Summary
Background
The management of HER2-positive metastatic breast cancer (HER2+ mBC) has rapidly evolved. We assessed the real-world efficacy and safety of trastuzumab deruxtecan (T-DXd) versus trastuzumab emtansine (T-DM1) and tucatinib by emulating two phase III trials.
Methods
We emulated two target trials using the French National Health Data System: T-DXd versus T-DM1 and T-DXd versus tucatinib, for second- and third-line HER2+ mBC treatment. We included patients from September 2020 to September 2023, and followed them until death or April 2024. We emulated treatment assignment randomization with inverse probability of treatment weighting. Efficacy outcomes included time to treatment discontinuation (TTD) and overall survival (OS). Safety outcomes included cause-specific hospitalizations.
Findings
In the second-line treatment emulation (n = 2931: 1633 T-DM1, 1298 T-DXd), T-DXd had longer TTD (median 14⋅1 versus 6⋅5 months; weighted hazard ratio, wHR [95% confidence interval, CI], 0⋅46 [0⋅42–0⋅51]) and OS (median not reached; wHR [95% CI], 0⋅66 [0⋅55–0⋅80]) than T-DM1. We observed more cases of interstitial lung disease in the T-DXd group. In the third-line treatment emulation (n = 2391: 566 tucatinib, 1825 T-DXd), T-DXd had longer TTD (median 11⋅8 versus 5⋅8 months; wHR [95% CI], 0⋅60 [0⋅53–0⋅68]) and OS (median 31⋅7 versus 26⋅6 months; wHR [95% CI], 0⋅79 [0⋅69–0⋅92]) than tucatinib. T-DXd tended to protect from cardiac disorders (wHR [95% CI], 0⋅44 [0⋅26–0⋅74]) while enhancing respiratory disorders occurrence (wHR [95% CI], 1⋅72 [1⋅03–2⋅89]).
Interpretation
In this real-world study, T-DXd was more effective than T-DM1 as a second-line treatment and tucatinib as a third-line treatment, in line with clinical trial results.
Funding
None.
Keywords: Target trial emulation, HER2-positive metastatic breast cancer, Trastuzumab deruxtecan, Tucatinib, Trastuzumab emtansine, French National Health Data System (SNDS)
Research in context.
Evidence before this study
Clinical trials have proven that trastuzumab deruxtecan (T-DXd) and tucatinib are key therapies for the treatment of HER2-positive metastatic breast cancer in advanced lines. However, real-world comparative effectiveness and safety data on these new treatments remain extremely limited, particularly in underrepresented populations such as elderly patients and those with comorbid conditions.
We conducted a PubMed search up to April 28, 2025, using the following search terms: ((T-DXd[Title/Abstract]) or (trastuzumab deruxtecan[Title/Abstract])) and ((trastuzumab emtansine[Title/Abstract]) OR (T-DM1[Title/Abstract]) OR (tucatinib[Title/Abstract])) and ((observational[Title/Abstract]) OR (real-life[Title/Abstract]) OR (real-world[Title/Abstract])). The search yielded only 27 records, with no observational study directly comparing T-DXd with the standard of care in the second line, trastuzumab emtansine (T-DM1), or T-DXd with tucatinib in the third line. Most publications were non-specific review articles or observational studies reporting on the use, effectiveness, or safety of a single treatment. These studies involved limited sample sizes.
Given the limited evidence on the comparative effectiveness and safety of new treatments for HER2-positive metastatic breast cancer, especially among real-world populations, which are different from those enrolled in clinical trials, a large-scale comparison of these treatments by emulating target trials in a real-world setting was necessary in a public health perspective.
Added value of this study
Following the framework of two-arm randomized clinical trials emulation, this study provides the first large-scale comparison of HER2-targeting treatments for the second- and third-line treatment of HER2-positive metastatic breast cancer. T-DXd was associated with improved treatment persistence and better survival outcomes compared with T-DM1 in the second-line setting and with tucatinib in the third-line setting. These advantages were consistently observed across all subgroups analyzed. The treatments showed distinct safety profiles: T-DXd was associated with a higher incidence of respiratory events, particularly interstitial lung disease; T-DM1 was linked to a greater frequency of hematological adverse events, while tucatinib was associated with an increased risk of cardiac disorders.
Implications of all the available evidence
Although the absolute outcomes achieved with T-DXd in real-world settings were less favorable than those reported in clinical trials, T-DXd demonstrated consistent relative effectiveness across all lines of therapy and patient subgroups, including those with brain metastases. These findings support its positioning as a reference treatment for the management of HER2-positive metastatic breast cancer. Future research should focus on optimizing treatment sequencing and developing strategies to prevent or enable early detection of major toxicities such as interstitial lung disease with T-DXd. In addition, the effectiveness and safety of new therapies should be evaluated in underrepresented populations, including elderly patients and those with significant comorbid conditions.
Introduction
Breast cancer remains a leading cause of cancer-related deaths in women worldwide, and therapeutic strategies therefore need to be improved.1 The continuing challenge of managing this disease, particularly its metastatic forms, has driven efforts to develop more targeted and effective treatments. For patients with human epidermal growth factor receptor 2 (HER2)-overexpressing cancers (HER2+ mBC), accounting for 15–20% of breast cancers, therapeutic care has improved substantially due to the advent of HER2-targeting treatments.2
Historical approaches to the treatment of HER2+ mBC included, as a first-line treatment, dual HER2 blockade with trastuzumab and pertuzumab, followed by second-line treatment with the antibody drug conjugate3 (ADC) trastuzumab emtansine (T-DM1). Third-line treatment included trastuzumab or lapatinib, combined with capecitabine.2
The recent approval of novel HER2-targeting treatments has improved patient outcomes. Third-line trastuzumab deruxtecan (T-DXd) treatment was shown to be more effective than the treatment of the physicians' choice in the DESTINY-Breast02 trial, reducing the risk of progression by 64% and that of death by 34%.4 Furthermore, as a second-line treatment, T-DXd was found to be significantly more effective than T-DM1 in the DESTINY-Breast03 trial, with decreases in the risks of progression and death of 72% and 36%, respectively.5 Furthermore, in the HER2CLIMB trial for third-line treatment, the tucatinib combination reduced the risks of progression and death by 43% and 27%, respectively.6 Based on these results, early-access programs were implemented for T-DXd and tucatinib in France, and these drugs have been integrated into the standard of care for HER2+ mBC as second- and third-line (or beyond) treatments, respectively.
Despite these advances, the real-world efficacy and safety of T-DXd and tucatinib remain underexplored.7, 8, 9, 10 Patients selected for inclusion in clinical trials differ from those treated in routine clinical practice, who tend to be older, with more comorbid conditions and a higher frequency of symptomatic brain metastases. Furthermore, there has been no direct comparison to date comparing T-DXd and tucatinib for the third-line treatment of HER2+ mBC.
