ABSTRACT
This study aimed to explore the association between ACMSD methylation level in peripheral blood and brain dynamic functional connectivity (dFC) patterns in adolescents with MDD. Sixty-seven drug-naive, first-episode adolescents with MDD (mean age 14.55 ± 1.38 years, 24 males [35.8%]) and twenty-three healthy controls (HCs, mean age 14.34 ± 1.47 years, 10 males [43.5%]) completed resting-state structural and functional magnetic resonance imaging. DNA samples were collected from peripheral venous blood. Joint and Individual Variation Explained (JIVE) method was used to explore the joint and independent components of four domains of environmental factors (life adverse events, LAE; family environment, FE; family functioning, FF; childhood chronic stress, CCS). Dynamic independent component analysis was used to compute dynamic functional connectivity between brain regions. Associations between ACMSD methylation, environment and brain dFC patterns were assessed. JIVE calculated one joint (JIVE-joint) and seven individual components (JIVE-LAE-1, JIVE-FE-1, JIVE-FE-2, JIVE-FF-1, JIVE-FF-2, JIVE-CCS-1, and JIVE-CCS-2). ACMSD methylation was negatively correlated with JIVE-joint (r = −0.304, p = 0.012) and JIVE-CCS-1 (r = −0.299, p = 0.014) but positively correlated with JIVE-CCS-2 (r = 0.248, p = 0.043). Greater ACMSD methylation was associated with increased dFC strength between the left lateral occipital cortex and right postcentral gyrus (PostCG; T[65] = 4.02, p < 0.001, p-FDR = 0.010) and between the left temporal occipital fusiform cortex and right PostCG (T[65] = 3.86, p < 0.001, p-FDR = 0.035) in adolescent MDD patients. Methylation value of the ACMSD gene is more likely to be influenced by childhood chronic stress. This study may provided a new perspective for future epigenetic research on adolescent MDD.
KEYWORDS: Adolescents, MDD, dFC patterns, environment, DNA methylation
Introduction
Major depressive disorder (MDD) is a debilitating psychiatric condition characterized by persistent depressed mood, marked anhedonia, and significantly impaired motivation [1]. Epidemiological studies indicate that approximately 40% of individuals experience their first episode of MDD prior to age 20 [1]. Compared with adult-onset MDD patients, adolescent-onset patients are at greater risk of symptom recurrence, psychiatric comorbidity, and suicidal behaviors [2,3]. This developmental period-specific disease burden highlights the urgent need to elucidate the mechanisms underlying adolescent MDD pathogenesis. However, the current understanding remains limited by substantial clinical heterogeneity across developmental stages and incomplete characterization of associated neurodevelopmental alterations.
Recently, neuroimaging using resting-state functional magnetic resonance (rs-fMRI) has emerged as a promising approach for investigating the neural circuit impairment of MDD [4], with functional connectivity (FC) representing a key research focus [5]. The functional connectome approach demonstrated in 2014 that brain FC changes dynamically over time [6,7]. Consequently, dynamic functional connectivity (dFC) analysis was developed to address the limitations of traditional approaches in capturing time-varying FC patterns. Although electroencephalogram (EEG) is more temporally efficient than rs-fMRI, which shows their ability for predicting depression [8,9], we selected fMRI for two key reasons: 1) Spatial Precision: fMRI provides whole-brain coverage at 2 mm isotropic resolution, enabling precise localization of ACMSD-related effects in subcortical regions (e.g., thalamus, hippocampus) that are poorly resolved by scalp EEG; 2) Frequency selection: FMRI-derived functional connectivity exhibits spatial reconfigurations or time-varying dynamics at infralow (< 0.1 Hz) speeds. Conversely, electrophysiological connectivity is based on cross-region coupling of fast oscillations (~1-100 Hz) [10]. And infralow speed was chosen to mitigate physiological noise (respiration/cardiac pulsation). Currently, dFC analysis using rs-fMRI provides valuable insights into brain network dynamics and has proven effective for characterizing FC alterations in MDD [11–13]. For example, studies have reported increased FC state transition frequencies in MDD patients [14]. Yao et al. identified two dFC connection strength patterns (weak and strong), with MDD-related alterations occurring primarily in weak connections [11]. Compared with both bipolar disorder patients and HCs, MDD patients presented increased dFC strength in the precentral gyrus [15].
Emerging evidence suggests that aminocarboxymuconate semialdehyde decarboxylase (ACMSD) plays an important role in MDD pathophysiology [16]. ACMSD is an important enzyme involved in regulating tryptophan degradation via the kynurenine pathway (KP) [17]. ACMSD modulates the inflammatory response by inhibiting the formation of quinolinic acid [18], a neurotoxic metabolite associated with MDD onset [19–22]. Moreover, ACMSD represents a promising therapeutic target for mitigating suicidal behavior by modulating excitotoxicity and neuroinflammation [23]. One plausible hypothesis posits that environmental factors (such as chronic stress) may lead to neurotoxic changes and rendering the glial-neuronal network vulnerable to MDD through activating KP, including elevated enzyme activity [24–26]. While ACMSD is an important enzyme in KP, little is known about its activity changes under chronic stress in MDD patients. As we all know, environment could regulate genes via epigenetic mechanisms, such as DNA methylation, thereby modulating phenotype [27]. And in our prior Epigenome-Wide Association Study (EWAS), we identified lower methylation value of ACMSD CpG site in drug-naïve, first-episode adolescent MDD patients compared to healthy controls [28]. For psychiatric disorders, which are closely related to brain development, there is still significant controversy as to whether DNA methylation levels in peripheral blood are representative of central DNA methylation levels. Although certain gene methylation sites have been found to be consistently altered in peripheral blood versus brain tissue (e.g., SLC6A4, BDNF genes) in previous EWAS [29], no study has yet demonstrated a strong concordance between brain tissue ACMSD methylation levels and peripheral blood ACMSD levels. Additionally, there are significant differences in gene methylation levels in different tissues subject to specific regulatory mechanisms and that there are significant differences in response to the environment [30–33]. However, the exploration of peripheral blood gene methylation in MDD patients which can lay a solid foundation for future biomarker identifying is indispensable. In this preliminary study, we aimed to initially identify the association between brain working patterns and blood ACMSD methylation levels, to screen brain regions for further exploration.
In this study, four environment domains (life adverse events, LAE; family environment, FE; family functioning, FF; childhood chronic stress, CCS) were selected based on established publications suggested to be associated with DNA methylation [34–36]. And these domains were shown to be the most common early adversity during childhood and adolescents, which also contribute to depression [37,38]. A meta-analysis revealed that individuals who experienced early-life adversity were more likely to develop depression before the age of 18 years old [39]. One study using machine learning to select features predicting adolescence depression found family environment to be a good predictor [40], which also are associated with centromedial amygdala functional connectivity alterations in adolescent depression [41]. While in adolescents with MDD, family functioning mediated the impact of all childhood trauma subtypes on depression severity [42]. Although these domains exist correlations [42] and overlaps on depression [43], most traditional studies, still focused on either single early-life environmental factor or analyzed multiple factors separately, neglecting these correlations and overlaps [44–46]. The cumulative risk approach was subsequently developed to quantify multiple life events in children and adolescents, partially addressing variable correlations [47]. However, this approach overemphasizes combined effects, potentially masking the distinct impacts of specific adversities on brain development. In addition, prior work also showed differential neurobiological effects of these domains, motivating their separate modeling rather than a cumulative index [38]. For example, stress exposure unique to adolescence was related to higher connectivity from the salience network to the cognitive networks [48], while family relationship was major contributors to variation in connectivity of salience, cingulo-opercular, ventral attention, subcortical and somatosensory-motor networks [49]. In this study, we used Joint and Individual Variation Explained (JIVE) [50,51], a multidimensional data reduction technique, to disentangle the shared and unique contributions of adversity domains. Unlike cumulative-risk models, JIVE explicitly models shared (e.g., generalized stress response) and domain-specific effects, which align with the nature effect of these environmental factors. We then analyzed the relationships among ACMSD methylation in peripheral blood, joint or individual adversity components, and brain dFC patterns in adolescent MDD patients, offering an integrated perspective on genetic‒environmental interactions.
