Abstract
The kynurenine (KYN) pathway is the major catabolic pathway for tryptophan in humans, producing several metabolites that influence health. In clinical settings, KYN levels serve as a valuable biomarker for the diagnosis and prognosis of inflammatory and neurological diseases. Nevertheless, KYN detection relies on mass spectrometry analysis, which requires specialized knowledge and expertise with high operational costs. The bacterial biosensor presents as a promising tool for rapid and cost-effective targeted substance detection due to its ease of genetic modification. Therefore, this study aimed to develop an engineered bacterial biosensor by integrating a genetic module in a plasmid designed for KYN detection harboured in an Escherichia coli chassis. The KYN biosensing component in the genetic module encodes a KYN pathway regulator (KynR) from Pseudomonas aeruginosa, driven by the PBAD arabinose-inducible promoter. Upon expression, KynR would bind to the exogenous KYN and the bacterial responding kyn promoter to express the downstream green fluorescent protein gene to emit a fluorescence signal. However, despite successful induction by arabinose and the presence of KYN, biosensors with different gene orientations and genetic components failed to produce a significant fluorescence signal. These findings suggest that the sensitivity of P. aeruginosa KynR is insufficient to detect physiological levels of KYN. Further exploration of alternative biological sensing components is warranted.
Keywords: biosensor, bioengineering, kynurenine, synthetic biology, tryptophan
Data Summary
The plasmid designs, bio-component sequences and raw data on bacterial growth and fluorescence production in this study are provided in the supplementary materials. The GenBank accession numbers for KYNvA and KYNvB sequences are PV413183 and PV413184, respectively.
Impact statement
This research aimed to develop bacterial biosensors intended for kynurenine (KYN) detection, a potential biomarker for various inflammatory and neurological diseases. Despite optimization of plasmid design and experimental methodology, the biosensors failed to generate a significant fluorescence signal in response to exogenous KYN. These findings highlight that the bacterial KYN pathway regulator may not provide sufficient sensitivity for biosensor construction to detect physiologically relevant KYN concentrations. Further research is required to explore the efficient biosensing elements to develop an applicable biosensor for screening physiological KYN levels in clinical settings.
Introduction
The kynurenine (KYN) pathway is the major catabolic route for the essential amino acid, tryptophan (Trp) in humans, accounting for ~95% of Trp catabolism [1]. Trp-KYN catabolism is initiated and rate-limited by either tryptophan 2,3-dioxygenase (TDO) (EC 1.13.11.11) or the two isoforms of indoleamine 2,3-dioxygenase (IDO1 and IDO2) (EC 1.13.11.52). KYN is further catabolized through multiple enzymatic reactions, generating several metabolites involved in physiological processes in humans, including kynurenic acid (KYNA), anthranilic acid (AA), 3-hydroxy-l-kynurenine, quinolinic acid (QA) and picolinic acid [2]. QA could be further used for the synthesis of nicotinamide adenine dinucleotide (NAD+), which is an essential cofactor involved in various redox reactions [1]. Since the KYN pathway occurs in various cell types, its dysregulation has been implicated in the pathogenesis of several diseases such as neuropsychiatric disorders, neurodegenerative diseases, autoimmune diseases and cancer [2,3]. For instance, high levels of serum KYN have been associated with the poor prognosis of patients with hepatocellular carcinoma [4]. Patients with schizophrenia also demonstrated increased concentrations of KYN (56–65 nM) and KYNA (1.8–2.3 nM) in their cerebrospinal fluid, compared to KYN (27–30 nM) and KYNA (1.3–1.4 nM) concentrations from healthy subjects [5]. Therefore, KYN and its metabolites may represent potential biomarkers for healthcare professionals to provide diagnosis and prognosis.
A biosensor is a transformative tool used for the measurement of a substance of interest. The main principle is based on biosensing by a bioreceptor to the targeted substance, which is then transduced by biological signals to emit quantifiable and interpretable signals [6]. Biosensors can be classified into several categories based on detection systems, transducers, technology and bioreceptors [7]. The common classification is based on the biosensing method and signal transduction perspectives, including catalytic enzyme reactions, protein receptor sensing, antigen–antibody interactions, aptamer-based recognition and whole-cell-based detection [8]. Biosensors can also be classified based on the mode of signal transduction, which depends on the operational methods of the transducer in converting these biosensing events into detectable signals, such as optical, electrochemical, thermal or gravimetric [6,7].
