Abstract
Functional neuroimaging studies implicate limbic and paralimbic activity in emotional responses, but few studies have sought to understand neurochemical mechanisms which modulate these responses. We have used positron emission tomography to measure μ-opioid receptor binding, and cerebral blood flow in the same subjects, and demonstrated that the baseline binding potential and the regional cerebral blood flow in the left inferior temporal pole are functionally related. Higher baseline μ-opioid receptor binding potential was associated with lower regional cerebral blood flow in this region during presentation of emotionally salient stimuli. This is consistent with an inhibitory/anxiolytic role of the endogenous opioid system in limbic regions of the temporal lobe and basal forebrain.
Neuroimaging studies have implicated temporal lobe and basal forebrain in the mediation of emotional responses. These large neuroanatomical regions contain central components of the limbic system, such as the amygdala and hippocampus, as well as associated limbic and paralimbic cortex (1). Functional neuroimaging studies, along with lesion data and animal research, have began to illuminate specific roles of limbic structures in various components of emotional processing, including the role of extended amygdala in salience (2, 3) and the role of orbitofrontal cortex in processing reward contingencies (4). A more precise understanding of the specific roles of limbic regions is essential for a better understanding of pathological conditions involving emotional dysregulation, e.g., psychiatric disorders. Functional studies of limbic structures in psychiatric disorders have just begun, but initial findings suggest that abnormal regional cerebral blood flow (rCBF) in these regions might be characteristic of specific diagnoses (5–7) or, alternatively, specific psychiatric symptoms, cutting across diagnostic boundaries (8, 9). Although such findings suggest hypo- or hyperactivity of limbic regions in a particular disorder, pathophysiologic processes giving rise to abnormal rCBF remain poorly understood. The study of neurotransmitter systems in vivo can provide an important link between cellular neurochemistry and functional neuroanatomy; although relatively few studies have attempted to make such links so far. The development of positron-emitting radiotracers designed for characterizing specific receptor sites, which can probe the neurochemistry of limbic systems in vivo, provides an attractive tool to combine with functional activation studies of limbic brain.
Work in our laboratory has implicated the extended amygdala in the mediation of aversive emotional responses, such as fear or disgust (2, 10, 11), consistent with both the animal literature (12, 13) and with other imaging studies (14, 15). Among the various neurotransmitter systems implicated in the regulation of limbic and paralimbic regions, the endogenous opioid system and μ-opioid receptors have been implicated in the modulation of fear (16), the suppression of affective defense behavior (17) and emotions (18) and in the regulation of the anxiolytic effects of benzodiazepines (19). High μ-opioid receptor density has been demonstrated in limbic and paralimbic regions, including the amygdala (20), by using a specific μ-opioid receptor PET ligand: [11C]carfentanil ([11C]CFN). We hypothesized that μ-opioid receptor binding and the rCBF response to emotional stimuli in amygdaloid region are related functionally, such that higher rCBF response in the amygdaloid region would be associated with lower μ-opioid receptor binding potential (BP) at baseline. To test this hypothesis, and to examine functional relationships between amygdaloid μ-opioid receptors and amygdaloid activation in response to aversive stimuli, we performed serial positron emission tomography (PET) studies in the same subjects. We obtained measures of μ-opioid receptor availability with [11C]CFN at baseline, and performed [15O]H2O blood flow activation studies, by using our previously validated activation paradigm with salient aversive stimuli.
Methods
Subjects.
Twelve healthy male volunteers were recruited from community advertisements (mean age = 45.8 ± 5.6 y). Subjects had no past or current history of chronic medical or neurological illness. Absence of an Axis I psychiatric disorder was verified by a structured clinical interview (21). All subjects gave written, informed consent after explanation of the experimental protocol, as approved by the local Institutional Review Board. Subjects were compensated $200 for their participation in this study.
Psychophysiology.
Skin conductance was recorded by using an MP-100 psychophysiological monitoring system (BioPac Systems, Santa Barbara, CA). Ag/AgCl electrodes filled with isotonic electrolyte jelly (KY jelly, Personal Product, Skillman, NJ) were attached to the volar surface of the second phalanx of the first and third fingers of the left hand. Waveform peak and integral during image presentation were analyzed, relative to a 1-sec baseline before image presentation.
[11C]CFN Protocol.
PET scans were begun at 9:00 a.m. by using a Siemens ECAT EXACT-scanner, while subjects were resting quietly, with eyes open. Subjects were positioned in the PET scanner gantry, using the orbito-meatal line as a reference line. A forehead restraint was used to reduce intrascan movement. [11C]CFN, a selective μ-opioid receptor radiotracer was synthesized at high specific activity (>1,000 Ci/mmol) (22) with minor modifications to improve its synthetic yield. Up to 18 mCi were administered, with a maximum mass injected of 0.03 μg/kg/study. This dose ensured that the compound was administered in true tracer quantities, therefore eliminating significant changes in receptor occupancy during the scan and physiological effects at the dose injected. The dose (55%) was injected as a bolus, with the remainder administered as a continuous infusion by using a computer-controlled, automated pump. Nineteen sets of PET scans in three-dimensional acquisition mode were acquired over 70 min with an increasing duration (30 sec up to 5 min). Scans were decay corrected and reconstructed by filtered back-projection by using a Hanning 0.5, in a 24 × 24 cm field-of-view, with scatter and attenuation correction (68Ge source). The dynamic scans were subsequently aligned to each other and reoriented to the intercommissural line (anterior commissure-posterior commissure line) by using fully automated algorithms (23).
