Abstract
To investigate the effect of cold plasma activated water (PAW) on pickled aquatic products, PAW was utilized to substitute salt water in the curing and processing of semi-dried golden pomfret. The results showed that the water content, salt content and TBARS value of the final dried golden pomfret cured with PAW were 1.11, 0.57 and 0.80 times that of the CK group respectively. A total of 11 kinds of free fatty acids were identified, the main fatty acids were palmitic acid (C16:0) and oleic acid (C18:1), the most polyunsaturated fatty acids were linoleic acid (C18:2), and docosahexaenoic acid (DHA, C22:6n-3).Through OPLS-DA and VIP analysis, a total of 58 volatile taste compounds were found, 19 distinctive flavor compounds including nonaldehyde, heptyl aldehyde,hexal, and others were acquired.The results demonstrated that PAW curing could increase characteristic flavor compounds and increase polyunsaturated fatty acid content in semi-dried golden pomfret.
Keywords: Low salt curing, Salt substitution, Cold plasma activated water, Semi-dried golden pomfret, Quality
Graphical abstract
Highlights
-
•
The salt water of curing solution was instead by PAW.
-
•
Higher water content and lower TBARS values were displayed in the PAW samples.
-
•
The PUFA content and nutritional indices of dietary fats were higher the PAW samples.
-
•
Nonaldehyde, heptyl aldehyde, and hexal were characteristic volatile compounds.
-
•
11 and 58 kinds FFA and volatile compounds were identified in PAW samples.
1. Introduction
One of the traditional aquatic products is semi-dried fish, which is highly favored by customers for its distinct flavor and taste. The water level of semi-dry aquatic products is appropriately regulated between 40 and 55 % (Qiu et al., 2019). To the maximum extent possible, semi-dry aquatic products give the product a distinctive curing flavor while preserving the primary features of fresh products. Semi-dried fish products have a stronger flavor than fresh fish products. Compared with dried fish products, they have a higher water content, which decrease manufacturing costs and energy consumption and offer a promising economic future (Qiu et al., 2019).With the increase of production of golden pomfret, semi-dry products is conducive to enriching the product types of golden pomfret market and promoting the economic development (Liu et al., 2023).
Curing is a technique employed to diminish water activity, impede bacterial proliferation, enhance the functional attributes of fish proteins, and prolong shelf life (Zeng et al., 2018). Cured products frequently using substantial quantities of sodium chloride, resulting in elevated sodium levels in the final product (Fraqueza et al., 2020). Prolonging consumption of high-salt foods can precipitate chronic conditions such as hypertension, hyperlipidemia, cardiovascular, and cerebrovascular diseases (Petit et al., 2019).As public awareness of healthy living increases, the demand for low-salt cured products rises, thus necessitating the development of such products within the food industry.
Plasma-activated water (PAW) can be obtained from direct treatment of cold plasma equipment to water, containing many active species such as hydrogen peroxide (H2O2), nitrate ion (NO3−), nitrite ion (NO2−), ozone (O3), hydroxyl radical (·OH), singlet oxygen (1O2), nitric oxide (NO−), and superoxide (O2−) etc.(Cai et al., 2023).It has been reported that cold plasma activated water can replace nitrite as curing agent in meat processing, to reduce the added and residual content of nitrite (Birania et al., 2022) When PAW was used in the processing of emulsified sausage (Jung et al., 2015) and pork breast (Yong et al., 2019), not only the color and flavor of the product was improved, but also the salt content was reduce.
In contrast to fresh fish, the effects of cold plasma on the physicochemical properties of dried fish products have not been extensively investigated. Zhiwen et al. (2023) treated dried fish with atmospheric cold plasma (CP) for 5 min. The muscle fibers broke and contracted, the microporous structure increased, the moisture content and hardness of the samples decreased. Effectively improving the storage stability, chewability and rehydration property of dried fish products. The characteristic odors of nonaldehyde, hexanal and octene-3-alcohol were enhanced by 2.13, 2.16 and 2.17 times respectively, and the results indicated that moderate lipid oxidation was conducive to the formation of characteristic volatiles. Choi et al. (2020)have demonstrated that a CP treatment duration of less than 30 min can effectively inactivate microorganisms in dried blackmouth fish (Lophiomus setigerus) by disrupting microbial cells, while preserving flavor and overall acceptability. Ke, Bai, Bai, et al. (2022) recognized that CP treatment enhanced the characteristic volatile compounds of dry-cured mackerel through the promotion of lipid oxidation. Additionally, Chen et al. (2018) found that CP treatment inhibited endogenous lipase or lipase-containing microorganisms in mackerel, thereby delaying both primary and secondary lipid oxidation and maintaining their sensory properties.
So far, still few studies about the application of PAW in pickled and dried aquatic products were published. It is still unclear what impact PAW as a curing condition will have on semi-dried fish products.Previous studies have shown that PAW can improve the quality of pickled or dried seafood. Therefore, semi-dried golden pomfret using PAW instead of saline solution were produced. The moisture, salt content, pH, TBARS, color, textural properties, free fatty acid content, and flavor profile of the final product were examined. The purpose of this study is to offer a theoretical framework for the substitution of salt water with PAW in the low-salt, semi-dried golden pomfret products.
2. Materials and methods
2.1. Semi-dried golden pomfret processing
Fresh golden pomfret (deceased,600-800 g) was purchased from a local market at Haikou, China, and translated to the laboratory on ice as soon as possible.The fish were removed the internal organs and cleaned, with cuts made every centimeter and the fish meat joined together.
Then divided into two groups randomly, with 15 golden pomfret in each group and 3 golden pomfret at each sampling point.Control group (CK): the fish cured with 4 % (w/v) salt water (salt was dissolved in water);PAW group: the fish was cured with 2 % (w/v) salt water (salt was dissolved in PAW).The two groups were cured at 4 °C, 80 %RH for 24 h with a turn every 6 h. After curing, the fish were translated to an oven for air drying controlling the temperature and humidity. The conditions were strictly controlled as follows: the temperature increased from 13.5 °C to 30 °C with an increasing rate of 1.5 °C every 8 h, while the humidity decreased from 75 % to 64 % with a reducing rate of 1 % every 8 h. The samples were taken for further analysis at the end of curing and dried for 24, 48, 72 and 96 h, respectively.
2.2. Preparation of PAW
PAW is prepared by a cold plasma jet system (PG-1000ZD, Nanjing Suman Plasma Engineering Research Institute Co., LTD.), and the plasma exciter is placed under the water surface for treatment, the condition is as follows: power 650 W, treatment time 90s, and the air flow rate is 0.9 L/min.
2.3. Determination of water content
Referring to the method of Kim et al. (2020) and making some modifications. The moisture content of samples was assessed by weight loss following 12 h of drying at 105 °C in the oven.
2.4. Determination of texture property
The texture changes of fish meat were measured with the TMS-PRO texture analyzer, and the fish (2 × 2 × 1 cm) from the back was carried out. Measurement parameters: Flat-bottom cylindrical probe TA41, pre-measurement rate of 30 mm/min, determination rate of 60 mm/min; measured speed 60 mm/min; compression degree 50 %; two measurement intervals of 5 s. Following each measurement, the probe was sanitized. At each sampling point, the back meat of three different golden pomfret fish from the same group was taken,and each sample was assessed in parallel three times to derive its average result.
2.5. Determination of chromatic aberration
A CR-10 color meter was selected, and the observation light source was CIE standard D65 light source. The sample CIE L*, a*, and b* was detected and the total color difference ΔE was calculated to measure the color change of the fillet. The calculation formula is as follows: ΔE = [(L*-L0*)2 + (a*-a0*)2 + (b*-b0*)2]1/2.
2.6. Determination of Thiobarbituric acid reactive substances
The Thiobarbituric Acid Reactive Substances(TBARS) values were determined according to the method described by Lan et al. (2023). The TBARS values were measured by detecting the absorption light value at 535 nm by spectrophotometry.
2.7. Determination of pH
The sample (2 g) and potassium chloride solution (18 mL) were homogenized at 15,000 g (PHS-3C, Shanghai Instrument & Electrical Scientific Instrument Co., LTD.) for 2 min, then the pH value was determined by pH meter. After washing, place the electrode in a standard buffer solution (pH 6.86 standard solution) and calibrate it at room temperature.
2.8. Determination of salt content
The salt content of semi-dried golden samples was determined by automatic titration (Yang et al., 2023). The samples (2 g) were grinded and mixed with water to 100 mL, and then transferred 0.5 mL 10 % potassium chromate indicator. The mixture was titrated with 0.1 mol/L silver nitrate standard solution.
2.9. Analysis of fatty acid content
The methods of Sang et al. (2024) was employed with minor modifications. Methyl esterification: the lipid samples were treated with 2 mL of methanol sulfate (1 %, v/v) for 30 min Subsequently, n-hexane (1 mL) solution were introduced and agitated for 1 min, followed to achieve a stratified solution. The upper organic layer was removed using aspiration, and the solvent was then evaporated. Prior to examination, the material was subjected to an organic filter membrane (0.22 μm).
The heating protocol was as follows: the temperature was sustained at 45 °C for 4min, thereafter elevated to 175 °C at a rate of 13 °C/min for 27 min, and finally raised to 215 °C at a rate of 4 °C/min for 35 min. The mass spectrum analysis qualitatively identified the fatty acid components, and the percentage content of each fatty acid was determined using peak area normalization.
