Abstract
Background and Objective:
Oxygen desaturation events occur commonly during Endobronchial endobronchial ultrasound (EBUS) guided-transbronchial needle aspiration (TBNA) performed under conscious sedation. We hypothesized that high-flow nasal cannula (HFNC) would be superior to conventional nasal cannula (NC) in preventing these hypoxemic episodes.
Methods:
We randomized consecutive subjects ≥18 years undergoing EBUS-TBNA to receive oxygen with HFNC or NC. The primary objective was to compare the proportion of subjects experiencing oxygen desaturation events (defined as SPO2 < 90% for at least 30 sec) during the EBUS-TBNA procedure between the two study arms. The key secondary outcomes were the number of desaturation events during the procedure and patient comfort on a visual analogue scale (VAS [0 mm-100 mm]).
Results:
We randomized 300 subjects (150 in each arm). The mean ± SD age of the study population (129 [43%] females) was 46.5 ± 14 years. The proportion of subjects experiencing clinically significant hypoxemic episodes was significantly (P < 0.0001) higher in NC (42.7% [64/150]) than in the HFNC (20% [30/150]) arm. The median nadir SPO2 was significantly lower in the NC arm than in HFNC (91% vs. 93%, P < 0.0001). The use of HFNC during EBUS-TBNA resulted in fewer desaturation events (mean difference [95% confidence interval], 0.55 [0.22-0.88]) and better patient comfort (mean difference in VAS, 7.1 [4.3 mm-9.9 mm]). We found no difference in the complication rates.
Conclusion:
HFNC during EBUS reduced the number of subjects experiencing clinically significant hypoxemia, the number of desaturation events, and improved patient comfort compared to conventional oxygen therapy.
Keywords: Bronchoscopy, hypoxia, lung cancer, sarcoidosis, tuberculosis
INTRODUCTION
Endobronchial ultrasound (EBUS)-guided transbronchial needle aspiration (TBNA) is routinely used to sample mediastinal lymph nodes.[1,2] The procedure is performed either under conscious sedation or general anaesthesia. At our centre, EBUS-TBNA is performed under conscious sedation using midazolam and fentanyl.[3,4] Hypoxemia is the most common complication encountered during bronchoscopy.[5,6] During EBUS-TBNA, oxygen supplementation is provided using nasal prongs (low flow system) to prevent desaturation.[7,8,9,10] However, the fraction of oxygen (FIO2) delivered by a low flow system such as a nasal cannula can vary according to an individual’s breathing pattern, causing a fall in pulse oximetric saturation (SPO2) below 90% during EBUS.[11,12] Most patients undergoing EBUS-TBNA have some underlying lung disease, including chronic obstructive pulmonary disease, interstitial lung disease, sarcoidosis, pneumonia, obesity hypoventilation syndrome, and others.[3,4,13,14,15,16,17,18,19] Hypoxemia during EBUS-TBNA is predominantly due to sedation-related hypoventilation but ventilation-perfusion mismatch may have a role especially in patients with underlying lung disease.[11]
The high-flow nasal cannula (HFNC) provides humidified, warmed 100% oxygen at a flow of 10-60 L per minute through a special nasal cannula. The device has been used extensively in intensive care settings in adults with hypoxemic respiratory failure and during bronchoscopy.[7,8,9,10,20,21,22,23] HFNC use during bronchoscopy improves partial pressure of oxygen (PaO2), prevents end-expiratory lung volume loss, and maintains the same tidal volumes at lower diaphragmatic effort than standard oxygen therapy.[22] A few studies have also evaluated HFNC during EBUS with conflicting results.[11,24,25] We hypothesized that oxygen supplementation through HFNC during EBUS-TBNA would have lesser frequency of clinically significant hypoxemic (peripheral pulse oximetric saturation [SPO2] ≤90%) events than conventional nasal cannula (NC). Herein, we report the results of a randomized controlled trial (RCT) comparing HFNC vs. NC in patients undergoing EBUS-TBNA.
METHODS
We conducted an investigator-initiated, open-label, RCT between 1 June 2022 and 31 December 2023 in the interventional bronchoscopy suite of the Department of Pulmonary Medicine of our Institute. No changes were made to the protocol after trial commencement.
Ethical statement
We performed the study per the principles outlined in the Declaration of Helsinki involving human subjects. The Institute Ethics Committee approved the study protocol. We obtained written informed consent from all study subjects. The first subject was enrolled on 12 June 2022, while the last was enrolled on 2 November 2023. The last patient was followed up on 3 December 2023. We have reported the results following the Consolidated Standards of Reporting Trials (CONSORT) statement.
