Abstract
Aims
Cardiac impairment in AL amyloidosis is the major determinant of survival. Treatment goals include reducing circulating light chains to improve organ function. Global longitudinal strain (GLS) is an independent predictor of survival and useful for assessing cardiac function before and after therapy. This study aimed to describe GLS change from baseline to one year post-treatment, identify factors associated with GLS improvement (GLS+), and evaluate its prognostic significance.
Methods and results
Ninety-seven patients with AL amyloidosis and cardiac stage II/III disease who underwent echocardiogram and haematologic evaluation at baseline and one year were included. GLS+ was defined as a 2.0%-point increase. A cardiac or B-type natriuretic peptide (BNP+) response was defined as a 30% reduction from baseline. Overall survival was measured from baseline echocardiogram to death. Of 97 patients, 62% had Stage II, 29% Stage IIIa, and 9% Stage IIIb disease. Baseline median left ventricular ejection fraction, GLS, and septal thickness were 65%, −14.9%, and 1.3 cm, respectively. GLS+ was observed in 36% of patients and BNP+ in 51%. Median overall survival was 113.4 months. The hazard ratio for survival was 0.42 in the GLS+ group and 0.46 in the BNP+ group, after adjusting for haematologic response.
Conclusion
GLS improvement post-treatment confers a significant survival benefit. This study supports GLS as an important marker for risk stratification and cardiac response.
Keywords: AL amyloidosis, Cardiomyopathy, Prognosis, Echocardiography, Global longitudinal strain
Graphical Abstract
Graphical Abstract.
Introduction
The goals of treatment in AL amyloidosis are to block the production of monoclonal light chains, prevent organ damage, and, if possible, reverse existing damage. Cardiac involvement, occurring in more than 50% of AL amyloid cases, is the major determinant of survival.1 As more therapeutic options become available, accurate monitoring and assessment tools to detect and quantify cardiac involvement are essential to evaluate their effectiveness and guide treatment decisions.
The current standard for assessing cardiac response post-treatment relies on a reduction in N-terminal pro-brain natriuretic peptide (NT-proBNP), a serum biomarker of myocardial stress.2 However, natriuretic peptide levels may not directly reflect myocardial function, as they can be influenced by factors such as intravascular volume overload or changes in renal function. Global longitudinal strain (GLS), an index of myocardial deformation, is a sensitive marker of myocardial function that has been shown to provide incremental prognostic value beyond circulating cardiac biomarkers in patients with AL amyloidosis.3–5 Previously, Cohen et al. from the UK National Amyloidosis Center6 demonstrated that improvement in GLS provided independent and additional prognostic value compared to improvements in NT-proBNP levels following plasma-directed therapy in patients with cardiac involvement. Their work was pivotal in identifying additional markers of cardiac response and predictors of survival. Given the importance of assessing cardiac improvement—a key factor in treatment decisions—it would be worthwhile to validate this finding in other populations of patients with AL amyloidosis to determine its broader applicability.
Thus, the current study aimed to examine the change in GLS from pre-treatment to 1 year after plasma-directed therapy in patients with baseline cardiac stage II, IIIa, or IIIb disease, as defined by the Boston University (BU) staging system. This system, which relies on B-type natriuretic peptide (BNP), is applicable in centres where NT-proBNP is unavailable and has proven to be highly informative and predictive of prognosis.7,8 The study also sought to identify clinical factors associated with GLS improvement and assess its prognostic significance.
Methods
Study design and patient selection
Two-hundred and eighteen consecutive patients with newly diagnosed biopsy-proven systemic AL amyloidosis, with positive troponin I and/or BNP classified as BU stage II, IIIa, or IIIb disease, evaluated at Memorial Sloan Kettering Cancer Center between May 2007 and January 2020, were identified, and included in the study. Patients with Stage I disease were not included due to the absence of elevated cardiac biomarkers, indicating a low likelihood of prognostically significant cardiac involvement. The BU staging system, based on the cut-off of troponin I (TnI) 0.1ng/mL and BNP 81 pg/L, consisted of the following definitions: Stage I—both Tn and BNP below the cut-offs; Stage II—either Tn or BNP above the cut-off; Stage IIIa—both Tn and BNP above the cut-offs with BNP < 700 pg/L; Stage IIIb—both Tn and BNP above the cut-offs with BNP ≥ 700 pg/L.8 Patients who were alive and had a 1 year follow-up echo and haematologic evaluation available were included in this analysis.
Baseline clinical, echocardiographic, and laboratory test results were collected prior to or within one month of the start of plasma cell-directed therapy. Clinical and treatment data were extracted from a prospectively maintained database of an ongoing Memorial Sloan Kettering Cancer Center Institutional Review Board approved protocol that collects clinical characteristics and outcomes of patients with systemic AL amyloidosis. Ethical approval was granted for this protocol.