2.10. Analysis of volatile organic compounds
Volatile chemicals in desiccated fish samples were analyzed using gas chromatography–mass spectrometry (GC/MS) following the methodology of Wang et al. (2022). Two grams of samples were introduced into a 15 mL extraction flask containing 10 μL of the internal standard, 2,4,6-trimethylpyridine (50 μg/mL).
The solid-phase microextraction (SPME) conditions: a 65 μm PDMS/DVB extraction head was used and adsorbed at 60 °C for 40 min, then inserted into the GC inlet for GC–MS analysis. GC conditions: HP-5MS elastic capillary column (30 m × 0.25 mm,0.25 μm).Heating procedure: the initial temperature is 40 °C, maintained for 2 min, increased to 160 °C at 4 °C/min, not maintained, and then increased to 250 °C at 10 °C/min, maintained for 5 min; The inlet temperature was 250 °C, and the flow rate of the carrier gas (He) was 20 mL/min. MS conditions: electron energy 70 eV, transmission line temperature 280 °C, ion source temperature 230 °C, mass scanning range m/z:30–350.
Qualitative: The extracted volatile components are qualitatively confirmed by the system's built-in NIST 02 and Wiley mass spectrometry data.
2.11. Determination of nitrate and nitrite content
The nitrate and nitrite concentrations in dried fish samples were quantified using High Performance Liquid Chromatography (HPLC). The sample (2 g) was weighed and placed in 150 mL, 80 mL of water was added into a corked conical bottle, ultrasonic extraction was performed for 30 min. After five minutes in a water bath set at 75 °C, remove it and allow it to come to room temperature. Mix thoroughly after transferring it to a 100 mL volumetric flask at a consistent volume. Use filter paper to filter it, then take a piece of the filtrate and centrifuge it for 15 min at 10, 000 rpm. Remove and save the supernatant. Then, using around 15 mL of spare solution, choose the 0.22 μm aqueous filter needle filter, C18 column (throw away the first 3 mL), or pass through the needle filter, C18 column, Ag column, and Na column in order of concentration of chloride ions (throw away the first 7 mL).
For testing, gather the eluent. The solid-phase extraction column must be turned on prior to sample processing. The Ag (1.0 mL) and Na (1.0 mL) columns were both activated by passing through 10 mL of water and letting them stand for 30 min. For a C18 column (1.0 mL), 10 mL of methanol and 15 mL of water should be run through the column in that order and allowed to activate for 30 min. The chromatographic column that is utilized is a high-capacity anion exchange column that has alkyl alcohol quaternary ammonium salt functional groups, divinylbenzene ethylstyrene copolymer matrix, and hydroxide selectivity. 4 mm × 250 mm (protected by 4 mm × 50 mm) or an ion chromatographic column with comparable performance are its parameters. With elution gradients of 6 mmol/L for 30 min, 70 mmol/L for 5 min, and 6 mmol/L for 5 min, with a flow rate of 10 mL/min, the potassium hydroxide solution has a concentration range of 6 mmol/L ∼ 70 mmol/L. With an injection volume of 50 uL (configurable as needed), the detector is either a UV detector (wavelength 226 nm) or a conductivity detector (detection cell temperature 35 °C).
2.12. Statistical analysis
The SPSS 20.0 Statistics software(SPSS Inc., Chicago, IL,USA)was used to analyze the difference significance of the data, and P < 0.05 was considered statistically significant. Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA) was analyzed using SIMCA-P 14.1 software (Umetrics, Umea, Sweden) and plotted using Origin 2024 software (Origin Lab, Inc., Northampton, MA, USA).
3. Results and discussion
3.1. Water content
The water content of both groups after curing exhibited no significant difference (P > 0.05), suggesting that the curing process had a negligible impact on the water content of the fish (Fig. 1). As the drying time increased, a downward trend was observed, and the content of the PAW group totally diminished by 20.64 % during the whole drying procedure, while the CK group was 24.99 %. Osmotic pressure caused by salt facilitated the loss of cellular fluid to the curing solution, and the air drying further expedited water loss, resulting in a decreased water content correlating with the prolonged time (Yang et al., 2020). After drying 96 h, the water content of the PAW group exceeded that of the CK group, suggesting that partial salt treatment more effectively preserves the water in golden pomfret. Concurrently, water content of air-dried products diminished as the salt concentration of curing solution increased, which was attributable to the water loss induced by the elevated osmotic pressure (Hwang et al., 2012; Yang et al., 2020).
Fig. 1.
Changes of water content of semi-dried golden pomfret products during processing.
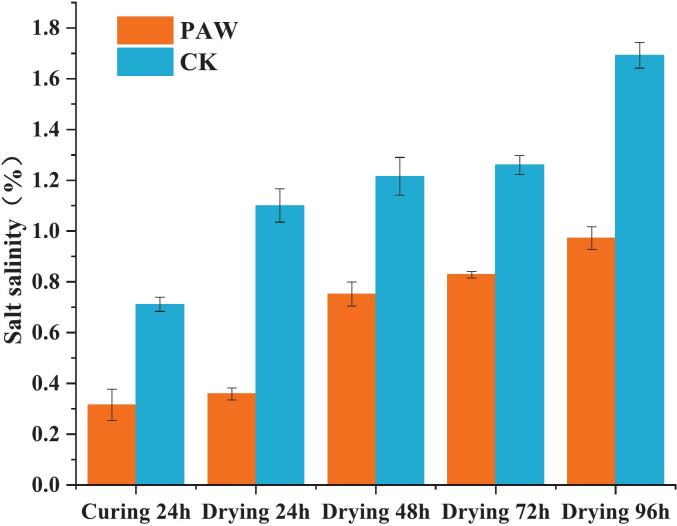
3.2. Salt content
The salt content in golden pomfret flesh escalated with prolonged drying processing(Fig. 2), which was consistent with the above results of water content. The elevation of salt content in fish was primarily attributed to the reduction of water content (Jo et al., 2020). After 96-h drying, the salt content in the final product of the PAW and CK groups was 0.97 % and 1.69 %, respectively, while the salt concentration of the curing solution in the PAW group was half of that in the CK group. The salt concentration of the curing solution resulted in osmotic pressure, which impelled the diffusion of chloride ions in the fish. What's more, the active ions in the PAW would enhance the curing process. Gao et al. (2023) utilized MgCl2, arginine, KCl, and other substances partially substituting NaCl to cure the chicken, and found a 49.7 % reduction of salt content and an improvement of flavor components. Ferreira et al. (2024) employed a NaCl-glass grass mixture to process traditional dry-cured pork belly, with decreasing salt content while maintaining microbial stability during refrigerated vacuum storage and generating specific aromatic compounds characteristic of dry-cured meat products.
Fig. 2.
Changes of salt content in semi-dried golden pomfret products during processing.
3.3. pH
The pH values of the two group samples were decreased first and then increased trends during the processing (Fig.3). the decrease in pH may result from the oxidative breakdown of fats, leading to the formation of fatty acids. Besides, as processing time was extended, the gradual oxidation and decomposition of protein in fish samples were cured, leading to the formation of alkaline substances such as ammonia and amines, which serve to neutralize certain acidic compounds (Jin et al., 2011). As reported that the pH of fermented fish Plaa-som was decreased from 6 to 4.5, when the curing salt increased from 6 % to 7 %. Therefore, the reduction of pH in fish was caused by the elevated salt content in the pickling solution (Paludan-Müller et al., 2002).
Fig. 3.
Changes of pH during processing of semi-dried golden pomfret products.
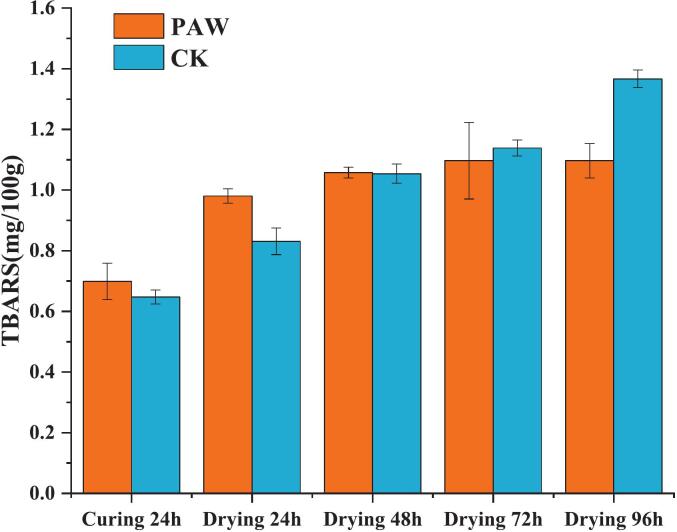
3.4. TBARS
As illustrated in Fig. 4, the TBARS values in both groups exhibited a significant increase (P < 0.05) with the prolongation of processing time. Notably, the PAW group demonstrated higher values compared to the control group after curing and drying for 24 h. The TBARS values in the two groups were equal, and after 72 h drying, the TBARS value of the CK group was progressively greater than that of the PAW group.It has been reported that the cold plasma treatment could enhance the TBARS values in herring fillets(Albertos et al., 2019) and turbot fish(Ke, Bai, Bai, et al., 2022). There were many active species in the PAW facilitating the lipid oxidation of the samples, but as the drying period increased, the active components in PAW were decreased to diminish, leading to a slow increase of lipid oxidation. In addition, the application of cold plasma treatment effectively inhibited endogenous lipase and lipase-containing microorganisms in fish products, thereby delaying both primary and secondary lipid oxidation(Chen et al., 2018). Elevated levels of sodium chloride have been demonstrated to compromise cell membrane integrity and facilitate the ingress of oxidants into the lipid matrix, enhancing the lipid oxidation (Lilian & Bragagnolo, 2017), besides, NaCl may facilitate the decomposition of hydroperoxides into secondary oxidation products and enhance the oxidation process (Wang et al., 2022).During the subsequent drying process, the NaCl content, lipase and the action of microorganisms are the main reasons promoting lipid oxidation in semi-dried golden pomfret.Therefore, the lipid oxidation rate of the CK group gradually exceeded that of the PAW group.
