Abstract
The Clara cells are nonciliated, nonmucous, secretory cells containing characteristic peptidergic granules; they constitute up to 80% of the epithelial cell population of the distal airways. Despite this exposed histotopology and abundance within the terminal airways where fluid secretion is of pivotal importance, the functional role of the Clara cells remained poorly understood. At the transcriptional, translational, and cellular levels, we provide evidence that the Clara cells are well equipped with the bioactive peptide guanylin and proteins of the cGMP-signaling system including guanylate cyclase C, cGMP-dependent protein kinase II, and cystic fibrosis transmembrane conductance regulator (CFTR) together with the two CFTR scaffolding proteins EBP50/NHERF and E3KARP/NHERF-2 that are essential for proper function of CFTR. Guanylin was localized to secretory granules underneath the apical membrane of Clara cells and was, in addition, detected in high concentrations in bronchoalveolar lavage fluid, predicting release of the peptide luminally into the bronchiolar airways. On the other hand, the guanylin-receptor guanylate cyclase C, CFTR, and proteins linked to CFTR activation and function were all confined to the adluminal membrane of Clara cells, implicating an intriguing air-side route of action of guanylin. Whole-cell patch-clamp recordings in the Clara cell line H441 revealed that guanylin activates CFTR Cl− conductance via the cGMP but not the cAMP-signaling pathway. Hence, in the critical location of distal airways in situ, the Clara cells may play the outstanding role of CFTR-dependent regulation of epithelial electrolyte/water secretion through a sophisticated paracrine/luminocrine mode of guanylin-induced CFTR activation.
Keywords: guanylin‖guanylate cyclase c‖lung‖cystic fibrosis‖electrolyte secretion
In the airway epithelia, regulated secretion of electrolyte/fluid is essential for the mucociliary clearance that is severely impaired in cystic fibrosis (CF; refs. 1 and 2), a lethal genetic disease caused by the loss of function of CF transmembrane conductance regulator (CFTR; refs. 1–3). In various epithelia, CFTR is a cAMP-regulated key protein of electrolyte/fluid secretion by acting as a Cl− channel and serving as regulator protein of various transporters including Cl−/HCO3− exchangers (anion exchangers, AEs) (4, 5). Notably, in the respiratory system CF manifests itself primarily in the small airways where the main epithelial cell type is the Clara cell (6, 7) of which the functional role in connection with CFTR and thus with electrolyte/fluid secretion largely remained enigmatic.
In looking for a potential intrinsic peptide of the Clara cells that may activate CFTR locally within the small conducting airways, we converged our studies on guanylin, a bioactive peptide originally isolated from the intestine (8), with particular focus on its route and mode of signaling. Guanylin is structurally related to heat-stable enterotoxins (STa), which cause secretory diarrhea when secreted into the intestine by pathogenic strains of Escherichia coli. Guanylate cyclase C (GC-C), originally identified as the STa-receptor, represents the genuine receptor for guanylin (9). In the intestine, stimulation of GC-C by guanylin or STa resulted in an intracellular increase of cGMP concentration and thus induced net epithelial chloride and water secretion (8–10), which was virtually eliminated in the intestinal epithelia of CF-mice (11) and of cGMP-dependent protein kinase II (cGKII)-deficient mice (12); hence, a functional CFTR has been considered crucial for the respective guanylin effect (10, 13) that apparently includes cGKII because it also phosphorylates and activates CFTR, which has been demonstrated paradigmatically in a rat intestinal cell line (IEC-CF7) (14, 15).
In the present study, we report on the expression of CFTR and proteins relevant or linked to CFTR function in Clara cells and on the functional significance of these cells in the air-side regulation of CFTR within the critical location of the terminal airways.
Materials and Methods
Expression Analyses in the Human and Rat Lung.
Lung tissues from rat and man (obtained during surgical resection) were separated from large airways by microdissection and prepared for reverse transcription (RT)-PCR analyses as described (13) by using the following primers and specifications given in 5′-3′ orientation: human guanylin (GenBank database accession no. M97496), positions (p.) 196–215 and 456–437; human GC-C (no. M73489), p. 10–29 and 140–121; human cGKII (no. X94612), p. 67–86 and 443–424; human CFTR (no. M28668), p. 4,464–4,483 and 4843–4824; human AE2 (no. X62137), p. 3,121–3,140 and 3,496–3,477; human EBP50/NHERF (no. AF036241), p. 1,313–1,332 and 1,621–1,602; and human E3KARP/NHERF-2 (no. AF035771), p. 686–705 and 991–972. Rat guanylin (no. M93005), p. 208–227 and 533–510; rat GC-C (no. M55636), p. 2,971–2,990 and 3,290–3,271; rat cGKII (no. Z36376), p. 1,280–1,299 and 1,579–1,560, rat CFTR (no. M89906), p. 921–940 and 1,231–1,212; and rat AE2 (no. J05166), p. 1,628–1,647 and 2,003–1,984. The amplification of genomic DNA was excluded by appropriate controls. Sequencing of all amplified PCR-products (MWG Biotech, Ebersberg, Germany) confirmed complete homology with the respective cDNAs.
