Abstract
Marek's disease virus (MDV) genetics has lagged behind that of other herpesviruses because of the lack of tools for the introduction of site-specific mutations into the genome of highly cell-associated oncogenic strains. Overlapping cosmid clones have been successfully used for the introduction of mutations in other highly cell-associated herpesviruses. Here we describe the development of overlapping cosmid DNA clones from a very virulent oncogenic strain of MDV. Transfection of these cosmid clones into MDV-susceptible cells resulted in the generation of a recombinant MDV (rMd5) with biological properties similar to the parental strain. To demonstrate the applicability of this technology for elucidation of gene function of MDV, we have generated a mutant virus lacking an MDV unique phosphoprotein, pp38, which has previously been associated with the maintenance of transformation in MDV-induced tumor cell lines. Inoculation of Marek's disease-susceptible birds with the pp38 deletion mutant virus (rMd5Δpp38) revealed that pp38 is involved in early cytolytic infection in lymphocytes but not in the induction of tumors. This powerful technology will speed the characterization of MDV gene function, leading to a better understanding of the molecular mechanisms of MDV pathogenesis. In addition, because Marek's disease is a major oncogenic system, the knowledge obtained from these studies may shed light on the oncogenic mechanisms of other herpesviruses.
Marek's disease virus (MDV), a highly cell-associated avian herpesvirus, is the etiological agent of Marek's disease (MD), a malignant T cell lymphoma (1–3). Since the late 1960s, MD has largely been controlled by vaccination with live attenuated or naturally avirulent strains (3–6). Nonetheless, field efficacy of existing vaccines is decreasing probably in large part as a result of the increasing virulence of MDV strains (7). An extensive knowledge about MDV gene function is crucial to the understanding of viral oncogenicity. The MDV genome codes for several unique proteins, some of which have been associated with the oncogenicity of the virus. Meq is the most extensively studied gene of MDV and it codes for a protein that shares significant homology to the jun/fos family of transcriptional factors (8). Meq is consistently expressed in all MDV-transformed cells, suggesting that it may play an important role in transformation (8). pp38 is a phosphoprotein expressed in both lytically infected and tumor cells (9). The function of this protein is still not clear but has been suggested to be involved in the maintenance of transformation (10). Understanding the role these proteins play in oncogenesis requires the introduction of mutations in the viral genome. The unavailability of the MDV genomic sequence and the strong cell-associated nature of the virus has, until now, hampered the ability to introduce site-specific mutations into the genome of oncogenic strains, making MDV genetics lag behind that of other herpesviruses.
Initial studies with purified MDV DNA showed that it is infectious in cell culture (11), indicating that cotransfection with a selectable marker could be used to generate MDV recombinants. Until recently, generation of mutant MDV viruses has routinely been done by the marker-rescue method (12–14). This method requires the introduction of a selectable marker in the mutant virus as well as several rounds of plaque purification. Because of the highly cell-associated nature of MDV, selection of recombinant viruses is an extremely laborious process, which can result in the introduction of spurious mutations elsewhere in the genome and sometimes attenuation of the selected recombinant virus. In addition, although the expression of a foreign marker gene has not impaired the oncogenic properties of the mutant viruses described thus far (13, 14), it is possible that introduction of a foreign gene in other regions of the genome might affect the viral phenotype.
Alternative methods for the generation of recombinant herpesviruses are the use of overlapping cosmid DNAs (15–18) and bacterial artificial chromosomes (BACs) (19–22). Advantages of these two methods are that plaque purification and insertion of selection markers are not required for the generation and identification of recombinant viruses. Attempts have been made by several laboratories to generate overlapping cosmid and BAC clones of oncogenic and nononcogenic strains of MDV. However, until now only the generation of an infectious BAC clone of an attenuated strain of MDV has been reported (23).
In the present report, we describe the successful construction of overlapping cosmid clones from a very virulent strain of MDV (Md5). To demonstrate the utility of this technology for studies of gene function, we generated a mutant virus lacking the MDV unique pp38 gene (rMd5Δpp38) and showed that in vivo, pp38 was not involved in tumorigenesis but played a role in early cytolytic infection in lymphocytes. Therefore, application of the technology described here will open the door to a better understanding of molecular mechanisms of MDV pathogenesis.
Materials and Methods
Construction of Recombinant Cosmid DNAs.