Echocardiography
The conventional two-dimensional (2D) and Doppler echocardiography protocols have been previously described.4 Briefly, the studies were performed using commercially available standard ultrasound scanners (Vivid E9, General Electric Medical Systems and iE33, Philips Medical Systems), according to the standardized American Society Echocardiography (ASE) protocol.9 Left ventricular ejection fraction (LVEF) was calculated using the modified Simpson’s method. Mitral inflow velocity pattern was recorded from the apical four-chamber view with the pulsed-wave Doppler sample volume positioned at the tips of the leaflets during diastole. Peak early filling (E-wave) and late diastolic filling (A-wave) velocities were measured, and their ratio (mitral E/A) derived. Doppler tissue imaging of the mitral annulus was performed with measurement of the early (e′) diastolic velocity at the lateral and medial annulus.
Strain measurement
The methods of image acquisition and post-processing of strain measurements have been previously described.4 Briefly, GLS measurements were performed offline using vendor independent 2D Cardiac Performance Analysis software (Tom Tec Imaging Systems, Unterschleissheim, Germany). All echocardiographic measurements were made with the operator blinded to the clinical and outcome data. The endocardial border was traced in end-diastole in the three standard apical views, which allowed the software to track myocardial movement throughout the cardiac cycle.10 After careful inspection, manual correction was performed if the myocardial tracking was suboptimal. Each view was divided into six segments, for a total of 18 segments representing the entire LV. Longitudinal strain curves were generated for each segment. GLS was calculated as the average value of the peak negative systolic strain values for all the segments within the three standard apical views.
Repeatability and reproducibility
Echocardiograms were analysed by a single experienced echocardiographer. To assess reproducibility, GLS measurements in 25 randomly selected patients were repeated on two occasions by the original reader to determine intra-observer variability and by a second experienced reader blinded to prior measurements to determine inter-observer variability. The mean difference in calculated GLS was −0.052 (95% CI −1.09 to +0.98) and −0.028% (95% CI −1.74% to +1.69%) for intra and inter-observer variabilities, respectively (see Supplementary material online, Figure S1).
Criteria for haematologic and cardiac response
The haematologic response at 1 year following plasma cell-directed therapy was defined according to the following published criteria11: (i) complete response (CR): normalization of the involved free light chain (FLC) to below the upper limit of normal, and negative serum and urine immunofixation; (ii) very good partial response (VGPR): a difference between involved and uninvolved light chains (dFLC) of <40 mg/L; (iii) partial response (PR): a dFLC decrease > 50%; and (iv) no response (NR): no significant dFLC change. A deeper light chain response (DR) has been defined as a difference between involved and uninvolved light chains (dFLC) < 10 mg/L. Cardiac or BNP response (BNP+) was defined based on a 30% reduction from pre-treatment as per definition of cardiac improvement.2,7 The absolute value of GLS was reported. Similar to the previously published study,6 GLS improvement (GLS+) was defined as an absolute 2.0%-point increase from baseline, a cut-off representing a meaningful change beyond measurement variability (see Supplementary material online, Figure S1).
Statistical analysis
Overall survival (OS) was defined as time from the baseline echocardiogram obtained within one month of treatment to death from any cause. Continuous variables are presented as median [interquartile range (IQR)] and categorical variables as number (percentage). Clinical characteristics and echocardiographic parameters were compared by group using the Wilcoxon signed rank or Kruskal–Wallis test for continuous variables and χ2 test or Fisher’s exact test for categorical variables. Correlation between BNP response and GLS response was calculated by Pearson correlation coefficients. Survival analysis was performed using the Kaplan–Meier method and a log-rank test was used to compare overall survival between groups of interest. Multivariable Cox proportional hazards models evaluated associations between overall survival with GLS improvement, BNP response, and GLS improvement or BNP response, adjusting for haematologic response and age. To compare the goodness-of-fit of the models, the Likelihood-Ratio test was performed between models. Statistical significance was defined as a two-sided P-value of <0.05. Bland–Altman plots were used to display inter-and intra-observe variabilities in GLS measurements. Statistical analyses were performed in R, version 3.5.2 (R Foundation for Statistical Computing), Stata, version 15.1 (StataCorp, LLC), and SAS Version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Patient characteristics
Two-hundred and eighteen AL amyloidosis patients with BU cardiac stage II-IIIb were identified. The clinical and echocardiographic characteristics of this baseline group are shown in Supplementary Tables. Fifty-seven patients died before reaching one year after starting treatment, and an additional 64 patients were lost to follow-up or had missing 1-year echo/GLS data. This resulted in a study group of 97 patients who fulfilled the criteria. Patients in the study group were similar to the patients lost to follow-up or missing 1 year echo with respect to the baseline clinical and echocardiographic characteristics. As expected, patients who died before reaching one year had more advanced disease with higher levels of TnI and BNP, and dFLC, as well as more impaired cardiac structure and function. The baseline clinical characteristics of the study group are shown in Table 1. Of 97 patients, 62% (60) had BU Stage II, 29% (28) had BU Stage IIIa, and 9% (9) had BU stage IIIb cardiac disease at baseline. The cardiovascular comorbidities included 51% (49) with a history of hypertension, 47% (46) with hyperlipidaemia, 20% (19) with chronic kidney disease, 18% (17) with clinical heart failure, and 9% (9) with coronary artery disease. The median troponin I level was 0.05 ng/mL, and the median BNP was 280 pg/mL, with 36% (35) of patients having a BNP ≥ 400 pg/mL.
