Abstract
Introduction
Bimekizumab (BKZ), a monoclonal antibody targeting interleukin (IL)-17A and IL-17F, has shown high efficacy in clinical trials. However, real-world data on its use in psoriatic arthritis (PsA) are limited. This study aimed to evaluate the effectiveness and safety of BKZ over 24 weeks in a real-world setting.
Methods
A retrospective, multicenter study was conducted at two Italian rheumatology centers, enrolling adult patients with PsA who initiated BKZ treatment between January 2023 and February 2025. Clinical data were collected at baseline, week 12, and week 24.
Results
Forty patients with PsA were included. Of these, 75% had failed at least two biologic and/or targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs) prior to BKZ and 25/40 (62.5%) had failed at least one IL-17A inhibitor (IL-17Ai). Among these 25 patients, 32% experienced a primary failure and 68% a secondary failure. The median baseline Disease Activity in Psoriatic Arthritis (DAPSA) score was 22.9 (17.5–27.2), decreasing to 6.0 (3.1–12.8) at week 24 (p < 0.001). By week 24, 72.5% achieved DAPSA low disease activity (LDA), and 25% achieved DAPSA remission. The median swollen joint count (SJC) decreased from 3.0 (0.8–5.3) to 0.0 (IQR 0.0–1.0), and median tender joint count (TJC) decreased from 4.5 (3.0–7.3) to 1.0 (0.0–2.0) (both p < 0.001). Pain visual analog scale (VAS) and Patient Global Assessment (PGA) improved significantly, from 7.0 (6.0–8.0) and 7.5 (6.5–8.0) at baseline to 2.0 (1.0–5.0) and 2.0 (1.0–4.5) at week 24 (both p < 0.001). Skin involvement also improved, with 51.5% achieving Psoriasis Area and Severity Index (PASI) 100 by week 24. The safety profile was favorable; 15% of patients developed mild oral candidiasis, none of which required treatment discontinuation.
Conclusion
BKZ demonstrated rapid and sustained improvements in PsA symptoms in a challenging real-world population, with a favorable safety profile.
Keywords: Psoriatic arthritis, b/tsDMARDs, Difficult-to-treat, Real-world evidence
Key Summary Points
| Why carry out this study? |
| Bimekizumab (BKZ) is a monoclonal antibody recently approved for the treatment of psoriatic arthritis (PsA), acting as a dual inhibitor of interleukin (IL)-17A and IL-17F. Despite robust efficacy demonstrated in clinical trials, real-world evidence (RWE) on BKZ remains limited. |
| The study aimed to evaluate the real-world effectiveness and safety of BKZ after 24 weeks of treatment. |
| What was learned from this study? |
| BKZ demonstrated robust efficacy, with 72.5% of patients achieving Disease Activity in Psoriatic Arthritis (DAPSA) low disease activity (LDA) and 25% attaining DAPSA remission by week 24. |
| These findings are particularly noteworthy given the difficult-to-treat characteristics of the cohort, with 75% of patients having previously failed at least two biologic and/or targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs) classes and over 60% having experienced prior failure with an IL-17A inhibitor. |
| Notably, BKZ proved effective even in patients previously treated with IL-17A inhibitors, particularly those who experienced a secondary loss of response. |
Introduction
Psoriatic arthritis (PsA) is a chronic, systemic, immune-mediated disease affecting approximately 1% of the general population and up to 30% of individuals with psoriasis (PsO) [1–3]. As a result of its multidomain nature, PsA presents with a broad spectrum of clinical phenotypes, involving peripheral arthritis, axial disease, enthesitis, dactylitis, and psoriasis involving the skin and nails. The frequency, type, and severity of affected domains can significantly influence treatment response and long-term outcomes [4].
Over the past two decades, the therapeutic landscape for PsA has expanded significantly with the introduction of biologic disease-modifying antirheumatic drugs (bDMARDs) and targeted synthetic DMARDs (tsDMARDs). Both the European Alliance of Associations for Rheumatology (EULAR) and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) guidelines recommend bDMARDs for patients with inadequate response to conventional synthetic DMARDs (csDMARDs) but do not specify a preferred mechanism of action. However, in patients with extensive cutaneous involvement, IL-17A, IL-17A/F, IL-23, and IL-12/23 inhibitors are particularly recommended [5, 6].
The IL-17 cytokine family plays a central role in the development of psoriatic disease [7, 8]. Among its six isoforms, IL-17A and IL-17F share approximately 50% sequence homology and can form both homodimers and heterodimers, contributing to tissue inflammation and bone remodeling [9]. While IL-17A is the most potent isoform, IL-17F is present at higher levels in psoriatic lesions and serum [9–11]. Notably, in vitro studies have shown that simultaneously inhibiting both IL-17A and IL-17F provides stronger anti-inflammatory effects than targeting either cytokine alone [10].
Bimekizumab (BKZ) is a novel humanized IgG1 monoclonal antibody that selectively inhibits both IL-17A and IL-17F, offering broader suppression of IL-17-driven inflammation compared to selective IL-17A inhibitors (IL-17Ai) [10, 12].
BKZ was previously approved for the treatment of moderate-to-severe plaque psoriasis, where it also demonstrated superior efficacy compared to secukinumab [13]. Furthermore, its efficacy and safety profile in psoriasis has been reinforced by long-term extension trials and real-world studies, which independently confirm its clinical benefits in clinical practice [14].
