Abstract
Objective:
To describe clinical, surgical, and cosmetic outcomes in patients with germline mutations undergoing endoscopic nipple- and skin-sparing mastectomy (eNSM) with immediate reconstruction.
Methods:
We conducted a retrospective review of 6 patients (11 breasts) treated between March 2022 and May 2024. All patients had confirmed BRCA1, BRCA2, CHEK2, or MUTYH mutations. Data on operative time, bleeding, specimen weight, reconstruction satisfaction (BRECON 31), complications, and recurrence were collected.
Results:
All surgeries were completed without conversion to open surgery. Mean age was 41 ± 9.2 years; mean surgical time was 115.6 ± 11.0 minutes; mean blood loss was 110 ± 70.9 mL. No complications were reported. BRECON 31 satisfaction score at 6 months was 15.3/16. Median follow-up was 13 months with no recurrences.
Conclusions:
eNSM is a feasible and effective option for patients with high-risk genetic mutations, offering oncologic safety and high satisfaction in our initial Latin American experience.
Keywords: Breast cancer, Endoscopic mastectomy, Genetic mutation, Minimally Invasive Surgery, Reconstruction
INTRODUCTION
Endoscopic mastectomy has recently emerged as a minimally invasive option for the management of breast cancer, offering the benefits of reduced surgical trauma and better cosmetic outcomes without compromising oncologic prognosis.1,2 This approach is especially relevant for patients carrying genetic mutations such as BRCA1, BRCA2, CHEK2, and other less frequent mutations, which significantly increase the lifetime risk of developing breast cancer. These cases often justify risk-reducing or more aggressive therapeutic interventions, but not necessarily with greater aesthetic impact, which remains a key factor in decision-making when considering breast reconstruction.3–5
In this context, the application of minimally invasive techniques (robotic and endoscopic) enables precise dissection with adequate oncologic control through a small hidden incision, while achieving better aesthetic outcomes and reducing psychological, sexual, and quality-of-life impacts. Furthermore, the integration of genetic evaluation into the diagnostic and therapeutic process has revolutionized the surgical approach, allowing for the selection of patients who may benefit from less invasive procedures with improved cosmetic results. Several studies have reported promising outcomes with endoscopic mastectomy, suggesting that, in experienced hands, this technique can match the oncologic efficacy of conventional surgery while improving patient satisfaction when combined with immediate reconstruction.6–9
Here, we present our initial experience with endoscopic mastectomy in genetically predisposed patients, detailing clinical management and surgical outcomes.
MATERIALS AND METHODS
We conducted a retrospective review of 6 patients (11 breast procedures) between March 2022 and May 2024. Inclusion criteria were: confirmed germline mutation, breast cancer or risk-reducing indication, eligibility for nipple- and skin-sparing surgery, and informed consent.
Exclusion Criteria
Candidates for breast-conserving surgery, refusal of immediate reconstruction, prior chest wall irradiation, heavy smoking, or significant previous breast incisions for any reason.
Surgical Technique
A 2.5- to 4.5-cm axillary incision was made to insert a single-port system. Subcutaneous flaps were raised, and tumescent solution with lidocaine and epinephrine was infiltrated. CO2 insufflation was maintained at 7 mmHg. Dissection was performed using monopolar cautery and 5-mm scissors with a Maryland forceps for traction. The dissection began in the superficial flap through the outer quadrants, progressing toward the nipple. Samples from the posterior portion of the nipple were obtained and sent for intraoperative cytopathological analysis. Anterior dissection continued completely to create sufficient space to proceed with posterior dissection over the pectoralis major. The gland was extracted through the incision. The procedure included:
Dissection through subcutaneous and prepectoral planes, preserving the integrity of the skin and muscle.
In cancer cases: Excision of the mammary tissue with disease-free margins, confirmed by histopathological examination.
Sentinel lymph node biopsy and/or axillary dissection using a dual technique.
Detailed documentation of operative time, estimated blood loss, and intraoperative assessments of surgical extent.
Reconstruction
Immediate reconstruction was performed using prepectoral or dual-plane pockets, skin flaps were evaluated clinically (thickness, tissue quality, and preservation of the subdermal plexus) and with perfusion-assessing devices (thermography and/or ICG). After achieving adequate hemostasis a 15 Fr drain was placed, and implants/expanders were inserted using a 'no touch' funnel-assisted technique. Closure was completed in two layers using Monocryl 3-0.
