Abstract
Cardiac amyloidosis, characterized by extracellular deposition of amyloid fibrils within the myocardium, is an increasingly recognized cause of heart failure. With the advent of disease-modifying therapies, imaging has become central to diagnosis, subtype differentiation, prognostication, and treatment monitoring. This review provides a comprehensive update on multimodality imaging in cardiac amyloidosis, emphasizing its clinical utility across the disease continuum. Echocardiography, technetium-labeled bone scintigraphy, amyloid-specific positron emission tomography, cardiac magnetic resonance, and cardiac computed tomography each contribute uniquely to detecting amyloid burden and assessing cardiac function. In addition to outlining a practical diagnostic approach, we highlight emerging imaging biomarkers for monitoring treatment response and predicting clinical outcomes. The integration of these modalities into clinical practice enhances diagnostic accuracy, enables individualized risk stratification, and supports optimized, evidence-based care for patients with cardiac amyloidosis.
Keywords: Cardiac amyloidosis, AL amyloidosis, ATTR amyloidosis, Echocardiography, Cardiac MRI, SPECT/CT, Amyloid PET, Scintigraphy
Introduction
Cardiac amyloidosis is a progressive and often fatal condition characterized by the deposition of amyloid fibrils in the extracellular space of the myocardium, leading to restrictive cardiomyopathy and heart failure. The condition most commonly arises from either immunoglobulin light chain (AL) amyloidosis or transthyretin (ATTR) amyloidosis, the latter of which includes hereditary (variant) and wild-type forms. Early and accurate diagnosis is crucial for managing cardiac amyloidosis because treatment strategies and prognostic outcomes vary significantly depending on the type and extent of amyloid deposition [1, 2].
Imaging modalities have become indispensable in the diagnosis, risk stratification, and treatment monitoring of cardiac amyloidosis [3, 4]. Advancements in imaging techniques have markedly improved the detection of amyloid infiltration, functional assessment, and therapeutic decision-making. Echocardiography, nuclear scintigraphy with single-photon emission computed tomography (SPECT) using bone-avid tracers, amyloid-specific positron emission tomography (PET), and cardiac magnetic resonance imaging (CMR) have emerged as key tools in the non-invasive evaluation of cardiac amyloidosis [5]. In addition, cardiac computed tomography (CCT) serves as a valuable alternative for patients who are ineligible for CMR and may facilitate opportunistic screening within clinical workflows [6].
This review summarizes the latest advancements in the imaging-based cardiac amyloidosis diagnosis, focusing on key developments of the past few years. Our goal is to provide a comprehensive update on how emerging technologies are shaping the future of cardiac amyloidosis diagnostics and potentially improving patient care.
Pathophysiology
Amyloid refers to an abnormal protein that arises when normally soluble proteins misfold and aggregate into insoluble, fibrillar structures. These fibrils deposit in tissues and organs, where they exhibit characteristic staining properties and distinctive appearances under microscopy. Because amyloid proteins are resistant to degradation, they accumulate progressively, leading to disruption of normal tissue architecture and eventual organ dysfunction [7]. The term “amyloid” was first introduced by Rudolf Virchow in the mid-nineteenth century. During autopsies, he observed abnormal deposits in organs such as the liver and spleen that reacted with iodine similarly to starch. Based on this reaction, he mistakenly believed the material to be carbohydrate in nature and coined the term “amyloid,” derived from the Latin word amylum, meaning starch [8].
Amyloidosis is a disorder characterized by the abnormal accumulation of amyloid proteins in tissues and organs. Systemic amyloidosis affects multiple organs, including the heart, kidneys, liver, and peripheral/autonomic nervous systems, often resulting in progressive dysfunction and poor outcomes. Among more than 30 identified amyloidogenic proteins, immunoglobulin light chains (AL amyloidosis) and transthyretin (ATTR amyloidosis) are the most clinically relevant in cardiac involvement. Cardiac amyloidosis is an infiltrative cardiomyopathy caused by amyloid fibril deposition in the myocardial interstitium, leading to increased wall thickness, impaired compliance, and both systolic and diastolic dysfunction [9].
In AL amyloidosis, a clonal population of plasma cells produces misfolded immunoglobulin light chains, which deposit in tissues including the heart. In ATTR amyloidosis, misfolded transthyretin (TTR), a hepatic transport protein, forms amyloid fibrils that accumulate in the heart. This condition includes two subtypes: hereditary (ATTRv), caused by TTR gene mutations, and wild-type (ATTRwt), which occurs sporadically in elderly individuals [10, 11].
Clinical presentation
The clinical presentation of cardiac amyloidosis is often insidious and non-specific, which can contribute to diagnostic delays. Common symptoms include progressive dyspnea, fatigue, peripheral edema, and orthostatic hypotension, reflecting restrictive cardiomyopathy and evolving heart failure. On physical examination, signs of right-sided heart failure are frequently noted, including jugular venous distention, hepatomegaly, ascites, and lower extremity edema. Several extracardiac manifestations may precede cardiac symptoms and aid in early detection. In AL amyloidosis, extracardiac features such as periorbital purpura and macroglossia can serve as diagnostic clues [12]. Carpal tunnel syndrome is a well-recognized red flag for wild-type transthyretin cardiomyopathy (ATTRwt-CM), often appearing 10–15 years before cardiac involvement. Studies indicate that 30–50% of patients with ATTRwt-CM have a history of bilateral carpal tunnel syndrome, often requiring surgical release. Other extracardiac red flags include lumbar spinal stenosis and spontaneous biceps tendon rupture, both of which may result from soft tissue amyloid deposition [13]. In addition, ATTRwt-CM is increasingly recognized in elderly patients with aortic stenosis (AS), particularly those undergoing transcatheter aortic valve replacement, with a reported prevalence of 12–16% [14, 15]. These patients often present with concentric left ventricular (LV) hypertrophy and preserved LV ejection fraction (LVEF), clinical features that may overlap with AS alone.
Treatment strategy
The management of cardiac amyloidosis depends primarily on the underlying amyloid subtype, AL-CM and ATTR-CM, and the severity of cardiac involvement. Timely and accurate typing is essential, as the prognosis and therapeutic approach differ markedly between subtypes.
In AL amyloidosis, the goal of treatment is to suppress the production of amyloidogenic light chains by targeting the underlying clonal plasma cell disorder. The current standard of care is a daratumumab-based regimen combining daratumumab, bortezomib, cyclophosphamide, and dexamethasone, which has shown significant hematologic and cardiac benefits in the phase III ANDROMEDA trial [16]. Selected patients with preserved performance status and limited organ involvement may benefit from autologous stem cell transplantation (ASCT), which can offer durable remission. Treatment response is assessed by hematologic markers such as serum free light chains and immunofixation, and by cardiac biomarkers including N-terminal pro-B-type natriuretic peptide (NT-proBNP) and troponins [17].
Therapeutic strategies for ATTR-CM have advanced dramatically in the last decade. Tafamidis, a TTR stabilizer, was the first disease-modifying therapy shown to reduce mortality and cardiovascular hospitalizations in both ATTRwt and ATTRv patients [18]. Acoramidis, another TTR stabilizer, has also demonstrated clinical benefit [19]. For patients with more advanced or rapidly progressive disease, gene-silencing therapies such as patisiran (a small interfering RNA agent) and inotersen (an antisense oligonucleotide) suppress hepatic TTR production and have shown efficacy in patients with ATTR amyloidosis with polyneuropathy, and there is growing evidence of cardiac benefits as well [18]. Most recently, in vivo CRISPR/Cas9-based gene editing has shown promising early-phase results, achieving sustained TTR suppression with a single intravenous dose [20].
For selected patients with end-stage cardiac amyloidosis and preserved extracardiac function, heart transplantation may be considered. In AL amyloidosis, transplantation is typically followed by ASCT or chemotherapy to prevent disease recurrence. In ATTRv, liver transplantation was historically used to remove the source of mutant TTR, but its role has declined with the advent of TTR silencers and stabilizers [21]. A multidisciplinary approach is essential to assess transplant candidacy and to optimize timing in relation to systemic disease control [22].
Assessment tools for cardiac amyloidosis
Accurate diagnosis and precise subtype classification are critical for appropriate therapeutic decision-making and prognostic evaluation in cardiac amyloidosis. This section reviews the principal diagnostic modalities, such as blood biomarkers, electrocardiography, and multimodal imaging, which together form the foundation of clinical assessment.
Blood biomarkers
Blood-based biomarkers are essential in the diagnostic evaluation of cardiac amyloidosis, providing early clues to myocardial involvement and aiding in the differentiation between subtypes. The most widely used biomarkers are natriuretic peptides, particularly BNP and NT-proBNP. These markers are released in response to myocardial wall stress and elevated intracardiac pressures, conditions that are common in cardiac amyloidosis due to restrictive physiology. NT-proBNP levels are generally higher in AL-CM than in ATTR-CM, reflecting the toxic effects of circulating light chains [23]. Cardiac troponins, particularly troponin T and troponin I, are also frequently elevated in cardiac amyloidosis due to direct myocardial injury from amyloid infiltration. In AL amyloidosis, elevated troponin levels are associated with worse prognosis and are incorporated into staging systems such as the Mayo Clinic staging algorithm [24].
In suspected AL amyloidosis, the serum free light chain (FLC) assay is indispensable. It quantifies kappa (κ) and lambda (λ) light chains, and the κ/λ ratio aids in detecting clonal plasma cell disorders. When combined with serum and urine immunofixation electrophoresis, these tests allow for the identification of monoclonal proteins, providing critical support for both diagnosis and response assessment [25, 26].
Electrocardiography
Electrocardiography (ECG) is a key tool in evaluating cardiac amyloidosis. Low-QRS voltage in limb leads, despite increased ventricular wall thickness on imaging, is a hallmark feature due to electrical insulation by amyloid fibrils [27–29]. Pseudoinfarct patterns, such as Q waves in the absence of coronary artery disease, are also characteristic and typically indicate subendocardial amyloid deposition [2]. Conduction abnormalities, including atrioventricular (AV) block and bundle branch blocks, are common and may require pacemaker implantation. Atrial fibrillation (AF) frequently occurs and can further reduce cardiac output in the context of restrictive physiology [30, 31]. Other nonspecific findings, such as ST-segment depression and T-wave inversion, may also suggest myocardial involvement [30].