More recently, BKZ received approval for the treatment of PsA, following its robust efficacy and safety results in two phase 3 trials—BE-OPTIMAL and BE-COMPLETE—achieving minimal disease activity (MDA) in approximately 50% of patients with PsA, regardless of prior biologic use, and showing comparable effectiveness to adalimumab, which served as reference arm [15, 16].
The aim of the present study is to provide additional real-world evidence (RWE) on the effectiveness of BKZ. Indeed, relying solely on randomized controlled trials (RCTs) has clear limitations, as their inclusion and exclusion criteria do not fully capture the complexity of patients encountered in routine clinical practice. In particular, patients enrolled in clinical trials are typically biologic-naïve or have failed no more than two biologic therapies as a result of lack of efficacy or intolerance, thereby excluding the difficult-to-treat (D2T) population [17, 18]. Additionally, the predominant disease domain in trials is usually peripheral arthritis with at least three active joints; patients with fewer than three active joints or with predominant enthesitis are generally not eligible for enrollment. Moreover, psoriatic disease is a heterogeneous condition, and patients are often co-managed by multiple specialists, especially because of frequent extra-articular involvement such as skin disease or the presence of comorbidities—features that are commonly seen in real-life clinical settings but rarely represented in trials as a result of their complexity of management.
Methods
This was a multicenter, retrospective study conducted between January 2023 and February 2025 in two Italian centers: Santa Maria della Misericordia Hospital (Udine) and G.B. Rossi University Hospital (Verona). The study was approved by the institutional review board (IRB) of each participating institution. Informed consent was obtained from each patient in accordance with the Declaration of Helsinki and with local guidelines for good clinical practice. This study was conducted in accordance with the Helsinki Declaration of 1964 and its later amendments.
Inclusion criteria were (1) a diagnosis of PsA and fulfilment of the Classification Criteria for Psoriatic Arthritis (CASPAR) [19]; (2) age ≥ 18 years; (3) initiation of BKZ treatment for active PsA with a minimum follow-up of 24 weeks, regardless of treatment persistence.
The aims of the study were (1) to assess the proportion of patients who achieved Disease Activity in Psoriatic Arthritis (DAPSA) low disease activity (LDA) [20] and DAPSA remission [21] at 24 weeks; (2) to evaluate the proportion of patients who achieved DAPSA LDA and DAPSA remission at week 24 after having failed a prior IL-17Ai.
Data Collection
Demographic and clinical data were retrospectively collected, including age, sex, smoking status, and anthropometric measurements (height and weight). Medical history was recorded, with a focus on comorbidities such as hypertension, diabetes, cardiovascular diseases, malignancies, obesity, and chronic kidney disease (CKD). PsA-related history was documented, including prior use of csDMARDs and b/tsDMARDs. Rheumatologic assessments were performed at baseline, week 12, and week 24, including swollen joint count (SJC), tender joint count (TJC), dactylitis count, and the Leeds Enthesitis Index (LEI). Additionally, patient-reported pain was recorded using a visual analog scale (VAS). Inflammatory markers, including C-reactive protein (CRP), were collected. Psoriasis severity was assessed using the Psoriasis Area and Severity Index (PASI), which was performed by dermatologists in patients followed in the combined dermatology–rheumatology outpatient clinic. Disease activity was evaluated using the DAPSA score, which was performed exclusively by the rheumatologist.
Statistical Analysis
The baseline demographic and clinical traits of all patients were recorded and entered into an anonymized database. Descriptive statistics were used to summarize patient characteristics and treatment outcomes. Continuous variables were expressed as medians with interquartile ranges (IQRs), while categorical variables were reported as frequencies and percentages. Changes in disease activity scores from baseline to week 12 and week 24 were analyzed to assess treatment effectiveness. Longitudinal changes in clinical variables were analyzed using linear mixed-effects models. Time was modeled as a fixed effect, while a random intercept was included for between-subject variability. Fixed-effect coefficients, standard errors (SEs), 95% confidence intervals (CIs), and p values were reported. For continuous variables, pairwise comparisons between time points were performed using estimated marginal means derived from the linear mixed model. For binary variables, pairwise comparisons between time points were made using the McNemar test. Bonferroni correction for multiple testing was applied. Analyses were conducted using the statistical software STATA 18.0.
Results
Patient Characteristics
Of the 58 patients initially considered, 40 were included in the final analysis based on having completed at least 24 weeks (6 months) of follow-up from the initiation of BKZ therapy. The remaining 18 patients were excluded because they had not yet reached the 6-month follow-up milestone at the time of data collection. The median age was 55.0 years (47.4–62.0), with female patients representing 50% of the cohort (20/40). The median BMI was 27.0 (24.0–29.3), with 6 out of 40 (15%) classified as obese. Full demographic details are summarized in Table 1.
Table 1.
