Abstract
Organoids have emerged as a transformative in vitro platform, offering reliable recapitulation of human tissue architecture and function compared to conventional two-dimensional (2D) cultures. Concurrently, engineered nanoparticles (NPs) have been integrated into organoid systems to enhance scaffold functionality and expand their application in drug delivery, toxicity screening, and disease modeling. Furthermore, decellularized extracellular matrix (dECM) has attracted wide attention for its application in organoid culture, as it provides tissue-specific biochemical and mechanical cues that more closely resemble the native niche, thereby promoting organoid maturation. This review summarizes recent studies that explore how NPs and dECM contribute to the growth and maturation of organoids. It further discusses their applications in therapeutic development and disease modeling, as well as emerging strategies toward refined organoid platforms. Lastly, we outlined how the combined utilization of NPs and dECM may further improve organoid research by enhancing both structural and functional complexity. Together, these approaches support the advancement for developing multifunctional organoid models with broad applicability in disease modeling, therapeutic screening, and regenerative medicine.
Keywords: Organoids, Nanoparticles, Decellularized extracellular matrix, Tissue engineering
Graphical abstract
1. Introduction
Organoids have revolutionized in vitro modeling by enabling the generation of three-dimensional (3D) mini-organs that recapitulate structural and functional properties of native tissues. Conventional two-dimensional (2D) cultures, which have been the standard for decades, lack spatial complexity and cell-to-cell interactions that are crucial for natural organ development and homeostasis. Organoids, in contrast, are derived from tissue-specific or pluripotent stem cells (PSCs) using precisely formulated cocktails of growth factors and supportive scaffolds [1,2]. Under these optimized conditions, the cells self-organize into 3D architectures that exhibit organ-specific features, such as apicobasal polarity, compartmentalization, and multilineage differentiation [3,4]. Due to these advantages, organoids are increasingly utilized not only as biomimetic alternatives to 2D cultures but also as ethically favorable partial substitutes for animal models [5,6]. They have been applied across various biomedical fields and are particularly valuable for studying complex disease processes [7]. For instance, they have proven useful in investigating pathophysiology, therapeutic responses, and drug safety in disease-specific contexts, such as neurodegenerative disorders, liver fibrosis, and cancer [[8], [9], [10], [11], [12]]. Despite these promising features, current organoid systems still face challenges in fully recapitulating the complexity and functionality of native tissues, highlighting the need for further refinement in their structural and environmental design [13].
Concurrently, advances in nanotechnology have introduced a range of engineered nanoparticles (NPs)—nanoscale materials typically ranging from 1 to 100 nm in size—that possess tunable physicochemical properties for biomedical applications [14,15]. These include inorganic (e.g., gold, silver, iron oxide), carbon-based (e.g., carbon nanotubes, graphene oxide), polymeric (e.g., nanocellulose, poly(L-lactic acid)), and lipid-based (e.g., extracellular vesicles, lipid nanoparticles) formulations [16,17]. Given their high surface-area-to-volume ratio, ease of functionalization (e.g., ligand or peptide conjugation), and ability to encapsulate diverse therapeutic or diagnostic agents, NPs have shown significant promise in therapeutic fields such as regenerative medicine and oncology [[18], [19], [20], [21]]. Importantly, when applied to organoid models, these features help overcome key limitations in complex 3D structures, such as restricted nutrient diffusion, inadequate mechanical support, and limited spatial control in imaging or drug delivery [2,22,23]. Furthermore, NP-integrated organoid platforms have shown increasing utility in translational applications, ranging from targeted therapy and biosensing to disease modeling and personalized treatment strategies [[24], [25], [26]].
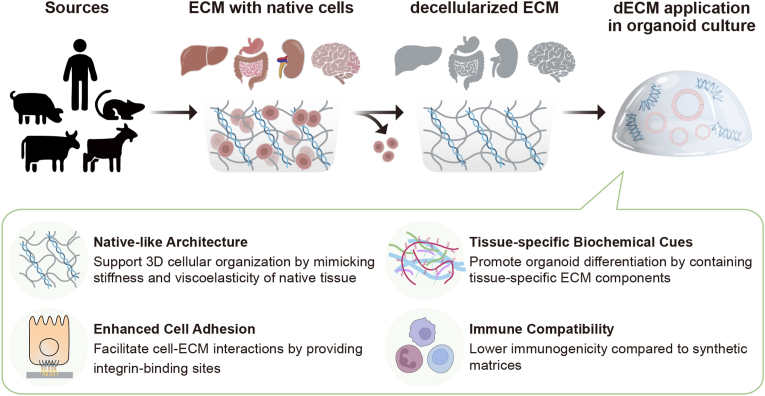
Although nanoparticles have extended the multifunctional capabilities of organoids, there remains a considerable gap in their ability to fully recapitulate the native microenvironment. Organoids require an extracellular matrix (ECM) that provides not only mechanical support but also essential biochemical cues that guide cell differentiation, polarization, and tissue organization [[27], [28], [29]]. Recently, the decellularized extracellular matrix (dECM) has emerged as a promising strategy to resolve the challenges associated with NP-based approaches in organoid culture [[30], [31], [32], [33]]. By removing cellular components and nucleic acids from native tissues, dECM retains essential ECM proteins, glycoproteins, and bioactive factors, providing a tissue-specific microenvironment that better supports in vitro tissue development and physiological functionality [34,35]. Unlike synthetic or commercial matrices such as Matrigel, dECM contains a repertoire of organ-specific signals and microstructures that are difficult to replicate artificially. Indeed, several studies have shown that culturing organoids in dECM not only leads to more reliable architectural development but also promotes enhanced functional maturation, as evidenced by increased albumin secretion in hepatic models and improved electrophysiological activity in cerebral models [36,37]. Considering these advantages, dECM can serve as a complementary strategy to nanoparticle-based systems, potentially addressing their biological limitations (Fig. 1).
Fig. 1.
Schematic illustration depicting the synergistic roles of nanoparticles (NPs) and decellularized extracellular matrix (dECM) in development of organoids through structural support, biochemical cues, and dynamic real-time monitoring.
This review provides a comprehensive overview of recent investigations into the application of nanoparticles and decellularized ECM in organoid research. We highlight how these materials have been employed to enhance the growth, maturation, and functional performance of organoids across various bioengineering strategies. Furthermore, we examine emerging evidence supporting the synergistic integration of NPs and dECM, emphasizing their potential to overcome current limitations and drive the development of more biomimetic and clinically translatable organoid models.
2. Nanoparticles (NPs) in organoid research: Recent progress and applications
Nanoparticles (NPs) have developed in parallel with organoid technologies, offering complementary solutions to challenges faced in each field. Nanostructured materials enhance the integrity of organoids through their structural, electrical, and physicochemical properties, while various engineered NPs have been utilized to improve organoid development and physiological functions [23,38]. Conversely, organoid models have introduced organotypic platforms that bridge the gap between preclinical testing and clinical translation in nanomedicine [25,[39], [40], [41]]. While recent work by Shi et al. [22] primarily explored the roles of nanomaterials in regulating stem cell fate and leveraging nanotechnologies to construct 3D microenvironments for personalized medicine, this review shifts focus toward the functional applications of nanoparticles in organoid systems. Specifically, we cover how various types of nanoparticles contribute to the growth and maturation of organoids, as well as their translational potential in therapeutic delivery, toxicity assessment, and mechanistic studies (Fig. 2).
Fig. 2.
Schematic overview of the NP applications in organoid models.
2.1. Nanoparticles for enhanced organoid growth and maturation
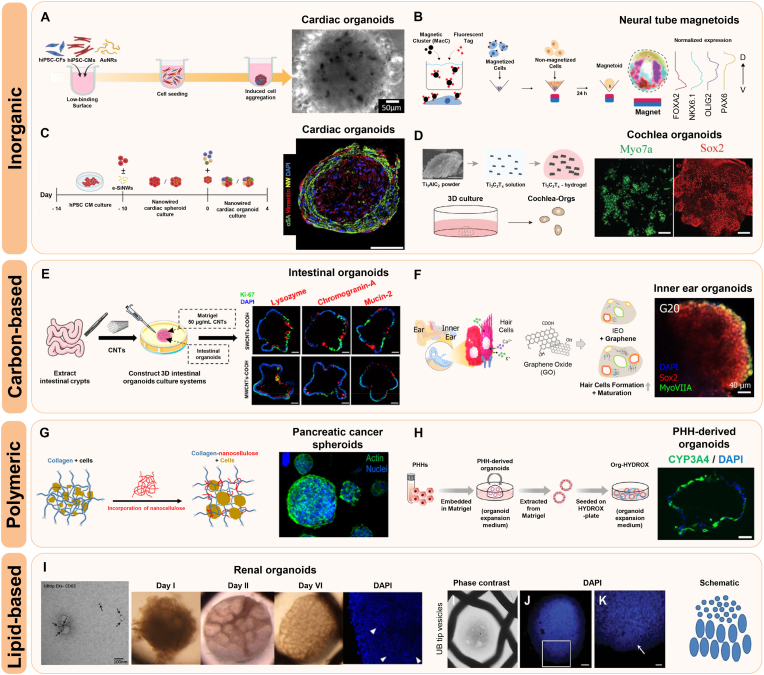
Organoids require microenvironments that closely recapitulate physiologically relevant conditions, including appropriate scaffold composition, mechanical stiffness, and spatiotemporally controlled molecular signals [1,2]. Nanoparticle-based approaches are regarded as promising strategies, providing mechanical reinforcement, controlled delivery of bioactive molecules, and improved cell-cell or cell-ECM interactions [[42], [43], [44]]. Through deliberate design and surface functionalization, nanoparticles can optimize the organoid niche, ultimately supporting more robust growth and functional maturation. To address these multifaceted requirements, different categories of nanoparticles—including inorganic, carbon-based, polymeric, and lipid-based—have been investigated for their utility in organoid culture (Table 1) (Fig. 3).
Table 1.
Nanoparticles used for the enhanced growth and maturation of organoids.
| Type | NPs | Characteristics | Functions in organoid models | Organoid types | Refs |
|---|---|---|---|---|---|
| Inorganic | AuNPs | Biocompatible; surface-conjugated with BDNF | Promote neuronal differentiation | Cerebral organoids | [51] |
| AuNRs | Improved cellular aggregation; scaffold-free organoid formation | Enhance electrical conductivity and intercellular interactions | Cardiac organoids | [52] | |
| Gold and iron oxide MNPs | Magnetic properties | Facilitate 3D levitation and innervation | Salivary glands organoids | [56,57] | |
| Fe3O4 MNPs | Magnetic properties | Guide asymmetric tissue growth and neural tube development | Neural tube magnetoids | [58] | |
| Fe3O4 MNPs | Magnetic properties; thermoresponsive | Reduce cryo-damage during freeze-thaw cycles | Heart organoids | [62] | |
| Silicate NPs | Mesoporous; low toxicity | Promote B cell proliferation and GC reactions | B cell follicle organoids | [65] | |
| Silicon nanowires | Electrically conductive | Improve electrical pacing and cardiac repair | Cardiac organoids | [67] | |
| Ti3C2Tx MXene | Electrically conductive; mechanical flexible | Promotes hair cell formation via mTOR signaling | Cochlear organoids | [71] | |
| Carbon-based | CNTs | Mechanical reinforcement; high surface area | Accelerate ECM degradation and mechanical signaling | Intestinal organoids | [75] |
| SCACs | Peptide-assembled; resembled with SWCNTs | Enhance ESC adhesion and neurogenesis | Forebrain organoids | [76] | |
| GO | Electrically conductive; high mechanical strength | Boosts hair cell maturation and function | Inner ear organoids | [80] | |
| Polymeric | NC | TEMPO-oxidized; biocompatible | Supports hepatocyte differentiation | Liver organoids | [93] |
| TEMPO-oxidized; RGD-functionalized | Promotes epithelial differentiation and maturation | Small intestinal organoids | [94,95] | ||
| Collagen-nanocellulose hybrid; thermoresponsive | Supports epithelial organization | Intestinal organoids | [96] | ||
| Collagen-nanocellulose hybrid | Enhances uniform spheroid growth | Pancreatic cancer cell spheroids | [97] | ||
| HYDROX | PSar-PLLA based nanofiber hydrogel; biodegradable | Facilitates hepatic differentiation and long-term culture | PHH-derived organoids | [101] | |
| Lipid-based | Exosomes | Kidney UB cell line derived vesicles; biocompatible | Enhance nephron patterning and survival | Renal organoids | [105] |
Abbreviations: AuNPs, gold nanoparticles; AuNRs, gold nanoribbons; MNPs, magnetic nanoparticles; CNTs, carbon nanotubes; SCACs, single-chain atomic crystals; GO, graphene oxide; NC, nanocellulose; BDNF, brain-derived neurotropic factor; GC, germinal center; mTOR, mammalian target of rapamycin; SWCNT, single-walled CNT; ESC, embyonic stem cell; UB, ureteric bud; PHH, primary human hepatocyte.
Fig. 3.
Representative examples of nanoparticles (NPs) used for organoid development. A Application of gold nanoribbons (AuNRs) for the formation of hiPSC-derived cardiac organoids. Reprinted with permission from Ref. [52] Copyright 2023, Royal Society of Chemistry. B Generation of human neural tube magnetoids and the expression patterning of neuronal markers. Reprinted with permission from Ref. [58] Copyright 2023, Springer Nature. C Sequential seeding of hPSC-derived cardiomyocytes and supporting cells onto an electrically conductive silicon nanowire (e-SiNW) scaffold. Reprinted with permission from Ref. [67] Copyright 2023, American Association for the Advancement of Science. D Fabrication of Ti3C2TxMXene hydrogel and representative confocal images showing hair cell and supporting cell markers in cochlear organoids. Reprinted with permission from Ref. [71] Copyright 2022, John Wiley and Sons. E Construction of intestinal organoids using single-walled (SWCNTs) and multi-walled carbon nanotubes (MWCNTs). Reprinted with permission from Ref. [75] Copyright 2021, American Chemical Society. F Generation and characteristics of graphene-inner ear organoids. Reprinted with permission from Ref. [80] Copyright 2023, American Chemical Society. G Nanocellulose-based collagen matrix supporting pancreatic cancer cell spheroids. Reprinted with permission from Ref. [97] Copyright 2024, American Chemical Society. H Incorporation of primary human hepatocyte (PHH)-derived organoids into a biodegradable 3D nanofiber hydrogel (HYDROX). Reprinted with permission from Ref. [101] Copyright 2024, Springer Nature. I Formation and cellular organization of renal organoids via internalization of exosomes derived from a ureteric bud (UBtip) cell line. Reprinted with permission from Ref. [105] Copyright 2018, John Wiley and Sons.