Study participants
We enrolled consecutive subjects ≥18 years undergoing EBUS-TBNA for the evaluation of undiagnosed mediastinal lymphadenopathy. We excluded subjects with any of the following: (1) oxygen supplementation required to achieve SPO2 >95%; (2) subjects with altered mentation; (3) systolic blood pressure (BP) ≥180 mmHg and diastolic BP ≥90 mmHg); (4) pregnancy, and (5) failure to provide informed consent.
Randomization and blinding
We randomized subjects 1:1 to one of the study groups. Group 1 received oxygen therapy through conventional nasal cannula (NC arm), while group 2 received the high-flow oxygen through a nasal cannula (HFNC arm). Randomization was computer-generated with variable-sized blocks between four and eight. We concealed the randomization sequence in an opaque sealed envelope. The envelopes were opened by the bronchoscopy technician not involved in the data analysis. We used StatsDirect software (StatsDirect Ltd Wirral, UK) to generate the randomization sequence. There was no blinding.
Sample size calculation
We assumed that 30% of subjects would experience episodic oxygen desaturation (SPO2 <90%) in the NC arm. To reduce the episodes by 15% in the HFNC arm, a sample size of 270 (135 in each arm) was required for 80% power at a significance level of 0.05. We enrolled 300 subjects to compensate for 10% dropout.
Study procedures
All the subjects underwent complete blood count, coagulation profile, liver and kidney function tests, chest radiograph and chest computed tomography (CT).
EBUS-TBNA procedure: After an overnight fast, 10% lignocaine solution was sprayed four times (10 mg/puff) over the oropharynx with an atomizer before the procedure. The subjects were administered intravenous midazolam (1-2 mg initially, and additional 1 mg doses) and fentanyl (25-50 µg initially with additional doses as required to maintain cough suppression) as directed by the bronchoscopist.[3,4]
The convex probe EBUS bronchoscope (BF-UC 180F; Olympus Medical Systems, Japan) with a 7.5 MHz convex transducer and a compatible endoscopic ultrasound scanner (EU-ME2; Olympus Medical Systems, Japan) was introduced transorally. We sprayed 2 mL aliquots of 1% lignocaine solution through the echo bronchoscope at the vocal cords, tracheal carina, and in the right and left main bronchus.[26] Additional lignocaine aliquots were instilled as needed to suppress cough at the operator’s discretion. Subjects were monitored for any adverse effects related to the use of the local anaesthetic and sedative drugs. Heart rate, respiratory rate, blood pressure, and SPO2 were monitored throughout the procedure. We assessed the depth of sedation using the Ramsay Sedation Scale (RSS) and maintained an RSS score of 2-3. The RSS is a numerical scale describing six levels of consciousness from 1 (subject anxious, agitated, and/or restless) through 6 (subjects exhibiting no response to light glabella tap or loud auditory stimulus).[3] Lymph nodes were punctured using a disposable, 22-gauge, dedicated EBUS-TBNA needle (Vizishot, NA-201SX-4022, Olympus Medical Systems, Japan) under sonographic and bronchoscopy visualization.
Oxygen supplementation in the NC arm: Oxygen therapy was delivered using a standard nasal cannula (Oxy Set, Romsons Scientific and Surgical, Agra, India). Five minutes before sedation the flow rate was kept at 1-2 L per minute. The flow was increased to maintain SPO2 ≥92% during the procedure, up to a maximum of 10 L per minute.
Oxygen supplementation in HFNC arm: HFNC (AIRVO2; Fisher and Paykel, Auckland, New Zealand) was started 5 min before the sedation at a flow rate of 30 L per minute and at a fraction of inspired oxygen (FIO2) of 0.30. Oxygen was titrated by increments of 10 L per minute to keep SPO2 ≥92% during the procedure. The temperature was kept at 37°C. The flow rate was maintained between 30 and 60 L per minute, depending on the patient’s tolerance. The size of the NC was selected to occlude two-thirds of the nostrils.
