Abstract
Phagocytes are a critical component of the innate immune response in humans and eliminate invading microorganisms through a process known as phagocytosis. Two distinct receptor-linked phagocytic pathways, one with Ab receptors (FcRs; FcR, Fc receptor) and the other complement receptors (CRs), mediate binding and ingestion of pathogens by human polymorphonuclear leukocytes (PMNs). Although progress has been made toward defining complex signal transduction processes that underlie phagocytosis in each pathway, very little is known about gene regulation during or after phagocytosis. Therefore, we used human oligonucleotide microarrays to identify changes in expression of 12,561 genes accompanying FcR- and CR-mediated phagocytosis. Eighty-four percent of 279 differentially expressed genes were induced or repressed 90 min after ingestion of Ab- and/or complement-opsonized particles. Unexpectedly, more than 30 of these genes encoded proteins involved in at least three distinct apoptotic pathways. Ninety-four differentially expressed cell fate-related genes were identified between 180 and 360 min after phagocytosis and most were induced or repressed by PMNs activated through both receptors simultaneously. By using flow cytometry, we found that FcR- and CR-mediated phagocytosis each promoted programmed cell death in human PMNs; however, phagocytosis mediated by the combination of FcRs and CRs induced apoptosis earlier than that by either receptor alone. Our results reveal distinct patterns of receptor-mediated gene expression that define complex inducible apoptotic pathways in activated PMNs. Most significantly, we discovered that programmed cell death is regulated at the level of gene expression. Thus, we hypothesize that gene regulation in PMNs facilitates resolution of inflammatory responses.
Polymorphonuclear leukocytes (PMNs) are a first line of defense against invading microorganisms. This innate defense mechanism depends on the ability of PMNs to ingest and subsequently eliminate pathogens by oxidative and nonoxidative processes (1). Ab receptors (FcRs; FcR, Fc receptor) and complement receptors (CRs) mediate phagocytosis by human PMNs (reviewed in refs. 2 and 3). Binding and ingestion of microorganisms initiate a complex series of signal transduction events, including activation of multiple protein tyrosine kinases and increased phosphoinositide and arachidonic acid metabolism (4–6). However, signal transduction pathways and other molecular processes activated during and after phagocytosis are incompletely defined.
Receptor activation in macrophages induces transcriptional events that subsequently mediate host cell responses (7). On the other hand, there is limited information on the regulation of patterns of gene expression in PMNs. Recent work from two laboratories has demonstrated that unstimulated PMNs synthesize mRNA and bacteria induce changes in gene expression in neutrophils, but these studies were limited in scope by the number of transcripts analyzed and by sensitivity of the assay systems (8, 9). Therefore, it is unknown whether transcriptional events in PMNs contribute to differences in FcR- and CR-mediated phagocytosis or to resolution of inflammatory responses. To gain new insights into changes in gene expression that accompany phagocytosis, we investigated global gene expression by human PMNs during FcR- and CR-mediated phagocytosis by using oligonucleotide microarrays. Our findings demonstrate that receptor-specific induction of genes ultimately determines cell fate, suggesting that resolution of inflammatory responses is mediated, at least in part, by gene expression in PMNs.
Materials and Methods
Detailed protocols are published as Supporting Methods on the PNAS web site, www.pnas.org.
Isolation of Human PMNs.
Human PMNs were isolated from venous blood of healthy individuals by using the method of Boyum (10) in accordance with a protocol approved by the Institutional Review Board for Human Subjects, National Institute of Allergy and Infectious Diseases. Briefly, fresh, heparinized blood was mixed 1:1 with 0.9% sodium chloride (Injection USP, Baxter Healthcare, Deerfield, IL) containing 3.0% Dextran T-500 (Amersham Pharmacia) and then incubated for 20 min at room temperature to sediment erythrocytes. The resulting leukocyte-rich supernatant was centrifuged at 550 × g for 10 min, and cells were resuspended in 35 ml of 0.9% sodium chloride (Baxter Healthcare). The leukocyte suspension was underlayed with 10 ml of Ficoll-Paque PLUS (1.077 g/liter, Amersham Pharmacia) and centrifuged for 25–30 min to separate PMNs from peripheral blood mononuclear cells (PBMCs). After aspiration of the PBMC layer (Ficoll-Paque–0.9% sodium chloride interface) and remaining supernatant, sides of the gradient tubes were swiped with sterile cotton swabs to remove any residual cells. After standard hypotonic lysis of erythrocytes [cells suspended in sterile water (Injection USP, Baxter Healthcare) for 20 s and then mixed immediately with an equal volume of 1.7% sodium chloride], purified PMNs were suspended in RPMI 1640 medium (GIBCO), buffered with 10 mM Hepes, and incubated on ice until used. The entire procedure for the purification of PMNs was performed at room temperature, and all reagents used contained <10.0 pg/ml endotoxin. PMNs in each preparation were enumerated visually on a hemocytometer in 2% acetic acid, and slides were routinely prepared and stained with a modified Wright-Giemsa (Sigma). Each PMN preparation contained 95–98% neutrophils, with the remaining cells being predominantly eosinophils. Contaminating PBMCs could not be found in PMN preparations by microscopy. However, we found that contaminating lymphocytes and monocytes account for 0.71 ± 0.26% and 0.11 ± 0.07% (n = 30 PMN preparations) of the cells in the PMN preparations, respectively, when analyzed by flow cytometry (FACsCalibur, BD Biosciences, San Jose, CA).