Demographic characteristics of patients enrolled in the study
| N = 40 | |
|---|---|
| Age | 55 (47.4–62.0) |
| Female | 20 (50.0) |
| BMI | 27 (24–29.3) |
| Smoking (current) | 17 (42.5) |
| Comorbidities | |
| CVD | 8 (20.0) |
| Diabetes | 6 (15.0) |
| Hypertension | 6 (15.0) |
| Malignancy | 0 (0) |
| CKD | 1 (2.5) |
| Obesity | 6 (15.0) |
| Dyslipidemia | 8 (20.0) |
| Predominant pattern of PsA | |
| Axial | 2 (5.0) |
| Peripheral | 38 (95.0) |
| Disease phenotype | |
| Oligoarticular | 31 (77.5) |
| Polyarticular | 7 (17.5) |
| Entheseal | 2 (5.0) |
| Type of psoriasis | |
| Vulgar | 36 (90.0) |
| Pustular | 2 (5) |
| Inverse | 2 (5) |
| Nail psoriasis | 17 (42.5) |
| History of dactylitis | 9 (22.5) |
| D2T-PsA | 30 (75.0) |
| N° of b/tsDMARDs failed | 3 (2–3.3) |
| N° of b/tsDMARDs classes failed | 2 (1.8–3) |
Data are presented as median (IQR) or n (%)
IQR interquartile range, BMI body mass index, CVD cardiovascular disease, CKD chronic kidney disease, D2T-PsA difficult-to-treat psoriatic arthritis, b/tsDMARDs biologic or targeted synthetic disease-modifying antirheumatic drugs
Most patients (35/40) were managed in combined dermatology–rheumatology clinics. In 31 of the 40 cases (77.5%), BKZ was initiated to address both active joint and skin involvement, while in the remaining 9 cases (22.5%), treatment was started primarily because of active joint disease. The most commonly adopted dosing regimen was 320 mg every 8 weeks, administered in 35 patients (87.5%). In these patients, treatment was initiated with an induction regimen, consisting of 320 mg administered at weeks 0, 4, 8, 12, and 16. At baseline, the median DAPSA score was 22.9 (17.9–28.7), SJC 3.0 (0.8–5.3), and TJC 4.5 (3.0–7.3). Dactylitis was present in 6 patients (15%), while median LEI was 0.0 (0.0–1.3). Only two patients (5%) were in DAPSA LDA at baseline and none in DAPSA remission. Vulgar psoriasis was the predominant skin phenotype, present in 36 out of 40 patients (90%) with a median PASI score of 13.0 (4.4–16.0). Nail involvement was documented in 17 out of 40 patients (42.5%),
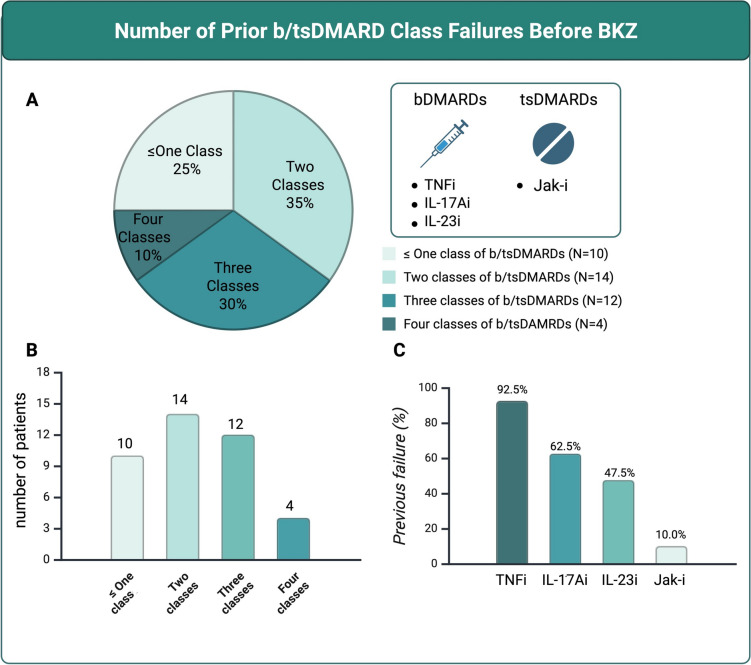
History of Previous b/tsDMARDs Failure
Prior to initiating BKZ, most patients had experienced multiple treatment failures. The median number of previously failed b/tsDMARDs was 3.0 (2.0–3.3), and the median number of failed mechanisms of action was 2.0 (1.8–3.0). Among the 40 patients included in the analysis, 30 (75%) had failed at least two different classes of b/tsDMARDs and were therefore classified as having D2T-PsA (Fig. 1A, B) [17, 18, 22–24]. Regarding prior biologic and targeted synthetic therapies, tumor necrosis factor inhibitors (TNFi) were the most frequently used (92.5%), followed by IL-17Ai (62.5%), while IL-23 inhibitors (IL-23i) and JAK inhibitors (Jak-i) were prescribed less frequently (Fig. 1C).
Fig. 1.
A, B Number of prior b/tsDMARD class failures before initiation of BKZ; C Previous b/tsDMARDs classes failed. bDMARDs biologic disease-modifying antirheumatic drugs, tsDMARDs targeted synthetic disease-modifying antirheumatic drugs, TNFi tumor necrosis factor inhibitors, IL17Ai interleukin-17A inhibitor, IL-23i interleukin-23 inhibitors, Jak-I Janus kinase inhibitors
Three out of 40 patients received BKZ as their first-line b/tsDMARD because of concomitant moderate to severe skin involvement. The treatment decision in all these cases was made within a combined dermatology–rheumatology clinic setting.
Effectiveness of Bimekizumab
At week 24, BKZ was maintained in 37 out of 40 patients (92.5%). Three patients (7.5%) discontinued BKZ because of lack of efficacy: one due to primary inefficacy, and two following disease reactivation at week 24 despite partial improvement at week 12.
Disease activity, as measured by DAPSA, showed a marked improvement, with scores decreasing from 22.9 (17.9–28.7) at baseline to 10.7 (6.9–13.6) at week 12 (p < 0.001), and further to 6.0 (3.1–12.8) at week 24 (p < 0.001) (Fig. 2). By week 24, 29 out of 40 patients (72.5%) had achieved at least LDA, and 10 out of 40 (25%) reached DAPSA remission.
Fig. 2.
