Abstract
Mutation rates at two expanded simple tandem repeat loci were studied in the germ line of first- and second-generation offspring of inbred male CBA/H, C57BL/6, and BALB/c mice exposed to either high linear energy transfer fission neutrons or low linear energy transfer x-rays. Paternal CBA/H exposure to either x-rays or fission neutrons resulted in increased mutation rates in the germ line of two subsequent generations. Comparable transgenerational effects were observed also in neutron-irradiated C57BL/6 and x-irradiated BALB/c mice. The levels of spontaneous mutation rates and radiation-induced transgenerational instability varied between strains (BALB/c>CBA/H>C57BL/6). Pre- and postmeiotic paternal exposure resulted in similar increases in mutation rate in the germ line of both generations of CBA/H mice, which together with our previous results suggests that radiation-induced expanded simple tandem repeat instability is manifested in diploid cells after fertilization. The remarkable finding that radiation-induced germ-line instability persists for at least two generations raises important issues of risk evaluation in humans.
One of the major challenges of modern genetics is to apply recent advances in mutation research to improve the accuracy of the estimates of genetic risks for humans. For example, it has been recognized recently that ionizing radiation not only increases mutation rates in the exposed somatic cells but also results in an elevated mutation rate many cell divisions after the initial irradiation damage (1). In principle, this radiation-induced genomic instability will contribute to the accumulation of oncogenic mutations in somatic cells and malignant transformations (2). If genomic instability is induced also in the germ line of exposed parents, then delayed transgenerational effects may be manifested in their offspring, therefore presenting greater delayed risk in human populations exposed to ionizing radiation.
We recently obtained the first experimental evidence that germ-line mutation rates in unexposed offspring of irradiated male mice do not return to the mutation rates seen in unexposed individuals but are maintained at levels similar to that of directly exposed males (3). These data were generated by using our approach for monitoring germ-line mutation in mice based on a set of hypervariable expanded simple tandem repeat (ESTR) DNA loci (4–7). Unstable ESTRs consist of homogenous arrays of short tandem repeats and show very high rates of spontaneous and radiation-induced germ-line mutations, observed as size changes in the alleles of these loci (4–10). Here we use ESTR loci to study the effects of high- and low-linear energy transfer (LET) exposure and sex and strain specificity on germ-line mutation rates in the first- and second-generation offspring of irradiated male mice.
Materials and Methods
Mouse Breeding and Irradiation.
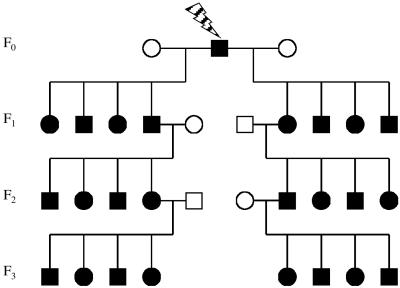
CBA/H, C57BL/6, and BALB/c inbred strains of mice from Harwell colonies were used in this study. Five CBA/H and three C57BL/6 males were given whole-body chronic irradiation of 0.4 Gy of fission neutrons (absorbed dose) using a 252Cf source with a dose-rate of 0.003 Gy⋅min−1. Seven CBA/H males and five BALB/c males were given whole-body acute irradiation of 2 and 1 Gy of x-rays, respectively (0.5 Gy⋅min−1, 250-kV constant potential, half-value layer 1.2 mm Cu). All CBA/H males subsequently were mated to untreated CBA/H females 3 and 6 weeks postirradiation; C57BL and BALB/c males were mated 6 weeks after exposure to control females from the same inbred strain to produce F1 offspring. F2 and F3 offspring were produced from the random mating of male and female F1 and F2 mice with control partners (Fig. 1). To ensure the random assignment of F1 and F2 parents, all genotyping was performed after the end of the three-generational breeding scheme. All animal procedures were carried out under guidance issued by the Medical Research Council and Home Office project.
Figure 1.
Design of the transgenerational study. The exposed male and its offspring are in black; control parents with no history of irradiation are in white.
DNA Isolation and ESTR Typing.
