Abstract
The generation of amyloid fibrils from an amyloidogenic polypeptide occurs by a nucleation-dependent process initiated in vitro by seeding the protein solution with preformed fibrils. This phenomenon is evidenced in vivo by the fact that amyloid protein A (AA) amyloidosis in mice is markedly accelerated when the animals are given, in addition to an inflammatory stimulus, an i.v. injection of protein extracted from AA amyloid-laden mouse tissue. Heretofore, the chemical nature of this “amyloid enhancing factor” (AEF) has not been definitively identified. Here we report that the active principle of AEF extracted from the spleen of mice with silver nitrate-induced AA amyloidosis was identified unequivocally as the AA fibril itself. Further, we demonstrated that this material was extremely potent, being active in doses <1 ng, and that it retained its biologic activity over a considerable length of time. Notably, the AEF was also effective when administered orally. Our studies have provided evidence that AA and perhaps other forms of amyloidosis are transmissible diseases, akin to the prion-associated disorders.
The amyloidoses represent a spectra of protein conformational diseases that result from the pathologic depositions as fibrils of over 20 biochemically diverse protein molecules, including immunoglobulins, polypeptide hormones, transport molecules, and acute phase reactants (1–4). These fibrillar aggregates are highly ordered with a predominant β-sheet secondary structure that allows inter-molecule hydrogen bonding, and consequently results in a highly stable product. Some of the amyloid proteins, e.g., transthyretin and lysozyme, have in their native form a high degree of β structure, whereas other proteins, e.g., islet amyloid polypeptide and Aβ protein, are predominantly in random coil. Despite their chemical differences, the fibrillar products of these components possess virtually identical tinctorial and ultrastructural properties (for review, see ref. 5). Although it is not well understood how soluble proteins adopt an aggregation-prone conformation, it has been shown that this process can be initiated in vitro by seeding a solution of precursor molecules with small amounts of fibrils formed from the same protein (for review, see ref. 6). This seeding phenomenon has been demonstrated experimentally for several types of amyloidogenic molecules including islet amyloid polypeptide (7), β-protein (8, 9), and lysozyme (10).
The serum amyloid A (SAA) protein is an acute phase apolipoprotein reactant. SAA is produced mainly by hepatocytes under regulation of interleukin-1, interleukin-6, and tumor necrosis factor α (for review see ref. 11). The plasma concentration of SAA is normally low (≈20 mg/liter) (12), but can increase to >1,000 mg/liter as a result of an inflammatory stimulus (13, 14). SAA can undergo cleavage to an ≈76-residue N-terminal cleavage product, designated amyloid protein A (AA), that is deposited systemically as amyloid in vital organs including the liver, spleen, and kidneys (15). Clinically, AA amyloidosis occurs in patients with rheumatoid arthritis and other chronic inflammatory diseases, and also can be induced experimentally in mice in which SAA concentrations are markedly increased by injections of silver nitrate, casein, or lipopolysaccharide (16, 17). Two to 3 weeks after the inflammatory stimulus, the animals develop systemic AA deposits as found in patients with AA amyloidosis (18, 19). This lag phase is dramatically shortened when mice are given, concomitantly, an i.v. injection of protein extracted from AA amyloid-laden mouse spleen or liver (20–25). The amyloidogenic accelerating activity of such preparations is termed “amyloid enhancing factor” (AEF); however, despite intensive efforts, the chemical nature of this material has not been defined (26–28). Among other possibilities, it has been suggested that AEF may be amyloid itself (29), a minute β strand-containing fragment of the amyloid fibril (25) or an early AA peptide–glycosaminoglycan complex (30). Based on our demonstration that fibrils formed from 10- to 20-mer synthetic peptides that corresponded to segments of amyloidogenic precursor proteins had AEF activity in vivo, we posited that the active principle in the AA amyloid extracts was the amyloid fibril itself (31, 32).
We have now substantiated our amyloid fibril seeding hypothesis through the demonstration that AEF prepared from AA-laden mouse liver consisted of proteins that are chemically identical to the AA molecule. This material was capable of accelerating amyloidosis even in femtomolar doses and retained its biologic activity over a considerable period. The AEF preparation was also effective in promoting amyloid formation when administered orally. The fact that AA amyloidosis can be accelerated by ingestion of a chemically related molecule that serves as a nucleation seed to promote fibrillogenesis provides direct experimental evidence of the mode of transmission of this disease process. In this respect, AA amyloidosis, mouse senile amyloidosis (and perhaps other amyloidoses), and the prion-associated diseases share a common pathogenic mechanism.
Materials and Methods
Animals.
Outbreed female 6- to 8-week-old NMRI mice were obtained from B & K Universal (Södertälje, Sweden) and housed in cages with free access to type R 36 pellets (Lactamin, Vadstena, Sweden) and drinking bottles containing tap water.
Source of AEF.
