Abstract
Neonatal hepatic steatosis (OMIM 228100) is a fatal condition of unknown etiology characterized by a pale and yellow liver and early postnatal mortality. In the present study, a deficit in adenosine-dependent metabolism is proposed as a causative factor. Physiologically, adenosine is efficiently metabolized to AMP by adenosine kinase (ADK), an enzyme highly expressed in liver. ADK not only ensures normal adenine nucleotide levels but also is essential for maintaining S-adenosylmethionine-dependent transmethylation processes, where adenosine, an obligatory product, has to be constantly removed. Homozygous Adk−/− mutants developed normally during embryogenesis. However, within 4 days after birth they displayed microvesicular hepatic steatosis and died within 14 days with fatty liver. Adenine nucleotides were decreased and S-adenosylhomocysteine, a potent inhibitor of transmethylation reactions, was increased in the mutant liver. Thus, a deficiency in adenosine metabolism is identified as a powerful contributor to the development of neonatal hepatic steatosis, providing a model for the rapid development of postnatally lethal fatty liver.
Neonatal hepatic steatosis (OMIM 228100) is a fatal condition characterized by a rapid microvesicular fat infiltration and enlargement of the liver, which shows a pale and yellowish coloration (1). In this condition, microvesicular fat infiltration, liver failure, coma, and finally death, is considered to be a consequence of severe impairment of mitochondrial function (2–4). So far, mitochondrial dysfunction related to hepatic steatosis has been associated (i) with genetic defects in the β-oxidation of fatty acids (5), (ii) with inhibition of β-oxidation by drugs (i.e., valproate) (3, 4), (iii) with defective carnitine-dependent transport of fatty acids (6), and (iv) with an impaired production of ATP (7).
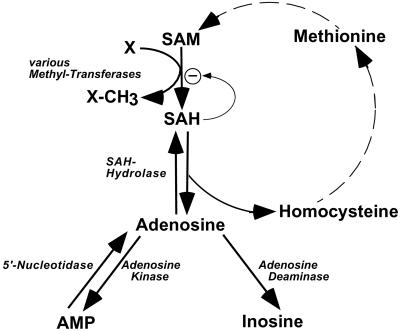
In the present study, a deficit in adenosine-dependent metabolism is proposed as a causative factor for the development of microvesicular hepatic steatosis. A deficiency in adenosine kinase (ADK; EC 2.7.1.20) (Fig. 1), the major adenosine-removing enzyme of postnatal liver, is expected to affect liver function on three different levels: (i) Availability of adenine nucleotides, (ii) disruption of the futile cycle between AMP and adenosine, and (iii) maintenance of transmethylation reactions.
Figure 1.
Pathways of adenosine metabolism. Adenosine is formed either by hydrolysis of AMP or by hydrolysis of SAH, which arises from the action of methyltransferases. Adenosine can be metabolized by ADA or ADK into inosine and AMP, respectively. Note the AMP/adenosine futile cycle, which is catalyzed by ADK and 5′-nucleotidase. SAM = S-adenosylmethionine; SAH = S-adenosylhomocysteine; X = methyl-group acceptor; X-CH3 = methylated compound X.
(i) Physiologically, adenosine is constantly recycled into the adenine nucleotide pool by ADK-mediated phosphorylation of adenosine to AMP. Because the metabolic flux rate in liver mitochondria is proportional to the amount of available adenine nucleotides, decreased levels of adenine nucleotides in the liver should lead to an impairment of mitochondrial function. Thus, a deficiency in ADK is expected to result in reduced levels of adenine nucleotides with a concomitant impairment of mitochondrial function.
(ii) Adenine nucleotides can be interconverted among each other and with adenosine according to the equilibrium: ATP ⇋ ADP ⇋ AMP adenosine (Fig. 1). In this sequence, the dephosphorylation of AMP by 5′-nucleotidase (EC 3.1.3.5) to adenosine and phosphate and the recycling of adenosine to AMP by ADK under the consumption of ATP forms a futile cycle leading to a loss of energy. It is assumed that this futile cycle is active in liver mitochondria (8, 9) at an estimated rate of at least 20 nmol per min per g of liver (10). The physiological role of this futile cycle is considered to enable cells by inhibition of their ADK activity to rapidly react to adverse conditions (i.e., hypoxia) with the release of adenosine, which is an important cell-protective molecule. Based on these considerations, a deficiency of ADK is expected to disrupt the AMP/adenosine futile cycle, thus leading to the accumulation of adenosine.
