Abstract
Background
Ramadan fasting alters the timing and content of food, including dietary vitamin K, and medication intake, potentially affecting stability of vitamin K antagonist (VKA) treatment and clinical outcomes.
Objectives
To assess population-level changes in VKA treatment quality and incidence of clinical events, including bleeding and venous and arterial thromboembolism, during Ramadan in the Netherlands.
Methods
Data from 17 Dutch anticoagulation clinics were linked to Statistics Netherlands. Prevalent VKA users in 2013-2019 with an immigration background who were likely to fast during Ramadan (ie, the Ramadan cohort) were studied from 2 months preceding Ramadan until 2 months after. During each 30-day interval, VKA treatment quality and risk of clinical events were assessed. A cohort of native Dutch VKA users was studied as negative control.
Results
The Ramadan cohort included 3835 VKA users (median age 65.8 years, 55.2% male). Frequency of international normalized ratio (INR) monitoring, INR variability, and time within target range remained similar across Ramadan. However, the proportion of supratherapeutic INRs was slightly higher during (18.9% ± 31.5% [mean ± SD]) and after Ramadan (month +1, 19.9% ± 32.7% and month +2, 19.7% ± 32.8%) compared with before (month –2, 18.1 ± 30.9% and month –1, 17.8 ± 31.5%). Meanwhile, there was a higher proportion of clinically relevant dose reductions during Ramadan (4.7%) than the other months (3.6%-4.3%). These were not observed in the native Dutch cohort (N = 139,207). Monthly risk of the composite of bleeding and thromboembolic events remained unchanged across Ramadan in both cohorts.
Conclusion
There were no clinically relevant population-level changes across Ramadan in VKA treatment quality and clinical outcomes, except for a slightly higher proportion of supratherapeutic INRs and dose reductions.
Keywords: 4-hydroxycoumarins, hemorrhage, international normalized ratio, Ramadan fasting, thromboembolism
Essentials
-
•
Ramadan-related changes in diet and medication intake may impact quality of vitamin K antagonist (VKA) therapy.
-
•
This cohort study assessed VKA treatment quality and clinical outcomes across Ramadan at a population level.
-
•
Supratherapeutic anticoagulation intensity and VKA dose reductions occurred slightly more often during Ramadan.
-
•
Rates of death, bleeding, and thromboembolic events remained unchanged during the Ramadan period.
1. Introduction
Although direct oral anticoagulants (DOACs) have replaced vitamin K antagonists (VKAs) as first-choice oral anticoagulant treatment for most common indications, including stroke prevention in atrial fibrillation (AF) and treatment or prevention of venous thromboembolism [[1], [2], [3]], VKAs remain indicated in patients with mechanical heart valves [4,5], (rheumatic) moderate-to-severe mitral stenosis [6], or antiphospholipid syndrome [[7], [8], [9], [10], [11]]. In addition, among those who were already receiving VKAs, switching to DOACs may not always be appropriate; for instance, in frail older AF patients, this switch was associated with increased bleeding risk [12]. Globally, VKAs are still commonly used, partly because of cost-related issues for DOACs [13,14]. Treatment with VKAs has some disadvantages, such as the narrow therapeutic window and multiple drug and food interactions [[15], [16], [17], [18], [19], [20]]. Changes in dietary intake of vitamin K affect their anticoagulant effect [[21], [22], [23], [24], [25], [26], [27]]. Therefore, patients treated with VKAs are usually managed by specialized anticoagulation clinics, which monitor the international normalized ratio (INR) and perform dose adjustments when necessary [16]. In addition, VKA users are advised to maintain a stable dietary vitamin K intake [16].
During Ramadan, the ninth month of the Islamic lunar calendar, adult Muslims are expected to fast, which includes refraining from eating and drinking, including oral medication intake, from dawn to sunset [28]. The month of Ramadan typically lasts for 29 to 30 days. Muslims are exempted when fasting may cause significant harm to their health [[29], [30], [31], [32]]. Changes in dietary vitamin K intake during Ramadan and timing of food and oral medication intake, which is limited to the predawn and meal at sunset [28,33], may cause fluctuations in stability of VKA treatment. Muslims on long-term anticoagulant treatment with warfarin or DOACs who fasted during Ramadan reported that self-guided modifications of oral anticoagulant intake occur frequently during Ramadan, including adjusted timing of intake and skipping or doubling doses, to adapt to the fasting times [34].
A limited number of studies have shown inconsistent results on the potential impact of Ramadan fasting on quality of VKA treatment and clinical outcomes [[35], [36], [37], [38], [39], [40], [41], [42], [43]]. A systematic review of studies comparing cohorts of patients before, during, and after Ramadan reported no significant change in time in therapeutic range (TTR) but a slightly higher mean INR during Ramadan and more frequent supratherapeutic INRs during and after it [43]. Nevertheless, these studies were limited by their sample sizes, and not all assessed the occurrence of clinical outcomes.
By identifying a cohort of VKA users who are likely to fast during Ramadan, we intend to overcome these limitations and assess potential population-level changes in VKA treatment quality and incidences of adverse clinical events, including bleeding and venous and arterial thromboembolic events, across Ramadan in the Netherlands.
2. Methods
2.1. Setting and data sources
In the Netherlands, patients treated with VKAs are managed by regional anticoagulation clinics [44]. Seventeen Dutch anticoagulation clinics provided detailed data on VKA treatment, including start and end dates of VKA therapy, treatment indications, VKA type and dose, INR target range, and INR results [45]. Data from these clinics were linked on an individual level to nationwide data from Statistics Netherlands (in Dutch, “Centraal Bureau voor de Statistiek” [CBS]) by sex, date of birth, postal code, and last known date to be alive. CBS provides nationwide data on personal characteristics [46], diagnoses made during hospital admissions in Dutch hospitals [[47], [48], [49], [50]], outpatient-dispensed medication prescriptions [51], cause of death [52], and date of death [53]. Diagnoses in the Dutch Hospital Data registry are coded according to the International Classification of Diseases (ICD) (ICD-9 for some diagnoses made from 2010 to 2012, and ICD-10 thereafter). Data on outpatient-dispensed medication prescriptions include year of dispensing and Anatomical Therapeutic Chemical code (4 digits) and for anticoagulants only, dispensing dates and anticoagulant type. More information on the data sources used is described in the Supplementary Methods.
2.2. Study population
Our primary study cohort, ie, the Ramadan cohort, comprised prevalent VKA users, treated at one of the participating anticoagulation clinics between 2013 and 2019, who were likely to fast during Ramadan. VKA users were classified as such based on an immigration background (first or second generation) from a country of origin where the Muslim population accounts for ≥85% of the total population (Supplementary Methods and Supplementary Table 1). During the study period, approximately 5% of the total population in the Netherlands identified as Muslim, with the largest groups originating from Turkey or Morocco [54,55]. We included adults (aged ≥18 years) who were already receiving VKA treatment ≥2 months before the start of Ramadan (d0) in a particular calendar year from 2013 to 2019. Exclusion criteria were 1) no registered dispensed VKA prescription or a registered dispensed DOAC prescription within 6 months before the start of follow-up (d0), 2) a type of VKA other than the 2 registered VKAs in the Netherlands (acenocoumarol and phenprocoumon), 3) missing INR target range at the last INR measurement before d0, or 4) country of origin imputed by CBS.
As a negative control, we similarly identified a cohort of VKA users with the Netherlands as their registered country of origin (ie, native Dutch cohort), applying the same inclusion and exclusion criteria. For both cohorts, if a VKA user was eligible for inclusion over multiple calendar years, only 1 calendar year was randomly selected for inclusion.
2.3. Study design
For each calendar year (2013-2019), follow-up started 2 months before the start of Ramadan (d0) and ended 2 months after the end of Ramadan (d5) (Figure 1). Thirty-day intervals were determined based on the start and end dates of Ramadan in a particular calendar year. Potential changes in quality of VKA treatment and the incidence of adverse clinical events across Ramadan were assessed by comparing these metrics across the 5 30-day observation periods within the same cohort (either the Ramadan cohort or the native Dutch cohort).
Figure 1.
Study design. For each calendar year during the study period (2013-2019), follow-up started 2 months before the start of Ramadan (d0) and ended 2 months after the end of Ramadan (d5). The 30-day intervals were determined based on the start and end dates of Ramadan in a particular calendar year. Note: d2 and d3 refer to the start date and end date of Ramadan in each calendar year, respectively, but to keep the interval at 30 days consistently, d3 of years 2015-2019 was the actual end date of Ramadan plus 1 more day.
2.4. Baseline characteristics
Baseline characteristics were collected at d0 and included age, sex, immigration background (ie, native Dutch, first or second-generation immigrant), INR target range, type of INR monitoring (eg, at home, outpatient, self-management), type of VKA (acenocoumarol or phenprocoumon), presence of comorbidities, CHA2DS2-VASc and HAS-BLED scores, and prior use of low molecular weight heparin or antiplatelet drugs (defined as ≥1 dispensed prescription within 6 months before d0). Comorbidities were identified by hospital diagnoses registered within 3 years before d0 (Supplementary Table 2).