Echocardiography
Echocardiography typically shows concentric LV thickening due to extracellular amyloid deposition rather than true hypertrophy. The myocardium may exhibit a granular or sparkling appearance on two-dimensional imaging [32, 33]. Right ventricular (RV) involvement may also be seen, with wall thickening, chamber dilation, and impaired systolic function [34].
Doppler imaging demonstrates a restrictive filling pattern, with an elevated early diastolic transmitral inflow velocity (E-wave), a diminished atrial contraction wave (A-wave), and an elevated E/A ratio. Tissue Doppler imaging further reveals markedly reduced mitral annular velocities, especially early diastolic (e') velocity, reflecting impaired compliance [35].
Speckle-tracking echocardiography allows for sensitive detection of subclinical myocardial dysfunction. A characteristic longitudinal strain pattern with preserved apical strain and reduced basal and mid-segmental strain, referred to as apical sparing, is highly specific for ATTR-CM [36]. However, its sensitivity in AL-CM is limited. A recent meta-analysis reported only moderate diagnostic accuracy of apical sparing in patients with AL-CM [37, 38].
Nuclear scintigraphy with bone-avid tracers
Scintigraphy using bone-avid radiotracers such as technetium-99m-labeled pyrophosphate (99mTc-PYP), hydroxymethylene diphosphonate (99mTc-HMDP), and 3,3-diphosphono-1,2-propanodicarboxylic acid (99mTc-DPD) become a cornerstone in the non-invasive diagnosis of ATTR-CM. These radiotracers have shown a high affinity for ATTR amyloid deposits in the myocardium, enabling effective imaging of cardiac involvement. The mechanism by which these radiotracers bind to amyloid deposits is not fully understood, but they are believed to have a high affinity for calcium-rich microcalcifications within amyloid fibrils [39]. Recent findings have shown an inverse correlation between the heart-to-contralateral lung (H/CL) ratio and the age of onset in patients with ATTRwt-CM, indicating different pathophysiological mechanisms depending on patient age [40].
99mTc-PYP is widely used in the United States, whereas 99mTc-HMDP and 99mTc-DPD are more common in Europe. In Japan, both 99mTc-PYP and 99mTc-HMDP are used clinically. When interpreted using the Perugini visual grading scale (Table 1), Grade 2 or 3 myocardial uptake—especially in the absence of a monoclonal protein—is highly specific for ATTR-CM (Fig. 1) [41]. These imaging techniques are particularly valuable for distinguishing ATTR-CM from AL-CM, which typically demonstrates little to no radiotracer uptake. In hereditary ATTR-CM, 99mTc-PYP scintigraphy also demonstrates moderate-to-high uptake [42]. Multiple studies and meta-analyses have demonstrated the high diagnostic accuracy of bone scintigraphy for detecting ATTR-CM [43, 44].
Table 1.
Perugini score for bone-avid radiotracer cardiac scintigraphy
| Score | Cardiac uptake (relative to bone) | Imaging findings |
|---|---|---|
| 0 | No myocardial uptake | Normal bone uptake; no visible myocardial tracer activity |
| 1 | Myocardial uptake < bone uptake | Faint myocardial uptake; ribs clearly more intense |
| 2 | Myocardial uptake = bone uptake | Myocardial uptake comparable to bone (e.g., ribs/sternum) |
| 3 | Myocardial uptake > bone uptake | Myocardial uptake clearly exceeds bone signal; ribs may appear faint |
The Perugini score is a visual grading system used to assess myocardial tracer uptake relative to adjacent bone (typically ribs or vertebrae) on planar images obtained 3 h post-injection. It is widely applied across various technetium-labeled tracers, including 99mTc-PYP, 99mTc-DPD, and 99mTc-HMDP. Grades 2 and 3, in the absence of a monoclonal gammopathy, are considered diagnostic of ATTR-CM, potentially eliminating the need for biopsy. Grade 1 is inconclusive and requires further evaluation with blood and urine tests to rule out AL-CM
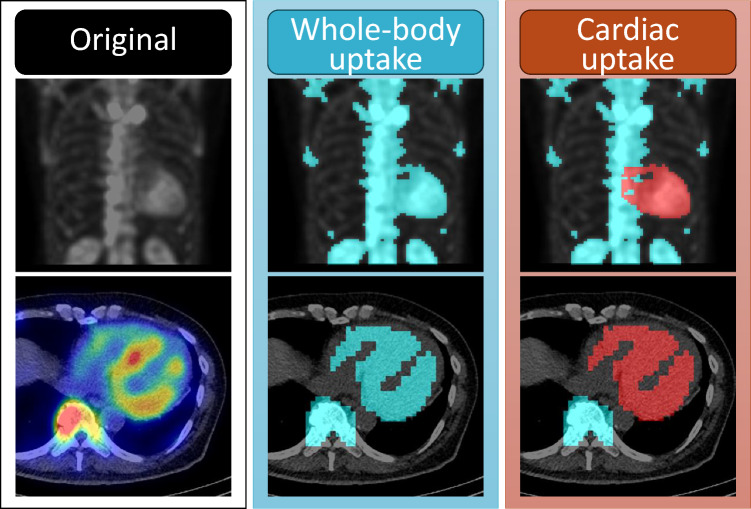
Fig. 1.
Representative case of transthyretin cardiac amyloidosis in a man in his 70 s. Planar images acquired at 1 and 3 h post-injection of 99mTc-pyrophosphate (PYP), along with corresponding axial and short-axis SPECT/CT images, are shown. There is intense radiotracer uptake in the left ventricular myocardium, with relatively reduced uptake in the apical region. Mild uptake is also observed in the right ventricle on SPECT images. Visual assessment corresponds to Grade 3 (uptake greater than rib uptake with mild rib uptake). Quantitative evaluation yielded a heart-to-contralateral lung ratio of 1.88 at 1 h and 1.78 at 3 h
A multicenter study evaluated the diagnostic performance of three technetium-labeled tracers for ATTR-CM and found that all three agents showed high accuracy [41]. When grade 2 or 3 myocardial uptake was present, the sensitivities were 94% for 99mTc-DPD, 84% for 99mTc-PYP, and 80% for 99mTc-HMDP, while specificities were 89%, 92%, and 100%, respectively. Despite slight numerical differences, the overall diagnostic performance was comparable across tracers, particularly when combined with an absence of a monoclonal protein, yielding a specificity and positive predictive value of 100% in all groups.
Interpretation pitfalls include false-positive uptake caused by residual blood pool activity, as well as uptake in adjacent structures such as ribs, cardiac valves, or areas of prior infarction. In particular, blood pool activity can result in misleading findings on early [1, 45]. Recent data have shown that 3-h imaging significantly improves diagnostic accuracy over 1-h imaging. Recent studies have demonstrated that 3-h imaging provides significantly greater diagnostic precision compared to 1-h protocols. Furthermore, SPECT or SPECT/CT imaging should be employed to localize myocardial tracer uptake and differentiate it from surrounding structures [46–48]. While semi-quantitative indices such as the H/CL ratio have traditionally supported interpretation, their reliability is limited by factors like region-of-interest placement, overlying bone structures, and residual blood pool activity. These limitations have led to recent guidelines emphasizing visual interpretation with SPECT and SPECT/CT, especially in patients with Perugini Grade 1–2 findings.
Although AL-CM typically shows little tracer uptake, moderate-to-high uptake (e.g., Grade 2 or 3) has been observed in some cases, particularly those with coexisting myocardial injury. These findings underscore the importance of comprehensive screening for M-protein using serum and urine immunofixation and FLC assays [49, 50].
Quantitative approaches using SPECT/CT are emerging as powerful tools for measuring amyloid burden. Metrics such as standardized uptake values (SUV), total tracer volume, and percentage of injected dose can support diagnosis and track treatment response [51]. New indices, including amyloid deposition volume (AmyDV) and total amyloid uptake (AmyDV × SUVmean), show high diagnostic accuracy in hybrid SPECT/CT imaging (Fig. 2) [52]. A simple index, the lateral wall-to-aorta (LW/Ao) ratio, recently demonstrated excellent performance (area under the curve [AUC] 0.99, sensitivity 100%, specificity 97.6%) and superior reproducibility compared to the H/CL ratio and Perugini scoring [53].
Fig. 2.
Quantitative 99mTc-PYP scintigraphy in in the same patient shown in Fig. 1. Intense myocardial radiotracer uptake was observed at 3 h post-injection. The top row shows maximum intensity projection (MIP) images: the left panel displays the original image; the middle highlights all voxels above 40% of the cardiac 40% of the SUVmax (in light blue coloration); and the right isolates cardiac-specific uptake from the high-uptake regions (in red coloration). The bottom row presents corresponding axial SPECT/CT fusion images in the same order. Quantitative analysis yielded the following values: SUVmax = 6.79; amyloid deposition volume = 416.4 mL; and total amyloid uptake = 1509.3 mL
Artificial intelligence (AI) applications in nuclear imaging offer further advances. AI-driven segmentation and SUV extraction have improved reproducibility and reduced observer variability. These tools correlate well with Perugini scores and cardiac biomarkers, offering promise for response monitoring and risk stratification [54, 55].
Amyloid-specific PET tracers
Amyloid PET is an emerging imaging modality that enables non-invasive visualization and quantification of myocardial amyloid deposits (Fig. 3). It uses radiotracers that bind specifically to amyloid fibrils. The first successful Aβ-selective PET tracer, 11C-Pittsburgh compound B (PiB), is a derivative of thioflavin, an amyloid-binding fluorescent dye initially developed for imaging cerebral amyloid in Alzheimer’s disease. Its discovery led to the development of 1⁸F-labeled tracers such as florbetaben, florbetapir, and flutemetamol, which have longer half-lives and are more suitable for broader clinical use [56, 57]. Although currently investigational in cardiac amyloidosis, growing evidence supports the diagnostic utility of amyloid PET [58, 59].
Fig. 3.
