Abstract
We grouped the fossil records of marine animal genera into suites defined by function and physiology. The stratigraphic coherence of the resulting diversity history indicates the importance of ecological structure in constraining taxonomic richness through time. The proportional representation of major functional groups was stably maintained for intervals as long as 200 million years, despite evolutionary turnover and changes in total diversity. Early Paleozoic radiations established stable ecosystem relationships, and thereafter only the great era-bounding mass extinctions were able to break patterns of incumbency, permitting the emergence of new community structures with distinct proportional diversity relationships.
Fossils provide insights into the evolution of animal morphology and function, whereas compilations of biostratigraphic data (refs. 1 and 2 and similar compilations) allow us to explore the history of biological diversity. Surprisingly, given the rich interpretational possibilities, attempts to integrate function and diversity through time have been limited, especially in studies of whole faunas (3–7).
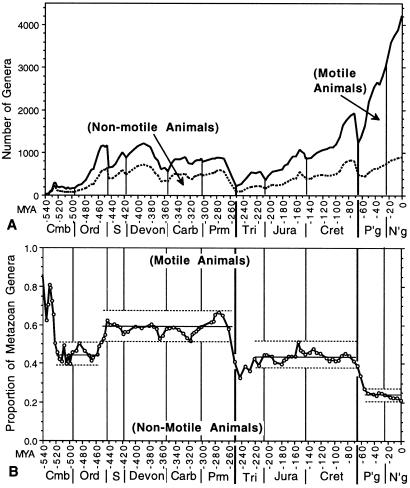
There is broad consensus on the overall trajectory of Phanerozoic marine animal diversity (refs. 7–10, but see refs. 11 and 12). This pattern is most famously depicted in Sepkoski's compilation of marine family diversity through time (13), but is equally clear in our tabulation of his data on marine genera (Fig. 1A). After several phases of diversification spanning the latest Proterozoic through Middle Ordovician, taxonomic richness of preserved marine animals fluctuated, but exhibited no secular trend toward either increase or decrease, for the next 200 million years (Myr). Following the catastrophic end-Permian extinction, marine diversity has increased nearly continuously for more than 200 Myr, with only transitory setbacks.
Figure 1.
Comparison of genus diversity and proportional diversity of motile (active) and nonmotile (passive) marine metazoans through the Phanerozoic Eon; groups designated in Table 1. (A) Genus diversity of the two groups. The data are number of genera (and subgenera of molluscs) crossing interval boundaries. The top line represents the total genus diversity of tabulated marine animals. The dotted line divides the nonmotile group (below) from the motile group (above). (B) The proportion of marine metazoan genus diversity comprising nonmotile taxa. (The proportion of motile marine genera is the complement.) Light horizontal lines bracket the range of proportion and the solid line indicates mean proportion over each of the four intervals of stability discussed in the text. Because our interest is in the long intervals of restricted range of proportion of nonmotile taxa rather than the determination of whether any one value is or is not statistically significantly different from another value, we do not show 95% confidence intervals around each datum. However, confidence intervals are all narrow (ranging from ±0.08 units, or 8%, for a Cambrian interval with only 149 genera to ±0.016 units for a Cenozoic interval with 3,018 genera). Confidence intervals for a majority of the data in each “stable interval” do not overlap with those in adjacent stable intervals, although individual extreme values in different stable intervals may have overlapping confidence intervals. More apropos for the subject of this paper, the Mann–Whitney U test demonstrates that the suite of proportions in each stable interval differs from that in the succeeding or preceding interval with P < 0.001. This is not surprising because the values in each stable interval do not overlap those of the adjacent intervals. There are many groups in each category (Table 1) and those taxa vary independently in diversity through time, with great diversity “turnover” within each stable interval. Diversity fluctuates or increases by a factor of two or more during each stable interval, more than enough to permit marked change in the proportional relations noted here, especially given the independence of the many lineages involved. Also, the transitions that do occur between stable intervals take place in as little time as two intervals (from the start of the Mid Caradoc to the end of the Late Caradoc, from the start of the Danian to the end of the Paleocene). These three factors together demonstrate that, although the data are from the time series of the history of marine diversity, autocorrelation alone was not a major factor in maintaining the narrow range of proportions characterizing each stable interval. To address any concerns about autocorrelation, however, we calculated the Mann–Whitney U test for every other datum in each stable interval (valid in this case because none of the values in any stable interval overlap with those of the adjacent intervals to which they are being compared, so summed ranks for any group of half the data in each interval would produce the same result). The adjacent stable intervals are still highly significantly different (P < 0.001). The same statistical points hold, in general, for the other two analyses (physiological grouping and proportion of predators) presented below.