AEF was prepared from the liver of mice with AgNO3-induced AA amyloidosis as described (31). Amyloid fibrils were extracted with distilled water according to a modification (33) of the method described by Pras et al. (34). Briefly, the tissue was first homogenized in 0.15 M NaCl and centrifuged at 15,000 × g for 30 min at 4°C, and the supernatant was discarded. This process was repeated 10 times with 0.15 M NaCl/0.05 M sodium citrate followed by distilled water. Pooled supernatants from the second and the third water extractions were used without further purification as the source of AEF. The protein content in this fibril preparation was 1.32 mg/ml, determined by using a protein assay kit (Bio-Rad).
Chemical Characterization of AEF.
Reversed-phase high-performance liquid chromatography (RP-HPLC) was performed by using a 4.6 × 220 mm Aquapore 300-Å C8 column (Brownlee, Norwalk, CT) connected to an ABI model 151 apparatus (Applied Biosystems). A linear gradient of 7–70% acetonitrile in 0.1% trifluoroacetic acid (TFA) was used to elute protein over a 45-min period. The effluent was monitored at 220 nm, and the resultant UV-absorbing material was dried in a vacuum centrifuge (Speed Vac, Savant Instruments, Farmingdale, NY). Amino acid sequence analyses were done by using an ABI model 494 Procise sequencer. Mass spectrometry was performed by using an ABI 173 capillary HPLC system with the output directed to the ion-spray of a PE-Sciex 150EX single-quadrupole mass spectrometer. The column (Brownlee 150 × 0.5 mm C18, 5-μm particle size) was maintained at 37°C, and the flow rate was 6 μl/min. The chromatography was performed with a gradient of 15–75% acetonitrile containing 0.1% TFA over a period of 2 h.
In Vivo Assay of AEF Activity.
Because we previously showed that our AEF preparation was active at a 1:64 dilution (31), we prepared a series of dilutions (ranging from 1:32 to 1:14,697,216) to test for AEF activity corresponding to a dose of 4 μg to 0.008 ng, respectively. Mice were divided into 20 experimental groups, with three to five animals in each group. Each mouse received a single injection of 0.1 ml of AEF diluted in distilled water into the lateral tail vein. Another group of five mice received 0.1 ml of distilled water alone. Immediately afterward, both treated and control animals were injected s.c. with 0.5 ml of a freshly prepared 1% solution of AgNO3 in distilled water; additional 0.1-ml doses of AgNO3 were given 7 and 14 days later. All animals were killed on day 16 by cervical dislocation. The spleens were removed and divided into two equal parts: one part was crushed, smeared between two glass slides, and air dried overnight, and the second was fixed in 10% buffered formalin and embedded in paraffin for microscopic analyses.
In another experiment, inflammation was induced with Escherichia coli-derived lipopolysaccharide (LPS) (serotype no. O26:B6; Sigma). Four mice were injected s.c. with 1.25 mg of LPS in 0.1 ml of 0.15 M NaCl. Simultaneously, 0.1 ml of AEF, diluted 1:64 (≈2 μg of protein) was given i.v.. The animals were killed after 2 days, and the spleens were examined for presence of amyloid.
The effect of guanidine⋅HCl denaturation on AEF activity was studied by dissolving 0.1 ml of lyophilized undiluted AEF in 0.1 ml of 6 M guanidine⋅HCl in 0.1 M Tris⋅HCl, pH 8.0, and after overnight incubation at room temperature, the solution was diluted to 10 ml with 0.15 M NaCl. Each of six mice was injected i.v. with 0.1 ml of this material (≈1.32 μg of protein) and s.c. with 0.5 ml of a 1% solution of AgNO3 in distilled water. For control purposes, an identical amount of lyophilized, undiluted AEF was resuspended in 9.9 ml of 0.15 M NaCl to which 0.1 ml 6 M guanidine⋅HCl was added, and six additional animals were treated as above. All animals were killed after 48 h and studied for the presence of splenic amyloid.
Serial Transmission of Amyloidosis.
Each of four mice was given an i.v. injection of 0.1 ml of AEF, diluted 1:10 in distilled water, and 0.5 ml of 1% AgNO3 s.c. The mice were killed after 2 days. One part of the spleen was taken for examination of amyloid deposits, and the rest was used for extraction of amyloid fibrils. The other portion of the spleen was treated exactly as described for AEF preparation with the difference that only water wash no. 3 was used for the transmission experiments. The four supernatants were adjusted to a protein concentration of 20–30 μg/ml with distilled water, and each supernatant was used as AEF on two new mice (0.1 ml per mouse), which were given AgNO3 as above. These eight mice were killed after 2 days, and the spleens were recovered and processed as above. From these extracts, three were chosen at random as sources of AEF, and each was given i.v. at doses of 2–3 μg to two new additional AgNO3-treated mice. The six animals were killed after 2 days, and the spleens were harvested for microscopic analyses.
