Abstract
Introduction
Studies suggest that early intervention with disease-modifying treatment for spinal muscular atrophy (SMA) might provide the best opportunity for optimal outcomes. One such treatment is onasemnogene abeparvovec, a gene replacement therapy with durable efficacy demonstrated in clinical trials, long-term studies, and real-world data (e.g., RESTORE registry).
Methods
A pooled post-hoc analysis was conducted to assess the early post-treatment impact of intravenous onasemnogene abeparvovec on motor function and event-free survival for symptomatic infants with SMA type 1 (i.e., non-sitters). Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND) scores and event-free survival were evaluated for patients enrolled in the START, STR1VE-US, and STR1VE-EU clinical trials.
Results
The pooled analysis set included 67 patients. Mean (SD) CHOP INTEND score at baseline was 29.3 (9.58) points. Rapid increases in mean CHOP INTEND of 7.0, 9.7, and 11.8 points were observed at 1, 2, and 3 months post-dose, respectively. At 6 months post-dose, 54/59 infants (91.5%) treated with onasemnogene abeparvovec achieved a clinically significant ≥ 4-point improvement in CHOP INTEND score from baseline, with a mean (SD) CHOP INTEND score of 44.3 (9.92) points. Patients who received onasemnogene abeparvovec had longer ventilation-free survival compared with natural history, with a statistically significant separation from the natural history cohort being maintained throughout follow-up.
Conclusions
Rapid and clinically significant improvements in motor function were observed for onasemnogene abeparvovec–treated patients with symptomatic SMA type 1. Early diagnosis and treatment are essential for timely restoration and preservation of motor neurons and maximal motor function improvement.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40120-025-00791-1.
Keywords: Gene therapy, Onasemnogene abeparvovec, Post-hoc analysis, Spinal muscular atrophy, Speed-to-effect
Key Summary Points
| Why carry out this study? |
| Spinal muscular atrophy (SMA) is a rare neurodegenerative disease that results in marked, progressive muscle weakness and atrophy and impacts bulbar and respiratory function in infancy. SMA type 1 is the most common and most severe phenotype, with symptoms usually appearing within the first 6 months of life. Without treatment, patients with SMA type 1 are not able to hold their head up or sit independently and usually do not survive without permanent ventilation by 2 years of age. |
| Clinical trials and long-term follow-up studies of onasemnogene abeparvovec have demonstrated improved survival, motor and bulbar function, and achievement of motor milestones for children with SMA. |
| We assessed early post-treatment impact of intravenous onasemnogene abeparvovec on motor function for symptomatic infants with SMA type 1, including changes measured by CHOP INTEND scores and time to permanent ventilation or death. |
| What was learned from the study? |
| Pooled clinical trial results demonstrated rapid and clinically significant improvements in ventilation-free survival and motor function for patients with SMA type 1 and two copies of the SMN2 gene treated with onasemnogene abeparvovec. |
| These findings corroborate the real-world data obtained from the RESTORE registry, which supports the effectiveness of onasemnogene abeparvovec in real-world settings and confirms that observed improvements are not limited to controlled clinical trial conditions. |
| Early diagnosis and treatment initiation are essential for timely restoration and preservation of SMN expression and motor function improvement, which aligns with the current understanding of SMA management. |
Introduction
Spinal muscular atrophy (SMA) is a rare monogenic motor neuron disease that is characterized by motor neuron death and progressive muscle weakness and atrophy, and manifests across a range of clinical subtypes [1–7]. SMA is caused by biallelic deletion or mutation of the survival motor neuron 1 (SMN1) gene, leading to deficiency of functional survival motor neuron (SMN) protein, which is critical for motor neuron development and viability [8–10]. The survival motor neuron 2 (SMN2) gene is a homologous gene with variable copy numbers, producing only small amounts of functional SMN protein [11]. The SMN2 gene copy number is the major SMA disease modifier and correlates inversely with disease severity [8].
Historically, SMA was classified based on age of symptom onset and maximal motor function achieved [12–16]. SMA type 0 is a very rare and severe type of SMA, classified as neonatal, with a history of reduced fetal movements and substantial weakness and hypotonia at birth [12]. Type 1 is the most common and most severe phenotype of SMA, with infants experiencing progressive weakness at birth or soon thereafter, resulting in the inability to hold their head up or to achieve independent sitting, and death or the need for permanent ventilation by 2 years of age if left untreated [1, 2, 13]. The median age at death or permanent ventilation for untreated infants with SMA type 1 and two copies of SMN2 is reported to be 10.5 (range 8.1–13.6) months [2]. Untreated patients with SMA type 2 also experience neuromuscular decline, although more gradual than that observed for patients with SMA type 1, and most achieve the ability to sit independently, but not stand independently [14, 15]. Untreated patients with SMA type 3 have even later symptom onset and achieve the ability to stand and walk independently although with increasing difficulty as patients age, while individuals with SMA type 4 resemble type 3, with symptoms usually occurring in adulthood, although it may have a juvenile origin [16–21]. However, newborn screening and SMA disease-modifying treatments, including nusinersen, onasemnogene abeparvovec, and risdiplam, are changing the way SMA is classified and described, with experts increasingly recommending that patients with SMA be reclassified by functional ability at the time of diagnosis—as non-sitters, sitters, and walkers—instead of SMA types 1, 2, 3, and 4 [22–25].
Onasemnogene abeparvovec is a one-time gene replacement therapy that delivers a fully functional copy of the human SMN1 gene to address the genetic root cause of SMA [26, 27]. Onasemnogene abeparvovec is designed for immediate and sustained expression of the SMN protein, allowing for rapid onset and durable therapeutic effect [28]. In clinical trials and long-term follow-up studies, intravenous onasemnogene abeparvovec has demonstrated efficacy and safety for patients with SMA type 1 or presymptomatic SMA and two or three SMN2 gene copies [27, 29–34]. In these trials, including START [27], STR1VE-US [30], and STR1VE-EU [31], symptomatic patients with SMA type 1 and two SMN2 gene copies who received onasemnogene abeparvovec demonstrated substantial improvements in event-free survival—free of permanent ventilation—and improved motor function, achievement of developmental motor milestones, and independence from respiratory and nutritional support compared with natural history [27, 30–34]. In addition, long-term safety and sustained efficacy has been demonstrated in ongoing studies for more than 5 years post-dose [7, 28, 29].