Phagocytosis Experiments and RNA Preparation/Gene Expression Analysis.
IgG and complement-coated 2.0 μM latex beads (LBs) (Polysciences) were prepared with standard methods, which are published as supporting information. Phagocytosis assays and those measuring reactive oxygen species (ROS) were performed with described methods (11).
For gene expression experiments, phagocytosis assays were performed with 107 human PMNs and 8 × 107 LB in wells of a 12-well tissue culture plate. At the indicated time points, cells were lysed directly with RLT buffer (Qiagen, Valencia, CA). Purified RNA was used to prepare labeled cRNA target (12 μg) for analysis on GeneChip Hu95A arrays (Affymetrix, Santa Clara, CA) according to standard GeneChip protocols (http://www.affymetrix.com/pdf/expression_manual.pdf). Experiments were performed in triplicate with three separate donors. Detailed information regarding data analysis is published as supporting information.
Taqman analysis of gene expression (triplicate samples) was performed with conditions used for the microarray analysis by using an ABI 7700 thermocycler (Applied Biosystems) as described (12).
Apoptosis Assays.
Early apoptosis was measured with the Annexin V-FITC Apoptosis Detection kit II (BD Biosciences). Apoptotic nuclei were detected with propidium iodide (PI) staining as described (13). DNA fragmentation in PMNs after phagocytosis was determined with an Apo-BRDU Apoptosis Detection kit (BD Biosciences).
Results and Discussion
Phagocytosis Mediated by FcRs and CRs.
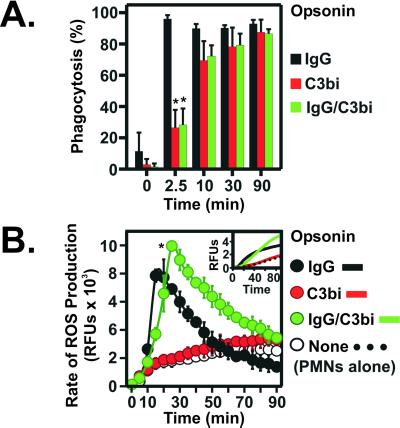
Opsonization of invading pathogens with Ab and/or complement facilitates their removal by phagocytic leukocytes. To investigate phagocytosis mediated by FcRs and CRs, we monitored ingestion of LBs opsonized with Ab (IgG LB), serum complement (C3bi LB), and Ab and serum complement (IgG/C3bi LB) by human PMNs (Fig. 1A). Ingestion mediated by FcRs was complete within 2.5 min and occurred more rapidly than that mediated by CRs or by the combination of FcRs and CRs (Fig. 1A). However, by 90 min all particles were similarly ingested (Fig. 1A).
Figure 1.
FcR- and CR-mediated phagocytosis in human PMNs. (A) PMNs were stimulated with IgG LB (IgG), C3bi LB (C3Bi), or IgG/C3bi LB (IgG/C3bi), and phagocytosis was quantitated as described in Materials and Methods. Results are the mean ± SD of 3–5 separate experiments. *, P < 0.01 vs. IgG LB. (B) ROS production during phagocytosis. Data are expressed as rate of ROS production over time and are the mean ± SD of 3–6 experiments. Inset illustrates representative plots of FcR-, CR-, and FcR/CR-stimulated ROS production. *, P < 0.01 vs. PMNs stimulated with IgG and C3bi LB.