Long-Term Effects of AEF.
Mice were divided into experimental groups with 4–10 mice in each group, and control groups with 2–8 mice. Mice were injected i.v. with 0.1 ml of undiluted AEF (132 μg of protein) or with 0.1 ml of distilled water. The animals were then given a s.c. injection of 0.5 ml of 1% AgNO3, given immediately afterward or at intervals ranging from 2 to 125 days later, and then killed 2, 8, or 16 days after the inflammatory stimulus. Two mice (controls) were given AEF alone and were killed 66 days later.
In another experiment, three groups of 16 mice each were injected i.v. with 0.1 ml of undiluted AEF, synthetic transthyretin (TTR)-(115–124) fibrils (31) or vehicle for fibril formation (10% acetic acid neutralized with concentrated NH4OH and diluted 1:10 with distilled water) (31). After 180 days, 8 mice in each group received inflammatory stimulation with AgNO3 as described above. All animals were killed 16 days later.
Induction of Amyloidosis by Oral AEF Administration.
To study the effect of oral administration of AEF, a group of 9 mice had this material added to their drinking water (1.32 mg/liter) for 5 weeks. After 3 of these weeks they received a s.c. injection of 0.5 ml of 1% AgNO3, followed by 0.1 ml of 1% AgNO3 after 4 weeks and 0.1 ml of 1% AgNO3 after 5 weeks. A control group of 10 mice were treated identically with AgNO3, but were given tap water alone. Both groups of animals were killed 48 h after the last AgNO3 treatment, and organ samples were taken for analysis.
Microscopy.
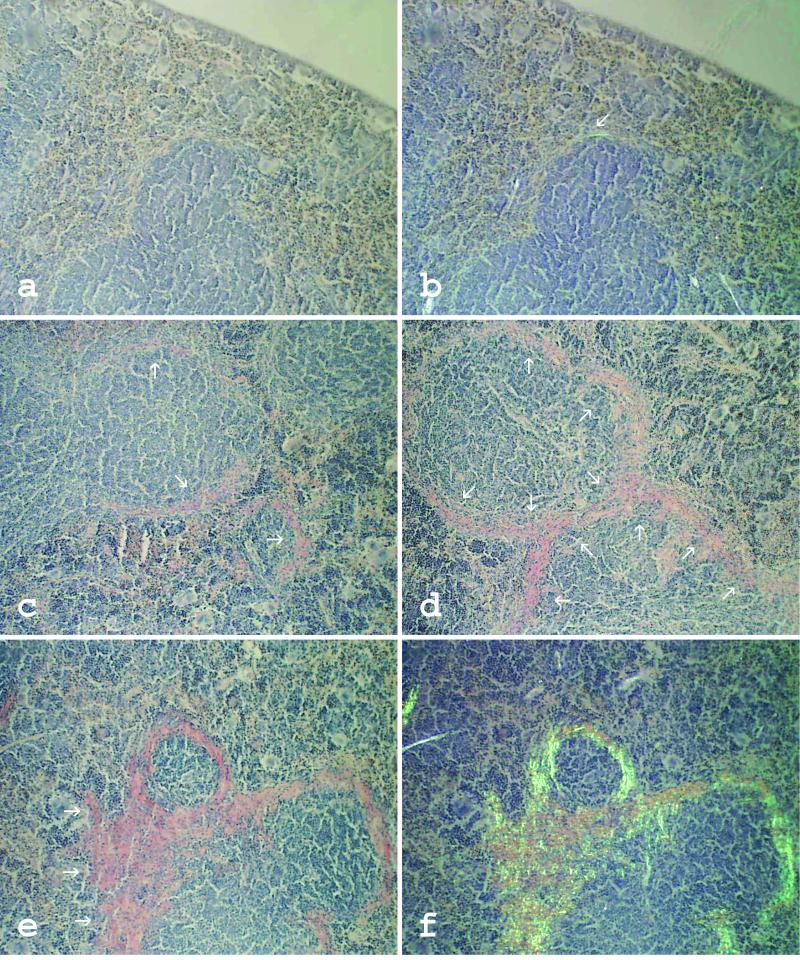
Air-dried amyloid smears and 10-μm-thick deparaffinized tissue sections were stained with alkaline Congo red, counterstained with hematoxylin, and examined by polarization microscopy. Amyloid grading was performed on spleen sections or spleen smears for each mouse separately as described (31). In brief, 1+ means trace of amyloid, 2+ means small amyloid deposits, 3+ means moderate amyloid deposits, and 4+ means extensive amyloid deposits (Fig. 1). For electron microscopy, AEF was diluted in distilled water, and droplets were placed on Formvar-coated copper grids, negatively contrasted with uranyl acetate, and viewed by using a Jeol 1200 electron microscope at 80 kV.
Figure 1.