In a pioneering attempt to identify substructure in this diversity record (13), Sepkoski performed a factor analysis on the numbers of families within classes to identify the classes that contributed most to total diversity at different times. The first three factors grouped the classes of marine animals into three faunal suites (termed “evolutionary faunas” by Sepkoski) that have successively dominated the marine realm over the past 543 Myr. No biological criteria were used to group taxa a priori, and the only unifying biological attribute suggested by the study was a similar level of evolutionary volatility among members of each fauna. Sepkoski used this idea to model the dynamics of faunal succession, using coupled logistic equations borrowed from population biology, with the assumptions that each fauna had an intrinsic rate of origination and diversity-dependent variation in rate of extinction (14–16). In that analysis, faunal succession emerged as an inevitable consequence of taxonomic birthrate and nothing else; mass extinctions perturbed diversity, but had no effect on the ultimate fate of each evolutionary fauna (16).
In this paper, we revisit diversity history, but from a functional perspective. Using Sepkoski's genus database (unpublished compilation of the stratigraphic ranges of 40,859 genera transferred to Bambach in May, 1996), we bin taxa a priori by using morphological and physiological criteria, rather than simply recognizing groups of taxa retrospectively through their changing contributions to total diversity. The purpose is to determine whether any consistent functional patterns are associated with diversity change through time.
Morphological/Functional Grouping of Taxa
We conducted two analyses based on features of functional morphology and physiology of adult organisms that can be inferred reliably from fossil remains. For the morphological/functional analysis we grouped most metazoan taxa as either passive (nonmotile) or active (motile) organisms (Table 1). Passive organisms are either sedentary benthos or nonswimming plankton. Active organisms include all taxa capable of independent and regular locomotion, by whatever means. Some of these may remain quiescent for extended periods (deep-burrowing bivalves, for example), but all can move if disturbed. Assignment of taxa to these groups is generally straightforward. We did not comb the genus lists for exceptions within the taxonomic subdivisions chosen, but those exceptions make up a minuscule fraction of the total. Over 95% of the marine metazoan genera in the Sepkoski database are included in our tabulation.
Table 1.
Higher taxa assigned to the two morphological/functional groups discussed in the text
| Passive (nonmobile) animals | Active (self-mobile) animals |
|---|---|
| Porifera, P, m | Various molluskan groups: Rostroconchs, Hyolithids, C |
| Archaeocyatha, C | Gastropods, p, M |
| Anthozoa (corals), P, m | Infaunal bivalves, p, M |
| Bryozoa, P, M | Cephalopods, P, m |
| Brachiopods, C, P | Polychaetes, C, p, M |
| Epifaunal (attached and cemented) bivalves, p, M | Arthropods, C, P, M |
| Pelmatazoan echinoderms, P | Echinoids, M |
| Graptolites, c, p | Conodonts, p |
| Tube-dwelling incertae sedis, c | Chordates, M |
Letters following taxon names identify the categories to which their containing classes were assigned in Sepkoski's 1981 analysis (13). C, factor I (the Cambrian Fauna); P, factor II (the Paleozoic Fauna); M, factor III (the Modern Fauna). The variation in factor loadings of different classes in Sepkoski's factor analysis is noted by uppercase letters (for major contribution to that evolutionary fauna) and lowercase letters (moderate contribution to that evolutionary fauna).
Our morphological/functional groupings represent two fundamentally distinct functional systems. Passive organisms necessarily depend on the direct delivery of food. Also, because they cannot move, passive animals must cope with environmental perturbation by endurance rather than migration to a more sheltered location. In contrast, active organisms can and do fulfill many different modes of life, from some that are as passive as nonmotile taxa to those involving rapid motion or long distance migration. In effect, the passive group represents one “superguild” (17), whereas the active group includes many functional types. Comparing the diversity of these groupings through time can, thus, reveal whether functional “flexibility” or variety is associated with the maintenance or increase of diversity.