SMA type 1 leads to rapid deterioration of motor neurons if left untreated [1, 2]. However, regardless of phenotype, SMA manifestations indicate an irreversible loss of motor neurons [35–38], which may be prevented or mitigated by early intervention. Studies have suggested that intervention with disease-modifying treatment at the youngest possible age and early in the disease course, potentially before symptoms occur, might provide the best opportunity for optimal outcomes [32, 38–41]. This analysis sought to assess the early post-treatment impact of intravenous onasemnogene abeparvovec on motor function and survival for symptomatic infants with SMA type 1.
Methods
Study Design
The objective of this pooled post-hoc analysis was to evaluate the relationship between treatment timing, time to treatment effect, and clinical outcomes for symptomatic infants with SMA type 1 and two SMN2 gene copies who underwent a one-time intravenous infusion of onasemnogene abeparvovec gene therapy at the approved therapeutic dose (1.1 × 1014 vector genomes (vg)/kg). Baseline patient demographics and characteristics, Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND) scores, and event-free survival were evaluated for a pooled population of patients enrolled in the phase 1 START [27] and phase 3 STR1VE-US [30] and STR1VE-EU [31] clinical trials. The details of START [27], STR1VE-US [30], and STR1VE-EU [31] have been previously published (Table 1).
Table 1.
Key clinical trial characteristics for START, STR1VE-US, and STR1VE-EU
| START [27] | STR1VE-US [30] | STR1VE-EU [31] | |
|---|---|---|---|
| Phase | 1 | 3 | 3 |
| End of follow-up | 24 months post-dosing | 18 months of age | 18 months of age |
| Age eligible for study enrollment | ≤ 9 months of age at beginning of study, changed to ≤ 6 months of age for remainder of study | < 6 months of age | < 6 months of age |
| Exclusion criteria associated with ventilatory support | Use of invasive ventilatory support (tracheotomy with positive pressure) or pulse oximetry (patients were allowed to be managed using non-invasive ventilator support for < 16 h per day at physician or study staff discretion | Tracheostomy/requirement of noninvasive ventilatory support averaging ≥ 6 h per day over the 7 days prior to the screening visit/during the screening period or requiring ventilatory support while awake over the 7 days prior to screening visit/during the screening period | Use of invasive ventilatory support (tracheotomy with positive pressure) or pulse oximetry < 95% saturation at screening; patients may be put on non-invasive ventilatory support for < 12 h per day at physician/trial staff discretion; use or requirement of non-invasive ventilatory support for ≥ 12 h per day in the 2 weeks prior to dosing |
| Exclusion criteria regarding additional SMA treatment(s) | Participation in recent SMA treatment clinical trial that, in the opinion of the Principal Investigator, creates unnecessary risks for gene transfer | Participation in a recent interventional SMA treatment clinical study or receipt of an investigational or commercial compound, product, or therapy intended to treat SMA | Participation in recent SMA treatment clinical trial (with the exception of observational cohort studies or non-interventional studies) or receipt of an investigational or commercial compound, product, or therapy administered with the intent to treat SMA (e.g., nusinersen, valproic acid) at any time prior to screening |
| Total number of patients | 12a | 22 | 33 |
| Study design | Non-randomized, single-arm, open-label | Open-label, single-arm, single-dose | Multicenter, single-arm, single-dose, open-label |
aA total of 15 patients were included in START, but 3 patients received a lower-than-therapeutic dose and were excluded from this pooled analysis
Ethical Compliance
The START [27], STR1VE-US [30], and STR1VE-EU [31] study protocols were approved by the institutional review boards at each participating institution (Table S1), and study procedures were conducted according to the principles outlined in the Declaration of Helsinki. All parents or guardians provided written informed consent before any study procedures were performed.
Baseline Demographics and Characteristics
Baseline demographic, clinical, and treatment characteristics for patients enrolled in START [27], STR1VE-US [30], and STR1VE-EU [31] were summarized descriptively for the pooled analysis set. Baseline descriptive statistics were generated using univariate analysis [means with standard deviations (SDs) and ranges or frequencies with percentages] on all available assessments from infants’ first visits. Missing observations were not imputed in the analysis of individual questions or items.
Motor Function
Improvement in motor function was quantified by change from baseline in CHOP INTEND total score, which ranges from 0 to 64 points, with higher scores indicating better motor abilities [42, 43]. In START [27], STR1VE-US [30], and STR1VE-EU [31], CHOP INTEND scores were measured at baseline and monthly through end of study. Patients were followed until 18 months of age in STR1VE-US [30] and STR1VE-EU [31] and until 24 months post-dosing in START [27]. Descriptive statistics for absolute and change values were reported for each visit [27, 30, 31]. Categorical variables were described as numbers and percentages, and continuous variables were described as mean or median and corresponding SD or range. Motor function was presented graphically using the CHOP INTEND scale for the pooled analysis set from the START [27], STR1VE-US [30], and STR1VE-EU [31] infants who received onasemnogene abeparvovec [42, 43]. For CHOP INTEND scores, analyses were descriptive only, and no hypothesis was considered for this pooled analysis. Mean change in first observation to last, mean monthly change, and time between assessments were assessed for CHOP INTEND for the pooled analysis set. Minimal clinically important difference was defined as a ≥ 4-point improvement in CHOP INTEND score between first and last visit.
Event-Free Survival
Descriptive statistics of infants surviving event free at 24 months was performed for the pooled analysis using percentages of patients in SMA type 1 cohorts and time to event-free survival (free of death or need for permanent ventilation) from START [27], STR1VE-US [30], and STR1VE-EU [31] clinical trials. Permanent ventilation was defined as at least 16 h of respiratory assistance per day continuously for at least 14 days in the absence of an acute, reversible illness or a perioperative state [27, 30, 31]. Event-free survival was assessed using a Kaplan–Meier analysis. Analyses were performed using SAS Version 9.4 (Cary, NC, USA). Survival curves for the pooled clinical trial cohort and the natural history cohort were compared using a log-rank test at a significance level of 0.05. Because these analyses were exploratory, no multiplicity adjustment was carried out.
