Abstract
The extraordinary diversity and ecological success of the social insects has been attributed to their ability to cope with the rich and often infectious microbial community inhabiting their nests and feeding sites. Mechanisms of disease control used by eusocial species include antibiotic glandular secretions, mutual grooming, removal of diseased individuals from the nest, and the innate and adaptive immune responses of colony members. Here we demonstrate that after a challenge exposure to the entomopathogenic fungus Metarhizium anisopliae, dampwood termites Zootermopsis angusticollis have higher survivorship when individuals develop immunity as group members. Furthermore, termites significantly improve their ability to resist infection when they are placed in contact with previously immunized nestmates. This “social transfer” of infection resistance, a previously unrecognized mechanism of disease control in the social insects, could explain how group living may improve the survivorship of colony members despite the increased risks of pathogen transmission that can accompany sociality.
Adapting to the infection risks from pathogenic bacteria, fungi, and other microbes that thrive in the nest environment has had primary importance in the remarkable diversification of the social insects (1, 2). Recent research has begun to reveal the pervasive impact of disease on the evolution of insect social organization, influencing colony and population genetics, demography, and mating systems (3, 4), among other attributes. Although the majority of studies have focused on bees and the dead wood and soil-nesting ants (Order Hymenoptera), termites (Order Isoptera) also inhabit decayed wood and soil and appear to be highly susceptible to fungi and other parasitic infections (ref. 5; a table detailing the incidence of pathogen and parasite infection in termites is posted at http://people.bu.edu/rrosenga/table1.htm). In addition to the diversity and pathogenicity of the microbial community of a termite colony, the maintenance of a homeothermic nest and the likelihood of disease transmission through social exchanges between parents and offspring, among offspring, and between mates can exacerbate pathogen-related mortality (6, 7). Termite adaptations that reduce disease susceptibility include mutual grooming scaled in frequency to pathogen prevalence (6, 7), the production of antibiotic secretions in exocrine glands and other exudates (8, 9), and the communication of information about the presence of pathogens in the nest (10). Additionally, termites significantly improve their physiological resistance to infection by mounting a humoral immune response after they are exposed to a nonlethal inoculum of a bacterial or fungal pathogen (11) and produce antibacterial peptides in their salivary glands (12). In our model termite species, Zootermopsis angusticollis, enhanced survivorship of immunized individuals is correlated with changes in the protein constituents of their hemolymph, suggesting humoral immunity. SDS/PAGE plasma analyses show that qualitative and quantitative changes in protein banding patterns occur with specificity after exposure to fungal spores or bacteria. Immune proteins identified in other insects (13–16), visible in the hemolymph of Z. angusticollis nymphs 3–7 days postimmunization, are absent in naïve termites. The dynamics of the immune response of Z. angusticollis and its associated hemolymph protein profile resemble immunization-related protective changes in the protein constituents of the phylogenetically related roaches (17).
Here we show that the level of immunocompetence attained by termites depends on association with colony members and that the disease resistance of individuals that have not experienced direct contact with a pathogen can be significantly enhanced through interactions with immunized nestmates. Our studies of the development of immunity in Z. angustocollis reveal novel social mechanisms of infection control.
Materials and Methods
Development of Immunocompetence in Isolated and Grouped Termites.
