Abstract
PAX6 is an evolutionarily conserved transcription factor that plays a critical role in vertebrate and invertebrate eye formation. Heterozygous null mutations in the PAX6 gene result in aniridia in humans and a distinct small eye syndrome in rodents. Vertebrates primarily express two alternatively spliced isoforms of Pax6 that differ by the presence or absence of exon 5a (e5A) that encodes an additional 14 aa residues within the paired domain. The e5a-containing isoform, PAX6(5a), is specific to and conserved in vertebrates. To determine the role of PAX6(5a), we have generated mice that lack e5a of the Pax6 gene. Unlike Pax6 null mice that exhibit anopthalmia with central nervous system defects and lethality, 5a isoform-null mice have iris hypoplasia and defects in the cornea, lens, and retina. Although invertebrates have structures that respond to light intensity and act to restrict light exposure of the eyes, a significant and distinct feature of the vertebrate eye is its ability to regulate the amount of incoming light through contractile pupils. This feature of the eye not only allows vertebrates to see in various light conditions but also enhances image resolution. The requirement of the 5a isoform in iris formation suggests that the evolution of this isoform contributed to advanced features of the vertebrate eye.
PAX6 is an essential and evolutionarily conserved transcription factor for eye formation (1–3). Heterozygous null mutations in the PAX6 gene result in distinct phenotypes in humans (aniridia) and rodents (small eye) (4, 5). Aniridia is a severe panocular disease that is characterized by lack of proper iris development, associated with cataracts, optic nerve hypoplasia, and glaucoma. Small eye syndrome is distinguished from aniridia by reduced eye size, cataracts, and poor lens development. Homozygous mutations in the PAX6 gene result in severe brain abnormalities, microencephaly, early postnatal death, and the absence of the eyes and nose in both humans (4) and rodents (5, 6).
The Pax6 locus encodes two products caused by alternative splicing of exon 5a (e5a) that adds an additional 14 aa residues within the paired domain (7, 8). The e5a-containing isoform, PAX6(5a), is specific to and conserved in vertebrates (9). Pax6 is expressed in the developing eye, nose, pancreas, and central nervous system (7, 10–13). PAX6 is a protein of 422 aa residues, and PAX6(5a) is 14 aa longer and exhibits unique DNA-binding properties (14). Structural analysis of PAX6 identified two DNA-binding domains (a paired domain at the amino terminus and a paired-like homeodomain located centrally), a glycine-rich region that links the two DNA-binding domains, and a transactivation domain at the carboxyl terminus. The paired domain of PAX6 is a bipartite DNA-binding motif and is believed to have two independent amino-terminal and carboxyl-terminal subdomains (15, 16). The paired domain can bind DNA alone or cooperatively by interacting with other DNA-binding domains, such as the homeodomain (17). Unlike other paired domains that bind DNA predominantly through their amino termini, the longer paired domain of the 5a isoform preferentially interacts with DNA through its carboxyl terminus. Deletion of the 30 amino-terminal residues from PAX6 or PAX2 yields a protein that mimics the DNA-binding properties of the 5a isoform (16). Thus, the insertion of 14 aa in the amino-terminal subdomain of the PAX6 paired region is thought to act as a molecular toggle to unmask the DNA-binding potential of the carboxyl-terminal subdomain. This finding suggests that PAX6 and PAX6(5a) may have unique roles.
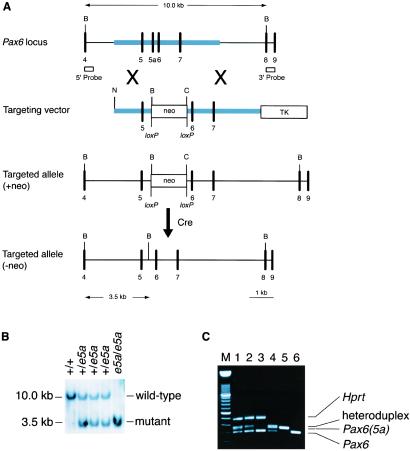
To determine the role of the 5a isoform, we deleted e5a of the Pax6 gene in embryonic stem (ES) cells by using a Cre/loxP gene targeting strategy (18). In this strategy, e5a is deleted and replaced by a loxP-flanked neomycin resistance selection cassette (neo) (Fig. 1A). The neo cassette is removed by crosses with CMV-Cre mice. Pax6(5a) null mice have iris hypoplasia, distinct defects in the cornea, lens, and retina.