Natural History Cohort
The results from the pooled analysis for event-free survival were compared with a natural history cohort that included 23 patients from the Pediatric Neuromuscular Clinical Research (PNCR) database [2, 30] and 16 patients from Network for Excellence in Neuroscience Clinical Trials (NeuroNEXT) [1, 32]. The PNCR and NeuroNEXT datasets provided valid patient-level control data that thoroughly described the natural history of patients with SMA type 1 absent use of any disease-modifying treatment (Table S2). The natural history cohort derived from these datasets was reflective and descriptive of the United States and European standard of care for infants affected by SMA, and was deemed appropriate for comparison with the clinical experience of patients who received onasemnogene abeparvovec in START [27], STR1VE-US [30], and STR1VE-EU [31]. The natural history cohort was logically matched to the onasemnogene abeparvovec cohort for regulatory submissions [44, 45]. Of note, the NeuroNEXT natural history study composite endpoint was death or tracheostomy [1], while the composite endpoint for the PNCR natural history [2], START [27], STR1VE-US [30], and STR1VE-EU [31] studies was death or permanent ventilation support (defined in the studies as tracheostomy or ≥ 16 h of ventilation per day for > 2 consecutive weeks in the absence of acute reversible illness or perioperative ventilation). These endpoints have been described in detail and published previously for the separate PNCR [2] and NeuroNEXT [1] cohorts, as well as in publications detailing the START [27], STR1VE-US [30], and STR1VE-EU [31] clinical trials.
Real-World Data Comparison
Real-world data support the findings from the pooled data from clinical trials [46] and enhance understanding of the effects of disease-modifying treatments for SMA. Therefore, a similar post-hoc analysis of CHOP INTEND scores was conducted using real-world data from the RESTORE registry [46, 47] to compare with the results of the pooled analysis of CHOP INTEND clinical trial data from START [27], STR1VE-US [30], and STR1VE-EU [31]. RESTORE is a prospective, multicenter, multinational, observational registry that gathers real-world data about long-term efficacy and safety of SMA treatments [47, 48]. Detailed methodology of RESTORE, including registry study design, ethical considerations, patient eligibility, data acquisition, variables assessed, and outcomes have been published [47, 48]. To confirm the results of the current pooled analysis and to best match the pooled patient population from the onasemnogene abeparvovec clinical trials [27, 30, 31], the inclusion criteria for the post-hoc RESTORE real-world data comparison analysis were: (1) treatment with onasemnogene abeparvovec at < 6 months of age; (2) SMA type 1; (3) ≤ 2 copies of SMN2; and (4) having CHOP INTEND scores at baseline and at least 6 months post-baseline.
Results
A total of 67 patients were included in the pooled analysis set: 12 from START, 22 from STR1VE-US, and 33 from STR1VE-EU [27, 30, 31]. A total of 15 patients were included in the START clinical trial [27], but only the patients from Cohort 2 (who received a one-time intravenous infusion of onasemnogene abeparvovec gene therapy at the approved therapeutic dose) were included. The three patients in Cohort 1 who received a lower-than-therapeutic dose were excluded from the current pooled analysis.
Patient Characteristics
Key baseline demographics and clinical characteristics are described in Table 2. Over half (n = 38/67; 56%) of patients in the pooled analysis set were women. At baseline, 20.9% (n = 14/67) of pooled patients required feeding support and 14.9% (n = 10/67) required ventilatory support. Mean (SD) disease duration at onasemnogene abeparvovec dosing (i.e., duration from symptom onset to dosing) for the pooled analysis set was 9.3 (4.63) (range 0.3–25.4) weeks.
Table 2.
Patient characteristics at baseline for START, STR1VE-US, STRIVE-EU, and the pooled analysis set
| START [27] (n = 12) | STR1VE-US [30] (n = 22) | STR1VE-EU [31] (n = 33) | Pooled analysis set (N = 67) | |
|---|---|---|---|---|
| Women, n (%) | 7 (58) | 12 (55) | 19 (58) | 38 (56.7) |
| Mean (range) weight at baseline, kg | 5.7 (3.6–8.4) | 5.8 (3.9–7.5) | 5.8 (4.2–8.4) | 5.8 (3.6–8.4) |
| Mean (range) age at symptom onset, months | 1.4 (0.0–3.0) | 1.9 (1.0–3.0) | 1.6 (0.0–4.0) | 1.7 (0.0–4.0) |
| Mean (range) age at genetic diagnosis, monthsa | 2.0 (0.0–4.5) | 1.8 (1.8–4.1) | 2.7 (0.9–5.1) | 2.2 (0.0–5.1) |
| Mean (range) age at dosing, months | 3.4 (0.9–7.9) | 3.7 (0.5–5.9) | 4.1 (1.8–6.0) | 3.8 (0.5–7.9) |
aOriginal published study data reported mean (range) age at genetic diagnosis reported in days: START [27] = 60.0 (0.0–136.0) days; STR1VE-US [30] = 56.1 (56.0–126.0); STR1VE-EU [31] = 81.3 (26.0–156.0). Results in days for the pooled analysis set = 65.8 (0.0–156.0) days. Formula used for calculation from days to months: divide the day time value by 30.417 and round to one decimal place
Baseline characteristics for the 39 infants with SMA type 1 in the natural history cohort and the 26 patients from the RESTORE registry real-world comparison cohort were similar to the pooled analysis set in age and genetic profile. Key baseline demographics and clinical characteristics for the natural history cohort are described in Table S3.
Motor Function
For the pooled analysis set, the mean (SD) CHOP INTEND score at baseline was 29.3 (9.58) (range 12.0–55.0) points (Table 3). Mean baseline scores (range) were similar across all three clinical trials [START, 28.9 (12.0–50.0) points; STR1VE-US, 32.0 (18.0–52.0) points; STR1VE-EU, 29.3 (12.0–55.0) points] [27, 30, 31]. At 1 month post-dose, mean CHOP INTEND score increased by 7.0 points from baseline. Increases continued through Month 6, with a mean increase of 15.4 points.
Table 3.