Typical examples of different degrees of splenic amyloid deposits stained with Congo red dye. Amyloid appears as orange material when studied without polarized light (a and c–e) and exhibits green birefringence when studied with polarized light (b and f). 1+, Very thin focal deposits at follicles, almost undetectable when studied without polarized light (a and b). 2+, More pronounced perifollicular amyloid deposits in limited areas of the spleen (c). 3+, Moderate amyloid deposits around most or all follicles (d). 4+, Extensive amyloid deposits localized around follicles but often forming continuous infiltration (e and f). (×100.)
Statistical Analysis.
For statistical comparison between groups, Fisher's exact test was used with INSTAT 2.01 software.
Results
Characterization of AEF.
The pooled second and third water supernatants from material extracted from AA-laden hepatic tissue contained amyloid fibrils as evidenced after Congo red staining by the green birefringent nature of the material when viewed by polarization microscopy and by the presence of ∼10-nm-thick unbranched fibrils when examined by electron microscopy (Fig. 2).
Figure 2.
Electron micrograph of AEF used in the study. The material consists of amyloid fibrils of typical appearance. (Original magnification, ×60,000.)
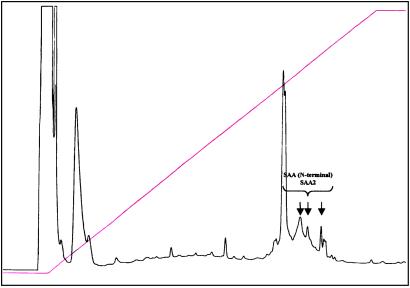
To determine the chemical composition of the AEF contained in the amyloid extract, 1 ml of uncentrifuged solution containing 1.32 mg of protein was subjected to reversed-phase HPLC. No increase in pressure occurred, indicating a lack of particulate material in the specimen. Approximately 90% of the recovered protein eluted as a single peak at an acetonitrile concentration of 52%; several additional smaller peaks were also present (Fig. 3). Fractions corresponding to the major as well as three minor peaks were collected, freeze-dried, reconstituted in water, and subjected to Edman degradation. In each case, the amino acid sequence of the first 16–18 terminal residues established by automatic (direct) analysis was identical to the N-terminal portion of the amyloidogenic precursor protein SAA1 (15).
Figure 3.
Elution pattern of AEF when run through a reversed-phase HPLC column. As indicated, the retarded peaks where identified as amyloid protein AA with N-terminal amino acid sequences identical to mouse SAA1.
For mass spectrometry, the AEF preparation (1.32 mg/ml) was diluted 10-fold with 20% acetonitrile in 0.1% TFA, and 20 μ l was injected directly into the capillary HPLC. Again, no pressure increase was noted. A single major peak with a pronounced trailing shoulder was detected by both ion current and UV absorbance. The peak contained peptides with masses corresponding most closely to the first 75–82 amino acid residues of SAA1, with the 82-mer species predominating. These masses were higher by 16 than the expected values, presumably because oxidation of the methionine residue at position 76, given the appropriate Mr of the trailing 75-mer peptide that lacks this residue.
Effect of Parenteral AEF Administration.
We have previously shown that our AEF preparation was active in vivo at a protein concentration of 0.02 mg/ml. To test the effect of lower doses of AEF, the sample was serially diluted in distilled water and injected i.v. in amounts decreasing from ≈4 μg to ≈0.008 ng. As summarized in Table 1, AEF in a dose as low as ≈0.015 ng was effective in accelerating amyloidogenesis in AgNO3-treated mice. There was no discernible difference in the extent of the perifollicular splenic amyloid deposits in animals that received >2 ng of material. Slightly less congophilic deposits were found in mice dosed with 0.12–1.0 ng of AEF, and at doses below 0.12 ng some mice did not develop amyloidosis. None of the control (non-AEF-treated) animals had detectable splenic amyloid (P < 0.0001). The four animals given AEF plus lipopolysaccharide (instead of AgNO3) also developed splenic amyloidosis after 2 days.
Table 1.
Splenic amyloid deposits in mice treated with AEF i.v. and AgNO3 s.c. compared with controls treated with H2O i.v. and AgNO3 s.c., killed on day 16
| AEF dose per mouse, μg | Incidence of mice with amyloid | Amyloid grade |
|---|---|---|
| 4 | 3/3 | 4+ |
| 2 | 3/3 | 4+ |
| 1 | 3/3 | 4+ |
| 0.5 | 3/3 | 4+ |
| 0.25 | 3/3 | 4+ |
| 0.125 | 3/3 | 4+ |
| 0.0625 | 3/3 | 4+ |
| 0.03125 | 3/3 | 4+ |
| 0.01562 | 3/3 | 4+ |
| 0.00781 | 3/3 | 4+ |
| 0.0039 | 5/5 | 4+ |
| 0.00195 | 3/3* | 4+ |
| 0.000976 | 5/5 | 3+ to 4+ |
| 0.000488 | 5/5 | 2+ to 4+ |
| 0.000244 | 5/5 | 2+ to 3+ |
| 0.000122 | 5/5 | 1+ to 2+ |
| 0.000061 | 2/5 | 1+ |
| 0.000030 | 4/4† | 1+ to 3+ |
| 0.000015 | 2/5 | 1+ |
| 0.000008 | 0/5 | – |
| 0 | 0 | – |
Two mice died at the beginning of the experiment.