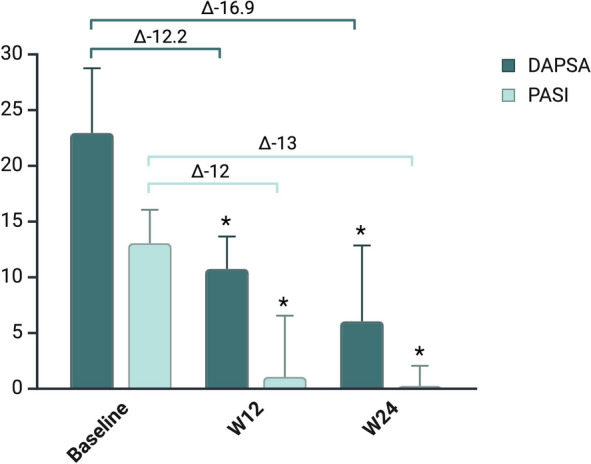
Trends in DAPSA and PASI during follow-up. DAPSA Disease Activity in Psoriatic Arthritis, PASI Psoriasis Area and Severity Index
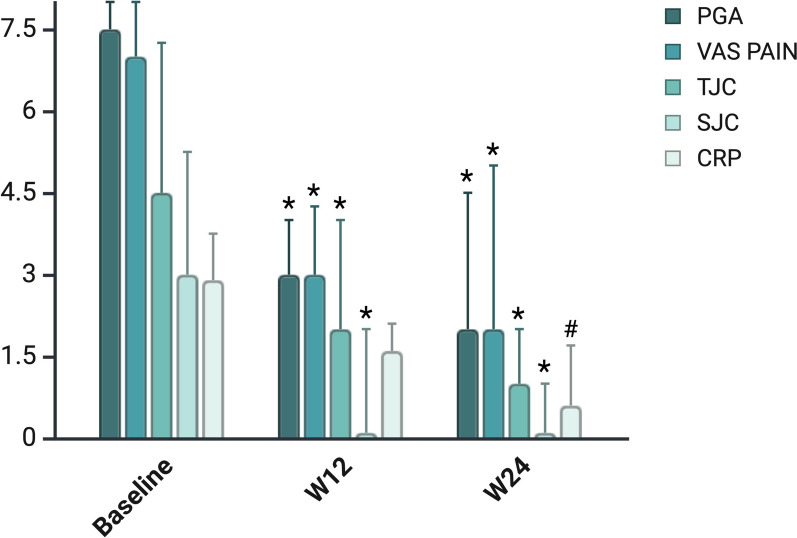
As shown in Fig. 3, treatment with BKZ led to a rapid and significant improvement in peripheral joint involvement. Median SJC decreased from 3.0 (0.8–5.3) at baseline to 0.0 (0.0–2.0) at week 12 and 0.0 (0.0–1.0) at week 24 (p < 0.001 for both). TJC showed a similar trend, dropping from 4.5 (3.0–7.3) at baseline to 2.0 (0.0–2.0) at week 12 and 1.0 (0.0–2.0) at week 24 (p < 0.001) (Table 2).
Fig. 3.
Trends in DAPSA variables over time. SJC swollen joint count, TJC tender joint count, VAS visual analog scale, PGA Patient Global Assessment, CRP C-reactive protein
Table 2.
Comparison of clinical and laboratory parameters during the study period
| Baseline | 12 weeks | 24 weeks | P value | |||
|---|---|---|---|---|---|---|
| N = 40 | N = 40 | N = 40 | Baseline vs 12 weeks | Baseline vs 24 weeks | 12 weeks vs 24 weeks | |
| Joint counts | ||||||
| Swollen (0–66) | 3.0 (0.8–5.3) | 0.0 (0.0–2.0) | 0.0 (0.0–1.0) | < 0.001* | < 0.001* | 1.000 |
| Tender (0–68) | 4.5 (3.0–7.3) | 2.0 (0.0–2.0) | 1.0 (0.0–2.0) | < 0.001* | < 0.001* | 1.000 |
| LEI | 0.0 (0.0–1.3) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.064 | 0.011* | 1.000 |
| Patients with dactylitis | 6 (15) | 1 (2.5) | 2 (5) | 0.011* | 0.062 | 1.000 |
| DAPSA | 22.9 (17.9–28.7) | 10.7 (6.9–13.6) | 6.0 (3.1–12.8) | < 0.001* | < 0.001* | 1.000 |
| PASI | 13.0 (4.4–16.0) | 1.0 (0.0–6.5) | 0.0 (0.0–2.0) | < 0.001* | < 0.001* | 0.143 |
| PASI 100 | NA | 11/33a (33.3) | 17/33 (51.5) | NA | NA | 0.007* |
| PASI 90 | NA | 13/33a (39.4) | 22 (66.6) | NA | NA | 0.225 |
| PASI 75 | NA | 14/33a (42.4) | 23/33a (69.7) | NA | NA | 0.278 |
| Pain VAS | 7.0 (6.0–8.0) | 3.0 (2.0–4.3) | 2.0 (1.0–5.0) | < 0.001* | < 0.001* | 1.000 |
| PGA | 7.5 (6.5–8.0) | 3.0 (2.0–4.0) | 2.0 (1.0–4.5) | < 0.001* | < 0.001* | 1.000 |
| CRP (mg/L) | 2.9 (0.2–3.8) | 1.6 (0.2–2.1) | 1.2 (1.3) [0.5] | 0.060 | 0.001* | 0.613 |
Data are presented as median (IQR) or n (%)
IQR interquartile range, LEI Leeds Enthesitis Index, DAPSA Disease Activity in Psoriatic Arthritis, PASI Psoriasis Area and Severity Index, VAS visual analog scale, PGA Patient Global Assessment, CRP C-reactive protein, NA not applicable
aSeven patients were excluded from this analysis because of a baseline PASI < 3
*Statistically significant
Although enthesitis was infrequent at baseline (as assessed by LEI), significant improvement was noted: by week 24, 9 out of 11 patients (81.9%) with enthesitis at baseline had achieved complete resolution (p = 0.011). Dactylitis, initially present in 6 patients (15%), resolved in nearly all cases by week 12, with 1 patient (2.5%) still showing signs of dactylitis at week 24 (p = 0.011).
