Abstract
The demonstration that angiogenesis is required for the growth of solid tumors has fueled an intense interest in the development of new therapeutic strategies that target the tumor vasculature. Here we report the development of an immune-based antiangiogenic strategy that is based on the generation of T lymphocytes that possess a killing specificity for cells expressing vascular endothelial growth factor receptors (VEGFRs). To target VEGFR-expressing cells, recombinant retroviral vectors were generated that encoded a chimeric T cell receptor comprised of VEGF sequences linked to intracellular signaling sequences derived from the ζ chain of the T cell receptor. After transduction of primary murine CD8 lymphocytes by such vectors, the transduced cells were shown to possess an efficient killing specificity for cells expressing the VEGF receptor, Flk-1, as measured by in vitro cytotoxicity assays. After adoptive transfer into tumor-bearing mice, the genetically modified cytotoxic T lymphocytes strongly inhibited the growth of a variety of syngeneic murine tumors and human tumor xenografts. An increased effect on in vivo tumor growth inhibition was seen when this therapy was combined with the systemic administration of TNP-470, a conventional angiogenesis inhibitor. The utilization of the immune system to target angiogenic markers expressed on tumor vasculature may prove to be a powerful means for controlling tumor growth.
Angiogenesis, or the recruitment of a new blood supply, is required for the growth of solid tumors (1), and accordingly there has been intense interest in the development of therapeutic strategies that target the tumor vasculature. Many of the most promising strategies examined to date involve the use of either small molecules (2–6) or soluble forms of endothelial growth factor receptors (7–9) to interfere with the further development of tumor vasculature. In addition to these “cytostatic” strategies (10), other approaches aimed at the direct destruction of the tumor vasculature have been described recently that make use of toxins (11–14) or thrombotic agents (15, 16) that have been conjugated to endothelial cell receptor ligands or antibodies. These latter “cytotoxic” strategies, in principle, could provide for the most potent and long-lasting inhibition of tumor growth, because they are potentially able to both prevent the formation of new vessels and destroy existing tumor vasculature.
In an effort to expand the potential power of such cytotoxic strategies further, we have considered a “cell-based” therapy aimed at both the immune-mediated destruction of tumor vasculature and the targeted delivery of biologically active gene products to sites of tumor and its associated vasculature. For this purpose, we have made use of chimeric T cell receptor (TCR) technology (17–19) in conjunction with gene transfer to generate cytotoxic T cells capable of recognizing and killing cells that express vascular endothelial growth factor VEGFR2, a receptor critically involved in the growth of tumor vessels, in an MHC-independent fashion. Here we describe the construction of specific recombinant retroviruses encoding such a chimeric receptor consisting of VEGF-coding sequences linked to the signaling ζ chain of the TCR, demonstrate the ability of primary T lymphocytes transduced by the vectors to efficiently and specifically kill VEGFR2-bearing cells in vitro, and report on the antitumor activity of the genetically modified cells after adoptive transfer.
Materials and Methods
Mice.
Mice were purchased from Taconic Farms, and all animal work was conducted at the Harvard Institutes of Medicine Animal Facility in accordance with institutional guidelines.
Cell Lines.
HeLa, B16.F10, and LS174T cells were obtained from the American Type Culture Collection. T241 (murine fibrosarcoma) and murine islet endothelial (MILE) cells (syngeneic with C57BL/6) were kindly provided by Judah Folkman (Children's Hospital, Boston). MILE cells were grown in DMEM supplemented with 10% inactivated fetal serum/10% Nu serum IV/10 ng/ml basic fibroblast growth factor (Becton Dickinson) in a 10% CO2 incubator. The CL96 cytotoxic T cell line was a kind gift of Uwe Altenschmidt (20). Cytotoxic T lymphocytes (CTLs) were maintained in T cell growth medium (TCGM): RPMI medium 1640 supplemented with 10% FCS/1 mM pyruvate/100 units/ml penicillin/100 μg/ml streptomycin/2 mM glutamine/20 mM Hepes/0.1 mM nonessential amino acids/64 μM 2-mercaptoethanol/2 ng/ml human recombinant IL-2 (Sigma).
Retroviral Vector Construction.
Murine VEGF-164 cDNA was a gift of Bruce Spiegelman (Dana–Farber Cancer Institute, Boston; ref. 21), murine CD8α cDNA was a gift of Dan Littman (New York University, New York), and murine TCR ζ chain cDNA was a gift of Bernd Groner (22). CMMP-VEGF-cTcR was created by fusing the murine VEGF-165 coding sequence to a human c-Myc epitope (EIKLISEED), the hinge region of murine CD8α, and the murine TCR ζ chain (22) using standard molecular biology techniques. CMMP-VEGF-cTcR del Z was generated by using a synthetic double-stranded oligonucleotide bearing BamHI and BspEI sticky ends to replace the 0.2-kb BamHI-BspEI fragment of CMMP-VEGF-cTcR. This oligonucleotide substituted a CAG codon (Q) for the first intracytoplasmic TAC codon (Y) and introduced a TAA stop codon 15 aa downstream, thus eliminating the C-terminal 100 amino acids. Murine Flk-1 cDNA was obtained from Ihor Lemischska (Princeton University, Princeton), and the entire Flk-1 coding sequence was inserted between the NcoI and BamHI sites in the SFG retroviral vector (SFG-Flk-1; ref. 23). Soluble Flk-Fc was created by cloning the Fc portion of murine IgG2a between the BsaBI and BamHI sites of SFG-Flk-1, thereby replacing the transmembrane and intracellular domain of Flk-1. The MR-1 gene encodes a single-chain monoclonal antibody directed against a mutant EGFRvIII receptor (24) and was assembled (25) by using synthetic oligonucleotides corresponding to the published cDNA sequence. MR-1 sequences were cloned between the XbaI and BglII sites into CMMP-VEGF-cTcR, replacing VEGF, to generate CMMP-MR-1-cTcR .
