Abstract
Introduction
Effectiveness and tolerability of plant-derived highly purified cannabidiol (CBD) in patients with Lennox–Gastaut syndrome (LGS), Dravet syndrome (DS), or tuberous sclerosis complex (TSC)-associated epilepsy in clinical practice in Germany were evaluated.
Methods
This multicenter, retrospective, chart review study analyzed patients with LGS, DS, or TSC-associated epilepsy receiving ≥ 1 dose of adjunctive CBD (Epidyolex® 100 mg/mL oral solution). Treatment characteristics, seizure outcomes, physician-rated Clinical Global Impression of Change (CGI-C), treatment retention rates, and adverse events (AEs) were analyzed ≤ 12 months.
Results
Among 202 patients identified (159 LGS; 34 DS; 9 TSC), median (interquartile range; range) age was 18.0 (7.9–32.0; 0.3–72.0) years, and median (range) number of prior and concomitant antiseizure medications was 6 (1–24) and 3 (1–7), respectively. Median target CBD dose was 11.1 mg/kg/day (17.6, 15.2, and 9.9 mg/kg/day in the < 6, 6–17, and ≥ 18 years subgroups, respectively). Responder rates (≥ 50% seizure reduction) for total seizures at 3 (n = 194) and 12 (n = 168) months were 43.3% (37.0–50.0% across ages) and 44.0% (37.0–52.5% across ages), respectively, and for generalized tonic–clonic seizures 54.3% (n = 94) (50.0–66.7% across ages) and 47.7% (n = 88) (37.8–66.7% across ages), respectively. Median (range) number of seizure days per month significantly decreased from 30 (0.3–30) to 18 (0–30) in the 3 months before the last 3 months of CBD treatment (p < 0.001). Any improvement in CGI-C was observed in 62% of patients. Of those with available data at 3 and 12 months, 89.6% and 67.1% remained on CBD, respectively. Retention was similar across age groups. AEs reported in ≥ 5% of patients were sedation and diarrhea.
Conclusions
In patients with LGS, DS, or TSC-associated epilepsy, adjunctive CBD was associated with a reduction in seizure frequency across age groups. CBD demonstrated tolerability consistent with its known profile, and 67% of patients remained on treatment at 12 months.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s40120-025-00788-w.
Keywords: Cannabidiol, Clinical Global Impression of Change, Developmental and epileptic encephalopathies, Dravet syndrome, Epilepsy, Lennox–Gastaut syndrome, Responder rate, Tuberous sclerosis complex
Key Summary Points
| Why carry out the study? |
| This study reports the real-world treatment characteristics, effectiveness, tolerability, and retention of adjunctive cannabidiol (CBD, Epidyolex®) among a large, multicenter cohort of children, adolescents, and adults with Lennox–Gastaut syndrome (LGS), Dravet syndrome (DS), or tuberous sclerosis complex (TSC)-associated epilepsy. |
| It is the largest real-world study to date to evaluate CBD solely in its indicated etiologies, and it also includes a large proportion of adult patients allowing, in contrast to many studies, analysis of the effectiveness of CBD in both adult and pediatric patients. |
| What was learned from the study? |
| Adjunctive CBD treatment was associated with reductions in seizure frequency across age groups analyzed. Kaplan–Meier estimated retention rates at 1 year were 62.9% for the overall population, and did not significantly differ across age groups, syndrome (LGS, DS, or TSC) or in patients with or without concomitant clobazam. This retrospective chart review study provides further insights into the effectiveness, tolerability, and retention of CBD in clinical practice, with a focus on the use of CBD in the indicated patient populations, and with a high number of adult patients. |
Digital Features
This article is published with digital features, including a graphical abstract, to facilitate understanding of the article. To view digital features for this article, go to 10.6084/m9.figshare.29270747.
Introduction
Lennox–Gastaut syndrome (LGS), Dravet syndrome (DS), and tuberous sclerosis complex (TSC)-associated epilepsy are characterized by seizures that are highly refractory to treatment with antiseizure medications (ASMs) [1]. The term developmental and epileptic encephalopathies (DEEs) encompasses this patient group and others who suffer from recurrent, severe, drug-resistant seizures of multiple types [1]. Seizures typically begin in infancy or early childhood. In addition to seizures, patients have neurodevelopmental impairments affecting cognition, behavior, speech, language, sleep, motor function, and growth [2]. DEEs are also associated with an increased risk of sudden unexpected death in epilepsy (SUDEP) [3]. DEEs have profound life-long impacts on the individuals and their families, together with a substantial burden on healthcare systems [4–8].
A plant-derived highly purified formulation of cannabidiol (CBD, Epidyolex®; 100 mg/mL oral solution) is approved by the European Medicines Agency (EMA) as an adjunctive therapy in patients aged ≥ 2 years of age, for seizures associated with LGS or DS in conjunction with clobazam (CLB), or as adjunctive therapy for seizures associated with TSC [9]. In the USA, it is approved by the Food and Drug Administration (FDA) for the treatment of seizures associated with LGS, DS, or TSC in patients ≥ 1 year of age [10]. The approvals are based on evidence from double-blind randomized controlled trials (RCTs) that demonstrated the efficacy of CBD as adjunctive therapy to standard ASMs in reducing drop seizures in LGS [11, 12], convulsive seizures in DS [13, 14], and TSC-associated seizures [15] compared with placebo. Subsequent open-label extension studies that included patients from these respective pivotal randomized controlled trials (RCTs) demonstrated sustained efficacy and tolerability in patients with LGS, DS, or TSC-associated seizures up to 156 weeks [16–18].
Observational, real-world studies can help to provide a further understanding of the effectiveness, tolerability, and safety of treatments in patients in clinical practice without the inherent limitations associated with the stringent eligibility criteria of RCTs [19]. A number of observational studies have evaluated the effectiveness and tolerability of CBD in real-world practice [20–30]; however, most of the studies included a substantial proportion of patients being treated with CBD for non-indicated etiologies. Here, we describe the real-world effectiveness, tolerability, and retention of CBD among a large, multicenter cohort of children, adolescents, and adults with LGS, DS, or TSC-associated epilepsy; this is the largest study to date to evaluate CBD solely in its indicated etiologies. This cohort also includes a large proportion of adult patients allowing, in contrast to many studies, analysis of the effectiveness of CBD in both adult and pediatric patients.
Methods
Study Design and Patients
This multicenter, retrospective, chart review study involved six centers across Germany located in Frankfurt am Main, Freiburg im Breisgau, Greifswald, Lingen (Münster), Marburg, and Radeberg. The analysis was approved by the ethics committee of the University of Frankfurt. Informed consent was waived because of the retrospective nature of the chart reviews. STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines were followed [31].
This was a retrospective chart review study, which included all patients at the study centers who had been treated with at least one adjunctive dose of highly purified CBD medicine (Epidyolex® 100 mg/mL oral solution), either as part of a compassionate use program or commercial supply from 2019 (when CBD was first available in Germany outside clinical trials) to 2023. Here we report the use of CBD in the indicated etiologies. We reviewed the charts of all patients seen in our respective institutions and identified all patients with LGS, DS, or TSC who had been treated with adjunctive CBD. Patients who had been treated with another non-pharmaceutical grade CBD formulation were not included. For the purposes of the data analysis, we then identified records which had at least 3 months of follow-up, including patients who discontinued during that time. Diagnosis of LGS and DS was based on the definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy [32], and of TSC based on the consensus statement of the International TSC Consensus Group [33]. A sub-analysis of this study involving patients with LGS or DS and treated exclusively with adjunct CBD and concomitant CLB has been published elsewhere [34].
Outcomes and Statistical Analysis
Using the medical records of the patients, baseline data were collected for etiology, sex, age at epilepsy onset and age at the start of CBD treatment, intellectual disability (none/not reported, mild, moderate, or severe), seizure frequency in the 3 months prior to CBD treatment (denoted baseline), prior and current treatment with other ASMs, and dose and titration of CBD. The mean and standard deviation (SD), and/or median and range or interquartile range (IQR) were calculated for continuous data; frequencies and percentages were calculated for categorical variables.
Patients were seen according to the clinical practice of the center, at regular intervals, typically every 3–6 months. Data were obtained from the medical records to assess outcomes at 3, 6, and 12 months after initiation of adjunctive CBD, based on the diaries for the respective timepoints or the visit closest to those timepoints. Follow-up data included target and maximal doses of CBD, seizure frequency, physician-rated Clinical Global Impression of Change (CGI-C) and CGI-C-behavior, adverse events (AEs), retention of CBD, and discontinuation of CBD according to the following reasons: AEs, lack of effectiveness, both AEs and lack of effectiveness, or not reported.