Activation Protocol.
Subjects returned to the PET suite in the early afternoon (≈12:30 p.m.) after having a standardized light lunch (sandwich and juice) with no caffeine content.
Stimuli.
Subjects viewed two sets of complex visual images and a third set of blank screens as a control condition. The images were selected from the standardized international affective picture system. One set contained negatively valenced, aversive images, such as facial mutilation, wounds, and dead bodies. A second set contained neutral images of common objects, which did not elicit strong emotions, such as faces with neutral expressions and benign scenes. Because processing of faces recruits specific brain regions (24), we matched these two sets with respect to the number of slides portraying faces and human figures. In addition, to control for potential effects of differential color composition, e.g., more red in aversive images, all images were rendered in black and white. The set of blank images consisted of a fixation cross on four different gray fields of varying saturation. We analyzed and matched the image sets, including the blanks, on overall luminance (photoshop 4.0, Adobe Systems, Mountain View, CA).
Scanning Sequence and Stimulus Presentation.
We acquired PET data, psychophysiological data, and subjective responses while subjects viewed images with aversive emotional content, neutral emotional content, or blank content, in a block design. Images were presented by using superlab (Cedrus, Phoenix) and displayed on an LCD computer monitor suspended 40 cm from the subject, at a 15° viewing angle. Fourteen images per block were displayed for 5 sec each, with no interstimulus interval. The first block always consisted of neutral stimuli without PET image acquisition to allow the subjects to adjust to the scanner and to determine the precise timing of radiotracer arrival to the brain. Acquired data consisted of two runs of the three conditions, in the same order, with this order counterbalanced across subjects.
Behavioral Task.
Subjects verbally rated the pictures for aversive content on a 5-point scale: from “1,” not at all unpleasant to “5,” extremely unpleasant. During the blank condition, subjects were instructed to say “3” each time the fixation cross blinked (every 5 sec). Subjects rated their emotions after each set of stimuli by using the modified positive affectivity–negative affectivity scale (PANAS) (25) to assess the overall emotional tone immediately after presentation of the stimuli.
[15O]H2O Data Acquisition.
A transmission scan was acquired before [15O]H2O scans, as described above. For emission scans, subjects were given an i.v. bolus injection of 10 mCi of [15O]H2O. Data were collected in three-dimensional acquisition mode, as a single 60-sec frame beginning 2 sec after the arrival of the radioactivity in the brain. Lights were dimmed and ambient noise (from cooling fans) was minimal. Scans were separated by 12 min. Stimuli presentation began 5 sec before image acquisition. Either preceding or following the six scans described above, four additional scans were acquired for each subject by using different conditions, the results of which will not be discussed here.
Analysis
[11C]CFN Data Processing.
Image data were transformed, on a pixel-by-pixel basis into two sets of parametric maps: (i) a tracer transport measure (K1 ratio), which is proportional to cerebral blood flow (tracer transport = blood flow x extraction), and (ii) a receptor-related measure. Tracer transport and binding measures were calculated by using a modified Logan plot (26) on a pixel-by-pixel basis with occipital cortex, an area devoid of μ-opioid receptors (20), as reference region. The Logan plot becomes linear by 10 min after the start of radiotracer administration with a slope proportional to the (Bmax/Kd) + 1 for this receptor site [distribution volume (DV) ratio], where Bmax/Kd is the receptor measure (referred to as “binding potential” or in vivo receptor availability in the text). K1 images were first aligned to the intercommissural line and nonlinearly warped to stereotactic coordinates, as previously described (23, 27) Using this transformation matrix from the K1 image, the coregistered DV ratio images were then anatomically normalized.
The measurements of μ-opioid receptor BP were obtained at baseline (≈150 min before the rCBF studies), and there is no reason to expect systematic rCBF changes (and hence changes in tracer transport) during the receptor quantification studies. CFN is relatively insensitive to blood flow changes induced during the receptor measurement, when appropriate kinetic techniques are used in the analyses. We have performed extensive computer simulations to determine the influence of changes in rCBF on BP, with most regions showing less than a 1% bias in Bmax/Kd with a 10% change in flow. In other words, a 5% increase in flow, in the typical range observed in cognitive challenges, caused a 0–0.5% positive bias in Bmax/Kd, independent of whether Bmax/Kd also increased, decreased, or remained unchanged. The total mass of CFN injected was 0.028 ± 0.004 g/kg/scan, ensuring that the compound was administered in tracer quantities, i.e., subpharmacological doses. We also calculated expected percentage occupancy of μ-opioid receptors at peak regional CFN concentrations by using the mass of CFN and the known concentration of μ-opioid receptors in the human brain (postmortem) (28, 29). For areas with high receptor binding, the average percentage occupancy was estimated to be 0.2–0.5%, These doses and receptor occupancies do not induce changes in physical state (e.g., side-effects), nor do they induce more subtle analgesic, respiratory, or pupilary changes.