2.1.1. Inorganic
Inorganic nanoparticles possess distinctive features, such as tunable structures, facile functionalization, and stable physicochemical properties, which collectively support improved organoid growth and maturation [[45], [46], [47]]. Gold nanoparticles (AuNPs) are widely used for their strong biocompatibility and versatile surface chemistry, designating them as ideal nanocarriers for the delivery of organoids [44,[48], [49], [50]]. AuNPs conjugated with brain-derived neurotrophic factor (BDNF) have been reported to enhance neuronal differentiation in cerebral organoids [51]. By incubating 25-day-cultured cerebral organoids with these BDNF-conjugated AuNPs, researchers achieved deep nanoparticle penetration without cytotoxicity, leading to upregulated expressions of neuronal differentiation genes and more mature cerebral organoids. In addition to zero-dimensional AuNPs, one-dimensional gold nanoparticles, such as nanoribbons (AuNRs), have been introduced for organoid systems. In a cardiac organoid model, AuNRs have been synthesized using a bi-surfactant system consisting of cetrimonium bromide (CTAB) and sodium oleate (NaOL), followed by PEGylating to improve biocompatibility and reduce cytotoxicity (Fig. 3A) [52]. When introduced to cardiac organoids, these AuNRs promoted cellular aggregation and scaffold-free formation of organoids, while enhancing electrical conductivity and intercellular interactions, thereby contributing to the development of functionally mature cardiac organoids.
Magnetic nanoparticles (MNPs) have also emerged as powerful tools for organizing 3D tissue constructs [[53], [54], [55]]. Adine et al. assembled innervated secretory epithelial organoids by magnetically levitating cells using gold and iron oxide NPs crosslinked with a poly-L-lysine biopolymer, demonstrating key aspects of salivary gland epithelial morphology and neural innervation [56,57]. Additionally, Abdel Fattah et al. introduced a novel platform termed "magnetoids," where localized magnetic clusters within neural organoids enabled spatiotemporally controlled mechanical stimulation (Fig. 3B) [58]. This internal actuation significantly guided asymmetric tissue growth, cytoskeletal remodeling, and dorsoventral patterning in neural tube development. Beyond their magnetic properties, iron oxide NPs have also been utilized for their thermoresponsive features in cryopreservation [[59], [60], [61]]. In nanowarming systems, Fe3O4 nanoparticles have been incorporated into cryoprotectants for organoids to reduce osmotic stress and inhibit ice formation during the freeze-thaw process [62]. Researchers investigated that this method significantly preserves cell viability and restores their physiological functions, such as beating ability and contractile force, in heart organoids following freeze-thaw cycles.
Silicon/silica nanoparticles (Si/SiO2NPs) have also been applied to organoid culture due to their mesoporous structure, easy availability, and biologically low toxicity [63,64]. To develop ex vivo immune organs, Purwada et al. introduced a B cell follicle organoid model by embedding B cells and engineered stromal cells in a gelatin-silicate NP hydrogel, designed to mimic germinal center (GC) reactions typically seen in secondary lymphoid organs [65,66]. In this environment, B cells rapidly upregulated GC markers and underwent antibody class switching, which are hallmarks of GC responses, indicating the potential of silicate-based materials for understanding complex immune processes. Furthermore, electrically conductive silicon nanowires (e-SiNWs) were incorporated into cardiac organoids to enhance electrical pacing and improve therapeutic efficacy for heart transplantation (Fig. 3C) [67]. When applied to ischemia/reperfusion (I/R)-injured rat hearts, the organoids significantly recovered contractile performance and cardiac functions.
In addition to gold, iron oxide, and silicate, other inorganic nanoparticles also show considerable promise for organoid applications. Recently, MXenes, an emerging 2D inorganic materials, have gained attention in tissue engineering due to their distinctive properties, such as high electrical conductivity and mechanical flexibility [[68], [69], [70]]. In a cochlea organoid model, Zhang et al. demonstrated that incorporating Ti3C2Tx MXenes into a Matrigel scaffold modulated the mechanical properties of the hydrogel and enhanced hair cell development via mammalian target of rapamycin (mTOR) signaling (Fig. 3D) [71]. The MXenes-Matrigel also facilitated robust synapse-like connections between hair cells and sensory neurons, suggesting the potential of MXene-based systems for advancing hearing-loss therapies.
2.1.2. Carbon-based
Carbon-based nanoparticles are characterized by mechanical robustness, high surface area, and adjustable chemical properties, all of which can support the process of 3D tissue formation and functional maturation [[72], [73], [74]]. Among them, carbon nanotubes (CNTs)—either single‐walled (SWCNTs) or multi-walled (MWCNTs)—play a remarkable role in intestinal organoids development by modulating both extracellular matrix (ECM) and intracellular metabolism (Fig. 3E) [75]. Researchers have shown that CNTs reduce ECM stiffness by upregulating matrix metalloproteinase (MMP) expression (e.g., MMP2, MMP7, and MMP9), thereby accelerating ECM degradation and enhancing mechanical signaling through the Piezo-p38 MAPK-YAP signaling pathways. Meanwhile, CNTs enter organoids, localize to mitochondria, and boost both oxidative phosphorylation (OXPHOS) and glycolysis, which in turn increases overall ATP production. These changes collectively promote cell proliferation and lineage differentiation in intestinal organoids, although SWCNTs have milder effects than MWCNTs. Furthermore, peptide-assembled single-chain atomic crystals (SCACs), which resembles with SWCNTs, have been applied to enhance human embryonic stem cell (ESC) adhesion and promote neuronal lineage differentiation [76]. During brain organoid development, SCACs have been shown to increase neural markers and drive more robust neuronal differentiation, presumably through providing both physical support and biochemical cues.
Graphene oxide (GO) has emerged as another carbon‐based 2D nanomaterial with high electrical conductivity, mechanical strength, and surface area [[77], [78], [79]]. By facilitating both cell-ECM and cell-cell interactions, GO boosts the differentiation of specialized cell types in inner ear organoids (IEOs), particularly sensory hair cells (Fig. 3F) [80]. In this model, GO incorporation was shown to upregulate gap junction proteins and ECM‐related signals, thereby accelerating hair cell formation and IEO maturity, as well as enabling more sensitive drug screening. These findings emphasize the potential of GO-based hybrids to enhance IEO models by better replicating their native structure and function.
2.1.3. Polymeric
Polymeric nanoparticles, composed of natural or synthetic macromolecules, are widely explored in tissue engineering due to their modifiable physical characteristics and minimal cytotoxicity [[81], [82], [83], [84], [85]]. Nanocellulose (NC) is a promising polymeric material extracted from cellulose, which is derived from various sources, including wood, algae, bacteria, and biomass [[86], [87], [88]]. This biomaterial comprises a range of nanoscale cellulose-based structures, with physical and chemical properties that vary depending on the origin, extraction method, and subsequent surface modifications. Among chemical treatments, 2,2,6,6-Tetramethylpiperidine-1-oxyl (TEMPO) oxidation is one of the most widely used strategies, introducing carboxylic groups onto the surface of cellulose nanofibrils to enhance colloidal stability, enable ionic cross-linking, and promote mechanically stable hydrogel formation [[89], [90], [91], [92]]. TEMPO-oxidized cellulose nanofibrils have been effectively applied in hepatic and intestinal organoid cultures, where they support hepatocyte differentiation and epithelial maturation, respectively (Fig. 3G) [[93], [94], [95]]. Moreover, functionalization with Arg-Gly-Asp (RGD) peptides and cationic cross-linkers such as Ca2+ and Mg2+ improves cell adhesion and mechanical stability, enabling sustained culture and passaging of organoids. Additionally, collagen-nanocellulose hybrid (COL-NC) scaffolds offer increased spatial uniformity and mechanical strength, promoting spheroid formation and epithelial organization in pancreatic and intestinal organoid models [96,97].
In addition to natural polymer hydrogels, synthetic polymer systems may offer even greater flexibility in engineering the biochemical and mechanical environment of organoids [[98], [99], [100]]. For example, HYDROX, a biodegradable 3D nanofiber hydrogel composed of poly(sarcosine) and poly(L-lactic acid) (PSar-PLLA), was developed for the culture of primary human hepatocyte (PHH)-derived organoids (Fig. 3H) [101]. Despite limited proliferation, HYDROX scaffolds significantly improved the expression of hepatic functional genes, such as ALB, CYP3A4, CK8, and HNF4α, and exhibited strong cytochrome P450 enzyme activity comparable to that in PHHs. Furthermore, hepatic organoids in this system maintained their functions for up to 35 days, suggesting their application for chronic hepatotoxicity assessment.
2.1.4. Lipid-based
In recent years, lipid-based nanoparticles, such as extracellular vesicles (EVs), liposomes, and solid lipid nanoparticles, have gained increasing attention in biomedical applications due to their intrinsic biocompatibility, biodegradability, and low immunogenicity [[102], [103], [104]]. While their application in organoid systems remains relatively limited, these nanocarriers hold considerable promises for supporting organoid development. A representative example involves the application of EVs as secondary inductive signals during kidney organogenesis (Fig. 3I) [105]. In this study, exosomes derived from an immortalized ureteric bud (UBtip) cell line were shown to be internalized by metanephric mesenchyme (MM) cells in a kidney organoid model, enhancing cellular organization and MM cell survival. Although some miRNAs associated with Wnt/β-catenin pathway inhibition were identified, the overall exosomal cargo favored activation of this pathway, indicating a supportive role in nephrogenesis. These findings underscore the potential of exosomes in developmental signaling and highlight the need for further research on lipid-based nanoparticles in organoid engineering.
2.2. Applications of NP-organoid systems
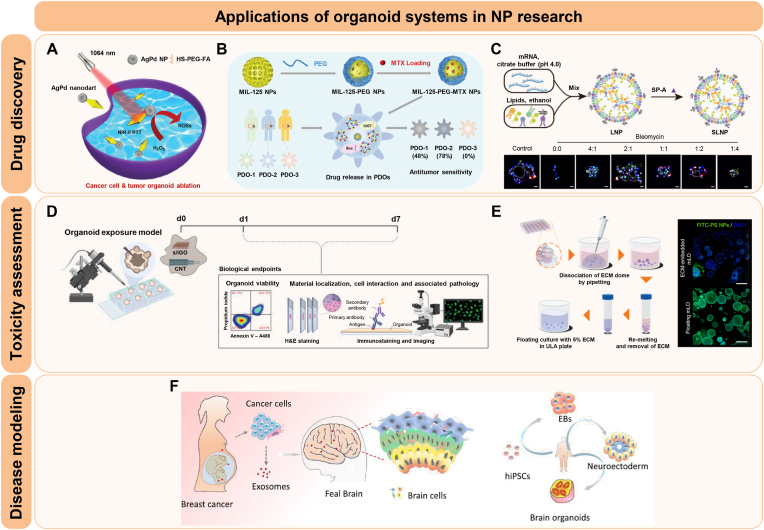
The integration of functional organoids and nanotechnology creates a powerful platform for advancing translational research. Herein, we introduce the biomedical applications of NP-Organoid systems, with a focus on drug delivery and therapeutic strategies, toxicity and safety assessments, and disease diagnosis and mechanistic research within organoid models (Fig. 4).
Fig. 4.
Applications of organoid systems in NP research. A Photothermal tumor ablation in cancer organoids using AgPd nanoparticles. Reprinted with permission from Ref. [108] Copyright 2024, John Wiley and Sons. B Evaluation of the anti-tumor efficacy of metal-organic framework (MOF) nanoparticles in patient-derived organoids (PDOs). Reprinted with permission from Ref. [107] Copyright 2024, American Chemical Society. C Assessment of a lipid nanoparticle (LNP)-based delivery system for pulmonary disease using alveolar organoid models. Reprinted with permission from Ref. [110] Copyright 2024, American Association for the Advancement of Science. D Toxicity evaluation of carbon-based NPs via microinjection in a human lung organoid model. Reprinted with permission from Ref. [116] Copyright 2024, Elsevier. E A novel method for nanotoxicity testing using floating culture for organoids. Reprinted with permission from Ref. [120] Copyright 2024, American Chemical Society. F Modeling breast cancer exosome-mediated fetal neurodevelopmental effects using brain organoids. Reprinted with permission from Ref. [127] Copyright 2022, Springer Nature.
2.2.1. Drug delivery and therapeutic strategies
Recent advances in nanomedicine have been further accelerated by the emergence of organoid systems, which closely resemble in vivo tissue environments for evaluating nanoparticle-based therapeutics [106,107]. These biomimetic models provide suitable tools for investigating drug responses, including therapeutic efficacy, toxicity, and underlying biological mechanisms of nanomedicine—particularly in complex diseases such as cancer and neurodegeneration. These approaches offer deeper insights that are often challenging to obtain using conventional 2D cultures or animal models.
A growing number of studies have demonstrated the potential of NPs in delivering anti-cancer therapeutics using organoid models. For instance, Zhang et al. synthesized multifunctional plasmonic “nanodarts” by depositing AgPd bimetallic tips on gold nanobipyramids and applied them to cancer cells and tumor organoids for photothermal-catalytic combined cancer cell ablation, achieving efficient tumor cell destruction (Fig. 4A) [108]. To further enhance functionality, the researchers selectively coated the AgPd tips with other nanoparticles, including zeolitic imidazolate framework-8 (ZIF-8) and TiO2, generating hybrid nanodarts with improved antimicrobial and structural properties. Similarly, nanosized metal-organic framework (MOF) nanoparticles were evaluated for their anti-tumor efficacy in a colorectal cancer (CRC) organoid model (Fig. 4B) [107]. Among three typical MOFs—ZIF-8, ZIF-67, and MIL-125—MIL-125 demonstrated superior biocompatibility in both intestinal and hepatic organoid models. When loaded with methotrexate (MTX), it showed efficient drug loading and release, resulting in significant anticancer effects in patient-derived CRC organoids. In another study, semiconducting polymer nanoparticles functionalized with hyaluronic acid (HA) were developed for CD44-targeted photothermal therapy in CRC organoids [109]. Despite limited tissue penetration, HA coating facilitated targeted retention at the periphery of organoids, allowing efficient heat-induced ablation and reduced tumor viability upon laser activation.