A very virulent strain of MDV, Md5 (24), was used for the construction of the overlapping cosmid clones. Viral DNA was purified from the cytoplasm of Md5 (passage 9)-infected duck embryo fibroblasts (DEFs) as described (25).
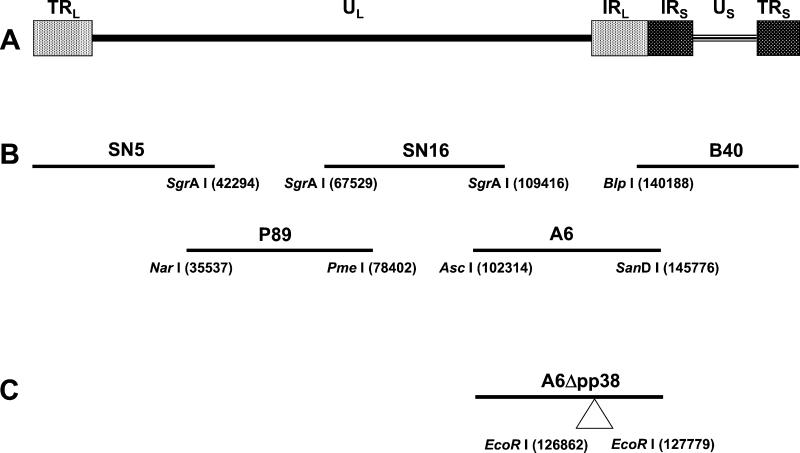
To generate overlapping fragments, viral DNA was digested with different restriction-enzyme combinations based on the MDV sequences (26, 27). The restriction enzymes used for cloning the overlapping cosmid DNAs and their locations in the viral genome were as follows: NarI (nucleotide 35537), SgrAI (nucleotides 42294, 67529, and 109416), PmeI (nucleotide 78402), AscI (nucleotide 102314), BlpI (nucleotide 140188), and SanDI (nucleotide 145776) (Fig. 1). After restriction-enzyme digestion, the DNA was blunt-ended with T4 DNA polymerase and ligated to NotI linkers. Ligated DNA was digested with NotI and inserted into NotI-digested SuperCos I cosmid vector (Stratagene). Ligation reactions were packaged with the Gigapack III Gold Packaging Extract (Stratagene) and inserted into HB101 Escherichia coli (Life Technologies, Rockville, MD). To identify bacterial clones containing correct viral DNA inserts, cosmid DNA was isolated, subjected to restriction enzyme digestion with different restriction enzymes, and the patterns obtained were compared with those estimated from the Md5 sequence (27). Clones with the correct restriction pattern for each region of the genome were selected and used for transfections.
Figure 1.
Organization of serotype 1 MDV viral genome. (A) The MDV genome consists of a unique long (UL) region flanked by inverted repeats, terminal repeat long (TRL), internal repeat long (IRL), and a unique short region (US) also flanked by two inverted repeats, internal repeat short (IRS) and terminal repeat short (TRS). (B) Schematic representation of the overlapping clones generated to reconstitute an infectious virus from a very virulent (vv) strain of MDV (Md5). The restriction enzymes used to generate the cosmid clones and their positions are indicated. (C) Location of EcoRI restriction sites in cosmid A6 used to delete the MDV-specific pp38 gene.
Mutagenesis of Cosmid A6.
Cosmid A6, containing the complete coding sequence of the MDV unique gene pp38, was used for the deletion of this gene by the RecA-assisted restriction endonuclease (RARE) cleavage method (28). Briefly, two oligonucleotides, pp38 ER3′ (5′-TAT TCG TAA AGG TGA GAA TTC GCT TAA TCT CCG CC-3′) and pp38 ER5′ (5′-TCG TGT TCT GCT TCG AAT TCC ATC ACC CCC TGC CG-3′), located at both ends of the pp38 gene, were used to protect two EcoRI sites (nucleotides 126862 and 127779) from methylation. The protected A6 cosmid was methylated with EcoRI methylase, digested with EcoRI, religated, packaged, and introduced into HB101 E. coli cells. A6 cosmid clones in which the pp38 gene had been deleted (A6Δpp38) were identified by the absence of a 917-bp fragment after digestion with EcoRI.
Transfections.