Patient-reported outcomes (PROs) also improved significantly. Pain VAS and PGA decreased from 7.0 (6.0–8.0) and 7.5 (6.5–8.0) at baseline to 3.0 (2.0–4.3) and 3.0 (2.0–4.0) at week 12 (p < 0.001), with further reductions at week 24 to 2.0 (1.0–5.0) and 2.0 (1.0–4.5), respectively (p < 0.001).
Skin involvement, as assessed by the PASI score, also improved markedly. The median PASI decreased from 13.0 (IQR 4.4–16.0) at baseline to 0.0 (IQR 0.0–2.0) at week 24 (p < 0.001).
Among the 33 patients with baseline PASI ≥ 3, complete skin clearance (PASI 100) was achieved in 11 (33.3%) at week 12, and in 17 (51.5%) by week 24.
Results from the linear mixed-effects model confirmed a statistically significant effect of time on multiple clinical and laboratory parameters (Table 3).
Table 3.
Linear mixed model analyses of clinical and laboratory parameters during the study period
| Linear mixed model coefficient | SE | 95% CI | P value | |
|---|---|---|---|---|
| Joint counts | ||||
| Swollen | ||||
| Time | − 1.26 | 0.20 | − 1.66, − 0.86 | < 0.001* |
| Tender | ||||
| Time | − 1.75 | 0.56 | − 2.35, − 1.15 | < 0.001* |
| LEI | ||||
| Time | − 0.24 | 0.08 | − 0.40, − 0.08 | 0.004* |
| DAPSA | ||||
| Time | − 9.88 | 2.34 | − 14.47, − 5.29 | < 0.001* |
| PASI | ||||
| Time | − 5.15 | 0.54 | − 6.23, − 4.08 | < 0.001* |
| Pain VAS | ||||
| Time | − 1.76 | 0.21 | − 2.18, − 1.34 | < 0.001* |
| PGA | ||||
| Time | − 2.08 | 0.23 | − 2.53, − 1.64 | < 0.001* |
| CRP (mg/L) | ||||
| Time | − 0.61 | 0.17 | − 0.94, − 0.27 | < 0.001* |
95% CI 95% confidence interval, SE standard error, LEI Leeds Enthesitis Index, DAPSA Disease Activity in Psoriatic Arthritis, PASI Psoriasis Area and Severity Index, VAS visual analog scale, PGA Patient Global Assessment, CRP C-reactive protein
*Statistically significant
Switch Intra-IL-17i Class
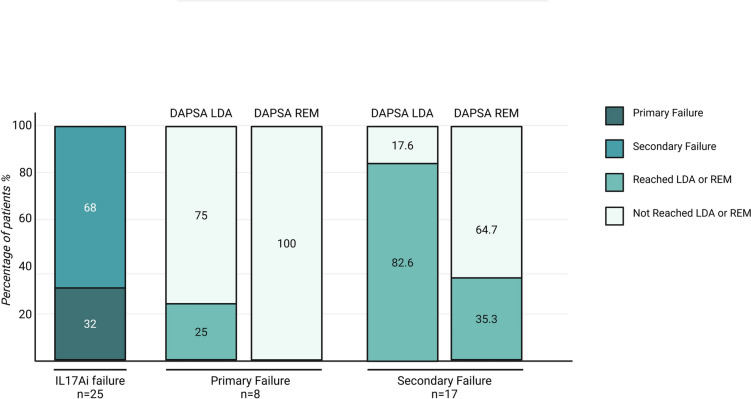
A total of 25 out of 40 (62.5%) patients had been previously treated with IL-17Ai, accounting for 30 treatment courses. Among these, 8 patients (32%) experienced at least one primary failure to IL-17Ai, while the remaining 17 out of 25 (68%) had secondary failures.
In the primary failure group, only 2 out of 8 patients (25%) achieved LDA at 24 weeks, and none reached remission. In contrast, among the 17 patients with only secondary failures, 14 (82.4%) achieved at least LDA at week 24, and 6 (35.3%) achieved DAPSA remission (Fig. 4).
Fig. 4.
Proportion of patients with primary or secondary failure to prior IL-17A inhibitors and rates of low disease activity and remission among them. DAPSA Disease Activity in Psoriatic Arthritis, LDA low disease activity, REM remission
At 24 weeks, the median DAPSA score was significantly higher in the primary failure group compared to the secondary failure group (19.3 [13.6–22.3] vs 4.1 [3.0–8.6], p = 0.002).
Safety Profile of Bimekizumab
No serious adverse events (SAEs) were reported during the follow-up period. The most commonly observed adverse event was oral candidiasis, occurring in 6 out of 40 patients (15%). All cases were classified as mild by the treating physicians. Importantly, none of these cases led to treatment discontinuation (Table 4).
Table 4.