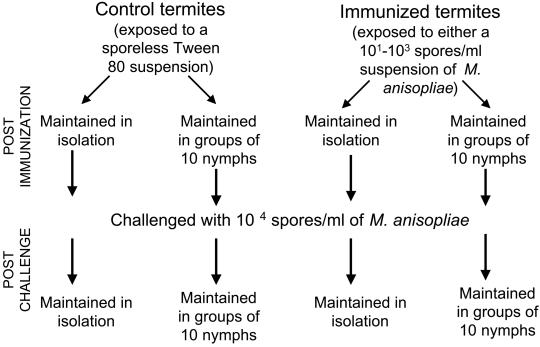
To determine whether the social environment influences the development of disease resistance, we conducted a series of experiments in which we allowed termites to develop immunity after receiving a nonlethal dosage of a fungal pathogen when nesting either in isolation or groups, and then measured the strength of their immune defense with a challenge exposure to a lethal dose of the same pathogen (Fig. 1). Using nymphs of Z. angusticollis from two stock colonies recently collected in the field, we exposed individuals (average age approximately 600 days) to either a 5 × 101, 5 × 102, or 5 × 103 spores/ml suspension of the entomopathogenic fungus Metarhizium anisopliae or a sporeless 0.1% Tween 80 suspension medium (controls). The three spore concentrations were chosen to ensure that exposure levels would be adequate to induce an immune response in both isolated termites, which rely only on their physiology to resist infection, as well as in grouped termites, which can use individual physiological responses in combination with social behaviors to reduce susceptibility (6). Nymphs were cold-immobilized and subsequently placed dorsally on a 3-μl droplet of either a spore solution or the control suspension medium at 4°C for 1 h. This procedure provided an effective method to control the quantity of inoculum received by each individual. Chilling was not a significant predictor of mortality (Wald Statistic = 2.1, P > 0.1). After exposure, termites were transferred to sterile Petri dishes lined with moistened filter paper (Whatman no. 1, 1 ml sterile water) and immediately divided into two treatment groups. In the first group, each nymph (n = 50 for controls and n = 50 for each spore concentration) was isolated for 10 days. In the second group, nymphs were maintained in groups of 10 (n = eight groups of 10 termites for controls and n = eight groups of 10 termites for each spore concentration). To permit the development of an immune response, we allowed 10 days to elapse (11), and each termite from the control and immunization treatments was then challenged with a 5 × 104 spore/ml direct dorsal exposure, using the procedure described above. After the challenge exposures, termites were transferred to new Petri dishes maintaining the same social and isolation treatments, and survivorship was recorded daily for 10 days. In addition to these two treatments, we challenged naïve termites (termites that originated from the same parent colonies but had no exposure to spores or the Tween 80 suspension medium) with the same 5 × 104 spores/ml solution and maintained them in isolation (n = 50 nymphs) or in groups of 10 (n = five groups). Survival censuses were conducted daily and dead termites were removed, surface-sterilized with 5.2% sodium hypochlorite, and plated on potato dextrose agar to confirm that M. anisopliae was the cause of mortality (6). Confirmation rates (18) varied from 52.2% to 96%, indicating that most termites died as a result of exposure to M. anisopliae. Comparisons of the survival parameters of termites in all treatments allowed us to evaluate the effect of isolation and grouping on disease resistance, as well as to confirm that the Tween 80 suspension medium had no immunizing effect.
Figure 1.
Schematic representation of methods used in studies designed to test the effect of isolation and grouping on the development of immunity.
Social “Transfer” of Immunity.
To test the hypothesis that naïve individuals can increase their resistance to infection through social association with immunized nestmates, we created groups composed of naïve and immunized termites in a 1:1 ratio and determined whether resistance could be transferred in this group context. Nymphs (n = 40) were immunized with a 6.5 × 103 spores/ml suspension of M. anisopliae. Seven days before exposure, these termites were fed colored filter paper (Whatman no. 1 moistened with 1 ml of a 0.1% Nile blue solution) to distinguish them from naïve nestmates. The dye had no effect on survivorship (Wald Statistic = 0.3, df = 1, P = 0.6). After immunization, five dyed termites were grouped with five naïve nestmates (unstained) in a Petri dish (n = eight replicates). Survivorship was recorded daily for 7 days. Controls (n = eight replicates) were established and counted in the same manner, but dyed termites were in this case exposed only to a sporeless Tween 80 suspension medium. On the seventh day postimmunization, termites in the experimental and control replicates were challenged with a lethal 6.5 × 104 spores/ml suspension [average spore viability (± SD) = 93.1 ± 14.1, n = 30 fields of vision]. All termites were isolated after the challenge exposure, allowing us to control for any reduction in disease susceptibility that might be caused by allogrooming. Survivorship postchallenge was recorded for 12 days and the survival distributions and hazard ratios of death were compared with those of newly established groups composed of either five dyed and five unstained termites exposed to a spore-free Tween 80 suspension (n = five replicates) or five dyed and five unstained nestmate nymphs exposed for the first time to the same 6.5 × 104 spores/ml challenge suspension (n = eight replicates). A total of 202 termites were challenged (n = 134 control and naïve termites that had no contact with the pathogen and n = 68 experimental exposures).
Statistics.