Peptides and Abs.
Immunogenic peptides were synthesized as C-terminal amides by using a standard fluorenylmethoxycarbonyl protocol and used for immunization of rabbits as described (13, 16, 17). The antisera generated and used here were K605 and K606, each directed against proguanylin-(101–115), K39 directed against proguanylin-(25–37), K42 directed against proguanylin-(34–46), K735 recognizing GC-C-(31–45) and K741 recognizing GC-C-(1009–1023), EG (1)-PKIIA directed against cGKII-(146–158), EG (2)-PKIIB directed against cGKII-(734–749), and EG (1)-AE2 and EG (2)-AE2 both directed against AE2-(1214–1236), EG (1)-NHE-RF1 recognizing EBP50/NHERF-(295–306), and EG (1)-NHE-RF2 recognizing E3KARP/NHERF-2-(274–285). The already successfully tested (13) mouse CFTR (mCFTR) mAb raised against β-galactosidase-CFTR exon 13 fusion protein came from Genzyme.
Western Blot Analyses and Cellular Localization Studies.
Extraction and preparation of proteins from the distal lung parenchymal tissues and from the intestine for gel electrophoresis and subsequent immunoblot experiments were performed according to the protocol published (13). Preadsorption of the respective Abs with homologous antigens confirmed the specificity of immunoreactions. For cellular and subcellular localization, tissue sections from the lung fixed in 4% buffered formalin or Bouin's fluid were immunostained as detailed (13, 16, 17). Subcellular localization by immunogold electron microscopy was performed as described (17). As published (13, 16, 17), staining specificities were checked by appropriate preadsorption controls. Note that the AE2 Abs localize the respective protein as expected at the basolateral membrane of parietal cells (13).
Detection and Quantification of Guanylin in Bronchoalveolar Lavage (BAL) Fluid.
BAL fluid samples were obtained directly from terminal airway branches by collecting on standardized conditions from patients (n = 5) with suspected lung diseases (one sarcoidosis, two allergic alveolitis, and two normal lungs) subjected to diagnostic bronchoscopy. Sterile 0.9% saline (100 ml) was instilled strictly into the fifth segment of the right lung and subsequently recovered by gentle suction. The unpooled samples were centrifuged at 1,000 × g for 10 min at 4°C, and the supernatants were filtered (0.45 μm) and extracted in octadecasilyl (C18) Sep-Pak cartridges (Waters) according to the protocol published (13). For detection of immunoreactive guanylin in BAL fluid, extracts of 10 ml of BAL fluid were separated on 16.5% tricine/SDS/polyacrylamide gels and immunoblotted (13) with guanylin Abs. Parallel analyses of lung and intestinal tissue extracts (50 μg of total protein) were performed for correlation. The concentration of guanylin in BAL fluid was ascertained with a guanylin-specific mAb (18) by ELISA measurements according to the established protocol (18).
Functional Role of Guanylin in Clara Cell CFTR Activation.
Initially, H441 Clara cells of human origin (ref. 19; a gift from A. F. Gazdar and G. Suske, Institute of Molecular Biology and Tumor Research, Philipps University) were grown in culture at 37°C in 5% CO2 in RPMI 1640 media (GIBCO) supplemented with 10% (vol/vol) heat-inactivated FBS, penicillin (100 units/ml), and streptomycin (100 μg/ml). These cells then were analyzed by RT-PCR with the same primer specifications (see above), confirming expression of all proteins under study which were localized with the respective Abs to these cells by immunofluorescence microscopy using Cy-2- and Cy-3-tagged second Abs (13). Subsequently, the H441 cells were analyzed by patch-clamp studies. Whole-cell current recordings were performed in the tight-seal whole-cell configuration on single cells and small cell clusters (≤5 cells). Cell capacitance and series resistance were compensated. Voltage ramps from −100 to +40 mV lasting 800 ms were applied from 0 mV holding potential every 3 s. Patch pipettes were filled with a KCl solution (145 mM KCl/1 mM MgCl2/2.0 mM MgATP/0.1 mM EGTA/10 mM glucose/10 mM Hepes, pH 7.1). The bath solution consisted of 140 mM NaCl, 4.7 mM KCl, 1 mM MgCl2, 1.3 mM CaCl2, 10 mM glucose, and 10 mM Hepes, pH 7.4. Measurements were carried out at room temperature (20–25°C). Mean values are given as mean ± SE.
Results and Discussion
Expression of Guanylin and Affiliated Signaling and Effector Proteins in the Rat and Human Lung.