Retrovirus Production.
293T cells were grown to 50–60% confluence in 150-mm plates and underwent a tripartite transfection with the following plasmids by using a standard calcium phosphate protocol (26): 35 μg of pMD-gag-pol (27), 35 μg of pMD-G (27), and 40 μg of either CMMP-VEGF-cTcR or CMMP-MR-1-cTcR. Viral supernatant was removed at 28 h after DNA addition, passed through a 0.45-μm filter (Nalge), and refrigerated. Concentrated viral stocks were prepared by centrifugation of viral supernatant in an SW28 rotor at 25,000 × g, 4°C, for 1.5 h. The supernatant was decanted, and 250 μl of TNE (50 mM Tris, pH 7.8/130 mM NaCl/1 mM EDTA, pH 8.0) was added. After sitting overnight at 4°C, virus was resuspended and stored at −80°C. The titers of unconcentrated and concentrated viral stocks on NIH 3T3 cells were ≈4 × 106 and 3 × 108 infectious particles per milliliter, respectively, as determined by fluorescence-activated cell sorter (FACS) analysis.
Retroviral Transduction of Cell Lines.
HeLa cells were transduced with CMMP-VEGF-cTcR retrovirus. After 3 days of culture, populations of HeLa cells expressing VEGF-cTcR were FACS-sorted by using anti-Myc antibody treatment followed by anti-mouse IgG2a-phycoerythrin. CL96 cells were transduced with either CMMP-VEGF-cTcR or CMMP-MR-1-cTcR as described below for primary splenocytes. Three days after transduction, single cell clones were established by limiting dilution in 96-well plates.
Splenocyte Harvest and Retroviral Transduction of CTLs.
Spleens were harvested and crushed through a 70-μm nylon filter. After red-cell lysis, CD8+ splenocytes were obtained by using negative selection columns (Cytovax Biotechnologies, Edmonton, AB, Canada). CTLs were seeded in 6-well plates precoated with 2 μg of anti-mouse CD28 and 2 μg of anti-mouse CD3e antibodies (PharMingen) per well. Three days later the CTLs were harvested and pooled, and 1 × 106 cells were transferred to conical tubes and centrifuged at 1,000 rpm. Supernatant was removed, leaving 100 μl to cover the cells. Fifty microliters of concentrated viral stock and 250 μl of PBS were added, and cells were resuspended and placed on ice for 3 h. Cells then were transferred to 12-well plates (precoated as described above), and 400 μl of TCGM containing 4 ng/ml IL-2 and 16 μg/ml polybrene was added to each well. After 6 h at 37°C, 1 ml of TCGM was added to each well. After an additional 12 h, the cells were washed with PBS and replated in precoated dishes. On days 3 and 5 posttransduction, cells were re-fed and split into uncoated 6-well dishes.
Southern Blot Analysis.
Five days after retroviral transduction, CD8+ splenocytes were harvested. Genomic DNA was prepared from viable cells and digested overnight with XbaI and BglII. Filters were incubated with the 32P-labeled ApaLI-AflIII 453-bp fragment of CMMP-VEGF-cTcR, washed, and exposed to film.
FACS Analysis.
High-titer retrovirus encoding the gene for a soluble form of Flk-1, Flk-Fc, were used to infect 293T cells. Conditioned medium from these transduced cells was collected and subjected to Western blot analysis by using either antibody directed against Flk-1 or 125I-labeled protein A to confirm the presence of both the Flk-1 and Fc portions of the fusion protein (data not shown). For FACS analysis of HeLa-VEGF-cTcR cells, 2 ml of conditioned medium from these transduced cells or control medium from untransduced 293T cells was used as the primary reagent. The cells then were washed and incubated with anti-mouse IgG2a-phycoerythrin (Chemicon) and analyzed. For the VEGF-cTcR/KDR-Fc binding study, VEGF-cTcR clone 2 cells were incubated with increasing amounts of human KDR-Fc (R & D Systems), washed twice with PBS, and stained with anti-human IgG1-FITC antibody. For analysis of transduced CTLs, cells were incubated successively with anti-human c-Myc antibody, biotinylated anti-mouse IgG, and streptavidin-phycoerythrin or only anti-mouse CD8 antibody conjugated to FITC (PharMingen).
In Vitro Cytotoxicity Assay.
Nontransduced B16.F10 cells or B16.F10 cells that were transduced with a retroviral construct encoding full-length Flk-1 were labeled with Cr51-sodium (NEN), washed with PBS, and incubated with varying amounts of primary CTLs (5 days posttransduction) in 96-well dishes for 8 h. MILE cells were seeded onto 12-well dishes at a density of 1.5 × 105 cells per well in endothelial cell medium. On the following day, the cells were overlaid with varying amounts of CTLs (4 days posttransduction) in TCGM and incubated for 5 h. Cell-free supernatants were harvested and analyzed in a scintillation counter (B16.F10 cells) or by using a standard dehydrogenase cytotoxicity kit (MILE cells) (Promega). In some experiments, MILE cells were preincubated with 10 μg/ml of either anti-Flk-1 antibodies or isotype control antibodies (PharMingen). Maximal release was calculated after incubating target cells in 1% Triton X-100.
