let you know… | We will call or email you within 5 days… |
Remove abstract medical terms
Abstract medical terms and jargon are confusing and lack specificity, which is a source of confusion.30 For example, “preventative healthcare”15 is an abstract medical term and should be replaced with more specific, concrete language, such as “routine well visits and screening to prevent future health problems.”
Avoid vague phrasing
Ambiguous and vague phrasing is commonly used in research and in clinical practice, which can serve as a barrier to comprehension.31 For example, instead of “participating in this study will not impact your relationship with other healthcare providers,” use “participating in this study will not impact the care you receive from your psychiatrist, therapist, and/or primary care provider.”
This is also a common issue when choosing phrasing for frequency. Words such as “regularly,” “commonly,” and “frequently” should be replaced with more concrete and specific terms. For example, instead of “we will check in with you regularly regarding your symptoms,” use “we will check in with you about once per week.”
Limit figures of speech
For example, “this study is for people that are feeling down or blue” should be revised with specifics, such as “In this study, we will study feelings of depression, sadness, and low mood.”
Provide examples
Institutional legal counsel sometimes requires abstract terminology to be included in a consent form. For example, “your specimens” is required by one of the consortium institutions and is not specific. Providing examples can help participants understand the meaning of the phrasing, such as “your specimens, like your blood or saliva.”
G. Structure information in a logical order.
Updated regulations require that informed consent “be organized and presented in a way that does not merely provide lists of isolated facts.”12 We provide a suggested structure below with headings that have been designed in a logical order to enhance readability:
Who is doing this research study?
What is this research study about?
- What will happen if I decide to be in the research study?
- What will the questions be about [if applicable]?
- How many times will I need to do something for the research study?
- How long will I stay in the study?
- Why would the research team contact me?
- What information, if any, will you collect from my medical records?
- How can I contact the research team or quit the study?
- What are the possible risks or benefits of this research study?
- Can anything bad happen if I am in the study?
- How will I benefit from being in the study?
- Will I get to see my results from this study?
- What will happen if I decide not to be in the study?
- How will my information be used and kept safe?
- How will my information be kept confidential or safe?
- Who may see my information? (privacy)
- How long will the research team keep my information?
- What if I want the team to stop using my information?
Will I be paid for being in the study?
- What are my choices?
- Think about it. (Take more time. Talk to friends, family, providers, etc.)
- Learn more. (Contact the study team with more questions.)