One mouse died at the beginning of the experiment.
Guanidine⋅HCl treatment of AEF abolished its activity, because none of the six animals treated with this denatured preparation (1.32 μg protein per mouse) developed amyloidosis. In contrast, all six control mice that received comparable doses of lyophilized but not denatured AEF had pronounced splenic amyloidosis after 2 days.
Effect of Oral AEF Administration.
Eight of nine mice that had AEF added to their drinking water for 21 days before and 14 days after AgNO3 injections developed extensive amyloid deposits in the perifollicular areas of the spleen, central hepatic veins, as well as in the small intestine mucous membrane, blood vessels, and adjacent connective tissue (Table 2). In addition, one mouse had cardiac and pancreatic endovascular congophilic deposits. No amyloid was detected in any organ of the AgNO3-injected animals that drank tap water alone during a similar time period (P < 0.0001).
Table 2.
Splenic amyloid deposits induced by oral treatment with AEF and s.c. AgNO3 injections, compared with controls treated with AgNO3 only
| Oral treatment | Calculated total AEF dose per mouse, mg | No. of AgNO3 injections | Duration of experiment after AgNO3 injections, days | Incidence of mice with amyloid | Amyloid grade |
|---|---|---|---|---|---|
| AEF | 0.2 | 3 | 16 | 8/9 | 2+ to 4+ |
| H2O | 0 | 3 | 16 | 0/10 | — |
Serial Transmission of AEF.
All four AEF/AgNO3-treated mice developed splenic amyloid deposits within 48 h. This material, when extracted from the spleens, also had AEF activity as evidenced by its identical effect when administered to another group of eight AgNO3-treated mice. Extracts of spleen from three randomly chosen mice in this second group again accelerated amyloidogenesis in a third group of six mice.
Long-Term Effect of AEF.
All mice that were given injections of AgNO3 at the time of i.v. injection of AEF or up to 180 days later developed amyloidosis as evidenced in Congo red-stained smears and sections of splenic tissue. Although the extent of amyloid deposition was most marked in those animals killed 16 days after AgNO3 treatment, amyloid was also found as early as 48 h after the inflammatory stimulus in all animals studied (Table 3). None of the animals that were injected with distilled water, vehicle, or synthetic TTR-(115–124) fibrils instead of AEF and given AgNO3 16 days before killing developed amyloidosis. Amyloid was not detected in animals given AEF or synthetic TTR-(115–124) fibrils alone.
Table 3.
Splenic amyloid deposits induced by i.v. treatment with AEF and AgNO3 injected s.c. 0–125 days after AEF administration, as compared with controls treated with H2O i.v. and AgNO3 s.c.
| Time delay, days | Duration of experiment after first AgNO3 injection
|
|||||||
|---|---|---|---|---|---|---|---|---|
| AEF
|
H2O
|
|||||||
| 2 days
|
8 days
|
16 days
|
16 days
|
|||||
| Incidence of mice with amyloid | Amyloid grade | Incidence of mice with amyloid | Amyloid grade | Incidence of mice with amyloid | Amyloid grade | Incidence of mice with amyloid | Amyloid grade | |
| 0 | 2/2 | 2+ | 2/2 | 3+ | 2/2 | 4+ | 0/2 | – |
| 2 | 2/2 | 2+ to 3+ | 2/2 | 3+ | 2/2 | 4+ | 0/2 | – |
| 4 | 2/2 | 1+ | 2/2 | 1+ | 2/2 | 4+ | 0/2 | – |
| 8 | 2/2 | 1+ to 3+ | 2/2 | 3+ to 4+ | 2/2 | 4+ | 0/2 | – |
| 16 | 2/2 | 1+ | 2/2 | 2+ to 4+ | 2/2 | 4+ | 0/2 | – |
| 25 | 2/2 | 1+ | 2/2 | 1+ | 2/2 | 4+ | 0/2 | – |
| 50 | 4/4 | 1+ to 3+ | 2/2 | 3+ | 4/4 | 4+ | 0/4 | – |
| 75 | 2/2 | 3+ to 4+ | – | – | 2/2 | 4+ | 0/2 | – |
| 125 | 2/2 | 1+ to 2+ | – | – | 2/2 | 4+ | 0/2 | – |
Discussion
Our chemical analyses of the AEF derived from extracts of AA-laden mouse spleen indicated that this material consisted exclusively of AA-related protein. We found no evidence of other amyloid-associated components, such as heparan sulfate proteoglycan or serum amyloid P component. The capability of our AEF preparation to accelerate amyloidogenesis was evidenced in AgNO3-treated mice, even in doses as low as ≈0.015 ng; this is the smallest amount of AEF reported to induce experimental AA amyloidosis (35). Further, the biologic effect was not dose-dependent; there was no difference in the extent of amyloid deposition in animals that received from 2.0 ng to 4.0 μg of AEF, and only a slight decrease in doses ranging from 0.24 to 1.0 ng. These results suggest that seeding efficiency depends on SAA rather than AEF concentrations. This conclusion is also consistent with the fact that AEF could also accelerate amyloidogenesis in lipopolysaccharide-stimulated mice.