Figure 1.
Generation of Pax6(5a) mutant mice. (A) Gene targeting strategy. Correct targeting will delete e5a, replacing it with a loxP-flanked neo cassette. The neo cassette is removed by crosses with CMV-Cre mice. Exons (numbered), vertical lines; homology used for targeting, thick line. neo, neomycin resistance expression cassette; TK, thymidine kinase expression cassette; B, BamH1; C, ClaI; N, NotI. The 5′ and 3′ external Southern probes are shown. (B) Southern blot analysis of tail DNA digested with BamHI hybridized with the 5′ external probe. (C) Reverse transcription–PCR analysis of total RNA from eyes of wild type (lane 1), e5a/+ (lane 2), and e5a/e5a (lane 3) mouse for Pax6 and Pax6(5a) isoform. Primers 5′-GCGGAGTGAATCAGCTTGGTGGTG and 5′-CTCCGATTGCCCTGGGTCTGATG encompassing e5a were used for amplification during PCR. Mouse hypoxanthine phosphoribosyltransferase (HPRT) gene was amplified to provide loading control for reactions. Plasmids containing Pax6(5a) and Pax6 alone (lanes 5 and 6) or a mixture of both (lane 4) were used as control to mark the respective products. Because of identical sequences in the flanking region of the PCR products, a heteroduplex is formed during PCR that migrates slower than either of the isoform. As expected, in e5a/e5a mice heteroduplex as well as band for 5a isoform are absent. M, 100-bp DNA marker ladder.
Materials and Methods
Targeted Deletion of e5a of the Pax6 Gene.
Pax6 genomic clones were isolated from a 129/SvEv mouse genomic library. The targeting event was designed to delete e5a, replacing it with a loxP-flanked PGKneobpA (neo) expression cassette (18). Correct targeting introduces novel BamHI and ClaI restriction sites to follow the targeting event by Southern blot analysis (Fig. 1A). The targeting vector was linearized at a unique NotI site and electroporated into AB1 ES cells (19). Correctly targeted clones were identified by Southern analysis using 5′ (Fig. 1B) and 3′ (not shown) external probes. Twenty targeted clones were identified among 192 colonies screened. Correctly targeted ES cell clones (clones H4 and H10) were injected into C57BL/6J (B6) blastocysts to generate chimeras. After germ-line transmission was confirmed by crosses with B6 females, the resulting progeny were bred with Cre-expressing mice (CMV-Cre) to remove the neo cassette (20). The removal of neo in the resulting pups was confirmed by PCR. Sequencing of PCR products was performed to confirm the expected structural changes. Mice were genotyped by Southern blot and/or PCR analysis with tail DNA. Both mouse lines (H4 and H10) gave similar results.
Histology.
Adult mouse eyes were processed for plastic embedding with a Leica-historesin embedding kit according to the manufacturer's instructions. Sections of 1.5 μm were cut by using a glass knife and Leica motorized microtome. Sections were stained according to the method described (21). Neonatal eyes were fixed in neutral-buffered formalin overnight at room temperature and embedded in paraffin. Sections (8 μm) were stained with hematoxylin and eosin.