Method
Participants
This study received ethics approval from the Ethics Committee of West China Hospital, Sichuan University (approved number: 2019–1002), and was prospectively registered with the Chinese Clinical Trial Registry (ChiCTR2000033402) on 31 May 2020. All participants and their legal guardians provided written informed consent following full disclosure of the study procedures. All adolescent MDD participants met the Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV) diagnostic criteria for MDD. Two board-certified psychiatrists conducted diagnostic assessments via the Chinese version of the Affective Disorders and Schizophrenia-Present and Lifetime Version (KSADS-PL) [52]. The inclusion criteria for all participants were to be 12–17 years old, right-handed, having at least an elementary school education, having a normal brain structure, and being able to understand the contents of the scales. We excluded subjects with a history of electroconvulsive treatment, severe physical disease, antipsychotic or other medication use, alcohol or drug abuse, or other axis I and axis II mental illness. Healthy controls (HCs) had no history of psychiatric disorders or suicidal behavior. Depression severity was evaluated via the 21-item Beck Depression Inventory (BDI; score range 0–63) [53], which was administered to all participants. Four domains of early-life adversity (life adverse events, LAE; family environment, FE; family functioning, FF; childhood chronic stress, CCS) were collected through the Adolescent Self-rating Life Events Checklist (ASLEC) [54], Family Environment Scale (FES) [55], Family Assessment Device (FAD) [56] and Childhood Chronic Stress Questionnaire (CCSQ) [57].
MRI protocol procedure
All participants underwent structural and resting-state functional magnetic resonance imaging (rs-fMRI) via two 3.0T MRI scanners (uMR790, United-Imaging Healthcare, Shanghai, China; Achieva, Philips Medical Systems, Best, Netherlands). The participants were instructed to (1) keep their eyes closed; (2) remain awake but relaxed; and (3) avoid focused thinking during scanning. Foam padding and earplugs were used to reduce the impact of head movement and scanner noise. During the scan, images were visually inspected for structural abnormalities, head motion and artifacts. After the scan, the participants were asked whether they fell asleep during the scan. Only images without the abovementioned influencing factors were retained for analysis. The detailed acquisition parameters are provided in additional file [see Additional File 1].
Functional image preprocessing
DICOM images were converted to the NIfTI format via MRIcron (https://www.nitrc.org/projects/mricron), with header information verified for consistency. All the converted images underwent manual quality inspection to exclude susceptibility artifacts, signal dropouts, and ghosting artifacts prior to preprocessing. Preprocessing and analysis pipelines were implemented in CONN toolbox version 22b (www.nitrc.org/projects/conn) [58] based on MATLAB R2022b (MathWorks, Natick, MA, USA) and SPM12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/).
Structural MRI preprocessing comprises (1) translation to (0,0,0) coordinates; (2) tissue segmentation (gray matter, GM; white matter, WM; cerebrospinal fluid, CSF); and (3) spatial normalization to MNI space. T1-weighted images served as the anatomical reference for segmentation, offering optimal gray‒white matter contrast. Functional preprocessing involved the following steps: realignment and unwarp (for motion and field map correction), translation of the image center (to the origin 0,0,0), slice-timing correction, outlier scan detection and scrubbing (using ART: artifact removal toolbox), spatial normalization to an MNI template (functional target resolution 2 mm) and functional smoothing (full width half maximum of 8 mm). The ART toolbox identified outliers with either (1) framewise displacement (FD) > 0.9 mm or (2) global blood oxygen level-dependent (BOLD) signal changes > 5 SD [59]. The reference BOLD signal image of each subject was calculated by averaging all scanned images (excluding outliers). To mitigate physiological noise (respiration/cardiac pulsation) and scanner drift, WM/CSF signals were regressed out, and a bandpass filter of 0.008–0.09 Hz was applied by default [60]. Post-preprocessing quality control included visual inspection and quantitative metrics for all participants.
Dynamic independent component analysis
Independent component analysis (ICA) enables signal-noise separation and enhances detection sensitivity for interindividual differences [61]. ‘Dynamicity’ here means estimate time-varying functional connectivity on a temporal scale, reflecting the time-varying characteristics of brain functional connectivity. Dyn-ICA matrices represent a measure of different modulatory circuits expression and rate of connectivity change between each pair of regions of interest (ROIs), characterized by the strength and sign of connectivity changes covarying with a given component timeseries. Unlike other dFC analyses in other software utilize independent component analysis, which is based on the sliding window method to calculate windowed average dFC value using a series of sequential sliding windows, dynamic independent component analysis (dyn-ICA) in CONN software calculates the connection strength between each pair of ROIs at any given time point. The ROIs comprised 132 regions from (1) the Harvard – Oxford cortical atlas (provided by the Harvard Center of Morphometric Analysis, Cambridge, Massachusetts) and (2) cerebellar parcellations, as implemented in CONN’s default atlas. The analysis included (1) ICA decomposition of dynamic ROI-to-ROI connectivity into 20 components and (2) temporal smoothing (30 kernels) via CONN’s default parameters. And FDR correction was applied at the cluster-level and connection-level, both the threshold were at p-FDR<0.05 using Benjamini – Hochberg method.
Gene methylation measurement
The ACMSD CpG site (cg26890182) being identified in our previous Epigenome-Wide Association Study (EWAS) were selected for measuring methylation values [28]. In addition, to further elucidate the biological significance of this CpG site, we searched for genomic annotation using the UCSC Genome Browser (GRCh37/hg19) and the NCBI database (National Center for Biotechnology Information) Illumina 850k gene methylation chip information. The result showed that this differentially methylated site is located within the coding region of the ACMSD gene (UCSC_RefGene_Accession: NM_138326; NM_001307983). Therefore, primers were designed according to this CpG site.
Peripheral blood samples were collected from all participants in EDTA-coated vacutainers and cryopreserved at −80°C within 30 minutes of collection. DNA was extracted from thawed samples via a commercial kit (Qiagen, Hilden, Germany), and DNA methylation was analyzed via the EpiTect Bisulfite Kit (Qiagen). PyroMark Assay Design 2.0 software was used to design the primers, which were synthesized by the Beijing Genomics Institute. And they had the following sequences: 5’- GGTTGGAGTTGGTTGAGTAGTT −3,’ 5’- ACTTTCCCCAAATCCAAATTAACACCC −3,’ and 5’- GTATAGATAGGAAAGAGTTT −3.’
PCR amplification was performed as follows. The reaction system was a 50 μl mixture, including 10 μl 5X GC buffer (KAPA), 10 mm dNTP, 2 μl template DNA, 1 U/μl Taq polymerase, 50 pM upstream primer, 50 pM down-stream primer and 34.8 μl H2O. The reaction started with 95°C for three minutes, followed by 40 cycles at 94°C for 30 s, 56°C for 30 s, and 72°C for 1 min, then 72°C for 7 min. Quantitative methylation analysis was performed via Pyro-Q-CpG software (v1.0.9, Qiagen). CpG site methylation levels were calculated as the percentage of methylated alleles relative to total alleles at each locus. ACMSD methylation levels were computed as the mean methylation across all CpG sites analyzed in the target genomic region.