- Yes, I want to be in the study
- No, I do not want to be in the study
H. Integrate visuals and/or multimedia.
At a minimum, consent materials should include both written and visual representations of the information needed to make a decision about participating in the research study. Other groups have explored using multimedia and vignettes to improve informed decision-making during the consent process,32,33 which may be a support learning tool as well. We recommend that researchers consider integrating visuals into consent materials and procedures and ensure that visuals meet standards for low vision (i.e., black text against white or yellow background; alt text on images for screen readers):
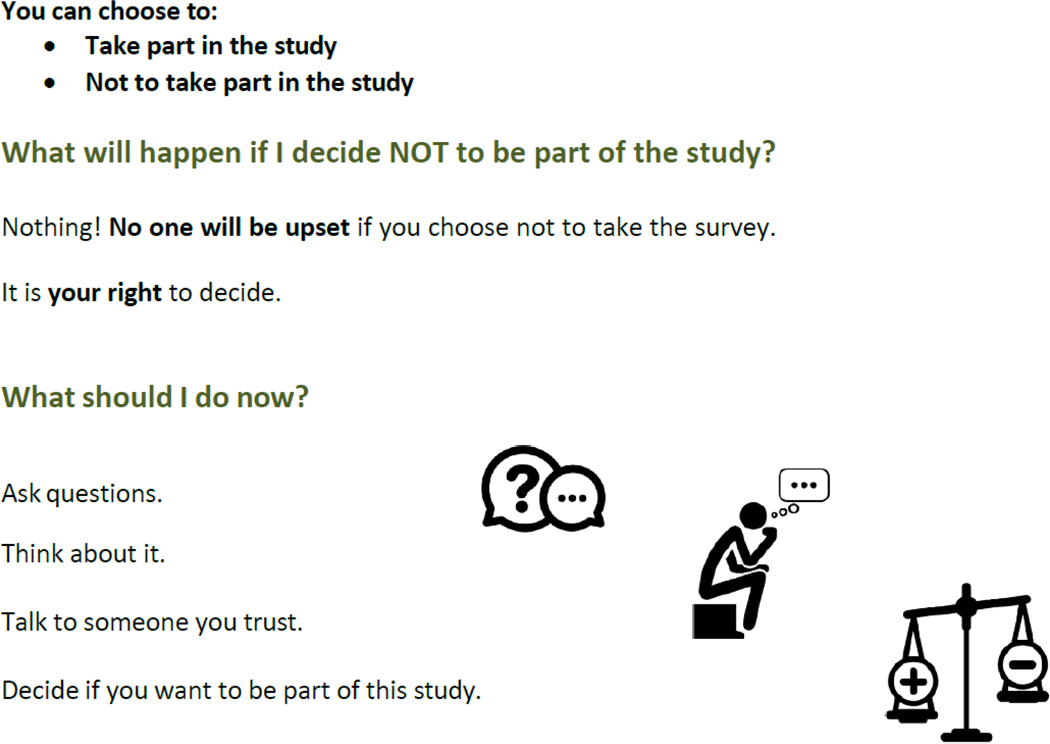
Add icons at important decision points and include visual understanding checks (e.g., having images that can be pointed to or places to write in questions or comments) to offer opportunities for nonverbal communication (see Fig. 1).15
Add photographs of the primary researchers and involved autistic partners to humanize the research process.
Use pictures or icons to better illustrate the people and places involved in participation, as well as the things participants will need to do, particularly if study procedures involve special equipment. Visual aids also add white space, which improves readability.
Consider multimedia34 to provide participants the key information of the research study to make an informed decision on joining the study. For example, create a video detailing what study participation will look like.
FIG. 1.
Example of using icons to enhance decision points.
I. Simplify and integrate authorization language, as needed.
In the United States, studies that use PHI (i.e., individually identifiable health information) need to comply with the privacy rules in the Health Insurance Portability and Accountability Act (HIPAA) of 1996.12 A Privacy Rule Authorization is an individual’s written or electronic signed permission to allow the use of or disclose the individual’s PHI. Sections 45 C.F.R. §164.508(c)(1) and (2) of the HIPAA Privacy Rule12 detail the Core Elements and Required Statements, respectively, of such an authorization. Researchers must include authorization in consent materials if the research study will access PHI. Institutional authorization forms may be particularly complex, with a large amount of legal-sounding text, and we have found that institutions may be especially hesitant to change such forms. However, researchers and institutions are ethically obligated to make authorization forms accessible.
We recommend that authorization is integrated into a consent document. Integrating authorization forms offers the following advantages for enhancing understandability:
It reduces the overall length of consent materials.
It helps contextualize authorization into a more coherent flow of information.
It reduces the chance that participants discount an authorization form and sign it without reading it.
Table 3 shows examples of how authorization language may be simplified and integrated into a combined form (see Supplementary Data S1 for a full example).
Table 3.