We next determined whether phagocytosis mediated by FcRs or CRs resulted in differences in ROS generation by PMNs. Phagocytosis mediated by FcRs alone elicited rapid and robust production of ROS by PMNs, which coincided with ingestion (Fig. 1B). Compared with FcR-mediated phagocytosis, ROS production by PMNs activated by the combination of FcRs and CRs was greater in magnitude and duration, suggesting receptor cooperativity (14) (Fig. 1B). In contrast, phagocytosis mediated by CRs alone elicited little or no ROS production, consistent with previous findings in monocytes (15) (Fig. 1B). These results indicate that events mediating activation of PMNs by FcRs or CRs proceed through distinct intracellular signal transduction pathways.
Changes in Gene Expression During Phagocytosis.
To gain insight into molecular determinants that mediate events accompanying phagocytosis, we used oligonucleotide microarrays to analyze global gene expression patterns in PMNs during and after receptor-mediated phagocytosis. We screened 12,561 human genes and determined that unstimulated human PMNs isolated from peripheral blood contain transcripts of ≈3,000 of these genes. Previous studies of baseline gene expression in PMNs by Itoh et al. (8) identified 748 different mRNA transcripts. All of the PMN transcripts identified as most abundant by Itoh et al. were correspondingly very highly expressed in our data set (not shown), conclusively demonstrating that PMNs express a large number of mRNA transcripts. Moreover, we found that most transcripts reported as low in abundance by Itoh et al. were moderately or highly expressed in our studies (data not shown). Therefore, the difference in numbers of identified genes between our studies and those of Itoh et al. is likely a result of the limits of their assay system (8).
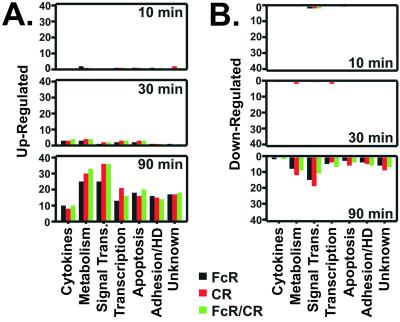
A limited number of genes (45) were differentially expressed during the first 30 min of FcR- or CR-mediated phagocytosis, a period when ingestion is completed and ROS production is maximal (compare Fig. 1 A and B with Fig. 2). In contrast, 256 unique genes were induced or repressed by 90 min after FcR-, CR-, and the combination of FcR and CR (FcR/CR)-mediated events (Fig. 2). Our finding that PMNs differentially regulate expression of a significant number of genes during cell activation is supported by previous studies of Subrahmanyam et al. (9).
Figure 2.
Differential gene expression during phagocytosis. (A and B) Human PMNs were stimulated with IgG LB (FcR), C3bi LB (CR), or IgG/C3bi LB (FcR/CR) for the indicated times, and gene expression was measured with Affymetrix human oligonucleotide microarrays. Data were analyzed with GENESPRING software version 4.0.4 (Silicon Genetics, Redwood City, CA). Genes induced (A) or repressed (B) after FcR- (black bars), CR- (red bars), or FcR/CR-mediated phagocytosis (green bars) were categorized by function and enumerated. HD, host defense.
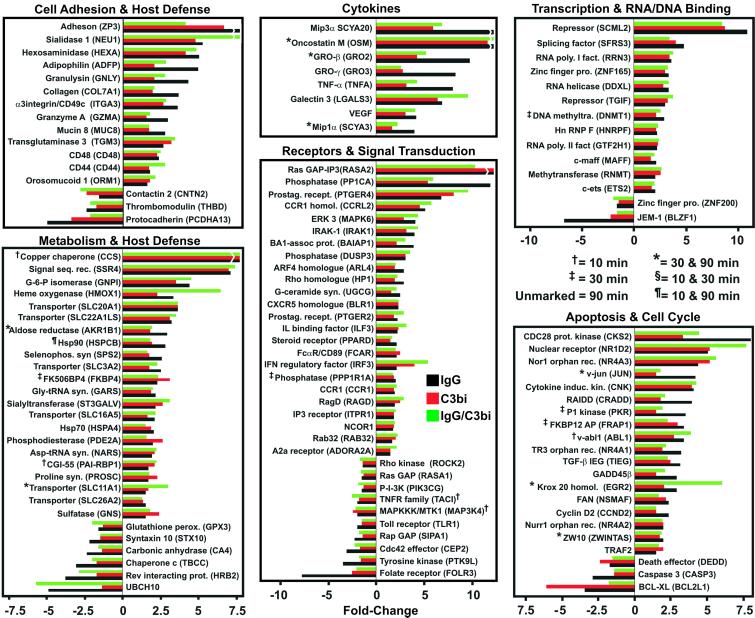
To facilitate subsequent analysis, differentially expressed genes were divided into several categories based on function (Fig. 2). Similar changes were observed in the expression patterns of 121 genes of known function following FcR-, CR-, or FcR/CR-mediated phagocytosis and most encoded proteins involved in signal transduction and metabolism (Fig. 3). Genes encoding eight cytokines and growth factors, including tumor necrosis factor-α, were also up-regulated, a finding consistent with the ability of PMNs to produce inflammatory mediators after activation (Fig. 3). In addition, at least 38 genes regulating apoptosis, cell cycle, and transcription were differentially expressed (Fig. 3). However, genes directly involved in host defense comprised only 10% of the genes that were differentially regulated (Fig. 3).