Statistical analysis
R 4.3.3 (https://www.r-project.org/) was used for statistical analysis. JIVE performs integrative dimension reduction of multiple variables in different domains (see the supplementary details of decomposition in [Additional File 1]) [50]. Five groups of latent variables (denoted as joint, LAE-individual, FE-individual, FF-individual and CCS-individual) were derived from LAE, FE, FF and CCS. These latent variables were uncorrelated by construction and thus could be used simultaneously in regression analyses as novel measures that capture either joint or individual information from the four domains [62,63]. JIVE analysis was performed with the r.jive package [51]. Pearson correlation was conducted between ACMSD methylation and JIVE components in adolescent MDD patients. Binary regression models were carried out to explore the main effect of ACMSD methylation on the risk of MDD, with the age, sex and education level of the subjects as covariates.
The ComBat method was used to harmonize these differences induced by scanner sites. ComBat both preserves biological variability and removes unwanted variation introduced by site [64,65]. ComBat harmonization was applied to neuroimaging data with scanner sites (3T Unitedimage/Philips) as batch variables; age, sex as protected biological covariates; group (MDD/HC) covariates were also included in ComBat to avoid removing biologically meaningful variance between adolescents MDD and HCs. And Principal Component Analysis (PCA) plot by site after Combat analysis was displayed in [Additional File 1]. The main effects of ACMSD methylation on the brain dFC pattern, as well as methylation by diagnostic interactions, were assessed via linear regression analyses in the CONN toolbox. And Leave-one-out cross-validation (LOOCV) analyses were conducted in R using ‘caret’ package to strengthen comprehensive stability.
Differences associated with p < 0.05 were considered significant. The false discovery rate (FDR) correction was used for multiple testing adjustments.
Results
Demographic and clinical characteristics
A total of 67 drug-naive, first-episode adolescent MDD patients (24 males, 43 females, mean age 14.55 ± 1.38) and 23 healthy controls (HCs; 10 males, 13 females, mean age 14.34 ± 1.47) completed rs-fMRI. Table 1 shows the demographic and clinical characteristics of the participants. There were no significant differences in age, sex, or education level distribution between the MDD patients and HCs (p > 0.05). The BDI score was significantly lower in HCs than in adolescents with MDD (t = −19.21, p < 0.001). The methylation value of the ACMSD in MDD patients was lower than that in HCs (t = 2.12, p = 0.038).
Table 1.
Sociodemographic and clinical characteristics of the participants.
| Healthy Controls(n = 23) | Adolescents MDD(n = 67) | t/ | p | |
|---|---|---|---|---|
| Sex, N(%) | 0.16 | 0.686 | ||
| Male | 10 (43%) | 24 (44%) | ||
| Female | 13 (57%) | 43 (56%) | ||
| Education, N(%) | 0.42 | 0.812 | ||
| Junior school | 15 (65%) | 42 (62%) | ||
| Senior school | 8 (35%) | 25 (38%) | ||
| MRI scan sites | 14.11 | <0.001 | ||
| uMR790 | 21(91%) | 29(42%) | ||
| Philips | 2(9%) | 38(58%) | ||
| Age, y | 14.34(1.47) | 14.55(1.38) | −0.59 | 0.562 |
| BDI score | 4.57(4.56) | 33.25(9.43) | −19.21 | <0.001 |
| ACMSD methylation | 72.97(6.53) | 69.29(9.09) | 2.12 | 0.038 |
| Life adverse events | ||||
| Interpersonal relationships | 7.09(4.73) | 12.76(6.33) | −4.53 | <0.001 |
| Study pressure | 7.22(4.51) | 10.05(5.94) | −2.39 | 0.021 |
| Punishment | 4.22(4.03) | 9.37(7.21) | −4.23 | <0.001 |
| Sense of loss | 3.13(3.48) | 3.18(3.19) | −0.06 | 0.953 |
| Healthy adaptation | 1.43(1.27) | 3.52(3.19) | −4.43 | <0.001 |
| Other adverse events | 2.57(2.25) | 7.31(4.31) | −6.73 | <0.001 |
| Family environment | ||||
| Cohesion | 7.87 (1.84) | 5.30 (2.78) | 5.02 | <0.001 |
| Expressiveness | 5.48 (1.83) | 4.48 (2.25) | 2.13 | 0.039 |
| Conflict | 3.39 (2.29) | 5.25 (2.13) | −3.42 | 0.002 |
| Independence | 5.13 (1.49) | 6.16 (2.25) | −2.50 | 0.015 |
| Achievement orientation | 6.74 (1.54) | 5.79 (2.51) | 2.13 | 0.037 |
| Intellectual | 5.83 (2.19) | 3.96 (2.61) | 3.36 | 0.002 |
| Active-recreational orientation | 6.65 (2.53) | 4.52 (2.64) | 3.44 | 0.001 |
| Moral-religious emphasis | 5.30 (1.55) | 4.87 (2.02) | 1.08 | 0.286 |
| Organization | 6.43 (2.11) | 5.51 (2.51) | 1.73 | 0.090 |
| Control | 3.83 (2.48) | 3.48 (2.03) | 0.61 | 0.548 |
| Family functioning | ||||
| Problem solving | 11.22(2.49) | 14.03(3.05) | −4.40 | <0.001 |
| Communication | 19.04(3.05) | 24.03(3.52) | −6.49 | <0.001 |
| Roles | 23.30(4.00) | 27.70(3.77) | −4.61 | <0.001 |
| Affective responsiveness | 13.13(2.91) | 16.78(2.83) | −5.22 | <0.001 |
| Affective involvement | 14.61(3.74) | 18.64(4.27) | −4.30 | <0.001 |
| Behavior control | 19.74(2.26) | 21.96(3.00) | −3.71 | <0.001 |
| Childhood chronic stress | ||||
| Peer bullying | 20.00 (6.71) | 25.07 (14.99) | −2.20 | 0.031 |
| Childhood abuse and neglect | 38.09 (14.33) | 54.10 (28.94) | −3.46 | <0.001 |
| Adverse childhood experience | 26.61 (10.31) | 38.06 (18.59) | −3.66 | <0.001 |
Abbreviations: MDD, major depressive disorder; BDI, Beck Depression Inventory.
JIVE results
A correlation heatmap between different variables of life adversity is shown in Figure 1. JIVE estimated that the optimal number of components is one for the joint (JIVE-joint), one for LAE (JIVE-LAE-1), two for FE (JIVE-FE-1, JIVE-FE-2), two for FF (JIVE-FF-1, JIVE-FF-2) and two for CCS (JIVE-CCS-1, JIVE-CCS-2). In LAE, FE, FF and CCS domains, the joint component explained 69.8%, 20.5%, 17.8% and 62.9%, respectively, of the total variation, and the independent components explained 9.4%, 38.2%, 44.5% and 28.3%, respectively, of the variation. However, 20.9%, 41.3%, 37.7% and 8.8% of the total variation in LAE, FE, FF and CCS, respectively, remained unexplained by the estimated JIVE model (see details in [Additional File 1]).
Figure 1.

The correlation heatmap between early-life environmental factors.
*p<0.05,**p<0.01,***p<0.001
Blue represents a positive correlation, red represents a negative correlation, and the darker the color represents a larger value of the correlation coefficient.