Responder rates were defined as the proportion of patients who achieved reductions in seizure frequency from baseline of ≥ 25%, ≥ 50%, ≥ 75%, and 100% (seizure free). Responder rates were assessed for both generalized tonic–clonic seizures (GTCS) alone and total seizures, which consisted of all seizure types including GTCS, atonic, atypical absence, focal impaired awareness, myoclonic, and tonic seizures. The percentage of patients with an increase of ≥ 25% or no change (≥ 0% and < 25%) in seizure frequency was also evaluated. In addition to the overall population, responder rates were also evaluated in the different age subgroups, i.e., preschool (below 6 years), school and adolescents (from 6 to 17 years), and adults (18 years and over). The assessment of responder rates was performed at 3 and 12 months in all patients from the initial 202 medical records who had available data, i.e., patients were excluded from the analysis if data on seizure frequency were missing from the patients’ medical records. Patients without follow-up data at 12 months were included so as not to overestimate the responder rate due to there being fewer patients with follow-up data.
Seizure days were defined as a day with a seizure irrespective of seizure type. The median number of seizure days per month was calculated for the 3 months before CBD treatment and the last 3 months of CBD treatment. The CGI-C completed at the last follow-up visit by physicians enabled rating of the overall clinical change and change in behavior during treatment from very much improved to very much worse on a 7-point categorical rating scale.
The proportion of patients remaining on CBD at 3, 6, and 12 months was calculated based on start and end treatment dates. As per the study design, all patients had 3 months follow-up data and therefore all patients were considered for the 3-month analysis. For the 6-month and 12-month visits, only patients who were started at least 6 or 12 months before the data analysis were considered (i.e., with 6- and 12-months follow-up data). One-year retention rates were also estimated using Kaplan–Meier (KM) survival curves, and differences in retention rates for age groups (< 6, 6–17, ≥ 18 years), the DEE syndromes (LGS, DS, and TSC), and the use of concomitant CLB (with and without concomitant CLB) were assessed. The proportions of patients discontinuing treatment over the entire 12-month period were also calculated. Of note, the discontinuation rate was calculated using the whole population as the denominator, whereas the proportion of patients remaining on CBD excluded patients from the denominator who had not yet reached 6 months follow-up or 12 months follow-up, and the KM analysis used standard censoring techniques to account for missing data; therefore the values differ between the respective analyses.
AEs were categorized according to whether they were related to sedation, gastrointestinal (GI) symptoms, psychobehavioral AEs, decreased weight/appetite, sialorrhea, sleep, central nervous system/ataxia, skin, elevated liver enzymes, and other.
The study protocol specified for descriptive statistics to be used to summarize baseline characteristics and treatment outcomes (responder rates, discontinuation rates). Changes in seizure days were compared using Wilcoxon signed-rank test, while group comparisons for retention rates were compared using log-rank test. No adjustments for multiplicity were made as analyses were exploratory, and p values should be interpreted accordingly. Statistical analyses were conducted using IBM SPSS Statistics, version 28 (IBM Corp., Armonk, NY, USA). All p values were considered as statistically significant at < 0.05.
Results
Patients
Overall, 202 patients with a diagnosis of LGS, DS, or TSC-associated epilepsy who had been treated with at least one dose of adjunctive CBD were included. Of these, 159 (78.7%) had a diagnosis of LGS, 34 (16.8%) of DS, and 9 (4.5%) of TSC. The baseline demographics and clinical characteristics of the overall population and for each syndrome are shown in Table 1. Overall, 116 patients (57.4%) were male. The median age at the start of treatment with CBD was 18.0 years (IQR 7.9–32.0; range 0.3–72.0); 14.4% were preschool children aged < 6 years, 35.1% were school children and adolescents aged 6–17 years, and 50.5% of the population were adults ≥ 18 years. The vast majority of patients (77.7%) had severe or moderate intellectual disability. In 15 patients, intellectual disability was not reported or was not evident in younger children before intellectual disability could be ascertained.
Table 1.
Patient characteristics at baseline
| Characteristic | Overall | LGS | DS | TSC |
|---|---|---|---|---|
| N = 202 | N = 159 | N = 34 | N = 9 | |
| Sex, n (%) | ||||
| Male | 116 (57.4) | 92 (57.9) | 19 (55.9) | 5 (55.6) |
| Female | 86 (42.6) | 67 (42.1) | 15 (44.1) | 4 (44.4) |
| Age at epilepsy onset, years | ||||
| Mean (SD) | 2.6 (3.4) | 3.1 (3.6) | 0.6 (0.5) | 1.7 (3.0) |
| Median (IQR) | 1.0 (0.5–3.0) | 2.0 (0.7–4.0) | 0.5 (0.4–0.7) | 0.4 (0.3–0.8) |
| Age at start of CBD treatment, years | ||||
| Mean (SD) | 21.0 (15.3) | 22.2 (15.7) | 17.0 (13.2) | 14.4 (11.1) |
| Median (IQR) | 18.0 (7.9–32.0) | 19.3 (8.8–33.0) | 13.9 (6.0–23.6) | 14.3 (6.0–21.8) |
| Range | 0.3–72.0 | 0.4–72 | 2.4–54.5 | 0.3–31.5 |
| Age categories, n (%) | ||||
| Preschool < 6 years | 29 (14.4) | 19 (11.9) | 8 (23.5) | 2 (22.2) |
| School and adolescents, 6–17 years | 71 (35.1) | 55 (34.6) | 12 (35.3) | 4 (44.4) |
| Adults ≥ 18 years | 102 (50.5) | 85 (53.5) | 14 (41.2) | 3 (33.3) |
| Intellectual disability, n (%) | ||||
| None or not reporteda | 15 (7.4) | 13 (8.2) | 2 (5.9) | 0 |
| Mild | 30 (14.9) | 23 (14.5) | 5 (14.7) | 2 (22.2) |
| Moderate | 59 (29.2) | 39 (24.5) | 15 (44.1) | 5 (55.6) |
| Severe | 98 (48.5) | 84 (52.8) | 12 (35.3) | 2 (22.2) |
| Seizure days per months, median (range) | ||||
| Overall | 30 (0.3–30) | 30 (1.0–30) | 10 (0.3–30) | 30 (10–30) |
| Preschool < 6 years | 30 (0.5–30) | 30 (4.0–30) | 11 (0.5–30) | 30 (30–30) |
| School and adolescents, 6–17 years | 30 (2.0–30) | 30 (2.0–30) | 12 (4.0–30) | 30 (30–30) |
| Adults ≥ 18 years | 23.5 (0.3–30) | 25.0 (1–30) | 8.0 (0.3–30) | 25.0 (10–30) |
| Number of prior and concomitant ASMs | ||||
| Prior ASMs, median (range) | 6 (1–24) | 6 (1–16) | 5 (1–24) | 3 (2–16) |
| Concomitant ASMs, median (range) | 3 (1–7) | 3 (1–7) | 3 (1–5) | 3 (2–4) |
| Concomitant CLBb, n (%) | 130 (64.3) | 102 (64.2) | 24 (70.6) | 4 (44.4) |
ASM antiseizure medication, CBD cannabidiol, CLB clobazam, DS Dravet syndrome, EU European Union, IQR interquartile range, LGS Lennox–Gastaut syndrome, SD standard deviation, TSC tuberous sclerosis complex
aPatients without intellectual disability were usually children before intellectual disability could be ascertained
bCBD is approved for seizures associated with LGS and DS in conjunction with CLB in the EU, but concomitant CLB is not stipulated for TSC-associated seizures
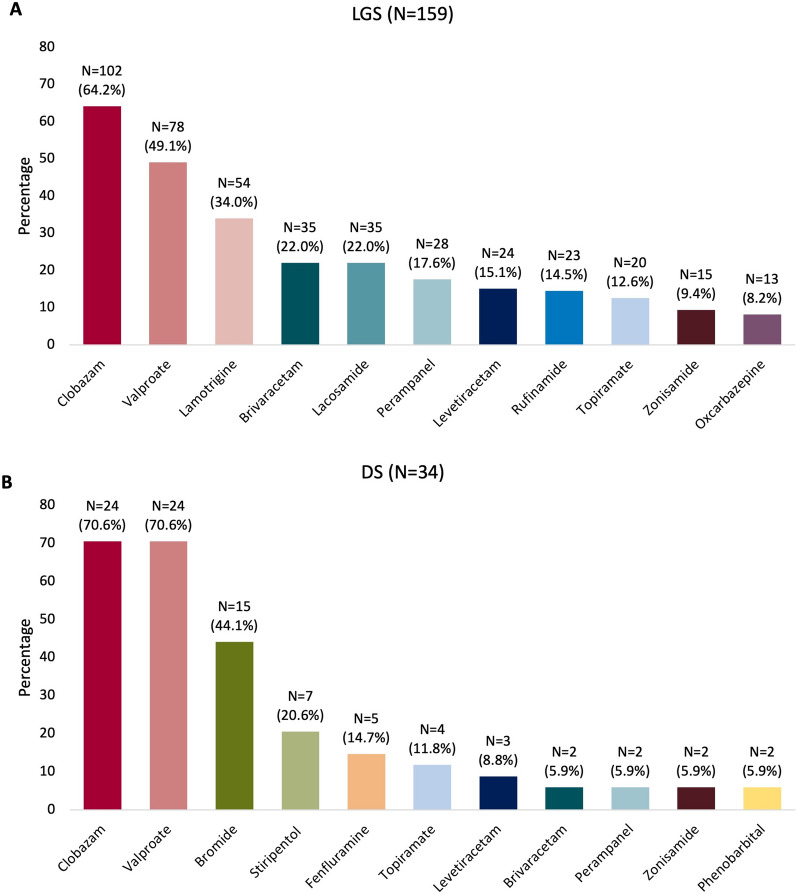
Patients were highly refractory to ASMs, with a median number of prior ASMs of 6 (range 1–24) and concomitant ASMs of 3 (range 1–7). As aligned with the EMA label, concomitant CLB was prescribed in the majority of patients with LGS (64.2%) or DS (70.6%). Apart from CLB, the ten most common concomitant ASMs in patients with LGS (in decreasing frequency) were valproate, lamotrigine, brivaracetam, lacosamide, perampanel, levetiracetam, rufinamide, topiramate, zonisamide, and oxcarbazepine (Fig. 1a). In patients with DS, CLB and valproate were also the most common concomitant ASMs, followed by bromide, stiripentol, fenfluramine, topiramate, levetiracetam, brivaracetam, perampanel, zonisamide, and phenobarbital (Fig. 1b). In the nine patients with TSC, the most frequent concomitant ASMs included valproate (n = 4), lamotrigine (n = 4), and vigabatrin (n = 4). Everolimus was used in three patients with TSC.