Figure 3.
Differentially expressed genes shared between FcR- and CR-mediated phagocytosis. Genes differentially expressed during and after FcR- (black bars), CR- (red bars), or FcR/CR-mediated phagocytosis (green bars) were categorized by function. Breaks in the bars indicate genes whose differential expression level is off-scale. Results are presented as the mean fold-induction or repression of genes from three experiments.
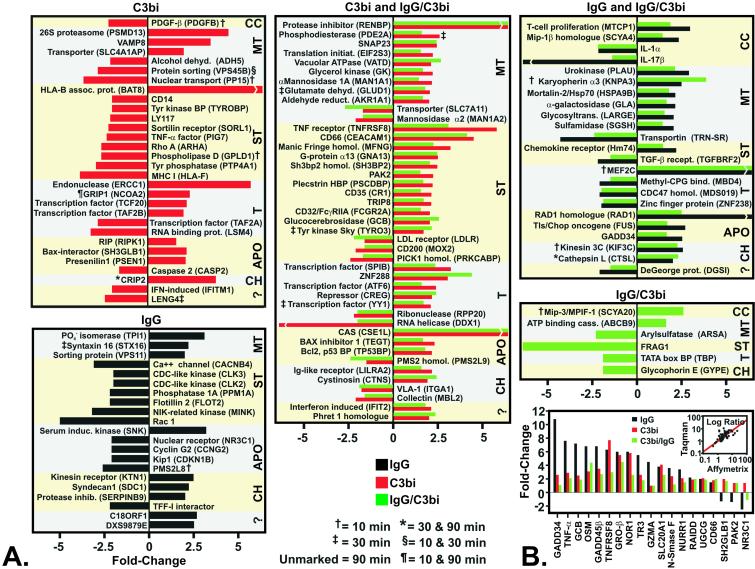
We next examined receptor-specific changes in gene expression after FcR- and CR-mediated phagocytosis (Fig. 4A). For genes whose expression level changed significantly after phagocytosis of IgG/C3bi LB and either C3bi- or IgG LB, it was presumed that gene induction or repression resulted from ligation of the receptor specific to the common ligand, i.e., CR or FcR, respectively. Forty-four differentially expressed genes were specific to FcR-activated PMNs and 73 were unique to CR-mediated phagocytosis (Fig. 4A). Compared with FcR-activated PMNs, there were 29 additional differentially expressed genes after CR-phagocytosis (Figs. 2 and 4A). Sixteen of these genes encoded receptors and key signal-transduction mediators including glucocerebrosidase (GCB), protein tyrosine kinase Sky (TYRO3), G protein α13 (GNA13), glycosylphosphatidylinositol (GPI)-phospholipase D1 (GPLD1), p21-activated kinase (PAK2), and rhoA (ARHA) (Fig. 4A). Of these genes, only GPLD1 was regulated very early (by 10 min) during phagocytosis (Fig. 4A). GPLD1 regulates surface expression of GPI-anchored proteins that mediate host responses, such as CD87 (16), by cleaving the GPI-linked membrane anchors of target proteins. Interaction of CD87 with CR3 promotes chemotaxis and motility in PMNs (17), and thus, down-regulation of GPLD1 in PMNs could modulate early phagocytosis. Recent studies from Palicz et al. (18) demonstrated that diacylglycerol and phosphatidic acid, both products of GPLD1 activity (19), directly activate the NADPH oxidase. Therefore, the potential effects of down-regulating GPLD1 during CR-mediated phagocytosis are consistent with our finding that CR-activated PMNs produce little or no ROS (Fig. 1B).
Figure 4.
Receptor-specific induction or repression of genes during phagocytosis. (A) Differentially expressed genes identified in both C3bi and IgG/C3bi (CR-specific) or IgG and IgG/C3bi (FcR-specific) stimulated PMNs are labeled as C3bi and IgG/C3bi and IgG and IgG/C3bi, respectively. CC, cytokines, chemokines, and growth factors; MT, metabolism and trafficking; ST, receptors and signal transduction; T, transcription and RNA/DNA binding; APO, apoptosis and cell cycle; CH, cell adhesion and host defense; ?, unknown function. (B) Taqman verification of microarray results. Genes (n = 19) identified as differentially transcribed by Affymetrix microarrays were selected for confirmation by Taqman real-time PCR. Inset illustrates the correlation (r = 0.81) between real-time PCR and microarray analyses.