Parental and mutant cosmid DNAs were digested with NotI to release the viral insert and purified by phenol chloroform extraction and ethanol precipitation before transfection. Five hundred nanograms of each cosmid DNA along with 2 μg of sheared salmon sperm DNA were used to transfect 60-mm dishes containing 1.2 × 106 DEF cells by the calcium phosphate procedure as described (29). Four days after transfection, cells were trypsinized and seeded into a 100-mm dish and monitored for cytopathic effects. Viral stocks were subsequently made in DEF cells for further analysis.
Protein Analysis.
For indirect immunofluorescence assay (IFA), DEF cells were grown on 35-mm dishes, infected with MDV, and fixed 4–5 days later. IFA was done as described with mAbs H19 (pp38-specific) and T81 (ribonucleotide reductase-specific) at 1/500 dilution, and cells were analyzed by fluorescence microscopy (30). Radioimmunoprecipitation assays (RIPAs) were carried out by using H19 (pp38-specific that also coprecipitates pp24) and 2BN90 (pp40-specific) mAbs as described (31, 32).
Southern Blots.
Md5, rMd5, and rMd5Δpp38 viral DNA was isolated from infected cells as described above. Five micrograms of each viral DNA were digested with EcoRI, separated on a 1% agarose Tris-borate/EDTA (TBE) gel, and transferred to nylon membranes. [32P]dCTP-labeled probes from total genomic viral DNA (SN5, P89, SN16, A6, and B40 cosmid DNA fragments) or from pp38 (917-bp fragment) were generated by random priming, and hybridization was carried out with standard protocols.
Growth Curves.
Growth characteristics of Md5, rMd5, and rMd5Δpp38 were studied as described (15). Briefly, 100 plaque-forming units (pfu) of the different viruses were inoculated onto DEF cells seeded on 25-mm flasks. On days 1, 2, 3, 4, and 5 after inoculation the infected cells were trypsinized, serial dilutions were inoculated onto fresh DEF cells seeded on 35-mm plates, and plaques of the different dilutions were counted 7 days later.
In Vivo Experiments.
Chickens used in the study were Marek's disease-susceptible F1 progeny (15 × 7) of Avian Disease and Oncology Laboratory line 15I5 males and line 71 females. In the first experiment, chickens from breeder hens vaccinated with all three serotypes of MDV (Ab-positive) were wing-banded at hatch, randomly sorted into four experimental groups, and held in modified Horsfall–Bauer isolators for the duration of the experiment. One of the lots consisting of 20 chickens remained as a noninoculated control group. The rest of the groups were inoculated intraabdominally with 750 plaque-forming units (pfu) of either Md5 (passage 8) or one of the recombinant viruses (rMd5, rMd5Δpp38). All groups had 19–21 birds per group except wild-type Md5, which had only 12 birds. All birds that died during the trial or were killed at the end of the experiment (8 weeks after inoculation) were necropsied and evaluated for gross and histological lesions. In the second experiment, chickens from unvaccinated breeder hens free of antibodies to all three MDV serotypes (Ab-negative) were wing-banded at hatch and randomly sorted into three experimental groups and held in modified Horsfall–Bauer isolators. One group served as a negative control, the other two groups were inoculated either with 2,000 pfus of rMd5 or rMd5Δpp38. To study early cytolytic infection, samples from lymphoid organs (spleen, thymus, and bursa of Fabricius) were collected from 3 birds per treatment group at 6 days after inoculation. The expression of glycoprotein B was evaluated by immunohistochemistry with 1AN86 mAb (33). Serum samples were collected 5 weeks after inoculation from 5 chickens per group to evaluate for sero-conversion by IFA (30).
Results
Generation of Infectious MDV by Using Overlapping Cosmid DNAs.
Overlapping cosmid clones spanning the entire viral genome were digested with EcoRI or BamHI to verify that they had the predicted restriction pattern based on the Md5 sequence (27). Cosmid clones (SN5, P89, SN16, A6, and B40) (Fig. 1) were digested with NotI and transfected into DEF cells. Four days after transfection, the cells were trypsinized and seeded into a larger dish, and 5 days later these cells were used to make viral stocks. Typical MDV plaques, resulting from recombination of overlapping cosmid DNA fragments, were observed 12–13 days after transfection. Southern blot analysis of wild-type Md5 and rMd5 DNA digested with EcoRI showed the same restriction pattern, indicating that there had been no rearrangement in the recombinant viral DNA (Fig. 2).
