Abstract
Suppressor of Cytokine Signaling 3 (SOCS3) is a critical regulator of cytokine signaling, primarily acting through the Janus Kinase (JAK)/Signal Transducer and Activator of Transcription (STAT) pathway. It plays a significant role in the development and progression of various malignancies. Abnormal expression of SOCS3 in cancer cells is linked to dysregulated cell growth, migration, and apoptosis, driven by cytokines and hormones. This aberrant expression makes SOCS3 a potential biomarker for tumor diagnosis, prognosis, and gene therapy. Targeting SOCS3 may offer innovative strategies for cancer treatment. This review provides a comprehensive overview of SOCS3's molecular structure, its biological functions in tumors, underlying molecular mechanisms, and therapeutic strategies targeting SOCS3.
Keywords: Suppressor of Cytokine Signaling 3, Malignant Tumors, Therapeutic Target, Immune Microenvironment, Immune signaling, Immune target, Immunotherapy
Introduction
Despite significant advances in cancer treatment, cancer remains a leading cause of human death [1]. Traditional treatments, such as chemotherapy and radiotherapy, often have limited therapeutic effects due to adverse reactions and acquired drug resistance [2]. The immune system plays a pivotal role in the surveillance and elimination of malignant tumors [3]. However, tumors often develop mechanisms to evade immune detection and suppression, necessitating the development of novel therapeutic strategies. A key component in understanding and manipulating the immune response is the identification of regulatory molecules that influence immune cell function and signaling pathways. Immunotherapy (IT) represents a significant achievement in cancer treatment [4]. It works by restarting the tumor immune cycle and restoring the body’s natural anti-tumor immune response. Currently, there are at least four main kinds of immunotherapy strategies, which include immune checkpoint inhibitors (ICIs) such as Programmed cell Death protein-1 (PD-1) and Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4), chimeric antigen receptor T-cell therapy, tumor vaccines, and peripatetic immunotherapy [5]. Although these therapies have been widely successful, enhancing clinical oncology outcomes, not all patients have benefited from it [6]. The clinical benefit of ICIs is quite variable among different solid tumor types and objective tumor responses and durable long-term disease control are seen in only 10–40% of unselected patients with these solid tumor types [7].
The tumor microenvironment (TME) includes tumor cells and its surrounding blood vessels, fibroblasts, immune cells (e.g., lymphocytes), bone marrow-derived suppressed cells (MDSCs), extracellular matrix(ECM), and signalling molecules (e.g., interleukin (IL)−1, interferon-gamma (IFN-γ) [8]. Tumors can influence the microenvironment by releasing extracellular signals and stimulating peripheral immune tolerance, while the immune cells in the microenvironment can affect the growth, proliferation, and evolution of cancer cells [9]. In the TME, a series of stepwise events are initiated in the cancer-immunity cycle that leads to effective killing of cancer cells [10]. However, various immune effector cells that are recruited and interacted with tumor cells are downregulated in response to tumor-derived signals. Meanwhile, activation of molecular mechanism that leads to apoptosis of antitumor effector cells also contributes to tumor escape [11]. Therefore, there is an urgent need for potential diagnostic markers and therapeutic methods to control tumor progression.
SOCS3 (Suppressor of Cytokine Signaling 3) is an intracellular protein that has emerged as a significant regulator of immune responses [12]. It functions primarily as a negative regulator of cytokine signaling pathways, including those mediated by interleukins and interferons [13]. By modulating these pathways, SOCS3 can influence the activation, proliferation, and effector functions of various immune cells, such as T cells, macrophages, and dendritic cells. Dysregulation of SOCS3 expression has been implicated in several diseases, including chronic inflammation and cancer, highlighting its importance in maintaining immune homeostasis [14]. Negative regulators of the cytokine signaling are important immune checkpoint molecules that regulate anti-tumor immunity [15]. SOCS protein family regulates signaling pathways and control the body’ s inflammatory and immune response by binding to cytokine receptors, JAK, and other signaling molecules [16]. SOCS3 is activated and released through the JAK/STAT signaling pathway, and its dysfunction has been shown to contribute to a variety of diseases, including immune disorders, metabolic diseases, and cancer [17]. The expression of SOCS3 is significantly altered in different types of tumor with different trends, thus SOCS3 could be a more effective and sensitive marker for persistent tumors [18]. In effect, SOCS3 may be a potential target for the diagnosis, treatment, and prognosis of tumors.
In this review, we will focus on the SOCS3 protein and the relationship to anti-tumor immunity. SOCS3 emerges as a crucial molecule in cancer development [19]. Currently, SOCS3 has been shown to be involved in embryonic development, inflammatory responses, immune regulation, and tumor progression [20]. Increased expression of SOCS3 in tumor tissues is associated with improved disease-free survival and overall survival rates, suggesting its potential as a novel and reliable biomarker for predicting cancer recurrence and mortality risk [21].
Moreover, the potential of SOCS3 as a biomarker for immunotherapy has garnered significant attention. Given its role in regulating immune responses, SOCS3 expression levels in tumor tissues or immune cells may serve as an indicator of the immune status within the tumor microenvironment [22]. This information could be crucial for predicting the efficacy of immunotherapeutic interventions, such as immune checkpoint blockade therapies. Elevated or altered SOCS3 expression might reflect an immunosuppressive environment, potentially influencing the response to treatment [12]. Therefore, understanding the mechanisms by which SOCS3 influences immune regulation and its potential as a biomarker is essential for developing targeted immunotherapies against malignant tumors. In this review, we will explore the multifaceted role of SOCS3 in immune regulation, its expression patterns in various malignancies, and its potential as a biomarker and therapeutic target in cancer immunotherapy. We aim to provide a comprehensive overview of the current knowledge and future directions for leveraging SOCS3 in the fight against cancer.
The Structure of SOCS3
The Structure and Biosynthesis of SOCS3
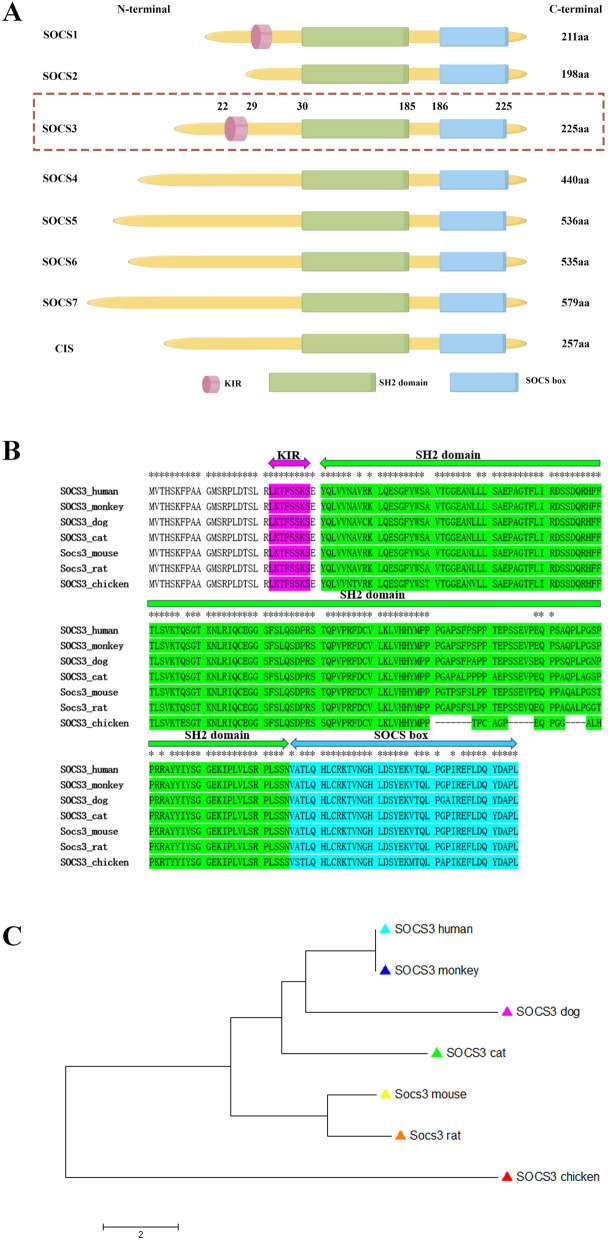
The SOCS family comprises eight members: Cytokine-inducible Src homology 2 protein (CIS) and SOCS2-SOCS7 [23–25]. SOCS3 is one of the most important members of the SOCS family. Its role as a potential negative regulator of signal transduction was first reported in 1998 [26]. It was discovered that human SOCS3 protein contains 225 amino acids with a molecular weight of 24.75 ku. The human SOCS3 Gene is located at 17q25.3 and spans 850 bp in length. Structurally, SOCS3 is composed of three key domains that contribute to its regulatory functions [27] (Fig. 1A).
Fig. 1.
The structure of SOCS protein and the evolutionary analysis of SOCS3 gene. A Illustration of the structure of the SOCS protein. The SOCS family is comprised of eight members. All eight members have a common SH2 domain, and a C-terminal SOCS box. Furthermore, SOCS1 and SOCS3 also have a region that inhibits kinases, known as the KIR. B, C Protein sequence alignment and neighbor-joining tree of SOCS3 genes for seven species. B Protein sequence alignment of SOCS3 genes for seven species. Asterisk (*) indicates identical amino acid. KIR, SH2 domain and SOCS box are shaded in magenta, chartreuse and cyan color. C Neighbor-joining tree of SOCS3 genes for seven species. The tree is drawn to scale and bar scale indicates 2 amino acids difference in branch length. Chicken SOCS3 gene is served as the outgroup to root the tree
Src homology 2 domain (SH2 domain) (Fig. 1A):
Central to the function of SOCS3, the SH2 domain binds specifically to phosphorylated tyrosine residues on target proteins, such as JAKs [28]. This binding is crucial for the inhibitory effects of SOCS3 on the JAK/STAT pathway, effectively blocking the signaling cascade and preventing the activation of downstream effectors [29]. Unlike the canonical SH2 domain found in many proteins, SH2 domains of SOCS3 proteins contain a unique N-terminal α-helical region, known as the extended SH2 (ESS) domain, that directly contacts residues crucial for phosphotyrosine binding [30].
N-terminal domain with Kinase Inhibitory Region (KIR) (Fig. 1A):
The N-terminal region of SOCS3 contains a kinase inhibitory region (KIR), which is unique to SOCS3 and SOCS1 [31]. This KIR directly binds to and inhibits Janus kinases (JAKs), thereby modulating the JAK/STAT signaling pathway [32]. This interaction enhances the inhibitory effect on downstream signaling, preventing the phosphorylation and activation of STAT proteins [33]. Additionally, immediately upstream of the ESS, SOCS3 containS KIR domain, a short amino acid stretch of 12 residues that acts as a pseudosubstrate, blocking the substrate binding groove of JAK to prevent detrimental kinase activity [34].
SOCS box (Fig. 1A):
Located at the C-terminal, the SOCS box is a conserved module of approximately 40 amino acids. It is involved in protein–protein interactions and plays a crucial role in the ubiquitination and degradation of target proteins via the ubiquitin–proteasome pathway [35]. Notably, the SOCS box of SOCS3 exhibits lower binding affinity with the E3 ubiquitin ligase protein Cullin-5 compared to other SOCS family members, indicating distinct regulatory mechanisms [35]. SOCS3 features a 35-residue PEST motif between the SH2 domain and SOCS box that enhances SOCS3 turnover, influencing not only its degradation pathway but also its intracellular stability [24].
SOCS3 gene is highly conserved within mammalian species (Fig. 1B). Human and monkey share the identical SOCS3 protein sequence (Fig. 1C). The largest sequence difference among mammalian species is no more than 5 amino acids (dog to mouse). The biosynthesis of SOCS3 is a tightly regulated process that involves multiple steps, starting from gene transcription to protein translation and post-translational modifications [36]. The expression of the SOCS3 gene is induced by various cytokines and growth factors, such as IL-6 and IFN-γ, which activate transcription factors like STAT3 and STAT1 to bind to the promoter region of the SOCS3 gene, initiating transcription [37]. The resulting pre-mRNA undergoes splicing, capping, and polyadenylation before being transported to the cytoplasm for translation. Once translated, SOCS3 undergoes post-translational modifications, including phosphorylation and ubiquitination, which are crucial for its stability and function [38, 39]. These modifications ensure that SOCS3 can effectively interact with target proteins such as JAKs, modulating the JAK/STAT signaling pathway [22].
Negative Feedback Regulation of SOCS3
In the resting state of the cell, the gene for SOCS3 is expressed at extremely low levels thus making it difficult to detect [40]. In many diseases, the activation of the JAK/STAT pathway induces varying degrees of SOCS3 gene expression in different kinds of cells and tissues [41–43]. SOCS3 protein expression is induced by activation of the JAK/STAT signaling pathway, in which negative feedback inhibits a variety of cytokines, including IL-6, IL- 23, and granulocyte colony-stimulating factor (G-CSF) [44–46]. SOCS3 protein is an important intracellular inhibitor associated with the body’ s immunity [47]. In order to avoid aberrant cell proliferation and tumorigenesis resulting from prolonged cytokine-mediated signaling, SOCS3 protein returns cells to their basal state by inhibiting signal transduction [48]. After being promoted for transcription and translation by STAT proteins, the SOCS3 protein further exercise its negative feedback regulatory effects on cytokines and their induced JAK/STAT pathways [49, 50].
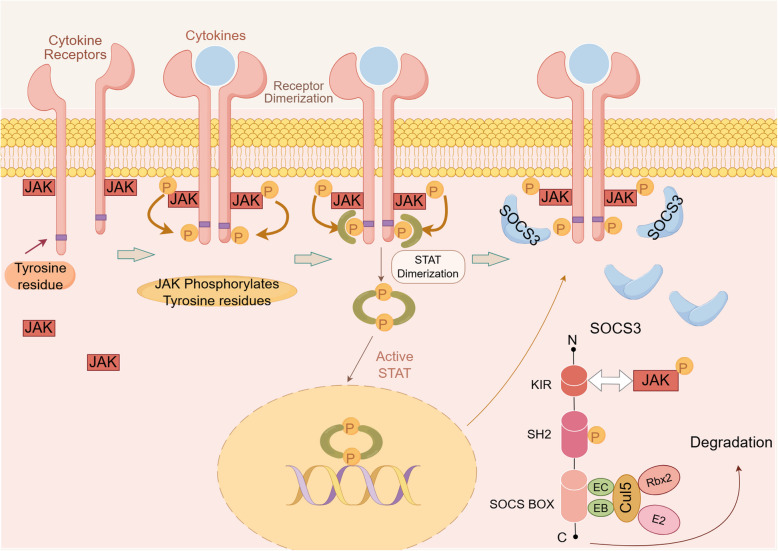
Within the SOCS family, SOCS3 functions as a key regulator in the negative feedback loop of the JAK/STAT signaling pathway [51, 52]. Among the SOCS subfamily, SOCS3 is the most effective negative regulator of pro-inflammatory cytokine signal transduction, triggering a feedback loop on the JAK/STAT pathway [53, 54] (Fig. 2). Upon JAK activation, the SOCS3-mediated negative regulatory loop is initiated. Firstly, the conserved C-terminal SOCS 3 box recruits Elongin B/C, Cullin 2, and Ring-box 2 to form a ubiquitin E3 ligase complex, promoting the proteasomal degradation of SH2-binding proteins. Subsequently, the SH2 domain competitively binds to the phosphorylated tyrosine sites of cytokine receptors, preventing STAT activation. Furthermore, the KIR structure in SOCS3 interacts with the phosphorylated tyrosine sites on JAK kinases, hindering STAT protein activation [55]. SOCS3 directly inhibits JAK1, JAK2, and TYK2. SOCS3 can also modulate other STAT pathways, such as STAT3 [56, 57] and STAT4 [58, 59]. Additionally, SOCS3 inhibits other signaling pathways like Wnt/mTOR [60], Phosphatidylinositol-3-Kinase (PI3K) [61, 62], and the Nuclear Factor kappa-light-chain-enhancer of activated B cells (NF-κB) cascade [63].
Fig. 2.