However, the finding that only 16% of the total number of differentially expressed genes are induced or repressed during early phagocytosis (10 and 30 min), coupled with the observation that few of these genes (10%) are directly involved in host defense, suggests that gene regulation plays a limited role in mediating immediate PMN responses such as phagocytosis, ROS production, and bactericidal activity. Therefore, most gene expression in circulating neutrophils or that regulated during PMN activation likely serves an alternative function.
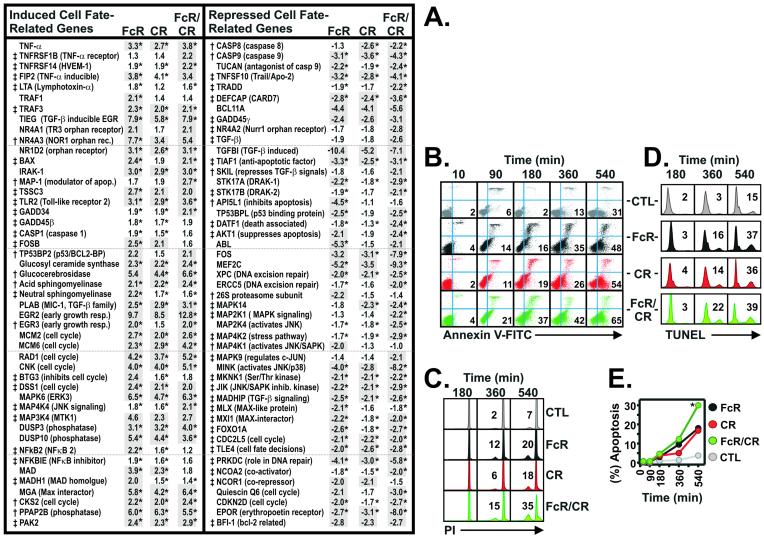
Differential Expression of Genes Determining Cell Fate.
There were similarities and differences between FcR- and CR-activated PMNs in the expression levels of specific genes governing cellular apoptosis and/or cell cycle (Figs. 3 and 4). Genes encoding several downstream effectors of apoptosis, including Caspases 4 and 10, DAXX, FADD, PUMA, and Apaf-3, were expressed but not differentially regulated (data not shown). In contrast, apoptosis-related genes such as RAIDD, GADD45β, TRAF2, DEDD, BCL-XL, PKR, CASP3, and FAN were induced or repressed in both FcR- and CR-activated PMNs (Fig. 3). Genes encoding four nuclear orphan receptors, TR3, NOR1, NURR1, and NR1D2, were up-regulated after FcR- and CR-mediated phagocytosis (Fig. 3). The latter findings are important because the orphan receptors TR3 and NOR1 have crucial roles in T cell apoptosis (20, 21), but expression of these genes has not been previously identified in PMNs or associated with PMN apoptosis. Several apoptosis-related and cell cycle genes were specifically induced or repressed by CR ligation, including cellular apoptosis susceptibility protein (CAS), caspase 2 (CASP2), bax inhibitor 1 (TEGT), bcl2-p53bp (TP53BP), RIP (RIPK1), bax interactor (SH3GLB1), and presenilin 1 (PSEN1) (Fig. 4A). FcR-specific genes that were differentially transcribed and related to cell fate included RAD1, GADD34, Cyclin G2, and the glucocorticoid receptor (NR3C1) (Fig. 4A). In addition, genes encoding granulysin and granzyme A, not identified previously in human PMNs but implicated in T cell apoptosis (22, 23), were up-regulated significantly (P = 0.03 and 0.05, respectively) in FcR-activated neutrophils (Fig. 3). Consistent with our findings, Subrahmanyam et al. (9) identified nine apoptosis-related genes differentially expressed by PMNs in response to bacteria, including up-regulation of GADD34 and GADD45β. Taken together, these findings suggest that unlike phagocytosis, PMN apoptosis is regulated at the level of gene expression.
Confirmation of Differentially Expressed Genes by Taqman Real-Time PCR.