Consistent with the typical location of Clara cells (6), we prepared distal lung parenchymal tissues from rat and man by microdissectional separation from large cartilaginous bronchial airways; RT-PCR analyses in the respective extracts revealed high expression not only of guanylin, guanylin-receptor GC-C, and cGKII, but also of CFTR and of the two scaffold proteins EBP50/NHERF and E3KARP/NHERF-2 that are essential for the CFTR function by polarization of CFTR within the cells and stabilization within the apical cell membrane (20, 21). In the same extracts, we identified expression of AE2 as the major epithelial isoform of Cl−/HCO3− exchangers (22) that are functionally coupled to CFTR (ref. 5; Fig. 1 A and C). At the translational level, the presence of all these proteins in the distal lung parenchyma was confirmed by Western blotting studies with a series of protein-domain-specific Abs yielding immunoreactive protein bands of correct molecular weights (Fig. 2) as deduced from the respective cDNAs (13).
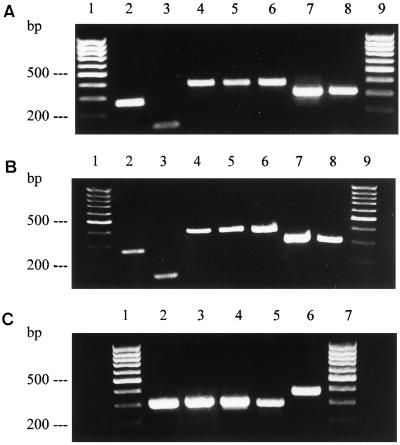
Figure 1.
RT-PCR analyses of the human lung (A), H441 Clara cells (B), and rat lung (C) reveal gene expression of guanylin (lanes 2), GC-C (lanes 3), cGKII (lanes 4), CFTR (lanes 5), AE2 (lanes 6), EBP50/NHERF (lanes 7 in A and B), and E3KARP/NHERF-2 (lanes 8 in A and B) with amplification products of correct molecular size. A 100-bp DNA ladder is indicated (A and B, lanes 1 and 9; C, lanes 1 and 7).
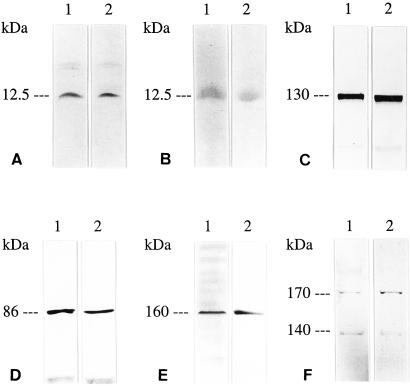
Figure 2.
Western blot analyses of guanylin (A and B), GC-C (C), cGKII (D), CFTR (E), and AE2 (F) in the rat intestine (lanes 1, positive control) and rat lung (lanes 2). Guanylin [Ab K42 (A), Ab K605, (B)], GC-C (Ab K735), cGKII [Ab EG (1)-PKIIA], CFTR (mouse CFTR mAb), and AE2 [Ab EG (1)-AE2] are present coincidently in extracts of the lung and intestine with expected molecular weights as indicated.
Localization of Guanylin in Clara Cells and Its Presence in Human Bronchoalveolar Lavage Fluid.
At the cellular level in human and rat lung tissues, immunohistochemical studies with various region-specific antisera consistently localized guanylin to the typical nonciliated secretory (Clara) cells within the epithelial line of small conducting airways (ref. 17; Fig. 3 A and B). By immunoelectron microscopy, this peptide was confined to a distinct population of Clara cell secretory granules, which were mostly concentrated at the supranuclear cell pole underneath the apical plasma membrane (data not shown; see ref. 17), predicting that guanylin is released into the airway lumen referring to the secretory characteristics of Clara cells (7). Because the guanylin receptor GC-C was exclusively localized to the airway adluminal cell membrane (see below), the assumed presence of the immunoreactive guanylin in the airway lumen was further analyzed. Western blotting studies with region-specific guanylin antisera indeed coincidentally identified the peptide of correct molecular weight (13, 17) in extracts of the human BAL fluid with the same immunoreactivity pattern as in lung tissue extracts (Fig. 4). To ascertain detectability and to assess the range of guanylin concentration in human BAL fluid, a sensitive ELISA with a detection sensitivity of 5 pmol/ml was developed by using a guanylin mAb already successfully used in ELISA measurements (18). In all BAL fluid samples tested (n = 5), a high concentration of guanylin [32.6 ± 15.9 nmol/ml (mean ± SE)] was measured, ranging from 18.9 to 50.4 nmol/ml BAL fluid sample, that obviously is considerably higher than the reported guanylin concentration in the circulation (23). Hence, the luminal presence of guanylin in terminal airways is basically consistent with the apical secretory characteristics of Clara cells (7) because they also may release the Clara cell protein CC10 luminally into the bronchiolar airways in high concentrations (24). Collectively, for the situation in situ, a luminocrine secretory and air-side acting mode of guanylin in Clara cells of small pulmonary airways is conclusive based on the presence of guanylin in BAL fluid and on its receptor GC-C confined to the air-side apical cell membrane domain.
