Abstract
Sanitary landfilling remains a cost-effective waste management strategy, employing engineered liners and leachate collection systems to mitigate environmental pollution. However, long-term degradation of compacted clay baseliners (CCLs) poses risks to environmental safety and groundwater quality. This study investigated seasonal and habitat-specific microbial communities within CCLs and leachate from the Pulau Burung Sanitary Landfill, Pinang, Malaysia, utilizing 16S rRNA gene amplicon sequencing and functional prediction via PICRUSt2. Triplicate samples were collected from leachate and baseliner layers (0–30 cm depth) during both rainy and dry seasons, alongside assessments of physicochemical properties and permeability. Significant seasonal differences (p < 0.05) were observed in the physicochemical profiles of leachate and baseliner samples. Baseliner microbiomes exhibited greater compositional stability and smaller beta-diversity shifts compared to the more dynamic leachate communities. Alpha diversity increased in both matrices during the dry season, although changes in baseliner richness were not statistically significant (p > 0.05). Microbial community shifts were primarily driven by seasonal variations in environmental parameters. Core phyla shared across both habitats included Pseudomonadota (31.15–45.88%), Bacillota (8.58–31.15%), Actinobacteriota (6.22–19.58%), Acidobacteriota (0.16–15.85%), Chloroflexota (0.85–13.84%), and Bacteroidota (1.38–12.74%). Additional phyla such as Patescibacteria (0.77–2.06%), Cyanobacteria (0.12–6.16%), Desulfobacterota (0.77–5.38%), and Verrucomicrobiota (0.59–2.33%) showed matrix-specific enrichment. Functional prediction revealed distinct enzyme profiles and metabolic pathway enrichment. Anaerobic genera such as Geobacter, Desulfuromonas, Desulfuromusa, Pseudopelobacter, Desulfotomaculum, Clostridium, Desulfitobacterium, Telmatospirillum, and Dethiobacter were associated with redox cycling and mineral-transforming processes, suggesting potential contributions to increased clay porosity and reduced structural integrity. These findings demonstrate the ecological and functional complexity of landfill microbiomes and their potential role in compromising barrier performance. The study recommends routine monitoring of microbial functional genes and the development of biogeochemically resilient clay blends or in situ biobarriers to enhance long-term containment efficacy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10532-025-10185-7.
Keywords: Waste management, Environmental safety, Sanitary landfill, Microbial community, 16S rRNA sequencing, Seasonal variations
Introduction
The increasing amount of municipal solid waste (MSW) poses a significant environmental threat due to the generation of leachate. Leachate, a contaminated liquid, can infiltrate groundwater and surface water, thereby threatening ecological quality due to its diverse composition of pollutants (Lindamulla et al. 2022). Landfilling remains a cost-effective waste management method that involves depositing solid waste in engineered landfills, which are structurally designed with liners, drainage systems, compacted clay, and capping layers to ensure environmental isolation and waste stabilization. Landfill operations are governed by comprehensive regulations and demand continuous environmental monitoring to maintain compliance and safeguard safety (Kumar 2021; Ozbay et al. 2021).
Landfill leachate is a complex mixture of chemicals, including organic acids, ammonium, sulfates, phosphates, heavy metals, and salts. Its composition can vary seasonally. During waste decomposition processes such as hydrolysis and acidogenesis, the pH level decreases, metals are released, and both biochemical and chemical oxygen demands increase (Abdel-Shafy et al. 2024). The dynamics of microbial communities and seasonal changes in landfills are influenced by the composition of leachate and its movement through the basal containment layers (Abiriga et al. 2021). Leachate plumes can spread over considerable distances through percolation, which is affected by factors such as climate, geology, waste characteristics, and groundwater flow (Zubova and Ivantsov 2024). The interaction between leachate and compacted baseliners is crucial for maintaining containment integrity. Engineered liners help reduce the migration of contaminants; however, factors such as pH, ionic strength, redox conditions, and microbial activity can affect the longevity, permeability, and bio-clogging of these liners (Safari and Valizadeh 2018).
Sanitary landfills employ engineered barriers such as cellular construction, composite liners, geomembranes, and compacted clay to contain waste, manage leachate, and prevent environmental contamination (Meyer-Dombard et al. 2020; Tang et al. 2018). Leachate collection systems and methane recovery further reduce environmental risks and support sustainable waste management. Low-permeability soil layers and geomembrane liners impede contaminant movement by restricting both advection and diffusion, thereby safeguarding groundwater and surface water. The effectiveness of these liners largely depends on their hydraulic conductivity, with regulatory standards typically requiring a minimum clay liner thickness of three feet (0.9 m) and a maximum hydraulic conductivity of 1 × 10⁻⁷ cm/s (Texas Commission on Environmental Quality 2015). In developed countries, advanced landfill designs and strict regulations ensure the safe and effective containment of solid waste. In this study, the term “baseliner” refers to the compacted clay liner at the landfill base that prevents leachate migration.
Research has shown that landfill baseliners can deteriorate over time, leading to breaches and the leakage of leachate into surrounding soils and aquifers (Regadío et al. 2015). Microbial activity plays a key role in this deterioration. Gilmour et al. (2022) demonstrated that indigenous iron-reducing microbes in compacted MX80 bentonite can survive extreme conditions, reducing Fe(III) to Fe(II), thereby potentially weakening the barrier's geomechanical integrity. Biofilm-forming genera such as Pseudomonas and Streptomyces promote the chelation of aluminium and iron minerals, altering their structure and permeability. Microorganisms drive biogeochemical cycles by producing acids, alkalis, and reducing agents that influence mineral solubility, thereby enhancing weathering and facilitating the mobilization of elements (Cuadros 2017; Mitzscherling et al. 2023; Wild et al. 2022). Iron-reducing bacteria (FeRB), including Geobacter and Shewanella, facilitate mineral transformations such as the conversion of smectite to illite, which alters clay permeability (Jaisi et al. 2011; Pentráková et al. 2013). Similarly, sulfate-reducing bacteria (SRB), such as Desulfovibrio and Desulfobacterales, generate sulfides that transform clay minerals, reduce their swelling capacity, and increase pore pressure, further compromising barrier effectiveness (Grigoryan et al. 2018; Ruiz-Fresneda et al. 2023). Post-construction microbial redox reactions influence metal speciation and mobility, while ongoing microbial processes drive clay mineral transformations through precipitation, weathering, and early soil formation (Cuadros 2017). Grigoryan et al. reported that fermentative and acid-producing groups, such as Clostridiaceae and Peptococcaceae, can decrease clay cohesion and enhance water diffusivity. Collectively, these microbially driven mechanisms progressively compromise the structural and functional integrity of landfill liner systems.
Recent studies have confirmed that compacted bentonite clays, which are critical components of deep geological repository barriers, can support resilient microbial communities. Engel et al. (2023) reported that sulfate-reducing bacteria (Desulfosporosinus, Desulfovibrio) persist on bentonite surfaces exposed to groundwater for up to five years, although they remain scarce within the bentonite core. Burzan et al. (2022) documented that aerobic microbes can survive deep within MX-80 bentonite under nominally anoxic conditions, demonstrating remarkable metabolic versatility. Vachon et al. (2021) showed that culture-based methods recover only a fraction of the microbial community, whereas DNA-based analyses revealed greater diversity, including sporulating bacteria such as Streptomyces and Bacillus, along with sulfate reducers and denitrifiers. Microcosm studies of uncompacted clays have identified context-dependent sulfide production by diverse taxa (Clostridia, Bacilli, Gammaproteobacteria, Deltaproteobacteria) active under lactate-rich, anoxic conditions (Grigoryan et al. 2018). Tang et al. (2018) demonstrated that Escherichia coli biofilms can alter the hydraulic properties of clay liners, affecting permeability and contaminant migration. Grigoryan et al. (2018), cited alongside Huang et al. (2018), confirmed microbial diversity and sulfide production in commercial clay microcosms, characterized using MPN, DGGE, and high-throughput 16S rRNA sequencing.
Seasonal fluctuations in clay properties closely linked to compaction, water absorption, and retention, and DNA extraction methods significantly affect microbial biomass, activity, and community structure, resulting in discernible seasonal dynamics (Chernov and Zhelezova 2020; Wu et al. 2018). Analyzing microbial communities in compacted clay liners has proven challenging due to the adsorption of nucleic acids by clay, which obstructs DNA recovery in low-biomass environments. Stroes-Gascoyne et al. (2010) showed that highly compacted bentonite, a common engineered barrier in deep geological repositories (DGRs), suppresses microbial culturability, an essential factor in resisting microbially influenced corrosion (MIC). Jalique et al. (2016) further demonstrated that spore-forming Gram-positive bacteria persist in eight-year-old compacted bentonite plugs, indicating dormancy as a survival mechanism under extreme conditions. Beaver et al. (2024) found that dry density and saturation strongly influence initial microbial activity and sulfide production: genera such as Pseudomonas, Bacillus, Cupriavidus, and Streptomyces thrived at lower dry densities before saturation, while higher compaction inhibited growth. These findings explained the consistently low biomass and poor DNA recovery observed in compacted clay liners. Conversely, advanced extraction techniques using desorption buffers and magnetic capture enhance DNA yields but may introduce contaminants that affect downstream analyses (Engel et al. 2019). High-throughput sequencing (HTS), particularly next-generation sequencing, has transformed soil microbial ecology by enabling culture-independent profiling of diverse and rare taxa and facilitating functional inference (Staley and Sadowsky 2016; Thompson et al. 2017). These approaches have revealed microbial diversity and function beyond the reach of traditional methods.
Although previous studies have explored microbial communities in clay liners from various engineered landfills, the seasonal dynamics within compacted clay liners of active sanitary landfills remain insufficiently studied. This research aims to investigate the seasonal variation and functional potential of microbial communities in compacted clay baseliners and leachate environments at an active sanitary landfill. Specifically, it seeks to identify microbial taxa and metabolic pathways that may compromise the integrity of compacted clay liners and inform the development of biogeochemically resilient landfill containment strategies. To achieve this, the study employs 16S rRNA gene amplicon sequencing to investigate seasonal shifts in microbial diversity and functional potential within compacted clay baseliners and landfill leachate at the Pulau Burung sanitary landfill. The specific objectives are to: (i) characterize the biodiversity, composition, and structure of microbial communities associated with baseliner degradation; (ii) assess seasonal variations in microbial community dynamics; and (iii) identify key functional genes and taxa that may compromise the structural integrity of clay liners.
Materials and methods
Study area
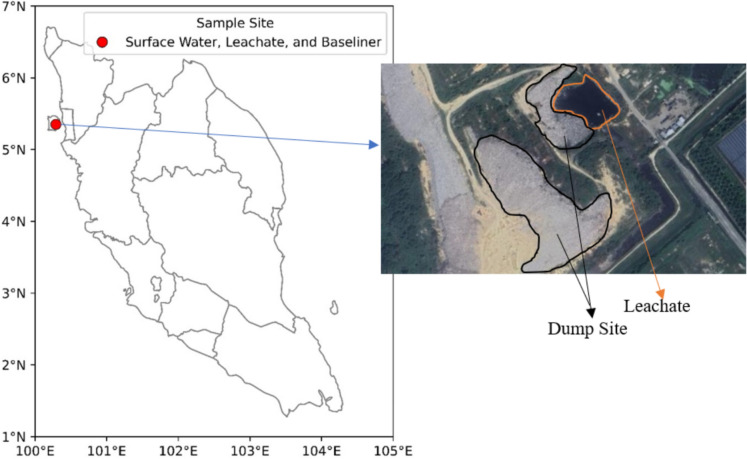
The study was conducted at the Pulau Burung Sanitary Landfill (PBSL), located in Nibong Tebal City, Pulau Pinang, Malaysia. The site spans 62.4 hectares and primarily manages municipal solid waste (Kamaruddin et al. 2016). The landfill, situated at coordinates 5° 35′ 64.2″ N and 100° 29′ 74.22″ E (Fig. 1), has been operational since 1991 and is classified as a Level III facility. Since 2012, it has been managed by Majlis Bandaraya Seberang Perai (MBSP) under a concession agreement to ensure regulatory compliance and efficient waste management (Kamaruddin et al. 2016). Leachate samples were collected from both the discharge point and the reservoir to assess the extent of leachate intrusion into the compacted clay liner. Baseliner samples were obtained along predetermined spatial gradients to ensure systematic coverage of the study area during the peak rainy season (September–November 2023) and the dry season (January–March 2024). Each sample type was collected twice per season, resulting in a total of 120 datasets.
Fig.1.