Representative PET imaging of transthyretin cardiac amyloidosis. Representative amyloid PET images from a male patient in his 80 s with wild-type transthyretin cardiac amyloidosis. The upper row shows images with 11C-Pittsburgh compound B (PiB), and the lower row shows images with 18F-florbetaben (FMM). From left to right: maximum intensity projection, axial PET/CT, and coronal PET/CT images. Both tracers demonstrate myocardial uptake predominantly in the basal segments of the left ventricular wall
A major advantage lies in its ability to directly detect amyloid deposits without the need for invasive procedures such as endomyocardial biopsy [60]. Additionally, amyloid PET holds potential for differentiating between various forms of amyloidosis, particularly ATTR-CM and AL-CM (Fig. 4), which require distinct treatments. A study by Rosengren et al. reported higher 11C-PiB uptake in AL-CM than in ATTR-CM, suggesting its potential for subtype differentiation despite the small sample size [61]. Although technetium-based scintigraphy is commonly used to detect ATTR-CM, PET imaging could provide enhanced accuracy and earlier detection of amyloid deposits. Furthermore, combining 11C-PiB PET with 99mTc-PYP or 99mTc-DPD scintigraphy may be useful for more detailed subtyping of amyloid pathology [62, 63]. Amyloid PET also offers the ability to quantify the myocardial amyloid burden, providing insight into disease severity and progression over time. Such quantification is especially important for monitoring how the disease evolves and responding to changes in the patient's condition [64].
Fig. 4.
Representative case of AL cardiac amyloidosis evaluated with 11C-Pittsburgh compound B (PiB) PET. A male patient in his 70 s was diagnosed with AL cardiac amyloidosis based on biopsy findings. Shown from left to right are maximum intensity projection (MIP), axial PET/CT, and coronal PET/CT images. Diffuse radiotracer uptake is evident in both the left and right ventricular myocardium, with mild uptake also observed in the atrial walls
While amyloid PET is considered a promising diagnostic tool for cardiac amyloidosis, a previous report noted limited visualization in some cases [64]. Therefore, optimized imaging protocols including appropriate acquisition timing are needed to enhance diagnostic performance.
Cardiac magnetic resonance imaging
CMR plays a vital role in the diagnosis and management of cardiac amyloidosis by offering comprehensive insights into both myocardial structure and function. One of its major strengths lies in its ability to characterize tissue composition with high precision using late gadolinium enhancement (LGE), T1 and T2 mapping, and extracellular volume (ECV) quantification (Fig. 5). In addition to these advanced tissue characterization capabilities, CMR also serves an important clinical role in ruling out cardiac amyloidosis and differentiating it from other forms of cardiomyopathy.
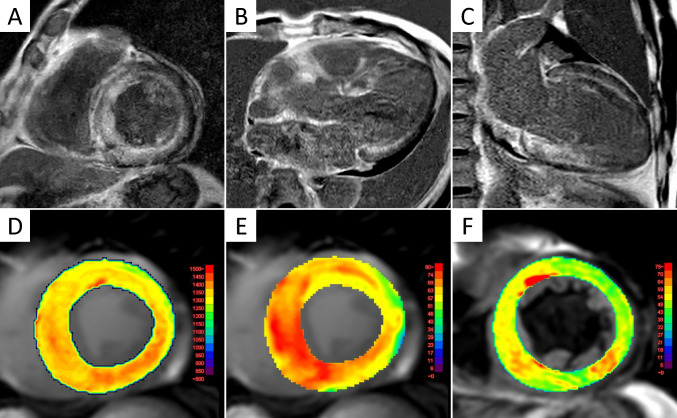
Fig. 5.
CMR findings in ATTRwt-CM. Cardiac magnetic resonance (CMR) images from a man in his 70 s with wild-type transthyretin cardiac amyloidosis (ATTRwt-CM). Late gadolinium enhancement (LGE) images in the left ventricular short-axis (A), 3-chamber (B), and 2-chamber (C) views show diffuse subendocardial LGE in the left ventricle, with additional LGE in the right ventricular wall and atrial walls. A dark blood pool sign, characterized by low signal intensity within the cardiac chambers, is also observed. Native T1 map (D) and extracellular volume (ECV) map (E) demonstrate markedly elevated values (yellow to red areas), consistent with widespread amyloid infiltration. T2 map (F) shows moderately prolonged T2 values
Recent meta-analyses evaluating the diagnostic performance of LGE, native T1, and ECV for cardiac amyloidosis using CMR have yielded consistent findings [65, 66]. ECV demonstrated the highest diagnostic accuracy, exhibiting superior sensitivity (90%) and specificity (91%) compared with native T1 (sensitivity: 87%, specificity: 88%) and LGE (sensitivity: 85%, specificity: 92%; significantly lower diagnostic odds ratio compared to ECV). On the other hand, native T1 does not require gadolinium-based contrast administration, making it particularly advantageous for assessing myocardial involvement in patients with renal impairment [67].
T2 mapping reflects myocardial water content, and thus elevated T2 values are indicative of myocardial edema or inflammation. Patients with cardiac amyloidosis generally exhibit increased myocardial T2 values, with AL-CM demonstrating higher values compared to ATTR-CM. Nonetheless, patients with ATTR-CM also exhibit elevated T2 values compared with healthy individuals [68, 69]. These findings suggest that T2 mapping may have clinical utility, particularly in the diagnosis of AL-CM [70]. On the other hand, recent studies have suggested that MRI-derived ECV may not accurately reflect the histological amyloid burden in ATTR-CM patients exhibiting prolonged T2 values, a limitation that warrants careful consideration [71, 72].
CMR allows precise evaluation of myocardial deformation through strain analysis using feature-tracking techniques on cine images. In patients with cardiac amyloidosis, global longitudinal strain (GLS) is often markedly impaired, providing incremental prognostic value beyond traditional parameters. Moreover, a pattern of relative apical sparing—characterized by more pronounced strain reduction at the basal and mid-ventricular segments compared to the apex—is frequently observed in cardiac amyloidosis and aids in differentiating it from other hypertrophic cardiac conditions such as hypertrophic cardiomyopathy and Anderson-Fabry disease [73]. This strain pattern commonly correlates with a base-to-apex gradient in LGE and ECV, reflecting regional variations in amyloid deposition [74]. However, the diagnostic accuracy of this relative apical sparing pattern alone is not consistently high and has been reported to be inferior to that of native T1 mapping and ECV [75]. The combination of T2 mapping and LGE has been reported to differentiate AL-CM from ATTR-CM with high accuracy, underscoring the clinical utility of a multiparametric approach [76].
In the field of CMR, AI-driven diagnostic approaches have garnered considerable attention. One reason is that LGE can exhibit diverse and often subtle patterns, making visual interpretation and pattern recognition challenging even for experienced clinicians. A convolutional neural network trained on LGE images demonstrated excellent performance in distinguishing cardiac amyloidosis from other cardiomyopathies, achieving a sensitivity of 95%, diagnostic accuracy of 88%, and an AUC of 0.982—comparable to expert human readers [77]. More recently, a multicenter study applied a Vision Transformer based deep learning model to cine MRI images for the diagnosis of cardiac amyloidosis, achieving AUCs of 0.954 in internal validation and 0.957 in external validation cohorts. Notably, this model proved particularly effective in cases with equivocal imaging findings, highlighting its potential as an objective second-opinion tool and a valuable aid in clinical decision-making [78]. The continued advancement of AI-based approaches holds great promise for enhancing the diagnostic accuracy and efficiency in the evaluation of cardiac amyloidosis [79].
Cardiac computed tomography
Although CCT is not indispensable for the diagnosis of cardiac amyloidosis, it serves as a useful alternative for myocardial tissue characterization and functional assessment, particularly when MRI is contraindicated or unavailable. Furthermore, when CCT is performed for other clinical indications, opportunistic evaluation of myocardial tissue may aid in the incidental detection or screening of cardiac amyloidosis. In clinical practice, CCT is mainly used for coronary assessment, while CMR remains the gold standard for myocardial tissue characterization. However, MRI has practical limitations, including long scan times, device-related restrictions, contrast contraindications in dialysis patients, and the need for technical expertise. In contrast, CCT is widely accessible and feasible in many settings. Recent advances now allow CCT to assess myocardial tissue via ECG-gated equilibrium-phase imaging, enabling evaluation of late iodine enhancement and ECV [6, 80, 81]. Notably, CT-derived ECV has proven particularly useful for detecting cardiac amyloidosis and is now recognized as an alternative to CMR in the Japanese guidelines for the diagnosis and management of cardiac amyloidosis [9]. Cardiac amyloidosis is frequently coexistent with conditions such as AS and AF. The addition of ECV assessment to transcatheter aortic valve replacement planning CCT enables effective detection of subclinical cardiac amyloidosis (Fig. 6) [82]. Recent studies have demonstrated that CT-ECV can reliably differentiate between isolated AS, AS with concomitant ATTR-CM, and isolated ATTR-CM with good diagnostic accuracy and defined threshold values [83]. Furthermore, a prospective study showed that incorporating ECV assessment into planning CT for catheter ablation in patients with atrial arrhythmias, in combination with clinical red flags, facilitates early identification of subclinical ATTR-CM [84]. Additionally, emerging evidence supports the utility of photon-counting CT for ECV evaluation in patients with cardiac amyloidosis, highlighting its potential for further advancement and clinical application in the near future [85, 86].
