Abstract
Background:
Biological mesh derived from porcine small intestinal submucosa (SIS) has a higher porosity and is more hydrophilic than tissue derived from bovine and cow dermal tissues. Therefore, we believe SIS mesh will lead to a milder inflammatory reaction than other, polypropylene and polypropylene-polydioxanone meshes, fewer adhesions, and less mesh shrinkage.
Methods:
Ninety rats were divided randomly into three groups: in group 1, polypropylene mesh was implanted; in group 2, polypropylene-polydioxanone; and in group 3, the SIS mesh. The meshes were fixed intra-abdominally, in the upper part of the abdomen. Ten animals from each group were sacrificed on days 7, 28, and 60 after the implantation. Relaparotomy was performed, with a left paramedial incision and the adhesions formed were assessed according to the Surgical Membrane Study Group (SMSG) score, along with the percentage of shrinkage of the mesh, and any inflammation.
Results:
There were no differences in terms of inflammatory reaction or the formation of adhesions between the meshes tested on the 7th day after implantation. However, the shrinkage of the SIS mesh was more expressed. On days 28 and 60, the SIS mesh caused less inflammatory reaction and formation of adhesions in relation to the other meshes tested. On day 60, there was no significant difference in the size of the meshes.
Conclusion:
This study confirmed that, despite conflicting views on biological mesh, SIS mesh results in less inflammatory reaction, less adhesion formation, and a lesser degree of shrinkage, and can take its place in hernia repair.
Keywords: Adhesion, Biological polypropylene, Inflammatory reaction, Mesh, Polydioxanonemesh
INTRODUCTION
Synthetic mesh is the standard for treatment of ventral hernias.1 The main advantages of synthetic mesh are its strength and price, but it causes significant formation of adhesions, and creates fistulas and stiffness in the abdomen.2
Modification of the surface of polypropylene (PP) mesh can reduce the tissue reaction and the formation of adhesions. Therefore, various composite meshes are being introduced into clinical practice in order to reduce the inflammatory response and the formation of adhesions. Polydioxanone shows a lower level of inflammation in comparison with polyglactin,3 and a mesh is being introduced into clinical practice that is a combination of PP-polydioxanone. There is also a composite mesh being introduced with large pores, and prolene-monocryl residual PP after absorption with monocryl filaments (pologlecaprone) in order to improve the biocompatibility of synthetic meshes.4
However, synthetic meshes shrink over time, which affects the recurrence rate. In 58% PP meshes with small pores, and in 5% PP meshes with large pores, there was a tendency for more severe shrinkage.5 The causal factors of mesh shrinkage have not been researched, but it is thought that mesh shrinkage is the result of the contraction and physiological healing of the wound, and the size of the pores and mass of the mesh, whose role is crucial.6
Biological mesh has been developed in order to overcome the weaknesses of these standard meshes.7 This mesh is developed from biological sources, such as human dermal allografts, cow or pig skin, or sheep’s stomachs.8,9 Biological mesh derived from porcine small intestinal submucosa (SIS) has a higher porosity and is more hydrophilic, than tissue derived from bovine and cow dermal tissues.10
Therefore, we believe that SIS mesh will lead to a milder inflammatory reaction than other, PP and PP-polydioxanone meshes, with fewer adhesions, and less mesh shrinkage.
MATERIALS AND METHODS
This experimental study was conducted at the Department of Pathophysiology of the Veterinary faculty of the University of Sarajevo. The study protocol was approved by the Ethics Committee for Animal Experiments of the University of Sarajevo.
We kept 90 Wistar Albino rats, weighing 100–150 grams, in normal laboratory conditions before the surgery, housed in cages and adapted to the standard laboratory conditions (temperature 20°C to 24°C; 12 hours light, 12 hours darkness). All the rats that underwent surgery fasted for 24 hours before the procedure. Ninety rats were randomly divided into three equal groups: in group 1 Prolene (PP) mesh (Ethicon, Somerville, NJ) was implanted, in group 2 Proceed (PP-polydioxanone) (Ethicon), and in group 3 the Biosis Vidasis biological mesh (porcine SIS) (Beijing Biosis Healing Biological Technology Co., China) was implanted.