[15O]H2O Data Processing.
Analysis first used a standardization process that enabled averaging of image data both within and across subjects. Automated routines normalized images proportionally to the mean of global activity (arbitrarily set to 1,000), and individual images within each subject were coregistered to the fourth scan to correct for head movement that may have occurred between scans (usually <2-mm translation and <2° rotation). A nonlinear transformation was applied to each image to standardize individual anatomy according to a reference brain atlas (23, 27, 30). Standardized images were smoothed with a three-dimensional 9-mm Gaussian filter, yielding a final, effective full-width half-maxima of ≈14 mm. Difference images were made by subtracting scans obtained in different conditions, and expressed as Z scores, using the between-subjects variance (31, 32).
[15O]H2O Data Analysis.
We searched for significant activations on the basis of regional a priori hypotheses as well as by an image-wide search unconstrained by regional predictions. Within the a priori defined volume (≈120 ml), which consisted of medial frontal cortex (including rostral anterior cingulate gyrus), bilateral temporal poles (including amygdaloid regions and associated cortex), hypothalamus, and ventral forebrain, we set a significance threshold of Z = 3.09 (0.001 probability of type I error, uncorrected). We accepted 3.1 > Z > 2.9 as trend-level activations (P < 0.005, uncorrected) in the region of interest, to permit comparisons with other published work. For the image-wide search, we reported activations that exceeded a corrected probability of P < 0.05. This generally corresponded to Z score of ≈4.4 for our image sets by using a statistical threshold estimated using the Euler characteristic (32), number of pixels in the gray matter, and the image smoothness (31).
Correlation Analysis.
Correlation coefficients for the CFN BP in the regions that demonstrated activation during the [15O]water studies were correlated with rCBF activity during stimuli presentation. Both the total rCBF and the contrasts (aversive minus blanks and nonaversive minus blanks) were examined. BP for volumes of interest (VOI) were calculated within 13.5-mm sphere (6 voxels) placed on the stereotactic coordinates of activation foci obtained in the activation studies. The VOI was also positioned on the anatomically normalized DV ratio binding maps from the CFN study. μ-Opioid receptor availability data are expressed as the mean + SD of Bmax/Kd values.
Results
Behavioral and Psychophysiological Results.
Results from the online subjective rating of aversiveness of the stimuli, and from the skin conductance recording, verified the intended manipulation of emotional content. Our subjects experienced the aversive pictures as significantly more disturbing than the nonaversive pictures (t = 6.41, df = 10, P < 0.0001) (Table 1). In concert, significantly larger increases in skin conductance (F = 9.0, df = 2, P < 0.01) were found for aversive stimuli as compared to neutral and blank conditions. The overall emotional tone during the presentation, as reflected in composite negative and positive valence factors from the modified PANAS (see Methods), also confirmed sustained differential emotional states during block presentations (Table 1). After the presentation of aversive sets, our subjects reported a more negative mood as compared to the nonaversive and blank sets (t = 3.96, df = 10, P < 0.005 and t = 4.05, df = 10, P < 0.005, respectively). Accordingly, the subjects scored their mood as more positive during the neutral set than during the aversive set (P < 0.07 trend level). We could not detect any behavioral or pharmacological effects of CFN administration that could affect the afternoon rCBF studies.
Table 1.
Behavioral and psychophysiologic measures: aversive, nonaversive, and blank conditions
| Aversive, mean ± SE | Nonaversive, mean ± SE | Blank, mean ± SE | |
|---|---|---|---|
| Subjective rating* | 3.03 ± 1.02 | 1.05 ± 0.12 | — |
| PANAS positive† | 3.12 ± 1.28 | 3.97 ± 1.99 | 3.09 ± 1.97 |
| PANAS negative‡ | 3.47 ± 1.91 | 1.14 ± 0.16 | 1.08 ± 0.18 |
| Skin conductance, integral§ | 31.44 ± 8.70 | 12.73 ± 5.34 | 8.14 ± 4.70 |
| Skin conductance, peak§¶ | 1.33 ± 0.28 | 0.55 ± 0.21 | 0.54 ± 0.18 |
P < 0.0001.
P < 0.07 nonaversive > aversive.
P < 0.0005.
In μmho.
P < 0.01 aversive > nonaversive and blanks.
Neuroimaging Results
[15O]H2O Studies.