Change in CHOP INTEND scores
| CHOP INTEND score, mean (SD) | ||||
|---|---|---|---|---|
| START [27] | STR1VE-US [30] | STR1VE-EU [31] | Pooled analysis set | |
| Baseline | (n = 12) | (n = 22) | (n = 33) | (n = 67) |
| Score | 28.2 (12.29) | 32.0 (9.69) | 27.9 (8.26) | 29.3 (9.58) |
| Month 1 | (n = 12) | (n = 22) | (n = 32) | (n = 66) |
| Score | 37.9 (12.72) | 38.9 (8.26) | 33.9 (8.56) | 36.3 (9.49) |
| Change from baseline | 9.8 (3.91) | 6.9 (5.35) | 6.0 (5.41) | 7.0 (5.26) |
| Month 2 | (n = 11) | (n = 22) | (n = 30) | (n = 63) |
| Score | 42.8 (13.36) | 41.2 (9.03) | 35.6 (9.91) | 38.8 (10.59) |
| Change from baseline | 14.7 (6.54) | 9.2 (5.88) | 8.1 (6.37) | 9.7 (6.58) |
| Month 3 | (n = 12) | (n = 22) | (n = 30) | (n = 64) |
| Score | 43.6 (13.47) | 43.7 (8.35) | 38.1 (9.04) | 41.1 (10.01) |
| Change from baseline | 15.4 (6.36) | 11.7 (6.40) | 10.3 (6.30) | 11.8 (6.52) |
| Month 4 | (n = 11) | (n = 21) | (n = 27) | (n = 59) |
| Score | 46.8 (13.55) | 44.1 (8.45) | 38.4 (7.89) | 42.0 (9.81) |
| Change from baseline | 17.6 (8.36) | 12.9 (6.18) | 11.4 (7.69) | 13.1 (7.55) |
| Month 5 | (n = 11) | (n = 21) | (n = 30) | (n = 62) |
| Score | 50.7 (8.59) | 46.7 (7.55) | 38.6 (7.29) | 43.5 (8.99) |
| Change from baseline | 21.1 (6.93) | 14.3 (6.61) | 11.9 (7.27) | 14.3 (7.65) |
| Month 6 | (n = 11) | (n = 20) | (n = 28) | (n = 59) |
| Score | 49.5 (13.95) | 46.3 (8.39) | 40.9 (8.00) | 44.3 (9.92) |
| Change from baseline | 21.4 (10.27) | 14.6 (7.04) | 13.6 (6.59) | 15.4 (7.95) |
CHOP INTEND Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders, SD standard deviation
Each month, a majority of patients who received gene therapy with onasemnogene abeparvovec achieved a clinically significant ≥ 4-point improvement in CHOP INTEND score from baseline (Fig. 1A). As early as 1 month post-dosing, the mean change in CHOP INTEND score for all trials included in the pooled analysis was ≥ 4 points, indicating a clinically significant change (Figure S1). The mean change continued to increase through Month 6, with 54/59 (91.5%) infants having a clinically significant change and a mean (SD) CHOP INTEND score of 44.3 (9.92) (Fig. 1B; Table 3).
Fig. 1.
CHOP INTEND score changes in the pooled analysis set.a Percentage of patients achieving an improvement of ≥ 4 points in CHOP INTEND score (A). Mean change and confidence intervals of CHOP INTEND score (B)b. CHOP INTEND Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders. aPooled onasemnogene abeparvovec cohort includes patients enrolled in START [27], STR1VE-US [30], and STR1VE-EU [31] clinical trials. bMean is represented by circles and lines represent confidence intervals. The red line represents a clinically significant change
Event-Free Survival
Patients who received onasemnogene abeparvovec had longer ventilation-free survival compared with natural history, with a substantial positive impact on event-free survival being observed (Table S4). The statistically significant separation from the natural history comparator group was maintained throughout the follow-up period. Kaplan–Meier plots for survival are provided in Fig. 2.
Fig. 2.
Kaplan–Meier plot of time-to-death or permanent ventilation for children who received onasemnogene abeparvovec compared with natural history.a PNCR Pediatric Neuromuscular Clinical Research, NeuroNEXT Network for Excellence in Neuroscience Clinical Trials. aThe pooled onasemnogene abeparvovec cohort included patients enrolled in START [27], STR1VE-US [30], and STR1VE-EU [31] clinical trials. The natural history cohort included patients from the PNCR database [2, 30, 31] and NeuroNEXT [1, 32]
Real-World Data Comparison
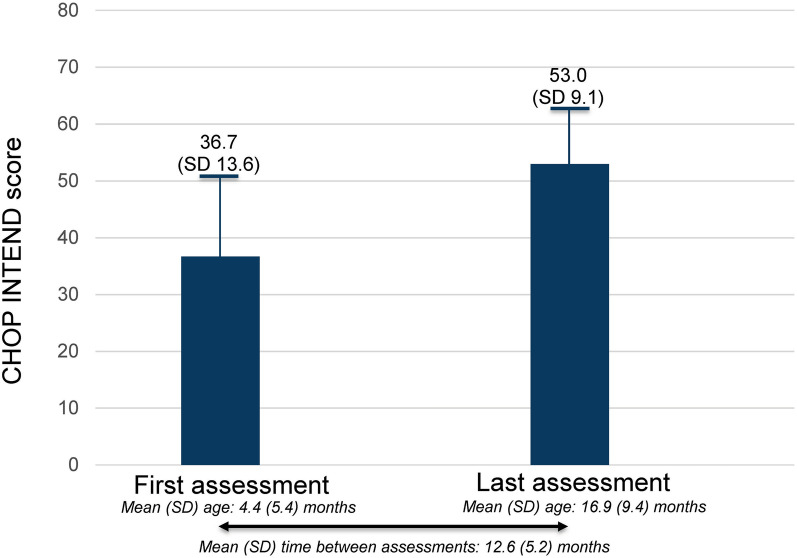
A total of 26 patients met the eligibility criteria for the real-world comparison post-hoc analysis of RESTORE [47, 48]. The findings from the current post-hoc pooled analysis of clinical trial data were comparable with those observed in the similar analysis of CHOP INTEND changes for patients in the real-world RESTORE registry, with a mean (SD) CHOP INTEND score change of 16.4 (10.1) points. (Fig. 3). In the post-hoc analysis of RESTORE, similar to the pooled post-hoc analysis of clinical trial data, 25/26 patients (96.2%) achieved a ≥ 4-point improvement in CHOP INTEND score from baseline. Mean (SD) age at first recorded CHOP INTEND score was 4.4 (5.4) months and age at last recorded score was 16.9 (9.4) months. Mean time (SD) between first and last CHOP INTEND scores was 12.6 (5.2) months.