(iii) S-adenosylmethionine (SAM)-dependent transmethylation reactions are essential for multiple vital biological functions. The transmethylation reactions differ in the nature of their substrates, but have a common end product, namely S-adenosylhomocysteine (SAH). Despite the diversity of the methyl acceptors and the specificity of the catalyzed reactions the mammalian methyltransferases share the property of product inhibition by SAH (11). Removal of SAH by SAH-hydrolase (EC 3.3.1.1) is therefore an essential step leading to the formation of adenosine, an obligatory product of SAM-dependent transmethylation reactions (Fig. 1). The thermodynamic equilibrium of the SAH-hydrolase reaction favors the synthesis of SAH, but physiologically the reaction proceeds in the hydrolytic direction because of the efficient hydrolysis of homocysteine under the formation of adenosine (11). The further metabolism of adenosine follows two pathways. During embryonic development, adenosine is mainly degraded to inosine by adenosine deaminase (ADA, EC 3.5.4.4), but this reaction is not pronounced in postnatal liver where ADA is expressed at very low levels (12). Postnatally, adenosine is converted to AMP by ADK (13).
ADK is present in most tissues with the highest expression level in liver (14), the organ in which 85% of all transmethylation reactions take place (15) (Fig. 1). On the basis of its low affinity for adenosine (Km = 0.2–2 μM), the phosphorylation of adenosine mediated by ADK is believed to be the primary route of postnatal adenosine metabolism (16). ADK is therefore an important downstream control point (i) for the recycling of adenine nucleotides, (ii) for regulating adenosine release by means of the AMP/adenosine futile cycle, and (iii) for the maintenance of transmethylation reactions (17). Dysfunction of adenosine metabolism may therefore be associated with a failure of liver function.
So far, no human disease has been linked to mutations in the Adk locus. The murine Adk gene was therefore disrupted to test the potential role of the adenosine futile cycle in the development of fatal neonatal hepatic steatosis.
Materials and Methods
Isolation of Adk-Targeted Embryonic Stem Cells.
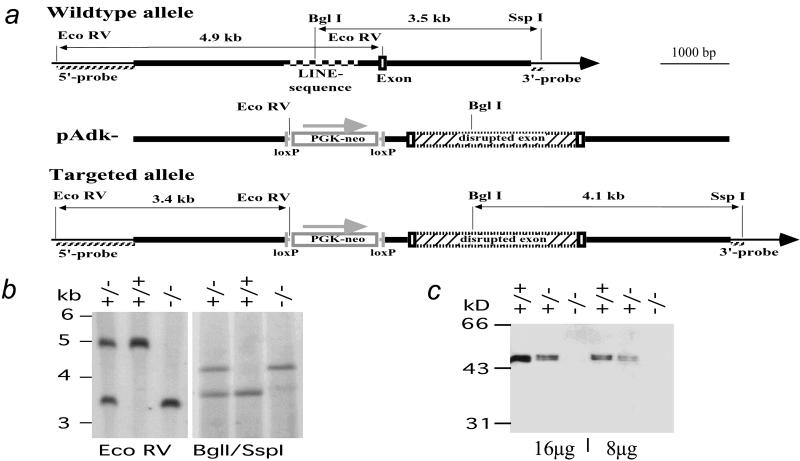
A 5.9-kb SspI/HindIII-fragment of the murine Adk gene (strain 129/JEms) that contained the exon corresponding to amino acids Gly-169 to Thr-225 of ADK was inserted into the BluescriptIIKS+ vector (Stratagene). The exon is located 5′ to the codon for the active site of the protein (Asp-300) (16). The coding sequence of the exon was disrupted by the in frame insertion of an expression cassette containing the cDNA for enhanced green fluorescent protein, an internal ribosomal entry site, and the cDNA for a tetracycline-regulated transactivator. Sequencing of the construct revealed an intronic 455-bp segment with 88% homology to the family of Line-1 (L1) retrotransposons (18) that consists of 4,800 members in mouse strain 129 (19). Because the frequent occurrence of homology to thousands of other murine gene loci would decrease the frequency of homologous recombination at the Adk locus, an 1,100-bp segment encompassing the LINE-1 element was removed and replaced by a PGKneobpA selection cassette (20) that was flanked with loxP sites. The resulting replacement-type gene targeting vector pAdk− was electroporated into the mEMS32 line (21) of embryonic stem cells. After selection for resistance to G418, 100 colonies were analyzed of which 4 clones were correctly targeted. For the analysis of targeted clones, DNA was extracted from the cells and digested with the restriction enzyme EcoRV, and Southern blot analysis was carried out with the 5′ probe (Fig. 2 a and b). Homologous recombination events were verified by probing a BglI/SspI restriction with the 3′ probe (Fig. 2 a and b) and a SspI restriction with a neo probe (data not shown).
Figure 2.