AF
| N = 40 | |
|---|---|
| Bimekizumab regimen | |
| 320 mg every 8 weeks | 35 (87.5) |
| 160 mg every 4 weeks | 5 (12.5) |
| AEs | 6 (15) |
| Serious AEs | 0 (0) |
| Candida | 6 (15) |
| Oral candidiasis | 6 (100) |
| Systemic candidiasis | 0 (0) |
Data are presented as n (%)
IQR interquartile range, AEs adverse events
Discussion
This RWE study provides valuable insights beyond those derived from RCTs, particularly in evaluating BKZ in a population of patients with PsA and active disease after multiple prior treatment failures. These patients, often excluded from RCTs as a result of strict inclusion criteria, represent a clinically relevant and increasingly common subgroup referred to as D2T-PsA [17, 18].
To date, the only available RWE on BKZ comes from two case series involving seven and six patients with PsA, respectively [25, 26]. This study represents the largest real-world cohort of patients with PsA treated with BKZ and demonstrates robust and rapid clinical improvements across musculoskeletal and cutaneous domains over a 24-week period. The favorable safety profile further supports its applicability in complex clinical settings. Notably, the majority of patients had previously failed at least two bDMARDs and/or tsDMARDs, emphasizing the refractory nature of the cohort.
Among the most clinically relevant findings is the performance of BKZ in patients previously exposed to IL-17Ai. While a therapeutic response in TNFi-experienced patients could be reasonably expected because of the distinct mechanism of action of BKZ, its efficacy in patients who had already failed IL-17A-targeted therapies was less certain. Despite this uncertainty, meaningful clinical benefit was observed in this subgroup. In particular, patients with a history of secondary failure to IL-17Ai responded well to BKZ, with more than 80% achieving DAPSA LDA and over one-third reaching DAPSA remission. These results suggest that dual inhibition of IL-17A and IL-17F may overcome mechanisms of resistance that emerge during long-term IL-17Ai monotherapy. On the other hand, patients with primary failure to IL-17A inhibition showed lower response rates. However, rather than indicating true non-responsiveness to IL-17 blockade, these outcomes may reflect the more severe and refractory disease course typically observed in this subgroup. In clinical practice, this suggests that while response rates may be attenuated, BKZ could still offer therapeutic benefit in selected patients even after a primary failure of IL-17A inhibition.
BKZ produced significant improvements in PROs, such as pain and global disease assessment, which were already evident by week 12. These findings align with three post hoc analyses showing substantial reductions in pain and fatigue as early as after a single injection [27–29].
The rapid effect of BKZ on PROs, resembling that observed with Jak-i [30, 31], raises the possibility that dual IL-17A/F inhibition may play a role in pain modulation beyond its anti-inflammatory effects. Preclinical evidence suggests that IL-17 may directly contribute to pain by increasing nerve sensitivity and promoting communication between nerve and glial cells in the spinal cord, highlighting a role for IL-17 in pain mechanisms independent from inflammation [32–34]. This concept is consistent with recent real-world studies reporting rapid improvements in PROs even in patients with minimal objective signs of inflammation—indicating a dissociation between symptom burden and traditional inflammatory markers [26, 30–34].
The majority of patients in this study were followed in integrated dermatology–rheumatology clinics, reflecting the complex, multidomain nature of PsA and the need for coordinated care [35, 36]. These were not only D2T patients in terms of joint disease—remaining active despite failure of at least two prior biologic therapies—but also individuals for whom skin involvement had a significant clinical and psychosocial impact. The concurrent presence of refractory articular and cutaneous disease necessitated shared management and justified the use of the dermatologic induction regimen of BKZ. This comprehensive approach may have contributed to the rapid and sustained clinical improvements observed. Remarkably, in this challenging population, more than half of those with significant baseline skin involvement achieved complete clearance at 24 weeks, underscoring the potential of dual IL-17A/F blockade to address the full spectrum of psoriatic disease in real-life practice.
The safety profile of BKZ was favorable and consistent with expectations for IL-17 pathway inhibition. Oral candidiasis was the most frequently reported adverse event, with all cases being classified as mild and not requiring discontinuation of therapy. Although the incidence was slightly higher than in clinical trials [16, 30], this may be related to the broader use of induction dosing in our cohort and the more complex clinical profiles of the patients, which were characterized by multiple comorbidities and prior exposure to several b/tsDMARDs.
This study has several limitations. Its relatively small sample size and retrospective design may affect the robustness and generalizability of our findings. The 24-week follow-up provides valuable insight into short-term effectiveness and safety but does not allow conclusions on long-term drug survival or delayed adverse events. Notably, most patients in our cohort required the dermatologic induction dosing regimen because of concomitant moderate-to-severe skin involvement, reflecting the shared dermatology–rheumatology nature of our outpatient clinic. This factor may have contributed to the high early response rates observed but could also limit the generalizability of our findings to standard rheumatology practice, where patients typically present with predominant joint involvement and milder skin disease.
Additionally, the low number of patients with enthesitis and dactylitis restricts our ability to evaluate BKZ’s effectiveness in these specific domains. Furthermore, while we use the term D2T-PsA throughout this manuscript, there is currently no universally accepted definition for this entity. We therefore adopted a pragmatic definition in line with those proposed in the literature [22–24]. Finally, as our cohort is characterized by failure of multiple b/tsDMARD classes and a high burden of comorbidities, it may not fully reflect the broader PsA population.
Conclusions
This multicenter RWE study supports the effectiveness and tolerability of BKZ in a challenging PsA population, including those with multiple prior biologic failures and, notably, prior IL-17Ai exposure. The results highlight the clinical relevance of dual IL-17A/F inhibition in real-life practice and support BKZ as a valuable treatment option even for patients with refractory PsA. Further prospective, long-term studies are needed to confirm these findings and optimize positioning of BKZ within the PsA treatment algorithm.