Genomic DNA was extracted from tails by using a standard phenol-chloroform technique. DNA profiles were produced by using two mouse-specific hypervariable single-locus ESTR probes, Ms6-hm and Hm-2, as described (5). Germ-line mutations at Ms6-hm and Hm-2 were defined as new-length alleles present in offspring; somatic mosaics with a third nonparental allele have not been included in the analysis.
Sequencing of Cdkn2a and Prkdc Genes.
PCR primers described in previous publications (11, 12) were used to identify functionally relevant polymorphisms within the genes encoding the proteins p16INK4a (Cdkn2a) and DNA-dependant protein kinase catalytic subunit (Prkdc). Two intronic regions of each of these genes containing the polymorphisms were PCR-amplified by using 0.2 mM dNTPs, 1 μM of primers, 25 ng of template DNA, 0.5 units of Taq polymerase (ABgene, Epsom, UK), 1× PCR buffer (ABgene), and 1.5 mM MgCl2. Amplification was performed in a total volume of 20 μl in thin-walled 96-well plates on an MJ DNA Engine PTC 220. After initial denaturation at 94°C for 5 min, PCRs were cycled at 94°C for 30 sec, 58–60°C for 30 sec, and 72°C for 1 min for 28 cycles, and ended with a 10-min incubation at 72°C. PCR products were cleaned by electroelution before sequencing by using an ABI PRISM BigDye Terminator cycle-sequencing ready-reaction kit. Reactions were run on an ABI 377 automated sequencer and analyzed by using FACTURA and AUTOASSEMBLER packages (all supplied by Perkin–Elmer/Applied Biosystems).
Results
Experimental Design.
The frequency of ESTR mutation was established in the F1, F2, and F3 offspring of irradiated males, which yielded germ-line mutation rates for the F0, F1, and F2 generations, respectively (Fig. 1). The number of mutations scored in each of the three subsequent generations was divided by the total number of offspring in that generation to give an estimate of the parental mutation rate (Table 1). Three experiments were designed. (i) To compare the transgenerational effects of high- and low-LET irradiations, mutation rates were measured in the germ line of offspring of CBA/H male mice exposed to either 0.4 Gy of high-LET fission neutrons or 2 Gy of low-LET x-rays. (ii) To evaluate the transgenerational effects of exposure of post- and premeiotic spermatogenic cells, mutation rates were established in the germ line of first-generation CBA/H offspring and their descendants conceived 3 and 6 weeks after the initial paternal exposure to neutrons and x-rays. The litters conceived during week 3 were derived from irradiated postmeiotic spermatids, whereas those conceived on week 6 were derived from irradiated premeiotic As spermatogonia (13). (iii) To compare the transgenerational effects between three inbred mouse strains, F1 and F2 germ-line mutation rates were compared between CBA/H, C57BL/6, and BALB/c mice. These strains have been shown to differ significantly in their radiosensitivity (14) and radiation-induced instability in somatic cells (15–17).
Table 1.
Mutation rates in the germ line of controls and offspring of irradiated males
| Strain, group* | No. of offspring | No. of mutations† | Mutation rate | Ratio to control | Prob.‡ |
|---|---|---|---|---|---|
| CBA/H | |||||
| Control (8♂, 8♀) | 76 | 22 (20) | 0.072 | — | — |
| Neutrons, 0.4 Gy | |||||
| F0, 3 weeks (5♂) | 18 | 1 (1) | 0.028 | 0.4 | 0.55 |
| F0, 6 weeks (5♂) | 43 | 18 (16) | 0.209 | 2.9 | 0.001 |
| F1 (7♂, 9♀) | 83 | 42 (30) | 0.253 | 3.5 | 2.1 × 10−7 |
| F2 (9♂, 7♀) | 84 | 33 (25) | 0.196 | 2.7 | 0.0002 |
| X-rays, 2 Gy | |||||
| F0, 3 weeks (7♂) | 18 | 4 (2) | 0.111 | 1.5 | 0.58 |
| F0, 6 weeks (7♂) | 47 | 18 (16) | 0.192 | 2.6 | 0.003 |
| F1 (13♂, 8♀) | 106 | 47 (39) | 0.222 | 3.1 | 2.1 × 10−6 |
| F2 (13♂, 11♀) | 157 | 75 (57) | 0.239 | 3.3 | 1.2 × 10−8 |
| C57BL/6 | |||||
| Control (4♂, 4♀) | 98 | 25 (21) | 0.064 | — | — |
| Neutrons, 0.4 Gy | |||||
| F0, 6 weeks (3♂) | 45 | 24 (22) | 0.267 | 4.2 | 5.5 × 10−7 |
| F1 (3♂, 5♀) | 58 | 18 (18) | 0.155 | 2.4 | 0.006 |
| F2 (5♂, 5♀) | 63 | 19 (19) | 0.151 | 2.4 | 0.006 |
| BALBc | |||||
| Control (11♂, 12♀) | 94 | 45 (43) | 0.120 | ||
| X-rays, 1 Gy | |||||
| F0, 6 weeks (5♂) | 32 | 18 (16) | 0.281 | 2.4 | 0.003 |
| F1 (5♂, 10♀) | 68 | 42 (36) | 0.309 | 2.6 | 2.7 × 10−6 |
| F2 (8♂, 6♀) | 86 | 43 (35) | 0.250 | 2.1 | 0.0003 |
The number of male and female parents is given in parentheses.