Homologous recombination into the murine Adk locus. (a) Restriction enzyme maps of the murine Adk wild-type allele, targeting vector pAdk-, and targeted allele. The thick lines indicate genomic sequences of the murine Adk gene homologous to the targeting vector. The thin lines in the wild type and targeted alleles indicate external Adk sequences not present in the targeting vector. In the wild-type sequence a checkered line marks the region with homology to mouse long interspersed nucleotide elements. Exon sequences, as well as the insertion cassette for the neomycin resistance gene (PGK-neo), are outlined as open boxes, loxP sites are outlined as vertical bars. PGK-neo is transcribed in the direction indicated by the arrow. The bicistronic expression cassette (hatched box) for the enhanced green fluorescent protein, the internal ribosomal entry site, and the tetracycline-dependent transactivator is fused in-frame to the Adk-specific exon, thus causing a disruption of the Adk gene. The hatched lines indicate the 5′ and 3′ flanking probes external to pAdk− and the neo-probe inside pAdk−. (b) Southern blot analysis of the murine Adk locus. Genomic DNA was digested with the restriction enzymes EcoRV (Left) or BglII + SspI (Right) and hybridized with the 5′ probe and 3′ probe, respectively. Hybridizing fragments are 4,959 bp (+, wild-type allele) and 3,389 bp (−, mutant allele) with EcoRV digestion, and are 3,549 bp (+) and 4,131 bp (−) with BglII/SspI digestion. The DNA size marker shown is a 1-kb ladder (Life Technologies). (c) Western blot analysis of liver extracts from E17.5 embryos from an Adk+/− × Adk+/− intercross. The 8- or 16-μg liver extracts from wild type (+/−), heterozygous (+/−), and homozygous (−/−) mutant embryos were probed with a polyclonal rabbit antiserum raised against recombinant mouse ADK.
Generation of Adk-Deficient Mice.
One correctly targeted clone was injected into C57BL/6 blastocysts, and five chimeras that transmitted the mutation to their offspring were generated. Chimeras were backcrossed with C57BL/6 mice. Offspring were genotyped by Southern analysis or by PCR using allele-specific primer sets. Mice heterozygous for the mutation were intercrossed but no homozygous mutants were found in weaned animals. Therefore, a colony of heterozygous Adk-mutant mice (Adktm1) was maintained on a mixed 129/JEms and C57BL/6 background.
Excision of the Neomycin Resistance Cassette.
Outcrossing Adk+/− mice with an E2a-Cre deleter mouse eliminated the loxP-flanked neomycin resistance cassette present in an intron of the Adk-deficient allele of Adk+/− mice (22) as was verified by Southern blot analysis (data not shown). After deletion of the neo cassette, Adk−/− mice were analyzed by Western blot analysis and found to be negative for ADK protein. In line with the ADK-deficiency, these mice were indistinguishable from Adk −/− mice before Cre-recombination.
Western Blot Analysis.
Aqueous protein extracts from embryonic day 17.5 (E17.5) livers were separated on a SDS/10% PAGE gel and blotted onto a nitrocellulose membrane according to standard procedures. The blots were probed with a polyclonal rabbit antiserum (own production) raised against recombinant mouse ADK.
Determination of the Body Temperature.
A thermistor [Thermometrics-P20, R (25°C) = 10 kΩ, maximum diameter = 0.5 mm] was introduced into the rectum of pups at postnatal day 4 (P4) for body temperature measurements. Temperature values were registered every minute. The separation of the pups from their mother and transfer into a new cage at room temperature was defined as time point t = 0. The registration of data were stopped when the body temperature reached 24°C or, alternatively, after a maximum of 30 min. The room temperature was 22.1 ± 0.2°C.
Histology.
Deeply anesthetized animals (from E17.5 to P14) were perfused transcardially with 4% paraformaldehyde (PFA) in physiological saline. Livers were postfixed in 4% PFA, embedded in paraffin, and sections of 25 μm were cut on a microtome and stained with hematoxylin and eosin.
Analysis of Liver Metabolites.
Pups at P2 from Adk+/− matings were killed by decapitation. As quickly as possible after killing, the liver was excised and two volumes of 0.4 M perchloric acid were added. The sample was then homogenized lightly and centrifuged at 14,000 × g for 2 min. The supernatant was quickly frozen in liquid nitrogen and stored at −80°C. The whole procedure from decapitation until probe freezing was performed within 5 min at a temperature of 4°C. The concentrations of SAM and SAH were determined by HPLC analysis as described previously (23). For the quantification of adenine nucleotides, the supernatants were diluted 1:100 in 10 mM Hepes, pH 7.7. ATP was quantified with a luciferase assay (ATP Bioluminescent Assay kit, Sigma). Immediately after adding 100 μl of the ATP-assay mix in dilutions of 1:5 to 1:15 to the liver extracts, the luminescence of the mixture, measured as counts per minute, was determined in a Packard Liquid Scintillation Analyzer (Model 2500TR) for 10 s. ATP solutions with known concentrations of ATP (1, 2, and 4 nmol/ml) were used as external standard. AMP and ADP were quantified similarly after phosphorylation to ATP by myokinase, pyruvate kinase, GTP, and phosphoenolpyruvate (all from Boehringer Mannheim).
Results
Disruption of the Adk Gene.