Treatment of Mice with Genetically Modified T Cells.
On day 0, tumor cells were implanted into the s.c. space on the right flank of recipient mice. On the days of treatment, CTLs (4–7 days posttransduction) were harvested, washed, resuspended in cold PBS, and injected in a volume of 300 μl into the retroorbital venous plexus. Mice were treated daily with 25,000 units of human recombinant IL-2 (Chiron) in 0.5 ml of PBS via i.p. injection. TNP-470 (30 mg/kg, TAP Holdings, Deerfield, IL) was injected s.c. (into a site remote from the tumor) every other day in 0.3 ml of PBS starting with the first day of CTL therapy. Starting volumes of tumors ranged from 40 to 80 mm3. All mice were killed when control mice reached a mean tumor volume of 2,000 mm3 or had extensive tumor ulceration.
Results
Generation of CD8 Lymphocytes Targeted to VEGFRs.
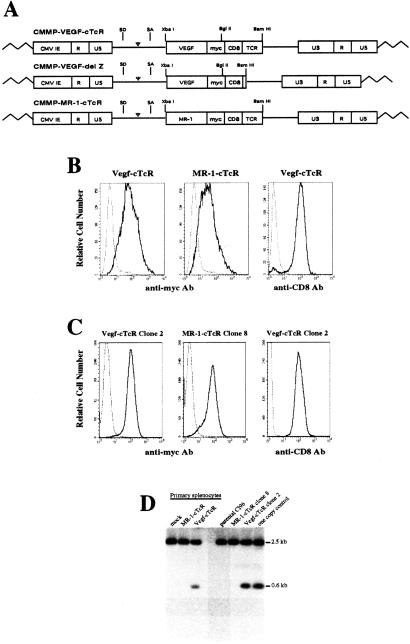
In a first step toward the generation of T lymphocytes possessing a killing specificity for VEGFRs, cDNA sequences derived from several sources were assembled to encode a chimeric TCR (termed VEGF-cTcR) composed of the entire coding region of VEGF-165 (21) followed by a human c-Myc epitope, a CD8α hinge region, and the transmembrane and signal-transducing domain of the ζ chain of the murine CD3–TCR complex (ref. 22; Fig. 1A). A second related chimeric TCR gene, termed VEGF-cTcR delZ, which encoded a truncated form of the TCR lacking the C-terminal 100 amino acids of the wild-type VEGF-cTcR, also was constructed. Lastly, a chimeric TCR (MR-1-cTcR) gene, which replaced the VEGF coding sequences present in VEGF-cTcR with sequences encoding a single-chain monoclonal antibody directed against the antigen, EGFRvIII (24), also was constructed. EGRFvIII is an epidermal growth factor receptor variant that is expressed on several solid tumor types but is not present in normal mice (24).
Figure 1.
Transduction of primary lymphocytes and cloned T cell lines by cTcR-expressing retroviral vectors. (A) Structure of retroviral vectors used in this study. VEGF-cTcR, VEGF chimeric TCR; MR-1-cTcR, MR-1 single-chain monoclonal antibody chimeric TCR; SD, splice donor; SA, splice acceptor; CMV IE, cytomegalovirus immediate early promoter; myc, human c-Myc epitope; CD8, CD8α hinge region; TCR, ζ chain of the TCR; LTR, long-terminal repeat. (B) FACS analysis of untransduced CTLs (dashed line) or CTLs transduced with VEGF-cTcR or MR-1-cTcR (solid line). Splenocytes transduced with VEGF-cTcR also were incubated with anti-CD8-FITC antibody (solid line) or an anti-rat IgG-FITC negative control antibody (dashed line). (C) FACS analysis of VEGF-cTcR clone 2 and MR-1-cTcR clone 8 cells. (D) Southern blot analysis of genomic DNA from transduced primary CD8+ splenocytes and CTL clones. Indicated is the expected 600-bp XbaI-BglII fragment containing the transgenic VEGF sequence and a background band that migrated as a 2.5-kb fragment. The one-copy control lane represents mock-transduced genomic DNA spiked with 12 pg of CMMP-VEGF-cTcR plasmid DNA, which correlates with an expected one copy of transgene per genome.
Each of the chimeric TCR genes was inserted into the retroviral vector CMMP (28), and high-titer recombinant virus encoding each of the gene products was produced. To generate primary murine CD8+ lymphocytes expressing the different chimeric TCR genes, a transduction protocol was developed that involved ex vivo preactivation of CTLs, coincubation of concentrated retroviral stock with activated CTLs on ice, and finally incubation of the CTL retrovirus suspension at 37°C in the presence of polybrene and IL-2 (see Materials and Methods for details). Transduced cells then were maintained in the presence of T cell activators (anti-CD3 and anti-CD28 antibodies) for an additional 3 days after retroviral transduction. After infection of primary lymphocytes with either CMMP-VEGF-cTcR or CMMP-MR-1-cTcR viruses, over 90% of the cells efficiently expressed the relevant transgene, as determined by FACS analysis, using antibody directed to the Myc epitope present in both transgenes (Fig. 1B). Transduction of primary T cells with the VEGF-cTCR-delZ also led to efficient cell surface expression (data not shown). Ninety-five percent of the transduced cells were CD8-positive 3 days postretroviral transduction (Fig. 1B), and after day 5 of culture over 98% of cells were CD8-positive (data not shown). After transduction, cTcR expression directly correlated with T cell activation status as determined by the expression of the high-affinity IL-2 receptor, CD25, and cell surface expression of the chimeric receptors was maintained for at least 8 days posttransduction (data not shown). Although this efficiency of gene transfer to primary lymphocytes has been reported by others (17, 29–32), the transduction protocol presented here is of particular interest in that it does not require either cocultivation with viral producer cells, multiple viral supernatant exposures, or antibiotic selection.
