Abstract
As repetitive acoustic stimulation and auditory conditioning do, electric stimulation of the primary auditory cortex (AI) evokes reorganization of the frequency map of AI, as well as of the subcortical auditory nuclei. The reorganization is caused by shifts in best frequencies (BFs) of neurons either toward (centripetal) or away from (centrifugal) the BF of stimulated cortical neurons. In AI of the Mongolian gerbil, we found that focal electrical stimulation evoked a centripetal BF shift in an elliptical area centered at the stimulated neurons and a centrifugal BF shift in a zone surrounding it. The 1.9-mm long major and 1.1-mm long minor axes of the elliptical area were parallel and orthogonal to the frequency axis, respectively. The width of the surrounding zone was 0.2–0.3 mm. Such “center-surround” reorganization has not yet been found in any sensory cortex except AI of the gerbil. The ellipse is similar to the arborization pattern of pyramidal neurons, the major source of excitatory horizontal connections in AI, whereas the surrounding zone is compatible to the arborization range of small basket cells (inhibitory neurons) in AI.
Keywords: frequency tuning‖hearing‖plasticity‖tonotopic map
The response properties of frequency-tuned neurons and/or the cochleotopic (frequency) map of the primary auditory cortex (AI) can be changed by repetitive acoustic stimulation (1, 2), auditory conditioning (3–9), learning of a discrimination task (10), focal electric stimulation of AI (1, 2, 11), or electric stimulation of the basal forebrain paired with acoustic stimulation (6, 12).
The response properties of frequency-tuned neurons and the cochleotopic map of the inferior colliculus (IC) also can be changed by repetitive acoustic stimulation (2, 13, 14), auditory conditioning (8, 9, 14), or focal electric stimulation of AI (2, 13, 15, 17).
In the big brown bat, Eptesicus fuscus, cortical and collicular changes in the cochleotopic map are caused by the shift in best frequency (BF) of single neurons toward the frequency of a repetitively delivered acoustic stimulus (1, 13, 14), the frequency of a conditioned sound (8, 9, 14), or the BF of electrically stimulated AI neurons (1, 11, 13, 15). BF shifts resulting in reorganization of the frequency map are basically the same regardless of the means that evoked them and are attributable to activity within the corticofugal system (8, 9, 14). This means that focal electric stimulation of AI activates the essential portion of the neural mechanism for reorganization (plasticity) of the central auditory system and that it can be an appropriate method for the exploration of the plasticity of neurons in the IC, medial geniculate body, and AI (18). BF shifts toward and away from the BF of electrically stimulated cortical neurons or the frequency of a repetitively delivered tone burst are called “centripetal” and “centrifugal” BF shifts, respectively (18).
A centripetal BF shift evoked by focal electric stimulation of AI has been found not only in the big brown bat, but also in the posterior division (nonspecialized portion) of AI of the mustached bat and AI of the Mongolian gerbil (11). Furthermore, centripetal shift of the receptive fields of neurons in the somatosensory cortex has been found in monkeys (19) and cats (20). Focal electric stimulation of AI also evokes centripetal shift in sound-duration tuning (15) of collicular neurons. Therefore, the corticofugal system modulates the functional organization of the IC not only in the frequency domain but also in the time domain. Centripetal reorganization is assumed to be widely shared with mammalian sensory systems.
Reorganization of the frequency map is, however, different between specialized and nonspecialized areas of the auditory cortex of the mustached bat (11, 18). The Doppler-shifted constant frequency (DSCF) and frequency modulation (FM)–FM areas of the auditory cortex of the mustached bat are specialized for processing either velocity and relative size information carried by ≈61 kHz sound (21) or target-distance information carried by a pair of FM sounds (23, 24), respectively. Focal electric stimulation of the DSCF or FM–FM area revokes centrifugal shift in the BFs of collicular, thalamic, (16, 25) and cortical (11) DSCF neurons or in the best delays of collicular and thalamic FM–FM neurons (26), respectively. The direction of these shifts in BF and best delay is opposite to that in BF observed in the posterior division of AI of the mustached bat (11). Such a difference appears to be related to corticofugal facilitation and lateral inhibition of auditory responses of subcortical auditory neurons, so that the following working hypothesis has been proposed (18). If the positive feedback evoking facilitation is strong and widespread to neighboring neurons and negative feedback evoking lateral inhibition is weak, a focal activation of the auditory cortex evokes a prominent centripetal BF shift in a large area that is surrounded by a narrow zone of centrifugal BF shift. On the contrary, if the positive feedback is highly focused and negative feedback is strong and widespread to neighboring neurons, a focal activation of the auditory cortex evokes a prominent centrifugal BF shift in a large area surrounding a narrow zone where a small centripetal BF shift is evoked. In AI, there are excitatory neurons for horizontal connections and inhibitory neurons for local connections (27, 28), so that facilitation and inhibition for BF shifts may also occur.