The number of singleton mutations is given in parentheses.
Prob., probability of difference from the control group (Fisher's exact test, two-tailed).
Mutation Induction in the Exposed F0 Males.
Premeiotic exposure of spermatogonia (6 weeks) to low-LET x-rays and high-LET fission neutrons resulted in elevated mutation rate in the germ line of all exposed males (Table 1). In contrast, postmeiotic exposure of spermatids (3 weeks) did not increase ESTR mutation rate in CBA/H males. Neither pre- nor postmeiotic irradiation affected the mutation rate in nonexposed F0 females (Fig. 2). These data therefore confirm our previous results on the stage specificity of mutation induction at mouse ESTR loci (5, 7).
Figure 2.
ESTR mutation rates in the germ line of male (open boxes) and female (hatched boxes) parents. The dates are given for controls, F0-exposed males, nonexposed females, and F1/F2 offspring of irradiated males. The 95% confidence intervals (CIs) for mutation rate estimated from the Poisson distribution and the probabilities of difference between male and female mutation rates in the F1 and F2 parents (Fisher's exact test, two-sided) within each generation are shown. The F0 data for premeiotic exposure (6 weeks after irradiation) are shown.
Germ-Line Instability in Male and Female Germ Lines.
Because all inbred strains showed multiallelism and heterozygosity at the Ms6-hm and Hm-2 loci, it was possible to establish the parental origin of mutant bands. The paternal and maternal mutation rates did not differ for the control parents (Fig. 2). Within each strain, male and female offspring from either the first (F1) or second (F2) generations born from irradiated F0 males showed similarly elevated mutation rates in their germ line. The elevated mutation rates observed in the germ line of F1 and F2 offspring were as high as those in the germ line of directly exposed F0 males. These results show that mutation rate in the germ line of F1 and F2 offspring of irradiated males is elevated significantly, and there is no difference in the transmission of instability through the male or female germ line. It therefore was possible to combine data across the sexes to produce single estimates for the control groups and the F1/F2 offspring of irradiated males (Table 1).
Effects of High- and Low-LET Exposure.