Study outcomes
The primary outcome was the proportion of subjects experiencing oxygen desaturation events (defined as SPO2 <90% for at least 30 sec) during the EBUS-TBNA procedure. The time was recorded using a stopwatch by an assistant not involved in the EBUS-TBNA procedure. Secondary outcomes were (i) SPO2 after pre-oxygenation for 5 min before the start of the procedure; (ii) number of desaturation events (SPO2 <90% for at least 30 sec) during the procedure; (iii) nadir SPO2 level during the procedure; (iv) patient comfort (0 mm being extreme discomfort and 100 mm, very comfortable) and bronchoscopist satisfaction (0 mm, being unsatisfied and 100 mm, very satisfied) assessed on a visual analogue scale (VAS); (v) VAS for patient-reported nasal dryness (0 mm = no dryness; 100 mm = severe dryness) and, (vi) complications including cardiac arrhythmia, hypotension, escalation of the level of care (hospitalization, airway intubation need for non-invasive or invasive mechanical ventilation), altered mentation, respiratory failure or cardiac arrest. We recorded patient-reported outcomes one hour after the EBUS procedure.
Cost-analysis: We performed a cost analysis from the hospital’s perspective to compare HFNC, and NC oxygen delivery strategies used during EBUS-TBNA. The cost components included: (1) Capital cost of the HFNC device, estimated at ₹2,00,000 (INR), amortized over three years. We perform 450 EBUS procedures per year i.e., 1350 procedures over three years. The per-patient share of device cost was thus ₹148.15; (2) Consumable cost: HFNC cannula cost was ₹2,500 per patient and standard nasal cannula cost was ₹200 per patient; (3) Cost of hospital-stay due to sustained hypoxemia: defined as requiring oxygen supplementation for ≥15 min post-procedure. We assumed each such patient incurred an additional 1 hour of monitored hospital stay, costed at ₹3,000 per hour (includes bed charges, man-power charges, equipment, and other miscellaneous charges). Costs of staffing, sedatives, procedural equipment, and bronchoscope use were assumed to be equivalent across groups and not included. We calculated total and per-patient costs for each arm (HFNC and NC) based on the observed frequency of sustained hypoxemia in our trial.
Systematic review: We identified randomized controlled trials comparing HFNC with conventional oxygen therapy for performing EBUS-TBNA. We searched the PubMed and Embase database using the free text terms: (endobronchial ultrasound, EBUS, EUS, endosonographic, bronchial ultrasound, curvilinear ultrasound) AND (high flow oxygen, hfnc, hfno, high flow oxygen with nasal cannula). We pooled the proportion of subjects experiencing oxygen desaturation events during the EBUS-TBNA procedure using a random effect model.
Statistical methods
We used the statistical package SPSS (version 22.0, IBM Corp., Armonk, NY, United States) to analyse data. Data are presented as mean (95% confidence intervals [95% CI]), median (interquartile range [IQR]), or number (percentage). We used an intention-to-treat analysis to report the study’s results. The difference between continuous and categorical variables was analysed using analysis of variance and Chi-square test. We used StatsDirect software version 2.8.0 to perform random effects meta-analysis. A P value ≤ 0.05 was considered statistically significant.
RESULTS
We screened 331 subjects, of whom 300 (150 in both study arms), were included in the current study [Figure 1]. The mean (95% CI) age of the study population (129 [43%] women) was 46.5 (36-57) years [Table 1]. The most common indication for the EBUS procedure was sarcoidosis followed by malignancy (lung or metastatic cancer), which was evenly distributed between the two study groups. The pre-procedure SPO2 was similar between the two study arms [Figure 2]. The dose of midazolam and fentanyl was similar in each arm with a median Ramsay sedation score of 2. We sampled a median of two lymph node stations per patient, and the number of lymph node stations sampled was similar in both the study arms [Table 1]. A median of six passes were performed per patient and the number of passes per patient was similar in each arm.
Figure 1.
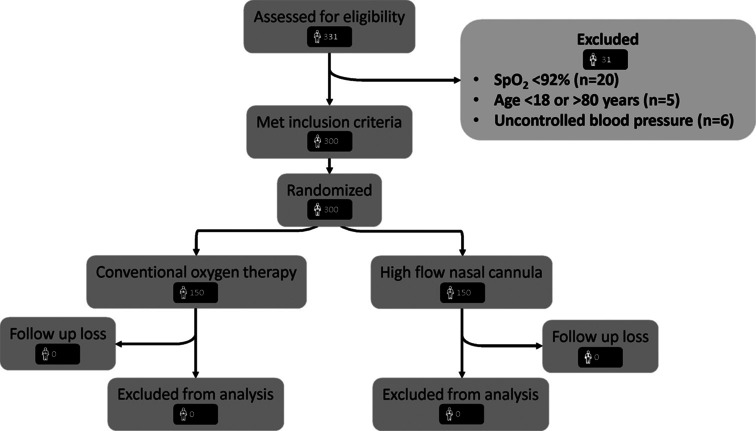
‘Consolidated Standards of Reporting Trials’ diagram depicting the flow of patients during the study
Table 1.