If the corticofugal system evokes facilitation and inhibition of subcortical neurons for processing auditory information and reorganizing the central auditory system, reorganization of AI of the Mongolian gerbil may consist of two types of areas: an area for centripetal BF shift at the center and an area for centrifugal BF shift in the surround. The aim of the current article is to report the data showing such a center-surround reorganization of the gerbil's AI.
Materials and Methods
Animal preparation and experimental procedures were exactly the same as those in our previous article (11). Eleven adult Mongolian gerbils (Meriones unguiculatus; 65.2 ± 1.8 g body weight) were used. Under anesthesia with ketamine (40 mg/kg body weight) and meditomidine (0.26 mg/kg body weight), the dorsal surface of the animal's skull was exposed, and four plastic sockets (1.5 mm in diameter and 2.0 mm in length) were attached onto the lateral sides of the skull with glue and dental cement. After the surgery (5–7 d), the animal was lightly anesthetized with the above drugs and placed in a polyethylene-foam body-mold suspended by an elastic band at the center of a soundproof room. The animal's head was immobilized by inserting metal rods into the sockets and adjusted to face directly at a loudspeaker located 74 cm away. Holes (50–100 μm in diameter) were made on the skull covering AI. Tungsten-wire electrodes (≈7 μm in tip diameter) for electric stimulation or recording action potentials were orthogonally inserted into AI through the holes. The protocol of our research was approved by the Animal Studies Committee of Washington University.
A tone burst (20 ms-long, 0.5-ms rise/decay time) was delivered at a rate of 5/s from the loudspeaker. The BF, minimum threshold, and frequency-tuning curve of a single neuron were first measured audiovisually. A computer-controlled frequency scan (22 time blocks, 200 ms long for each) was then delivered, in which the frequency of the tone burst was shifted in steps of a given frequency (0.04–0.5 kHz) across the BF of the neuron. The amplitude of tone bursts in the scan was set at 10 dB above the minimum threshold of a given neuron. Identical frequency scans were repeated at a rate of 1/5 s.
A pair of tungsten-wire microelectrodes (≈7 μm in tip diameter; 150-μm apart, one proximal to the other) was inserted into cortical layer V (700–800 μm in depth). Action potentials originating from 2–3 single neurons were recorded and the BFs of the neurons were measured. Then they were electrically stimulated through the pair of electrodes. The electric stimulation was a 6.2-ms long train of four monophasic electric pulses (100 nA, 0.2-ms duration, 2.0-ms intervals). The train was delivered at a rate of 10/s for 30 min.
Action potentials of a single cortical neuron were selected with an amplitude-window discriminator (BAK Electronic, Rockville, MD; model DLS-1), and an action potential of the neuron was stored on the screen of a digital storage oscilloscope at the beginning of data acquisition. The responses of a cortical neuron to 50 identical frequency scans were displayed as an array of peri-stimulus-time histograms as a function of frequency. Acquisition of peri-stimulus-time histograms was continued as long as action potentials visually matched the template, the stored action potential of the neuron. Data were stored on a computer hard drive and analyzed off-line for BF shifts. If a shifted BF and frequency-response curve did not shift back by more than 50% of the change, the data were excluded from the analysis. In stable recordings, all BFs and curves shifted by the electric stimulation recovered by >50%. This recovery itself helped to prove that the shift was significant.