Sample authorization language
| Component | Sample Accessible Language | Location to Integrate into a Consent Form |
|---|---|---|
| The name(s) or other specific identification of the person(s) or class of persons who may use the PHI or to whom the covered entity may make the requested disclosure. | Who is doing this research? <Names of investigators and institutional affiliations, along with pictures> Who may see my information? We will not tell anyone which responses were yours. However, there are some exceptions: • We may have to report abuse to Adult Protective Services if you tell us that you or someone else is being abused. • Someone from our research team may ask you more questions about your safety if you tell us that you are actively planning to hurt yourself or anyone else. • We may need to tell someone in your local emergency system so that they can help you if you or someone else are in severe danger. • Certificate of Confidentiality • We may have to release (give) information to others. o For example, there is a chance that someone may want to check that we are doing things the way we should be. (This is called an “audit.”) o We may need to give the information to: i. The group of people at our university who make sure the research is ethical (the Institutional Review Board) ii. Other approved researchers ii. The agency who pays for the study (the National Institute for Mental Health) iii. Other agencies that oversee research (for example, the Office of Human Research Protection) o If that happens, we will try not to give information that could identify you. |
Introduction Information Use and Privacy |
| Description of PHI to be used or disclosed | What information, if any, will you collect from my medical records? We may get additional information from your electronic medical records about: • Your medical diagnoses related to autism or any physical or mental health conditions • The healthcare services you have used. |
Study Activities |
| Authorization expiration date or event | How long will the research team keep my information? We would like your permission to keep your study data and contact information indefinitely (forever). |
Information Use and Privacy |
| The individual’s right to revoke their Authorization and the exceptions to the right to revoke and a description of how the individual may revoke Authorization | What if I want the research team to stop using my information? You can decide you do not want us to keep your study data at any time. You will need to let us know by contacting the principal investigator. We will stop using or sharing your information on the day that we get your message. We may have already used or shared information we collected before we knew that you had changed your mind. |
Information Use and Privacy |
| Notice of the covered entity’s ability or inability to condition treatment, payment, enrollment, or eligibility for benefits on the Authorization, and, if applicable, consequences of refusing to sign the Authorization. | You do not have to allow the use and disclosure (sharing) of your health information in the study. However, if you do not allow it, you cannot be in the study. You choose not to join the study or decide to stop at any time. That will not affect your ability to receive health care or insurance coverage. |
Information Use and Privacy |
Guidelines for improving consent procedures
Consent is an ongoing process, and changes to consent materials are incomplete without equal consideration to consent procedures. The ongoing nature of informed consent is particularly important for autistic people and people with intellectual disability, whose learning styles often benefit from repetition of content especially in studies with longitudinal, tiered, or complex research designs. We recommend that researchers intentionally design virtual and in-person environments to accommodate for cognitive and sensory needs of research participants. These consent procedures should enhance understanding across heterogeneous support needs.
Box 2 provides an overview of the guidelines for the consent process.
Box 2. Guidelines for Consent Process.
Minimize environmental and executive functioning barriers.
Provide options for a range of communication needs.
Offer decision-making supports.
Consider whether a competency assessment is necessary.
Consider people with decisional impairment.
A. Minimize environmental and executive functioning barriers.
Virtual and in-person environments should be designed to make the consent process more inclusive of autistic people and people with intellectual disability. We recommend the following considerations and environmental adaptations:
Create a private, quiet environment16: When possible, conduct consent in a private room with limited distractions. Avoid busy waiting rooms or public spaces. Minimize harsh lighting, noise, and strong smells. During virtual consent calls, offer participants the option to turn off their camera once eligibility criteria has been confirmed.
Limit distractions and clutter16: Crowded and constrained spaces or rooms that are overly cluttered may negatively impact the sensory experience. Virtual backgrounds may reduce clutter and other distractions.
Maximize predictability16: Uncertainty and unpredictability can be distressing and confusing and exacerbate sensitivities. Providing information in advance can help foster an inclusive environment for research and consent processes.
Offer sensory supports and adjustments: Offer sensory tools or adjustments, such as headphones, fidgets, weighted supports, and other sensory tools that may promote coping with an unfamiliar environment.
Allow the use of electronic consent, as applicable: Minimize the need for in-person or complicated procedures to provide written consent (e.g., do not ask participants to download, sign, and upload documents). Allow participants to waive written consent or provide consent electronically.
B. Provide options for a range of communication needs.
Consent procedures should proactively plan for multiple options for communication, with research staff trained on ways to present consent information in a variety of modalities. We recommend research teams design protocols this way in advance rather than placing the onus on the research participants to request accommodations.
Create a visual presentation: Design a visual presentation (e.g., a document with visual aids, PowerPoint presentation, booklet, multimedia) to guide the informed consent process and be more inclusive to visual learners.
Allow for different communication styles: Use of augmentative and alternative communication (AAC) devices, nondevice AAC (e.g., writing, letter-boarding), and nonverbal communication, such as head shaking, nodding, pointing, or shrugging, should be permissible responses for people when obtaining informed consent. Consider including decision-making visuals in consent forms, which introduce opportunities for individuals to communicate by pointing. Offer options for asynchronous communication and participation.