Results and Discussion
To generate e5a null mice, we deleted e5a of the Pax6 gene in ES cells by using a Cre/loxP gene targeting strategy. In this strategy, e5a is deleted and replaced by a loxP-flanked neomycin resistance selection cassette (neo) (Fig. 1A). In contrast to mice heterozygous for a Pax6 null allele, mice heterozygous for the e5a deletion with the neo cassette located between exons 5 and 6 did not develop the small eye phenotype, indicating that the modifications within intron 5 did not block the expression of the PAX6 isoform. These mice were then bred with mice expressing Cre recombinase to remove the neo cassette. The structure of the modified locus was verified by Southern blot (Fig. 1B) and reverse transcription–PCR (RT-PCR) analysis (Fig. 1C). In the modified allele 274-bp sequence from 617 nt 3′ of the end of exon 5 to 71 nt 5′ of exon 6 including 42-bp exon 5 is replaced by a DNA fragment that contains loxP sequence, part of polylinker, and is flanked by BamHI and ClaI restriction sites (5′-GGATCCCCTCGAGGGACCTAATAACTTCGTATAGCATACATTATACGAAGTTATATTAAGGGTTATTGAATATGATCGGAATTGGGCTGCAGGAATTCGATATCAAGCTTATCGAT-3′). RT-PCR analysis of adult eye mRNA encoded by the mutated gene, followed by sequencing did not reveal any abnormality in the structure or expression of the Pax6 isoform (Fig. 1C). Replacing exon that contributes to alternative splicing with a foreign sequence within a gene, in this case loxP, raises the possibility of negative effect on expression. However, our data did not reveal any noticeable difference in expression of the gene. Recently, a similar strategy of cre/loxP-mediated, exon-specific targeting was used to determine the role of splice variants of the Wilms' tumor gene (22). Like our results no effect of loxP sequence was observed on the total expression of Wilm's tumor gene. About 12% mice of C57BL/6 strain develop spontaneous ocular defects (23, 24). Founder mice in this study were crossed with Cre-expressing transgenic mice that were of C57BL/6 strain to remove neo. To rule out the phenotypic effects that may be contributed because of the C57BL/6 genetic component, we used a total of nine chimeric mice from two different ES cell clones that were crossed to five different Cre-expressing mice and examined more than 20 animals born from different crosses for each group. None of the wild-type animals showed any abnormal phenotype.
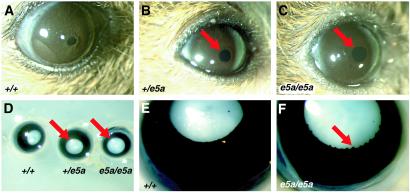
The gross morphology and histology of the adult eyes of wild-type (+/+) mice and their littermates that were heterozygous (e5a/+) or homozygous (e5a/e5a) for the Pax6 e5a deletion were analyzed. Both e5a/+ and e5a/e5a animals had iris hypoplasia (Fig. 2 A–D). It was noticed that although e5a/e5a animals had iris hypoplasia, they still responded to light. Therefore, for consistency eyes from 6–8 different animals were examined under identical bright light. Also, identical light conditions were used to take photographs of the eyes. Similarly eyes from 5–6 animals from each group were fixed and examined. Results within a group were consistent. In addition to hypoplasia, it was consistently observed that the iris in the pupil region of e5a/e5a mice was irregular compared with wild type (Fig. 2 E and F). This irregularity was not observed in e5a/+ mice. Thus, the 5a isoform is essential for normal iris formation and morphology.
Figure 2.