2.5. Study outcomes
The quality of VKA treatment was assessed within each 30-day period, with 1 period corresponding to Ramadan. We studied the following metrics: median INR value, median recommended average dose, ≥1 clinically relevant dose increases or reductions, proportions of INRs within/below/above the target range, proportions of INRs ≥5 or ≥8, percentage of time in/below/above the target range (TTR, TBR, and TAR, respectively), and INR variability. A clinically relevant dose adjustment was defined as a change of ≥10% in the recommended average VKA dose prescribed at 2 consecutive INR measurements. TTR, TBR, and TAR were calculated according to the Rosendaal method, which assumes a linear relationship between consecutive INR measurements and divides the time with linearly interpolated INR values within, below or above the INR target range by the total number of observation days [56]. INR variability was expressed by the variance growth rate (VGR) according to Cannegieter, which reflects the deviation in INR value between 2 consecutive measurements [57]. Only individuals receiving VKA treatment at the start of a 30-day observation period and with ≥2 INR measurements within a certain 30-day period (where 1 INR measurement was allowed to be recorded in the previous observation period) were included in this calculation. In addition, we evaluated the frequency of INR monitoring, the time interval between 2 consecutive INR measurements, and switch to another type of VKA (according to anticoagulation clinic records) or DOAC (according to outpatient-dispensed prescriptions).
Within each 30-day observation period, we determined if adverse clinical events occurred, including 1) major/clinically relevant bleeding, 2) arterial thromboembolism (ischemic stroke, transient ischemic attack [TIA], myocardial infarction, and other arterial thromboembolism), 3) the composite of venous and arterial thromboembolic and bleeding events, and 4) all-cause mortality. These events were identified by first in-hospital diagnoses (restricted to primary or main diagnosis) and primary causes of death (Supplementary Table 2).
Individuals were followed from d0 until 2 months after the end of Ramadan or until a registered DOAC dispensing, registered DOAC use according to anticoagulation clinic records, discontinuation of VKA therapy, a clinical event of interest, or date of death, whichever occurred first.
2.6. Statistical analyses
Summary statistics of the baseline characteristics as well as the metrics for the quality of VKA treatment are presented as mean ± SD, median (25th-75th percentile, IQR), or frequency (percentage). To assess differences in the continuous metrics for the quality of VKA treatment across Ramadan within the same cohort, a linear mixed-effects model was employed. The 5 observation periods were treated as a 5-level categorical variable with the first period as reference (ie, month −2 prior to Ramadan), and a subject-specific random intercept was included to account for within-subject correlations over time. Model parameters were estimated via restricted maximum likelihood, with 95% CIs derived by the Wald method.
For clinical outcomes, 30-day cumulative incidences were estimated by the cumulative incidence competing risks method or Kaplan–Meier method (for all-cause mortality) within each of the 5 observations periods (ie, month −2 prior to Ramadan, month −1 prior to Ramadan, Ramadan month, month +1 after Ramadan, month +2 after Ramadan). Incidence rates were calculated by dividing the number of first events by total observation time, with 95% CIs according to the chi-squared distribution.
We performed a subgroup analysis among VKA users in the Ramadan cohort with Morocco as their registered country of origin. This was supported by a survey study from The Netherlands Institute for Social Research indicating that the vast majority of Dutch Moroccan Muslims fasted every day during Ramadan (87%), while lower participation rates were reported for Muslims with other backgrounds [58]. In addition, participation was higher among first-generation than second-generation immigrants.
Analyses were performed in R (version 4.4.0, R Core Team) [59] with the packages dplyr [60], tableone [61], survival [62], cmprsk [63], and lme4 [64].
2.7. Ethics approval
This study used deidentified, retrospective administrative data from population registries and routine care in the Netherlands. Consequently, it was exempt from ethics approval under the Dutch Medical Research Involving Human Subjects Act (WMO), and individual participant consent was waived. The Scientific Committee of the Department of Clinical Epidemiology of the Leiden University Medical Centre approved the study protocol (No. A232). Results based on a frequency <10 were masked in line with CBS’s privacy policy.
3. Results
3.1. Study population
Among 239,980 VKA users between 2013 and 2019, a total of 4734 VKA users were considered eligible for inclusion in the Ramadan cohort as well as 172,591 for the native Dutch cohort. After applying the exclusion criteria, a total of 3835 VKA users were included in the Ramadan cohort and 139,207 in the native Dutch cohort (Figure 2).
Figure 2.
Flowchart of the Ramadan cohort and Native Dutch cohort. aThe date d0 refers to the date 60 days before the start of Ramadan in a calendar year. bDetails about the identification of the Ramadan cohort are presented in the Supplementary Methods. cBased on data on the latest INR measurement record within 6 weeks before d0. In the data, the other registered types of anticoagulants included warfarin, fluindione, direct oral anticoagulant, or unknown. DOAC, direct oral anticoagulant; INR, international normalized ratio; VKA, vitamin K antagonist.
The majority of VKA users in the Ramadan cohort were of Moroccan (38.9%) or Turkish (34.4%) origin and were first-generation immigrants (95.9%) (Table 1). The Ramadan cohort had a median age of 65.8 (IQR, 55.0-74.9) years, and 55.2% of VKA users were male. Most patients were managed with INR target ranges of 2.0 to 3.0 or 2.5 to 3.5 (89.0%) and used acenocoumarol (82.1%). The most commonly registered indications for VKA treatment were AF (56.3%) and other valvular heart disease (18.2%), followed by venous thromboembolism (15.5%). The median CHA2DS2-VASc and HAS-BLED scores were 2 (IQR, 1-3) and 1 (IQR, 1-2), respectively. Based on in-hospital diagnoses within 3 years before d0, 3.3% had a history of ischemic stroke/TIA and 6.2% of major bleeding (Supplementary Table 3).
Table 1.
Baseline characteristics of study cohorts.
| Cohort | Ramadan cohort (N = 3835) | Native Dutch cohort (N = 139,207) |
|---|---|---|
| Age (y), median (IQR) | 65.8 (55.0-74.9) | 76.7 (68.4-84.0) |
| Female, n (%) | 1717 (44.8) | 62,764 (45.1) |
| Immigration background, n (%) | ||
| Native Dutch | 0 (0) | 139,207 (100) |
| First-generation immigrant | 3678 (95.9) | 0 (0) |
| Second-generation immigrant | 157 (4.1) | 0 (0) |
| Country of origin, n (%) | ||
| Netherlands | 0 (0) | 139,207 (100) |
| Morocco | 1492 (38.9) | 0 (0) |
| Turkey | 1321 (34.4) | 0 (0) |
| Indonesia | 272 (7.1) | 0 (0) |
| Iraq | 143 (3.7) | 0 (0) |
| Other | 607 (15.8) | 0 (0) |
| Calendar year of d0a, n (%) | ||
| 2013 | 406 (10.6) | 18,527 (13.3) |
| 2014 | 503 (13.1) | 18,771 (13.5) |
| 2015 | 567 (14.8) | 18,728 (13.5) |
| 2016 | 579 (15.1) | 19,733 (14.2) |
| 2017 | 551 (14.4) | 20,669 (14.8) |
| 2018 | 541 (14.1) | 18,244 (13.1) |
| 2019 | 688 (17.9) | 24,535 (17.6) |
| INR target range, n (%) | ||
| 2.0-3.0 | 1637 (42.7) | 71,580 (51.4) |
| 2.5-3.5 | 1776 (46.3) | 58,622 (42.1) |
| 3.0-4.0 | 394 (10.3) | 7876 (5.7) |
| Other | 28 (0.7) | 1129 (0.8) |
| Type of VKA, n (%) | ||
| Acenocoumarol | 3149 (82.1) | 107,178 (77.0) |
| Phenprocoumon | 686 (17.9) | 32,029 (23.0) |
| Type of INR monitoring, n (%) | ||
| At home measurement | 1447 (37.7) | 50,983 (36.6) |
| Nursing home | <10b | 2020 (1.5) |
| Outpatient | 1993 (52.0) | 65,756 (47.2) |
| Self-management | 57 (1.5) | 5859 (4.2) |
| Self-measurement | 306 (8.0) | 12,481 (9.0) |
| Unknown | Maskedb | 2108 (1.5) |
| Registered VKA treatment indicationc, n (%) | ||
| Atrial fibrillation | 2158 (56.3) | 98,640 (70.9) |
| Stroke/transient ischemic attack | 109 (2.8) | 4781 (3.4) |
| Systemic thromboembolism/peripheral arterial disease | 90 (2.3) | 2826 (2.0) |
| Heart failure | 168 (4.4) | 2787 (2.0) |
| Venous thromboembolism | 596 (15.5) | 21,768 (15.6) |
| Mechanical heart valve | 537 (14.0) | 7305 (5.2) |
| Biovalve prosthesis | 75 (2.0) | 2825 (2.0) |
| Rheumatic mitral stenosis | <10b | 139 (0.1) |
| Other mitral valve disease | 53 (1.4) | 980 (0.7) |
| Aortic valve disease | 26 (0.7) | 1058 (0.8) |
| Other valve disease | 697 (18.2) | 10,090 (7.2) |
| Coronary artery disease | 96 (2.5) | 3548 (2.5) |
| Cardiomyopathy | 269 (7.0) | 4827 (3.5) |
| Antiphospholipid syndrome/thrombophilia | <10b | 92 (0.1) |
| Other | 338 (8.8) | 12,440 (8.9) |
| CHA2DS2-VASc score, median (IQR) | 2 (1-3) | 2 (1-3) |
| HAS-BLED scored, median (IQR) | 1 (1-2) | 1 (1-2) |
INR, international normalized ratio; VKA, vitamin K antagonist.