Results
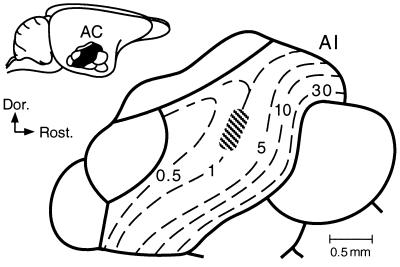
In AI of the gerbil, frequencies between 0.2 and 43 kHz are systematically mapped along the caudo-rostral axis, and frequencies from 0.5 to 5 kHz are over-represented (ref. 29; Fig. 1). The BFs of electrically stimulated cortical neurons ranged between 0.88 and 1.2 kHz (mean ± SE: 1.10 ± 0.09 kHz, n = 97) and those of recorded cortical neurons ranged between 0.1 and 37.0 kHz (4.62 ± 3.8 kHz, n = 338). Electric stimulation of cortical neurons evoked a BF shift of other cortical neurons located near those stimulated. When stimulated and recorded neurons were tuned to 1.00 and 1.60 kHz, respectively (Fig. 2Aa1), electric stimulation suppressed the response of the recorded neuron at 1.60 kHz (Fig. 2Ab2) and facilitated its response at 1.20 kHz (Fig. 2Ac2). As a result, the BF and the frequency-response curve of the neuron shifted toward the BF of the stimulated neurons (Fig. 2Aa2). In other words, centripetal BF shift was evoked. When stimulated and recorded neurons were tuned to 1.10 and 1.45 kHz, respectively (Fig. 2Ba1), the electric stimulation suppressed response of the recorded neuron at 1.45 kHz (Fig. 2Bb2) and facilitated its response at 1.65 kHz (Fig. 2Bc2). As a result, the BF and frequency-response curve of the neuron shifted away from the BF of the stimulated neuron (Fig. 2Ba2). In other words, centrifugal BF shift was evoked. The shifted BF and changed responses returned to the control condition 180 min after the electric stimulation (a4, b4, and c4 in Fig. 2 A and B).
Figure 1.
Frequency map of the AI of the Mongolian gerbil. Dashed lines: iso-BF lines [based on Thomas et al. (29)]. Electric stimulation was delivered to cortical neurons within the shaded area. (Inset) Dorsolateral view of the gerbil brain. The auditory cortex (AC) consists of seven areas. The black area is the AI.
Figure 2.
Shifts in frequency-response curve evoked by cortical electric stimulation. (Aa and Ba) Array of peristimulus-time cumulative histograms representing frequency-response curves. (Ab, Ac, Bb, and Bc) Peristimulus-time histograms representing responses at BF in the control (BFc) or shifted (BFs) condition. (A) The BF at 1.60 kHz shifted toward the 1.00 kHz BF of the stimulated cortical neurons (centripetal BF shift). (B) The BF at 1.45 kHz shifted away from the 1.10-kHz BF of the stimulated cortical neurons (centrifugal BF shift). (Aa2 and Ba2, arrow) The BF of the stimulated cortical neurons. (Aa and Ba) The BFs of the recorded cortical neuron in the control ●, shifted ×, and recovered ○ conditions, respectively. 1–4: The data obtained before, and 30, 80, and 180 min after the onset of electric stimulation. The horizontal bars at the bottom of b and c represent 20 ms-long tone bursts.
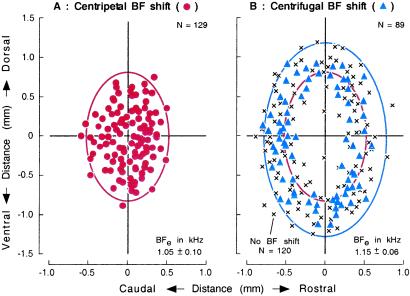
Whether a recorded neuron showed a centripetal or centrifugal BF shift depended on the distance between the recorded and stimulated neurons along the surface of AI. Neurons showing a centripetal BF shift were distributed in an elliptical area centered at the stimulated neurons that were tuned to ≈1.1 kHz on the average (Fig. 3A). Their confidence ellipse (based on Mahalanobis's equation P > 0.95) crossed the rostro-caudal (x) axis at approximately ±0.56 mm and the 1.1-kHz iso-BF line (y) axis at approximately ±0.95 mm. On the other hand, neurons showing centrifugal BF shift distributed in a zone surrounding the elliptical area (Fig. 3B). The confidence ellipse crossed the x axis at approximately ±0.72 mm and the y axis at approximately ±1.23 mm. This surround was 0.20–0.30 mm wide. In this surrounding zone, many neurons (≈57%) did not show a BF shift (see Discussion).