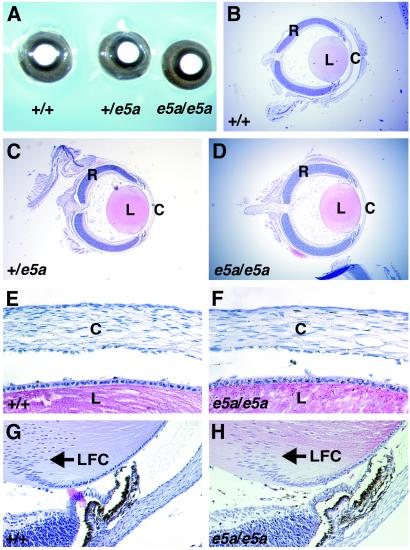
Aniridia in Pax6(5a) mutant mice. (A–C) Eyes of live adult mice. Both e5a/+ and e5a/e5a mutant mice have hypoplasia of the iris with a larger pupil (arrows) relative to +/+ mice. (D) Whole-mount view of the eyes of adult mice after fixation. The iris is the black area and the lens is white. Both e5a/+ and e5a/e5a mutant eyes have iris hypoplasia (arrows). (E and F) Close-up view of an e5a/e5a mutant eye (F) showing an irregular iris (arrow) in the pupil region compared with the regular and smooth iris of a +/+ control (E). (Magnifications: A–C, ×20; D, ×40; E and F, ×60.)
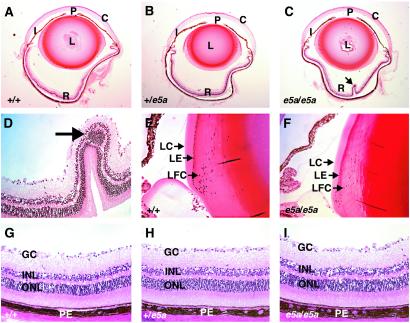
The retina is a highly organized, layered structure that lines the posterior inner surface of the eye. The eyes of e5a-null mice had focal retinal abnormalities (Fig. 3 A–C). The retinas of the e5a-null mice consistently showed papillated structures, and many times the outer nuclear layer aggregated within the papillated structure (Fig. 3 C and D). The number of these papillated structure varied between 1 and 2 in various samples analyzed, and they were mostly located adjacent to the optic nerve. The inner and outer nuclear layers, inner and outer segments of photoreceptors, and ganglion cell layers were present and appeared normal (Fig. 3 G–I) in the areas that were not papillated. No papillated structures were found in the eyes of e5a/+ mice. These data indicate that the 5a isoform is essential for normal retinal structure.
Figure 3.
Histological analysis of plastic-embedded adult eyes. (A–C) Midsagittal sections showing the entire eye. C, cornea; I, iris; L, lens; P, pupil; R, retina. The e5a/e5a eye has an enlarged pupil and peri-retinal space and a papillated structure (arrow) in the retina. (D) Closer view of the papillated structure shown in C, showing the retinal fold and cluster of inner nuclear layer cells (arrow). Equator of the lenses of +/+ (E) and e5a/e5a (F) eyes. The mutant lens shows relatively loose packing of the elongated lens fiber cells (LFC) and reduced numbers of newly differentiated lens epithelium (LE) cells in the transition zone in comparison to the wild type. LC, lens capsule. (G–I) The retinal layers appear normal in wild-type and mutant eyes. GC, ganglionic cells; INL, inner nuclear layer; ONL, outer nuclear layer; PE, pigmented epithelium. (Magnifications: A–C, ×25; D–I, ×200.)
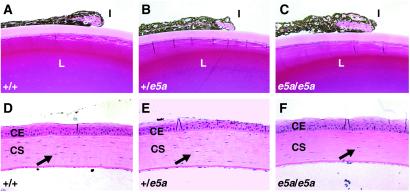
Pax6 activity in the lens primordium is required for lens formation and correct placement of retinal cells in the eye (25). Although the gross morphology of the lenses of adult e5a-null mice appeared normal, a closer examination revealed 37% fewer lens fiber cells in the epithelial to fiber transition zone than in their wild-type littermates (Fig. 3 E and F). In addition, the cells in the epithelial to fiber transition zone were less elongated, wider, and relatively less compact in the e5a-null mice. Furthermore, the iris cells of the e5a-null mice were less compact (Fig. 4 A–C) and the corneal stroma had fewer (30%) keratocytes (Fig. 4 D–F). Higher magnification examination of iris revealed spongy structure with empty spaces where no tissue was detected. It is possible that these spaces were created because of nonoptimal growth of connective tissue. The e5a/+ mice showed intermediate compactness of the iris cells but the numbers of keratocytes were nearly similar to the wild-type littermates. These results indicate that the 5a isoform also regulates lens, iris, and corneal cell behavior.