The results of our studies demonstrate unequivocally that the biologically active component of AEF responsible for the amyloid accelerating activity in the experimental mouse model is the AA amyloid fibril fragment itself. Normally, the N-terminal amyloidogenic portion of SAA is bound to high-density lipoprotein (HDL), and thus protected from interaction with AA fibrils (36). However, when the serum concentration of this amyloidogenic protein is greatly increased as a result of an inflammatory stimulus, not all SAA molecules are bound to HDL (37), and thus would be free to interact with AA-derived fibril seeds. As a consequence of this interaction, we posit that SAA undergoes a conformational change leading to the formation of oligomeric intermediates, protofibrils, and ever-expanding amyloid deposits. The new fibrils themselves may act as nuclei and enhance the efficiency of the seed. The proposed mechanism can explain the lack of dose dependence between the amount of AEF administered and the extent of amyloid deposition, as well as the lack of activity of the AEF preparation in mice in the absence of an inflammatory stimulus. Furthermore, our experiments that demonstrated that AEF activity could be serially transferred provide further evidence for a seeding mechanism. The loss of effect of the AEF preparation after denaturation is also in agreement with a conformation-dependent process.
Our experimental data support the concept that AA amyloidosis is an autonucleation-dependent process (38, 39), analogous to that described for scrapie proteins (8, 40, 41). Aggregation of prions into amyloid fibrils results from a conformational change in the normal, cellular prion protein (PrPC) caused by an interaction with the structurally abnormal PrPSc (scrapie) conformer (42). As in the case of AA-derived AEF fibril seeds, PrPSc protein is pathologically active in minute doses (43), and its pathologic effect can be transmitted orally (44, 45).
We have also shown that AEF can retain its amyloid accelerating activity even when administered 6 months before the inflammatory stimulus. Similarly, a long latency period between exposure to the PrPSc seed and evident fibrillar pathology has been demonstrated in the prion-associated illnesses (for review, see ref. 46). Synthetic TTR-(115–124) fibrils, which exert AEF effect when administered together with an inflammatory stimulus (31), had no sustained effect, probably depending on instability in vivo.
The most notable finding in our study was the demonstration that AA-derived AEF can accelerate amyloidogenesis when given orally. Although AA amyloidogenesis was shown to be enhanced by oral administration of a splenic extract from an AA mouse, this material was reported not to contain AA protein (47). However, given the extremely potent effect of our AEF, we attribute this response to the presence of trace amounts of AA-fibrillar material in the preparation used. Recently, it has been reported that AApoAII amyloidosis in mice could also be accelerated by oral administration of AApoAII fibrillar extracts (48). Although this phenomenon occurred only in a mouse strain expressing an amyloid-prone form of apolipoprotein AII, oral transmission of AEF may be possible in different forms of amyloid diseases, if basic requisites for fibril formation are present, such as sufficient concentration of an amyloidogenic precursor protein.
Whether ingestion or inhalation of AEF-like material showing a high content of β-pleated sheet structure that occurs in nature, e.g., silk (49), can be a causative factor in amyloidosis and other protein folding diseases remains to be established.
Acknowledgments
We thank Dr. Robert Kisilevsky for valuable advice. A.S. is an American Cancer Society Clinical Research Professor. This work was supported by the Swedish Medical Research Council (Project No. 5941), the Swedish Association against Rheumatism, the “Förenade Liv” Mutual Group Life Insurance Company (Stockholm), U.S. Public Health Service Research Grant CA 10056 from the National Cancer Institute, and the Aslan Foundation.