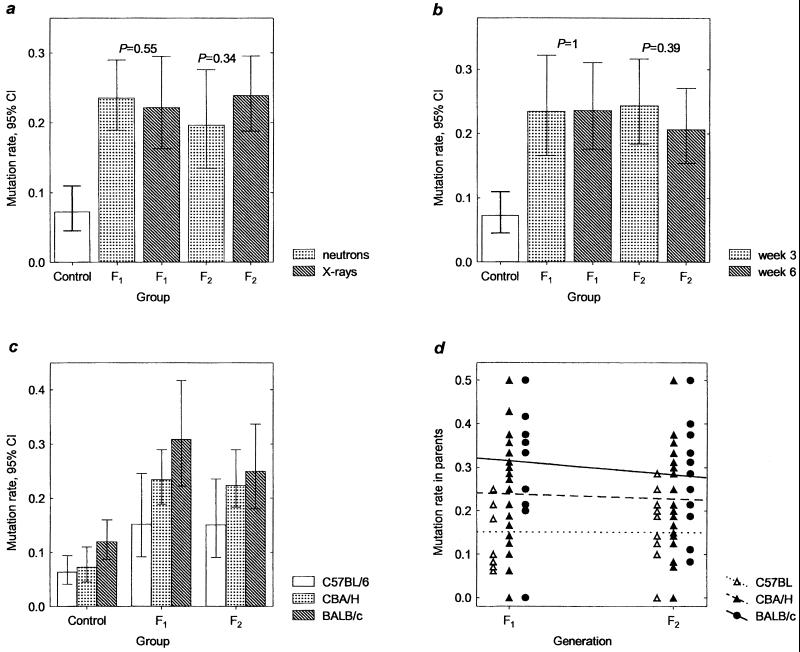
All offspring of CBA/H male mice exposed to either 0.4 Gy of high-LET fission neutrons or 2 Gy of low-LET x-rays showed significant increases in germ-line mutation rates (Table 1). The magnitude of transgenerational increase for these two doses of high- and low-LET irradiations was similar, suggesting that they both can induce germ-line instability in the F1/F2 offspring of irradiated males (Fig. 3a). The data for both types of exposure therefore were combined for further analyses.
Figure 3.
Transgenerational increases in ESTR germ-line mutation rate. (a) Mutation rates in the germ line of F1 and F2 offspring CBA/H male mice exposed to either 0.4 Gy of fission neutrons or 2 Gy of x-rays. (b) Mutation rates in the germ line of F1 offspring of CBA/H male mice conceived 3 and 6 weeks after paternal exposure to ionizing radiation and in the germ line of pre- and postmeiotically exposed grandfathers (F2). Aggregated data for the offspring of male mice exposed to x-rays and fission neutrons are given. The probabilities of difference between mutation rates within each generation are shown (a and b). (c) Comparison of spontaneous mutation rates and transgenerational effects in the germ line of three inbred strains of mice. (d) Individual mutation rates in the germ line of F1 and F2 parents of exposed males.
Stage Specificity for Transgenerational Instability.
We have compared mutation rates in the germ line of F1 offspring of CBA/H male mice conceived 3 and 6 weeks after exposure to fission neutrons and x-rays (Fig. 3b). The magnitude of transgenerational increases for both postmeiotic (3 weeks) and premeiotic (6 weeks) exposures was similar, and mutation rates for the second-generation offspring of pre- and postmeiotically exposed grandfathers also were similar.
Strain Specificity for Transgenerational Instability.
The combined data for the CBA/H mice germ-line mutation rates were compared with those for the C57BL/6 and BALB/c strains (Table 1 and Fig. 3c). An increase in germ-line mutation rates was observed in the F1 and F2 offspring of all inbred mice; however, the magnitude of both spontaneous mutation rates and transgenerational instability clearly varied between strains. The highest and lowest mutation rates were detected in BALB/c and C57BL/6 mice strains respectively.
By using direct sequencing, we screened the BALB/c, CBA/H, and C57BL/6 mice for the allelic variants of the Cdkn2a and Prkdc genes previously reported in the BALB/c mice (11, 12). The Harwell BALB/c colony contains a C → A transversion at base 134 (His → Pro) and a G → A transition at base 232 (Val → Ile) within the Cdkn2a gene. Also, two changes were confirmed in the Prkdc gene: a C → T transition at base 6,418 (Agr → Cys) and an A → G transition at base 11,530 (Met → Val). These polymorphisms were not found in the CBA/H and C57BL/6 mice and are exactly as reported (11, 12).
Transgenerational Instability in the F1 and F2 Parents.
The frequency of ESTR mutation was established in the F2 and F3 litters, which provided estimates of mutation rates for the germ line of F1 and F2 individuals (Fig. 3d). Most F1 and F2 parents showed an elevated mutation rate, which resulted in a relatively homogeneous distribution of rates within each strain (χ2 test for homogeneity of the Poisson distribution, P > 0.95, data not shown). By using the two-way ANOVA, the homogeneity of mutation rate (arc-transformed) for all offspring of irradiated parents also was tested and showed that whereas mutation rates differed significantly between strains (F = 6.97, df = 2,118, P = 0.001), they remained similar between generations within a strain (F = 0.61, df = 1,118, P = 0.4350; interaction F = 0.01, df = 2,118, P = 0.99). Thus, within a strain there is no decrease in ESTR germ-line mutation rate from the first to the second generation.