Inasmuch as expression of many of these genes has not been identified previously in PMNs, we used Taqman real-time PCR to verify changes in expression detected by microarray analysis (Fig. 4B). We selected 19 genes representative of the entire microarray data set for confirmation by Taqman (Fig. 4B). These genes comprised six different functional categories and included up- and down-regulated genes and receptor-specific or shared genes. There was good correlation between Taqman and microarray gene expression profiles, consistent with previous studies (Fig. 4B) (24). Importantly, we confirmed several unexpected findings, including induction of genes encoding TR3, NOR1, and granzyme A (Fig. 4B).
Phagocytosis Induces Expression of Cell Fate-Related Genes.
Based on our finding that multiple apoptosis-related genes were differentially regulated late in phagocytosis (90 min), we hypothesized that induction or repression of cell fate-related genes would be accentuated at later time points. Remarkably, 869 unique genes were differentially transcribed between 3 and 6 h after receptor-mediated phagocytosis (data not shown). Of these genes, 94 cell fate-related genes were identified, including those encoding proteins involved in tumor necrosis factor receptor-, transforming growth factor-β-, and Toll-like receptor-mediated apoptosis, and proteins regulating ceramide synthesis and signaling (Fig. 5A). Compared with FcR- or CR-activated PMNs, phagocytosis mediated by the combination of both receptors (FcR/CR) induced changes in the expression levels of cell fate-related genes that were greatest in both magnitude and number (Fig. 5A). Eighty-three percent of the total number of cell fate genes identified were induced or repressed at least 2-fold by FcR/CR-mediated phagocytosis, compared with 76.1% and 52.2%, for FcR- and CR-activated PMNs, respectively (Fig. 5A). Induction and repression of multiple apoptosis-related inhibitors by either receptor alone and the combination of receptors underscore the complexity of the signal transduction pathways and proteins involved in regulated cell fate. Taken together, these findings suggest that phagocytosis mediated by both receptors simultaneously in human PMNs induces apoptosis more rapidly than that by either receptor alone.
Figure 5.
Phagocytosis-induced apoptosis in PMNs. (A) Induction or repression of cell fate-related genes in PMNs after phagocytosis was determined 180 (†) and 360 (‡) min after phagocytosis as indicated. Genes differentially expressed at least 2-fold are shaded in gray. *, P < 0.05 for stimulated (FcR, CR, and FcR/CR) vs. unstimulated PMNs. (B) Flow cytometric analysis of early apoptosis during phagocytosis. PMNs with exposed phosphatidylserine (early apoptosis) bound Annexin V-FITC (lower right quadrants); upper quadrants, propidium iodide staining. Percent staining is indicated within the quadrant, and data are representative of six separate experiments. (C) After phagocytosis, chromatin condensation in apoptotic PMNs was determined by flow cytometry. Percent of apoptotic nuclei is indicated within the histogram panels. (D) After phagocytosis, late apoptosis (DNA fragmentation) in PMNs was detected with a modified terminal deoxynucleotidyltransferase dUTP nick end-labeling assay. Percent DNA fragmentation is indicated within each histogram panel. Results are representative of three separate experiments. (E) Quantitation of six apoptosis (chromatin condensation) experiments. *, P < 0.01 vs. FcR-, CR-phagocytosis, and unstimulated PMNs (CTL).
Phagocytosis Induces Apoptosis in Human PMNs.
To confirm that gene expression after phagocytosis was linked to the induction of programmed cell death in activated human neutrophils, we measured apoptosis in PMNs after receptor-mediated phagocytosis. Staining of plasma membrane phosphatidylserine by Annexin V, an indicator of early apoptosis, demonstrated that phagocytosis mediated by FcRs, CRs, and the combination of FcRs and CRs (FcR/CR) each induced apoptosis in PMNs (Fig. 5B). However, FcR/CR-mediated phagocytosis induced apoptosis more rapidly than that by either receptor alone (Fig. 5B). Indicators of mid-late apoptosis, chromatin condensation, and DNA fragmentation confirmed that apoptosis occurred earlier in FcR/CR-activated PMNs (P < 0.01) (Fig. 5 C–E). These observations are consistent with studies demonstrating that receptor cooperativity enhances other PMN functions, e.g., respiratory burst (14) (Fig. 1B). The finding that FcR and CR ligation induced apoptosis is in agreement with two earlier reports, although no comparison was made between receptors or with cooperating receptors (25, 26). The induction of apoptosis in all cells coincided precisely with the time at which specific apoptosis-related genes were differentially expressed during phagocytosis (compare Figs. 3–5A with 5 B–E). Furthermore, the observation that apoptosis occurred most rapidly in PMNs after FcR/CR-mediated phagocytosis correlated directly with the finding that expression of cell-fate genes was greatest in magnitude in those cells (Fig. 5). Therefore, gene expression after phagocytosis not only accurately predicted cell fate but also defined precisely the mechanism and signal transduction pathway for activation-induced apoptosis in human PMNs (Fig. 6).