Fig. 1.
Concomitant clobazam and the most common other concomitant ASMs at initiation of CBD in patients with a LGS and b DS. ASM antiseizure medication, CBD cannabidiol, DS Dravet syndrome, LGS Lennox–Gastaut syndrome
Within clinical practice in Germany, the starting dose of CBD is typically titrated to a target dose (based on clinical experience and patient/caregiver preferences), and subsequently further titrated to a maximum dose if needed based on tolerability and clinical response. In this retrospective chart review study, the median start, target, and maximal doses of CBD in the total population were 3.3 mg/kg/day, 11.1 mg/kg/day, and 16.8 mg/kg/day, respectively. The respective values across age groups were 3.8 mg/kg/day, 17.6 mg/kg/day, and 19.5 mg/kg/day for < 6 years; 3.3 mg/kg/day, 15.2 mg/kg/day, and 20.0 mg/kg/day for 6–17 years; and 3.3 mg/kg/day, 9.9 mg/kg/day, and 11.9 mg/kg/day for ≥ 18 years.
Effectiveness Outcomes
Patient disposition and definitions of the analysis population are shown in Fig S1. Responder rates were calculated in all patients with data available, and comprehensive details of the number of patients who had seizure reductions, no changes, and seizure increases are provided in Table S1, together with the respective denominators and responder rate calculations at 3 and 12 months.
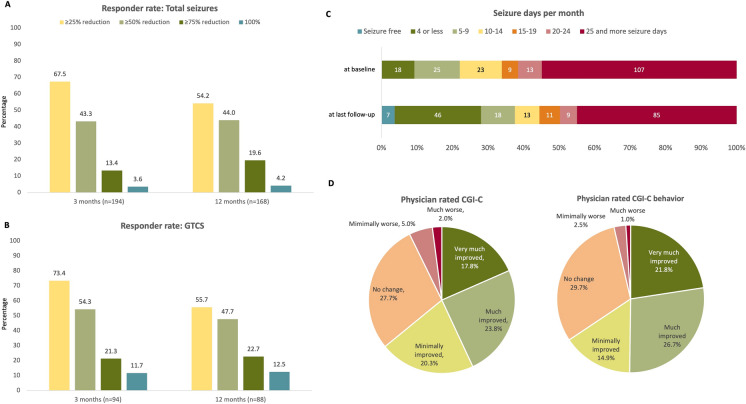
Responder rates at 3 and 12 months (i.e., the percentage of patients with ≥ 25%, ≥ 50%, ≥ 75%, and 100% reductions in the frequency of seizures from baseline) for total seizures and GTCS are shown in Fig. 2 and Table S1. At 3 months of treatment with CBD (n = 194 with available seizure frequency at 3-month follow-up), a ≥ 50% reduction in total seizures was achieved by 43.3% of patients (37.0–50.0% across age groups) (Fig. 2a and Table 2), while 26.3% of patients experienced no effect on total seizures and 6.2% had increases in seizure frequency (Table S1). At 12 months (n = 168 who had reached 12 months of treatment and had seizure frequency data available), 44.0% of patients had a ≥ 50% reduction (37.0–52.5% across age groups) (Fig. 2a and Table 2). Overall, at 12 months, 33.9% had discontinued, 11.3% had no change, and one patient (0.6%) had a seizure increase (Table S1).
Fig. 2.
Responder rates at 3 and 12 months for a total seizures and b generalized tonic–clonic seizures (GTCS); for details, please refer to Table S1. c Number and percentage of patients according to seizure days per month across seven incremental categories at baseline and at the last follow-up after initiation of CBD. Data were unavailable for four patients at baseline and four patients at the last follow-up. d Physician-rated Clinical Global Impression of Change overall and for behavior assessed at the last visit; data were unavailable for seven patients. CBD cannabidiol, CGI-C Clinical Global Impression of Change, GTCS generalized tonic–clonic seizures
Table 2.
CBD responder rates and proportion of patients remaining on CBD, overall, and according to age groups
| Overall N = 202 |
Preschool < 6 years N = 29 |
School and adolescents 6–17 years N = 71 |
Adults ≥ 18 years N = 102 |
|
|---|---|---|---|---|
| Responder ratesa,b: ≥ 50% reduction in total seizures, n/N (%) | ||||
| 3 months | 84/194 (43.3) | 10/27 (37.0) | 34/68 (50.0) | 40/99 (40.4) |
| 12 months | 74/168 (44.0) | 12/26 (46.2) | 32/61 (52.5) | 30/81 (37.0) |
| Responder ratesa: ≥ 50% reduction in generalized tonic–clonic seizures, n/Nc (%) | ||||
| 3 months | 51/94 (54.3) | 8/12 (66.7) | 20/36 (55.6) | 23/46 (50.0) |
| 12 months | 42/88 (47.7) | 8/12 (66.7) | 17/31 (54.8) | 17/45 (37.8) |
| Proportion of patients remaining on CBD (retention rated), n/N (%) | ||||
| 3 months | 181/202 (89.6) | 24/29 (82.8) | 68/71 (95.8) | 89/102 (87.3) |
| 6 months | 149/187 (79.7) | 22/29 (75.9) | 52/65 (80.0) | 75/93 (80.6) |
| 12 months | 116/173 (67.1) | 18/27 (66.7) | 44/62 (71.0) | 54/84 (64.3) |
CBD cannabidiol
aDefined as reductions in the frequency of seizures of ≥ 50% at 3 months and 12 months compared with the 3-month retrospective baseline
bFor the calculation of responder rates, all patients from the initial 202 medical records were considered if they had available data. Please refer to Fig. S1 and Table S1 for further details of the denominator
cN = number of patients who experienced a generalized tonic–clonic seizure (GTCS) in the 3 months prior to start of CBD, and with data available. Please refer to Table S1 for further details of the denominator
dFor the calculation of retention rates, all 202 patients were considered for the 3-month visit as per the study inclusion criteria. For the 6-month and 12-month visits, only patients who were started at least 6 or 12 months before the data analysis were considered. Please refer to Fig. S1 for further details of the denominator
In patients with GTCS at baseline (3 months prior to starting CBD, n = 94), a ≥ 50% reduction in GTCS was achieved by 54.3% of patients at 3 months (50.0–66.7% across age groups) and 47.7% of patients at 12 months (37.8–66.7% across age groups) (Fig. 2b and Table 2). Overall, 21.3% and 9.1% of patients experienced no change in GTCS at 3 and 12 months, respectively, and 5.3% and 1.1%, respectively, had increases in GTCS (Table S1).