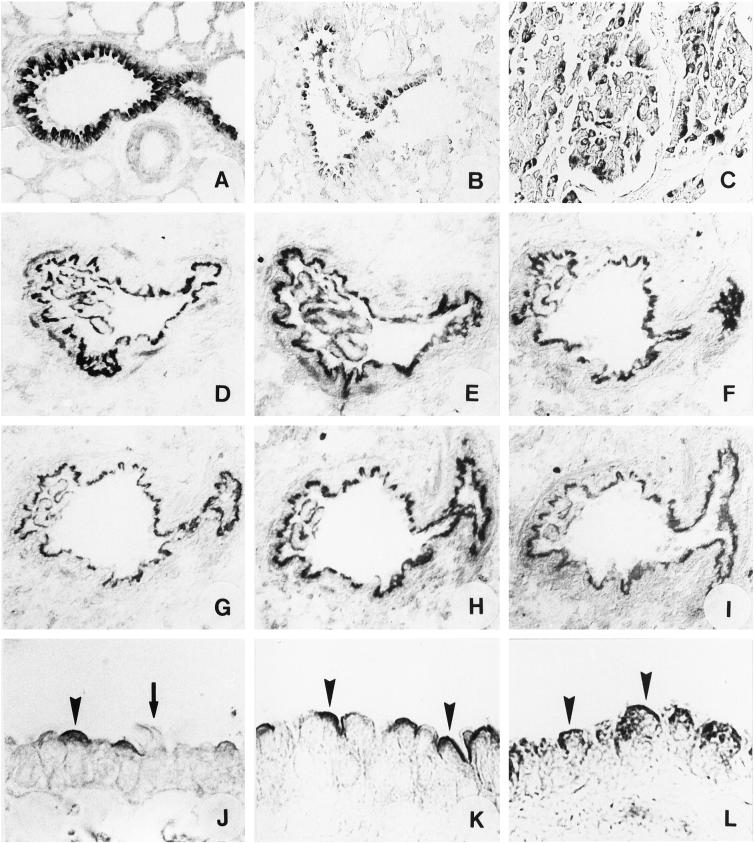
Figure 3.
Immunohistochemical characterization of Clara cells in small conducting airways. Tissue sections of rat (A) and human lung (B) show strong guanylin immunostaining in the epithelial line of Clara cells. Serial tissue sections of rat lung display immunolocalization of GC-C (D), cGKII (E), CFTR (F), AE2 (G), EBP50/NHERF (H), and E3KARP/NHERF-2 (I) to Clara cells. (J–L) Tissue sections of rat lung immunostained for GC-C (J), cGKII (K), and CFTR (L) demonstrate localization to the air-side apical membrane of Clara cells (arrowheads). Neighbored ciliated airway epithelial cells lack immunoreactivity (arrow in J). (Magnification: A, C, and D–I, ×180; B, ×90; J–L, ×360.) (C) Localization of guanylin immunoreactivity in pulmonary adenocarcinomas is demonstrated reflecting a representative documentation of pulmonary adenocarcinomas from patients (n = 20; 18 males and 2 females) identified and diagnosed by conventional hematoxylin/eosin and periodic acid/Schiff reagent staining.
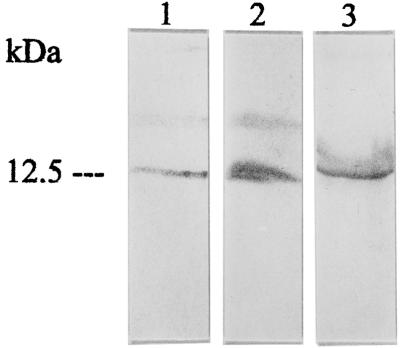
Figure 4.
Correlating Western blot analyses of immunoreactive guanylin in extracts of the intestine (lane 1, positive control), lung (lane 2), and BAL fluid (lane 3) with the Ab K605. Note the comigration of the respective guanylin immunoreactivities.
Clara Cell Apical Membrane Domain: Adluminal Localization of Receptor and Effector Proteins in the Small Airways.
Conceivable with the presence and high concentration of immunoreactive guanylin in the airway lumen (BAL fluid), immunohistochemical studies localized the guanylin-receptor GC-C exclusively to the apical cell membrane domain of Clara cells (Fig. 3 D and J). This unique polarized site of GC-C location unequivocally defines an air-side binding and activation of the receptor by guanylin.