Location of Pulau Burung Sanitary Landfill, Malaysia, and sampling stations
Sampling for baseliner and leachate samples
Ten baseliner samples (1 kg each) were aseptically collected in triplicate from the surface to the subsurface at the edge of the landfill, following the ASTM E1197-14 standard. Permeability was tested using the falling head method. Samples were aseptically collected from a depth of 0–30 cm using a manual soil auger to ensure consistent depth control and minimize the risk of cross-contamination; the auger was cleaned between each sampling event. The samples were placed in sterile containers, appropriately labeled, and transported to the laboratory for analysis. Sample codes were assigned as 1PBLS1–10 for the rainy season and 2PBLS1–10 for the dry season, following ASTM E1197-14 guidelines. Ten leachate samples (1 L each), labeled 1PBLE1–10 for the rainy season and 2PBLE1–10 for the dry season, were collected using pre-sterilized containers treated with 70% ethanol for 24 h and rinsed with sterile distilled water. Each sample type was collected twice per season, resulting in a total of 120 datasets. These containers were submerged in reservoirs using mesh-covered funnels to prevent contamination. Three replicates were obtained for each sample type and season. All samples were sealed in polythene bags, transported at 4 °C, and stored at − 80 °C until further processing.
Sample analysis
Chemical analyses included measurements of alkalinity (HCO₃⁻, CO₃2⁻), chloride (Cl⁻), sulfate (SO42⁻), nitrite (NO₂⁻), phosphate (PO43⁻), ammonium (NH4+), and biochemical oxygen demand (BOD), using absorbance-based calibration curves and titrimetric methods (Radojevic and Bashkin 2015). Physical parameters, including pH, temperature, dissolved oxygen, salinity, total dissolved solids (TDS), electrical conductivity, and redox potential, were measured using a YSI Professional Plus multi-parameter meter (HI-9828). Quality assurance and control (QA/QC) protocols were rigorously applied, incorporating field blanks to detect contamination, duplicate samples to assess precision, and standard reference materials for calibration and validation.
Baseliner (100 g) and leachate (500 mL) samples were designated as 1PBLS1–3 and 2PBLS1–3 for compacted clay baseliners, and 1PBLLE1–3 and 2PBLLE1–3 for leachate. Genomic DNA was extracted from 0.39 g of baseliner clay using the PrimeWay Soil DNA Extraction Kit (1st BASE, KIT-9060), and from 250 mL of leachate using the DNeasy® PowerWater® Kit (QIAGEN, Cat. No. 14900–50-NF), following the manufacturer’s protocols. All samples were transported on ice and stored at 4 °C to preserve integrity (Environmental Protection Policy (EPP) 2018).
Amplicon sequencing and analysis
Purified gDNAs were amplified targeting the 16S rRNA V3-V4 region using locus-specific primers with Illumina overhang adapters for quality control (5′-TCGTCGGCAGCGTGTATAAGAGACAG-3′, and 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAG-3′). PCR reactions used REDiant 2X PCR Master Mix (1st BASE) and KOD-Multi and Epi® (Toyobo), with dual indices added via the Illumina Nextera XT Index Kit v2. Libraries were quality-checked using the Agilent Bioanalyzer 2100 and Helixyte Green™, normalised, pooled, and sequenced on an Illumina MiSeq with a v3 600-cycle kit, including > 10% PhiX spike-in (2 × 300 bp). Amplicon sequence variants (ASVs) were generated using DADA2 v1.18 (Callahan et al. 2016), with Cutadapt v3.5 for trimming (Martin 2011), SILVA nr database v138.1 for chimera screening and taxonomy assignment, MUSCLE 3.8 for alignment and FastTree2 for phylogenetic analysis (Price et al. 2010) Statistical analyses were performed using R version 3.6.1, including alpha diversity indices (Chao1, Shannon, Simpson), LEfSe, Kruskal–Wallis tests, ALDEx2, and multivariate analyses. The predicted relative abundance of MetaCyc pathways and gene content, combined with ASV abundance data, was used to estimate gene family abundances (Enzyme Commission numbers) using PICRUSt2. Beta diversity between liner and leachate samples was assessed using non-metric multidimensional scaling (NMDS) based on Bray–Curtis distances at the amplicon sequence variant (ASV) level. Physicochemical parameters of leachate and baseliner samples were analysed using t-tests and Spearman's correlation, with significance at p < 0.05. Analysis was conducted in Python 3.12.
Results
Leachate and baseliner environmental parameters
Leachate plume characterization within the baseliner was assessed using independent t-tests (Table 1) and Spearman’s rank correlation (Fig. 2a–d). The results summarize how leachate physicochemical parameters influence the baseliner across seasons. This analysis highlights seasonal variability in leachate composition and its potential impact on the structural integrity of the landfill baseliner. Physicochemical parameters of leachate and baseliner samples were analyzed using independent t-tests to assess mean differences, and Spearman’s rank correlation (Fig. 2a–d) to evaluate monotonic relationships, with statistical significance set at p < 0.05.
Table 1.
Comparison of physicochemical parameters between leachate and baseliner during dry and rainy seasons
| Dry season comparison | p-Value | Rainy season comparison | p-Value |
|---|---|---|---|
| Temp_LD vs Temp_BD | 0.0001 | Temp_LR vs Temp_BR | 0.0001 |
| EC_LD vs EC_BD | 0.001 | EC_LR vs EC_BR | 0.0001 |
| TDS_LD vs TDS_BD | 0.0001 | TDS_LR vs TDS_BR | 0.0001 |
| SAL_LD vs SAL_BD | 0.017 | SAL_LR vs SAL_BR | 0.121 |
| REDOX_LD vs REDOX_BD | 0.0001 | REDOX_LR vs REDOX_BR | 0.004 |
| pH_LD vs pH_BD | 0.0001 | pH_LR vs pH_BR | 0.0101 |
| NH4+_LD vs NH4+_BD | 0.003 | NH4+_LR vs NH4+_BR | 0.0001 |
| PO43⁻_LD vs PO43⁻_BD | 0.0001 | PO43⁻_LR vs PO43⁻_BR | 0.0001 |
| SO42⁻ _LD vs SO42⁻ _BD | 0.333 | SO42⁻_LR vs SO42⁻_BR | 0.0001 |
| ALK_LD vs ALK_BD | 0.329 | ALK_LR vs ALK_BR | 0.838 |
BD baseliner dry season, BR baseliner rainy season, LD leachate dry season, LR leachate rainy season
Fig. 2.
Heatmap of seasonal Spearman correlation of a landfill leachate and baseliner Chemical parameters during the dry season, b landfill leachate and baseliner parameters during the rainy season, c landfill leachate and baseliner physical parameters during the rainy season, d landfill leachate and baseliner physical parameters during the dry season
As shown in Table 2, baseline temperature remained stable across seasons (p > 0.05), with an average of approximately 28.9 °C. In contrast, pH levels declined from near-neutral values during the rainy season to more acidic conditions in the dry season. Alkalinity, ammonium, and phosphate exhibited minimal seasonal variation (p > 0.05), whereas electrical conductivity, redox potential, and salinity significantly increased during the dry period (p < 0.05), indicating elevated ionic activity. Sulfate concentrations nearly doubled, suggesting enhanced mobilization. Overall, most physicochemical parameters were elevated in the dry season, except for pH and total dissolved solids (TDS), which showed a decrease.
Table 2.
Seasonal comparison of physicochemical parameters for baseliner and leachate
| Baseliner seasonal comparison | p-Value | Leachate seasonal comparison | p-Value |
|---|---|---|---|
| Temp_BR vs Temp_BD | 0.788 | Temp_LR vs Temp_LD | 0.0091 |
| pH_BR vs pH_BD | 0.0001 | pH_LR vs pH_LD | 0.0001 |
| EC_BR vs EC_BD | 0.0001 | EC_LR vs EC_LD | 0.0001 |
| TDS_BR vs TDS_BD | 0.070 | TDS_LR vs TDS_LD | 0.004 |
| SAL_BR vs SAL_BD | 0.0001 | SAL_LR vs SAL_LD | 0.438 |
| REDOX_BR vs REDOX_BD | 0.0001 | REDOX_LR vs REDOX_LD | 0.0001 |
| NH4+_BD vs NH4+_BR | 0.176 | TSS_LR vs TSS_LD | 0.0001 |
| PO43⁻_BD vs PO43⁻_BR | 0.143 | NH4+_LR vs NH4+_LD | 0.0001 |
| SO42⁻ _BD vs SO42⁻ _BR | 0.029 | PO43⁻_LR vs PO43⁻_LD | 0.0001 |
| ALK_BD vs ALK_BR | 0.611 | SO42⁻ _LR vs SO42⁻ _LD | 0.0001 |
| ALK_LR vs ALK_LD | 0.0001 | ||
| CL_LR vs CL_LD | 0.0001 |
BD baseliner dry season, BR baseliner rainy season, LD leachate dry season, LR leachate rainy season
Microbial community characteristics: alpha diversity analysis and alpha diversity metrics
This study profiled microbial communities in landfill leachate and baseliner using high-throughput 16S rRNA (V3–V4) amplicon sequencing to enable taxonomic classification and assess alpha diversity. After adapter trimming, read merging, and quality filtering, 1,047,633 high-quality sequences were obtained from 12 samples: 432,878 from the rainy season and 497,174 from the dry season. Both leachate and baseliner metagenomes exhibited high alpha diversity (Table 3). Denoising and clustering at 98% similarity yielded diverse amplicon sequence variants, revealing a rich microbial community. Leachate and baseliner samples had comparable sequencing depth, each producing approximately 11,375 high-quality reads. Rarefaction curves (Fig. 3) confirmed sufficient sequencing depth, supporting robust microbial community characterization and indicating increased alpha diversity during the dry season. ASV distribution revealed seasonal shifts in microbial diversity. In the rainy season, leachate samples (1PBLLE1–3) contained 205,690 ASVs, while liner samples (1PBLS1–3) had 227,188. In the dry season, leachate samples (2PBLLE1–3) showed greater richness with 260,467 ASVs, compared to 236,707 in baseliner samples (2PBLS1–3).
Table 3.
Seasonal comparison of the Chao1, ACE, Shannon, and Simpson diversity indices between leachate and Baseliner
| Index |
1PBLLE Rainy mean |
2PBLLE Dry Mean | p-value | 1PBLS Rainy Mean | 2PBLS Dry Mean | p-value |
|---|---|---|---|---|---|---|
| Chao1 | 595.58 | 436.65 | 0.100 | 755.05 | 777.4 | 1.00 |
| ACE | 596.67 | 437.45 | 0.100 | 757.01 | 777.88 | 1.00 |
| Shannon | 5.08 | 4.65 | 0.100 | 5.52 | 5.42 | 1.00 |
| Simpson | 0.983 | 0.973 | 0.100 | 0.987 | 0.981 | 0.70 |
| Merged | 239,440 | 289,529 | 254,578 | 264,086 | ||
| Rarefied | 205,690 | 260,467 | 227,188 | 236,707 | ||
| No. of species observed | 1,307 | 1,784 | 2,261 | 2328 |
Fig. 3.
Rarefaction curves for the beta Alpha diversity in the leachate and liner. The colored lines represent the observed amplicon sequencing variants across different seasons
Alpha diversity profiling of leachate and baseliner microbial communities revealed consistently high richness, diversity, and evenness across both seasons. DADA2 processing generated high-quality amplicon sequence variants, with coverage estimators (Chao1, ACE, Shannon, Simpson) confirming sufficient sequencing depth for robust characterization (Table 3). Leachate samples (1PBLLE1, 2PBLLE) exhibited seasonal shifts: the dry season showed lower species richness (Chao1, ACE), reduced Shannon diversity, and higher Simpson dominance, reflecting harsher environmental conditions. Although Mann–Whitney tests were not statistically significant (p = 0.1), the trend suggests greater diversity during the rainy season. In contrast, baseliner samples (1PBLS, 2PBLS) showed no significant seasonal variation in diversity indices (species richness and Shannon: p = 1.00; Simpson: p = 0.7), indicating that seasonal factors did not substantially affect microbial diversity.