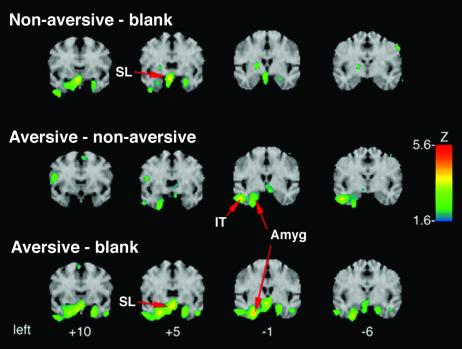
All complex visual stimuli (both aversive and nonaversive) activated the sublenticular extended amygdala (SLEA) region as compared to the blank condition, replicating our previous findings (Table 2 and Fig. 1). Aversive stimuli, as compared to blanks, activated an area in the left amygdala, which was not present in the nonaversive minus the blank condition. A direct comparison of aversive and nonaversive conditions revealed an additional activation peak in the infero-temporal pole. This activation focus extended at least 8 mm on the A–P plane from the lateral nucleus of the amygdala posteriorly to inferior temporal gyrus (see Fig. 1). All three activation peaks reported here fell within the a priori regions of interest containing limbic structures implicated in emotional processing.
Table 2.
Activation foci to aversive, nonaversive, and blank conditions
| Region | Aversive–blank
|
Nonaversive–blank
|
Aversive–nonaversive
|
|||
|---|---|---|---|---|---|---|
| x, y, z* | Z score† | x, y, z* | Z score† | x, y, z* | Z score† | |
| Limbic regions | ||||||
| Sublenticular area (SI‡) | −1, 3, −11 | 3.75 | −3, 8, −11 | 3.79 | ||
| L infer temporal cortex | 46, −4, −25 | 3.75 | ||||
| L amygdala | −26, 1, −27 | 4.02 | −24, 1, −29 | 2.92 | ||
| Prefrontal cortex | ||||||
| Dorsomedial cortex | −1, 55, 29 | 4.46 | [−3, 53, 29 | 3.13] | ||
| L dorsolateral cortex | −24, 48, 36 | 3.97 | ||||
| Visual cortex | ||||||
| Cuneus/lingual gyrus | −6, −85, 4 | 12.37 | −1, −80, 0 | 11.48 | ||
| L mid-occipital gyrus | −33, −85, 7 | 6.09 | ||||
| R fusiform gyrus | 37, −49, −18 | 4.62 | 35, −46, −18 | 4.41 | ||
| 37, −71, −11 | 5.64 | |||||
| L fusiform gyrus | −33, −73, −14 | 6.38 | ||||
Stereotactic coordinates from Talairach and Tournoux atlas (46), left/right, anterior/posterior, and superior/inferior, respectively.
For a priori area of interest, foci are listed with Z > 3.09 (P < 0.001, uncorrected); outside a priori regions, foci are listed with Z > 4.0.
SI, substancia innominata/extended amygdala.
Figure 1.
Activity increases in limbic regions. The activity increase for nonaversive pictures (top row) and aversive pictures (bottom row) relative to the blank condition, and aversive relative to nonaversive pictures (middle row), in coronal planes. For all comparisons, activation is depicted as Z scores on the color scale, displayed for Z > 1.65 (uncorrected probability of P < 0.05), mapped onto a reference MRI atlas. Numbers below the bottom row correspond to millimeters anterior/posterior of the anterior commissure. The red arrows point to the peak activity in SLEA region, amygdala, and inferior temporal region.
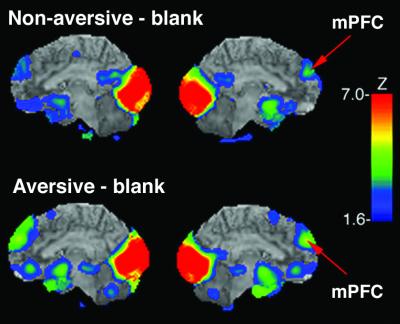
Outside of limbic regions, activation occurred in the dorso-medial prefrontal cortex (mPFC) (Brodmann area 9) in the aversive and nonaversive conditions as compared with the blank condition, and in the left dorso-lateral prefrontal cortex in the aversive minus blank condition (Fig. 2. Table 2). During the aversive condition, there was a negative correlation (r = −0.58, P < 0.06 trend level significance) between the rCBF in the dorso-mPFC (VOI peak coordinates x = 1, y = 55, z = 29) and the normalized rCBF in the left amygdala only (VOI peak coordinates x = 24, y = 1, z = −29). In the visual cortex, the comparisons of aversive and neutral conditions with the blank condition both yielded two peaks in the cuneus/lingual gyrus, and one in the fusiform gyrus (Table 2).
Figure 2.