Acknowledgements
We thank the participants of the study.
Medical Writing/Editorial Assistance
Language editing support was provided by ChatGPT (OpenAI, San Francisco, CA, USA) and was limited to refining the English language. No content or conceptual assistance was provided. The authors are fully responsible for all scientific content.
Author Contribution
Alen Zabotti: Investigation, conceptualization, writing original draft, review and editing, writing, supervision, visualization; Nicola Cabas: Investigation, conceptualization, writing original draft, review and editing, visualization. Andrea Guiotto: Investigation, visualization; Maria De Martino: Formal analysis, visualization; Giuseppe Stinco: Supervision, visualization; Paolo Gisondi: Investigation, supervision, visualization; Enzo Errichetti: Investigation, supervision, visualization; Ivan Giovannini: Visualization, supervision; Luca Idolazzi: Investigation, supervision; Francesco Bellinato: Visualization; Beatrice Gabrielli: Visualization; Maurizio Rossini: Supervision, review and editing, visualization; Luca Quartuccio: Conceptualization, writing, supervision, review and editing, visualization.
Funding
No funding or sponsorship was received for this study or publication of this article.
Data Availability
Data generated and analyzed during the current study are available upon reasonable request to the corresponding author.
Declarations
Conflict of Interest
Alen Zabotti is an Editorial Board member of Rheumatology and Therapy. Alen Zabotti was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Nicola Cabas, Maria De Martino, Luca Idolazzi, Andrea Guiotto, Francesco Bellinato, Ivan Giovannini, Beatrice Gabrielli, Paolo Gisondi, Enzo Errichetti, Maurizio Rossini, Giuseppe Stinco, Luca Quartuccio declare no conflict of interest relevant for this study.
Ethical Approval
The study was approved by the institutional review board (IRB) of each participating institution. Informed consent was obtained from each patient in accordance with the Declaration of Helsinki and with local guidelines for good clinical practice. This study was conducted in accordance with the Helsinki Declaration of 1964 and its later amendments.
Footnotes
Alen Zabotti and Nicola Cabas contributed equally to this work.
References
- 1.Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med. 2017;376:957–70. 10.1056/NEJMra1505557. [DOI] [PubMed] [Google Scholar]
- 2.Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80(1):251-265.e19. 10.1016/j.jaad.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 3.Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005. 10.1136/ard.2004.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogdie A, Hur P, Liu M, et al. Effect of multidomain disease presentations on patients with psoriatic arthritis in the corrona psoriatic arthritis/spondyloarthritis registry. J Rheumatol. 2021;48(5):698–706. 10.3899/jrheum.200371. [DOI] [PubMed] [Google Scholar]
- 5.Coates LC, Soriano ER, Corp N, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol. 2022;18(8):465–79. 10.1038/s41584-022-00798-0. (Erratum in: Nat Rev Rheumatol. 2022 Dec;18(12):734. 10.1038/s41584-022-00861-w). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gossec L, Kerschbaumer A, Ferreira RJO, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2023 update. Ann Rheum Dis. 2024;83(6):706–19. 10.1136/ard-2024-225531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veale DJ, Fearon U. The pathogenesis of psoriatic arthritis. Lancet. 2018;391(10136):2273–84. 10.1016/S0140-6736(18)30830-4. [DOI] [PubMed] [Google Scholar]
- 8.Blauvelt A, Chiricozzi A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin Rev Allergy Immunol. 2018;55(3):379–90. 10.1007/s12016-018-8702-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansen C, Usher PA, Kjellerup RB, Lundsgaard D, Iversen L, Kragballe K. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol. 2009;160(2):319–24. 10.1111/j.1365-2133.2008.08902.x. [DOI] [PubMed] [Google Scholar]
- 10.Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77(4):523–32. 10.1136/annrheumdis-2017-212127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iznardo H, Puig L. Dual inhibition of IL-17A and IL-17F in psoriatic disease. Ther Adv Chronic Dis. 2021;12(12):20406223211037850. 10.1177/20406223211037846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;21(11):1894. 10.3389/fimmu.2020.01894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385(2):142–52. 10.1056/NEJMoa2102383. [DOI] [PubMed] [Google Scholar]
- 14.Potestio L, Ruggiero A, Martora F, Megna M. Long-term efficacy and safety of bimekizumab in real-world setting: a 52-week prospective study. Arch Dermatol Res. 2024;317(1):102. 10.1007/s00403-024-03594-w. [DOI] [PubMed] [Google Scholar]
- 15.McInnes IB, Asahina A, Coates LC, et al. Bimekizumab in patients with psoriatic arthritis, naive to biologic treatment: a randomised, double-blind, placebo-controlled, phase 3 trial (BE OPTIMAL). Lancet. 2023;401(10370):25–37. 10.1016/S0140-6736(22)02302-9. [DOI] [PubMed] [Google Scholar]
- 16.Merola JF, Landewé R, McInnes IB, et al. Bimekizumab in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor-α inhibitors: a randomised, double-blind, placebo-controlled, phase 3 trial (BE COMPLETE). Lancet. 2023;401(10370):38–48. 10.1016/S0140-6736(22)02303-0. [DOI] [PubMed] [Google Scholar]