C. Offer decision-making supports.
Simply sharing an approved consent document for independent reading and signature does not support informed consent and decision making. There are many opportunities to embed decision making supports within consent procedures. For example, we recommend that researchers consider the following supports:
Share consent materials for review before meeting with the prospective participant: This allows the prospective participant to review the form ahead of time when and where they are comfortable. Moreover, researchers should highlight that, if desired, the individual can also discuss the information with someone of their choosing.
Allow for a support person: Allow individuals to invite a person of their choice to accompany them through the consent process, if desired. In those cases, researchers should check to make sure that the support person is not unduly influencing the person’s choice to join the research study.
Provide clear expectations and concrete choices: Social or visual stories can provide predictability and support consent decision making. Clear, concrete choices should be provided, including the option to think about it, talk to others about it, and respond later. See Figure 1 and Supplementary Data S1.
Allow multiple meetings to review consent information and answer questions: People may benefit from additional time to consider the decision to join a research study. Study teams can offer additional meeting times to emphasize voluntariness and allow time for processing the decision.
- Use consent companions: Consent companions are simplified overviews or quick guides of the study. These quick guides should be clear, concise, and use visuals. They can be used during consent meetings to help structure the meeting with visual cues, effectively convey study information, and highlight important decision-making points. Consent companions need to be approved by the IRB and can serve as a support throughout all research procedures. Generally, they include:
- A brief overview of the study.
- The key steps of the research study with concrete details.
- Relevant visual aids that functionally illustrate each step of the study procedures.
- A brief reminder of risks, benefits, and voluntary participation.
D. Consider whether a competency assessment is necessary.
Autistic people and people with intellectual disability are considered among those who may experience impaired decision-making capacity.35 Potential research participants must have the ability to understand the purpose of the research study, the potential risks and benefits, and make an independent, voluntary choice of whether to join the research study without pressure or coercion.36 Much of this is the researchers responsibility to provider accessible and understandable information to the potential participant. In some cases, a competency assessment may be necessary to determine whether the potential participant can consent to a research study on their own. However, not all research studies in these populations should require assessment of consent capacity, nor do all autistic people or people with intellectual disability need to be presumed incompetent.6,37 Researchers should consider whether a competency assessment is necessary based on the details of the research study, anticipated participant population, and the individual needs of each potential participant.
E. Consider people with decisional impairment.
When appropriate and necessary, assessments of consent capacity must be individualized to the complexity of the research participation decision. The consent capacity assessment must be accessible and not hold people with disabilities to higher standards of understanding than others. In the case of people subject to guardianship (a legal process in which a person is found legally incapable of making major life decisions), researchers may need to make decisions about their eligibility and, when eligible, how to responsibly include them. In some cases of guardianship, the court orders do not extend to research participation considerations. We recommend the considerations below to begin this process though the list is not exhaustive. See McDonald and colleagues for a systematic review of consent to research among adults with intellectual disability.36
Pursue meaningful assent: When possible, contact the individual to assess their interest prior to receiving the guardian’s consent. Researchers should talk directly to and privately with the individual in their effort to pursue meaningful assent.
Consider the person’s wants: Instruct guardians to consider what the person subject to guardianship wants themselves related to the research study goals, procedures, and risk/benefit.
Integrate consent and assent documents: Rather than creating separate documents, create a single consent and assent document. An integrated form provides accessible information in full about the study to both parties.
Conclusions
Consent (including authorization to use and disclose PHI when applicable) is a foundational and required process when conducting research. Ongoing consent processes protect a person’s autonomy to decide whether to participate in research. Yet, there are decades of research indicating that consent materials and procedures threaten ethical conduct due to the complexity and lack of accessibility. Standard boilerplate consent materials often utilized by institutions typically require high literacy, rely upon scientific jargon, and are too complicated and complex. This inherently limits the reach and ethics of research for all people. To our knowledge, this is the first article to address this topic specifically for autism research.