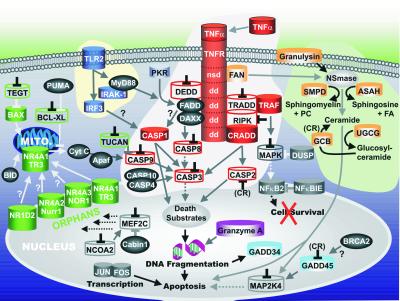
Figure 6.
Model of phagocytosis-induced cell fate in human PMNs. Apoptosis-related genes identified in this study were categorized into signaling pathways involved in cell fate; tumor necrosis factor-receptor (red), ceramide synthesis (light orange), Toll-like receptor signaling (dark blue), mitochondrial (green), or nuclear (gray). Genes up-regulated are illustrated as solid cylinders, genes down-regulated are illustrated as open cylinders, and genes expressed but neither induced nor repressed are depicted by dark gray spheres. Genes down-regulated also contain an inverted black “T” and in some instances repression or induction by a specific receptor is indicated (in parentheses). Solid lines/arrows indicate positive effector functions; dotted lines/arrows indicate the absence of effector functions; gray lines ending with an inverted T indicate block of function. The model is based on gene and protein functions reported in the literature, but has been modified to fit our data set.
Conclusions
Signaling mechanisms governing the ability of PMNs to ingest and kill microorganisms and events that mediate resolution of the inflammatory response remain relatively obscure. Importantly, our studies have generated an understanding of global gene expression patterns in PMNs both for characterized genes and for those genes not previously reported to play a role in neutrophil biology or whose functions are unknown. Furthermore, we have identified and elucidated complex genetic pathways for programmed cell death in human PMNs (Fig. 6). Most significantly, we discovered that neutrophil apoptosis is regulated at the level of gene expression, a finding that has important implications in host defense and pathogenesis of inflammatory diseases. The rapid induction of apoptosis in activated neutrophils and their subsequent removal from inflammatory sites by macrophages is likely important for two reasons. First, apoptosis in PMNs prevents damage to healthy tissues that would occur after necrotic cell lysis (a result of the release of toxic granule contents), thus preventing further inflammation. Second, apoptosis in leukocytes is a critical event for termination of the cellular immune response. For example, abnormalities in the control of cell fate and cessation of inflammation likely play a role in the formation of granulomas in inflammatory disorders such as chronic granulomatous disease (27, 28). The observation that inflammatory mediators produce antiapoptotic effects (29) coupled with recent reports describing phagocytosis of pathogens as proapoptotic (30) underscores the importance of regulated cell fate in leukocytes. However, few studies have focused on regulation of activation-induced apoptosis in PMNs, and the specific genes involved in PMN apoptosis have remained mostly uncharacterized until now. A thorough understanding of the molecular pathways that determine cell fate in PMNs will provide further insight into the pathogenesis of inflammatory diseases and infection processes, resulting potentially in novel therapeutic strategies.
Supplementary Material
Acknowledgments
We thank R. Lempicki and J. Yang (National Cancer Institute, National Institutes of Health, Frederick, MD) for performing the RNA hybridizations and scanning of the Affymetrix GeneChips; G. Bammert and B. Gowen for technical assistance; and J. M. Musser, W. M. Nauseef, and H. L. Malech for critical review of the manuscript.