In addition to the transduction of primary murine CD8+ lymphocytes, the CMMP-VEGF-cTcR and CMMP-MR-1-cTcR viruses also were used to transduce CL96 cells, a murine CTL line (20), and two stable cell clones, VEGF-cTcR clone 2 and MR-1-cTcR clone 8, were isolated and expanded. As shown in Fig. 1C, these clones demonstrated efficient transgene expression even after several months in culture. Southern blot analysis of both transduced primary lymphocytes and CL96 cells indicated that the transduced CTLs contained approximately one copy of transgene per cell (Fig. 1D).
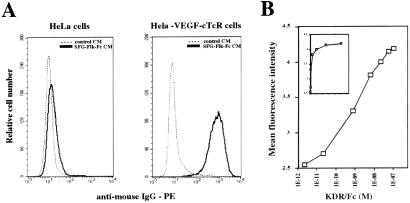
To assess whether the VEGF-cTcR encoded by the CMMP vector was capable of recognizing Flk-1, a FACS-based assay was used to measure directly the binding of a soluble form of Flk-1 (Flk-Fc) to CMMP-VEGF-cTcR-transduced cells. As shown in Fig. 2A, soluble Flk-Fc efficiently bound to HeLa cells expressing VEGF-cTcR but not to nontransduced HeLa cells. To facilitate the repeated measurements of the affinity of binding of Flk-Fc to cell surface VEGF-cTcR, Cl96 cells expressing the VEGF-cTcR gene (VEGF-cTcR clone 2 cells) rather than transduced primary cells were used next in a binding assay involving incubation of the cells with varying amounts of purified KDR-Fc followed by the subsequent addition of an FITC-labeled secondary antibody (see Materials and Methods). FACS analysis of the mean fluorescence of bound secondary antibody indicated that a half-maximal shift in mean fluorescence was achieved in the presence of 2 nM KDR-Fc (Fig. 2B). This binding affinity is comparable to that reported for the association of native VEGF and Flk-1 (0.1–0.5 nM; refs. 33 and 34). This affinity is noteworthy in light of our use of human rather than murine VEGFR2 in the binding assay (because of the commercial availability of KDR-Fc) and in light of previous studies that suggested that VEGF normally binds to Flk-1 as a homodimer in a head-to-tail configuration (35). As expected, binding of KDR-Fc to the VEGF-cTcR clone 2 cells was inhibited in a dose-dependent manner by VEGF, and there was no appreciable binding of soluble KDR-Fc to a CTL clone stably expressing MR-1-cTcR (MR-1-cTcR clone 8 cells; data not shown).
Figure 2.
Assessment of binding of Flk-1 to cTcR-expressing cells. (A) FACS analysis demonstrating the binding of soluble Flk-Fc only to HeLa cells expressing VEGF-cTcR. CM, conditional medium. (B) Binding of soluble human KDR-Fc to a CTL clone expressing VEGF-cTcR (VEGF-cTcR clone 2). Cells were incubated with increasing concentrations of soluble KDR-Fc, washed, incubated with anti-human IgG-FITC, and subjected to FACS analysis. The data are plotted on a logarithmic scale. (Insert) Plot of the data on a linear scale to demonstrate saturable binding.
VEGF-cTcR CTLs Specifically Lyse Flk-1-Expressing Cells in Vitro.
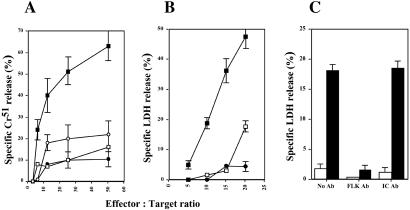
To determine whether primary CTLs transduced with the VEGF-cTcR construct could recognize and kill syngeneic cells expressing Flk-1, CTLs were incubated with either B16.F10 melanoma cells (which do not express Flk-1) or B16.F10 cells genetically modified to express Flk-1. In a standard in vitro cytotoxicity assay, CTLs transduced with the VEGF-cTcR construct but not the MR-1-cTcR construct specifically and efficiently lysed only the Flk-1-expressing B16.F10 cells (Fig. 3A). Moreover, there was no difference in nonspecific cell death between the VEGF-cTcR CTLs and MR-1-CTLs when parental B16.F10 cells were used as cellular targets (Fig. 3A). To assess whether VEGF-cTcR CTLs could lyse syngeneic, activated endothelial cells that naturally express Flk-1, the CTLs were incubated with MILE cells (36). As shown in Fig. 3B, CTLs transduced by the VEGF-cTcR construct but not the MR-1-cTcR construct demonstrated specific and efficient in vitro cytotoxicity against MILE cells in a dose-dependent manner. As expected, CTLs that express on their surface the VEGF-TcR lacking cytoplasmic signaling sequences (VEGF-cTcR delZ) showed no significant cell killing in cytotoxicity assays except at the highest effector-to-target ratio. The specific killing observed using the MILE cells depended on the presence of Flk-1 on the surface of the endothelial cells as preincubation of the cells with monoclonal antibodies directed against Flk-1 (but not isotype-matched antibodies) led to a 5–10-fold reduction in MILE-cell killing by VEGF-cTcR CTLs (Fig. 3C). Again, no appreciable killing was observed by using T cells expressing VEGF-cTcR delZ (open bars).