Additional table [see Additional file 1] shows the direction (±, positive/negative direction) and squares of the loadings of each variable on the joint JIVE components. For each component, the squares of the loadings added up to 100%. Therefore, their magnitude could be interpreted as the proportional importance of the corresponding variable for that specific component. We focused on variable loads with more than 5% proportional variation. JIVE-joint was distinguished by interpersonal relationship (-, 13.0%), study pressure (-, 7.3%), punishment (-, 13.9%), childhood abuse and neglect (−. 22.3%) and childhood adverse experiences (-, 10.0%). The direction (±) and squares of loadings for the estimated individual JIVE components are shown in the additional file [see Additional file 1].
Associations between ACMSD methylation and JIVE components
Pearson correlation was conducted between ACMSD methylation and JIVE components in adolescent MDD patients. ACMSD methylation was negatively correlated with JIVE-joint (r = −0.304, p = 0.012) and JIVE-CCS-1 (r = −0.299, p = 0.014) but positively correlated with JIVE-CCS-2 (r = 0.248, p = 0.043) (Figure 2). And the results of correlation between other JIVE components and ACMSD methylation were added in [Additional File 1].
Figure 2.
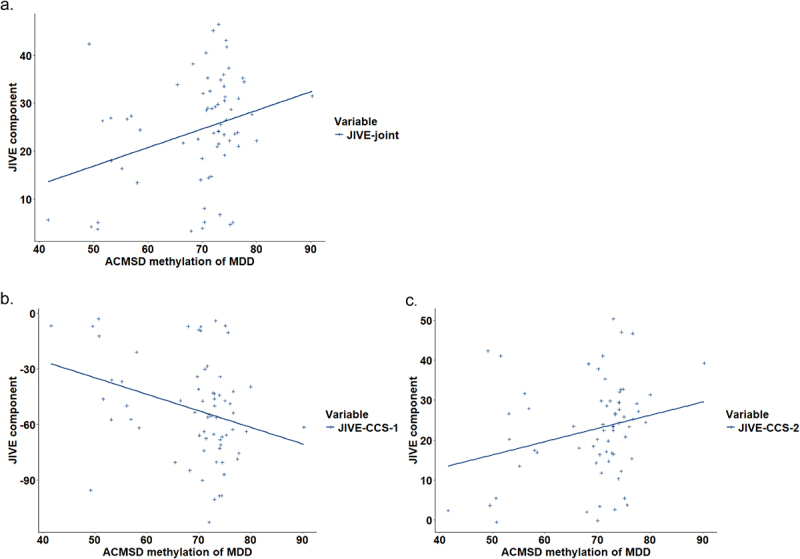
The correlation between ACMSD methylation and JIVE components in adolescents MDD.
a. the correlation between ACMSD methylation and JIVE-joint component; b. the correlation between ACMSD methylation and the first JIVE individual component of childhood chronic stress; c. the correlation between ACMSD methylation and the second JIVE individual component of childhood chronic stress.
Associations between ACMSD methylation and the risk of MDD
Binary regression models were carried out to explore the main effect of ACMSD methylation on the risk of MDD in adolescents, with the age, sex and education level of the participants as covariates. ACMSD methylation was significantly associated with the risk of MDD (OR = 0.908, 95% CI, 0.814–0.981).
Associations between ACMSD methylation and dFC patterns
The main effects of ACMSD methylation on the brain dFC pattern, as well as methylation by diagnostic interactions, were assessed via linear regression analyses in the CONN toolbox in full sample, adolescent MDD and healthy controls (Table 2). There was a significant main effect of ACMSD methylation on dynamic connectivity between the left inferior division of the lateral occipital cortex (iLOC) and the precuneus (T[88] = −4.63, p < 0.001, p-FDR = 0.002), indicating that greater methylation was associated with decreased dynamic connectivity regardless of diagnosis. In addition, the diagnosis*methylation interaction also significantly influenced the dynamic connectivity between the left iLOC and the precuneus (T[87] = −4.37, p < 0.001, p-FDR = 0.004).
Table 2.
Results of ACMSD effects on dynamic functional connectivity patterns and leave-one-out cross validation.
| Sample | connections | T | p | p-FDR | RMSE | MAE |
|---|---|---|---|---|---|---|
| Full sample | ||||||
| main effect of ACMSD methylation | Left iLOC-precuneus | −4.63 | <0.001 | 0.002 | 0.15 | 0.13 |
| diagnosis*ACMSD methylation interaction | Left iLOC-precuneus | −4.37 | <0.001 | 0.004 | 0.19 | 0.16 |
| Adolescents MDD | left iLOC – right PostCG | 4.02 | <0.001 | 0.010 | 0.12 | 0.10 |
| left TOFusC – right PostCG | 3.86 | <0.001 | 0.035 | 0.12 | 0.09 | |
| HCs | vermis 8 - right FO | −3.76 | 0.001 | 0.033 | 0.21 | 0.20 |
| vermis 8 -right CO | −3.73 | 0.001 | 0.033 | 0.24 | 0.22 | |
| vermis 8 -right IC | −3.65 | 0.002 | 0.033 | 0.15 | 0.13 | |
| vermis 8 -left SMA | −3.53 | 0.002 | 0.033 | 0.22 | 0.21 | |
| right OP – left PO | 4.05 | 0.001 | 0.049 | 0.10 | 0.08 | |
| right OP – right FO | 3.82 | 0.001 | 0.049 | 0.12 | 0.09 | |
| right OP -right PO | 3.77 | 0.001 | 0.049 | 0.10 | 0.07 |
Abbreviation: iLOC, inferior division of the lateral occipital cortex; PostCG, postcentral gyrus; TOFusC, temporal occipital fusiform cortex; FO, frontal operculum cortex; CO, central operculum cortex; IC, insular cortex; SMA, supplementary motor cortex; OP, occipital pole; PO, parietal operculum cortex.
In adolescent MDD patients only, greater ACMSD methylation was associated with increased dFC strength between the left iLOC and right postcentral gyrus (PostCG; T[65] = 4.02, p < 0.001, p-FDR = 0.010) and between the left temporal occipital fusiform cortex and right PostCG (T[65] = 3.86, p < 0.001, p-FDR = 0.035).
In healthy adolescents only, greater ACMSD methylation was associated with decreased dFC strength between vermis 8 and the right frontal operculum cortex (FO; T[21] = −3.76, p = 0.001, p-FDR = 0.033), between vermis 8 and the right central operculum cortex (T[21] = −3.73, p = 0.001, p-FDR = 0.033), between vermis 8 and the right insular cortex (T[21] = −3.65, p = 0.002, p-FDR = 0.033), and between vermis 8 and the left supplementary motor cortex (T[21] = −3.53, p = 0.002, p-FDR = 0.033). Greater ACMSD methylation was associated with increased dFC strength between the right occipital pole (OP) and left parietal operculum cortex (PO; T[21] = 4.05, p = 0.001, p-FDR = 0.049), between the right OP and right FO (T[21] = 3.82, p = 0.001, p-FDR = 0.049), and between the right OP and right PO (T[21] = 3.77, p = 0.001, p-FDR = 0.049).
Discussion
In this study, we revealed that the peripheral ACMSD methylation was associated with brain dFC patterns in adolescent MDD patients, which may suggest that ACMSD methylation may be a key indicator of MDD in adolescents. Previous studies have confirmed that single nucleotide polymorphisms (SNPs) of ACMSD play important roles in the pathogenesis of MDD [16,22,23,66], but no study focused on epigenetic modification. To our knowledge, this is the first study to explore the association between ACMSD methylation and the brain structure or function of MDD patients.