Abbreviations
- AA
amyloid protein A
- AEF
amyloid enhancing factor
- SAA
serum amyloid A
- TFA
trifluoroacetic acid
- TTR
transthyretin
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Westermark P, Araki S, Benson M D, Cohen A S, Frangione B, Masters C L, Saraiva M J, Sipe J D, Husby G, Kyle R A, Selkoe D. Amyloid. 1999;6:63–66. doi: 10.3109/13506129908993290. [DOI] [PubMed] [Google Scholar]
- 2.Häggqvist B, Näslund J, Sletten K, Westermark G T, Mucchiano G, Tjernberg L O, Nordstedt C, Engström U, Westermark P. Proc Natl Acad Sci USA. 1999;96:8669–8674. doi: 10.1073/pnas.96.15.8669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vidal R, Révész T, Rostagno A, Kim E, Holton J, Bek T, Bojsen-Møller M, Braendgaard H, Plant G, Ghiso J, Frangione B. Proc Natl Acad Sci USA. 2000;97:4920–4925. doi: 10.1073/pnas.080076097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghiso J, Vidal R, Rostagno A, Mead S, Révész T, Plant G, Frangione B. Ann NY Acad Sci. 2000;903:129–137. doi: 10.1111/j.1749-6632.2000.tb06359.x. [DOI] [PubMed] [Google Scholar]
- 5.Sipe J D, Cohen A S. J Struct Biol. 2000;130:88–98. doi: 10.1006/jsbi.2000.4221. [DOI] [PubMed] [Google Scholar]
- 6.Rochet J C, Lansbury P T., Jr Curr Opin Struct Biol. 2000;10:60–68. doi: 10.1016/s0959-440x(99)00049-4. [DOI] [PubMed] [Google Scholar]
- 7.Ashburn T, Lansbury P T., Jr J Am Chem Soc. 1993;115:11012–11013. [Google Scholar]
- 8.Jarrett J T, Lansbury P T., Jr Cell. 1993;77:1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- 9.Esler W P, Stimson E R, Ghilardi J R, Lu Y A, Felix A M, Vinters H V, Mantyh P W, Lee J P, Maggio J E. Biochemistry. 1996;35:13914–13921. doi: 10.1021/bi961302+. [DOI] [PubMed] [Google Scholar]
- 10.Morozova-Roche L A, Zurdo J, Spencer A, Noppe W, Recerveur V, Archer D B, Joniau M, Dobson C M. J Struct Biol. 2000;130:339–351. doi: 10.1006/jsbi.2000.4264. [DOI] [PubMed] [Google Scholar]
- 11.Baumann H, Gauldie J. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 12.Hijmans W, Sipe J D. Clin Exp Immunol. 1979;35:96–100. [PMC free article] [PubMed] [Google Scholar]
- 13.De Beer F C, Mallya R K, Fagan E A, Lanham J G, Hughes G R, Pepys M B. Lancet. 1982;2:231–234. doi: 10.1016/s0140-6736(82)90321-x. [DOI] [PubMed] [Google Scholar]
- 14.Cunnane G, Grehan S, Geoghegan S, McCormack C, Shields D, Whitehead A S, Bresnihan B, Fitzgerald O. J Rheumatol. 2000;27:58–63. [PubMed] [Google Scholar]
- 15.Husby G, Marhaug G, Dowton S B, Sletten K, Sipe J D. Amyloid. 1994;1:119–137. [Google Scholar]
- 16.Skinner M, Shirahama T, Benson M D, Cohen A S. Lab Invest. 1977;36:420–427. [PubMed] [Google Scholar]
- 17.Ishihara T. Acta Pathol Jpn. 1973;23:439–464. doi: 10.1111/j.1440-1827.1973.tb01217.x. [DOI] [PubMed] [Google Scholar]
- 18.Teilum G. Acta Pathol Microbiol Scand. 1964;61:21–45. doi: 10.1111/apm.1964.61.1.21. [DOI] [PubMed] [Google Scholar]
- 19.Sipe J D, McAdam K P, Uchino F. Lab Invest. 1978;38:110–114. [PubMed] [Google Scholar]
- 20.Werdelin O, Ranløv P. Acta Pathol Microbiol Scand. 1966;68:1–18. doi: 10.1111/apm.1966.68.1.1. [DOI] [PubMed] [Google Scholar]
- 21.Hultgren M K, Druet R L, Janigan D T. Am J Pathol. 1967;50:943–955. [PMC free article] [PubMed] [Google Scholar]
- 22.Janigan D T. In: Amyloidosis. Mandema E, Ruinen L, Scholten J H, Cohen A S, editors. Groningen, The Netherlands: Excerpta Medica Foundation; 1968. pp. 303–315. [Google Scholar]
- 23.Willerson J T, Gordon J K, Talal N, Barth W F. Arthritis Rheum. 1969;12:232–240. doi: 10.1002/art.1780120311. [DOI] [PubMed] [Google Scholar]
- 24.Kisilevsky R, Boudreau L. Lab Invest. 1983;48:53–59. [PubMed] [Google Scholar]