Schematic representation of the JAK/STAT pathway activated by cytokines to promote SOCS3 protein expression. The cytokine binds to its receptor and induces JAK phosphorylation. Tyrosine residues are phosphorylated upon activation of JAK, followed by recruitment of STAT proteins. STAT proteins bound to tyrosine residues are phosphorylated and form dimers that translocate into the nucleus and subsequently facilitate transcription and translation of the SOCS3 gene. SOCS3 protein binds to specific receptor-JAK complexes to control cytokine signaling through direct inhibition of kinases
Regulation of SOCS3 in Malignant Tumors
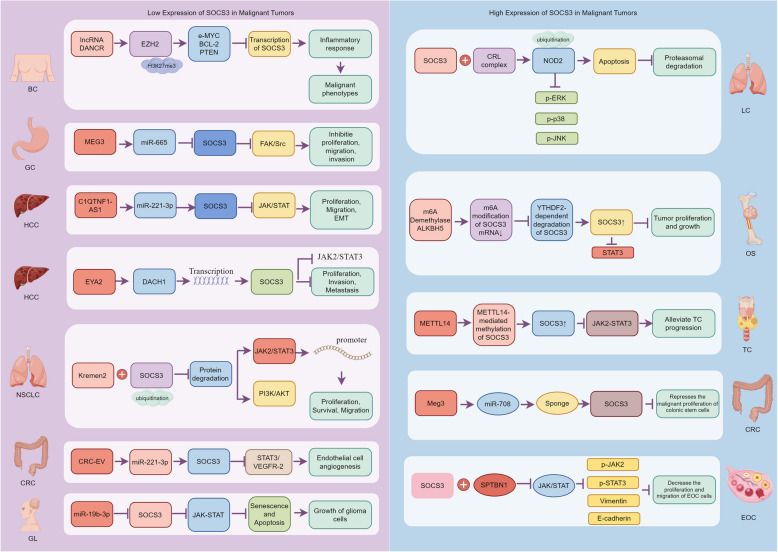
This section of the review examines the complex regulatory mechanisms of SOCS3 in the context of oncology. SOCS3 plays a pivotal role as a modulator in the pathogenesis of various cancers, with its expression being regulated by intricate genetic, epigenetic, and post-transcriptional mechanisms (Fig. 3).
Fig. 3.
Mechanistic roles of SOCS3 in the progression of various malignant tumors. This figure illustrates the differential roles of SOCS3 in the development and progression of multiple types of malignant tumors, categorized into those with low and high SOCS3 expression
Genetic Regulation
Dai et al. showed that SOCS3 was useful for the diagnosis, staging, histological subtyping, prognosis, and therapeutic response in several types of cancer in the context of immuno-oncology via multiple mechanisms, and that genetic or epigenetic alterations in SOCS3 frequently predict a poor prognosis [64]. Overexpression of SOCS3 reverses the enhancement of Chemokine (C-X-C motif) ligand 10 (CXCL10) levels in tumors mediated by microRNA-9 (miR-9), delaying the heightened recruitment of immunosuppressive cells and reducing tumor aggressiveness and progression [65]. Patients with hepatocellular carcinoma (HCC) with low PDL-1 and high SOCS3 expression had a better prognosis based on their pT stage (p < 0.05). The co-expression of low PDL-1 and high SOCS3 may be a superior independent prognostic marker for patients with HCC [64]. Overexpression of SOCS3 enhanced further resistin-stimulated growth of PC-3 cells, whereas silencing SOCS3 antagonized resistin-increased cell growth. Further PCa tissue analysis demonstrated higher levels of RETN, TLR4, SOCS3, and SOCS5 mRNAs in cancer tissues than benign prostate hyperplasia and indicated positive correlations among RETN, TLR4, and SOCS5. This suggests that SOCS5, TLR4, and, to a lesser extent, SOCS3 can mediate the mitogenic effect of resistin on PC-3 PCa cells [62]. Overexpression of SOCS3 rescues multiple malignant phenotypes induced by long non-coding RNA differentiation antagonizing non-protein coding RNA (DANCR) in both normal breast epithelial MCF10A and breast cancer MCF7 cells, including cell viability, invasion, Epithelial-Mesenchymal Transition (EMT), and cancer stemness, demonstrating that SOCS3 functions as a tumor suppressor in breast cancer [66].
Silencing SOCS3 significantly abolishes the upregulation of Krüppel-like factor 14 (KLF14) on tumor cell proliferation and invasion [67]. Knockdown of SOCS3 restores the proliferation and stemness of liver cancer stem cells (LCSCs) that are inhibited by the depletion of METTL3 [68]. Notably, intestinal cell-specific Aryl Hydrocarbon Receptor (AhR) knockout (KO) reduces responsiveness to IL22 and compromises DNA damage response after exposure to carcinogen, in part due to the enhancement of SOCS3 expression. Deletion of SOCS3 increases levels of pSTAT3 in AhR KO organoids, and phenocopies the effects of IL22 treatment on wild-type (WT) organoid growth [69]. Silencing of SOCS3 expression in Monocyte-derived Dendritic Cells (moDCs) resulted in increased release of both pro- and anti-inflammatory cytokines, upregulation of PD-L1, and decreased T-cell proliferation. This shows that H. pylori induces SOCS3 via an autocrine loop involving the T4SS and TNF-α and p38 signaling. Moreover, Sarajlic et al. demonstrate that high levels of SOCS3 in DCs dampen PD-L1 expression on Dendritic cells (DCs), which in turn drives T-cell proliferation [70]. Chen found that SPTBN1 and SOCS3 were positively co-expressed in Epithelial Ovarian Cancer (EOC) patients. SOCS3 overexpression or JAK2 inhibition decreased the proliferation and migration of EOC cells as well as the expression of p-JAK2, p-STAT3 and Vimentin, which were enhanced by the downregulation of SPTBN1, while E-cadherin expression was also reversed. It was also verified in mouse embryonic fibroblasts (MEFs) that loss of SPTBN1 activated the JAK/STAT3 signaling pathway with suppression of SOCS3. This result suggests that SOCS3 mediates the inhibitory effects of SPTBN1 on the progression of epithelial ovarian cancer by blocking the JAK/STAT3 signaling pathway [71].
Epigenetic Regulation
High methylation of SOCS3 is associated with Hepatitis B virus (HBV)-related HCC, with SOCS3 hypermethylation in tumor tissues being much higher than in non-tumor tissues, suggesting that SOCS3 may be a therapeutic target for HCC [72]. The presence of SOCS3 promoter methylation in H2228 cells as well as in three cases of seven EML4-ALK-positive lung cancer tissues. The expression level of SOCS3 significantly increased in H2228 cells after 5'-Aza-dC treatment. The aerrant methylation of the SOCS3 promoter region in EML4-ALK (+) H2228 cells and lung cancer tissues may be significantly involved in the pathogenesis of EML4-ALK-positive lung cancer [73]. The mRNA expression of SOCS3 was downregulated in OS tissues and negatively correlated with THAP9-AS1 expression in tumors. Functionally, ectopic expression of THAP9-AS1 promoted cell proliferation, migration, and invasion and inhibited apoptosis, and this phenomenon could be reversed by SOCS3 [74]. Cytokine signaling is a crucial mechanism in the development and progression of gastric cancer, with STAT3 signaling potentially playing a central role in gastritis-associated tumorigenesis. As an activator of phosphorylated (p-) STAT3 expression in non-tumorous gastric mucosa from early gastric cancer patients, SOCS3 methylation is associated with sustained p-STAT3 overexpression and enhanced epithelial cell proliferation, exerting tumor-suppressive effects [75]. In non-neoplastic gastric mucosa (non-NGM), SOCS3 promoter methylation was detected in 17/51 patients (33.3%) with early Gastric cancer (GC). In those patients, the non-NGM labeling indices of both Ki67 and p-STAT3 were significantly higher compared with that in patients with early GC without SOCS3 methylation. SOCS3 methylation is associated with continuous p-STAT3 overexpression and enhanced epithelial cell proliferation in non-NGM of patients with early GC [75]. SOCS3 methylation mediated by DNA Methyltransferases (DNMTs) promotes the occurrence and development of Acute Myeloid Leukemia (AML) and can be used as a potential biomarker for the diagnosis and efficacy evaluation of AML [76]. Bioinformatics analysis combined with validation experiments determined that m6A was modified by Methyltransferase-like Protein 3 (METTL3)-targeting SOCS3 mRNA [68]. METTL3 had side effects regarding the stability of SOCS3 mRNA. SOCS3 overexpression impaired and SOCS3 depletion facilitated the development of LCSCs via the JAK2/STAT3 pathway [68]. Furthermore, METTL3 depletion suppressed proliferation and stemness in LCSCs, which was restored by SOCS3 knockdown or colivelin treatment. METTL3 facilitated the stemness and tumorigenicity of LCSCs by modifying SOCS3 mRNA with m6A [68].
Post-transcriptional Regulation
siRNA-Mediated Regulation of SOCS3
Inhibition of SOCS3 using small interfering RNA (siRNA) reduces the proliferation of Glioblastoma multiforme (GBM) cell lines, and high expression of SOCS3 is associated with poor prognosis [52]. SOCS3 siRNA reverses the inhibitory effects of miR-483-5p inhibitors on migration and invasion in the Triple Negative Breast Cancer (TNBC) cell line BT-549 [77]. Similarly, si-SOCS3 transfected macrophages boosted Breast Cancer (BC) cell migration and invasion in a positive feedback manner [78].
MicroRNA-Mediated Regulation of SOCS3
Disruption of SOCS3 reverses the inhibitory effects of miR-455-5p inhibitors on the malignant potential of breast cancer cells [79]. Furthermore, studies confirm that miR-9 can directly reduce SOCS3 expression by targeting its 3′UTR, thereby promoting the recruitment of Myeloid-Derived Suppressor Cells (MDSCs) in breast cancer regions, suggesting that miR-9 fosters MDSC development by disrupting SOCS3 in the context of breast cancer [51]. As a downstream target of miR-501-3p, SOCS3 inhibits colorectal cancer cell proliferation, migration, and invasion while promoting apoptosis [80]. Mechanistically, exosomal miR-191-5p directly inhibited the SOCS3 expression in macrophages and aggravated macrophage M2 polarization [78]. SOCS3 was proved as a target of miR-30a-5p and could activate mTOR/P70S6K pathway to repress autophagy [81]. MiR-9 targets SOCS3, thereby inhibiting its expression and promoting the recruitment of G-MDSCs in tumors via the CCL5/CCR5 axis. Conversely, SOCS3 overexpression reverses the miR-9-mediated increase in CXCL10 levels within tumors. Given that elevated CXCL10 expression is associated with the recruitment of immunosuppressive cells and enhanced tumor invasiveness, these findings underscore the pivotal role of the miR-9/SOCS3 axis in orchestrating the tumor microenvironment [65]. Functionally, miR-452-5p knockdown restrained RB cell proliferation, invasion, EMT, and facilitated cell apoptosis. Mechanistically, SOCS3 knockdown restored the inhibitory effects of miR-452-5p knockdown on RB cells. Also, miR-452-5p knockdown increased SOCS3 protein levels, and decreased phosphorylated JAK2, phosphorylated signal transducer and activator of transcription 3/STAT3 in vivo [82]. SOCS3 was the direct target of miR-455-5p and was down-regulated in breast cancer. Interference with SOCS3 reversed the inhibitory effect of the miR-455-5p inhibitor on breast cancer cells'malignant potential [79]. The glioma patients exhibited overexpressed miR-19b-3p and poorly-expressed SOCS3. SOCS3 was identified as a target gene of miR-19b-3p through dual-luciferase reporter gene assay. miR-19b-3p repressed SOCS3 expression and activated the JAK-STAT signaling pathway. Furthermore, miR-19b-3p inhibition promoted apoptosis and senescence, and suppressed cell proliferation through inactivation of the JAK-STAT signaling pathway and up-regulation of SOCS3 [52]. Colon cancer increased miR-501-3p and DNA methyltransferase 1 and downregulated SOCS3 and SET domain containing 7. miR-151-3p inhibited SET domain containing 7, upregulating DNA methyltransferase 1. Increased promoter methylation by DNA methyltransferase 1 decreased SOCS3 expression. M2-EVs with miR-501-3p regulated the SET domain containing 7/DNA methyltransferase 1/SOCS3 axis to induce apoptosis and colon cancer cell growth, invasion, and migration [80]. miR-708 appears to promote malignant proliferation of colonic stem cells by targeting SOCS3/STAT3 signaling [83]. Additionally, SOCS3 overexpression reversed the effects of miR-483-5p mimic on the proliferation, migration, invasion and inflammation of BT-549 cells [77]. SOCS3 was a downstream target of miR-221-3p, and up-regulation of miR-221-3p choked SOCS3 and activated JAK2/STAT3 [84]. MiR-203 can regulate the proliferation and apoptosis of the pancreatic cancer cells by targeting the inhibited SOCS3 expression and regulating the JAK-STAT pathway activity [85].
Circular RNA-Mediated Regulation of SOCS3
Circular RNA (Circ)ANKRD28 regulated tumor cell progression and cisplatin(DDP) sensitivity through the miR-221-3p/SOCS3 axis [86]. Exogenous circ0003356 expression and miR-668-3p silencing suppressed the migration, viability, proliferation, epithelial to EMT and invasion of GC cells and enhanced apoptosis. Targeting of miR-668-3p by circ0003356 was confirmed through binding assays and SOCS3 was identified as a downstream target of miR-668-3p. The impacts of circ0003356 on cell proliferation, apoptosis, migration, invasion and EMT were reversed by miR-668-3p up-regulation or SOCS3 down-regulation in GC cells [87]. RIP and dual-luciferase reporter assay demonstrated that has-circ0001785 could regulate the SOCS3 by sponging miR-942. In general, circular RNA has-circ0001785 inhibits the proliferation, migration and invasion of BC cells by modulating the miR-942/SOCS3 signaling axis [88]. CircANKIB1 promoted miR-19b expression, inhibited the expression of the downstream target gene SOCS3, and then activated the STAT3 pathway. When cotransfected with circANKIB1 siRNA, and miR-19b mimics, the expression of SOCS3 and the phosphorylation level of STAT3 did not change significantly. When cotransfected with circANKIB1 siRNA and miR-19b mimics or SOCS3 siRNA, the cell proliferation, apoptosis, and invasion levels did not change significantly, suggesting that circ_ANKIB1 could affect the STAT3 pathway and osteosarcoma cell growth and invasion by enhancing the regulation of miR-19b on the downstream target gene SOCS3 [89]. CircTADA2A-E6 preferentially acted as a miR-203a-3p sponge to restore the expression of miRNA target gene SOCS3, resulting in a less aggressive oncogenic phenotype [90]. Long Intergenic Non-Protein Coding RNA 893(Linc00893) acted as a sponge for miR-3173-5p and inhibited its activity, which in turn regulated the SOCS3/JAK2/STAT3 signaling axis.
RNA Binding Protein-Dependent Regulation of SOCS3 mRNA Stability
RNA binding proteins (RBPs) play a crucial role in the post-transcriptional regulation of gene expression by modulating mRNA stability, splicing, transport, translation, and degradation [91]. In the context of SOCS3, RBPs can significantly impact its mRNA stability, thereby influencing its expression levels and functional outcomes in cancer [92]. RBPs can bind to specific sequences or structural elements within the 3'or 5'untranslated regions (UTRs) of SOCS3 mRNA, affecting its stability and degradation rates. For instance, MEX3C increased JAK2/STAT3 pathway activity by downregulating SOCS3. MEX3C interacted with the 3’UTR of SOCS3 and recruited CNOT7 to ubiquitinate and accelerate decay of SOCS3 mRNA. Treatment with MEX3C-specific antisense oligonucleotide (ASO) significantly inhibited JAK2/STAT3 pathway activation, suppressing HCC migration in vitro and metastasis in vivo [92]. TNF-α increases SOCS3 expression by stabilizing SOCS3 mRNA. Activation of the Mitogen-Activated Protein Kinase Kinase 6 (MKK6)/p38MAPK-cascade is required for TNF-α-mediated stabilization of SOCS3 mRNA and results in enhanced SOCS3 protein expression [93]. In fibroblasts or macrophages deficient for MAPK-activated protein kinase 2 (MK2), a downstream target of the MKK6/p38MAPK cascade, basal SOCS3-expression is strongly reduced and TNF-α-induced SOCS3-mRNA stabilization is impaired, indicating that MK2 is crucial for the control of SOCS3 expression by p38MAPK-dependent signals [93]. TNF-α regulates SOCS3 expression on the level of mRNA stability via activation of the MKK6/p38MAPK cascade [93].
SOCS3 and Cancer Heterogeneity
SOCS3 expression and its downstream signaling pathways are significantly associated with cancer heterogeneity across various types of malignancies. This heterogeneity is evident in the differential expression patterns of SOCS3 and its impact on disease progression, prognosis, and therapeutic response.
In early T precursor acute lymphoblastic leukemia (ETP-ALL), a high-risk subtype of T-ALL, SOCS3 expression levels are lower compared to other ALL subtypes. This reduced expression is linked to the high aggressiveness and poor prognosis of ETP-ALL. The study by Soumyadeep et al. highlights the role of SOCS3 in modulating the developmental propensity of ETP-ALL blasts, influencing their lineage-specific characteristics and therapeutic response [94]. SOCS3 expression varies between different genotypes of hepatitis C virus (HCV)-infected cells. Marcello et al. demonstrates that HCV genotype 1b-infected cells exhibit higher SOCS3 expression compared to genotype 2-infected cells. This difference is associated with insulin resistance and non-response to antiviral therapy, highlighting the role of SOCS3 in mediating metabolic and inflammatory pathways in HCC [95]. SOCS3 expression levels differ among histopathological subtypes of non-small cell lung carcinoma (NSCLC), with squamous cell carcinoma (SCC) showing higher expression compared to large cell carcinoma (LCC). These differences may serve as potential diagnostic markers for distinguishing between NSCLC subtypes and could influence therapeutic strategies [96]. In breast cancer, SOCS3 expression is differentially regulated across molecular subtypes. Triple-negative breast cancer (TNBC) exhibits lower SOCS3 expression compared to other subtypes, such as luminal A, luminal B, and HER2-enriched cancers. This downregulation in TNBC is associated with its aggressive behavior and poor prognosis [97].