A microbial biosensor is a whole-cell-based biosensor, employing micro-organisms such as bacteria, fungi, protozoa or viruses as the biosensing element [7,9]. The biosensing process in a microbial biosensor occurs through the physiologic or metabolic responses of microbes upon exposure to the targeted substance [9]. For instance, a whole-cell-based Escherichia coli biosensor was constructed to detect heavy metals by engineering the bacteria with a metal-sensitive plasmid. Upon sensing the target metal, the promoter of the metal-sensitive gene (i.e. the zntA gene) is activated, driving the expression of the reporter gene (i.e. the lux gene), which then emits an optical signal [10]. Microbial biosensors offer several advantages, including robust production of different modifications, a broad range of substance detection and ease of genetic engineering to achieve optimal effectiveness [9,11]. E. coli is one of the most widely used chassis for microbial biosensors due to its well-defined genetic profiles, rapid proliferation rate and suitability for genetic modification [12]. An example of the adoption of E. coli-based biosensors is the detection of bile salts in serum samples from hepatic transplant patients. This biosensor model employed the TcpH/TcpP regulatory system in Vibrio cholerae for bile salt sensing, which was integrated into E. coli to enable the detection of bile salts, a biomarker for liver dysfunction [13]. This sheds light on promising avenues for developing cost-effective biomedical devices for biomarker screening and diagnostic testing.
The current standard method for KYN detection in the biomedical field relies on expensive analytic chemistry methods, e.g. mass spectrometry (MS), which is highly technically demanding and time-consuming. The development of a microbial biosensor may present an alternative option for rapid and convenient detection. Interestingly, only a few bacterial families, such as Pseudomonadaceae and Bacillaceae, possess their own KYN pathway [14]. Among these bacteria, the KYN pathway in Pseudomonas aeruginosa is the most recognized and characterized bacterial KYN pathway. The KYN regulon in P. aeruginosa contains the kynR gene that encodes the KYN pathway regulator (KynR), which serves a crucial role in sensing exogenous KYN. In the presence of KYN, KynR can activate the expression of the downstream gene kynB, which encodes a formamidase. However, the role of KYN in the activation of kynA, which encodes TDO, remains unclear, as the kynA promoter exhibits constitutive activity in a kynA′-lacZ transcriptional fusion. Interestingly, KynR binds to the kynA promoter only in the presence of KYN (Fig. 1) [15]. Transcription of these enzymes contributes to bacterial virulence by promoting Pseudomonas quinolone signal (PQS) production by converting KYN into AA via kynureninase, allowing AA in turn to be converted into PQS. Therefore, the enzymes TDO, formamidase and kynureninase are essential for the KYN pathway in P. aeruginosa and PQS production and are orchestrated by KynR [14].
Fig. 1. KYN regulon consists of kynR, kynA, kynB and kynU, which encode KynR, TDO, formamidase and kynureninase, respectively. KynR detects the exogenous KYN and subsequently activates the kynA and kynB promoters, inducing the expression of these enzymes responsible for KYN metabolism in P. aeruginosa, consequently leading to the production of PQS.
In this study, we integrated P. aeruginosa KynR regulon into KYN detecting modules with fluorescent output in E. coli chassis as a biosensor for KYN detection. Despite several attempts with different designs and verification, biosensors failed to produce a significant fluorescence signal in the presence of physiological levels of KYN, suggesting the sensitivity of P. aeruginosa KynR is insufficient and KynR may not function in the E. coli chassis. Further exploration of alternative KYN sensing and responding components is needed to develop convenient biosensors for KYN sensing.
Methods
Plasmid designs
Our pilot biosensors were pMA-KYN and pMK-KYN. The pMA-KYN biosensor is an E. coli containing plasmid designed to detect exogenous KYN and transmit a fluorescence signal. The plasmid comprises kynR, the kynA promoter (PkynA) and gfp, with a constitutively expressed promoter ProD to drive kynR expression. The pMK-KYN biosensor was modified from the pMA-KYN biosensor with modifications, including the substitution of the kynA promoter with the kynB promoter (PkynB) and replacement of gfpmut3 as the reporter gene (National Center for Biotechnology Information[NCBI] GenBank: ABF74540.1), which encodes the green fluorescence protein (GFPmut3). The kynR gene, kynA promoter and kynB promoter sequences were derived from P. aeruginosa PA14 [16]. The selected promoter regions of kynA (−208 bp to+20 bp relative to the ATG of kynA) and kynB (−289 bp to+89 bp relative to the ATG of kynB) were incorporated into the designs, and their binding to the KynR was confirmed by the electrophoretic mobility shift assay [15].