The Adk-gene was disrupted by homologous recombination in the murine Adk-locus (Fig. 2 a and b). The lack of ADK protein in Adk−/− genotypes was demonstrated by Western blotting of liver cell extracts derived from E17.5 fetuses from an Adk+/− intercross (Fig. 2c) by using a polyclonal rabbit antiserum raised against recombinant ADK. In wild-type mice, a double band was detected in the size range of 44 to 46 kDa being consistent with two alternatively spliced products (24). As expected, livers from heterozygous Adk+/− embryos contained approximately 50% of the ADK protein levels of wild type; samples from Adk+/− (16-μg protein) resulted in bands of the same intensity as half the sample from Adk+/+ (8-μg protein) when probed with the ADK-specific antiserum. In contrast, no ADK specific protein was detected in liver extracts from homozygous Adk−/− mutants. These findings demonstrated that a null allele was introduced into the Adk gene by homologous recombination, leading to ADK-deficiency in homozygous mutant mice.
Life Span of Adk−/− Mice.
In Adk−/− mice no major impact on embryonic development was apparent. The distribution of the genotypes at gestational day 16.5 was in the expected range with 20.6% Adk+/+, 55.9% Adk+/−, and 23.5% Adk−/− (n = 34 embryos analyzed). Homozygous mutant embryos taken at this (E16.5) and other stages (E10.5 to E18.5) appeared morphologically normal in their development. Hematoxylin and eosin staining in whole body sections of E10.5, E12.5, E14.5, E16.5, and E18.5 embryos (n = 3 for each gestational day and genotype) revealed no structural differences between homozygous mutants and wild-type littermates. Thus, the ADK-deficiency did not appear to cause an apparent abnormality in prenatal development.
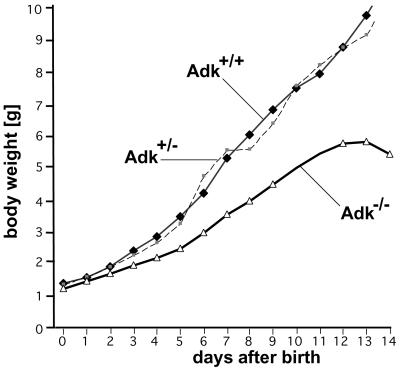
The homozygous mutants usually died within the first 8 days after birth (88%) with around a third of the Adk−/− pups dying before P4 (35%) (Table 1). Only occasionally, Adk−/− mice reached an age of up to 14 days (12%). Homozygous Adk−/− mutants displayed a deficit in postnatal development from P3 onwards despite normal feeding and nursing by the mother animal. The postnatal retardation in body weight development (Fig. 3) became evident (i) by a reduced weight gain of the mutants (470 mg per day from P5 until P12 compared with 750 mg per day of wild-type pups), and (ii) by a delay in the time to eye opening (P 14 in Adk−/− pups compared with P 11 in wild-type). It is important to note that heterozygous mutants did not differ from wild-type littermates in their weight development and life expectancy.
Table 1.
Life expectancy of Adk−/− mice
| Genotype life expectancy | −/−(n = 17) | +/−(n = 33) | +/+ (n = 18) |
|---|---|---|---|
| up to 4 days | 35% | — | — |
| 5 to 8 days | 53% | — | — |
| 9 to 14 days | 12% | — | — |
| more than 14 days | — | 100% | 100% |
Pups (n = 68) from matings of Adk+/− mice were genotyped and their life expectancy was followed for a period of at least 14 days. The percentage of surviving animals is given.
Figure 3.
Growth kinetic of offspring of Adk+/− intercrosses. The body weight of Adk+/+, Adk+/−, and Adk−/− pups was followed during a 14-day period. The population size at the time of birth (P0; n = 17 for each genotype) dropped to n = 2 at P14 for homozygous mutants (Table 1). Note that heterozygous offspring did not differ from wild-type littermates in their growth curve. Points are given as means ± SD.
Postnatal Microvesicular Liver Steatosis in ADK-Deficiency.
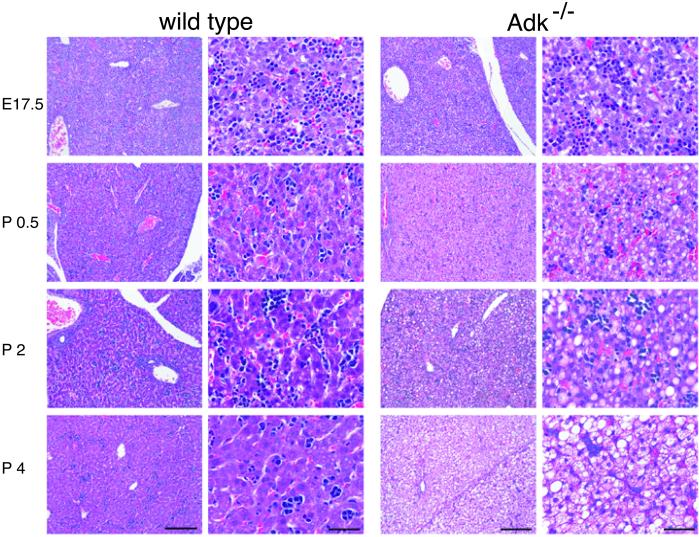
The perinatal development of the wild-type liver followed the normal pattern progressing from a heterogeneous structure with areas of extramedullary hematopoiesis that was apparent by the presence of erythrocytic or granulocytic precursor cells at E17.5 into a tightly organized dense tissue at P4 (Fig. 4, wild-type). This characteristic change in the cellular architecture of liver reflects the transition from extramedullary to medullar hematopoiesis around the time of birth. The detailed histological analysis of the livers from homozygous mutants and wild-type littermates performed at different perinatal time points (E17.5, P0.5, P2, P4, P6, and P14) revealed that the livers of Adk−/− mice were morphologically normal at E17.5 (Fig. 4, ADK−/−). However, 12 h after birth (P0.5) the first morphological differences became evident in the form of microvesicular steatosis. Macrovesicular steatosis began to develop at P2 with the entire cytoplasm frequently occupied by fat and a displacement of the nucleus to the periphery of the cell. Finally, at day P4 the mutant liver showed a homogenous mixture of micro- and macrovesicular steatosis without any lobular predominance. No accompanying hepatocellular necrosis, inflammatory cells, or indications for bile duct proliferation or bilary stasis were apparent. At P7 the pale color of the homozygous mutant liver strikingly contrasted to the dark red liver of the wild-type pups (Fig. 5).