Median (range) seizure days per month significantly decreased from 30 (0.3–30) in the 3 months before CBD treatment to 18 (0–30) in the last 3 months of CBD treatment (p < 0.001). Figure 2c shows the number of seizure days per month from baseline to the last follow-up, showing a shifting trend towards more patients experiencing fewer seizure days per month.
At the last follow-up visit, 125 (61.9%) patients were minimally (n = 41, 20.3%), much (n = 48, 23.8%), or very much improved (n = 36, 17.8%) according to physicians CGI-C (Fig. 2d). Fifty-six patients (27.7%) had no change, while 10 (5.0%) patients were minimally worse, four (2.0%) were much worse, and none were rated as very much worse. Data were unavailable for seven (3.5%) patients (Fig. 2d). According to the CGI-C-behavior, 128 patients (63.4%) were either minimally improved (n = 30, 14.9%), much improved (n = 54, 26.7%), or very much improved (n = 44, 21.8%); 60 (29.7%) patients had no change, five (2.5%) were minimally worse, two (1.0%) much worse, and none were rated as very much worse. Data were unavailable for seven (3.5%) patients (Fig. 2d).
Retention Rates
All patients had follow-up data at 3 months (as per the study design), 187 patients had follow-up data at 6 months, and 173 patients at 12 months (Fig. S1). The proportion of patients remaining on CBD at the respective timepoints was 89.6% (181/202) at 3 months, 79.7% (149/187 with follow-up data) at 6 months, and 67.1% (116/173 with follow-up data) at 12 months (Table 2; Fig S1). These were similar across pediatric and adult groups (Table 2).
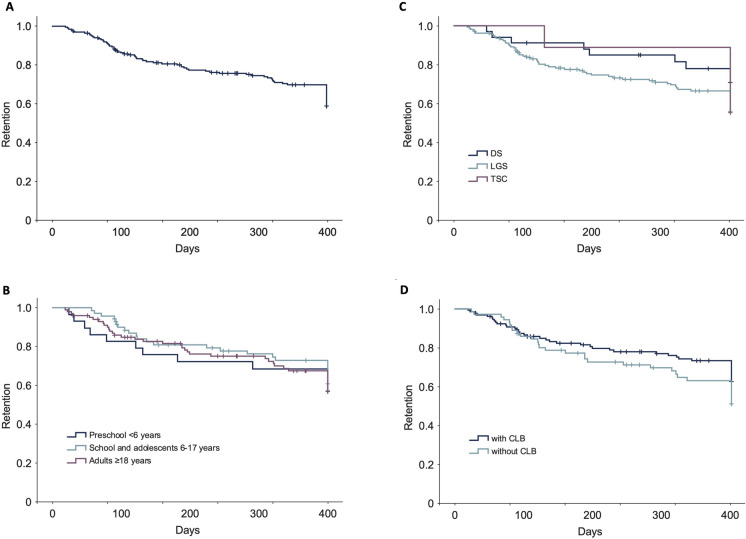
Retention times assessed using KM survival curves are shown in Fig. 3. Estimated retention rates from KM analysis at 1 year were 62.9% for the overall population and were not significantly different across age groups (58.6%, 64.8%, and 62.7% in the < 6 years, 6–17 years, and ≥ 18 years subgroups, respectively; log-rank p = 0.799), according to syndrome diagnosis (61.0%, 73.5%, and 55.6% in patients with LGS, DS, or TSC respectively; log-rank p = 0.291), or in patients treated with or without concomitant CLB (66.9% and 55.6%, respectively; log-rank p = 0.154).
Fig. 3.
Retention time assessed using Kaplan–Meier survival curves for a all patients, and according to b age groups, c DEE, and d with and without concomitant CLB. CLB clobazam, DEE developmental and epileptic encephalopathy, DS Dravet syndrome, LGS Lennox–Gastaut syndrome, TSC tuberous sclerosis complex
Adverse Events and Treatment Discontinuations
An overview of the AEs is shown in Table 3. Overall, 84 (41.6%) patients experienced an AE. The most common AEs (≥ 5%) across the categories were sedation/apathy (n = 41, 20.3%) and GI (n = 28, 13.9%, particularly diarrhea [n = 27, 13.4%]). In addition, those reported in ≥ 2% of patients across the categories were psychobehavioral AEs (n = 11, 5.4%, particularly irritability/aggressiveness [n = 5, 2.5%]), decreased weight/appetite (n = 9, 4.5%, particularly lack of appetite [n = 8, 4.0%]), and sialorrhea (n = 4, 2.0%).
Table 3.
Adverse events
| Adverse event, N (%) | Overall N = 202 |
Discontinuations N = 202 |
|---|---|---|
| Overall | 84 (41.6) | – |
| Sedation and apathy | 41 (20.3) | 11 (5.4) |
| Sedation | 40 (19.8) | 10 (5.0) |
| Apathy | 1 (< 1) | 1 (< 1) |
| GI symptoms | 28 (13.9) | 8 (4.0) |
| Diarrhea | 27 (13.4) | 8 (4.0) |
| Vomiting | 3 (1.5) | 0 |
| Constipation | 1 (< 1) | 1 (< 1) |
| Psychobehavioral | 11 (5.4) | 4 (2.0) |
| Irritability/aggressiveness | 5 (2.5) | 1 (< 1) |
| Behavior disorder | 3 (1.5) | 1 (< 1) |
| Concentration disorder | 2 (1) | 1 (< 1) |
| Psychological | 1 (< 1) | 0 |
| Psychomotor slowing | 1 (< 1) | 1 (< 1) |
| Restlessness | 1 (< 1) | 1 (< 1) |
| Social withdrawal | 1 (< 1) | 1 (< 1) |
| Weight loss/lack of appetite | 9 (4.5) | 4 (2.0) |
| Lack of appetite | 8 (4.0) | 3 (1.5) |
| Weight loss | 3 (1.5) | 2 (1.0) |
| Sialorrhea | 4 (2.0) | 0 |
| CNS/ataxia | 5 (2.5) | 1 (< 1) |
| Dizziness | 3 (1.5) | 1 (< 1) |
| Ataxia | 2 (1) | 0 |
| Tremor | 1 (< 1) | 0 |
| Sleep | 3 (1.5) | 2 (1.0) |
| Sleep disorder | 3 (1.5) | 2 (1.0) |
| Skin | 2 (1.0) | 1 (< 1) |
| Acne | 1 (< 1) | 1 (< 1) |
| Rash | 1 (< 1) | 0 |
| Elevated liver enzymesa | 2 (1.0) | 0 |
| Other | 0 | |
| Dry mouth | 1 (< 1) | 0 |
| Thrombocytopenia | 1 (< 1) | 0 |
| Increased seizures | 1 (< 1) | 1 (< 1) |
If more than one adverse event in the category (i.e., sedation, GI symptoms, behavior, etc.) was reported by the same patient, it was only counted once to prevent double counting
CNS central nervous system, GI gastrointestinal
aElevated liver enzymes that were reported as an AE in the medical notes; a detailed analysis of routine lab values was not performed
Of the 202 patients in the whole cohort, 57 (28.2%) discontinued over the 12-month period, due to lack of treatment effectiveness (n = 28, 13.9%), AEs (n = 13, 6.4%), both treatment effectiveness and AEs (n = 9, 4.5%), or other (n = 7, 3.5%). Among those 22 patients with AEs leading to discontinuation, sedation/apathy was reported by 11 (5.4%), GI symptoms by eight (4.0%), psychobehavioral AEs by four (2.0%), and decreased weight/appetite by four (2.0%).
Discussion
In this multicenter chart review study in Germany with a large cohort of patients with severe drug refractory LGS, DS, or TSC, adjunctive CBD treatment was associated with reductions in seizure frequency across age groups and sustained treatment retention for up to 12 months irrespective of age, syndrome, or concomitant CLB use. In addition, adjunctive CBD had a safety profile similar to that previously observed in clinical trials [11–14, 16–18]. As far as we are aware, this is the largest real-world study to date in terms of patient numbers to report on the use of CBD in the indicated patient populations, and provides further insights based on age groups with a high number of adult patients.
In this study, responder rates, retention rates and the AE profile were generally in-line with observations from each of the individual indications studied in the open-label extension studies in patients with LGS, DS, and TSC (Table S2) [16, 17, 35], and other real-world studies (Table 4) [20–28, 36]. It should be noted that there was heterogeneity between studies, including CBD dose, concomitant ASMs, number of patients, proportions of patients with different syndromes (including non-indicated treatment-resistant epilepsies [TREs]), and duration of treatment and follow-up.
Table 4.