Table 1.
Baseline clinical characteristics (N = 97)
| Male | 60 (62) |
| Age, years | 62 (57–69) |
| BMI, kg/m2 | 26.3 (23.0–30.4) |
| SBP, mmHg | 116 (103–133) |
| DBP, mmHg | 73 (64–79) |
| HR, b.p.m. | 82 (71–90) |
| Troponin I, ng/mL | 0.05 (0.05–0.14) |
| BNP, pg/mL | 280 (167–504) |
| Serum creatinine, mg/dL | 1.0 (0.8–1.3) |
| BU | |
| II | 60 (62) |
| IIIa | 28 (29) |
| IIIb | 9 (9) |
| History of hypertension | 49 (51) |
| Hyperlipidaemia | 46 (47) |
| Chronic kidney disease | 19 (20) |
| Clinical heart failure | 17 (18) |
| Coronary artery disease | 9 (9) |
| dFLC, mg/dL | 18 (7–45) |
| BNP ≥ 400, pg/mL | 35 (36) |
Values are n (%) or median (interquartile range).
BU, Boston University; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; BNP, brain natriuretic peptide; dFLC, difference between involved and uninvolved light chains.
The median (IQR) creatinine was 1.0 (0.8–1.3) mg/dL. Forty-six patients (47%) received high dose melphalan with autologous stem cell transplant and the remaining 51 patients (53%) received bortezomib based therapy. The baseline echocardiographic findings, which are listed in Table 2, included a median LVEF of 65% (IQR 59, 70), GLS of −14.9% (IQR −12.1, −17.7), and septal thickness of 1.3 cm (IQR 1.1, 1.5).
Table 2.
Baseline echocardiographic characteristics
| n | Median (interquartile range) | |
|---|---|---|
| GLS% | 97 | −14.9 (−12.1, −17.7) |
| LVEF % | 101 | 65 (59, 70) |
| IVS thickness, cm | 96 | 1.3 (1.1, 1.5) |
| Mitral E/A ratio | 89 | 1.0 (0.8, 1.5) |
| Lateral e′, cm/s | 82 | 6.4 (5.2, 8.0) |
| Septal e′, cm/s | 80 | 5.3 (4.2, 6.9) |
| Average E/e′ | 84 | 14.9 (12.0, 18.9) |
| Deceleration time, s | 78 | 0.19 (0.16, 0.24) |
| LA volume index, mL/m2 | 90 | 31.8 (23.4, 39.3) |
| TAPSE, mm | 73 | 2.0 (1.7, 2.3) |
| RV TDI, cm/s | 53 | 14.0 (12.0, 15.7) |
| Stroke volume, ml | 81 | 65.3 (48.6, 80.9) |
| Stroke volume index, mL/m2 | 81 | 35.2 (27.8, 41.2) |
| Cardiac output, L/min | 81 | 4.9 (4.0, 5.9) |
| Cardiac index, L/min/m2 | 81 | 2.6 (2.2, 3.0) |
GLS, global longitudinal strain; LVEF, left ventricular ejection fraction; IVS, interventricular septal thickness; LA, left atrial volume; TAPSE, tricuspid annular plane systolic excursion; RV TDI, right ventricular tissue Doppler imaging.
Assessment at 1 year after treatment
Haematologic and cardiac response
At the 1-year mark, all patients underwent a follow-up evaluation to assess haematologic and cardiac response, as well as an echocardiogram. The haematologic response included 38 (39%) CR, 32 (33%) VGPR, 16 (16%) PR and 11 (11%) NR, as well as 39 (40%) DR. A cardiac response or a BNP response (BNP+), defined by a 30% reduction from baseline, was seen in 49 (51%) patients.
GLS improvement
GLS improvement (GLS+), defined by an absolute 2.0%-point increase from baseline, was seen in 35 (36%) patients. Table 3 compares the clinical characteristics of patients with GLS improvement (GLS+) and those without (GLS−). The GLS(+) patients compared to GLS(−) were younger (60 vs. 64 years; P = 0.034), were more frequently women (54% vs. 29%, P = 0.014), and had a lower baseline GLS (−13.5% vs. −16.1%; P = 0.003). There was no difference in the BU stage between the two groups. The dFLC at 1 year was lower in the GLS(+) group compared to the GLS(−) group (1.10 mg/dL vs. 1.79 mg/dL; P = 0.081), and the CR rate was close to 50% in the GLS(+) group compared to 34% in the GLS(−) group. However, these differences did not reach statistical significance. A cardiac response, or BNP(+), was significantly higher in the GLS(+) than the GLS(−) group. We further evaluated the patients based upon a combination of BNP response and GLS improvement (Table 4). Twenty-nine (30%) patients had both GLS improvement and BNP response (GLS+/BNP+), and 42 (43%) patients had no GLS improvement or BNP response (GLS−/BNP−). Patients with both BNP response and GLS improvement (GLS+/BNP+) had a lower dFLC at 1 year (1.01 mg/dL) compared to patients with GLS+ or BNP+ response (1.51 mg/dL) or no GLS improvement and BNP response (GLS−/BNP−) (2.50 mg/dL; P = 0.045).
Table 3.