Figure 3.
VEGF-cTcR T cells specifically lyse cells expressing Flk-1. (A) Primary VEGF-cTcR CTLs (squares) or MR-1-cTcR CTLs (circles) were incubated with either B16.F10 cells that either expressed (■ and ●, respectively) or did not express (□ and ○, respectively) Flk-1 at varying effector-to-target ratios, and cell lysis was determined by using a standard Cr51 release assay. (B) Primary VEGF-cTcR CTLs (■), VEGF-cTcR del Z CTLs (□), or MR-1-cTcR CTLs (●) were incubated with adherent MILE cells, and lysis was determined by using a standard dehydrogenase (LDH) release assay. (C) MILE cells were preincubated with no antibodies, anti-Flk-1 antibodies, or isotype control (IC) antibodies before incubation with CTLs expressing VEGF-cTcR (filled bars) or VEGF-cTcR delZ (open bars) in a 5-h cytotoxicity assay at an effector-to-target ratio of 15:1. Each data point reflects the mean of six independent determinations.
VEGF-cTcR CTLs Suppress Tumor Growth in Vivo.
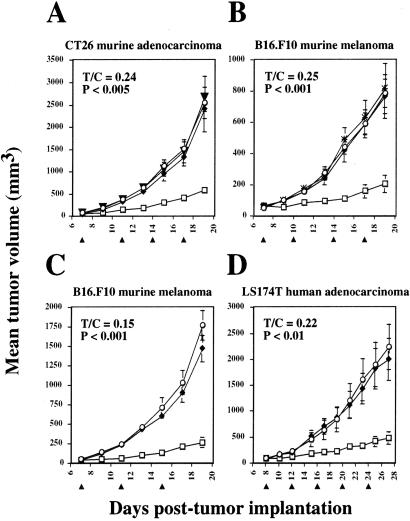
To determine whether adoptive transfer of syngeneic VEGF-cTcR CTLs could inhibit tumor growth, primary CTLs were injected intravenously into either BALB/c mice bearing CT26 murine colon adenocarcinomas or C57BL/6 mice bearing B16.F10 murine melanomas. Cytotoxic T-lymphocytes transduced with the VEGF-cTcR construct but not the MR-1-construct inhibited the growth of CT26 and B16.F10 tumors by 76 (Fig. 4A) and 75% (Fig. 4B), respectively, as determined by the ratio of the size of the tumors in the treated mice to that of the control mice (T/C ratio measured at day 19 postimplantation). Signaling through the ζ chain of VEGF-cTcR was required for antitumor activity, because CTLs expressing VEGF-cTcR delZ had no impact on tumor growth (Fig. 4B). In all experiments, exogenous IL-2 was administered daily (37) beginning with the first day of CTL therapy. Although IL-2 had no independent antitumor effect (Fig. 4A), its coadministration with the CTLs was essential to achieve significant therapeutic effect (Fig. 4B; ref. 38). To achieve the observed level of inhibition, repeated administrations of chimeric T cells were required at days 7, 11, 14, and 17, and tumor growth resumed after cessation of the injection of cells and cytokine (data not shown.)
Figure 4.
Adoptive immunotherapy using genetically modified CTLs. (A) CT26 cells (5 × 105, n = 6 per group) were implanted s.c. on BALB/c mice. (B) B16.F10 cells (7 × 105, n = 4 per group) were implanted on C57BL/6 mice. B16.F10 cells (5 × 105, n = 4 per group) (C) or LS174T cells (1 × 106, n = 7 per group) (D) were implanted on C57BL/6 nude mice. On the days indicated with an arrowhead, mice were treated with 5 × 106–9 × 106 VEGF-cTcR CTLs (□), MR-1-cTcR CTLs (♦), PBS (○), PBS with no exogenous IL-2 (▾), or VEGF-cTcR del Z (X). Daily i.p. injections of IL-2 started on the first day of CTL infusion (except for the group indicated with the inverted triangle in B). Tumor volume was calculated by using the formula width2 × length × 0.52, and the SEM is indicated with error bars. The ratio of the tumor volumes of the VEGF-cTcR CTL-treated mice to the PBS control mice (T/C) was determined for the last time point.
In a second series of studies, we asked whether VEGF-cTcR-expressing Cl96 cells possessed antitumor activity after adoptive transfer comparable to transduced primary cells. To enable the evaluation of the activity of the chimeric TCR against both murine and human tumors, tumor-bearing nude mice were used. As shown in Fig. 4 C and D, VEGF-cTcR clone 2 cells suppressed the growth of B16.F10 melanomas by 85% (Fig. 4C) and LS174T human colon adenocarcinomas by 78% (Fig. 4D), whereas the adoptive transfer of control MR-1-cTcR clone 8 cells led to no significant antitumor effects (Fig. 4 C and D).