Survival parameters used in statistical evaluations included the survival distribution, percent survival at the end of the census period, median survival time (LT50), and the hazard ratio of death, using the Cox Proportional Regression analysis to generate the Wald Statistic. Regression models included the following variables: nesting treatment (group/isolation comparisons), naïve/immunized nestmate association, spore concentration during immunization, and colony of origin (where applicable). The hazard function characterized the instantaneous rate of death at a particular time, given that the individuals survived up to that point, while controlling for the effect of the other variables on survival (19, 20). This process provided a relative measure of the degree of susceptibility to disease across the 10 different treatments while controlling for the effects of the concentration of the immunizing dose and colony of origin of termites. The survival distributions for all treatments were computed and analyzed with the Breslow Statistic. Although survival analysis allows for multiple comparisons, we adjusted the α-value of significance accordingly (P ≤ 0.008; ref. 21) to provide a more conservative analysis. All P values thus calculated were highly significant (P < 0.00001).
Immunizing spore concentration (101, 102, and 103 spores/ml) used in the nesting treatment studies produced no significant mortality relative to controls in those termites that were maintained in groups during the immunization, although termites grouped after a 103 spores/ml immunization had lower survival than Tween 80 controls (Breslow Statistic = 4.7, P = 0.03; P ≤ 0.008 after correcting for multiple comparisons). Termites maintained in isolation during the immunization showed significantly higher mortality than controls when exposed to a 103 spores/ml immunizing exposure (Breslow Statistic = 28.5, P < 0.0001). Isolated nymphs exposed to a 101 and 102 spores/ml suspension had no significant mortality relative to Tween 80 controls (Wald Statistic = 4.8 and 0.5, respectively, P > 0.03; threshold for significance is P ≤ 0.008 after correcting for multiple comparisons). Overall, after controlling for the effects of colony, treatment, and cold exposure, the level of initial exposure to the immunizing dose was not a significant predictor of mortality (Wald Statistic = 0.51, 0.41, and 0.01 for 101, 102, and 103 spores/ml, respectively, P > 0.5, Cox Proportional Regression) relative to Tween 80 controls. Thus, survival data for immunized termites could be pooled within the grouped and isolated treatments.
Results
Immunocompetence of Isolated and Grouped Termites.
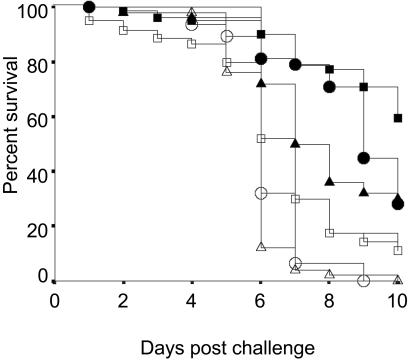
A Cox Proportional Regression analysis showed that nesting treatment was a significant and independent predictor of termite survival (Wald Statistic = 213, df = 9, P < 0.0001). After controlling for the effect of colony of origin, immunizing spore concentration, and cold exposure and assessing mortality in reference to the treatment that had the highest survivorship (individuals immunized with a 103 spores/ml solution while grouped (Table 1), pairwise statistical comparisons revealed that termites immunized with 101–103 spores/ml of M. anisopliae that were nesting in social groups and then challenged had significantly higher survivorship than naïve termites that were challenged and maintained in groups or control termites that were challenged and maintained in groups (Fig. 2, Table 1). Also, immunized termites nesting in isolation while they developed immunity and subsequently challenged had significantly higher survival than naïve isolated termites and control isolated termites that were challenged (Fig. 2, Table 1). Furthermore, the survival of immunized—grouped and challenged termites was significantly greater than that of immunized—isolated and challenged termites (Fig. 2, Table 1).
Table 1.