Fig. 3.
CHOP INTEND score changes from patients in the RESTORE registry [48]. CHOP INTEND Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders, SD standard deviation
Discussion
This post-hoc analysis of pooled clinical trial data from three clinical trials (START [27], STR1VE-US [30], and STR1VE-EU [31]) demonstrates rapid and clinically significant improvements in event-free survival and motor function for symptomatic patients with SMA type 1 and two copies of the SMN2 gene who were treated with onasemnogene abeparvovec. Specifically, mean CHOP INTEND score increases of 7 points were observed at 1 month post-dose and increases of more than 15 points were observed at 6 months post-dose. Patients who received onasemnogene abeparvovec had longer ventilation-free survival compared with natural history, and this statistically significant separation from the natural history comparator group was maintained throughout the follow-up period.
Although there was no natural history comparison for motor function assessment in this post-hoc analysis, the findings are supported by natural history data in the literature. According to natural history, patients with SMA type 1 rarely achieve or maintain CHOP INTEND scores > 40 points [1, 2, 13]. In the current analysis, the mean (SD) CHOP INTEND score for patients in the pooled analysis set who were treated with onasemnogene abeparvovec increased from less than 40 points at baseline to more than 40 points by Month 3 [41.1 (10.01) points], with scores increasing through Month 6 [44.3 (9.92) points]. This increase in CHOP INTEND scores (7.0 and 15.4 points at 1 and 6 months post-dose, respectively) demonstrates the rapid positive impact that onasemnogene abeparvovec treatment had on the expected early motor function decline for infants with SMA type 1. This improvement in motor function with gene therapy, which is in contrast with the natural history of motor function decline for patients with SMA, was also observed for patients treated with onasemnogene abeparvovec who had improved mean CHOP INTEND scores by 22.7 points, compared with a decline of 8.9 points in untreated natural history patients [32]. Additional post-hoc analyses including a natural history comparison may further confirm the early post-treatment impact of onasemnogene abeparvovec on motor function.
Knowledge of disease-modifying treatments for SMA has been largely based on clinical trial data, which is limited most notably by narrow eligibility criteria and limited follow-up duration [46]. However, real-world treatment with onasemnogene abeparvovec engages patients with different criteria from those found in clinical trials, and this real-world data provides a heterogeneous presentation of patients not observed in clinical trials [27, 30, 31, 33, 34, 49–51]. The findings of this post-hoc analysis of pooled clinical trial data were corroborated by the real-world data obtained from the RESTORE registry [47, 48]. Despite associated limitations, real-world data, including experience from RESTORE, has been crucial in filling knowledge gaps and demonstrating that onasemnogene abeparvovec is associated with improvements in motor, bulbar, and pulmonary function in a varied SMA patient population and over an extended period of observation [51–57]. In addition to the motor function improvements observed in the post-hoc comparison population (n = 26), the full population of patients in RESTORE, which includes patients with SMA types 1, 2, and 3, event-free survival was dramatically improved compared with natural history. Event-free survival of 93.7% was reported for 79 patients with two copies of SMN2 post-treatment at 1 year in the RESTORE registry (as of data cutoff of May 23, 2022) [47].
The pathophysiology of SMA is characterized by rapid and irreversible loss of motor neurons; early intervention is necessary to maximize patient outcomes [22, 23, 33, 34, 58–61]. Greater efficacy from disease-modifying treatments, such as onasemnogene abeparvovec, for patients with SMA, is likely to come when treatment is initiated prior to disease onset, before disease progression claims non-replicating cells such as neurons [40, 41, 62]. Although the START [27], STR1VE-US [30], and STR1VE-EU [31] clinical studies did not record whether newborn screening was conducted for the patient populations studied, preclinical and clinical studies have demonstrated the benefits of newborn screening and early treatment for SMA [63]. Real-world data and clinical trials indicate that newborn screening allows infants to be diagnosed and assessed sooner, to score higher on the CHOP INTEND scale earlier, and to demonstrate overall greater improvements with timely treatment [33, 34, 48, 51, 64, 65]. For example, in the RESTORE study, patients identified through newborn screening with CHOP INTEND scores of less than 40 points demonstrated substantial improvement following onasemnogene abeparvovec treatment, as evidenced by increases in CHOP INTEND measurements [47]. In addition, the SPR1NT clinical trial of onasemnogene abeparvovec in two- and three-copy patients revealed that newborns who were treated presymptomatically achieved greater developmental motor milestones at earlier time points compared with patients who were not treated and patients who received treatment following onset of symptoms [33, 34]. The SPR1NT study demonstrated motor milestone improvements within 3 months of treatment with onasemnogene abeparvovec, and CHOP INTEND scores for children in SPR1NT were similar to those of healthy peers [1, 33, 34]. CHOP INTEND scores for all children in the SPR1NT two-copy patient group remained greater than 40 3 months post-treatment. This status was never achieved by untreated patients with SMA type 1 who were older than 6 months [1, 33, 34]. A similar analysis of the early post-treatment impact of onasemnogene abeparvovec for patients diagnosed by newborn screening, as well as additional analyses examining the relationship between age at treatment initiation or baseline motor function, would increase understanding of the benefits of early intervention and speed-to-effect for SMA type 1 following onasemnogene abeparvovec gene replacement therapy.
This post-hoc pooled analysis had a few limitations. First, the sample size of this pooled analysis from three clinical trials (START [27], STR1VE-US [30], and STR1VE-EU [31]) was small (N = 67). Second, the single-arm design of the pooled clinical trials had no comparator group [27, 30, 31] and was instead compared with natural history cohorts. For the Kaplan–Meier analysis, there was no statistical adjustment for the different studies included. As for the comparison with RESTORE real-world data for CHOP INTEND, it should also be noted that regular assessments are not performed in RESTORE, so the speed to effect may not be well reflected, because assessments follow standard of care according to health care provider discretion [47, 48]. In addition, a lack of training in use of the CHOP INTEND scale at each of the registry sites in RESTORE may have limited the interpretation of RESTORE data [47, 48].