Baseline clinical, demographic, and procedural details of the study participants
| NC arm (n=150) | HFNC arm (n=150) | Total (n=300) | P | |
|---|---|---|---|---|
| Age in years | 47.5 (36-59) | 45 (36-55) | 46.5 (36-57) | 0.405 |
| Female sex, n (%) | 66 (44) | 63 (42) | 129 (43) | 0.726 |
| Clinical indication, n (%) | 0.725 | |||
| Sarcoidosis | 72 (48) | 74 (49.3) | 146 (48.7) | |
| Tuberculosis | 29 (19.3) | 24 (160) | 53 (17.7) | |
| Malignancy including staging | 45 (30) | 45 (30) | 90 (30) | |
| Others | 4 (2.7) | 7 (4.7) | 11 (3.7) | |
| Pre-procedure SPO2 | 100 (98-100) | 98 (97-99) | 98 (97-99) | 0.720 |
| SPO2 after preoxygenation | 100 (98-100) | 99 (99-100) | 100 (99-100) | 0.897 |
| Number of lymph nodes sampled | 2 (2-3) | 2 (2-3) | 2 (2-3) | 0.829 |
| Total number of passes | 6 (6-7) | 6 (5.8-7) | 6 (6-7) | 0.123 |
| Duration of EBUS, minutes | 20 (15-24) | 20 (15-25) | 20 (15-25) | 0.471 |
| Dose of midazolam, mg | 2 (2-3) | 2 (2-3) | 2 (2-3) | 0.110 |
| Dose of fentanyl, µg | 50 (50-50) | 50 (50-50) | 50 (50-50) | 0.687 |
| Ramsay sedation score | 2 (2-3) | 2 (2-3) | 2 (2-3) | 0.702 |
All values are represented as median (interquartile range) or number (percentage)
Figure 2.

Peripheral pulse oximetric saturation (SPO2) before (A), during (B), and after (C) the endobronchial ultrasound-guided transbronchial needle aspiration procedure in the conventional oxygen therapy (COT) and the high-flow nasal cannula (HFNC) group. *represents statistically significant difference between the two study groups
Primary outcome: The proportion of subjects experiencing clinically significant hypoxemia was 42.7% in the NC arm and was significantly higher than in the HFNC arm [Table 2]. The use of HFNC led to a 23% absolute risk reduction in desaturation events.
Table 2.
Primary and secondary outcomes
| NC arm (n=150) | HFNC arm (n=150) | Estimate difference (95% confidence interval) | P | |
|---|---|---|---|---|
| Primary outcome | ||||
| Proportion of subjects with desaturation events, n (%) | 64 (42.7) | 30 (20) | 0.23 (0.12-0.32) | <0.0001 |
| Secondary outcomes | ||||
| Nadir SPO2 | 91 (87-94) | 93 (91-97) | 1.85 (0.05-3.65) | <0.0001 |
| Number of desaturation events | 0 (0-2) | 0 (0-0) | 0.55 (0.22-0.88) | <0.0001 |
| Number of titration events | 0 (0-1) | 0 (0-0) | −0.03 (−0.35-0.29) | 0.015 |
| VAS for bronchoscopist satisfaction, mm | 89 (78.8-92) | 91 (81-98) | 4.41 (0.58-8.24) | <0.0001 |
| VAS for patient comfort, mm | 87 (78-92) | 94 (89-98) | 7.1 (4.29-9.91) | <0.0001 |
| VAS for nasal dryness | 3 (2-9) | 2 (1-7) | 0.03 (−1.64-1.70) | 0.023 |
| Post-procedure SPO2 | 95 (92-97) | 94 (92-97) | 0.18 (−0.79-1.15) | 0.953 |
| Complications, n (%) | 21 (14) | 12 (8) | 0.06 (−0.01-0.13) | 0.189 |
| Sustained hypoxemia | 17 (11.3) | 7 (4.7) | 0.07 (0.01-0.13) | 0.033 |
| Chest pain | 2 (1.3) | 4 (2.7) | −0.01 (−0.05-0.02) | 0.409 |
| Pneumothorax | 1 (0.7) | 1 (0.7) | 0 (−0.03-0.03) | 1.000 |
| Pneumomediastinum | 1 (0.7) | 0 | 0.01 (−0.02-0.04) | 0.317 |
All values are expressed as median (interquartile range) or number (percentage)
Secondary outcomes: The use of HFNC during EBUS-TBNA resulted in fewer desaturation events, fewer oxygen titration manoeuvres, higher mean SPO2 during procedure, better patient comfort, and better operator satisfaction [Table 2 and Figure 2]. Although the overall complication rate was similar in the two arms, subjects in the HFNC arm had less risk of experiencing sustained hypoxemia (requiring oxygen supplementation for at least 15 min) post-procedure [Table 2]. None of the patients required escalation of care or hospitalization. We recorded no deaths.