Figure 4.
Hematoxylin and eosin staining of livers during the perinatal development from E17.5 to P4. In wild-type mice the liver developed normally with hepatocytes displaying a normal size with centrally located nuclei. A compact and homogeneous tissue mass was formed at P4. In ADK-deficient mice (ADK−/−) the livers are morphologically normal at E17.5 but develop micro- and macrovesicular diffuse panlobular steatosis (intracellular lipid accumulation) after birth, which appears as cytoplasmic pallor. Postnatal liver cells contained fine lipid vesicles, which increased in size and became confluent during the following days (P2, P4). There was no inflammation, no development of cirrhosis, and no evidence of liver cell necrosis. Mallory bodies were not encountered. Original magnification (objective): first and third column, ×10; second and fourth column, ×40. Original microscopical magnification: first and third column, ×100; second and fourth column, ×400. Bars in first and third column, 200 μm; bars in second and fourth column, 50 μm.
Figure 5.
Overview of livers from Adk+/+ (Left) and Adk−/− (Right) mice at P7. (Scale bar, 1 cm.)
The liver from the mutant animal was of the same size as that of wild-type mice, although the overall body size of the mutants was reduced by 20% compared with controls. No additional histological signs were seen at P14 compared with P7. Periodic acid Schiff staining for glycogen was negative in the mutants as well as in the wild-type control at all developmental stages (data not shown). It is noteworthy that the extramedullary hematopoiesis did not seem to be influenced by the mutation. Hematopoietic cells (dark blue, Fig. 4) displayed a similar appearance in homozygous mutants and wild-type littermates and showed the same age-dependent decrease in prevalence after birth. The pattern of hematoxylin and eosin staining in sections of heart, lung, thymus, spleen, kidney, intestine, and brain of Adk−/− pups indicated no cellular abnormalities in these organs. Adk+/− pups had normal livers comparable to wild type (not shown).
Elevated SAH Levels and Decreased Adenine Nucleotide Levels in ADK Deficiency.
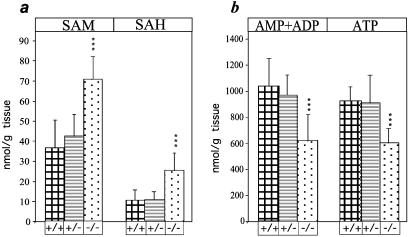
An impairment in adenosine recycling was expected to be accompanied with elevated levels of SAH. In heterozygous offspring (n = 12), the levels of SAH and SAM were comparable to those of wild-type littermates (n = 12) as tested at P2 (Fig. 6a). However, in Adk−/− mice both SAH and SAM levels in liver were significantly elevated (SAH: 25.2 ± 8.7 nmol/g liver in Adk−/− compared with 10.8 ± 5 nmol/g in wild type; SAM: 70.9 ± 11 nmol/g liver in Adk−/− compared with 36.9 ± 13.8 nmol/g in wild type; n = 12 for each group) (Fig. 6a). In line with an impairment of the recycling of adenosine to AMP, the concentrations of total adenine nucleotides were decreased in homozygous Adk−/− mutants. The levels of ATP as well as the sum of AMP and ADP in liver were lower than those in wild-type littermates (ATP: 604 ± 109 nmol/g liver in Adk−/− compared with 924 ± 103 nmol/g in wild-type; total AMP + ADP: 620 ± 194 nmol/g liver in Adk−/− compared with 1,037 ± 204 nmol/g in wild-type; n = 12 for each group) (Fig. 6b). The adenine nucleotide levels in the livers of heterozygous littermates were not different from those of wild types.
Figure 6.
Adenosine-related metabolites in livers. The concentrations of SAM and SAH (a), and of the sum of AMP and ADP (b) as well as the concentration of ATP were determined in liver extracts from Adk+/+(+/+), Adk+/− (+/−), and Adk−/− (−/−) mice at P2. ***, P < 0.0001, Student's t test.