Figure 3.
Distributions of cortical neurons that exhibited centripetal or centrifugal BF shift. (A) Centripetal BF shift, ●. (B) Centrifugal BF shift, ▴ and no BF shift, ×. Locations of recorded neurons along the cortical surface are plotted relative to that of stimulated cortical neurons at the origin of the coordinates. x and y axes, directions parallel or orthogonal to the frequency axis of the AI. Data are pooled from 16 hemispheres of 11 animals. BFe, BF of electrically stimulated cortical neurons. Ellipse, area for centripetal or centrifugal BF shifts.
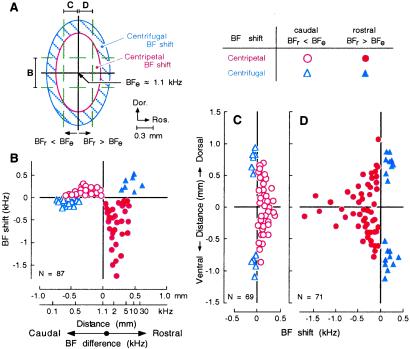
The amount of BF shift varied depending on the distance and the BF difference between recorded and stimulated neurons. BF shifts measured in a 0.6-mm wide horizontal zone containing the electrically stimulated neurons at its center (zone B in Fig. 4A) were pooled because the edge of the ellipse in this zone was somewhat parallel to the 1.1-kHz iso-BF line. The pooled data were plotted as a function of the distance between stimulated and recorded neurons along the frequency axis. The great majority of neurons within ±0.45 mm from the 1.1-kHz iso-BF line showed centripetal BF shift, and the amounts of their BF shifts were nonmonotonically related to the distance (Fig. 4B, circles). The largest negative centripetal BF shift occurred at 0.21 mm rostral to the 1.1-kHz iso-BF line (corresponding to a BF 1.8 kHz higher than 1.1 kHz), whereas the largest positive centripetal BF shift occurred at 0.27 mm caudal (corresponding to a BF 0.36 kHz lower than 1.1 kHz). The largest positive centrifugal BF shift occurred at 0.48 mm rostral to the 1.1-kHz iso-BF line, whereas the largest negative centrifugal BF shift occurred at 0.62 mm caudal (Fig. 4B, triangles). Both centripetal and centrifugal BF shifts were significantly larger on the rostral side than on the caudal side (P < 0.05). However, there was no difference in distance for evoking a BF shift between the rostral and caudal sides (Fig. 4B).
Figure 4.
Amount of BF shift in a zone parallel or orthogonal to the frequency axis of the AI. (A) Confidence ellipses for neurons that showed centripetal (open area) or centrifugal BF shift (shaded area) (see Fig. 3). The directions and amounts of BF shifts of neurons in the rostro-caudal (B) and dorso-ventral (C and D) zones in A were respectively plotted in B–D as a function of distance along the cortical surface. BFe, BF of electrically stimulated cortical neuron and BFr, BF of recorded cortical neuron. See Inset at the top right for symbols.
BF shifts measured within a 0.30-mm wide vertical zone caudal or rostral to the 1.1-kHz iso-BF line (C and D in Fig. 4A) were pooled because the edge of the ellipse in this zone was somewhat parallel to the frequency axis. The pooled data were plotted as a function of distance between stimulated and recorded neurons along the 1.1-kHz iso-BF line. The amount of BF shift was larger on the rostral side of the 1.1-kHz iso-BF line (Fig. 4D) than on its caudal side (Fig. 4C). Centripetal and centrifugal BF shifts both occurred symmetrically in amount on the dorsal and ventral sides of the stimulated cortical neurons. Within ±0.60 mm, the great majority of the neurons showed centripetal BF shift, and there was a tendency that the farther the distance away from the stimulated neurons along the iso-BF line, the smaller the centripetal BF shift. Large BF shifts (1.5–1.8 kHz) occurred within 0.10 mm. This finding indicated that BF shift was the largest along the frequency axis crossing the electrically stimulated cortical neurons. Centrifugal BF shift occurred at distances between 0.5 and 1.1 mm from the stimulated neurons (Fig. 4 C and D).