In this French nationwide study, we emulated two target trials from real-world data for patients with HER2+ mBC, to compare the efficacy and safety of T-DXd with those of T-DM1 for second-line treatment, and the tucatinib combination for third-line treatment.
Methods
The results are reported according to the STROBE guidelines. We followed the framework proposed by Hernan and Robins11,12 to emulate two-arm randomized clinical trials (the target trials) comparing (a) T-DXd with T-DM1 for the second-line treatment of HER2+ mBC and (b) T-DXd with tucatinib combination therapy for the third-line treatment of HER2+ mBC.
We first specified the framework for the target trial emulation to address our research questions (see Supplementary Material and Supplementary Table S1) regarding the comparative efficacy and safety of T-DXd relative to T-DM1 and tucatinib. We then specified how we would use observational data to emulate components of the protocol and to conduct the corresponding analyses.
Emulation of the target trials from SNDS data
Data source and characterization of the patients
We used data from the French National Health Data System (SNDS). The SNDS covers almost the totality (>99%) of the French population—68 million residents. Each person is identified by a unique and anonymous number. The SNDS records comprehensive outpatient and inpatient reimbursements. The SNDS also contains sociodemographic data and medical indications for hospital-level deliveries of expensive or early-access program drugs.13, 14, 15, 16, 17, 18
Patients with no prior history of treatment with T-DM1 (ATC L01FD03), T-DXd (ATC L01FD04) or tucatinib (ATC L01EH02) beginning these treatments from 30 September 2020 to 30 September 2023 were included and followed until their death or 30 April 2024 (last date for which data were available at the time of analysis). Tucatinib was assumed to be used in combination with trastuzumab and capecitabine, as this is the only scheme in which tucatinib is reimbursed in France. The maximum follow-up duration was 18 months for second-line treatments and 36 months for third-line treatments. The first delivery of treatment defined the index date for the study participants.
We restricted our analysis to patients with a diagnosis of breast cancer identified from hospital discharges and long-term disease records for the 10 years preceding the index date. The occurrence and location of the metastases were obtained from hospital discharge records for the five years preceding inclusion (Supplementary Tables S2 and S3). Visceral disease at baseline was defined as the presence of any recording of metastasis at a location other than bone or lymph nodes.
Metastatic stage, HER2-positive subtype and line of treatment were defined according to the medical indication recorded at dispensation of T-DM1 or T-DXd, together with the patients' prior medical history, as in previous studies.18,19 Tucatinib is only reimbursed for patients treated for HER2+ mBC as a third-line treatment or beyond.
Baseline medical history was assessed over the five years preceding inclusion in the study. We noted all radiotherapy, breast surgery, endocrine therapy (used as a proxy for hormone receptor status), intravenous or per os chemotherapy and HER2-targeting therapies (Supplementary Tables S4 and S5). Baseline comorbid conditions were identified with the diseases and expenditures mapping tool developed by the French National Health Insurance and described elsewhere.20,21
Treatment discontinuation was defined as a period of 90 days with no delivery of treatment. The date of treatment discontinuation was defined as the last date of administration plus 30 days (or, if the patient died, the date of death). The time-to-treatment discontinuation (TTD), used as imperfect proxy for progression-free survival (PFS), was defined as the time from the index date to the date of treatment discontinuation. Overall survival (OS) was defined as the time from the index date to death or the end date of the study.
Serious adverse events were defined as overnight hospitalizations for major health issues occurring during treatment (Supplementary Table S6).
Statistical analysis
Second- and third-line treatment emulations were carried out in the same way. We accounted for non-random treatment allocation by using stabilized inverse probability of treatment weighting for the average treatment effect in the whole population (IPTW).22 A directed acyclic graph (DAG), developed using DAGitty (https://dagitty.net/), was used to identify potential confounders and precision variables for inclusion in the propensity score model, based on clinical and causal hypotheses (Supplementary Table S7, Supplementary Figure S1). In the intention-to-treat (ITT) analysis, the treatment strategy was assessed at baseline. In the per-protocol analysis, the treatment strategy was evaluated at each treatment delivery and patients were censored and weighted at treatment crossover, through the inverse probability of censoring weighting (IPCW) method. We did not include any time-varying covariate in the censoring model, as we considered that baseline covariates included in the model were the main predictors of disease progression, and thus, potential crossover.
Weighted Kaplan–Meier models were used to obtain the median overall survival (mOS) and time-to-treatment discontinuation (mTTD) for each treatment group. Weighted Cox proportional hazards regression models were used to estimate hazard ratios for death, treatment discontinuation and serious adverse events. Restricted mean-survival time (RSMT) was computed to estimate the absolute mean difference in treatment duration and survival between the groups compared.23 We computed the e-value to quantify the minimum strength of association that an unmeasured confounder would need to have with the exposure and the outcome, in order to explain the observed association.24 The complete statistical methodology and additional sensitivity and subgroup analyses are described in Supplementary Materials.
Ethics approval
The French National Health Data System (SNDS) is a strictly anonymous database set, including all mandatory health insurance reimbursement data of the French population, in particular data from the processing of healthcare reimbursements (electronic or paper treatment sheet) and data from health establishments (PMSI).
EPI-PHARE has permanent regulatory access to the data from the French National Health Data System (SNDS) via its constitutive bodies ANSM and CNAM, in application of the provisions of the French Decree No. 2016-1871 of December 26, 2016 relating to the processing of personal data called the “National Health Data System”,15 the French law articles Art. R. 1461-1316 and R. 1461–1417 from the French Public Health Code and the French Data Protection Authority (CNIL) decision CNIL-2016-316.18.
In accordance with the permanent regulatory access granted to EPI-PHARE via ANSM and CNAM, this work did not require any specific opinion from the French Ethical and Scientific Committee for Research, Studies and Evaluations health (CESREES) nor approval from the CNIL.
All requests in the database were made by duly authorized people.
Role of the funding source
The authors are employees of the French National Health Insurance Organization (CNAM), the French National Agency for Medicines and Health Product Safety (ANSM), and the Avec Groupe Hospitalier Mutualiste and received no specific funding for this study.
Results
The study flow chart is presented in Supplementary Figure S2.
Second-line treatment emulation: T-DXd versus T-DM1
The first target trial emulation—comparing T-DXd and T-DM1 for second-line treatment—included 2931 patients, 1298 and 1633 having initiated T-DXd treatment and T-DM1 treatment, respectively. The detailed characteristics of the patients at baseline, before weighting, are summarized in Table 1. Mean age at treatment initiation was similar between the groups, at 59⋅2 years (standard deviation, SD, 12⋅4) for the T-DXd group and 60⋅6 years (SD, 13⋅2) for the T-DM1 group. The prevalence of brain metastases was higher in the T-DXd group (29⋅4%, n = 382) than in the T-DM1 group (24⋅4%, n = 399). In addition, T-DXd users had a higher frequency of prior treatment with per os chemotherapy (15⋅8%, n = 205, versus 8⋅3%, n = 136) and tyrosine kinase inhibitors (6⋅5%, n = 85, versus 1⋅9%, n = 31) (Table 1).