Figure 4.
Histological analysis of the iris and cornea. (A–C) The iris of e5a/e5a and e5a/+ mice is less compact than the iris of +/+ mice. In addition, the corneal stroma of e5a/e5a mice have fewer keratocytes (arrows) than e5a/+ and +/+ mice. CE, corneal epithelium; CS, corneal stroma; I, iris; L, lens. (Magnifications: ×200.)
It has been shown that PAX6(5a) is expressed in the eye at about one-tenth the level of PAX6 during embryonic development (14, 16, 26, 27). However, a recent study has shown that the expression levels of the two isoforms in the lens are similar after birth (28). Therefore, we analyzed the eyes of newborn e5a-null mice. We found that the eyes of newborn (2 days old) +/+, e5a/+, and e5a/e5a mice were grossly and histologically similar (Fig. 5). This finding suggests that the 5a isoform does not have a significant role in embryonic eye development, apparently because of low expression levels, but that its up-regulation after birth is required later for the differentiation and/or maintenance of adult eye structures.
Figure 5.
Analysis of the eyes of newborn mice. (A) Whole-mount view of eyes of newborn mice after fixation. There are no detectable differences in the irises of mutant and wild-type mice. (B–H) Histological analysis of paraffin-embedded eyes from newborn mice. No significant differences were detected between the eyes of the mutant and wild-type mice. C, cornea; L, lens; LFC, lens fiber cells; R, retina. (Magnifications: A, ×40; B–D, ×25; E–H, ×200.)
It appears that the ratio of the two PAX6 isoforms is critical for the normal development and function of vertebrate eyes. Changes in the ratio of the two isoforms correlate with a distinct human ocular syndrome (16). In addition, overexpression of human PAX6(5a) in the lenses of transgenic mice disrupts the lens fiber cells, causing cataracts (29). Azuma et al. (30) identified four patients with missense mutations within e5a. Although all four patients had nystagmus, corneal opacity, and foveal hypoplasia their phenotypes were not identical. Two patients had mild micropthalmos. Other abnormalities that were not common in these patients included cataract, peripheral iridocorneal adhesion, and microcornea. We have not noticed any corneal opacity or nystagmus in either e5a/+ or e5a/e5a mice so far. In the light of the fact that heterozygous mutations in Pax6 gene exhibit different effects in mice and humans, but have similar effects in homozygous condition, difference in the phenotype of e5a/+ mice and the patients is not surprising. It will be interesting to know the effect of homozygous mutations in e5a if we ever find one. Humans and mice with mutations in Pax6 show abnormality in brain and pancreas (12, 31). However, Azuma et al. (30) reported that, except for one, patients with mutations in e5a had normal growth and intelligence. In the study of anatomy of other organs in e5a/e5a mice where Pax6 is expressed we have noticed differences in the anatomy of the pancreas; however, these results will be part of a separate study (R.M., S.S., R.R.B., and G.F.S., unpublished work).
As discussed above, e5a/+ mice have relatively mild phenotype compared with humans with heterozygous mutations in e5a. Recently, Favor et al. (32) and Lyon et al. (33) reported hypomorphic alleles, namely Sey4Neu, Sey7Neu, and Seycoop of Pax6. Unlike Sey1neu heterozygous mice that have severe small eye phenotype and show a high degree of corneal opacity, Sey4Neu, Sey7Neu mice in heterozygous condition have milder reduction in eye size and have none to a relatively small degree of corneal opacity. The e5a/+ mice show mild iris hypoplasia, but unlike hypomorphic Sey4Neu, in Sey7Neu alleles no reduction in eye size or any instance of corneal opacity was noticed.