RESULTS
See Table 1 for clinical and surgical characteristics of the included patients.
Table 1.
Summary of Clinical and Surgical Characteristics of Patients Undergoing Endoscopic Nipple-Sparing Mastectomy and Immediate Reconstruction
| # | Age | Genetic Mutation | Satisfaction (out of 16) | Follow-up (months) | Preoperative Therapy | Side | Surgical Time (min) | Tumor Size | Molecular Subtype | Type of Reconstruction | Specimen Weight (g) | Blood Loss (ml) | Complications |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 38 | BRCA1 | 11/16 | 25 | No | Right | 115 | NA | NA | Tissue expander | 170 | 100 | No |
| Left | 120 | NA | NA | Tissue expander | 210 | 100 | No | ||||||
| 2 | 41 | CHEK2 | 13/16 | 35 | No | Right | 100 | 24 × 10 mm | Luminal A | Tissue expander | 246 | 200 | No |
| 3 | 40 | BRCA2 | 16/16 | 12 | Neoadjuvant chemotherapy | Right | 125 | 23 × 19 + 9 × 10 mm | Luminal B | Tissue expander | 400 | <50 | No |
| Left | 125 | NA | NA | Direct implant | 420 | <50 | No | ||||||
| 4 | 26 | BRCA1 | 16/16 | 14 | No | Right | 98 | NA | NA | Tissue expander | 148 | 50 | No |
| Left | 120 | NA | NA | Tissue expander | 150 | 30 | No | ||||||
| 5 | 52 | MUTYH | 16/16 | 7 | Neoadjuvant chemotherapy | Right | 119 | NA | NA | Tissue expander | 220 | 180 | No |
| Left | 100 | 23 mm | Triple negative | Tissue expander | 230 | 250 | No | ||||||
| 6 | 49 | MUTYH | 16/16 | 11 | Neoadjuvant chemotherapy | Right | 121 | 11 × 8 mm | HER2 | Tissue expander | 159.8 | 100 | No |
| Left | 128 | NA | NA | Tissue expander | 165 | 100 | No |
Variables include age, genetic mutation, satisfaction score, follow-up time, type of preoperative therapy, laterality, operative time, tumor size, molecular subtype, reconstruction method, specimen weight, estimated blood loss, and complications. Demographics and surgical characteristics of patients undergoing endoscopic mastectomy. NA, not available; HER2, human epidermal growth factor receptor 2.
Clinical and Demographic Data
The group of patients analyzed displayed marked heterogeneity in both demographic and clinical characteristics. Ages ranged from young adulthood to middle age, with a mean age of approximately 41 years. Genetic evaluation revealed the presence of classical mutations such as BRCA1 and BRCA2, along with other alterations (e.g., CHEK2 and MUTYH mutations) commonly associated with a strong family history of breast cancer. Tumor presentation showed considerable variability in lesion size, with specific dimensions documented in several cases (e.g., 2.4 × 1 cm, 23 × 19 mm and 9 × 10 mm). These demographic and clinical characteristics provide a comprehensive view of the patients’ risk profiles and the diversity in tumor presentation among those undergoing this innovative surgical approach.
All bilateral cases in this series were conducted in a single operative session. Each breast was treated independently using the same standardized endoscopic technique, followed by immediate reconstruction on each side with either an implant or tissue expander, based on intraoperative assessment of flap viability. All surgeries were completed successfully without conversion. Mean age was 41 ± 9.2 years. Specimen weight averaged 230 ± 95.7 g, operative time was 115.6 ± 11.0 minutes, and blood loss was 110 ± 70.9 mL. No seromas or nipple necrosis occurred. Hospital stay was a maximum of 2 nights.
Cosmetic satisfaction was evaluated at 6 months using the BRECON 31 scale, yielding a mean satisfaction score of 15.3/16. Follow-up included clinical evaluation and labs every 3 months. Overall survival was 100%, with no local or distant recurrence observed during a median disease-free interval of 13 months (range 7–35; mean 17.7 months) (Figures 1 and 2).
Figure 1.
Patient with neurofibromatosis and BRCA1 mutation (A) Day of the surgery. (B) Four weeks after surgery (note how the arm naturally hides the incision).