Fig. 1A shows the genus diversity of these two groupings through the Phanerozoic. Summed together, the diversity histories of the two groups display the consensus diversity pattern, but we focus here on the changing proportional contributions of the groups to that total diversity.
Fig. 1B shows the proportion of the marine fauna comprising passive taxa (active animals make up the complement). A clear four-phase faunal succession emerges, with different, but persistent, relationships of overall diversity, diversification rate, and proportional representation characterizing each successive interval. The majority of individual ratios in each of the four intervals discussed below are significantly different from those of adjacent intervals, and the suites of ratios in each interval are highly significantly different from adjacent intervals, as noted in the caption to Fig. 1B. The results display a stratigraphic coherence equal to that of Sepkoski's three evolutionary faunas (13), but the pattern reported here is based on a priori biological binning of taxa rather than a posteriori aggregation.
The first 100 Myr of the Paleozoic include the Cambrian “Explosion,” the interval when many phyla and classes first appeared in the fossil record; a Middle and Late Cambrian diversity plateau; and the Ordovician radiation, an interval of renewed diversification when the classes that dominate Sepkoski's Paleozoic Fauna became diverse and those in his Cambrian Fauna began to fade (Fig. 1).
The precise stratigraphic ranges of many Early Cambrian genera remain uncertain (18, 19), making detailed claims about early diversity patterns risky. Our biological understanding of the Cambrian fauna is also limited because many forms belong to extinct clades. However, it is a fairly straightforward process to evaluate whether most taxa were passive (nonmotile) or active (motile). Therefore, we can follow function/morphology from the Cambrian onward.
Because of the variety of sedentary tubular “small shelly” fossils and sponge-like archaeocyathans (which may be taxonomically over-split), passive animals dominate compilations of Early Cambrian diversity. With the onset of trilobite diversification in the Atdabanian, however, and, especially, with the collapse of archaeocyathans in the late Early Cambrian, passive and active animals became nearly equal in diversity. For the ensuing 60-Myr interval, from the Middle Cambrian through the Llandeilian (mid-Middle Ordovician; Fig. 1), proportional diversity remained within a narrow range (mean 44.3% nonmotile taxa, n = 14), despite the transition from the low and nearly stable diversity of the later Cambrian plateau to the Ordovician radiation. Although genus-level diversity increased by over 600% during this interval, the proportion of passive to active animals did not trend outside the range established in the Middle and Late Cambrian; neither did proportional diversity ever rise into the range it would occupy from the Late Caradocian (end Middle Ordovician) through the Permian.
As total genus diversity leveled off in the Caradocian, active animals actually began to lose genera. In consequence, the proportional representation of passive animals increased, establishing a new diversity dominance of passive animals by the end of the Ordovician Period. It remains unclear whether late Ordovician mass extinction completed this transition or simply strengthened a change already, by and large, accomplished. The proportion of passive animals shifted sharply higher in the extinction, but for the three previous intervals it had already been above any proportional representation seen in the Middle Cambrian through Llandeilian. In any event, the proportion of total metazoan diversity comprising passive taxa remained near 60% (mean 58%, n = 36) through the bulk of the post-Caradoc Paleozoic, with only brief excursions of a few percentage points above or below that balance (Fig. 1B).
The end-Permian mass extinction reduced diversity dramatically, with passive taxa suffering markedly higher extinction than the active taxa—essentially reversing the diversity relationship of the two groups. This proportion did not stay fixed, however; our data reveal passive taxa diversified faster than the active taxa as both groups recovered in the Triassic. The proportion of passive taxa, which was less than 38% in the earliest Triassic, reached 43% by the middle of the Late Triassic (end of the Carnian) and remained near that level (mean 44%, n = 26) for the rest of the Mesozoic, even as diversity of both active and passive taxa continued to increase (Fig. 1). During the Mesozoic and Cenozoic the proportional diversity of passive taxa has never been as great as the lowest values recorded from the Late Ordovician through the rest of the Paleozoic.
The end-Cretaceous extinction event did not have the same selective effect as the end-Permian event. Diversity decreased almost equally in both groups, and passive taxa still comprised about 39% of the diversity of metazoan genera at the start of the Cenozoic. During the Paleocene (earliest Paleogene), however, active taxa diversified far more rapidly than passive taxa. By the start of the Eocene, active taxa comprised 73% of metazoan diversity and passive taxa only 27%. During the rest of the Cenozoic this 3:1 proportional relationship (not seen at any previous time) has stayed remarkably stable, given the continuous increase in diversity recorded over the last 55 Myr.