Apnea and Deficits in Thermoregulation in Adk−/− Pups.
During the first 2 weeks of life, homozygous mutant pups displayed intermittent periods (up to 20 s) of apnea up to two times per hour as determined by visual inspection. In addition, in Adk−/− pups separated from their mother during their first week of life, the skin temperature dropped more rapidly compared with littermate controls. At room temperature (22°C), the core body temperature of Adk−/− mutants dropped faster than in wild type (elapsed time to 24°C body temperature in Adk−/−: 15.6 ± 3.7 min; in wild type: 26.3 ± 4.1 min; n = 7 for each group). Thus, ADK-deficiency is associated with a deficit in thermoregulation in developing pups.
Discussion
In the present study a complete absence of ADK led to abnormalities in hepatic adenosine metabolism and to the development of acute, severe neonatal hepatic steatosis. The histological analysis of Adk−/− livers revealed a rapid accumulation of fat, beginning with the microvesicular form of steatosis at P0.5 (Fig. 4). The development of a completely steatotic liver during the first 4 postnatal days in Adk−/− mice is consistent with the high postnatal expression of ADK in this tissue in wild-type mice (25). Histological analysis of Adk−/− pups revealed consistently that the liver was the only organ affected at this stage of postnatal development. Microvesicular steatosis is considered to be indicative for mitochondrial dysfunction and deficiencies in fatty acid transport and oxidation (2, 26). At the transition from a previously glucose-based energy source in the fetus to fatty acids as a major source of energy in the neonate, mitochondrial function is critical for the energy metabolism (27). In newborn homozygous Adk−/− mice, two abnormalities of hepatic metabolism may contribute to the development of microvesicular steatosis: ATP depletion and transmethylation reactions.
ATP-Depletion.
Given the existence of an AMP/adenosine futile cycle in healthy liver mitochondria (Fig. 1) (8–10), a disruption of ADK is likely to lead to a loss of all three adenine nucleotides in favor of the accumulation and release of adenosine. As the analysis of metabolites from homogenates from Adk−/− livers has shown, the levels of the adenine nucleotides (AMP + ADP) and ATP were reduced to 60% and 65%, respectively, in comparison to wild-type controls (Fig. 6b). Because the homogenates were derived from intact livers, it cannot be excluded that these differences may be more pronounced in certain subcellular compartments such as mitochondria. The resulting deficiency of adenine nucleotides, including ATP, in the postnatal liver is expected to lead to a reduction of the mitochondrial metabolic capacity including an impairment of mitochondrial lipid metabolism.
Transmethylation Reactions.
An inhibition of methyltransferases is considered to be one of the causes of fatty liver, as the liver is the organ in which 85% of all transmethylation reactions occur (15). To assess the possible contribution of an inhibition of transmethylation reactions to the liver pathology in ADK-deficiency the liver metabolites SAH and SAM were analyzed. The increase of the SAH level (2.3-fold) in Adk−/− livers (Fig. 6a) indicates a corresponding shift of the equilibrium of the SAH-hydrolase reaction toward accumulation of SAH, which is a powerful inhibitor of SAM-dependent transmethylation reactions (28, 29). Although the SAH/SAM ratio is not significantly altered in ADK-deficiency compared with control animals, a slight inhibition of transmethylation reactions may be indicated by the concomitant increase of SAM (1.9-fold, Fig. 6a). In a related study, as a consequence of the perfusion of rat livers with adenosine, intracellular SAH was increased and the rates of various methyltransferase reactions were reduced, which in turn resulted in elevated levels of SAM (30).
The possible contribution of various metabolic defects to the etiology of fatty liver may best be illustrated by the comparison of three different mouse models of hepatic steatosis: cystathionine β bynthase (CBS)-deficient mice, ADA-deficient mice, and phosphatidylethanolamine N-methyltransferase (PEMT)-deficient mice.
CBS-Deficient Mice.
A mild form of postnatal microvesicular steatosis has been described in CBS-deficient mice (31). In this model of homocyst(e)inemia, elevated levels of plasma homocysteine (40 times higher than in controls) have been described. In contrast to the rapid fat accumulation in the liver of newborn Adk−/− mice (within 4 days after birth), in CBS-deficient mice the hepatic steatosis developed around 21 days after birth, whereas their livers were completely normal at P7 (31). The discrepancy of the respective phenotypes with regard of the mutation-linked elevation of metabolites (plasma homocysteine 40-fold in CBS deficiency; liver SAH 2.3-fold in ADK deficiency) point to the possibility that, in Adk−/− mice, elevated SAH levels are not likely to be the only factor contributing to fat accumulation of the liver.
ADA-Deficient Mice.