To ensure the expression of kynR, the replacement of the constitutive ProD promoter with the inducible PBAD promoter was performed. The developed plasmid designs utilized for KYN detection in this study were categorized into two versions based on their promoters: KYNvA, consisting of the kynA promoter, and KYNvB, consisting of the kynB promoter (Fig. 2). Both versions contain their truncated downstream gene ORFs (20 bp in the 5′ end for kynA and 89 bp in the 5′ end for kynB) to ensure the native promoters remain intact for KynR binding. These promoters were engineered with gfpmut3. The biosensor operation hinges on the promoter PBAD, which is inducible by arabinose. The arabinose induction would enable kynR expression, allowing KynR to sense exogenous KYN, subsequently activating either the PkynA-gfpmut3 or PkynB-gfpmut3 gene to produce a fluorescence signal. Strong DT16 and T7 transcriptional terminators [17] and Shine–Dalgarno sequence (AGGAGG) for E. coli ribosome binding are also integrated into the biosensor designs (Fig. 2). Genetic modules were synthesized, cloned into pBR322ori high-copy number plasmid pBAD24 and transformed into E. coli DH10B (Thermo Fisher Scientific, Waltham, MA, US). Plasmid sequences are available in Material S1, available in the online Supplementary Material.
Fig. 2. KYNvA (top) and KYNvB (bottom) biosensors employed the PBAD promoter to induce the kynR expression through arabinose induction. KynR would sense the exogenous KYN and activate the kynA or kynB promoter to drive the expression of the reporter gene to produce the fluorescence signals.
Culture medium and conditions
The pilot biosensors were cultured overnight on Luria–Bertani (LB) agar supplemented with ampicillin 100 µg⋅ml−1 for pMA-KYN and kanamycin 50 µg⋅ml−1 for pMK-KYN at 37 °C. A fresh colony was inoculated into LB broth supplemented with ampicillin 100 µg⋅ml−1 for pMA-KYN and kanamycin 50 µg⋅ml−1 for pMK-KYN and incubated at 37 °C with shaking at a speed of 200 r.p.m.
KYNvA and KYNvB were cultured on LB agar plates supplemented with carbenicillin 50 µg⋅ml−1 and incubated overnight at 37 °C. A fresh colony was inoculated into LB broth supplemented with carbenicillin 50 µg⋅ml−1 and incubated overnight at 37 °C with a shaking speed of 200 r.p.m. before further experimentation.
RNA extraction and reverse transcription PCR
The overnight culture of KYNvA and KYNvB was subcultured to an optical density at a wavelength of 600 nm (OD600) of around 0.06–0.08 and incubated for a further 2 h until it reached OD600 of 0.3–0.8. The culture was split into three groups: 1% (w/v) arabinose (Thermo Fisher Scientific) induction, 0.5% (w/v) arabinose induction and no arabinose induction, followed by a further incubation at 37 °C for 2 h. Bacterial cells were harvested by centrifugation at 3,800 g, and the supernatant was discarded. The pellet was resuspended in 120 µl spheroplasting lysis buffer (spheroplasting/26% (w/v) raffinose buffer, 1 mg⋅ml−1 lysosyme, 250 U mutanolysin) and incubated at 37 °C for 5 min. The lysate was transferred to a screw-cap tube containing 250 µl iced zirconia beads (Thermo Fisher Scientific) and homogenized using TissueLyser LT (QIAGEN, Hilden, Germany) at 50 Hz for 6 min. After homogenization, the lysate was centrifuged at 12,000 g for 1 min, followed by the addition of 350 µl PureLink™ lysis buffer (Thermo Fisher Scientific). RNA purification was then performed according to the manufacturer’s protocol for the PureLinkTM RNA Mini Kit (Thermo Fisher Scientific).
High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific) was employed to conduct reverse transcription PCR according to the manufacturer’s protocol. The primers used for kynR amplification were as follows: forward, CCATGGTACATGCCCCTGGAC, reverse, GCAGGTCGACTCTAGATCAACTCTTCAGG, with a 477 bp product. The reverse transcription PCR products were examined by 1% (w/v) agarose gel electrophoresis at 85V for 40 min to confirm the expression of kynR RNA.
Biosensor characterization
Following overnight incubation of the pilot biosensors, the culture was subcultured to an OD600 of 0.05 (OD490 of 0.1 for pMA-KYN). The experiment was divided into five groups, each receiving 1, 5, 10 or 100 µM of KYN (Sigma-Aldrich, Darmstadt, Germany) or no KYN in a black micro clear 96-well plate (Greiner Bio-One, Stonehouse, UK).