In summary, then, the proportional representation of active and passive taxa in ancient oceans does not track total diversity in any simple way. Rather, the record is characterized by four long intervals of stability separated by brief transitions.
Physiological/Anatomical Grouping
In previous research, Knoll and others (20, 21) proposed and sought to test a novel kill mechanism for end-Permian mass extinction. The kill mechanism, hypothesized on geological grounds, was catastrophic CO2 increase (resulting in hypercapnia). The plausibility of this mechanism was tested by dividing the suite of marine genera alive in the Permian into two groups based on attributes of anatomy and physiology in the adult animals that could be inferred reliably from fossils. Group I consisted of animals characterized by low rates of metabolism, limited internal circulation, gas exchange across little differentiated or undifferentiated body surfaces, and massive investment in CaCO3 skeletons—features expected to increase vulnerability to hypercapnic stress. Of Group I genera present in the Late Permian, 79% disappeared at the P-Tr boundary. In contrast, Group II comprised animals expected to be less vulnerable to hypercapnia, animals characterized by relatively high metabolic demand, well developed gills and circulatory systems that aid in physiological regulation, and skeletons limited in mass or made of materials other than CaCO3. Group II lost only 27% of its genera at the end of the Permian Period.
The strong selectivity of the end-Permian mass extinction may not have been related equally to each of the physiological and anatomical attributes that differentiate the two groups. Nor is it likely that CO2 stress was chronic throughout the Phanerozoic. Nonetheless, Groups I and II identify distinct and biologically coherent suites of animals based on physiological responses to certain types of environmental perturbation. Animals in Group I can be regarded as open systems, vulnerable to or “unbuffered” against a range of chemically related physiological stresses. In contrast, those in Group II comprise closed, physiologically “buffered” systems expected to be less vulnerable to ambient chemical insult. We combined all fossil taxa with the same sets of traits and charted their Phanerozoic diversity histories. Taxa for which we can make no confident physiological interpretation because living representatives are few or absent were grouped separately. Table 2 lists the three groups of taxa, which we term physiologically unbuffered, physiologically buffered, and physiologically indeterminate. We treat only the two physiologically understood groups in this analysis.
Table 2.
Higher taxa assigned to each of the three physiological groups discussed in the text
| Physiologically “unbuffered” taxa | Physiologically “buffered” taxa | Physiologically indeterminate taxa |
|---|---|---|
| Calcareous forams | Textulariina | Archaeocyatha |
| Calcareous sponges, p, m | Radiolaria | Conularida |
| Anthozoa (corals), P, m | Siliceous sponges, p, M | Problematica |
| Stenolaemata, P, m | Ctenostomata, M | Inarticulata (brachiopoda), C |
| Cheilostomata, M | Prosobranchia, P, M | Tergomya, C |
| Articulata (brachiopoda), P | Opisthobranchia, P, M | Hyolitha, C |
| Epifaunal bivalvia, P, M | Infaunal bivalvia, P, M | Helcionellida |
| Ammonoidea, P, m | Nautiloidea, P, m | Mollusca incertae sedis |
| Belemnitida, P, m | Polychaeta, C, p, M | Rostroconchia |
| Ostracoda, P | Eurypterida, c, p | Arthropoda incertae sedis |
| Rhombifera, p | Malacostraca, c, M | Trilobita, C |
| Diploporita, p | Conodontophorida, c, p | Archaeocopida (ostracoda), P |
| Blastoidea, p | Agnathan chordates | |
| Crinoidea, P | Placodermi and acanthodii | |
| Echinoidea, M | Chondrichthyes, M | |
| Graptolithina, c, p | Osteichthyes, M |
Details of criteria for assignment available in ref. 21. Letters following taxon names identify the categories to which their containing classes were assigned in Sepkoski's 1981 analysis (13), as noted in Table 1. Boldface indicates the highest loadings. No letter means the group was not explicitly designated in Sepkoski's analysis.