Fitting with this membrane-specific localization of GC-C, both cGKII and CFTR also were confined exclusively to the apical membrane of Clara cells (Fig. 3 E, F, K, and L). The presence of these proteins in a common (apical) membrane domain argues for a morphofunctional coupling of air-side GC-C stimulation and CFTR activation in Clara cells. Indeed, as emerged from studies in intestinal epithelial cells, targeting of cGKII to the apical membrane and its spatial proximity to CFTR is obviously required for an immediate phosphorylation and activation of CFTR (14, 15). Moreover, affiliated to the apical presence of CFTR, we localized two CFTR-scaffolding proteins—i.e., EBP50/NHERF and E3KARP/NHERF-2—equally to the adluminal membrane domain of Clara cells (Fig. 3 H and I). The coexistence of CFTR and of the scaffold proteins in a given cell type and particularly in a defined functional membrane domain has been considered decisive for the proper function of CFTR because the apical targeting and correct membrane localization of CFTR is ensured by the PDZ-interaction with both scaffolding proteins (20). To establish the functional integrity of CFTR, the scaffolding proteins mediate association of CFTR with ezrin and protein kinase (20, 21) and its anchoring to the actin cytoskeleton (20). This actin filament organization and interaction is crucial for activation of the CFTR Cl− channel function via cAMP as demonstrated in melanoma cells (25); the drastic inhibition of STa-induced Cl− secretion in intestinal T84 cells after polymerization of actin and prevention of dynamic reorganization of microfilaments by phalloidin underlines the outstanding role of the cytoskeleton also for the cGMP-mediated Cl− secretion pathway (26).
In addition to its Cl− channel function, CFTR may serve as regulator protein for the epithelial Cl−/HCO3− AE to promote epithelial HCO3− secretion (5). Among the various AEs, AE2 has been stressed as the major epithelial isoform (22). This exchanger protein as well is localized to Clara cells and exclusively confined to the apical membrane (Fig. 3G), the site of CFTR residence. This particular coexistence in given functional membrane domain is in accordance with the immediate regulatory interaction of CFTR and AE (5) in secretory epithelia and with the CFTR-dependence of apical HCO3− secretion (10) arguing for AE2 as that epithelial CFTR-regulated Cl−/HCO3− exchanger isoform of Clara cells for secretion of HCO3− in terminal airways; besides normal Cl−, the secretion of HCO3− substantially contributes to the physical characteristics and rheological properties of the airway mucus (27).
Conclusively, the expression of GC-C, cGKII, CFTR, AE2, and of the CFTR-scaffolding proteins EBP50/NHERF and E3KARP/NHERF-2 in Clara cells indicates that these cells are thoroughly equipped with functionally interacting protein modules for CFTR activation and electrolyte secretion; their common polarized localization stresses the air-side apical membrane of Clara cells as the pivotal domain of signal reception, regulatory activity, and electrolyte/fluid secretory capacity.
Effect of Guanylin on Clara Cells: cGMP-Mediated Activation of CFTR Cl− Conductance.
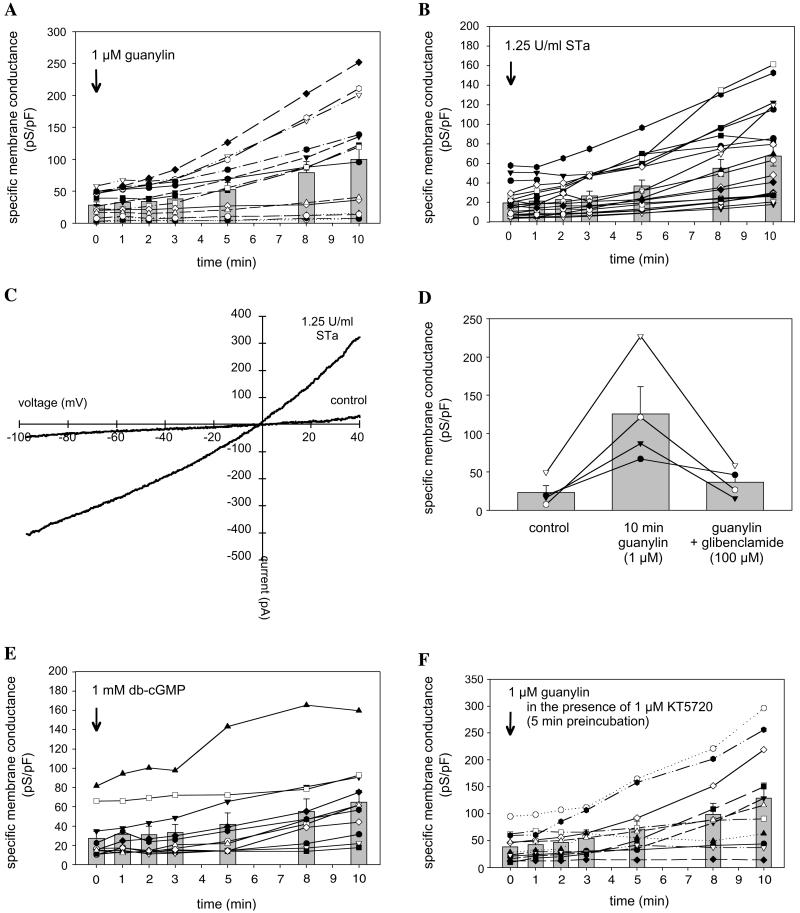