Survivorship of termites in isolation and group treatments after a challenge exposure to spores of M. anisopliae
| Spore concentration of inoculum, spores/ml
|
|||||
|---|---|---|---|---|---|
| Controls | 101 | 102 | 103 | Naïve | |
| Isolated | 6 days | 6 days | 7 days | 7 days | 6 days |
| 0% | 6.5% | 14.6% | 17.6% | 0% | |
| 10.1×** | 6.9×** | 5.7×** | 5.6×** | 13.2×** | |
| Grouped | 9 days | >10 days | >10 days | >10 days | 7 days |
| 28.1% | 59.5% | 70.1% | 67.1% | 30.0% | |
| 2.7×** | 1.3× (NS) | 0.89× (NS) | N/A | 3.5×** | |
| Breslow Statistic/P | 67.4/** | 50.3/** | 58.5/** | 34.2/** | 37.9/** |
Values of LT50 (line 1), percent survival at day 10 postchallenge (line 2) and hazard ratios of death (line 3) are given. Death hazard ratios are expressed as a multiple (×) of the maximum survival value (103 immunization, kept in groups). P compares significance between Breslow Statistics for survival distributions of isolated vs. grouped termites.
= significant at P < 0.0001; NS = not significant; N/A = not applicable (reference group for death hazard comparisons).
Figure 2.
Postchallenge survival distributions of Z. angusticollis nymphs in various social and isolation treatments. Grouped during immunization: (■ = data pooled across the 101, 102, and 103 spores/ml immunization treatments), challenged, grouped. Isolated during immunization: (□ = data pooled across the 101, 102, and 103 spores/ml immunization treatments), challenged, isolated. Naïve, challenged, and grouped (▴); naïve, challenged, isolated (▵); Tween 80 controls, challenged, grouped (●); Tween 80 controls, challenged, isolated (○).
Social Transfer of Immunity.
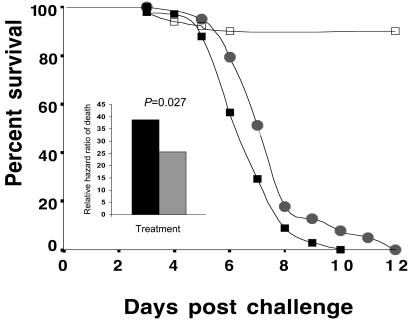
Statistical evaluation of the survivorship of control naïve termites and naïve termites that had nested with immunized nestmates revealed that treatment was a significant and independent predictor of mortality (Wald Statistic = 56.4, df = 3, P < 0.0001). This finding indicated that naïve nymphs that associated with immunized nymphs were significantly less susceptible to a lethal challenge exposure. Naïve termites exposed for the first time to a lethal spore concentration challenge had 38.8 times the hazard ratio of death, relative to termites exposed only to a Tween 80 suspension [the reference group that had the lowest mortality; Wald Statistic = 55.1, df = 1, P < 0.0001, 95% confidence interval (CI) (14.7–101.9)]. Naïve termites that had contact with immunized nestmates, however, had a 13-fold reduction in susceptibility [25.8 times the hazard ratio of death of the reference group; Wald Statistic = 42.3, df = 1, P < 0.0001, 95% CI (9.7–68.0); Fig. 3]. These “socially immunized” termites were 34.2% less susceptible to a lethal infection than termites that were not in contact with immunized siblings [Wald Statistic = 4.9, df = 1, P = 0.027; 95% CI (1.04–2.16); Fig. 3]. The time course of survival of socially immunized nymphs was also significantly greater than that of naïve termites that encountered the lethal challenge spore exposure for the first time (Breslow Statistic = 8.4, P < 0.005; Fig. 3).
Figure 3.
Survival distributions of Tween 80 controls (□), naïve/challenged nymphs (■), and socially immunized/challenged nymphs ( ). (Inset) Histogram illustrating the relative hazard ratios of death of naïve/challenged termites (black bar) and naïve termites placed in social contact with previously immunized nestmates (gray bar). Both treatments had hazard ratios significantly different from controls (see text).
). (Inset) Histogram illustrating the relative hazard ratios of death of naïve/challenged termites (black bar) and naïve termites placed in social contact with previously immunized nestmates (gray bar). Both treatments had hazard ratios significantly different from controls (see text).