Activity increases in cortical regions. The figure shows the activity increase for aversive and nonaversive pictures relative to blanks mapped onto surface rendering maps from reference MRI atlas. For each comparison medial view is depicted. The red arrows point to the peak activity in the mPFC. For all comparisons, activation is depicted as Z scores on the color scale, displayed for Z > 1.65
μ-Opioid Receptor Availability.
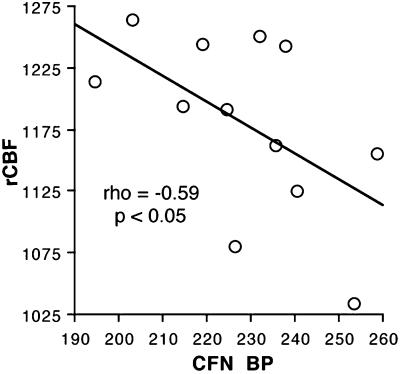
To examine the hypothesis regarding limbic μ-opioid receptors, we examined relationships between baseline μ-opioid receptor BP and the cerebral blood flow response to salient stimuli. The mean BP within the VOI identified in the rCBF studies were 1.13 ± 0.38 in the SLEA region, 1.28 ± 0.22 in left amygdala, and 1.28 ± 0.20 in inferior temporal region. Based on our a priori hypothesis, we examined correlations between BP and rCBF only in the three limbic regions that exhibited significant activation in response to emotional stimuli. In support of our hypothesis, we found significant negative correlation (Spearman ρ = −0.59, P = 0.04) between μ-opioid receptor availability in inferior temporal pole (Fig. 3) at baseline and rCBF during emotionally salient stimulus presentation (aversive and nonaversive stimulus conditions) (Fig. 4). No other significant correlation was noted between rCBF and BP.
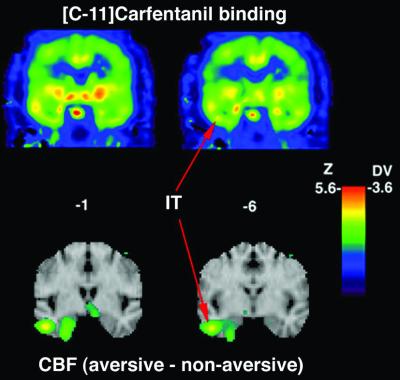
Figure 3.
CFN DV and [15O]water activation map for aversive–nonaversive contrast. (Upper) Coronal sections from a representative DV image (anatomically normalized to Talairach space). (Lower) Activation maps for the aversive–nonaversive contrast mapped onto surface rendering maps from reference MRI atlas. The red arrows indicate the peak for the inferotemporal region activation used to place a spherical region of interest on the normalized CFN DV images. The activity increase and the CFN DV values within this spherical region of interest were used for the correlation analysis.
Figure 4.
Baseline μ-opioid receptor BP in inferotemporal region is negatively correlated with the rCBF during the presentation of emotionally salient stimuli. Normalized CFN DV values within 13.5 mm regions of interest sphere in inferotemporal region are plotted against normalized activity counts obtained for the same region
Discussion
To identify neurotransmitter systems that might modulate limbic activation, we tested the relationship between the rCBF response to emotional stimuli and baseline μ-opioid receptor BP. Confirming our hypothesis, we demonstrated a negative correlation between rCBF in the inferior temporal pole and CFN binding. This association between lower CFN BP at baseline and higher rCBF during emotional stimuli presentation is consistent with an inhibitory or anxiolytic role for the endogenous opioid system in this limbic region, which has strong projections to the adjacent amygdaloid nuclei (1). In other words, higher baseline μ-opioid BP was associated with lower rCBF during emotional stimuli presentation, potentially reducing the neural intensity of the response. To our knowledge, this is the first evidence linking regional cerebral blood flow reflecting neural activity and the binding capacity of an endogenous neurotransmitter in the same limbic region.
The involvement of endogenous opioid systems in stress, antinociception, and emotional responses has been demonstrated in multiple studies (for a recent review, see ref. 33). Consistent with the proposed inhibitory or anxiolytic role for μ-opioid receptors in amygdaloid regions, Sugita and North (34) have demonstrated in animals that activation of μ-opioid receptors hyperpolarizes ≈50% of lateral amygdaloid neurons. Helmstetter et al. (35) demonstrated that μ-opioid receptors in basolateral amygdala modulate transmission in neural circuits involved in stress-related hypoalgesia. Similarly, an inhibitory role for μ-opioid receptors has been demonstrated in related regions, such as ventrolateral periaqueductal gray (36) and ventral pallidum (37). Using PET to measure rCBF, Casey et al. (38), demonstrated that a μ-opioid agonist attenuates thalamic and cortical responses to noxious stimulation in humans. μ-Opioid receptors have been colocalized with both N-methyl-d-aspartate (NMDAR1) (39) and γ-aminobutyric acid (40) receptors in limbic regions. This suggests that the inhibitory effects of μ-opioid receptors can be exerted both by direct mechanisms, such as hyperpolarization, or by indirect mechanisms, such as modulation of γ-aminobutyric acid and/or glutamatergic transmission.