This study revealed for the first time the methylation level of ACMSD gene in patients with adolescent depression (MDD). The significant correlation between methylation levels and childhood stress provides a new direction for understanding the epigenetic mechanisms of childhood adversity that lead to MDD. Although no prior work found direct relationship between childhood stress and ACMSD methylation, they did find a close relationship between chronic stress and other DNA methylation. One review discovered stress-associated epigenetic changes in the following genes correlated with depression: NRC31, SLCA4, BDNF, SKA2, OXTR, LINGO3, POU3F1 and ITGB1 [67]. Demethylation of FKBP5 gene was linked to increased stress-dependent gene transcription followed by a long-term dysregulation of the stress hormone system [68]. Previous studies have shown that inflammation is closely related to chronic stress, especially the physical or social threats encountered in early childhood [69]. Also, the relationship between ACMSD and inflammation is also closely related [70]. Overall, inflammation may play a key role in this process. Although there have been no previous studies directly linking the ACMSD gene to childhood stress, previous reviews have summarized that the kynurenine pathway plays an important role in the pathophysiological mechanisms of depression induced by chronic stress [24]. Additionally, the IDO gene, which is also a key gene in the kynurenine pathway alongside the ACMSD gene, has been found to activate proinflammatory factors, potentiating neuroinflammation and deregulating other physiological mechanisms related to chronic stress and MDD [71]. This provides supplementary supporting evidence for our current study. However, future research is still needed to provide direct evidence linking the ACMSD gene and childhood chronic stress to validate the preliminary findings of our current study. There are several possible reasons for the weak association of other individual components like family environment, family functioning and life adverse events with ACMSD methylation: 1) Individual differences due to sample size. The total number of adolescents with depression in this study was 67, which is in line with the sample size recommended by our current mainstream neuroimaging studies [72,73], but further expansion of the sample size is still needed to minimize the presence of bias, and the present study is only a preliminary exploratory study, and our group is still in the process of collecting samples and expanding the sample size, and in the future we hope to take a further step to address the problems of the current study. problems of the current study; 2) As we stated in the limitations of this paper, the fewer methylation sites of the ACMSD gene in this study were the ones that were observed to be significantly different in the adolescent MDD group and healthy controls in our group’s previous EWAS, and the CpG sites of the whole gene were not explored, which may also contribute to the weak association between other JIVE environmental components and gene methylation; 3) In previous studies, different studies have explored different results. For example, one longitudinal study found that maternal stress during pregnancy was associated with methylation levels of the PLAGL1, HYMAI, BRD2, and ERC2 genes in the cord blood of newborns or in the peripheral blood of infants after 1 year of life [74], whereas another meta-study did not find that any DNA methylation levels in the cord blood of newborns were associated with maternal stress during pregnancy [75]. Both studies used EWAS but found mutually controversial results, which suggested a controversy still existed.
The lateral occipital cortex, postcentral gyrus and fusiform cortex are important brain regions involved in ACMSD methylation changes in adolescent MDD patients in this study. The lateral occipital cortex is associated with visual processing processes [76]. Yuan et al. reported that structural changes in the lateral occipital cortex are related to worse visual cognitive processing in MDD patients [77]. Moreover, previous studies revealed that visual processing regions are involved in the pathogenesis of MDD [78]. Based on these findings, LOC may play a crucial role in the pathophysical process of stress-induced depression. Previous studies have also shown that individuals with MDD have abnormal gray matter volume or thickness in the left postcentral gyrus [79,80]. The postcentral gyrus, which processes tactile information, potentially affects pain perception and processing in depressed patients [81]. Among female patients with MDD, those with greater psychological resilience had greater activation in the postcentral gyrus [82]. The fusiform gyrus, which is involved in emotion processing and expression recognition, shows function impairments that are positively correlated with depression severity [83,84]. One study revealed that FC in the left temporal occipital fusiform gyrus and the left caudate was associated with the different microbiota taxa between HCs and depressed patients [85]. In addition, a review revealed that several taxa and their mechanisms of action may be related to depression via the communication of peripheral inflammation to the brain [86], which could provide indirect evidence for this study.
This study has the following limitations. First, our sample size is relatively small, which suggests that the reliability and accuracy of the results should be viewed with caution. However, this study could still provide evidence for the importance of ACMSD methylation in adolescent MDD patients. In subsequent studies, we will increase the sample size to ensure the reliability of the results. Second, our study is a cross-sectional study, which means that we cannot explore the causality between dFC patterns and DNA methylation or environmental factors. In the future, we should continue to observe the effects of the methylation level of ACMSD on the treatment response of patients. Third, we mentioned that the inflammatory response may play an important role in ACMSD methylation changes among adolescents MDD with chronic stress. Unfortunately, we did not test for the inflammatory factors or white blood cell counts when we initially collected fresh plasma. Previous literatures on extracting white blood cells or cytokines from frozen blood [87,88], mentioned that white blood cell levels or cytokine levels may undergo activity changes under long-term frozen storage conditions, increasing the probability of false positives. Therefore, we did not re-test cytokine or white blood cell levels in the plasma we stored, which is our limitation in this study. Future studies should test whether ACMSD methylation is sensitive to inflammatory markers, particularly in brain-relevant contexts (e.g., neuroinflammation). Fourth, in this study, we measured the gene methylation levels in the peripheral blood of the subjects. However, whether the peripheral blood methylation level can represent the brain methylation level is still controversial. While blood and brain methylation levels can correlate for some genes, ACMSD-specific concordance remains understudied. Also, there was no specific literature to identify if transcript levels and enzymatic activity of ACMSD in brain vs peripheral blood is same. In the future, we will incorporate the results of this study to explore the correlation between specific brain tissue ACMSD methylation levels and specific depression-like behavior in animal experiments, to identify the reliability of ACMSD methylation as a biomarker for depression. Lastly, we did not investigate whether changes in these methylation sites continued to influence gene expression and function in this study, which is one of our limitations. Additionally, to our knowledge, there are currently no downstream validation studies on this relationship. This study is currently a simple correlation study on the methylation levels of the ACMSD gene in peripheral blood and brain dynamic functional connectivity patterns. Future studies are needed to identify ACMSD methylation as a validate biomarker.
Conclusion
In this study, the application of a novel dimension-reduction technique demonstrated joint and individual dimensions of early-life environmental factors related to ACMSD methylation values in MDD patients. And ACMSD methylation in peripheral blood was found to be significantly associated with brain dFC patterns in adolescent MDD patients. This study may provided a new perspective for future epigenetic research on adolescent MDD.
Supplementary Material
Acknowledgments
MR: Conceptualization, Methodology, Software, Writing- original draft, Writing- review and editing. MJ: Data curation, Writing- review and editing. ZW: Data curation. HZ: Data curation. YT: Data curation. HX: Data curation. SZ: Data curation. FD: Data curation. HZ: Data curation. XT: Data curation. XF: Data curation. LY: Data curation, Writing- review and editing, Supervision.
Funding Statement
This work was supported by the National Key R&D Program of the Chinese Science & Technology Department [2023YFE0118600], the National Natural Science Foundation of China [81801357], the Science and Technology Department of Sichuan Province [2022YFS0351 and 2020JDKP0013], the Health Commission of Sichuan Province [21PJ020], and the Science and Technology Department of Chengdu [2019-YF05-00284-SN]. These funding agencies were not involved in any of the research processes.
Disclosure statement
The funders had no further role in the study design, collection, analysis, interpretation of data, in the writing of the report or in the decision to submit the paper for publication. And the authors have nothing to disclose.
Availability of data and materials
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data are in controlled access data storage at West China Hospital of Sichuan University.