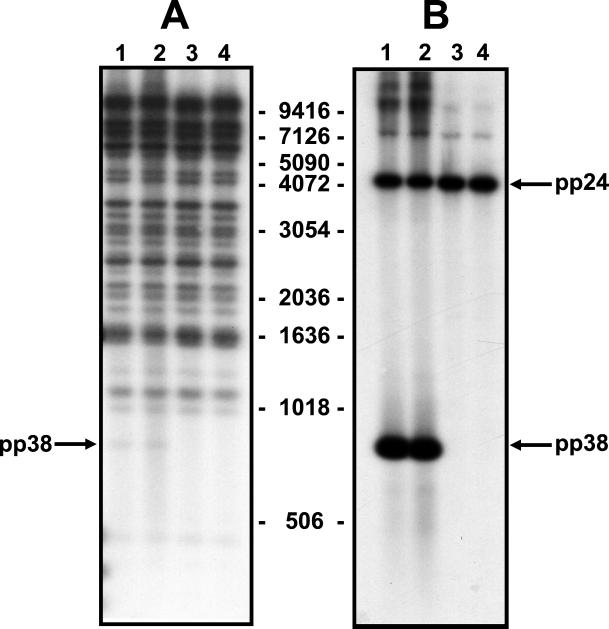
Figure 2.
Southern blot analysis of DNA from wild-type Md5 (lane 1), recombinant rMd5 (lane 2), and pp38 deletion mutants, rMd5Δpp38-19 (lane 3) and rMd5Δpp38-24 (lane 4). Viral DNA isolated from nucleocapsid preparations was digested with EcoRI and probed with all five radiolabeled cosmids (A) or a pp38 probe (B). The pp38 probe hybridizes to the pp38- and pp24-containing band, because both genes share 195 nucleotides. The size of the DNA markers is indicated in kb.
Biological Characterization of rMd5.
In vitro characterization of rMd5 showed that the growth properties of this recombinant virus were the same as wild-type Md5 (Fig. 3). To compare the pathogenic properties of rMd5 and wild-type Md5, both viruses were inoculated into Ab-positive chickens at day of age. At termination (8 weeks after inoculation), no significant differences were found in the pathogenesis of the rMd5 and the original low-passage Md5. Results showed that 65% (rMd5) and 41% (wild-type Md5) of the chickens developed gross visceral tumors. In addition, 100% of the chickens of both groups showed gross enlargement of the peripheral nerves (Table 1). These data show that the rMd5 virus generated from overlapping cosmid clones has similar pathogenic phenotype as wild-type Md5.
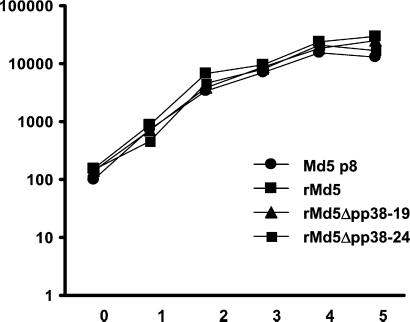
Figure 3.
In vitro growth properties of Md5, rMd5, and rMd5Δpp38. DEFs were infected with the indicated viruses, and infected cells were harvested on days 1, 2, 3, 4, and 5 after infection and titered on fresh DEF. Day 0 indicates the titer of the virus in the inoculum. The experiment was performed in duplicate, and the titer (logarithm of the mean number of plaque-forming units per dish) is indicated.
Table 1.
Pathological lesions of 15 × 7 birds inoculated with wild-type (Md5) and recombinant (rMd5, rMd5Δpp38) MDV viruses
| Virus | Gross lesions†
|
Microscopic lesions‡
|
|
|---|---|---|---|
| Visceral | Nerve | Vagus nerve | |
| None | 0/20 (0)* | 0/20 (0) | ND§ |
| rMd5Δpp38-19 | 1/19 (5.3) | 5/19 (26) | 9/19 (47) |
| rMd5Δpp38-24 | 0/21 (0) | 6/21 (28) | 11/21 (52) |
| rMd5 | 13/20 (65) | 20/20 (100) | ND |
| Md5 p8 | 5/12 (41) | 12/12 (100) | ND |
Data in parentheses indicate percentage of birds affected.
Visceral lesions indicate gross tumors, and nerve lesions are gross enlargements of the vagus, sciatic, or the brachial plexus.
Lymphocytic infiltration of the vagus nerve.
Not all the nerves were examined microscopically. ND, not determined.
Generation of pp38 Deletion Mutant rMd5 Virus (rMd5Δpp38).