Cost analysis: Among 300 patients (150 per arm), sustained hypoxemia requiring extended post-procedural monitoring occurred in 7 HFNC and 17 NC patients.
The total cost for the HFNC arm was ₹4,18,222, driven by ₹22,222 in amortized device cost, ₹3,75,000 for single-use cannulae, and ₹21,000 for additional hospital stay, yielding a per-patient cost of ₹2,788. In contrast, the NC arm incurred a total of ₹81,000, ₹30,000 for cannulae and ₹51,000 for hypoxemia-related hospital stay, with a per-patient cost of ₹540.
Systematic review: We found three RCTs comparing conventional oxygen therapy vs. HFNC for oxygen supplementation during EBUS-TBNA [Table 3]. On pooling previous studies and the current study, we found that conventional oxygen therapy had significantly higher odds (odds ratio, 6.2 [95% CI, 2.2-17.2]) of causing hypoxemia compared to HFNC [Figure 3].
Table 3.
Randomized controlled trials comparing high flow nasal cannula (HFNC) and conventional oxygen therapy (SOT) for oxygen supplementation during endobronchial ultrasound guided transbronchial needle aspiration
| Author/year | Number of patients | Comparator | HFNC used | Primary outcome | Secondary outcomes | Comment |
|---|---|---|---|---|---|---|
| Douglas N et al.[11] 2018 | 60 | SOT via bite block (flow rate 10-15 L/minute) | OptiFlow THRIVE with 100% FIO2 and a flow rate of 30-70 L/minute | Proportion of patients with desaturation (SPO2 <90%): HFNC vs. SOT, 4/30 vs. 10/30, P=0.07 | SPO2 after preoxygenation was significantly higher in HFNC vs. SOT arm. No difference in the lowest SPO2 during the EBUS procedure | A small sample size could not detect the difference between the two strategies. Used 100% FIO2 in the HFNC group. Performed both radial and convex probe EBUS. |
| Irfan M et al.[24] 2021 | 40 | Nasal prongs | OptiFlow THRIVE | Drop in SPO2 during procedure was significantly different by 7.7% points in the two arms | SPO2 was 9.2 mmHg lower in the SOT arm vs. HFNC arm. The proportion of patients with desaturation (SPO2 <90%): HFNC vs. SOT, 1/20 vs. 11/20, P<0.001. There was no difference in safety outcomes or willingness to undergo a repeat procedure | Small sample size. |
| Ucar EY et al.[25] 2021 | 170 | Nasal cannula at 5 L/minute | HFNC at a flow rate of 35 L/minute and FIO2 of 40% | Proportion of patients with desaturation (SPO2 <90%): HFNC vs. SOT, 5/85 vs. 26/85, P<0.001 | SPO2 was significantly higher in HFNC arm at the end of procedure. More patients in the SOT arm reported procedural discomfort. | Used fixed flow rates and FIO2. |
| Current study | 300 | Nasal cannula | HFNC 30-60 L/minute, FIO2 21-100. OptiFlow AIRVO-2 | Proportion of patients with desaturation (SPO2 <90%): HFNC vs. SOT, 30/150 vs. 64/150, P<0.001 | Nadir SPO2, number of desaturation events, and need for titration significantly differed, favouring the HFNC arm. Complication rates were similar. Both operator and patients reported better comfort with HFNC | Single centre, ETCO2 not measured. |
ETCO2: end-tidal carbon dioxide; FIO2: fraction of inspired oxygen; SPO2: peripheral pulse oximetric saturation
Figure 3.