The overnight culture of KYNvA and KYNvB was subcultured and incubated until reaching an OD600 of 0.3–0.8 before arabinose induction. The experiment was divided into three groups, consisting of 1% (w/v) arabinose induction, 0.5% (w/v) arabinose induction and no arabinose induction. They were then further incubated at 37 °C for an additional 2 h. Subsequently, each group was divided into two subgroups: one subgroup received 1 mM KYN addition, while the other subgroup did not receive KYN. In a black micro clear 96-well plate, 200 µl of each subgroup was added per well in triplicate. The LB broth alone was used as the negative control, while E. coli DH5α harbouring the plasmid pUC18T-mini-Tn7T-Tp-gfpmut3 (NCBI GeneBank: DQ493881) served as the positive control for fluorescence detection [18].
The OD absorbance and fluorescence were measured at excitation and emission wavelengths of 485 nm and 528 nm, respectively, at 0, 2 and 24 h using the Biotek Synergy HT Microplate Reader (Marshall Scientific, Hampton, NH, USA). The experiments were conducted in three separate biological trials.
Fluorescence microscopy
Biosensor strains (pMK-KYN, KYNvA and KYNvB) were cultured for 24 h under the conditions described above. Following incubation, 10 µl of each culture was mounted on a glass slide and covered with a coverslip. Samples were imaged using a fluorescence microscope at 60× magnification. Brightfield and green fluorescence images (excitation/emission at 488/510 nm) were captured. The pUC18T-mini-Tn7T-Tp-gfpmut3 strain was used as a positive control for GFP expression.
Statistical analysis
Data obtained from the biosensor characterization were recorded in Microsoft Excel 16 (Microsoft, Redmond, WA, USA) for subsequent analysis. Graph generation and statistical analysis were performed using GraphPad Prism 10 (GraphPad Software Inc., La Jolla, CA, USA). The one-way ANOVA was conducted for comparison among the groups. The independent t-test was used for comparison between groups, with a P-value less than 0.05 considered statistically significant.
Results
Pilot biosensors
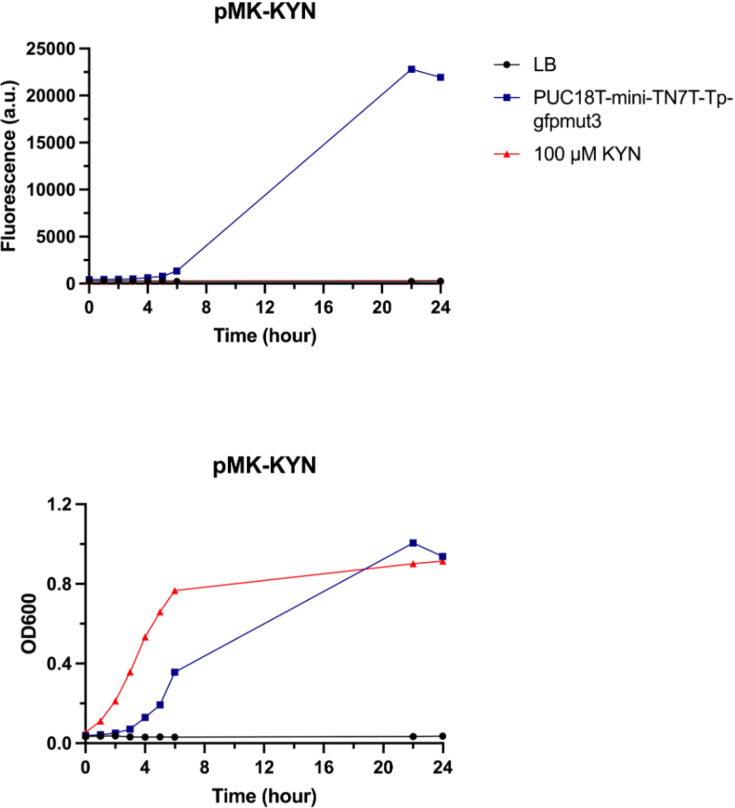
The results obtained from pMA-KYN did not show any discernible fluorescence signal after 12 h in the 1, 5 or 10 µM KYN groups. The transmitted signals exhibited similarity to those observed in the group lacking KYN addition (Fig. 3). Similarly, the pMK-KYN biosensor did not show a remarkable fluorescence signal upon addition of 100 µM KYN over a 24-h period (Fig. 4). The pUC18T-mini-Tn7T-Tp-gfpmut3 was used as fluorescence production control and demonstrated a stronger signal compared to the pMK-KYN biosensor from 5 to 24 h. Raw data of bacterial growth and fluorescence production are available in Material S2. It is important to note that M9 minimal medium was attempted to use as an alternative to LB medium to reduce background noise and to improve the signal-to-noise ratio for fluorescent detection; however, the pilot biosensor showed a similar fluorescence signal when compared to M9 minimal medium alone, which has been provided in Material S2. Additionally, the ProD promoter failed to drive kynR expression as indicated by reverse transcription PCR (Fig. S1).