Fig. 4.
Changes of TBARS during processing of semi-dried golden pomfret products.
3.5. Color parameter
Table 1 showed that the L* values were gradually diminished, while the b* and ΔE values steadily escalated. The L* and b* values in the PAW group consistently exceeded those in the CK group, but the ΔE values in the CK group surpassed those in the PAW group during the drying processing. During the processing of semi-dried golden pomfret, both protein and lipid were oxidated, which resulted in a reduction in brightness and an increase in yellowness (Hui et al., 2023). Simultaneously, both reactive oxygen and nitrogen species contained in PAW might interact with myoglobin, leading to a yellowish discoloration of fish. Upon completion of drying, the PAW-treated samples exhibited higher L* and b* values, suggesting that the partial substitution with PAW of curing solution significantly enhanced the color of semi-dried golden pomfret.
Table 1.
Color changes of semi-dried golden pomfret products during processing.
| Sample | group | L* | a* | b* | ΔE |
|---|---|---|---|---|---|
| Curing-24 h | PAW | 50.72 ± 0.63aA | 0.27 ± 0.13aA | 2.44 ± 0.70bA | 1.65 ± 0.41c |
| CK | 47.13 ± 1.77bB | −0.77 ± 0.28bB | 1.86 ± 0.45bB | 4.54 ± 1.16b | |
| Drying-24 h | PAW | 50.46 ± 1.41aA | −0.05 ± 0.49bcA | 2.53 ± 0.28bA | 2.15 ± 0.76bc |
| CK | 46.52 ± 1.37bB | −1.37 ± 0.27cB | 2.16 ± 0.56bB | 5.30 ± 1.10b | |
| Drying-48 h | PAW | 48.11 ± 0.27bA | −0.71 ± 0.43cdA | 2.71 ± 0.49bA | 3.69 ± 0.46b |
| CK | 46.20 ± 0.94bcB | −1.70 ± 0.44cdB | 2.36 ± 0.46bB | 5.72 ± 0.96b | |
| Drying-72 h | PAW | 45.34 ± 2.47cA | −1.07 ± 0.97deA | 2.93 ± 0.35bA | 6.49 ± 1.51a |
| CK | 44.50 ± 1.45cdB | −2.02 ± 0.22deB | 2.40 ± 0.36bB | 7.32 ± 1.26a | |
| Drying-96 h | PAW | 44.37 ± 1.52cA | −1.73 ± 0.39eA | 4.85 ± 1.93aA | 7.98 ± 2.12a |
| CK | 44.19 ± 1.06dA | −2.27 ± 0.59eB | 4.61 ± 2.45aB | 8.41 ± 1.23a |
Note: Different lowercase letters indicate significant differences among each group of samples at different sampling time points (p < 0.05), while different uppercase letters indicate significant differences among the treatment groups at the same sampling time point (p < 0.05). The annotations in the subsequent charts are the same.
3.6. Texture property
Table 2 showed that the texture changes of golden pomfret were obvious during the processing. The hardness of samples was increased as the processing time increased, as the water lost and salt content increased resulting in the muscle fiber shrinks. Besides, these changes also induced the stickiness decrease of fish (Gao, Cheng, et al., 2024). After curing, the hardness and chewiness of the PAW group were significantly diminished compared to the CK group (P < 0.05). It has been proved that the hardness and chewiness of food augment with elevated salt concentrations during the curing process (Zeng et al., 2018). Increasing salt content in the fish can diminish the activity of endogenous enzymes that degrade tissue structural components in fish muscles, leading to increased protein contraction (Thorarinsdottir et al., 2011) This was one of the reasons that the PAW group samples showed higher hardness. Consequently, the PAW substitute salt water plays an obvious role in reducing hardness and cohesiveness and is useful in keeping the softness of fish.
Table 2.
Texture characteristics changes of semi-dried golden pomfret during processing.
| Sample | Group | Hardness /g | Elasticity /mm | Cohesion | Adhesiveness /g | Masticatory /mJ |
|---|---|---|---|---|---|---|
| Curing-24 h | PAW | 123.9 ± 20.87c | 1.95 ± 0.07c | 47.4 ± 3.05d | 0.90 ± 0.07c | 0.28 ± 0.04b |
| CK | 136.3 ± 10.03c | 2.33 ± 0.29b | 60.0 ± 10.97d | 1.60 ± 0.68d | 0.49 ± 0.11b | |
| Drying-24 h | PAW | 146.1 ± 15.18bc | 2.54 ± 0.13b | 93.8 ± 11.16c | 2.34 ± 0.23b | 0.49 ± 0.07a |
| CK | 164.6 ± 24.98c | 2.86 ± 0.27a | 94.0 ± 14.37c | 2.64 ± 0.54cd | 0.61 ± 0.12ab | |
| Drying-48 h | PAW | 150.9 ± 28.12bc | 2.78 ± 0.12b | 107.4 ± 21.94c | 2.96 ± 0.66b | 0.59 ± 0.18a |
| CK | 173.2 ± 31.35bc | 3.23 ± 0.41a | 111.8 ± 17.16c | 3.58 ± 0.86c | 0.74 ± 0.15a | |
| Drying-72 h | PAW | 220.0 ± 13.92b | 3.31 ± 0.48b | 146.4 ± 36.04b | 4.88 ± 1.60a | 0.64 ± 0.11a |
| CK | 207.3 ± 47.35b | 3.26 ± 0.35a | 153.6 ± 7.95b | 4.90 ± 0.49b | 0.68 ± 0.08a | |
| Drying-96 h | PAW | 289.1 ± 40.71a | 2.85 ± 0.14a | 178.8 ± 31.60a | 5.00 ± 0.71a | 0.58 ± 0.10a |
| CK | 341.3 ± 14.63a | 3.23 ± 0.45a | 212.8 ± 37.59a | 6.72 ± 1.23a | 0.61 ± 0.12ab |
3.7. Fatty acid content
Golden pomfret as a deep-sea fish with high lipid content, is easily oxidation into free fatty acids during air drying (Gil & Gil, 2015). Free fatty acids, as precursors of volatile molecules, can significantly influence the flavor development of a product (Kang et al., 2013). As shown in Table 3, 11 kinds of free fatty acids were identified throughout the processing of semi-dried golden pomfret, which was consistent with the results of free fatty acid composition observed in the golden pomfret processed by traditional marination method (Wang et al., 2022). The total amount of monounsaturated fatty acids (MUFA) indented was higher than that of saturated fatty acids (SFA) and polyunsaturated fatty acids (PUFA) during the whole process. Simultaneously, notable disparities in SFA and PUFA were observed between the two groups after the curing (P < 0.05). Throughout the drying process, the total SFA content of the PAW group was markedly reduced (P < 0.05), whereas the levels of MUFA and PUFA were dramatically elevated (P < 0.05).
Table 3.
Fatty acid content(%)changes of semi-dried golden pomfret products during processing.