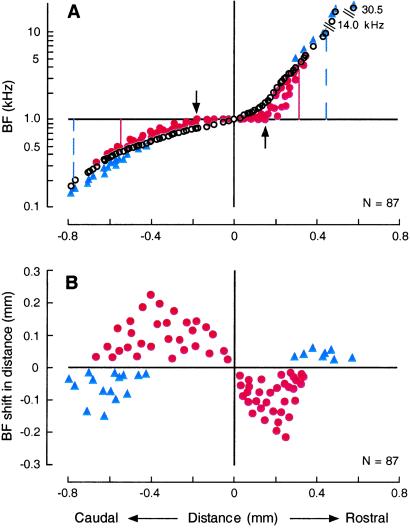
In the normal (i.e., control) condition, a BF systematically increased from the caudal end to the rostral end of AI (Fig. 5A, ○). There was no sign that 1.1 kHz was over-represented. However, BF shift evoked by electric stimulation of 1.1-kHz-tuned cortical neurons resulted in the prominent over-representation of 1.1-kHz sound, so that the iso-1.1-kHz BF line changed into a 0.33-mm wide band (Fig. 5A, ●). A small under-representation also occurred for frequencies lower than 0.52 kHz and higher than 4.7 kHz (Fig. 5A, ▴).
Figure 5.
Change in the frequency axis (A) and amount of BF shift expressed in millimeters (B). (A) Over- (between the arrows) and under- (between the vertical lines) representations evoked by cortical electric stimulation. Distribution of BFs along the caudo-rostral axis of the AI was studied before (○) and after (● and ▴) electric stimulation of 1.1-kHz-tuned cortical neurons. (B) Distributions of centripetal (●) and centrifugal (▴) BF shifts expressed in millimeters along the caudo-rostral axis of the AI.
Because a BF systematically changed along the caudo-rostral axis of AI, the amount of BF shifts shown in Fig. 4B could be expressed in mm (Fig. 5B). When expressed in this manner, the maximum amount of centripetal BF shift in mm was 0.21 or 0.22 mm on the rostral side and on the caudal side, respectively. Therefore, the prominent asymmetrical BF shift in kHz (Fig. 4B) was related to the nonlinear frequency axis in AI: a small BF change/unit distance below 1.1 kHz and a large BF change/unit distance above 1.1 kHz.
Discussion
Centripetal and Centrifugal BF Shifts.
The amount of BF shifts we observed was related to differences in both distance and BF between recorded and stimulated cortical neurons. Iso-BF lines are not necessarily parallel to each other and show some variations among animals (22, 29). Accordingly, in our data pooled from 11 animals, the area for the centripetal BF shift overlapped with the surround for the centrifugal BF shift, and neurons showing centrifugal BF shift were intermixed with those showing no BF shift. Irrespective of these overlap and intermixture, our present data clearly show center-surround reorganization and favor the hypothesis stating that facilitation and lateral inhibition, respectively, evoke centripetal and centrifugal BF shifts (18).
In AI of the big brown bat (1, 2) and the nonspecialized posterior division of AI of the mustached bat (11), cortical electric stimulation evokes a centripetal BF shift around the stimulated cortical neurons and a centrifugal BF shift at the rostral or caudal edge of the centripetal BF shift area. However, in previous studies, the number of neurons showing centrifugal BF shift was so small that the center-surround reorganization was not clear. In rats (12), electric stimulation of the basal forebrain paired with an acoustic stimulus evokes a centripetal BF shift. Centrifugal BF shift has not been reported in any species of animals except the above two species of bats. In our previous studies of the gerbil AI (11), we found 65 centripetal BF shifts and two centrifugal BF shifts, so that center-surround reorganization was unclear. However, it became clear in our present detailed studies. Therefore, it is expected that center-surround reorganization will also be found in the auditory cortex of other species of animals by further studies. In rats, a train of tone bursts paired with electric stimulation of dopaminergic neurons in the ventral tegmental area evokes over-representation of the frequency of these tones and under-representation of frequencies lower or higher than that in AI (30). However, it has not yet been shown whether this under-representation is partly caused by centrifugal BF shifts.