Comparison of clinical characteristics of patients based upon GLS improvement
| Parameter | GLS+ N = 35 | GLS− N = 62 | P-value |
|---|---|---|---|
| Age (years) | 60 (55, 65) | 64 (59, 69) | 0.034 |
| Female | 19 (54) | 18 (29) | 0.014 |
| BU | |||
| Stage II | 24 (69) | 36 (58) | 0.324 |
| Stage IIIA | 7 (20) | 21 (34) | |
| Stage IIIB | 4 (11) | 5 (8) | |
| dFLC (mg/dL) baseline | 12.84 (2.71, 52.06) | 20.89 (9.26, 44.86) | 0.194 |
| Baseline GLS (%) | 13.5 (11.4, 16.4) | 16.1 (13.3, 18.5) | 0.003 |
| Baseline LVEF (%) | 66.0 (61.1, 69.6) | 64.3 (58.3, 71.0) | 0.677 |
| Baseline troponin (ng/mL) | 0.05 (0.05, 0.13) | 0.05 (0.05, 0.16) | 0.856 |
| Baseline BNP (pg/mL) | 336 (219, 611) | 257 (162, 467) | 0.122 |
| Autologous SCT | 20 (57) | 26 (42) | 0.15 |
| dFLC (mg/dL) 1 year | 1.10 (0.31, 1.98) | 1.79 (0.62, 5.66) | 0.081 |
| Complete response | 17 (49) | 21 (34) | 0.154 |
| Deep response | 15 (43) | 24 (39) | 0.689 |
| BNP response | 29 (83%) | 20 (32%) | <0.001 |
Values are n (%) or median (interquartile range).
GLS+, GLS improvement defined by a 2%-point increase; GLS−, no GLS improvement defined by <2%-point increase; BU, Boston University; dFLC, difference between involved and uninvolved light chains; LVEF, left ventricular ejection fraction; BNP, brain natriuretic peptide.
Table 4.
Comparison of clinical characteristics of patients based upon GLS and BNP response
| Parameter | GLS+/BNP+; N = 29 | GLS+ or BNP+; N = 26 | GLS− and BNP− N = 42 | P-value |
|---|---|---|---|---|
| Age (years) | 60 (55, 65) | 61.5 (58, 69) | 64 (59, 69) | 0.192 |
| Female | 14 (48) | 11 (42) | 12 (29) | 0.214 |
| BU | ||||
| Stage II | 21 (72) | 13 (50) | 26 (62) | 0.265 |
| Stage IIIA | 5 (17) | 9 (35) | 14 (33) | |
| Stage IIIB | 3 (10) | 4 (15) | 2 (5) | |
| dFLC (mg/dL) baseline | 12.84 (2.71, 52.06) | 19.36 (6.37, 43.25) | 20.25 (10.28, 56.50) | 0.472 |
| dFLC (mg/dL) 1 year | 1.01 (0.31, 1.75) | 1.51 (0.62, 3.64) | 2.50 (0.75, 5.83) | 0.045 |
| Complete response | 14 (48) | 11 (42) | 13 (31) | 0.325 |
| Deep response | 14 (48) | 10 (38) | 15 (36) | 0.602 |
| Baseline GLS (%) | 14.2 (11.4, 16.4) | 14.6 (11.9, 17.9) | 16.4 (13.9, 19.0) | 0.010 |
| Baseline LVEF (%) | 66.2 (61.1, 69.0) | 62.5 (57.0, 70.0) | 64.8 (60.0, 71.0) | 0.511 |
| Baseline troponin (ng/mL) | 0.05 (0.05, 0.11) | 0.09 (0.05, 0.14) | 0.05 (0.05, 0.16) | 0.675 |
| Baseline BNP (pg/mL) | 336 (219, 562) | 323 (194, 498) | 212 (148, 431) | 0.066 |
Values are n (%) or median (interquartile range).
GLS+, GLS improvement defined by a 2%-point increase; GLS−, no GLS improvement defined by <2%-point increase; BNP+, cardiac response defined by a 30% reduction from pre-treatment; BNP−, no cardiac response or <30% reduction from pre-treatment; BU, Boston University; dFLC, difference between involved and uninvolved light chains; LVEF, left ventricular ejection fraction; BNP, brain natriuretic peptide.