In addition to cancer research, organoid systems have also been applied to explore nanotherapeutics targeting complex diseases that are difficult to model using conventional systems. For idiopathic pulmonary fibrosis (IPF), inhalable mucus-penetrating mRNA-lipid nanoparticles (LNPs) promoted alveolar type 2 (AT2)-to-AT1 epithelial cell differentiation and supported alveolar regeneration, as demonstrated in an alveolar organoid model (Fig. 4C) [110]. This platform also enabled the evaluation of co-delivered BMP4 and CYB5R3 mRNAs, which restored mitochondrial function and attenuated fibrotic remodeling.
In myocardial ischemia-reperfusion injury (IRI), Poh et al. reported that mesenchymal stem cell-derived small extracellular vesicles (MSCs-EVs) significantly reduced apoptosis and improved contractile recovery in human cardiac organoids, indicating their cardioprotective effects [111]. Metabolomic analysis further showed that EV treatment modulated unsaturated very long-chain fatty acids (VLCFAs), lipids associated with oxidative stress, thereby reducing cell death and promoting functional recovery of cardiac tissue.
Additionally, in a neurodegenerative disease model, Sol to B-Mica Powder (STB-MP), mica-derived nanoparticles, were applied to Alzheimer's disease (AD) patient-derived cortical brain organoids, resulting in a significant reduction in Aβ plaque accumulation without inducing cytotoxicity [112]. Mechanistically, STB-MP exerted its therapeutic effects by suppressing proinflammatory cytokine levels and γ-secretase activity, while concurrently promoting autophagy through the inhibition of mTOR expression within the AD brain organoid model. Collectively, these findings emphasize the utility of organoid platforms for evaluating nanoparticle-based delivery systems, therapeutic efficacy, and mechanistic action across diverse diseases.
2.2.2. Toxicity and safety assessments
To expand the clinical applicability of nanoparticles, accurate toxicity and safety evaluation are essential. Organoid models are increasingly utilized in nanotoxicity screening due to their ability to replicate native tissue complexity [[113], [114], [115]]. For example, a human lung organoid model was used to evaluate pulmonary response to carbon-based nanoparticles by microinjecting graphene oxide (GO) and multi-walled carbon nanotubes (MWCNTs) into the lumen of the organoids, mimicking inhalation exposure (Fig. 4D) [116]. Consistent with in vivo pulmonary fibrosis models, GO exhibited no significant level of toxicity, while MWCNTs induced fibrotic remodeling, evidenced by epithelial thickening and elevated mesenchymal marker expression in the organoid system. These results reveal the relevance of lung organoids in assessing pulmonary toxicity and fibrogenic responses to nanoparticles.
Importantly, organoid models are increasingly used to investigate NP-induced neurotoxicity. For example, Huang et al. applied cerebral organoids from human induced pluripotent stem cells (hiPSCs) to evaluate the neurodevelopmental toxicity of silver nanoparticles (AgNPs) [117]. Exposure to low and high concentrations over a 7-day period revealed that higher doses of AgNPs caused critical cilia morphology disruption, impaired cytoskeletal integrity, and suppressed neuronal marker expression in a dose-dependent manner. While brain organoids represent a promising model for assessing NP-induced neurotoxicity, this study also emphasizes the importance of integrating in vitro blood-brain barrier (BBB) models through organ-on-a-chip or 3D-printed microfluidic technologies to address limitations and better simulate in vivo neurovascular environments. In another study, hiPSC-derived 3D brain organoids expressing cortical layer proteins were used to assess the neurotoxicity of zinc oxide nanoparticles (ZnO NPs) [118]. In this study, high concentrations of Zn NPs induced cytotoxicity primarily through defective autophagy and intracellular Zn ion accumulation, rather than oxidative stress. Fluorescence micro-optical sectioning tomography demonstrated that the outer organoid layers, directly exposed to NPs, showed reduced LC3B protein expression and increased micronuclei formation, while autophagy inhibition further increased cytotoxicity and Zn ion buildup, indicating the importance of autophagy in mediating ZnO NP-induced neurotoxicity.
Hepatotoxicity evaluation using organoid platforms has also provided valuable insights into NP-induced liver injury. In one study, human liver organoids and a rat convulsion model were utilized to investigate the potential hepatotoxic effects of aspartic acid-coated magnesium oxide nanoparticles (MgO NPs) and the antiepileptic drug valproate, both individually and in combination [119]. The combined exposure induced marked liver toxicity, as evidenced by decreased cell viability, reduced ATP production, elevated ROS levels in vitro, and increased serum liver enzyme activities in vivo. These findings showed that organoid models can detect the effects of compound-nanoparticle interactions that may not be captured by conventional in vitro systems. Building on the use of organoid-based hepatotoxicity assays, Lee et al. developed a novel floating culture system for liver organoids that bypass the limitations of conventional ECM-embedded methods (Fig. 4E) [120]. This method produced uniformly sized organoids with preserved hepatic differentiation markers, while significantly enhancing the internalization of nanoparticles such as gold and polystyrene nanoparticles. Notably, cytotoxic effects of zinc oxide (ZnO) nanoparticles were evident only in floating organoids, as ECM-embedded organoids impeded nanoparticle access and failed to reflect toxicity. These results establish the floating system as a more effective and reliable strategy for standardized safety evaluation of nanoparticles.
In addition to determining cytotoxicity, organoid models can also assess the biocompatibility of NPs by evaluating the maintenance of tissue-specific functions, structural integrity, and inflammatory response. Iqbal et al. synthesized biocompatible magnetic Fe-TiO2 nanorods via solvothermal and thermal decomposition methods, and assessed biocompatibility by both conventional 2D cell culture and 3D organoid models [121]. Similarly, Leite et al. tested the neurotoxicity and biocompatibility of gold nanoparticles (AuNPs) and polylactic acid nanoparticles (PLA-NPs) using human 3D brain models [122]. They demonstrated that AuNPs, particularly PEGylated forms, induced mitochondrial dysfunction, activated oxidative stress genes, and altered cytokine profiles, whereas PLA-NPs maintained cellular physiology in the organoid models. These results show that multicellular 3D brain models are valuable tools not only for evaluating the safety of nanoparticles but also comprehensively assessing their biocompatibility and further indicate that these NPs represent a safer option for CNS drug delivery. Collectively, these studies suggest the versatility of organoid platforms for comprehensive assessment of nanoparticle safety and biocompatibility across diverse organ systems.
2.2.3. Disease diagnosis and mechanistic studies
The application of NP-organoid models is expanding to facilitate the development of novel diagnostic strategies and to elucidate the pathophysiology of complex diseases. For instance, Parkinson's disease (PD) is a progressive neurodegenerative disorder characterized by the accumulation of α-synuclein and subsequent neurotoxicity [[123], [124], [125]]. Consequently, the early and accurate detection of this condition remains a major clinical challenge, emphasizing the necessity for advanced diagnostic strategies that involve sophisticated techniques, such as nanoparticle-based methods and patient-derived organoid systems. Lee et al. developed peptide-imprinted poly(hydroxymethyl 3,4-ethylenedioxythiophene) (PEDOT) nanotube sensors for electrochemical detection of α-synuclein, a key biomarker in PD [126]. The sensing device demonstrated high sensitivity, with a detection limit of 4.0 pM, and were successfully applied to culture medium collected from patient-derived midbrain organoids. This organoid-based system allowed noninvasive, real-time monitoring of α-synuclein expression, supporting its utility in modeling disease-associated protein aggregation and facilitating early diagnostic screening.
In another example, Cui et al. developed a brain organoid-on-a-chip model to study the developmental effects of breast cancer-derived exosomes on the fetal brain (Fig. 4F) [127]. When exposed to exosomes from breast cancer cells (MCF-7), the hiPSC-derived brain organoids exhibited enhanced expression of stemness markers (OCT4, NANOG) and forebrain development markers (PAX6, FOXG1), indicating aberrant maintenance of pluripotency and altered regional identity. Subsequent RNA sequencing further revealed the enrichment of genes associated with breast cancer, medulloblastoma, and other tumorigenic pathways. As a result, these findings suggested that the exposure of tumor-derived exosomes may trigger carcinogenic transformation and impair early neurodevelopment. These results imply that exosomal communication from maternal tumors could cause neurodevelopmental risks to the fetus, which highlights the importance of organoid-chip systems in modeling complex tumor-host interactions.
In summary, nanoparticles—spanning inorganic, carbon-based, polymeric, and lipid-based categories—have been extensively integrated into organoid platforms to reinforce structural and biochemical microenvironments. They also enhance electrical and mechanical coupling, and deliver bioactive or therapeutic agents that promote growth, maturation, and functionality related to diseases. These combined systems have demonstrated utility in targeted drug delivery, photothermal and catalytic therapies, standardized nanotoxicity assessment, and precise disease diagnostics. Despite these advances, several challenges constrain the translational potential of NP-organoid systems. First, NP-based scaffolds lack tissue specificity and basement-membrane cues, which limits lineage stabilization and functional maturation [93]. Second, ECM-mimetic niches with tissue-specific stiffness and viscoelasticity are required to recapitulate in vivo-like responses and to improve the predictivity of NP safety and efficacy assessment [128]. Third, the transport and retention of nanoparticles are often heterogeneous, with diffusion bottlenecks that drive peripheral accumulation [120]. Incorporating ECM components can mitigate these issues by supplying native proteins and basement-membrane motifs, enabling control over stiffness and viscoelastic properties, and providing similar architecture and poroelasticity that promote more uniform NP penetration and application.
3. Decellularized extracellular matrix (dECM): Current results in organoid applications
As organoid systems advance toward greater functional maturity, decellularized extracellular matrix (dECM) serves as a versatile biomaterial that enhances organoid development and supports broader application in translational research. This section reviews recent results in dECM-based scaffolds for diverse organoid systems, with a focus on tissue-specific applications and bioengineering strategies to improve the maturation of organoids, scalability, and disease modeling (Fig. 5).
Fig. 5.
Schematic illustration of dECM-based scaffold engineering for the organoid applications.
3.1. Tissue-specific applications of dECM in organoids
The composition of decellularized ECM varies significantly by tissue and organ of origin, providing a unique biochemical and structural niche for each organoid type. Leveraging tissue-specific dECM enables enhanced lineage commitment, functional maturation, and physiological behavior in organoid cultures. This section summarizes recent studies that employ dECM derived from liver, gastrointestine, kidney, and central nervous system (CNS) to support the development of corresponding organoid systems (Table 2) (Fig. 6).
Table 2.
Tissue-specific applications of decellularized ECM for organoid development.
| Organoid types | Source of dECM | Advantages of dECM | Refs |
|---|---|---|---|
| Human hepatic organoids | Sheep liver dECM | Enhanced hepatic functions (albumin secretion, urea synthesis, CYP3A4 activity) through co-culture with hepatocarcinoma cells, MSCs, and HUVECs in LEMgel | [129] |
| Human intrahepatic cholangiocyte organoids | Human and porcine liver dECM | Supported organoid formation and maintained hepatic and biliary markers (ALB, KRT19) comparable to Matrigel despite slower proliferation | [130] |
| Mouse ductal organoids | Rat liver dECM | Preserved ductal architecture and bile transport functionality; serotonin enrichment | [131] |
| Human endodermal organoids | SI mucosa dECM | Maintained cell viability and differentiation across multiple passages with preserved ECM proteins and Matrigel-like mechanics | [36] |
| Human gastrointestinal organoids | Porcine stomach and small intestine dECM | Preserved lineage marker expression and differentiation potential in both gastric and intestinal organoids | [132] |
| Human kidney organoids | Human and Porcine renal cortex-derived dECM | Promoted nephron-like structure formation and expression of renal and endothelial markers in hPSC-derived kidney assembloids | [133] |
| Human kidney organoids | Porcine Kidney dECM | Supported vascular network formation and disease modeling (e.g., Fabry nephropathy) via bioactive renal microenvironment | [134] |
| Human kidney organoids | Mouse kidney dECM | Induced mesenchymal-to-epithelial transition and nephron marker expression (Nephrin, WT1), supporting CKD drug screening | [135] |
| Human brain organoids | Porcine brain dECM | Enabled cerebral organoid formation with neuroepithelial structure and expression of SOX2, Nestin, and MAP2 | [136] |
| Human spinal cord organoids | Rat brain dECM | Improved regional marker expression and dorsoventral patterning in spinal cord organoids from hiPSCs | [137] |
| Human spinal cord organoids | Human placenta dECM | Enhanced dorsoventral organization and laminar marker expression (PAX6, PAX7, and FOXA2), promoting spinal cord-like structure | [138] |
Abbreviations: dECM, decellularized extracellular matrix; SI, small intestine; LEMgel, liver-derived dECM hydrogel; CKD, chronic kidney disease.
Fig. 6.
Examples of dECM used for organoid development. A Formation of intrahepatic cholangiocyte organoids (ICOs) using liver-derived extracellular matrix (LECM) gels. Reprinted with permission from Ref. [130] Copyright 2022, Elsevier. B Development of gastric and intestinal organoids using stomach and intestinal dECM hydrogels. Reprinted with permission from Ref. [132] Copyright 2022, Springer Nature. C Generation of kidney assembloids supported by decellularized human kidney ECM hydrogels. Reprinted with permission from Ref. [133] Copyright 2024, John Wiley and Sons. D Maturation of cerebral organoids using brain-derived ECM hydrogels. Reprinted with permission from Ref. [136] Copyright 2021, Public Library of Science.