Because the physiologically indeterminate group dominated Cambrian and Early Ordovician diversity, we start our analysis in the Caradocian, at the end of the Ordovician Radiation. At this time, physiologically well characterized taxa comprise a strong majority (70%) of all marine diversity recorded in Sepkoski's dataset. Physiologically understood taxa reached 86% of the total fauna by the Mid Devonian and generally exceeded 90% from the Early Carboniferous onward. Although there is considerable “cross-linkage” of the morphological and anatomical/physiological analyses, they use different criteria to parse taxa, do not comprise identical lists of higher taxa, and are not necessarily parallel.
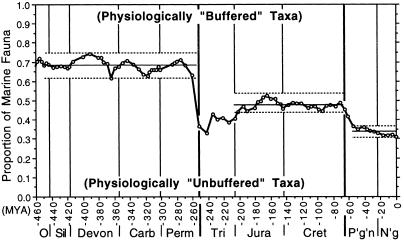
Mirroring the results of the morphological parsing, the diversity ratio of physiologically unbuffered to buffered taxa remained close to 2:1 over the 200-Myr interval from the Caradocian to the Guadelupian in the later Permian (Fig. 2). Physiologically unbuffered taxa comprise between 63 and 74% of physiologically understood genera throughout this interval, despite continual turnover and strong fluctuations in total diversity.
Figure 2.
The proportion of tabulated marine genera comprising physiologically unbuffered taxa of marine organisms from the Caradocian (Late Mid Ordovician) to the Recent. The proportion of physiologically buffered marine organisms is the complement. Taxa were assigned to the two groups based on predicted sensitivity to hypercapnia, as noted in the text (see Table 2). Light horizontal lines bracket the range of proportion and the solid line shows the mean proportion over each of the three post-Caradoc intervals of stability discussed in the text.
The end-Permian extinction (20, 21) was selectively catastrophic for unbuffered taxa (as expected, given the basis of this analysis), resulting in a transient reversal in proportional dominance between the physiologically unbuffered and buffered groups (Fig. 2). However, this new proportional structure did not persist, nor did the relationship between the two groups revert to the Paleozoic pattern. The end-Permian extinction event “broke the mold” of Paleozoic diversity structure for taxa grouped by physiological parameters, just as it had for taxa grouped by morphological/functional criteria. In its wake, the physiologically buffered group ended up as the diversity-dominants in the Early Triassic, with over 60% of the total genera. Because the physiologically unbuffered taxa diversified at a slightly faster rate, however, the two groups had become nearly equal in diversity by the Early Jurassic. Thereafter, the two groups diversified in lockstep until the end of the Cretaceous, maintaining a nearly stable diversity ratio close to 1:1 for 135 Myr (Fig. 2).
The end-Cretaceous extinction did not have the same selectivity as the end-Permian event (Fig. 2); however, it destroyed the Mesozoic pattern of proportional diversity, just as it had for the morphological/functional grouping. During the Cenozoic both groups resumed diversification, but now at markedly different rates. The physiologically buffered group, which accounted for only 30% of total diversity during the Paleozoic and about 50% during most of the Mesozoic, dominated diversity in Cenozoic faunas. And although the rates of diversification of the two groups were different in the Cenozoic, they became proportionally linked during the Paleocene and have remained close to a 1:2 balance over the past 55 Myr (Fig. 2).
A Note on Sampling Bias
The Paleozoic and post-Paleozoic intervals of stable proportional diversity are interesting because they represent both times of nontrending total diversity and times of diversity increase. There is some concern that the Mesozoic and, especially, Cenozoic records may be influenced by the “pull of the Recent” (22), which is likely to increase systematically toward the present. However, data on within habitat species richness (23) show that Neogene fossil assemblages average twice the species richness of Cretaceous assemblages, confirming that observed Cenozoic diversity increase does not simply reflect biases such as an increase in preserved rock volume. Also, although Alroy and others (11) recently suggested that the Cenozoic diversity increase may not be as large as currently thought, the techniques used in their analyses turn out to not account for all sources of diversity (24). Other views include those of Jackson and Johnson (12), who have recently argued that diversity may have increased more than recorded in the Sepkoski database. Regardless of the outcome of debate about overall Cenozoic diversity history, the pattern of proportional diversity should not be subject to stratigraphic sampling bias. Most Mesozoic and Cenozoic fossils belong to well skeletonized taxa that have reliable fossil records (25–28). Thus, any effect from the “pull of the Recent” should extend across all taxa, affecting the data proportionately.