Our guidelines—based on our experience over multiple research studies—propose enhancements to the consent process that are critical to conducting ethical research that is inclusive of all people, especially those from marginalized backgrounds. Our guidelines emphasize the importance of multiple modes to deliver content, such as auditory and visual, in order to design materials that are accessible to people with a wide range of abilities, communication needs, and experiences.38 Consortium members found that improving consent processes required involvement of all constituents. By involving HRPP professionals, study consent materials were more easily approved, and in some institutions, leadership was more open to widespread adoption of the revised template language across the university. These guidelines have improved materials across numerous studies with participants that had varying conditions, communication preferences, and support needs.
Throughout, we have emphasized consent as a process, rather than a form or a formality. This framing is intentional, and understanding consent in this way is an important part of making consent more inclusive and accessible. Forms are often static documents, which cannot be adapted to individual needs outside of a specific revision process. As a result, when consent is understood primarily in terms of independently reading a document and completing a form, it can exclude people for whom the standard consent form is not accessible. Researchers and HRPP professionals inaccurately assume that someone who needs modifications to the boilerplate consent language is unable to consent and ineligible to participate in research.6 When consent is instead understood as a process, the emphasis can shift to creating an environment and approach that allows prospective participants to give consent, making it easier to improve access. Thus, approaching consent as a process is a necessary first step to adopting the practices outlined in this paper.
However, our recommendations are not exhaustive or without limitations. When implementing these guidelines, specific institutional policies will also need to be considered. While these guidelines are a starting point for creating accessible consent materials and procedures, and an improvement on the status quo, it is important to acknowledge that there may not be a single version of a consent form or process that works for all people. Furthermore, although our experience suggests these practices will support universal improvements to consent processes, our consortium focused specifically on improving informed consent processes within the narrow context of autism and intellectual disability, and they may not adequately address specific needs of other marginalized groups. In addition, laws surrounding research with individuals whose consent capacity may be limited vary between states and other jurisdictions and require additional legal considerations.36
In conclusion, it is essential to design consent materials and procedures that maximize understanding and reduce barriers. The benefits of accessible informed consent materials and procedures are not limited to autism and intellectual disability research. Clear and understandable consent forms and procedures benefit everyone, and likely it especially benefits marginalized groups of people who may already be disproportionally excluded from research.36 We must educate all human subjects’ researchers and HRPP professionals on the importance of inclusivity in consent materials. This article and supporting materials offer a guide and we recommend that researchers use these guides to tailor informed consent materials and procedures for individual populations.
Supplementary Material
Community Brief.
Why is this topic important?
The consent process is how researchers share information with people so they can decide if they want to be in a research study. However, the information shared is often difficult to read and studies often do not offer accommodations. People may not truly understand the risks of the study they agree to be in, or they may not be allowed to make their own decisions about taking part in a study because they cannot understand the study information. Improving consent documents and processes is critical for people to make an informed decision about being in research.
What is the purpose of this article?
We created guidelines for improving consent materials and protocols. Our guidelines aim to support accessible and inclusive informed consent processes in autism and intellectual disability research.
What personal or professional perspectives do the authors bring to this topic?
We put together a “consortium” or a group of teams. Our consortium includes teams of neurodivergent (e.g., autistic, attention-deficit/hyperactivity disorder, acquired neurodivergence) and neurotypical researchers, community members, and advocates. Members bring both lived and professional experiences working to improve inclusive and ethical research procedures. We used participatory methods because lived experience is central to respectful, impactful science that is tailored to the needs of specific populations.
What did the researchers do?
We created guidelines for improving informed consent materials and processes for research with autistic people and people with intellectual disability. We based these guidelines on our experiences working with our universities on multiple research studies with autistic people and people with intellectual disability. The guidelines were acceptable both to the community and our universities.
What do the authors recommend?
Improve the understandability of consent materials:
Start with consent checklists instead of templates.
Improve written language and formatting.
Use participant-centered language.
Use precise and concrete phrasing.
Structure documents in a logical order.
Integrate visuals and/or multimedia.
Simplify and integrate authorization language, as needed.
Create accessible consent procedures:
Minimize environmental and executive functioning barriers.
Allow the use of electronic consents, as applicable.
Provide options for a range of communication needs.
Support decision making.
Consider whether a consent competency assessment is necessary.
Consider people with decisional impairment.
