Abstract
Surgical gloves are a staple in every surgeon’s daily routine, yet their full lifecycle is not always well understood. This paper outlines the journey of a surgical glove from manufacturing to disposal, with particular emphasis on clinically relevant properties such as durability, perforation rates, and allergy risk. It begins with a review of the historical context of sterile surgical gloves, followed by a detailed overview of the manufacturing process and the materials used, including latex and various synthetic alternatives. These various materials may differ in barrier protection, fit, tactile sensitivity, and allergenic potential. Data presented here suggests that synthetic alternatives to latex, while hypoallergenic, may be more prone to microperforations or decreased dexterity. The logistics of glove sourcing and inventory management are also examined, providing insights to help surgical teams and hospital administrators prepare for supply chain disruptions, such as those experienced during the COVID-19 pandemic. Finally, best practices for glove disposal and the environmental impact of surgical gloves are explored. By examining the clinical and logistical aspects of glove use, this article offers insights to optimize surgical safety, resource management, and sustainability.
BACKGROUND
Sterile surgical gloves are now a universal requirement in modern operating theaters; however, their early adoption was slow. Before surgical gloves becoming widespread, surgeons operated with bare hands and relied on emerging aseptic techniques such as hand disinfection and instrument sterilization to reduce infection risk.1 Medical gloves have a documented history that extends as far as 1758, with obstetricians having worn animal membrane-based gloves.2 In 1844, Charles Goodyear introduced vulcanized rubber to the market, which provided a more durable potential material for surgical gloves. However, these were not introduced until 1889 by William Stewart Halsted, a pioneer at Johns Hopkins Hospital, who instituted rubber gloves to prevent his scrub nurse from getting antiseptic-induced dermatitis.2 Before the 1890s, surgeons who wore gloves primarily did so to protect themselves and their staff from skin infections such as furuncles or sepsis from contaminated wounds.1 In 1899, Joseph Colt Bloodgood, Halsted’s first chief resident, reported an almost 100% decline in postoperative infections among patients undergoing hernia repair with surgeons who wore gloves.3
Even with these reported advantages, universal gloving in the operating room (OR) was not the standard for many years. By the early 1900s, gloves had become more common in major academic centers in German-speaking countries and the United States (U.S.), but adoption in nonacademic and community hospitals lagged.1 Photographs and written accounts from the early 20th century show that gloves were still not used routinely in many settings. During the World War II era, gloves became standard practice in military and high-volume surgical environments, accelerating broader adoption.1 By the 1950s, gloves had become a universal standard in surgical practice.1
Today, surgical gloves are a vital barrier to pathogens for both patients and surgeons. Although they are critically important in the OR, few surgeons are aware of the complex processes involved in rendering these gloves sterile and ready for the OR. Their quality depends on numerous factors, including materials, sterilization methods, and handling practices. This article traces the journey of a surgical glove from production to disposal, emphasizing the critical steps required to make gloves sterile and safe for use.
METHODS
This narrative review was conducted at a single academic institution. A literature search was performed using PubMed and Google Scholar in February 2025. Search terms included combinations of “surgical gloves,” “surgical glove suppliers,” “latex gloves,” “synthetic gloves,” “glove manufacturing,” “glove perforation,” “double gloving,” “glove disposal,” “surgical gloves cost,” “surgical glove sensitivity,” “surgical gloves environmental impact,” “surgical waste,” “glove waste,” “latex glove allergies,” “surgical glove sterilization,” and “sustainability in operating rooms.” We included published peer-reviewed articles, review papers, regulatory reports, and manufacturer documents that addressed glove materials, performance, allergic risks, logistics, disposal, and environmental impact. Studies were selected based on relevance to clinical practice or glove lifecycle stages. Additional references were identified through citation tracking.
SUPPLIERS OF SURGICAL GLOVES
The U.S. heavily relies on imports for its surgical glove supply. In 2019 alone, the U.S. imported some $437M worth of surgical gloves including $207M from Malaysia, the biggest single exporter.4 Domestic production contributes minimally to the U.S.’s annual surgical glove needs. Major suppliers operating in the U.S. often source surgical gloves from manufacturers in Malaysia and Thailand, then distribute them under their own brands. These partnerships allow U.S. suppliers to offer a broad selection of surgical gloves across various materials and thicknesses. A summary of major U.S. suppliers and the types of gloves they offer is shown in Table 1.
TABLE 1.