Ethics approval and consent to participate
This study received ethics approval from the Biomedical Ethics Committee of West China Hospital, Sichuan University (approved number: 2019–1002), and was prospectively registered with the Chinese Clinical Trial Registry (ChiCTR2000033402) in 31 May 2020. All participants and their legal guardians provided written informed consent following full disclosure of the study procedures.
Supplementary Information
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15592294.2025.2560339
References
- [1].Malhi GS, Mann JJ.. Depression. Lancet. 2018;392(10161):2299–15. doi: 10.1016/S0140-6736(18)31948-2 [DOI] [PubMed] [Google Scholar]
- [2].Viswanathan M, Wallace IF, Cook Middleton J, et al. Screening for depression and suicide risk in children and adolescents: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2022;328(15):1543–1556. doi: 10.1001/jama.2022.16310 [DOI] [PubMed] [Google Scholar]
- [3].Viswanathan M, Wallace I, Middleton JC, et al. U.S. Preventive Services Task Force evidence syntheses, formerly systematic evidence reviews. In: Meera Viswanathan, editor. Screening for depression, anxiety, and suicide risk in children and adolescents: an evidence review for the US Preventive Services Task Force. Rockville (MD): Agency for Healthcare Research and Quality (US); 2022. p. 547. [PubMed] [Google Scholar]
- [4].Alexandros Lalousis P, Wood S, Reniers R, et al. Transdiagnostic structural neuroimaging features in depression and psychosis: a systematic review. Neuroimage: Clin. 2023;38:103388. doi: 10.1016/j.nicl.2023.103388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tse NY, Ratheesh A, Ganesan S, et al. Functional dysconnectivity in youth depression: systematic review, meta-analysis, and network-based integration. Neurosci Biobehav Rev. 2023;153:105394. doi: 10.1016/j.neubiorev.2023.105394 [DOI] [PubMed] [Google Scholar]
- [6].Calhoun VD, Miller R, Pearlson G, et al. The chronnectome: time-varying connectivity networks as the next frontier in fMRI data discovery. Neuron. 2014;84(2):262–274. doi: 10.1016/j.neuron.2014.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Marchitelli R, Paillère-Martinot ML, Bourvis N, et al. Dynamic functional connectivity in adolescence-onset major depression: relationships with severity and symptom dimensions. Biol Psychiatry Cognit Neurosci Neuroimaging. 2022;7(4):385–396. doi: 10.1016/j.bpsc.2021.05.003 [DOI] [PubMed] [Google Scholar]
- [8].Elnaggar K, El-Gayar MM, Elmogy M.. Depression detection and diagnosis based on electroencephalogram (EEG) analysis: a systematic review. Diagnostics (Basel). 2025;15(2):210. doi: 10.3390/diagnostics15020210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Han S, Li XX, Wei S, et al. Orbitofrontal cortex-hippocampus potentiation mediates relief for depression: a randomized double-blind trial and TMS-EEG study. Cell Rep Med. 2023;4(6):101060. doi: 10.1016/j.xcrm.2023.101060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wirsich J, Giraud AL, Sadaghiani S. Concurrent EEG- and fMRI-derived functional connectomes exhibit linked dynamics. Neuroimage. 2020;219:116998. doi: 10.1016/j.neuroimage.2020.116998 [DOI] [PubMed] [Google Scholar]
- [11].Yao Z, Shi J, Zhang Z, et al. Altered dynamic functional connectivity in weakly-connected state in major depressive disorder. Clin Neurophysiol. 2019;130(11):2096–2104. doi: 10.1016/j.clinph.2019.08.009 [DOI] [PubMed] [Google Scholar]
- [12].Zhu DM, Yang Y, Zhang Y, et al. Cerebellar-cerebral dynamic functional connectivity alterations in major depressive disorder. J Affect Disord. 2020;275:319–328. doi: 10.1016/j.jad.2020.06.062 [DOI] [PubMed] [Google Scholar]
- [13].Jing R, Lin X, Ding Z, et al. Heterogeneous brain dynamic functional connectivity patterns in first-episode drug-naive patients with major depressive disorder. Hum Brain Mapp. 2023;44(8):3112–3122. doi: 10.1002/hbm.26266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hoheisel L, Kambeitz-Ilankovic L, Wenzel J, et al. Alterations of functional connectivity dynamics in affective and psychotic disorders. Biol Psychiatry Cognit Neurosci Neuroimaging. 2024;9(8):765–776. doi: 10.1016/j.bpsc.2024.02.013 [DOI] [PubMed] [Google Scholar]
- [15].Pang Y, Zhang H, Cui Q, et al. Combined static and dynamic functional connectivity signatures differentiating bipolar depression from major depressive disorder. Aust N Z J Psychiatry. 2020;54(8):832–842. doi: 10.1177/0004867420924089 [DOI] [PubMed] [Google Scholar]
- [16].Sha Q, Escobar Galvis ML, Madaj ZB, et al. Dysregulated placental expression of kynurenine pathway enzymes is associated with inflammation and depression in pregnancy. Brain Behav Immun. 2024;119:146–153. doi: 10.1016/j.bbi.2024.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yoshino J. Acmsd: a novel target for modulating NAD(+) homeostasis. Trends Endocrinol Metab. 2019;30(4):229–232. doi: 10.1016/j.tem.2019.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Schwarcz R, Bruno JP, Muchowski PJ, et al. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13(7):465–477. doi: 10.1038/nrn3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Grant RS, Coggan SE, Smythe GA. The physiological action of picolinic acid in the human brain. Int J Tryptophan Res. 2009;2:71–79. doi: 10.4137/IJTR.S2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Trepci A, Sellgren CM, Pålsson E, et al. Central levels of tryptophan metabolites in subjects with bipolar disorder. Eur Neuropsychopharmacol. 2021;43:52–62. doi: 10.1016/j.euroneuro.2020.11.018 [DOI] [PubMed] [Google Scholar]
- [21].Schwarcz R, Whetsell WO, Mangano RM. Quinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat brain. Science (New Y, NY). 1983;219(4582):316–318. doi: 10.1126/science.6849138 [DOI] [PubMed] [Google Scholar]
- [22].Yilmaz NS, Sen B, Karadag RF, et al. A kynurenine pathway enzyme aminocarboxymuconate-semialdehyde decarboxylase may be involved in treatment-resistant depression, and baseline inflammation status of patients predicts treatment response: a pilot study. J Neural Transm (Vienna). 2022;129(12):1513–1526. doi: 10.1007/s00702-022-02553-x [DOI] [PubMed] [Google Scholar]
- [23].Brundin L, Sellgren CM, Lim CK, et al. An enzyme in the kynurenine pathway that governs vulnerability to suicidal behavior by regulating excitotoxicity and neuroinflammation. Transl Psychiatry. 2016;6(8):e865. doi: 10.1038/tp.2016.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Won E, Kim YK. Stress, the autonomic nervous system, and the immune-kynurenine pathway in the etiology of depression. Curr Neuropharmacol. 2016;14(7):665–673. doi: 10.2174/1570159X14666151208113006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Myint AM, Kim YK. Network beyond IDO in psychiatric disorders: revisiting neurodegeneration hypothesis. Prog Neuro-Psychopharmacol Biol Psychiatry. 2014;48:304–313. doi: 10.1016/j.pnpbp.2013.08.008 [DOI] [PubMed] [Google Scholar]
- [26].Wang B, Lian YJ, Su WJ, et al. Hmgb1 mediates depressive behavior induced by chronic stress through activating the kynurenine pathway. Brain Behav Immun. 2018;72:51–60. doi: 10.1016/j.bbi.2017.11.017 [DOI] [PubMed] [Google Scholar]