Survival analysis
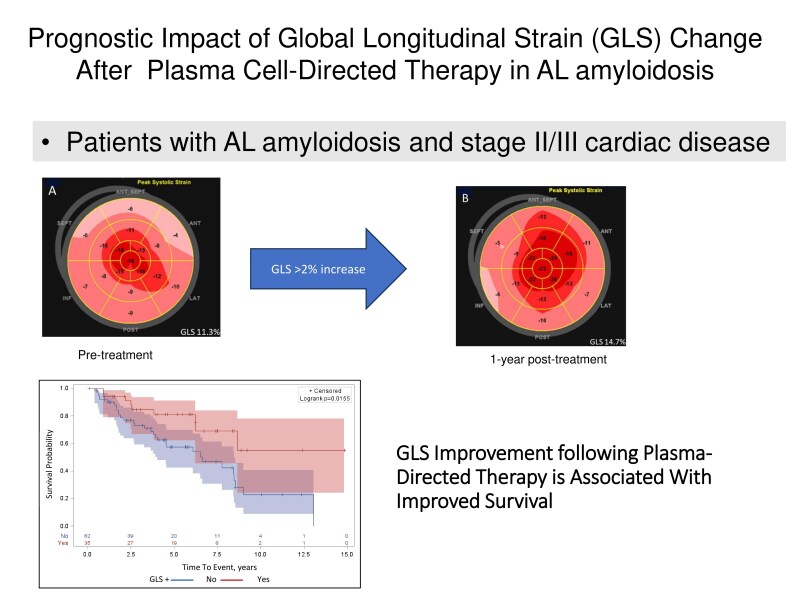
The median follow-up time after the baseline echocardiogram was 64.7 months (IQR: 37.1–91.8). Among survivors with AL amyloidosis at one year following treatment, the median overall survival (OS) was 113.4 months, with no significant difference between patients with baseline BU stage II and III (a, b combined) disease (OS 105.3 months and 114.6 months, respectively P = 0.7). Median OS was greater in patients who achieved complete response (CR) or deep response (DR) compared to those who did not achieve CR or DR (120.2 vs. 90.2 months, respectively; P = 0.02) (Figure 1). The median OS was also greater in the GLS−positive (GLS [+]) group compared to the GLS-negative (GLS [−]) group (not reached for GLS [+] vs. 91.8 months for GLS [−]; P = 0.02) (Graphical Abstract). Similarly, the median OS was greater in the BNP-positive (BNP [+]) group compared to the BNP-negative (BNP [−]) group (not reached for BNP [+] vs. 90.2 months for BNP [−]; P = 0.006) (Figure 2). The hazard ratio (HR) for OS was 0.42 [95% confidence interval (CI): 0.20–0.89] in the GLS [+] group and 0.46 (95% CI: 0.23–0.91) in the BNP [+] group after adjusting for DR. Adjusting for age had no effect on any of the models. Patients with either GLS [+] or BNP [+] (HR = 0.25; 95% CI: 0.09–0.73; P = 0.011) or both GLS [+] and BNP [+] (HR = 0.42; 95% CI: 0.19–0.89; P = 0.024) had significantly improved survival compared to those without GLS improvement or BNP response (Table 5). From the likelihood-ratio test, Model 4, which included both BNP and GLS, demonstrated significantly better goodness-of-fit compared to Model 3 with BNP only (P = 0.016) and Model 1 with DR only (P = 0.020), supporting the incremental value of GLS in predicting survival.
Figure 1.
Kaplan–Meier survival curves based on haematologic response at 1 year following treatment. dFLC, difference between involved and uninvolved light chains. Patients who achieved deep response showed improved survival than patients who did not (median overall survival 120.2 vs. 90.2 months, respectively, P = 0.02). Product-limit survival estimates with number of subjects at risk and 95% confidence limits.
Figure 2.
Kaplan–Meier survival curves based on BNP response at one year following treatment. Patients with a positive BNP response showed significantly better survival than patients with a negative BNP response (median overall survival 90.2 months for BNP(−) and not reached for BNP(+), P = 0.006), Product-limit survival estimates with number of subjects at risk and 95% confidence limits. BNP, B-type natriuretic peptide.
Table 5.
Multivariable Cox proportional hazards models of survival with haematologic response and cardiac response as dependent covariates
| Model 1 Hazard ratio 95% CI |
P-value | Model 2 Hazard ratio 95% CI |
P-value | Model 3 Hazard ratio 95% CI |
P-value | Model 4 Hazard ratio 95% CI |
P-value | |
|---|---|---|---|---|---|---|---|---|
| Deep response | 0.44 (0.22, 0.89) | 0.022 | 0.46 (0.23, 0.92) | 0.028 | 0.52 (0.26, 1.06) | 0.071 | 0.50 (0.25, 1.02) | 0.055 |
| GLS+/− | 0.42 (0.20, 0.89) | 0.024 | ||||||
| BNP+/− | 0.46 (0.23, 0.91) | 0.025 | ||||||
| GLS and BNP | ||||||||
| −/− | Ref | 0.011 | ||||||
| −/+ or +/− | 0.25 (0.09, 0.73) | 0.024 | ||||||
| +/+ | 0.42 (0.19, 0.89) |
With overall survival as the outcome, Model 1 included deep response as the dependent covariate, Model 2 included deep response and GLS improvement as the dependent covariates, Model 3 included deep response and BNP improvement as the dependent covariates, and Model 4 included deep response and integrated GLS and BNP improvement (GLS+ and BNP+ vs. GLS+ or BNP+ vs. GLS− and BNP−) as the dependent covariates.
CI, confidence interval; GLS+, GLS improvement defined by a 2%-point increase; GLS−, no GLS improvement defined by <2%-point increase; BNP+, cardiac response defined by a 30% reduction from pre-treatment; BNP−, no cardiac response or <30% reduction from pre-treatment.