3.1.1. Liver organoids
Decellularized liver-derived scaffolds offer tissue-specific biochemical and mechanical cues that support the growth and maturation of hepatic organoids. Saheli et al. developed a sheep liver-derived dECM hydrogel (LEMgel), prepared using SDS and Triton X-100, which exhibited suitable pore size and viscoelasticity for hepatic organoids [129]. When co-cultured with hepatocarcinoma cells, mesenchymal stem cells (MSCs), and human umbilical vein endothelial cells (HUVECs), the resulting hepatic organoids showed enhanced hepatic functions, including increased albumin secretion, urea production, and CYP3A4 enzyme activity. Cholangiocytes, another type of liver epithelial cells, have been used to generate intrahepatic cholangiocyte organoids (ICOs) in hydrogels derived from human and porcine liver dECM (HLECM and PLECM) (Fig. 6A) [130]. Despite a slight decrease in proliferation rate in LECM, these collagen-rich hydrogels supported the formation of organoids and maintained hepatic and biliary marker expression (e.g., ALB, KRT19) comparable to Matrigel. Similarly, rat liver-derived dECM scaffolds were used to generate functional ductal organoids (FDOs) from primary mouse cholangiocytes, preserving biliary architecture and ECM proteins [131]. These scaffolds supported polarized duct-like structures with active bile transport, and metabolomic analysis revealed serotonin enrichment, suggesting a role in biliary development and scaffold bioactivity.
3.1.2. Gastrointestinal organoids
Decellularized gastrointestinal (GI) tissue-derived hydrogels provide organ-specific extracellular matrices that support the formation and differentiation of gastric and intestinal organoids. A porcine small intestine (SI) dECM hydrogel processed by detergent-enzymatic treatment retained key ECM proteins, including collagens, elastin, and glycosaminoglycans (GAGs), and exhibited gelation kinetics and rheological properties comparable to Matrigel [36]. This matrix enabled the culture of human endodermal organoids, such as stomach and small intestinal organoids, and maintained their viability and the expression of differentiation markers over multiple passages. Likewise, stomach- and intestine-specific ECM hydrogels (SEM and IEM), prepared by non-ionic detergent (Triton X-100) decellularization, preserved tissue-specific biochemical composition and exhibited a higher elastic modulus than those prepared using an ionic detergent (sodium deoxycholate; SDC) (Fig. 6B) [132]. Both gastric and intestinal organoids cultured in these hydrogels expressed lineage-specific markers comparable to or higher than those observed in Matrigel, while maintaining consistent stemness and differentiation potential.
3.1.3. Kidney organoids
Kidney dECM hydrogels, by providing a native-like renal microenvironment, contribute significantly to the structural maturation of kidney organoids. For example, porcine and human renal cortex dECM hydrogels were shown to support the differentiation of human pluripotent stem cell (hPSC)-derived renal organoids (Fig. 6C) [133]. These hydrogels facilitated the maturation of nephron progenitor cells (NPCs), leading to the generation of kidney organoids with distinct segmented nephron-like structures. Bulk RNA-seq analysis showed that the resulting organoids had elevated levels of both renal differentiation markers and endothelial markers, prompting further investigation into kidney-endothelial assembloid formation to more closely replicated in vivo renal architecture. In addition, porcine kidney dECM exhibits mechanical properties suitable for 3D organoid culture [134]. This approach not only enhanced vascular network formation but also enabled disease modeling, such as Fabry nephropathy, suggesting the potential of ECM-based scaffolds. Furthermore, mouse renal extracellular matrix (rECM) gels have been demonstrated to support the differentiation of hiPSC-derived renal organoids [135]. In this model, the mesenchymal-to-epithelial transition and the expression of nephron markers (e.g., Nephrin, WT1) were observed, indicating its potential utility for drug screening in chronic kidney disease (CKD).
3.1.4. CNS organoids
To better understand the functional and pathological complexity of central nervous system (CNS), a range of neural organoid models have been developed. To further promote their maturation and acquisition of human-specific features, dECMs obtained from tissue-specific niches have been employed as supportive scaffolds. Simsa et al. demonstrated that hydrogels prepared from decellularized adult porcine brain extracellular matrix (B-ECM), composed primarily of retained collagens but lacking several brain-specific proteoglycans, could successfully support the formation of human embryonic stem cell (hESC)-derived cerebral organoids (Fig. 6D) [136]. These organoids exhibited comparable expressions of neural progenitor and neuronal markers (e.g., SOX2, Nestin, MAP2) and developed characteristic neuroepithelial structures, indicating that B-ECM may serve as a tissue-specific alternative to Matrigel for the generation of cerebral organoids. In another approach, rat decellularized brain extracellular matrix hydrogel (DBECMH) has been shown to facilitate the generation of hiPSC-derived spinal cord organoids, with enhanced expression of regional spinal cord markers, including both dorsal and ventral patterning genes [137]. Moreover, a novel thermosensitive hydrogel fabricated from decellularized human placenta ECM (DPECMH), enriched with laminin, fibronectin, collagen I, and elastin, provided a human-specific and biocompatible microenvironment for the generation of spinal cord organoids [138]. Compared to Matrigel, organoids cultured in DPECMH exhibited significantly improved dorsoventral organization and increased expression of key laminar markers such as PAX6, PAX7, and FOXA2, supporting the formation of more mature spinal cord-like structures.
Taken together, these findings reveal the critical role of tissue-specific dECM hydrogels in promoting the development of organoid systems that more closely resemble in vivo tissue architecture and function.
3.2. Advances in dECM-based organoid models
While dECM-based scaffolds hold great promise for organoid development, several hurdles still limit their widespread adoption. First, native-like mechanical and biochemical cues are often insufficiently replicated, prompting the investigation of advanced engineering approaches—such as matrix remodeling, 3D bioprinting, and microfluidic systems—to better mimic in vivo microenvironments and guide tissue organization. Second, the absence of standardized decellularization protocols across tissue sources results in batch-to-batch variability and poor reproducibility of the formation of organoids [[139], [140], [141]]. Finally, tissue- and tumor-specific dECM scaffolds are increasingly paired with patient-derived cells to create organoid disease models and drug-testing platforms that replicate in vivo pathology, yet their broader use still depends on precisely tuning ECM composition and mechanical cues while establishing scalable, reproducible fabrication protocols. This section delineates recent bioengineering strategies aimed at overcoming current limitations and enhancing the functional applicability of dECM-based organoid models (Fig. 7).
Fig. 7.
Engineering Strategies for dECM-Based Scaffolds. A Fabrication of organoid-laden dECM bioink using a blue light-induced bioprinting method. Reprinted with permission from Ref. [148] Copyright 2022, John Wiley and Sons. B Bioprinting and application of vascularized organoid models for screening patient-specific chemotherapeutic responses. Reprinted with permission from Ref. [150] Copyright 2023, John Wiley and Sons. C Microfluidic culture of brain organoids using brain dECM hydrogel in a dynamic flow-based device. Reprinted with permission from Ref. [37] Copyright 2021, Springer Nature. D Optimization of decellularization protocols for human lung organoids using bovine lung dECM. Reprinted with permission from Ref. [157] Copyright 2023, American Chemical Society. E Decellularized matrices derived from normal or neoplastic peritoneum, repopulated with peritoneal metastases (PM)-derived organoids. Reprinted with permission from Ref. [164] Copyright 2022, Oxford University Press.
3.2.1. Advanced engineering strategies and mechanical modulation
To improve the biological relevance and functional performance of organoid systems, scaffold materials must replicate the structural, biochemical, and mechanical properties of native tissues. Recent studies have focused on bioengineering techniques, such as matrix remodeling, 3D bioprinting, and microfluidic integration, combined with tissue-specific decellularized ECM to better guide the differentiation and spatial organization of organoids [[142], [143], [144], [145], [146]]. As one matrix remodeling strategy, Xu et al. introduced thrombospondin-1 (THBS1) into hepatic dECM hydrogels to create a dynamically tunable microenvironment for hepatocyte organoids [147]. This remodeling activated the YAP/TAZ signaling pathway, upregulated reprogramming transcription factors such as KLF4 and SOX2, and induced a progenitor-like state in hepatocytes. Transplantation of these reprogrammed hepatocyte organoids into a hepatectomy mouse model significantly enhanced liver regeneration, demonstrating the therapeutic potential of viscoelastic control in dECM scaffolds.
The integration of tissue-specific dECM into 3D bioprinting platforms has enabled the construction of organoid scaffolds with defined architecture and biomimetic properties. For example, a photocrosslinkable bioink composed of porcine small intestine dECM, gelatin methacrylate (GelMA), and hyaluronic acid supported 3D bioprinting of intestinal organoids while preserving stemness (Fig. 7A) [148]. Layer-by-layer printing allowed co-culture with intestinal subepithelial myofibroblasts and macrophages, which promoted intestinal stem cell (ISC) proliferation and niche-specific differentiation. Upon transplantation into NSG mice, the printed constructs facilitated epithelial regeneration, highlighting the therapeutic potential of dECM-based bioinks. In parallel, Choi et al. and Kim et al. modeled tumor-specific microenvironments by developing vascularized lung and gastric cancer organoids using 3D bioprinting with organ-specific dECM bioinks [149,150]. Choi et al. utilized porcine lung dECM with patient-derived tumor cells, fibrotic lung fibroblasts, and endothelial cells to generate lung cancer organoids that retained oncogenic profiles and resistance to EGFR-targeted therapies under fibrotic conditions [149]. Likewise, Kim et al. employed stomach-derived dECM to bioprint patient-derived gastric cancer organoids (PDOs) with perfusable vasculature, accurately recapitulating the PDO molecular subtype-specific responses to VEGFR2-targeted therapy (Fig. 7B) [150].
Microfluidic systems have been increasingly applied to organoid models to enhance nutrient exchange, reduce hypoxia, and provide physiologically dynamic environments [[151], [152], [153], [154]]. In a brain organoid model, Cho et al. incorporated decellularized human brain ECM (BEM) into a pump-free microfluidic device, providing brain-specific biochemical cues while enhancing organoid viability and reducing batch-to-batch variability (Fig. 7C) [37]. This 3D culture system not only supported structural and electrophysiological maturation of brain organoids but also promoted cell proliferation and reduced apoptosis by enabling continuous nutrient and oxygen exchange through microfluidic perfusion. In liver applications, a microfluidic system was also combined with liver dECM scaffolds to create vascularized liver organoids [155]. Co-cultured with induced hepatic (iHep) cells and endothelial cells under continuous flow, the device enhanced cell-cell and cell-matrix interactions while reducing tissue apoptosis. This combination improved vascularization, hepatic metabolic function, and drug response predictability, supporting its application in pharmacological testing.
3.2.2. Standardization and biomanufacturing of dECM scaffolds
Standardizing decellularization protocols is crucial for ensuring reproducibility and scalability in dECM-based organoid systems. In practice, decellularization is carried out as a sequence of physical, chemical, and enzymatical steps [31,33,156]. Physical methods, such as freeze-thaw cycling, mechanical oscillation, superhigh pressure, and electroporation, promote cellular disruption and reagent exchange while, in thick tissues, preserving three-dimensional and vascular architecture. Chemical reagents are chosen to balance efficacy with ECM preservation: ionic detergents (e.g., SDS, sodium deoxycholate) enable aggressive cell lysis, whereas non-ionic detergents (e.g., Triton X-100, Tween-20) and zwitterionic surfactants (e.g., CHAPS) allow gentler extraction; adjuncts such as hyper/hypotonic buffers, selective acids/bases, alcohols for delipidation, chelators, and oxidants used as needed. Enzymatic finishing with proteases (e.g., trypsin, dispase) and nucleases (DNase, RNase) removes residual adhesions and nucleic acids. Parameters including reagent concentration, temperature, exposure time, and perfusion rate are optimized by each tissue to maximize cellular clearance while preserving collagen/elastin networks, glycosaminoglycans (GAGs), basement-membrane proteins, and matrix-bound growth factors.
However, variability among protocols and source tissues still limits batch-to-batch consistency and scale-up, both of which are critical for the practical use of dECM scaffolds. Kim et al. addressed this challenge by developing gastrointestinal (GI) tissue-derived ECM hydrogels that not only preserved essential ECM components but also demonstrated minimal lot-to-lot variation, providing a reliable alternative to Matrigel for long-term culture or organoids [132]. Their optimized non-ionic detergent-based protocol notably supported the robust formation of gastric and intestinal organoids, indicating the potential of tissue-specific dECM in clinical and industrial settings. In parallel, Kuşoğlu et al. compared four decellularization methods for bovine lung tissue, revealing method-dependent changes in ECM composition, stiffness, and viscoelasticity (Fig. 7D) [157]. By investigating the distinct effects of each protocol on lung cancer cells and patient-derived organoids, this study suggested the need for reproducible methods that preserve key biochemical and mechanical cues. Taken together, both papers reveal the importance of advanced decellularization strategies to achieve consistent, high-quality dECM scaffolds across different types of tissue.
3.2.3. Disease modeling and drug testing
Tissue-specific dECM scaffolds have been increasingly adopted to construct organoid-based disease models that more faithfully recapitulate in vivo pathology. In tumor organoid systems, fine-tuning dECM composition is critical for accurately replicating the metastatic microenvironment, which is essential for modeling tumor progression and therapeutic responses [143,[158], [159], [160], [161]]. Extending this concept to organ-specific metastasis, patient-derived cholangiocarcinoma organoids cultured on decellularized human lung or lymph nodes ECM exhibited tissue-specific regulation of epithelial-to-mesenchymal transition (EMT) and cancer stem cell populations. These organoids also showed donor- and tumor-dependent differences in migratory and proliferative behavior, indicating that organ-specific dECM plays a critical role in metastatic outgrowth [162]. Chen et al. developed a liver-derived dECM (L-dECM) scaffold to construct a HepG2-based tumor organoid-like tissue model [163]. The L-dECM scaffold not only enhanced hepatic function but also promoted the EMT, an initial response in tumorigenesis triggered by signals from the surrounding microenvironment. Moreover, the model exhibited differential drug sensitivities, suggesting its utility for in vitro anticancer drug screening and EMT-focused studies. Similarly, Varinelli et al. demonstrated that dECM derived from patient neoplastic peritoneum, characterized by increased stiffness and altered composition, enhanced the growth of metastatic colorectal organoids and mimicked in vivo tumor behavior (Fig. 7E) [164]. Under simulated hyperthermic intraperitoneal chemotherapy (HIPEC) treatment—a heated chemotherapy approach commonly used for abdominal cancers—this ECM reduced the sensitivity of organoids to standard anticancer drugs, indicating its role in promoting treatment resistance. In a breast cancer model, an autologous dECM derived from MCF-7 cells (CD-dECM), combined with rat tendon collagen type I, provided an oncogenic microenvironment that significantly enhanced the viability and proliferation of organoids [165]. This hydrogel also promoted the formation of larger spheroids and supported the development of breast cancer organoids with improved morphological integrity and epithelial organization.