Conclusions
This post-hoc analysis of pooled clinical trial results demonstrates rapid and clinically significant improvements in ventilation-free survival and motor function for patients with SMA type 1 and two copies of the SMN2 gene who were treated with onasemnogene abeparvovec. Specifically, a mean increase of 7 points in the CHOP INTEND score was observed at 1 month post-dose and increases of more than 15 points were observed at 6 months post-dose. These findings further support the effectiveness evidence obtained from the RESTORE registry, which supports the effectiveness of onasemnogene abeparvovec in real-world settings and confirms that observed improvements are not limited to controlled clinical trial conditions. These findings also contrast sharply with the natural history of progressive motor function decline for patients with SMA. Early diagnosis and prompt treatment initiation are essential for timely restoration and preservation of SMN expression and motor function improvement, which aligns with the current understanding of SMA management. Future analyses should include additional measures of early efficacy, larger pooled patient populations, and exploration of the speed of post-treatment effect of other disease-modifying treatments for SMA.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to thank the clinical trial investigators and site coordinators and, most importantly, all the patients, families, and caregivers for their willingness to participate in these studies, which were sponsored by Novartis Pharma AG, Basel, Switzerland.
Medical Writing, Editorial, and Other Assistance
Writing and editing assistance, including preparation of a draft manuscript under the direction and guidance of the authors, incorporating author feedback, and manuscript submission, was provided by Wynne Dillon, MS (Kay Square Scientific, Newtown Square, PA, USA). This support was funded by Novartis Pharma AG, Basel, Switzerland.
Author Contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published. Walter Toro: analysis and interpretation of data, participation in development and critical review of the manuscript, approval of final version of manuscript and its submission for publication, accountability for the accuracy and integrity of the work. Sandra P. Reyna: analysis and interpretation of data, participation in development and critical review of the manuscript, approval of final version of manuscript and its submission for publication, accountability for the accuracy and integrity of the work. Nayla Mumneh: critical review of the manuscript, approval of final version of manuscript and its submission for publication, accountability for the accuracy and integrity of the work. Shannon Ritter: conceptualization, analysis and interpretation of data, participation in development and critical review of the manuscript, approval of final version of manuscript and its submission for publication, accountability for the accuracy and integrity of the work. Anish Patel: critical review of the manuscript, approval of final version of manuscript and its submission for publication, accountability for the accuracy and integrity of the work. Omar Dabbous: critical review of the manuscript, approval of final version of manuscript and its submission for publication, accountability for the accuracy and integrity of the work. All authors have read and approved this manuscript and agree with its submission.
Funding
This analysis was funded by Novartis Pharma AG, Basel, Switzerland. Sponsorship for this study and the Rapid Service Fee were funded by Novartis Pharma AG.
Data Availability
The data sets used and analyzed during the present study are available from the corresponding author on reasonable request.
Declarations
Conflict of Interest
Walter Toro, Sandra P. Reyna, and Nayla Mumneh are employees of Novartis and own stock/other equities. Shannon Ritter, Anish Patel, and Omar Dabbous were employees of Novartis at the time of this study and own stock/other equities. Anish Patel is currently an employee of Nanoscope Therapeutics Inc. Omar Dabbous is currently an employee of Komar Pharmaceuticals and Curestack.
Ethical Approval
The START [27], STR1VE-US [30], and STR1VE-EU [31] study protocols were approved by the institutional review boards at each participating institution (Table S1), and study procedures were conducted according to the principles outlined in the Declaration of Helsinki. The RESTORE registry is being conducted in accordance with established research principles, local treatment practices and regulations, and guidelines of the International Council on Harmonisation. All parents or guardians provided written informed consent before any study procedures were performed.
Footnotes
Prior Presentation: These data have been presented as a poster at the World Muscle Society’s 2023 congress, held 3–7 October 2023, in Charleston, South Carolina, United States.
References
- 1.Kolb SJ, Coffey CS, Yankey JW, et al. Natural history of infantile-onset spinal muscular atrophy. Ann Neurol. 2017;82:883–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finkel RS, McDermott MP, Kaufmann P, et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology. 2014;83:810–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neil EE, Bisaccia EK. Nusinersen: a novel antisense oligonucleotide for the treatment of spinal muscular atrophy. J Pediatr Pharmacol Ther. 2019;24:194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finkel RS, Mercuri E, Darras BT, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. 2017;377:1723–32. [DOI] [PubMed] [Google Scholar]
- 5.Lefebvre S, Bürglen L, Reboullet S, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–65. [DOI] [PubMed] [Google Scholar]
- 6.Wirth B. Spinal muscular atrophy: in the challenge lies a solution. Trends Neurosci. 2021;44:306–22. [DOI] [PubMed] [Google Scholar]
- 7.Shell RD, McGrattan KE, Hurst-Davis R, et al. Onasemnogene abeparvovec preserves bulbar function in infants with presymptomatic spinal muscular atrophy: a post-hoc analysis of the SPR1NT trial. Neuromuscul Disord. 2023;33:670–6. [DOI] [PubMed] [Google Scholar]
- 8.Verhaart IEC, Robertson A, Wilson IJ, et al. Prevalence, incidence and carrier frequency of 5q-linked spinal muscular atrophy—a literature review. Orphanet J Rare Dis. 2017;12:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coovert DD, Le TT, McAndrew PE, et al. The survival motor neuron protein in spinal muscular atrophy. Hum Mol Genet. 1997;6:1205–14. [DOI] [PubMed] [Google Scholar]
- 10.Fallini C, Bassell GJ, Rossoll W. Spinal muscular atrophy: the role of SMN in axonal mRNA regulation. Brain Res. 2012;1462:81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burghes AHM, McGovern VL. Genetics of spinal muscular atrophy. In: Boulis N, O’Connor S, Donsante A, editors. Molecular and cellular therapies for motor neuron diseases. London: Academic Press; 2017. p. 121–39. [Google Scholar]
- 12.Farooq FT, Holcik M, MacKenzieet A. Spinal muscular atrophy: classification, diagnosis, background, molecular mechanism and development of therapeutics. In: Kishore A, editor. Neurodegenerative diseases. London: IntechOpen; 2013. p. 561–79. [Google Scholar]