The variances in baseline μ-opioid receptor binding found here could reflect a between subjects difference in the concentration of the μ-opioid receptor, or differences in the baseline release of its endogenous ligand(s), that competes with our radiotracer. Based on our data alone, we cannot distinguish between these two possibilities; however, in both cases, lower binding capacity potentially reflects fewer binding sites available for endogenous ligand during emotional stimulation. If, as proposed above, the role of endogenous opioids is to inhibit activation in these regions, lower μ-opioid BP could lead to lower capacity to modulate responses to aversive stimuli. Alternatively, μ-opioid receptors could be located on the inhibitory interneurons, as has been demonstrated in the hippocampus (41). In this case, lower binding capacity, reflecting higher release of endogenous opioids at baseline, could lead to stronger inhibition of interneurons, and as a result, weaker inhibition during emotional stimulation. Both presynaptic and somatodendritic μ-opioid receptors have been identified in limbic regions, suggesting that these alternative interpretations are possible. Future studies will be needed to elucidate the precise mechanisms involved in μ-opioid modulation of limbic activity.
Salient stimuli (both aversive and nonaversive), compared to a blank condition, activated the SLEA region, extending our previous findings (2), to an older cohort of male subjects, and further supporting the validity of emotional activation paradigm used. These findings are also consistent with the role of the SLEA region in processing general emotional salience, as hypothesized from our earlier work (I. Liberzon, L. R. Decker, and S. F. Taylor, unpublished work). Nevertheless, the limited anatomic resolution of this technique leaves some uncertainty as to the exact neural systems generating observed responses because of the presence of various neuronal populations in basal forebrain and hypothalamus. For example, we cannot rule out that activity in the cholinergic neurons of the corticopetal system, contributed to the activation in SLEA. However, because blood flow activation generally represents activity from terminal projections (42), it is less likely that we would see a significant contribution from cell bodies in SLEA.
We found the correlation between CFN BP and rCBF in the left inferior temporal region, raising the question as to the significance of this laterality. However, it is important to note that we tested only for correlations in the three limbic regions that exhibited significant activation; therefore we cannot draw any conclusions about any possible correlations on the right because we did not test for any. The left lateralization of the activation focus is of potential interest, although we noted both left-lateralized and bilateral activation of limbic temporal lobe structures in separate studies conducted in our laboratory (2, 10, 11). Therefore, we think that interpretations about lateralized function from this single experiment would be premature because it could stem from true laterality differences, the issue of power, or from other methodological reasons.
In addition to the activation focus in the SLEA, activation in a more caudal amygdaloid area was present during the presentation of aversive stimuli. We have previously observed activation caudal to the SLEA in the amygdala, proper, in response to aversive stimuli (ref. 2 and I. Liberzon, L. R. Decker, and S. F. Taylor, unpublished work). The present data confirm this association and are consistent with a hypothesized rostro-caudal gradient, within extended amygdala, for appetitive and aversive stimuli. We also have found activation of the amygdala by nonaversive stimuli in other studies (11). Thus it is possible that the amygdala (or a subsets of nuclei within this complex structure) can activate for generally salient stimuli, in addition to more specific responses to aversive stimuli. Complex visual stimuli, compared to blanks, also modulated activity in cortical regions: mPFC (-BA 9), visual cortex (Cuneus/lingual and fusiform gyrae), and dorsolateral prefrontal cortex (BA, aversive stimuli only). Activation of visual cortex represents the expected effect of processing complex visual stimuli. mPFC has been consistently activated in numerous emotional activation studies (43, 44) and may play a role in cognitive–emotional interactions. We proposed earlier that the mPFC might play a compensatory or inhibitory role during emotional activation of amygdaloid regions.‖ The negative correlation between regional blood flow in the mPFC and the left amygdala provides empirical evidence in support of this hypothesis.
The current study replicates and further extends our earlier findings on the role of the extended amygdala (like SLEA) and the mPFC in processing of emotional stimuli. Most importantly, it demonstrates a novel link between limbic activity in response to emotionally salient stimuli and μ-opioid receptor BP, consistent with the inhibitory/anxiolytic role of limbic μ-opioid systems in emotional responses. The elucidation of potential neuromodulators regulating the responsiveness of the amygdaloid regions to emotional stimuli has direct relevance to psychiatric conditions, such as posttraumatic stress disorder (PTSD) or depression. At least two groups, including our own (6, 7), have demonstrated an exaggerated amygdaloid response in PTSD patients, and abnormalities in the function of endogenous opioid systems in PTSD have been proposed (46). The present data suggest mechanisms that could underlie exaggerated responses of limbic regions in PTSD, offering potential explanations for PTSD symptoms and even new treatment strategies. Lastly, these findings demonstrate the utility of combining PET receptor binding measures with blood flow activation studies, allowing us to proceed from testing hypotheses about nonspecific neuroanatomy to investigating more specific cellular mechanisms.