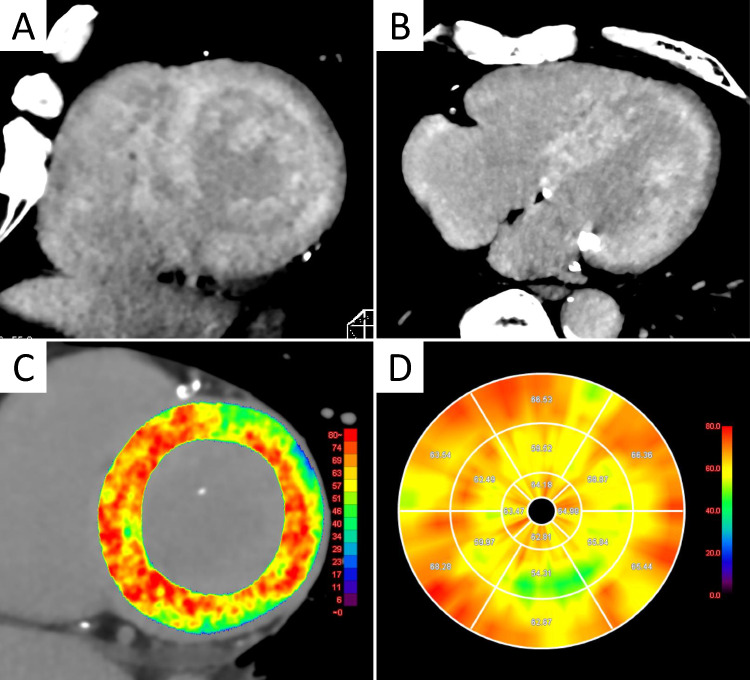
Fig. 6.
CT findings in ATTRwt-CM. A man in his 70 s with wild-type transthyretin cardiac amyloidosis (ATTRwt-CM). The patient was scheduled for transcatheter aortic valve replacement (TAVR) due to severe aortic stenosis. Preprocedural planning CT with additional late iodine enhancement (LIE) imaging revealed diffuse LIE in the left ventricular (LV) wall (A, B). CT-derived extracellular volume (ECV) maps demonstrated markedly elevated ECV values (yellow to red areas in C, D). Subsequent evaluation confirmed concomitant ATTRwt-CM
Diagnostic approach to cardiac amyloidosis
An accurate and timely diagnostic approach is essential for cardiac amyloidosis, given the significant differences in prognosis and treatment between AL and ATTR subtypes. This section outlines a stepwise diagnostic algorithm that integrates clinical findings, laboratory testing, and multimodality imaging to guide appropriate subtype identification and management (Fig. 7) (Table 2) [32].
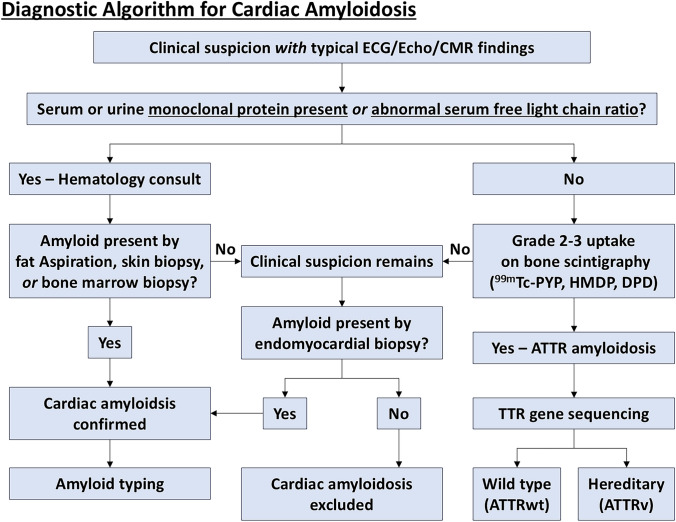
Fig. 7.
Diagnostic algorithm for cardiac amyloidosis. This flowchart is modified from Hanna et al. [114]. Patients with typical echocardiographic or cardiac magnetic resonance imaging (CMR) findings of cardiac amyloidosis should undergo evaluation for serum and urine protein electrophoresis with immunofixation and serum free light chain assay to assess light chain (AL) amyloidosis. If AL amyloidosis can be ruled out, Grade 2–3 myocardial uptake on bone scintigraphy using 99mTc-pyrophoshate (PYP), 99mTc-hydroxymethylene diphosphonate (HMDP), or 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid (DPD) can lead to a diagnosis of transthyretin (ATTR) cardiac amyloidosis without a tissue biopsy. SPECT/CT or fusion of SPECT and CT images is recommended to localize myocardial uptake and to exclude blood pool artifacts
Table 2.
Imaging differences between ATTR and AL cardiac amyloidosis
| Modality | ATTR amyloidosis | AL amyloidosis |
|---|---|---|
| 99mTc-labeled phosphate scintigraphy | Strong myocardial uptake (Perugini grade 2–3); high H/CL ratio (≥ 1.5 at 1 h); low false-positive rate when monoclonal protein is excluded | Minimal or absent uptake; Perugini grade 0–1; false positives rare but possible, especially with myocardial injury or concurrent pathologies |
| PET (18F-florbetapir, 18F-florbetaben, 18F-flutemetamol, 11C-PiB) | Mild to moderate myocardial uptake; variable depending on genotype (ATTRwt vs. ATTRv) | Strong and diffuse myocardial uptake; high sensitivity and specificity; superior to scintigraphy in some AL cases |
| CMR | Subendocardial or diffuse LGE; very high ECV (> 45–50%); mildly elevated native T1 and T2 values | Global or transmural LGE; elevated native T1 and markedly prolonged T2 (inflammation/edema); increased ECV; less frequent apical sparing pattern |
| CCT | LIE; increased CT-derived ECV; applicable in TAVR/ablation planning | May show LIE and increased ECV; less validated and less commonly used in AL amyloidosis evaluation |
ATTR, transthyretin amyloidosis; AL, light chain amyloidosis; PET, positron emission tomography; CMR, cardiac magnetic resonance; CCT, cardiac computed tomography; H/CL, heart-to-contralateral lung ratio; LGE, late gadolinium enhancement; ECV, extracellular volume; T1/T2, MRI relaxation times; PiB, Pittsburgh Compound B; LIE, late iodine enhancement; CAD, coronary artery disease; PYP, pyrophosphate; ATTRwt, wild-type ATTR; ATTRv, variant (hereditary) ATTR
The diagnostic process begins with clinical suspicion, which should be raised in patients with unexplained LV hypertrophy, particularly in the absence of hypertension or valvular disease. Additional red flags include heart failure with preserved LVEF, low-QRS voltage on ECG despite thickened ventricular walls on echocardiography, and extracardiac manifestations such as bilateral carpal tunnel syndrome, lumbar spinal stenosis, and spontaneous biceps tendon rupture. AF with concentric LV hypertrophy or elderly patients undergoing evaluation for AS should also prompt consideration of cardiac amyloidosis, as these populations have an elevated prevalence of ATTR-CM [87].
Upon clinical suspicion, the next step is to confirm the presence of amyloid deposition. This is typically achieved by Congo red staining of tissue biopsies viewed under polarized light microscopy, which reveals characteristic apple-green birefringence. While endomyocardial biopsy remains the gold standard, less invasive options such as abdominal fat pad aspiration, salivary gland, or rectal mucosa biopsy can often be used. However, in many cases of ATTR-CM, especially wild-type forms, a non-biopsy diagnostic pathway can be pursued. Regardless of biopsy findings, amyloid typing is essential. In all patients with suspected cardiac amyloidosis, laboratory testing should include serum and urine protein electrophoresis with immunofixation and a serum FLC assay to detect the presence of monoclonal protein. A positive result indicates AL amyloidosis and necessitates urgent hematologic evaluation and treatment due to its aggressive nature [41].
If AL amyloidosis is excluded by the absence of monoclonal protein, the diagnostic algorithm shifts toward confirmation of ATTR-CM. This involves bone scintigraphy using technetium-labeled tracers such as 99mTc- PYP, 99mTc-DPD, or 99mTc-HMDP, depending on regional availability. Interpretation of the scan relies on the Perugini grading system, which compares cardiac uptake to that of adjacent bone. Grades 0 and 1 are considered negative or indeterminate, while Grade 2 (equal to bone) and Grade 3 (greater than bone with reduced bone signal) are strongly suggestive of ATTR-CM. In the appropriate clinical context, a diagnosis of ATTR-CM can be made without tissue biopsy. This approach is endorsed by current international guidelines and is reflected in the 2020 Japanese Circulation Society (JCS) guidelines, which permit a non-biopsy diagnosis of wild-type ATTR-CM (ATTRwt-CM) when all of the following criteria are fulfilled: [1] Grade 2 or 3 myocardial uptake on bone scintigraphy; [2] no evidence of monoclonal protein on serum/urine immunofixation and serum FLC assay; and [3] absence of pathogenic TTR gene mutations. Importantly, alternative causes of radiotracer accumulation—such as myocardial infarction, cardiac sarcoidosis, or valvular/annular calcification—must also be excluded [9, 41, 88].
In cases where findings are equivocal (e.g., Grade 1 uptake), or when both monoclonal protein and radiotracer uptake are present (possible dual pathology), further assessment with CMR or endomyocardial biopsy may be necessary. CMR provides valuable information on myocardial tissue characteristics, including LGE, native T1 values, and ECV, all of which support the diagnosis and stratification of amyloid burden [71].
Monitoring treatment response in cardiac amyloidosis
Imaging plays a central role in assessing treatment response in cardiac amyloidosis, offering a non-invasive means to monitor disease activity, track therapeutic efficacy, and inform prognosis. Recent advances in echocardiography, nuclear scintigraphy, and CMR have enhanced the precision and reproducibility of such assessments (Table 3).
Table 3.
Treatment response by imaging modality
| Modality | Key parameters | Response indicators |
|---|---|---|
| Echocardiography | GLS, RV free wall strain, diastolic function | Improved GLS or RV strain, improved diastolic filling |
| SPECT/CT | SUV, % Injected Dose, SUV volume | ↓SUV, ↓SUV volume, ↓% Injected Dose (especially with tafamidis or RNAi therapy) |
| CMR | ECV, T1/T2 mapping, LGE, strain (GLS) | ↓ECV, ↓T2 (if edema resolves), improved GLS |
| CCT | CT-derived ECV, delayed iodine enhancement | ↓ ECV or stable values over time |
GLS, global longitudinal strain; RV, right ventricle; SPECT/CT, single-photon emission computed tomography/computed tomography; SUV, standardized uptake value; RNAi, ribonucleic acid interference; CMR, cardiovascular magnetic resonance; CCT, cardiac computed tomography; ECV, extracellular volume; T1/T2, MRI relaxation times; LGE, late gadolinium enhancement; CT, computed tomography
While echocardiography is widely used in clinical practice, its role in treatment response monitoring is evolving. Speckle-tracking echocardiography allows for the assessment of GLS and RV free wall strain, both of which are sensitive to functional changes in cardiac amyloidosis. Improvements in RV strain have been associated with cardiac response in AL-CM, and GLS may detect subclinical dysfunction earlier than LVEF in ATTR-CM [89]. While further validation is needed, echocardiographic strain metrics are increasingly integrated into multiparametric follow-up protocols.