Parental Origin of Alleles and Mutation in the F1 Generation.
Each F1 offspring contained alleles derived from the irradiated F0 father and nonirradiated F0 mother (Fig. 1). In most cases it was possible to establish whether ESTR mutation, occurring in the F1 generation and detected in the F2 offspring, happened in the allele derived from the irradiated F0 father or nonirradiated F0 mother. Among 149 de novo mutants transmitted from the F1 parents to their offspring, 63 were traced clearly to the allele derived from the irradiated F0 father, and 53 were derived from the nonirradiated F0 mother. The origin of the remaining 35 mutants could not be established unambiguously. We conclude that ESTR instability in the F1 germ line affects alleles derived from both irradiated and nonirradiated F0 parents.
ESTR mutation rates in the germ line of the two groups of F1 parents both with and without mutations derived from irradiated fathers were compared. One-way ANOVA analysis showed that at both ESTR loci, mutation rates in the germ line of F1 parents containing mutations transmitted from the irradiated fathers were similar to those without paternal mutations (Ms6-hm: F = 0.85, df = 1,57, P = 0.36; Hm-2: F = 0.11, df = 1,57, P = 0.74). We therefore conclude that the presence of mutations transmitted from the irradiated fathers could not affect the ESTR stability of F1 parents.
Discussion
The analysis of ESTR mutation in the germ line of first- and second-generation offspring of irradiated males has revealed that (i) germ-line mutation rates were elevated significantly in both generations of all inbred strains studied; (ii) no differences could be observed in the transmission of instability through the male or female germ line; (iii) exposure to 2-Gy x-rays and 0.4 Gy of fission neutrons resulted in similar increases in both generations of CBA/H mice; (iv) the extent of transgenerational increase clearly varied with the different strains; and (v) germ-line mutation rates were elevated equally in the F1 and F2 offspring.
To date, only a limited number of studies have addressed the issue of transgenerational changes in the mammalian germ line (18–20). They have shown elevated rates of mortality and malformation in the grand-offspring of exposed males providing indirect evidence for an elevated mutation rate within the first generation. In contrast, the results from our pilot study provide direct experimental evidence for an elevated germ-line mutation rate in the first-generation offspring of neutron-irradiated male mice (3), and we have confirmed this result here.
We also have now analyzed the efficiency of high- and low-LET paternal exposure on the transgenerational instability in the F1 and F2 offspring within CBA/H inbred mice. High-LET radiation produces highly complex and localized initial DNA damage, which is qualitatively different to the sparse damage produced by low-LET radiation. It has been proposed that these differences in damage patterns may result in the unique final biological effects of these different radiation sources (21). However, it seems that both irradiations are capable of inducing genomic instability in somatic cells, although some studies have failed to detect the effects of low-LET exposure (reviewed in ref. 22). Our results show that paternal exposure to either high-LET fission neutrons or low-LET x-rays results in increases in germ-line mutation rates in both generations and therefore are consistent with the data on somatic cells.
In a number of our previous studies we have shown that postmeiotic exposure to ionizing radiation does not affect ESTR germ-line mutation rate in the directly exposed males (5, 7). The lack of mutation induction at this stage has been explained by assuming that spontaneous and radiation-induced mutation at ESTR loci most probably occurs in diploid cells but not in haploid postmeiotic cells. In contrast, we now have shown that a similar elevated mutation rate is found in the germ line of F1 offspring of exposed males conceived from either postmeiotic (3 weeks) or premeiotic (6 weeks) stages of spermatogenesis, and this elevated mutation rate persists to the germ line of the second generation (Fig. 3b). Thus, the destabilization of the F1 germ line occurs after fertilization regardless of the stage of spermatogenesis exposed to radiation, and the radiation-induced signal also persists and destabilizes the F2 germ line. Several recent publications report conflicting data on the efficiency of postmeiotic paternal exposure and the effects that it has on the transgenerational mutation processes (23–28). This apparent discrepancy in findings remains unexplained, and future work should directly address in greater detail the issue of the stage specificity of transgenerational effects.