The Surgical Procedure
The rats were anaesthetized with ketamine (50 mg/kg), secured and placed on the operating table in the supine position, using adhesive tape. The animals’ abdomens were shaved and disinfected with a povidone-iodine solution, and dried with gauze. Laparotomy was undertaken by means of a medial incision about 4 cm in length. Then a full-thickness, abdominal midline fascia muscle peritoneum defect was created, measuring 3 × 2 cm.
Implantation of a mesh the same size as the defect was performed in the upper part of the abdomen and fixed to all the layers of the stomach wall with Prolene (Ethicon) 4-0. The incisions were closed with 3-0 continuous sutures. No antibiotic therapy was used before or after the surgery. During the observation period the animals were monitored and underwent clinical testing in order to observe any local or systemic complications.
Parameters of Monitoring
Ten animals from each group were sacrificed on days 7, 28, and 60 after the implantation. In order to view the whole abdomen, relaparotomy was performed with a left paramedial incision.
Formation of Adhesions
Formation of adhesions was assessed on the basis of the Surgical Membrane Study Group (SMSG) score (Table 1) after opening the abdomen.
Table 1.
Adhesion Score according to the Surgical Membrane Study Group
| Adhesion Characteristics | Score |
|---|---|
| Extent of site involvement | |
| None | 0 |
| <25% | 1 |
| <50% | 2 |
| <75% | 3 |
| 100% | 4 |
| Type | |
| None | 0 |
| Filmy, transparent, avascular | 1 |
| Opaque, translucent, avascular | 2 |
| Opaque, capillaries present | 3 |
| Opaque, larger vessels present | 4 |
| Tenacity | |
| None | 0 |
| Adhesion falls apart | 1 |
| Adhesion lysed with traction | 2 |
| Adhesion required with sharp dissection | 3 |
Mesh Shrinkage
Mesh shrinkage is defined as a relative loss of surface area in comparison with the original size of the mesh (%). After removing the mesh, it was measured, and the percentage of shrinkage determined in relation to its initial size.
Inflammatory Reaction
Histological testing was undertaken at the Polyclinic for Laboratory Diagnostics of the University Clinical Center Tuzla. Part of the tissue from the mesh was subjected to histological testing. Segments of tissue were fixed in formalin, moulded in paraffin blocks and sliced into 4-μm thick slices, and stained by the HE method for histopathological testing in order to establish the presence of the tissue reaction to the implanted mesh. Inflammatory reactions were graded semiquantitatively as mild, moderate and severe inflammations.
Statistical Analysis of Data
The results were expressed as the mean value and standard deviation. Shapiro-Wilk’s test was used to assess the data distribution. The Kruskal-Wallis test was used to compare inflammatory reactions, SMSG scores and the size of the mesh. Post hoc analysis was performed using Dunn’s post hoc test (with the Bonferroni correction). Differences at the level of P < .05 were considered statistically significant. Processing, graphic presentation of data, and testing of statistical hypotheses were performed using the program for statistical analysis of data “R.”
RESULTS
There were no intra- or postoperative mortalities, or complications related to the anesthesia or infections of the implanted prosthetic materials.
Inflammatory Reaction
The Kruskal-Wallis test showed that there was no statistically significant difference in the degree of inflammation between the three meshes being compared after 7 days (χ2 = 1.856 and P = .395).
The Kruskal-Wallis test showed statistically significant difference in the degree of inflammation between the three meshes after 28 days (χ2 = 7.4203 and P = .0245). After that, Dunn’s post hoc test was performed (with the Bonferroni correction) which showed that there was statistically significant difference between the SIS and the Prolene mesh, Z = −2.71, which in this case indicated that the SIS mesh had lower values in comparison with the Prolene mesh (P = .021).