Two other concerns require comment. One is the possibility of systematic change in preservation potential that might create the trend we observe. Some taphonomic windows have indeed changed through time, notably a secular decline in skeletal silicification (29); however, such changes would likely bias preservation against lightly calcified motile organisms. Much of the actual observed increase in motile taxa occurs among fish and malacostracans, taxa that have a generally lower preservation potential than heavily skeletonized sedentary taxa. Moreover, in a review of Lagerstätten that preserve soft parts, Bambach (23) found no general increase in nonskeletal taxa during the Phanerozoic.
A second concern is that secular change within the higher taxa considered might work against our observations. But the known shifts of this sort [the increase in motile over nonmotile gastropods noted by Linsley (30) and the evolution of the motile comatulid crinoids in the post-Paleozoic are examples] enhance, not reduce, the trend we document. Thus, changing preservational biases would serve to bias the record against the patterns documented here and known functional shifts within higher taxa make our conclusions conservative.
Discussion
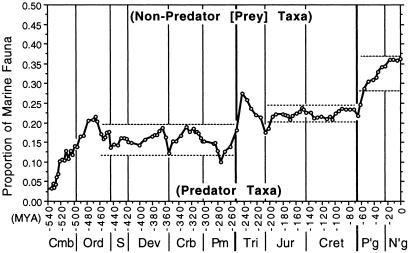
At least three general questions are raised by our observations of faunal succession. (i) Why was there an apparent ceiling on diversity in the Paleozoic, but no similar limitation evident in younger eras? (ii) What maintains the proportionality of diversity within narrow limits over long intervals of time between groups defined on either functional/morphological or anatomical/physiological grounds? (iii) Why does this proportionality change only at the ends of the two Early Paleozoic radiations and after the great era-bounding mass extinctions? We suggest that the answers to all three questions relate to evolutionary inertia imparted by the ecological structure of marine communities. Note that the groupings of taxa used here are biologically informative, but they are not the only subdivisions possible. The primary importance of ecological constraint and the breaking of incumbency by novel radiations or mass extinction will likely be observable in other functionally informed parsings of diversity data as well. To cite just one example, Bambach (31) has shown that the proportional representation of predators through time conforms to the stratigraphic pattern documented in this paper (Fig. 3).
Figure 3.
The proportion of tabulated marine genera comprising predators through the Phanerozoic Eon. [The proportion of nonpredator (“prey”) taxa is the complement; data discussed in detail in ref. 31.] Light horizontal lines bracket the range of proportion over the three post-Caradoc intervals of stability of proportion similar to those discussed for the functional and physiological groupings documented in the text. Note that the y-axis scale only extends to 0.50, not 1.00 as in Figs. 1B and 2. Predators are generally motile taxa, which as a group increase in proportion of metazoan diversity at the expense of nonmotile forms (Fig. 1B). Nonpredator taxa also include motile organisms, however, so changes in the proportional representation of predators do not simply reflect the changing proportion of motile taxa.
The diversity relationships observed here appear as a global balance between groupings of disparate taxa, but they are, in fact, composites—the sum of local ecosystems, where the principal determinants of the pattern must lie (32). One important control appears to be incumbency, the constraints that existing populations impose on the survival probability of new variants (33–36). At some level, incumbency of the groups that determine ecosystem structure must underlie both the Paleozoic plateau of total diversity and the long intervals of fixed proportionality of diversity, present even when total diversity was changing.
New stable systems appear after globally pervasive evolutionary events, either major radiations of new taxa or recovery from devastating extinctions. As discussed below, the two post-Paleozoic intervals of faunal stability appear to reflect the rebuilding of ecosystems following mass extinction. Paleozoic stable intervals may have been somewhat influenced by the Botomian and end-Ordovician mass extinctions, but they also reflect “de novo ” ecosystem construction in the Early Cambrian and the addition of diversity from new and functionally varied taxa during the Ordovician radiation. In both instances entirely new ecosystem constituents became important parts of the biota, facilitating the development of novel community structures in all habitats.