Interaction of SOCS3 with the Tumor Microenvironment
The TME has garnered increasing attention due to its crucial role in tumor immunosuppression, distant metastasis, local drug resistance, and response to targeted therapies [98, 99]. The TME is a highly complex system comprising tumor cells, infiltrating immune cells (such as macrophages [100], dendritic cells [101], and lymphocytes [102]), cancer-associated stromal cells (e.g., cancer-associated fibroblasts [103]), the extracellular matrix [104], and various signaling molecules. Recent studies have found that SOCS3 had a higher correlation with T follicular helper cells (Tfh cells), iTregs, and macrophages. In ESCA, SOCS3 was significantly related to macrophages, the infiltration score, and NK cells [105]. This section reviews the regulation of SOCS3 in the TME on T lymphocytes, dendritic cells, myeloid-derived suppressor cells, macrophages, natural killer cells, etc. (Fig. 4).
Fig. 4.
Expression and regulation of SOCS3 on tumor cell and various immune cells. The expression of SOCS3 on tumor cell is upregulated by transcriptional regulation, post-transcriptional regulation, and post-translational modification. In addition, SOCS3 limits anti-tumor immunity through various immune cell types
Expression of SOCS3 in Tumor Cells and Its Impact on Proliferation, Invasion, Metastasis, and Drug Resistance
SOCS3 plays an inhibitory role in the initiation and progression of various types of human cancers, exerting significant influence in regulating oncogene expression and guiding tumor development [106] (Table 1). It can serve as a potential biomarker for tumor diagnosis and treatment [48].
Table 1.
Review of SOCS3 expression and function in various malignant tumors
| Type of cancer | In Vitro/In Vivo/ Clinical |
Cell lines | Expression | upstream molecular | egulated target genes | Functions | Reference |
|---|---|---|---|---|---|---|---|
| Acute myelocytic leukemia | In Vitro + Clinical | THP-1; U937 | low expression | DNMT3a | JAK2/STAT | Inhibiting the JAK2/STAT pathway can reduce the proliferation of leukemia cell lines and increase apoptosis | [76] |
| Clinical | - | low expression | STAT3 | Inhibit cell proliferation | [42] | ||
| Breast Cancer | In Vitro + Clinical | MDA-MB-453; SKBR3; MDA-MB-231; MCF-7 | low expression | miR-191-5p | M2-Subtype Macrophages | Inhibit M2 polarization of macrophages and suppress the tumor microenvironment | [78] |
|
In Vitro + in Vivo |
MCF-7; MDA-MB-231 | low expression | miR-9 | CCL5/CCR5 | Overexpression of SOCS3 reverses the promotive effect of miR-9 on CXCL10 content in tumors, delays the high recruitment of immunosuppressive cells, and reduces tumor invasiveness and progression | [65] | |
| Both | EO771 | low expression | miR-155 | STAT3/NF-κB、Wnt/mTOR | Suppressing tumor growth contributes to an immunosuppressive tumor microenvironment | [60] | |
|
In Vitro + in Vivo |
MCF-7 | low expression | Low expression of SOCS3 is significantly associated with poor prognosis in breast cancer patients | [107] | |||
| Both | MDA-MB-231; MDA-MB-468; MCF10A; THP-1 | low expression | KLF14 | RhoA/Rock/STAT3 | Silencing SOCS3 significantly abolishes the inhibitory effect of KLF14 upregulation on tumor cell proliferation, invasion, and M2 polarization of macrophages | [67] | |
| In Vitro + Clinical | MCF-10、BT-20; MDA-MB-231; MDA-MB-468; BT-549 | low expression | miR-483-5p | SOCS3 silencing reversed the inhibitory effects of miR-483-5p on cell proliferation, apoptosis, migration, invasion, and inflammatory factor secretion | [77] | ||
| Both | MCF10A; MCF7; T47D; MDA-MB-231; MDA-MB-468 | - | DANCR | The inhibition of SOCS3 has been shown to be a Tumor suppressor in 46breast cancer | [66] | ||
|
In Vitro + in Vivo |
T47D; MCF-7; MDA-MB-453; MDA-MB-231 | low expression | miR-942 | High levels of SOCS3 are associated with a favorable prognosis | [88] | ||
| In Vitro + Clinical | MCF-7; MDA-MB-231; T47D; BT20; BT549; SKBR3; MDA-MB-157; MDA-MB-435; MDA-MB-436; MDA-MB-468; MCF-10A; 293 T | low expression | miR-203a-3p | Patients with lower SOCS3 levels have a poorer prognosis | [90] | ||
| Lung cancer | In Vitro + Clinical | H1299; H1437 | low expression | - | JAK2/STAT3 | Inhibit the growth and metastasis of tumors in the body | [56] |
| Both | IMR90; MRC5; H1299; A549; H460; H1703; H4006; H358; HEK293T | low expression | Kremen2 | EGFR、 STAT3 | Inhibit the growth and metastasis of tumors in the body | [57] | |
|
In Vitro + in Vivo |
HEK293T; A549; H460; H1299; H2009; H1975; H3255 | high expression | NOD2 | Regulate cancer characteristics such as vitality and migration; serve as a key regulator of NOD2 ubiquitination and degradation | [108] | ||
|
In Vitro + in Vivo |
HBE; A549; H1299; THP-1 | low expression | miR-1290 | Overexpression of SOCS3 reversed the upregulation of p-STAT3 induced by miR-1290 in THP-1 cells; Overexpression of SOCS3 reversed the promotion of miR-1290 on macrophage polarization towards the M2 phenotype, which is related to tumor progression and metastasis | [109] | ||
| Pancreatic cancer |
In Vitro + in Vivo |
HPDE67; PANC-1; CFPAC-1; BxPC-3; ASPC-1; PACA-2; SW1990; HEK293T | low expression | miR-491-5p | - | Inhibit cancer cell proliferation, migration, and gemcitabine resistance | [110] |
| Both | PSC; PANC-1; HEK-293 T | low expression | LncRNA UCA1 | EZH2 | Inhibit cancer cell proliferation | [111] | |
| Clinical | low expression | MiR-203 | JAK-STAT | Regulate the proliferation and apoptosis of pancreatic cancer cells by modulating JAK-STAT pathway activity | [85] | ||
| In Vitro | HPDE6-C7; PANC-1 | low expression | MiR-221 | Inhibit cancer cell proliferation | [112] | ||
| Rectal cancer | In Vitro | HEK-293 T; FHC; Caco-2; SW480; HT29 | lncRNA CCAT1 | Suppress tumorigenesis | [113] | ||
| Prostate cancer | Vitro | PC-3; LNCaP-FGC; DU145; U937 | low expression | TLR4 | PI3K/AKT 、JAK2 | The function of SOCS3 is necessary for the stimulation of cancer cell growth by resistin. Knocking down SOCS3 inhibits the growth of PC-3 cells stimulated by resistin. Overexpression of SOCS3 enhances the growth of PC-3 cells stimulated by resistin | [62] |
| Both | PC-3; DU145; VCaP; LNCaP; RWPE-1 | low expression | miR-3173-5p | JAK2/STAT3 | Impeded the proliferation, epithelial-mesenchymal transition (EMT), and migration capabilities of PCa cells, and inhibited the tumorigenesis of PCa cells in nude mice | [114] | |
| Both | PC3; DU145; TRAMP-C1 | low expression | IL-30 | Inhibit cancer cell proliferation | [50] | ||
| Melanoma | In Vitro + vivo | C918MUM-2B | low expression | PTK6 | SOCS3 can inhibit UM tumorigenesis both in vivo and in vitro. It inhibits the proliferation, migration, and invasion of UM cells | [115] | |
| Gastric cancer | Both | AGS; HGC-27; GES-1 | low expression | miR-668-3p | Inhibited the migration, viability, proliferation, epithelial-mesenchymal transition, and invasion of GC cells, while enhancing cellular apoptosis | [87] | |
|
In Vitro + Clinical |
AGS; NCI-N87 | low expression | lncRNA AC125807.2 | SOCS3 exhibits a significant tumor-suppressing effect in GC | [116] | ||
| Both | BGC-823; SGC-7901; MKN-28; GES-1 | low expression | miR-665 | FAK/Src | SOCS3 exhibits a significant tumor-suppressing effect in GC | [117] | |
| Glioma | Both | HEB; A172; SF188; LN229; T98G; U251 | high expression | miR-19b-3p | JAK-STAT | SOCS3 inactivates the JAK-STAT signaling pathway to inhibit glioma cell proliferation, thereby promoting senescence and apoptosis | [52] |
| In Vitro | A172; U-87MG; U-373MG | high expression | Reduce the proliferation of glioblastoma | [118] | |||
| T-ALL/LBL |
In Vitro + Clinical |
thymocytes | low expression | IL-7、KAK/STAS | Hypermethylation of SOCS3 can contribute to its dysregulation in other malignancies; SOCS3 emerges as a potential therapeutic target for patients with constitutively activated JAK/STAT pathway | [41] | |
| Liver cancer | Both | HCCLM6, Huh7, PLC/PRF/5, L-O2 | low expression | MEX3C | Restoration of SOCS3 expression significantly reversed the enhanced ECM degradation, invasiveness, and motility promoted by MEX3C overexpression; it also mitigated MEX3C-induced tumor metastasis in vivo | [92] | |
| Both | Huh-7-EYA2 | low expression | EYA2 | JAK/STAT | Patients with HCC who have high expression of SOCS3 have a good prognosis | [119] | |
| Both | MIHA, HepG2, Huh7 | low expression | JAK/STAT | Upregulation of SOCS3 inhibited the proliferation rate of HCC cells, as well as the invasion and migration of HepG2 and Huh7 cells; overexpression of SOCS3 led to enhanced apoptosis | [120] | ||
| In Vitro | SMMC-7721, BEL-7404 | low expression | miR-650 | JAK/STAT3 | Low expression of SOCS3 in liver cancer patients may be associated with tumorigenesis and metastasis | [121] | |
| In vivo | low expression | FXR | STAT3 | Inhibited the proliferation of HCC cells | [122] | ||
|
In Vitro + Clinical |
Huh7, HepG2, PLC/PRF/5, l-02, HCCLM3, SMMC7721, Hep3B | low expression | CTCF | Inhibited the proliferation of HCC cells | [123] | ||
|
In Vitro + Vivo |
MIHA, HepG2, Huh7 | low expression | miR-221-3p | JAK/STAT | Inhibited the proliferation of HCC cells | [120] | |
| Colon cancer | Both | HEK 293 T, FHC, SW480, LS174T, HCT116 | low expression | DNMT1 | SOCS3 inhibits the proliferation, migration, and invasion of colon cancer cells | [80] | |
|
In Vitro + Clinical |
HCT116 | low expression | miR-183-5p | PD-L1 | SOCS3 inhibits the proliferation of colon cancer cells | [124] | |
| Colorectal cancer | Both | L-Wnt 3 A, DLD1, RKO, IMEC | low expression | miR-708 | SOCS3 can inhibit the development of CRC and indicates a better prognosis for CRC patients | [83] | |
|
In Vitro + Clinical |
HCT116, SW480, HT29 | low expression | microR-4449 | SOCS3 inhibits the proliferation of colon cancer cells | [125] | ||
| Thyroid cancer |
In Vitro + Clinical |
Nthy-ori-3, K1, BCPAP, FTC133, TPC1 | low expression | miR-221、miR-222 | STAT3 | Inhibits the proliferation of thyroid cancer cells | [126] |
| Osteosarcoma |
In Vitro + Clinical |
143B, MG63, SaOS-2, U2OS, hFOB1.19 | low expression | THAP9-AS1 | SOCS3 inhibits the number of tumor cells that migrate and invade | [74] | |
|
In Vitro + Vivo |
143B, Saos2, THP-1 | low expression | miR-221-3p | JAK2/STAT3 | Inhibition of the expression of downstream gene SOCS3, along with phosphorylation of STAT3, promotes the growth and invasion of OS cells | [84] | |
|
In Vitro + Vivo |
HFOB1.19, C28/I2, U2O, MG63 | low expression | miR-30d-5p | JAK2/STAT3 | Inhibits the proliferation of osteosarcoma cells | [127] | |
| In Vitro | hFOB 1.19, MG-63, 143B, Saos-2, U-2 OS, HOS | low expression | circ_ANKIB1 | Inhibits the proliferation of osteosarcoma cells | [89] | ||
| Cholangiocarcinoma | Both | HCCC9810, QBC939, RBE, HUCCT1, HiBEC | 中low expression | miR-30a-5p | SOCS3 may be a suppressor gene for CCA | [128] | |
| Ovarian cancer |
In Vitro + Vivo |
OVA | low expression | hsa_circ_0007874 | Having anti-proliferation and anti-metastasis effects | [129] | |
|
In Vitro + Vivo |
A2780, HO8910 | low expression | SPTBN1 | JAK/STAT3 | Inhibit the proliferation of ovarian cancer cells | [71] |
Increased SOCS3 expression within breast cancer cells effectively impedes tumor proliferation. Overexpression of SOCS3 promotes the proliferation, migration, and invasion capabilities of lung cancer cells [108]. Protein expression level of SOCS3 was frequently higher in tumor tissues than adjacent normal tissues. SOCS3 interacts with Nucleotide-binding oligomerization domain-containing protein 2(NOD2) and SOCS3 ubiquitinates NOD2 directly [108]. Overexpressed SOCS3 inhibits the expression of breast cancer stem cell and EMT-related genes, as well as STAT3 pathway activation, in both mice and humans [107]. Glycochenodeoxycholate promotes the metastasis of gallbladder cancer cells by inducing epithelial to mesenchymal transition via activation of SOCS3/JAK2/STAT3 signaling pathway [130]. High SOCS3 expression was more inclined to poor prognosis and was positively correlated with main immune cell infiltration in almost each cancer type, especially in colon cancer. Compared with the colon primary tumor, lung metastasis harbored higher CD163 and SOCS3 expression, and high SOCS3 expression was more likely to be associated with high CD163 expression in lung metastasis [20]. Provision of recombinant SOCS3 loaded within synthetic liposomes inhibited proliferation and survival of lung adenocarcinoma cells in vitro as well as malignant transformation of normal endothelial Cells (ECs) [129]. METTL14 silencing could inhibit IL-6-induced HaCaT cell viability, cell cycle progression and inflammation response, while SOCS3 overexpression also suppressed METTL14-induced HaCaT cell viability, cell cycle progression and inflammation [131].
Either SOCS3 silencing and activation of RhoA/Rock/STAT3 signaling dramatically restrained the regulatory roles of KLF14 overexpression in breast cancer invasion and M2 macrophages polarization [67]. Mechanically, eyes Absent Homolog 2 (EYA2) combined with Dachshund Homolog 1 (DACH1) to transcriptionally regulate SOCS3 expression, thus suppressing the progression of HCC via SOCS3-mediated blockade of the JAK/STAT signaling pathway [119]. Up-regulation of SOCS3, mediated by the overexpression of C1QTNF1-AS1 through down-regulation of miR-221-3p, inhibits HCC cell proliferation, migration, and invasion, and promotes apoptosis via the JAK/STAT signaling pathway [120]. SOCS3 was down-regulated in Intra cholangiocarcinoma (ICC) and Extrahepatic Cholangiocarcinoma (ECC) tissues and negatively regulated by miR-30a-5p. The inhibition of SOCS3 could largely rescue the inhibitory effect of miR-30a-5p inhibition on Cholangiocarcinoma (CCA) cells proliferation. In clinical, up-regulated miR-30a-5p expression was correlated with large tumor size in both ICC and ECC cohorts. MiR-665 was upregulated and SOCS3 was downregulated in gastric adenocarcinoma tissues and cells [128]. Downregulation of SOCS3 or miR-760 overexpression restored the migration and proliferation ability of SKOV3 or A2780 cells overexpressing has-circ0007874. Downregulation of SOCS3 restored the proliferation and migration in miR-760 knockdown SKOV3 and A2780 cells [117].
Expression of SOCS3 in Tumor Microenvironment and Its Functional Impact
SOCS3 in Dendritic Cells
Dendritic cells (DCs) are a heterogeneous population of leukocytes, consisting of distinct subsets, whose potent ability to initiate and regulate adaptive immune responses is fundamental to successful antitumor immune responses [132]. Tumor-specific immune responses are initiated, programmed, and regulated by DCs, which have the unique ability to capture antigens and subsequently process them into peptides. These peptides are then presented to naive T cells in lymphoid tissues via major histocompatibility complex molecules [133].
SOCS3 plays a role in regulating the tolerogenic capacity of DCs. High levels of SOCS3 in DCs inhibit the expression of PD-L1 on DCs, thereby driving T cell proliferation [70]. High levels of SOCS3 in DCs dampen PD-L1 expression on DCs, which in turn drives T-cell proliferation [70]. Tyrosine-phosphorylated ITIMs will bind the SOCS3, which drives indoleamine 2,3-dioxygenase 1 (IDO1) proteasomal degradation and shortens the enzyme half-life [134].