Beyond oncology, tissue-specific dECM has also been applied to enhance organoid maturation and vascularization. Kidney dECM hydrogels supported the generation of hPSC-derived kidney organoids with extensive vascular networks and their own endothelial cells, as well as more mature glomerular structures with greater similarity to human kidney tissues [134]. This approach also enabled efficient recapitulation of Fabry nephropathy with vasculopathy using GLA-knockout hPSCs, and, upon transplantation into mouse kidney, promoted endothelial cell recruitment from the host and maintained vascular integrity with more organized slit diaphragm-like structures. While substantial progress has been made in tumor-focused dECM-organoid systems, studied addressing other disease types remain limited, highlighting the potential of diverse tissue-specific dECM platforms for developing organoid-based disease modeling.
Collectively, tissue-specific dECM hydrogels and bioinks provide organotypic biochemical and mechanical cues that support lineage specification, polarization, electrophysiological maturation, and durable function across liver, gastrointestinal, kidney, and CNS organoids. When coupled with matrix remodeling, 3D bioprinting, and microfluidic perfusion, dECM scaffolds enable defined architecture, multicellular co-culture, and disease models with greater translational relevance for drug testing and regenerative medicine. However, several challenges still hinder their application in organoid culture. Batch-to-batch variability and growth-factor sequestration can shift biochemical composition and distort signaling gradients, yielding inconsistent exposure [[166], [167], [168]]. Mechanical properties can also drift during culture, complicating controlled transport and functional readouts. Furthermore, limited shelf stability and GMP readiness impede scale-up and standardization. In this context, nanoparticles offer complementary functions, including matrix-anchored or stimuli-responsive delivery to standardize exposure and nanoscale reinforcement to stabilize stiffness and poroelasticity. Accordingly, integrating dECM with NP-based components is a rational next step to improve reproducibility, safety, and clinical translatability, providing the basis for the integration strategies outlined in Section 4.
4. Synergistic integration of NPs and dECM: Current challenges and prospects
Nanoparticles (NPs) and decellularized extracellular matrix (dECM) each advance organoid engineering—NPs by enabling structural support and controlled delivery and sensing, and dECM by supplying tissue-specific biochemical and mechanical cues. Their collaboration can compensate for their respective limitations identified in Sections 2, 3, creating more resembled microenvironments with defined delivery kinetics, stabilized stiffness and poroelasticity, and reliable quantitative measurements. Although the evidence upon this is still emerging, convergent results from various tissue and scaffold engineering point to a clear synergy in mechanical integrity, biocompatibility, and functional versatility, positioning NP-dECM integration as a practical route for next-generation organoid platforms (Fig. 8).
Fig. 8.
Synergistic Potential of NP-dECM scaffolds in cell culture. Representative examples of NP-dECM hybrid scaffolds applied in cell culture systems, combined with bioengineering technologies to enhance lineage-specific differentiation and functional maturation (Top). A bone-mimetic scaffold integrating nanostructured calcium phosphate oligomers (CPO) and bone-derived dECM facilitates sustained ion release and osteoinductive signaling, supporting in vitro mineralization of bone organoids (Middle). Reprinted with permission from (150) Copyright 2025, Elsevier. C A 3D-printed hybrid scaffold composed of magnesium hydroxide nanoparticles (MH-NPs), decellularized bone matrix (DBM) microparticles, and PLGA promotes stage-specific modulation of osteogenesis of BMSCs. Reprinted with permission from (152) Copyright 2024, John Wiley and Sons. A tumor-specific NP–dECM scaffold combining cellulose nanoparticles and gastric tissue-derived dECM tunes matrix stiffness to replicate native tumor microenvironments, enabling gastric cancer cell culture in a 3D-bioprinted platform (Bottom). Reprinted with permission from (153) Copyright 2021, Frontiers Media S.A.
A few studies have explored the combination of tissue-specific dECM and nanoparticles to engineer organoid scaffolds with enhanced mechanical integrity, immunocompatibility, and tissue-specific bioactivity. Gai et al. developed a bone-mimetic hydrogel by assembling bone-derived dECM, salmon DNA, and nanostructured calcium phosphate oligomers (CPO) into a composite matrix tailored for the formation of bone organoids [169]. Designed to emulate the native bone niche, this hydrogel created a synergistic microenvironment that supported osteogenic and angiogenic differentiation of bone marrow-derived mesenchymal stromal cells (BMSCs), driven by the osteoinductive properties of dECM, structural reinforcement from DNA, and sustained calcium ion release from CPO. Consequently, this scaffold enabled in vitro mineralization and in vivo vascularization, highlighting the translational potential of NP-dECM synergy in organoid engineering.
Beyond current organoid models, several NP-dECM scaffolds developed in tissue engineering research exhibit characteristics that could be translated to organoid culture and maturation. For instance, silver nanoparticle (AgNP)-funtionalized xenogeneic pancreas dECM scaffolds demonstrated reduced immunogenicity and promoted vascular integration in vivo [170]. As a cross-linking agent, AgNPs contributed antibacterial, anti-inflammatory, and collagen-binding properties, While the pancreas-derived ECM provided tissue-specific biochemical cues, together supporting mechanical stability and inducing M2 macrophage polarization. In addition, Yuan et al. engineered a 3D-printed hybrid scaffold incorporating magnesium hydroxide nanoparticles (MH-NPs), decellularized bone matrix microparticles (DBM-MPs), and polylactic acid-glycolic acid (PLGA) to recreate a osteoimmune niche [171]. This construct enabled stage-specific modulation of inflammation, angiogenesis, and osteogenesis, offering insights for future strategies in bone organoid research. In another approach, Kim et al. generated a hybrid bioink using cellulose nanoparticles (CN) and gastric dECM to tune matrix stiffness and replicate a tumor-specific environment for gastric cancer cell culture [172]. While not yet applied to organoids, such NP-dECM systems reveal the versatility and future potential of these scaffolds across a wide range of organoid applications.
In addition to providing structural support, NPs offer functional advantages that expand the analytical capabilities of dECM-based organoid systems. In a representative study, Cho et al. combined brain-derived decellularized extracellular matrix (BEM) with a microfluidic device to improve the maturation of human brain organoids [37]. To monitor oxygen dynamics within the organoids, they introduced Pt(II) meso-tetra(pentafluorophenyl)porphine (PtTFPP)-conjugated poly(urethane acrylate nonionomer) (PUAN) nanoparticles as phosphorescent oxygen sensors. This enabled real-time spatial mapping of oxygen distribution and demonstrated improved oxygen penetration under microfluidic conditions. These findings provide evidence for how NP-ECM integration can be harnessed not only for structural enhancement but also for real-time physiological assessment, broadening the utility of organoid platforms in functional studies.
Collectively, while current evidence on NP-dECM synergy in organoid systems remains scarce, converging findings from related scaffold-engineering studies suggest several plausible molecular bases, including NP-mediated modulation of biochemical signaling, sustained release of bioactive ions or molecules, and nanoscale architectural tuning that enhances ECM-cell receptor engagement. Exploring these mechanisms in organoids will be a promising direction for future research and will guide the rational design of hybrid scaffolds with maximized synergistic effects. These scaffolds could enhance structural reinforcement, dynamic sensing, immune modulation, and tissue-specific guidance, thereby improving organoid maturation for applications in drug testing and regenerative medicine.
5. Conclusion and future perspectives
Organoid technology has revolutionized in vitro modeling by enabling the previse replication of human tissue architecture and function. Nanoparticles (NPs) and decellularized extracellular matrix (dECM) have independently advanced in this field: NPs offer structural integrity, precise control over biochemical delivery, and real-time sensing, while dECM provides tissue-specific biochemical and mechanical cues that conventional matrices lack. Recent efforts combining NPs with dECM have begun to demonstrate synergistic benefits, resulting in multifunctional scaffolds that support organoid maturation, facilitate physiological monitoring, and expand analytical versatility. These NP-dECM systems are currently being explored across various bioengineering applications and show considerable promise for future translation into advanced scaffolds for organoid culture.
Translating NP-dECM hybrid platforms into clinical and industrial use requires overcoming several key challenges. Scalability remains a major hurdle, as the preparation of dECM is labor-intensive, variable in biochemical composition and mechanical properties across laboratories [31,33,156], and the incorporation of NPs demands precise control over size distribution, surface chemistry, and loading efficiency to ensure reproducibility [22,38]. Achieving GMP standards will require automated, high-throughput manufacturing pipelines capable of producing large quantities with consistent performance. Clear understanding of the regulatory pathways for these hybrid biomaterial-drug systems is also essential to guide preclinical and clinical studies. Importantly, in personalized medicine, combining patient-specific ECM with tailored nanoparticles can better replicate individual disease characteristics, improve prediction of therapeutic responses, and thereby enhance the efficiency of clinical transition. Addressing these issues through harmonized protocols, validated analytical methods, and early regulatory engagement will be critical to developing NP-dECM organoid scaffolds from experimental tools to clinically relevant platforms.
In addition to these translational considerations, the continued evolution of NP-dECM organoid platforms will depend on the application of cutting-edge technologies that can resolve current limitations and expand their functional scope. Recent developments in nanoparticle engineering have enabled the design of materials with controlled size, shape, and surface properties, facilitating precise delivery biosensing, and structural reinforcement in organoid systems [22,25,38,173]. Notably, emerging strategies are being explored to overcome the obstacles of toxic nanoparticles for broader biomedical use, including biodegradable coatings, bioinspired surface modifications, and controlled-release construction that minimize off-target effects [174,175]. Furthermore, applying computational modeling and artificial intelligence (AI) with organoid research, such as AI-driven scaffold design, in silico prediction of NP-cell interactions, and automated analysis of functional assays, provides powerful opportunities to accelerate optimization and expand the range of NP-dECM applications [[176], [177], [178]].
All together, these innovations will not only address current barriers but also unlock new capabilities for NPs and dECM in organoid models, enabling more efficient drug discovery, disease modeling, and precision medicine. With ongoing technological progress, NPs-dECM integration holds high potential for advancing organoid systems into clinically and industrially applicable platforms. As these hybrid scaffolds continue to be refined, organoid models are expected to become core technologies for bridging the gap between experimental research and therapeutic application, ultimately accelerating the translation of innovative biomedical strategies to clinical use.
CRediT authorship contribution statement
Sang-Ji Lee: Writing – review & editing, Writing – original draft, Visualization, Project administration, Investigation, Conceptualization. Jae-Yong Cho: Writing – review & editing. Tae-Hyun Heo: Writing – review & editing. Dae Hyeok Yang: Writing – review & editing. Heung Jae Chun: Writing – review & editing. Jeong-Kee Yoon: Writing – review & editing. Gun-Jae Jeong: Writing – review & editing, Writing – original draft, Visualization, Supervision, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Grant No. RS-2023-00237493) and the Ministry of Trade, Industry and Energy (MOTIE), Republic of Korea (Grant No. RS-2024-00424600 and RS-2024-00433255).
Data availability
No data was used for the research described in the article.