We offer accessible language, checklists, and consent document examples. We recommend that researchers work with their universities or other research institutions to make changes to university-wide templates and guidance. We highlight the importance of developing strong community partnerships and including community members in attempts to make research more inclusive.
How will these recommendations help autistic adults and people with intellectual disability now or in the future?
Making an informed decision to participate in research is a right. However, researchers often use complex informed consent language in disabling environments. These guidelines encourage researchers to partner with community members and representatives of research institutions to change informed consent forms and processes. These guidelines also encourage researchers to evaluate and make changes to consent procedures to be more inclusive. We believe such efforts can improve the ethical inclusion of autistic people and people with intellectual disability in research.
Acknowledgments
The authors would like to acknowledge Kathryn Schuff for her work as the OHSU IRB chair to support all the highly modified consent materials and procedures. The changes recommended in this guidelines paper would not have been possible without her leadership. The authors also would like to express our gratitude to all the research participants and community members who have participated in the projects that lead to these guidelines.
Funding Information
This article was supported by the NCATS under Award Number KL2TR001856, NICHD under Award Number L30HD109969 (K.B.B.), and NIH under Award Numbers 5P50MH130957–03 (K.B.B.), R01MH121407 (PI: C.N.), R01HD105655 (PI: W.H.-J.), R34MH111536–01 (PI: C.N.), R21MH112038–01 (PI: D.R.), K23 MH123934 (PI: A.V.K.), R01HD108701 (PI: M.S., K.M.). K.B.B. was also supported by the Supporting Our Scientists program funded by the University of Pittsburgh and Doris Duke Charitable Foundation (2021382-OF). K.E.M. and M.S. were partially supported by NICHD/NHGRI grant R01HD108701.
Footnotes
Authorship Confirmation Statement
K.B.B. led the consortium and wrote the initial draft of the article. K.T.M., A.V.K., K.M., I.M., K.B., E.R., and C.N. contributed sections to the article. C.N. provided guidance and supervision throughout. All WIRE Consortium authors provided feedback and approval to the guidelines detailed in this article. All authors were involved in the creation of consent materials described in guidelines and/or the creation of the guidelines. All authors provided critical feedback to the article and approved this article before submission. This article has been submitted solely to this journal and is not published, in press, or submitted elsewhere.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Disclosure Statement
The authors of this article have reported no financial interests or potential conflicts of interest.
References
- 1.Walker R National Advisory Council on Minority Health and Health Disparities (NACMHD) Working Group on Persons Living with Disabilities. NIMHD; 2023. [Google Scholar]
- 2.Bottema-Beutel K, Kapp SK, Lester JN, Sasson NJ, Hand BN. Avoiding ableist language: Suggestions for autism researchers. Autism Adulthood. 2021;3(1):18–29; doi: 10.1089/aut.2020.0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Botha M, Frost DM. Extending the minority stress model to understand mental health problems experienced by the autistic population. Soc Ment Health. 2020;10(1):20–34; doi: 10.1177/2156869318804297 [DOI] [Google Scholar]
- 4.Abbott S, Mcconkey R. The barriers to social inclusion as perceived by people with intellectual disabilities. J Intellect Disabil. 2006;10(3):275–287; doi: 10.1177/1744629506067618 [DOI] [PubMed] [Google Scholar]
- 5.Eisenman LT, Rolón-Dow R, Freedman B, Davison A, Yates N. “Disabled or Not, People Just Want to Feel Welcome”: Stories of Microaggressions and Microaffimations from College Students with Intellectual Disability. Critical Education. EBSCOhost. December 1, 2020. Available from: https://openurl.ebsco.com/contentitem/gcd:147472244?sid=ebsco:plink:crawler&id=ebsco:gcd:147472244 [Last accessed: January 30, 2024]. [Google Scholar]
- 6.McDonald KE, Schwartz AE, Sabatello M. Eligibility criteria in NIH-funded clinical trials: Can adults with intellectual disability get in? Disabil Health J. 2022;15(4):101368; doi: 10.1016/j.dhjo.2022.101368 [DOI] [PubMed] [Google Scholar]
- 7.DeCormier Plosky W, Ne’eman A, Silverman BC, et al. Excluding people with disabilities from clinical research: Eligibility criteria lack clarity and justification. Health Aff (Millwood). 2022;41(10):1423–1432; doi: 10.1377/hlthaff.2022.00520 [DOI] [PubMed] [Google Scholar]