During embryogenesis, ADA is the major adenosine-degrading enzyme with particularly high expression levels in the placenta (32). Mice with a disruption of the Ada gene (28, 29) die in the embryonic stage with severe embryonic hepatic necrosis (28), cell death in the small intestine, and incomplete expansion of the lungs in rare life-born pups (29). This phenotype was attributed to the formation of cytotoxic derivatives of 2′-deoxyadenosine (28, 29), which are potent inhibitors of SAH hydrolase (33). As a consequence, in Ada−/− embryos the levels of SAH and SAM in liver were increased (5- to 6-fold and 2-fold, respectively) compared with wild type, indicating an inhibition of transmethylation reactions (29). In contrast to Ada−/− mice, in Adk−/− mice the fetal metabolism of adenosine is not expected to be affected. It is only after birth that the regulation of the adenosine level in most parts of the body becomes dependent on ADK-expression. After birth, ADA-expression shifts to cells lining the alimentary canal (34). In keeping with this notion, no histological signs of liver damage were apparent in Adk−/− embryos as analyzed between E12.5 and E18.
PEMT-Deficient Mice.
The disruption of the Pemt gene (35) led to a rapid microvesicular hepatic steatosis provided choline was not offered by the diet (36). Large amounts of phosphatidylcholine are required for the secretion of very-low-density lipoproteins from the postnatal liver (37). An impairment of hepatic choline biosynthesis would be one factor contributing to the liver pathology in Adk−/− pups.
In conclusion, we have generated mice carrying the disrupted Adk gene. A deficiency in adenosine metabolism was identified as a powerful contributor to the development of neonatal hepatic steatosis, providing a model for the rapid development of postnatally lethal fatty liver. The homozygous ADK-null mice will be helpful in increasing our understanding of the pathophysiology of hepatic steatosis, as well as for developing new means of its treatment.
Acknowledgments
We thank Elizabeth M. Simpson for the generous gift of mJAX391 cells and Heiner Westphal for providing the E2a-Cre mice. This work was supported by Swiss National Science Foundation Grants 31-46965.96 and 31-59′109.99 (to D.B.) and 32-55657.98 (to B.F.), and by the National Center for Competence in Research on Neural Plasticity and Repair. V.Z. was funded by the Cloëtta Foundation.
Abbreviations
- ADK
adenosine kinase
- SAM
S-adenosylmethionine
- SAH
S-adenosylhomocysteine
- ADA
adenosine deaminase
- En
embryonic day n
- Pn
postnatal day n
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Satran L, Sharp H L, Schenken J R, Krivit W. J Pediatr. 1969;75:39–46. doi: 10.1016/s0022-3476(69)80099-5. [DOI] [PubMed] [Google Scholar]
- 2.Burt A D, Mutton A, Day C P. Semin Diagn Pathol. 1998;15:246–258. [PubMed] [Google Scholar]
- 3.Fromenty B, Berson A, Pessayre D. J Hepatol. 1997;26:13–22. doi: 10.1016/s0168-8278(97)82328-8. [DOI] [PubMed] [Google Scholar]
- 4.Fromenty B, Pessayre D. J Hepatol. 1997;26:43–53. doi: 10.1016/s0168-8278(97)80496-5. [DOI] [PubMed] [Google Scholar]
- 5.Boles R G, Buck E A, Blitzer M G, Platt M S, Cowan T M, Martin S K, Yoon H, Madsen J A, Reyes-Mugica M, Rinaldo P. J Pediatr. 1998;132:924–933. doi: 10.1016/s0022-3476(98)70385-3. [DOI] [PubMed] [Google Scholar]
- 6.Brivet M, Boutron A, Slama A, Costa C, Thuillier L, Demaugre F, Rabier D, Saudubray J M, Bonnefont J P. J Inherit Metab Dis. 1999;22:428–441. doi: 10.1023/a:1005552106301. [DOI] [PubMed] [Google Scholar]
- 7.Krahenbuhl S. Pharmacol Ther. 1993;60:1–38. doi: 10.1016/0163-7258(93)90020-e. [DOI] [PubMed] [Google Scholar]
- 8.Bontemps F, Vincent M F, Van den Berge G. Biochem J. 1993;290:671–677. doi: 10.1042/bj2900671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bontemps F, Mimouni M, Van den Berghe G. Biochem J. 1993;290:679–684. doi: 10.1042/bj2900679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bontemps F, Van den Berghe G, Hers H G. Proc Natl Acad Sci USA. 1983;80:2829–2833. doi: 10.1073/pnas.80.10.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finkelstein J D. Eur J Pediatr. 1998;157 Suppl. 2:S40–S44. doi: 10.1007/pl00014300. [DOI] [PubMed] [Google Scholar]