Key observational studies (conducted in > 20 patients) of CBD in patients with treatment-resistant epilepsies including LGS, DS, or TSC-associated epilepsy
| Study | Study design | Patient population | Responder rates for total seizuresa | Retention/discontinuation | AEs (≥ 2% where reported) |
|---|---|---|---|---|---|
| Current study |
Retrospective multicenter study six centers in Germany Median target CBD dose: 11.1 mg/kg/day |
N = 202 Median age (range): 18.0 (0.5–72.0) LGS: 159 (78.7%) DS: 34 (16.8%) TSC: 9 (4.5%) < 6 years: 14.4% 6–17 years: 35.1% ≥ 18 years: 50.5% |
≥ 50% reduction at 3 months Total: 43.3% < 6 years: 37.0% 6 to < 18 years: 50.0% ≥ 18 years: 40.4% ≥ 50% reduction at 12 months Total: 44.0% < 6 years: 46.2% 6 to < 18 years: 52.5% ≥ 18 years: 37.0% |
12-month retention rate (calculated): 69.8% 12-month retention rate (KM): 62.9% Discontinuations over study period: 28.2% |
AEs: 41.6% Sedation: 19.8% Diarrhea: 13.4%, Lack of appetite: 4.0% Irritability/aggressiveness: 2.5% Sialorrhea: 2.0% |
| Sub-study of the current study in patients with LGS or DS treated with CBD + CLB [34] |
Retrospective multicenter study Six centers in Germany Study duration: up to 12 months Median target CBD dose: 11.1 mg/kg/day CLB: 100% |
N = 126 Median age (range): 21.7 (1–65.0) LGS: 102 (81.0%) DS: 24 (19.0%) < 6 years: 11.9% 6–17 years: 31.7% ≥ 18 years: 56.3% |
≥ 50% reduction at 3 months Total: 47.5% < 6 years: 35.7% 6 to < 18 years: 52.6% ≥ 18 years: 47.1% ≥ 50% reduction at 12 months Total: 45.5% < 6 years: 46.1% 6 to < 18 years: 44.4% ≥ 18 years: 46.2% |
12-month retention rate (calculated): 69.8% 12-month retention rate (KM): 67.5% Discontinuations over study period: 25.4% |
AEs: 45.2% Sedation: 23.8% Diarrhea: 10.3%, Lack of appetite: 4.0% Irritability/aggressiveness: 4.0% Sialorrhea: 3.2% Dizziness: 2.4% Weight loss: 2.4% |
| Calonge 2024 [36] |
Retrospective single center study in France Median duration of follow-up of 24 months Maximal treatment dose (mg/kg/day) (median and interquartile range): LGS, DS, or TSC: 10.7 (8.63–12.4) Other TREs: 11.1 (8.92–14.1) |
N = 91 LGS, DS, or TSC: n = 32 Mean (SD) age: 29.5 (7.57) Other TREs: Mean (SD) age: 31.3 (10.1) |
> 50% reduction of seizure frequency (timeframe: NR) LGS, DS, or TSC: 31.3% Other TREs: 35.6% |
12-month retention rate LGS, DS, or TSC: 75.0% Other TREs: 74.6% Discontinuations over 1 year: 25.3% |
LGS, DS, or TSC/ other TREs AEs: 62.5%/40.7% Sleepiness: 50.0%/22.0% Behavioral disorders: 6.3%/8.5% Increased seizures: 6.3%/8.5% Liver balance disturbance: 6.3%/1.7% |
| Kühne 2023 [22] |
Retrospective multicenter study 16 centers in Germany Median treatment duration of 15.8 months (range 3 days to 5.9 years, IQR 6.9 months to 2.2 years) Median starting dose: 3.2 mg/kg/day Median end dose: 19.0 mg/kg/day in children and 13.3 mg/kg/day in adults |
N = 311 Median age (range): 11.3 (0–72) Children and adolescents: 75.6% Adults: 24.4% LGS: 44 (18.7%) DS: 20 (8.5%) TSC: 12 (3.9%) Other: 235 (68.9%) |
> 50% reduction at median treatment duration of 15.8 months: Total: 30.5% Children: 31.1% Adults: 27.9% |
Retention: NR Discontinuations over study period: 30.5% |
AEs: 46.9% Reduced vigilance and tiredness: 24.1% GI: 25.1% Psychiatric symptoms: 4.1% |
| Georgieva 2023 [20] |
Single-center retrospective review conducted at the University of Wisconsin Health in Madison, WI, USA Average time on therapy: 20 months Mean (SD) initial and maintenance doses were 5.3 mg/kg/day (1.3) and 15.3 mg/kg/day (5.8), respectively |
N = 108 Mean age: 20.3 years (SD: 13.1, range 2 to 63) Pediatric: 57 (52.8%) Adults: 51 (47.2%) LGS: 86 (79.6%) DS: 5 (4.6%) TSC: 3 (2.8%) Other: 14 (12.9%) |
NR |
Retention (average time on therapy 20 months): 76.9% Discontinuations over study period: 23.1% Retention in: Pediatric patients: 77.2% Adults: 76.5% |
AEs: 46.3% Drowsiness/sedation/fatigue/sleepiness: 16.6% Sleep disturbances: 6.5% Diarrhea: 4.6% Worsened seizures: 4.6% Sialorrhea: 4.6% Aggression: 3.7% Decreased appetite: 3.7% Liver function test elevations: 2.7% Rash: 2.7% |
| Iannone 2021 [21] |
Italian EAP: 30 epilepsy centers in Italy Mean (SD) treatment duration: 8.7 (4.1) months Follow-up: 12 months CBD dose: NR |
Safety population (N = 93) Mean age (SD): 21.4 (13.5) Children: 49.5% Adults: 50.5% LGS: 63 (67.7%) DS: 30 (32.3%) TSC: 0 Effectivenessb (N = 82) Mean age (SD): 21.0 (13.1) Children: 47.6% Adults: 52.4% LGS: 55 (67.1%) DS: 27 (32.9%) |
12-month follow-up (51/82 patients, 62.2%): ≥ 50% reduction: 49.0% |
12-month retention rate (KM): 68.5% Discontinuations over study period: 31.2% The survival distribution was statistically significantly different for age, X2 = 7.38, p = 0.007 (80.4% retention rate for patients ≥ 18 years), whereas no statistical significance was reached for diagnosis X2 = 3.04, p = 0.06 (82.1% retention rate for DS). Notably, when considering the diagnosis in the age subgroups, pediatric patients with DS have a higher retention rate than patients with LGS (X2 = 9.96, p = 0.002), whereas no difference was observed in adult patients (X2 = 0.03, p = 0.87) |
AEs: 51.6% Somnolence: 22.6% Diarrhea:11.8% Elevated liver enzymes: 10.7% Loss of appetite: 8.6% |
| Laux 2019 [23]c |
US EAP: 25 USA-based, independent epilepsy centers Median (IQR) treatment duration: 80.1 (20.7–107.7) weeks Follow-up: 144 weeks Median (IQR) dose: 21 mg/kg/day (15–25) at 12 weeks and 25 mg/kg/day (21–25) at 96 weeks |
N = 152 Mean age (range): 12.8 (1.7–51) LGS: 94 (61%) DS: 58 (39%) TSC: NA |
≥ 50% reduction at 12 weeks (n = 118): 46% ≥ 50% reduction at 96 weeks (n = 57): 49% |
Retention rate: 76% |
AEs: 91% Somnolence: 30% Convulsion: 24% Diarrhea: 24% URTI: 20% Decreased appetite: 16% Fatigue: 16% Pyrexia: 16% Vomiting: 13% |
| Patel 2021 [24] |
Retrospective chart review at one center in the USA of patients enrolled in the Massachusetts General Hospital's open-label EAP Median duration: 45.5 (range 34–60) months Mean CBD dose at last visit: 32.2 mg/kg/day (median = 30.5; IQR = 22) |
N = 54 Median age (range): 13 (1–51) Possible LGS: 1 (1.9%) DS: 2 (3.7%) TSC: 13 (24.1%) Other: 31 (57.4%) Uncertain etiology: 6 (including possible LGS) (11.1%) |
≥ 50% reduction at 12 months: 41.7% ≥ 50% reduction over study period 69.7% in patients with seizure data available (N = 33); 42.6% over all subjects |
n = 34 (63.0%) remained on treatment during the 5-year tracking period |
AEs: Drowsiness: n = 44 Diarrhea: n = 22 Ataxia: n = 15 Elevated liver function tests: n = 9 Fatigue: n = 4 Loose stool: n = 3 Appetite suppression: n = 3 |
| Perriguey 2023 [25] |
Retrospective chart review at two centers in France Median follow-up duration: 12 months (range 1–27) Median daily dose reached during follow-up: 12.3 mg/kg/day (range 2.2–26.2) |
N = 51 in Group A: epileptic encephalopathies Median age (range): 25 (18–54) Adults: 100% LGS: 36 (71%) DS: 10 (20%) TSC: 0 Other: 5 (9%) |
≥ 50% reduction in Group A: 29.4% | NR |
Group A (epileptic encephalopathies, N = 51) + Group B (focal or multifocal epilepsy, N = 22): AEs: 55% (47% Group A and 72.7% Group B) Somnolence: 19.2% Seizure aggravation: 11% Digestive disorders: nausea, diarrhea, constipation, abdominal pain: 9.6% Decreased appetite/weight loss: 9.6% Asthenia: 6.8% Ataxia/dizziness: 6.8% Aggressiveness/behavioral disturbances: 6.8% Hematologic disorders (thrombopenia, macrocytosis, anemia): 4.1% |
| Tzadok 2024 [26] |
Retrospective chart review at five centers in Israel Median treatment duration: 9.0 months (range 0.5–48.0) Median purified CBD dose was 12.5 mg/kg (range 2.5–20.0) |
N = 139 Median age (range): 12 (2.3–29.2) Children and adolescents: 77% Adults: 22% LGS: 52 (37.4%) DS: 23 (16.5%) TSC: 23 (16.5%) Other: 41 (29.5%) |
Median treatment duration: 9.0 months (range 0.5–48.0) ≥ 50% reduction (n = 53): 41.1% |
12-month retention rate (KM): 62% Discontinuations over study period: 30.2% |
AEs: 28.1% Irritability: 20.9% Drowsiness: 12.9% Increased appetite: 4.3% Decreased appetite: 2.9% |
| Vicino 2023 [27] |
Retrospective observational study one center in Italy Study duration: up to 12 months Mean dose of CBD: 9 mg/kg/day |