Our study demonstrates that PAX6(5a) plays a distinct role in postnatal iris formation and is critical for the structural integrity of the cornea, lens, and retina. Finally, the vertebrate eye is capable of regulating the amount of incoming light that strikes the retina through a contractile iris. This feature of the eye not only allows vertebrates to see in various light conditions but also enhances image resolution. The requirement of PAX6(5a) in iris development suggests that the evolution of this PAX6 isoform contributed to advanced features of the vertebrate eye.
Acknowledgments
We thank A. Bradley for ES and STO cell lines and R. Johnson and C. Pressman for help with plastic sectioning. This work was supported by grants from the National Institutes of Health (to G.F.S. and R.R.B.) and funds from the Barnts family (to R.R.B.). DNA sequencing and veterinary resources were supported by a National Institutes of Health Cancer Center Support (Core) Grant and a National Eye Institute Center Support Grant.
Abbreviations
- e5a
exon 5a
- ES
embryonic stem
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Niimi T, Seimiya M, Kloter U, Flister S, Gehring W J. Development (Cambridge, UK) 1999;126:2253–2260. doi: 10.1242/dev.126.10.2253. [DOI] [PubMed] [Google Scholar]
- 2.Halder G, Callaerts P, Gehring W J. Science. 1995;267:1788–1792. doi: 10.1126/science.7892602. [DOI] [PubMed] [Google Scholar]
- 3.Hanson I, Van Heyningen V. Trends Genet. 1995;11:268–272. doi: 10.1016/s0168-9525(00)89073-3. [DOI] [PubMed] [Google Scholar]
- 4.Glaser T, Jepeal L, Edwards J G, Young S R, Favour J, Maas R L. Nat Genet. 1994;7:463–471. doi: 10.1038/ng0894-463. , and erratum (1994) 8, 203. [DOI] [PubMed] [Google Scholar]
- 5.Hill R E, Favor J, Hogan B L, Ton C C, Saunders G F, Hanson I M, Prosser J, Jordan T, Hastie N D, van Heyningen V. Nature (London) 1991;354:522–525. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- 6.Schmahl W, Knoedlseder M, Favor J, Davidson D. Acta Neuropathol. 1993;86:126–135. doi: 10.1007/BF00334879. [DOI] [PubMed] [Google Scholar]
- 7.Ton C C, Hirvonen H, Miwa H, Weil M M, Monaghan P, Jordan T, van Heyningen V, Hastie N D, Meijers-Heijboer H, Drechsler M. Cell. 1991;67:1059–1074. doi: 10.1016/0092-8674(91)90284-6. [DOI] [PubMed] [Google Scholar]
- 8.Walther C, Guenet J L, Simon D, Deutch U, Jostes B, Goulding M D, Plachov D, Balling R, Gruss P. Genomics. 1991;11:424–434. doi: 10.1016/0888-7543(91)90151-4. [DOI] [PubMed] [Google Scholar]
- 9.Miles C, Elgar G, Coles E, Kleinjan D J, van Heyningen V, Hastie N. Proc Natl Acad Sci USA. 1998;95:13068–13072. doi: 10.1073/pnas.95.22.13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walther C, Gruss P. Development (Cambridge, UK) 1991;113:1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- 11.Turque N, Plaza S, Radvanyi F, Carriere C, Saule S. Mol Endocrinol. 1994;8:929–938. doi: 10.1210/mend.8.7.7984154. [DOI] [PubMed] [Google Scholar]
- 12.St-Onge L, Sosa-Pineda B, Chowdhury K, Mansouri A, Gruss P. Nature (London) 1997;387:406–409. doi: 10.1038/387406a0. [DOI] [PubMed] [Google Scholar]