Seasonal and matrix-specific beta diversity differences in microbial communities
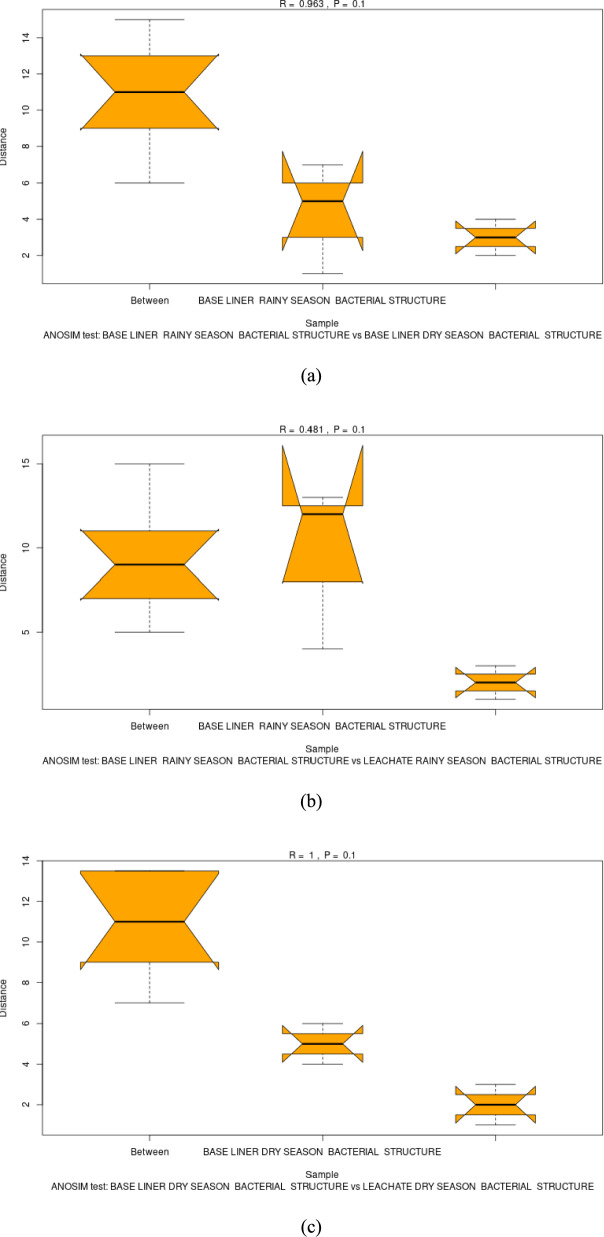
Figure 4a summarizes ANOSIM and pairwise PERMANOVA tests (Table S2) used to assess seasonal and matrix-specific differences in microbial communities. In the baseliner, season had a strong effect (ANOSIM R = 0.963, p < 0.01), explaining approximately one-third of the variance (PERMANOVA R2 = 0.35). Figure 4b shows moderate separation (ANOSIM R = 0.481) between baseliner and leachate communities during the rainy season, likely due to increased hydrological connectivity. In contrast, Fig. 4c reveals complete separation (ANOSIM R = 1) between these communities in the dry season. However, despite this strong divergence, the p-value of 0.1 indicates the difference is not statistically significant between seasons. For leachate, PERMANOVA (R2 = 12.944, p = 0.763) suggested that seasonal variability accounts for a substantial portion of community variance, influencing diversity without fully restructuring the taxa.
Fig. 4.
Box plots of the ANOSIM results on landfill leachate and liner microbial community composition in individual terrain groups and between the groups (a). Compare the baseliner rainy season and baseliner dry season (b). Compare the baseliner and leachate conditions during the rainy season. c Compare the baseliner and leachate conditions during the dry season
Beta diversity of the microbial community in landfill leachate and the baseliner
To assess the influence of seasonal variation on microbial community composition, 3D Principal Coordinate Analysis (PCoA3D) based on Bray–Curtis dissimilarity was performed (Fig. 5). Axis 1 (18.63%) explained the greatest variation in bacterial community structure, indicating the primary dissimilarity pattern, while Axis 2 (12.4%) and Axis 3 (11.29%) accounted for additional variation, collectively capturing over 42% of total dissimilarity across samples. NMDS (Figure S2) with pairwise tests showed a low stress value (0.0939), indicating robust ordination and clear separation between baseliner and leachate samples, influenced by season and sample type. Matrices are key drivers of bacterial community differences. Within the leachate cluster, slight seasonal separation was evident, with dry and rainy season samples clustering more closely to each other than to baseliner samples. In contrast, baseliner samples exhibited clear seasonal separation with greater overall spread. The NMDS plot visually confirmed distinct microbial communities between matrices and highlighted explanatory variables and seasonal variation within each.
Fig. 5.
Eigenvalues in PCoA3d represent the dissimilarities between samples PCoA 3d
Microbial Community Analysis of Leachate and Baseliner Environments.
Taxonomic characterisation of microbial communities across baseliner and leachate environments
Taxonomic classification of 16S rRNA gene amplicons, based on the SILVA nr reference database (v138.1), revealed a taxonomically diverse prokaryotic community in both landfill leachate and compacted clay baseliner samples. In total, 65 phyla, 183 classes, 454 orders, 793 families, and approximately 2,200 unique species-level taxa were identified. Amplicon sequence variant (ASV) abundance profiling revealed ten dominant phyla (Table S3), reflecting substantial variation in microbial community composition and sequencing depth across environmental compartments.
Microbial communities play a fundamental role in ecological processes within landfill environments, influencing waste degradation, gas production, and leachate composition. In this study, domain-level profiling revealed that bacteria were predominant, accounting for 89.9% to 98.2% of total sequences across all samples. Archaea comprised between 1% and 9.1%, with a pronounced seasonal signal observed in archaeal abundance (Fig. 1). Baseliner samples during the rainy season (Fig. 3a) exhibited elevated archaeal representation (1.4%–3.1%) compared to leachate samples (Fig. 3b) (0.9%–2.1%) and dry season baseliner samples (0.1%–2.7%). Archaeal community structure varied across seasons and sample types. During the rainy season (Fig. S3a), baseliner samples were dominated by Euryarchaeota, Halobacterota, and Nanoarchaeota, suggesting active methanogenic and halophilic processes. In contrast, dry season leachate samples (Fig. 3b) were dominated by Halobacterota, Thermoplasmatota, Nanoarchaeota, and Euryarchaeota, while dry season baseliner samples (Fig. 3c) showed enrichment in Crenarchaeota, Nanoarchaeota, and Halobacterota. These shifts reflect the influence of seasonal hydrology and redox conditions on archaeal ecology.
Phylum-level bacterial profiling (Table S4a and b) revealed pronounced seasonal and matrix-specific shifts in community composition. During the rainy season, leachate samples were dominated by Pseudomonadota (30.21 ± 5.54%; formerly Proteobacteria), Bacillota (31.15 ± 0.40%; formerly Firmicutes), Patescibacteria (2.06 ± 0.50%; also known as Patescibacteriota), Verrucomicrobiota (1.35 ± 0.70%), and Desulfobacterota (5.38 ± 2.04%). In contrast, baseliner samples were enriched in Actinobacteriota (19.58 ± 4.32%), Chloroflexota (13.84 ± 6.98%; formerly Chloroflexi), Bacteroidota (12.74 ± 7.55%), Cyanobacteria (4.50 ± 1.25%), and Acidobacteriota (0.16 ± 0.15%), alongside a diverse assemblage of low-abundance phyla. The presence of Chloroflexota and Cyanobacteria in leachate suggests anaerobic degradation potential and a phototrophic legacy, whereas baseliner enrichment in Desulfobacterota, Verrucomicrobiota, and Patescibacteria indicates a structured, redox-adapted subsurface consortium. During the dry season, leachate communities shifted toward increased representation of Bacillota (23.49 ± 1.22%), Bacteroidota (7.37 ± 0.54%), Cyanobacteria (6.16 ± 5.08%), Patescibacteria (1.05 ± 0.17%), and Desulfobacterota (3.59 ± 0.57%). Baseliner assemblages exhibited elevated levels of Pseudomonadota (45.88 ± 4.17%), Actinobacteriota (16.23 ± 4.80%), Chloroflexota (8.81 ± 5.50%), and Verrucomicrobiota (2.33 ± 1.94%), with consistent dominance of Acidobacteriota (15.85 ± 5.58%). Unclassified or low-abundance phyla accounted for approximately 7.29 ± 1.47% in the rainy season and 8.54 ± 6.81% in the dry season. These spatial and temporal shifts reflect seasonal variations in water flow, redox conditions, and nutrient availability, highlighting the dynamic nature of microbial communities across landfill compartments.
Seasonal variations and comparisons of microbial communities in leachate and baseliner environments
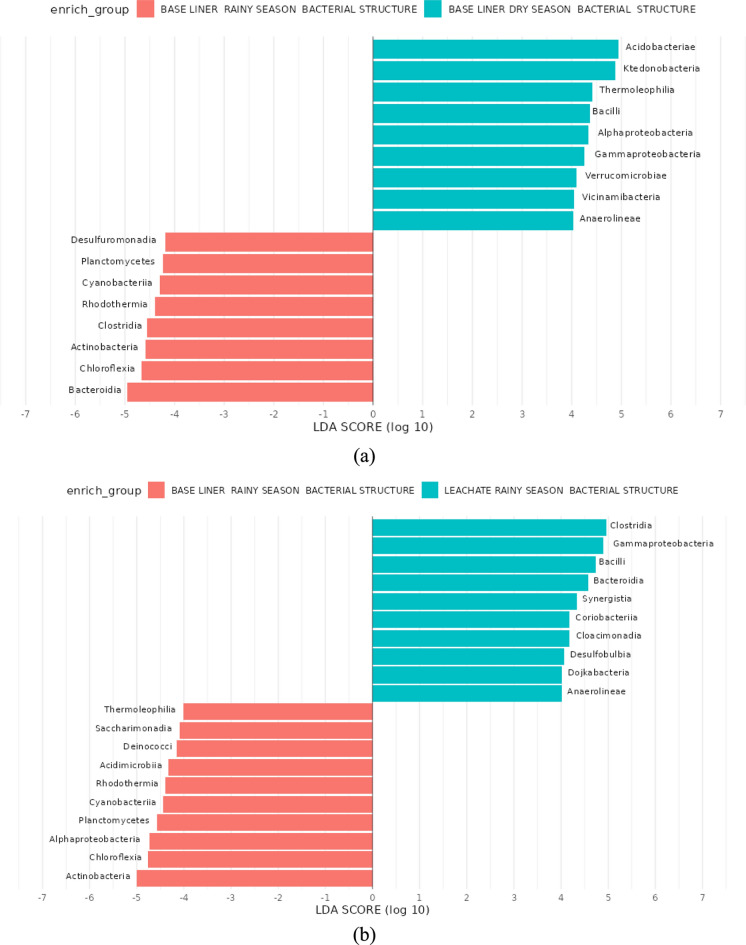
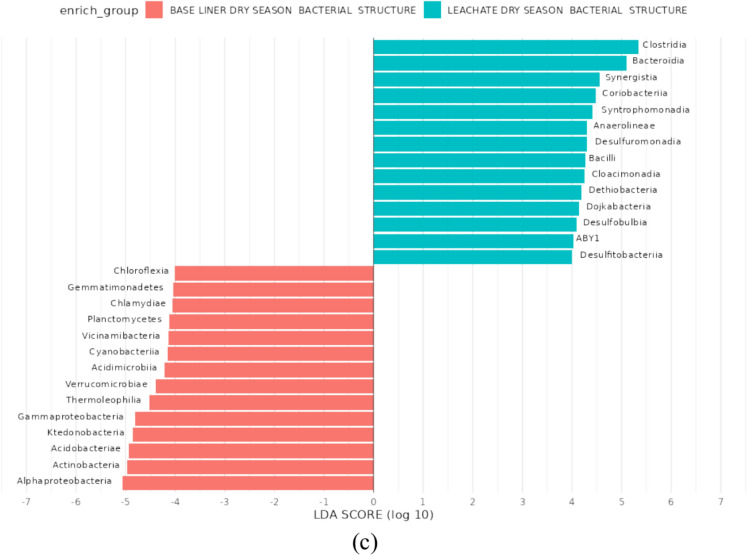
This study evaluated seasonal shifts in microbial community structure and taxonomic composition across landfill leachate and compacted clay baseliner matrices. High-resolution profiling was conducted using 16S rRNA gene amplicon sequencing, followed by biomarker discovery through Linear Discriminant Analysis Effect Size (LEfSe) to identify taxa significantly enriched under different conditions. LEfSe analysis (Fig. 6) identified differentially abundant classes with LDA scores (log₁₀) > 2 as potential biomarkers (Malinowska et al. 2022). Taxonomic classification using the SILVA v138.1 reference database revealed 65 prokaryotic biomarkers exhibiting significant seasonal variation. These biomarkers were categorized by environmental compartment and seasonal prevalence: 17 classes showed differential abundance between baseliner samples from the dry and rainy seasons; 28 classes differed significantly between leachate and baseliner samples during the rainy season; and 20 biomarkers distinguished leachate from baseliner samples in the dry season.
Fig. 6.
A- c LEfSe analysis (LDA) Score diagram shows differentially abundant taxa. Linear discriminant analysis (LDA) score = 2(Log10). a enrich group baseliner rainy and dry season bacterial structure (b) enrich group baseliner rainy season and leachate rainy bacterial structure. c Baseliner dry season and leachate dry season bacterial structure
During the rainy season, the baseliner microbial community was enriched in classes such as Desulfuromonadia, Planctomycetes, Cyanobacteriia, Rhodothermia, Clostridia, Actinobacteria, Chloroflexia, and Bacteroidia. In contrast, dry season baseliner samples exhibited increased representation of Acidobacteriae, Ktedonobacteria, Thermoleophilia, Alphaproteobacteria, Gammaproteobacteria, Verrucomicrobiae, Vicinamibacteria, Anaerolineae, and Bacilli, suggesting adaptations to reduced moisture and nutrient fluxes.