Table 1.
Unweighted baseline characteristics of the second-line treatment study population.
| T-DXd n = 1298 |
T-DM1 n = 1633 |
All n = 2931 |
|
|---|---|---|---|
| Year of inclusion | |||
| 2020 | 8 (0⋅6) | 227 (13⋅9) | 235 (8⋅0) |
| 2021 | 53 (4⋅1) | 844 (51⋅7) | 897 (30⋅6) |
| 2022 | 474 (36⋅5) | 430 (26⋅3) | 904 (30⋅8) |
| 2023 | 763 (58⋅8) | 132 (8⋅1) | 895 (30⋅5) |
| Mean age (SD), years | 59⋅2 (12⋅4) | 60⋅6 (13⋅2) | 60⋅0 (12⋅8) |
| Median age (IQR), years | 59⋅0 (51⋅0–68⋅0) | 60⋅0 (51⋅0–71⋅0) | 60⋅0 (51⋅0–70⋅0) |
| Age categories, years | |||
| 18–49 | 283 (21⋅8) | 331 (20⋅3) | 614 (20⋅9) |
| 50–59 | 383 (29⋅5) | 460 (28⋅2) | 843 (28⋅8) |
| 60–69 | 341 (26⋅3) | 395 (24⋅2) | 736 (25⋅1) |
| ≥70 | 291 (22⋅4) | 447 (27⋅4) | 738 (25⋅2) |
| Sex: female | 1292 (99⋅5) | 1622 (99⋅3) | 2914 (99⋅4) |
| Deprivation index, quintiles | |||
| 1 (least deprived) | 237 (18⋅3) | 288 (17⋅6) | 525 (17⋅9) |
| 2 | 238 (18⋅3) | 268 (16⋅4) | 506 (17⋅3) |
| 3 | 271 (20⋅9) | 332 (20⋅3) | 603 (20⋅6) |
| 4 | 253 (19⋅5) | 351 (21⋅5) | 604 (20⋅6) |
| 5 (most deprived) | 243 (18⋅7) | 321 (19⋅7) | 564 (19⋅2) |
| 0 (not estimated) | 28 (2⋅2) | 56 (3⋅4) | 84 (2⋅9) |
| Missing | 28 (2⋅2) | 17 (1⋅0) | 45 (1⋅5) |
| Type of hospital facility | |||
| University/regional center | 208 (16⋅0) | 205 (12⋅6) | 413 (14⋅1) |
| Cancer center | 374 (28⋅8) | 349 (21⋅4) | 723 (24⋅7) |
| Small center/other | 324 (25⋅0) | 526 (32⋅2) | 850 (29⋅0) |
| Private center | 392 (30⋅2) | 553 (33⋅9) | 945 (32⋅2) |
| Duration of breast cancer | |||
| ≤3 years | 537 (41⋅4) | 786 (48⋅1) | 1323 (45⋅1) |
| 3–8 years | 519 (40⋅0) | 604 (37⋅0) | 1123 (38⋅3) |
| >8 years | 242 (18⋅6) | 243 (14⋅9) | 485 (16⋅5) |
| Local or unspecific treatments (past 5 years) | |||
| Breast surgery | 685 (52⋅8) | 868 (53⋅2) | 1553 (53⋅0) |
| Radiotherapy | 813 (62⋅6) | 1015 (62⋅2) | 1828 (62⋅4) |
| IV chemotherapy | 477 (36⋅7) | 546 (33⋅4) | 1023 (34⋅9) |
| Per os chemotherapy | 205 (15⋅8) | 136 (8⋅3) | 341 (11⋅6) |
| Endocrine therapy | 804 (61⋅9) | 917 (56⋅2) | 1721 (58⋅7) |
| HER2 targeting therapy (past 5 years) | |||
| Trastuzumab alone | 459 (35⋅4) | 605 (37⋅0) | 1064 (36⋅3) |
| Tyrosine kinase inhibitor | 85 (6⋅5) | 31 (1⋅9) | 116 (4⋅0) |
| Lapatinib | 31 (2⋅4) | 24 (1⋅5) | 55 (1⋅9) |
| Tucatinib | 60 (4⋅6) | 7 (0⋅4) | 67 (2⋅3) |
| Metastases | 1105 (85⋅1) | 1313 (80⋅4) | 2418 (82⋅5) |
| Lymph node | 345 (26⋅6) | 405 (24⋅8) | 750 (25⋅6) |
| Bone | 721 (55⋅5) | 851 (52⋅1) | 1572 (53⋅6) |
| Digestive | 521 (40⋅1) | 582 (35⋅6) | 1103 (37⋅6) |
| Pulmonary | 410 (31⋅6) | 517 (31⋅7) | 927 (31⋅6) |
| Brain | 382 (29⋅4) | 399 (24⋅4) | 781 (26⋅6) |
| Duration of active metastatic disease | |||
| ≤1 year | 382 (29⋅4) | 498 (30⋅5) | 880 (30⋅0) |
| 1–4 years | 562 (43⋅3) | 691 (42⋅3) | 1253 (42⋅7) |
| >4 years | 161 (12⋅4) | 124 (7⋅6) | 285 (9⋅7) |
| No active metastases | 193 (14⋅9) | 320 (19⋅6) | 513 (17⋅5) |
| Comorbid condition | 574 (44⋅2) | 751 (46⋅0) | 1325 (45⋅2) |
| Cardiac | 174 (13⋅4) | 251 (15⋅4) | 425 (14⋅5) |
| Diabetes | 128 (9⋅9) | 158 (9⋅7) | 286 (9⋅8) |
| Respiratory | 108 (8⋅3) | 144 (8⋅8) | 252 (8⋅6) |
| Neurologic | 38 (2⋅9) | 62 (3⋅8) | 100 (3⋅4) |
| Psychiatric | 57 (4⋅4) | 101 (6⋅2) | 158 (5⋅4) |
| IMID | 36 (2⋅8) | 48 (2⋅9) | 84 (2⋅9) |
| Liver/pancreas | 47 (3⋅6) | 47 (2⋅9) | 94 (3⋅2) |
| Obesity | 137 (10⋅6) | 188 (11⋅5) | 325 (11⋅1) |
| Tobacco use | 140 (10⋅8) | 167 (10⋅2) | 307 (10⋅5) |
Figures are presented in numbers (percentages), unless stated otherwise.
T-DM1: trastuzumab emtansine; T-DXd: trastuzumab deruxtecan; SD: standard deviation; IQR: interquartile range; HER2: human epidermal growth factor receptor 2; IV: intravenous; IMID: immune-mediated inflammatory disease (including rheumatoid arthritis, spondyloarthritis, and inflammatory bowel diseases).