Fig. 3. Fluorescence produced by the pilot biosensor, pMA-KYN, in conditions of no KYN, 1 µM, 5 µM, 10 µM KYN and LB, which served as the negative control (top). There were no remarkable fluorescence signals observed from any group, despite the increase in bacteria numbers over time (bottom).
Fig. 4. The pilot biosensor, pMK-KYN, was the developed variant of the pMA-KYN biosensor, which exhibited growth over time (bottom). However, it failed to produce a remarkable fluorescence signal as anticipated, in contrast to pUC18T-mini-TN7T-Tp-gfpmut3, which served as a positive control, showing a strong fluorescence signal from 5 to 24 h (top).
kynR expression under arabinose induction
Due to the lack of KynR expression under the ProD promoter, the PBAD-driven system was adopted and incorporated into KYNvA and KYNvB, enabling tighter control and inducible expression for systematic evaluation. To determine the optimal arabinose concentration for inducing the PBAD promoter to drive kynR expression in KYNvA and KYNvB biosensors, three conditions were examined: no arabinose, 0.5% (w/v) arabinose and 1% (w/v) arabinose. The reverse transcription PCR products were analysed on agarose gel electrophoresis and showed that only 1% (w/v) arabinose successfully induced PBAD to drive kynR expression, as illustrated by the presence of a distinct band corresponding to the expected size (477 bp) (Fig. 5). Conversely, 0.5% (w/v) arabinose failed to induce the PBAD promoter, showing no observable band similar to the condition without arabinose induction (Fig. 5).
Fig. 5. The kynR expression was confirmed by reverse transcription PCR, as indicated by a distinct band (477 bp). The kynR expression was successfully induced by 1% (w/v) arabinose for both KYNvA (left) and KYNvB (right). In contrast, no observable bands were present under 0.5% (w/v) arabinose or in the absence of arabinose induction.
Functional characterization to detect KYN
KYNvA and KYNvB biosensors were employed to detect the exogenous KYN at a concentration of 1 mM under varying levels of arabinose induction (0, 0.5 and 1%). Bacterial cell growth was monitored by measuring OD600 to ensure that fluorescence production was not affected by impaired growth (Fig. 6). Despite arabinose induction and confirmed bacterial growth, no remarkable fluorescence signal was detected in either biosensor in a 24-h period (Fig. 6). A one-way ANOVA revealed no significant differences in fluorescence production among groups at 24 h for KYNvA, F (6, 14) = 0.5, P=0.73, and KYNvB, F (6, 14) = 0.5, P=0.79. Moreover, the fluorescence signals observed were comparable to those of the control groups without KYN addition at 24 h for KYNvA with 1% (w/v) arabinose induction, t (4) = 0.01, P=0.99, and KYNvB with 1% (w/v) arabinose induction, t (4) = 0.30, P=0.78 (Fig. 6).
Fig. 6. Despite the bacterial growth, KYNvA (top) and KYNvB (bottom) failed to generate significant fluorescence signals upon the induction of 1% (w/v) arabinose and the addition of 1 mM KYN. There was no significant difference in fluorescence intensities compared to the groups lacking KYN in the 24 h period (KYNvA, P=0.99; KYNvB, P=0.78). No significant differences in fluorescence production were observed among groups at 24 h (KYNvA, P=0.73, KYNvB, P=0.79).
To verify the fluorescence signal, a positive control, E. coli pUC18T-mini-Tn7T-Tp-gfpmut3, which harbours a constitutive gfpmut3, demonstrated a high intensity of fluorescence signal at 24 h without arabinose (M=51,036, sd=4,507). However, KYNvA and KYNvB with 1% (w/v) arabinose and 1 mM KYN exhibited significantly lower fluorescence signals compared to E. coli pUC18T-mini-Tn7T-Tp-gfpmut3 at 24 h, with KYNvA: t (4) = 19.29, P=0.0026; KYNvB: t (4) = 19.29, P=0.0026 (Fig. 7). Furthermore, the fluorescence intensities of KYNvA and KYNvB did not differ significantly from the LB control at 24 h, with KYNvA: t (7) = 0.12, P=0.91; KYNvB: t (7) = 0.17, P=0.87 (Fig. 7). These findings indicate that neither KYNvA nor KYNvB functioned as presumed even in the presence of 1 mM KYN and 1% (w/v) arabinose induction, with no observable differences compared to conditions without KYN and LB alone. Raw data of bacterial growth and fluorescence production are available in Material S2.