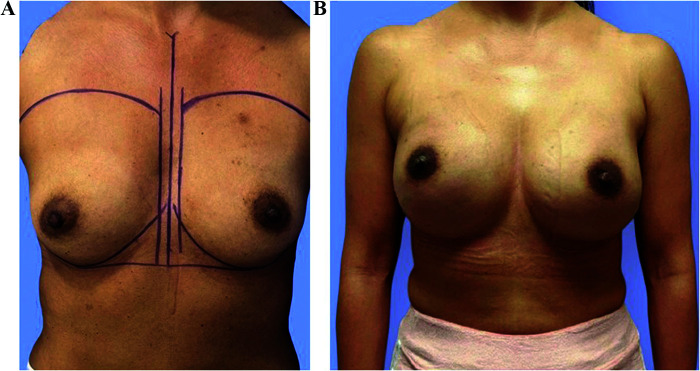
Figure 2.
Patient BRCA1 mutation (A) before and (B) four weeks after reconstruction.
DISCUSSION
This is, to our knowledge, the first report of routine single-port endoscopic mastectomy in genetically predisposed patients in America. Our results demonstrate acceptable operative time and blood loss, even when considering diverse anatomies and patient profiles.
Including patients with non-BRCA mutations like CHEK2 and MUTYH broadens the applicability of this approach, suggesting that this procedure may benefit a more heterogeneous group of high-risk patients, many of whom present a strong familial predisposition to breast cancer. This highlights the importance of integrating genetic data into surgical planning, allowing treatment to be personalized not only for oncologic safety but also for optimizing aesthetic outcomes and postoperative quality of life.
Published studies show endoscopic surgery may match conventional surgery in control and survival, while improving recovery.10
Moreover, the high aesthetic satisfaction reported through the BRECON test and the absence of complications underscore the positive impact of this technique on the overall patient experience. In recent years, more women carrying high-risk mutations such as BRCA1, BRCA2, PALB2, CHEK2, and others have chosen prophylactic mastectomy not only to reduce cancer risk but also for psychological reassurance. Meta-analyses show bilateral risk-reducing mastectomy lowers lifetime breast-cancer incidence by up to 95% in mutation carriers while improving anxiety and satisfaction scores.11 Nevertheless, the decision is deeply personal and multifactorial, balancing oncologic benefit with body-image concerns, recovery time and reconstructive expectations. Patient-specific anatomical factors—breast volume, ptosis, prior surgery or syndromic conditions—must guide surgical planning. In this setting, endoscopic nipple- and skin-sparing mastectomy (eNSM) provides a personalized, minimally invasive route that combines oncologic safety with superior aesthetic and psychological outcomes, especially valuable for young women and those with strong family history.4
Although the technique requires specific skills and careful patient selection, breast size is not an absolute contraindication. However, larger volumes may demand greater surgical experience and occasionally a slightly longer incision to allow safe specimen extraction. With precise handling of endoscopic equipment and appropriate training, our experience demonstrates that excellent outcomes in terms of oncologic safety and aesthetics are achievable and are expected to improve further along the learning curve.2,4,12
We acknowledge that the small sample size and short follow-up period limit the generalizability of our findings. Thus, prospective studies involving a larger cohort and longer follow-up are necessary to confirm the durability of outcomes in terms of recurrence, aesthetic results, and medium- to long-term quality of life.13
CONCLUSION
Minimally invasive breast surgery is becoming standard in various regions. To our knowledge, based on the current literature, we are the only center in Latin America performing this procedure routinely. Our experience supports the safety and effectiveness of single-port endoscopic mastectomy in patients with high-risk mutations.
In experienced hands, this technique achieves oncologic and risk-reduction outcomes comparable to conventional surgery, while offering significant advantages in terms of aesthetics and quality of life. Looking ahead, it is imperative to foster international collaborations and establish dedicated training centers to facilitate the widespread adoption of this technique.
Footnotes
Acknowledgments: We would like to thank the entire Breast Surgery, Anesthesiology, and Pathology Departments at Hospital H+ for their support. Special thanks to Dr. Denisse Sepúlveda for reviewing the manuscript.
Disclosure: none.
Conflict of interests: none.
Funding sources: none.