Addressing the functional role of guanylin in the small airways, the H441 Clara cell line of human origin (19) was analyzed. As RT-PCR and immunohistochemical analyses revealed, these cells express all of the proteins necessary for cGMP-dependent CFTR activation, which were also detected in Clara cells in situ (Figs. 1B and 5 A–H). Patch-clamp recordings on single cells and small cell clusters showed that H441 cells respond to stimulation with a mixture of forskolin (10 μM) and 3-isobutyl-1-methylxanthine (1 mM), which causes a rise in the cytosolic cAMP concentration with a long-lasting increase in the chloride whole-cell conductance. A similar increase in the whole-cell conductance could be observed when H441 cells were stimulated with either guanylin or the guanylin-related E. coli enterotoxin STa (Fig. 6 A and B). The specific membrane conductance of H441 cells, determined in the range of −60 to 0 mV holding potential, increased from 28.2 ± 5.2 pS/pF (n = 14) under control condition (t = 0 min) to 52.4 ± 10.9 pS/pF 5 min after application of guanylin to the bath solution (Fig. 6A). A further increase in the membrane conductance to 99.7 ± 22.1 pS/pF was observed 10 min after guanylin application. Similar current activation is given when the guanylin-related peptide STa was used for cell stimulation (Fig. 6B). From the figures it can be noticed that there was a considerable variability in the specific membrane conductance of individual cells both under control conditions and in the presence of agonists. This variability reflects differences in the channel density and might be caused by cell heterogeneity in cell culture. Because cells were used in a nonconfluent layer, the cell cycle status of cells used in the experiments might vary. Guanylin- and STa-induced currents had a reversal potential near to 0 mV when 140 mM chloride was present in both the bath and the pipette solution. The currents showed slight outward rectification at positive membrane potentials (Fig. 6C). Chloride selectivity was demonstrated with anion exchange experiments by using methanesulfonate instead of chloride in the extracellular solution. The replacement of chloride by methanesulfonate caused a shift in the reversal potential of guanylin-induced whole-cell currents from ≈0 mV to 35.3 ± 3.4 mV (n = 5). Guanylin-evoked membrane currents were significantly blocked by extracellular application of the sulfonylurea glibenclamide (Fig. 6D) that at high doses inhibits CFTR Cl− conductance (28), assuming that the chloride currents obtained in H441 cells are at least in part caused by CFTR Cl− channel activity. Guanylin-induced current activation could be mimicked by the membrane permeant cGMP-analog db-cGMP (1 mM), indicating that the intracellular messenger mediating the current activation is cGMP (Fig. 6E). To exclude crossactivation of the cAMP-dependent PKA pathway, the specific PKA inhibitor KT5720 (1 μM, preincubation for 5 min) was used which, notably, did not prevent guanylin-evoked current activation in H441 Clara cells (Fig. 6F). These data clearly indicate that in Clara cells guanylin serves as an regulator of the CFTR Cl− channel function through a PKA-independent, cGMP-mediated activation pathway, virtually involving a cGMP-dependent protein kinase that, by its high expression and colocalization with CFTR in the same membrane domain of Clara cells, is cGKII rather than cGKI. This is further substantiated by the fact that in other cell systems not cGKI but cGKII serves as activator of CFTR (14, 15). Notably, cGKI is primarily attributed to smooth muscle, platelets, and cerebellum (14). The importance of cGKII as a protein module in the coupling to epithelial Cl− conductance is further underlined by studies in the intestine of cGKII-deficient mice (12).
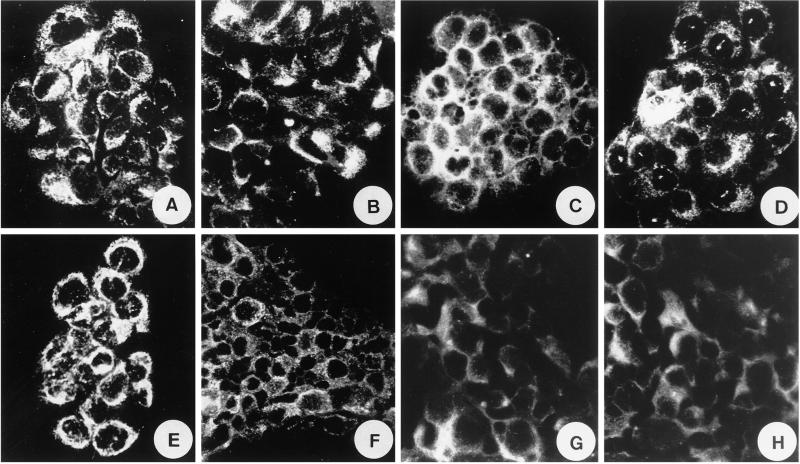
Figure 5.