Quantitative SPECT/CT using bone-avid tracers such as 99mTc-PYP and 99mTc-DPD allows for objective monitoring of amyloid burden in ATTR-CM. In a prospective study, patients treated with tafamidis demonstrated significant reductions in total SUV, SUV volume, and percent injected dose over a 6- to 12-month period—equating to an approximate 7.7% monthly decline in amyloid tracer uptake [51]. Similar results were observed in patients receiving RNA interference therapies (e.g., patisiran, inotersen), with significant reductions in myocardial SUV retention indices [90]. These SUV metrics correlate well with clinical severity, biomarkers, and CT-derived ECV [54, 91]. Moreover, higher tracer uptake has been linked to worse outcomes, such as reduced LVEF and increased heart failure hospitalizations [55]. A recent observational study following tafamidis-treated ATTR-CM patients for 6 months revealed that progression was most frequently detected in the imaging domain, particularly via worsening diastolic function and strain values—often preceding clinical symptoms or biomarker elevation [92].
Amyloid PET also plays a role in monitoring the response to treatment. As treatments aim to reduce or stabilize amyloid deposits, PET imaging can provide a visual and quantitative assessment of how amyloid accumulation changes during therapy. This allows clinicians to gauge the effectiveness of the treatment and make informed decisions about ongoing care strategies [58, 93].
In patients with AL-CM, multiparametric CMR has become a cornerstone for evaluating therapeutic response. Among the most promising imaging biomarkers, ECV mapping and T2 relaxation time have gained attention for their ability to reflect changes in myocardial amyloid burden and tissue characteristics. In a prospective study of 111 patients, reductions in both LV and RV ECV were observed following chemotherapy among those who achieved favorable hematologic and cardiac biomarker responses. Improvements in RV strain and slight increases in T2 values were also reported, suggesting potential reversal of myocardial edema and functional recovery [94]. Another prospective study confirmed that reductions in LV and RV ECV correlated with hematologic response and improvements in biomarkers, T2 values, and strain parameters, further supporting the role of ECV as an imaging marker of amyloid burden regression and myocardial recovery in AL-CM [95]. In ATTRv-CM treated with patisiran, serial CMR assessments showed significant reductions in ECV over 12 months, validating its utility as a surrogate marker for amyloid regression [96]. Similarly, a recent study demonstrated that ECV remained stable or showed mild improvement over a 12-month period in patients with ATTR-CM treated with tafamidis, indicating disease stabilization [97, 98]. Furthermore, in the first-in-human phase 1 trial of NI006—a monoclonal antibody targeting amyloid fibrils—dose-dependent reductions in ECV were observed after 3 to 6 months of therapy, underscoring the value of ECV as a sensitive early indicator of anti-amyloid treatment efficacy [99].
Prognostic imaging markers in cardiac amyloidosis
Non-invasive imaging plays a pivotal role in the prognostic assessment of cardiac amyloidosis, providing insights into myocardial infiltration, functional impairment, and overall disease burden. Recent advances across echocardiography, nuclear imaging, CMR, and CCT have identified several imaging biomarkers that independently predict adverse outcomes and assist in risk stratification (Table 4).
Table 4.
Prognostic markers in cardiac amyloidosis imaging
| Modality | Prognostic markers |
|---|---|
| Echocardiography |
Apical sparing pattern: suggests better preserved function Reduced RV free wall strain: predicts worse outcomes Myocardial work indices and GLS dispersion: associated with adverse prognosis |
| SPECT/CT |
High myocardial SUVmax or retention index: associated with worse outcomes RV uptake: linked to greater disease burden and mortality Correlates with ECV and LV mass on CMR |
| CMR |
Elevated ECV: associated with reduced survival Prolonged T2 relaxation time: independent predictor of mortality High native T1 values: associated with poor outcome Reduced GLS and LA strain: predictive of MACE |
ATTR, transthyretin amyloidosis; AL, light-chain amyloidosis; SPECT/CT, single-photon emission computed tomography/computed tomography; CMR, cardiovascular magnetic resonance; ECV, extracellular volume; T1/T2, MRI relaxation times; SUV, standardized uptake value; SUVmax, maximum standardized uptake value; GLS, global longitudinal strain; LA, left atrial; MACE, major adverse cardiovascular events; LV, left ventricle; RV, right ventricle
Prognostic assessment using speckle-tracking echocardiography has shown that relative apical sparing of longitudinal strain, a characteristic feature of cardiac amyloidosis, is associated with longer survival [100]. Patients with more pronounced apical-to-basal strain gradients exhibit better preserved myocardial function and improved outcomes. Moreover, RV free wall strain and myocardial work indices, including the apical-to-basal work ratio and longitudinal strain dispersion, provide incremental prognostic value beyond conventional parameters such as ejection fraction and wall thickness [101–103].
While initially developed for diagnosis, bone-avid tracer imaging using 99mTc-PYP or 99mTc-DPD has been increasingly applied in prognostic contexts. Quantitative metrics such as SUVmax, myocardial retention indices, and total cardiac SUV load have been associated with clinical status, functional capacity, and mortality risk [54]. Importantly, elevated RV uptake or diffuse myocardial distribution have been linked to more advanced disease and worse outcomes [104]. SUV metrics obtained from SPECT/CT correlate well with myocardial ECV on CMR and LV mass, suggesting their broader value beyond visual Perugini grading [91]. These findings support the integration of quantitative SPECT/CT into risk models, especially in settings where CMR is contraindicated.
CMR-derived myocardial ECV has emerged as one of the most robust predictors of prognosis [105]. In AL-CM, studies have shown that elevated ECV values, particularly above 47–50%, are strongly associated with reduced survival, irrespective of hematologic response [106, 107]. Complementary large-scale data have established baseline ECV as an independent predictor of mortality: patients with ECV > 40% had poor outcomes unless a deep hematologic response was achieved, while those with ECV < 30% had favorable prognosis regardless of treatment response [107]. Furthermore, T2 prolongation (> 44 ms) has been shown to be independently associated with increased mortality, even after adjusting for LGE, LVEF, and hematologic response in AL-CM. This finding suggests that persistent myocardial inflammation or edema may indicate subclinical disease activity despite biochemical remission [106]. Recent studies have also emphasized the prognostic value of combining strain, ECV, and perfusion imaging. Quantitative stress perfusion MRI detects microvascular dysfunction in AL-CM and improves survival prediction when combined with ECV [108]. GLS and ECV have also shown strong associations with major adverse cardiac events, outperforming traditional staging systems such as Mayo stage when incorporated into multiparametric models [109].
In ATTR-CM, CMR also provides robust prognostic markers. A landmark study demonstrated that ECV values exceeding 59% were strongly predictive of adverse outcomes, with native T1 mapping also associated with disease severity and prognosis [110]. In contrast to AL-CM, T2 values in ATTR-CM have not been shown to predict mortality, underscoring distinct pathophysiologic mechanisms between subtypes. Moreover, a recent meta-analysis synthesizing data from CMR studies confirmed that ECV, native T1, and LGE are independently associated with survival, with ECV showing the highest pooled hazard ratio for mortality prediction [111].
Recent advances in CCT have enabled the quantification of CT-derived ECV, which also shows prognostic utility. In two prospective studies, higher CT-ECV was independently associated with all-cause mortality and adverse cardiac events in patients with suspected or confirmed cardiac amyloidosis [112, 113]. These findings support the role of CT as a practical alternative when CMR is contraindicated.
Conclusion
Cardiac amyloidosis represents a diagnostic and therapeutic challenge due to its heterogeneous presentation and variable prognosis. Advances in multimodality imaging have transformed its evaluation by enabling early detection, non-invasive subtype differentiation, and objective monitoring of treatment response. Integrating these imaging tools into routine clinical practice and clinical trials will be essential for advancing personalized care and improving outcomes in patients with cardiac amyloidosis.
Acknowledgements
The authors declare that they have no acknowledgements to disclose.
Author contributions
O.M. conceived the study, performed the literature review, and drafted the manuscript. O.M., S.O, T.N., T.A., Y.O. were responsible for the design and structure of the review article. N.T. critically revised the manuscript for important intellectual content. All authors reviewed the final version of the manuscript and approved its submission.
Data availability
The datasets used and/or analyzed in the current study are available from the corresponding author upon request.