After inverse probability of treatment weighting, all baseline covariates were well balanced (standardized differences < 0⋅1) (Supplementary Table S8, Supplementary Figure S3).
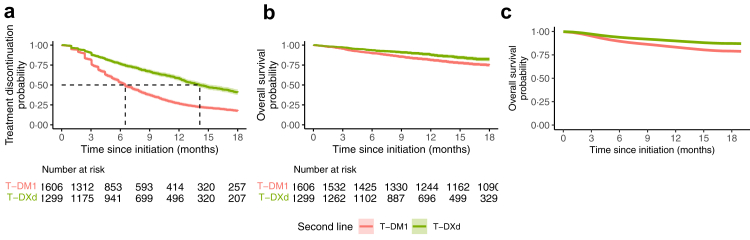
After weighting, the mTTD was 14⋅1 months (95% confidence interval CI, 13⋅2–15⋅5) in the T-DXd group and 6⋅5 months (95% CI, 6⋅2–7⋅0) in the T-DM1 group (Fig. 1a). The weighted hazard ratio (wHR) of treatment discontinuation associated with T-DXd exposure relative to T-DM1 exposure was 0⋅46 (95% CI, 0⋅42–0⋅51) (Fig. 2, Supplementary Table S9). This corresponds to a mean difference of 4·0 months of treatment between T-DXd and T-DM1 at 18 months of follow-up.
Fig. 1.
Weighted Kaplan–Meier survival curves comparing T-DXd and T-DM1 as second-line treatments. T-DM1: trastuzumab emtansine; T-DXd: trastuzumab deruxtecan. (a) Survival without treatment discontinuation after weighting on the inverse probability of treatment. (b) Intention-to-treat Overall survival after weighting on the inverse probability of treatment. (c) Per protocol Overall Survival after weighting on the inverse probability of treatment and the inverse probability of censoring, we did not provide the patients at risk for this curve, as it is based on modeling only.
Fig. 2.
Weighted estimates of efficacy and safety outcomes of initiating T-DXd compared to T-DM1 as second-line treatments. T-DM1: trastuzumab emtansine; T-DXd: trastuzumab deruxtecan; TTD: time to treatment discontinuation; OS: overall survival; ITT: intention-to-treat effect, i.e., the effect of treatment strategy assignment whatever occurs next; per protocol effect: the effect of treatment strategy assignment in adherent patients; historical cohort: comparison sur T-DM1 initiators in 2016–2017; wHR: weighted hazard ratio; 95% CI: 95% confidence interval.
In the ITT survival analysis, median OS was not reached, with 177 deaths (13⋅6%) in the T-DXd group and 395 deaths (24⋅2%) in the T-DM1 group (Fig. 1b). T-DXd decreased the risk of death relative to T-DM1 (wHR: 0⋅66, 95% CI, 0⋅55–0⋅80) (Fig. 2, Supplementary Table S9). This corresponds to an e-value of 2⋅4 and a mean survival difference of 2⋅1 months between T-DXd and T-DM1 at 18 months of follow-up.
During follow-up, 41⋅7% (n = 681) of patients initially treated with T-DM1 switched to T-DXd, whereas only 7⋅5% (n = 97) of patients switched from T-DXd to T-DM1. In the per-protocol analysis adjusting for this crossover, T-DXd was associated with a survival advantage, with a wHR of 0⋅57 (95% CI, 0⋅39–0⋅97) (Fig. 1c, Fig. 2, and Supplementary Table S9).
Further analyses comparing T-DXd with a historical cohort of T-DM1 users (treatment initiated in 2016 or 2017) showed a consistent benefit in terms of overall survival, with a wHR of 0⋅59 (95% CI, 0⋅49–0⋅71) (Fig. 2, Supplementary Table S10). Subgroup analyses showed consistent results of T-DXd among all groups considered. The reduced risk of death, or treatment discontinuation associated with T-DXd, compared to TDM-1, was observed independent of age (<65 versus ≥65 years), hormone receptor status, and the presence or absence of visceral disease or brain metastases. Particularly, among patients with baseline brain metastases, T-DXd was associated with an important lower risk of treatment discontinuation (wHR: 0⋅40; 95% CI, 0⋅33–0⋅49) and of death (wHR: 0⋅46; 95% CI, 0⋅33–0⋅64) (Supplementary Figures S4 and S5).
In terms of safety, we found no significant and clinically relevant difference between T-DXd and T-DM1 use. Severe interstitial lung disease concerned 3 patients in the T-DM1 group and 11 patients in the T-DXd group (Fig. 2, Supplementary Tables S11 and S12).
Third-line treatment emulation: T-DXd versus the tucatinib combination
For the second target trial emulation—comparing T-DXd and tucatinib for third-line treatment—2391 patients were included, 1825 and 566 having initiated T-DXd treatment and tucatinib treatment, respectively. The detailed characteristics of the patients at baseline are summarized in Table 2. Age was similar between the groups, with a mean age at inclusion of 60⋅2 years (SD, 12·4) in the T-DXd group and 58⋅2 years (SD, 12⋅0) in the tucatinib group. The prevalence of brain metastasis was higher in the tucatinib group than in the T-DXd group (57⋅1%, n = 323 versus 29⋅3%, n = 534) (Table 2).
Table 2.
Unweighted baseline characteristics of the third-line treatment study population.