Discussion
In this single-centre retrospective cohort analysis of patients with AL amyloidosis and cardiac stage II, IIIA, and IIIB disease, GLS improvement at 1 year following plasma cell-directed therapy was shown to be a strong predictor of survival. Among survivors at 1-year post-treatment, GLS improvement was observed in 36% of patients, occurring across all three cardiac stages. This improvement was associated with a 60% reduction in the risk of death, independent of haematologic response and age.
Global longitudinal strain (GLS) is a measure of the longitudinal contraction of cardiac motion. It serves as a marker for subendocardial function and can detect subclinical dysfunction at an early stage, as longitudinally oriented fibres, primarily located in the subendocardium, are highly sensitive and vulnerable to myocardial disease.10,12 GLS has been shown to be superior to LVEF and wall motion score in predicting all-cause mortality among patients undergoing an echocardiogram for suspected or known left ventricular (LV) impairment in the general population, as well as in specific cardiovascular conditions.13,14 The role of GLS as a strong and independent prognostic marker in patients with newly diagnosed AL amyloidosis has been well established.5,15 The findings of this study further expand the role of GLS by demonstrating the strong prognostic value of GLS improvement following treatment. Cohen et al.6 reported GLS improvement at 12 months following plasma cell-directed therapy in 32% of patients with cardiac involvement who achieved CR, which provided incremental prognostic value beyond the BNP response. In the current study, GLS improvement was observed in 36% of the patients, and it was more common in patients with greater haematologic response. The GLS(+) group had a lower dFLC at 1 year than the GLS(−) group, which is consistent with the prevailing notion that improvement in organ function is correlated with the quality of haematologic response. However, among patients with GLS+, no significant difference was observed between those who achieved CR and those who did not. These results suggest that lowering serum FLC, even without reaching the defined threshold of complete remission, may contribute to improved cardiac function. Supporting this observation, a previous study demonstrated a simultaneous drop in serum FLCs and NT-proBNP after three cycles of chemotherapy.16 There is evidence suggesting that soluble pre-fibrillar amyloidogenic light chains themselves exert direct cytotoxic effects on cardiomyocytes, and the ultimate cardiac damage may result from the combination of amyloid burden and/or direct toxicity caused by the light chains.17,18 In addition, there is likely heterogeneity in the proteotoxicity of amyloidogenic light chains, which may contribute to the variability in the association between light chain levels and the degree of cardiac dysfunction observed among AL amyloidosis patients.16,19 Thus, it is conceivable that reducing circulating light chains could lead to GLS improvement, even in cases where the haematologic response is less than complete remission.
Another remarkable finding of the study is the impressive observed survival rate among patients who made it to 1 year, especially those who showed GLS improvement or BNP response, with a respective reduction in the risk of death by 58% and 54%. While this study was unable to demonstrate an additional prognostic benefit of GLS improvement when combined with BNP response due to the small sample size of patients exhibiting both BNP and GLS improvement, Cohen et al.6 demonstrated an incremental value when GLS was combined with NT pro BNP. Given the association of GLS improvement with improved survival, further investigation is needed to determine whether GLS improvement could guide the intensity and duration of plasma cell-directed therapy, particularly for patients at risk of treatment-related toxicity.
It is interesting to note that among patients who reached 1 year on treatment, baseline biomarker-based staging no longer predicted their survival. While there were only nine patients with cardiac stage IIIb disease, GLS improvement was noted across all three stages suggesting that recovery of cardiac function is possible even among patients with advanced disease. The baseline GLS was lower in the GLS(+) group compared to the GLS(−) group. This difference in baseline GLS may be attributed to the fact that patients with low GLS succumbed to their condition before the 12-month assessment, while only those who showed improvement in GLS survived to the 1-year follow-up. Another possible explanation for this finding is that patients with normal or near-normal GLS at baseline had little room for improvement. As a result, when comparing those who improved vs. those who did not, they may not appear to have improved. Since the same criteria were applied to individuals with normal baseline values, the effect size on mortality for GLS(+) vs. GLS(−) may, in fact, be underestimated, as some of these baseline-normal individuals would be classified as GLS(−).
The findings of this study support and expand upon the current data regarding the role of GLS improvement as a prognostically significant marker following plasma-directed therapy.6,20 This study is distinct from prior publications in that the assessment of GLS improvement included all patients with a prognostically significant increase in circulating cardiac biomarker levels, as determined by the conventional staging system commonly used for risk stratification. Furthermore, the utilization of a BNP-based staging system in this study enhanced its overall applicability, considering that some centres do not provide NT-proBNP testing.
Study limitations
The study is limited by its single centre and retrospective design, with missing data points at 1 year due to patients lost to follow-up. However, the baseline characteristics of those lost to follow-up were similar to those included in the study. Patients who died before reaching 1 year following treatment were excluded, given that the focus of the paper is examining the significance of the GLS at one year.