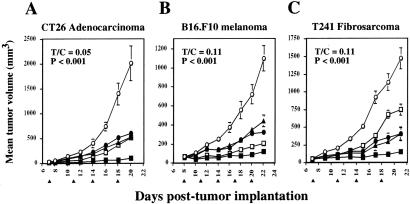
Lastly, to determine whether the antitumor efficacy of VEGF-cTcR primary CTLs could be enhanced by the addition of a conventional angiogenesis inhibitor, immunocompetent tumor-bearing mice were treated with a combination of the CTLs and a fumagillin analog, TNP-470, in three separate preexisting tumor models (Fig. 5 A–C). When TNP-470 was combined with the VEGF-cTcR CTLs, growth of CT26 adenocarcinomas, B16.F10 melanomas, and T241 fibrosarcomas was inhibited by 95, 89, and 90%, respectively. In comparison, treatment with TNP-470 alone suppressed the growth by 70, 71, and 73%, respectively, whereas those treated with the VEGF-cTcR CTLs alone were inhibited by 74, 81, and 49%, respectively (Fig. 5 A–C). In contrast, the addition of nonspecific CTLs to TNP-470 therapy was no more efficacious than treatment with TNP-470 alone (Fig. 5 A–C). Mice treated with TNP-470 alone displayed a weight loss of up to 10% of total body weight and occasionally had mild skin breakdown at the TNP-470 injection site, but no further toxicity was noted in mice that concomitantly received CTL therapy (data not shown).
Figure 5.
Effects of the combined treatment of genetically modified CTLs and TNP-470 on tumor growth. (A) CT26 adenocarcinoma cells (5 × 105, n = 4 per group) were implanted s.c. on BALB/c mice. B16.F10 melanoma cells (7 × 105, n = 4 per group) (B) or T241 fibrosarcoma cells (5 × 105, n = 3 per group) (C) were implanted on C57BL/6 mice. On the days indicated with an arrowhead, mice were treated with 5 × 106–10 × 106 VEGF-cTcR CTLs (□), VEGF-cTcR CTLs + TNP-470 (■), MR-1-cTcR CTLs + TNP-470 (▴), PBS + TNP-470 (●), or PBS (○). Mice were treated with TNP-470 every other day and IL-2 every day starting on the first day of CTL therapy. Tumor measurements and analyses were as described for Fig. 4. T/C, ratio of the tumor volumes of the VEGF-cTcR CTL-treated mice to the PBS control mice.
Discussion
The studies reported here indicate that the adoptive transfer of primary T cells possessing a killing specificity for VEGFR2 results in the potent inhibition of tumor growth. Critical to our studies was the use of chimeric TCR technology, which makes it possible to generate cytotoxic T cells possessing an MHC-independent killing specificity for virtually any antigen (18, 19, 22). Although in previous studies chimeric TCRs have been generated against either tumor-specific or tumor-associated antigens (17, 19, 22, 29, 31, 38–40), our studies have made use of the technology to direct an immune response to what is essentially a “self-antigen” expressed primarily, although not exclusively, on proliferating endothelial cells. Although the generation of a conceptually similar chimeric receptor based on a single-chain antibody to flk-1 has been reported recently (41), our report documents therapeutic activity in vivo.
On the basis of the in vitro cytotoxicity observed with VEGF-cTcR-bearing T cells and the dependence of the therapeutic efficacy of the genetically modified cells on a chimeric TCR capable of signaling, it is very likely that the antitumor activity indeed is caused by the cytotoxic activity of the cells. However, other mechanisms are possible, including the release of cytotoxic or inhibitory cytokines in the milieu of the tumor neovascular network or interference with the incorporation of new endothelial cells into the vascular network. Although our efforts to date to directly observe the destruction of tumor vasculature through measurements of microvessel density have been unsuccessful (unpublished results), recent studies suggest that measurements of microvessel density may not always be informative of successful angiogenic blockade, because successful inhibition of tumor angiogenesis can lead to a simultaneous decrease in tumor mass and tumor microvessels that results in no apparent change in microvessel density (J. Folkman, unpublished observations). Clearly, additional studies are necessary to fully understand the mechanistic bases underlying the inhibition of tumor growth observed.
Although the studies presented here represent an important “proof-of-principle” for the eventual development of immune-based antiangiogenic therapies, there are a variety of issues regarding such a therapy that need to be addressed more fully in the future. First, from the standpoint of toxicity, it is worth noting that our experiments indicated that animals treated with T cells expressing the VEGF-cTcR exhibited no obvious toxicity with respect to changes in weight, general appearance, or behavior despite repeated CTL infusions. Flk-1, the target of the chimeric receptor, is known to be expressed at some level in several normal tissues including the retina (37), kidney (42), and pancreas (43). Additional detailed studies of those specific tissues/organs are needed to define the levels of Flk-1 required for efficient recognition by chimeric receptor-bearing cells and to evaluatemore fully the potential toxicities of the therapy as currently developed. In this regard, it may be important to consider the generation of cTcRs that target gene products that are more specifically expressed on tumor neovasculature (44).