| Fatty acid | group | Curing 24 h | Drying 24 h | Drying 48 h | Drying 72 h | Drying 96 h |
|---|---|---|---|---|---|---|
| C14:0 | PAW | 2.80 ± 0.29aA | 2.81 ± 0.13aA | 2.72 ± 0.13aA | 2.29 ± 0.01bB | 2.26 ± 0.01bA |
| CK | 2.74 ± 0.19aA | 2.88 ± 0.01aA | 2.52 ± 0.08bA | 2.48 ± 0.03bA | 2.33 ± 0.10bA | |
| C16:0 | PAW | 31.04 ± 0.47aA | 27.85 ± 0.47bA | 26.86 ± 0.13bcA | 26.33 ± 0.67cA | 26.31 ± 0.52cA |
| CK | 27.37 ± 0.99abB | 28.23 ± 0.57aA | 26.96 ± 0.67bA | 25.74 ± 0.38cA | 26.78 ± 0.32bcA | |
| C16:1 | PAW | 3.64 ± 0.15aA | 3.30 ± 0.13bcA | 3.41 ± 0.06bA | 3.01 ± 0.02dB | 3.13 ± 0.05cdA |
| CK | 3.63 ± 0.16aA | 3.46 ± 0.03bA | 3.10 ± 0.09cB | 3.24 ± 0.04cA | 3.10 ± 0.03cA | |
| C17:1 | PAW | 0.90 ± 0.12bA | 1.22 ± 0.38abA | 1.15 ± 0.03abA | 1.45 ± 0.05aA | 1.10 ± 0.12abA |
| CK | 0.88 ± 0.17bA | 0.83 ± 0.11bA | 1.38 ± 0.26aA | 1.04 ± 0.19bB | 0.90 ± 0.69bA | |
| C18:0 | PAW | 4.41 ± 0.15aA | 4.6 ± 0.18aA | 4.33 ± 0.28aA | 4.47 ± 0.13aA | 4.53 ± 0.16aA |
| CK | 3.79 ± 0.18cB | 4.25 ± 0.07bB | 4.78 ± 0.14aA | 4.25 ± 0.12bA | 4.23 ± 0.04bB | |
| C18:1 | PAW | 30.84 ± 0.59aA | 30.71 ± 0.04aA | 29.81 ± 0.51bB | 30.87 ± 0.37aA | 31.35 ± 0.35aA |
| CK | 30.25 ± 0.35bA | 28.82 ± 0.05cB | 31.35 ± 0.45aA | 31.52 ± 0.16aA | 30.45 ± 0.26bB | |
| C18:2 | PAW | 20.84 ± 0.29cB | 21.95 ± 0.24bB | 23.22 ± 0.53aA | 23.22 ± 0.31aB | 23.20 ± 0.33aB |
| CK | 24.28 ± 0.25abA | 23.95 ± 0.12bA | 22.05 ± 0.35cB | 23.96 ± 0.18bA | 24.59 ± 0.19aA | |
| C20:1 | PAW | 1.56 ± 0.05dB | 1.71 ± 0.02cB | 1.83 ± 0.09bA | 1.99 ± 0.05aA | 2.04 ± 0.02aB |
| CK | 1.89 ± 0.03cA | 1.95 ± 0.02bA | 1.68 ± 0.02dA | 1.96 ± 0.03bA | 2.15 ± 0.05aA | |
| C18:3n-3 | PAW | 1.40 ± 0.01bA | 1.44 ± 0.02bA | 1.56 ± 0.04aA | 1.60 ± 0.04aA | 1.60 ± 0.04aA |
| CK | 1.46 ± 0.07bcA | 1.41 ± 0.04cA | 1.51 ± 0.06bA | 1.62 ± 0.01aA | 1.61 ± 0.03aA | |
| C20:2n-6 | PAW | 1.29 ± 0.03dB | 1.42 ± 0.03cB | 1.67 ± 0.05bA | 1.76 ± 0.03aB | 1.74 ± 0.02aB |
| CK | 1.50 ± 0.09cA | 1.62 ± 0.02bA | 1.65 ± 0.02bA | 1.86 ± 0.02aA | 1.79 ± 0.01aA | |
| C22:6n-3 | PAW | 1.88 ± 0.23cA | 3.00 ± 0.37abA | 3.44 ± 0.28aA | 2.99 ± 0.14abA | 2.73 ± 0.15bA |
| CK | 2.21 ± 0.31bA | 2.60 ± 0.01abA | 3.03 ± 0.56aA | 2.33 ± 0.11bB | 2.07 ± 0.10bB | |
| ∑SFA | PAW | 38.24 ± 0.88aA | 35.26 ± 0.45bA | 33.91 ± 0.17bcA | 33.10 ± 0.83cA | 33.12 ± 0.69cA |
| CK | 33.90 ± 1.01bB | 35.35 ± 0.07aA | 34.26 ± 0.65abA | 32.47 ± 0.42cA | 33.34 ± 0.43bcA | |
| ∑MUFA | PAW | 36.94 ± 0.76abA | 36.94 ± 0.32abA | 36.20 ± 0.84bA | 37.32 ± 0.45abA | 37.62 ± 0.51aA |
| CK | 36.65 ± 0.42bA | 35.06 ± 0.02cB | 37.51 ± 0.28aA | 37.76 ± 0.19aA | 36.60 ± 0.24bB | |
| ∑PUFA | PAW | 25.41 ± 0.09cB | 27.80 ± 0.13bB | 29.89 ± 0.86aA | 29.58 ± 0.43aA | 29.26 ± 0.41aA |
| CK | 29.45 ± 0.72aA | 29.58 ± 0.09aA | 28.23 ± 0.87bA | 29.77 ± 0.23aA | 30.05 ± 0.30aA |
In this study, the palmitic acid (C16:0) had the highest content among the saturated fatty acids, while the oleic acid (C18:1) was the most one of monounsaturated fatty acids. Besides, the contents of linoleic acid (C18:2) and DHA (C22:6n-3) were significantly higher (P < 0.05) than other polyunsaturated fatty acids. After curing, the palmitic acid (C16:0) content in the PAW group exceeded that of the CK group by 3.67 %, while the SFA content was 4.34 % higher and the PUFA content was 4.04 % lower in the PAW group compared to the CK group.
After 96 h of drying, the SFA and PUFA showed no significant difference between the two groups. During the drying period, the extent of lipid oxidation was increased gradually to produce more short-chain saturated fatty acids. Both palmitic acid (C16:0) and oleic acid (C18:1) were the predominant free fatty acids during the pickling and drying processes of pomfret. After curing 24 h, there was no notable difference in palmitic acid (C16:0) content of the two groups. Palmitic acid(C16:0) has been recognized as a volatile component in fish; but it does not significantly influence product odor (Sang et al., 2024). The amount of oleic acid (C18:1) in the PAW group was markable increased after drying, potentially attributable to the pronounced lipid oxidation observed in the CK group, for it can be oxidized into octanaldehyde and nonanaldehyde flavor compounds (Chen et al., 2023).
DHA (C22:6n-3) is a crucial fatty acid within the polyunsaturated fatty acid (PUFA) category, for it is related to the decreased incidence of cardiovascular disease in humans and is essential for sustaining fundamental physiological activities (Valentini et al., 2020). DHA is crucial for preserving cell membrane permeability, facilitating proper cell metabolism, and ensuring energy supply, however, it is considered to play a minimal influence on food flavor development (Wen et al., 2023). In this study, the DHA in the PAW group was markedly lower than the CK group after 24 h curing, this may cause by the susceptible response of DHA to the reactive species in the PAW (Wang, Cai, et al., 2024). The DHA content in the PAW group was increased after drying, and it was 31.88 % higher than that in the CK group after 96 h drying. Therefore, the PAW group is good in preserving DHA in the samples and enhancing the nutritious composition of semi-dried golden pomfret.
Linoleic acid (C18:2) was another predominant polyunsaturated fatty acid identified in the semi-dried fish, it was 3.44 % lower in the PAW group after curing for 24 h. during the drying period, its content was increased and exceeded that of the CK group at the end of drying. In the 24 h of curing, the free radicals in PAW may react with the linoleic acid (C18:2) to cause oxidative degradation. As the disappear of the free radicals in the PAW, the enhanced oxidation process of linoleic acid in the PAW became relatively slow, compared to the CK group in the drying time. What's more, the linoleic acid accumulation could improve the generation of hexaldehyde, to result in a more pronounced grassy flavor in fermented fish products (Wang et al., 2022).
Fig. 5 displayed the notable differences in fatty acid contents of semi-dried golden pomfret during processing, the percentage of saturated fatty acids (SFA) in the PAW group was markedly greater than that in the CK group after curing 24 h. PAW, containing reactive species such as ROS, particularly O3, •OH, and 1O2, which could target the double bonds of unsaturated fatty acids leading to their cleavage and degradation, facilitated the oxidation and destruction of unsaturated fatty acids in the samples during the curing period (Wang, Wang, et al., 2024). During the curing, the double bonds of unsaturated fatty acids were cleaved to yield short-chain saturated fatty acids (Sang et al., 2024), as a result, the total content of SFA was increased comparable.However, the application of cold plasma treatment effectively inhibited endogenous lipase and lipase-containing microorganisms in fish products, thereby delaying both primary and secondary lipid oxidation (Chen et al., 2018). Consequently, during the subsequent drying process, the rate of lipid oxidation in the PAW group was significantly reduced compared to that observed in the control group and during the curing process.As a result, more PUFA and MUFA in the free fatty acids produced during the drying period could be retained, leading to an increase in the percentage content of PUFA and MUFA in the PAW group in the later stage.Meanwhile, an increase in NaCl concentration is known to enhance the oxidation of unsaturated fatty acids (Cao et al., 2025).Given that higher NaCl levels were present in the CK group, this would further promote oxidative reactions involving unsaturated fatty acids during drying processes.
Fig. 5.
Fatty acids content of semi-dried golden pomfret during processing.
Generally, the dietary fats are fatty acids that could play positive or negative roles in disease preventing and treating, and their nutritional and/or medicinal value is often determined with a nutritional index (Chen & Liu, 2020). As shown in Table 4, the nutritional indices of PUFA/SFA, HH, and HPI of final product in PAW group were slightly higher than those in CK group, while the IA indices in PAW group was slightly lower with no significant difference. It is considered that the fish lipid quality (FLQ) was the main nutritional indicator for fish lipids (Senso et al., 2007), therefore, the FLQ was calculated in our study. After 96 h drying, the FLQ index of PAW group was 0.29 higher than that of CK group with a significant difference (P < 0.05), which indicated that the lipid nutrition of semi-dried golden pomfret processed by PAW instead of salt water was effectively improved. Above all, PAW partial instead of partial salt water can enhance the breakdown of polyunsaturated fatty acids in the lipid oxidation. Moderate lipid oxidation improved the synthesis and preservation of high-quality free fatty acids during the processing of semi-dried golden pomfret.