Acknowledgments
This work was supported by the Department of Veterans Affairs and Department of Defense Traumatic Stress Initiative Merit Award (to I.L. and L.M.F.), and by Ann Arbor Veterans Affairs Health Medical System.
Abbreviations
- PET
positron emission tomography
- BP
binding potential
- rCBF
regional cerebral blood flow
- SLEA
sublenticular extended amygdala
- VOI
volumes of interest
- CFN
carfentanil
- PTSD
posttraumatic stress disorder
- mPFC
medial prefrontal cortex
- DV
distribution volume
- PANAS
positive affectivity–negative affectivity scale
Footnotes
Phan, K., Liberzon, I. & Taylor, S. (2000) Soc. Neurosci. Abstr. 2, 755.17.
References
- 1.Paxinos G. The Human Nervous System. San Diego: Academic; 1990. [Google Scholar]
- 2.Liberzon I, Taylor S F, Fig L M, Decker L R, Koeppe R A, Minoshima S. Neuropsychopharmacology. 2000;23:508–516. doi: 10.1016/S0893-133X(00)00157-3. [DOI] [PubMed] [Google Scholar]
- 3.Whalen P J, Rauch S L, Etcoff N L, McInerney S C, Lee M B, Jenike M A. J Neurosci. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Doherty J, Kringelbach M L, Hornak J, Andrews C, Rolls E T. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- 5.Drevets W C. Ann NY Acad Sci. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- 6.Liberzon I, Taylor S F, Amdur R, Jung T D, Chamberlain K R, Minoshima S, Koeppe R A, Fig L M. Biol Psychiatry. 1999;45:817–826. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- 7.Rauch S L, Whalen P J, Shin L M, McInerney S C, Macklin M L, Lasko N B, Orr S P, Pitman R K. Biol Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 8.Liberzon I, Taylor S F, Fig L M, Koeppe R A. Depress Anxiety. 1996;4:146–150. doi: 10.1002/(SICI)1520-6394(1996)4:3<146::AID-DA9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 9.Rauch S L, Jenike M A, Alpert N M, Baer L, Breiter H C, Savage C R, Fischman A J. Arch Gen Psychiatry. 1994;51:62–70. doi: 10.1001/archpsyc.1994.03950010062008. [DOI] [PubMed] [Google Scholar]
- 10.Taylor S F, Liberzon I, Fig L M, Decker L R, Minoshima S, Koeppe R A. Neuroimage. 1998;8:188–197. doi: 10.1006/nimg.1998.0356. [DOI] [PubMed] [Google Scholar]
- 11.Taylor S F, Liberzon I, Koeppe R A. Neuropsychologia. 2000;38:1415–1425. doi: 10.1016/s0028-3932(00)00032-4. [DOI] [PubMed] [Google Scholar]
- 12.Davis M. In: The Amydala: Neurolbiological Aspects of Emotion, Memory and Mental Dysfunction. Aggleton J P, editor. New York: Wiley–Liss; 1992. pp. 255–395. [Google Scholar]
- 13.LeDoux J E, Cicchetti P, Xagoraris A, Romanski L M. J Neurosci. 1990;10:1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LaBar K S, Gatenby J C, Gore J C, LeDoux J E, Phelps E A. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- 15.Morris J S, Ohman A, Dolan R J. Proc Natl Acad Sci USA. 1999;96:1680–1685. doi: 10.1073/pnas.96.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Good A J, Westbrook R F. Behav Neurosci. 1995;109:631–641. doi: 10.1037//0735-7044.109.4.631. [DOI] [PubMed] [Google Scholar]
- 17.Shaikh M B, Lu C L, Siegel A. Brain Res. 1991;559:109–117. doi: 10.1016/0006-8993(91)90293-5. [DOI] [PubMed] [Google Scholar]
- 18.Graeff F G. Braz J Med Biol Res. 1994;27:811–829. [PubMed] [Google Scholar]
- 19.Kang W, Wilson S P, Wilson M A. Neuropsychopharmacology. 2000;22:77–88. doi: 10.1016/S0893-133X(99)00090-1. [DOI] [PubMed] [Google Scholar]
- 20.Frost J J, Douglass K H, Mayberg H S, Dannals R F, Links J M, Wilson A A, Ravert H T, Crozier W C, Wagner H N., Jr J Cereb Blood Flow Metab. 1989;9:398–409. doi: 10.1038/jcbfm.1989.59. [DOI] [PubMed] [Google Scholar]
- 21.Spitzer R L, Williams J B W, Gibbon M, First M B. Structured Clinical Interview for DSM-III-R. Washington, DC: Am. Psych. Press; 1990. [DOI] [PubMed] [Google Scholar]