The date d0 refers to the date 60 days before the start of Ramadan in a particular calendar year.
Cells containing <10 individuals are masked according to Statistics Netherlands’ privacy policy. To prevent recalculation of cells <10, sometimes corresponding cells are additionally masked.
All indications for VKA treatment that have been registered in the data are presented, regardless of whether an indication became valid before d0 (because of data availability). One or more indications can be present.
Calculated without the term “labile INR.”
Compared with the Ramadan cohort, the native Dutch cohort was older (median age 76.7 [IQR, 68.4-84.0] years) and had a different distribution of indications for VKA treatment (ie, 70.9% AF, 7.2% other valvular heart disease; Table 1). Additional baseline characteristics of the study cohorts, such as relevant comorbidities and prior use of antithrombotic drugs, are displayed in Supplementary Tables 3 and 4.
3.2. Quality of VKA treatment across Ramadan
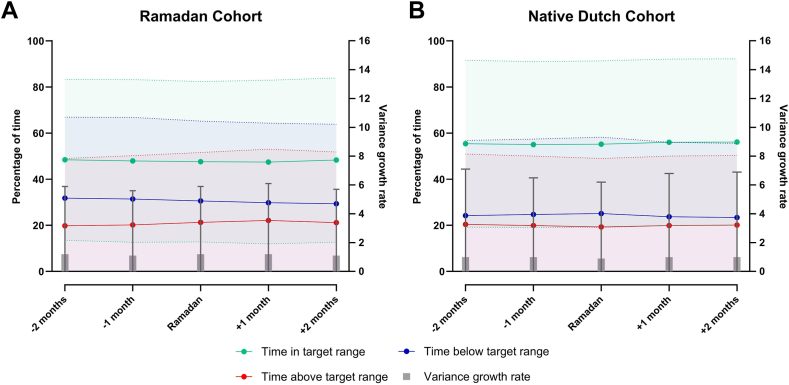
In the Ramadan cohort, TTR remained low but stable across Ramadan (Figure 3A), with a mean TTR during Ramadan of 47.6 ± 34.8 and the lowest TTR during the month after Ramadan (47.4 ± 35.5) (Table 2). The mean proportion of INRs within target range was 51.2% ± 41.0% during Ramadan, which was slightly lower compared with the other months (ranging from 51.5% to 53.0%), although not different than the first observation period (ie, month −2 prior to Ramadan; difference of −0.4% [95% CI, −2.3% to 1.5%]). Both during and after Ramadan (Ramadan month, month +1, and month +2), a higher mean proportion of INRs was above target range (18.9% ± 31.5%, 19.9% ± 32.7%, 19.7% ± 32.8%, respectively) compared with the months preceding Ramadan (month −2, 18.1% ± 30.9% and month −1, 17.8% ± 31.5%). Compared to the first observation period, the mean proportions of INRs above target were on average 0.8%, 1.8%, and 1.6% higher, respectively, during Ramadan and the next 2 months. INR variability remained similar across Ramadan, with a mean VGR of 1.2 ± 4.7 during Ramadan.
Figure 3.
Quality of vitamin K antagonist treatment across Ramadan. Quality of vitamin K antagonist treatment for each 30-day period during follow-up is expressed as percentage of time in, above, and below target range (TTR, TAR, and TBR) and by the variance growth rate, which is a measure for international normalized ratio variability. TTR, TAR, and TBR were calculated according to the Rosendaal method. For all metrics, mean and SD are displayed separately for the (A) Ramadan and (B) native Dutch cohorts.
Table 2.
Vitamin K antagonist treatment across Ramadan.
| Cohort |
Ramadan cohort |
Native Dutch cohort |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Observation period | −2 month | −1 month | Ramadan | +1 month | +2 month | -2 month | -1 month | Ramadan | +1 month | +2 month |
| Number of INR measurements | 3835d | 3718d | 3633d | 3531d | 3459d | 139,207d | 135,402d | 132,036d | 128,779d | 125,851d |
| Median (IQR) | 2.0 (1.0-3.0) | 1.0 (1.0-2.0) | 1.0 (1.0-2.0) | 1.0 (1.0-2.0) | 1.0 (1.0-2.0) | 1.0 (1.0-2.0) | 1.0 (1.0-2.0) | 1.0 (1.0-2.0) | 1.0 (1.0-2.0) | 1.0 (1.0-2.0) |
| Mean interval between INR measurement (d) | 3422d | 3051d | 2864d | 2674d | 2509d | 127,345d | 115,712d | 112,304d | 109,611d | 106,174d |
| Median (IQR) | 16.3 (10.5-24.5) | 16.0 (10.8-22.0) | 16.3 (10.5-22.5) | 16.3 (11.0-23.0) | 17.0 (11.3-23.5) | 18.0 (12.0-28.0) | 17.5 (12.0-28.0) | 17.5 (13.0-28.0) | 18.7 (13.7-28.0) | 19.0 (13.5-28.0) |
| Median INR value | 3427d | 3212d | 3009d | 2811d | 2684d | 127,410d | 122,520d | 119,490d | 116,501d | 112,972d |
| Median (IQR) | 2.60 (2.20-3.10) | 2.60 (2.20-3.10) | 2.60 (2.20-3.10) | 2.60 (2.20-3.20) | 2.65 (2.20-3.20) | 2.70 (2.30-3.10) | 2.60 (2.30-3.10) | 2.60 (2.30-3.05) | 2.65 (2.30-3.10) | 2.65 (2.30-3.10) |
| Mean ± SD | 2.71 ± 0.79 | 2.70 ± 0.74 | 2.71 ± 0.78 | 2.74 ± 0.79 | 2.74 ± 0.77 | 2.74 ± 0.68 | 2.72 ± 0.67 | 2.70 ± 0.66 | 2.73 ± 0.68 | 2.73 ± 0.68 |
| Fixed effect β (95% CI) | 0 (Reference) | −0.017 (−0.050 to 0.016) | −0.004 (−0.038 to 0.030) | 0.021 (−0.014 to 0.056) | 0.013 (−0.023 to 0.048) | 0 (Reference) | −0.019 (−0.024 to −0.015) | −0.035 (−0.040 to −0.030) | −0.011 (−0.015 to −0.006) | −0.005 (−0.010 to 0.000) |
| Median recommended average dosea | 3423d | 3208d | 3007d | 2807d | 2680d | 127,300d | 122,413d | 119,390d | 116,400d | 112,881d |
| Median (IQR) | 2.00 (1.29-2.91) | 2.00 (1.29-2.89) | 2.00 (1.28-2.90) | 2.00 (1.27-2.91) | 1.94 (1.27-2.86) | 1.78 (1.00-2.57) | 1.77 (1.01-2.57) | 1.75 (1.00-2.57) | 1.75 (1.00-2.57) | 1.75 (1.00-2.57) |
| Mean ± SD | 2.21 ± 1.30 | 2.22 ± 1.31 | 2.19 ± 1.29 | 2.20 ± 1.29 | 2.18 ± 1.28 | 1.93 ± 1.16 | 1.93 ± 1.16 | 1.92 ± 1.15 | 1.91 ± 1.15 | 1.90 ± 1.14 |
| Fixed effect β (95% CI) | 0 (Reference) | 0.001 (−0.008 to 0.011) | −0.012 (−0.021 to −0.002) | −0.021 (−0.031 to −0.011) | −0.024 (−0.034 to −0.014) | 0 (Reference) | −0.006 (−0.007 to −0.005) | −0.009 (−0.011 to −0.008) | −0.015 (−0.017 to −0.014) | −0.022 (−0.024 to −0.021) |
| ≥1 clinically relevant dose increaseb | 3417d | 3045d | 2855d | 2666d | 2501d | 127,143d | 115,440d | 112,066d | 109,384d | 105,952d |
| n (%) | 126 (3.7) | 94 (3.1) | 91 (3.2) | 73 (2.7) | 72 (2.9) | 4911 (3.9) | 4132 (3.6) | 3868 (3.5) | 3430 (3.1) | 3182 (3.0) |
| ≥1 clinically relevant dose reductionb | 3417d | 3045d | 2855d | 2666d | 2501d | 127,143d | 115,440d | 112,066d | 109,384d | 105,952d |
| n (%) | 146 (4.3) | 112 (3.7) | 135 (4.7) | 107 (4.0) | 90 (3.6) | 5766 (4.5) | 4811 (4.2) | 4485 (4.0) | 4378 (4.0) | 4137 (3.9) |