Table 4.
Fatty acid nutritional indices changes of semi-dried golden pomfret products during processing.
| Index | group | Sample |
||||
|---|---|---|---|---|---|---|
| Curing 24 h | Drying 24 h | Drying 48 h | Drying 72 h | Drying 96 h | ||
| Polyunsaturated fatty acid/ saturated Fatty acid ratio (PUFA/SFA) |
PAW | 0.66 ± 0.01cB | 0.79 ± 0.01bB | 0.88 ± 0.03aA | 0.89 ± 0.02aA | 0.88 ± 0.02aA |
| CK | 0.87 ± 0.03abA | 0.84 ± 0.01bA | 0.83 ± 0.02bA | 0.92 ± 0.01aA | 0.90 ± 0.01aA | |
| Index of atherogenicity (IA) | PAW | 0.68 ± 0.02aA | 0.60 ± 0.01bB | 0.57 ± 0.02bcA | 0.53 ± 0.01cA | 0.53 ± 0.01cA |
| CK | 0.58 ± 0.02abB | 0.61 ± 0.01aA | 0.56 ± 0.01bcA | 0.52 ± 0.01cA | 0.54 ± 0.01cA | |
| Hypocholesterolemic /hypercholesterolemic ratio (HH) |
PAW | 1.66 ± 0.03cB | 1.91 ± 0.03bA | 2.02 ± 0.08abA | 2.11 ± 0.05aA | 2.12 ± 0.04aA |
| CK | 1.99 ± 0.06bcA | 1.87 ± 0.01cB | 2.02 ± 0.04bA | 2.17 ± 0.02aA | 2.07 ± 0.02abA | |
| Health-promoting index (HPI) | PAW | 1.48 ± 0.04cB | 1.65 ± 0.03bA | 1.75 ± 0.07abA | 1.88 ± 0.04aA | 1.89 ± 0.03aA |
| CK | 1.73 ± 0.06bcA | 1.62 ± 0.01cB | 1.77 ± 0.04abA | 1.89 ± 0.02aA | 1.85 ± 0.03abA | |
| Fish lipid quality/flesh lipid quality (FLQ) |
PAW | 1.88 ± 0.13cA | 2.99 ± 0.21abA | 3.44 ± 0.16aA | 2.99 ± 0.08abA | 2.73 ± 0.09bA |
| CK | 2.21 ± 0.18bA | 2.60 ± 0.01abB | 3.03 ± 0.32aA | 2.07 ± 0.06bB | 2.44 ± 0.11bB | |
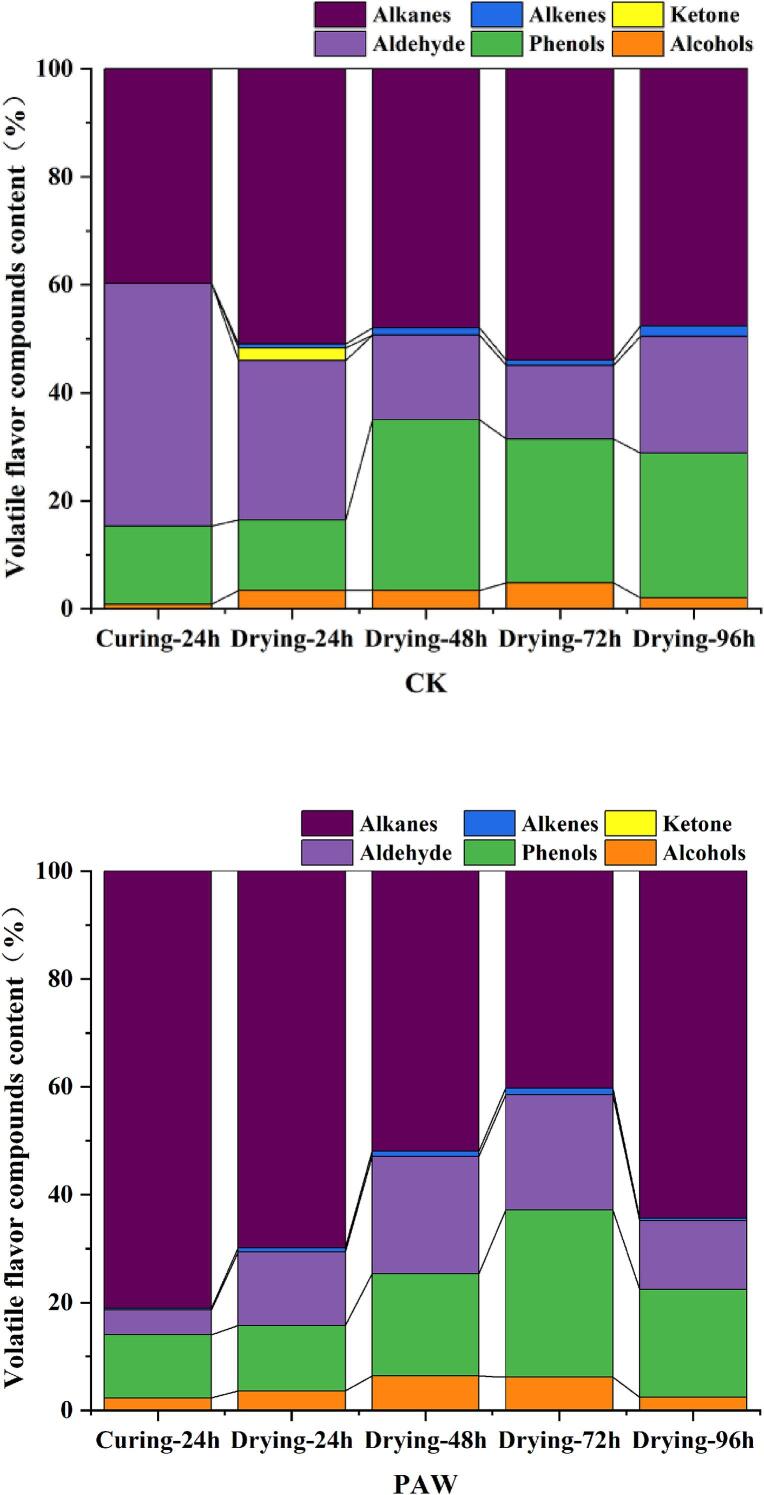
3.8. Volatile flavor compounds
Endogenous lipase could induce the degradation of lipids to generate free fatty acids that can be further oxidized to aldehydes, alcohols, ketones, acids, alkanes, and other flavor compounds (Tian et al., 2020). As shown in Table 5, a total of 60 volatile components in the semi-dried good pomfret were identified, including 27 alkanes, 12 aldehydes, 7 esters, 6 alcohols, 4 olefins,1 ketone, and 1 phenol and 2 others. The relative contents of volatile compounds in semi-dried golden pomfret were displayed in Fig.6. During the whole processing, the changes of compounds contents were as follows: alcohols (0.89–6.42 %), aldehydes (4.62–44.89 %), phenols (11.71–31.60 %), ketones (0–2.28 %), olefins (0.30–1.91 %), olefins (0.30–1.91 %), and alkanes (39.77–81.06 %). The relative contents of both aldehydes and alcohols in the two groups were significantly higher (P < 0.05) than others, for they were the main flavor formation sources of semi-dried golden pomfret.
Table 5.
Volatile flavor compounds changes of semi-dried golden pomfret products during processing.
| group | Sample | Volatile flavor compounds |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| alcohols | phenols | aldehyde | ketone | esters | alkenes | alkanes | other | ||
| PAW | Curing-24 h | 3 | 1 | 4 | 0 | 2 | 1 | 32 | 1 |
| Drying-24 h | 3 | 1 | 6 | 0 | 0 | 1 | 19 | 0 | |
| Drying-48 h | 3 | 1 | 5 | 0 | 0 | 1 | 25 | 0 | |
| Drying-72 h | 2 | 1 | 6 | 0 | 2 | 2 | 24 | 0 | |
| Drying-96 h | 2 | 1 | 4 | 0 | 0 | 1 | 18 | 0 | |
| CK | Curing-24 h | 1 | 1 | 8 | 0 | 1 | 0 | 14 | 1 |
| Drying-24 h | 1 | 1 | 7 | 1 | 1 | 1 | 17 | 1 | |
| Drying-48 h | 1 | 1 | 5 | 0 | 1 | 1 | 24 | 0 | |
| Drying-72 h | 2 | 1 | 4 | 0 | 0 | 1 | 27 | 0 | |
| Drying-96 h | 1 | 1 | 5 | 0 | 0 | 2 | 21 | 0 | |
Fig. 6.
Volatile flavor compounds content(%)changes of semi-dried golden pomfret products during processing.