Comparative seasonal analysis revealed distinct biomarker signatures between leachate and baseliner matrices. During the rainy season, the baseliner was enriched with microbial classes including Actinomycetia (formerly Actinobacteria), Chloroflexia, Alphaproteobacteria, Planctomycetia (formerly Planctomycetes), Cyanobacteriia, Acidimicrobiia (formerly Acidimicrobia), Deinococci, Saccharimonadia, Thermoleophilia, and Rhodothermia. In contrast, leachate samples were dominated by Clostridia, Gammaproteobacteria, Bacilli, Synergistia, Coriobacteriia, Bacteroidia, Cloacimonadia, Desulfobulbia, Dojkabacteria, and Anaerolineae. These compositional differences likely reflect the contrasting physicochemical conditions between the compacted clay matrix of the baseliner and the chemically reactive, nutrient-rich environment of the leachate. During the dry season, microbial richness in the baseliner remained relatively stable; however, community composition shifted, with increased representation of Ktedonobacteria, Verrucomicrobiae, Vicinamibacteria, Chlamydiia, and Gemmatimonadetes. Conversely, the leachate underwent substantial restructuring, marked by the emergence of dominant classes including Syntrophomonadia, Desulfuromonadia, Dethiobacteria, and Desulfitobacteriia. These shifts underscore the influence of environmental stressors on microbial community assembly and functional dynamics.
During the dry season, leachate biomarkers were predominantly represented by Bacteroidia, Clostridia, and Synergistia. In contrast, baseline samples were characterized by the presence of Acidobacteriia (GTDB-standardized from Acidobacteriae), Alphaproteobacteria, Actinomycetia (updated from Actinobacteria), and Ktedonobacteria. During the rainy season, the microbial composition of the leachate shifted markedly, with Clostridia, Gammaproteobacteria, and Bacilli emerging as dominant classes, while the baseliner was enriched in Chloroflexia, Alphaproteobacteria, Actinobacteria, and Bacteroidia. Across all samples and seasons, certain taxa, including Clostridia, Desulfuromonadia, Bacteroidia, Gammaproteobacteria, Bacilli, and Anaerolineae, were consistently detected and served as core environmental biomarkers, indicative of microbial resilience and functional versatility in response to fluctuating environmental stressors. These taxa may play pivotal roles in biogeochemical cycling and adaptation within landfill ecosystems. In contrast, rare taxa such as Vicinamibacteria were detected at low relative abundance, representing a minor but ecologically relevant component of microbial diversity within the compacted clay liner environment. Their sporadic presence may reflect specialized niches or stochastic colonization under seasonally constrained conditions.
Functional prediction and microbial metabolic potential in baseliner and leachate samples
The functional potential and metabolic capabilities of bacterial communities in landfill baseliner and leachate environments were inferred using PICRUSt2 (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States 2). This tool employed a hidden-state prediction algorithm to reconstruct functional profiles from 16S rRNA gene amplicon data, linking taxonomy to gene functions and metabolic pathways via curated reference databases (Matchado et al. 2023). In this study, PICRUSt2 predictions were mapped to the Kyoto Encyclopedia of Genes and Genomes (KEGG) database to evaluate the relative abundance of metabolic pathways and enzymes.
The analysis identified 2,402 distinct Enzyme Commission (EC) numbers, each representing a unique enzymatic function (Martínez Cuesta et al. 2015). Oxidoreductases were the most abundant enzyme class, followed by hydrolases, transferases, isomerases, ligases, and lyases, indicating a dominance of redox-related metabolic processes typical of anaerobic landfill conditions. Figure 7 illustrates the distribution of EC numbers across sample types and highlights functionally distinct microbial biomarkers in liner versus leachate matrices. To explore functional and compositional differences, multivariate ordination analyses including Redundancy Analysis (RDA), Principal Coordinate Analysis (PCoA), Canonical Correspondence Analysis (CCA), and Non-metric Multidimensional Scaling (NMDS) were conducted. These methods revealed distinct clustering of microbial communities by sample type and season, demonstrating that both environmental factors and habitat strongly shape microbial composition and functional gene profiles.
Fig. 7.
Cluster distribution of communities between the dry and rainy seasons
Ordination plots further emphasized ecological differentiation between leachate and baseliner microbiomes. Clustering reflected functionally similar microbial assemblages, while separation indicated divergent metabolic profiles. As shown in Fig. 7, baseliner samples consistently clustered together across seasons, suggesting a stable microbial community supported by the relatively uniform physicochemical conditions of the compacted clay matrix. Conversely, leachate samples exhibited greater seasonal variability, particularly during the dry season, reflecting shifts in community structure and functional potential driven by fluctuating nutrient levels, redox gradients, and moisture content.
Figure 8a and b present comparative bar plots (log₁₀ scale) illustrating the relative abundances of enzyme-coding functional genes across six major enzyme classes: oxidoreductases, transferases, hydrolases, lyases, isomerases, and ligases in leachate and baseliner samples across different seasons. These enzyme classes represent key metabolic functions predicted by PICRUSt2, with functional annotations based on Enzyme Commission (EC) numbers. Enzymes with high relative abundance were identified as functionally significant biomarkers within the microbial communities, as detailed in Table S5. Seasonal and environmental variations between sample types were evident in the differential distribution of predicted functional genes. Leachate samples, particularly during the dry season, exhibited increased abundance of EC-annotated enzymes associated with stress response and nutrient cycling. In contrast, liner samples maintained a more stable enzymatic profile across seasons, likely due to the relatively constant physicochemical conditions of the compacted clay liner. These patterns underscore the functional adaptability of microbial communities to changing hydrological and physicochemical conditions.
Fig. 8.
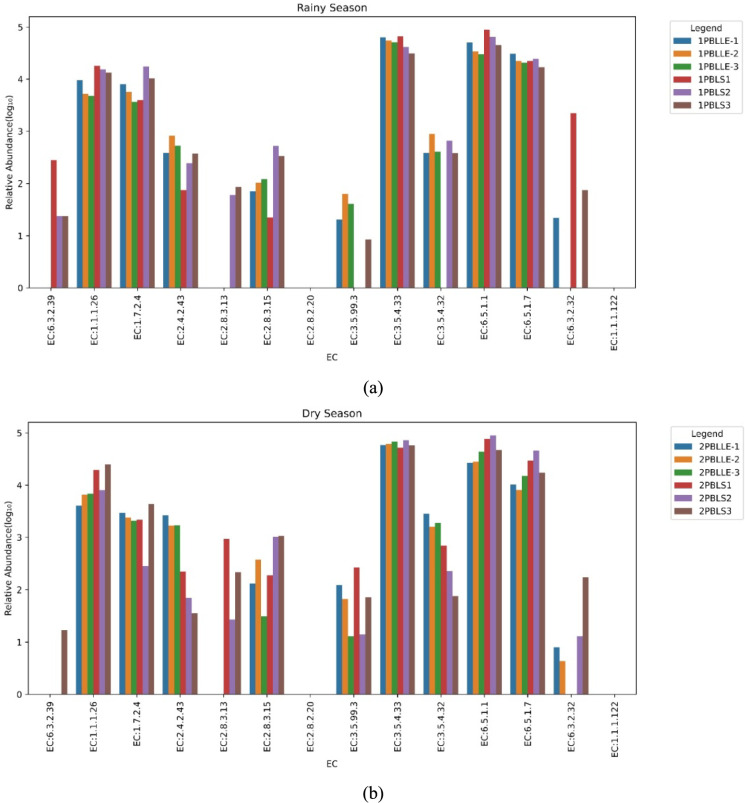
Distribution and abundances of EC in liner and leachate samples, highlighting differences in enzyme functional potential within the microbial communities during (a) Rainy season and (b) Dry season
Certain enzyme-coding genes, such as Aerobactin Synthase (EC:6.3.2.39), Succinate-Hydroxymethylglutarate CoA-Transferase (EC:2.8.3.13), and Succinyl-CoA:(R)-Benzylsuccinate CoA-Transferase (EC:2.8.3.15), were more abundant in baseliner samples across both seasons. In contrast, enzymes such as Nitrous Oxide Reductase (EC:1.7.2.4), Lipid IV(A) 4-amino-4-deoxy-L-arabinosyltransferase (EC:2.4.2.43), Hydroxydechloroatrazine Ethylaminohydrolase (EC:3.5.99.3), and 8-Oxoguanine Deaminase (EC:3.5.4.32) were predominantly present in the leachate samples. These findings suggest that these enzymes played critical roles in the degradation of organic matter within their respective environments. Enzymes such as Glyoxylate Reductase (EC:1.1.1.26), tRNA (adenine (34)) Deaminase (EC:3.5.4.33), DNA Ligase (ATP) (EC:6.5.1.1), DNA Ligase (ATP, ADP, or GTP) (EC:6.5.1.7), and Coenzyme gamma-F420-2: alpha-L-glutamate Ligase (EC:6.3.2.32) were abundant in both liner and leachate samples, indicating shared metabolic capabilities across these environments. The Enzyme Commission (EC) prediction results (Fig. 8a, b) revealed distinct variations in functional gene clusters. Aerobactin Synthase (EC:6.3.2.39) and EC:2.8.3.13 were exclusively identified in baseliner samples, whereas genes associated with EC:3.5.99.3, EC:3.5.4.32, and EC:2.8.3.15 exhibited lower relative abundances, reflecting reduced enzymatic activity and specialized roles under varying environmental conditions and evenness.
Discussion
Recent studies have characterized microbial diversity in engineered environments using both molecular techniques (e.g., 16S rRNA gene amplicon sequencing) and other methods. Although compacted clay liners (CCLs) are critical containment components in sanitary landfills, comprehensive surveys of their autochthonous seasonal microbial communities under actual landfill conditions remain limited. Most existing research has focused on engineered liner systems, with detailed investigations of native microbial assemblages within operational sanitary landfill settings still scarce. A key challenge contributing to this paucity is the difficulty of extracting high-quality DNA from clay-rich matrices such as bentonite, where strong adsorption of DNA to mineral surfaces hinders nucleic acid recovery. While chemical blocking agents can facilitate DNA desorption, their potential effects on microbial community composition and downstream bioinformatics analyses are not yet fully understood (Beaver et al. 2024; Engel et al. 2019; Vachon et al. 2021).
Seasonal analysis of physicochemical parameters revealed the critical role of the baseliner in preventing contaminant migration and shaping microbial dynamics. Microbial proliferation in the baseliner could be influenced by its distinct environmental conditions, which independently structured microbial communities across seasons. Community richness in both leachate and baseliner samples was positively associated with elevated concentrations of physicochemical parameters during the dry season. Baseliner samples consistently exhibited slightly higher species diversity and evenness across both seasons. These findings suggest that the functional diversity and resilience of landfill microbial communities are governed by seasonal dynamics, with a decline during the rainy season and an increase during the dry season (Tables 3a and 3b). Environmental variables, particularly temperature, pH, redox potential, phosphate, ammonium, alkalinity, sulfate, chloride, nitrite, carbonate (CO₃2⁻), and salinity, could be key drivers of bacterial alpha and beta diversity (Tables 3a, 3b; Online Resources Table S1a and S1b).
Seasonal fluctuations, especially elevated nutrient concentrations during the dry season, significantly influenced microbial community structure and functional potential. This was evident from alpha diversity metrics, which showed significantly higher Shannon and Simpson indices in the dry season (p < 0.05), and from ordination analyses (NMDS, PCoA3D), which revealed distinct seasonal shifts in microbial composition despite overall structural stability. These environmental dynamics influenced microbial metabolic functions, as reflected in clustering patterns and shifts in the abundance of functional genes. Microbial communities demonstrated functional plasticity, with resilient taxa becoming dominant under changing conditions, driving compositional reorganization and seasonal transitions in community assemblages (Chen et al. 2024; Jiang et al. 2024).
Seasonal variations in microbial communities within baseliner samples, as revealed by PCoA 3D eigenvalues and NMDS analyses, were subtle yet discernible. Fluctuations in physicochemical parameters appeared to drive minor shifts in community structure, even though the baseliner system maintained stable ecosystem functions that supported consistent inorganic nutrient levels. However, elevated sulfate, salinity, and variable redox conditions may impose selective pressures, favoring more resilient and tolerant taxa depending on the intensity of these stressors (Li et al. 2021; Wang et al. 2021). Significant seasonal pH variations were observed (Table S1a), shifting from neutral during the rainy season to slightly acidic in the dry season. This acidification reflects increased acidogenesis, marked by elevated concentrations of organic acids and volatile fatty acids (VFAs), which are key intermediates in anaerobic digestion (Cheah et al. 2019; Sanchez-Monedero et al. 2018). Lower pH conditions promote the proliferation of acidophilic microbes, increasing diversity among acid-tolerant taxa while suppressing non-acidophilic populations. pH fluctuations may also alter the chemical stability and physical properties of the baseliner, affecting its moisture content and structural integrity. These changes enhance organic matter decomposition in the leachate, further influencing microbial dynamics and biogeochemical cycling (Faraghati et al. 2019).