- 25.Niewold T A, Hol P R, van Andel A C, Lutz E T, Gruys E. Lab Invest. 1987;56:544–549. [PubMed] [Google Scholar]
- 26.Axelrad M A, Kisilevsky R, Willmer J, Chen S J, Skinner M. Lab Invest. 1982;47:139–146. [PubMed] [Google Scholar]
- 27.Shirahama T, Miura K, Ju S T, Kisilevsky R, Gruys E, Cohen A S. Lab Invest. 1990;62:61–68. [PubMed] [Google Scholar]
- 28.Alizadeh-Khiavi K, Normand J, Chronopoulos S, Ali A, Ali-Khan Z. Virchows Arch A Pathol Anat Histopathol. 1992;420:139–148. doi: 10.1007/BF02358805. [DOI] [PubMed] [Google Scholar]
- 29.Shirahama T, Lawless O J, Cohen A S. Proc Soc Exp Biol Med. 1969;130:516–519. doi: 10.3181/00379727-130-33594. [DOI] [PubMed] [Google Scholar]
- 30.Snow A D, Kisilevsky R, Stephens C, Anastassiades T. Lab Invest. 1987;56:665–675. [PubMed] [Google Scholar]
- 31.Ganowiak K, Hultman P, Engström U, Gustavsson Å, Westermark P. Biochem Biophys Res Commun. 1994;199:306–312. doi: 10.1006/bbrc.1994.1229. [DOI] [PubMed] [Google Scholar]
- 32.Johan K, Westermark G T, Engström U, Gustavsson Å, Hultman P, Westermark P. Proc Natl Acad Sci USA. 1998;95:2558–2563. doi: 10.1073/pnas.95.5.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skinner M, Shirahama T, Cohen A S, Deal C L. Prep Biochem. 1982;12:461–476. doi: 10.1080/10826068208070597. [DOI] [PubMed] [Google Scholar]
- 34.Pras M, Schubert M, Zucker-Franklin D, Rimon A, Franklin E C. J Clin Invest. 1968;47:924–933. doi: 10.1172/JCI105784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shtrasburg S, Livneh A, Gal R, Pras M. Clin Exp Rheumatol. 1996;14:37–42. [PubMed] [Google Scholar]
- 36.Kirschner D A, Elliott-Bryant R, Szumowski K E, Gonnermann W A, Kindy M S, Sipe J D, Cathcart E S. J Struct Biol. 1998;124:88–98. doi: 10.1006/jsbi.1998.4047. [DOI] [PubMed] [Google Scholar]
- 37.Hajri T, Elliott-Bryant R, Sipe J D, Liang J S, Hayes K C, Cathcart E S. Biochim Biophys Acta. 1998;1394:209–218. doi: 10.1016/s0005-2760(98)00109-x. [DOI] [PubMed] [Google Scholar]
- 38.Hawkins P N, Pepys M B. Clin Exp Immunol. 1990;81:325–328. doi: 10.1111/j.1365-2249.1990.tb03339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hawkins P N, Herbert J, McBride A, Hutchinson W L, Pepys M B. In: Amyloid and Amyloidosis. Kyle R A, Gertz M A, editors. Rochester, MN: Parthenon; 1998. pp. 387–389. [Google Scholar]
- 40.Gajdusek D C. J Neuroimmunol. 1988;20:95–110. doi: 10.1016/0165-5728(88)90140-3. [DOI] [PubMed] [Google Scholar]
- 41.Harper J D, Lansbury P T., Jr Annu Rev Biochem. 1997;66:385–407. doi: 10.1146/annurev.biochem.66.1.385. [DOI] [PubMed] [Google Scholar]
- 42.Cohen F E, Prusiner S B. Annu Rev Biochem. 1998;67:793–819. doi: 10.1146/annurev.biochem.67.1.793. [DOI] [PubMed] [Google Scholar]
- 43.Gibbs C J, Jr, Asher D M, Kobrine A, Amyx H L, Sulima M P, Gajdusek D C. J Neurol Neurosurg Psychiatry. 1994;57:757–758. doi: 10.1136/jnnp.57.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beekes M, Baldauf E, Diringer H. J Gen Virol. 1996;77:1925–1934. doi: 10.1099/0022-1317-77-8-1925. [DOI] [PubMed] [Google Scholar]
- 45.Bons N, Mestre-Frances N, Belli P, Cathala F, Gajdusek D C, Brown P. Proc Natl Acad Sci USA. 1999;96:4046–4051. doi: 10.1073/pnas.96.7.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wisniewski T, Aucouturier P, Soto C, Frangione B. Amyloid. 1998;5:212–224. doi: 10.3109/13506129809003848. [DOI] [PubMed] [Google Scholar]
- 47.Elliott-Bryant R, Cathcart E S. Clin Immunol Immunopathol. 1998;88:65–69. doi: 10.1006/clin.1998.4555. [DOI] [PubMed] [Google Scholar]
- 48.Xing Y, Nakamura A, Chiba T, Kogishi K, Matsushita T, Li F, Guo Z, Hosokawa M, Mori M, Higuchi K. Lab Invest. 2001;81:493–499. doi: 10.1038/labinvest.3780257. [DOI] [PubMed] [Google Scholar]