The data presented here show elevated germ-line mutation rates in the F1 and F2 offspring of initially irradiated males belonging to three inbred strains CBA/H, C57BL/6 and BALB/c, which clearly demonstrates that transgenerational instability is not restricted to one particular inbred strain of mice and shows significant interstrain variation in transgenerational instability. Previous studies of these three inbred strains of mice also have revealed profound differences in their response to ionizing radiation, showing that BALB/c and CBA mice are significantly more radiosensitive and show higher levels of radiation-induced genomic instability in somatic cells than C57BL/6 mice (12, 14–17). It has been suggested also that the high level of radiation-induced genomic instability in BALB/c mice could be attributed to the strain-specific amino acid substitutions at the Cdkn2a and Prkdc genes (11, 12). Our data showing low spontaneous germ-line mutation rate and radiation-induced transgenerational instability within the C57BL/6 strain with significantly higher values for the CBA/H and BALB/c strains are consistent with these observations.
The results of this study also provide important clues relating to the possible mechanisms of transgenerational instability. If the transgenerational signal inducing instability in the F1 germ line was direct radiation-induced DNA damage within the ESTR loci themselves, then mutations in the F1 germ line should occur predominantly in the damaged allele transmitted to them from the irradiated F0 fathers. Because equally elevated mutation rates at both alleles derived from the irradiated F0 fathers and the unexposed F0 mothers were detected in the germ line of F1 offspring, this explanation can be excluded, and a global elevation of mutation rate is inferred. The persistence of elevated mutation rates in the germ line of two consecutive generations rules out the possibility that transgenerational effects are caused by radiation-induced mutations at any specific set of genes in the exposed F0 males. For example, mutations in some DNA-repair genes transmitted from irradiated males to their offspring potentially could affect genome stability in the first generation. However, the further mating of F1 offspring to control animals should result in Mendelian segregation of wild-type and mutant alleles, causing an overall reduction of mutation rate in the second generation. On the contrary, mutation rates in the germ line of both generations are similar and significantly exceed those for the control parents, clearly implicating an epigenetic mechanism for the transgenerational instability.
Finally our results may have far-reaching implications for the evaluation of genetic risks of ionizing radiation. If germ-line instability is able to persist in populations for several generations after the initial exposure to ionizing radiation, this instability would lead to a significant increase in mutation load. Because ESTRs are noncoding loci with no apparent function, the germ-line mutations studied here can be considered selectively neutral, and it may be possible to argue that instability at these loci should not affect the fitness of population. However, recent studies provide strong evidence for health-related transgenerational effects, affecting predisposition to cancer, mortality, somatic mutation, fertility, and behavior (Table 2). Altogether, these data raise the important issue of the delayed transgenerational effects of ionizing radiation for humans, providing, for example, a plausible explanation for the apparent leukemia cluster near Sellafield nuclear plant (34).
Table 2.
Transgenerational changes in the offspring of male mice and rats exposed to ionizing radiation or chemical mutagens
| Strain | Paternal exposure | Generation | Endpoint | Effect* | Ref. |
|---|---|---|---|---|---|
| Mice | |||||
| ICR | X-rays (216 rad) | F1 | Lung tumors after treatment with urethane | Elevated | 29 |
| SHR | X-rays (4.2 Gy) | F1, F2 | Skin tumors after treatment with phorbol 12-myristate 13-acetate | Elevated | 27 |
| CBA/H | Injection with 239Pu | F1 | Lympho-hemopoietic malignancies after treatment with methylnitrosourea | Elevated | 30 |
| DBA2 | Injection with 55Fe | F1 | Lympho-hemopoietic malignancies after treatment with methylnitrosourea | Elevated | 23 |
| CBA | Injection with 239Pu | F1 | Pre-/postimplantation loss among offspring | Elevated | 18 |
| HLG/Zte | X-rays (1 Gy) | F1 | Pre-/postimplantation loss and malformations among offspring | Elevated | 19 |
| CBA/H | Neutrons (0.4 Gy) | F1 | Germ line mutation rate at two ESTR loci | Elevated | 3 |
| C57BL/6 | X-rays (1–4 Gy) | F1 | Somatic mutation rate at lacI gene | Elevated | 26 |
| C57BL/6J | X-rays (1 Gy) | F1 | Somatic reversions at pun locus | Elevated | 31 |
| CD1 | γ-rays (1 Gy) | F1 | Proliferation of early embryonic cells in offspring | Decreased | 24 |
| CD1 | γ-rays (1 Gy) | F1 | Fertilization rate for spermatozoa | Decreased | 25 |
| CD1 | γ-rays (1 Gy) | F3 | Levels of p53 and p21waf1 | Elevated | 32 |
| Rats | |||||
| Sprague | Cyclophosphamide | F1 | Pre-/postimplantation loss and malformations among offspring | Elevated | 20 |
| Swiss | X-rays (4.5 Gy) | F1 | Chromosome aberrations after treatment with x-rays or cyclophosphamide | Elevated | 28 |
| Wistar | Cyclophosphamide | F1, F2 | Learning capacity | Decreased | 33 |
Compared with the offspring of nonexposed parents.