| INR variability (VGR) | 3422d | 3051d | 2864d | 2674d | 2509d | 127,345d | 115,712d | 112,304d | 109,611d | 106,174d |
| Mean ± SD | 1.2 ± 4.7 | 1.1 ± 4.5 | 1.2 ± 4.7 | 1.2 ± 4.9 | 1.1 ± 4.6 | 1.0 ± 6.1 | 1.0 ± 5.5 | 0.9 ± 5.3 | 1.0 ± 5.8 | 1.0 ± 5.9 |
| Fixed effect β (95% CI) | 0 (Reference) | −0.124 (−0.339 to 0.091) | −0.031 (−0.250 to 0.189) | 0.070 (−0.154 to 0.294) | −0.106 (−0.334 to 0.122) | 0 (Reference) | −0.056 (−0.099 to −0.013) | −0.117 (−0.160 to −0.073) | −0.038 (−0.082 to 0.005) | −0.012 (−0.056 to 0.032) |
| Proportion of INRs within target range, % | 3427d | 3212d | 3009d | 2811d | 2684d | 127,410d | 122,520d | 119,490d | 116,501d | 112,972d |
| Mean ± SD | 51.5 ± 40.9 | 53.0 ± 41.9 | 51.2 ± 41.0 | 51.9 ± 41.8 | 51.9 ± 42.0 | 59.4 ± 41.1 | 60.0 ± 41.5 | 60.3 ± 41.4 | 60.9 ± 41.3 | 60.9 ± 41.4 |
| Fixed effect β (95% CI) | 0 (Reference) | 1.449 (−0.434 to 3.332) | −0.419 (−2.337 to 1.500) | 0.295 (−1.662 to 2.252) | 0.289 (−1.695 to 2.274) | 0 (Reference) | 0.741 (0.438-1.043) | 1.098 (0.793-1.402) | 1.611 (1.305-1.918) | 1.642 (1.333-1.952) |
| Proportion of INRs below target range, % | 3427d | 3212d | 3009d | 2811d | 2684d | 127,410d | 122,520d | 119,490d | 116,501d | 112,972d |
| Mean ± SD | 30.4 ± 37.4 | 29.2 ± 38.0 | 29.9 ± 37.7 | 28.2 ± 37.4 | 28.4 ± 37.6 | 22.3 ± 34.4 | 22.4 ± 35.0 | 22.8 ± 35.3 | 21.6 ± 34.3 | 21.3 ± 34.3 |
| Fixed effect β (95% CI) | 0 (Reference) | −1.085 (−2.738 to 0.568) | −0.308 (−1.993 to 1.377) | −1.951 (−3.671 to −0.231) | −1.764 (−3.509 to −0.020) | 0 (Reference) | 0.038 (−0.206 to 0.283) | 0.426 (0.180-0.672) | −0.766 (−1.014 to −0.519) | −0.990 (−1.240 to −0.740) |
| Proportion of INRs above target range, % | 3427d | 3212d | 3009d | 2811d | 2684d | 127,410d | 122,520d | 119,490d | 116,501d | 112,972d |
| Mean ± SD | 18.1 ± 30.9 | 17.8 ± 31.5 | 18.9 ± 31.5 | 19.9 ± 32.7 | 19.7 ± 32.8 | 18.3 ± 31.8 | 17.6 ± 31.7 | 16.9 ± 31.0 | 17.6 ± 31.7 | 17.8 ± 31.8 |
| Fixed effect β (95% CI) | 0 (Reference) | −0.307 (−1.787 to 1.173) | 0.802 (−0.705 to 2.310) | 1.783 (0.247-3.320) | 1.586 (0.028-3.144) | 0 (Reference) | −0.772 (−1.008 to −0.536) | −1.512 (−1.750 to −1.275) | −0.823 (−1.062 to −0.584) | −0.623 (−0.864 to −0.382) |
| Proportion of INRs ≥5, % | 3427d | 3212d | 3009d | 2811d | 2684d | 127,410d | 122,522d | 119,493d | 116,504d | 112,978d |
| Mean ± SD | 2.7 ± 11.7 | 2.3 ± 10.6 | 2.7 ± 11.8 | 3.0 ± 12.2 | 2.6 ± 11.7 | 1.9 ± 9.5 | 1.8 ± 9.2 | 1.7 ± 9.0 | 1.8 ± 9.3 | 1.8 ± 9.3 |
| Proportion of INRs ≥8, % | 3427d | 3212d | 3009d | 2811d | 2684d | 127,410d | 122,522d | 119,493d | 116,504d | 112,978d |
| Mean ± SD | 0.3 ± 3.4 | 0.2 ± 2.7 | 0.2 ± 3.1 | 0.2 ± 3.4 | 0.1 ± 2.1 | 0.3 ± 3.3 | 0.2 ± 3.0 | 0.2 ± 3.0 | 0.2 ± 3.0 | 0.2 ± 3.2 |
| Time in target range, % | 3400d | 3041d | 2852d | 2653d | 2496d | 126,350d | 115,261d | 111,666d | 109,051d | 105,748d |
| Mean ± SD | 48.4 ± 34.9 | 47.9 ± 35.3 | 47.6 ± 34.8 | 47.4 ± 35.5 | 48.3 ± 35.6 | 55.4 ± 36.2 | 55.0 ± 36.0 | 55.2 ± 36.2 | 56.0 ± 36.1 | 56.1 ± 36.1 |
| Fixed effect β (95% CI) | 0 (Reference) | −0.370 (−1.958 to 1.217) | −0.669 (−2.292 to 0.954) | −0.977 (−2.635 to 0.681) | 0.065 (−1.624 to 1.755) | 0 (Reference) | −0.068 (−0.332 to 0.195) | 0.418 (0.151-0.685) | 1.076 (0.808-1.345) | 1.210 (0.939-1.481) |
| Time below target range, % | 3400d | 3041d | 2852d | 2653d | 2496d | 126,350d | 115,261d | 111,666d | 109,051d | 105,748d |
| Mean ± SD | 31.8 ± 35.1 | 31.4 ± 35.4 | 30.6 ± 34.6 | 29.8 ± 34.5 | 29.4 ± 34.4 | 24.2 ± 32.6 | 24.7 ± 32.7 | 25.1 ± 33.1 | 23.7 ± 32.3 | 23.4 ± 32.1 |
| Fixed effect β (95%CI) | 0 (Reference) | −0.404 (−1.943 to 1.135) | −1.245 (−2.820 to 0.330) | −1.842 (−3.451 to −0.232) | −2.264 (−3.904 to −0.624) | 0 (Reference) | 0.326 (0.096-0.555) | 0.564 (0.331-0.797) | −0.728 (−0.962 to −0.494) | −1.000 (−1.236 to −0.764) |
| Time above target range, % | 3400d | 3041d | 2852d | 2653d | 2496d | 126,350d | 115,261d | 111,666d | 109,051d | 105,748d |
| Mean ± SD | 19.8 ± 29.2 | 20.2 ± 30.0 | 21.3 ± 30.3 | 22.1 ± 30.9 | 21.2 ± 30.6 | 20.4 ± 30.5 | 19.9 ± 30.1 | 19.3 ± 29.7 | 19.9 ± 30.1 | 20.1 ± 30.2 |
| Fixed effect β (95% CI) | 0 (Reference) | 0.364 (−1.039 to 1.768) | 1.423 (−0.010 to 2.855) | 2.202 (0.739-3.665) | 1.259 (−0.231 to 2.749) | 0 (Reference) | −0.613 (−0.838 to −0.388) | −1.360 (−1.588 to −1.132) | −0.695 (−0.924 to −0.465) | −0.534 (−0.766 to −0.303) |
| Changed VKA type | 3416d | 3041d | 2851d | 2665d | 2496d | 127,096d | 115,343d | 111,978d | 109,265d | 105,835d |
| n (%) | <10c | <10c | <10c | <10c | <10c | 120 (0.1) | 141 (0.1) | 97 (0.1) | 95 (0.1) | 73 (0.1) |
| Switch to DOACs | 3835d | 3718d | 3633d | 3531d | 3459d | 139,207d | 135,402d | 132,036d | 128,779d | 125,851d |
| n (%) | 18 (0.5) | 12 (0.3) | 12 (0.3) | Maskedc | <10c | 438 (0.3) | 352 (0.3) | 337 (0.3) | 306 (0.2) | 258 (0.2) |
DOAC, direct oral anticoagulant; INR, international normalized ratio; VGR, variance growth rate; VKA, vitamin K antagonist.
Recommended average dose is expressed as number of tablets, where 1 tablet of acenocoumarol contains 1 mg and 1 tablet of phenprocoumon contains 3 mg.
A clinically relevant dose increase or reduction was defined as a change of ≥10% in the average daily VKA dose prescribed at 2 consecutive INR measurements.
Cells containing <10 individuals were masked according to Statistics Netherlands’ privacy policy. To prevent recalculation of cells <10, sometimes corresponding cells are additionally masked.
Number of individuals included in calculation for particular 30-day period.