In a nonspecialized auditory cortex such as the gerbil's AI, reorganization is predominantly based on centripetal BF shift. A centripetal BF shift would result in over-representation of a particular value of a parameter characterizing an acoustic signal (1, 2, 11, 13, 14). A specialized auditory cortex such as the DSCF area of the mustached bat over-represents particular values of frequency in a narrow range in the natural condition (21, 22). In the DSCF area, reorganization is predominantly based on centrifugal BF shift, which would increase contrast in neural representation of a particular value of frequency characterizing an acoustic signal (16, 25).
Receptive-Field Shift in Nonauditory Sensory Systems.
In monkeys (19), excess stimulation of a digit evokes over-representation of that digit in the primary somatosensory cortex. This over-representation is caused by centripetal shift of the receptive fields of cortical neurons representing a cutaneous area around that digit. Thus far, centrifugal shift of a receptive field has not been reported for reorganization of the somatosensory system. In cats, electric stimulation of the primary visual cortex evokes lateral inhibition associated with focused facilitation in the lateral geniculate nucleus (31) as in the auditory system of the mustached bat (16, 25, 26). However, shift in receptive field has not been reported.
Neurons in the primary visual cortex have excitatory inputs surrounded by inhibitory inputs (32, 33). However, the diameter of the area for excitatory inputs (≈0.25 mm) is almost one-half of the minor axis of the elliptical area for a centripetal BF shift in the gerbil's AI (≈0.60 mm). The estimated density of horizontal axon collaterals of pyramidal neurons in the visual cortex is approximately one-half of that in the auditory cortex (34). Because the most basic cortical neural circuit (27, 35) and the corticothalamic feedback loop are presumably shared by the auditory (36), visual (37), and somatosensory (38) cortices, it is likely that center-surround reorganization also may be shared by these sensory systems.
Anatomical Correlates to Centripetal and Centrifugal BF Shifts.
Axon collaterals of pyramidal neurons in the primary auditory, somatosensory, visual, and motor cortices extend ≈1,500 μm (27) for the integration of neural signals and the synchronization of the activities of distant neurons (39, 40). Horizontal excitatory connections are mostly under tonic inhibition produced by GABAergic interneurons (41). The balance between excitatory and inhibitory influences on single neurons is the primary determinant of the representation of the somatosensory (42), visual (40), and motor cortices (43). In the somatosensory cortex, expansion of representation of a body surface caused by training (10) or cortical electric stimulation (19), especially its rapid change, is the result of unmasking, i.e., conversion of subthreshold excitatory-horizontal connections to suprathreshold connections. In a slice preparation of AI, synaptic efficiency between horizontal fibers is highly modifiable (44). In the bat's AI, BF shifts evoked by cortical electric stimulation occur within several minutes and disappears within 180 min after the cessation of the stimulation (Fig. 2; 1, 2). This short-term plasticity is probably not caused by to a morphological change such as axonal sprouting or dendritic spine proliferation, but by a change in efficiency of existing synapses.
Horizontal arborizations of pyramidal neurons for excitation are classified into short- and long-range projections. The former is up to 0.6 mm long and omnidirectional, whereas the latter is several millimeters long and preferentially parallel to iso-BF contour lines (45–48). The minor and major axes of the ellipse for centripetal BF shift may, respectively, be related to the short- and long-range projections. The recurrent fibers of pyramidal neurons form an intracortical positive feedback loop. In the big brown bat, electric stimulation of AI evokes a BF shift in the IC, which is very similar to cortical BF shift (2), and the cortical BF shift greatly depends on the collicular BF shift (8, 9). Because centripetal BF shift in the IC is evoked by the corticofugal system (9, 13, 14, 18), the recurrent circuit within AI and the corticofugal feedback loop both presumably play an important role in cortical plasticity.
The recurrent fibers of pyramidal neurons spread over 0.6 to several millimeters within the cortex and form minute connections with inhibitory interneurons. Most inhibitory interneurons (small basket cells) in AI radially project 0.2–0.3 mm, forming uniformly dense arbors (28, 49). The surrounding zone for centrifugal BF shift was 0.56–0.95 mm away from the stimulated cortical neurons and 0.15–0.30 mm wide. Therefore, the inhibitory interneurons activated by the recurrent fibers may produce lateral inhibition that evokes a centrifugal BF shift.