References
- 1.Tang X.-Y., Wu S., Wang D., et al. Human organoids in basic research and clinical applications. Signal Transduct. Targeted Ther. 2022;7:168. doi: 10.1038/s41392-022-01024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yi S.A., Zhang Y., Rathnam C., et al. Bioengineering approaches for the advanced organoid research. Adv. Mater. 2021;33:45. doi: 10.1002/adma.202007949. 2007949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brassard J.A., Lutolf M.P. Engineering stem cell self-organization to build better organoids. Cell Stem Cell. 2019;24:6 860–876. doi: 10.1016/j.stem.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Co J.Y., Margalef-Català M., Monack D.M., et al. Controlling the polarity of human gastrointestinal organoids to investigate epithelial biology and infectious diseases. Nat. Protoc. 2021;16:11 5171–5192. doi: 10.1038/s41596-021-00607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park G., Rim Y.A., Sohn Y., et al. Replacing animal testing with stem cell-organoids : advantages and limitations. Stem Cell Reviews and Reports. 2024;20:6 1375–1386. doi: 10.1007/s12015-024-10723-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen C., Teng Y. Is it time to start transitioning from 2D to 3D cell culture? Front. Mol. Biosci. 2020;7 doi: 10.3389/fmolb.2020.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang S., Hu H., Kung H., et al. Organoids: the current status and biomedical applications. MedComm. 2023;4 doi: 10.1002/mco2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lancaster M.A., Renner M., Martin C.-A., et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:7467 373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grenier K., Kao J., Diamandis P. Three-dimensional modeling of human neurodegeneration: brain organoids coming of age. Mol. Psychiatr. 2020;25:2 254–274. doi: 10.1038/s41380-019-0500-7. [DOI] [PubMed] [Google Scholar]
- 10.Wray S. Modelling neurodegenerative disease using brain organoids. Semin. Cell Dev. Biol. 2021;111:60–66. doi: 10.1016/j.semcdb.2020.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Tuveson D., Clevers H. Cancer modeling meets human organoid technology. Science. 2019;364:6444 952–955. doi: 10.1126/science.aaw6985. [DOI] [PubMed] [Google Scholar]
- 12.Guan Y., Enejder A., Wang M., et al. A human multi-lineage hepatic organoid model for liver fibrosis. Nat. Commun. 2021;12:1–6138. doi: 10.1038/s41467-021-26410-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrews M.G., Kriegstein A.R. Challenges of organoid research. Annu. Rev. Neurosci. 2022;45:23–39. doi: 10.1146/annurev-neuro-111020-090812. 45, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinnear C., Moore T.L., Rodriguez-Lorenzo L., et al. Form follows function: nanoparticle shape and its implications for nanomedicine. Chem. Rev. 2017;117:17 11476–11521. doi: 10.1021/acs.chemrev.7b00194. [DOI] [PubMed] [Google Scholar]
- 15.Verma J., Warsame C., Seenivasagam R.K., et al. Nanoparticle-mediated cancer cell therapy: basic science to clinical applications. Cancer Metastasis Rev. 2023;42:3 601–627. doi: 10.1007/s10555-023-10086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeong G.J., Castels H., Kang I., et al. Nanomaterial for skeletal muscle regeneration. Tissue Eng. Regener. Med. 2022;19:2 253–261. doi: 10.1007/s13770-022-00446-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altammar K.A. A review on nanoparticles: characteristics, synthesis, applications, and challenges. Front. Microbiol. 2023;14 doi: 10.3389/fmicb.2023.1155622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anselmo A.C., Mitragotri S. Nanoparticles in the clinic: an update. Bioeng Transl Med. 2019;4 doi: 10.1002/btm2.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Afzal O., Altamimi A.S.A., Nadeem M.S., et al. Nanoparticles in drug delivery: from history to therapeutic applications. Nanomaterials. 2022;12(24):4494. doi: 10.3390/nano12244494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gavas S., Quazi S., Karpiński T.M. Nanoparticles for cancer therapy: current progress and challenges. Nanoscale Res. Lett. 2021;16 doi: 10.1186/s11671-021-03628-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francis W.R., Liu Z., Owens S.E., et al. Role of hypoxia inducible factor 1α in cobalt nanoparticle induced cytotoxicity of human THP-1 macrophages. Biomaterials Translational. 2021;2:2 143. doi: 10.12336/biomatertransl.2021.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi Y., Han X., Zou S., et al. Nanomaterials in organoids: from interactions to personalized medicine. ACS Nano. 2024;18:49 33276–33292. doi: 10.1021/acsnano.4c13330. [DOI] [PubMed] [Google Scholar]
- 23.Abdel Fattah A.R., Ranga A. Nanoparticles as versatile tools for mechanotransduction in tissues and organoids. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao D.-K., Liang J., Huang X.-Y., et al. Organoids technology for advancing the clinical translation of cancer nanomedicine. WIREs Nanomedicine and Nanobiotechnology. 2023;15:5 e1892. doi: 10.1002/wnan.1892. [DOI] [PubMed] [Google Scholar]
- 25.Mahapatra C., Lee R., Paul M.K. Emerging role and promise of nanomaterials in organoid research. Drug Discov. Today. 2022;27(3):890–899. doi: 10.1016/j.drudis.2021.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Liang J., Zhao D.-K., Yin H.-M., et al. Combinatorial screening of nanomedicines in patient-derived cancer organoids facilitates efficient cancer therapy. Nano Today. 2025;61 doi: 10.1016/j.nantod.2025.102665. [DOI] [Google Scholar]
- 27.Tortorella I., Argentati C., Emiliani C., et al. The role of physical cues in the development of stem cell-derived organoids. Eur. Biophys. J. 2022;51:2 105–117. doi: 10.1007/s00249-021-01551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hussey G.S., Dziki J.L., Badylak S.F. Extracellular matrix-based materials for regenerative medicine. Nat. Rev. Mater. 2018;3:7 159–173. doi: 10.1038/s41578-018-0023-x. [DOI] [Google Scholar]
- 29.Rezakhani S., Gjorevski N., Lutolf M.P. Extracellular matrix requirements for gastrointestinal organoid cultures. Biomaterials. 2021;276 doi: 10.1016/j.biomaterials.2021.121020. [DOI] [PubMed] [Google Scholar]
- 30.Zhu L., Yuhan J., Yu H., et al. Decellularized extracellular matrix for remodeling bioengineering organoid's microenvironment. Small. 2023;19:25. doi: 10.1002/smll.202207752. 2207752. [DOI] [PubMed] [Google Scholar]
- 31.Guo X., Liu B., Zhang Y., et al. Decellularized extracellular matrix for organoid and engineered organ culture. J. Tissue Eng. 2024;15 doi: 10.1177/20417314241300386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C., An N., Song Q., et al. Enhancing organoid culture: harnessing the potential of decellularized extracellular matrix hydrogels for mimicking microenvironments. J. Biomed. Sci. 2024;31:1. doi: 10.1186/s12929-024-01086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moura B.S., Monteiro M.V., Ferreira L.P., et al. Advancing tissue decellularized hydrogels for engineering human organoids. Adv. Funct. Mater. 2022;32:29. doi: 10.1002/adfm.202202825. 2202825. [DOI] [Google Scholar]
- 34.Brown M., Li J., Moraes C., et al. Decellularized extracellular matrix: new promising and challenging biomaterials for regenerative medicine. Biomaterials. 2022;289 doi: 10.1016/j.biomaterials.2022.121786. [DOI] [PubMed] [Google Scholar]
- 35.Kort-Mascort J., Flores-Torres S., Peza-Chavez O., et al. Decellularized ECM hydrogels: prior use considerations, applications, and opportunities in tissue engineering and biofabrication. Biomater. Sci. 2023;11:2 400–431. doi: 10.1039/D2BM01273A. [DOI] [PubMed] [Google Scholar]
- 36.Giobbe G.G., Crowley C., Luni C., et al. Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture. Nat. Commun. 2019;10:1 5658. doi: 10.1038/s41467-019-13605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho A.-N., Jin Y., An Y., et al. Microfluidic device with brain extracellular matrix promotes structural and functional maturation of human brain organoids. Nat. Commun. 2021;12:1–4730. doi: 10.1038/s41467-021-24775-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen C., Zhang Z.J., Li X.X., et al. Intersection of nanomaterials and organoids technology in biomedicine. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1172262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nikonorova V.G., Chrishtop V.V., Mironov V.A., et al. Advantages and potential benefits of using organoids in nanotoxicology. Cells. 2023;12(4):610. doi: 10.3390/cells12040610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li M., Gong J., Gao L., et al. Advanced human developmental toxicity and teratogenicity assessment using human organoid models. Ecotoxicol. Environ. Saf. 2022;235 doi: 10.1016/j.ecoenv.2022.113429. [DOI] [PubMed] [Google Scholar]
- 41.Mattei F., George J.T., Jolly M.K. Editorial: organoids, organs-on-chip, nanoparticles and in silico approaches to dissect the tumor-immune dynamics and to unveil the drug resistance mechanisms to therapy in the tumor microenvironment. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1253551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Septiadi D., Crippa F., Moore T.L., et al. Nanoparticle–cell interaction: a cell mechanics perspective. Adv. Mater. 2018;30:19. doi: 10.1002/adma.201704463. 1704463. [DOI] [PubMed] [Google Scholar]
- 43.Li J., Mao H., Kawazoe N., et al. Insight into the interactions between nanoparticles and cells. Biomater. Sci. 2017;5:2 173–189. doi: 10.1039/C6BM00714G. [DOI] [PubMed] [Google Scholar]
- 44.Chandrakala V., Aruna V., Angajala G. Review on metal nanoparticles as nanocarriers: current challenges and perspectives in drug delivery systems. Emergent Materials. 2022;5:6 1593–1615. doi: 10.1007/s42247-021-00335-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Q., Kim Y.-J., Im G.-B., et al. Inorganic nanoparticles applied as functional therapeutics. Adv. Funct. Mater. 2021;31:12. doi: 10.1002/adfm.202008171. 2008171. [DOI] [Google Scholar]
- 46.Fallert L., Urigoitia-Asua A., Cipitria A., et al. Dynamic 3D in vitro lung models: applications of inorganic nanoparticles for model development and characterization. Nanoscale. 2024;16:23 10880–10900. doi: 10.1039/D3NR06672J. [DOI] [PubMed] [Google Scholar]
- 47.Henrique R.B.L., Lima R.R.M., Monteiro C.A.P., et al. Advances in the study of spheroids as versatile models to evaluate biological interactions of inorganic nanoparticles. Life Sci. 2022;302 doi: 10.1016/j.lfs.2022.120657. [DOI] [PubMed] [Google Scholar]
- 48.Zhang R., Kiessling F., Lammers T., et al. Clinical translation of gold nanoparticles. Drug Delivery and Translational Research. 2023;13:2 378–385. doi: 10.1007/s13346-022-01232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cunha L., Coelho S.C., Pereira M.d.C., et al. Nanocarriers based on gold nanoparticles for epigallocatechin gallate delivery in cancer cells. Pharmaceutics. 2022;14(3):491. doi: 10.3390/pharmaceutics14030491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kostka K., Sokolova V., El-Taibany A., et al. The application of ultrasmall gold nanoparticles (2 nm) functionalized with doxorubicin in three-dimensional normal and glioblastoma organoid models of the blood–brain barrier. Molecules. 2024;29:11 2469. doi: 10.3390/molecules29112469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park S.B., Cho H.-J., Moon S.R., et al. Gold nanoparticle-assisted delivery of brain-derived neurotrophic factor to cerebral organoids. Nano Res. 2022;15:4 3099–3105. doi: 10.1007/s12274-021-3975-x. [DOI] [Google Scholar]
- 52.Patino-Guerrero A., Esmaeili H., Migrino R.Q., et al. Nanoengineering of gold nanoribbon-embedded isogenic stem cell-derived cardiac organoids. RSC Adv. 2023;13:25 16985–17000. doi: 10.1039/D3RA01811C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caleffi J.T., Aal M.C.E., Gallindo H.d.O.M., et al. Magnetic 3D cell culture: state of the art and current advances. Life Sci. 2021;286 doi: 10.1016/j.lfs.2021.120028. [DOI] [PubMed] [Google Scholar]
- 54.Kim J.A., Choi J.-H., Kim M., et al. High-throughput generation of spheroids using magnetic nanoparticles for three-dimensional cell culture. Biomaterials. 2013;34:34 8555–8563. doi: 10.1016/j.biomaterials.2013.07.056. [DOI] [PubMed] [Google Scholar]
- 55.Marovič N., Ban I., Maver U., et al. Magnetic nanoparticles in 3D-printed scaffolds for biomedical applications. Nanotechnol. Rev. 2023;12:1. doi: 10.1515/ntrev-2022-0570. [DOI] [Google Scholar]
- 56.Ferreira J.N., Hasan R., Urkasemsin G., et al. A magnetic three-dimensional levitated primary cell culture system for the development of secretory salivary gland-like organoids. J. Tissue Eng. Regen. Med. 2019;13:3 495–508. doi: 10.1002/term.2809. [DOI] [PubMed] [Google Scholar]
- 57.Adine C., Ng K.K., Rungarunlert S., et al. Engineering innervated secretory epithelial organoids by magnetic three-dimensional bioprinting for stimulating epithelial growth in salivary glands. Biomaterials. 2018;180:52–66. doi: 10.1016/j.biomaterials.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 58.Abdel Fattah A.R., Kolaitis N., Van Daele K., et al. Targeted mechanical stimulation via magnetic nanoparticles guides in vitro tissue development. Nat. Commun. 2023;14:1–5281. doi: 10.1038/s41467-023-41037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lewis J.K., Bischof J.C., Braslavsky I., et al. The grand challenges of organ banking: proceedings from the first global summit on complex tissue cryopreservation. Cryobiology. 2016;72(2):169–182. doi: 10.1016/j.cryobiol.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 60.Manuchehrabadi N., Gao Z., Zhang J., et al. Improved tissue cryopreservation using inductive heating of magnetic nanoparticles. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aah4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Etheridge M.L., Xu Y., Rott L., et al. RF heating of magnetic nanoparticles improves the thawing of cryopreserved biomaterials. Technology. 2014;2 doi: 10.1142/s2339547814500204. [DOI] [Google Scholar]
- 62.Lee S.-G., Kim J., Seok J., et al. Development of heart organoid cryopreservation method through Fe3O4 nanoparticles based nanowarming system. Biotechnol. J. 2024;19:1. doi: 10.1002/biot.202300311. 2300311. [DOI] [PubMed] [Google Scholar]