Increased cytokine IL-10 in the immune microenvironment has been reported to increase SOCS3 expression and IL-10 secretion by DCs, which prolong Treg cell function. Treg cells, in turn, contribute to T-cell exhaustion, which mount ineffective immune responses against cancer. SOCS3 + DCs also direct T-cell polarization towards a Th2 phenotype [13]. Additionally, T-cell effector responses can be hampered by tolerogenic DCs, as antigen presentation in SOCS3 + DCs is disrupted in tumors, with lower expression of MHC class II and co-stimulatory molecules [135]. Along with a higher expression of the DC maturation markers CD40, CD86 and MHC-II, IL-6/STAT3 is markedly upregulated in the SOCS3 siRNA-treated DCs after exposure to C. albicans as compared with control DCs. This enhanced Th17 cell differentiation induced by siSOCS3-treated DCs in presence of C. albicans can be partly offset when anti-IL-6 antibody is added into the co-culture [136]. Suppression of SOCS3 alone also has the potential to fully activate DCs maturation. SOCS3 silencing particularly increases Th17 differentiation. Socs3 deficiency in DCs also promotes induction of Foxp3 + Tregs, which is dependent on higher production of TGF-β from Socs3 −/− DCs [130].
SOCS3 operate in negative feedback loops to terminate cytokine signaling through JAK/STAT pathway, which regulates neuronal growth and immune cell differentiation (e.g., neuronal cells, T cells and DCs). Also, SOCS3 has been assumed to decrease the antigen-presenting function of DCs. While SOCS3 binds to gp130, a common receptor for signal transduction via interleukin-6 (IL-6), or JAK1 and JAK2, the signal transduction for DCs activation are then subsequently inhibited. The host immune system includes negative feedback mechanisms to modulate immune response, of which DCs use SOCS to regulate the strength of immune response by a variety of cytokine activations and antigen-presenting processes. Silencing of SOCS3 improves the diffusion capacity of DCs. DC diffusion to the lymph nodes is an indispensable step in immune surveillance. In the lymph nodes, DCs make contact with a vast repertoire of naïve Agspecific T cells and mediate T cells through an increased expression of CCR7 and decreased expression of CCR6. The mechanisms underlying the promotion of DC migration by SOCS3 silencing might be due in part to the induced expression of CCR7. SOCS3 silencing promotes the secretion of IL-6, IL-17 in DCs that leads to an IL-6-mediated Th17 cell proliferation [136].
SOCS3 in Myeloid-Derived Suppressor Cells
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of pathologically activated cells with potent immunosuppressive properties [137]. In the context of cancer, MDSCs are abnormally generated and recruited to the TME, contributing to the establishment of an immunosuppressive microenvironment that promotes tumor immune evasion [138]. MDSCs are a critical component of the immunosuppressive network [139]. SOCS3, a known feedback inhibitor of the JAK/STAT3 signaling pathway, participates in the differentiation of early myeloid-derived suppressor cells (eMDSCs) [140]. The generation of eMDSCs is dependent on SOCS3 deficiency and is associated with differentiation arrest in the myeloid lineage [56]. Sustained inhibition of SOCS3 and abnormal hyperactivation of the JAK/STAT signaling pathway promote the insitu accumulation of eMDSCs and their immunosuppressive capacity in vitro [140].
The CCL5/CCR5 axis has been shown to be involved in MDSC recruitment, and blocking this axis can reduce the content of granulocytic myeloid-derived suppressor cells (G-MDSCs) in tumor tissues [141]. Gu et al. found that SOCS3 significantly inhibits CCL5 levels, and high expression of SOCS3 in breast cancer cells can inhibit tumor growth and effectively hinder MDSCs, particularly G-MDSCs [65]. The overexpression of miR-9 in breast cancer cells significantly increased the recruitment of G-MDSCs to the tumor microenvironment. SOCS3 was identified as direct targets of miR-9, and it was confirmed that miR-9 recruited G-MDSCs by activating the CCL5/CCR5 signaling axis. MiR-9 can promote chemotactic enrichment of G-MDSCs in tumors through the SOCS3/CCL5/CCR5 signaling axis, thereby promoting breast cancer proliferation. SOCS3 inhibit tumor proliferation and MDSC recruitment [60]. SOCS3 deletion in myeloid cells produced higher levels of CD11b + Gr-1 + MDSCs in prostate tumors. The studies about MDSCs in prostate cancer demonstrated that SOCS3 negatively regulated the development and function of MDSCs by inhibiting STAT3 activation. Moreover, SOCS3-deficient mice elevated Gr-1 + CD11b + MDSCs in tumors and exhibited heightened STAT3 activation. Besides, it was previously demonstrated that significant suppression of SOCS3 in human e-MDSCs induced persistent activation of the JAK/STAT signaling pathway and expression of downstream functional genes, such as IDO, IL-10, and TGF-β, which produce immunosuppressive microenvironments locally [140].
SOCS3 in Cancer-Associated Fibroblasts
Over the past decade, the concept of cancer-associated fibroblasts (CAFs) as immunosuppressive cells has been widely accepted. CAFs are the most prominent and abundant cell population in the TME, accounting for nearly 70% of cells in tumor tissues [142]. CAFs have a wide range of functions, such as secreting inflammatory ligands, growth factors, and extracellular matrix (ECM) proteins, thereby enhancing drug resistance, immune antagonism, and tumor development [143]. In addition, CAFs actively participate in cancer progression through complex interactions with other cell types in the TME [144].
The cytokine leptin, an important regulator of crosstalk between breast cancer cells and CAFs, is associated with the development of breast cancer. The farnesoid X receptor (FXR) exerts tumor-suppressive effects in various tumors. FXR agonist GW4064, which inhibits leptin signaling synthesis, affects the tumor-promoting activity of CAFs in breast malignancies. These effects depend on the ability of activated FXR to increase SOCS3 inhibitor expression, thereby inhibiting leptin-activated signaling and downregulating leptin target genes. Studies using MCF-7 cells and in vivo xenotransplantation showed that GW4064 administration significantly reduced tumor growth. Interestingly, tumors treated with GW4064 exhibited strong SOCS3 staining intensity, suggesting that FXR ligands may represent a novel potential anticancer therapy by blocking the tumor-supporting role of activated fibroblasts in the breast microenvironment through SOCS3 [145]. SPTBN1 exerts anticancer effects in various tumors and is involved in chemotherapy resistance in epithelial ovarian cancer (EOC). Studies have found that SPTBN1 and SOCS3 are co-expressed positively in EOC patients. Overexpression of SOCS3 or JAK2 inhibition reduces the proliferation and migration of EOC cells, as well as the expression of p-JAK2, p-STAT3, and vimentin, which is enhanced by downregulating SPTBN1. Additionally, the expression of E-cadherin is reversed. The absence of SPTBN1 in MEFs activates the JAK/STAT3 signaling pathway while inhibiting SOCS3. SPTBN1 inhibits EOC progression by blocking the SOCS3-mediated JAK/STAT3 signaling pathway [71].
SOCS3 on Macrophages
Tumor-Associated Macrophages (TAMs) are crucial components of the TME. Cytokines and chemokines released through interactions between TAMs and tumor cells significantly stimulate anti-apoptotic, hyperproliferative, and metastatic responses [146]. TAMs can be categorized into three subpopulations: classical subtype M0 (non-polarized or neutral), M1 (anti-tumor), and M2 (tumor-promoting) macrophages. SOCS3 expression was positively correlated with macrophages and neutrophils, but negatively correlated with B cells and tumour purity [147].
SOCS3 as a regulator of macrophage activation and M1 polarization via the STAT pathway, and indicate a protective influence of SOCS3 on macrophage inflammatory responses. SOCS3 is implicated in regulating innate immune responses and in attenuating pro-inflammatory cytokine signals, leading to inhibition of STAT activation. SOCS3 as a modulator of macrophage polarization, and show that SOCS3 deficiency skews macrophages towards the M1 phenotype [148]. In M1-activated human monocyte-derived macrophages, SOCS3 silencing, using short interfering RNA technology, resulted in a decreased expression of proinflammatory markers and an increased expression of M2 macrophage markers [149].
Enhanced infiltration of M2-like macrophages can induce tumor metastasis and exacerbate cancer progression [100]. Jun Ni et al. found that overexpression of SOCS3 prevented miR-191-5p-transfected macrophages from undergoing M2-like polarization, and SOCS3-transfected macrophages promoted breast cancer cell migration and invasion in a positive feedback manner [78]. Studies have shown that the use of exosomes derived from epidermal stem cells (EpiSC EXO) reduced SOCS3 expression. The abundant miR-203a-3p present in EpiSC EXO specifically binds to SOCS3 and activates the JAK2/STAT3 signaling pathway to induce M2 macrophage polarization [150]. The SOCS3/STAT3 signaling pathway has been identified as participating in upregulating MFG-E8-induced M2 polarization [151]. Exosomal miR-1290 derived from low-oxygen lung adenocarcinoma (LUAD) cells activates the STAT3 signaling pathway by targeting SOCS3 to promote M2 macrophage polarization [109]. Extracellular vesicles derived from M2 macrophages containing miR-501-3p regulate the SET domain, which contains the axis of SET7/DNA methyltransferase 1/SOCS3, to promote the occurrence of colon cancer [80]. EOC-derived exosomal miR-222-3p can downregulate SOCS3 in macrophages and activate the SOCS3/STAT3 signaling pathway, thereby inducing macrophage translation into the M2 phenotype and promoting the progression of EOC [106]. It was reported that SOCS3 inhibits STAT3 activity, preserving the characteristics of M1-type macrophages, and without the presence of SOCS3, macrophages are polarized to the M2 phenotype. MiR-1290 promotes M2 macrophage polarization through regulation of the SOCS3/STAT3 pathway [109]. Recently published data suggest that SOCS3-deficient mouse macrophages are converted to alternatively activated M2-type [152]. In macrophages, Socs3 deficiency resulted in the conversion of the effects of IL-6 to those of IL-10, which is a potent inhibitor of macrophages and DCs [153].
SOCS3 in T cells
Helper T (Th) cells are the largest subset of T cells and an essential component of the TME. Th cells play a pivotal role in regulating adaptive immune responses in epithelial tissues by secreting various cytokines that attract and regulate the functions of various other immune cells [154]. Regulatory T cells (Tregs) can suppress antitumor immunity in two stages, impeding immune surveillance against cancer development and hindering the host's effective antitumor immune response [155]. Tregs are the"culprits"that help tumor cells evade human immune surveillance. Tumor cells can use Tregs to achieve immune evasion. SOCS3 inhibits the JAK-STAT pathway, enhancing the efficacy of oncolytic adenoviruses by promoting viral replication and T cell activation [156]. The interaction between Th17 cells and Tregs is crucial in regulating autoimmunity and cancer [157]. High expression of SOCS3 has been shown to inhibit Th17 cell differentiation and reduce IL-17 production [158]. Overexpression of SOCS3 reverses miR-22-induced Tregs recruitment to tumor cells, and clinically, the gradually increasing Tregs infiltration during advanced skin squamous cell carcinoma progression is negatively correlated with SOCS3 abundance [159].
The deletion of the SOCS3 in T cells potentiates anti-tumor immune responses by conferring the anti-tumorigenic function of IL-6. In Socs3-deficient CD8 + T cells, IL-6 upregulates the expression of type I interferon (IFN)-regulated genes and enhances the anti-tumor effector function of T cells. SOCS3 knockdown in human chimeric antigen receptor T (CAR-T) cells exhibits a strong anti-tumor response in humanized mice. The downregulation of Tcf7, a key transcription factor for maintaining stemness, in Socs3 −/− CD8 cells contributes to the accelerated differentiation toward effector cells. It should be noted that the deletion of SOCS3 does not prevent CD8 T cells from exhaustion, as evidenced by the continuous growth of B16-OVA tumors even after transfer of SOCS3-deficient T cells [158]. SOCS3 has been shown to negatively regulate IL-23-mediated STAT3 phosphorylation and Th17 polarization, which could bind to both the IL-17A and IL-17F promoter [160].
SOCS3 in T cells regulates Th1/2/17 differentiation. SOCS3 also suppresses Th17 development because SOCS3 inhibits STAT3, which is essential for Th17 development.
STAT3 induce both SOCS3 and retinoic acid receptor-related orphan nuclear receptor γ (RORγt) to initiate Th17 cell differentiation which is inhibited by SOCS3, a negative feedback regulator of STAT3 [153]. T cell specific Socs3 deletion protected mice from both chronic and acute experimental autoimmune uveitis (EAU) through an increase in regulatory T cells [161]. Increased SOCS3 expression promotes (1) IL-4-producing T-cell clones, (2) IL-17-producing primary T cells, T-cell clones, and TH17 precursor cells, and (3) decreased proliferation of T cells in vitro. IL-7-induced inhibition of SOCS3 expression leads to reduced proportions of IL-17-producing TH17 cells [162]. In addition to CD4 T helper cells, SOCS3 also affects Foxp3 + Treg cells. Retroviral expression of SOCS3 constrained the effector function and expansion of Treg cells, demonstrating an inhibitory effect of SOCS3 on Treg cell. Thus, SOCS3 interferes with Treg cell differentiation and function [163]. Enhanced action of SOCS3 may promote allergic responses, because a recent analysis has indicated that transgenic SOCS3 expression in T cells inhibits Th1 development and promotes Th2 development. Enhanced Th2 development could be due to suppression of Th1 because IL-12 mediated Th1 differentiation is impaired by SOCS3 overexpression [31]. Tumor-derived IL-6 impaired the differentiation of myeloid cells and promoted the accumulation of e-MDSCs by inhibiting SOCS3 expression and persistently activating the JAK/STAT signaling pathway. IL-6-related dysfunction of the SOCS3 feedback loop accelerated the growth and metastasis of mammary carcinoma by affecting myeloid differentiation and attenuating the T cell-based immune surveillance [140]. Additionally, SOCS3 acts as an inhibitor of cytokine signaling, governing the differentiation of CD4 + T cells and the development of CD8 + T cells, alongside its role in modulating innate immune cells and influencing tumorigenesis [164].
NK Cells
Natural killer cells (NK cells) are cytotoxic lymphocytes that accumulate in the tumor microenvironment and are generally considered to have antitumor effects [165]. A study observing tumor samples of triple-negative breast cancer found a unique Socs3-high CD11b-CD27-immature NK cell subset [166]. In acute lymphoblastic leukemia, SOCS3 mRNA expression is significantly reduced, while overexpression of SOCS3 may upregulate the expression of NKG2D ligands MICA and MICB on the surface of Jurkat cells by negatively regulating the JAK/STAT signaling pathway, thereby promoting the killing ability of NK cells [167]. IL-17A inhibits IL-15-induced STAT5 phosphorylation by upregulating SOCS3 in NK cells, thereby inhibiting the terminal maturation of NK cells and limiting their antitumor and antiviral activities, thus promoting tumorigenesis and metastasis [168]. In Esophageal carcinoma (ESCA), SOCS3 was significantly related to macrophages, the infiltration score, and NK cells [105]. Ad-SOCS3 (40 MOI) could suppress IL-6 production in DU-145 cells and PD-L1 expression induced by IFN-γ in TRAMP-C2 cells, and increased the NK cell sensitivity of Prostate cancer cells [169]. Ad-SOCS3 therapy extended the lifespan of NK cells infiltrating Castration-Resistant Prostate Cancer (CRPC) tumors, thereby boosting their ability to fight against the tumor [170].
SOCS3 in Immune Modulation within the Tumor Microenvironment
SOCS3 is a crucial regulatory molecule that modulates numerous cytokine signals within the body, playing a pivotal role in various physiological regulatory processes [106]. The regulatory mechanisms of SOCS3 are primarily mediated through its interactions with key signaling pathways and cytokine receptors, which in turn influence immune cell function and tumor progression [171]. In breast cancer, IL-6-related dysfunction in the SOCS3 feedback loop accelerates tumor growth and metastasis by influencing myeloid differentiation and weakening T-cell-based immune surveillance [140]. This occurs because SOCS3 normally inhibits the JAK/STAT pathway, which is activated by IL-6. When SOCS3 is downregulated, IL-6 signaling becomes uncontrolled, leading to increased STAT3 activation [140]. This uncontrolled activation promotes tumor growth and suppresses antitumor immune responses [140]. In HCC, decreased SOCS3 expression leads to reduced IL-10 and TGF-β levels, which are crucial for maintaining anti-inflammatory mechanisms [172]. The absence of SOCS3 allows for sustained STAT3 activation, which promotes tumor cell survival and proliferation while inhibiting immune surveillance [172]. In gastric cancer, restoration of SOCS3 expression via gene delivery methods effectively inhibits STAT activity, thereby modulating cancer cell proliferation, apoptosis, migration, invasion, and epithelial-mesenchymal transition (EMT) [173]. This highlights the importance of SOCS3 in maintaining a balanced cytokine network within the tumor microenvironment [87]. Additionally, in colon cancer, PCSK9 functions as an oncogene by downregulating SOCS3 expression and activating the JAK2/STAT3 signaling pathway This downregulation of SOCS3 allows for uncontrolled cytokine signaling, promoting tumor development and progression [174]. CCR5 blockade suppresses melanoma development through inhibition of IL-6-Stat3 pathway via upregulation of SOCS3 [175]. SOCS3 also plays a critical role in regulating Toll-like receptor (TLR) and IL-1 signaling in myeloid cells (e.g., macrophages) by inhibiting the TNF receptor-associated factor 6 (TRAF6)-TGF-β/activated kinase 1 (TAK1) transcription complex [176]. This inhibition modulates the negative regulation of IL-6 signaling induced by TLR signaling. Furthermore, SOCS3 differentially affects IL-6 and IL-10 signaling, both of which are STAT3-dependent. SOCS3 can bind to the IL-6 receptor (IL-6R) and suppress STAT3 function, thereby inhibiting IL-6 signaling while allowing IL-10 signaling to proceed [177]. Increased abundance of SOCS3 interfered with γc cytokine signaling, including IL-7 and IL-2, resulting in impaired thymopoiesis—which depends on IL-7, and diminished generation of Foxp3 + CD25 + Treg cells—which requires IL-2, respectively [163].