Contributor Information
Guillermo G. Peralta-Castillo, Breast Division, Hospital H+, Querétaro, Mexico. (Drs. Peralta, Cornejo, Valencia, and Bajonero); Breast Surgery Department, Breast Queretaro, Hospital Ángeles Centro Sur, Querétaro, Mexico. (Drs. Peralta, Bajonero, and Valladares).
Luis Miguel Cornejo-Mota, Breast Division, Hospital H+, Querétaro, Mexico. (Drs. Peralta, Cornejo, Valencia, and Bajonero); Plastic and Reconstructive Surgery Department, Breast Queretaro, Hospital Ángeles Centro Sur, Querétaro, Mexico. (Drs. Cornejo and Valencia).
Luis César Valencia-García, Breast Division, Hospital H+, Querétaro, Mexico. (Drs. Peralta, Cornejo, Valencia, and Bajonero); Plastic and Reconstructive Surgery Department, Breast Queretaro, Hospital Ángeles Centro Sur, Querétaro, Mexico. (Drs. Cornejo and Valencia).
Paulina Bajonero-Canónico, Breast Division, Hospital H+, Querétaro, Mexico. (Drs. Peralta, Cornejo, Valencia, and Bajonero); Breast Surgery Department, Breast Queretaro, Hospital Ángeles Centro Sur, Querétaro, Mexico. (Drs. Peralta, Bajonero, and Valladares).
Claudirocy Marely Valladares Yañez, Breast Surgery Department, Breast Queretaro, Hospital Ángeles Centro Sur, Querétaro, Mexico. (Drs. Peralta, Bajonero, and Valladares).
References:
- 1.Blay L, Larrañaga I, York E, Rabadán L. Endoscopic mastectomy in 10 steps. Cir Esp (Engl Ed). 2024;102(2):103. [DOI] [PubMed] [Google Scholar]
- 2.Peralta-Castillo GG, Cavazos-García R, Eulalia-Hernández E, et al. Mastectomía endoscópica por puerto único: técnica quirúrgica y primer caso en México. Cir Cir. 2020;88(Suppl 2):108–112. [DOI] [PubMed] [Google Scholar]
- 3.Lawrenson T, Blackmore T, Norman K, et al. Key factors in the decision-making process for mastectomy or reconstruction: a qualitative analysis. Breast. 2024;73:103600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rathat G, Blay L, Bakenga J, et al. Scarless preventive surgery. Int J Gynaecol Obstet. 2023;163(2):701–702. [DOI] [PubMed] [Google Scholar]
- 5.Panchal H, Pilewskie ML, Sheckter CC, et al. National trends in contralateral prophylactic mastectomy. J Surg Oncol. 2019;119(1):79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai HW, Chen ST, Lin YJ, et al. Minimal access breast surgery: 10-year experience. Front Oncol. 2021;11:739144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mok CW, Lai HW. Endoscopic-assisted surgery in breast cancer: 20-year review. Breast. 2019;46:144–156. [DOI] [PubMed] [Google Scholar]
- 8.Kim JH, Ryu JM, Bae SJ, et al. ; Korea Robot-endoscopy Minimal Access Breast Surgery Study Group. Minimal access vs conventional nipple-sparing mastectomy: a comparative study. JAMA Surg. 2024;159(10):1177–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wan A, Liang Y, Chen L, et al. Association of long-term oncologic prognosis with minimal access breast surgery vs conventional breast surgery. JAMA Surg. 2022;157(12):e224711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith J, Doe A, Zhang L, et al. Endoscopic mastectomy in breast cancer: a review. J Surg Oncol. 2021;118(3):123–130. [Google Scholar]
- 11.Ludwig KK, Neuner J, Butler A, Geurts JL, Kong AL. Risk reduction and survival benefit of prophylactic surgery in BRCA mutation carriers, a systematic review. Am J Surg. 2016;212(4):660–669. [DOI] [PubMed] [Google Scholar]
- 12.Lai HW, Mok CW, Chang YT, et al. Endoscopic breast-conserving surgery: outcomes and aesthetic evaluation. Eur J Surg Oncol. 2020;46(8):1374–1380. [Google Scholar]
- 13.Brown A, Lee C, Torres M, et al. Minimally invasive techniques in breast surgery. Breast J. 2019;25(5):640–646. [Google Scholar]