- 13.Mercuri E, Lucibello S, Perulli M, et al. Longitudinal natural history of type I spinal muscular atrophy: a critical review. Orphanet J Rare Dis. 2020;15:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carson VJ, Young M, Brigatti KW, et al. Nusinersen by subcutaneous intrathecal catheter for symptomatic spinal muscular atrophy patients with complex spine anatomy. Muscle Nerve. 2022;65:51–9. [DOI] [PubMed] [Google Scholar]
- 15.Muntoni F, Bertini E, Comi G, et al. Long-term follow-up of patients with type 2 and non-ambulant type 3 spinal muscular atrophy (SMA) treated with olesoxime in the OLEOS trial. Neuromuscul Disord. 2020;30:959–69. [DOI] [PubMed] [Google Scholar]
- 16.Annoussamy M, Seferian AM, Daron A, et al. Natural history of type 2 and 3 spinal muscular atrophy: 2-year NatHis-SMA study. Ann Clin Transl Neurol. 2021;8:359–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chabanon A, Seferian AM, Daron A, et al. Prospective and longitudinal natural history study of patients with type 2 and 3 spinal muscular atrophy: baseline data NatHis-SMA study. PLoS ONE. 2018;13: e0201004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coratti G, Cutrona C, Pera MC, et al. Motor function in type 2 and 3 SMA patients treated with nusinersen: a critical review and meta-analysis. Orphanet J Rare Dis. 2021;16:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufmann P, McDermott MP, Darras BT, et al. Prospective cohort study of spinal muscular atrophy types 2 and 3. Neurology. 2012;79:1889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mercuri E, Finkel R, Montes J, et al. Patterns of disease progression in type 2 and 3 SMA: Implications for clinical trials. Neuromuscul Disord. 2016;26:126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chong LC, Gandhi G, Lee JM, et al. Drug discovery of spinal muscular atrophy (SMA) from the computational perspective: a comprehensive review. Int J Mol Sci. 2021;22:8962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finkel RS, Benatar M. Pre-symptomatic spinal muscular atrophy: a proposed nosology. Brain. 2022;145:2247–9. [DOI] [PubMed] [Google Scholar]
- 23.Tizzano EF. Treating neonatal spinal muscular atrophy: a 21st century success story? Early Hum Dev. 2019;138: 104851. [DOI] [PubMed] [Google Scholar]
- 24.Mercuri E, Finkel RS, Muntoni F, et al. Diagnosis and management of spinal muscular atrophy: part 1: recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord. 2018;28:103–15. [DOI] [PubMed] [Google Scholar]
- 25.Wirth B, Karakaya M, Kye MJ, et al. Twenty-five years of spinal muscular atrophy research: from phenotype to genotype to therapy, and what comes next. Annu Rev Genom Hum Genet. 2020;21:231–61. [DOI] [PubMed] [Google Scholar]
- 26.Al-Zaidy S, Pickard AS, Kotha K, et al. Health outcomes in spinal muscular atrophy type 1 following AVXS-101 gene replacement therapy. Pediatr Pulmonol. 2019;54:179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendell JR, Al-Zaidy S, Shell R, et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377:1713–22. [DOI] [PubMed] [Google Scholar]
- 28.Dean R, Dabbous O, Weidlich D, et al. Body of evidence for onasemnogene abeparvovec in spinal muscular atrophy supports long-term duration of effect without relapse. Value Health. 2022;25:1922–3. [DOI] [PubMed] [Google Scholar]
- 29.Mendell JR, Al-Zaidy SA, Lehman KJ, et al. Five-year extension results of the phase 1 start trial of onasemnogene abeparvovec in spinal muscular atrophy. JAMA Neurol. 2021;78:834–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Day JW, Finkel RS, Chiriboga CA, et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy in patients with two copies of SMN2 (STR1VE): an open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021;20:284–93. [DOI] [PubMed] [Google Scholar]
- 31.Mercuri E, Muntoni F, Baranello G, et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy type 1 (STR1VE-EU): an open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021;20:832–41. [DOI] [PubMed] [Google Scholar]
- 32.Al-Zaidy SA, Kolb SJ, Lowes L, et al. AVXS-101 (onasemnogene abeparvovec) for SMA1: comparative study with a prospective natural history cohort. J Neuromuscul Dis. 2019;6:307–17. [DOI] [PubMed] [Google Scholar]
- 33.Strauss KA, Farrar MA, Muntoni F, et al. Onasemnogene abeparvovec for pre-symptomatic infants with two copies of SMN2 at risk for spinal muscular atrophy type 1: the Phase III SPR1NT trial. Nat Med. 2022;28:1381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strauss KA, Farrar MA, Muntoni F, et al. Onasemnogene abeparvovec for pre-symptomatic infants with three copies of SMN2 at risk for spinal muscular atrophy: the Phase III SPR1NT trial. Nat Med. 2022;28:1390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calucho M, Bernal S, Alías L, et al. Correlation between SMA type and SMN2 copy number revisited: an analysis of 625 unrelated Spanish patients and a compilation of 2834 reported cases. Neuromuscul Disord. 2018;28:208–15. [DOI] [PubMed] [Google Scholar]
- 36.Blaschek A, Kölbel H, Schwartz O, et al. Newborn screening for SMA - can a wait-and-see strategy be responsibly justified in patients with four SMN2 copies? J Neuromuscul Dis. 2022;9:597–605. [DOI] [PubMed] [Google Scholar]
- 37.Müller-Felber W, Vill K, Schwartz O, et al. Infants diagnosed with spinal muscular atrophy and 4 SMN2 copies through newborn screening—opportunity or burden? J Neuromuscul Dis. 2020;7:109–17 (Erratum in: J Neuromuscul Dis 2021;8:335–336). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glascock J, Sampson J, Haidet-Phillips A, et al. Treatment algorithm for infants diagnosed with spinal muscular atrophy through newborn screening. J Neuromuscul Dis. 2018;5:145–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Servais L, Farrar M, Finkel RS, et al. Nusinersen demonstrates greater efficacy in infants with shorter disease duration: end of study results from the ENDEAR study in infants with spinal muscular atrophy (SMA). Neuromuscul Disord. 2017;27:S211. [Google Scholar]
- 40.De Vivo DC, Bertini E, Swoboda KJ, et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: interim efficacy and safety results from the Phase 2 NURTURE study. Neuromuscul Disord. 2019;29:842–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Servais L, Bertini E, Al-Muhaizea M, et al. RAINBOWFISH: a study of risdiplam in infants with presymptomatic spinal muscular atrophy (SMA). Neuromuscul Disord. 2021;31:S48. [Google Scholar]
- 42.Glanzman AM, Mazzone E, Main M, et al. The Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND): test development and reliability. Neuromuscul Disord. 2010;20:155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glanzman AM, McDermott MP, Montes J, et al. Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND). Pediatr Phys Ther. 2011;23:322–6. [DOI] [PubMed] [Google Scholar]
- 44.Novartis Gene Therapies EU Ltd. Modul 4A, 5q-assoziierte spinale Muskelatrophie mit biallelischer Mutation von SMN1 und klinischer Diagnose SMA Typ 1 oder bis zu 3 SMN2-Kopien [Module 4A, 5q-associated spinal muscular atrophy with biallelic mutation of SMN1 and clinical diagnosis of SMA type 1 or up to 3 SMN2 copies] [Article in German]. 2021. https://www.g-ba.de/downloads/92-975-4738/2021_05_12_Modul4A_Onasemnogen_Abeparvovec.pdf. Accessed 13 Feb 2025.