Salinity is a key environmental factor influencing bacterial community structure and function. Elevated salinity imposes selective pressure, suppressing sensitive taxa, reducing biomass, and lowering alpha diversity, while favoring halotolerant and halophilic microorganisms (Li et al. 2021). Seasonal fluctuations in alpha diversity in leachate samples (Table 3) reflect the dynamic influence of salinity and other environmental variables between the rainy and dry seasons (Table S1b). The present findings align with established patterns, showing a clear inverse relationship between salinity and microbial diversity. UPGMA clustering based on microbial community composition revealed distinct seasonal groupings of baseliner and leachate samples (Figure S5), further supported by ordination analyses (Figs. 4 and S4), which indicated compositional shifts linked to seasonal changes in environmental parameters. During the dry season, reduced salinity coincided with higher alpha diversity, suggesting a relaxation of selective pressure on salt-tolerant genera such as Marinobacter, Paracoccus, and Thermovirga.
This pattern is consistent with previous studies showing that salinity modulates microbial diversity and function by altering the abundance of genes encoding glycosyl transferases and glycoside hydrolases, thereby affecting pathways involved in carbon degradation, nitrogen cycling, and nutrient turnover (Yang et al. 2021a, b; Zhang et al. 2021). Figure S5 also illustrates seasonal microbial composition in the baseliner, with Comamonas and Aeromicrobium predominating during the rainy season. Both genera are adapted to low-salinity, non-halophilic environments and thrive at neutral to slightly alkaline pH levels (6.5–7.7). Their prevalence correlates with reduced salinity and stable pH conditions during the rainy season (Table S1a), underscoring the role of physicochemical factors in shaping microbial community structure.
Conversely, the observed decrease in microbial diversity within landfill baseliner (CCLs) during the rainy season is likely attributable to the clay matrix’s physical and hydraulic properties. Vachon et al. (2021) reported that highly compacted bentonite clay inhibits microbial growth by reducing water activity and swelling upon saturation, which reduces pore space and restricts nutrient and oxygen transport. The relatively stable physicochemical conditions observed during the rainy season (Table 1) reflect the CCL’s buffering capacity, characterized by low hydraulic conductivity and limited leachate infiltration. These properties underscore the baseliner’s effectiveness as a barrier, minimizing contaminant migration while maintaining structural integrity (Nugroho et al. 2021)..
This geotechnical stability, driven by the compaction and swelling behavior of the liner, creates a controlled subsurface microenvironment that supports specialized microbial consortia. These communities are likely dominated by taxa adapted to hydrologically constrained and nutrient-limited conditions. As a result, a more uniform and functionally resilient microbial community persists across seasons, exhibiting diverse metabolic capabilities inferred from predicted gene functions (Fig. 8). The baseliner bacterial community showed greater compositional stability and minor seasonal diversity shifts, as evidenced by the tighter clustering of baseliner samples compared to leachate samples in ordination and UPGMA clustering analyses. This functional redundancy and compositional stability suggest that compacted clay liners harbor a core microbiome adapted to persist under fluctuating environmental pressures and engineered containment conditions. This aligns with the fact that sanitary landfill baseliners harbor a resilient core microbiome that has adapted to endure fluctuating environmental conditions and engineered containment barriers.
Figure S5 illustrates that environmental variables exerted a stronger influence on leachate samples than on baseliner samples, resulting in greater heterogeneity and seasonal fluctuations in microbial alpha diversity. Notably, leachate samples exhibited more pronounced seasonal shifts in microbial diversity than baseliner samples. Phylogenetic clustering and ordination analyses confirmed distinct microbial community structures, with clear separation based on sample matrix and season. Figure S6 further demonstrates that leachate communities are less diverse and less even, dominated by a few abundant taxa, indicative of a more selective environment. In contrast, baseliner communities display greater diversity and evenness, suggesting a broader range of microbial taxa with more balanced relative abundances.
Community composition differences between experimental groups were evaluated using ANOSIM to assess beta-diversity separation (Tang et al. 2016). This study quantified seasonal and habitat-specific differences in beta-diversity and community composition shifts using ANOSIM and pairwise PERMANOVA on ASV–level Bray–Curtis distance matrices. Baseliner samples formed distinct clusters separate from leachate samples, indicating matrix-dependent microbiomes. Within baseliner samples, strong seasonal separation was observed (ANOSIM R = 0.963, p < 0.01; PERMANOVA R2 = 0.35), reflecting compositional shifts driven by seasonal variation and baseliner characteristics. During the rainy season, leachate samples clustered closer to baseliner samples (ANOSIM R = 0.481, p < 0.05), likely due to increased hydrological connectivity, whereas dry-season leachate samples diverged markedly (ANOSIM R = 1.0, p < 0.01). Both environments exhibited seasonally dynamic beta-diversity, with varying degrees of overlap corresponding to environmental stability and diversity-abundance patterns. These findings are consistent with NMDS and 3D PCoA analyses, which similarly revealed distinct seasonal clustering of leachate and liner microbial communities. These results contrast with earlier studies reporting that leachate significantly disrupts microbial diversity and community assembly, often indicating stress-induced shifts (K. Zhang et al. 2020). Microbial taxa serve as sensitive bioindicators of soil health, rapidly responding to contamination and reflecting ecosystem stress (Gu et al. 2022).
The distribution of common bacterial phyla associated with landfills during both the rainy and dry seasons reflects the typical microbiomes found in landfills and compacted clay baseliners. Proteobacteria, in particular, play a central role due to their metabolic versatility and key functions in nutrient cycling and biogeochemical processes (Chitthan et al. 2023; Köchling et al. 2015). Although archaeal communities in landfill baseliners remain underexplored, 16S rRNA gene analysis in this study revealed increased relative abundances of Nanoarchaeota, Crenarchaeota, and Halobacterota in baseliner samples. In contrast, leachate metagenomes were primarily dominated by Thermoplasmatota, Nanoarchaeota, and Euryarchaeota, indicating matrix-specific archaeal assemblages likely shaped by contrasting physicochemical conditions. These primarily methanogenic archaea drive methane production under anoxic conditions and support nutrient cycling, highlighting their ecological resilience and adaptation to landfill environments (Petitjean et al. 2015).
Pairwise comparisons of dominant microbial phyla between landfill leachate and baseliner metagenomes using STAMP identified ten core taxa shared across both environments. Indicator species analysis (Figure S4) highlighted Pseudomonadota, Synergistota, Bacillota, and low-abundance Atribacterota (formerly Calidatribacterota) as signature taxa in leachate samples during the rainy season. Atribacterota are known for anaerobic fermentation of complex organic matter, producing substrates essential for syntrophic methanogens (Jiao et al. 2024; Li et al. 2020; (Katayama et al. 2020). In contrast, Actinobacterota, Acidobacteriota, Planctomycetota, and Chloroflexota predominated in baseliner samples, reflecting adaptation to stable, oligotrophic environments. low-abundance Deinococcota were also detected, indicating stress resilience, but there is no evidence that they actively participate in organic matter degradation or nutrient cycling within clay environments. These phyla contribute to carbon turnover and nutrient cycling through diverse metabolic capabilities suited to clay-bound, low-nutrient habitats (Dragone et al. 2024; Jaeger et al. 2024; Lopez-Fernandez et al. 2015).
Seasonal changes significantly influenced microbial community composition in both matrices. During the dry season, leachate samples showed minor shifts, with Bacillota, Bacteroidota, and Synergistota contributing to anaerobic hydrolysis and fermentation, while Halobacterota, though less studied, may reflect halophilic adaptation (Ke et al. 2022; Yang et al. 2021a, b). Conversely, baseliner samples favored Pseudomonadota, which support biofilm formation and mineral interactions (Mitzscherling et al. 2023), along with Actinobacteriota and Acidobacteriota, which facilitate organic matter turnover and carbon cycling. Adapted to oligotrophic conditions, Acidobacteriota may also contribute to structural cohesion, though their functional activity in compacted clay remains poorly understood (Bhatti et al. 2017; de Castro et al. 2013). These groups likely form the core microbiome, vital for maintaining ecosystem function amid changing conditions (Jiang et al. 2024; Oliverio et al. 2017).
LEfSe biomarkers (Fig. 6a–c) were analyzed using PICRUSt2 to predict functional potential through MetaCyc pathways. Predictive profiling highlighted enriched nitrogen and sulfur metabolism, consistent with dominant anaerobic respiratory processes in landfill leachate and liner systems. Seasonal fluctuations in environmental variables modulated microbial biomarkers, altering nutrient availability and promoting the proliferation of rare taxa such as Vicinamibacteria and Dojkabacteria, particularly during the dry season (Hu et al. 2024; McReynolds et al. 2025). Resilient bioindicator classes distinguished microbial community structures between rainy and dry seasons, demonstrating adaptability to changes in moisture, nutrient levels, temperature, and precipitation. Metagenomic analysis further identified sulfur-cycling biomarkers, notably enriched taxa from the classes Desulfobulbia and Dethiobacteria, known for anaerobic sulfur reduction (Simon and Kroneck 2013. In contrast, Desulfitobacteriia are associated with reductive dehalogenation and sulfite reduction (Villemur et al. 2006). These groups also harbor diverse carbohydrate-active enzymes (CAZymes), including cellulases, glycoside hydrolases, glucokinase, and gluconeogenic enzymes, underscoring their roles in carbohydrate metabolism and polysaccharide degradation (Chen et al. 2021; Zheng et al. 2021).
A high relative abundance of carbohydrate-active taxa was detected, including Clostridia and Bacteroidia, anaerobic degraders that ferment sugars into organic acids and alcohols in oxygen-limited leachate (Nair et al. 2024). Acidobacteriae produce exopolysaccharides that aid in soil aggregation, nutrient retention, and resilience to osmotic and nutrient stress, and support carbohydrate turnover (Kalam et al. 2020). Stress-tolerant generalists such as Gemmatimonadetes and Ktedonobacteria have been reported to persist in dry, nutrient-poor environments, contributing to slow carbon turnover (Mucsi et al. 2024; Yabe et al. 2017). Anaerolineae, common in anaerobic digesters and leachate, play key roles in complex carbohydrate degradation (Chelliapan et al. 2019), while Syntrophomonadia specialize in fatty acid degradation via syntrophic interactions. Thermoleophilia and Verrucomicrobiae can adapt to arid soils and complex matrices, contributing to polysaccharide degradation and carbon cycling (Marasco et al. 2021).
In leachate samples, Clostridia, Coriobacteriia, Bacteroidia, and Anaerolineae dominated carbohydrate metabolism, facilitating cellulose hydrolysis, fermentative sugar breakdown, and central carbon flux flux (Lanigan et al. 2019). Across both baseliner and leachate microbiomes, sequences encoding glycolytic enzymes such as pyruvate and glyceraldehyde-3-phosphate dehydrogenases were linked to Chlamydiae, Chloroflexia, Syntrophomonadia, and Bacilli, indicating strong functional capacity for central carbohydrate metabolism. Pathways related to fermentation, hydrogen, protein, and iron metabolism were abundant across sample matrices and seasons, with increased activity during the dry season. These classes contribute unique traits that enhance ecosystem adaptability and resilience, consistent with the ecological “insurance effect” observed across terrestrial, aquatic, and host-associated microbiomes (Jousset et al. 2017).
The analysis identified distinct biomarker enrichment within the compacted liner environment, predominantly comprising oligotrophic and stress-adapted taxa such as Ktedonobacteria, Verrucomicrobiae, Vicinamibacteria, Chlamydiae, and Gemmatimonadetes. The leachate microbiome exhibited pronounced temporal variability, dominated by anaerobic and syntrophic taxa such as Syntrophomonadia, Desulfuromonadia, Dethiobacteria, and Desulfitobacteriia organisms implicated in fatty acid degradation, extracellular electron transfer, and haloalkaline adaptation, respectively (Soares et al. 2024; Sorokin and Merkel 2022). This pattern reflects strong spatial structuring and habitat specificity in the landfill environment. Additionally, low-abundance taxa representing less than 0.1% of the total microbial community harbor extensive genetic and functional potential. These low abundance organisms may enhance ecosystem resilience by contributing to functional redundancy, thereby providing an ecological insurance mechanism under fluctuating or stressful environmental conditions (Collingro et al. 2020; Lynch and Neufeld 2015; Pascoal et al. 2021).