The Kruskal-Wallis test showed that there was a statistically significant difference after 60 days (χ2 = 15,425 and P = .0004472). After that, Dunn’s post hoc test was performed (with the Bonferroni correction). There was a statistically significant difference between SIS and Prolene mesh and SIS and Proceed mesh after 60 days (Z = −3.78 and Z = −2.81), which indicates that the SIS group had lower values than the Prolene and Proceed groups, which can be seen in Table 2, because the median for SIS mesh is lower than the medians for the other two meshes (Figures 1–3).
Table 2.
Inflammatory Reaction, SMSG Score, and Size of Mesh during the Days Tested
| Inflammatory Reaction Scored | SMSG Score | Size of Mesh | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 7 Days | 28 Days | 60 Days | 7 Days | 28 Days | 60 Days | 7 Days | 28 Days | 60 Days | |
| SIS mesh | |||||||||
| N | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Mean | 5.4 | 3 | 3.1 | 475.8 | 349.9 | 422.2 | |||
| Median | 3 | 2 | 1 | 5 | 2.5 | 4 | 459 | 348.5 | 432 |
| SD | 2.46 | 3.3 | 2.28 | 63.11 | 39.55 | 44.62 | |||
| 95% CI for mean | 3.64–7.16 | 0.63–5.36 | 1.47–4.73 | 430.65–520.94 | 321.61–378.19 | 390.28–454.12 | |||
| Range | 1 | 1 | 1 | 7 | 8 | 6 | 192 | 122 | 140 |
| Minimum | 2 | 1 | 0 | 3 | 0 | 0 | 408 | 286 | 336 |
| Maximum | 3 | 2 | 1 | 10 | 8 | 6 | 600 | 408 | 476 |
| Proceed mesh | |||||||||
| N | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Mean | 4 | 3.3 | 8.9 | 563.5 | 338 | 340.5 | |||
| Median | 3 | 2 | 2 | 3.5 | 3 | 9 | 600 | 428.5 | 362 |
| SD | 1.33 | 2.32 | 2.47 | 48.4 | 160.26 | 81.5 | |||
| 95% CI for mean | 3.04–4.95 | 1.64–4.95 | 7.13–10.66 | 528.87–598.12 | 223.35–452.64 | 282.19–398.80 | |||
| Range | 1 | 0 | 2 | 4 | 8 | 8 | 114 | 400 | 256 |
| Minimum | 2 | 2 | 1 | 3 | 0 | 3 | 486 | 50 | 144 |
| Maximum | 3 | 2 | 3 | 7 | 8 | 11 | 600 | 450 | 400 |
| Prolene mesh | |||||||||
| N | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Mean | 5.5 | 3.9 | 4.5 | 590 | 432.4 | 395.5 | |||
| Median | 3 | 2 | 2 | 5.5 | 4.5 | 4 | 600 | 462.5 | 421.5 |
| SD | 2.72 | 3.07 | 1.27 | 31.62 | 116.16 | 101.99 | |||