- 8.Botha M. Academic, activist, or advocate? angry, entangled, and emerging: A critical reflection on autism knowledge production. Front Psychol. 2021;12:727542; doi: 10.3389/fpsyg.2021.727542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milton D, Mills R, Pellicano E. Ethics and autism: Where is the autistic voice? commentary on Post et al et al. J Autism Dev Disord. 2014;44(10):2650–2651; doi: 10.1007/s10803-012-1739-x [DOI] [PubMed] [Google Scholar]
- 10.Wisgalla A, Hasford J. Four reasons why too many informed consents to clinical research are invalid: A critical analysis of current practices. BMJ Open. 2022;12(3):e050543; doi: 10.1136/bmjopen-2021-050543 [DOI] [Google Scholar]
- 11.Bazzano LA, Durant J, Brantley PR. A modern history of informed consent and the role of key information. Ochsner 2021;21(1):81–85; doi: 10.31486/toj.19.0105 [DOI] [Google Scholar]
- 12.Department of Health US and Human Services. Federal Policy for the Protection of Human Subjects (‘Common Rule’). Regulations, 45 CFR Part 46. [Google Scholar]
- 13.Nebeker C, Gholami M, Kareem D, Kim E. Applying a digital health checklist and readability tools to improve informed consent for digital health research. Front Digit Health. 2021;3:690901; doi: 10.3389/fdgth.2021.690901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reinert C, Kremmler L, Burock S, et al. Quantitative and qualitative analysis of study-related patient information sheets in randomised neuro-oncology phase III-trials. Eur J Cancer. 2014;50(1):150–158; doi: 10.1016/j.ejca.2013.09.006 [DOI] [PubMed] [Google Scholar]
- 15.Nicolaidis C, Raymaker DM, McDonald KE, et al. Creating accessible survey instruments for use with autistic adults and people with intellectual disability: Lessons learned and recommendations. Autism Adulthood. 2020;2(1):61–76; doi: 10.1089/aut.2019.0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manning C, Williams G, MacLennan K. Sensory-inclusive spaces for autistic people: We need to build the evidence base. Autism. 2023;27(6):1511–1515; doi: 10.1177/13623613231183541 [DOI] [PubMed] [Google Scholar]
- 17.Israel BA, Schulz AJ, Parker EA, Becker AB. Review of community-based research: Assessing partnership approaches to improve public health. Annu Rev Public Health. 1998;19:173–202; doi: 10.1146/annurev.publhealth.19.1.173 [DOI] [PubMed] [Google Scholar]
- 18.University of Utah. IRB New Consent Guidance. 2018. Available from: https://irb.utah.edu/informed-consent/consent-video.php [Last accessed: June 18, 2024].
- 19.Lage C, Smith ES, Lawson RP. A meta-analysis of cognitive flexibility in autism spectrum disorder. Neurosci Biobehav Rev. 2024;157:105511; doi: 10.1016/j.neubiorev.2023.105511 [DOI] [PubMed] [Google Scholar]
- 20.Demetriou EA, Lampit A, Quintana DS, et al. Autism spectrum disorders: A meta-analysis of executive function. Mol Psychiatry. 2018;23(5):1198–1204; doi: 10.1038/mp.2017.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horner SD, Surratt D, Juliusson S. Improving readability of patient education materials. J Community Health Nurs. 2000;17(1):15–23; doi: 10.1207/S15327655JCHN1701_02 [DOI] [PubMed] [Google Scholar]
- 22.Wang LW, Miller MJ, Schmitt MR, Wen FK. Assessing readability formula differences with written health information materials: Application, results, and recommendations. Res Social Adm Pharm. 2013;9(5):503–516; doi: 10.1016/j.sapharm.2012.05.009 [DOI] [PubMed] [Google Scholar]
- 23.Autistic Self Advocacy Network. One Idea Per Line: A Guide to Making Easy Read Resources. 2021; 65. Available from: https://autisticadvocacy.org/resources/accessibility/easyread/ [Last accessed: June 18, 2024]. [Google Scholar]
- 24.Butterworth J Upgoer Five: Difficult Stuff in Simple Words. Guardian; January 19, 2013. Available from: https://www.theguardian.com/science/life-and-physics/2013/jan/19/upgoerfive-particlephysics [Last accessed: June 18, 2024]. [Google Scholar]
- 25.Luterman S Plain Language Translation of Disability Visibility First-Person Stories from the Twenty-First Century . Disability Visibility Project. Available from: https://disabilityvisibilityproject.com/ [Last accessed: June 18, 2024]. [Google Scholar]
- 26.Everyday Words for Public Health Communication. The CDC Clear Communication Index. Centers for Disease Control and Prevention; September 22, 2023. Available from: https://www.cdc.gov/ccindex/everydaywords/index.html [Last accessed: June 18, 2024]. [Google Scholar]
- 27.MRCT Center of Brigham and Women’s Hospital and Harvard. Clinical Research Glossary. Health Literacy in Clinical Research. Available from: https://mrctcenter.org/health-literacy/tools/overview/glossary/ [Last accessed: July 2, 2024].