Major Surgical Glove Suppliers Operating in the United States and Their Product Characteristics
| Manufacturer (HQ Location) | Surgical Glove Brands | Materials Offered | Glove Thickness Offered |
|---|---|---|---|
| Ansell (Iselin, NJ) | Gammex, Encore | Latex, Neoprene, Polyisoprene | Micro, Standard, Orthopedic |
| Cardinal Health (Dublin, OH) | Protexis | Latex, Neoprene, Polyisoprene | Micro, Standard, Orthopedic |
| Mölnlycke Health (Norcross, GA) | Biogel | Latex, Neoprene, Polyisoprene | Micro, Standard, Orthopedic |
| Medline (Northfield, IL) | SensiCare | Latex, Neoprene, Polyisoprene | Micro, Standard, Orthopedic |
| Mckesson (Irving, TX) | Confiderm | Latex, Neoprene, Polyisoprene, Nitrile | Micro, Standard, Orthopedic |
Information was compiled from publicly available manufacturer websites, product catalogs, and medical supply distributors as of April 2025. Listed companies have major U.S. operations or distribution centers. Glove thickness categories (micro, standard, and orthopedic) are based on manufacturer classifications. HQ = U.S. operational headquarters, not necessarily global HQ or manufacturing location.
MANUFACTURING PROCESS
Surgical glove production starts with harvesting or synthesizing raw materials. Natural rubber latex gloves are derived from the sap of the Hevea brasiliensis tree, primarily grown in Thailand and Indonesia, which together supply approximately 60% of the world’s natural rubber.5,6 Latex is harvested by tapping the tree bark and collecting a milky fluid composed of 30% to 35% cis-1,4 polyisoprene (cis-PI) suspended in water.7
Synthetic alternatives include neoprene, polyisoprene, and nitrile gloves. Neoprene (polychloroprene rubber) is made by emulsion polymerization of chloroprene monomers.5 Polyisoprene gloves use synthetic cis-PI rubber, which shares the same chemical backbone as natural rubber but is produced artificially via polymerizing isoprene monomers.5 Nitrile gloves are made from acrylonitrile butadiene rubber.8
Both synthetic and natural latex gloves undergo a similar series of steps to create the final product. First, clean glove molds are dipped into a coagulant solution and then dried.9 Next, the coagulant-coated glove molds are dipped into liquid latex or a synthetic alternative compound. After dipping, glove molds pass through sequential heated ovens to dry and vulcanize the rubber, a curing process that cross-links the polymer chains to provide elasticity and strength.5
To ensure high purity, gloves undergo 1 or more water leaching stages before the final cure to extract residual proteins and chemicals.5 Because modern surgical gloves are powder-free, manufacturers use chlorination or polymer coatings as a final treatment to prevent sticking. Alternatively, a thin layer of polyurethane or silicone may be applied to achieve the same effect.
After the curing and coating, gloves are rinsed and dried in warm air to remove any residues. Next, surgical gloves are sterilized usually by gamma irradiation or ethylene oxide gas to achieve the required sterility assurance level.10,11 The sterility assurance level for surgical gloves is 10⁻⁶, meaning the probability of a viable microorganism is fewer than one in a million.12 Gloves are individually packed in sterile pairs, sealed, and labeled with lot information before being boxed for distribution.
Throughout and after manufacturing, rigorous quality control tests ensure compliance with regulatory standards. These include visual inspection and mechanical testing of batch samples. A key test is the water leak test, where gloves are filled with water to detect pinhole leaks. In the U.S., surgical gloves must meet American Society for Testing and Materials (ASTM) D3577, which specifies dimensions (length and thickness), tensile strength, elongation, and pinhole defect limits.13 Only batches that meet the acceptable quality level for defects are released. In 2023, Malaysia, China, and Thailand were the top global exporters of surgical gloves, with export values of approximately $1.86B, $1.31B, and $797M, respectively.14 The manufacturing process is summarized in Figure 1.
FIGURE 1.
Manufacturing process of a surgical glove: the production of surgical gloves involves a multistep process beginning with material collection, followed by processing and refinement. The material is then molded into glove form, cured and dried through heating, and subjected to leaching and cleaning to remove impurities. Afterward, gloves undergo sterilization to achieve sterility assurance. Finally, the gloves are packaged and distributed for clinical use.