Declarations
Conflict of interest
Dr. Takashi Norikane is conducting a researcher-initiated clinical study on 18F-FMM PET/CT in patients with ATTR-CM, supported by Nihon Medi-Physics Co., Ltd., which has provided both the radiopharmaceutical (18F-FMM) and research funding. The other authors declare no financial conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cuddy SAM, Falk RH. Amyloidosis as a systemic disease in context. Can J Cardiol. 2020;36(3):396–407. [DOI] [PubMed] [Google Scholar]
- 2.Bonderman D, Pölzl G, Ablasser K, Agis H, Aschauer S, Auer-Grumbach M, et al. Diagnosis and treatment of cardiac amyloidosis: an interdisciplinary consensus statement. Wien Klin Wochenschr. 2020;132(23–24):742–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorbala S, Cuddy S, Falk RH. How to image cardiac amyloidosis: a practical approach. JACC Cardiovasc Imaging. 2020;13(6):1368–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamaki N, Manabe O. Current status and perspectives of nuclear cardiology. Ann Nucl Med. 2024;38(1):20–30. [DOI] [PubMed] [Google Scholar]
- 5.Ionescu TM, Jalloul W, Stolniceanu CR, Iacob R, Grecu LP, Stătescu AM, et al. Transthyretin cardiac amyloidosis: a review of the nuclear imaging findings with emphasis on the radiotracers mechanisms. Ann Nucl Med. 2021;35(9):967–93. [DOI] [PubMed] [Google Scholar]
- 6.Gatti M, De Filippo O, Cura Curà G, Dusi V, Di Vita U, Gallone G, et al. Diagnostic accuracy of late iodine enhancement on cardiac CT for myocardial tissue characterization: a systematic review and meta-analysis. Eur Radiol. 2025;35(6):3054–67. [DOI] [PubMed] [Google Scholar]
- 7.Bart NK, Bianchi G, Cuddy SAM, Goyal P, Griffin JM, Hummel SL, et al. Cardiac amyloidosis in older adults with a focus on frailty: JACC: advances expert consensus. JACC Adv. 2025;4(6 Pt 1): 101784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sipe JD, Cohen AS. Review: history of the amyloid fibril. J Struct Biol. 2000;130(2–3):88–98. [DOI] [PubMed] [Google Scholar]
- 9.Kitaoka H, Izumi C, Izumiya Y, Inomata T, Ueda M, Kubo T, et al. JCS 2020 guideline on diagnosis and treatment of cardiac amyloidosis. Circ J. 2020;84(9):1610–71. [DOI] [PubMed] [Google Scholar]
- 10.Stern LK, Kittleson MM. Updates in cardiac amyloidosis diagnosis and treatment. Curr Oncol Rep. 2021;23(4):47. [DOI] [PubMed] [Google Scholar]
- 11.Ihne S, Morbach C, Obici L, Palladini G, Störk S. Amyloidosis in heart failure. Curr Heart Fail Rep. 2019;16(6):285–303. [DOI] [PubMed] [Google Scholar]
- 12.Gertz MA. Immunoglobulin light chain amyloidosis diagnosis and treatment algorithm 2018. Blood Cancer J. 2018;8(5): 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aldinc E, Campbell C, Gustafsson F, Beveridge A, Macey R, Marr L, et al. Musculoskeletal manifestations associated with transthyretin-mediated (ATTR) amyloidosis: a systematic review. BMC Musculoskelet Disord. 2023;24(1):751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castaño A, Narotsky DL, Hamid N, Khalique OK, Morgenstern R, DeLuca A, et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J. 2017;38(38):2879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scully PR, Treibel TA, Fontana M, Lloyd G, Mullen M, Pugliese F, et al. Prevalence of cardiac amyloidosis in patients referred for transcatheter aortic valve replacement. J Am Coll Cardiol. 2018;71(4):463–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kastritis E, Palladini G, Minnema MC, Wechalekar AD, Jaccard A, Lee HC, et al. Daratumumab-based treatment for immunoglobulin light-chain amyloidosis. N Engl J Med. 2021;385(1):46–58. [DOI] [PubMed] [Google Scholar]
- 17.Bianchi G, Zhang Y, Comenzo RL. AL amyloidosis: current chemotherapy and immune therapy treatment strategies. JACC Cardiooncol. 2021;3(4):467–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007–16. [DOI] [PubMed] [Google Scholar]
- 19.Gillmore JD, Judge DP, Cappelli F, Fontana M, Garcia-Pavia P, Gibbs S, et al. Efficacy and safety of acoramidis in transthyretin amyloid cardiomyopathy. N Engl J Med. 2024;390(2):132–42. [DOI] [PubMed] [Google Scholar]
- 20.Fontana M, Solomon SD, Kachadourian J, Walsh L, Rocha R, Lebwohl D, et al. CRISPR-Cas9 gene editing with Nexiguran Ziclumeran for ATTR cardiomyopathy. N Engl J Med. 2024;391(23):2231–41. [DOI] [PubMed] [Google Scholar]
- 21.Kittleson MM, Ruberg FL, Ambardekar AV, Brannagan TH, Cheng RK, Clarke JO, et al. 2023 ACC expert consensus decision pathway on comprehensive multidisciplinary care for the patient with cardiac amyloidosis: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2023;81(11):1076–126. [DOI] [PubMed] [Google Scholar]
- 22.Grogan M, Scott CG, Kyle RA, Zeldenrust SR, Gertz MA, Lin G, et al. Natural history of wild-type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol. 2016;68(10):1014–20. [DOI] [PubMed] [Google Scholar]
- 23.De Michieli L, Cipriani A, Iliceto S, Dispenzieri A, Jaffe AS. Cardiac troponin in patients with light chain and transthyretin cardiac amyloidosis. JACC Cardiooncol. 2024;6(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Colby C, et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30(9):989–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mollee P, Merlini G. Free light chain testing for the diagnosis, monitoring and prognostication of AL amyloidosis. Clin Chem Lab Med. 2016;54(6):921–7. [DOI] [PubMed] [Google Scholar]
- 26.Bochtler T, Hegenbart U, Heiss C, Benner A, Cremer F, Volkmann M, et al. Evaluation of the serum-free light chain test in untreated patients with AL amyloidosis. Haematologica. 2008;93(3):459–62. [DOI] [PubMed] [Google Scholar]
- 27.Cipriani A, De Michieli L, Porcari A, Licchelli L, Sinigiani G, Tini G, et al. Low QRS voltages in cardiac amyloidosis: clinical correlates and prognostic value. JACC Cardiooncol. 2022;4(4):458–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mussinelli R, Salinaro F, Alogna A, Boldrini M, Raimondi A, Musca F, et al. Diagnostic and prognostic value of low QRS voltages in cardiac AL amyloidosis. Ann Noninvasive Electrocardiol. 2013;18(3):271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ota S, Izumiya Y, Kitada R, Nishi T, Taruya A, Wada T, et al. Diagnostic significance of paradoxical left ventricular hypertrophy in detecting cardiac amyloidosis. IJC Heart Vasc. 2023;49: 101279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartnett J, Jaber W, Maurer M, Sperry B, Hanna M, Collier P, et al. Electrophysiological manifestations of cardiac amyloidosis. JACC Cardiooncol. 2021;3(4):506–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shinzato K, Takahashi Y, Yamaguchi T, Otsubo T, Nakashima K, Yoshioka G, et al. Atrial amyloidosis identified by biopsy in atrial fibrillation: prevalence and clinical presentation. Eur Heart J. 2025. 10.1093/eurheartj/ehaf332 [DOI] [PMC free article] [PubMed]
- 32.Dorbala S, Ando Y, Bokhari S, Dispenzieri A, Falk RH, Ferrari VA, et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: part 1 of 2-evidence base and standardized methods of imaging. Circ Cardiovasc Imaging. 2021;14(7): e000029. [DOI] [PubMed] [Google Scholar]
- 33.Agrawal T, Nagueh SF. Echocardiographic assessment of cardiac amyloidosis. Heart Fail Rev. 2022;27(5):1505–13. [DOI] [PubMed] [Google Scholar]
- 34.Liang S, Liu Z, Li Q, He W, Huang H. Advance of echocardiography in cardiac amyloidosis. Heart Fail Rev. 2023;28(6):1345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cuddy SAM, Chetrit M, Jankowski M, Desai M, Falk RH, Weiner RB, et al. Practical points for echocardiography in cardiac amyloidosis. J Am Soc Echocardiogr. 2022;35(9):A31–40. [DOI] [PubMed] [Google Scholar]
- 36.Phelan D, Collier P, Thavendiranathan P, Popović ZB, Hanna M, Plana JC, et al. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart. 2012;98(19):1442–8. [DOI] [PubMed] [Google Scholar]
- 37.Huang PN, Liu YN, Cheng XQ, Liu HY, Zhang J, Li L, et al. Relative apical sparing obtained with speckle tracking echocardiography is not a sensitive parameter for diagnosing light-chain cardiac amyloidosis. Quant Imaging Med Surg. 2024;14(3):2357–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee CY, Nabeshima Y, Kitano T, Yang LT, Takeuchi M. Diagnostic accuracy and prognostic value of relative apical sparing in cardiac amyloidosis—systematic review and meta-analysis. Circ J. 2024;89(1):16–23. [DOI] [PubMed] [Google Scholar]
- 39.Mori A, Saito Y, Nakamura K, Iida T, Akagi S, Yoshida M, et al. Microcalcification and 99mTc-pyrophosphate uptake without increased bone metabolism in cardiac tissue from patients with transthyretin cardiac amyloidosis. Int J Mol Sci. 2023. 10.3390/ijms24031921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanaya H, Shiraishi S, Ogasawara K, Iwashita K, Sakamoto F, Takashio S, et al. Inverse correlation between age of onset and myocardial amyloid deposition quantified by (99m)Tc-PYP scintigraphy in patients with wild-type transthyretin amyloid cardiomyopathy. Ann Nucl Med. 2024;38(9):744–53. [DOI] [PubMed] [Google Scholar]
- 41.Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133(24):2404–12. [DOI] [PubMed] [Google Scholar]
- 42.Guo H, Wu S, Xiang X, Wang S, Fang Z, Ye Q, et al. Performance of (99m)Tc-PYP scintigraphy in the diagnosis of hereditary transthyretin cardiac amyloidosis. Ann Nucl Med. 2024;38(4):288–95. [DOI] [PubMed] [Google Scholar]
- 43.Treglia G, Glaudemans AWJM, Bertagna F, Hazenberg BPC, Erba PA, Giubbini R, et al. Diagnostic accuracy of bone scintigraphy in the assessment of cardiac transthyretin-related amyloidosis: a bivariate meta-analysis. Eur J Nucl Med Mol Imaging. 2018;45(11):1945–55. [DOI] [PubMed] [Google Scholar]