SOCS3 in Immune Modulation
SOCS3 plays a crucial role in modulating systemic immune responses by interacting with multiple signaling pathways, such as JAK/STAT [20], PI3K/AKT [178], and NF-κB [179]. These pathways collectively influence the function of various immune cells, thereby shaping the overall immune response [180]. SOCS3 is a critical regulator of the JAK/STAT signaling pathway, which is central to cytokine signaling and immune cell activation [156]. The SH2 domain of SOCS3 binds to phosphorylated tyrosine residues on JAKs or cytokine receptors, directly inhibiting JAK kinase activity via its KIR [13]. This inhibition prevents the activation of downstream STAT proteins, thereby modulating immune responses. For instance, in T cells, SOCS3 inhibits the JAK/STAT pathway, reducing the production of pro-inflammatory cytokines such as IFN-γ and IL-17, and promoting the differentiation of Tregs [158]. In macrophages, SOCS3 suppresses the JAK/STAT pathway, limiting the production of pro-inflammatory cytokines like TNF-α and IL-6, and promoting an anti-inflammatory phenotype [149]. This dual role of SOCS3 in T cells and macrophages underscores its importance in balancing immune responses and preventing excessive inflammation. SOCS3 also modulates the PI3K/AKT pathway, which is crucial for cell survival, proliferation, and metabolism. By inhibiting the PI3K/AKT pathway, SOCS3 can influence the function of immune cells such as MDSCs. In breast cancer, SOCS3 deficiency leads to sustained activation of the PI3K/AKT pathway in MDSCs, enhancing their immunosuppressive function and contributing to tumor progression [140]. Conversely, overexpression of SOCS3 can inhibit the PI3K/AKT pathway, reducing the immunosuppressive activity of MDSCs and enhancing antitumor immunity. This highlights the potential of SOCS3 as a therapeutic target for modulating the immune microenvironment in cancer. The NF-κB pathway is a key regulator of inflammation and immune cell activation. SOCS3 can modulate the NF-κB pathway, influencing the production of inflammatory cytokines and the activation of immune cells. In DCs, SOCS3 inhibits the NF-κB pathway, reducing the expression of co-stimulatory molecules such as CD80 and CD86, and limiting T cell activation [136]. This regulation is crucial for maintaining immune homeostasis and preventing excessive inflammation within the tumor microenvironment. Additionally, SOCS3's modulation of the NF-κB pathway in macrophages can influence their polarization state, shifting them towards an anti-inflammatory phenotype. The anti-tumorigenic impacts of SOCS3 can be partly exerted by controlling the MAPK pathway [170]. The p44/p42 MAPK pathway was activated in prostate cancer cells in the presence of fibroblast growth factor-2 (FGF-2) [181]. In prostate cancer cells, SOCS3 limited the FGF-2-related signaling pathway and prevented the phosphorylation of p44/p42 MAPK resulting in a decrease in prostate cancer cell proliferation [181]. Decreased SOCS3 expression increased MAPK phosphorylation and cell proliferation, while SOCS3 overexpression decreased cell proliferation and migration [181].
Potential of SOCS3 as a Therapeutic Target for Malignant Tumors
The investigation of novel therapeutic targets within the intricate landscape of the tumor microenvironment constitutes a pivotal frontier in oncology research. SOCS3, a central regulator of cytokine signaling pathways, has been identified as a promising candidate in this context [182]. Correlations between its expression levels and the prognosis of various malignancies indicate that SOCS3 may serve a dual function as both a tumor suppressor and an immune response modulator [46]. This section examines the evidence supporting SOCS3 as a viable therapeutic target, evaluates the relationship between its expression and tumor outcomes, and explores therapeutic strategies aimed at targeting SOCS3 to augment anti-tumor immunity and enhance treatment efficacy (Table 2).
Table 2.
The Role of SOCS3 in Targeted Therapy
| Type of cancer | In Vitro/In Vivo/ Clinical |
Combination | Mechanism of Action | Reference |
|---|---|---|---|---|
| T-cell neoplasms |
In Vitro + Clinical |
Ruxolitinib + SOCS3↑ | JAK/STAT↓ | [41] |
| AML | In Vitro | TQ + SOCS3↑ | JAK/STAT↓ | [183] |
| HCC | In Vitro | SOCS3↓ + pomegranate | IL-6-STAT3 ↓ | [184] |
| In Vivo | OCA + SOCS3↓ | STAT3↓ | [122] | |
|
In Vitro + In Vitro |
Arsenic and benzo[a]pyrene + SOCS3↓ → | Akt, Erk1/2 ↑ | [121] | |
| BC | In Vivo | UTMD + SOCS3↑ | STAT↓ | [107] |
|
In Vitro + In Vitro + Clinical |
KLF14 + SOCS3↑ | RhoA/Rock/STAT3 ↓ | [67] | |
| CRC | In Vivo | AhR + SOCS3↑ | IL22↓ | [69] |
| In Vivo |
Evolocumab + SOCS3↑ PCSK9 + SOCS3↓ |
PCSK9↓; JAK2/STAT3↑ | [174] | |
|
In Vitro + Clinical |
FU and OXA + SOCS3↓ | PD-L1↓ | [124] | |
|
In Vitro + In Vivo |
OCA + SOCS3↑ → | JAK2/STAT3↓ | [185] | |
| PDAC |
In Vitro + Clinical |
Gemcitabine + SOCS3↓ → | EZH2↑ | [111] |
Correlation of SOCS3 Expression with Tumor Prognosis
Studies have demonstrated that SOCS3 is an independent prognostic factor for patients with HCC [172]. HCC is a leading cause of cancer-related deaths, primarily caused by chronic inflammatory responses. As damage progresses, Socs3 expression decreases, and further reductions in IL-10 and TGF-β indicate disruption of anti-inflammatory mechanisms. Differential regulation of cytokine inhibitors and inflammatory cytokines may play a role in the initiation and progression of liver injury leading to HCC [172]. High methylation of SOCS3 is associated with HBV-related HCC, with SOCS3 hypermethylation in tumor tissues being much higher than in non-tumor tissues, suggesting that SOCS3 may be a therapeutic target for HCC [72]. In addition, SOCS3 is significantly downregulated in glioma tissues and is significantly correlated with World Health Organization (WHO) grade, Karnofsky Performance Status (KPS) score, and glioma tumor size [118]. Analysis of human patient genomic databases indicates that SOCS3 expression serves as a positive prognostic marker for breast cancer [186]. High levels of SOCS3 correlate with early tumor stages and favorable clinical outcomes in breast cancer. Research shows that reduced SOCS3 mRNA expression levels in breast cancer tissue samples are associated with improved relapse-free survival rates [187] and indicate favorable prognoses for breast cancer patients [88]. SOCS3 is lowly expressed in HCC, and high SOCS3 serves as a better predictive marker for HCC patients [188]. Low SOCS3 expression is an indicator of poor prognosis in gastric cancer [189].
Therapeutic Strategies Targeting SOCS3
SOCS3 is a prognostic gene for gliomas, which is increased in glioblastomas but also exerts tumor-suppressive effects. Inhibition of SOCS3 using siRNA reduces the proliferation of GBM cell lines [52]. The siRNA-mediated silencing of gene expression has shown promise for the development of novel therapeutic strategies to treat autoimmune diseases. Delivery of siRNA targeting SOCS3 can promptly and effectively activate DCs and yet avoid an overlong activation of those DCs [136]. Furthermore, KLF14 positively activates SOCS3 transcription, subsequently blocking the activation of the RhoA/Rock/STAT3 signaling pathway [67]. The silencing of SOCS3 and the activation of the RhoA/Rock/STAT3 signaling pathway significantly inhibit the regulatory effects of KLF14 overexpression on breast cancer invasion and M2 macrophage polarization [67]. By modulating the SOCS3/RhoA/Rock/STAT3 signaling, KLF14 collaboratively suppresses breast cancer cell invasion and M2 macrophage polarization, offering a potential new therapeutic target for cancer [67]. IL-6R Ab effectively reversed SOCS3 suppression, recovered T cell immunity, and abolished IL-6-related differentiation block of myeloid cells in vitro and vivo. Consistently, CCR5 blockade inhibited IL-6-STAT3 pathway via SOCS3, and anti-CCR5 antibody treatment could inhibit the growth of tumor via upregulated SOCS3 to decrease MDSCs accumulation and immunosuppressive capacity in vivo. Therefore, it may be a practical scheme to upregulate the SOCS3 expression to inhibit MDSCs accumulation and reverse immunosuppression [140]. CCR5 blockade also induces SOCS3 upregulations, and anti-CCR5 antibody fails to suppress expression of p-Stat3, MMP9, and IL-6 in cells transfected with SOCS3 SiRNA. Thus, CCR5 blockade suppresses melanoma development through inhibition of IL-6-Stat3 pathway via upregulation of SOCS3 [175].
SOCS3KDCAR-T cells exhibited superior control of target cells compared with WT CAR-T cells. Thus, the deletion of SOCS3 in T cells confers an anti-tumorigenic role to IL-6, a cytokine that typically promotes tumor growth. This approach shows potential for cancer therapy and expands the possibilities in the field [158]. IL-6R Ab effectively reversed SOCS3 suppression, recovered T cell immunity, and abolished IL-6-related differentiation block of myeloid cells in vitro and vivo. It may be a practical scheme to upregulate the SOCS3 expression to inhibit MDSCs accumulation and reverse immunosuppression [140]. The synthetic FXR agonist GW4064, inhibiting leptin signaling, affects the tumor-promoting activities of CAFs in breast malignancy. GW4064 inhibited growth, motility and invasiveness induced by leptin as well as by CAF-conditioned media in different breast cancer cell lines. These effects rely on the ability of activated FXR to increase the expression of the SOCS3 leading to inhibition of leptin-activated signaling and downregulation of leptin-target genes [145]. PF act via enhancing SOCS3 to inhibit STAT3/PD-L1 signaling and subsequently restore T cell sensitivity to kill tumor cells. The current findings support the potential use of PF as an immune checkpoint inhibitor targeting PD-L1 to prevent HCC development [162].
Preclinical Studies of SOCS3-Targeted Therapies
Ultrasound-Targeted Microbubble Destruction (UTMD), a novel gene delivery method and targeted drug delivery system, has enhanced the transfection efficiency of SOCS3 [107]. UTMD and liposome-mediated SOCS3 have reduced the viability, proliferation, migration, and invasion of Breast Cancer Stem Cells (BCSCs), arrested the cell cycle, inhibited the formation of spheroids, and delayed tumor growth in mice [107]. UTMD-mediated SOCS3 exhibits promising therapeutic effects on breast cancer, providing new experimental evidence for its treatment [107]. IL-22 signaling plays a vital role in maintaining gastrointestinal epithelial barrier function, cell proliferation, and protecting intestinal stem cells from genotoxic insults. The aryl hydrocarbon receptor (AhR), a ligand-activated transcription factor, promotes IL-22 production in intestinal immune cells by regulating SOCS3 [69]. IL-22 treatment induces STAT3 phosphorylation, inhibits colonic organoid growth, and promotes colonic cell proliferation in vivo [69]. The absence of SOCS3 increases pSTAT3 levels in intestinal cell-specific AhR knockout organoids and phenocopies the effects of IL-22 treatment on wild-type (WT) organoid growth. AhR regulates colonic epithelial cell responses to IL-22 by upregulating SOCS3 expression, thereby inhibiting carcinogen-associated colon tumorigenesis, providing a rationale for targeting AhR as a means to improve inflammatory bowel disease (IBD) and reduce colon cancer risk [69]. Piperlongumine (PL) upregulates the protein levels of SOCS3 inhibitors, inactivates the JAK2/STAT3 pathway, and decreases miRNA-30d-5p levels, inhibiting osteosarcoma cell growth, migration, invasion, epithelial-mesenchymal transition, and promoting apoptosis [127]. Under hypoxic conditions, exosomes secreted by hypoxia-induced pancreatic stellate cells deliver lncRNA UCA1 to pancreatic cancer cells, where UCA1 recruits EZH2 and modulates histone methylation levels in the SOCS3 gene region, enhancing pancreatic cancer resistance to gemcitabine [111]. STAT3 induce both SOCS3 and RORγt to initiate Th17 cell differentiation which is inhibited by SOCS3, a negative feedback regulator of STAT3. HO-1 induction as a potential therapeutic strategy for EA treatment by reducing STAT3 phosphorylation, STAT3-SOCS3-mediated Th2/Th17 immune responses, and ultimate allergic airway inflammation [190]. KLF14 suppressed breast cancer cell invasion and M2 macrophage polarization through modulating SOCS3/RhoA/Rock/STAT3 signaling, and these findings would provide a new potential target against breast cancer [67]. MiR-455-5p promotes breast cancer progression by targeting the SOCS3 pathway and may be a potential therapeutic targ benzo[a]pyrene et for breast cancer [79]. MiR-19b-3p facilitates glioma progression via activation of the JAK-STAT signaling pathway by targeting SOCS3, highlighting a novel therapeutic target for glioma treatment [52]. Linc00893 suppresses the progression of PCa cells through targeting miR-3173-5p/SOCS3/JAK2/STAT3 axis. Our data uncovers a novel tumor-suppressor role of Linc00893 in PCa, which may serve as a potential strategy for targeted therapy in PCa [114]. Obeticholic acid (OCA) promoted SOCS3 transcription by enhancing the binding of FXR to the FXRE/IR9 of the SOCS3 promoter. This research demonstrates that targeting FXR and improving its function might be a promising strategy for CRC treatment [185]. Studies have shown that Thymoquinone (TQ) significantly enhances the re-expression of SHP-1 and CSS-3 through de-methylation, inhibits the enzyme activity of JAK/STAT signaling, and thus inhibits MV4-11 cells, and may be a promising therapeutic candidate for patients with AML [183].
Clinical Studies of SOCS3-Targeted Therapies
Administration of pomegranate peel extract (PPE) components to significantly reduces SOCS3 levels in HCC, slowing tumor growth. In HCC, treatment with PPE significantly slowed the proliferation of human liver cancer cell line HepG2, markedly reduced SOCS3 levels, induced apoptosis by inhibiting MAPK, JAK/STAT3, β-catenin/NOTCH, and SOCS3 signaling pathways, and sensitized HCC cells to ionizing radiation, demonstrating considerable therapeutic potential against HCC [184]. Evolocumab, a selective inhibitor of PCSK9, inhibits intestinal adenoma formation and oncogenesis in antigen-presenting cells [83]. miR-183-5p upregulates PD-L1 by targeting and downregulating SOCS3 expression. Combined treatment with Fluorouracil (FU) and Oxaliplatin (OXA) significantly downregulates miR-183-5p expression, subsequently downregulating SOCS3 and PD-L1, inhibiting malignant biological behaviors [124]. OCA may attenuate the development and progression of nonalcoholic steatohepatitis (NASH)-dependent HCC by disrupting the SOCS3/Jak2/STAT3 pathway, suggesting the potential use of FXR agonists in NASH-related diseases, even in later stages, to prevent progression to more aggressive HCC [122]. A combination of Ad-SOCS3 gene therapy and NK cell immunotherapy could be a powerful treatment option for advanced CRPC overexpressing pSTAT3 [170]. Intratumoral injection of SOCS3 liposomes attenuated tumor growth in a lung cancer xenograft model. This work identifies AM-derived vesicular SOCS3 as an endogenous antitumor mechanism that is disrupted within the tumor microenvironment and whose rescue by synthetic liposomes can be leveraged as a potential therapeutic strategy for lung cancer [129].
Quercetin (QU) inhibits Th17 cell differentiation by targeting the peroxisome proliferator-activated receptor γ to regulate the SOCS3/STAT3 axis, exerting an anti-arthritic effect [191]. Paeoniflorin may inhibit STAT3/PD-L1 signaling by enhancing SOCS3, increasing CD8(+) T cells to trigger T cell-mediated antitumor immune responses and restoring T cell sensitivity to kill tumor cells [49]. Qinzhu Liangxue (QZLX) could significantly enhance the expression level of SOCS3, while inhibit the level of METTL14, and p-STAT3/STAT3. In addition, QZLX inhibits METTL14-induced HaCaT cell viability, cell cycle progression, and inhibits the level of IL-1β, IL-6, and IL-8 [131].