Acknowledgments
We thank A. J. Jeffreys, C. L. Yauk, J. D. H. Stead, and an anonymous reviewer for the helpful suggestions. This work was supported by grants from the Wellcome Trust (to Y.E.D.) and the Leukaemia Research Fund (to M.A.P.).
Abbreviations
- ESTR
expanded simple tandem repeat loci
- LET
linear energy transfer
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Morgan W F, Day J P, Kaplan M I, McGhee E M, Limoli C L. Radiat Res. 1996;146:247–258. [PubMed] [Google Scholar]
- 2.Little JB. Carcinogenesis. 2000;21:397–404. doi: 10.1093/carcin/21.3.397. [DOI] [PubMed] [Google Scholar]
- 3.Dubrova Y E, Plumb M, Gutierrez B, Boulton E, Jeffreys A J. Nature (London) 2000;405:37. doi: 10.1038/35011135. [DOI] [PubMed] [Google Scholar]
- 4.Dubrova Y E, Jeffreys A J, Malashenko A M. Nat Genet. 1993;5:92–94. doi: 10.1038/ng0993-92. [DOI] [PubMed] [Google Scholar]
- 5.Dubrova Y E, Plumb M, Brown J, Fennelly J, Bois P, Goodhead D, Jeffreys A J. Proc Natl Acad Sci USA. 1998;95:6251–6255. doi: 10.1073/pnas.95.11.6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubrova Y E, Plumb M, Brown J, Boulton E, Goodhead D, Jeffreys A J. Mutat Res. 2000;453:17–24. doi: 10.1016/s0027-5107(00)00068-3. [DOI] [PubMed] [Google Scholar]
- 7.Barber R, Plumb M A, Smith A G, Cesar C E, Boulton E, Jeffreys A J, Dubrova Y E. Mutat Res. 2000;457:79–91. doi: 10.1016/s0027-5107(00)00130-5. [DOI] [PubMed] [Google Scholar]
- 8.Kelly R, Bulfield G, Collick A, Gibbs M, Jeffreys A J. Genomics. 1989;5:844–856. doi: 10.1016/0888-7543(89)90126-2. [DOI] [PubMed] [Google Scholar]
- 9.Gibbs M, Collick A, Kelly R, Jeffreys A J. Genomics. 1993;17:121–128. doi: 10.1006/geno.1993.1292. [DOI] [PubMed] [Google Scholar]
- 10.Bois P, Williamson J, Brown J, Dubrova Y E, Jeffreys A J. Genomics. 1998;49:122–128. doi: 10.1006/geno.1998.5228. [DOI] [PubMed] [Google Scholar]
- 11.Zhang S, Ramsay E S, Mock B A. Proc Natl Acad Sci USA. 1998;95:2429–2434. doi: 10.1073/pnas.95.5.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu Y, Okayashi R, Weil M M, Silver A, McCarthy M, Zabriskie R, Long S, Cox R, Ullrich R L. Cancer Res. 2001;61:1820–1824. [PubMed] [Google Scholar]
- 13.Searle A G. Adv Radiat Biol. 1974;4:131–207. [Google Scholar]
- 14.Roderick T H. Radiat Res. 1963;20:631–639. [PubMed] [Google Scholar]
- 15.Watson G E, Lorimore S A, Clutton S M, Kadhim M A, Wright E G. Int J Radiat Biol. 1997;71:497–503. doi: 10.1080/095530097143824. [DOI] [PubMed] [Google Scholar]
- 16.Mothersill C E, O'Malley K J, Murphy D M, Seymour C B, Lorimore S A, Wright E G. Carcinogenesis. 1999;20:2273–2278. doi: 10.1093/carcin/20.12.2273. [DOI] [PubMed] [Google Scholar]