- 63.Naidu S., Pandey J., Mishra L.C., et al. Silicon nanoparticles: synthesis, uptake and their role in mitigation of biotic stress. Ecotoxicol. Environ. Saf. 2023;255 doi: 10.1016/j.ecoenv.2023.114783. [DOI] [PubMed] [Google Scholar]
- 64.O'Farrell N., Andrew H., R B., Horrocks Silicon nanoparticles: applications in cell biology and medicine. Int. J. Nanomed. 2006;1:4 451–472. doi: 10.2147/nano.2006.1.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Purwada A., Jaiswal M.K., Ahn H., et al. Ex vivo engineered immune organoids for controlled germinal center reactions. Biomaterials. 2015;63:24–34. doi: 10.1016/j.biomaterials.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gatto D., Brink R. The germinal center reaction. J. Allergy Clin. Immunol. 2010;126:5 898–907. doi: 10.1016/j.jaci.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 67.Tan Y., Coyle R.C., Barrs R.W., et al. Nanowired human cardiac organoid transplantation enables highly efficient and effective recovery of infarcted hearts. Sci. Adv. 2023;9 doi: 10.1126/sciadv.adf2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cui D., Kong N., Ding L., et al. Ultrathin 2D titanium carbide MXene (Ti3C2T) nanoflakes activate WNT/HIF-1α-Mediated metabolism reprogramming for periodontal regeneration. Adv. Healthcare Mater. 2021;10 doi: 10.1002/adhm.202101215. 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao X., Wang L.-Y., Tang C.-Y., et al. Smart Ti3C2Tx MXene fabric with fast humidity response and joule heating for healthcare and medical therapy applications. ACS Nano. 2020;14:7 8793–8805. doi: 10.1021/acsnano.0c03391. [DOI] [PubMed] [Google Scholar]
- 70.Chao M., He L., Gong M., et al. Breathable Ti3C2Tx MXene/Protein nanocomposites for ultrasensitive medical pressure sensor with degradability in solvents. ACS Nano. 2021;15:6 9746–9758. doi: 10.1021/acsnano.1c00472. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Z., Gao S., Hu Y.-N., et al. Ti3C2TxMXene composite 3D hydrogel potentiates mTOR signaling to promote the generation of functional hair cells in cochlea organoids. Adv. Sci. 2022;9 doi: 10.1002/advs.202203557. 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y., Dayton P., Yang X., et al. Toxicity and efficacy of carbon nanotubes and graphene: the utility of carbon-based nanoparticles in nanomedicine. Drug Metab. Rev. 2014;46:2 232–246. doi: 10.3109/03602532.2014.883406. [DOI] [PubMed] [Google Scholar]
- 73.Khajavinia A., El-Aneed A. Carbon-based nanoparticles and their surface-modified counterparts as MALDI matrices. Anal. Chem. 2023;95:1 100–114. doi: 10.1021/acs.analchem.2c04537. [DOI] [PubMed] [Google Scholar]
- 74.Ayanda O.S., Mmuoegbulam A.O., Okezie O., et al. Recent progress in carbon-based nanomaterials: critical review. J. Nanoparticle Res. 2024;26:106. doi: 10.1007/s11051-024-06006-2. 5. [DOI] [Google Scholar]
- 75.Bao L., Cui X., Wang X., et al. Carbon nanotubes promote the development of intestinal organoids through regulating extracellular matrix viscoelasticity and intracellular energy metabolism. ACS Nano. 2021;15:10 15858–15873. doi: 10.1021/acsnano.1c03707. [DOI] [PubMed] [Google Scholar]
- 76.Kim N.H., Chae S., Yi S.A., et al. Peptide-assembled single-chain atomic crystal enhances pluripotent stem cell differentiation to neurons. Nano Lett. 2023;23:15 6859–6867. doi: 10.1021/acs.nanolett.3c00966. [DOI] [PubMed] [Google Scholar]
- 77.Itoo A.M., Vemula S.L., Gupta M.T., et al. Multifunctional graphene oxide nanoparticles for drug delivery in cancer. J. Contr. Release. 2022;350:26–59. doi: 10.1016/j.jconrel.2022.08.011. [DOI] [PubMed] [Google Scholar]
- 78.Shahriari S., Murali S., Santosh P., et al. Graphene and graphene oxide as a support for biomolecules in the development of biosensors. Nanotechnol. Sci. Appl. 2021;14:197–220. doi: 10.2147/NSA.S334487. null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yildiz G., Bolton-Warberg M., Awaja F. Graphene and graphene oxide for bio-sensing: general properties and the effects of graphene ripples. Acta Biomater. 2021;131:62–79. doi: 10.1016/j.actbio.2021.06.047. [DOI] [PubMed] [Google Scholar]
- 80.Park S., Kim Y.J., Sharma H., et al. Graphene hybrid inner ear organoid with enhanced maturity. Nano Lett. 2023;23:12 5573–5580. doi: 10.1021/acs.nanolett.3c00988. [DOI] [PubMed] [Google Scholar]
- 81.Beach M.A., Nayanathara U., Gao Y., et al. Polymeric nanoparticles for drug delivery. Chem. Rev. 2024;124:9 5505–5616. doi: 10.1021/acs.chemrev.3c00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Elmowafy M., Shalaby K., Elkomy M.H., et al. Polymeric nanoparticles for delivery of natural bioactive agents: recent advances and challenges. Polymers. 2023;15(5 1123) doi: 10.3390/polym15051123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Castro K.C.d., Martins C.J., N M.G., Campos Drug-loaded polymeric nanoparticles: a review. International Journal of Polymeric Materials and Polymeric Biomaterials. 2022;71:1 1–13. doi: 10.1080/00914037.2020.1798436. [DOI] [Google Scholar]
- 84.Li X., Sheng S., Li G., et al. Research progress in hydrogels for cartilage organoids. Adv. Healthcare Mater. 2024;13:22. doi: 10.1002/adhm.202400431. 2400431. [DOI] [PubMed] [Google Scholar]
- 85.Wang F., Gu Z., Yin Z., et al. Cell unit-inspired natural nano-based biomaterials as versatile building blocks for bone/cartilage regeneration. J. Nanobiotechnol. 2023;21(1):293. doi: 10.1186/s12951-023-02003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Klemm D., Kramer F., Moritz S., et al. Nanocelluloses: a new family of nature-based materials. Angew. Chem. Int. Ed. 2011;50:24 5438–5466. doi: 10.1002/anie.201001273. [DOI] [PubMed] [Google Scholar]
- 87.Thomas B., Raj M.C., K. B A., et al. Nanocellulose, a versatile green platform: from biosources to materials and their applications. Chem. Rev. 2018;118:24 11575–11625. doi: 10.1021/acs.chemrev.7b00627. [DOI] [PubMed] [Google Scholar]
- 88.Jonoobi M., Oladi R., Davoudpour Y., et al. Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: a review. Cellulose. 2015;22:2 935–969. doi: 10.1007/s10570-015-0551-0. [DOI] [Google Scholar]
- 89.Nikolits I., Radwan S., Liebner F., et al. Hydrogels from TEMPO-Oxidized nanofibrillated cellulose support in vitro cultivation of encapsulated human mesenchymal stem cells. ACS Appl. Bio Mater. 2023;6:2 543–551. doi: 10.1021/acsabm.2c00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sharma P., Pal V.K., Kaur H., et al. Exploring the TEMPO-oxidized nanofibrillar cellulose and short ionic-complementary peptide composite hydrogel as biofunctional cellular scaffolds. Biomacromolecules. 2022;23:6 2496–2511. doi: 10.1021/acs.biomac.2c00234. [DOI] [PubMed] [Google Scholar]
- 91.Lan X., Ma Z., Szojka A.R.A., et al. TEMPO-Oxidized cellulose nanofiber-alginate Hydrogel as a bioink for Human Meniscus tissue engineering. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.766399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Isogai A., Saito T., Fukuzumi H. TEMPO-oxidized cellulose nanofibers. Nanoscale. 2011;3:1 71–85. doi: 10.1039/C0NR00583E. [DOI] [PubMed] [Google Scholar]
- 93.Krüger M., Oosterhoff L.A., van Wolferen M.E., et al. Cellulose nanofibril Hydrogel Promotes Hepatic Differentiation of human liver organoids. Adv. Healthcare Mater. 2020;9:6. doi: 10.1002/adhm.201901658. 1901658. [DOI] [PubMed] [Google Scholar]
- 94.Curvello R., Garnier G. Cationic cross-linked nanocellulose-based matrices for the growth and recovery of intestinal organoids. Biomacromolecules. 2021;22:2 701–709. doi: 10.1021/acs.biomac.0c01510. [DOI] [PubMed] [Google Scholar]
- 95.Curvello R., Kerr G., Micati D.J., et al. Engineered plant-based Nanocellulose hydrogel for small intestinal organoid growth. Adv. Sci. 2021;8:1. doi: 10.1002/advs.202002135. 2002135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Curvello R., Alves D., Abud H.E., et al. A thermo-responsive collagen-nanocellulose hydrogel for the growth of intestinal organoids. Mater. Sci. Eng. C. 2021;124 doi: 10.1016/j.msec.2021.112051. [DOI] [PubMed] [Google Scholar]
- 97.Curvello R., Raghuwanshi V.S., Wu C.M., et al. Nano- and microstructures of collagen-nanocellulose hydrogels as engineered extracellular matrices. ACS Appl. Mater. Interfaces. 2024;16:1 1370–1379. doi: 10.1021/acsami.3c10353. [DOI] [PubMed] [Google Scholar]
- 98.Casalini T., Rossi F., Castrovinci A., et al. A perspective on polylactic acid-based polymers use for nanoparticles synthesis and applications. Front. Bioeng. Biotechnol. 2019;7 doi: 10.3389/fbioe.2019.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.PLGA-Based Nanoparticles as Cancer Drug Delivery Systems Asian Pac. J. Cancer Prev. APJCP. 2014;15(2):517–535. doi: 10.7314/apjcp.2014.15.2.517. [DOI] [PubMed] [Google Scholar]
- 100.da Luz C.M., Boyles M.S.P., Falagan-Lotsch P., et al. Poly-lactic acid nanoparticles (PLA-NP) promote physiological modifications in lung epithelial cells and are internalized by clathrin-coated pits and lipid rafts. J. Nanobiotechnol. 2017;15 doi: 10.1186/s12951-016-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tong Y., Ueyama-Toba Y., Yokota J., et al. Efficient hepatocyte differentiation of primary human hepatocyte-derived organoids using three dimensional nanofibers (HYDROX) and their possible application in hepatotoxicity research. Sci. Rep. 2024;14:1. doi: 10.1038/s41598-024-61544-y. 10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu Y., Fourniols T., Labrak Y., et al. Surface modification of lipid-based nanoparticles. ACS Nano. 2022;16:5 7168–7196. doi: 10.1021/acsnano.2c02347. [DOI] [PubMed] [Google Scholar]
- 103.Mehta M., Bui T.A., Yang X., et al. Lipid-Based nanoparticles for Drug/gene delivery: an overview of the production techniques and difficulties encountered in their industrial development. ACS Mater. Au. 2023;3:6 600–619. doi: 10.1021/acsmaterialsau.3c00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Patel P., Garala K., Singh S., et al. Lipid-based nanoparticles in delivering bioactive compounds for improving therapeutic efficacy. Pharmaceuticals. 2024;17(3):329. doi: 10.3390/ph17030329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Krause M., Rak-Raszewska A., Naillat F., et al. Exosomes as secondary inductive signals involved in kidney organogenesis. J. Extracell. Vesicles. 2018;7 doi: 10.1080/20013078.2017.1422675. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang Y.S., Zhang Y.-N., Zhang W. Cancer-on-a-chip systems at the frontier of nanomedicine. Drug Discov. Today. 2017;22(9):1392–1399. doi: 10.1016/j.drudis.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li D., Zhang R., Le Y., et al. Organoid-Based assessment of metal–organic framework (MOF) nanomedicines for Ex vivo cancer therapy. ACS Appl. Mater. Interfaces. 2024;16:26 33070–33080. doi: 10.1021/acsami.4c05172. [DOI] [PubMed] [Google Scholar]
- 108.Zhang H., Lu Y., Zhang R., et al. Synthesis of multifunctional plasmonic nanodarts through one-end deposition on gold nanobipyramids for tumor organoid ablation and antimicrobial applications. Adv. Funct. Mater. 2024;34 doi: 10.1002/adfm.202405588. 44. [DOI] [Google Scholar]
- 109.McCarthy B., Cudykier A., Singh R., et al. Semiconducting polymer nanoparticles for photothermal ablation of colorectal cancer organoids. Sci. Rep. 2021;11:1–1532. doi: 10.1038/s41598-021-81122-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang Y., Zhang J., Liu Y., et al. Realveolarization with inhalable mucus-penetrating lipid nanoparticles for the treatment of pulmonary fibrosis in mice. Sci. Adv. 2024;10 doi: 10.1126/sciadv.ado4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Poh B.M., Liew L.C., Soh Y.N.A., et al. MSC-Derived small extracellular vesicles exert cardioprotective effect through reducing VLCFAs and apoptosis in human Cardiac organoid IRI model. Stem Cell. 2024;42:5 416–429. doi: 10.1093/stmcls/sxae015. [DOI] [PubMed] [Google Scholar]
- 112.Kim N.G., Jung D.J., Jung Y.-K., et al. The effect of a novel mica nanoparticle, STB-MP, on an alzheimer's disease patient-induced PSC-Derived cortical brain organoid model. Nanomaterials. 2023;13(5):893. doi: 10.3390/nano13050893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Astashkina A.I., Jones C.F., Thiagarajan G., et al. Nanoparticle toxicity assessment using an in vitro 3-D kidney organoid culture model. Biomaterials. 2014;35:24 6323–6331. doi: 10.1016/j.biomaterials.2014.04.060. [DOI] [PubMed] [Google Scholar]
- 114.Gerbolés A.G., Galetti M., Rossi S., et al. Three-Dimensional bioprinting of organoid-based scaffolds (OBST) for long-term nanoparticle toxicology investigation. Int. J. Mol. Sci. 2023;24:7 6595. doi: 10.3390/ijms24076595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang H., Niu S., Guo M., et al. Applications of 3D organoids in toxicological studies: a comprehensive analysis based on bibliometrics and advances in toxicological mechanisms. Arch. Toxicol. 2024;98:8 2309–2330. doi: 10.1007/s00204-024-03777-4. [DOI] [PubMed] [Google Scholar]
- 116.Issa R., Lozano N., Kostarelos K., et al. Functioning human lung organoids model pulmonary tissue response from carbon nanomaterial exposures. Nano Today. 2024;56 doi: 10.1016/j.nantod.2024.102254. [DOI] [Google Scholar]
- 117.Huang Y., Guo L., Cao C., et al. Silver nanoparticles exposure induces developmental neurotoxicity in hiPSC-derived cerebral organoids. Sci. Total Environ. 2022;845 doi: 10.1016/j.scitotenv.2022.157047. [DOI] [PubMed] [Google Scholar]
- 118.Liu L., Wang J., Zhang J., et al. The cytotoxicity of zinc oxide nanoparticles to 3D brain organoids results from excessive intracellular zinc ions and defective autophagy. Cell Biol. Toxicol. 2023;39:1 259–275. doi: 10.1007/s10565-021-09678-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mekky G., Seeds M., Diab A.E.-A.A., et al. The potential toxic effects of magnesium oxide nanoparticles and valproate on liver tissue. J. Biochem. Mol. Toxicol. 2021;35 doi: 10.1002/jbt.22676. 3. [DOI] [PubMed] [Google Scholar]
- 120.Baek A., Kwon I.H., Lee D.-H., et al. Novel organoid culture System for improved safety assessment of nanomaterials. Nano Lett. 2024;24:3 805–813. doi: 10.1021/acs.nanolett.3c02939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Iqbal M.Z., Luo D., Akakuru O.U., et al. Facile synthesis of biocompatible magnetic titania nanorods for T1-magnetic resonance imaging and enhanced phototherapy of cancers. J. Mater. Chem. B. 2021;9:33 6623–6633. doi: 10.1039/D1TB01097B. [DOI] [PubMed] [Google Scholar]