Discussion
We recorded a higher survivorship among nymphs that developed immunity as members of a group in comparison to nymphs that were experimentally “vaccinated” in the same way but were then isolated. This finding indicates that the development of immunity depends on the social environment. The nature of the dependency is intriguing: were the observed differences in survival between isolated and group-nesting termites the result of a social enhancement of immunocompetence in the grouped individuals or were isolated nymphs at some physiological disadvantage that lowered their resistance to an immune challenge? All termites are eusocial, and isolation may adversely affect an individual's ability to mount an immune defense because of a lack of social interactions that might directly or indirectly impact immunological competence. Although our experiments do not allow us to discriminate cause and effect in the lower immune response of isolated nymphs, prior research and extant variation in termite social organization may offer some important insights. The full spectrum of colonial life in termites does not encompass a solitary existence, but termites do have a continuum of sociality marked by the absence of a sterile worker caste in basal groups that include species such as Zootermopsis (22). Previous studies on other lower termites suggest that removal from the parent colony does not appear to affect metamorphosis, caste differentiation, and feeding. Grassé and Noirot (23) compared caste differentiation in isolated and grouped Kalotermes flavicollis. Their results indicated that the survival, metamorphosis, and reproductive ability of termites raised individually in small glass tubes were not dramatically different from termites reared in colonies. Feeding and nutrition appeared to be normal; isolated termites could molt into neotenic (supplementary) reproductive forms and females laid eggs. This latter result in particular suggests that isolated termites may not have been nutritionally handicapped. Our experiments on isolated Z. angusticollis were completed in 20–22 days, a small portion of their lifespan (24), and immunity develops within approximately the first 3–5 days of isolation (11). During this brief isolation period, the ability to generate an immune response may not have been significantly compromised. In fact, the encapsulation responses of isolated and grouped termites do not differ significantly. When we challenged termites with a nylon thread (0.1 mm diameter, 2 mm length) inserted beneath the ventral abdominal cuticle of isolated (n = 30) and grouped (n = three groups of 10 individuals) nymphs and measured the degree of encapsulation after 4 days, we found no significant differences between treatments in the average total number [25.0 ± 14.0 (mean ± SD) vs. 21.9 ± 11.1, respectively; Mann–Whitney U = 372.5, z = −0.7, P = 0.4] and size (18.7 ± 9.6 vs. 19.0 ± 9.2 μm; Mann–Whitney U = 217682, z = −1.3, P = 0.2) of melanin deposits on the implant. There was no mortality in either treatment. Additionally, the survival of unexposed isolated Z. angusticollis is age-specific: only third-instar larvae have appreciable mortality (43% survivorship after 15 days of isolation in comparison to 97%, 90%, 97%, and 100% survival for instars IV, V, VI and nymphs, respectively) (20). In the present study we used nymphs that had 100% survivorship when isolated.
Lower termites such as Z. angusticollis are characterized by an extraordinary degree of reproductive plasticity; all individuals in a colony are capable of differentiating into reproductively competent forms (25). Because lower termites appear to survive well in isolation and individuals retain their reproductive options within the context of a social group, selection may not have reduced individual physiological immunity as the ancestral lower termites, which were likely to have been similar in biology to Zootermopsis, evolved socially. Perhaps termites retained the disease-resistance adaptations inherited from their close solitary relatives, the roaches (17), and then augmented their ability to control infection through social behavior.
Although the use of isolated termites as a form of cell or organ culture for social insect research requires further investigation, our studies demonstrate that either the physiological immunocompetence of individual Z. angusticollis nymphs is augmented by colonial life or that lower termites, despite their reproductive plasticity, are nevertheless socially integrated to such a high degree that even brief separation from the parent colony can depress the immune response. Either hypothesis suggests a significant reliance on sociality for the maintenance of disease resistance but implies different mechanisms of group infection control. If the development of immunity in isolated nymphs is not negatively impacted by separation from the parent colony, then disease-reducing features of group life such as the removal of spores through allogrooming, the “social vaccination” of nestmates or the social transfer of immunity could complement the infection control mechanisms of individuals. In contrast, if the immune system of individual termites is compromised outside of the natural social milieu, then group mechanisms of pathogen resistance may have evolved because they reduced the costs of maintaining individual immunity. In these scenarios, the proximate and ultimate causes of “socially enhanced” or “individually deficient” resistance are equally interesting and significant.
Independent of the mechanism responsible for the decreased ability of isolated nymphs to resist an immune challenge, our second series of experiments demonstrated that the immunity of naïve termites improved after they were placed in association with immunized nestmates. The difference in survivorship was caused by more than the nutritional environment because termites in both treatments had the ability to engage in social food transfer. Additionally, even at the conservative ratio of immunized/naïve termites of 1:1, interactions with immunized nestmates conferred on naïve individuals a significant survivorship benefit. The magnitude of this survivorship benefit (in terms of days of survival) is likely a function of the ratio of immunized/naïve termites, and we imagine resistance benefits would be extended when the ratio is biased in favor of immunized individuals.