Given the lack of GLS standardization and the inter-vendor variability in strain measurements, the cut-off value used in the present study to define GLS improvement may not be applicable when using a different vendor system. However, TomTec is platform-neutral software used by many centres, which reduces variation resulting from different vendor platforms. Similar to the study by Cohen et al.,6 our study also utilized a 2.0% change in GLS as a meaningful cut-off, which was also found to be prognostic, despite variations in GLS measurement techniques and software used. This study was unable to assess the effects of the treatment regimen on the study outcome, as there was a higher usage of SCT compared to other studies and the lack of specific data, such as dosage, on the regimens patients received. Additionally, this study was conducted prior to the advent of more recent, effective therapies, such as daratumumab-based treatment. It is conceivable that the predictive value of GLS on outcomes observed in this study may differ in the era of contemporaneous treatment. Further evaluation of determinants of GLS improvement and validation of the correlation between GLS improvement and clinical outcomes in large cohorts of patients treated with contemporary therapy would be warranted.
Conclusions
In summary, GLS improvement following plasma cell-directed therapy among patients with AL amyloidosis conferred a significant survival benefit, even in those with advanced disease, supporting its role as an important prognostic marker. GLS improvement was more likely to occur in patients with lower dFLC levels after treatment; however, further investigation is needed to define other determinants of GLS improvement. These findings suggest that GLS improvement may serve as an important marker for risk stratification and possibly treatment decision-making in AL amyloidosis patients undergoing therapy. Further investigation through a large multicentre trial is warranted.
Lead author biography

Jennifer Liu is the Chief of Cardiology at the Memorial Sloan Kettering Cancer Center and a Professor of Medicine at Weill Cornell Medical College. She is a recognized Cardio-Oncology specialist with expertise in advanced echocardiographic imaging. Her clinical and research endeavours focus on the role of advanced imaging in diagnosing and managing cancer therapy-related cardiac dysfunction and systemic amyloidosis with cardiac involvement. She has led several expert consensus documents that establish practice standards for the use of echocardiography in cardiotoxicity monitoring. She has served on the ACC Cardio-Oncology Leadership Council and the editorial board of JACC: CardioOncology. Currently, she is a member of the Board of Directors of the American Society of Echocardiography.
Supplementary Material
Contributor Information
Kristine H Jang, Department of Cardiology, Stony Brook University Hospital, Stony Brook, NY 11794, USA.
Anthony F Yu, Cardiology Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA; Department of Medicine, Weill Cornell Medical College, New York, NY 10065, USA.
Heather Landau, Department of Medicine, Weill Cornell Medical College, New York, NY 10065, USA; Adult Bone Marrow Transplant Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA.
Xiaoyue Ma, Dept of Biostatistics, Weill Cornell Medical College, New York, NY 10065, USA.
Richard K Cheng, Division of Cardiology, University of Washington, Seattle, WA 98195, USA.
Mathew S Mauer, Division of Cardiology, Columbia University Vagelos College of Physicians and Surgeons, New York, NY 10032, USA.
Katherine Lee Chuy, Cardiology Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA.
Daniel Lenihan, St Francis Healthcare, Cape Girardeau, MO 63703, USA; International Cardio-Oncology Society, Tampa, FL 33606, USA.
Ji Can Yang, Department of Cardiology, Northwell Health—Southshore University Hospital, Bay Shore Island, NY 11706, USA.
Carol L Chen, Cardiology Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA; Department of Medicine, Weill Cornell Medical College, New York, NY 10065, USA.
Jennifer E Liu, Cardiology Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA; Department of Medicine, Weill Cornell Medical College, New York, NY 10065, USA.
Data availability
The datasets generated and/or analysed during this study are available from the corresponding author upon reasonable request.
Supplementary material
Supplementary material is available at European Heart Journal Open online.
Funding
National Cancer Institute Cancer Center Support Grant P30CA008748 (A.F.Y., H.L., C.L.C., J.E.L.).
References
- 1. Muchtar E, Gertz MA, Kumar SK, Lacy MQ, Dingli D, Buadi FK, Grogan M, Hayman SR, Kapoor P, Leung N, Fonder A, Hobbs M, Hwa YL, Gonsalves W, Warsame R, Kourelis TV, Russell S, Lust JA, Lin Y, Go RS, Zeldenrust S, Kyle RA, Rajkumar SV, Dispenzieri A. Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: cracking the glass ceiling of early death. Blood 2017;129:2111–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Palladini G, Dispenzieri A, Gertz MA, Kumar S, Wechalekar A, Hawkins PN, Schönland S, Hegenbart U, Comenzo R, Kastritis E, Dimopoulos MA, Jaccard A, Klersy C, Merlini G. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol 2012;30:4541–4549. [DOI] [PubMed] [Google Scholar]