- 13.Grindley J C, Davidson D R, Hill R E. Development (Cambridge, UK) 1995;121:1433–1442. doi: 10.1242/dev.121.5.1433. [DOI] [PubMed] [Google Scholar]
- 14.Kozmik Z, Czerny T, Busslinger M. EMBO J. 1997;16:6793–6803. doi: 10.1093/emboj/16.22.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Czerny T, Schaffner G, Busslinger M. Genes Dev. 1993;7:2048–2061. doi: 10.1101/gad.7.10.2048. [DOI] [PubMed] [Google Scholar]
- 16.Epstein J A, Glaser T, Cai J, Jepeal L, Walton D S, Maas R L. Genes Dev. 1994;8:2022–2034. doi: 10.1101/gad.8.17.2022. [DOI] [PubMed] [Google Scholar]
- 17.Jun S, Desplan C. Development (Cambridge, UK) 1996;122:2639–2650. doi: 10.1242/dev.122.9.2639. [DOI] [PubMed] [Google Scholar]
- 18.Nagy A. Genesis. 2000;26:99–109. [PubMed] [Google Scholar]
- 19.McMahon A P, Bradley A. Cell. 1990;62:1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- 20.Arango N A, Lovell-Badge R, Behringer R R. Cell. 1999;99:409–419. doi: 10.1016/s0092-8674(00)81527-5. [DOI] [PubMed] [Google Scholar]
- 21.Pressman C L, Chen H, Johnson R L. Genesis. 2000;26:15–25. [PubMed] [Google Scholar]
- 22.Hammes A, Guo J K, Lutsch G, Leheste J R, Landrock D, Ziegler U, Gubler M C, Schedl A. Cell. 2001;106:319–329. doi: 10.1016/s0092-8674(01)00453-6. [DOI] [PubMed] [Google Scholar]
- 23.Robinson M L, Holmgren A, Dewey M J. Exp Eye Res. 1993;56:7–16. doi: 10.1006/exer.1993.1003. [DOI] [PubMed] [Google Scholar]
- 24.Smith R S, Roderick T H, Sundberg J P. Lab Anim Sci. 1994;44:551–560. [PubMed] [Google Scholar]
- 25.Ashery-Padan R, Marquardt T, Zhou X, Gruss P. Genes Dev. 2000;14:2701–2711. doi: 10.1101/gad.184000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richardson J, Cvekl A, Wistow G. Proc Natl Acad Sci USA. 1995;92:4676–4680. doi: 10.1073/pnas.92.10.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaworski C, Sperbeck S, Graham C, Wistow G. Biochem Biophys Res Commun. 1997;240:196–202. doi: 10.1006/bbrc.1997.7623. [DOI] [PubMed] [Google Scholar]
- 28.Zhang W, Cveklova K, Oppermann B, Kantorow M, Cvekl A. Mol Vision. 2001;7:1–5. [PMC free article] [PubMed] [Google Scholar]
- 29.Duncan M K, Kozmik Z, Cveklova K, Piatigorsky J, Cvekl A. J Cell Sci. 2000;113:3173–3185. doi: 10.1242/jcs.113.18.3173. [DOI] [PubMed] [Google Scholar]
- 30.Azuma N, Yamaguchi Y, Handa H, Hayakawa M, Kanai A, Yamada M. Am J Hum Genet. 1999;65:656–663. doi: 10.1086/302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sisodiya S M, Free S L, Williamson K A, Mitchell T N, Willis C, Stevens J M, Kendall B E, Shorvon S D, Hanson I M, Moore A T, van Heyningen V. Nat Genet. 2001;28:214–216. doi: 10.1038/90042. [DOI] [PubMed] [Google Scholar]
- 32.Favor J, Peters H, Hermann T, Schmahl W, Chaterjee B, Neuhauser-Klaus A, Sandulache R. Genetics. 2001;159:1689–1700. doi: 10.1093/genetics/159.4.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lyon M F, Bogani D, Boyd Y, Guillot P, Favor J. Mol Vision. 2000;6:199–203. [PubMed] [Google Scholar]