Figure 8 illustrates distinct enzymatic profiles between microbial communities inhabiting the landfill liner and leachate, reflecting divergent metabolic functional potentials. Taxonomic diversity supports the synthesis of a broad spectrum of metabolic enzymes, each adapted to specific substrate types. Seasonal fluctuations in community composition further modulate enzymatic activity, demonstrating the dynamic responsiveness of microbial metabolic pathways to environmental variability (Wani et al. 2022). Spatial and temporal heterogeneity in microbial assemblages across liner and leachate systems is primarily driven by key environmental variables, including nutrient availability, moisture content, redox potential, and salinity, which differentiate the two habitats. These factors are closely associated with soil properties and exert a more pronounced influence on microbial community structure than soil moisture alone. Additionally, shifts in community composition along moisture gradients underscore taxon-specific sensitivities to hydrological changes, highlighting the complex interplay of environmental drivers that shape microbial diversity and functional capacity (Long et al. 2016).
Metagenomic profiling revealed significant variation in enzyme abundance across samples. Elevated levels of Aerobactin synthase (EC 6.3.2.39) and Succinate hydroxymethylglutarate CoA-transferase (EC 2.8.3.13) in baseliner samples across seasons likely reflect site- and season-specific metabolic adaptations, including siderophore-mediated iron acquisition and specialized CoA transfer reactions. In contrast, leachate samples consistently exhibited high relative abundances across diverse enzyme classes, indicative of metabolically versatile and dynamic microbial communities. Fluctuations in baseliner, such as pH, emerged as key environmental drivers influencing microbial composition and enzymatic activity. Functional gene profiling further revealed enrichment of genes associated with protein biosynthesis, DNA repair, and replication during both rainy and dry seasons. Notably, genes encoding tRNA-specific deaminase (EC 3.5.4.33), DNA ligases (EC 6.5.1.7 and EC 6.5.1.1), and oxidative stress response enzymes such as superoxide dismutase, catalase, and glutathione reductase were highly abundant in both leachate and baseliner metagenomes. These genomic signatures demonstrate the adaptive strategies employed by landfill microbiomes to mitigate oxidative stress, manage nutrient limitations, and maintain essential cellular functions under fluctuating environmental conditions.
Functional gene analysis revealed significant enrichment of glyoxylate reductase (EC 1.1.1.26) and nitrous oxide reductase (EC 1.7.2.4), demonstrating their roles in organic carbon turnover and greenhouse gas mitigation. Glyoxylate reductase catalyzes the reduction of glyoxylate to glycolate, a key reaction in the glyoxylate cycle and carbon assimilation (Schneider et al. 2014). Nitrous oxide reductase facilitates the exclusive conversion of N₂O to N₂, completing the denitrification pathway and mitigating greenhouse gas emissions (Lee et al. 2022). Additionally, the abundance of genes associated with xenobiotic degradation, nutrient cycling, and DNA repair highlights the functional resilience of landfill microbial communities under environmental stress (Ratzke and Gore 2018). Seasonal metagenomic profiling of baseliner samples further identified the presence of dissimilatory iron-reducing and sulfate-reducing bacteria, indicating active redox processes and microbial adaptation to fluctuating geochemical conditions.
The microbial communities detected in baseliner encompass taxa known to perform key biogeochemical functions, including sulfur and iron cycling, organic matter degradation, and redox transformations. Genera detected in the microbial community (Table S6) included Geobacter, Desulfuromonas, Desulfuromusa, and Pseudopelobacter (class Desulfuromonadia), Desulfotomaculum and Clostridium (class Clostridia), Desulfitobacterium (class Desulfitobacteriia), Telmatospirillum (class Alphaproteobacteria), members of the class Dethiobacteria, and the class Bacilli. Among these, the genera within the class Desulfuromonadia, notably Geobacter, Desulfuromonas, Desulfuromusa, and Pseudopelobacter, are well-known dissimilatory iron and sulfur reducers that can reduce ferric iron (Fe(III)) as well as various sulfur compounds (Langwig et al. 2022). Desulfitobacterium (class Desulfitobacteriia) engages in dissimilatory sulfur transformations, influencing iron mineral dissolution and reprecipitation (Comensoli et al. 2018). The genus Desulfotomaculum (class Clostridia) can reduce both sulfate and ferric iron in subsurface environments, linking anaerobic respiration to organic matter degradation (G. Yang et al. 2016). Clostridium spp. mediate Fe(III) reduction during fermentative metabolism, highlighting metabolic versatility in iron cycling (List et al. 2019). The Alphaproteobacterial genus Telmatospirillum exhibits non-conventional iron-reducing pathways through non-classical electron transfer pathways (Gagen et al. 2019). Dethiobacter (class Dethiobacteria). generate H₂S through sulfate, sulfur, or thiosulfate reduction pathways (Melton et al. 2017). Sulfide production by these anaerobic taxa in compacted clay liners can alter clay mineralogy, decrease pH, and drive microbially influenced corrosion (MIC) (Hadi et al. 2023) thereby compromising liner integrity.
The study’s findings, based on metagenomic analysis, provide evidence that diverse microbial consortia actively colonize sanitary landfill liners designed to contain leachate plumes. These microbes utilize various metabolic pathways and enzymes to survive the extreme compaction and nutrient-poor conditions of the liner. Microbial activity, particularly genes encoding hydrolytic and redox enzymes, can gradually degrade the baseliner's integrity despite its reliance on low hydraulic conductivity and structural cohesion. Enzymatic mineral solubilization, organic acid production, and redox cycling disrupt geochemical stability by increasing porosity, reducing cohesion, and dissolving iron-bearing minerals. This study predicts that such microbial transformations could gradually compromise the liner’s physical barrier, endangering its long-term efficiency without proper monitoring and mitigation. To address this, the study recommends incorporating microbial functional gene monitoring, especially genes linked to redox cycling and mineral degradation, into seasonal landfill management. It also suggested developing microbial-resistant clay blends or in-situ biobarriers to improve long-term liner durability and sustainability. A key limitation of this research is the lack of direct experimental assessment of microbial impacts on the integrity and performance of compacted clay liners. Future investigations should focus on evaluating how microbial activity influences liner durability under real-world conditions.
Conclusion
This study investigated the bacterial community structure, identified discriminant taxa, and analyzed ecological function changes in landfill baseliners and leachate using next-generation sequencing (NGS). Significant differences in microbial diversity and evenness were observed between baseliner and leachate samples, influenced by seasonal and physicochemical variations. Dominant and low-abundance played a notable role in shaping community profiles, while environmental factors independently drove changes in composition, abundance, and functional capabilities.
Microbial richness and evenness displayed remarkable stability; however, species abundance varied significantly with seasonal changes, reflecting microbial heterogeneity. The microbial communities within the liners demonstrated resilience and functional potential, with minimal seasonal variation, attributed to high diversity and abundance. Importantly, no evidence of cross-contamination was found between leachate and baseliner microbial communities, emphasizing the baseliner's ability to support diverse and functionally robust assemblages. These findings provide valuable insights into the dual role of microbial communities in landfill ecosystems, offering opportunities to enhance liner integrity and ecosystem management. The results can inform policies and strategic decisions for optimizing sanitary landfill operations and preserving environmental sustainability.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are deeply grateful to Universiti Sains Malaysia for their financial support and to the Pulau Burung Sanitary Landfill (PBSL) in Nibong Tebal City, Malaysia, for providing access to their facility for sampling and Apical Scientific Lab Malaysia for Metagomics and bioinformatics analyses, which were critical to the study's success.
Author contributions
George Obinna Akuaka, Hazzeman Haris, Kamarul Zaman Zarkasi, Go Furusawa, and Nyok-Sean Lau contributed to the study's conception and design. George Obinna Akuaka prepared materials and collected data, and Vine Nwabuisi Madukpe, Go Furusawa, and Nyok-Sean Lau performed data analyses. George Obinna Akuaka wrote the first draft of the manuscript, and all authors commented on previous versions. All authors read and approved the final manuscript.
Funding
Open access funding provided by The Ministry of Higher Education Malaysia and Universiti Sains Malaysia.
Data availability
The data used in this work has been deposited with National Center Biotechnology International NCBI with Accession reference No: PRJNA1201362 and SRA Submission ID: SUB14944080.
Declarations
Competing interests
The authors have no competing interests or financial affiliations relevant to the content of this article to declare.
Ethical Approval
This declaration is not applicable, as this study does not involve human images, human data, or the use of animals.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdel-Shafy HI, Ibrahim AM, Al-Sulaiman AM, Okasha RA (2024) Landfill leachate: sources, nature, organic composition, and treatment: an environmental overview. Ain Shams Eng J 15(1):102293. 10.1016/j.asej.2023.102293 [Google Scholar]
- Abiriga D, Jenkins A, Alfsnes K, Vestgarden LS, Klempe H (2021) Spatiotemporal and seasonal dynamics in the microbial communities of a landfill-leachate contaminated aquifer. FEMS Microbiol Ecol. 10.1093/femsec/fiab086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver RC, Vachon MA, Tully CS, Engel K, Spasov E, Jeffrey Binns W, Noël JJ, Neufeld JD (2024) Impact of dry density and incomplete saturation on microbial growth in bentonite clay for nuclear waste storage. J Appl Microbiol. 10.1093/jambio/lxae053 [DOI] [PubMed] [Google Scholar]
- Bhatti AA, Haq S, Bhat RA (2017) Actinomycetes benefaction role in soil and plant health. Microb Pathog 111:458–467. 10.1016/j.micpath.2017.09.036 [DOI] [PubMed] [Google Scholar]
- Burrell PC, O’Sullivan C, Song H, Clarke WP, Blackall LL (2004) Identification, detection, and spatial resolution of Clostridium populations responsible for cellulose degradation in a methanogenic landfill leachate bioreactor. AEM 70(4):2414–2419. 10.1128/AEM.70.4.2414-2419.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzan N, Murad LR, Frutschi M, Janowczyk A, Reddy B, Rance A, Diomidis N, Bernier-Latmani R (2022) Growth and persistence of an aerobic microbial community in Wyoming Bentonite MX-80 despite anoxic in situ conditions. Front Microbiol. 10.3389/fmicb.2022.858324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP (2016) DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13(7):581–583. 10.1038/nmeth.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi R, Altman T, Billington R, Dreher K, Foerster H, Fulcher CA, Holland TA, Keseler IM, Kothari A, Kubo A, Krummenacker M, Latendresse M, Mueller LA, Ong Q, Paley S, Subhraveti P, Weaver DS, Weerasinghe D, Zhang P, Karp PD (2014) The MetaCyc database of metabolic pathways and enzymes, and the BioCyc collection of pathway/genome databases. Nucleic Acids Res 42(D1):D459–D471. 10.1093/nar/gkt1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah YK, Vidal-Antich C, Dosta J, Mata-Álvarez J (2019) Volatile fatty acid production from mesophilic acidogenic fermentation of organic fraction of municipal solid waste and food waste under acidic and alkaline pH. Environ Sci Pollut Res Int 26(35):35509–35522. 10.1007/s11356-019-05394-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelliapan S, Abdullah N, Danish Ahmad M, Ahmad I (2019) Sanitary landfill is a solution in solid waste management or a silent threat to environment: Malaysian scenario sanitary landfill is a solution in solid waste management or a silent threat to environment: Malaysian scenario article history. Open Int J Inform 7. https://www.researchgate.net/publication/341651210