GLOVE MATERIALS AND PROPERTIES
Each of the materials used for surgical gloves has their own benefits and drawbacks. Latex gloves have long been the gold standard as they provide the best fit and tactile sensitivity but carry a risk of allergic reactions to proteins found in natural latex.5 A systematic review found that latex allergy affects 4.32% of healthcare workers.15 Clinical manifestations range from mild to severe and may include irritant and allergic contact dermatitis, urticaria, angioedema, rhinitis, conjunctivitis, bronchospasm, and anaphylaxis.16 Latex gloves also pose a risk to patients, particularly in the OR, where they may be exposed for prolonged periods in a confined environment. For example, the use of latex products in patients with a latex allergy can trigger severe reactions, including perioperative anaphylaxis, a life-threatening complication.17 Latex gloves are the primary source of airborne latex allergens in surgical settings, and transitioning to latex-free gloves has been shown to reduce aeroallergen levels by more than tenfold.18 To reduce the risk of allergic reactions, many surgical centers have partially or fully transitioned to synthetic alternatives.
Nitrile gloves are a hypoallergenic synthetic option that offers excellent chemical resistance, durability, and puncture resistance.19,20 However, nitrile gloves are generally stiffer than latex gloves and have a greater risk of microperforations due to their stiffness.21 As a result, despite their widespread use in healthcare, nitrile gloves are less commonly utilized in surgical procedures. Neoprene gloves, another latex-free alternative, stand out due to their chemical resistance, temperature resistance, and toughness. The minimum and maximum operating temperatures of these gloves are −25 °C and 93 °C, respectively.5 Reports of the tactility and sensitivity of neoprene gloves are generally good, but some users feel that they are stiffer than their latex or polyisoprene alternatives.5
Polyisoprene gloves are made from synthetic cis-PI rubber, the same material that is found in natural latex rubber. Because they share the same chemical properties, they provide a very similar fit and feel to natural rubber latex without the risk of latex allergy due to the lack of allergenic proteins.5,22,23 The downside of polyisoprene gloves is that they are significantly more expensive than the alternatives. Types of surgical glove material are displayed in Table 2.
TABLE 2.
Pros and Cons of Various Surgical Glove Materials
| Latex | Nitrile | Neoprene | Polyisoprene | |
|---|---|---|---|---|
| Structure | Cis-IP natural rubber | Acrylonitrile butadiene | Chloroprene | Cis-IP synthetic rubber |
| Pros | Fit and tactile sensitivity | Hypoallergenic; chemical resistance, durability, and puncture resistance | Chemical resistance, temperature resistance, and durability | Similar fit and feel of natural rubber latex without the risk of latex allergy |
| Cons | Risk of allergic reactions to latex proteins | Generally considered to be stiffer than latex of polyisoprene alternatives | Some users feel that they are stiffer than their latex or polyisoprene alternatives | Significantly more expensive than the alternatives |
Despite the benefits of synthetic alternatives, a complete transition away from latex gloves presents potential drawbacks. One study found that a standardized puncture test through nitrile and neoprene gloves showed a tenfold increase in bacterial passage compared to latex.21 Additionally, neoprene and polyisoprene surgical gloves are generally more expensive, leading to higher upfront costs for hospitals. This higher upfront cost may be a barrier for surgery-intensive hospitals, which often operate under tight cost-efficiency initiatives. In such settings, supply expenses can represent up to 30% to 40% of total hospital expenditures, making glove cost a meaningful factor in institutional decision-making.24 However, this cost may be offset by a reduction in healthcare worker disability due to latex allergy, as one study noted.25 The costs of different types of surgical gloves are displayed in Table 3.
TABLE 3.
Cost Per Pair of Surgical Gloves by Material, Texture, and Thickness
| Average Cost Per 1 Pair (USD) | Price Range Per 1 Pair (USD) | |
|---|---|---|
| Material type | ||
| Polyisoprene | $3.17 | $2.06–$5.19 |
| Latex | $1.77 | $1.10–$ 3.58 |
| Neoprene | $2.47 | $2.14–$2.74 |
| Nitrile | $1.64 | $1.56–$1.71 |
| Texture | ||
| Smooth | $ 2.23 | $1.13–$3.91 |
| Microroughened | $2.44 | $1.19–$3.99 |
| Textured | $1.85 | $1.28–$3.38 |
| Thickness | ||
| Micro | $2.67 | $1.57–$3.81 |
| Standard | $2.08 | $1.28–$3.95 |
| Orthopedic | $2.23 | $1.91–$2.48 |
The average glove costs were calculated using data from various online distributors, which offered products from multiple suppliers. Gloves classified as micro had thicknesses ranging from 0.15 to 0.18 mm, standard gloves ranged from 0.19 to 0.25 mm, and orthopedic gloves had thicknesses greater than 0.30 mm.