The increase in individual disease resistance associated with grouping could be attributed to the removal of fungal spores on the cuticle of nestmates through mutual grooming (6), or caused by other, more subtle interactions that could lower disease susceptibility. For example, nestmates may vary in their degree of immunoresponsiveness and some individuals could attain higher levels of resistance relatively soon after an exposure to a pathogen whereas others may have a delayed development of immunity. In nature, termites inhabiting one portion of a nest may receive an immunizing dosage to a pathogen whereas nestmates living in a more distant gallery system might lack such an exposure, but later contact one another and receive an immunizing inoculum during their interactions. In either case, individual variation in immunocompetence could produce heterogeneous groupings of naïve or susceptible termites and their more resistant siblings.
Ecological and phylogenetic factors such as the utilization of wood as a food source, symbiont transfer, developmental plasticity, shifts in dependent care from parents to offspring, cycles of inbreeding and outbreeding, philopatric reproduction, and disease may have contributed to the evolution of eusociality in termites (25, 26). Sociality compounds disease risks caused by increased rates of infection transmission (27, 28) and genetic relatedness (3, 4, 29), but there is little evidence that grouping lowers pathogen-related death hazards. In some gregarious lepidopteran species, grouping appears to stimulate pathogen resistance mechanisms, including the prophenoloxidase enzyme cascade and cuticular melanization (30), and in honey bees “fever” (elevated nest temperature) is generated as a colony-level response to prevent chalk brood, which is caused by a heat-sensitive fungus (31). Recently, a transgenerational enhancement of immunity was demonstrated in the bumblebee Bombus terrestris (32). Male sexual offspring showed elevated hemolymph phenoloxidase, which is involved in melanization and the encapsulation of parasites, when their parent colony was challenged by injections of lipopolysaccharide. Here we have shown that interaction between immunized and naïve nestmates can improve the disease resistance of individuals that have not been directly exposed to a fungal pathogen, indicating that the immunocompetence of colony members can be modulated through the social immunization of nestmates. In Z. angusticollis, the mechanisms that underscore this increase in colony-level immunocompetence may involve exposure to sublethal dosages of active spores during social interactions, contact with inactivated spores distributed through mutual grooming, or the transfer of immune factors or promoters through trophallactic exchanges. Sociality may thus reduce the cost of maintaining pathogen resistance in individual colony members (33) and provide a collective mechanism that reduces the risk of infection transfer, which may be prevalent in densely packed termite nests.
Acknowledgments
We thank Dr. Ken Wilson and anonymous reviewers for their critical reading of the manuscript and the administrators of the Redwood East Bay Regional Park District and Pebble Beach Corporation for allowing us to collect termites. Stephanie Chu provided useful data on termite encapsulation rates. This research was supported by National Science Foundation Grants IBN-9632134 and IBN-0116857.