- Chen D, Sun W, Xiang S, Zou S (2021) High-Throughput Sequencing Analysis of the Composition and Diversity of the Bacterial Community in Cinnamomum camphora Soil. Microorganisms, 10(1):72. 10.3390/microorganisms10010072 [DOI] [PMC free article] [PubMed]
- Chen CZ, Li P, Liu L, Sun YJ, Ju WM, Li ZH (2024) Seasonal variations of microbial communities and viral diversity in fishery-enhanced marine ranching sediments: insights into metabolic potentials and ecological interactions. Microbiome 12(1):209. 10.1186/s40168-024-01922-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernov TI, Zhelezova AD (2020) The dynamics of soil microbial communities on different timescales: a review. Eurasian Soil Sci 53(5):643–652. 10.1134/S106422932005004X [Google Scholar]
- Chitthan V, Banu N, Thajuddin N, Dhanasekaran D (2023) Sampling and identification of toxic cyanobacteria in the landfill leachate. In: Protocols for cyanobacteria sampling and detection of cyanotoxin, pp 53–60. Springer, Singapore. 10.1007/978-981-99-4514-6_7
- Collingro A, Köstlbacher S, Horn M (2020) Chlamydiae in the environment. Trends Microbiol 28(11):877–888. 10.1016/j.tim.2020.05.020 [DOI] [PubMed] [Google Scholar]
- Comensoli L, Maillard J, Kooli W, Junier P, Joseph E (2018) Soluble and solid iron reduction assays with Desulfitobacterium hafniense. Bio Protoc. 10.21769/BioProtoc.3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadros J (2017) Clay minerals interaction with microorganisms: a review. Clay Miner 52(2):235–261. 10.1180/claymin.2017.052.2.05 [Google Scholar]
- de Castro VHL, Schroeder LF, Quirino BF, Kruger RH, Barreto CC (2013) Acidobacteria from oligotrophic soil from the Cerrado can grow in a wide range of carbon source concentrations. Can J Microbiol 59(11):746–753. 10.1139/cjm-2013-0331 [DOI] [PubMed] [Google Scholar]
- Dragone NB, Hoffert M, Strickland MS, Fierer N (2024) Taxonomic and genomic attributes of oligotrophic soil bacteria. ISME Commun. 4(1). 10.1093/ismeco/ycae081 [DOI] [PMC free article] [PubMed]
- Engel K, Coyotzi S, Vachon MA, McKelvie JR, Neufeld JD (2019) Validating DNA extraction protocols for bentonite clay. mSphere. 10.1128/mSphere.00334-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel K, Ford SE, Binns WJ, Diomidis N, Slater GF, Neufeld JD (2023) Stable microbial community in compacted bentonite after 5 years of exposure to natural granitic groundwater. mSphere. 10.1128/msphere.00048-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Environmental Protection Policy (EPP) (2018) Environmental Protection (Water) Policy 2009-Monitoring and Sampling Manual: Sampling design and preparation. https://environment.des.qld.gov.au/__data/assets/pdf_file/0024/90735/sampling-design-quality-control-for-water-and-sediment-sampling.pdf
- Faraghati SN, Sadeghpour MH, Ghadimi A, Kolaei H (2019) Effect of PH changes on the geotechnical properties of clay liners in landfill. In: ARCE, vol 1, Issue 1. www.arce.ir
- Freches A, Fradinho JC (2024) The biotechnological potential of the Chloroflexota phylum. Appl Environ Microbiol. 10.1128/aem.01756-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagen EJ, Zaugg J, Tyson GW, Southam G (2019) Goethite reduction by a neutrophilic member of the Alphaproteobacterial genus Telmatospirillum. Front Microbiol. 10.3389/fmicb.2019.02938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour KA, Davie CT, Gray N (2022) Survival and activity of an indigenous iron-reducing microbial community from MX80 bentonite in high temperature / low water environments with relevance to a proposed method of nuclear waste disposal. Sci Total Environ 814:152660. 10.1016/j.scitotenv.2021.152660 [DOI] [PubMed] [Google Scholar]
- Greening C, Carere CR, Rushton-Green R, Harold LK, Hards K, Taylor MC, Morales SE, Stott MB, Cook GM (2015) Persistence of the dominant soil phylum Acidobacteria by trace gas scavenging. Proc Natl Acad Sci U S A 112(33):10497–10502. 10.1073/pnas.1508385112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryan AA, Jalique DR, Medihala P, Stroes-Gascoyne S, Wolfaardt GM, McKelvie J, Korber DR (2018) Bacterial diversity and production of sulfide in microcosms containing uncompacted bentonites. Heliyon 4(8):e00722. 10.1016/j.heliyon.2018.e00722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Feng K, Li Y, Li Q (2022) Microbial characteristics of the leachate-contaminated soil of an informal landfill site. Chemosphere 287. 10.1016/j.chemosphere.2021.132155e00722 [DOI] [PubMed]
- Hadi J, Greneche JM, Wersin P, Koho P, Pastina B (2023) Determination of sulfide consumption by Fe-bearing components of bentonites. Clay Miner 71(5):577–599. 10.1007/s42860-023-00254-4 [Google Scholar]
- Hu H, Kristensen JM, Herbold CW, Pjevac P, Kitzinger K, Hausmann B, Dueholm MKD, Nielsen PH, Wagner M (2024) Global abundance patterns, diversity, and ecology of Patescibacteria in wastewater treatment plants. Microbiome 12(1):55. 10.1186/s40168-024-01769-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Chen Q, Deng M, Japenga J, Li T, Yang X, He Z (2018) Heavy metal pollution and health risk assessment of agricultural soils in a typical peri-urban area in southeast China. J Environ Manag. 207:159–168. 10.1016/j.jenvman.2017.10.072 [DOI] [PubMed]
- Jaeger ACH, Hartmann M, Conz RF, Six J, Solly EF (2024) Prolonged water limitation shifts the soil microbiome from copiotrophic to oligotrophic lifestyles in scpScots/scp pine mesocosms. Environ Microbiol Rep 16(1). 10.1111/1758-2229.13211 [DOI] [PMC free article] [PubMed]
- Jaisi DP, Eberl DD, Dong H, Kim J (2011) The formation of illite from nontronite by mesophilic and thermophilic bacterial reaction. Clay Miner 59(1):21–33. 10.1346/CCMN.2011.0590105 [Google Scholar]
- Jalique DR, Stroes-Gascoyne S, Hamon CJ, Priyanto DG, Kohle C, Evenden WG, Wolfaardt GM, Grigoryan AA, McKelvie J, Korber DR (2016) Culturability and diversity of microorganisms recovered from an eight-year old highly-compacted, saturated MX-80 Wyoming bentonite plug. Appl Clay Sci 126:245–250. 10.1016/j.clay.2016.03.022
- Jiang H, Chen X, Li Y, Chen J, Wei L, Zhang Y (2024) Seasonal dynamics of soil microbiome in response to dry–wet alternation along the Jinsha River Dry-hot Valley. BMC Microbiol 24(1):496. 10.1186/s12866-024-03662-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao JY, Ma SC, Salam N, Zhou Z, Lian Z. H, Fu L, Chen Y, Peng CH, OuYang YT, Fan H, Li L, Yi Y, Zhang JY, Wang JY, Liu L, Gao L, Oren A, Woyke T, Dodsworth JA., Cheng L (2024) Cultivation of novel Atribacterota from oil well provides new insight into their diversity, ecology, and evolution in anoxic, carbon-rich environments. Microbiome, 12(1). 10.1186/s40168-024-01836-7 [DOI] [PMC free article] [PubMed]
- Jousset A, Bienhold C, Chatzinotas A, Gallien L, Gobet A, Kurm V, Küsel K, Rillig MC, Rivett DW, Salles JF, Van Der Heijden MGA, Youssef NH, Zhang X, Wei Z, Hol GWH (2017) Where less may be more: How the rare biosphere pulls ecosystems strings. In ISME J 11(4):853–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalam S, Basu A, Ahmad I, Sayyed RZ, El-Enshasy HA, Dailin DJ, Suriani NL (2020) Recent understanding of Soil Acidobacteria and their ecological significance: a critical review. Front Microbiol. 10.3389/fmicb.2020.580024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamaruddin MA, Yusoff MS, Aziz HA, Alrozi R (2016) Current status of Pulau Burung Sanitary Landfill leachate treatment, Penang, Malaysia. In: AIP Conference Proceedings. 10.1063/1.4965070
- Katayama T, Nobu MK, Kusada H, Meng XY, Hosogi N, Uematsu K, Yoshioka H, Kamagata Y, Tamaki H (2020) Isolation of a member of the candidate phylum ‘Atribacteria’ reveals a unique cell membrane structure. Nat Commun. 11(1):6381. 10.1038/s41467-020-20149-5 [DOI] [PMC free article] [PubMed]
- Ke H, Li J, Zhang X, Hu J (2022) Bacterial community structure and predicted metabolic function of landfilled municipal solid waste in China. Sustainability 14(6):3144. 10.3390/su14063144 [Google Scholar]
- Köchling T, Sanz JL, Gavazza S, Florencio L (2015) Analysis of microbial community structure and composition in leachates from a young landfill by 454 pyrosequencing. Appl Microbiol Biotechnol 99(13):5657–5668. 10.1007/s00253-015-6409-4 [DOI] [PubMed] [Google Scholar]
- Kostka JE, Dalton DD, Skelton H, Dollhopf S, Stucki JW (2002) Growth of iron(III)-reducing bacteria on clay minerals as the sole electron acceptor and comparison of growth yields on a variety of oxidized iron forms. AEM 68(12):6256–6262. 10.1128/AEM.68.12.6256-6262.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar MV (2021) A review on municipal solid waste disposal by sanitary landfilling method. IJRR 8(10):520–530. 10.52403/ijrr.20211066 [Google Scholar]
- Langwig MV, De Anda V, Dombrowski N, Seitz KW, Rambo IM, Greening C, Teske AP, Baker BJ (2022) Large-scale protein-level comparison of Deltaproteobacteria reveals cohesive metabolic groups. ISME J 16(1):307–320. 10.1038/s41396-021-01057-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanigan N, Kelly E, Arzamasov AA, Stanton C, Rodionov DA, van Sinderen D (2019) Transcriptional control of central carbon metabolic flux in Bifidobacteria by two functionally similar, yet distinct LacI-type regulators. Sci Rep 9(1):17851. 10.1038/s41598-019-54229-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Lin B, Lei Z (2022) Nitrous oxide emission mitigation from biological wastewater treatment – a review. Bioresour Technol 362:127747. 10.1016/j.biortech.2022.127747 [DOI] [PubMed] [Google Scholar]
- Li D, Yang B, Yang C, Zhang Z, Hu M (2021) Effects of salt content on desiccation cracks in the clay. Environ Earth Sci 80(19):671. 10.1007/s12665-021-09987-8 [Google Scholar]
- Li L, Liu Z, Zhang M, Meng D, Liu X, Wang P, Li X, Jiang Z, Zhong S, Jiang C, Yin H (2020) Insights into the Metabolism and Evolution f the Genus Acidiphilium , a Typical Acidophile in Acid Mine Drainage. MSystems. 5(6). 10.1128/mSystems.00867-20 [DOI] [PMC free article] [PubMed]
- Lindamulla L, Nanayakkara N, Othman M, Jinadasa S, Herath G, Jegatheesan V (2022) Municipal solid waste landfill leachate characteristics and their treatment options in tropical countries. Curr Pollut Rep 8(3):273–287. 10.1007/s40726-022-00222-x [Google Scholar]
- List C, Hosseini Z, Lederballe Meibom K, Hatzimanikatis V, Bernier-Latmani R (2019) Impact of iron reduction on the metabolism of Clostridium acetobutylicum. Environ Microbiol 21(10):3548–3563. 10.1111/1462-2920.14640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long XE, Wang J, Huang Y, Yao H (2016) Microbial community structures and metabolic profiles response differently to physiochemical properties between three landfill cover soils. Environ Sci Pollut Res Int 23(15):15483–15494. 10.1007/s11356-016-6681-6 [DOI] [PubMed] [Google Scholar]
- Lopez-Fernandez M, Cherkouk A, Vilchez-Vargas R, Jauregui R, Pieper D, Boon, N, Sanchez-Castro I, Merroun ML (2015) Bacterial Diversity in Bentonites, Engineered Barrier for Deep Geological Disposal of Radioactive Wastes. Microb Ecol 70(4):922–935. 10.1007/s00248-015-0630-7 [DOI] [PubMed]
- Lynch MDJ, Neufeld JD (2015) Ecology and exploration of the rare biosphere. Nat Rev Microbiol 13(4):217–229. 10.1038/nrmicro3400 [DOI] [PubMed] [Google Scholar]
- Malinowska AM, Kok DE, Steegenga WT, Hooiveld GJEJ, Chmurzynska A (2022) Human gut microbiota composition and its predicted functional properties in people with western and healthy dietary patterns. Eur J Nutr 61(8):3887–3903. 10.1007/s00394-022-02928-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marasco R, Fusi M, Rolli E, Ettoumi B, Tambone F, Borin S, Ouzar H, Boudabous A, Sorlini C, Cherif A, Adani F, Daffonchio D (2021) Aridity modulates belowground bacterial community dynamics in olive tree. Environ Microbiol 23(10):6275–6291. 10.1111/1462-2920.15764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. Embnet J 17(1):10. 10.14806/ej.17.1.200 [Google Scholar]