Meanwhile, there was a higher proportion of clinically relevant dose reductions during Ramadan (4.7%) than the months before (3.7%-4.3%) and after Ramadan (3.6%-4.0%). In contrast, the proportion of clinically relevant dose increases was lower during the months after Ramadan (2.7%-2.9%) than the pre- and Ramadan period (3.1%-3.7%). However, the median recommended average VKA dose did not differ across Ramadan. The frequency of INR monitoring was also similar across the different periods with a median of 1 measurement per month (IQR, 1-2) and a median of approximately 16 days between consecutive INR measurements.
In comparison, VKA treatment quality metrics in the negative control native Dutch cohort remained similar across Ramadan, including time and percentage of INRs within target range, and VGR (Figure 3B and Table 2). The mean TTR during the Ramadan month was 55.2 ± 36.2 and on average 60.3% ± 41.4% of INRs were within target range.
3.3. Clinical events across Ramadan
A low number of bleeding events was observed among the Ramadan cohort during the 5-month study period. The 30-day cumulative incidence of major and clinically relevant bleeding during Ramadan was 2.63 (95% CI, 1.37-4.74) per 1000 (Table 3). For the composite outcome of bleeding, venous and arterial thromboembolic events, the 30-day cumulative incidence per 1000 was numerically the highest during Ramadan, at 5.26 (95% CI, 3.34-8.01), compared to the 2 preceding months (4.95; 95% CI, 3.10-7.62 and 3.67; 95% CI, 2.12-6.05, respectively) or the 2 months after Ramadan (3.70; 95% CI, 2.14-6.10 and 3.72; 95% CI, 2.15-6.13, respectively), although with overlapping CIs (Figure 4A). A consistent pattern was observed when evaluated by incidence rates (Table 3). Cumulative 30-day all-cause mortality was similar across the 5 observation periods, ranging from 0.34 to 0.69 per 1000.
Table 3.
Incidence of clinical outcomes across Ramadan.
| Cohort |
Ramadan cohort |
Native Dutch cohort |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Observation period | −2 month | −1 month | Ramadan | +1 month | +2 month | -2 month | -1 month | Ramadan | +1 month | +2 month |
| No. at risk | 3835 | 3813 | 3800 | 3779 | 3760 | 139,207 | 138,079 | 136,940 | 135,785 | 134,651 |
| Major/clinically relevant bleeding | ||||||||||
| No. of events | <10a | <10a | 10 | <10a | <10a | 273 | 266 | 235 | 264 | 235 |
| IR (95% CI), per 100 PYs | Maskeda | Maskeda | 3.22 (1.54-5.92) | Maskeda | Maskeda | 2.40 (2.12-2.70) | 2.36 (2.08-2.66) | 2.10 (1.84-2.39) | 2.38 (2.10-2.68) | 2.13 (1.87-2.43) |
| 30-d cumulative incidence, per 1000 (95% CI) | Maskeda | Maskeda | 2.63 (1.37-4.74) | Maskeda | Maskeda | 1.96 (1.74-2.21) | 1.93 (1.71-2.17) | 1.72 (1.51-1.95) | 1.94 (1.72-2.19) | 1.75 (1.53-1.98) |
| Ischemic stroke/TIA/myocardial infarction/arterial thromboembolism | ||||||||||
| No. of events | 12 | 11 | <10a | <10a | 12 | 324 | 337 | 338 | 299 | 284 |
| IR (95% CI), per 100 PYs | 3.82 (1.98-6.68) | 3.52 (1.76-6.31) | Maskeda | Maskeda | 3.90 (2.02-6.82) | 2.85 (2.55-3.17) | 2.99 (2.68-3.32) | 3.02 (2.71-3.36) | 2.69 (2.40-3.02) | 2.58 (2.29-2.90) |
| 30-d cumulative incidence, per 1000 (95% CI) | 3.13 (1.73-5.36) | 2.88 (1.55-5.06) | Maskeda | Maskeda | 3.19 (1.76-5.47) | 2.33 (2.09-2.59) | 2.44 (2.19-2.71) | 2.47 (2.22-2.74) | 2.20 (1.96-2.46) | 2.11 (1.88-2.37) |
| Composite of bleeding, venous and arterial thromboembolism | ||||||||||
| No. of events | 19 | 14 | 20 | 14 | 14 | 621 | 624 | 597 | 591 | 553 |
| IR (95% CI), per 100 PYs | 6.06 (3.65-9.46) | 4.49 (2.45-7.53) | 6.44 (3.93-9.94) | 4.53 (2.47-7.60) | 4.56 (2.49-7.64) | 5.46 (5.04-5.91) | 5.53 (5.11-5.99) | 5.34 (4.92-5.78) | 5.33 (4.91-5.78) | 5.03 (4.62-5.47) |
| 30-d cumulative incidence per 1000 (95%CI) | 4.95 (3.10-7.62) | 3.67 (2.12-6.05) | 5.26 (3.34-8.01) | 3.70 (2.14-6.10) | 3.72 (2.15-6.13) | 4.46 (4.12-4.82) | 4.52 (4.18-4.88) | 4.36 (4.02-4.72) | 4.35 (4.01-4.71) | 4.11 (3.78-4.46) |
| All-cause mortality | ||||||||||
| No. of events | 22 | 13 | 21 | 19 | 26 | 1,128 | 1,139 | 1,155 | 1,134 | 1,117 |
| IR (95% CI), per 100 PYs | 7.00 (4.39-10.60) | 4.16 (2.21-7.11) | 6.75 (4.18-10.31) | 6.13 (3.69-9.58) | 8.45 (5.52-12.38) | 9.90 (9.33-10.50) | 10.08 (9.51-10.69) | 10.31 (9.72-10.92) | 10.21 (9.62-10.82) | 10.14 (9.56-10.75) |
| 30-d cumulative incidence, per 1000 (95%CI) | 0.57 (0.33-0.81) | 0.34 (0.16-0.53) | 0.55 (0.32-0.79) | 0.50 (0.28-0.73) | 0.69 (0.43-0.96) | 0.81 (0.76-0.86) | 0.82 (0.78-0.87) | 0.84 (0.79-0.89) | 0.84 (0.79-0.88) | 0.83 (0.78-0.88) |
IR, incidence rate; PYs, person-years; TIA, transient ischemic attack.
Cells containing <10 individuals are masked according to Statistics Netherlands’ privacy policy. To prevent recalculation of cells <10, sometimes corresponding cells are additionally masked.
Figure 4.
30-day cumulative incidence of clinical outcomes across Ramadan. 30-day cumulative incidences for both (A) the composite of bleeding and thromboembolic events and (B) all-cause mortality with corresponding 95% CIs are depicted separately for the native Dutch (orange) and Ramadan (blue) cohorts. The 5 observation periods are displayed as: month −2 prior to Ramadan, month −1 prior to Ramadan, Ramadan month, month +1 after Ramadan, and month +2 after Ramadan. Thromboembolic events included venous thromboembolism, ischemic stroke, transient ischemic attack, myocardial infarction, and other arterial thromboembolism.
In line with this result, the risk of adverse clinical events remained stable across Ramadan in the native Dutch cohort, with 30-day cumulative incidences ranging from 4.11 to 4.52 per 1000 for the composite of bleeding and venous and arterial thromboembolism, and from 0.81 to 0.84 per 1000 for all-cause mortality (Figure 4B).
3.4. Subgroup analysis: Moroccan cohort
The subgroup analysis restricted to VKA users in the Ramadan cohort with a Moroccan background (n = 1492) also demonstrated a higher proportion of clinically relevant dose reductions during the Ramadan month (4.3%) than the other months (range, 2.7%-3.6%), as well as a lower proportion of clinically relevant dose increases (1.6% during Ramadan vs 1.9%-3.8% in other months; Supplementary Table 5). In addition, the mean proportion of supratherapeutic INRs was numerically the highest during Ramadan (20.4% ± 33.0%), with a difference of 0.8% (95% CI, −1.6% to 3.2%) compared with the first observation period. A similar pattern was observed for the percentage of time spent above the target range. TTRs in the 5 observation periods were generally low, with a mean of 46.6 ± 34.8 during the Ramadan month. INR variability remained similar across Ramadan, with a mean of 1.3 ± 6.1 during Ramadan.
4. Discussion
Our cohort study examined the quality of VKA treatment and clinical outcomes across Ramadan at a population level in the Netherlands. In the Ramadan cohort, no apparent differences were observed in TTR across Ramadan. The percentage of INRs above target range was slightly higher during and after Ramadan than before Ramadan. Notably, a higher percentage of VKA users received a clinically relevant dose reduction during Ramadan than in the consecutive months, while a lower percentage received a relevant dose increase after Ramadan. INR variability remained stable over the 5-month follow-up. We observed similar incidences of clinical outcomes, including all-cause mortality, bleeding, and thromboembolic events, across Ramadan.
As survey data from The Netherlands Institute for Social Research indicated higher participation in Ramadan fasting among Dutch Moroccan Muslims compared to other Muslim groups in the Netherlands [58], we performed a subgroup analysis among VKA users with a Moroccan background, the results of which were similar to our primary analysis. We also studied a negative control native Dutch cohort, and as expected, no apparent changes were observed in either VKA treatment quality or risk of adverse clinical events across Ramadan.