- 17.Singla S, Ribeiro A, Torgutalp M, Mease PJ, Proft F. Difficult-to-treat psoriatic arthritis (D2T PsA): a scoping literature review informing a GRAPPA research project. RMD Open. 2024;10(1):e003809. 10.1136/rmdopen-2023-003809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ribeiro AL, Singla S, Chandran V, et al. Deciphering difficult-to-treat psoriatic arthritis (D2T-PsA): a GRAPPA perspective from an international survey of healthcare professionals. Rheumatol Adv Pract. 2024;8(3): rkae074. 10.1093/rap/rkae074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54(8):2665–73. 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- 20.Schoels MM, Aletaha D, Alasti F, Smolen JS. Disease activity in psoriatic arthritis (PsA): defining remission and treatment success using the DAPSA score. Ann Rheum Dis. 2016;75(5):811–8. 10.1136/annrheumdis-2015-207507. [DOI] [PubMed] [Google Scholar]
- 21.Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis. 2010;69(1):48–53. 10.1136/ard.2008.102053. [DOI] [PubMed] [Google Scholar]
- 22.Perrotta FM, Scriffignano S, Ciccia F, Lubrano E. Clinical characteristics of potential ‘difficult-to-treat’ patients with psoriatic arthritis: a retrospective analysis of a longitudinal cohort. Rheumatol Ther. 2022;9(4):1193–201. 10.1007/s40744-022-00461-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Philippoteaux C, Marty-Ane A, Cailliau E, et al. characteristics of difficult-to-treat psoriatic arthritis: a comparative analysis. Semin Arthritis Rheum. 2023;63:152275. 10.1016/j.semarthrit.2023.152275. [DOI] [PubMed] [Google Scholar]
- 24.Vassilakis KD, Papagoras C, Fytanidis N, et al. Identification and characteristics of patients with potential difficult-to-treat psoriatic arthritis: exploratory analyses of the Greek PsA registry. Rheumatology (Oxford). 2024;63(9):2427–32. 10.1093/rheumatology/keae263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaiopoulos AG, Michelakis I, Katsimbri P, et al. Real-World Data From the Joint Psoriasis-Psoriatic Arthritis Clinic (PPAC) on the efficacy of bimekizumab in patients with psoriasis and psoriatic arthritis. Int J Dermatol. 2025. 10.1111/ijd.17654. [DOI] [PubMed] [Google Scholar]
- 26.Zabotti A, Cabas N, Giovannini I, et al. Bimekizumab in biologics-refractory psoriatic arthritis: a real-life analysis from a combined dermatology-rheumatology clinic. Clin Cosmet Investig Dermatol. 2024;1(17):1553–6. 10.2147/CCID.S467832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gossec L, Orbai AM, de Wit M, et al. Effect of bimekizumab on patient-reported disease impact in patients with psoriatic arthritis: 1-year results from two phase 3 studies. Rheumatology (Oxford). 2024;63(9):2399–410. 10.1093/rheumatology/keae277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Husni ME, Mease PJ, Merola JF, et al. Bimekizumab provided rapid improvements in patient-reported symptoms and health-related quality of life in patients with active psoriatic arthritis: pooled 16-week results from two phase 3 studies. RMD Open. 2024;10(3):e004464. 10.1136/rmdopen-2024-004464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mease PJ, Asahina A, Gladman DD, et al. Effect of bimekizumab on symptoms and impact of disease in patients with psoriatic arthritis over 3 years: results from BE ACTIVE. Rheumatology (Oxford). 2023;62(2):617–28. 10.1093/rheumatology/keac353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McInnes IB, Ostor AJK, Mease PJ, et al. Effect of upadacitinib on reducing pain in patients with active psoriatic arthritis or ankylosing spondylitis: post hoc analysis of three randomised clinical trials. RMD Open. 2022;8(1):e002049. 10.1136/rmdopen-2021-002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogdie A, de Vlam K, McInnes IB, et al. Efficacy of tofacitinib in reducing pain in patients with rheumatoid arthritis, psoriatic arthritis or ankylosing spondylitis. RMD Open. 2020;6(1):e001042. 10.1136/rmdopen-2019-001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Segond von Banchet G, Boettger MK, König C, Iwakura Y, Bräuer R, Schaible HG. Neuronal IL-17 receptor upregulates TRPV4 but not TRPV1 receptors in DRG neurons and mediates mechanical but not thermal hyperalgesia. Mol Cell Neurosci. 2013;52:152–60. 10.1016/j.mcn.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Richter F, Natura G, Ebbinghaus M, et al. Interleukin-17 sensitizes joint nociceptors to mechanical stimuli and contributes to arthritic pain through neuronal interleukin-17 receptors in rodents. Arthritis Rheum. 2012;64(12):4125–34. 10.1002/art.37695. [DOI] [PubMed] [Google Scholar]
- 34.Gao SJ, Liu L, Li DY, et al. Interleukin-17: a putative novel pharmacological target for pathological pain. Curr Neuropharmacol. 2024;22(2):204–16. 10.2174/1570159X21666230811142713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savage L, Tinazzi I, Zabotti A, Laws PM, Wittmann M, McGonagle D. Defining pre-clinical psoriatic arthritis in an integrated dermato-rheumatology environment. J Clin Med. 2020;9(10):3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Idolazzi L, Zabotti A, Fassio A, et al. The ultrasonographic study of the nail reveals differences in patients affected by inflammatory and degenerative conditions. Clin Rheumatol. 2019;38(3):913–20. 10.1007/s10067-019-04437-0. (Erratum in: Clin Rheumatol. 2020 Apr;39(4):1369. 10.1007/s10067-020-04926-7). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data generated and analyzed during the current study are available upon reasonable request to the corresponding author.