Immunocytochemical characterization of H441 Clara cells. These cells exhibit immunoreactivities for guanylin [Ab K605 (A) and Ab K42 (B)], GC-C (C), cGKII (D), CFTR (E), AE2 (F), EBP50/NHERF (G), and E3KARP/NHERF-2 (H).
Figure 6.
Patch-clamp whole cell current recordings in H441 cells. Guanylin (A) and the guanylin-related peptide STa (B) produce an increase in the specific membrane conductance of H441 cells. The rise in the membrane conductance is because of activation of chloride channels that, at positive membrane potentials, generate a slightly outward-rectifying current (C). Guanylin-induced currents can be inhibited with glibenclamide (P < 0.01) (D). Application of dibutyryl (db)-cGMP mimics the effect of guanylin (E). Inhibition of PKA with KT5720 does not prevent current activation (F). The different symbols connected by lines indicate individual experiments. Mean values ± SE are represented as bars. The rise in the whole-cell conductance elicited by either guanylin or STa was already significant 1 min after peptide application (P < 0.01, paired Student's t test). When db-cGMP was used, the P value at 1 min was 0.03 and at 3 min was <0.01.
Conclusions
At present, the function of the Clara cells is discussed controversially but mainly correlated to a protective role against oxidative stress and inflammatory response in the respiratory tract and in production of surfactant in terminal airways (24). However, the present data conclusively reveal a particular function of Clara cells in the cGMP-mediated regulation of CFTR in small conducting airways implicating regulation of epithelial electrolyte/fluid secretion in this critical location through an intriguing air-side route of activation of CFTR by guanylin; this clearly differs from the basolateral bloodside activation route mediated by cAMP and PKA (20). Hence, the immediate accessibility of the Clara cells through the airways opens up new strategies in specific therapies or targeted gene transfer approaches in CF, particularly in view of the fact that Clara cells may serve as airway stem cells contributing considerably to the proliferation compartment of normal respiratory epithelia in humans (29). The potential role of Clara cells in carcinogenesis of pulmonary epithelia by dedifferentiation into pulmonary adenocarcinomas is well documented (30). In this respect, we could identify guanylin immunoreactivity in all (n = 20) pulmonary adenocarcinomas studied by immunohistochemistry (Fig. 3C), which in a prospective view may predict guanylin as significant marker in the diagnosis and histopathology of these tumors.
Acknowledgments
We thank Drs. A. F. Gazdar and G. Suske for providing the H441 Clara cells and U. Wagner for providing BAL fluids. The expert technical assistance of C. Merte-Grebe and T. Seitz is gratefully acknowledged.
Abbreviations
- CF
cystic fibrosis
- CFTR
CF transmembrane conductance regulator
- GC-C
guanylate cyclase C
- cGKI and II
cGMP-dependent protein kinase I and II
- AE
anion exchanger
- STa
heat-stable enterotoxin
- RT
reverse transcription
- BAL
bronchoalveolar lavage
- PK
protein kinase
- p.
positions
References
- 1.Quinton P M. Physiol Rev. 1999;79:3–22. doi: 10.1152/physrev.1999.79.1.S3. [DOI] [PubMed] [Google Scholar]
- 2.Pilewski J M, Frizzell R A. Physiol Rev. 1999;79:S215–S255. doi: 10.1152/physrev.1999.79.1.S215. [DOI] [PubMed] [Google Scholar]
- 3.Riordan J R, Rommens J M, Kerem B S, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou J L, et al. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 4.Schwiebert E M, Benos D J, Egan M E, Stutts M J, Guggino W B. Physiol Rev. 1999;79:145–166. doi: 10.1152/physrev.1999.79.1.S145. [DOI] [PubMed] [Google Scholar]
- 5.Lee M G, Wigley W C, Zeng W, Noel L E, Marino C R, Thomas P J, Muallem S. J Biol Chem. 1999;274:3414–3421. doi: 10.1074/jbc.274.6.3414. [DOI] [PubMed] [Google Scholar]
- 6.Plopper C G. Am Rev Respir Dis. 1983;128:S37–S41. doi: 10.1164/arrd.1983.128.2P2.S37. [DOI] [PubMed] [Google Scholar]
- 7.Stinson S F, Loosli C G. J Anat. 1978;127:291–298. [PMC free article] [PubMed] [Google Scholar]