Forest plot depicting the pooled analysis of proportion of patients, who experience hypoxemia during endobronchial ultrasound
DISCUSSION
During EBUS-TBNA, oxygen supplementation using HFNC significantly reduced the number of subjects experiencing hypoxemic episodes compared to conventional oxygen therapy using NC. Only four patients (95% CI, 3-8) need to be treated with HFNC to prevent a patient experiencing a significant hypoxemic episode. HFNC use during EBUS-TBNA reduced the number of desaturation events, required fewer adjustments, and led to better patient comfort and operator satisfaction than conventional oxygen therapy. On a pooled analysis of all the RCTs conducted till date, conventional oxygen therapy had six times higher odds of causing hypoxemia than HFNC for oxygen delivery during EBUS procedure.
Three previous RCTs evaluating HFNC for EBUS-TBNA reported conflicting results [Table 3].[11,24,25] Douglous et al.,[11] compared HFNC with conventional oxygen therapy in sixty patients undergoing either curvilinear or radial EBUS and found no difference in the proportion of subjects experiencing desaturation <90%. The study had a small sample size, used different sedative agents (propofol, midazolam, alfentanil, fentanyl, and remifentanil), and had a different patient profile. Interestingly, on a per-protocol analysis, the use of HFNC significantly reduced the proportion of patients experiencing clinically relevant desaturation.[11] In another study of 40 patients, HFNC use led to fewer patients experiencing desaturation below 90% than standard oxygen therapy.[24] A recent study of 170 patients also found fewer patients experiencing desaturation events <90% with HFNC than conventional oxygen, like the current study.[25]
What are the clinical implications of the study? We found that HFNC led to an absolute risk reduction of 23% of clinically significant desaturation events, with the number-needed-to-treat being four. Further, the number of desaturation events was lower, and the median SPO2 was higher with HFNC use. Although the HFNC strategy incurred higher upfront and per-patient costs, it was associated with a significantly lower incidence of hypoxemic events, less frequent sustained hypoxemia, and improved patient comfort. These findings suggest a potential clinical and operational advantage, especially in high-volume centres, despite the higher unit cost. Further, due to better tolerance and improved patient and operator comfort, we could perform EBUS-TBNA using conscious sedation and avoid general anaesthesia. About one-third of the EBUS procedures were performed for diagnosing and staging lung cancer. We used the AIRVO-2 device to deliver HFNC, which allows for the titration of FIO2 and flow rates, unlike the THRIVE device used in previous studies. The THRIVE device provides near 100% FIO2 and does not allow FIO2 titration, which can cause oxygen wastage and less optimal humidification. The AIRVO-2 device, on the other hand, provides better humidification that improves patient comfort and procedure tolerance. Only two patients in the current study in HFNC arm required FIO2 of ≥90%, and most subjects required FIO2 of 30% (data not shown). Furthermore, we observed that more patients in the HFNC appeared to be discharged earlier than NC patients. However, this requires further study.
Our study has a few limitations. The study was performed at a single, high-volume centre. We could not perform blinding of operators and study participants due to the study’s nature. Finally, we did not record end-tidal carbon dioxide or other parameters to study markers of ventilatory adequacy, such as partial pressure of carbon dioxide in blood. To our knowledge, the current study is the largest, comparing two strategies for providing oxygen supplementation during EBUS-TBNA.
In conclusion, HFNC led to a 23% absolute risk reduction of clinically relevant hypoxemic episodes during EBUS procedures performed using conscious sedation. HFNC may be used in high volume centres performing EBUS-TBNA under conscious sedation for added patient comfort.
Author contributions
ISS- conceived the idea, data collection, patient management, performed statistical analysis, drafted and revised the manuscript
SJ- data collection, patient management, drafted and revised the manuscript
NG- interpretation of cytology specimens, and revised the manuscript
SD- patient management, and revised the manuscript
KTP- patient management, and revised the manuscript
AB- interpretation of histopathology specimens, and revised the manuscript
PG- interpretation of histopathology specimens, and revised the manuscript
ANA- patient management, and revised the manuscript
VM- patient management, and revised the manuscript
RA- provided intellectual content to the manuscript, patient management, and revised the manuscript
ISS- guarantor of the paper, taking responsibility for the integrity of the work as a whole, from inception to published article.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We sincerely thank Fischer and Paykel, Auckland, New Zealand for providing us with the HFNC machine on loan, which was used for conducting the current trial.
Funding Statement
Nil.