Overall, we observed only minor Ramadan-related changes in the quality of VKA treatment. A possible explanation could be that anticoagulation clinics anticipate possible Ramadan-related changes in vitamin K and tablet intake when monitoring patients who participate in Ramadan fasting. In addition, in this patient group, physicians may be sooner inclined to perform dose adjustments upon a slight INR increase. In line with this hypothesis, we observed that a higher proportion of VKA users received a clinically relevant dose reduction during the month of Ramadan than in consecutive months. In addition, no such time-related pattern was observed across Ramadan in the native Dutch cohort, where the percentage of VKA users who received a clinically relevant dose reduction was also slightly lower compared to the Ramadan cohort.
However, possible changes over time unrelated to Ramadan could have contributed to our observations, such as seasonal variability in VKA stability. For example, respiratory tract infections, with or without antibiotic exposure, and fever are associated with increased risk of supratherapeutic INRs [65,66]. To account for possible time-related factors possibly affecting our study outcomes, we additionally identified a native Dutch cohort. As most time-related changes observed in the Ramadan cohort, such as changes in proportions of relevant dose adjustments and proportions of INRs within or above target range, were not observed in the negative control native Dutch cohort, this argues against a major impact of time-related factors.
Our study contributes to the existing literature on the quality of VKA treatment during Ramadan. A study among Muslim warfarin users who were clinically assessed to be fit to fast (n = 30) performed weekly INR measurements during a 3-month study period (ie, before, during, and after Ramadan), the results of which were masked to patients and their physicians [35]. The mean INR increased during Ramadan compared with the month before Ramadan and returned to the pre-Ramadan level thereafter [35]. Changes were also observed in the TTR, which was lower during and after Ramadan compared with the month preceding Ramadan, with a substantial increase in TAR during the Ramadan month and TBR during the post-Ramadan month [35]. This study was not powered to assess possible effects of Ramadan fasting on clinical outcomes. Other studies also reported more frequent supratherapeutic INRs during the Ramadan and post-Ramadan month [41] and an increase in the mean INR and lower TTR during and after Ramadan compared with before Ramadan [40].
Our study is strengthened by its design, comparing the study outcomes before, during, and after Ramadan in the same cohort of VKA users who were likely to fast during Ramadan, thereby limiting bias due to (time-fixed) confounding. The native Dutch cohort served as a negative control, examining possible changes over time unrelated to Ramadan. However, several limitations need to be taken into account when interpreting our study findings. First, we identified a cohort of VKA users who were likely to fast during Ramadan based on the proxy of immigration background combined with country of origin because no individual-level data on religious practice or fasting behavior were available. Specifically, we defined this as being born in (or having parents from) a country where ≥85% of the population identifies as Muslim. We acknowledge that this approach carries a risk of cultural stereotyping by inferring religious practice from family origin.
Moreover, our pragmatic proxy for potential fasting could have led to misclassification in both the Ramadan cohort and the native Dutch cohort, ie, patients in the Ramadan cohort might not have fasted during Ramadan and vice versa. In addition, some VKA users might be exempted from fasting because of their health status and therefore might not have fasted during Ramadan. As this misclassification would primarily dilute the results, the observed Ramadan-related changes would present an underestimation of the true effect. A qualitative study performed among Muslims with diabetes in the Netherlands of Moroccan or Turkish descent explored factors involved in the decision-making process about whether to fast during Ramadan [67]. Many participants indicated that deciding to refrain from fasting is difficult, can lead to feelings of guilt and shame, and that there is uncertainty about whether conditions are severe enough to be exempted from fasting [67]. Furthermore, studies among Muslims with diabetes indicate that fasting against advice from healthcare professionals may occur [68,69]. Although some VKA users might be exempted from fasting, changes in diet, timing of food and oral medication intake, sleep pattern, and physical activity may still occur during Ramadan, especially when surrounded by fasting relatives [67]. In addition, we expect the amount of misclassification in the native Dutch cohort to be minimal, as only a very low proportion of native Dutch inhabitants (estimated at about 0.2%) consider themselves Muslim [58].
Second, clinical events that did not result in a hospital admission and were diagnosed in primary care, such as deep vein thrombosis, TIA, or bleeding events, were missing, which might have led to an underestimation of the true event rates. However, as we observed the cohorts both before, during, and after Ramadan, this misclassification may not have affected comparisons over time.
The increase in the average percentage of supratherapeutic INRs during and after Ramadan we observed is in line with previous studies, possibly attributable to a decrease in dietary vitamin K intake and changes in timing of VKA intake, and might possibly place patients at increased risk of bleeding. Although we did not observe changes in incidences of adverse clinical outcomes across Ramadan, these estimates likely present an underestimation. Nevertheless, clinical guidelines or recommendations specifically addressing VKA management during Ramadan fasting are lacking, whereas these type of guidelines are available for diabetes [70], chronic kidney disease [71], and for broader cardiovascular disease management [33]. A survey conducted in 7 countries reported that patients with diabetes who received Ramadan-specific diabetes education before Ramadan better adhered to Ramadan-specific diabetes management recommendations than patients who did not receive such education, such as altering drug dosage and timing before Ramadan, blood glucose monitoring during fasting, and breaking the fast if symptoms of hypo- or hyperglycemia develop [72].
In conclusion, in a cohort of VKA users who were likely to fast during Ramadan in the Netherlands, both VKA treatment quality and risks of adverse clinical events remained largely unchanged across Ramadan, except for a slightly higher proportion of supratherapeutic INRs during Ramadan together with more frequent dose reductions. These findings could serve to increase awareness for possible Ramadan-related changes in patients treated with VKAs. Healthcare professionals involved may more frequently ask VKA users whether they plan to participate in Ramadan fasting, consider more intensive INR monitoring, or anticipate possible Ramadan-related changes in VKA dosing. Future research should prioritize collecting self-reported fasting status to improve the accuracy of exposure assessment and better capture religious and personal heterogeneity.
Acknowledgments
The authors thank the Federation of Dutch Anticoagulation Clinics and Statistics Netherlands for making the data available.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
E.K.K., Q.C., N.v.R., S.C.C., and M.J.H.A.K. designed the study. R.K.S., N.M.W., C.E.A.D., M.B., A.J.t.C.-H., M.C.N., A.D.M.S., P.M., and F.A.K. critically revised the research protocol. Q.C. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. R.K.S., N.M.W., C.E.A.D., M.B., A.J.t.C.-H., M.C.N., A.D.M.S., P.M., and F.A.K. were responsible for obtaining the data from participating anticoagulation clinics. E.K.K. drafted the initial version of the manuscript. All authors contributed to the interpretation of the data, critically revised the manuscript and approved the final version.
Relationship disclosure
A.D.M.S. has received speaker and consultancy fees from Amaryn, Astra, Boehringer-Ingelheim, NovoNordisk, Sanofi, and Viatris. F.A.K. has received research support from Bayer, BMS, BSCI, AstraZeneca, Angiodynamics, MSD, Leo Pharma, Actelion, Farm-X, The Netherlands Organisation for Health Research and Development, the Dutch Thrombosis Foundation, the Dutch Heart Foundation, and the Horizon Europe Program, all outside this project and paid to his institution. M.J.H.A.K. has received research support from The Netherlands Organisation for Health Research and Development, the Dutch Thrombosis Foundation, and the Horizon Europe Program, and speaker’s fees from Roche, all outside this project and paid to her institution. All other authors have nothing to declare.
Data availability
This study used nonpublic microdata from Statistics Netherlands and the Federation of Dutch Anticoagulation Clinics. Under certain conditions, these data are accessible for statistical and scientific research. For additional information: microdata@cbs.nl and/or fnt@fnt.nl
Footnotes
Handling Editor: Professor Michael Makris
Eva K. Kempers and Qingui Chen contributed equally to this work.