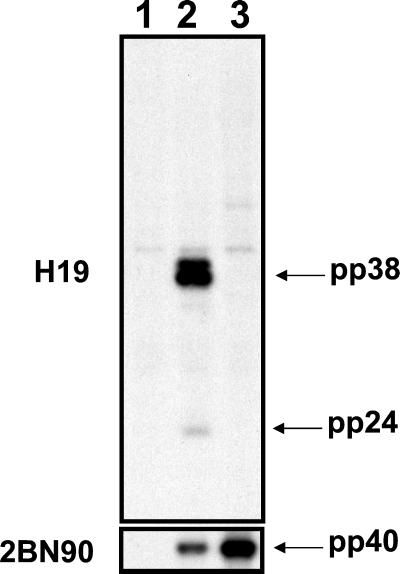
The MDV-specific pp38 gene was deleted from cosmid A6 by using the RecA-assisted restriction endonuclease cleavage method. The recombinant cosmid, A6Δpp38 (Fig. 1), was transfected into DEF cells in conjunction with parental SN5, P89, SN16, and B40 cosmid clones. rMd5Δpp38 plaques were evident 12–13 days after transfection. To confirm that the pp38 gene was not expressed, transfected cells were examined by IFA with H19 (pp38-specific) and T81 (ribonucleotide reductase-specific) mAbs. As expected, H19 did not stain the plaques induced by the rMd5Δpp38 mutant virus but plaques induced by the rMd5 virus were stained. On the other hand, T81 stained plaques from both rMd5 and rMd5Δpp38 viruses (data not shown). The results of the IFA were confirmed by performing a radioimmunoprecipitation assay of radiolabeled DEF-infected lysates prepared from rMd5 and rMd5Δpp38 viruses with H19 and 2BN90 mAbs (Fig. 4). It is important to note that, as expected, in the absence of pp38, pp24 is not coprecipitated by H19 (34). In addition, Southern blot analysis of DEF cells infected with two rMd5Δpp38 mutant viruses (derived from two independently generated mutant cosmid clones, 19 and 24), digested with EcoRI and probed with total viral DNA and a pp38-specific probe, confirmed the deletion of a 917-bp fragment corresponding to the pp38 gene and absence of rearrangement in the viral genome (Fig. 2). However, although the presence of additional mutations elsewhere in the genome could not be ruled out, the generation of two independently generated mutant viruses with the same phenotype makes it unlikely.
Figure 4.
Immunoprecipitation analysis of DEFs uninfected (lane 1) and infected with recombinant rMd5 (lane 2) and pp38 deletion mutant rMd5Δpp38-19 (lane 3). Immunoprecipitation of [35S]methionine-labeled cells was done with mAbs specific to pp38 (H19; Top) or pp40 (2BN90; Bottom). H19 not only immunoprecipitates pp38 but also coprecipitates pp24 in rMd5-infected cells. As expected, in the absence of pp38 (rMd5Δpp38), pp24 is not precipitated by H19. On the other hand, 2BN90 immunoprecipitates pp40 in both virus lysates.
Biological Characterization of rMd5Δpp38.
To determine whether the deletion of the pp38 gene had any effect on virus replication in vitro, the growth rate of two rMd5Δpp38 viruses (clones 19 and 24) were compared with those of rMd5 and wild-type Md5 in DEF as indicated in Materials and Methods. As seen in Fig. 3, the growth characteristics of all four viruses was similar, indicating that the absence of pp38 had no effect in viral replication in vitro.
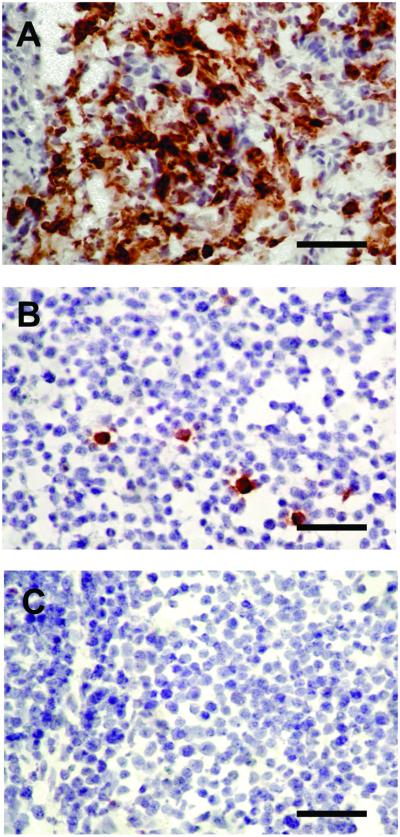
To determine the role of pp38 in early cytolytic infection, rMd5 and rMd5Δpp38 viruses were inoculated into MDV maternal Ab-negative, susceptible chickens at day of age. Results showed that at 6 days after inoculation, there was significant expression of glycoprotein B in the thymus (data not shown) and bursa of Fabricius of rMd5-inoculated birds but not in rMd5Δpp38-inoculated birds, showing that pp38 is involved in early cytolytic infection in lymphocytes (Fig. 5). Although very little viral replication was observed in lymphoid organs of rMd5Δpp38-inoculated chickens, all chickens tested sero-converted by 5 weeks after inoculation (data not shown).