Aldehydes are the degradation products of lipid oxidation and protein degradation as well as amino acid decarboxylation and deamidation (Li et al., 2022). Aldehydes possess a fatty flavor with a low sensory threshold, and even in trace concentrations, they can significantly influence the odor of fish (Benet et al., 2015). In this study, 12 aldehydes were identified, mainly including hexanal, heptanal, benzaldehyde, phenylacetaldehyde, nonanal, and capraldehyde. It has been proved that the contents of hexanal and nonanal linear aldehydes increased during the fermentation process was beneficial to improve the flavor of fermented golden pomfret (Wang et al., 2022). Hexanal with a grassy aroma, is formed from the cleavage of hydroperoxides produced by the auto-oxidation of linoleic acid (Benet et al., 2015). However, high levels of hexanal in meat products may cause a putrefactive odor (Benet et al., 2015). In this study, the nonanal in the PAW group was higher than that in CK group after curing 24 h, and it was reduced gradually till less than that in CK group as the drying time extending. Usually, it is considered that the nonanal was formed from the oxidation of oleic acid (C18:1) (Lilian & Bragagnolo, 2017). The reactive components in PAW, especially the reactive oxygen species (O3, 1O2,H2O2 and O2−), could enhance the oxidation in fish, promote the decomposition of fatty acids and proteins to produce more types and quantities of volatile flavor compounds (Wang, Wang, et al., 2024), which improve more aldehyde and alcohol substances generated in the PAW cured fish. Salt can enhance the activity of lipoxygenase in meat to facilitate the breakdown of unsaturated fatty acids and other lipids, hence increasing the amount of volatile components, However, too much salt can inhibit the lipoxygenase activity (Lilian & Bragagnolo, 2017). Therefore, the optimal decrease of sodium chloride content is beneficial to the formation of fish flavor.
Alcohols can be generated through the oxidation and breakdown of lipids and/or carbonyl reduction. Six alcohols were identified in the PAW group, but only two alcohols were identified in the CK group. Trans-2-Methylcyclopentanol, 2-methyldecanol, linalool, and 2-hexyl-1-decanol were just identified in the PAW curing samples. 1-Octen-3-ol showed the highest content, which is formed by the β- oxidation of linoleic acid and is rich in mushroom flavor with a low flavor threshold (Gao, Zhang, et al., 2024), played a significant contribution to the overall flavor profile of the samples. A notable positive correlation between 1-octene-3-ol and the lipid oxidation index TBARS has been reported (Sang et al., 2024). A lower oxidation degree was found in the PAW group samples as the above results shown, which is consistent with the lower contents of 1-octene-3-ol in PAW than in the CK group.
A total of 27 distinct hydrocarbons were identified, all of them had a relatively low content in all the samples. Hydrocarbon compounds are mainly derived from the homolysis of alkoxy radicals of fatty acids (Lilian & Bragagnolo, 2017). The hydrocarbon compounds were considered with no contribution to the overall flavor of fish samples, though many kinds of them are present, because of their high threshold.
OPLS-DA is an analytical method that visualizes data through correlations between data and quantifies the degree of difference between samples (Chen et al., 2024). As shown in Fig. 7, the OPLS-DA scores for the PAW group are: R2X = 0.991, R2Y = 0.998, Q2 = 0.998, indicating that the developed model has high data accuracy. The R2 (0.107) and Q2 (−0.766) were below the retention threshold of 1.0 (Fig. 7A), and the intercept of the regression line for model Q2 with the horizontal axis was negative, suggesting the absence of overfitting in the model.
Fig. 7.
(A) Cross-substitution plot of 200 permutation tests; (B)Score plot of OPLS-DA; (C)Distribution of VIP values.
Fig. 7B showed that the samples were distinctly categorized, where the samples of Drying-24 h and Drying-48 h occupied the second quadrant, the samples of Drying-72 h and Drying-96 h were located in the third quadrant, and the Curing-24 h sample was positioned in the first quadrant, illustrating a substantial alteration in the overall volatility of the samples across five sampling points. The volatile flavor of samples varies significantly across different processing stages.
Based on the variable importance prediction (VIP) score of OPLS-DA model, the primary volatile flavor components were screened as differential markers in different samples. Volatiles with a VIP value higher than 1 in the screening model were deemed typical scent components. The volatile compounds with a VIP value over 1 encompass phenylethanol, nonanal, benzaldehyde, heptanal, (E)-tetradecan-2-enal, trans-2-decenoal, linalool, decanal, phenylacetaldehyde, and hexanal, among others, and 19 chemicals were identified as typical odors through the integration of key odor assessment (Fig. 7C).
3.9. Active compounds identified by OAV
The intensity of odor is closely associated with the concentration of volatile flavor compounds and sensory thresholds. The Odor Activity Value (OAV) serves as a tool for identifying key volatile flavor substances in food (Pang et al., 2019). The contribution of differential markers to the overall flavor profile of tilapia fillets can be assessed by calculating the OAV values of these volatile flavor compounds. Substances with an OAV greater than 1 are considered key flavor components that significantly contribute to the overall taste; their contribution is directly proportional to their respective OAV values(Schoenauer & Schieberle, 2019).From the differential markers identified in semi-dry golden pomfret fillets, four key volatile flavor substances were selected based on an OAV threshold of ≥0.1: caproal, heptanal, nonal, and decal. In contrast, other differential markers such as phenylethanol, benzaldehyde, (E)-tetradecane-2-enal, trans-2-decenal, and linalool exhibited OAV values≤0.1 and were therefore excluded from further analysis.
Aldehydes primarily arise from lipid oxidation reactions. The OAV values for several critical aldehyde compounds are presented in Table 6. Hexanal imparts both fishy and grassy odors; heptanal and decanal provide fresh and fruity notes; while nonanal offers floral and citrus aromas. Notably, heptanaldehyde and nonanal exhibit additive effects that enhance the development of fishy odors in aquatic products. When present in excessive amounts, they may impart undesirable fishy smells that diminish consumer acceptability (Xu et al., 2021).In this study's PAW group at Curing for 24 h and Drying for 96 h conditions, the OAV values for heptanal and nonal were found to be lower-significantly so-compared to those observed in the control group. Under the cold plasma excitation medium, not only can the desirable flavor of semi-dry golden pomfret be preserved, but also the production of fishy odors can be significantly minimized.
Table 6.
Changes in OAV of aroma compounds with OAV>1 in semi-dried golden pomfret during processing.
| Compounds | Aroma description | Threshols(ug/kg) | OAV |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PAW |
CK |
|||||||||||
| Curing 24 h | Drying 24 h | Drying 48 h | Drying 72 h | Drying 96 h | Curing 24 h | Drying 24 h | Drying 48 h | Drying 72 h | Drying 96 h | |||
| Hexanal | Fishy,grassy(Zhang, Gao, et al., 2022) | 4.5 | 5.33 | 5.55 | 7.78 | 8.44 | 7.78 | 4.89 | 5.11 | 8.44 | 13.11 | 19.56 |
| Heptanal | Fruity(Zhang, Chen, et al., 2022) | 3 | 26.67 | 6.33 | 2.33 | 0 | 0 | 29.67 | 7 | 0 | 0 | 0 |
| Nonal | Citrus and fatty flavor, floral fragrance (Cai et al., 2021) | 1 | 1.2 | 1.09 | 1.6 | 1.5 | 0.88 | 4.6 | 1.7 | 1.1 | 1.5 | 2.5 |
| Decanoaldehyde | Fat aroma and fruity flavor(Zhang, Chen, et al., 2022) | 1 | 0.66 | 2.7 | 0.93 | 0.7 | 0.34 | 7.7 | 2.3 | 0.88 | 0.87 | 0.9 |
3.10. Nitrate and nitrite content
Nitrite is an important additive for improving color and flavor in meat products, as well as for inhibiting microbial growth and oxidation (Jo et al., 2020). Although nitrite is carcinogenic, at present, there is no safe additive that can completely replace it. PAW was investigated as a potential substitute of nitrite in the curing process, for nitrite ions were contained (Lee et al., 2018). In this study, both NO2− and NO3− were identified after curing 24 h and drying 24 h, while none of them were measured in the final product. When the nitrite is combined with the pigment in meat, its detectable amount is rapidly decreased (Inguglia et al., 2020). The contents of nitrate and nitrite were quickly diminished and below the minimal detection level in the semi-dried golden pomfret,demonstrating that PAW was safe enough for curing product processing.(See Table 7)
Table 7.
Contents of NO2− and NO3− of semi-dried golden pomfret during processing.
| Group |
PAW |
CK |
||
|---|---|---|---|---|
| Concentration (mg/Kg) | NO2− | NO3− | NO2− | NO3− |
| Curing 24 h | 10.17 | 2.99 | – | 2.39 |
| Drying 24 h | 7.37 | 2.36 | – | 1.89 |
| Drying 48 h | – | – | – | – |
| Drying 72 h | – | – | – | – |
| Drying 96 h | – | – | – | – |
4. Conclusion
The PAW as a substitution for salt showed satisfactory results in the semi-dried golden pomfret. Samples with PAW curing had high L* and b* values, reduced hardness, lower salinity, and superior sensory quality. The PAW enhanced the oxidation of lipids during the curing procedure, while the promoting effect gradually disappeared as drying time extended, resulting in a substantial increase of MUFA and PUFA. A total of 19 compounds were identified as typical odors in the semi-dried golden pomfret, including nonylaldehyde, heptyl aldehyde, (E)-tetradecan-2-enal, trans-2-decenal, linalool, decylaldehyde, and caproaldehyde. The PAW has enriched the volatile compounds in the samples, especially hexaldehyde, 1-octene-3-ol, and nonylaldehyde. Therefore, PAW as a partial substitute for saltwater effectively reduced the salinity of semi-dried golden pomfret and helpful in keeping its sensory quality and physicochemical property. Consequently, PAW is a potential safe substitute of salt, has an applicable ability in pickled aquatic products.