Most of the post-Paleozoic increase in marine animal diversity is nested within motile animals and, in the physiological parsing, within physiologically well buffered taxa. This observation can be rationalized in terms of the greater anatomical and physiological potential of these animals for invading and using ecospace. Despite the expected evolutionary versatility of these taxa, however, changes in proportional representation in the post-Paleozoic are confined in both analyses to the immediate aftermaths of era-ending mass extinctions. This strongly suggests that diversity is constrained not only by the incumbency of well adapted clades, but also by the persistence of guild structure at the community level (4, 17). In this view, the two great era-bounding mass extinctions are not simply perturbations with limited lasting effects on diversity, as inferred from Sepkoski's original three-faunal analysis (16). Rather, they engendered fundamental reorganization of the ecological structure on which diversity is built and maintained. Apparently, only the near total disruption of this biological fabric opens the door to reorganization. [Bottjer et al. (37) have proposed that mass extinctions knocked ecosystems back to some previously existing level of organization (see also ref. 38). In contrast, our data indicate that in the wake of era-closing mass extinctions, ecology was reorganized in ways that differed substantially from either the immediate or remote past.]
Note, however, that extinction events as such did not create a new stable balance in proportional diversity. The end-Permian and end-Cretaceous events left the proportional relations between groups at very different levels, but in neither case did the diversity ratio of survivors characterize the bulk of the succeeding era. New stable faunal relationships must have been established during the postextinction recovery intervals, as expanding populations of survivors began to interact in new ways, just as happened with the initial metazoan radiations in the Early Paleozoic. At these times of ecological reorganization, morphological, functional, and physiological potential could be realized.
For example, the benthic suspension-feeders that radiated after the Permo-Triassic extinction were, on average, larger and of higher nutritional quality than their dominant Paleozoic counterparts, perhaps facilitating diversification of specialized predators (39). Co-evolutionary interactions related to specialized feeding, the related “evolutionary arms race” that accelerated in the late Mesozoic and Cenozoic (4, 5, 40), and, perhaps, increasing nutritional contributions to the oceans from diversifying flowering plants (6) may combine to explain the continuing post-Paleozoic increase in taxonomic richness. New community types may also reflect the evolution of increased specialization, further contributing to increase in diversity (4, 6, 17, 26).
On the time scales studied by population biologists, feedback between ecology and evolution is widely acknowledged. The diversity relationships reported here—of long term continuity in the dynamic equilibrium between functionally distinct biotic systems and the uniqueness of the two events that altered the balance of that equilibrium—indicate that ecological/evolutionary feedback is just as important on the geological time scales observed by paleontologists. In highlighting the importance of functional and metabolic characters along with ecological structure in the long term diversification of marine faunas, paleontological investigations of diversity history provide a potentially fertile meeting ground for research on organismic structure and function, macroecology (41), and macroevolution.
Acknowledgments
This paper began as a collaborative effort with the late Jack Sepkoski. We thank Christine Janis and David Sepkoski for authorizing the posthumous inclusion of Jack as an author. Arnold Miller and Douglas Erwin made useful suggestions for improving the manuscript. We thank Arnold Miller and Charles Marshall for advice and assistance with the statistical analyses. A.H.K. acknowledges support from the NASA Astrobiology Institute.