N = 42 Mean age (SD) 36.1 (10.9) years LGS: 18 (42.8%) DS: 5 (11.9%) TSC: 1 (2.4%) Other: 18 (42.9%) |
> 30% to < 80% seizure reduction: 3 months: 23% 12 months: 41% |
12-month retention rate: 71.4% Discontinuations at 3 months: 85.7% |
AEs: 52.3% Drowsiness: 36.5% Diarrhea: 9.8% |
| Villanueva 2022 [28] |
Spanish EAP: 14 centers in Spain Study duration: up to 12 months Median CBD doses: 10 mg/kg/day at 3 months to 12.3 mg/kg/day at 12 months, and 10.2 mg/kg/day (430 mg/day) at the last visit |
N = 102 Mean age (range): 15.9 (0.9–58.2) < 15 years: 58.4% ≥ 15 years: 41.6% LGS: 60 (58.8%) DS: 12 (11.8%) Other: 30 (29.4%) |
≥ 50% reduction: 3 months: 37.1% 12 months: 38.9% LGS: 3 months: 31.4% 12 months: 40.6% DS: 3 months: 55.6% 12 months: 33.3% |
12-month retention rate: 61.4% Discontinuations over study period: 33.3% |
AEs: 66.7% Somnolence: 34.3% Diarrhea: 12.7% Decreased appetite: 6.9% Irritability: 6.9% Restlessness/agitation/nervousness: 5.9% |
Studies were identified using keywords in PubMed by one reviewer and data were extracted by one reviewer. Key studies up to January 2025 are included; the studies presented represent an overview and not all observational studies evaluating CBD have been included. Bold font highlights 12-month responder and retention rates
AE adverse event, CBD cannabidiol, DS Dravet syndrome, EAP early access program, GI gastrointestinal, IQR interquartile range, KM Kaplan–Meier, LGS Lennox–Gastaut syndrome, NR not reported, SD standard deviation, TRE treatment-resistant epilepsy, TSC tuberous sclerosis complex, URTI upper respiratory tract infection
aFor total seizures unless otherwise stated
bAt least 3 months of treatment
cOther publications including Szaflarski et al. 2023 (Szaflarski JP, Devinsky O, Lopez M, et al. Long-term efficacy and safety of cannabidiol in patients with treatment-resistant epilepsies: Four-year results from the expanded access program. Epilepsia. 2023;64:619–629) have reported on the US EAP across TREs, but patients with LGS, DS, and TSC only constitute 14%, 8%, and 4% of the overall population, respectively. Therefore, the Laux 2019 publication in patients with DS and LGS is the most appropriate publication for the US EAP
The doses prescribed in our study were generally in accordance with the EMA recommended dosing of 5 mg/kg/day starting dose and a 10 mg/kg/day maintenance dose up to a maximum of 20 mg/kg/day in patients with LGS or DS, and 25 mg/kg/day in patients with TSC-associated epilepsy [9]. Patients were concomitantly treated with varying numbers and types of concomitant ASMs. In-line with the EMA label, the majority of patients with LGS and DS, although not all, were treated with concomitant CLB. The reasons for a patient not being prescribed CLB are not known but could include prior discontinuation due to lack of effectiveness or poor tolerability, patient/caregiver preference, and/or physician recommendation, e.g., that CLB may not be needed if CBD is providing a good response. Data were not collected on dose responses or changes to the CBD dose or changes to concomitant ASMs in relation to managing response and AEs, as these decisions were tailored according to the patient’s characteristics, their experiences, and patient/caregiver and physician preferences.
Herein, CBD demonstrated effectiveness across age groups and similar retention; however, it is important to note that the study was not designed to compare outcomes across age groups. In particular, there were only 29 (14.4%) preschool children < 6 years. A retrospective, single-center study in the USA, Georgieva et al. 2023, reported similar retention rates in children and adults to the current study, with 77.2% of pediatric patients (n = 57) and 76.5% of adult patients (n = 51) remaining on CBD over an average of 20 months [20]. In contrast, Iannone et al. 2021, reporting data from an Italian expanded access program (EAP), found that the retention rate for adults was significantly higher than for pediatric patients, although the authors stated that the size of the population may have been a limitation for this analysis [21]. Meanwhile, US EAP data in patients with TREs demonstrates no significant differences in seizure frequency reduction at all time points up to 2 years between children and adults, although adults had a significantly greater improvement in seizure severity at 1 year [37]. However, the authors speculated that this improvement in seizure severity could be due to the natural variance in the severity data collected [37]. Furthermore, Calonge et al. 2024 has also recently reported positive effectiveness of CBD in adults with LGS, DS, or TSC (n = 32) and in adults with other TREs (n = 59) [36].
In the present study, there was a trend for improved retention in patients with concomitant CLB versus those without, although the difference was not statistically significant. These retention data appear to suggest that CLB-induced AEs (i.e., somnolence and sedation) are manageable, and indeed, sedation was reported as an AE in 20% of patients, leading to discontinuation in 5% of patients. Of note, a separate sub-analysis of the current study was performed in patients with LGS and DS who were treated concomitantly with adjunctive CBD and CLB [34] (Table 4). The responder rates were marginally numerically higher, while AEs, particularly sedation, were also marginally higher, although such numerical comparisons should be interpreted with caution (Table 4). However, overall, these data may suggest that, if tolerated, it could be worth considering adding CLB to CBD if the patient has not been responding to CBD alone. However, if not tolerated, several studies (including systematic reviews and meta-analyses of the RCTs versus placebo) have robustly demonstrated that CBD is also effective without CLB [38–41].
The data in the present study that focused on the indicated etiologies appears to be comparable with outcomes from other studies in patients with a high proportion of non-indicated etiologies (Table 4). Of interest, studies have shown that CBD may be effective as an adjunctive therapy across multiple seizure types [42], and across a range of different TREs, regardless of the underlying etiology [22, 23, 29, 36, 43]. This observation is supported by mechanism of action data that suggest CBD may exert its antiseizure effects through multiple mechanisms, including the modulation of intracellular Ca2+ (via G protein-coupled receptor 55 [GPR55] and transient receptor potential vanilloid 1 [TRPV1]) and modulation of adenosine-mediated signaling [44]. Although the antiseizure mechanisms are still being elucidated, these particular targets are not thought to be specific to distinct TRE syndromes [44].
Patients with LGS, DS, and TSC have significant neurodevelopmental comorbidities, including behavioral and psychiatric problems and sleep disorders, and some ASMs have been reported to exacerbate these symptoms [45]. In the present study, in-line with other studies of CBD (Tables S2, 4), psychobehavioral AEs were not commonly observed, with only five patients (2.5%) experiencing irritability/aggressiveness, three (1.5%) sleep disorder, three (1.5%) behavior disorder, and two (1.0%) concentration disorder. Furthermore, although we did not comprehensively evaluate non-seizure outcomes and quality of life, the physicians rated the majority of patients (> 60%) as showing improvement, both overall and for behavior, using the CGI-C scale. In this respect, caregivers of patients with LGS and DS who were treated with CBD have reported improvements in some non-seizure-outcomes including alertness, cognition and executive function, emotional functioning, language and communication, activities of daily living, sleep, and physical functioning [46]. Furthermore, improvements in seizure outcomes and comorbidities have been associated with improvements in health-related quality of life [47], with research continuing to explore the complex interplay between all three outcomes.