- 17.Ponnaiya B, Cornforth M N, Ullrich R L. Radiat Res. 1997;147:121–125. [PubMed] [Google Scholar]
- 18.Luning K G, Frolen H, Nilsson A. Mutat Res. 1976;34:539–542. doi: 10.1016/0027-5107(76)90229-3. [DOI] [PubMed] [Google Scholar]
- 19.Pils S, Muller W-U, Streffer C. Mutat Res. 1999;429:85–92. doi: 10.1016/s0027-5107(99)00101-3. [DOI] [PubMed] [Google Scholar]
- 20.Hales B F, Crosman K, Robaire B. Teratology. 1992;45:671–678. doi: 10.1002/tera.1420450612. [DOI] [PubMed] [Google Scholar]
- 21.Goodhead D T. Health Phys. 1988;55:231–240. doi: 10.1097/00004032-198808000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Limoli C L, Ponnaiya B, Corcoran J J, Giedzinski E, Kaplan M I, Hartmann A, Morgan W F. Adv Space Res. 2000;25:2107–2117. doi: 10.1016/s0273-1177(99)01062-5. [DOI] [PubMed] [Google Scholar]
- 23.Hoyes K P, Lord B I, McCann C, Hendry J H, Morris I D. Radiat Res. 2001;156:488–494. doi: 10.1667/0033-7587(2001)156[0488:teoppc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 24.Wiley L M, Baulch J E, Raabe O G, Straume T. Radiat Res. 1997;148:145–151. [PubMed] [Google Scholar]
- 25.Burruel V R, Raabe O G, Wiley L M. Mutat Res. 1997;381:59–66. doi: 10.1016/s0027-5107(97)00148-6. [DOI] [PubMed] [Google Scholar]
- 26.Luke G A, Riches A C, Bryant P E. Mutagenesis. 1997;12:147–152. doi: 10.1093/mutage/12.3.147. [DOI] [PubMed] [Google Scholar]
- 27.Vorobtsova I E, Aliyakparova L M, Anisimov V N. Mutat Res. 1993;287:207–216. doi: 10.1016/0027-5107(93)90013-6. [DOI] [PubMed] [Google Scholar]
- 28.Vorobtsova I E. Mutagenesis. 2000;15:33–38. doi: 10.1093/mutage/15.1.33. [DOI] [PubMed] [Google Scholar]
- 29.Nomura T. Mutat Res. 1983;121:59–65. doi: 10.1016/0165-7992(83)90087-8. [DOI] [PubMed] [Google Scholar]
- 30.Lord B I, Woolford L B, Wang L, McDonald D, Lorimore S A, Papworth D, Wright E G, Scott D. Br J Cancer. 1998;78:301–311. doi: 10.1038/bjc.1998.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carls N, Schiestl R H. Carcinogenesis. 1999;20:2351–2354. doi: 10.1093/carcin/20.12.2351. [DOI] [PubMed] [Google Scholar]
- 32.Baulch J E, Raabe O G, Wiley L M. Mutagenesis. 2001;16:17–23. doi: 10.1093/mutage/16.1.17. [DOI] [PubMed] [Google Scholar]
- 33.Auroux M R, Dulioust E J B, Nawar N N Y, Yacoub S G, Mayaux M J, Schwartz D, David G. J Androl. 1998;9:153–159. doi: 10.1002/j.1939-4640.1988.tb01027.x. [DOI] [PubMed] [Google Scholar]
- 34.Gardner M J, Snee M P, Hall A J, Powell C A, Downes S, Terrell J D. Br Med J. 1990;300:423–429. doi: 10.1136/bmj.300.6722.423. [DOI] [PMC free article] [PubMed] [Google Scholar]