- [27].Booij L, Wang D, Lévesque ML, et al. Looking beyond the DNA sequence: the relevance of DNA methylation processes for the stress-diathesis model of depression. Phil Trans R Soc Lond Ser B, Biol Sci. 2013;368(1615):20120251. doi: 10.1098/rstb.2012.0251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tao Y, Zhang H, Jin M, et al. Co-expression network of mRNA and DNA methylation in first-episode and drug-naive adolescents with major depressive disorder. Front Psychiatry. 2023;14:1065417. doi: 10.3389/fpsyt.2023.1065417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].van den Oord EJ, Clark SL, Xie LY, et al. A whole methylome CpG-SNP association study of psychosis in blood and brain tissue. Schizophr Bull. 2016;42(4):1018–1026. doi: 10.1093/schbul/sbv182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Walton E, Hass J, Liu J, et al. Correspondence of DNA methylation between blood and brain tissue and its application to schizophrenia research. Schizophr Bull. 2016;42(2):406–414. doi: 10.1093/schbul/sbv074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].McCartney DL, Hillary RF, Conole ELS, et al. Blood-based epigenome-wide analyses of cognitive abilities. Genome Biol. 2022;23(1):26. doi: 10.1186/s13059-021-02596-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Conole ELS, Robertson JA, Smith HM, et al. Epigenetic clocks and DNA methylation biomarkers of brain health and disease. Nat Rev Neurol. 2025;21(8):411–421. doi: 10.1038/s41582-025-01105-7 [DOI] [PubMed] [Google Scholar]
- [33].Koetsier J, Cavill R, Reijnders R, et al. Blood-based multivariate methylation risk score for cognitive impairment and dementia. Alzheimers Dement. 2024;20(10):6682–6698. doi: 10.1002/alz.14061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kaufman J, Wymbs NF, Montalvo-Ortiz JL, et al. Methylation in OTX2 and related genes, maltreatment, and depression in children. Neuropsychopharmacology. 2018;43(11):2204–2211. doi: 10.1038/s41386-018-0157-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chiarella J, Schumann L, Pomares FB, et al. Dna methylation differences in stress-related genes, functional connectivity and gray matter volume in depressed and healthy adolescents. J Affect Disord. 2020;271:160–168. doi: 10.1016/j.jad.2020.03.062 [DOI] [PubMed] [Google Scholar]
- [36].Diez I, Larson AG, Nakhate V, et al. Early-life trauma endophenotypes and brain circuit-gene expression relationships in functional neurological (conversion) disorder. Mol Psychiatry. 2021;26(8):3817–3828. doi: 10.1038/s41380-020-0665-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Choi JK, Teshome T, Smith J. Neighborhood disadvantage, childhood adversity, bullying victimization, and adolescent depression: a multiple mediational analysis. J Affect Disord. 2021;279:554–562. doi: 10.1016/j.jad.2020.10.041 [DOI] [PubMed] [Google Scholar]
- [38].Russell JD, Heyn SA, Peverill M, et al. Traumatic and adverse childhood experiences and developmental differences in psychiatric risk. JAMA Psychiatry. 2024;82(1):66. doi: 10.1001/jamapsychiatry.2024.3231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yu Z, Cao Y, Shang T, et al. Depression in youths with early life adversity: a systematic review and meta-analysis. Front Psychiatry. 2024;15:1378807. doi: 10.3389/fpsyt.2024.1378807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hawes MT, Schwartz HA, Son Y, et al. Predicting adolescent depression and anxiety from multi-wave longitudinal data using machine learning. Psychol Med. 2023;53(13):6205–6211. doi: 10.1017/S0033291722003452 [DOI] [PubMed] [Google Scholar]
- [41].Bao W, Gao Y, Feng R, et al. Negative family and interpersonal relationship are associated with centromedial amygdala functional connectivity alterations in adolescent depression. Eur Child Adolesc Psychiatry. 2024;33(12):4195–4204. doi: 10.1007/s00787-024-02456-0 [DOI] [PubMed] [Google Scholar]
- [42].Du Y, Liu J, Lin R, et al. The mediating role of family functioning between childhood trauma and depression severity in major depressive disorder and bipolar disorder. J Affect Disord. 2024;365:443–450. doi: 10.1016/j.jad.2024.08.155 [DOI] [PubMed] [Google Scholar]
- [43].Rinne GR, Mahrer NE, Guardino CM, et al. Childhood family stress modifies the association between perinatal stressful life events and depressive symptoms. J Fam Psychol. 2023;37(4):432–442. doi: 10.1037/fam0001076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Foulkes L, Blakemore SJ. Studying individual differences in human adolescent brain development. Nat Neurosci. 2018;21(3):315–323. doi: 10.1038/s41593-018-0078-4 [DOI] [PubMed] [Google Scholar]
- [45].Modabbernia A, Janiri D, Doucet GE, et al. Multivariate patterns of brain-behavior-environment associations in the adolescent brain and cognitive development study. Biol Psychiatry. 2021;89(5):510–520. doi: 10.1016/j.biopsych.2020.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Webb EK, Stevens JS, Ely TD, et al. Neighborhood resources associated with psychological trajectories and neural reactivity to reward after trauma. JAMA Psychiatry. 2024;81(11):1090. doi: 10.1001/jamapsychiatry.2024.2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Chad-Friedman E, Botdorf M, Riggins T, et al. Early childhood cumulative risk is associated with decreased global brain measures, cortical thickness, and cognitive functioning in school-age children. Dev Psychobiol. 2021;63(2):192–205. doi: 10.1002/dev.21956 [DOI] [PubMed] [Google Scholar]
- [48].Sacu S, Hermann A, Banaschewski T, et al. The long-term correlates of developmental stress on whole-brain functional connectivity during emotion regulation. Transl Psychiatry. 2025;15(1):152. doi: 10.1038/s41398-025-03374-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Gao Y, Feng R, Ouyang X, et al. Multivariate association between psychosocial environment, behaviors, and brain functional networks in adolescent depression. Asian J Psychiatr. 2024;95:104009. doi: 10.1016/j.ajp.2024.104009 [DOI] [PubMed] [Google Scholar]
- [50].Lock EF, Hoadley KA, Marron JS, et al. Joint and individual variation explained (JIVE) for integrated analysis of multiple data types. Ann Appl Stat. 2013;7(1):523–542. doi: 10.1214/12-AOAS597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].O’Connell MJ, Lock EF. R.jive for exploration of multi-source molecular data. Bioinformatics. 2016;32(18):2877–2879. doi: 10.1093/bioinformatics/btw324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kaufman J, Birmaher B, Brent D, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997. Jul 01;36(7):980–988. [DOI] [PubMed] [Google Scholar]
- [53].Jackson-Koku G. Beck depression inventory. Occup Med. 2016;66(2):174–175. doi: 10.1093/occmed/kqv087 [DOI] [PubMed] [Google Scholar]
- [54].Liu X, Liu L, Yang J, et al. Establishment and reliability and validity test of adolescent life events scale. Shangdong Psychiatry. 1997;10:15–19. [Google Scholar]
- [55].Boyd CP, Gullone E, Needleman GL, et al. The family environment scale: reliability and normative data for an adolescent sample. Fam Process. 1997;36(4):369–373. doi: 10.1111/j.1545-5300.1997.00369.x [DOI] [PubMed] [Google Scholar]
- [56].Staccini L, Tomba E, Grandi S, et al. The evaluation of family functioning by the family assessment device: a systematic review of studies in adult clinical populations. Fam Process. 2015;54(1):94–115. doi: 10.1111/famp.12098 [DOI] [PubMed] [Google Scholar]