Besides differences in material composition, there is variability in glove texture and thickness. Although there is no universal terminology for different types of textured gloves made by manufacturers, they can be categorized into three general categories: smooth, textured, and microroughened. Smooth gloves have no added texture. Textured gloves, available as finger-textured or fully textured, provide enhanced grip. Microroughened gloves, which are less textured than textured gloves, offer a subtle texture that balances grip and sensitivity. Overall, texture helps enhance grip during wet or slippery conditions, and current evidence does not indicate that surface texture significantly impairs touch sensitivity.26
Thickness also varies, with standard gloves typically ranging from 0.18 to 0.25 mm thick, aiming to balance durability and flexibility.27 Microthickness gloves, often called “micro” or ultrathin surgical gloves, are designed to be thinner than standard surgical gloves to maximize tactile sensitivity.27,28 In general, they are about 20% thinner than regular latex surgical gloves, which translate to roughly 0.15 to 0.20 mm thick at the fingertips. The ASTM D3577 regulatory requirements specify a minimum of ~0.10 mm for surgical glove fingers.13 One study reported that microthickness gloves provided similar sensitivity as no gloves on grating orientation task, a measure of tactile spatial acuity.28 Surgeons performing microsurgical procedures often prefer ultrathin gloves to maintain high tactile sensitivity when handling delicate tissues and performing precise suturing.27 Orthopedic surgical gloves are typically 50% thicker to withstand the rigors of orthopedic surgery, such as handling bone fragments and power tools, and to reduce the risk of ruptures.28 In practice, many orthopedic gloves have fingertip thickness in the range of roughly 0.25 to 0.33 mm. This increased thickness provides greater durability and resistance to perforations, although it may reduce tactile sensitivity. However, one study reported no significant difference in sensitivity between orthopedic and standard gloves.28
Double-gloving systems involve wearing two pairs of gloves for added protection. Double gloving, with either two pairs of standard gloves or a perforation indicator system, has been shown to significantly reduce glove perforations and surgical site infections compared with single gloves.29 Using a perforation system, which involves using a different colored glove underneath, has been shown to provide the timeliest identification of perforation, therefore minimizing risk of infection.30 Although there are concerns regarding tactile sensitivity and manual dexterity with double-gloving, research shows that double-gloving does not significantly impair either. For example, one study reported that there was no compromise to dexterity and 2-point discrimination when double-gloving.31
LOGISTICS AND INVENTORY MANAGEMENT
Surgical gloves are shipped from manufacturers to target locations based on the internationally recognized standard set by the International Organization for Standardization 11607, which outlines how to package and test sterile medical devices. In essence, packaging must withstand the rigors of distribution without tearing or losing its microbial barriers. Glove packaging materials are often validated using stress tests highlighted in the ASTM D4169 guidelines. Food and Drug Administration regulatory guidance highlights controlling the temperature, moisture, and physical protection during glove transport/storage.32 Best practices include using climate-controlled trucks or warehouses.
To preserve integrity after arrival at the target location, sterile surgical gloves should be stored in clean, limited-access areas with controlled temperature and humidity. Guidelines for sterile supply storage recommend an ambient temperature around 22 to 26 °C and relative humidity not exceeding ~60%.33 Most evidence-based guidelines agree that sterile supplies must be kept off the floor and away from walls/ceiling leaks, in well-ventilated conditions that guard against moisture and temperature extremes. Gloves should remain sealed in their sterile packaging until use. Gloves come with expiration dates set by manufacturers according to ASTM D7160/D7161 standards for glove aging.32
To prevent shortages or expired stock, hospitals and surgical centers use proactive inventory management strategies to ensure glove supplies meet demand without running out or expiring. A key practice is setting par levels—a minimum on-hand quantity for each glove size/type—based on usage rates. Stock is replenished when it nears these levels so that routine usage (and even minor surges) can be covered. The Joint Commission notes that healthcare organizations must stock sterile supplies at optimal levels and in accessible locations, while also ensuring nothing has expired or been compromised.34
Before the COVID-19 pandemic, many hospitals relied on lean, just-in-time inventory (keeping only a limited supply on site to reduce costs). However, the pandemic revealed that such minimal inventories could not handle sudden spikes in demand.35 Industry analysts recommend building more resilient supply chains, such as holding extra glove inventory or having rapid access to emergency stockpiles to prevent shortages during pandemics or supplier disruptions. One report suggests dual sourcing should be used for routine purchasing of key supplies to reduce the impact on supply chain disruption.36 Another report outlined the importance of healthcare facilities to train their healthcare professionals on management strategies during critical situations. The report also recommended that local healthcare facilities keep a 6-month supply on hand and routinely refresh it to prevent expiration.37 Ultimately, managers must balance maintaining sufficient supply for emergencies with avoiding excess inventory that could expire.