The critical role of patient demographics and genetic backgrounds in shaping the efficacy of SOCS3-targeted therapies is highlighted by recent studies. In a study on colon cancer, the combination of FU and OXA was shown to suppress tumor progression through the miR-183-5p/SOCS3 axis and downregulation of PD-L1. The study included 40 patients with an average age of 56.25 years, with 70% being male and 30% being female. Tissue specimens were obtained from CRC patients who were admitted to Yunnan Cancer Hospital from August 2018 to December 2019. The patients did not receive chemotherapy or radiotherapy before surgery. The results indicated that the combination therapy significantly reduced tumor size and improved overall survival rates. However, further analysis revealed that younger patients (< 50 years) and those with lower baseline PD-L1 expression levels had better responses to the treatment, with a higher complete response rate (40%) compared to older patients (20%) and those with higher PD-L1 expression (10%). At the same time, miR-183-5p overexpression remitted PD-L1 downregulation induced by SOCS3 overexpression via targeting downregulated SOCS3. Further experiments found that silencing SOCS3 promoted cell proliferation and the expression of chemokines and immune escape related proteins, inhibited cell apoptosis and facilitated cells in G2/M phrase. Treating HCT116 cells which silencing SOCS3 with FU and OXA could restore the promotion effect of knockdown of SOCS3 on the malignant biological behavior of HCT116 cells [124]. Another study on prostate cancer investigated the effects of CRISPR/Cas9-mediated deletion of Interleukin-30 (IL-30) on Tumor growth and mortality. The study involved 198 patients with high-grade locally advanced prostate cancer, with a mean age of 65 years. The results showed that IL-30 deletion significantly reduced tumor size, inhibited tumor progression, and prolonged survival. Notably, patients with a high baseline expression of SOCS3 had a better prognosis, with a median progression-free survival (PFS) of 48 months compared to 24 months for those with low SOCS3 expression. Additionally, the study found that patients with a lower Gleason score (≤ 7) had a higher response rate (60%) to IL-30 deletion compared to those with a higher Gleason score (≥ 8) (30%) [50].
Conclusion
SOCS3 emerges as a pivotal regulator of cytokine signaling pathways and plays a crucial role in both tumor development and immune regulation. By modulating key signaling pathways such as JAK/STAT, SOCS3 can influence the activation, proliferation, and function of various immune cells, thereby shaping the TME. SOCS3's ability to modulate cytokine signaling places it at the intersection of immune regulation and tumor biology. Within the TME, SOCS3 can inhibit the activity of immunosuppressive cells, such as MDSCs and Tregs, while enhancing the function of antitumor immune cells, including CTLs and NK cells. This dual role underscores SOCS3's significance in balancing the immune response within the TME. Both in vitro and in vivo experiments have demonstrated the existence of different targeted drugs based on the role of SOCS3 in various signaling pathways and immune molecules across different cancers. Furthermore, the overexpression or silencing of SOCS3 in combination with chemotherapeutic drugs exerts varying effects on prognosis and survival rates among different tumor types. This review has provided a comprehensive overview of SOCS3's molecular structure, biological functions in tumors, underlying mechanisms, and its potential as a therapeutic target. We firmly believe that future research on the role of SOCS3 in tumors will positively impact the diagnosis and treatment of cancer.
As our understanding of SOCS3's role in tumor immunoregulation deepens, personalized treatment strategies based on individual patient characteristics, including genetic profiles and TME features, hold great promise. By analyzing the tumor genome, epigenome, and immune microenvironment, we can predict patient responses to SOCS3-targeted therapies and tailor treatment plans accordingly. Moreover, identifying biomarkers that predict therapeutic response will be essential for selecting patients who are most likely to benefit from SOCS3-targeted interventions, thereby enhancing treatment efficacy and safety.
In summary, SOCS3's critical role in immune regulation within the TME highlights its potential as a therapeutic target for cancer. Future research should focus on unraveling the complex interplay between SOCS3, immune cells, and tumor cells, optimizing SOCS3-targeted therapies, and exploring personalized treatment strategies to improve outcomes for cancer patients.
Acknowledgements
This work was supported by Shanghai 2021"Science and Technology Innovation Action Plan"Medical Innovation Research Special Project (21Y11923000); Shanghai "Rising Stars of Medical Talents” Youth Development Program: Youth Medical Talents-Specialist Program 2023-33).
Authors’ Contributions
Y. Z: Writing—original draft, Writing—review & editing, Investigation, Data curation. ML. C, MN Y, YL Y: Writing—review & editing, Supervision, Project administration, Conceptualization. HF C: Project administration, Conceptualization.
Funding
This work was supported by Shanghai 2021"Science and Technology Innovation Action Plan"Medical Innovation Research Special Project (21Y11923000); Shanghai "Rising Stars of Medical Talents” Youth Development Program: Youth Medical Talents-Specialist Program 2023-33).
Data Availability
No datasets were generated or analysed during the current study.
Declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent to Participate
The manuscript has been approved for publication by all authors.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yulian Yin, Email: killua018yyl@163.com.
Hongfeng Chen, Email: chhfluk@126.com.
References
- 1.Shiravand Y, Khodadadi F, Kashani SMA, et al. Immune Checkpoint Inhibitors in Cancer Therapy. Curr Oncol. 2022;29(5):3044–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gavas S, Quazi S, Karpiński TM. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res Lett. 2021;16(1):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lv B, Wang Y, Ma D, et al. Immunotherapy: Reshape the Tumor Immune Microenvironment. Front Immunol. 2022;13:844142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernstock JD, Kang KD, Klinger NV, et al. Targeting oncometabolism to maximize immunotherapy in malignant brain tumors. Oncogene. 2022;41(19):2663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17(8):807–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donisi C, Pretta A, Pusceddu V, et al. Immunotherapy and Cancer: The Multi-Omics Perspective. Int J Mol Sci. 2024 ;25(6). 10.3390/ijms25063563. [DOI] [PMC free article] [PubMed]
- 7.Yu Y, Zeng D, Ou Q, et al. Association of Survival and Immune-Related Biomarkers With Immunotherapy in Patients With Non-Small Cell Lung Cancer: A Meta-analysis and Individual Patient-Level Analysis. JAMA Netw Open. 2019;2(7): e196879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimozaki K, Nakayama I, Hirota T, et al. Current Strategy to Treat Immunogenic Gastrointestinal Cancers: Perspectives for a New Era. Cells. 2023;12(7). 10.3390/cells12071049. [DOI] [PMC free article] [PubMed]
- 9.Pan C, Liu H, Robins E, et al. Next-generation immuno-oncology agents: current momentum shifts in cancer immunotherapy. J Hematol Oncol. 2020;13(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salvagno C, Mandula JK, Rodriguez PC, et al. Decoding endoplasmic reticulum stress signals in cancer cells and antitumor immunity. Trends Cancer. 2022;8(11):930–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spranger S. Mechanisms of tumor escape in the context of the T-cell-inflamed and the non-T-cell-inflamed tumor microenvironment. Int Immunol. 2016;28(8):383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jafarzadeh A, Jafarzadeh Z, Nemati M, et al. The Interplay Between Helicobacter pylori and Suppressors of Cytokine Signaling (SOCS) Molecules in the Development of Gastric Cancer and Induction of Immune Response. Helicobacter. 2024;29(3):e13105. [DOI] [PubMed] [Google Scholar]
- 13.Morelli M, Madonna S, Albanesi C. SOCS1 and SOCS3 as key checkpoint molecules in the immune responses associated to skin inflammation and malignant transformation. Front Immunol. 2024;15:1393799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian N, Cheng H, Du Y, et al. Cannabinoid receptor 2 selective agonist alleviates systemic sclerosis by inhibiting Th2 differentiation through JAK/SOCS3 signaling. J Autoimmun. 2024;147:103233. [DOI] [PubMed] [Google Scholar]
- 15.Bidgood GM, Keating N, Doggett K, et al. SOCS1 is a critical checkpoint in immune homeostasis, inflammation and tumor immunity. Front Immunol. 2024;15:1419951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai L, Han Y, Yang Z, et al. Identification and validation of SOCS1/2/3/4 as potential prognostic biomarkers and correlate with immune infiltration in glioblastoma. J Cell Mol Med. 2023;27(15):2194–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.La Manna S, Lopez-sanz L, Mercurio FA, et al. Chimeric Peptidomimetics of SOCS 3 Able to Interact with JAK2 as Anti-inflammatory Compounds. ACS Med Chem Lett. 2020;11(5):615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elliott J, Suessmuth Y, Scott LM, et al. SOCS3 tyrosine phosphorylation as a potential bio-marker for myeloproliferative neoplasms associated with mutant JAK2 kinases. Haematologica. 2009;94(4):576–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou Q, Li C, Chong Y, et al. Comprehensive single-cell and bulk transcriptomic analyses to develop an NK cell-derived gene signature for prognostic assessment and precision medicine in breast cancer. Front Immunol. 2024;15:1460607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Yang Z, Chen B, et al. SOCS3 as a potential driver of lung metastasis in colon cancer patients. Front Immunol. 2023;14:1088542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng X, Shao J, Wei S, et al. Prognostic Significance of SOCS3 in Patients With Solid Tumors: A Meta-Analysis. Front Surg. 2021;8:802143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang P, Pei B, Yi C, et al. The role of suppressor of cytokine signaling 3 in inflammatory bowel disease and its associated colorectal cancer. Biochim Biophys Acta Mol Basis Dis. 2025;1871(2):167578. [DOI] [PubMed] [Google Scholar]
- 23.Starr R, Willson TA, Viney EM, et al. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387(6636):917–21. [DOI] [PubMed] [Google Scholar]
- 24.Yoshimura A, Ohkubo T, Kiguchi T, et al. A novel cytokine-inducible gene CIS encodes an SH2-containing protein that binds to tyrosine-phosphorylated interleukin 3 and erythropoietin receptors. Embo j. 1995;14(12):2816–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hilton DJ, Richardson RT, Alexander WS, et al. Twenty proteins containing a C-terminal SOCS box form five structural classes. Proc Natl Acad Sci U S A. 1998;95(1):114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicholson SE, Hilton DJ. The SOCS proteins: a new family of negative regulators of signal transduction. J Leukoc Biol. 1998;63(6):665–8. [DOI] [PubMed] [Google Scholar]
- 27.Zhou X, Jiang Z, Zou Y, et al. Role of SOCS3 in the Jak/stat3 pathway in the human placenta: different mechanisms for preterm and term labor. Acta Obstet Gynecol Scand. 2015;94(10):1112–7. [DOI] [PubMed] [Google Scholar]
- 28.Bergamin E, Wu J, Hubbard SR. Structural basis for phosphotyrosine recognition by suppressor of cytokine signaling-3. Structure. 2006;14(8):1285–92. [DOI] [PubMed] [Google Scholar]
- 29.Funakoshi-tago M, Tsuruya R, Ueda F, et al. Tyrosine-phosphorylated SOCS3 negatively regulates cellular transformation mediated by the myeloproliferative neoplasm-associated JAK2 V617F mutant. Cytokine. 2019;123:154753. [DOI] [PubMed] [Google Scholar]
- 30.Babon JJ, Mcmanus EJ, Yao S, et al. The structure of SOCS3 reveals the basis of the extended SH2 domain function and identifies an unstructured insertion that regulates stability. Mol Cell. 2006;22(2):205–16. [DOI] [PubMed] [Google Scholar]
- 31.Tamiya T, Kashiwagi I, Takahashi R, et al. Suppressors of cytokine signaling (SOCS) proteins and JAK/STAT pathways: regulation of T-cell inflammation by SOCS1 and SOCS3. Arterioscler Thromb Vasc Biol. 2011;31(5):980–5. [DOI] [PubMed] [Google Scholar]
- 32.Yasukawa H, Ohishi M, Mori H, et al. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol. 2003;4(6):551–6. [DOI] [PubMed] [Google Scholar]
- 33.Wang B, Wangkahart E, Secombes CJ, et al. Insights into the Evolution of the Suppressors of Cytokine Signaling (SOCS) Gene Family in Vertebrates. Mol Biol Evol. 2019;36(2):393–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ushiki T, Huntington ND, Glaser SP, et al. Rapid Inflammation in Mice Lacking Both SOCS1 and SOCS3 in Hematopoietic Cells. PLoS ONE. 2016;11(9):e0162111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Babon JJ, Sabo JK, Zhang JG, et al. The SOCS box encodes a hierarchy of affinities for Cullin5: implications for ubiquitin ligase formation and cytokine signalling suppression. J Mol Biol. 2009;387(1):162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim W, Seo MK, Kim YJ, et al. Role of the suppressor of cytokine signaling-3 in the pathogenesis of Graves’ orbitopathy. Front Endocrinol (Lausanne). 2025;16:1527275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J, Wei X, Xie Y, et al. Long non-coding RNA-MIR181A1HG acts as an oncogene and contributes to invasion and metastasis in gastric cancer. Oncogene. 2025;44(20):1517–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wen Y, Wang J, Duan M, et al. SIRT1-Mediated Regulation of STAT3/SOCS3 Inhibits Insulin Resistance in Trophoblast Cells and Improves Gestational Diabetes Mellitus. Faseb j. 2025;39(9):e70620. [DOI] [PubMed] [Google Scholar]
- 39.Wang W, Ding Y, Yu C, et al. Kinsenoside attenuates ER stress and inhibits inflammatory responses through IL-10/STAT/SOCS3 pathway in chronic pain relief. Neuropharmacology. 2025;273:110463. [DOI] [PubMed] [Google Scholar]
- 40.Alston CI, Dix RD. Reduced frequency of murine cytomegalovirus retinitis in C57BL/6 mice correlates with low levels of suppressor of cytokine signaling (SOCS)1 and SOCS3 expression within the eye during corticosteroid-induced immunosuppression. Cytokine. 2017;97:38–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lahera A, Lopez-nieva P, Alarcón H, et al. SOCS3 deregulation contributes to aberrant activation of the JAK/STAT pathway in precursor T-cell neoplasms. Br J Haematol. 2023;201(4):718–24. [DOI] [PubMed] [Google Scholar]
- 42.Liu K, Wu Z, Chu J, et al. Promoter methylation and expression of SOCS3 affect the clinical outcome of pediatric acute lymphoblastic leukemia by JAK/STAT pathway. Biomed Pharmacother. 2019;115:108913. [DOI] [PubMed] [Google Scholar]
- 43.Shang AQ, Wu J, Bi F, et al. Relationship between HER2 and JAK/STAT-SOCS3 signaling pathway and clinicopathological features and prognosis of ovarian cancer. Cancer Biol Ther. 2017;18(5):314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Babon JJ, Varghese LN, Nicola NA. Inhibition of IL-6 family cytokines by SOCS3. Semin Immunol. 2014;26(1):13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang X, Jin J, Peng X, et al. Simvastatin inhibits IL-17 secretion by targeting multiple IL-17-regulatory cytokines and by inhibiting the expression of IL-17 transcription factor RORC in CD4+ lymphocytes. J Immunol. 2008;180(10):6988–96. [DOI] [PubMed] [Google Scholar]
- 46.Yu H, Liu Y, Mcfarland BC, et al. SOCS3 Deficiency in Myeloid Cells Promotes Tumor Development: Involvement of STAT3 Activation and Myeloid-Derived Suppressor Cells. Cancer Immunol Res. 2015;3(7):727–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams JJL, Alotaiq N, Mullen W, et al. Interaction of suppressor of cytokine signalling 3 with cavin-1 links SOCS3 function and cavin-1 stability. Nat Commun. 2018;9(1):168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dai L, Tao Y, Shi Z, et al. SOCS3 Acts as an Onco-immunological Biomarker With Value in Assessing the Tumor Microenvironment, Pathological Staging, Histological Subtypes, Therapeutic Effect, and Prognoses of Several Types of Cancer. Front Oncol. 2022;12:881801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao M, Zhang D, Jiang C, et al. Paeoniflorin inhibits hepatocellular carcinoma growth by reducing PD-L1 expression. Biomed Pharmacother. 2023;166:115317. [DOI] [PubMed] [Google Scholar]