- 28.Orelus PW (Ed.). Affirming Language Diversity in Schools and Society: Beyond linguistic apartheid. New York: Routledge. [Google Scholar]
- 29.Nicolaidis C, Raymaker D, McDonald K, et al. Collaboration strategies in nontraditional community-based participatory research partnerships: Lessons from an academic–community partnership with autistic self-advocates. Prog Community Health Partnersh. 2011;5(2):143–150; doi: 10.1353/cpr.2011.0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gotlieb R, Praska C, Hendrickson MA, et al. Accuracy in patient understanding of common medical phrases. JAMA Netw Open. 2022;5(11):e2242972; doi: 10.1001/jamanetwork open.2022.42972 [DOI] [Google Scholar]
- 31.Codish S, Shiffman RN. A model of ambiguity and vagueness in clinical practice guideline recommendations. AMIA Annu Symp Proc. 2005;2005:146–150. [PMC free article] [PubMed] [Google Scholar]
- 32.Afolabi MO, Bojang K, D’Alessandro U, et al. Multimedia informed consent tool for a low literacy African research population: Development and pilot-testing. J Clin Res Bioeth. 2014;5(3):178; doi: 10.4172/2155-9627.1000178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCormack LA, Wylie A, Moultrie R, et al. Supporting informed clinical trial decisions: Results from a randomized controlled trial evaluating a digital decision support tool for those with intellectual disability. PLoS One. 2019;14(10):e0223801; doi: 10.1371/journal.pone.0223801 [DOI] [Google Scholar]
- 34.Furberg RD, Raspa M, Wheeler AC, McCormack LA, Bailey DB. A digital health app to assess decisional capacity to provide informed consent: Protocol for a randomized controlled trial. JMIR Res Protoc. 2018;7(11):e10360; doi: 10.2196/10360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDonald KE, Gibbons C, Conroy N, Olick RS. Facilitating the inclusion of adults with intellectual disability as direct respondents in research: Strategies for fostering trust, respect, accessibility and engagement. J Appl Res Intellect Disabil. 2022;35(1):170–178; doi: 10.1111/jar.12936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDonald KE, Schwartz AE, Dinerstein R, Olick R, Sabatello M. Responsible inclusion: A systematic review of consent to social-behavioral research with adults with intellectual disability in the US. Disabil Health J. 2024;17(4):101669; doi: 10.1016/j.dhjo.2024.101669 [DOI] [PubMed] [Google Scholar]
- 37.National Institute of Health Central Resource for Grants and Funding Information. Research Involving Individuals with Questionable Capacity to Consent: Points to Consider. 2009. Available from: https://nihodoercomm.az1.qualtrics.com/jfe/form/SV_eypqaXlx2j1IY9T?Q_CHL=si&Q_CanScreenCapture=1. [Last accessed: April 21, 2024].
- 38.Burgstahler S Universal Design of Instruction. DO-IT, University of Washington: Box 355670, Seattle, WA 98195–5670; 2001. Available from: https://eric.ed.gov/?id=ED468709 [Last accessed: September 29, 2023]. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.