Figure 5.
Immunohistochemical analysis of bursa of Fabricius. MDV maternal Ab-negative chickens were inoculated with rMd5 (A), rMd5Δpp38 (B), or mock-inoculated (C). Bursa of Fabricius were harvested 6 days after inoculation and stained with glycoprotein B mAb. Antigen expression is severely impaired in rMd5Δpp38, showing that pp38 is involved in early cytolytic infection in lymphocytes. (Bar = 100 μm.)
To compare the pathogenic properties of rMd5 and rMd5Δpp38, viruses were inoculated into Ab-positive chickens at day of age. At termination (8 weeks after inoculation), significant differences were found in the pathogenesis of rMd5Δpp38 compared with rMd5. Results showed that 13/20 (65%) of the birds inoculated with rMd5 developed gross visceral tumors, whereas only 1/19 (5.3%) of the rMd5Δpp38 (clone 19)-inoculated birds developed a gross ovarian tumor. Gross enlargement of the peripheral nerves could be detected in less than 28% of the birds inoculated with rMd5Δpp38 compared with 100% in rMd5-inoculated birds. However, histological examination of nerves indicated that about half of the birds inoculated with rMd5Δpp38 showed lymphoid infiltration (Table 1). These results indicate that pp38 is dispensable for tumor formation even though it is involved in early cytolytic infection in lymphocytes.
Discussion
MDV genetics has lagged behind that of other herpesviruses because of the lack of efficient tools for the introduction of specific mutations into the viral genome of oncogenic strains. Because of these difficulties, most MDV genes have been characterized only by in vitro methods (8, 9, 35, 36). To overcome these limitations, we have generated overlapping cosmid clones spanning the entire genome of a very virulent oncogenic strain of MDV (Md5). Transfection of MDV-susceptible cells with these overlapping cosmid clones resulted in the rescue of a recombinant virus (rMd5) with biological properties similar to that of the parental Md5 strain.
To validate the usefulness of this procedure, we have generated a mutant virus lacking the unique MDV gene pp38 (rMd5Δpp38). This gene codes for a 38-kDa phosphoprotein, which is expressed in lytically infected cells as well as in lymphoblastoid cell lines and tumors (37–39). By using oligodeoxynucleotides complementary to the translation initiation site of pp38 gene, Xie et al. (10) were able to inhibit lymphoblastoid cell proliferation, suggesting that pp38 might be involved in maintenance of transformation. However, a pp38 homologue gene is also present in nononcogenic serotype 2 strains (40), thus, its direct role in transformation is questionable. In vitro growth characteristics of the rMd5Δpp38 viruses were similar to those of parental rMd5, showing that pp38 is not essential for replication in cultured cells (Fig. 3). On the other hand, this virus was severely impaired for its ability to replicate in lymphoid organs as evidenced by lack of glycoprotein B expression at 6 days after inoculation, which is the peak of early MDV replication (Fig. 5). Although rMd5Δpp38 was severely impaired for in vivo replication, the virus retained a low level of oncogenicity and thus demonstrated that pp38 was dispensable for tumor induction. Our results add important information to the role of pp38 in MDV pathogenesis and show that although in vitro characterization of viral genes may provide important information about their function, generation of mutant viruses is essential to study gene function in vivo.