Fig. 7. E. coli pUC18T-mini-Tn7T-Tp-gfpmut3 served as the positive control, exhibiting significantly higher fluorescence intensity than KYNvA (P=0.0026) and KYNvB (P=0.0026) under 1% (w/v) arabinose and 1 mM KYN at 24 h. LB was used as the negative control, displaying fluorescence intensity comparable to KYNvA (P=0.91) and KYNvB (P=0.87).
Discussion
The biosensor design in this study utilized the kynR gene from the KYN regulon of P. aeruginosa as the biological sensing component for the selective detection of exogenous KYN. KynR acts as a crucial transcriptional regulator for the KYN pathway in P. aeruginosa, upregulating the expression of genes kynA, kynB and kynU in response to KYN, which encode TDO, formamidase and kynureninase, respectively [14,15]. The transcription of kynA and kynB genes was demonstrated to be regulated dependently by KynR [15]. Additionally, KYN serves as a co-inducer, enabling KynR to bind to the kynA promoter to activate gene expression. On the contrary, the kynB promoter does not require KYN for activation, as it was shown that KynR can bind to the kynB promoter in the absence of KYN [15]. Therefore, our study developed biosensors based on the current available knowledge of the bacterial KYN regulatory mechanisms to examine whether KYNvA, KYNvB or both can produce a detectable signal upon the presence of KYN.
The pilot biosensors were designed to operate under the constitutive expression of the ProD promoter. Both pMA-KYN and pMK-KYN failed to produce strong fluorescence signals. The biosensor cells from pMK-KYN were also directly observed under fluorescence microscopy; however, they did not exhibit any detectable fluorescence signal compared to pUC18T-mini-Tn7T-Tp-gfpmut3 (Fig. S2). Moreover, reverse transcription PCR was performed to confirm the expression of kynR, indicating that the constitutive ProD promoter failed to drive kynR expression (Fig. S1). Therefore, the biosensor was developed for KYNvA and KYNvB using the inducible PBAD promoter to activate kynR expression. The PBAD promoter was selected for its tight regulation by arabinose, remaining repressed in the absence of arabinose and strongly activated when arabinose is present [19]. Additionally, the PBAD promoter has been shown to function effectively even in the presence of glucose, as demonstrated in a previous study [20]. Our results demonstrated that kynR expression was not activated without arabinose induction or 0.5% (w/v) arabinose, as no expected band was observed from the reverse transcription PCR products (Fig. 5). However, in the presence of 1% (w/v) arabinose, it can successfully induce the PBAD promoter, driving kynR expression in both KYNvA and KYNvB, as indicated by the distinct bands from the reverse transcription PCR products (Fig. 5). Given that E. coli is a well-characterized model organism in which transcription and translation are generally well-coupled and efficient, the likelihood of poor translation or protein instability of KynR is considered relatively low.
In our pilot biosensor model, KYN at concentrations of 1, 5, 10 and 100 µM was examined to characterize the function of the biosensor. However, the pilot biosensor failed to produce a strong fluorescence signal in response to these concentrations. Following the unsuccessful results obtained from the pilot biosensor, the current version of biosensors, KYNvA and KYNvB, was developed. To enhance responsiveness, the highest tested concentration of 1 mM KYN was used for functional characterization. Unfortunately, even with this high KYN concentration at 1 mM, both KYNvA and KYNvB exhibited fluorescence signals similar to the control groups without KYN addition (Fig. 6). Moreover, these biosensors were confirmed to have a negligible amount of fluorescence production through comparison with E. coli pUC18T-mini-Tn7T-Tp-gfpmut3, revealing that KYNvA and KYNvB produced significantly lower fluorescence signals (Fig. 7). These KYNvA and KYNvB biosensors also lacked visible fluorescence when observed directly under a fluorescence microscope (Fig. S2).
Although KynR binding to the kynA and kynB promoters was confirmed in gel shift assays [15], the results suggest that KynR from P. aeruginosa may not function properly to detect KYN in the E. coli chassis, resulting in insignificant fluorescence signals in KYNvA and KYNvB. In contrast, Knoten and coworkers demonstrated that 1 mM KYN could induce the expression of lacZ in the KynR expression plasmid in E. coli, as measured by β-galactosidase activity [15]. While LacZ-based reporter benefits from enzymatic amplification, they are less practical for rapid and convenient detection. To develop a versatile biosensor suitable for biomedical applications, a GFP-based biosensor was employed in this study due to its advantage for real-time monitoring. Nevertheless, the weak fluorescence signals in KYNvA and KYNvB may be attributed to GFP relying on proper protein folding and chromophore maturation, which may result in low sensitivity to detect weak transcriptional activation. However, the absence of fluorescence under direct microscopic observations suggests that this is unlikely due to GFP instability or degradation. Given these results, other upstream factors such as suboptimal KynR activity may contribute to limiting biosensor functionality.