The online version contains supplementary material available at https://doi.org/10.1016/j.rpth.2025.103010
Supplementary material
References
- 1.Van Gelder I.C., Rienstra M., Bunting K.V., Casado-Arroyo R., Caso V., Crijns H.J.G.M., et al. ESC Scientific Document Group. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2024;45:3314–3414. doi: 10.1093/eurheartj/ehae176. [DOI] [PubMed] [Google Scholar]
- 2.Joglar J.A., Chung M.K., Armbruster A.L., Benjamin E.J., Chyou J.Y., Cronin E.M., et al. Peer Review Committee Members. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2024;149:e1–e156. doi: 10.1161/CIR.0000000000001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ortel T.L., Neumann I., Ageno W., Beyth R., Clark N.P., Cuker A., et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020;4:4693–4738. doi: 10.1182/bloodadvances.2020001830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eikelboom J.W., Connolly S.J., Brueckmann M., Granger C.B., Kappetein A.P., et al. RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206–1214. doi: 10.1056/NEJMoa1300615. [DOI] [PubMed] [Google Scholar]
- 5.Wang T.Y., Svensson L.G., Wen J., Vekstein A., Gerdisch M., Rao V.U., et al. Apixaban or warfarin in patients with an On-X mechanical aortic valve. NEJM Evid. 2023;2 doi: 10.1056/EVIDoa2300067. [DOI] [PubMed] [Google Scholar]
- 6.Connolly S.J., Karthikeyan G., Ntsekhe M., Haileamlak A., El Sayed A., El Ghamrawy A., et al. INVICTUS Investigators. Rivaroxaban in rheumatic heart disease-associated atrial fibrillation. N Engl J Med. 2022;387:978–988. doi: 10.1056/NEJMoa2209051. [DOI] [PubMed] [Google Scholar]
- 7.Otto C.M., Nishimura R.A., Bonow R.O., Carabello B.A., Erwin J.P., 3rd, Gentile F., et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e72–e227. doi: 10.1161/CIR.0000000000000923. [DOI] [PubMed] [Google Scholar]
- 8.Vahanian A., Beyersdorf F., Praz F., Milojevic M., Baldus S., Bauersachs J., et al. ESC/EACTS Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561–632. doi: 10.1093/eurheartj/ehab395. [DOI] [PubMed] [Google Scholar]
- 9.Bejjani A., Khairani C.D., Assi A., Piazza G., Sadeghipour P., Talasaz A.H., et al. When direct oral anticoagulants should not be standard treatment: JACC state-of-the-art review. J Am Coll Cardiol. 2024;83:444–465. doi: 10.1016/j.jacc.2023.10.038. [DOI] [PubMed] [Google Scholar]
- 10.Khairani C.D., Bejjani A., Piazza G., Jimenez D., Monreal M., Chatterjee S., et al. Direct oral anticoagulants vs vitamin K antagonists in patients with antiphospholipid syndromes: meta-analysis of randomized trials. J Am Coll Cardiol. 2023;81:16–30. doi: 10.1016/j.jacc.2022.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tektonidou M.G., Andreoli L., Limper M., Amoura Z., Cervera R., Costedoat-Chalumeau N., et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. 2019;78:1296–1304. doi: 10.1136/annrheumdis-2019-215213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joosten L.P.T., van Doorn S., van de Ven P.M., Köhlen B.T.G., Nierman M.C., Koek H.L., et al. Safety of switching from a vitamin K antagonist to a non-vitamin K antagonist oral anticoagulant in frail older patients with atrial fibrillation: results of the FRAIL-AF randomized controlled trial. Circulation. 2024;149:279–289. doi: 10.1161/CIRCULATIONAHA.123.066485. [DOI] [PubMed] [Google Scholar]
- 13.Bayer V., Kotalczyk A., Kea B., Teutsch C., Larsen P., Button D., et al. Global oral anticoagulation use varies by region in patients with recent diagnosis of atrial fibrillation: the GLORIA-AF phase III registry. J Am Heart Assoc. 2022;11 doi: 10.1161/JAHA.121.023907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ageno W., Caramelli B., Donadini M.P., Girardi L., Riva N. Changes in the landscape of anticoagulation: a focus on direct oral anticoagulants. Lancet Haematol. 2024;11:e938–e950. doi: 10.1016/S2352-3026(24)00281-3. [DOI] [PubMed] [Google Scholar]
- 15.Ansell J., Hirsh J., Hylek E., Jacobson A., Crowther M., Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2008;133:160S–198S. doi: 10.1378/chest.08-0670. [DOI] [PubMed] [Google Scholar]
- 16.De Caterina R., Husted S., Wallentin L., Andreotti F., Arnesen H., Bachmann F., et al. Vitamin K antagonists in heart disease: current status and perspectives (Section III). Position paper of the ESC Working Group on Thrombosis--Task Force on Anticoagulants in Heart Disease. Thromb Haemost. 2013;110:1087–1107. doi: 10.1160/TH13-06-0443. [DOI] [PubMed] [Google Scholar]
- 17.Juurlink D.N. Drug interactions with warfarin: what clinicians need to know. CMAJ. 2007;177:369–371. doi: 10.1503/cmaj.070946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hylek E.M., Go A.S., Chang Y., Jensvold N.G., Henault L.E., Selby J.V., et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med. 2003;349:1019–1026. doi: 10.1056/NEJMoa022913. [DOI] [PubMed] [Google Scholar]
- 19.Schein J.R., White C.M., Nelson W.W., Kluger J., Mearns E.S., Coleman C.I. Vitamin K antagonist use: evidence of the difficulty of achieving and maintaining target INR range and subsequent consequences. Thromb J. 2016;14:14. doi: 10.1186/s12959-016-0088-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hylek E.M., Skates S.J., Sheehan M.A., Singer D.E. An analysis of the lowest effective intensity of prophylactic anticoagulation for patients with nonrheumatic atrial fibrillation. N Engl J Med. 1996;335:540–546. doi: 10.1056/NEJM199608223350802. [DOI] [PubMed] [Google Scholar]
- 21.Lurie Y., Loebstein R., Kurnik D., Almog S., Halkin H. Warfarin and vitamin K intake in the era of pharmacogenetics. Br J Clin Pharmacol. 2010;70:164–170. doi: 10.1111/j.1365-2125.2010.03672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan T., Wynne H., Wood P., Torrance A., Hankey C., Avery P., et al. Dietary vitamin K influences intra-individual variability in anticoagulant response to warfarin. Br J Haematol. 2004;124:348–354. doi: 10.1046/j.1365-2141.2003.04787.x. [DOI] [PubMed] [Google Scholar]
- 23.Cushman M., Booth S.L., Possidente C.J., Davidson K.W., Sadowski J.A., Bovill E.G. The association of vitamin K status with warfarin sensitivity at the onset of treatment. Br J Haematol. 2001;112:572–577. doi: 10.1046/j.1365-2141.2001.02635.x. [DOI] [PubMed] [Google Scholar]
- 24.Lubetsky A., Dekel-Stern E., Chetrit A., Lubin F., Halkin H. Vitamin K intake and sensitivity to warfarin in patients consuming regular diets. Thromb Haemost. 1999;81:396–399. [PubMed] [Google Scholar]
- 25.de Assis M.C., Rabelo E.R., Avila C.W., Polanczyk C.A., Rohde L.E. Improved oral anticoagulation after a dietary vitamin K-guided strategy: a randomized controlled trial. Circulation. 2009;120:1115–1122. doi: 10.1161/CIRCULATIONAHA.109.849208. [DOI] [PubMed] [Google Scholar]
- 26.Franco V., Polanczyk C.A., Clausell N., Rohde L.E. Role of dietary vitamin K intake in chronic oral anticoagulation: prospective evidence from observational and randomized protocols. Am J Med. 2004;116:651–656. doi: 10.1016/j.amjmed.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 27.Couris R., Tataronis G., McCloskey W., Oertel L., Dallal G., Dwyer J., et al. Dietary vitamin K variability affects International Normalized Ratio (INR) coagulation indices. Int J Vitam Nutr Res. 2006;76:65–74. doi: 10.1024/0300-9831.76.2.65. [DOI] [PubMed] [Google Scholar]
- 28.Grindrod K., Alsabbagh W. Managing medications during Ramadan fasting. Can Pharm J (Ott) 2017;150:146–149. doi: 10.1177/1715163517700840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beshyah S. Fasting during the month of ramadan for people with diabetes: Medicine and fiqh united at last. Ibnosina J Med Biomed Sci. 2009;01:58–60. [Google Scholar]
- 30.Ghouri N., Hussain S., Mohammed R., Beshyah S.A., Chowdhury T.A., Sattar N., et al. Diabetes, driving and fasting during Ramadan: the interplay between secular and religious law. BMJ Open Diabetes Res Care. 2018;6 doi: 10.1136/bmjdrc-2018-000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.al-Kalbi I. 2nd ed. Dar al-Kitab al-Arabi; Beirut: 1989. al-Qawanin al-Fiqhiyyah. [Google Scholar]
- 32.Az-Zarqa M. 3rd ed. Dar al-Qalam; Damascus: 1993. Sharh Al-Qawa‘id Al-Fiqhiyyah; pp. p33–p34. [Google Scholar]
- 33.Akhtar A.M., Ghouri N., Chahal C.A.A., Patel R., Ricci F., Sattar N., et al. Ramadan fasting: recommendations for patients with cardiovascular disease. Heart. 2022;108:258–265. doi: 10.1136/heartjnl-2021-319273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Batarfi A., Alenezi H., Alshehri A., Balelah S., Kazim H., Algthami M., et al. Patient-guided modifications of oral anticoagulant drug intake during Ramadan fasting: a multicenter cross-sectional study. J Thromb Thrombolysis. 2021;51:485–493. doi: 10.1007/s11239-020-02218-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai Y.F., Cheen M.H., Lim S.H., Yeo F.H., Nah S.C., Kong M.C., et al. The effects of fasting in Muslim patients taking warfarin. J Thromb Haemost. 2014;12:349–354. doi: 10.1111/jth.12496. [DOI] [PubMed] [Google Scholar]