The study has some limitations. Foremost, retrospective chart reviews have inherent limitations including uncontrolled study design, potential for missing/incomplete data, and variability in data collection. For example, there were some missing data regarding total seizures and GTCS that may have impacted the calculation of responder rates. In addition, seizure data obtained from seizure diaries were reliant on the accuracy of input from caregivers and potentially adults with some degree of intellectual disability. Furthermore, the study was not specifically designed to statistically compare the subgroups of age, the epilepsy syndrome, or CLB use; in particular across the age groups, there were only 29 patients in the preschool category, and the majority of patients were diagnosed with LGS, with very few patients with TSC. Finally, routine clinical practice differs from country to country, e.g., in Germany, bromide is an available ASM used in 15 (44%) patients with DS in our study. As such, results from the present study may not be fully comparable with real-world use observed in other countries.
Conclusion
In patients from clinical practice in Germany with drug refractory LGS, DS, or TSC, treatment with adjunctive CBD was associated with a reduction in seizure frequency and sustained treatment retention for up to 12 months across age groups, broadly aligned with observations in individual indications in open-label extension studies of CBD [16, 17, 35]. The adverse event profile in this population of indicated etiologies was also generally consistent with the known safety profile of CBD [9, 11–15]. Moreover, data are broadly consistent with reports from EAPs and other post-marketing real-world observational studies in indicated and non-indicated epilepsy etiologies [20–28, 36]. These data provide further insights into the effectiveness, tolerability, and retention of adjunctive CBD in clinical practice including effectiveness among a large cohort of adults.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Medical Writing, Editorial, and Other Assistance
Under the direction of the authors, Amanda Prowse, PhD, of Lochside Medical Communications (Glasgow, UK) provided medical writing, which was funded by Jazz Pharmaceuticals, Inc., in accordance with Good Publication Practice (GPP) 2022 guidelines. Support for the graphical abstract was provided by Emma Melchor, PhD (Emma Melchor Illustration, Glasgow, UK), funded by Jazz Pharmaceuticals, Inc.
Author Contributions
Adam Strzelczyk: Conceptualization, Data curation, Formal analysis, Funding acquisition, Writing—original draft. Kerstin Alexandra Klotz: Conceptualization, Investigation, Writing—review and editing. Thomas Mayer: Conceptualization, Investigation, Writing—review and editing. Felix von Podewils: Conceptualization, Investigation, Writing—review and editing. Susanne Knake: Conceptualization, Investigation, Writing—review and editing. Gerhard Kurlemann: Conceptualization, Investigation, Writing—review and editing. Luise Herold: Conceptualization, Investigation, Writing—review and editing. Ilka Immisch: Conceptualization, Investigation, Writing—review and editing. Elisa Buhleier: Conceptualization, Data curation, Investigation, Writing—review and editing. Felix Rosenow: Conceptualization, Investigation, Writing—review and editing. Susanne Schubert-Bast: Conceptualization, Investigation, Writing—review and editing.
Funding
The project was funded by GW Pharmaceuticals, Ltd. (now a part of Jazz Pharmaceuticals, Ltd). Medical writing assistance, and the journal’s Rapid Service Fee, were funded by Jazz Pharmaceuticals, Inc.
Data Availability
The datasets supporting the findings of this study are available within the article, its supplementary material, or on request from the corresponding author (AS). The datasets generated during and/or analyzed during the current study are not publicly available due their containing information that could compromise the privacy of the patients.
Declarations
Conflict of Interest
Adam Strzelczyk received personal fees and grants from Angelini Pharma, Biocodex, Desitin Arzneimittel, Eisai, Jazz Pharmaceuticals, Longboard, Neuraxpharm, Stoke Therapeutics, Takeda, UCB Pharma, and UNEEG medical. Kerstin Alexandra Klotz received speaker’s honoraria from Biocodex, Eisai, Jazz Pharmaceuticals, and UCB Pharma. Thomas Mayer received speaker’s honoraria from Jazz Pharmaceuticals, UCB, and Angelin Pharma. Felix von Podewils received personal fees and grants from Angelini Pharma, Desitin Arzneimittel, Eisai, Jazz Pharmaceuticals, UCB Pharma, Nutricia Milupa GmbH, Neuraxpharm, and Bial. Susanne Knake received speaker’s honoraria from Bial, Desitin Arzneimittel, Eisai, Jazz Pharmaceuticals, Merck Serono, and UCB. Gerhard Kurlemann received speaker’s honoraria from Angelini Pharma, Desitin Arzneimittel, Eisai, Jazz Pharmaceuticals, Takeda, UCB Pharma, Neuraxpharm, Stada Arzneimittel, Precisis GmbH, and Alexion Pharmaceuticals. Luise Herold and Elisa Buhleier have no conflicts of interest to disclose. Ilka Immisch received personal fees from Angelini Pharma, Jazz Pharmaceuticals, and Precisis. Felix Rosenow received personal fees and grants from Angelini Pharma, Desitin Arzneimittel, Dr. Schär Deutschland GmbH, Eisai GMBH, Jazz Pharmaceuticals, Nutricia Milupa GmbH, Takeda, UCB Pharma, and Vitaflo Deutschland GmbH. Susanne Schubert-Bast received personal fees and grants from Angelini Pharma, Biocodex, Biomarin, Desitin Arzneimittel, Eisai, Jazz Pharmaceuticals, Takeda, and UCB Pharma.
Ethics Approval
The analysis was approved by the ethics committee of the University of Frankfurt. Informed consent was waived because of the retrospective nature of the chart reviews.
References
- 1.Raga S, Specchio N, Rheims S, Wilmshurst JM. Developmental and epileptic encephalopathies: recognition and approaches to care. Epileptic Disord. 2021;23:40–52. [DOI] [PubMed] [Google Scholar]
- 2.Berg AT, Gaebler-Spira D, Wilkening G, et al. Nonseizure consequences of Dravet syndrome, KCNQ2-DEE, KCNB1-DEE, Lennox–Gastaut syndrome, ESES: a functional framework. Epilepsy Behav. 2020;111:107287. [DOI] [PubMed] [Google Scholar]
- 3.Donnan AM, Schneider AL, Russ-Hall S, Churilov L, Scheffer IE. Rates of status epilepticus and sudden unexplained death in epilepsy in people with genetic developmental and epileptic encephalopathies. Neurology. 2023;100:e1712–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallop K, Lloyd AJ, Olt J, Marshall J. Impact of developmental and epileptic encephalopathies on caregivers: a literature review. Epilepsy Behav. 2021;124:108324. [DOI] [PubMed] [Google Scholar]
- 5.Strzelczyk A, Lagae L, Wilmshurst JM, et al. Dravet syndrome: a systematic literature review of the illness burden. Epilepsia Open. 2023;8:1256–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strzelczyk A, Zuberi SM, Striano P, Rosenow F, Schubert-Bast S. The burden of illness in Lennox–Gastaut syndrome: a systematic literature review. Orphanet J Rare Dis. 2023;18:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sullivan J, Deighton AM, Vila MC, et al. The clinical, economic, and humanistic burden of Dravet syndrome—a systematic literature review. Epilepsy Behav. 2022;130:108661. [DOI] [PubMed] [Google Scholar]
- 8.Zöllner JP, Franz DN, Hertzberg C, et al. A systematic review on the burden of illness in individuals with tuberous sclerosis complex (TSC). Orphanet J Rare Dis. 2020;15:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Medicines Agency. Epidyolex® 100 mg/ml oral solution: summary of product characterisitics. 2025. https://www.ema.europa.eu/en/documents/product-information/epidyolex-epar-product-information_en.pdf. Accessed 26 June 2025.
- 10.US Food and Drug Administration. Epidiolex® Prescribing Information. 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/210365s021lbl.pdf. Accessed 26 June 2025.