CRediT authorship contribution statement
Wentao Deng: Writing – review & editing, Writing – original draft, Investigation, Formal analysis, Data curation. Yuanyuan Wang: Investigation, Formal analysis, Data curation. Tingting Yang: Writing – review & editing, Investigation, Formal analysis. Lixian Zeng: Methodology, Investigation. Tengfei Fu: Supervision, Funding acquisition, Conceptualization. Gaohao Liao: Methodology, Investigation. Zhenzhen Xu: Methodology, Investigation. Liming Zhang: Writing – review & editing, Conceptualization. Jiamei Wang: Supervision, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported by Hainan Provincial Natural Science Foundation of China (32060568) and the International Scientific and Technological Cooperation and Research and Development Projects in Hainan Province (GHYF2025009)
Data availability
Data will be made available on request.
References
- Albertos I., Martin-Diana A.B., Cullen P.J., Tiwari B.K., Shikha Ojha K., Bourke P., Rico D. Shelf-life extension of herring (Clupea harengus) using in-package atmospheric plasma technology. Innovative Food Science & Emerging Technologies. 2019;53:85–91. doi: 10.1016/j.ifset.2017.09.010. [DOI] [Google Scholar]
- Benet I., Guàrdia M.D., Ibañez C., Solà J., Arnau J., Roura E. Analysis of SPME or SBSE extracted volatile compounds from cooked cured pork ham differing in intramuscular fat profiles. LWT - Food Science and Technology. 2015;60:393–399. doi: 10.1016/j.lwt.2014.08.016. [DOI] [Google Scholar]
- Birania S., Attkan A.K., Kumar S., Kumar N., Singh V.K. Cold plasma in food processing and preservation: A review. Journal of Food Process Engineering. 2022;45 doi: 10.1111/jfpe.14110. [DOI] [Google Scholar]
- Cai L., Ao Z., Tang T., Tong F., Wei Z., Yang F.…Mai K. Characterization of difference in muscle volatile compounds between triploid and diploid crucian carp. Aquaculture Reports. 2021;20 doi: 10.1016/j.aqrep.2021.100641. [DOI] [Google Scholar]
- Cai Z., Wang J., Wang Y., Sang X., Zeng L., Deng W., Zhang J. Effect of different process conditions on the physicochemical and antimicrobial properties of plasma-activated water. Plasma Science and Technology. 2023;25 doi: 10.1088/2058-6272/acde34. [DOI] [Google Scholar]
- Cao P., Yilin L., Huang Q., Liu S., Ding Y., Zhou X., Ke Z. Influence of cold plasma treatment on NaCl diffusion and consequent effects on the flavor of dry-cured grass carp fillets. Food Chemistry. 2025;492 doi: 10.1016/j.foodchem.2025.145651. [DOI] [PubMed] [Google Scholar]
- Chen F., Shen L., Shi X., Yi Deng Y., Qiao W.W., Xiong G.…Shi L. Characterization of flavor perception and characteristic aroma of traditional dry-cured fish by flavor omics combined with multivariate statistics. LWT. 2023;173 doi: 10.1016/j.lwt.2022.114240. [DOI] [Google Scholar]
- Chen J., Liu H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. International Journal of Molecular Sciences. 2020;21:5695. doi: 10.3390/ijms21165695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Wang S.Z., Chen J.Y., Chen D.Z., Deng S.G., Bin X. Effect of cold plasma on maintaining the quality of chub mackerel (Scomber japonicus): Biochemical and sensory attributes. Journal of the Science of Food and Agriculture. 2018;99(1):39–46. doi: 10.1002/jsfa.9138. [DOI] [PubMed] [Google Scholar]
- Chen Q., Yang X., Liu S., Hong P., Zhou C., Zhong S.…Chen K. Changes in protein and volatile flavor compounds of low-salt wet-marinated fermented Golden Pomfret during processing. Food Chemistry. 2024;456 doi: 10.1016/j.foodchem.2024.140029. [DOI] [PubMed] [Google Scholar]
- Choi M.-S., Jeon E.B., Kim J.Y., Choi E.H., Lim J.S., Choi J., Park S.Y. Impact of non-thermal dielectric barrier discharge plasma on Staphylococcus aureus and Bacillus cereus and quality of dried blackmouth angler (Lophiomus setigerus) Journal of Food Engineering. 2020;278 doi: 10.1016/j.jfoodeng.2020.109952. [DOI] [Google Scholar]
- Ferreira I., Caro I., Mateo J., Kasaiyan A., Leite A., Vasconcelos L.…Teixeira A. Quality changes due to refrigerated storage in a traditional dry-cured pork belly salted with glasswort or KCl as partial substitutes for NaCl. Journal of the Science of Food and Agriculture. 2024;104:8748–8755. doi: 10.1002/jsfa.13701. [DOI] [PubMed] [Google Scholar]
- Fraqueza M.J., Laranjo M., Alves S., Fernandes M.H., Agulheiro-Santos A.C., Fernandes M.J.…Elias M. Dry-cured meat products according to the smoking regime: Process optimization to control polycyclic aromatic hydrocarbons. Foodss. 2020;9:91. doi: 10.3390/foods9010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F., Zhang K., Wang D., Xia L., Yue G., Tian J., Jin Y. Effect of lactobacillus helveticus IMAUJBH1 on fat and volatile flavor substances in fermented mutton sausages. Food Chemistry: X. 2024;21 doi: 10.1016/j.fochx.2024.101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Cheng S., Sun X., Bai Y., Xiaobo Y., Zeng X.…Han M. Combination of contact ultrasound and infrared radiation for improving the quality and flavor of air-dried beef during hot air drying. Ultrasonics Sonochemistry. 2024;110 doi: 10.1016/j.ultsonch.2024.107047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z., Liu X., Zhao S., Wei Q., Dou M. Effect of the partial substitution of NaCl with blended KCl, MgCl2 and arginine on sensory profiles and storage characteristics of cooked marinated chicken. Journal of Food Measurement and Characterization. 2023;17:5948–5958. doi: 10.1007/s11694-023-02098-x. [DOI] [Google Scholar]
- Gil A., Gil F. Fish, a Mediterranean source of n-3 PUFA: Benefits do not justify limiting consumption. British Journal of Nutrition. 2015;113:S58–S67. doi: 10.1017/S0007114514003742. [DOI] [PubMed] [Google Scholar]
- Hui T., Fang Z., Ma Q., Hamid N., Li Y. Effect of cold atmospheric plasma-assisted curing process on the color, odor, volatile composition, and heterocyclic amines in beef meat roasted by charcoal and superheated steam. Meat Science. 2023;196 doi: 10.1016/j.meatsci.2022.109046. [DOI] [PubMed] [Google Scholar]
- Hwang C.-C., Lin C.-M., Kung H.-F., Huang Y.-L., Hwang D.-F., Yi-Cheng S., Tsai Y.-H. Effect of salt concentrations and drying methods on the quality and formation of histamine in dried milkfish (Chanos chanos) Food Chemistry. 2012;135:839–844. doi: 10.1016/j.foodchem.2012.05.035. [DOI] [PubMed] [Google Scholar]
- Inguglia E.S., Oliveira M., Burgess C.M., Kerry J.P., Tiwari B.K. Plasma-activated water as an alternative nitrite source for the curing of beef jerky: Influence on quality and inactivation of listeria innocua. Innovative Food Science & Emerging Technologies. 2020;59 doi: 10.1016/j.ifset.2019.102276. [DOI] [Google Scholar]
- Jin G., Zhang J., Xiang Y., Lei Y., Wang J. Crude lipoxygenase from pig muscle: Partial characterization and interactions of temperature, NaCl and pH on its activity. Meat Science. 2011;87:257–263. doi: 10.1016/j.meatsci.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Jo K., Lee S., In Yong H., Choi Y.-S., Jung S. Nitrite sources for cured meat products. LWT - Food Science and Technology. 2020;129 doi: 10.1016/j.lwt.2020.109583. [DOI] [Google Scholar]
- Jung S., Kim H.J., Park S., In Yong H., Choe J.H., Jeon H.-J.…Jo C. The use of atmospheric pressure plasma-treated water as a source of nitrite for emulsion-type sausage. Meat Science. 2015;108:132–137. doi: 10.1016/j.meatsci.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Kang G., Cho S., Seong P., Park B., Kim S., Kim D.…Park K. Effects of high pressure processing on fatty acid composition and volatile compounds in Korean native black goat meat. Meat Science. 2013;94:495–499. doi: 10.1016/j.meatsci.2013.03.034. [DOI] [PubMed] [Google Scholar]
- Ke Z., Bai Y., Bai Y., Chu Y., Saiqi G., Xiang X.…Zhou X. Cold plasma treated air improves the characteristic flavor of dry-cured black carp through facilitating lipid oxidation. Food Chemistry. 2022;377 doi: 10.1016/j.foodchem.2021.131932. [DOI] [PubMed] [Google Scholar]