- 50.Sorrentino C, D’antonio L, Ciummo SL, et al. CRISPR/Cas9-mediated deletion of Interleukin-30 suppresses IGF1 and CXCL5 and boosts SOCS3 reducing prostate cancer growth and mortality. J Hematol Oncol. 2022;15(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang M, Zhang W, Zhang R, et al. Cancer exosome-derived miR-9 and miR-181a promote the development of early-stage MDSCs via interfering with SOCS3 and PIAS3 respectively in breast cancer. Oncogene. 2020;39(24):4681–94. [DOI] [PubMed] [Google Scholar]
- 52.Li T, Ge H, Yang Q, et al. Oncogenic role of microRNA-19b-3p-mediated SOCS3 in glioma through activation of JAK-STAT pathway. Metab Brain Dis. 2023;38(3):945–60. [DOI] [PubMed] [Google Scholar]
- 53.Tan P, Peng M, Liu D, et al. Suppressor of cytokine signaling 3 (SOCS3) is related to pro-inflammatory cytokine production and triglyceride deposition in turbot (Scophthalmus maximus). Fish Shellfish Immunol. 2017;70:381–90. [DOI] [PubMed] [Google Scholar]
- 54.Sood V, Lata S, Ramachandran VG, et al. Suppressor of Cytokine Signaling 3 (SOCS3) Degrades p65 and Regulate HIV-1 Replication. Front Microbiol. 2019;10:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghazawi FM, Faller EM, Parmar P, et al. Suppressor of cytokine signaling (SOCS) proteins are induced by IL-7 and target surface CD127 protein for degradation in human CD8 T cells. Cell Immunol. 2016;306–307:41–52. [DOI] [PubMed] [Google Scholar]
- 56.Zhang XM, Liu TY, Li SQ, et al. SOCS3 protein expression predicts the responses of advanced non-small cell lung cancer patients to platinum-based chemotherapy. Ann Transl Med. 2023;11(2):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun Y, Gao Y, Dong M, et al. Kremen2 drives the progression of non-small cell lung cancer by preventing SOCS3-mediated degradation of EGFR. J Exp Clin Cancer Res. 2023;42(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hwang JY, Holland JE, Valenteros KB, et al. Dissociating STAT4 and STAT5 Signaling Inhibitory Functions of SOCS3: Effects on CD8 T Cell Responses. Immunohorizons. 2019;3(11):547–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuan Y, Lin D, Feng L, et al. Upregulation of miR-196b-5p attenuates BCG uptake via targeting SOCS3 and activating STAT3 in macrophages from patients with long-term cigarette smoking-related active pulmonary tuberculosis. J Transl Med. 2018;16(1):284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang W, Li X, Jiang M, et al. SOCS3 deficiency-dependent autophagy repression promotes the survival of early-stage myeloid-derived suppressor cells in breast cancer by activating the Wnt/mTOR pathway. J Leukoc Biol. 2023;113(5):445–60. [DOI] [PubMed] [Google Scholar]
- 61.Wan J, Che Y, Kang N, et al. SOCS3 blocks HIF-1α expression to inhibit proliferation and angiogenesis of human small cell lung cancer by downregulating activation of Akt, but not STAT3. Mol Med Rep. 2015;12(1):83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu CW, Peng HY, Siao AC, et al. Resistin stimulates PC-3 prostate cancer cell growth through stimulation of SOCS3 and SOCS5 genes. Exp Biol Med (Maywood). 2023;248(20):1695–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan Z, Yang W, Parkitny L, et al. Deficiency of Socs3 leads to brain-targeted EAE via enhanced neutrophil activation and ROS production. JCI Insight. 2019;5(9). 10.1172/jci.insight.126520. [DOI] [PMC free article] [PubMed]
- 64.Ailia M J, Heo J, Yoo S Y. Navigating through the PD-1/PDL-1 Landscape: A Systematic Review and Meta-Analysis of Clinical Outcomes in Hepatocellular Carcinoma and Their Influence on Immunotherapy and Tumor Microenvironment. Int J Mol Sci. 2023;24(7). 10.3390/ijms24076495. [DOI] [PMC free article] [PubMed]
- 65.Gu X, Wei F, Tong J, et al. MiR-9 promotes G-MDSC recruitment and tumor proliferation by targeting SOCS3 in breast cancer. Faseb j. 2024;38(1):e23388. [DOI] [PubMed] [Google Scholar]
- 66.Zhang KJ, Tan XL, Guo L. The long non-coding RNA DANCR regulates the inflammatory phenotype of breast cancer cells and promotes breast cancer progression via EZH2-dependent suppression of SOCS3 transcription. Mol Oncol. 2020;14(2):309–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chu J, Hu XC, Li CC, et al. KLF14 alleviated breast cancer invasion and M2 macrophages polarization through modulating SOCS3/RhoA/Rock/STAT3 signaling. Cell Signal. 2022;92:110242. [DOI] [PubMed] [Google Scholar]
- 68.Hu J, Chen K, Hong F, et al. METTL3 facilitates stemness properties and tumorigenicity of cancer stem cells in hepatocellular carcinoma through the SOCS3/JAK2/STAT3 signaling pathway. Cancer Gene Ther. 2024;31(2):228–36. [DOI] [PubMed] [Google Scholar]
- 69.Han H, Davidson LA, Fan YY, et al. Loss of aryl hydrocarbon receptor suppresses the response of colonic epithelial cells to IL22 signaling by upregulating SOCS3. Am J Physiol Gastrointest Liver Physiol. 2022;322(1):G93-g106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sarajlic M, Neuper T, Vetter J, et al. H. pylori modulates DC functions via T4SS/TNFα/p38-dependent SOCS3 expression. Cell Commun Signal. 2020;18(1):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen M, Zeng J, Chen S, et al. SPTBN1 suppresses the progression of epithelial ovarian cancer via SOCS3-mediated blockade of the JAK/STAT3 signaling pathway. Aging (Albany NY). 2020;12(11):10896–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng H, Yan Y, Cheng J, et al. Association between SOCS3 hypermethylation and HBV-related hepatocellular carcinoma and effect of sex and age: A meta-analysis. Medicine (Baltimore). 2021;100(43):e27604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu C, Li Y, Dong Y, et al. Methylation Status of the SOCS3 Gene Promoter in H2228 Cells and EML4-ALK-positive Lung Cancer Tissues. Zhongguo Fei Ai Za Zhi. 2016;19(9):565–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang S, Wang B, Liu C, et al. THAP9-AS1 Promotes Tumorigenesis and Reduces ROS Generation through the JAK2/STAT3 Signaling Pathway by Increasing SOCS3 Promoter Methylation in Osteosarcoma. Oxid Med Cell Longev. 2021;2021:5620475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fukui H, Watari J, Zhang X, et al. Phosphorylated STAT3 expression linked to SOCS3 methylation is associated with proliferative ability of gastric mucosa in patients with early gastric cancer. Oncol Lett. 2020;19(5):3542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang X, Zhang K, Zhang J, et al. DNMTs-mediated SOCS3 methylation promotes the occurrence and development of AML. Eur J Haematol. 2024;112(3):439–49. [DOI] [PubMed] [Google Scholar]
- 77.Ren J, Xu G, Sun H, et al. Inhibition of miR-483-5p improves the proliferation, invasion and inflammatory response of triple-negative breast cancer cells by targeting SOCS3. Exp Ther Med. 2021;22(4):1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ni J, Xi X, Xiao S, et al. Tumor Cell-Derived Exosomal miR-191-5p Activates M2-Subtype Macrophages Through SOCS3 to Facilitate Breast Cancer. Mol Biotechnol. 2024;66(5):1314–25. [DOI] [PubMed] [Google Scholar]
- 79.Li X, Peng B, Li J, et al. Unleashing Breast Cancer Progression: miR-455-5p’s Targeting of SOCS3 Drives Proliferation, Migration, and Invasion. Protein Pept Lett. 2023;30(12):992–1000. [DOI] [PubMed] [Google Scholar]
- 80.Ding Y, Zhao H, Niu W, et al. M2 Macrophage-Derived Extracellular Vesicles Containing MicroRNA-501-3p Promote Colon Cancer Progression Through the SETD7/DNMT1/SOCS3 Axis. Dis Colon Rectum. 2023;66(12):e1234–45. [DOI] [PubMed] [Google Scholar]
- 81.Huang Q, Li Y, Chen Z, et al. Bushenhuoluo Decoction improves polycystic ovary syndrome by regulating exosomal miR-30a-5p/ SOCS3/mTOR/NLRP3 signaling-mediated autophagy and pyroptosis. J Ovarian Res. 2024;17(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu L, Huang G, Hong H, et al. MiR-452-5p facilitates retinoblastoma cell growth and invasion via the SOCS3/JAK2/STAT3 pathway. J Biochem Mol Toxicol. 2023;37(12):e23501. [DOI] [PubMed] [Google Scholar]
- 83.Zhang S, Ji WW, Wei W, et al. Long noncoding RNA Meg3 sponges miR-708 to inhibit intestinal tumorigenesis via SOCS3-repressed cancer stem cells growth. Cell Death Dis. 2021;13(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu W, Long Q, Zhang W, et al. miRNA-221-3p derived from M2-polarized tumor-associated macrophage exosomes aggravates the growth and metastasis of osteosarcoma through SOCS3/JAK2/STAT3 axis. Aging (Albany NY). 2021;13(15):19760–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lin XM, Chen H, Zhan XL. MiR-203 regulates JAK-STAT pathway in affecting pancreatic cancer cells proliferation and apoptosis by targeting SOCS3. Eur Rev Med Pharmacol Sci. 2019;23(16):6906–13. [DOI] [PubMed] [Google Scholar]
- 86.Song S, Shi Y, Zeng D, et al. circANKRD28 inhibits cisplatin resistance in non-small-cell lung cancer through the miR-221-3p/SOCS3 axis. J Gene Med. 2023;25(4):e3478. [DOI] [PubMed] [Google Scholar]
- 87.Li WD, Wang HT, Huang YM, et al. Circ_0003356 suppresses gastric cancer growth through targeting the miR-668-3p/SOCS3 axis. World J Gastrointest Oncol. 2023;15(5):787–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li Z, Zheng J, Lin W, et al. Circular RNA hsa_circ_0001785 inhibits the proliferation, migration and invasion of breast cancer cells in vitro and in vivo by sponging miR-942 to upregulate SOCS3. Cell Cycle. 2020;19(21):2811–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Du YX, Guo LX, Pan HS, et al. Circ_ANKIB1 stabilizes the regulation of miR-19b on SOCS3/STAT3 pathway to promote osteosarcoma cell growth and invasion. Hum Cell. 2020;33(1):252–60. [DOI] [PubMed] [Google Scholar]
- 90.Xu JZ, Shao CC, Wang XJ, et al. circTADA2As suppress breast cancer progression and metastasis via targeting miR-203a-3p/SOCS3 axis. Cell Death Dis. 2019;10(3):175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shi D L. RNA-Binding Proteins as Critical Post-Transcriptional Regulators of Cardiac Regeneration. Int J Mol Sci. 2023;24(15). 10.3390/ijms241512004. [DOI] [PMC free article] [PubMed]
- 92.Xiao Y, Li Y, Shi D, et al. MEX3C-Mediated Decay of SOCS3 mRNA Promotes JAK2/STAT3 Signaling to Facilitate Metastasis in Hepatocellular Carcinoma. Cancer Res. 2022;82(22):4191–205. [DOI] [PubMed] [Google Scholar]
- 93.Ehlting C, Lai WS, Schaper F, et al. Regulation of suppressor of cytokine signaling 3 (SOCS3) mRNA stability by TNF-alpha involves activation of the MKK6/p38MAPK/MK2 cascade. J Immunol. 2007;178(5):2813–26. [DOI] [PubMed] [Google Scholar]
- 94.Mukherjee S, Kar A, Paul P, et al. In Silico Integration of Transcriptome and Interactome Predicts an ETP-ALL-Specific Transcriptional Footprint that Decodes its Developmental Propensity. Front Cell Dev Biol. 2022;10:899752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Persico M, Russo R, Persico E, et al. SOCS3 and IRS-1 gene expression differs between genotype 1 and genotype 2 hepatitis C virus-infected HepG2 cells. Clin Chem Lab Med. 2009;47(10):1217–25. [DOI] [PubMed] [Google Scholar]
- 96.Pastuszak-lewandoska D, Domańska-senderowska D, Antczak A, et al. The Expression Levels of IL-4/IL-13/STAT6 Signaling Pathway Genes and SOCS3 Could Help to Differentiate the Histopathological Subtypes of Non-Small Cell Lung Carcinoma. Mol Diagn Ther. 2018;22(5):621–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Khalil AI, Zaazou MMT. Characterization of suppressors of cytokine signaling 3 expression in invasive breast carcinoma: differences in immunohistochemically defined molecular subtypes. Egyptian J Pathol. 2020;40(1):108–15. [Google Scholar]
- 98.Giraldo NA, Sanchez-salas R, Peske JD, et al. The clinical role of the TME in solid cancer. Br J Cancer. 2019;120(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mao X, Xu J, Wang W, et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer. 2021;20(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Boutilier A J, Elsawa S F. Macrophage Polarization States in the Tumor Microenvironment. Int J Mol Sci. 2021;22(13). 10.3390/ijms22136995. [DOI] [PMC free article] [PubMed]
- 101.Fu C, Jiang A. Dendritic Cells and CD8 T Cell Immunity in Tumor Microenvironment. Front Immunol. 2018;9:3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Grisaru-tal S, Rothenberg ME, Munitz A. Eosinophil-lymphocyte interactions in the tumor microenvironment and cancer immunotherapy. Nat Immunol. 2022;23(9):1309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang H, Yue X, Chen Z, et al. Define cancer-associated fibroblasts (CAFs) in the tumor microenvironment: new opportunities in cancer immunotherapy and advances in clinical trials. Mol Cancer. 2023;22(1):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen C, Wang Z, Ding Y, et al. Tumor microenvironment-mediated immune evasion in hepatocellular carcinoma. Front Immunol. 2023;14:1133308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang X, Tian M, Lin Y, et al. Characterization of the Roles of Suppressor of Cytokine Signaling-3 in Esophageal Carcinoma. Hum Gene Ther. 2023;34(11–12):495–517. [DOI] [PubMed] [Google Scholar]
- 106.Dai L, Li Z, Tao Y, et al. Emerging roles of suppressor of cytokine signaling 3 in human cancers. Biomed Pharmacother. 2021;144:112262. [DOI] [PubMed] [Google Scholar]
- 107.Tang X, Hao N, Zhou Y, et al. Ultrasound targeted microbubble destruction-mediated SOCS3 attenuates biological characteristics and epithelial-mesenchymal transition (EMT) of breast cancer stem cells. Bioengineered. 2022;13(2):3896–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jeong IH, Yun JK, Jin JO, et al. E3 ligase SOCS3 regulates NOD2 expression by ubiquitin proteasome system in lung cancer progression. Cell Oncol (Dordr). 2024;47(3):819–32. [DOI] [PubMed] [Google Scholar]
- 109.Gu J, Yang S, Wang X, et al. Hypoxic lung adenocarcinoma-derived exosomal miR-1290 induces M2 macrophage polarization by targeting SOCS3. Cancer Med. 2023;12(11):12639–52. 10.1002/cam4.5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wu R, Su Z, Zhao L, et al. Extracellular Vesicle-Loaded Oncogenic lncRNA NEAT1 from Adipose-Derived Mesenchymal Stem Cells Confers Gemcitabine Resistance in Pancreatic Cancer via miR-491-5p/Snail/SOCS3 Axis. Stem Cells Int. 2023;2023:6510571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chi Y, Xin H, Liu Z. Exosomal lncRNA UCA1 Derived From Pancreatic Stellate Cells Promotes Gemcitabine Resistance in Pancreatic Cancer via the SOCS3/EZH2 Axis. Front Oncol. 2021;11:671082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xie J, Wen JT, Xue XJ, et al. Retraction Note: MiR-221 inhibits proliferation of pancreatic cancer cells via down regulation of SOCS3. Eur Rev Med Pharmacol Sci. 2024;28(5):1639. [DOI] [PubMed] [Google Scholar]
- 113.Liu F, Wang Y, Cao Y, et al. Transcription factor B-MYB activates lncRNA CCAT1 and upregulates SOCS3 to promote chemoresistance in colorectal cancer. Chem Biol Interact. 2023;374:110412. [DOI] [PubMed] [Google Scholar]
- 114.Yu C, Fan Y, Zhang Y, et al. LINC00893 inhibits the progression of prostate cancer through miR-3173-5p/SOCS3/JAK2/STAT3 pathway. Cancer Cell Int. 2022;22(1):228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu B, Yao X, Zhang C, et al. PTK6 inhibits autophagy to promote uveal melanoma tumorigenesis by binding to SOCS3 and regulating mTOR phosphorylation. Cell Death Dis. 2023;14(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Al Saqri A, Malgundkar SH, Al Kindi F, et al. SOCS3 gene silencing does not occur through methylation and mutations in gastric cancer. Hum Cell. 2022;35(4):1114–25. [DOI] [PubMed] [Google Scholar]
- 117.Tang H, Long Q, Zhuang K, et al. miR-665 promotes the progression of gastric adenocarcinoma via elevating FAK activation through targeting SOCS3 and is negatively regulated by lncRNA MEG3. J Cell Physiol. 2020;235(5):4709–19. [DOI] [PubMed] [Google Scholar]