- 12.Mohamedali K A, Guicherit O M, Kellems R E, Rudolph F B. J Biol Chem. 1993;268:23728–23733. [PubMed] [Google Scholar]
- 13.Guranowski A, Montgomery J A, Cantoni G L, Chiang P K. Biochem. 1981;20:110–115. doi: 10.1021/bi00504a019. [DOI] [PubMed] [Google Scholar]
- 14.Andres C M, Fox I H. J Biol Chem. 1979;254:11388–11393. [PubMed] [Google Scholar]
- 15.Finkelstein J D, Martin J J. J Biol Chem. 1986;261:1582–1587. [PubMed] [Google Scholar]
- 16.Mathews I I, Erion M D, Ealick S E. Biochemistry. 1998;37:15607–15620. doi: 10.1021/bi9815445. [DOI] [PubMed] [Google Scholar]
- 17.Kowaluk E A, Bhagwat S S, Jarvis M F. Curr Pharmaceut Des. 1998;4:403–416. [PubMed] [Google Scholar]
- 18.Hardies S C, Wang L, Zhou L, Zhao Y, Casavant N C, Huang S. Mol Biol Evol. 2000;17:616–628. doi: 10.1093/oxfordjournals.molbev.a026340. [DOI] [PubMed] [Google Scholar]
- 19.DeBerardinis R J, Goodier J L, Ostertag E M, Kazazian H H., Jr Nat Genet. 1998;20:288–290. doi: 10.1038/3104. [DOI] [PubMed] [Google Scholar]
- 20.Soriano P, Montgomery C, Geske R, Bradley A. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- 21.Simpson E M, Linder C C, Sargent E E, Davisson M T, Mobraaten L E, Sharp J J. Nat Genet. 1997;16:19–27. doi: 10.1038/ng0597-19. [DOI] [PubMed] [Google Scholar]
- 22.Lakso M, Sauer B, Mosinger J, B, Lee E J, Manning R W, Yu S-H, Mulder K L, Westphal H. Proc Natl Acad Sci USA. 1992;89:6232–6236. doi: 10.1073/pnas.89.14.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loehrer F M, Angst C P, Brunner F P, Haefeli W E, Fowler B. Nephrol Dial Transplant. 1998;13:656–661. doi: 10.1093/ndt/13.3.656. [DOI] [PubMed] [Google Scholar]
- 24.McNally T, Helfrich R J, Cowart M, Dorwin S A, Meuth J L, Idler K B, Klute K A, Simmer R L, Kowaluk E A, Halbert D N. Biochem Biophys Res Com. 1997;231:645–650. doi: 10.1006/bbrc.1997.6157. [DOI] [PubMed] [Google Scholar]
- 25.Snyder F F, Lukey T. Biochim Biophys Acta. 1982;696:299–307. doi: 10.1016/0167-4781(82)90061-6. [DOI] [PubMed] [Google Scholar]
- 26.Day C P, James O F. Hepatol. 1998;27:1463–1466. doi: 10.1002/hep.510270601. [DOI] [PubMed] [Google Scholar]
- 27.Chalmers R A, Stanley C A, English N, Wigglesworth J S. J Pediat. 1997;131:220–225. doi: 10.1016/s0022-3476(97)70157-4. [DOI] [PubMed] [Google Scholar]
- 28.Wakamiya M, Blackburn M R, Jurecic R, McArthur M J, Geske R S, Cartwright J, Mitani K, Vaishnav S, Belmont J W, Kellems R E, et al. Proc Natl Acad Sci USA. 1995;92:3673–3677. doi: 10.1073/pnas.92.9.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Migchielsen A A J, Breuer M L, van Roon M A, te Riele H, Zurcher C, Ossendorp F, Toutain S, Hershfield M S, Berns A, Valerio D. Nat Genet. 1995;10:279–287. doi: 10.1038/ng0795-279. [DOI] [PubMed] [Google Scholar]
- 30.Duerre J A, Briske-Anderson M. Biochim Biophys Acta. 1981;678:275–282. doi: 10.1016/0304-4165(81)90217-8. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe M, Osada J, Aratani Y, Kluckman K, Reddick R, Malinow M R, Maeda N. Proc Natl Acad Sci USA. 1995;92:1585–1589. doi: 10.1073/pnas.92.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blackburn M R, Wakamiya M, Caskey C T, Kellems R E. J Biol Chem. 1995;270:23891–23894. doi: 10.1074/jbc.270.41.23891. [DOI] [PubMed] [Google Scholar]
- 33.Hershfield M S. J Biol Chem. 1979;254:22–25. [PubMed] [Google Scholar]
- 34.Chinsky J M, Ramamurthy V, Fanslow W C, Ingolia D E, Blackburn M R, Shaffer K T, Higley H R, Trentin J J, Rudolph F B, Knudsen T B, et al. Differentiation. 1990;42:172–183. doi: 10.1111/j.1432-0436.1990.tb00759.x. [DOI] [PubMed] [Google Scholar]
- 35.Walkey C J, Donohue L R, Bronson R, Agellon L B, Vance D E. Proc Natl Acad Sci USA. 1997;94:12880–12885. doi: 10.1073/pnas.94.24.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walkey C J, Yu L, Agellon L B, Vance D E. J Biol Chem. 1998;273:27043–27046. doi: 10.1074/jbc.273.42.27043. [DOI] [PubMed] [Google Scholar]
- 37.Fong D G, Nehra V, Lindor K D, Buchman A L. Hepatol. 2000;32:3–10. doi: 10.1053/jhep.2000.8978. [DOI] [PubMed] [Google Scholar]