- 44.Wu Z, Yu C. Diagnostic performance of CMR, SPECT, and PET imaging for the detection of cardiac amyloidosis: a meta-analysis. BMC Cardiovasc Disord. 2021;21(1): 482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saitou T, Aikawa T, Manabe O, Fujimoto S, Matsue Y, Nagase A, et al. Lateral planar imaging of 99mTc-pyrophosphate scintigraphy in patients with suspected transthyretin cardiac amyloidosis. Ann Nucl Cardiol. 2024;10(1):29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nichols KJ, Yoon SY, Van Tosh A, Palestro CJ. 1-hour versus 3-hour 99mTc-PYP imaging to evaluate suspected cardiac transthyretin amyloidosis. Medicine (Baltimore). 2023;102(20): e33817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saitou T, Aikawa T, Manabe O, Nagase A, Kudo T, Oyama-Manabe N. Comparison of 1-h with 3-h planar (99m)Tc-pyrophosphate scintigraphy in patients with suspected transthyretin cardiac amyloidosis using SPECT as a reference standard. Ann Nucl Med. 2023;37(2):99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khor YM, Cuddy SAM, Singh V, Falk RH, Di Carli MF, Dorbala S. Tc bone-avid tracer cardiac scintigraphy: role in noninvasive diagnosis of transthyretin cardiac amyloidosis. Radiology. 2023;306(2): e221082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quarta CC, Zheng J, Hutt D, Grigore SF, Manwani R, Sachchithanantham S, et al. 99mTc-DPD scintigraphy in immunoglobulin light chain (AL) cardiac amyloidosis. Eur Heart J Cardiovasc Imaging. 2021;22(11):1304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poterucha TJ, Elias P, Bokhari S, Einstein AJ, DeLuca A, Kinkhabwala M, et al. Diagnosing transthyretin cardiac amyloidosis by technetium Tc 99m pyrophosphate: a test in evolution. JACC Cardiovasc Imaging. 2021;14(6):1221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Godoy-Rivas C, Elsadany M, Jaiswal A, Weissler-Snir A, Arora S, Duvall WL. Single-photon emission computed tomography/computed tomography quantification of Tc-99m pyrophosphate uptake to assess tafamidis treatment response in transthyretin cardiac amyloidosis. J Nucl Cardiol. 2024;42: 102056. [DOI] [PubMed] [Google Scholar]
- 52.Matsuda N, Otsuka H, Otani T, Azane S, Kunikane Y, Otomi Y, et al. New quantitative indices of cardiac amyloidosis with 99mTc-pyrophosphate scintigraphy. Jpn J Radiol. 2023;41(4):428–36. [DOI] [PubMed] [Google Scholar]
- 53.Takaishi T, Kisohara M, Horino R, Kaneko H, Hotta N, Mizuno K, et al. A quantitative SPECT/CT metric for diagnosing transthyretin cardiac amyloidosis: multicenter study on biopsy-confirmed cases. Eur J Nucl Med Mol Imaging. 2025. 10.1007/s00259-025-07294-z [DOI] [PubMed]
- 54.Kessler L, Fragoso Costa P, Kersting D, Jentzen W, Weber M, Lüdike P, et al. Quantitative 99mTc-DPD-SPECT/CT assessment of cardiac amyloidosis. J Nucl Cardiol. 2023;30(1):101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller RJH, Cadet S, Mah D, Pournazari P, Chan D, Fine NM, et al. Diagnostic and prognostic value of technetium-99m pyrophosphate uptake quantitation for transthyretin cardiac amyloidosis. J Nucl Cardiol. 2021;28(5):1835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uzuegbunam BC, Librizzi D, Hooshyar Yousefi B. PET radiopharmaceuticals for Alzheimer’s disease and Parkinson’s disease diagnosis, the current and future landscape. Molecules. 2020. 10.3390/molecules25040977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dietemann S, Nkoulou R. Amyloid PET imaging in cardiac amyloidosis: a pilot study using (18)F-flutemetamol positron emission tomography. Ann Nucl Med. 2019;33(8):624–8. [DOI] [PubMed] [Google Scholar]
- 58.Hong Z, Xue S, Yu J, Calabretta R, Haberl D, Jiang Z, et al. Total-body 11C-PIB PET/CT imaging of systemic amyloidosis: inter-organ connectivity in cardiac amyloidosis for prognostic insights. Eur J Nucl Med Mol Imaging. 2025. 10.1007/s00259-025-07308-w. [DOI] [PubMed] [Google Scholar]
- 59.Okuyama C, Inuzuka Y, Takeuchi Y, Asagoe K, Kagawa S, Ito M, et al. Imaging of cardiac amyloidosis using dynamic (18)F-FPYBF-2 positron emission tomography. Ann Nucl Med. 2025;39(4):398–403. [DOI] [PubMed] [Google Scholar]
- 60.Aimo A, Ferrari Chen YF, Castiglione V, Passino C, Genovesi D, Giorgetti A, et al. Positron emission tomography in cardiac amyloidosis: current evidence and future directions. Heart Fail Rev. 2025;30(3):605–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosengren S, Skibsted Clemmensen T, Tolbod L, Granstam SO, Eiskjær H, Wikström G, et al. Diagnostic accuracy of [11C]PIB positron emission tomography for detection of cardiac amyloidosis. JACC Cardiovasc Imaging. 2020;13(6):1337–47. [DOI] [PubMed] [Google Scholar]
- 62.Takasone K, Katoh N, Takahashi Y, Abe R, Ezawa N, Yoshinaga T, et al. Non-invasive detection and differentiation of cardiac amyloidosis using 99mTc-pyrophosphate scintigraphy and 11C-Pittsburgh compound B PET imaging. Amyloid. 2020;27(4):266–74. [DOI] [PubMed] [Google Scholar]
- 63.Hong Z, Spielvogel CP, Xue S, Calabretta R, Jiang Z, Yu J, et al. Enhanced diagnostic and prognostic assessment of cardiac amyloidosis using combined 11C-PiB PET/CT and 99mTc-DPD scintigraphy. Eur J Nucl Med Mol Imaging. 2025. 10.1007/s00259-025-07157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Papathanasiou M, Kessler L, Carpinteiro A, Hagenacker T, Nensa F, Umutlu L, et al. F-Flutemetamol positron emission tomography in cardiac amyloidosis. J Nucl Cardiol. 2022;29(2):779–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pan JA, Kerwin MJ, Salerno M. Native T1 mapping, extracellular volume mapping, and late gadolinium enhancement in cardiac amyloidosis: a meta-analysis. JACC Cardiovasc Imaging. 2020;13(6):1299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang TKM, Brizneda MV, Kwon DH, Popovic ZB, Flamm SD, Hanna M, et al. Reference ranges, diagnostic and prognostic utility of native T1 mapping and extracellular volume for cardiac amyloidosis: a meta-analysis. J Magn Reson Imaging. 2021;53(5):1458–68. [DOI] [PubMed] [Google Scholar]
- 67.Baggiano A, Boldrini M, Martinez-Naharro A, Kotecha T, Petrie A, Rezk T, et al. Noncontrast magnetic resonance for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging. 2020;13(1 Pt 1):69–80. [DOI] [PubMed] [Google Scholar]
- 68.Kotecha T, Martinez-Naharro A, Treibel TA, Francis R, Nordin S, Abdel-Gadir A, et al. Myocardial edema and prognosis in amyloidosis. J Am Coll Cardiol. 2018;71(25):2919–31. [DOI] [PubMed] [Google Scholar]
- 69.Ridouani F, Damy T, Tacher V, Derbel H, Legou F, Sifaoui I, et al. Myocardial native T2 measurement to differentiate light-chain and transthyretin cardiac amyloidosis and assess prognosis. J Cardiovasc Magn Reson. 2018;20(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grazzini G, Pradella S, Bani R, Fornaciari C, Cappelli F, Perfetto F, et al. The role of T2 mapping in cardiac amyloidosis. Diagnostics. 2024. 10.3390/diagnostics14101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kidoh M, Oda S, Takashio S, Morioka M, Kuyama N, Oguni T, et al. MRI-extracellular volume fraction versus histological amyloid load in cardiac amyloidosis: the importance of T2 mapping. Circ Cardiovasc Imaging. 2025;18(5): e017427. [DOI] [PubMed] [Google Scholar]
- 72.Yamaguchi S, Oda S, Kidoh M, Hayashi H, Takashio S, Usuku H, et al. Cardiac MRI T1 and T2 mapping as a quantitative imaging biomarker in transthyretin amyloid cardiomyopathy. Acad Radiol. 2024;31(2):514–22. [DOI] [PubMed] [Google Scholar]
- 73.Williams LK, Forero JF, Popovic ZB, Phelan D, Delgado D, Rakowski H, et al. Patterns of CMR measured longitudinal strain and its association with late gadolinium enhancement in patients with cardiac amyloidosis and its mimics. J Cardiovasc Magn Reson. 2017;19(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim JY, Hong YJ, Han K, Lee HJ, Hur J, Kim YJ, et al. Regional amyloid burden differences evaluated using quantitative cardiac MRI in patients with cardiac amyloidosis. Korean J Radiol. 2021;22(6):880–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Korthals D, Chatzantonis G, Bietenbeck M, Meier C, Stalling P, Yilmaz A. CMR-based T1-mapping offers superior diagnostic value compared to longitudinal strain-based assessment of relative apical sparing in cardiac amyloidosis. Sci Rep. 2021;11(1):15521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kravchenko D, Isaak A, Zimmer S, Öztürk C, Mesropyan N, Bischoff LM, et al. Parametric mapping using cardiovascular magnetic resonance for the differentiation of light chain amyloidosis and transthyretin-related amyloidosis. Eur Heart J Cardiovasc Imaging. 2024;25(10):1451–61. [DOI] [PubMed] [Google Scholar]
- 77.Martini N, Aimo A, Barison A, Della Latta D, Vergaro G, Aquaro GD, et al. Deep learning to diagnose cardiac amyloidosis from cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2020;22(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cockrum J, Nakashima M, Ammoury C, Rizkallah D, Mauch J, Lopez D, et al. Leveraging a vision transformer model to improve diagnostic accuracy of cardiac amyloidosis with cardiac magnetic resonance. JACC Cardiovasc Imaging. 2025;18(3):278–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abdalla Ibrahim FI, Hussen Ali MG, Awad Ali MH, Abdalwahab Abdallah ABA, Elnoor Mohammed NG, Elhaj A, et al. The role of artificial intelligence in the detection of cardiac amyloidosis: a systematic review. Cureus. 2025;17(2): e78488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Han D, Lin A, Kuronuma K, Gransar H, Dey D, Friedman JD, et al. Cardiac computed tomography for quantification of myocardial extracellular volume fraction: a systematic review and meta-analysis. JACC Cardiovasc Imaging. 2023;16(10):1306–17. [DOI] [PubMed] [Google Scholar]