| 95% CI for mean | 3.55–7.44 | 1.72–6.09 | 3.59–5.41 | 567.37–612.62 | 349.30–515.49 | 322.54–468.46 | |||
| Range | 1 | 1 | 1 | 11 | 8 | 4 | 100 | 380 | 309 |
| Minimum | 2 | 2 | 1 | 0 | 0 | 3 | 500 | 180 | 204 |
| Maximum | 3 | 3 | 2 | 11 | 8 | 7 | 600 | 560 | 513 |
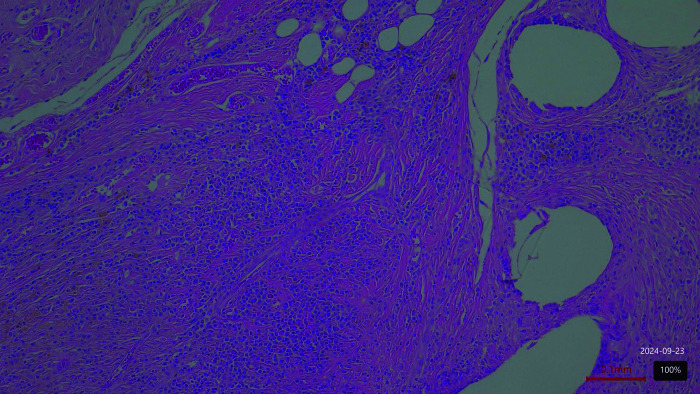
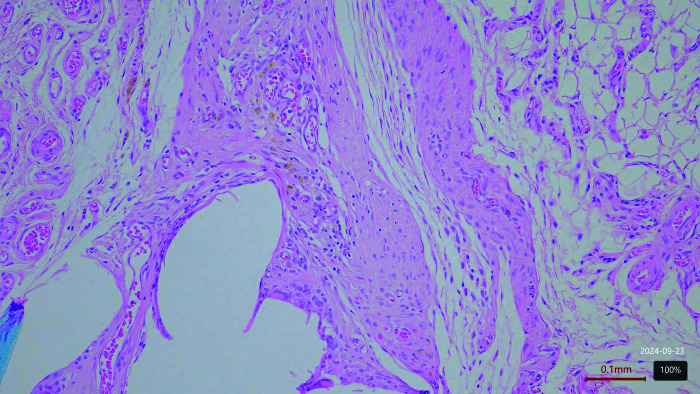
Figure 1.
Proceed mesh. Moderate inflammation on day 60 postimplantation.
Figure 2.
Prolene mesh. Moderate inflammation on day 60 postimplantation.
Figure 3.
Biological mesh. Mild inflammation on day 60 postimplantation.
Formation of Adhesions
The Kruskal-Wallis test showed that there was no statistically significant difference in the scores between the three meshes after 7 (χ2 = 4.282 and P = .117) and 28 days (χ2 = 0.685 and P = .7098), but after 60 days that there was a statistically significant difference (χ2 = 14.361 and P = .000761). After that, Dunn’s post hoc test was performed (with the Bonferroni correction). There was a statistically significant difference between the SIS and the Proceed mesh (P ≤ .001). This indicates that the values for the SIS mesh were significantly lower than for the Proceed mesh (Z = –3.52). There was no statistically significant difference between the SIS and the Prolene mesh (P = 1.00). There was a statistically significant difference between the Proceed and the Prolene groups, where the Proceed values were significantly higher than the Prolene mesh group (P = .0088) (Figure 4).
Figure 4.
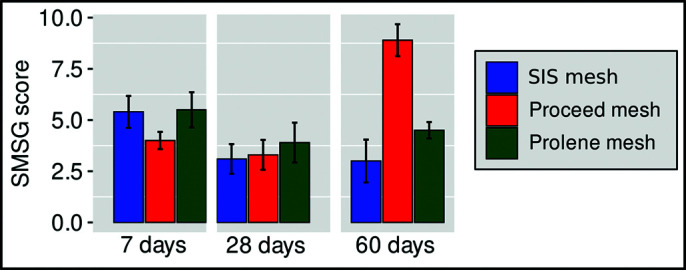
SMSG Score on days 7, 28, and 60 after implantation of meshes.
The Size of the Mesh
The Kruskal-Wallis test (χ2 = 16.013 and P = .000333) indicated that there was a statistically significant difference in the size of the mesh after 7 days. After that Dunn’s post hoc test was performed (with the Bonferroni correction). There was a statistically significant difference between the SIS and the Proceed mesh (P < .001), and the SIS and the Prolene mesh (P < .004). The values for the SIS mesh were significantly lower than for the Proceed (Z = −2.91) and the Prolene mesh (Z = −3.83). There was no statistically significant difference between the Proceed mesh and the Prolene mesh (Table 2 and Figure 5).