Acknowledgments
We thank N. Laleman and K. K. Ohlemiller for comments on the manuscript. Our research has been supported by a research grant from the National Institute on Deafness and Other Communicative Disorders (DC 00175).
Abbreviations
- AI
primary auditory cortex
- BF
best frequency
- IC
inferior colliculus
- DSCF
Doppler-shifted constant frequency
- FM
frequency modulation
References
- 1.Chowdhury S A, Suga N. J Neurophysiol. 2000;83:1856–1863. doi: 10.1152/jn.2000.83.4.1856. [DOI] [PubMed] [Google Scholar]
- 2.Ma X, Suga N. J Neurophysiol. 2001;85:1078–1087. doi: 10.1152/jn.2001.85.3.1078. [DOI] [PubMed] [Google Scholar]
- 3.Disterhoft J F, Olds J. J Neurophysiol. 1972;35:665–679. doi: 10.1152/jn.1972.35.5.665. [DOI] [PubMed] [Google Scholar]
- 4.Diamond D M, Weinberger N M. Brain Res. 1986;372:357–360. doi: 10.1016/0006-8993(86)91144-3. [DOI] [PubMed] [Google Scholar]
- 5.Edeline J M, Weinberger N M. Behav Neurosci. 1993;107:82–103. doi: 10.1037//0735-7044.107.1.82. [DOI] [PubMed] [Google Scholar]
- 6.Bakin J S, Weinberger N M. Proc Natl Acad Sci USA. 1996;93:11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohl F W, Scheich H. Eur J Neurosci. 1996;8:1001–1017. doi: 10.1111/j.1460-9568.1996.tb01587.x. [DOI] [PubMed] [Google Scholar]
- 8.Gao E, Suga N. Proc Natl Acad Sci USA. 2000;97:8081–8086. doi: 10.1073/pnas.97.14.8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji W, Gao E, Suga N. J Neurophysiol. 2001;86:211–225. doi: 10.1152/jn.2001.86.1.211. [DOI] [PubMed] [Google Scholar]
- 10.Recanzone G H, Schreiner C E, Merzenich M M. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakai M, Suga N. Proc Natl Acad Sci USA. 2001;98:3507–3512. doi: 10.1073/pnas.061021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilgard M P, Merzenich M M. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- 13.Gao E, Suga N. Proc Natl Acad Sci USA. 1998;95:12663–12670. doi: 10.1073/pnas.95.21.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma X, Suga N. Proc Natl Acad Sci USA. 2001;98:14060–14065. doi: 10.1073/pnas.241517098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan W, Suga N. Nat Neurosci. 1998;1:54–58. doi: 10.1038/255. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Suga N. J Neurophysiol. 2000;84:325–333. doi: 10.1152/jn.2000.84.1.325. [DOI] [PubMed] [Google Scholar]
- 17.Jen P H-S, Chen Q C, Sun X D. J Comp Physiol A. 1998;183:683–697. doi: 10.1007/s003590050291. [DOI] [PubMed] [Google Scholar]
- 18.Suga N, Gao E, Zhang Y, Ma X, Olsen J F. Proc Natl Acad Sci USA. 2000;97:11807–11814. doi: 10.1073/pnas.97.22.11807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Recanzone G H, Merzenich M M, Dinse H R. Cereb Cortex. 1992;2:181–196. doi: 10.1093/cercor/2.3.181. [DOI] [PubMed] [Google Scholar]
- 20.Kano M, Iino K, Kano M. NeuroReport. 1991;2:77–80. doi: 10.1097/00001756-199102000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Suga N, Jen P H-S. Science. 1976;194:542–544. doi: 10.1126/science.973140. [DOI] [PubMed] [Google Scholar]
- 22.Suga N, Manabe T. J Neurophysiol. 1982;47:225–255. doi: 10.1152/jn.1982.47.2.225. [DOI] [PubMed] [Google Scholar]
- 23.Suga N, O'Neill W E. Science. 1979;203:351–353. doi: 10.1126/science.482944. [DOI] [PubMed] [Google Scholar]