- 81.Zhang H, Guo H, Liu G, Wu C, Ma Y, Li S, et al. CT for the evaluation of myocardial extracellular volume with MRI as reference: a systematic review and meta-analysis. Eur Radiol. 2023;33(12):8464–76. [DOI] [PubMed] [Google Scholar]
- 82.Oda S, Kidoh M, Takashio S, Inoue T, Nagayama Y, Nakaura T, et al. Quantification of myocardial extracellular volume with planning computed tomography for transcatheter aortic valve replacement to identify occult cardiac amyloidosis in patients with severe aortic stenosis. Circ Cardiovasc Imaging. 2020;13(5): e010358. [DOI] [PubMed] [Google Scholar]
- 83.Kidoh M, Oda S, Tabata N, Kuyama N, Oguni T, Takashio S, et al. CT-derived extracellular volume fraction in aortic stenosis, cardiac amyloidosis, and dual pathology. Eur Heart J Cardiovasc Imaging. 2025;26(3):509–17. [DOI] [PubMed] [Google Scholar]
- 84.Yamasaki H, Kondo H, Shiroo T, Iwata N, Masuda T, Makita T, et al. Efficacy of computed tomography-based evaluation of myocardial extracellular volume combined with red flags for early screening of concealed cardiac amyloidosis in patients with atrial fibrillation. Circ J. 2024;88(7):1167–75. [DOI] [PubMed] [Google Scholar]
- 85.Aquino GJ, O’Doherty J, Schoepf UJ, Ellison B, Byrne J, Fink N, et al. Myocardial characterization with extracellular volume mapping with a first-generation photon-counting detector CT with MRI reference. Radiology. 2023;307(2): e222030. [DOI] [PubMed] [Google Scholar]
- 86.Popp S, Beitzke D, Strassl A, Kronberger C, Kammerlander A, Duca F, et al. Evaluation of extracellular volume and coronary artery disease in cardiac amyloidosis using photon-counting CT. Investig Radiol. 2025. 10.1097/RLI.0000000000001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maurer MS, Bokhari S, Damy T, Dorbala S, Drachman BM, Fontana M, et al. Expert consensus recommendations for the suspicion and diagnosis of transthyretin cardiac amyloidosis. Circ Heart Fail. 2019;12(9): e006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Singh V, Falk R, Di Carli MF, Kijewski M, Rapezzi C, Dorbala S. State-of-the-art radionuclide imaging in cardiac transthyretin amyloidosis. J Nucl Cardiol. 2019;26(1):158–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Usuku H, Takashio S, Yamamoto E, Yamada T, Egashira K, Morioka M, et al. Prognostic value of right ventricular global longitudinal strain in transthyretin amyloid cardiomyopathy. J Cardiol. 2022;80(1):56–63. [DOI] [PubMed] [Google Scholar]
- 90.Rettl R, Calabretta R, Duca F, Binder C, Kronberger C, Willixhofer R, et al. Reduction in 99mTc-DPD myocardial uptake with therapy of ATTR cardiomyopathy. Amyloid. 2024;31(1):42–51. [DOI] [PubMed] [Google Scholar]
- 91.Scully PR, Morris E, Patel KP, Treibel TA, Burniston M, Klotz E, et al. DPD quantification in cardiac amyloidosis: a novel imaging biomarker. JACC Cardiovasc Imaging. 2020;13(6):1353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ney S, Gertz RJ, Pennig L, Nies RJ, Holtick U, Völker LA, et al. Multiparametric monitoring of disease progression in contemporary patients with wild-type transthyretin amyloid cardiomyopathy initiating tafamidis treatment. J Clin Med. 2024;13(1):284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang X, Shen K, Zhang Y, Gao Y, Liu B, Guo Y, et al. Molecular stratification of light-chain cardiac amyloidosis with. JACC Cardiovasc Imaging. 2025;18(3):323–36. [DOI] [PubMed] [Google Scholar]
- 94.Guo Y, Li X, Gao Y, Shen K, Lin L, Wang J, et al. Light-chain cardiac amyloidosis: cardiac magnetic resonance for assessing response to chemotherapy. Korean J Radiol. 2024;25(5):426–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Martinez-Naharro A, Patel R, Kotecha T, Karia N, Ioannou A, Petrie A, et al. Cardiovascular magnetic resonance in light-chain amyloidosis to guide treatment. Eur Heart J. 2022;43(45):4722–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fontana M, Martinez-Naharro A, Chacko L, Rowczenio D, Gilbertson JA, Whelan CJ, et al. Reduction in CMR derived extracellular volume with Patisiran indicates cardiac amyloid regression. JACC Cardiovasc Imaging. 2021;14(1):189–99. [DOI] [PubMed] [Google Scholar]
- 97.Takashio S, Morioka M, Ishii M, Morikawa K, Hirakawa K, Hanatani S, et al. Clinical characteristics, outcome, and therapeutic effect of tafamidis in wild-type transthyretin amyloid cardiomyopathy. ESC Heart Fail. 2023;10(4):2319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kuyama N, Takashio S, Oguni T, Yamamoto M, Hirakawa K, Ishii M, et al. Cardiac biomarker change at 1 year after tafamidis treatment and clinical outcomes in patients with transthyretin amyloid cardiomyopathy. J Am Heart Assoc. 2024;13(10): e034518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Garcia-Pavia P, Aus dem Siepen F, Donal E, Lairez O, van der Meer P, Kristen AV, et al. Phase 1 trial of antibody NI006 for depletion of cardiac transthyretin amyloid. N Engl J Med. 2023;389(3):239–50. [DOI] [PubMed] [Google Scholar]
- 100.Huntjens PR, Zhang KW, Soyama Y, Karmpalioti M, Lenihan DJ, Gorcsan J. Prognostic utility of echocardiographic atrial and ventricular strain imaging in patients with cardiac amyloidosis. JACC Cardiovasc Imaging. 2021;14(8):1508–19. [DOI] [PubMed] [Google Scholar]
- 101.Istratoaie S, Bourg C, Lee KC, Marut B, Antonelli J, L’official G, et al. Right ventricular free wall strain predicts transthyretin amyloidosis prognosis as well as biomarker-based staging systems. Eur Heart J Cardiovasc Imaging. 2025;26(2):239–48. [DOI] [PubMed] [Google Scholar]
- 102.Ozbay B, Satyavolu BS, Rearick C, Soman P, Katz WE, Sezer A, et al. Right ventricular strain improves the echocardiographic diagnosis and risk stratification of transthyretin cardiac amyloidosis among other phenotypes of left ventricular hypertrophy. J Am Soc Echocardiogr. 2024;37(10):947–59. [DOI] [PubMed] [Google Scholar]
- 103.Clemmensen TS, Eiskjær H, Ladefoged B, Mikkelsen F, Sørensen J, Granstam SO, et al. Prognostic implications of left ventricular myocardial work indices in cardiac amyloidosis. Eur Heart J Cardiovasc Imaging. 2021;22(6):695–704. [DOI] [PubMed] [Google Scholar]
- 104.Porcari A, Fontana M, Canepa M, Biagini E, Cappelli F, Gagliardi C, et al. Clinical and prognostic implications of right ventricular uptake on bone scintigraphy in transthyretin amyloid cardiomyopathy. Circulation. 2024;149(15):1157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cai S, Haghbayan H, Chan KKW, Deva DP, Jimenez-Juan L, Connelly KA, et al. Tissue mapping by cardiac magnetic resonance imaging for the prognostication of cardiac amyloidosis: a systematic review and meta-analysis. Int J Cardiol. 2024;403: 131892. [DOI] [PubMed] [Google Scholar]
- 106.Li X, Guo Y, Shen K, Huang S, Gao Y, Lin L, et al. Comprehensive prognosis assessment of cardiovascular magnetic resonance parametric mapping in light chain amyloidosis. J Cardiovasc Magn Reson. 2024;27(1): 101135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Porcari A, Masi A, Martinez-Naharro A, Razvi Y, Patel R, Ioannou A, et al. Redefining cardiac involvement and targets of treatment in systemic immunoglobulin AL amyloidosis. JAMA Cardiol. 2024;9(11):982–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tang L, Zhao W, Li K, Tian L, Zhou X, Guo H, et al. Assessing microvascular dysfunction and predicting long-term prognosis in patients with cardiac amyloidosis by cardiovascular magnetic resonance quantitative stress perfusion. J Cardiovasc Magn Reson. 2024;27(1): 101134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Clerc OF, Cuddy SAM, Jerosch-Herold M, Benz DC, Katznelson E, Canseco Neri J, et al. Myocardial characteristics, cardiac structure, and cardiac function in systemic light-chain amyloidosis. JACC Cardiovasc Imaging. 2024;17(11):1271–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Martinez-Naharro A, Kotecha T, Norrington K, Boldrini M, Rezk T, Quarta C, et al. Native T1 and extracellular volume in transthyretin amyloidosis. JACC Cardiovasc Imaging. 2019;12(5):810–9. [DOI] [PubMed] [Google Scholar]
- 111.Boretto P, Patel NH, Patel K, Rana M, Saglietto A, Soni M, et al. Prognosis prediction in cardiac amyloidosis by cardiac magnetic resonance imaging: a systematic review with meta-analysis. Eur Heart J Open. 2023;3(5):oead092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gama F, Rosmini S, Bandula S, Patel KP, Massa P, Tobon-Gomez C, et al. Extracellular volume fraction by computed tomography predicts long-term prognosis among patients with cardiac amyloidosis. JACC Cardiovasc Imaging. 2022;15(12):2082–94. [DOI] [PubMed] [Google Scholar]
- 113.Deux JF, Nouri R, Tacher V, Zaroui A, Derbel H, Sifaoui I, et al. Diagnostic value of extracellular volume quantification and myocardial perfusion analysis at CT in cardiac amyloidosis. Radiology. 2021;300(2):326–35. [DOI] [PubMed] [Google Scholar]
- 114.Hanna M, Ruberg FL, Maurer MS, Dispenzieri A, Dorbala S, Falk RH, et al. Cardiac scintigraphy with technetium-99m-labeled bone-seeking tracers for suspected amyloidosis: JACC review topic of the week. J Am Coll Cardiol. 2020;75(22):2851–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed in the current study are available from the corresponding author upon request.