Figure 5.
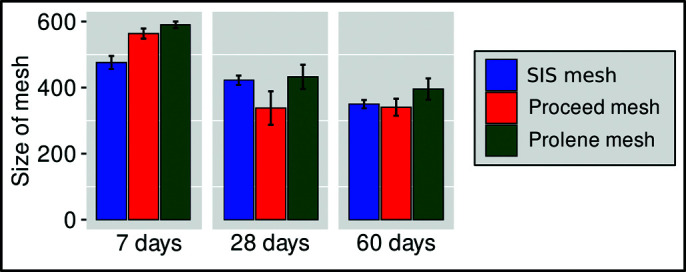
Size of meshes on days 7, 28, and 60 after implantation.
The Kruskal-Wallis test showed that there was no statistically significant difference in the size of the mesh after 28 days (χ2 = 3.17 and P = .204) and 60 days (χ2 = 4.88 and P = .0867) (Table 2 and Figure 5).
DISCUSSION
This experimental study conducted in physiological conditions, using the uncontaminated rat model, showed that the use of biological mesh in intraperitoneal conditions is possible.
Inflammatory reaction and the formation of adhesions can lead to potential complications. The degree of mesh shrinkage is also important.
The inflammatory reaction reached its peak on the seventh day after surgery, when there was no difference in the inflammatory reaction itself or the formation of adhesions between the SIS, Proceed and Prolene meshes. However, on the other tested days, 28 and 60, the SIS mesh had caused less inflammatory reaction and less formation of adhesion than the other two meshes.
Biological meshes are bio-compatible and produce a smaller inflammatory reaction in the body11 but the mesh has slightly greater shrinkage than synthetic mesh. This greater shrinkage was only observed on the 7th day. This, however, was less than the composite mesh, which shows that the biological mesh tested has good characteristics. On day 60 there was no significant difference in the size of the meshes.
Biological mesh has been promoted as resistant to infection and for the fact that it allows the wound to heal.12 Its development was prompted precisely by the complications that arise when using permanent meshes.13 Moreover, it also leads to significantly less adhesion and inflammatory reaction, as shown by this study.
Semiresorbable biological hybrid meshes show a lower degree of recurrence in comparison with completely resorbable meshes.14 It is said that their mechanical strength is lower than synthetic meshes.15 However, cross-linking the tested mesh lengthens its structural integrity (supporting fibroblast growth and resistance to collagenase enzymes).
The formation of adhesion was less than with the other two meshes tested. The antiadhesion layer that is placed on certain types of composite meshes is unnecessary.
Recent studies have shown a good response from strengthening biological meshes by embroidering with either PP or polyglycolic acid16 and it is possible to further improve the biological mesh tested. The relative benefits and risks in this manufacturing process have been widely discussed.17 SIS mesh has a higher porosity and is more hydrophilic, more easily wetted by body fluids and can better contact the repaired tissue than tissue derived from bovine and cow dermal tissues.10 Due to its excellent bioactivity, biocompatibility and biodegradability, this type of biological mesh is the optimal choice for staple line reinforcement patches.18
The major weakness of the cost of biological meshes is frequently mentioned. However, it has been shown that total hospital expenses are only 1.5 times greater.19 The surgeon’s choice of suitable mesh should be based on the expected surgical results and not the cost alone.20
This study has many limitations. A biological SIS mesh was compared with standard prolene and composite meshes, so that the conclusions of this study relate only to SIS biological meshes and not other types of biological mesh. Proportionally, the implanted meshes were much thicker in the rats than in humans. All the meshes were placed intraperitoneally, whilst in clinical conditions this would not be the case. Moreover, we were not able to provide a blinded independent surgeon for assessment of adhesions and the size of the meshes.21
For an evidence-based approach in the selection of the most appropriate mesh, and in future experimental studies, it may be useful to use the same mesh tissue integration index, focusing on the visible tissue ingrowth, the formation of adhesions, and mesh shrinkage. Its is also important also to test the mechanical strength of SIS mesh, what we did not do.