- 118.Yu Y, Sung SK, Lee CH, et al. SOCS3 is Related to Cell Proliferation in Neuronal Tissue: An Integrated Analysis of Bioinformatics and Experiments. Front Genet. 2021;12:743786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu ZK, Li C, Zhang RY, et al. EYA2 suppresses the progression of hepatocellular carcinoma via SOCS3-mediated blockade of JAK/STAT signaling. Mol Cancer. 2021;20(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lin J. C1QTNF1-AS1 regulates the occurrence and development of hepatocellular carcinoma by regulating miR-221-3p/SOCS3. Hepatol Int. 2021;15(2):526. [DOI] [PubMed] [Google Scholar]
- 121.Ge Y, Gu P, Wang W, et al. Benzo[a]pyrene stimulates miR-650 expression to promote the pathogenesis of fatty liver disease and hepatocellular carcinoma via SOCS3/JAK/STAT3 cascades. J Mol Cell Biol. 2021;13(8):556–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Attia YM, Tawfiq RA, Gibriel AA, et al. Activation of FXR modulates SOCS3/Jak2/STAT3 signaling axis in a NASH-dependent hepatocellular carcinoma animal model. Biochem Pharmacol. 2021;186:114497. [DOI] [PubMed] [Google Scholar]
- 123.Wei L, Liu Q, Huang Y, et al. Knockdown of CTCF reduces the binding of EZH2 and affects the methylation of the SOCS3 promoter in hepatocellular carcinoma. Int J Biochem Cell Biol. 2020;120:105685. [DOI] [PubMed] [Google Scholar]
- 124.Tu C, Wang Y, Cheng X, et al. The Combination Therapy of Fluorouracil and Oxaliplatin Suppress the Progression of Colon Cancer Through miR-183-5p/SOCS3 Axis and Downregulating PD-L1. Cancer Manag Res. 2021;13:1999–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yan Z, Hong S, Song Y, et al. microR-4449 Promotes Colorectal Cancer Cell Proliferation via Regulation of SOCS3 and Activation of STAT3 Signaling. Cancer Manag Res. 2021;13:3029–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ye T, Zhong L, Ye X, et al. miR-221-3p and miR-222-3p regulate the SOCS3/STAT3 signaling pathway to downregulate the expression of NIS and reduce radiosensitivity in thyroid cancer. Exp Ther Med. 2021;21(6):652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hu Y, Luo X, Zhou J, et al. Piperlongumine inhibits the progression of osteosarcoma by downregulating the SOCS3/JAK2/STAT3 pathway via miR-30d-5p. Life Sci. 2021;277:119501. [DOI] [PubMed] [Google Scholar]
- 128.Zhang JW, Wang X, Li GC, et al. MiR-30a-5p promotes cholangiocarcinoma cell proliferation through targeting SOCS3. J Cancer. 2020;11(12):3604–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li L, Yu P, Zhang P, et al. Upregulation of hsa_circ_0007874 suppresses the progression of ovarian cancer by regulating the miR-760/SOCS3 pathway. Cancer Med. 2020;9(7):2491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Masuzaki R, Kanda T, Sasaki R, et al. Suppressors of Cytokine Signaling and Hepatocellular Carcinoma. Cancers (Basel). 2022;14(10):2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen Y, Miao X, Xiang Y, et al. Qinzhu Liangxue inhibits IL-6-induced hyperproliferation and inflammation in HaCaT cells by regulating METTL14/SOCS3/STAT3 axis. J Ethnopharmacol. 2023;317:116809. [DOI] [PubMed] [Google Scholar]
- 132.Marciscano AE, Anandasabapathy N. The role of dendritic cells in cancer and anti-tumor immunity. Semin Immunol. 2021;52:101481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stevens D, Ingels J, Van Lint S, et al. Dendritic Cell-Based Immunotherapy in Lung Cancer. Front Immunol. 2020;11:620374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Albini E, Rosini V, Gargaro M, et al. Distinct roles of immunoreceptor tyrosine-based motifs in immunosuppressive indoleamine 2,3-dioxygenase 1. J Cell Mol Med. 2017;21(1):165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yan BY, Garcet S, Gulati N, et al. Novel immune signatures associated with dysplastic naevi and primary cutaneous melanoma in human skin. Exp Dermatol. 2019;28(1):35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shi D, Yang J, Wang Q, et al. SOCS3 ablation enhances DC-derived Th17 immune response against Candida albicans by activating IL-6/STAT3 in vitro. Life Sci. 2019;222:183–94. [DOI] [PubMed] [Google Scholar]
- 137.Li K, Shi H, Zhang B, et al. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct Target Ther. 2021;6(1):362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kumar V, Patel S, Tcyganov E, et al. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016;37(3):208–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mehraj U, Dar AH, Wani NA, et al. Tumor microenvironment promotes breast cancer chemoresistance. Cancer Chemother Pharmacol. 2021;87(2):147–58. [DOI] [PubMed] [Google Scholar]
- 140.Zhang W, Jiang M, Chen J, et al. SOCS3 Suppression Promoted the Recruitment of CD11b(+)Gr-1(-)F4/80(-)MHCII(-) Early-Stage Myeloid-Derived Suppressor Cells and Accelerated Interleukin-6-Related Tumor Invasion via Affecting Myeloid Differentiation in Breast Cancer. Front Immunol. 2018;9:1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Korbecki J, Kojder K, Simińska D, et al. CC Chemokines in a Tumor: A Review of Pro-Cancer and Anti-Cancer Properties of the Ligands of Receptors CCR1, CCR2, CCR3, and CCR4. Int J Mol Sci. 2020;21(21). 10.3390/ijms21218412. [DOI] [PMC free article] [PubMed]
- 142.Zhang H, Jiang H, Zhu L, et al. Cancer-associated fibroblasts in non-small cell lung cancer: Recent advances and future perspectives. Cancer Lett. 2021;514:38–47. [DOI] [PubMed] [Google Scholar]
- 143.Biffi G, Tuveson DA. Diversity and Biology of Cancer-Associated Fibroblasts. Physiol Rev. 2021;101(1):147–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen Y, Mcandrews KM, Kalluri R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol. 2021;18(12):792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Giordano C, Barone I, Vircillo V, et al. Activated FXR Inhibits Leptin Signaling and Counteracts Tumor-promoting Activities of Cancer-Associated Fibroblasts in Breast Malignancy. Sci Rep. 2016;6:21782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sarode P, Zheng X, Giotopoulou GA, et al. Reprogramming of tumor-associated macrophages by targeting β-catenin/FOSL2/ARID5A signaling: A potential treatment of lung cancer. Sci Adv. 2020;6(23):eaaz6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang W, Cai Z, Liang D, et al. Immune Cell-Related Genes in Juvenile Idiopathic Arthritis Identified Using Transcriptomic and Single-Cell Sequencing Data. Int J Mol Sci. 2023;24(13). 10.3390/ijms241310619. [DOI] [PMC free article] [PubMed]
- 148.Gordon P, Okai B, Hoare JI, et al. SOCS3 is a modulator of human macrophage phagocytosis. J Leukoc Biol. 2016;100(4):771–80. [DOI] [PubMed] [Google Scholar]
- 149.Qin H, Holdbrooks AT, Liu Y, et al. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J Immunol. 2012;189(7):3439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yang H, Xu H, Wang Z, et al. Analysis of miR-203a-3p/SOCS3-mediated induction of M2 macrophage polarization to promote diabetic wound healing based on epidermal stem cell-derived exosomes. Diabetes Res Clin Pract. 2023;197:110573. [DOI] [PubMed] [Google Scholar]
- 151.Ren J, Zhu B, Gu G, et al. Schwann cell-derived exosomes containing MFG-E8 modify macrophage/microglial polarization for attenuating inflammation via the SOCS3/STAT3 pathway after spinal cord injury. Cell Death Dis. 2023;14(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ashenafi S, Aderaye G, Bekele A, et al. Progression of clinical tuberculosis is associated with a Th2 immune response signature in combination with elevated levels of SOCS3. Clin Immunol. 2014;151(2):84–99. [DOI] [PubMed] [Google Scholar]
- 153.Xin P, Xu X, Deng C, et al. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int Immunopharmacol. 2020;80:106210. [DOI] [PubMed] [Google Scholar]
- 154.Boieri M, Malishkevich A, Guennoun R, et al. CD4+ T helper 2 cells suppress breast cancer by inducing terminal differentiation. J Exp Med. 2022;219(7). 10.1084/jem.20201963. [DOI] [PMC free article] [PubMed]
- 155.Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression - implications for anticancer therapy. Nat Rev Clin Oncol. 2019;16(6):356–71. [DOI] [PubMed] [Google Scholar]
- 156.Yan D, Li G, Yuan Y, et al. SOCS3 inhibiting JAK-STAT pathway enhances oncolytic adenovirus efficacy by potentiating viral replication and T-cell activation. Cancer Gene Ther. 2024;31(3):397–409. [DOI] [PubMed] [Google Scholar]
- 157.Knochelmann HM, Dwyer CJ, Bailey SR, et al. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell Mol Immunol. 2018;15(5):458–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mise-omata S, Ando M, Srirat T, et al. SOCS3 deletion in effector T cells confers an anti-tumorigenic role of IL-6 to the pro-tumorigenic cytokine. Cell Rep. 2023;42(8):112940. [DOI] [PubMed] [Google Scholar]
- 159.Yuan S, Zhu T, Wang J, et al. miR-22 promotes immunosuppression via activating JAK/STAT3 signaling in cutaneous squamous cell carcinoma. Carcinogenesis. 2023;44(7):549–61. [DOI] [PubMed] [Google Scholar]
- 160.Gao Y, Zhao H, Wang P, et al. The roles of SOCS3 and STAT3 in bacterial infection and inflammatory diseases. Scand J Immunol. 2018;88(6):e12727. [DOI] [PubMed] [Google Scholar]
- 161.Sobah ML, Scott AC, Laird M, et al. Socs3b regulates the development and function of innate immune cells in zebrafish. Front Immunol. 2023;14:1119727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kleinsteuber K, Heesch K, Schattling S, et al. SOCS3 promotes interleukin-17 expression of human T cells. Blood. 2012;120(22):4374–82. [DOI] [PubMed] [Google Scholar]
- 163.Luckey MA, Kim TH, Prakhar P, et al. SOCS3 is a suppressor of γc cytokine signaling and constrains generation of murine Foxp3(+) regulatory T cells. Eur J Immunol. 2020;50(7):986–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chen Y, Zhou X, Xie Y, et al. Establishment of a Seven-Gene Signature Associated with CD8(+) T Cells through the Utilization of Both Single-Cell and Bulk RNA-Sequencing Techniques in Clear Cell Renal Cell Carcinoma. Int J Mol Sci. 2023;24(18). 10.3390/ijms241813729. [DOI] [PMC free article] [PubMed]
- 165.Villegas FR, Coca S, Villarrubia VG, et al. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer. 2002;35(1):23–8. [DOI] [PubMed] [Google Scholar]
- 166.Thacker G, Henry S, Nandi A, et al. Immature natural killer cells promote progression of triple-negative breast cancer. Sci Transl Med. 2023;15(686):eab14414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tang B, Li YG, Cheng L, et al. Expression Level of SOCS3 in Acute Lymphoblastic Leukemia Cells Affects the Cytotoxicity of NK Cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2022;30(2):400–6. [DOI] [PubMed] [Google Scholar]
- 168.Wang X, Sun R, Hao X, et al. IL-17 constrains natural killer cell activity by restraining IL-15-driven cell maturation via SOCS3. Proc Natl Acad Sci U S A. 2019;116(35):17409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yoneda T, Kunimura N, Kitagawa K, et al. Overexpression of SOCS3 mediated by adenovirus vector in mouse and human castration-resistant prostate cancer cells increases the sensitivity to NK cells in vitro and in vivo. Cancer Gene Ther. 2019;26(11–12):388–99. [DOI] [PubMed] [Google Scholar]
- 170.Jafarzadeh A, Zandvakili R, Jafarzadeh Z, et al. Dysregulated expression of the suppressors of cytokine signaling (SOCS) contributes to the development of prostate cancer. Pathol Res Pract. 2024;262:155558. [DOI] [PubMed] [Google Scholar]
- 171.Chikuma S, Kanamori M, Mise-omata S, et al. Suppressors of cytokine signaling: Potential immune checkpoint molecules for cancer immunotherapy. Cancer Sci. 2017;108(4):574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ashfaq I, Sheikh N, Fatima N, et al. Inhibition of anti-inflammatory pathway through suppressors of cytokine signalling (Socs2/Socs3) in the initiation of hepatocellular carcinoma. Saudi J Biol Sci. 2022;29(8):103348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Matsumura S, Nakamori M, Tsuji T, et al. Oncolytic virotherapy with SOCS3 enhances viral replicative potency and oncolysis for gastric cancer. Oncotarget. 2021;12(4):344–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yang K, Zhu J, Luo HH, et al. Pro-protein convertase subtilisin/kexin type 9 promotes intestinal tumor development by activating Janus kinase 2/signal transducer and activator of transcription 3/SOCS3 signaling in Apc(Min/+) mice. Int J Immunopathol Pharmacol. 2021;35:20587384211038344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tang Q, Jiang J, Liu J. CCR5 Blockade Suppresses Melanoma Development Through Inhibition of IL-6-Stat3 Pathway via Upregulation of SOCS3. Inflammation. 2015;38(6):2049–56. [DOI] [PubMed] [Google Scholar]
- 176.Martino N, Bossardi Ramos R, Chuy D, et al. SOCS3 limits TNF and endotoxin-induced endothelial dysfunction by blocking a required autocrine interleukin-6 signal in human endothelial cells. Am J Physiol Cell Physiol. 2022;323(2):C556-c569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hill GR, Kuns RD, Raffelt NC, et al. SOCS3 regulates graft-versus-host disease. Blood. 2010;116(2):287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yang J, Jiang H, Fu Q, et al. Blue light photobiomodulation induced apoptosis by increasing ROS level and regulating SOCS3 and PTEN/PI3K/AKT pathway in osteosarcoma cells. J Photochem Photobiol B. 2023;249:112814. [DOI] [PubMed] [Google Scholar]
- 179.Wang J, Zhou H, Han Y, et al. SOCS3 methylation in synergy with Reg3A overexpression promotes cell growth in pancreatic cancer. J Mol Med (Berl). 2014;92(12):1257–69. [DOI] [PubMed] [Google Scholar]
- 180.Li Y, De Haar C, Peppelenbosch MP, et al. SOCS3 in immune regulation of inflammatory bowel disease and inflammatory bowel disease-related cancer. Cytokine Growth Factor Rev. 2012;23(3):127–38. [DOI] [PubMed] [Google Scholar]
- 181.Puhr M, Santer FR, Neuwirt H, et al. SOCS-3 antagonises the proliferative and migratory effects of fibroblast growth factor-2 in prostate cancer by inhibition of p44/p42 MAPK signalling. Endocr Relat Cancer. 2010;17(2):525–38. [DOI] [PubMed] [Google Scholar]
- 182.Babon JJ, Kershaw NJ, Murphy JM, et al. Suppression of cytokine signaling by SOCS3: characterization of the mode of inhibition and the basis of its specificity. Immunity. 2012;36(2):239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.AL-Rawashde F A, Johan M F, Taib W R W, et al. Thymoquinone Inhibits Growth of Acute Myeloid Leukemia Cells through Reversal SHP-1 and SOCS-3 Hypermethylation: In Vitro and In Silico Evaluation. Pharmaceuticals (Basel). 2021;14(12). 10.3390/ph14121287. [DOI] [PMC free article] [PubMed]
- 184.Elbakry MMM, Elbakary NM, Hagag SA, et al. Pomegranate Peel Extract Sensitizes Hepatocellular Carcinoma Cells to Ionizing Radiation, Induces Apoptosis and Inhibits MAPK, JAK/STAT3, β-Catenin/NOTCH, and SOCS3 Signaling. Integr Cancer Ther. 2023;22:15347354221151020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Li S, Xu Z, Guo J, et al. Farnesoid X receptor activation induces antitumour activity in colorectal cancer by suppressing JAK2/STAT3 signalling via transactivation of SOCS3 gene. J Cell Mol Med. 2020;24(24):14549–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Li G, Xu J, Wang Z, et al. Low expression of SOCS-1 and SOCS-3 is a poor prognostic indicator for gastric cancer patients. J Cancer Res Clin Oncol. 2015;141(3):443–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lin X, Lv J, Ge D, et al. Heme oxygenase-1 alleviates eosinophilic inflammation by inhibiting STAT3-SOCS3 signaling. Pediatr Pulmonol. 2020;55(6):1440–7. [DOI] [PubMed] [Google Scholar]
- 188.Chen L, Huang X, Zhang W, et al. Correlation of PD-L1 and SOCS3 Co-expression with the Prognosis of Hepatocellular Carcinoma Patients. J Cancer. 2020;11(18):5440–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Li Y, Hodge J, Liu Q, et al. TFEB is a master regulator of tumor-associated macrophages in breast cancer. J Immunother Cancer. 2020;8(1). 10.1136/jitc-2020-000543. [DOI] [PMC free article] [PubMed]
- 190.Sun M, Tang C, Liu J, et al. Comprehensive analysis of suppressor of cytokine signaling proteins in human breast Cancer. BMC Cancer. 2021;21(1):696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yang Y, Shi GN, Wu X, et al. Quercetin Impedes Th17 Cell Differentiation to Mitigate Arthritis Involving PPARγ-Driven Transactivation of SOCS3 and Redistribution Corepressor SMRT from PPARγ to STAT3. Mol Nutr Food Res. 2022;66(12): e2100826. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.