DISPOSAL AFTER USE
Proper disposal of surgical gloves is important to prevent contamination, infection spread, and environmental harm. To safely remove contaminated gloves, the CDC recommends grasping the outside of one glove with the opposite gloved hand and peeling it off, ensuring it is held in the still-gloved hand. Next, the fingers of the ungloved hand should be slid under the wrist of the remaining glove, carefully peeling it off inside out to contain contamination. This technique minimizes the risk of self-contamination during glove removal.38
Discarding surgical gloves depends on the type of procedure and the state of the gloves after use. Surgical gloves are considered medical waste if they have blood, bodily fluids, or infectious agents. If contaminated, surgical gloves are to be placed in designated biohazard containers. Standards of handling medical waste are outlined in Occupational Safety and Health Administration (OSHA) Bloodborne Pathogens Standard (29 CFR 1910.1030), which mandates proper labeling, puncture resistance, leak-proof design, and secure closure of biohazard bins to prevent worker exposure to infectious materials before disposal.39 When OSHA conducts an inspection addressing regulated waste concerns, compliance with the Bloodborne Pathogens Standard is evaluated on a case-by-case basis. If OSHA determines that sufficient evidence exists that the standard has been violated, a citation carrying monetary penalties may be issued to the employer.39 Surgical gloves that have not been exposed to contamination can be treated as regular waste and be disposed of accordingly.
Standards regarding disposal of medical waste after it is collected for the hospital are primarily regulated by state environmental and health departments.40 After collection, the medical waste is processed at a designated facility by one of various methods. Some common methods of disposal include incineration, thermal treatment, autoclaving, electropyrolysis, and chemical mechanical systems.40
OTHER CONSIDERATIONS
Though surgical gloves are a necessity in protecting both healthcare workers and patients, they have significant detrimental effects on the environment. Their environmental impact comes from emissions that are released during production and waste that is generated after use.
The production of rubber products, including sterile surgical gloves, involves the emission of volatile organic compounds such as dichloromethane, carbon disulfide, and styrene, which contribute to both photochemical ozone formation and cardiogenic risks.41 The manufacturing process contributes to the majority of the environmental burden, accounting for an average of 64.37% of the total impact for sterile latex gloves and 60.48% for nonlatex sterile gloves.42
A recent life cycle assessment study found that sterile gloves had a more significant adverse impact across multiple categories including climate change, ozone depletion, and resource use compared with nonsterile examination gloves. Sterile surgical gloves have a climate change impact that is 11.6 times higher than nonsterile gloves due to the extra steps required in their manufacturing.42
Beyond the manufacturing process, gloves (including surgical and non-surgical glove types) substantially contribute to environmental waste. During the COVID-19 pandemic alone, it was reported that around 65 billion gloves were being used monthly.43 Discarded gloves undergo physiochemical changes when exposed to environmental conditions such as ultraviolet weathering. This leads to the release of microparticles, organic matter, and heavy metals into the environment, further contributing to pollution.44
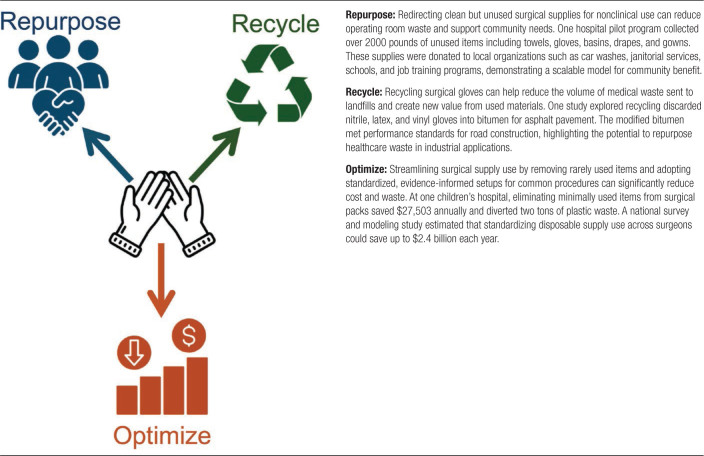
Due to the significant environmental harm, there are emerging recycling initiatives aimed at reducing their impact. One such example involves recycling disposable latex and nitrile gloves into bitumen, a sticky, tar-like binder used in asphalt pavement.45 In another study, a pilot program collected clean, nonreusable surgical supplies, including surgical gloves, and repurposed them for community benefit. One example included donating surgical towels to local car washes and cleaning businesses.46 Although these examples demonstrate methods to reduce surgical waste, widespread adoption is difficult. The immediate cost associated with implementing sustainable waste management practices can be high, and the long-term benefits are not readily apparent. This includes the cost of setting up recycling programs, training staff, and ensuring compliance with new protocols.47 However, adoption of sustainable practices has the potential to lower costs and benefit the environment long term. Table 4 outlines specific strategies to reduce surgical waste.