However, this study confirmed that the biological mesh tested has advantages despite the previous conflicting views on the use of biological mesh.22 The SIS mesh showed that, with a lower level of inflammatory reaction and formation of adhesion, but also the reduced level of shrinkage and its lower cost, it can take its place in hernia repair. For surgeons who are looking for options to avoid the unwanted consequences of standard synthetic meshes, biological meshes are an option.20
Footnotes
Disclosure: none.
Conflict of interests: none.
Funding sources: none.
Data availability: the data that support the findings of this study are available from the corresponding author, upon reasonable request.
Contributor Information
Samir Delibegovic, Surgical Department, University Clinical Centre Tuzla, Bosnia and Herzegovina. (Drs. Delibegovic, Idrizovic, and Kuralic); Faculty of Medicine, University of Tuzla, Tuzla, Bosnia and Herzegovina. (Drs. Delibegovic, Mustedanagic, and Cickusic).
Enes Idrizovic, Surgical Department, University Clinical Centre Tuzla, Bosnia and Herzegovina. (Drs. Delibegovic, Idrizovic, and Kuralic).
Muhamed Katica, Veterinary Faculty, University of Sarajevo, Bosnia and Herzegovina. (Drs. Katica and Katica).
Jasminka Mustedanagic, Faculty of Medicine, University of Tuzla, Tuzla, Bosnia and Herzegovina. (Drs. Delibegovic, Mustedanagic, and Cickusic); Polyclinic for Laboratory Diagnostics, University Clinical Centre Tuzla, Bosnia and Herzegovina. (Drs. Mustedanagic and Cickusic).
Elmir Cickusic, Faculty of Medicine, University of Tuzla, Tuzla, Bosnia and Herzegovina. (Drs. Delibegovic, Mustedanagic, and Cickusic); Polyclinic for Laboratory Diagnostics, University Clinical Centre Tuzla, Bosnia and Herzegovina. (Drs. Mustedanagic and Cickusic).
Aida Katica, Veterinary Faculty, University of Sarajevo, Bosnia and Herzegovina. (Drs. Katica and Katica).
Haris Kuralic, Surgical Department, University Clinical Centre Tuzla, Bosnia and Herzegovina. (Drs. Delibegovic, Idrizovic, and Kuralic).
References:
- 1.Mazzola Poli de Figueiredo S, Tastaldi L, Mao R-MD, et al. Biologic versus synthetic mesh in open ventral hernia repair: a systematic review and meta-analysis of randomized controlled trials. Surgery. 2023;173(4):1001–1007. [DOI] [PubMed] [Google Scholar]
- 2.Vorst AL, Kaoutzanis C, Carbonell AM, Franz MG. Evolution and advances in laparoscopic ventral and incisional hernia repair. World J Gastrointest Surg. 2015;7(11):293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacEwan M, Jeng L, Kovács T, Sallade E. Clinical application of bioresorbable, synthetic, electrospun matrix in wound healing. Bioengineering (Basel). 2022;10(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribeiro WG, Rodrigues DVS, Atta FFM, et al. Comparative study of peritoneal adhesions after intraperitoneal implantation in rats of meshes of polypropylene versus polypropylene/poliglecaprone versus polyester/porcine collagen. Acta Cir Bras. 2019;34(6):e201900603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanprasert P, Tepmalai K, Chakrabandhu B, et al. Collagen deposition and inflammatory response associated with macroporous mesh shrinkage in incisional hernia repair: a rat model. J Invest Surg. 2022;35(8):1635–1647. [DOI] [PubMed] [Google Scholar]
- 6.Klinge U, Park JK, Klosterhalfen B. The ideal mesh? Pathobiology. 2013;80(4):169–175. [DOI] [PubMed] [Google Scholar]