- 36.Addad F., Amami M., Ibn Elhadj Z., Chakroun T., Marrakchi S., Kachboura S. Does Ramadan fasting affect the intensity of acenocoumarol-induced anticoagulant effect? Br J Haematol. 2014;166:792–794. doi: 10.1111/bjh.12897. [DOI] [PubMed] [Google Scholar]
- 37.Awiwi M.O., Yagli Z.A., Elbir F., Aglar A.A., Guler E., Vural U. The effects of Ramadan fasting on patients with prosthetic heart valve taking warfarin for anticoagulation. J Saudi Heart Assoc. 2017;29:1–6. doi: 10.1016/j.jsha.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yildirim E., Secen O., Uku O., Nail Bilen M., Kutlu Karadag M. Is fasting for Ramadan safe in patients with mechanical cardiac valves? J Heart Valve Dis. 2017;26:200–204. [PubMed] [Google Scholar]
- 39.Mzoughi K., Zairi I., Fennira S., Kamoun S., Jnifene Z., Ben Moussa F., et al. Effect of Ramadan fasting on acenocoumarol-induced antocoagulant effect. Ann Biol Clin (Paris) 2017;75:513–518. doi: 10.1684/abc.2017.1264. [DOI] [PubMed] [Google Scholar]
- 40.Zghal Mghaieth F., Bonkano A., Boudiche S., Ayari J., Ben Mansour N., Rekik B., et al. Oral anticoagulation therapy using acenocoumarol during the month of Ramadan: a comparative study between fasting and non-fasting patients. Tunis Med. 2019;97:1177–1186. [PubMed] [Google Scholar]
- 41.Sridharan K., Al Banna R., Qader A.M., Husain A. Does fasting during Ramadan influence the therapeutic effect of warfarin? J Clin Pharm Ther. 2021;46:86–92. doi: 10.1111/jcpt.13254. [DOI] [PubMed] [Google Scholar]
- 42.Alwhaibi A., Alenazi M., Alwagh F., Al-Ghayhab A., Alghadeer S., Bablghaith S., et al. Does Ramadan fasting disrupt international normalised ratio control in warfarin-treated medically stable patients? Int J Clin Pract. 2021;75 doi: 10.1111/ijcp.14796. [DOI] [PubMed] [Google Scholar]
- 43.Rabea E.M., Abbas K.S., Awad D.M., Elgoweini N.H., El-Sakka A.A., Mahmoud N.H., et al. Does Ramadan fasting affect the therapeutic and clinical outcomes of warfarin? a systematic review and meta-analysis. Eur J Clin Pharmacol. 2022;78:755–763. doi: 10.1007/s00228-022-03281-7. [DOI] [PubMed] [Google Scholar]
- 44.Breukink-Engbers W.G. Monitoring therapy with anticoagulants in The Netherlands. Semin Thromb Hemost. 1999;25:37–42. doi: 10.1055/s-2007-996422. [DOI] [PubMed] [Google Scholar]
- 45.Toorop M.M.A., Chen Q., Kruip M.J.H.A., van der Meer F.J.M., Nierman M.C., Faber L., et al. Switching from vitamin K antagonists to direct oral anticoagulants in non-valvular atrial fibrillation patients: does low time in therapeutic range affect persistence? J Thromb Haemost. 2022;20:339–352. doi: 10.1111/jth.15592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Centraal Bureau voor Statistiek Persoonskenmerken van alle in de Gemeentelijke Basis Administratie (GBA) ingeschreven personen, gecoördineerd, ODISSEI Portal, V1. 2021. [DOI]
- 47.Centraal Bureau voor Statistiek Diagnosen behorend bij ziekenhuisopnamen Landelijke Basisregistratie Ziekenhuiszorg, ODISSEI Portal, V1. 2019. [DOI]
- 48.Centraal Bureau voor Statistiek Ziekenhuisopnamen Landelijke Basisregistratie Ziekenhuiszorg, ODISSEI Portal, V1. 2019. [DOI]
- 49.Centraal Bureau voor Statistiek Ziekenhuisopnamen voor RA-gebruik, ODISSEI Portal, V1. 2012. [DOI]
- 50.Centraal Bureau voor Statistiek Diagnosen behorend bij ziekenhuisopnamen voor RA-gebruik, ODISSEI Portal, V1. 2012. [DOI]
- 51.Centraal Bureau voor Statistiek Verstrekkingen van geneesmiddelen op 4 posities ATC-code aan personen, ODISSEI Portal, V1. 2020. [DOI]
- 52.Centraal Bureau voor Statistiek Doodsoorzaken van personen die bij overlijden inwoners waren van Nederland, ODISSEI Portal, V1. 2013. [DOI]
- 53.Centraal Bureau voor Statistiek Datum van overlijden van personen die ingeschreven staan in de Gemeentelijke Basisadministratie (GBA), ODISSEI Portal, V1. 2018. [DOI]
- 54.Centraal Bureau voor Statistiek Religieuze betrokkenheid; persoonskenmerken. 2025. https://opendata.cbs.nl/statline/#/CBS/nl/dataset/82904NED/table?ts=1748442682751
- 55.Statistics Netherlands The Netherlands in numbers. 2024. https://longreads.cbs.nl/the-netherlands-in-numbers-2024/what-are-the-major-religions/
- 56.Rosendaal F.R., Cannegieter S.C., van der Meer F.J., Briët E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236–239. [PubMed] [Google Scholar]
- 57.van Leeuwen Y., Rosendaal F.R., Cannegieter S.C. Prediction of hemorrhagic and thrombotic events in patients with mechanical heart valve prostheses treated with oral anticoagulants. J Thromb Haemost. 2008;6:451–456. doi: 10.1111/j.1538-7836.2007.02874.x. [DOI] [PubMed] [Google Scholar]
- 58.Huijnk W. De religieuze beleving van moslims in Nederland. 2018. https://www.scp.nl/publicaties/publicaties/2018/06/07/de-religieuze-beleving-van-moslims-in-nederland
- 59.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2024. R: A language and environment for statistical computing. [Google Scholar]
- 60.Wickham H., François R., Henry L., Müller K., Vaughan D. dplyr: A Grammar of Data Manipulation. 2023. https://CRAN.R-project.org/package=dplyr R package version 1.1.4.
- 61.Yoshida K., Bartel A. tableone: Create 'Table 1' to Describe Baseline Characteristics with or without Propensity Score Weights. 2022. https://cran.r-project.org/package=tableone R package version 0.13.2.
- 62.Therneau T. A Package for Survival Analysis in R. 2024. https://CRAN.R-project.org/package=survival R package version 3.6-4.
- 63.Gray B. cmprsk: Subdistribution Analysis of Competing Risks. 2024. https://cran.r-project.org/package=cmprsk R package version 2.2-12.
- 64.Bates D., Mächler M., Bolker B., Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 65.Clark N.P., Delate T., Riggs C.S., Witt D.M., Hylek E.M., Garcia D.A., et al. Warfarin-Associated Research Projects and Other Endeavors Consortium. Warfarin interactions with antibiotics in the ambulatory care setting. JAMA Intern Med. 2014;174:409–416. doi: 10.1001/jamainternmed.2013.13957. [DOI] [PubMed] [Google Scholar]
- 66.Self T.H., Oliphant C.S., Reaves A.B., Richardson A.M., Sands C.W. Fever as a risk factor for increased response to vitamin K antagonists: a review of the evidence and potential mechanisms. Thromb Res. 2015;135:5–8. doi: 10.1016/j.thromres.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 67.Bouchareb S., Chrifou R., Bourik Z., Nijpels G., Hassanein M., Westerman M.J., et al. “I am my own doctor”: a qualitative study of the perspectives and decision-making process of Muslims with diabetes on Ramadan fasting. PLoS One. 2022;17 doi: 10.1371/journal.pone.0263088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Babineaux S.M., Toaima D., Boye K.S., Zagar A., Tahbaz A., Jabbar A., et al. Multi-country retrospective observational study of the management and outcomes of patients with Type 2 diabetes during Ramadan in 2010 (CREED) Diabet Med. 2015;32:819–828. doi: 10.1111/dme.12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peeters B., Mehuys E., Van Tongelen I., Van Bever E., Bultereys L., Avonts D., et al. Ramadan fasting and diabetes: an observational study among Turkish migrants in Belgium. Prim Care Diabetes. 2012;6:293–296. doi: 10.1016/j.pcd.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 70.Hassanein M., Afandi B., Yakoob Ahmedani M., Mohammad Alamoudi R., Alawadi F., Bajaj H.S., et al. Diabetes and Ramadan: practical guidelines 2021. Diabetes Res Clin Pract. 2022;185:109185. doi: 10.1016/j.diabres.2021.109185. [DOI] [PubMed] [Google Scholar]
- 71.Malik S., Bhanji A., Abuleiss H., Hamer R., Shah S.H., Rashad R., et al. Effects of fasting on patients with chronic kidney disease during Ramadan and practical guidance for healthcare professionals. Clin Kidney J. 2021;14:1524–1534. doi: 10.1093/ckj/sfab032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ahmedani M.Y., Alvi S.F. Characteristics and Ramadan-specific diabetes education trends of patients with diabetes (CARE): a multinational survey (2014) Int J Clin Pract. 2016;70:668–675. doi: 10.1111/ijcp.12820. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study used nonpublic microdata from Statistics Netherlands and the Federation of Dutch Anticoagulation Clinics. Under certain conditions, these data are accessible for statistical and scientific research. For additional information: microdata@cbs.nl and/or fnt@fnt.nl