- Martínez Cuesta S, Rahman SA, Furnham N, Thornton JM (2015) The classification and evolution of enzyme function. Biophys J 109(6):1082–1086. 10.1016/j.bpj.2015.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matchado MS, Rühlemann M, Reitmeier S, Kacprowski T, Frost F, Haller D, Baumbach J, List M (2024) On the limits of 16S rRNA gene-based metagenome prediction and functional profiling. Microb Genom 10(2):001203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JE, Allison HE, McCarthy AJ (2010) Composition of the landfill microbial community as determined by application of domain- and group-specific 16S and 18S rRNA-targeted oligonucleotide probes. AEM 76(4):1301–1306. 10.1128/AEM.01783-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds E, Elshahed MS, Youssef NH (2025) An ecological-evolutionary perspective on the genomic diversity and habitat preferences of the Acidobacteriota. Microb Genom. 10.1099/mgen.0.001344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton ED, Sorokin DY, Overmars L, Lapidus AL, Pillay M, Ivanova N, del Rio TG, Kyrpides NC, Woyke T, Muyzer G (2017) Draft genome sequence of Dethiobacter alkaliphilus strain AHT1T, a gram-positive sulfidogenic polyextremophile. Stand Genomic Sci 12(1):57. 10.1186/s40793-017-0268-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Dombard DR, Bogner JE, Malas J (2020) A review of landfill microbiology and ecology: a call for modernization with ‘next generation’ technology. Front Microbiol 11:1127. 10.3389/fmicb.2020.01127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitzscherling J, Genderjahn S, Schleicher AM, Bartholomäus A, Kallmeyer J, Wagner (2023) Clay-associated microbial communities and their relevance for a nuclear waste repository in the Opalinus Clay rock formation. MicrobiolOpen. 10.1002/mbo3.1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucsi M, Borsodi AK, Megyes M, Szili-Kovács T (2024) Response of the metabolic activity and taxonomic composition of bacterial communities to mosaically varying soil salinity and alkalinity. Sci Rep 14(1):7460. 10.1038/s41598-024-57430-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushinski RM, Zhou Y, Gentry TJ, Boutton TW (2018) Bacterial metataxonomic profile and putative functional behavior associated with C and N cycle processes remain altered for decades after forest harvest. Soil Biol Biochem 119:184–193. 10.1016/j.soilbio.2018.01.008 [Google Scholar]
- Nair GR, Kooverjee BB, de Scally S, Cowan DA, Makhalanyane TP (2024) Changes in nutrient availability substantially alter bacteria and extracellular enzymatic activities in Antarctic soils. FEMS Microbiol Ecol. 100(6). 10.1093/femsec/fiae071 [DOI] [PMC free article] [PubMed]
- Nugroho SA, Wibisono G, Ongko A, Mauliza AZ. (2021). Effects of High Plasticity and Expansive Clay Stabilization with Lime on UCS Testing in Several Conditions. JCEF, 1000(1000). 10.22146/jcef.59438
- Oliverio AM, Bradford MA, Fierer N (2017) Identifying the microbial taxa that consistently respond to soil warming across time and space. Glob Chang Biol 23(5):2117–2129. 10.1111/gcb.13557 [DOI] [PubMed] [Google Scholar]
- Ozbay G, Jones M, Gadde M, Isah S, Attarwala T (2021) Design and operation of effective landfills with minimal effects on the environment and human health. J Environ Public Health 2021:1–13. 10.1155/2021/6921607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoal F, Costa R, Magalhães C (2021) The microbial rare biosphere: current concepts, methods and ecological principles. FEMS Microbiol Ecol. 10.1093/femsec/fiaa227 [DOI] [PubMed] [Google Scholar]
- Pentráková L, Su K, Pentrák M, Stucki JW (2013) A review of microbial redox interactions with structural Fe in clay minerals. Clay Miner 48(3):543–560. 10.1180/claymin.2013.048.3.10 [Google Scholar]
- Petitjean C, Deschamps P, López-García P, Moreira D (2015) Rooting the domain archaea by phylogenomic analysis supports the foundation of the new kingdom proteoarchaeota. Genome Biol Evol 7(1):191–204. 10.1093/gbe/evu274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MN, Dehal PS, Arkin AP (2010) FastTree 2—Approximately maximum-likelihood trees for large alignments. PLoS ONE 5(3):e9490. 10.1371/journal.pone.0009490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radojevic M, Bashkin VN (2015) Practical environmental analysis, 2nd edn. Royal Society of Chemistry. https://www.amazon.com/Practical-Environmental-Analysis-Miroslav-Radojevic-ebook/dp/B017WDD626. Accessed 16 Aug 2025
- Ratzke C, Gore J (2018) Modifying and reacting to the environmental pH can drive bacterial interactions. PLoS Biol 16(3):e2004248. 10.1371/journal.pbio.2004248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regadío M, Ruiz AI, Rodríguez-Rastrero M, Cuevas J (2015) Containment and attenuating layers: an affordable strategy that preserves soil and water from landfill pollution. Waste Manag 46:408–419. 10.1016/j.wasman.2015.08.014 [DOI] [PubMed] [Google Scholar]
- Ruiz-Fresneda MA, Martinez-Moreno MF, Povedano-Priego C, Morales-Hidalgo M, Jroundi F, Merroun ML (2023) Impact of microbial processes on the safety of deep geological repositories for radioactive waste. Front Microbiol. 10.3389/fmicb.2023.1134078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safari E, Valizadeh R (2018) Analysis of biological clogging potential in a simulated compacted clay liner subjected to high-strength leachate infiltration. Int J Environ Sci Technol 15(5):1029–1038. 10.1007/s13762-017-1445-5 [Google Scholar]
- Sakai S, Inokuma K, Nakashimada Y, Nishio N (2008) Degradation of glyoxylate and glycolate with ATP synthesis by a thermophilic anaerobic bacterium, Moorella sp. strain HUC22-1. Appl Environ Microbiol 74(5):1447–1452. 10.1128/AEM.01421-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Monedero MA, Cayuela ML, Roig A, Jindo K, Mondini C, Bolan N (2018) Role of biochar as an additive in organic waste composting. Bioresour Technol 247:1155–1164. 10.1016/j.biortech.2017.09.193 [DOI] [PubMed] [Google Scholar]
- Schneider LK, Wüst A, Pomowski A, Zhang L, Einsle O (2014) No laughing matter: the unmaking of the greenhouse gas dinitrogen monoxide by nitrous oxide reductase, pp 177–210. 10.1007/978-94-017-9269-1_8 [DOI] [PubMed]
- Sha H, Liu Z, Sun Y, Wang Y, Wang X, Zheng JMY, He X (2023) Leachate leakage enhances the microbial diversity and richness but decreases Proteobacteria and weakens stable microbial ecosystem in landfill groundwater. Water Res 243:120321. 10.1016/j.watres.2023.120321 [DOI] [PubMed] [Google Scholar]
- Soares R, Fonseca BM, Nash BW, Paquete CM, Louro RO (2024) A survey of the Desulfuromonadia “cytochromome” provides a glimpse of the unexplored diversity of multiheme cytochromes in nature. BMC Genomics 25(1):982. 10.1186/s12864-024-10872-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin DY, Merkel AY (2022) Dethiobacteria class. Nov. In: Whitman WB (ed) Bergey’s manual of systematics of Archaea and Bacteria. Wiley, New York, pp 1–3 [Google Scholar]
- Staley C, Sadowsky MJ (2016) Application of metagenomics to assess microbial communities in water and other environmental matrices. Mar Biol Assoc UK 96(1):121–129. 10.1017/S0025315415001496 [Google Scholar]
- Stroes-Gascoyne S, Hamon CJ, Maak P, Russell S (2010) The effects of the physical properties of highly compacted smectitic clay (bentonite) on the culturability of indigenous microorganisms. Appl Clay Sci 47(1–2):155–162. 10.1016/j.clay.2008.06.010 [Google Scholar]
- Tang Q, Gu F, Zhang Y, Zhang Y, Mo J (2018) Impact of biological clogging on the barrier performance of landfill liners. J Environ Manage 222:44–53. 10.1016/j.jenvman.2018.05.039 [DOI] [PubMed] [Google Scholar]
- Tang Q, Wang HY, Chen H, Li P, Tang X, Katsumi T (2016) Long-term hydraulic conductivity of compacted clay permeated with landfill leachates. JGS Spec Publ 2(53):1845–1848. 10.3208/jgssp.CHN-52
- Texas Commission on Environmental Quality (2015) Industrial solid waste management: Nonhazardous industrial solid waste landfills (Draft Technical Guideline No. 3). https://www.tceq.texas.gov/downloads/permitting/waste-permits/ihw/docs/tg3.pdf?
- Thompson LR, Sanders JG, McDonald D, Amir A, Ladau J, Locey KJ, Prill RJ, Tripathi A, Gibbons SM, Ackermann G, Navas-Molina JA, Janssen S, Kopylova E, Vázquez-Baeza Y, González A, Morton JT, Mirarab S, Xu ZZ, Jiang L, Zhao H (2017) A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 551(7681):457–463. 10.1038/nature24621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachon MA, Engel K, Beaver RC, Slater GF, Binns WJ, Neufeld JD (2021) Fifteen shades of clay: distinct microbial community profiles obtained from bentonite samples by cultivation and direct nucleic acid extraction. Sci Rep 11(1):22349. 10.1038/s41598-021-01072-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemur R, Lanthier M, Beaudet R, Lépine F (2006) The Desulfitobacterium genus. FEMS Microbiol Rev. 30(5):706–733. 10.1111/j.1574-6976.2006.00029.x [DOI] [PubMed]
- Wang K, Ma JY, Li MY, Qin YS, Bao XC, Wang CC, Cui DL, Xiang P, Ma LQ (2021) Mechanisms of Cd and Cu induced toxicity in human gastric epithelial cells: oxidative stress, cell cycle arrest and apoptosis. Sci Total Environ 756:143951. 10.1016/j.scitotenv.2020.143951 [DOI] [PubMed] [Google Scholar]
- Wani AK, Akhtar N, Sher F, Navarrete AA, Américo-Pinheiro JHP (2022) Microbial adaptation to different environmental conditions: molecular perspective of evolved genetic and cellular systems. Arch Microbiol 204(2):144. 10.1007/s00203-022-02757-5 [DOI] [PubMed] [Google Scholar]
- Wild B, Gerrits R, Bonneville S (2022) The contribution of living organisms to rock weathering in the critical zone. Npj Mater Degrad 6(1):98. 10.1038/s41529-022-00312-7 [Google Scholar]
- Wu H, Xiong DH, Xiao L, Zhang S, Yuan Y, Su ZA, Zhang BJ, Yang D (2018) Effects of vegetation coverage and seasonal change on soil microbial biomass and community structure in the dry-hot valley region. J Mt Sci 15(7):1546–1558 [Google Scholar]
- Xiao T, Li P, Pan Z, Wang J (2022) Effect of compaction condition on the water retention capacity, microstructure and its evolution during drying of compacted loess. Geoenviron Disasters. 10.1186/s40677-022-00229-y [Google Scholar]
- Yabe S, Sakai Y, Abe K, Yokota A (2017) Diversity of Ktedonobacteria with Actinomycetes-Like Morphology in Terrestrial Environments. M&E. 32(1):61–70. 10.1264/jsme2.ME16144 [DOI] [PMC free article] [PubMed]
- Yang C, Lv D, Jiang S, Lin H, Sun J, Li K, Sun J (2021a) Soil salinity regulation of soil microbial carbon metabolic function in the Yellow River Delta, China. Sci Total Environ 790:148258. 10.1016/j.scitotenv.2021.148258 [DOI] [PubMed] [Google Scholar]
- Yang G, Guo J, Zhuang L, Yuan Y, Zhou S (2016) Desulfotomaculum ferrireducens sp. nov., a moderately thermophilic sulfate-reducing and dissimilatory Fe(III)-reducing bacterium isolated from compost. Int J Syst Evol Microbiol 66(8):3022–3028. 10.1099/ijsem.0.001139 [DOI] [PubMed] [Google Scholar]
- Yang S, Li L, Peng X, Song L (2021b) Leachate microbiome profile reveals bacteria, archaea and eukaryote dynamics and methanogenic function during solid waste decomposition. Bioresour Technol 320:124359. 10.1016/j.biortech.2020.124359 [DOI] [PubMed] [Google Scholar]
- Zhang K, Delgado-Baquerizo M, Zhu YG, Chu H (2020) Space is more important than season when shaping soil microbial communities at a large spatial scale. Msyst. 10.1128/msystems.00783-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Qi L, Li W, Hu BX, Dai Z (2021) Bacterial community variations with salinity in the saltwater-intruded estuarine aquifer. Sci Total Environ 755:142423. 10.1016/j.scitotenv.2020.142423 [DOI] [PubMed] [Google Scholar]
- Zheng Y, Maruoka M, Nanatani K, Hidaka M. Abe N, Kaneko J, Sakai Y, Abe K, Yokota A, Yabe S (2021) High cellulolytic potential of the Ktedonobacteria lineage revealed by genome-wide analysis of CAZymes. JBB, 131(6):622–630. 10.1016/j.jbiosc.2021.01.008 [DOI] [PubMed]
- Zubova N, Ivantsov A (2024) Modeling of leachate propagation in a municipal solid waste landfill foundation. Fluid Dyn Mater Process 20(6):1407–1424. 10.32604/fdmp.2024.051130 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this work has been deposited with National Center Biotechnology International NCBI with Accession reference No: PRJNA1201362 and SRA Submission ID: SUB14944080.