- 3. Lee Chuy K, Drill E, Yang JC, Landau H, Hassoun H, Nahhas O, Chen CL, Yu AF, Steingart RM, Liu JE. Incremental value of global longitudinal strain for predicting survival in patients with advanced AL amyloidosis. JACC CardioOncol 2020;2:223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pun SC, Landau HJ, Riedel ER, Jordan J, Yu AF, Hassoun H, Chen CL, Steingart RM, Liu JE. Prognostic and added value of two-dimensional global longitudinal strain for prediction of survival in patients with light chain amyloidosis undergoing autologous hematopoietic cell transplantation. J Am Soc Echocardiogr 2018;31:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buss SJ, Emami M, Mereles D, Korosoglou G, Kristen AV, Voss A, Schellberg D, Zugck C, Galuschky C, Giannitsis E, Hegenbart U, Ho AD, Katus HA, Schonland SO, Hardt SE. Longitudinal left ventricular function for prediction of survival in systemic light-chain amyloidosis: incremental value compared with clinical and biochemical markers. J Am Coll Cardiol 2012;60:1067–1076. [DOI] [PubMed] [Google Scholar]
- 6. Cohen OC, Ismael A, Pawarova B, Manwani R, Ravichandran S, Law S, Foard D, Petrie A, Ward S, Douglas B, Martinez-Naharro A, Chacko L, Quarta CC, Mahmood S, Sachchithanantham S, Lachmann HJ, Hawkins PN, Gillmore JD, Fontana M, Falk RH, Whelan CJ, Wechalekar AD. Longitudinal strain is an independent predictor of survival and response to therapy in patients with systemic AL amyloidosis. Eur Heart J 2022;43:333–341. [DOI] [PubMed] [Google Scholar]
- 7. Lilleness B, Doros G, Ruberg FL, Sanchorawala V. Establishment of brain natriuretic peptide—based criteria for evaluating cardiac response to treatment in light chain (AL) amyloidosis. Br J Haematol 2020;188:424–427. [DOI] [PubMed] [Google Scholar]
- 8. Lilleness B, Ruberg FL, Mussinelli R, Doros G, Sanchorawala V. Development and validation of a survival staging system incorporating BNP in patients with light chain amyloidosis. Blood 2019;133:215–223. [DOI] [PubMed] [Google Scholar]
- 9. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt J-U. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 10. Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, Galderisi M, Marwick T, Nagueh SF, Sengupta PP, Sicari R, Smiseth OA, Smulevitz B, Takeuchi M, Thomas JD, Vannan M, Voigt J-U, Zamorano JL. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr 2011;24:277–313. [DOI] [PubMed] [Google Scholar]
- 11. Kastritis E, Palladini G, Minnema MC, Wechalekar AD, Jaccard A, Lee HC, Sanchorawala V, Gibbs S, Mollee P, Venner CP, Lu J, Schönland S, Gatt ME, Suzuki K, Kim K, Cibeira MT, Beksac M, Libby E, Valent J, Hungria V, Wong SW, Rosenzweig M, Bumma N, Huart A, Dimopoulos MA, Bhutani D, Waxman AJ, Goodman SA, Zonder JA, Lam S, Song K, Hansen T, Manier S, Roeloffzen W, Jamroziak K, Kwok F, Shimazaki C, Kim J-S, Crusoe E, Ahmadi T, Tran NP, Qin X, Vasey SY, Tromp B, Schecter JM, Weiss BM, Zhuang SH, Vermeulen J, Merlini G, Comenzo RL. Daratumumab-based treatment for immunoglobulin light-chain amyloidosis. N Engl J Med 2021;385:46–58. [DOI] [PubMed] [Google Scholar]
- 12. Smiseth OA, Torp H, Opdahl A, Haugaa KH, Urheim S. Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J 2016;37:1196–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Singh A, Voss WB, Lentz RW, Thomas JD, Akhter N. The diagnostic and prognostic value of echocardiographic strain. JAMA Cardiol 2019;4:580–588. [DOI] [PubMed] [Google Scholar]
- 14. Stanton T, Leano R, Marwick TH. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging 2009;2:356–364. [DOI] [PubMed] [Google Scholar]
- 15. Barros-Gomes S, Williams B, Nhola LF, Grogan M, Maalouf JF, Dispenzieri A, Pellikka PA, Villarraga HR. Prognosis of light chain amyloidosis with preserved LVEF: added value of 2D speckle-tracking echocardiography to the current prognostic staging system. JACC Cardiovasc Imaging 2016;10:398–407. [DOI] [PubMed] [Google Scholar]
- 16. Palladini G, Lavatelli F, Russo P, Perlini S, Perfetti V, Bosoni T, Obici L, Bradwell AR, D'Eril GM, Fogari R, Moratti R, Merlini G. Circulating amyloidogenic free light chains and serum N-terminal natriuretic peptide type B decrease simultaneously in association with improvement of survival in AL. Blood 2006;107:3854–3858. [DOI] [PubMed] [Google Scholar]
- 17. Merlini G, Palladini G. Light chain amyloidosis: the heart of the problem. Haematologica 2013;98:1492–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sapp V, Jain M, Liao R. Viewing extrinsic proteotoxic stress through the lens of amyloid cardiomyopathy. Physiology (Bethesda) 2016;31:294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abraham RS, Geyer SM, Price-Troska TL, Allmer C, Kyle RA, Gertz MA, Fonseca R. Immunoglobulin light chain variable (V) region genes influence clinical presentation and outcome in light chain-associated amyloidosis (AL). Blood 2003;101:3801–3808. [DOI] [PubMed] [Google Scholar]
- 20. Salinaro F, Meier-Ewert HK, Miller EJ, Pandey S, Sanchorawala V, Berk JL, Seldin DC, Ruberg FL. Longitudinal systolic strain, cardiac function improvement, and survival following treatment of light-chain (AL) cardiac amyloidosis. Eur Heart J Cardiovasc Imaging 2016;18:1057–1064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during this study are available from the corresponding author upon reasonable request.