- 24.O'Neill W E, Suga N. Science. 1979;203:69–73. doi: 10.1126/science.758681. [DOI] [PubMed] [Google Scholar]
- 25.Yan J, Suga N. Science. 1996;273:1100–1103. doi: 10.1126/science.273.5278.1100. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Suga N, Yan J. Nature (London) 1997;387:900–903. doi: 10.1038/43180. [DOI] [PubMed] [Google Scholar]
- 27.Szentagothai J. Brain Res. 1975;95:475–496. doi: 10.1016/0006-8993(75)90122-5. [DOI] [PubMed] [Google Scholar]
- 28.Prieto J J, Peterson B A, Winer J A. J Comp Neurol. 1994;344:349–382. doi: 10.1002/cne.903440304. [DOI] [PubMed] [Google Scholar]
- 29.Thomas H, Tillein J, Heil P, Scheich H. Eur J Neurosci. 1993;5:882–897. doi: 10.1111/j.1460-9568.1993.tb00940.x. [DOI] [PubMed] [Google Scholar]
- 30.Bao S, Chan V T, Merzenich M M. Nature (London) 2001;412:79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- 31.Tsumoto T, Creutzfeldt O D, Legendy C R. Exp Brain Res. 1978;32:345–364. doi: 10.1007/BF00238707. [DOI] [PubMed] [Google Scholar]
- 32.Dalva M B, Weliky M, Katz L C. Neuron. 1997;19:871–880. doi: 10.1016/s0896-6273(00)80968-x. [DOI] [PubMed] [Google Scholar]
- 33.Hirsch J A, Gilbert C D. J Neurosci. 1991;11:1800–1809. doi: 10.1523/JNEUROSCI.11-06-01800.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kudoh M, Shibuki K. J Neurosci. 1997;17:9458–9465. doi: 10.1523/JNEUROSCI.17-24-09458.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Creutzfeldt O D. Naturwissenschaften. 1977;64:507–517. doi: 10.1007/BF00483547. [DOI] [PubMed] [Google Scholar]
- 36.Huffman R F, Henson O W., Jr Brain Res Rev. 1990;15:295–323. doi: 10.1016/0165-0173(90)90005-9. [DOI] [PubMed] [Google Scholar]
- 37.Murphy P C, Duckett S G, Sillito A M. Science. 1999;286:1552–1554. doi: 10.1126/science.286.5444.1552. [DOI] [PubMed] [Google Scholar]
- 38.Krupa D J, Ghazanfar A A, Nicolelis M A. Proc Natl Acad Sci USA. 1999;96:8200–8205. doi: 10.1073/pnas.96.14.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buonomano D V, Merzenich M M. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 40.Gilbert C D. Physiol Rev. 1998;78:467–485. doi: 10.1152/physrev.1998.78.2.467. [DOI] [PubMed] [Google Scholar]
- 41.Jones E G. Cereb Cortex. 1993;3:361–372. doi: 10.1093/cercor/3.5.361-a. [DOI] [PubMed] [Google Scholar]
- 42.Merzenich M M, Jenkins W M. J Hand Ther. 1993;6:89–104. doi: 10.1016/s0894-1130(12)80290-0. [DOI] [PubMed] [Google Scholar]
- 43.Donoghue J P. Curr Opin Neurobiol. 1995;5:749–754. doi: 10.1016/0959-4388(95)80102-2. [DOI] [PubMed] [Google Scholar]
- 44.Buonomano D V. J Neurosci. 1999;19:6748–6754. doi: 10.1523/JNEUROSCI.19-16-06748.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reale R A, Brugge J F, Feng J Z. Proc Natl Acad Sci USA. 1983;80:5449–5453. doi: 10.1073/pnas.80.17.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winer J A. J Comp Neurol. 1984;229:476–496. doi: 10.1002/cne.902290404. [DOI] [PubMed] [Google Scholar]
- 47.Matsubara J A, Phillips D P. J Comp Neurol. 1988;268:38–48. doi: 10.1002/cne.902680105. [DOI] [PubMed] [Google Scholar]
- 48.Ojima H, Honda C N, Jones E G. Cereb Cortex. 1991;1:80–94. doi: 10.1093/cercor/1.1.80. [DOI] [PubMed] [Google Scholar]
- 49.Hendry S H, Jones E G. Brain Res. 1991;543:45–55. doi: 10.1016/0006-8993(91)91046-4. [DOI] [PubMed] [Google Scholar]