To improve the overall therapeutic efficacy of T cells directed toward the tumor vasculature, the use of chimeric receptors capable of providing signals for costimulation has been explored (45–47) in an effort to generate cells capable of long-term persistence in vivo and evaluating combination therapies involving the administration of other antiangiogenic and cytotoxic agents in conjunction with targeted CTL therapy. In addition, because the form of cytotoxic T cell therapy we have described provides a means of targeting genetically modified cells to sites of tumor and tumor vasculature, the use of cTcR-bearing cells further engineered to secrete additional gene products possessing antiangiogenic and/or other antitumor activities may further enhance the antitumor activity of these cells.
Acknowledgments
We thank Dr. Judah Folkman for helpful discussions and reviewing the manuscript. This work was supported by the Howard Hughes Medical Institute, the Brain Tumor Society, and National Cancer Institute Grants CA83772-02 and CA73133-01.
Abbreviations
- VEGF
vascular endothelial growth factor
- TCR
T cell receptor
- VEGFR
VEGF receptor
- MILE
murine islet endothelial
- CTL
cytotoxic T lymphocyte
- FACS
fluorescence-activated cell sorter
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Folkman J. J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 2.Vajkoczy P, Menger M D, Vollmar B, Schilling L, Schmiedek P, Hirth K P, Ullrich A, Fong T A. Neoplasia. 1999;1:31–41. doi: 10.1038/sj.neo.7900006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kusaka M, Sudo K, Matsutani E, Kozai Y, Marui S, Fujita T, Ingber D, Folkman J. Br J Cancer. 1994;69:212–216. doi: 10.1038/bjc.1994.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laird A D, Vajkoczy P, Shawver L K, Thurnher A, Liang C, Mohammadi M, Schlessinger J, Ullrich A, Hubbard S R, Blake R A, et al. Cancer Res. 2000;60:4152–4160. [PubMed] [Google Scholar]
- 5.Mendel D B, Schreck R E, West D C, Li G, Strawn L M, Tanciongco S S, Vasile S, Shawver L K, Cherrington J M. Clin Cancer Res. 2000;6:4848–4858. [PubMed] [Google Scholar]
- 6.Strawn L M, McMahon G, App H, Schreck R, Kuchler W R, Longhi M P, Hui T H, Tang C, Levitzki A, Gazit A, et al. Cancer Res. 1996;56:3540–3545. [PubMed] [Google Scholar]
- 7.Kuo C J, Farnebo F, Yu E Y, Christofferson R, Swearingen R A, Carter R, von Recum H A, Yuan J, Kamihara J, Flynn E, et al. Proc Natl Acad Sci USA. 2001;98:4605–4610. doi: 10.1073/pnas.081615298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldman C K, Kendall R L, Cabrera G, Soroceanu L, Heike Y, Gillespie G Y, Siegal G P, Mao X, Bett A J, Huckle W R, Thomas K A, Curiel D T. Proc Natl Acad Sci USA. 1998;95:8795–8800. doi: 10.1073/pnas.95.15.8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aiello L P, Pierce E A, Foley E D, Takagi H, Chen H, Riddle L, Ferrara N, King G L, Smith L E. Proc Natl Acad Sci USA. 1995;92:10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brower V. Nat Biotechnol. 1999;17:963–968. doi: 10.1038/13654. [DOI] [PubMed] [Google Scholar]
- 11.Backer M V, Budker V G, Backer J M. J Controlled Release. 2001;74:349–355. doi: 10.1016/s0168-3659(01)00346-7. [DOI] [PubMed] [Google Scholar]
- 12.Olson T A, Mohanraj D, Roy S, Ramakrishnan S. Int J Cancer. 1997;73:865–870. doi: 10.1002/(sici)1097-0215(19971210)73:6<865::aid-ijc17>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 13.Ramakrishnan S, Olson T A, Bautch V L, Mohanraj D. Cancer Res. 1996;56:1324–1330. [PubMed] [Google Scholar]
- 14.Ramakrishnan S, Wild R, Nojima D. Methods Mol Biol. 2001;166:219–234. doi: 10.1385/1-59259-114-0:219. [DOI] [PubMed] [Google Scholar]
- 15.Burrows F J, Thorpe P E. Pharmacol Ther. 1994;64:155–174. doi: 10.1016/0163-7258(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 16.Burrows F J, Thorpe P E. Proc Natl Acad Sci USA. 1993;90:8996–9000. doi: 10.1073/pnas.90.19.8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altenschmidt U, Klundt E, Groner B. J Immunol. 1997;159:5509–5515. [PubMed] [Google Scholar]
- 18.Romeo C, Seed B. Cell. 1991;64:1037–1046. doi: 10.1016/0092-8674(91)90327-u. [DOI] [PubMed] [Google Scholar]
- 19.Eshhar Z, Waks T, Gross G, Schindler D G. Proc Natl Acad Sci USA. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marcucci F, Waller M, Kirchner H, Krammer P. Nature (London) 1981;291:79–81. doi: 10.1038/291079a0. [DOI] [PubMed] [Google Scholar]
- 21.Claffey K P, Wilkison W O, Spiegelman B M. J Biol Chem. 1992;267:16317–16322. [PubMed] [Google Scholar]