- 22.Dannals R F, Ravert H T, Frost J J, Wilson A A, Burns H D, Wagner H N., Jr Int J Appl Radiat Isot. 1985;36:303–306. doi: 10.1016/0020-708x(85)90089-4. [DOI] [PubMed] [Google Scholar]
- 23.Minoshima S, Koeppe R A, Fessler J A, Mintun M A, Berger K L, Taylor S F, Kuhl D E. Quantification of Brain Function, Tracer Kinetics and Image Analysis in Brain PET. Amsterdam: Elsevier; 1993. pp. 409–415. [Google Scholar]
- 24.Haxby J V, Ungerleider L G, Horwitz B, Maisog J M, Rapoport S I, Grady C L. Proc Natl Acad Sci USA. 1996;93:922–927. doi: 10.1073/pnas.93.2.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watson D, Clark L A, Tellegen A. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 26.Logan J, Fowler J S, Volkow N D, Wang G J, Ding Y S, Alexoff D L. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Minoshima S, Koeppe R A, Frey K A, Kuhl D E. J Nucl Med. 1994;35:1528–1537. [PubMed] [Google Scholar]
- 28.Gabilondo A M, Meana J J, Garcia-Sevilla J A. Brain Res. 1995;682:245–250. doi: 10.1016/0006-8993(95)00333-l. [DOI] [PubMed] [Google Scholar]
- 29.Gross-Isseroff R, Dillon K A, Israeli M, Biegon A. Brain Res. 1990;530:312–316. doi: 10.1016/0006-8993(90)91301-v. [DOI] [PubMed] [Google Scholar]
- 30.Minoshima S, Berger K L, Lee K S, Mintun M A. J Nucl Med. 1992;33:1579–1585. [PubMed] [Google Scholar]
- 31.Friston K J, Frith C D, Liddle P F, Frackowiak R S. J Cereb Blood Flow Metab. 1991;11:690–699. doi: 10.1038/jcbfm.1991.122. [DOI] [PubMed] [Google Scholar]
- 32.Worsley K J, Evans A C, Marrett S, Neelin P. J Cereb Blood Flow Metab. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- 33.Vaccarino A L, Olson G A, Olson R D, Kastin A J. Peptides. 1999;20:1527–1574. doi: 10.1016/s0196-9781(99)00166-7. [DOI] [PubMed] [Google Scholar]
- 34.Sugita S, North R A. Brain Res. 1993;612:151–155. doi: 10.1016/0006-8993(93)91655-c. [DOI] [PubMed] [Google Scholar]
- 35.Helmstetter F J, Bellgowan P S, Poore L H. J Pharmacol Exp Ther. 1995;275:381–388. [PubMed] [Google Scholar]
- 36.Wiedenmayer C P, Barr G A. Behav Neurosci. 2000;114:125–136. doi: 10.1037//0735-7044.114.1.125. [DOI] [PubMed] [Google Scholar]
- 37.Napier T C, Mitrovic I. Ann NY Acad Sci. 1999;877:176–201. doi: 10.1111/j.1749-6632.1999.tb09268.x. [DOI] [PubMed] [Google Scholar]
- 38.Casey K L, Svensson P, Morrow T J, Raz J, Jone C, Minoshima S. J Neurophysiol. 2000;84:525–533. doi: 10.1152/jn.2000.84.1.525. [DOI] [PubMed] [Google Scholar]
- 39.Gracy K N, Svingos A L, Pickel V M. J Neurosci. 1997;17:4839–4848. doi: 10.1523/JNEUROSCI.17-12-04839.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Svingos A L, Moriwaki A, Wang J B, Uhl G R, Pickel V M. J Neurosci. 1997;17:2585–2594. doi: 10.1523/JNEUROSCI.17-07-02585.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drake C T, Milner T A. Brain Res. 1999;849:203–215. doi: 10.1016/s0006-8993(99)01910-1. [DOI] [PubMed] [Google Scholar]
- 42.Mata M, Fink D J, Gainer H, Smith C B, Davidsen L, Savaki H, Schwartz W J, Sokoloff L. J Neurochem. 1980;34:213–215. doi: 10.1111/j.1471-4159.1980.tb04643.x. [DOI] [PubMed] [Google Scholar]
- 43.Lane R D, Fink G R, Chau P M, Dolan R J. NeuroReport. 1997;8:3969–3972. doi: 10.1097/00001756-199712220-00024. [DOI] [PubMed] [Google Scholar]
- 44.Teasdale J D, Howard R J, Cox S G, Ha Y, Brammer M J, Williams S C, Checkley S A. Am J Psychiatry. 1999;156:209–215. doi: 10.1176/ajp.156.2.209. [DOI] [PubMed] [Google Scholar]
- 45.Pitman R K, van der Kolk B A, Orr S P, Greenberg M S. Arch Gen Psychiatry. 1990;47:541–544. doi: 10.1001/archpsyc.1990.01810180041007. [DOI] [PubMed] [Google Scholar]
- 46.Talairach J, Tournoux P. A Co-Planar Stereotaxic Atlas of a Human Brain. Stuttgart: Thiem; 1988. [Google Scholar]