TABLE 4.
Strategies to Reduce Surgical Waste
The costs associated with the procurement, storage, and disposal of surgical gloves are significant. In 2022, the surgical glove market size was estimated at 2B USD, with North America making up 37% of the market share alone.48 Research has suggested sustainable strategies to reduce costs. One pilot study at a children’s hospital showed that removing minimally used items from surgical packs saves $27,503 annually and diverts 2 tons of plastic waste from landfills.49 Another study investigating the variability in the use of disposable surgical supplies estimated that optimizing the use of these supplies, including surgical gloves, could lead to saving up to $2.4B annually across all US surgeons.50
CONCLUSION
Surgical gloves play an essential role in protecting patients and surgeons, but their effectiveness depends heavily on strict adherence to standards throughout production, transportation, storage, and disposal. Although necessary, surgical gloves contribute significantly to environmental pollution and waste. Implementing sustainable practices could reduce their environmental impact, minimize waste, and offer cost savings over the long term.
Footnotes
Disclosure: The authors declare that they have nothing to disclose.
REFERENCES
- 1.Schlich T. Negotiating technologies in surgery: the controversy about surgical gloves in the 1890s. Bull Hist Med. 2013;87:170–197. [DOI] [PubMed] [Google Scholar]
- 2.Lee KP. Caroline Hampton Halsted and the origin of surgical gloves. J Med Biogr. 2020;28:64–66. [DOI] [PubMed] [Google Scholar]
- 3.Lathan SR. Caroline Hampton Halsted: the first to use rubber gloves in the operating room. Proc (Bayl Univ Med Cent). 2010;23:389–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WITS. World Integrated Trade Solution United States Surgical Gloves (401511) important by country in 2019. 2019. Available at: https://wits.worldbank.org/trade/comtrade/en/country/USA/year/2019/tradeflow/Imports/partner/ALL/nomen/h5/product/401511? Accessed February 12, 2025. [Google Scholar]
- 5.Lovato MJ, Del Valle LJ, Puiggalí J, et al. Performance-enhancing materials in medical gloves. J Funct Biomater. 2023;14:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh AK, Liu W, Zakari S, et al. A global review of rubber plantations: impacts on ecosystem functions, mitigations, future directions, and policies for sustainable cultivation. Sci Total Environ. 2021;796:148948. [DOI] [PubMed] [Google Scholar]
- 7.Jędruchniewicz K, Ok YS, Oleszczuk P. COVID-19 discarded disposable gloves as a source and a vector of pollutants in the environment. J Hazard Mater. 2021;417:125938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herkins A, Dey S, Conroy D, et al. Nitrile glove composition and performance—substandard properties and inaccurate packaging information. PLoS One. 2024;19:e0312891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groves R, Welche P, Routh AF. The coagulant dipping process of nitrile latex: investigations of former motion effects and coagulant loss into the dipping compound. Soft Mat. 2023;19:468–482. [DOI] [PubMed] [Google Scholar]
- 10.Aquinio KAS. Sterilization by gamma irradiation. In: Adrovic F, ed. Gamma Radiation. InTech; 2012. [Google Scholar]
- 11.Rutala WA, Weber DJ. Sterilization of 20 billion medical devices by ethylene oxide (ETO): consequences of ETO closures and alternative sterilization technologies/solutions. Am J Infect Control. 2023;51:A82–A95. [DOI] [PubMed] [Google Scholar]
- 12.Dunkelberg H, Schmelz U. Determination of the efficacy of sterile barrier systems against microbial challenges during transport and storage. Infect Control Hosp Epidemiol. 2009;30:179–183. [DOI] [PubMed] [Google Scholar]
- 13.ASTM I. Standard specification for rubber surgical gloves. ASTM D-3577-09. 2009. [Google Scholar]