| T-DXd n = 1825 |
Tucatinib n = 566 |
All n = 2391 |
|
|---|---|---|---|
| Year of inclusion | |||
| 2020 | 172 (9⋅4) | 1 (0⋅2) | 173 (7⋅2) |
| 2021 | 711 (39⋅0) | 338 (59⋅7) | 1049 (43⋅9) |
| 2022 | 590 (32⋅3) | 167 (29⋅5) | 757 (31⋅7) |
| 2023 | 352 (19⋅3) | 60 (10⋅6) | 412 (17⋅2) |
| Mean age (SD), years | 60⋅2 (12⋅8) | 58⋅2 (12⋅0) | 59⋅7 (12⋅6) |
| Median age (IQR), years | 60⋅0 (51⋅0–69⋅0) | 59⋅0 (50⋅0–67⋅0) | 59⋅0 (51⋅0–69⋅0) |
| Age categories, years | |||
| 18–49 | 370 (20⋅3) | 141 (24⋅9) | 511 (21⋅4) |
| 50–59 | 535 (29⋅3) | 156 (27⋅6) | 691 (28⋅9) |
| 60–69 | 464 (25⋅4) | 169 (29⋅9) | 633 (26⋅5) |
| ≥70 | 456 (25⋅0) | 100 (17⋅7) | 556 (23⋅3) |
| Sex: female | 1803 (98⋅8) | 564 (99⋅6) | 2367 (99⋅0) |
| Deprivation index, quintiles | |||
| 1 (least deprived) | 337 (18⋅5) | 121 (21⋅4) | 458 (19⋅2) |
| 2 | 318 (17⋅4) | 121 (21⋅4) | 439 (18⋅4) |
| 3 | 354 (19⋅4) | 117 (20⋅7) | 471 (19⋅7) |
| 4 | 367 (20⋅1) | 91 (16⋅1) | 458 (19⋅2) |
| 5 (most deprived) | 374 (20⋅5) | 102 (18⋅0) | 476 (19⋅9) |
| 0 (not estimated) | 46 (2⋅5) | 10 (1⋅8) | 56 (2⋅3) |
| Missing | 29 (1⋅6) | 4 (0⋅7) | 33 (1⋅4) |
| Duration of breast cancer | |||
| ≤3 years | 466 (25⋅5) | 149 (26⋅3) | 615 (25⋅7) |
| 3–8 years | 864 (47⋅3) | 288 (50⋅9) | 1152 (48⋅2) |
| >8 years | 495 (27⋅1) | 129 (22⋅8) | 624 (26⋅1) |
| Local or unspecific treatments (past 5 years) | |||
| Breast surgery | 1011 (55⋅4) | 360 (63⋅6) | 1371 (57⋅3) |
| Radiotherapy | 1267 (69⋅4) | 461 (81⋅4) | 1728 (72⋅3) |
| IV chemotherapy | 858 (47⋅0) | 251 (44⋅3) | 1109 (46⋅4) |
| Per os chemotherapy | 616 (33⋅8) | 349 (61⋅7) | 965 (40⋅4) |
| Endocrine therapy | 1154 (63⋅2) | 312 (55⋅1) | 1466 (61⋅3) |
| HER2 targeting therapy (past 5 years) | |||
| Pertuzumab | 1420 (77⋅8) | 452 (79⋅9) | 1872 (78⋅3) |
| Trastuzumab alone | 1125 (61⋅6) | 426 (75⋅3) | 1551 (64⋅9) |
| Lapatinib | 302 (16⋅5) | 79 (14⋅0) | 381 (15⋅9) |
| Metastases | 1565 (85⋅8) | 514 (90⋅8) | 2079 (87⋅0) |
| Lymph node | 518 (28⋅4) | 170 (30⋅0) | 688 (28⋅8) |
| Bone | 1025 (56⋅2) | 316 (55⋅8) | 1341 (56⋅1) |
| Digestive | 651 (35⋅7) | 231 (40⋅8) | 882 (36⋅9) |
| Pulmonary | 647 (35⋅5) | 193 (34⋅1) | 840 (35⋅1) |
| Brain | 534 (29⋅3) | 323 (57⋅1) | 857 (35⋅8) |
| Duration of metastatic disease | |||
| ≤1 year | 226 (12⋅4) | 63 (11⋅1) | 289 (12⋅1) |
| 1–4 years | 894 (49⋅0) | 313 (55⋅3) | 1207 (50⋅5) |
| >4 years | 445 (24⋅4) | 138 (24⋅4) | 583 (24⋅4) |
| No active metastases | 260 (14⋅2) | 52 (9⋅2) | 312 (13⋅0) |
| Comorbid condition | 790 (43⋅3) | 260 (45⋅9) | 1050 (43⋅9) |
| Cardiac | 248 (13⋅6) | 76 (13⋅4) | 324 (13⋅6) |
| Diabetes | 163 (8⋅9) | 48 (8⋅5) | 211 (8⋅8) |
| Respiratory | 121 (6⋅6) | 37 (6⋅5) | 158 (6⋅6) |
| Neurologic | 82 (4⋅5) | 39 (6⋅9) | 121 (5⋅1) |
| Psychiatric | 87 (4⋅8) | 36 (6⋅4) | 123 (5⋅1) |
| IMID | 45 (2⋅5) | 13 (2⋅3) | 58 (2⋅4) |
| Liver/pancreas | 82 (4⋅5) | 15 (2⋅7) | 97 (4⋅1) |
| Obesity | 174 (9⋅5) | 49 (8⋅7) | 223 (9⋅3) |
| Tobacco use | 174 (9⋅5) | 71 (12⋅5) | 245 (10⋅2) |
Figures are presented in numbers (percentages), unless stated otherwise.
T-DM1: trastuzumab emtansine; T-DXd: trastuzumab deruxtecan; SD: standard deviation; IQR: interquartile range; HER2: human epidermal growth factor receptor 2; IV: intravenous; IMID: immune-mediated inflammatory disease (including rheumatoid arthritis, spondyloarthritis, and inflammatory bowel diseases).
After inverse probability of treatment weighting, all baseline covariates were well balanced (standardized differences < 0⋅1) (Supplementary Table S13, Supplementary Figure S6).
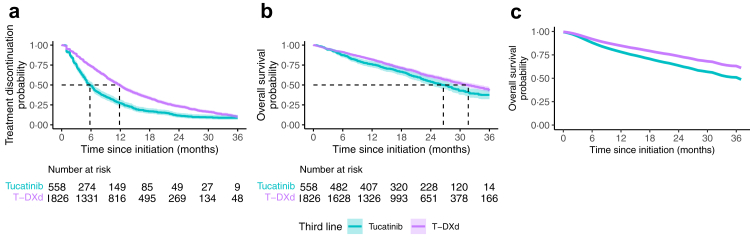
After weighting, the mTTD was 11⋅8 months (95% CI, 11⋅3–12⋅4) in the T-DXd group and 5⋅8 months (95% CI, 5⋅1–6⋅6) in the tucatinib group (Fig. 3a). The wHR of treatment discontinuation associated with T-DXd exposure relative to tucatinib exposure was 0⋅60 (95% CI, 0⋅53–0⋅68) (Fig. 4, Supplementary Table S14). This corresponds to a mean difference of 5⋅0 months of treatment between T-DXd and the tucatinib combination at 36 months of follow-up.
Fig. 3.
Weighted Kaplan–Meier survival curves comparing T-DXd and tucatinib as third-line treatments. T-DXd: trastuzumab deruxtecan. (a) Survival without treatment discontinuation after weighting on the inverse probability of treatment. (b) Intention-to-treat Overall survival after weighting on the inverse probability of treatment. (c) Per protocol Overall Survival after weighting on the inverse probability of treatment and the inverse probability of censoring, we did not provide the patients at risk for this curve, as it is based on modeling only.
Fig. 4.
Weighted estimates of efficacy and safety outcomes of initiating T-DXd compared to tucatinib as third-line treatments. T-DXd: trastuzumab deruxtecan; TTD: time to treatment discontinuation; OS: overall survival; ITT: intention-to-treat effect, i.e., the effect of treatment strategy assignment whatever occurs next; per protocol effect: the effect of treatment strategy assignment in adherent patients; wHR: weighted hazard ratio; 95% CI: 95% confidence interval.