- 45.AveXis. PNCR and NeuroNEXT Database Report, Final Report 01 June 2018 (RPT-806) [CONFIDENTIAL]. 2018.
- 46.Liu F, Panagiotakos D. Real-world data: a brief review of the methods, applications, challenges and opportunities. BMC Med Res Methodol. 2022;22:287 (Erratum in: BMC Med Res Methodol. 2023;23:109). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Servais L, Day JW, De Vivo DC, et al. Real-world outcomes in patients with spinal muscular atrophy treated with onasemnogene abeparvovec monotherapy: findings from the RESTORE registry. J Neuromuscul Dis. 2024;11:425–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finkel RS, Day JW, De Vivo DC, et al. RESTORE: a prospective multinational registry of patients with genetically confirmed spinal muscular atrophy—rationale and study design. J Neuromuscul Dis. 2020;7:145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Finkel RS, Darras BT, Mendell JR, et al. Intrathecal onasemnogene abeparvovec for sitting, nonambulatory patients with spinal muscular atrophy: phase I ascending-dose study (STRONG). J Neuromuscul Dis. 2023;10:389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McMillan HJ, Baranello G, Farrar MA, et al. Safety and efficacy of IV onasemnogene abeparvovec for pediatric patients with spinal muscular atrophy: the phase 3b SMART study. Neurology. 2025;104: e210268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swoboda KJ, Kissel JT, Crawford TO, et al. Perspectives on clinical trials in spinal muscular atrophy. J Child Neurol. 2007;22:957–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D’Silva AM, Holland S, Kariyawasam D, et al. Onasemnogene abeparvovec in spinal muscular atrophy: an Australian experience of safety and efficacy. Ann Clin Transl Neurol. 2022;9:339–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Erdos J, Wild C. Mid- and long-term (at least 12 months) follow-up of patients with spinal muscular atrophy (SMA) treated with nusinersen, onasemnogene abeparvovec, risdiplam or combination therapies: a systematic review of real-world study data. Eur J Paediatr Neurol. 2022;39:1–10. [DOI] [PubMed] [Google Scholar]
- 54.Weiß C, Ziegler A, Becker LL, et al. Gene replacement therapy with onasemnogene abeparvovec in children with spinal muscular atrophy aged 24 months or younger and bodyweight up to 15kg: an observational cohort study. Lancet Child Adolesc Health. 2022;6:17–27. [DOI] [PubMed] [Google Scholar]
- 55.Bitetti I, Lanzara V, Margiotta G, et al. Onasemnogene abeparvovec gene replacement therapy for the treatment of spinal muscular atrophy: a real-world observational study. Gene Ther. 2023;30:592–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Friese J, Geitmann S, Holzwarth D, et al. Safety monitoring of gene therapy for spinal muscular atrophy with onasemnogene abeparvovec a single centre experience. J Neuromuscul Dis. 2021;8:209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kirschner J, Butoianu N, Goemans N, et al. European ad-hoc consensus statement on gene replacement therapy for spinal muscular atrophy. Eur J Paediatr Neurol. 2020;28:38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Govoni A, Gagliardi D, Comi GP, et al. Time is motor neuron: therapeutic window and its correlation with pathogenetic mechanisms in spinal muscular atrophy. Mol Neurobiol. 2018;55:6307–18. [DOI] [PubMed] [Google Scholar]
- 59.Ramdas S, Servais L. New treatments in spinal muscular atrophy: an overview of currently available data. Expert Opin Pharmacother. 2020;21:307–15. [DOI] [PubMed] [Google Scholar]
- 60.Dangouloff T, Hiligsmann M, Deconinck N, et al. Financial cost and quality of life of patients with spinal muscular atrophy identified by symptoms or newborn screening. Dev Med Child Neurol. 2023;65:67–77. [DOI] [PubMed] [Google Scholar]
- 61.Hjartarson HT, Nathorst-Böös K, Sejersen T. Disease modifying therapies for the management of children with spinal muscular atrophy (5q SMA): an update on the emerging evidence. Drug Des Devel Ther. 2022;16:1865–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sumner CJ, Crawford TO. Early treatment is a lifeline for infants with SMA. Nat Med. 2022;28:1348–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Sanctis R, Coratti G, Pasternak A, et al. Developmental milestones in type I spinal muscular atrophy. Neuromuscul Disord. 2016;26:754–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pane M, Berti B, Capasso A, ITASMAc Group, et al. Onasemnogene abeparvovec in spinal muscular atrophy: predictors of efficacy and safety in naïve patients with spinal muscular atrophy and following switch from other therapies. EClinicalMedicine. 2023;59:101997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kariyawasam DS, D’Silva AM, Sampaio H, et al. Newborn screening for spinal muscular atrophy in Australia: a non-randomised cohort study. Lancet Child Adolesc Health. 2023;7:159–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets used and analyzed during the present study are available from the corresponding author on reasonable request.