It is important to note that physiological KYN concentrations are considerably lower than those concentrations used in laboratory experiments, typically ranging from 1.3 to 2.4 µM in human plasma and serum [21]. In healthy individuals, KYN concentrations in saliva were even lower, ~0.04–0.08 µM [22]. Furthermore, in inflammatory conditions such as the acute phase of the coronavirus disease 2019 (COVID-19), serum KYN concentrations were significantly increased; however, the concentrations were around 2.0–19.6 µM [23]. The KYN concentrations considered useful as a prognostic biomarker in patients with hepatocellular carcinoma were also relatively low, ~2.6 µM [4].
This information suggests that a biosensor intended for KYN detection in clinical settings would require a minimum detection threshold in the micromolar range. The biosensors in this study were developed aiming for screening KYN levels with high sensitivity and rapid detection capabilities for biomedical applications. Therefore, the biosensor based on P. aeruginosa KynR may not be fully functional in an E. coli background, which results from a lack of the key Pseudomonas factors or chaperones to facilitate KYN sensing by KynR. Furthermore, the extremely low fluorescence signals observed across all biosensors in this study suggest that the current E. coli-based biosensor lacks sufficient sensitivity to detect physiologically relevant KYN levels and generate a robust signal. Additionally, while E. coli lacks its own KYN pathway, Han and coworkers demonstrated that aspartate aminotransferase, a protein isolated from E. coli, exhibits KAT activity, enabling the catabolism of KYN to KYNA [24]. This enzymatic activity may interfere with KynR sensing. Our study also suggests the complexity of the KYN regulatory mechanism in P. aeruginosa, which may require additional investigation. To develop more effective biosensors for this purpose, further exploration of alternative biological sensing components is warranted. Alternatively, the biomimetic liposomes containing key sensing factors and components from Pseudomonas may provide a convenient biosensing approach without bacterial virulence concern.
Supplementary material
Acknowledgements
The authors acknowledge Katya Kozhevnikova and Philip Hardy for their technical support in the laboratory.
Abbreviations
- AA
anthranilic acid
- KYN
kynurenine
- KYNA
kynurenic acid
- KynR
KYN pathway regulator
- LB
Luria–Bertani
- PQS
Pseudomonas quinolone signal
- QA
quinolinic acid
- TDO
tryptophan 2,3-dioxygenase
- Trp
tryptophan
Footnotes
Funding: This research is supported by the Rosetrees Trust (Seedcorn2022/100007). C.-Y.C. and J.C. thank the National Institute for Health and Care Research Newcastle Biomedical Research Centre (NIHR Newcastle BRC) for the infrastructural support of their research labs in the School of Dental Sciences at Newcastle University. P.C. thanks Newcastle University and Mahidol University for his PhD studentship. W.C. thanks Applied Microbiology International for the Summer Student Placement Scholarship. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. The funding agencies have no role in the preparation of this manuscript.
Ethical statement: This study did not involve any human or animal subjects.
Author contributions: Conceptualization: C.-Y.C. Formal analysis: P.C. Funding acquisition: C.-Y.C. Investigation: P.C., H.A. and W.C. Methodology: C.-Y.C. and P.C. Supervision: C.-Y.C. and J.C. Writing – original draft: P.C. Writing – review and editing: P.C., H.A., W.C., J.C. and C.-Y.C.
Contributor Information
Pisit Charoenwongwatthana, Email: P.Charoenwongwatthana2@newcastle.ac.uk.
Halah Ahmed, Email: Halah.Ahmed@newcastle.ac.uk.
Wojciech Cajdler, Email: W.M.Cajdler1@newcastle.ac.uk.
Jamie Coulter, Email: Jamie.Coulter2@newcastle.ac.uk.
Chien-Yi Chang, Email: chienyi.chang@newcastle.ac.uk.