In the ITT analysis, median OS was 31⋅7 months (95% CI, 29⋅6–33⋅9) in the T-DXd group versus 26⋅6 months (95% CI, 23⋅6–29⋅7) in the tucatinib group, with 725 deaths (39⋅7%) in the T-DXd group and 286 deaths (50⋅5%) in the tucatinib group (Fig. 3b). T-DXd decreased the risk of death relative to tucatinib (wHR: 0⋅79; 95% CI, 0⋅69–0⋅92) (Fig. 4, Supplementary Table S14). This corresponds to an e-value of 1⋅85 and a mean survival difference of 2⋅0 months between T-DXd and the tucatinib combination at 36 months of follow-up.
During follow-up, 51⋅8% (n = 293) of patients who began tucatinib treatment switched to T-DXd, and 31⋅3% (n = 517) in the T-DXd group switched to tucatinib treatment. Accounting for this crossover in the per-protocol analysis, the wHR of death associated with T-DXd exposure relative to tucatinib was 0⋅64 (95% CI, 0⋅43–1⋅08) (Fig. 3c, Fig. 4, and Supplementary Table S14).
Subgroup analyses showed consistent results of T-DXd across multiple patient groups. The reduced risk of death or treatment discontinuation associated with T-DXd, compared to tucatinib, was observed, regardless of age (<65 versus ≥65 years), hormone receptor status, and the presence or absence of brain metastases or visceral disease (Supplementary Figures S7 and S8).
In terms of safety, tucatinib exposure was associated with a higher risk of cardiac disorder (wHR T-DXd versus tucatinib: 0⋅44, 95% CI, 0⋅26–0⋅74). T-DXd exposure was associated with a higher risk of respiratory disorder (wHR T-DXd versus tucatinib: 1⋅72, 95% CI, 1⋅03–2⋅89). In particular, severe interstitial lung disease was not observed in the tucatinib group while 16 patients suffered from this event in the T-DXd group (Fig. 4, Supplementary Table S15).
Discussion
In these emulated trials based on observational data for patients with HER2+ mBC, initiating treatment with T-DXd, compared to T-DM1 in the second line, and to tucatinib in the third line, was found to be beneficial in terms of treatment continuation and survival outcomes. These benefits were sustained in all the subgroups explored. In terms of safety signals, as expected, T-DXd was associated with a higher risk of respiratory conditions, including interstitial lung disease in particular,25 than treatment with T-DM1 or tucatinib. However, patients treated with T-DXd had a lower risk of cardiac diseases than those treated with tucatinib.
This is the first observational study comparing T-DXd and T-DM1 for the second-line treatment of HER2+ mBC, and T-DXd and tucatinib for the third-line treatment of HER2+ mBC. Building on our previous work,18,19,26 this study is also the largest observational study to investigate the use of T-DXd or tucatinib for the treatment of HER2+ mBC. Indeed, we included 3123 new users of T-DXd (whereas previous descriptive studies on T-DXd included between 29 and 459 patients7,8,27, 28, 29) and 566 new users of tucatinib (versus 216 in a study by Kaufman et al.10 and 101 in a study by Frenel et al.30).
We found that T-DXd decreased the risk of treatment discontinuation by 54% relative to T-DM1 for second-line treatment, with a median TTD (considered as a proxy for PFS) for of 14⋅1 months for T-DXd versus 6⋅5 months for T-DM1 (wHR: 0⋅46; 95% CI, 0⋅42–0⋅51). T-DXd also decreased the risk of death by 44% relative to T-DM1 (ITT OS wHR: 0⋅66; 95% CI, 0⋅55–0⋅80). These findings are consistent with the relative effect results reported for the DESTINY-Breast03 trial, which compared T-DXd to T-DM1 (PFS HR: 0⋅33, 95% CI, 0⋅26–0⋅43; OS HR: 0⋅64, 95% CI, 0⋅47–0⋅97).31
The difference observed between our study and clinical trials regarding absolute medians of PFS and TTD can be explained by the less selective real-world setting, in which the T-DXd users might have a longer treatment history, compared to that of a clinical trial,32 or by the difference in definitions between PFS and TTD.
In addition, T-DXd decreased the risk of treatment discontinuation by 40% relative to tucatinib for third-line treatment (TTD wHR: 0⋅60; 95% CI, 0⋅53–0⋅68), and the risk of death by 21% (ITT OS wHR: 0⋅79; 95% CI, 0⋅69–0⋅92). To our knowledge, this is the first study to compare tucatinib and T-DXd directly as third-line treatment options for HER2+ metastatic breast cancer.
The presence of brain metastases in patients with HER2+ mBC presents significant clinical challenges. In our study, the proportion of patients with brain metastases was high in both the T-DXd group (29⋅4%) and the T-DM1 group (24⋅4%). By contrast, this proportion was only 15–16% in DESTINY-Breast03 trial, in which the brain metastases included were asymptomatic. Despite this potentially unfavorable baseline characteristic, T-DXd was consistently more effective than T-DM1 in this patient population. These findings are consistent with a pooled analysis of the DESTINY trials, which reported intracranial responses to T-DXd.33,34 A smaller difference in efficacy was observed relative to tucatinib, which has been shown to be effective for the management of brain metastases.35
The safety results obtained were mostly in line with those of clinical trials and pharmacovigilance studies.4,5,36 Our study captured only serious adverse events requiring overnight hospitalization, a proxy for grade 3 or higher potential adverse events, which may explain the lower incidence of adverse effects reported here than in clinical trials. We chose to increase the specificity of the detection of events, a frequent target in observational studies. The expected increase in the risk of respiratory conditions in T-DXd users, including ILD in particular, relative to patients treated with T-DM1 is consistent with the findings of previous studies identifying interstitial lung disease as a the major adverse event associated with T-DXd use.5,31 We found that the incidence of serious ILD was relatively low in T-DXd users (<1%), probably due to improvements in the expertise of prescribers and the close monitoring of patients included in early-access programs. This highlights the need for vigilant monitoring and strategies for managing adverse effects.25 However, patients treated with T-DXd had a lower risk of hematological disorders than those treated with T-DM1, and a lower risk of cardiac diseases than those treated with tucatinib. Cardiotoxicity, a known adverse event associated with HER2-targeted agents, was observed in 4% of the patients treated with tucatinib. This incidence contrasts with the less than 1% reported in the HER2CLIMB phase III trial, which excluded patients with comorbid cardiac conditions.37
Strengths & limitations
The primary strength of our study was the large population size, made possible by the comprehensive nature of the SNDS database, which covers the entire French population. It provides complete information on hospitalizations, dates of death, and treatment deliveries, making it possible to estimate time to treatment discontinuation and overall survival accurately. Furthermore, we had access to the medical indications documented for each treatment delivery, enabling us to distinguish between the different indications for the multi-indication treatments analyzed. Our study was constructed with a target trial emulation framework, mimicking randomization within real-world data, thereby ensuring comparability between the exposure groups at baseline. This approach reduced bias and supported causal inference by balancing baseline characteristics and specifying the start of follow-up and assigned treatment group. We also ensured that this study was as close as possible to clinical trial conditions and as exhaustive as possible by performing two separate overall survival analyses: an intention-to-treat analysis of real-world efficacy regardless of event occurring after treatment initiation, and a per-protocol analysis estimating the theoretical efficacy for “pure” exposure with no crossover.