- 14.Bank W. Surgical rubber gloves (HS 401511) export data, 2023. 2025. Available at: https://wits.worldbank.org/trade/comtrade/en/country/ALL/year/2023/tradeflow/Exports/partner/WLD/product/401511. Accessed February 15, 2025. [Google Scholar]
- 15.Bousquet J, Flahault A, Vandenplas O, et al. Natural rubber latex allergy among health care workers: a systematic review of the evidence. J Allergy Clin Immunol. 2006;118:447–454. [DOI] [PubMed] [Google Scholar]
- 16.Toraason M, Sussman G, Biagini R, et al. Latex allergy in the workplace. Toxicol Sci. 2000;58:5–14. [DOI] [PubMed] [Google Scholar]
- 17.Manian DV, Volcheck GW. Perioperative anaphylaxis: evaluation and management. Clin Rev Allergy Immunol. 2022;62:383–399. [DOI] [PubMed] [Google Scholar]
- 18.Elliott BA. Latex allergy: the perspective from the surgical suite. J Allergy Clin Immunol. 2002;110:S117–S120. [DOI] [PubMed] [Google Scholar]
- 19.Rego A, Roley L. In-use barrier integrity of gloves: latex and nitrile superior to vinyl. Am J Infect Control. 1999;27:405–410. [DOI] [PubMed] [Google Scholar]
- 20.Patel HB, Fleming GJP, Burke FJT. Puncture resistance and stiffness of nitrile and latex dental examination gloves. Br Dent J. 2004;196:695–700; discussion 685; quiz 707. [DOI] [PubMed] [Google Scholar]
- 21.Bardorf MH, Jäger B, Boeckmans E, et al. Influence of material properties on gloves’ bacterial barrier efficacy in the presence of microperforation. Am J Infect Control. 2016;44:1645–1649. [DOI] [PubMed] [Google Scholar]
- 22.Osman MO, Jensen SL. Surgical gloves: current problems. World J Surg. 1999;23:630–7; discussion 637. [DOI] [PubMed] [Google Scholar]
- 23.Ansell Healthcare Products LLC. A Guide to Medical Glove Selection. Ansell; 2017. [Google Scholar]
- 24.Abdulsalam Y, Schneller E. Hospital supply expenses: an important ingredient in health services research. Med Care Res Rev. 2019;76:240–252. [DOI] [PubMed] [Google Scholar]
- 25.Phillips VL, Goodrich MA, Sullivan TJ. Health care worker disability due to latex allergy and asthma: a cost analysis. Am J Public Health. 1999;89:1024–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson RL, Smith HM, Duncan CM, et al. Factors that influence the selection of sterile glove brand: a randomized controlled trial evaluating the performance and cost of gloves. Can J Anaesth. 2013;60:700–708. [DOI] [PubMed] [Google Scholar]
- 27.Man T, Jiang J, Schulz M, et al. Surgical experience and different glove wearing conditions affect tactile sensibility. Heliyon. 2022;8:e12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riegel TO, Zellner EM, Hedlund CS, et al. Evaluation of sterile glove usage on digital tactile sensitivity using the Grating Orientation Task. Front Vet Sci. 2024;11:1401130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanner J, Parkinson H. Double gloving to reduce surgical cross‐infection. Cochrane Database Syst Rev. 2006:CD003087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Twomey CL. Double gloving: a risk reduction strategy. Jt Comm J Qual Saf. 2003;29:369–378. [DOI] [PubMed] [Google Scholar]
- 31.Fry DE, Harris WE, Kohnke EN, et al. Influence of double-gloving on manual dexterity and tactile sensation of surgeons. J Am Coll Surg. 2010;210:325–330. [DOI] [PubMed] [Google Scholar]
- 32.FDA. Medical glove guidance manual: guidance for industry and FDA Staff. 2008. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/medical-glove-guidance-manual. Accessed March 6, 2025. [Google Scholar]
- 33.Commission TJ. Sterile supply storage requirements (ASHRAE Standard 170). 2025. Available at: https://www.jointcommission.org/standards/standard-faqs/ambulatory/environment-of-care-ec/000001275/#:~:text=ASHRAE%20Standard%20170,SUPPLY%20areas%20includes%20the%20following. Accessed February 16, 2025. [Google Scholar]