REFERENCES
- 1.Damaraju V, Gupta N, Saini M, Dhooria S, Prasad KT, Gupta P, et al. The utility of WhatsApp-based off-site evaluation for rapid cytology of EBUS-TBNA samples. Cytopathology. 2023;34:43–7. doi: 10.1111/cyt.13188. [DOI] [PubMed] [Google Scholar]
- 2.Prasad KT, Muthu V, Sehgal IS, Dhooria S, Singh N, Gupta N, et al. Endosonographic characteristics of mediastinal lymph nodes for predicting malignancy in high tuberculosis burden settings: A study of 774 subjects. Expert Rev Respir Med. 2022;16:1011–5. doi: 10.1080/17476348.2022.2118717. [DOI] [PubMed] [Google Scholar]
- 3.Dhooria S, Sehgal IS, Gupta N, Aggarwal AN, Behera D, Agarwal R. Diagnostic yield and complications of EBUS-TBNA performed under bronchoscopist-directed conscious sedation: Single center experience of 1004 subjects. J Bronchology Interv Pulmonol. 2017;24:7–14. doi: 10.1097/LBR.0000000000000332. [DOI] [PubMed] [Google Scholar]
- 4.Kumari R, Jain K, Agarwal R, Dhooria S, Sehgal IS, Aggarwal AN. Fixed dexmedetomidine infusion versus fixed-dose midazolam bolus as primary sedative for maintaining intra-procedural sedation during endobronchial ultrasound-guided transbronchial needle aspiration: A double blind randomized controlled trial. Expert Rev Respir Med. 2021;15:1597–604. doi: 10.1080/17476348.2021.1918000. [DOI] [PubMed] [Google Scholar]
- 5.Muthu V, Ram B, Sehgal IS, Dhooria S, Prasad KT, Aggarwal AN, et al. Major complications encountered during 9979 flexible bronchoscopies performed under local anesthesia over 8 years. Lung India. 2022;39:384–7. doi: 10.4103/lungindia.lungindia_37_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sehgal IS, Kaur G, Gupta N, Dhooria S, Prasad KT, Bal A, et al. A Randomized control trial comparing the yield of bronchoalveolar lavage using three different techniques in patients undergoing flexible bronchoscopy (BAL-3T) J Bronchology Interv Pulmonol. 2024;31:e0979. doi: 10.1097/LBR.0000000000000979. [DOI] [PubMed] [Google Scholar]
- 7.Sharluyan A, Osona B, Frontera G, Brandstrup KB, Figuerola J, Sanz-Ruiz I, et al. High flow nasal cannula versus standard low flow nasal oxygen during flexible bronchoscopy in children: A randomized controlled trial. Pediatr Pulmonol. 2021;56:4001–10. doi: 10.1002/ppul.25655. [DOI] [PubMed] [Google Scholar]
- 8.Simon M, Braune S, Frings D, Wiontzek AK, Klose H, Kluge S. High-flow nasal cannula oxygen versus non-invasive ventilation in patients with acute hypoxaemic respiratory failure undergoing flexible bronchoscopy--A prospective randomised trial. Crit Care. 2014;18:712. doi: 10.1186/s13054-014-0712-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang W, Wang JL, Fu S, Zhou JM, Zhu YJ, Cai SN, et al. Incidence of oxygen desaturation using a high-flow nasal cannula versus a facemask during flexible bronchoscopy in patients at risk of hypoxemia: A randomised controlled trial. BMC Pulm Med. 2022;22:389. doi: 10.1186/s12890-022-02188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang W, Yuan X, Shen Y, Wang J, Xie K, Chen X. Optimal flow of high-flow nasal cannula oxygenation to prevent desaturation during sedation for bronchoscopy: A randomized controlled study. Ther Adv Respir Dis. 2024;18:17534666241246637. doi: 10.1177/17534666241246637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douglas N, Ng I, Nazeem F, Lee K, Mezzavia P, Krieser R, et al. A randomised controlled trial comparing high-flow nasal oxygen with standard management for conscious sedation during bronchoscopy. Anaesthesia. 2018;73:169–76. doi: 10.1111/anae.14156. [DOI] [PubMed] [Google Scholar]
- 12.Takakuwa O, Oguri T, Asano T, Fukuda S, Kanemitsu Y, Uemura T, et al. Prevention of hypoxemia during endobronchial ultrasound-guided transbronchial needle aspiration: Usefulness of high-flow nasal cannula. Respir Investig. 2018;56:418–23. doi: 10.1016/j.resinv.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Dhooria S, Mehta RM, Madan K, Vishwanath G, Sehgal IS, Chhajed PN, et al. A multicenter study on the utility of EBUS-TBNA and EUS-B-FNA in the diagnosis of mediastinal lymphoma. J Bronchology Interv Pulmonol. 2019;26:199–209. doi: 10.1097/LBR.0000000000000552. [DOI] [PubMed] [Google Scholar]