- 11.Devinsky O, Patel AD, Cross JH, et al. Effect of cannabidiol on drop seizures in the Lennox–Gastaut syndrome. N Engl J Med. 2018;378:1888–97. [DOI] [PubMed] [Google Scholar]
- 12.Thiele EA, Marsh ED, French JA, al. Cannabidiol in patients with seizures associated with Lennox–Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391:1085–96. [DOI] [PubMed] [Google Scholar]
- 13.Miller I, Scheffer IE, Gunning B, et al. Dose-ranging effect of adjunctive oral cannabidiol vs placebo on convulsive seizure frequency in Dravet Syndrome: a randomized clinical trial. JAMA Neurol. 2020;77:613–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet Syndrome. N Engl J Med. 2017;376:2011–20. [DOI] [PubMed] [Google Scholar]
- 15.Thiele EA, Bebin EM, Bhathal H, et al. Add-on cannabidiol treatment for drug-resistant seizures in tuberous sclerosis complex: a placebo-controlled randomized clinical trial. JAMA Neurol. 2021;78:285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel AD, Mazurkiewicz-Bełdzińska M, Chin RF, et al. Long-term safety and efficacy of add-on cannabidiol in patients with Lennox–Gastaut syndrome: results of a long-term open-label extension trial. Epilepsia. 2021;62:2228–39. [DOI] [PubMed] [Google Scholar]
- 17.Scheffer IE, Halford JJ, Miller I, et al. Add-on cannabidiol in patients with Dravet syndrome: results of a long-term open-label extension trial. Epilepsia. 2021;62:2505–17. [DOI] [PubMed] [Google Scholar]
- 18.Thiele E, Bebin EM, Filloux F, et al. Long-term safety and efficacy of add-on cannabidiol (CBD) for seizures associated with tuberous sclerosis complex (TSC): 3-year results from GWPCARE6 open-label extension (OLE) (P14–1.004). Neurology. 2023;100:2500. [Google Scholar]
- 19.de Simon L, Laura C, Neil M. Creating and using real-world evidence to answer questions about clinical effectiveness. BMJ Health Care Inform. 2015;22:368. [DOI] [PubMed] [Google Scholar]
- 20.Georgieva D, Langley J, Hartkopf K, et al. Real-world, long-term evaluation of the tolerability and therapy retention of Epidiolex® (cannabidiol) in patients with refractory epilepsy. Epilepsy Behav. 2023;141:109159. [DOI] [PubMed] [Google Scholar]
- 21.Iannone LF, Arena G, Battaglia D, et al. Results from an Italian expanded access program on cannabidiol treatment in highly refractory Dravet syndrome and Lennox–Gastaut syndrome. Front Neurol. 2021;12:673135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kühne F, Becker LL, Bast T, et al. Real-world data on cannabidiol treatment of various epilepsy subtypes: a retrospective, multicenter study. Epilepsia Open. 2023;8:360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laux LC, Bebin EM, Checketts D, et al. Long-term safety and efficacy of cannabidiol in children and adults with treatment resistant Lennox–Gastaut syndrome or Dravet syndrome: expanded access program results. Epilepsy Res. 2019;154:13–20. [DOI] [PubMed] [Google Scholar]
- 24.Patel S, Grinspoon R, Fleming B, et al. The long-term efficacy of cannabidiol in the treatment of refractory epilepsy. Epilepsia. 2021;62:1594–603. [DOI] [PubMed] [Google Scholar]
- 25.Perriguey M, Succar ME, Clément A, et al. High-purified cannabidiol efficacy and safety in a cohort of adult patients with various types of drug-resistant epilepsies. Rev Neurol (Paris). 2023;180:147–53. [DOI] [PubMed] [Google Scholar]
- 26.Tzadok M, Gur-Pollack R, Florh H, et al. Real-life experience with purified cannabidiol treatment for refractory epilepsy: a multicenter retrospective study. Pediatr Neurol. 2024;150:91–6. [DOI] [PubMed] [Google Scholar]
- 27.Vicino W, Muccioli L, Pondrelli F, et al. Real-world experience with cannabidiol as add-on treatment in drug-resistant epilepsy. Seizure. 2023;111:39–41. [DOI] [PubMed] [Google Scholar]
- 28.Villanueva V, García-Ron A, Smeyers P, et al. Outcomes from a Spanish Expanded Access Program on cannabidiol treatment in pediatric and adult patients with epilepsy. Epilepsy Behav. 2022;137:108958. [DOI] [PubMed] [Google Scholar]
- 29.Szaflarski JP, Bebin EM, Comi AM, et al. Long-term safety and treatment effects of cannabidiol in children and adults with treatment-resistant epilepsies: expanded access program results. Epilepsia. 2018;59:1540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szaflarski JP, Devinsky O, Lopez M, et al. Long-term efficacy and safety of cannabidiol in patients with treatment-resistant epilepsies: four-year results from the expanded access program. Epilepsia. 2023;64:619–29. [DOI] [PubMed] [Google Scholar]
- 31.Elm EV, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zuberi SM, Wirrell E, Yozawitz E, et al. ILAE classification and definition of epilepsy syndromes with onset in neonates and infants: position statement by the ILAE Task Force on nosology and definitions. Epilepsia. 2022;63:1349–97. [DOI] [PubMed] [Google Scholar]
- 33.Northrup H, Aronow ME, Bebin EM, et al. Updated international tuberous sclerosis complex diagnostic criteria and surveillance and management recommendations. Pediatr Neurol. 2021;123:50–66. [DOI] [PubMed] [Google Scholar]
- 34.Strzelczyk A, Schubert-Bast S, von Podewils F, et al. Real-world experience of cannabidiol in conjunction with clobazam for the treatment of seizures associated with Lennox–Gastaut syndrome and Dravet syndrome: results from a retrospective multicentre chart review in Germany. Epilepsy Behav. 2025;166:110302. [DOI] [PubMed] [Google Scholar]
- 35.Thiele EA, Bebin EM, Filloux F, et al. Long-term cannabidiol treatment for seizures in patients with tuberous sclerosis complex: an open-label extension trial. Epilepsia. 2022;63:426–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calonge Q, Besnard A, Bailly L, et al. Cannabidiol treatment for adult patients with drug-resistant epilepsies: a real-world study in a tertiary center. Brain Behav. 2024;14:e70122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaston TE, Ampah SB, Martina Bebin E, et al. Long-term safety and efficacy of highly purified cannabidiol for treatment refractory epilepsy. Epilepsy Behav. 2021;117:107862. [DOI] [PubMed] [Google Scholar]
- 38.Devinsky O, Thiele EA, Wright S, et al. Cannabidiol efficacy independent of clobazam: meta-analysis of four randomized controlled trials. Acta Neurol Scand. 2020;142:531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gunning B, Mazurkiewicz-Bełdzińska M, Chin RFM, et al. Cannabidiol in conjunction with clobazam: analysis of four randomized controlled trials. Acta Neurol Scand. 2021;143:154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nabbout R, Arzimanoglou A, Auvin S, et al. Retrospective chart review study of use of cannabidiol (CBD) independent of concomitant clobazam use in patients with Lennox–Gastaut syndrome or Dravet syndrome. Seizure. 2023;110:78–85. [DOI] [PubMed] [Google Scholar]
- 41.Lattanzi S, Trinka E, Striano P, et al. Cannabidiol efficacy and clobazam status: a systematic review and meta-analysis. Epilepsia. 2020;61:1090–8. [DOI] [PubMed] [Google Scholar]
- 42.Flamini RJ, Comi AM, Bebin EM, et al. Efficacy of cannabidiol in convulsive and nonconvulsive seizure types associated with treatment-resistant epilepsies in the Expanded Access Program. Epilepsia. 2023;64:e156–63. [DOI] [PubMed] [Google Scholar]
- 43.Devinsky O, Verducci C, Thiele EA, et al. Open-label use of highly purified CBD (Epidiolex®) in patients with CDKL5 deficiency disorder and Aicardi, Dup15q, and Doose syndromes. Epilepsy Behav. 2018;86:131–7. [DOI] [PubMed] [Google Scholar]
- 44.Reddy DS. Therapeutic and clinical foundations of cannabidiol therapy for difficult-to-treat seizures in children and adults with refractory epilepsies. Exp Neurol. 2023;359: 114237. [DOI] [PubMed] [Google Scholar]
- 45.Strzelczyk A, Schubert-Bast S. Psychobehavioural and cognitive adverse events of anti-seizure medications for the treatment of developmental and epileptic encephalopathies. CNS Drugs. 2022;36:1079–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berg AT, Dixon-Salazar T, Meskis MA, Danese SR, Le NMD, Perry MS. Caregiver-reported outcomes with real-world use of cannabidiol in Lennox–Gastaut syndrome and Dravet syndrome from the BECOME survey. Epilepsy Res. 2024;200:107280. [DOI] [PubMed] [Google Scholar]
- 47.Strzelczyk A, Kurlemann G, Bast T, et al. Exploring the relationships between composite scores of disease severity, seizure-freedom and quality of life in Dravet syndrome. Neurol Res Pract. 2022;4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the findings of this study are available within the article, its supplementary material, or on request from the corresponding author (AS). The datasets generated during and/or analyzed during the current study are not publicly available due their containing information that could compromise the privacy of the patients.