Abbreviations
- FcR
Fc-receptor
- CR
complement receptor
- PMN
polymorphonuclear leukocyte
- LB
latex beads
- ROS
reactive oxygen species
References
- 1.Nauseef W M, Clark R A. In: Basic Principles in the Diagnosis and Management of Infectious Diseases. Mandell G L, Bennett J E, Dolin R, editors. New York: Churchill Livingstone; 2000. pp. 89–112. [Google Scholar]
- 2.Daeron M. Annu Rev Immunol. 1997;15:203–234. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 3.Sengelov H. Crit Rev Immunol. 1995;15:107–131. [PubMed] [Google Scholar]
- 4.Greenberg S, Chang P, Silverstein S C. J Exp Med. 1993;177:529–534. doi: 10.1084/jem.177.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox D, Tseng C C, Bjekic G, Greenberg S. J Biol Chem. 1999;274:1240–1247. doi: 10.1074/jbc.274.3.1240. [DOI] [PubMed] [Google Scholar]
- 6.Lennartz M R, Brown E J. J Immunol. 1991;147:621–626. [PubMed] [Google Scholar]
- 7.McDonald P P, Fadok V A, Bratton D, Henson P M. J Immunol. 1999;163:6164–6172. [PubMed] [Google Scholar]
- 8.Itoh K, Okubo K, Utiyama H, Hirano T, Yoshii J, Matsubara K. Blood. 1998;92:1432–1441. [PubMed] [Google Scholar]
- 9.Subrahmanyam Y V, Yamaga S, Prashar Y, Lee H H, Hoe N P, Kluger Y, Gerstein M, Goguen J D, Newburger P E, Weissman S M. Blood. 2001;97:2457–2468. doi: 10.1182/blood.v97.8.2457. [DOI] [PubMed] [Google Scholar]
- 10.Boyum A. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- 11.DeLeo F R, Allen L A, Apicella M, Nauseef W M. J Immunol. 1999;163:6732–6740. [PubMed] [Google Scholar]
- 12.Chaussee M S, Watson R O, Smoot J C, Musser J M. Infect Immun. 2001;69:822–831. doi: 10.1128/IAI.69.2.822-831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicoletti I, Migliorati G, Pagliacci M C, Grignani F, Riccardi C. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 14.Zhou M J, Brown E J. J Cell Biol. 1994;125:1407–1416. doi: 10.1083/jcb.125.6.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright S D, Silverstein S C. J Exp Med. 1983;158:2016–2023. doi: 10.1084/jem.158.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilhelm O G, Wilhelm S, Escott G M, Lutz V, Magdolen V, Schmitt M, Rifkin D B, Wilson E L, Graeff H, Brunner G. J Cell Physiol. 1999;180:225–235. doi: 10.1002/(SICI)1097-4652(199908)180:2<225::AID-JCP10>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 17.Gyetko M R, Sitrin R G, Fuller J A, Todd R F, 3rd, Petty H, Standiford T J. J Leukocyte Biol. 1995;58:533–538. doi: 10.1002/jlb.58.5.533. [DOI] [PubMed] [Google Scholar]
- 18.Palicz A, Foubert T R, Jesaitis A J, Marodi L, McPhail L C. J Biol Chem. 2001;276:3090–3097. doi: 10.1074/jbc.M007759200. [DOI] [PubMed] [Google Scholar]
- 19.Tsujioka H, Takami N, Misumi Y, Ikehara Y. Biochem J. 1999;342:449–455. [PMC free article] [PubMed] [Google Scholar]
- 20.Youn H D, Sun L, Prywes R, Liu J O. Science. 1999;286:790–793. doi: 10.1126/science.286.5440.790. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Kolluri S K, Gu J, Dawson M I, Cao X, Hobbs P D, Lin B, Chen G, Lu J, Lin F. Science. 2000;289:1159–1164. doi: 10.1126/science.289.5482.1159. [DOI] [PubMed] [Google Scholar]
- 22.Gamen S, Hanson D A, Kaspar A, Naval J, Krensky A M, Anel A. J Immunol. 1998;161:1758–1764. [PubMed] [Google Scholar]
- 23.Beresford P J, Xia Z, Greenberg A H, Lieberman J. Immunity. 1999;10:585–594. doi: 10.1016/s1074-7613(00)80058-8. [DOI] [PubMed] [Google Scholar]
- 24.Smoot L M, Smoot J C, Graham M R, Somerville G A, Sturdevant D E, Migliaccio C A, Sylva G L, Musser J M. Proc Natl Acad Sci USA. 2001;98:10416–10421. doi: 10.1073/pnas.191267598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gamberale R, Giordano M, Trevani A S, Andonegui G, Geffner J R. J Immunol. 1998;161:3666–3674. [PubMed] [Google Scholar]
- 26.Coxon A, Rieu P, Barkalow F J, Askari S, Sharpe A H, von Andrian U H, Arnaout M A, Mayadas T N. Immunity. 1996;5:653–666. doi: 10.1016/s1074-7613(00)80278-2. [DOI] [PubMed] [Google Scholar]
- 27.Henderson W R, Klebanoff S J. J Biol Chem. 1983;258:13522–13527. [PubMed] [Google Scholar]
- 28.Chin T W, Stiehm E R, Falloon J, Gallin J I. J Pediatr. 1987;111:349–352. doi: 10.1016/s0022-3476(87)80452-3. [DOI] [PubMed] [Google Scholar]
- 29.Lee A, Whyte M K, Haslett C. J Leukocyte Biol. 1993;54:283–288. [PubMed] [Google Scholar]
- 30.Watson R W, Redmond H P, Wang J H, Condron C, Bouchier-Hayes D. J Immunol. 1996;156:3986–3992. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.