Recently, a tissue culture-attenuated strain of MDV (41) has been cloned into a BAC to generate an infectious virus (23). Although the MDV BAC clone was used to show that glycoproteins E and I are essential for cell-to-cell spread in cultured cells (42), it could not be used to study pathogenesis, because it was derived from a nononcogenic strain. Therefore, the cosmid clones described here are, currently, the only tool available to introduce site-specific mutations into oncogenic MDV without the need for repeated rounds of plaque purification and introduction of marker genes. Generation of additional MDV mutant viruses will allow for the studying of the role of individual MDV genes in replication, latency, and transformation. Because MDV is a major oncogenic system and in vivo characterization of mutant viruses now can be easily carried out, a better understanding of the mechanism of MDV transformation may also shed light on the transformation mechanisms of other herpesviruses.
Acknowledgments
We thank G. F. Kutish and D. L. Rock for providing the complete DNA sequence of MDV before publication. We also thank B. Coulson for excellent technical assistance and J. I. Cohen for helpful suggestions and advice during this study.
Abbreviations
- MDV
Marek's disease virus
- BAC
bacterial artificial chromosome
- DEF
duck embryo fibroblast
- IFA
immunofluorescence assay
References
- 1.Churchill A E, Biggs P M. Nature (London) 1967;215:528–530. doi: 10.1038/215528a0. [DOI] [PubMed] [Google Scholar]
- 2.Nazerian K, Solomon J J, Witter R L, Burmester B R. Proc Soc Exp Biol Med. 1968;127:177–182. doi: 10.3181/00379727-127-32650. [DOI] [PubMed] [Google Scholar]
- 3.Calnek B W, Witter R L. In: Diseases of Poultry. Calnek B W, Barnes H J, Beard C W, McDougald L R, Saif Y M, editors. Ames, IA: Iowa State Univ. Press; 1997. pp. 369–413. [Google Scholar]
- 4.Churchill A E, Chubb R C, Baxendale W. J Gen Virol. 1969;4:557–564. doi: 10.1099/0022-1317-4-4-557. [DOI] [PubMed] [Google Scholar]
- 5.Okazaki W, Purchase H G, Burmester B R. Avian Dis. 1970;14:413–429. [PubMed] [Google Scholar]
- 6.Rispens B H, VanVloten H J, Mastenbroek N, Maas H J L, Schat K A. Avian Dis. 1972;16:108–125. [PubMed] [Google Scholar]
- 7.Witter R L. Avian Dis. 1997;41:149–163. [PubMed] [Google Scholar]
- 8.Jones D, Lee L, Liu J L, Kung H J, Tillotson J K. Proc Natl Acad Sci USA. 1992;89:4042–4046. doi: 10.1073/pnas.89.9.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui Z Z, Lee L F, Liu J L, Kung H J. J Virol. 1991;65:6509–6515. doi: 10.1128/jvi.65.12.6509-6515.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie Q, Anderson A S, Morgan R W. J Virol. 1996;70:1125–1131. doi: 10.1128/jvi.70.2.1125-1131.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson M R, Coussens P M. Virology. 1991;185:673–680. doi: 10.1016/0042-6822(91)90538-m. [DOI] [PubMed] [Google Scholar]
- 12.Bublot M, Laplace E, Audonnet J C. Acta Virol. 1999;43:181–185. [PubMed] [Google Scholar]
- 13.Parcells M S, Anderson A S, Morgan T W. J Virol. 1995;69:7888–7898. doi: 10.1128/jvi.69.12.7888-7898.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parcells M S, Lin S F, Dienglewicz R L, Majerciak V, Robinson D R, Chen H C, Wu Z, Dubyak G R, Brunovskis P, Hunt H D, et al. J Virol. 2001;75:5159–5173. doi: 10.1128/JVI.75.11.5159-5173.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen J I, Seidel K E. Proc Natl Acad Sci USA. 1993;90:7376–7380. doi: 10.1073/pnas.90.15.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehsani M E, Abraha T W, Netherland-Snell C, Mueller N, Taylor M M, Holwerda B. J Virol. 2000;74:8972–8979. doi: 10.1128/jvi.74.19.8972-8979.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunningham C, Davison A J. Virology. 1993;197:116–124. doi: 10.1006/viro.1993.1572. [DOI] [PubMed] [Google Scholar]