- 14.Sehgal IS, Bal A, Dhooria S, Gupta N, Ram B, Aggarwal AN, et al. Predictors of successful yield of transbronchial lung biopsy in patients with sarcoidosis. J Bronchology Interv Pulmonol. 2018;25:31–6. doi: 10.1097/LBR.0000000000000439. [DOI] [PubMed] [Google Scholar]
- 15.Prasad KT, Muthu V, Sehgal IS, Dhooria S, Sharma A, Gupta N, et al. Utility of endobronchial ultrasound-guided transbronchial needle aspiration in HIV-infected patients with undiagnosed intrathoracic lymphadenopathy. Lung India. 2018;35:379–83. doi: 10.4103/lungindia.lungindia_480_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhooria S, Sehgal IS, Gupta N, Bal A, Prasad KT, Aggarwal AN, et al. A randomized trial evaluating the effect of 10 versus 20 revolutions inside the lymph node on the diagnostic yield of EBUS-TBNA in subjects with sarcoidosis. Respiration. 2018;96:464–71. doi: 10.1159/000490192. [DOI] [PubMed] [Google Scholar]
- 17.Sehgal IS, Dhooria S, Gupta N, Bal A, Ram B, Aggarwal AN, et al. Impact of Endobronchial Ultrasound (EBUS) training on the diagnostic yield of conventional transbronchial needle aspiration for lymph node stations 4R and 7. PLoS One. 2016;11:e0153793. doi: 10.1371/journal.pone.0153793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muthu V, Gupta N, Dhooria S, Sehgal IS, Bal A, Aggarwal AN, et al. A Prospective, randomized, double-blind trial comparing the diagnostic yield of 21- and 22-gauge aspiration needles for performing endobronchial ultrasound-guided transbronchial needle aspiration in sarcoidosis. Chest. 2016;149:1111–3. doi: 10.1016/j.chest.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Dhooria S, Sehgal IS, Aggarwal AN, Agarwal R. Convex-probe endobronchial ultrasound: A decade of progress. Indian J Chest Dis Allied Sci. 2016;58:21–35. [PubMed] [Google Scholar]
- 20.Frat JP, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372:2185–96. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 21.Sklar MC, Mohammed A, Orchanian-Cheff A, Del Sorbo L, Mehta S, Munshi L. The impact of high-flow nasal oxygen in the immunocompromised critically Ill: A systematic review and meta-analysis. Respir Care. 2018;63:1555–66. doi: 10.4187/respcare.05962. [DOI] [PubMed] [Google Scholar]
- 22.Longhini F, Pelaia C, Garofalo E, Bruni A, Placida R, Iaquinta C, et al. High-flow nasal cannula oxygen therapy for outpatients undergoing flexible bronchoscopy: A randomised controlled trial. Thorax. 2022;77:58–64. doi: 10.1136/thoraxjnl-2021-217116. [DOI] [PubMed] [Google Scholar]
- 23.Saksitthichok B, Petnak T, So-Ngern A, Boonsarngsuk V. A prospective randomized comparative study of high-flow nasal cannula oxygen and non-invasive ventilation in hypoxemic patients undergoing diagnostic flexible bronchoscopy. J Thorac Dis. 2019;11:1929–39. doi: 10.21037/jtd.2019.05.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irfan M, Ahmed M, Breen D. Assessment of high flow nasal cannula oxygenation in endobronchial ultrasound bronchoscopy: A randomized controlled trial. J Bronchology Interv Pulmonol. 2021;28:130–7. doi: 10.1097/LBR.0000000000000719. [DOI] [PubMed] [Google Scholar]
- 25.Yilmazel Ucar E, Araz Ö, Kerget B, Akgun M, Saglam L. Comparison of high-flow and conventional nasal cannula oxygen in patients undergoing endobronchial ultrasonography. Intern Med J. 2021;51:1935–9. doi: 10.1111/imj.15001. [DOI] [PubMed] [Google Scholar]
- 26.Kaur H, Dhooria S, Aggarwal AN, Gupta D, Behera D, Agarwal R. A randomized trial of 1% vs 2% lignocaine by the spray-as-you-go technique for topical anesthesia during flexible bronchoscopy. Chest. 2015;148:739–45. doi: 10.1378/chest.15-0022. [DOI] [PubMed] [Google Scholar]