- 8.Currie M G, Fok K F, Kato J, Moore R J, Hamra F K, Duffin K L, Smith C E. Proc Natl Acad Sci USA. 1992;89:947–951. doi: 10.1073/pnas.89.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garbers D L. Cell. 1992;71:1–4. doi: 10.1016/0092-8674(92)90258-e. [DOI] [PubMed] [Google Scholar]
- 10.Seidler U, Blumenstein I, Kretz A, Viellard-Baron D, Rossmann H, Colledge W H, Evans M, Ratcliff R, Gregor M. J Physiol (London) 1997;505:411–423. doi: 10.1111/j.1469-7793.1997.411bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuthbert A W, Hickman M E, MacVinish L J, Evans M J, Colledge W H, Ratcliff R, Seale P W, Humphrey P P A. Br J Pharmacol. 1994;112:31–36. doi: 10.1111/j.1476-5381.1994.tb13024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfeifer A, Aszodi A, Seidler U, Ruth P, Hofmann F, Fässler R. Science. 1996;274:2082–2086. doi: 10.1126/science.274.5295.2082. [DOI] [PubMed] [Google Scholar]
- 13.Kulaksiz H, Schmid A, Hönscheid M, Eissele R, Klempnauer J, Cetin Y. Histochem Cell Biol. 2001;115:131–145. doi: 10.1007/s004180000244. [DOI] [PubMed] [Google Scholar]
- 14.Lohmann S M, Vaandrager A B, Smolenski A, Walter U, de Jonge H R. Trends Biochem Sci. 1997;22:307–312. doi: 10.1016/s0968-0004(97)01086-4. [DOI] [PubMed] [Google Scholar]
- 15.Vaandrager A B, Smolenski A, Tilly B C, Houtsmuller A B, Ehlert E M E, Bot A G M, Edixhoven M, Boomaars W E M, Lohmann S M, de Jonge H R. Proc Natl Acad Sci USA. 1998;95:1466–1471. doi: 10.1073/pnas.95.4.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cetin Y, Kuhn M, Kulaksiz H, Adermann K, Bargsten G, Grube D, Forssmann W G. Proc Natl Acad Sci USA. 1994;91:2935–2939. doi: 10.1073/pnas.91.8.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cetin Y, Kulaksiz H, Redecker P, Bargsten G, Adermann K, Grube D, Forssmann W G. Proc Natl Acad Sci USA. 1995;92:5925–5929. doi: 10.1073/pnas.92.13.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin S, Adermann K, Forssmann W G, Kuhn M. Endocrinology. 1999;140:5022–5029. doi: 10.1210/endo.140.11.7103. [DOI] [PubMed] [Google Scholar]
- 19.Suske G, Lorenz W, Klug J, Gazdar A F, Beato M. Gene Expression. 1992;2:339–352. [PMC free article] [PubMed] [Google Scholar]
- 20.Kleizen B, Braakman I, de Jonge H R. Eur J Cell Biol. 2000;79:544–556. doi: 10.1078/0171-9335-00078. [DOI] [PubMed] [Google Scholar]
- 21.Sun F, Hug M J, Lewarchik C M, Yun C, Bradbury N A, Frizzell R A. J Biol Chem. 2000;275:14360–14366. doi: 10.1074/jbc.275.19.14360. [DOI] [PubMed] [Google Scholar]
- 22.Alper S L. Cell Physiol Biochem. 1991;4:265–281. [Google Scholar]
- 23.Kuhn M, Kulaksiz H, Cetin Y, Frank M, Nold R, Arnold R, Boker K, Bischoff S C, Manns M P, Forssmann W G. Eur J Clin Invest. 1995;25:899–905. doi: 10.1111/j.1365-2362.1995.tb01964.x. [DOI] [PubMed] [Google Scholar]
- 24.Shijubo N, Itoh Y, Shigehara K, Yamaguchi T, Itoh K, Shibuya Y, Takahashi R, Ohchi T, Ohmichi M, Hiraga Y, Abe S. Eur Respir J. 2000;16:414–419. doi: 10.1034/j.1399-3003.2000.016003414.x. [DOI] [PubMed] [Google Scholar]
- 25.Prat A G, Cunningham C C, Jackson G R, Borkan S C, Wang Y, Ausiello D A, Cantiello H F. Am J Physiol. 1999;277:C1160–C1169. doi: 10.1152/ajpcell.1999.277.6.C1160. [DOI] [PubMed] [Google Scholar]
- 26.Matthews J B, Awtrey C S, Thompson R, Hung T, Tally K J, Madara J C. Am J Physiol. 1993;265:G370–G378. doi: 10.1152/ajpgi.1993.265.2.G370. [DOI] [PubMed] [Google Scholar]
- 27.Trout L, King M, Feng W, Inglis S K, Ballard S T. Am J Physiol. 1998;274:L258–L263. doi: 10.1152/ajplung.1998.274.2.L258. [DOI] [PubMed] [Google Scholar]
- 28.Sheppard D N, Welsh M J. J Gen Physiol. 1992;100:573–591. doi: 10.1085/jgp.100.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boers J E, Ambergen A W, Thunnissen F B. Am J Respir Crit Care Med. 1999;159:1585–1591. doi: 10.1164/ajrccm.159.5.9806044. [DOI] [PubMed] [Google Scholar]
- 30.Linnoila R I, Szabo E, DeMayo F, Witschi H, Sabourin C, Malkinson A. Ann NY Acad Sci. 2000;923:249–267. doi: 10.1111/j.1749-6632.2000.tb05534.x. [DOI] [PubMed] [Google Scholar]