- 18.van Zijl M, Quint W, Briaire J, de Rover T, Gielkens A, Berns A. J Virol. 1988;62:2191–2195. doi: 10.1128/jvi.62.6.2191-2195.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brune W, Messerle M, Koszinowski U H. Trends Genet. 2000;16:254–259. doi: 10.1016/s0168-9525(00)02015-1. [DOI] [PubMed] [Google Scholar]
- 20.Smith G A, Enquist L W. Proc Natl Acad Sci USA. 2000;97:4873–4878. doi: 10.1073/pnas.080502497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horsburgh B C, Hubinette M M, Tufaro F. Methods Enzymol. 1999;306:337–352. doi: 10.1016/s0076-6879(99)06022-x. [DOI] [PubMed] [Google Scholar]
- 22.Borst E M, Hahn G, Koszinowski U H, Messerle M. J Virol. 1999;73:8320–8329. doi: 10.1128/jvi.73.10.8320-8329.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schumacher D, Tischer B K, Fuchs W, Osterrieder N. J Virol. 2000;74:11088–11098. doi: 10.1128/jvi.74.23.11088-11098.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witter R L, Sharma J M, Fadly A M. Avian Dis. 1980;24:210–232. [Google Scholar]
- 25.Wesley R D, Tuthill A E. Prev Vet Med. 1984;2:53–62. [Google Scholar]
- 26.Lee L F, Wu P, Sui D, Ren D, Kamil J, Kung H J, Witter R L. Proc Natl Acad Sci USA. 2000;97:6091–6096. doi: 10.1073/pnas.97.11.6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tulman E R, Afonso C L, Lu Z, Zsak L, Rock D L, Kutish G F. J Virol. 2000;74:7980–7988. doi: 10.1128/jvi.74.17.7980-7988.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrin L J, Camerini-Otero R D. Science. 1991;254:1494–1497. doi: 10.1126/science.1962209. [DOI] [PubMed] [Google Scholar]
- 29.Moriuchi H, Moriuchi M, Smith H A, Straus S E, Cohen J I. J Virol. 1992;66:7303–7308. doi: 10.1128/jvi.66.12.7303-7308.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee L F, Liu X, Witter R L. J Immunol. 1983;130:1003–1006. [PubMed] [Google Scholar]
- 31.Silva R F, Lee L F. Virology. 1984;136:307–320. doi: 10.1016/0042-6822(84)90167-3. [DOI] [PubMed] [Google Scholar]
- 32.Lee L F. Avian Dis. 1993;37:561–567. [PubMed] [Google Scholar]
- 33.Gimeno I M, Witter R L, Hunt H D, Lee L F, Reddy S M, Neumann U. Vet Pathol. 2001;38:491–503. doi: 10.1354/vp.38-5-491. [DOI] [PubMed] [Google Scholar]
- 34.Cui Z, Qin A, Lee L F, Wu P, Kung H J. Acta Virol. 1999;43:169–173. [PubMed] [Google Scholar]
- 35.Liu J L, Lee L F, Ye Y, Qian Z, Kung H J. J Virol. 1997;71:3188–3196. doi: 10.1128/jvi.71.4.3188-3196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu P, Reed W M, Lee L F. Arch Virol. 2001;146:983–992. doi: 10.1007/s007050170130. [DOI] [PubMed] [Google Scholar]
- 37.Cui Z Z, Yan D, Lee L F. Virus Genes. 1990;3:309–322. doi: 10.1007/BF00569038. [DOI] [PubMed] [Google Scholar]
- 38.Ikuta K, Nakajima K, Naito M, Ann S H, Ueda S, Kato S, Hirai K. Int J Cancer. 1985;35:257–264. doi: 10.1002/ijc.2910350219. [DOI] [PubMed] [Google Scholar]
- 39.Nakajima K, Ikuta K, Naito M, Ueda S, Kato S, Hirai K. J Gen Virol. 1987;68:1379–1389. doi: 10.1099/0022-1317-68-5-1379. [DOI] [PubMed] [Google Scholar]
- 40.Izumiya Y, Jang H K, Ono M, Mikami T. Curr Top Microbiol Immunol. 2001;255:191–221. doi: 10.1007/978-3-642-56863-3_8. [DOI] [PubMed] [Google Scholar]
- 41.Dudnikov L A, Witter R L. In: 6th International Symposium on Marek's Disease. Schat K A, Morgan R M, Parcells M S, Spencer J L, editors. Montreal, QC, Canada: Am. Assoc. Avian Pathol.; 2000. pp. 249–255. [Google Scholar]
- 42.Schumacher D, Tischer B K, Reddy S M, Osterrieder N. J Virol. 2001;75:11307–11318. doi: 10.1128/JVI.75.23.11307-11318.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]