These findings rely on several hypothetical estimations and modeling approaches. While robustness was partially assessed through sensitivity analyses, the results are subject to the assumptions inherent in the modeling frameworks employed.
One limitation of our study was the impossibility of controlling for unmeasured variables, leading to potential residual confounding, which may affect the validity of our findings. In particular, we were not able to distinguish between active and inactive brain metastases. Although the e-values show some robustness to unmeasured confounding, it remains possible that such residual confounding may have influenced the reported associations. However, ICD-10 discharge recordings, used to assess the presence of metastases, indicate significant diagnoses, allowing us to hypothesize that patients with metastatic recordings were actively treated for brain metastases. A further limitation of this study was the relatively short follow-up period for the second-line treatment emulation, which was censored at 18 months post-inclusion, due to the more recent approval of the second-line indication for T-DXd. These results should be updated when more long-term follow-up data become available. Moreover, we did not adjust for multiple comparisons in safety analyses; therefore, these findings must be interpreted with caution. Many analyses on safety events showed non-significant differences. Given the low number of events for several safety outcomes, we cannot exclude the possibility that the study was not powered enough to rule out actual differences. In fact, safety analyses should be interpreted as highly exploratory.
In addition, some data were not available, including actual healthcare utilization (e.g., in the private or outpatient setting), and we had to make assumptions to approximate actual prescription patterns in the context of a real-world study, using data from the SNDS. Finally, this study was not designed to determine the optimal therapeutic sequence for HER2-positive metastatic breast cancer, and further studies are therefore required to address this issue.
Ultimately, the absolute outcomes obtained with T-DXd in the real world are less favorable than those obtained in clinical trials, but T-DXd nevertheless had a consistently high relative efficacy across all treatment lines and patient subgroups (including patients with brain metastases in particular), supporting its use as a reference treatment for the management of HER2-positive metastatic breast cancer.
Contributors
Literature search: HJ, ADM, and NH. Study design: HJ, ADM, IM, and NH. Data extraction and collection: HJ and DD. Statistical analysis: HJ. Interpretation of the results: HJ, ADM, and NH. Drafting of the manuscript: HJ and NH. Revision of the manuscript: all. Supervision: NH and MZ.
HJ, NH, and MZ confirm that they had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. HJ, NH, and MZ take final responsibility for the decision to submit the manuscript for publication.
Data sharing statement
The study was registered on the study register of EPI-PHARE concerning studies from SNDS data under the reference T-2024-06-520.
In accordance with data protection legislation and the French regulation, the authors are not allowed to release or make public the data from the SNDS. However, any person or structure, public or private, for-profit or nonprofit, is able to access SNDS data in order to carry out a study, research or an evaluation in the public interest, upon authorization from the French Data Protection Office (CNIL), via the French Health Data Hub (https://www.health-data-hub.fr/).
All information for data subject is available on the EPI-PHARE website at https://www.epi-phare.fr/en/regulation-snds/.
Declaration of interests
The authors have no conflict of interest to report.
Acknowledgement
We thank Emilie Lanoy (Unité de Recherche Clinique de l'HEGP, Inserm CIC-EC1418) for her assistance with causal statistical analysis.
Footnotes
For the French translation of the abstract see the Supplementary Material.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2025.101455.
Appendix A. Supplementary data
References
- 1.Loibl S., Poortmans P., Morrow M., Denkert C., Curigliano G. Breast cancer. Lancet. 2021;397(10286):1750–1769. doi: 10.1016/S0140-6736(20)32381-3. [DOI] [PubMed] [Google Scholar]
- 2.Loibl S., Gianni L. HER2-positive breast cancer. Lancet. 2017;389(10087):2415–2429. doi: 10.1016/S0140-6736(16)32417-5. [DOI] [PubMed] [Google Scholar]
- 3.Thomas A., Teicher B.A., Hassan R. Antibody–drug conjugates for cancer therapy. Lancet Oncol. 2016;17(6):e254–e262. doi: 10.1016/S1470-2045(16)30030-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.André F., Hee Park Y., Kim S.B., et al. Trastuzumab deruxtecan versus treatment of physician's choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2023;401(10390):1773–1785. doi: 10.1016/S0140-6736(23)00725-0. [DOI] [PubMed] [Google Scholar]
- 5.Cortés J., Kim S.B., Chung W.P., et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022;386(12):1143–1154. doi: 10.1056/NEJMoa2115022. [DOI] [PubMed] [Google Scholar]
- 6.Curigliano G., Mueller V., Borges V., et al. Tucatinib versus placebo added to trastuzumab and capecitabine for patients with pretreated HER2+ metastatic breast cancer with and without brain metastases (HER2CLIMB): final overall survival analysis. Ann Oncol. 2022;33(3):321–329. doi: 10.1016/j.annonc.2021.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Botticelli A., Caputo R., Scagnoli S., et al. Real-world outcomes of trastuzumab deruxtecan in patients with HER2+ metastatic breast cancer: the DE-REAL study. Oncologist. 2023;29(4):303–310. doi: 10.1093/oncolo/oyad308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petit T., Hajjaji N., Antoine E., et al. Trastuzumab deruxtecan in previously treated HER2-positive metastatic or unresectable breast cancer: real-life data from the temporary use authorization program in France. Cancer Med. 2024;13(9) doi: 10.1002/cam4.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dannehl D., Jakob D., Mergel F., et al. The efficacy of sacituzumab govitecan and trastuzumab deruxtecan on stable and active brain metastases in metastatic breast cancer patients—a multicenter real-world analysis. ESMO Open. 2024;9(5) doi: 10.1016/j.esmoop.2024.102995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaufman P.A., Neuberger E., Schwartz N.R.M., et al. Real-world patient characteristics, treatment patterns, and clinical outcomes associated with tucatinib therapy in HER2-positive metastatic breast cancer. Front Oncol. 2023;13 doi: 10.3389/fonc.2023.1264861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hernán M.A., Robins J.M. Using big data to emulate a target trial when a randomized trial is not available: table 1. Am J Epidemiol. 2016;183(8):758–764. doi: 10.1093/aje/kwv254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernán M.A., Wang W., Leaf D.E. Target trial emulation: a framework for causal inference from observational data. JAMA. 2022;328(24):2446–2447. doi: 10.1001/jama.2022.21383. [DOI] [PubMed] [Google Scholar]