- Kim B.-S., Boung-Jun O., Lee J.-H., Yoon Y.S., Lee H.-I. Effects of various drying methods on physicochemical characteristics and textural features of yellow croaker (Larimichthys Polyactis) Foods. 2020;196 doi: 10.3390/foods9020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan W., Zhang B., Liu L., Tianting P., Zhou Y., Xie J. Slightly acidic electrolyzed water–slurry ice: shelf-life extension and quality maintenance of mackerel (Pneumatophorus japonicus) during chilled storage. Journal of the Science of Food and Agriculture. 2023;103:3787–3798. doi: 10.1002/jsfa.12269. [DOI] [PubMed] [Google Scholar]
- Lee J., Jo K., Lim Y., Jeon H.J., Choe J.H., Jo C., Jung S. The use of atmospheric pressure plasma as a curing process for canned ground ham. Food Chemistry. 2018;240:430–436. doi: 10.1016/j.foodchem.2017.07.148. [DOI] [PubMed] [Google Scholar]
- Li R., Geng C., Xiong Z., Yingying Cui E., Liao L.P., Jin W., Wang H. Evaluation of protein degradation and flavor compounds during the processing of xuan’en ham. Journal of Food Science. 2022;87:3366–3385. doi: 10.1111/1750-3841.16242. [DOI] [PubMed] [Google Scholar]
- Lilian R.B.M., Bragagnolo N. Influence of salt on lipid oxidation in meat and seafood products: A review. Food Research International. 2017;94:90–100. doi: 10.1016/j.foodres.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Liu J., Mai R., Liu P., Guo S., Yang J., Bai W. Flavor formation in dry-cured fish: Regulation by microbial communities and endogenous enzymes. Foods. 2023;12(16):3020. doi: 10.3390/foods12163020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paludan-Müller C., Madsen M., Sophanodora P., Gram L., Møller P.L. Fermentation and microflora of plaa-som, a Thai fermented fish product prepared with different salt concentrations. International Journal of Food Microbiology. 2002;73:61–70. doi: 10.1016/S0168-1605(01)00688-2. [DOI] [PubMed] [Google Scholar]
- Pang X., Yu W., Cao C., Yuan X., Qiu J., Kong F., Wu J. Comparison of Potent Odorants in Raw and Ripened Pu-Erh Tea Infusions Based on Odor Activity Value Calculation and Multivariate Analysis: Understanding the Role of Pile Fermentation. Journal of Agricultural and Food Chemistry. 2019;67(47):13139–13149. doi: 10.1021/acs.jafc.9b05321. [DOI] [PubMed] [Google Scholar]
- Petit G., Jury V., de Lamballerie M., Duranton F., Pottier L., Martin J.-L. Salt intake from processed meat products: Benefits, risks and evolving practices. Comprehensive Reviews in Food Science and Food Safety. 2019;18:1453–1473. doi: 10.1111/1541-4337.12478. [DOI] [PubMed] [Google Scholar]
- Qiu L., Zhang M., Tang J., Adhikari B., Cao P. Innovative technologies for producing and preserving intermediate moisture foods: A review. Food Research International. 2019;116:90–102. doi: 10.1016/j.foodres.2018.12.055. [DOI] [PubMed] [Google Scholar]
- Sang X., Wang Y., Wang J., Cai Z., Zeng L., Deng W.…Jiang Z. Effects of gas composition on the lipid oxidation and fatty acid concentration of Tilapia fillets treated with In-package atmospheric cold plasma. Foods. 2024;13:165. doi: 10.3390/foods13010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenauer S., Schieberle P. Characterization of the key aroma compounds in the crust of soft pretzels by application of the Sensomics concept. Journal of Agricultural and Food Chemistry. 2019;67(25):7110–7119. doi: 10.1021/acs.jafc.9b02601. [DOI] [PubMed] [Google Scholar]
- Senso L., Suárez M.D., Ruiz-Cara T., García-Gallego M. On the possible effects of harvesting season and chilled storage on the fatty acid profile of the fillet of farmed gilthead sea bream (Sparus aurata) Food Chemistry. 2007;101:298–307. doi: 10.1016/j.foodchem.2006.01.036. [DOI] [Google Scholar]
- Thorarinsdottir K.A., Arason S., Sigurgisladottir S., Gunnlaugsson V.N., Johannsdottir J., Tornberg E. The effects of salt-curing and salting procedures on the microstructure of cod (Gadus morhua) muscle. Food Chemistry. 2011;126:109–115. doi: 10.1016/j.foodchem.2010.10.085. [DOI] [Google Scholar]
- Tian X., Li Z.J., Chao Y.Z., Zhong Qin W., Zhou M.X., Xiao S.T.…Zhe J. Evaluation by electronic tongue and headspace-GC-IMS analyses of the flavor compounds in dry-cured pork with different salt content. Food Research International. 2020;137 doi: 10.1016/j.foodres.2020.109456. [DOI] [PubMed] [Google Scholar]
- Valentini J., Da Silva A.S., Fortuoso B.F., Reis J.H., Gebert R.R., Griss L.G.…Tavernari F.C. Chemical composition, lipid peroxidation, and fatty acid profile in meat of broilers fed with glycerol monolaurate additive. Food Chemistry. 2020;330 doi: 10.1016/j.foodchem.2020.127187. [DOI] [PubMed] [Google Scholar]
- Wang Y., Cai Z., Sang X., Deng W., Zeng L., Wang J., Zhang J. LC-MS-based lipidomics analyses of alterations in lipid profiles of Asian sea bass (Lates calcarifer) induced by plasma-activated water treatment. Food Research International. 2024;177 doi: 10.1016/j.foodres.2023.113866. [DOI] [PubMed] [Google Scholar]
- Wang Y., Wang J., Cai Z., Sang X., Deng W., Zeng L., Zhang J. Combined of plasma-activated water and dielectric barrier discharge atmospheric cold plasma treatment improves the characteristic flavor of Asian sea bass (Lates calcarifer) through facilitating lipid oxidation. Food Chemistry. 2024;443 doi: 10.1016/j.foodres.2023.113866. [DOI] [PubMed] [Google Scholar]
- Wang Y., Wang H., Yanyan W., Xiang H., Zhao Y., Chen S.…Li L. Insights into lipid oxidation and free fatty acid profiles to the development of volatile organic compounds in traditional fermented golden pomfret based on multivariate analysis. LWT - Food Science and Technology. 2022;171 doi: 10.1016/j.lwt.2022.114112. [DOI] [Google Scholar]
- Wen Y.-Q., Xue C.-H., Zhang H.-W., Li-Li X., Wang X.-H., Bi S.-J.…Jiang X.-M. Concomitant oxidation of fatty acids other than DHA and EPA plays a role in the characteristic off-odor of fish oil. Food Chemistry. 2023;404 doi: 10.1016/j.foodchem.2022.134724. [DOI] [PubMed] [Google Scholar]
- Xu Yanshun, Yunyi Yang, Cikun Liu, Yingying Sun, Xinyi Wen and Wenshui Xia.(2021)."Modification Of volatile profiles of silver carp surimi gel by immersion treatment with hydrogen peroxide (H2O2)." International Journal of Food Science and Technology 56(11): 5726–5737. Doi: 10.1111/ijfs.15133. [DOI]
- Yang B., Liu Y., Sang Y., Sun J. Bacterial diversity and physicochemical properties of low-salt shrimp paste fermented at different temperatures. LWT - Food Science and Technology. 2023;187 doi: 10.1016/j.lwt.2023.115277. [DOI] [Google Scholar]
- Yang W., Shi W., Yinghong Q., Wang Z., Shen S., Ludan T.…Han W. Research on the quality changes of grass carp during brine salting. Food Science & Nutrition. 2020;8:2968–2983. doi: 10.1002/fsn3.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong H.I., Lee S.H., Kim S.Y., Park S., Park J., Choe W., Jo C. Color development, physiochemical properties, and microbiological safety of pork jerky processed with atmospheric pressure plasma. Innovative Food Science & Emerging Technologies. 2019;53:78–84. doi: 10.1016/j.ifset.2017.09.005. [DOI] [Google Scholar]
- Zeng P., Ruan Q., Zhang Y., Ning Z., Zhang Y., Cheng J., Wang X. Effect of drying temperature and presalting methods on the quality and N-nitrosamine formation of dried mud carp (Cirrhinus molitorella) Journal of Food Processing and Preservation. 2018;42 doi: 10.1111/jfpp.13703. [DOI] [Google Scholar]
- Zhang M., Chen M., Fang F., Cuncun F., Xing S., Qian C.…Jin C. Effect of sous vide cooking treatment on the quality, structural properties and flavor profile of duck meat. International Journal of Gastronomy and Food Science. 2022;29 doi: 10.1016/j.ijgfs.2022.100565. [DOI] [Google Scholar]
- Zhang X., Gao P., Xia W., Jiang Q., Liu S., Xu Y. Characterization of key aroma compounds in low-salt fermented sour fish by gas chromatography-mass spectrometry, odor activity values, aroma recombination and omission experiments. Food Chemistry. 2022;397 doi: 10.1016/j.foodchem.2022.133773. [DOI] [PubMed] [Google Scholar]
- Zhiwen S., Aonan L., Shixian Y., Wu J., Wu F., Yongle L., Xianghong L. Moderate protein degradation and lipid oxidation induced by cold plasma and its effect on the quality of dried fish products. Journal of Food Composition and Analysis. 2023;123 doi: 10.1016/j.jfca.2023.105636. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.