References
- 1.Wilson E O. The Insect Societies. Cambridge, MA: Belknap; 1971. [Google Scholar]
- 2.Hölldobler B, Wilson E O. The Ants. Cambridge, MA: Harvard Univ. Press; 1990. [Google Scholar]
- 3.Hamilton W D. In: Animal Societies: Theories and Facts. Ito Y, Brown J L, Kikkawa J, editors. Tokyo: Japanese Science Society; 1987. pp. 81–102. [Google Scholar]
- 4.Schmid-Hempel P. Parasites in Social Insects. Princeton: Princeton Univ. Press; 1998. [Google Scholar]
- 5.Hendee E C. In: Termites and Termite Control. Kofoid C A, editor. Berkley, CA: Berkley University; 1934. , pp. 105–116. [Google Scholar]
- 6.Rosengaus R B, Maxmen A B, Coates L E, Traniello J F A. Behav Ecol Sociobiol. 1998;44:124–134. [Google Scholar]
- 7.Rosengaus R B, Traniello J F A, Lefebrve M L, Carlock D M. Ethol Ecol Evol. 2000;12:419–433. [Google Scholar]
- 8.Rosengaus R B, Guldin M R, Traniello J F A. J Chem Ecol. 1998;24:1697–1706. [Google Scholar]
- 9.Rosengaus R B, Lefebvre M L, Traniello J F A. J Chem Ecol. 2000;26:21–39. [Google Scholar]
- 10.Rosengaus R B, Jordan C, Lefebvre M L, Traniello J F A. Naturwissenschaften. 1999;86:544–548. doi: 10.1007/s001140050672. [DOI] [PubMed] [Google Scholar]
- 11.Rosengaus R B, Chen T, Brown J J, Traniello J F A. Naturwissenschaften. 1999;86:588–591. doi: 10.1007/s001140050672. [DOI] [PubMed] [Google Scholar]
- 12.Lamberty M, Zachary D, Lanot R, Bordereau Ch, Roberts A, Hoffmann J A, Bulet P. J Biol Chem. 2001;276:4085–4092. doi: 10.1074/jbc.M002998200. [DOI] [PubMed] [Google Scholar]
- 13.Hughes J A, Hurlbert R E, Rupp R A, Spence K D. J Insect Physiol. 1983;29:625–632. [Google Scholar]
- 14.Dunn P E. BioScience. 1990;40:738–744. [Google Scholar]
- 15.Hultmark D. Trends Genet. 1993;9:178–184. doi: 10.1016/0168-9525(93)90165-e. [DOI] [PubMed] [Google Scholar]
- 16.Phipps D J, Chadwick J S, Leeder R G, Aston W P. Dev Comp Immunol. 1989;13:103–111. doi: 10.1016/0145-305x(89)90025-6. [DOI] [PubMed] [Google Scholar]
- 17.Faulhauber L M, Karp R D. Immunology. 1992;75:378–381. [PMC free article] [PubMed] [Google Scholar]
- 18.Rosengaus R B, Traniello J F A. Sociobiology. 1997;30:185–195. [Google Scholar]
- 19.SPSS. Advanced Statistics Manual: SPSS/PC+ 4.0. Chicago: SPSS; 1990. [Google Scholar]
- 20.Rosengaus R B, Traniello J F A. Behav Ecol Sociobiol. 2001;50:546–556. doi: 10.1007/s00265-022-03203-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rice W R. Evolution. 1989;43:223–225. doi: 10.1111/j.1558-5646.1989.tb04220.x. [DOI] [PubMed] [Google Scholar]
- 22.Shellman-Reeve J S. In: The Evolution of Social Behavior in Insects and Arachnids. Choe J C, Crespi B J, editors. Cambridge, U.K.: Cambridge Univ. Press; 1997. pp. 52–93. [Google Scholar]
- 23.Grassé P-P, Noirot C. Insectes Sociaux. 1960;7:323–331. [Google Scholar]
- 24.Yin C M. Ph.D. thesis. Saskatoon, Canada: University of Saskatchewan; 1972. [Google Scholar]
- 25.Thorne B L. Annu Rev Ecol Syst. 1997;28:27–54. [Google Scholar]
- 26.Rosengaus R B, Traniello J F A. Proc Natl Acad Sci USA. 1993;90:6641–6645. doi: 10.1073/pnas.90.14.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freeland W J. Ecology. 1979;60:719–728. [Google Scholar]
- 28.Nunn C L, Gittleman J L, Antonovics J. Science. 2000;290:1168–1170. doi: 10.1126/science.290.5494.1168. [DOI] [PubMed] [Google Scholar]
- 29.Baer B, Schmid-Hempel P. Nature (London) 1999;397:151–154. [Google Scholar]
- 30.Reeson A F, Wilson K, Gunn A, Hails R S, Goulson D. Proc R Soc London Ser B. 1998;265:1787–1791. [Google Scholar]
- 31.Starks P T, Blackie C A, Seeley T D. Naturwissenschaften. 2000;87:229–231. doi: 10.1007/s001140050709. [DOI] [PubMed] [Google Scholar]
- 32.Moret Y, Schmid-Hempel P. Nature (London) 2001;414:506. doi: 10.1038/35107138. [DOI] [PubMed] [Google Scholar]
- 33.Moret Y, Schmid-Hempel P. Science. 2000;290:1166–1168. doi: 10.1126/science.290.5494.1166. [DOI] [PubMed] [Google Scholar]