- 13.Tuppin P., Rudant J., Constantinou P., et al. Value of a national administrative database to guide public decisions: from the système national d’information interrégimes de l'Assurance Maladie (SNIIRAM) to the système national des données de santé (SNDS) in France. Rev Epidemiol Sante Publique. 2017;65:S149–S167. doi: 10.1016/j.respe.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Swital M., Drouin J., Miranda S., Bakchine S., Botton J., Dray-Spira R. Use of multiple sclerosis disease-modifying therapies during pregnancy in France: nationwide study between 2010 and 2021. Mult Scler. 2024;30(2):227–237. doi: 10.1177/13524585231223395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jourdain H., Hoisnard L., Sbidian E., Zureik M. Persistence and safety of anti-TNF biosimilars versus originators in immune-mediated inflammatory diseases: an observational study on the French National Health Data System. RMD Open. 2024;10(1) doi: 10.1136/rmdopen-2023-003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roland N., Neumann A., Hoisnard L., et al. Use of progestogens and the risk of intracranial meningioma: national case-control study. BMJ. 2024;384 doi: 10.1136/bmj-2023-078078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rios P., Herlemont P., Fauque P., et al. Medically assisted reproduction and risk of cancer among offspring. JAMA Netw Open. 2024;7(5) doi: 10.1001/jamanetworkopen.2024.9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jourdain H., Mansouri I., Di Meglio A., Zureik M., Haddy N. Utilization and safety of trastuzumab emtansine (T-DM1): a nationwide population-based study using the French National Health Data System. ESMO Real World Data and Digit Oncol. 2024;4 [Google Scholar]
- 19.Jourdain H., Di Meglio A., Mansouri I., Desplas D., Zureik M., Haddy N. Use and outcomes of trastuzumab deruxtecan in HER2-positive and HER2-low metastatic breast cancer in a real-world setting: a nationwide cohort study. ESMO Open. 2024;9(12) doi: 10.1016/j.esmoop.2024.104083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Semenzato L., Botton J., Drouin J., et al. Chronic diseases, health conditions and risk of COVID-19-related hospitalization and in-hospital mortality during the first wave of the epidemic in France: a cohort study of 66 million people. Lancet Reg Health Eur. 2021;8 doi: 10.1016/j.lanepe.2021.100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rachas A., Gastaldi-Ménager C., Denis P., et al. The economic burden of disease in France from the National Health Insurance Perspective: the healthcare expenditures and conditions mapping used to prepare the French social security funding act and the public health act. Med Care. 2022;60(9):655–664. doi: 10.1097/MLR.0000000000001745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desai R.J., Franklin J.M. Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: a primer for practitioners. BMJ. 2019;367 doi: 10.1136/bmj.l5657. [DOI] [PubMed] [Google Scholar]
- 23.Royston P., Parmar M.K.B. The use of restricted mean survival time to estimate the treatment effect in randomized clinical trials when the proportional hazards assumption is in doubt. Stat Med. 2011;30(19):2409–2421. doi: 10.1002/sim.4274. [DOI] [PubMed] [Google Scholar]
- 24.Haneuse S., VanderWeele T.J., Arterburn D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA. 2019;321(6):602–603. doi: 10.1001/jama.2018.21554. [DOI] [PubMed] [Google Scholar]
- 25.Wekking D., Porcu M., Pellegrino B., et al. Multidisciplinary clinical guidelines in proactive monitoring, early diagnosis, and effective management of trastuzumab deruxtecan (T-DXd)-induced interstitial lung disease (ILD) in breast cancer patients. ESMO Open. 2023;8(6) doi: 10.1016/j.esmoop.2023.102043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jourdain H., Albin N., Monard A., Desplas D., Zureik M., Haddy N. Trastuzumab deruxtecan in human epidermal growth factor receptor 2-positive metastatic gastric cancer in a real-world setting: a nationwide cohort study. Clin Transl Gastroenterol. 2024;15(12) doi: 10.14309/ctg.0000000000000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazaratos A.M., Dankner M., Hamouda A., et al. The real-world clinical outcomes of heavily pretreated HER2+ and HER2-low metastatic breast cancer patients treated with trastuzumab deruxtecan at a single centre. Curr Oncol. 2024;32(1):1. doi: 10.3390/curroncol32010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fabi A., Rossi A., Caputo R., et al. Real life outcome analysis of breast cancer brain metastases treated with Trastuzumab Deruxtecan. NPJ Precis Oncol. 2025;9(1):22. doi: 10.1038/s41698-025-00801-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearson J., Khan A., Bhogal T., et al. A comparison of the efficacy of trastuzumab deruxtecan in advanced HER2-positive breast cancer: active brain metastasis versus progressive extracranial disease alone. ESMO Open. 2023;8(6) doi: 10.1016/j.esmoop.2023.102033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frenel J.S., Zeghondy J., Guérin-Charbonnel C., et al. Tucatinib combination treatment after trastuzumab-deruxtecan in patients with ERBB2-positive metastatic breast cancer. JAMA Netw Open. 2024;7(4) doi: 10.1001/jamanetworkopen.2024.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hurvitz S.A., Hegg R., Chung W.P., et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet. 2023;401(10371):105–117. doi: 10.1016/S0140-6736(22)02420-5. [DOI] [PubMed] [Google Scholar]
- 32.Hong Y.D., Jansen J.P., Guerino J., et al. Comparative effectiveness and safety of pharmaceuticals assessed in observational studies compared with randomized controlled trials. BMC Med. 2021;19(1):307. doi: 10.1186/s12916-021-02176-1. https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-021-02176-1 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harbeck N., Ciruelos E., Jerusalem G., et al. Trastuzumab deruxtecan in HER2-positive advanced breast cancer with or without brain metastases: a phase 3b/4 trial. Nat Med. 2024;30(12):3717–3727. doi: 10.1038/s41591-024-03261-7. https://www.nature.com/articles/s41591-024-03261-7 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.André F., Cortés J., Curigliano G., et al. A pooled analysis of trastuzumab deruxtecan in patients with human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer with brain metastases. Ann Oncol. 2024;35(12):1169–1180. doi: 10.1016/j.annonc.2024.08.2347. [DOI] [PubMed] [Google Scholar]
- 35.Lin N.U., Murthy R.K., Abramson V., et al. Tucatinib vs placebo, both in combination with trastuzumab and capecitabine, for previously treated ERBB2 (HER2)-positive metastatic breast cancer in patients with brain metastases: updated exploratory analysis of the HER2CLIMB randomized clinical trial. JAMA Oncol. 2023;9(2):197–205. doi: 10.1001/jamaoncol.2022.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cecco S., Puligheddu S., Fusaroli M., et al. Emerging toxicities of antibody-drug conjugates for breast cancer: clinical prioritization of adverse events from the FDA adverse event reporting system. Target Oncol. 2024;19(3):435–445. doi: 10.1007/s11523-024-01058-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murthy R.K., Loi S., Okines A., et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382(7):597–609. doi: 10.1056/NEJMoa1914609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.