References
- 1.Badawy AA-B. Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int J Tryptophan Res. 2017;10:1178646917691938. doi: 10.1177/1178646917691938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savitz J. The kynurenine pathway: a finger in every pie. Mol Psychiatry. 2020;25:131–147. doi: 10.1038/s41380-019-0414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanaka M, Szabó Á, Vécsei L. Redefining roles: a paradigm shift in tryptophan–kynurenine metabolism for innovative clinical applications. Int J Mol Sci. 2024;25:12767. doi: 10.3390/ijms252312767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bekki S, Hashimoto S, Yamasaki K, Komori A, Abiru S, et al. Serum kynurenine levels are a novel biomarker to predict the prognosis of patients with hepatocellular carcinoma. PLoS One. 2020;15:e0241002. doi: 10.1371/journal.pone.0241002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linderholm KR, Skogh E, Olsson SK, Dahl M-L, Holtze M, et al. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr Bull. 2012;38:426–432. doi: 10.1093/schbul/sbq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhalla N, Jolly P, Formisano N, Estrela P. Introduction to biosensors. Essays Biochem. 2016;60:1–8. doi: 10.1042/EBC20150001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naresh V, Lee N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors. 2021;21:1109. doi: 10.3390/s21041109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alhadrami HA. Biosensors: classifications, medical applications, and future prospective. Biotechnol Appl Biochem. 2018;65:497–508. doi: 10.1002/bab.1621. [DOI] [PubMed] [Google Scholar]
- 9.Su L, Jia W, Hou C, Lei Y. Microbial biosensors: a review. Biosens Bioelectron. 2011;26:1788–1799. doi: 10.1016/j.bios.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Kim Y, Choi H, Shin WH, Oh J-M, Koo S-M, et al. Development of colorimetric whole-cell biosensor for detection of heavy metals in environment for public health. Int J Environ Res Public Health. 2021;18:12721. doi: 10.3390/ijerph182312721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Souza SF. Microbial biosensors. Biosens Bioelectron. 2001;16:337–353. doi: 10.1016/s0956-5663(01)00125-7. [DOI] [PubMed] [Google Scholar]
- 12.Blake S. Development of genetically engineered microbial biosensors. Biosens Bioelectron. 2024;15:430. [Google Scholar]
- 13.Chang H-J, Zúñiga A, Conejero I, Voyvodic PL, Gracy J, et al. Programmable receptors enable bacterial biosensors to detect pathological biomarkers in clinical samples. Nat Commun. 2021;12:5216. doi: 10.1038/s41467-021-25538-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurnasov O, Jablonski L, Polanuyer B, Dorrestein P, Begley T, et al. Aerobic tryptophan degradation pathway in bacteria: novel kynurenine formamidase. FEMS Microbiol Lett. 2003;227:219–227. doi: 10.1016/S0378-1097(03)00684-0. [DOI] [PubMed] [Google Scholar]
- 15.Knoten CA, Hudson LL, Coleman JP, Farrow JM, 3rd, Pesci EC. KynR, a Lrp/AsnC-type transcriptional regulator, directly controls the kynurenine pathway in Pseudomonas aeruginosa. J Bacteriol. 2011;193:6567–6575. doi: 10.1128/JB.05803-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee DG, Urbach JM, Wu G, Liberati NT, Feinbaum RL, et al. Genomic analysis reveals that Pseudomonas aeruginosa virulence is combinatorial. Genome Biol. 2006;7:R90. doi: 10.1186/gb-2006-7-10-r90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandez-Rodriguez J, Moser F, Song M, Voigt CA. Engineering RGB color vision into Escherichia coli. Nat Chem Biol. 2017;13:706–708. doi: 10.1038/nchembio.2390. [DOI] [PubMed] [Google Scholar]
- 18.Choi KH, Schweizer HP. mini-Tn7 insertion in bacteria with single attTn7 sites: example Pseudomonas aeruginosa. Nat Protoc. 2006;1:153–161. doi: 10.1038/nprot.2006.24. [DOI] [PubMed] [Google Scholar]
- 19.Guzman LM, Belin D, Carson MJ, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lo TM, Chng SH, Teo WS, Cho HS, Chang MW. A two-layer gene circuit for decoupling cell growth from metabolite production. Cell Syst. 2016;3:133–143. doi: 10.1016/j.cels.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Metri NJ, Butt AS, Murali A, Steiner-Lim GZ, Lim CK. Normative data on serum and plasma tryptophan and kynurenine concentrations from 8089 individuals across 120 studies: a systematic review and meta-analysis. Int J Tryptophan Res. 2023;16:11786469231211184. doi: 10.1177/11786469231211184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurgan Ş, Önder C, Balcı N, Akdoğan N, Altıngöz SM, et al. Influence of periodontal inflammation on tryptophan-kynurenine metabolism: a cross-sectional study. Clin Oral Investig. 2022;26:5721–5732. doi: 10.1007/s00784-022-04528-4. [DOI] [PubMed] [Google Scholar]
- 23.Bizjak DA, Stangl M, Börner N, Bösch F, Durner J, et al. Kynurenine serves as useful biomarker in acute, long- and post-COVID-19 diagnostics. Front Immunol. 2022;13:1004545. doi: 10.3389/fimmu.2022.1004545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han Q, Fang J, Li J. Kynurenine aminotransferase and glutamine transaminase K of Escherichia coli: identity with aspartate aminotransferase. Biochem J. 2001;360:617–623. doi: 10.1042/0264-6021:3600617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.