- 7.Kao AM, Arnold MR, Augenstein VA, Heniford BT. Prevention and treatment strategies for mesh infection in abdominal wall reconstruction. Plast Reconstr Surg. 2018;142(3 Suppl):149S–155S. [DOI] [PubMed] [Google Scholar]
- 8.Bochicchio GV, Garcia A, Kaufman J, et al. Evaluating the impact of technique and mesh type in complicated ventral hernia repair: a prospective randomized multicenter controlled trial. J Am Coll Surg. 2019;228(4):377–390. [DOI] [PubMed] [Google Scholar]
- 9.Doussot A, Abo-Alhassan F, Derbal S, et al. Indications and outcomes of a cross-linked porcine dermal collagen mesh (Permacol) for complex abdominal wall reconstruction: a multicenter audit. World J Surg. 2019;43(3):791–797. [DOI] [PubMed] [Google Scholar]
- 10.Xia L, Chen Y, Men F, et al. Comparative study on physical properties of different tissue/derived collagen biomaterials. Material Sci. 2017;7:431–439. [Google Scholar]
- 11.Wang S, Kim T, Zhu D. Hernia mesh and hernia repair. A review. Eng Regen. 2020;1:19–33. [Google Scholar]
- 12.Hassan AM, Asaad M, Liu J, Offodile AC, 2nd, Butler CE. Xenogeneic mesh provides safe and durable long-term outcomes in abdominal wall reconstruction of high-risk centers for disease control and prevention class III and IV defects. Ann Surg. 2022;3(2). [Google Scholar]
- 13.Fitzgerald JF, Kumar AS. Biologic versus synthetic mesh reinforcement: what are the pros and cons? Clin Colon Rectum Surg. 2014;27:140–148. [Google Scholar]
- 14.Goetz M, Jurczyk M, Junger H, et al. Semiresorbable biologic hybrid meshes for ventral abdominal hernia repair in potentially contaminated settings: lower risk of recurrence. Updates Surg. 2022;74(6):1995–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elango S, Perumalsamy S, Ramachandran K, Vadodaria K. Mesh materials and hernia repair. Biomedicine (TaipBei). 2017;7(3):16. [Google Scholar]
- 16.Overbeck N, Nagvajara GM, Ferzoco S, et al. In vivo evaluation of a reinforced ovine biologic: a comparative study to available hernia mesh repair materials. Hernia. 2020;24(6):1293–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ball CG, Kirkpatrick AW, Stuleanu T, et al. Is the type of mesh relevant in the prevention of recurrence following abdominal wall reconstruction? A randomized controlled trial. Cn J Surg. 2022;65:E451–E549. [Google Scholar]
- 18.Jing W, Huang Y, Feng J, et al. The clinical effectiveness of staple line reinforcement with different matrix used in surgery. Front Bioeng Biotechnol. 2023;11:1178619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katzen M, Ayuso SA, Sacco J, et al. Outcomes of biologic versus synthetic mesh in CDC class 3 and 4 open abdominal wall reconstruction. Surg Endosc. 2023;37(4):3073–3083. [DOI] [PubMed] [Google Scholar]
- 20.Morales-Conde S, Berrevoet F, Jorgensen LN, et al. Establishing peer consensus about the use of long-term biosynthetic absorbable mesh for hernia (grades 2-3) as the standard of care. World J Surg. 2022;46:2996–3004. [DOI] [PubMed] [Google Scholar]
- 21.Patiniott P, Stagg B, Karatassas A, Maddern G. Developing a hernia mesh tissue integration index using a porcine model – A pilot study. Front Surg. 2020;7:600195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Köckerling F, Alam NN, Antoniou SA, et al. What is the evidence for the use of biologic or biosynthetic meshes in abdominal wall reconstruction? Hernia. 2018;22(2):249–269. [DOI] [PMC free article] [PubMed] [Google Scholar]