- 34.Commission TJ. Quick safety issue 65: managing packaged sterile supplies and devices. 2025. Available at: https://www.jointcommission.org/resources/news-and-multimedia/newsletters/newsletters/quick-safety/quick-safety-issue-65/#:~:text=Managing%20commercially%20prepared%20sterile%20supplies,while%20protecting%20them%20from%20contamination. Accessed February 16, 2025. [Google Scholar]
- 35.Rebmann T, Vassallo A, Holdsworth JE. Availability of personal protective equipment and infection prevention supplies during the first month of the COVID-19 pandemic: a national study by the APIC COVID-19 task force. Am J Infect Control. 2021;49:434–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christian MD, Devereaux AV, Dichter JR, et al. ; Task Force for Mass Critical Care. Introduction and executive summary: care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest. 2014;146(4 Suppl):8S–34S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weber DJ, Malani AN, Shenoy ES, et al. Society for Healthcare Epidemiology of America position statement on pandemic preparedness for policymakers: mitigating supply shortages. Infect Control Hosp Epidemiol. 2024;45:813–817. [DOI] [PubMed] [Google Scholar]
- 38.(CDC) CfDCaP. Isolation precautions: appendix A—figure. Available at: https://www.cdc.gov/infection-control/hcp/isolation-precautions/appendix-a-figure.html. Accessed February 18, 2025. [Google Scholar]
- 39.(OSHA) OSaHA. OSHA standard interpretation: disposal of contaminated medical waste. Available at: https://www.osha.gov/laws-regs/standardinterpretations/2009-06-02. Accessed February 16, 2025. [Google Scholar]
- 40.(EPA) USEPA. Medical Waste. 2025. Available at: https://www.epa.gov/rcra/medical-waste. Accessed February 18, 2025. [Google Scholar]
- 41.Huang H, Wang Z, Dai C, et al. Volatile organic compounds emission in the rubber products manufacturing processes. Environ Res. 2022;212:113485. [DOI] [PubMed] [Google Scholar]
- 42.Jamal H, Lyne A, Ashley P, et al. Non-sterile examination gloves and sterile surgical gloves: which are more sustainable? J Hosp Infect. 2021;118:87–95. [DOI] [PubMed] [Google Scholar]
- 43.Prata JC, Silva AL, Walker TR, et al. COVID-19 pandemic repercussions on the use and management of plastics. Environ Sci Technol. 2020;54:7760–7765. [DOI] [PubMed] [Google Scholar]
- 44.Wang Z, An C, Lee K, et al. Physicochemical change and microparticle release from disposable gloves in the aqueous environment impacted by accelerated weathering. Sci Total Environ. 2022;832:154986. [DOI] [PubMed] [Google Scholar]
- 45.Gedik A, Ozcan O, Ozcanan S. Recycling COVID-19 health care wastes in bitumen modification: a case of disposable medical gloves. Environ Sci Pollut Res Int. 2023;30:74977–74990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bae JH, Ravinal L, Barth E, et al. The “6th R” of sustainability: repurposing operating room waste for community benefit. Am J Surg. 2024;238:115930. [DOI] [PubMed] [Google Scholar]
- 47.Azouz S, Boyll P, Swanson M, et al. Managing barriers to recycling in the operating room. Am J Surg. 2019;217:634–638. [DOI] [PubMed] [Google Scholar]
- 48.Research GV. Surgical gloves market size, share, & trends analysis report by material (natural rubber, nitrile), by end-use, by sterility, by form, by distribution, by usage, by region, and segment forecasts, 2023 - 2030. 2023. GVR-4-68040-069-7. Available at: https://www.grandviewresearch.com/industry-analysis/surgical-gloves-market-report#. Accessed February 16, 2025. [Google Scholar]
- 49.Cunningham AJ, Krakauer K, Schofield C, et al. Reducing disposable surgical items: decreasing environmental impact and costs at a children’s hospital, a pilot study. J Surg Res. 2023;288:309–314. [DOI] [PubMed] [Google Scholar]
- 50.Baxter NB, Yoon AP, Chung KC. Variability in the use of disposable surgical supplies: a surgeon survey and life cycle analysis. J Hand Surg. 2021;46:1071–1078. [DOI] [PubMed] [Google Scholar]