- 122.Leite P.E.C., Pereira M.R., Harris G., et al. Suitability of 3D human brain spheroid models to distinguish toxic effects of gold and poly-lactic acid nanoparticles to assess biocompatibility for brain drug delivery. Part. Fibre Toxicol. 2019;16 doi: 10.1186/s12989-019-0307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bloem B.R., Okun M.S., Klein C. Parkinson's disease. Lancet. 2021;397:10291 2284–2303. doi: 10.1016/S0140-6736(21)00218-X. [DOI] [PubMed] [Google Scholar]
- 124.Tolosa E., Garrido A., Scholz S.W., et al. Challenges in the diagnosis of Parkinson's disease. Lancet Neurol. 2021;20(5):385–397. doi: 10.1016/S1474-4422(21)00030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Calabresi P., Di Lazzaro G., Marino G., et al. Advances in understanding the function of alpha-synuclein: implications for Parkinson's disease. Brain. 2023;146:9 3587–3597. doi: 10.1093/brain/awad150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee M.-H., Liu K.-T., Thomas J.L., et al. Peptide-Imprinted Poly(hydroxymethyl 3,4-ethylenedioxythiophene) nanotubes for detection of α Synuclein in human brain organoids. ACS Appl. Nano Mater. 2020;3:8 8027–8036. doi: 10.1021/acsanm.0c01476. [DOI] [Google Scholar]
- 127.Cui K., Chen W., Cao R., et al. Brain organoid-on-chip system to study the effects of breast cancer derived exosomes on the neurodevelopment of brain. Cell Regen. 2022;11:1–7. doi: 10.1186/s13619-021-00102-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Eivazzadeh-Keihan R., Sadat Z., Lalebeigi F., et al. Effects of mechanical properties of carbon-based nanocomposites on scaffolds for tissue engineering applications: a comprehensive review. Nanoscale Adv. 2024;6:2 337–366. doi: 10.1039/D3NA00554B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Saheli M., Sepantafar M., Pournasr B., et al. Three-dimensional liver-derived extracellular matrix hydrogel promotes liver organoids function. J. Cell. Biochem. 2018;119:6 4320–4333. doi: 10.1002/jcb.26622. [DOI] [PubMed] [Google Scholar]
- 130.Willemse J., van Tienderen G., van Hengel E., et al. Hydrogels derived from decellularized liver tissue support the growth and differentiation of cholangiocyte organoids. Biomaterials. 2022;284 doi: 10.1016/j.biomaterials.2022.121473. [DOI] [PubMed] [Google Scholar]
- 131.Chen J., Ma S., Yang H., et al. Generation and metabolomic characterization of functional ductal organoids with biliary tree networks in decellularized liver scaffolds. Bioact. Mater. 2023;26:452–464. doi: 10.1016/j.bioactmat.2023.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kim S., Min S., Choi Y.S., et al. Tissue extracellular matrix hydrogels as alternatives to Matrigel for culturing gastrointestinal organoids. Nat. Commun. 2022;13:1–1692. doi: 10.1038/s41467-022-29279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Garreta E., Moya-Rull D., Marco A., et al. Natural hydrogels support kidney Organoid generation and promote in vitro angiogenesis. Adv. Mater. 2024;36 doi: 10.1002/adma.202400306. 34. [DOI] [PubMed] [Google Scholar]
- 134.Kim J.W., Nam S.A., Yi J., et al. Kidney decellularized extracellular matrix enhanced the vascularization and maturation of human kidney organoids. Adv. Sci. 2022;9 doi: 10.1002/advs.202103526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nag S., Boyd A.S. Decellularization of mouse kidneys to generate an extracellular matrix gel for Human induced pluripotent stem cell derived renal organoids. Organ. 2023;2:1 66–78. [Google Scholar]
- 136.Simsa R., Rothenbücher T., Gürbüz H., et al. Brain organoid formation on decellularized porcine brain ECM hydrogels. PLoS One. 2021;16 doi: 10.1371/journal.pone.0245685. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wu W., Liu Y., Liu R., et al. Decellularized brain extracellular matrix hydrogel aids the Formation of human spinal-cord organoids recapitulating the complex three-dimensional Organization. ACS Biomater. Sci. Eng. 2024;10:5 3203–3217. doi: 10.1021/acsbiomaterials.4c00029. [DOI] [PubMed] [Google Scholar]
- 138.Wang Z., Liu R., Liu Y., et al. Human Placenta decellularized extracellular Matrix Hydrogel promotes the generation of human spinal cord organoids with dorsoventral Organization from human induced pluripotent stem cells. ACS Biomater. Sci. Eng. 2024;10:5 3218–3231. doi: 10.1021/acsbiomaterials.4c00067. [DOI] [PubMed] [Google Scholar]
- 139.Milton L.A., Davern J.W., Hipwood L., et al. Liver click dECM hydrogels for engineering hepatic microenvironments. Acta Biomater. 2024;185:144–160. doi: 10.1016/j.actbio.2024.06.037. [DOI] [PubMed] [Google Scholar]
- 140.Chan W.W., Yu F., Le Q.B., et al. Towards biomanufacturing of cell-derived matrices. Int. J. Mol. Sci. 2021;22:21 11929. doi: 10.3390/ijms222111929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hoshiba T. Cultured cell-derived decellularized extracellular matrix (cultured cell-derived dECM): future applications and problems — a mini review. Current Opinion in Biomedical Engineering. 2021;17 doi: 10.1016/j.cobme.2020.100256. [DOI] [Google Scholar]
- 142.Jin H., Xue Z., Liu J., et al. Advancing organoid engineering for tissue regeneration and biofunctional reconstruction. Biomater. Res. 2024;28:16. doi: 10.34133/bmr.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shao Y., Wang J., Jin A., et al. Biomaterial-assisted organoid technology for disease modeling and drug screening. Mater. Today Bio. 2025;30 doi: 10.1016/j.mtbio.2024.101438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.McWilliam R.H., Chang W., Liu Z., et al. Three-dimensional biofabrication of nanosecond laser micromachined nanofibre meshes for tissue engineered scaffolds. Biomater. Transl. 2023;4:2 104–114. doi: 10.12336/biomatertransl.2023.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Song S., Zhang J., Fang Y., et al. Nerve–bone crosstalk manipulates bone organoid development and bone regeneration: a review and perspectives. Organoid Research. 2025;1:1. doi: 10.36922/or.8294. [DOI] [Google Scholar]
- 146.Zhao J., Shen F., Sheng S., et al. Cartilage-on-chip for osteoarthritis drug screening. Organoid Research. 2025;1:1. doi: 10.36922/or.8461. [DOI] [Google Scholar]
- 147.Xu Z.-Y., Wang M., Shi J.-Y., et al. Engineering a dynamic extracellular matrix using thrombospondin-1 to propel hepatocyte organoids reprogramming and improve mouse liver regeneration post-transplantation. Mater. Today Bio. 2025;32 doi: 10.1016/j.mtbio.2025.101700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xu Z.Y., Huang J.J., Liu Y., et al. Extracellular matrix bioink boosts stemness and facilitates transplantation of intestinal organoids as a biosafe Matrigel alternative. Bioeng. Transl. Med. 2023;8 doi: 10.1002/btm2.10327. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Choi Y.M., Lee H., Ann M., et al. 3D bioprinted vascularized lung cancer organoid models with underlying disease capable of more precise drug evaluation. Biofabrication. 2023;15:3. doi: 10.1088/1758-5090/acd95f. [DOI] [PubMed] [Google Scholar]
- 150.Kim J., Kim J., Gao G., et al. Bioprinted organoids platform with tumor vasculature for implementing precision personalized medicine targeted towards gastric cancer. Adv. Funct. Mater. 2024;34:11. doi: 10.1002/adfm.202306676. 2306676. [DOI] [Google Scholar]
- 151.Liu H., Gan Z., Qin X., et al. Advances in microfluidic technologies in organoid research. Adv. Healthcare Mater. 2024;13:21. doi: 10.1002/adhm.202302686. 2302686. [DOI] [PubMed] [Google Scholar]
- 152.Saorin G., Caligiuri I., Rizzolio F. Microfluidic organoids-on-a-chip: the future of human models. Semin. Cell Dev. Biol. 2023;144:41–54. doi: 10.1016/j.semcdb.2022.10.001. [DOI] [PubMed] [Google Scholar]
- 153.Park B., Park J., Han S., et al. Advances in organoid-on-a-chip for recapitulation of human physiological events. Mater. Today. 2025;84:75–94. doi: 10.1016/j.mattod.2025.02.002. [DOI] [Google Scholar]
- 154.Chauhdari T., Zaidi S.A., Su J., et al. Organoids meet microfluidics: recent advancements, challenges, and future of organoids-on-chip. 2025;4:1 71–88. doi: 10.1007/s44164-025-00086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jin Y., Kim J., Lee J.S., et al. Vascularized liver organoids generated using induced hepatic tissue and dynamic liver-specific microenvironment as a drug testing platform. Adv. Funct. Mater. 2018;28:37. doi: 10.1002/adfm.201801954. 1801954. [DOI] [Google Scholar]
- 156.Zhu L., Yuhan J., Yu H., et al. Decellularized extracellular matrix for remodeling bioengineering organoid's microenvironment. Small. 2023;19 doi: 10.1002/smll.202207752. 25 e2207752. [DOI] [PubMed] [Google Scholar]
- 157.Kuşoğlu A., Yangın K., Özkan S.N., et al. Different decellularization methods in bovine lung tissue reveals distinct biochemical composition, stiffness, and viscoelasticity in reconstituted hydrogels. ACS Appl. Bio Mater. 2023;6:2 793–805. doi: 10.1021/acsabm.2c00968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Spagnol G., Sensi F., De Tommasi O., et al. Patient derived organoids (PDOs), extracellular matrix (ECM), tumor microenvironment (TME) and drug screening: state of the art and clinical implications of ovarian cancer organoids in the era of precision medicine. Cancers. 2023;15(7):2059. doi: 10.3390/cancers15072059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.van Tienderen G.S., Rosmark O., Lieshout R., et al. Extracellular matrix drives tumor organoids toward desmoplastic matrix deposition and mesenchymal transition. Acta Biomater. 2023;158:115–131. doi: 10.1016/j.actbio.2022.11.038. [DOI] [PubMed] [Google Scholar]
- 160.Ferreira L.P., Gaspar V.M., Mendes L., et al. Organotypic 3D decellularized matrix tumor spheroids for high-throughput drug screening. Biomaterials. 2021;275 doi: 10.1016/j.biomaterials.2021.120983. [DOI] [PubMed] [Google Scholar]
- 161.Jin H., Yang Q., Yang J., et al. Exploring tumor organoids for cancer treatment. APL Mater. 2024;12:6. doi: 10.1063/5.0216185. [DOI] [Google Scholar]
- 162.van Tienderen G.S., van Beek M.E.A., Schurink I.J., et al. Modelling metastatic colonization of cholangiocarcinoma organoids in decellularized lung and lymph nodes. Front. Oncol. 2023;12 doi: 10.3389/fonc.2022.1101901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chen Z., Long L., Wang J., et al. Constructing Tumor organoid-like tissue for reliable drug screening using liver-decellularized extracellular Matrix scaffolds. ACS Omega. 2024;9:5 5888–5898. doi: 10.1021/acsomega.3c09265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Varinelli L., Guaglio M., Brich S., et al. Decellularized extracellular matrix as scaffold for cancer organoid cultures of colorectal peritoneal metastases. J. Mol. Cell Biol. 2022;14:11. doi: 10.1093/jmcb/mjac064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tevlek A. The role of decellularized cell derived extracellular matrix in the establishment and culture of in vitro breast cancer tumor model. Biomed. Mater. 2024;19:2. doi: 10.1088/1748-605X/ad2378. 025037. [DOI] [PubMed] [Google Scholar]
- 166.Kim M., Kang D., Han H., et al. Light-activated decellularized extracellular matrix-based bioinks for enhanced mechanical integrity. Mater. Today Bio. 2025;32 doi: 10.1016/j.mtbio.2025.101859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ostadi Y., Khanali J., Tehrani F.A., et al. Decellularized extracellular matrix scaffolds for soft tissue augmentation: from host–scaffold interactions to bottlenecks in clinical translation. Biomater. Res. 2024;28:71. doi: 10.34133/bmr.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Biehl A., Gracioso Martins A.M., Davis Z.G., et al. Towards a standardized multi-tissue decellularization protocol for the derivation of extracellular matrix materials. Biomater. Sci. 2023;11:2 641–654. doi: 10.1039/D2BM01012G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gai T., Zhang H., Hu Y., et al. Sequential construction of vascularized and mineralized bone organoids using engineered ECM-DNA-CPO-based bionic matrix for efficient bone regeneration. Bioact. Mater. 2025;49:362–377. doi: 10.1016/j.bioactmat.2025.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Qiu H., Zhang L., Wang D., et al. Silver nanoparticles improve the biocompatibility and reduce the immunogenicity of xenogeneic scaffolds derived from decellularized pancreas. Cell. Reprogr. 2022;24:1 38–47. doi: 10.1089/cell.2021.0071. [DOI] [PubMed] [Google Scholar]
- 171.Yuan Y., Xu Y., Mao Y., et al. Three birds, one stone: an osteo-microenvironment stage-regulative scaffold for bone defect repair through modulating early Osteo-Immunomodulation, middle neovascularization, and later osteogenesis. Adv. Sci. 2024;11:6. doi: 10.1002/advs.202306428. 2306428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kim J., Jang J., Cho D.-W. Controlling cancer cell behavior by improving the stiffness of gastric tissue-decellularized ECM bioink with cellulose nanoparticles. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.605819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Abdel Fattah A.R., Kolaitis N., Van Daele K., et al. Local actuation of organoids by magnetic nanoparticles. bioRxiv. 2022 doi: 10.1101/2022.03.17.484696. 2022.03.17.484696. [DOI] [Google Scholar]
- 174.Ly P.-D., Ly K.-N., Phan H.-L., et al. Recent advances in surface decoration of nanoparticles in drug delivery. Front. Nanotechnol. 2024;6 doi: 10.3389/fnano.2024.1456939. [DOI] [Google Scholar]
- 175.Costoya J., Surnar B., Kalathil A.A., et al. Controlled release nanoplatforms for three commonly used chemotherapeutics. Mol. Aspect. Med. 2022;83 doi: 10.1016/j.mam.2021.101043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Subbotina J., Rouse I., Lobaskin V. In silico prediction of protein binding affinities onto core–shell PEGylated noble metal nanoparticles for rational design of drug nanocarriers. Nanoscale. 2023;15:32 13371–13383. doi: 10.1039/D3NR03264G. [DOI] [PubMed] [Google Scholar]
- 177.Bai L., Wu Y., Li G., et al. AI-enabled organoids: construction, analysis, and application. Bioact. Mater. 2024;31:525–548. doi: 10.1016/j.bioactmat.2023.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Smirnova L., Caffo B.S., Gracias D.H., et al. Organoid intelligence (OI): the new frontier in biocomputing and intelligence-in-a-dish. Front. Sci. 2023;1 doi: 10.3389/fsci.2023.1017235. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.