Abbreviation
- Myr
million years
References
- 1.Sepkoski J J., Jr . A Compendium of Fossil Marine Families. 2nd Ed. Milwaukee Pub. Mus., Milwaukee, WI: Contrib. in Biol. and Geol. 83; 1992. [PubMed] [Google Scholar]
- 2.Benton M J, editor. The Fossil Record 2. London: Chapman & Hall; 1993. [Google Scholar]
- 3.Thayer C W. In: Biotic Interactions in Recent and Fossil Benthic Communities. Tevesz M, McCall P, editors. New York: Plenum; 1983. pp. 479–625. [Google Scholar]
- 4.Bambach R K. In: Phanerozoic Diversity Patterns. Valentine J W, editor. Princeton, NJ: Princeton Univ. Press; 1985. pp. 191–253. [Google Scholar]
- 5.Vermeij G J. Evolution and Escalation. Princeton, NJ: Princeton Univ. Press; 1987. [Google Scholar]
- 6.Bambach R K. Geobios. 1999;32:131–144. [Google Scholar]
- 7.Sepkoski J J, Jr, Bambach R K, Raup D M, Valentine J W. Nature (London) 1981;293:435–437. [Google Scholar]
- 8.Sepkoski J J., Jr Paleobiology. 1993;19:43–51. doi: 10.1017/s0094837300012306. [DOI] [PubMed] [Google Scholar]
- 9.Benton M J. Science. 1995;268:52–58. doi: 10.1126/science.7701342. [DOI] [PubMed] [Google Scholar]
- 10.Sepkoski J J., Jr J Paleontol. 1997;71:533–539. doi: 10.1017/s0022336000040026. [DOI] [PubMed] [Google Scholar]
- 11.Alroy J, Marshall C R, Bambach R K, Bezusko K, Foote M, Fürsich F T, Hansen T A, Holland S M, Ivany L C, Jablonski D, et al. Proc Nat Acad Sci USA. 2001;98:6261–6266. doi: 10.1073/pnas.111144698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson J B C, Johnson K G. Science. 2001;293:2401–2404. doi: 10.1126/science.1063789. [DOI] [PubMed] [Google Scholar]
- 13.Sepkoski J J., Jr Paleobiology. 1981;7:36–53. [Google Scholar]
- 14.Sepkoski J J., Jr Paleobiology. 1978;4:223–251. [Google Scholar]
- 15.Sepkoski J J., Jr Paleobiology. 1979;5:222–251. [Google Scholar]
- 16.Sepkoski J J., Jr Paleobiology. 1984;10:246–267. [Google Scholar]
- 17.Bambach R K. In: Biotic Interactions in Recent and Fossil Benthic Communities. Tevesz M, McCall P, editors. New York: Plenum; 1983. pp. 719–746. [Google Scholar]
- 18. Porter, S. (2001) Lethaia, in press.
- 19.Babcock L. PaleoBios. 2001;21,Suppl.:27. [Google Scholar]
- 20.Knoll A H, Bambach R K, Canfield D E, Grotzinger J P. Science. 1996;273:452–457. [PubMed] [Google Scholar]
- 21. Bambach, R. K. & Knoll, A. H. (2001) Paleobiology, in press.
- 22.Raup D M. Bull Carnegie Mus Hat Hist. 1979;13:85–91. [Google Scholar]
- 23.Bambach R K. Paleobiology. 1977;3:152–167. [Google Scholar]
- 24. Bush, A. M., Markey, M. J. & Marshall, C. R. (2002) Paleobiology, in press.
- 25.Schopf T J M. Paleobiology. 1978;4:261–270. [Google Scholar]
- 26.Valentine J W. Paleobiology. 1989;15:83–94. [Google Scholar]
- 27.Donovan S K, Paul C R C, editors. The Adequacy of the Fossil Record. New York: Wiley; 1998. [Google Scholar]
- 28.Foote M, Sepkoski J J., Jr Nature (London) 1999;398:415–417. doi: 10.1038/18872. [DOI] [PubMed] [Google Scholar]
- 29.Schubert J K, Kidder D L, Erwin D H. Geology. 1997;25:1031–1034. [Google Scholar]
- 30.Linsley R M. Malacologia. 1978;17:193–206. [Google Scholar]
- 31.Bambach R K. In: The Fossil Record of Predation. Kowalewski M, Kelley P H, Dodson P, editors. Vol. 7: Paleontological Soc. Papers; 2002. , in press. [Google Scholar]
- 32.Sepkoski J J., Jr Paleobiology. 1988;14:221–234. doi: 10.1017/s0094837300011969. [DOI] [PubMed] [Google Scholar]
- 33.Valentine J W. Paleobiology. 1980;6:444–450. [Google Scholar]
- 34.Rosenzweig M L, McCord R D. Paleobiology. 1991;17:202–213. [Google Scholar]
- 35.Roy K. Paleobiology. 1996;22:436–452. [Google Scholar]
- 36.Jablonski D, Sepkoski J J., Jr Ecology. 1996;77:1367–1378. [PubMed] [Google Scholar]
- 37.Bottjer D J, Schubert J K, Droser M L. Geol Soc (London) Spec Publ. 1996;102:1–13. [Google Scholar]
- 38.Van Valen L. Nature (London) 1984;307:50–52. [Google Scholar]
- 39.Bambach R K. Paleobiology. 1993;19:372–397. [Google Scholar]
- 40.Vermeij G J. Paleobiology. 1977;3:245–258. [Google Scholar]
- 41.Brown J H. Macroecology. Chicago: Univ. of Chicago Press; 1995. [Google Scholar]