- 22.Moritz D, Wels W, Mattern J, Groner B. Proc Natl Acad Sci USA. 1994;91:4318–4322. doi: 10.1073/pnas.91.10.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matthews W, Jordan C T, Gavin M, Jenkins N A, Copeland N G, Lemischka I R. Proc Natl Acad Sci USA. 1991;88:9026–9030. doi: 10.1073/pnas.88.20.9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wikstrand C J, Hale L P, Batra S K, Hill M L, Humphrey P A, Kurpad S N, McLendon R E, Moscatello D, Pegram C N, Reist C J, et al. Cancer Res. 1995;55:3140–3148. [PubMed] [Google Scholar]
- 25.Stemmer W P, Crameri A, Ha K D, Brennan T M, Heyneker H L. Gene. 1995;164:49–53. doi: 10.1016/0378-1119(95)00511-4. [DOI] [PubMed] [Google Scholar]
- 26.Soneoka Y, Cannon P M, Ramsdale E E, Griffiths J C, Romano G, Kingsman S M, Kingsman A J. Nucleic Acids Res. 1995;23:628–633. doi: 10.1093/nar/23.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ory D S, Neugeboren B A, Mulligan R C. Proc Natl Acad Sci USA. 1996;93:11400–11406. doi: 10.1073/pnas.93.21.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein C, Bueler H, Mulligan R C. J Exp Med. 2000;191:1699–1708. doi: 10.1084/jem.191.10.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwu P, Yang J C, Cowherd R, Treisman J, Shafer G E, Eshhar Z, Rosenberg S A. Cancer Res. 1995;55:3369–3373. [PubMed] [Google Scholar]
- 30.Costa G L, Benson J M, Seroogy C M, Achacoso P, Fathman C G, Nolan G P. J Immunol. 2000;164:3581–3590. doi: 10.4049/jimmunol.164.7.3581. [DOI] [PubMed] [Google Scholar]
- 31.Darcy P K, Haynes N M, Snook M B, Trapani J A, Cerruti L, Jane S M, Smyth M J. J Immunol. 2000;164:3705–3712. doi: 10.4049/jimmunol.164.7.3705. [DOI] [PubMed] [Google Scholar]
- 32.Hagani A B, Riviere I, Tan C, Krause A, Sadelain M. J Gene Med. 1999;1:341–351. doi: 10.1002/(SICI)1521-2254(199909/10)1:5<341::AID-JGM58>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 33.Millauer B, Wizigmann-Voos S, Schnurch H, Martinez R, Moller N P, Risau W, Ullrich A. Cell. 1993;72:835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- 34.Quinn T P, Peters K G, De Vries C, Ferrara N, Williams L T. Proc Natl Acad Sci USA. 1993;90:7533–7537. doi: 10.1073/pnas.90.16.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiesmann C, Fuh G, Christinger H W, Eigenbrot C, Wells J A, de Vos A M. Cell. 1997;91:695–704. doi: 10.1016/s0092-8674(00)80456-0. [DOI] [PubMed] [Google Scholar]
- 36.Arbiser J L, Moses M A, Fernandez C A, Ghiso N, Cao Y, Klauber N, Frank D, Brownlee M, Flynn E, Parangi S, Byers H R, Folkman J. Proc Natl Acad Sci USA. 1997;94:861–866. doi: 10.1073/pnas.94.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheever M A, Chen W. Immunol Rev. 1997;157:177–194. doi: 10.1111/j.1600-065x.1997.tb00982.x. [DOI] [PubMed] [Google Scholar]
- 38.Hekele A, Dall P, Moritz D, Wels W, Groner B, Herrlich P, Ponta H. Int J Cancer. 1996;68:232–238. doi: 10.1002/(SICI)1097-0215(19961009)68:2<232::AID-IJC16>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 39.Brocker T, Karjalainen K. Adv Immunol. 1998;68:257–269. doi: 10.1016/s0065-2776(08)60561-1. [DOI] [PubMed] [Google Scholar]
- 40.McGuinness R P, Ge Y, Patel S D, Kashmiri S V, Lee H S, Hand P H, Schlom J, Finer M H, McArthur J G. Hum Gene Ther. 1999;10:165–173. doi: 10.1089/10430349950018968. [DOI] [PubMed] [Google Scholar]
- 41.Kershaw M H, Westwood J A, Zhu Z, Witte L, Libutti S K, Hwu P. Hum Gene Ther. 2000;11:2445–2452. doi: 10.1089/10430340050207939. [DOI] [PubMed] [Google Scholar]
- 42.Feng D, Nagy J A, Brekken R A, Pettersson A, Manseau E J, Pyne K, Mulligan R, Thorpe P E, Dvorak H F, Dvorak A M. J Histochem Cytochem. 2000;48:545–556. doi: 10.1177/002215540004800412. [DOI] [PubMed] [Google Scholar]
- 43.Christofori G, Naik P, Hanahan D. Mol Endocrinol. 1995;9:1760–1770. doi: 10.1210/mend.9.12.8614412. [DOI] [PubMed] [Google Scholar]
- 44.St. Croix B, Rago C, Velculescu V, Traverso G, Romans K E, Montgomery E, Lal A, Riggins G J, Lengauer C, Vogelstein B, Kinzler K W. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- 45.Beecham E J, Ma Q, Ripley R, Junghans R P. J Immunother. 2000;23:631–642. doi: 10.1097/00002371-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 46.Finney H M, Lawson A D, Bebbington C R, Weir A N. J Immunol. 1998;161:2791–2797. [PubMed] [Google Scholar]
- 47.Hombach A, Sent D, Schneider C, Heuser C, Koch D, Pohl C, Seliger B, Abken H. Cancer Res. 2001;61:1976–1982. [PubMed] [Google Scholar]