- [57].Hu G, Su P, Sun Y, et al. Development and reliability and validity assessment of childhood chronic stress questionnaire. Chin J School Health. 2015;36(1):70–73. [Google Scholar]
- [58].Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- [59].Power JD, Mitra A, Laumann TO, et al. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hallquist MN, Hwang K, Luna B. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. Neuroimage. 2013;82:208–225. doi: 10.1016/j.neuroimage.2013.05.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Monakhova YB, Rutledge DN. Independent components analysis (ICA) at the “cocktail-party” in analytical chemistry. Talanta. 2020;208:120451. doi: 10.1016/j.talanta.2019.120451 [DOI] [PubMed] [Google Scholar]
- [62].Kuligowski J, Pérez-Guaita D, Sánchez-Illana Á, et al. Analysis of multi-source metabolomic data using joint and individual variation explained (JIVE). Analyst. 2015;140(13):4521–4529. doi: 10.1039/C5AN00706B [DOI] [PubMed] [Google Scholar]
- [63].Kang SJ, Leroux A, Guo W, et al. Integrative modeling of accelerometry-derived sleep, physical activity, and circadian rhythm domains with current or remitted major depression. JAMA Psychiatry. 2024. JUN;12(9):e241321. doi: 10.1001/jamapsychiatry.2024.1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8(1):118–127. doi: 10.1093/biostatistics/kxj037 [DOI] [PubMed] [Google Scholar]
- [65].Bayer JMM, Thompson PM, Ching CRK, et al. Site effects how-to and when: an overview of retrospective techniques to accommodate site effects in multi-site neuroimaging analyses. Front Neurol. 2022;13:923988. doi: 10.3389/fneur.2022.923988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Chen DT, Cheng SW, Chen T, et al. Identification of genetic variations in the NAD-related pathways for patients with major depressive disorder: a case-control study in Taiwan. J Clin Med. 2022;11(13):3622. doi: 10.3390/jcm11133622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Park C, Rosenblat JD, Brietzke E, et al. Stress, epigenetics and depression: a systematic review. Neurosci Biobehav Rev. 2019;102:139–152. doi: 10.1016/j.neubiorev.2019.04.010 [DOI] [PubMed] [Google Scholar]
- [68].Klengel T, Mehta D, Anacker C, et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. 2013;16(1):33–41. doi: 10.1038/nn.3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. 2014;140(3):774–815. doi: 10.1037/a0035302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Liu YJ, Kimura M, Li X, et al. Acmsd inhibition corrects fibrosis, inflammation, and DNA damage in MASLD/MASH. J Hepatol. 2025;82(2):174–188. doi: 10.1016/j.jhep.2024.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Bertollo AG, Mingoti MED, Ignácio ZM. Neurobiological mechanisms in the kynurenine pathway and major depressive disorder. Rev Neurosci. 2025;36(2):169–187. doi: 10.1515/revneuro-2024-0065 [DOI] [PubMed] [Google Scholar]
- [72].Cremers HR, Wager TD, Yarkoni T, et al. The relation between statistical power and inference in fMRI. PLOS ONE. 2017;12(11):e0184923. doi: 10.1371/journal.pone.0184923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Szucs D, Ioannidis JP. Sample size evolution in neuroimaging research: an evaluation of highly-cited studies (1990–2012) and of latest practices (2017–2018) in high-impact journals. Neuroimage. 2020;221:117164. doi: 10.1016/j.neuroimage.2020.117164 [DOI] [PubMed] [Google Scholar]
- [74].Abrishamcar S, Zhuang BC, Thomas M, et al. Association between maternal perinatal stress and depression and infant DNA methylation in the first year of life. Transl Psychiatry. 2024;14(1):445. doi: 10.1038/s41398-024-03148-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Rijlaarsdam J, Pappa I, Walton E, et al. An epigenome-wide association meta-analysis of prenatal maternal stress in neonates: a model approach for replication. Epigenetics. 2016;11(2):140–149. doi: 10.1080/15592294.2016.1145329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Mullin CR, Steeves JK. TMS to the lateral occipital cortex disrupts object processing but facilitates scene processing. J Cogn Neurosci. 2011;23(12):4174–4184. doi: 10.1162/jocn_a_00095 [DOI] [PubMed] [Google Scholar]
- [77].Yuan L, Chu Z, Chen X, et al. Structural neuroimaging and molecular signatures of drug-naive depression with melancholic features. Depress Anxiety. 2024;2024(1):9680180. doi: 10.1155/2024/9680180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Zhao Y, Chen L, Zhang W, et al. Gray matter abnormalities in non-comorbid medication-naive patients with major depressive disorder or social anxiety disorder. EBioMedicine. 2017;21:228–235. doi: 10.1016/j.ebiom.2017.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Zorlu N, Cropley VL, Zorlu PK, et al. Effects of cigarette smoking on cortical thickness in major depressive disorder. J Psychiatr Res. 2017;84:1–8. doi: 10.1016/j.jpsychires.2016.09.009 [DOI] [PubMed] [Google Scholar]
- [80].Bore MC, Liu X, Huang X, et al. Common and separable neural alterations in adult and adolescent depression - evidence from neuroimaging meta-analyses. Neurosci Biobehav Rev. 2024;164:105835. doi: 10.1016/j.neubiorev.2024.105835 [DOI] [PubMed] [Google Scholar]
- [81].Pang X, Wu D, Wang H, et al. Cortical morphological alterations in adolescents with major depression and non-suicidal self-injury. Neuroimage: Clin. 2024;44:103701. doi: 10.1016/j.nicl.2024.103701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Wang Y, Huang J, Zheng H, et al. Resting-state activity and functional connectivity of insula and postcentral gyrus related to psychological resilience in female depressed patients: a preliminary study. J Affect Disord. 2024;352:509–516. doi: 10.1016/j.jad.2024.02.076 [DOI] [PubMed] [Google Scholar]
- [83].Li Y, Richardson RM, Ghuman AS. Posterior fusiform and midfusiform contribute to distinct stages of facial expression processing. Cereb Cortex (New Y, NY: 1991). 2019;29(7):3209–3219. doi: 10.1093/cercor/bhy186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Wu QZ, Li DM, Kuang WH, et al. Abnormal regional spontaneous neural activity in treatment-refractory depression revealed by resting-state fMRI. Hum Brain Mapp. 2011;32(8):1290–1299. doi: 10.1002/hbm.21108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Tsai CF, Chuang CH, Tu PC, et al. Interaction of the gut microbiota and brain functional connectivity in late-life depression. J Psychiatry Neurosci: Jpn. 2024;49(5):E289–e300. doi: 10.1503/jpn.240050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Simpson CA, Diaz-Arteche C, Eliby D, et al. The gut microbiota in anxiety and depression - a systematic review. Clin Psychol Rev. 2021;83:101943. doi: 10.1016/j.cpr.2020.101943 [DOI] [PubMed] [Google Scholar]
- [87].Keustermans GC, Hoeks SB, Meerding JM, et al. Cytokine assays: an assessment of the preparation and treatment of blood and tissue samples. Methods. 2013;61(1):10–17. doi: 10.1016/j.ymeth.2013.04.005 [DOI] [PubMed] [Google Scholar]
- [88].Jammes M, Contentin R, Audigié F, et al. Effect of pro-inflammatory cytokine priming and storage temperature of the mesenchymal stromal cell (MSC) secretome on equine articular chondrocytes. Front Bioeng Biotechnol. 2023;11:1204737. doi: 10.3389/fbioe.2023.1204737 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data are in controlled access data storage at West China Hospital of Sichuan University.


