Abstract
Advances in biomarker-driven therapies for patients with non-small cell lung cancer (NSCLC) provide both opportunities to improve the treatment and thus outcomes for patients and pose new challenges for equitable care delivery. Over the last decade, the continuing development of new biomarker-driven therapies and evolving indications for their use have intensified the importance of inter-disciplinary communication and coordination for patients with or suspected to have lung cancer. Multi-disciplinary teams are challenged with completing comprehensive and timely biomarker testing and navigating the constantly evolving evidence base for a complex and time-sensitive disease. This guide provides context for the current state of comprehensive biomarker testing for NSCLC, reviews how biomarker testing integrates within the diagnostic continuum for patients, and illustrates best practices and common pitfalls that influence the success and timeliness of biomarker testing using a series of case scenarios.
Keywords: Carcinoma, Non-Small-Cell Lung, Biomarkers, Interdisciplinary Communication, Precision Medicine
Introduction
Lung cancer is the oncologic public health challenge of this age. In 2022, lung cancer is estimated to account for 13% of new cancer diagnoses and 22% of all cancer deaths in the United States.1 Since most lung cancer is diagnosed at an advanced stage (defined as stage IIIB or greater), lung cancer care delivery is complex and increasingly demands the early interaction of experts from multiple disciplines to: 1) identify its possible presence and extent through radiologic studies; 2) obtain tissue for diagnosis, staging, and comprehensive biomarker testing; and 3) and make treatment decisions based on testing results. Optimal treatment for patients with advanced lung cancer increasingly involves biomarker-directed systemic therapy which requires a multi-disciplinary approach, aligning proceduralists (defined as a pulmonologist, surgeon, or interventional radiologist), pathologists, and oncologists.
The rising complexity of care delivery emanates directly from the expanding array of treatment options, which creates the opportunity for significantly better patient outcomes and prolonged survival. Paradoxically, this opportunity to achieve better outcomes also raises the adverse consequences of sub-optimal care. Appropriately deploying biomarker-driven therapies (immunotherapy and targeted agents) for both resectable and advanced non-small cell lung cancer (NSCLC) is particularly complex because the process depends on an accurate diagnosis and stage, as well as access to timely and comprehensive biomarker testing. Acknowledging the challenge of increasing complexity, and based on stakeholder feedback, the American Cancer Society National Lung Cancer Roundtable has developed this guide to promote understanding of the importance of the steps needed to achieve comprehensive biomarker testing and suggests best practices for efficient testing for patients with advanced NSCLC.
Rationale for Comprehensive Biomarker Testing for Patients with Advanced NSCLC
Nearly half of patients with NSCLC present with metastatic disease (involvement beyond regional lymph nodes) and need treatment with systemic chemotherapy, immunotherapy, or targeted agents.1 Biomarker-directed therapy has revolutionized NSCLC care by providing dramatically better patient-centered outcomes, including prolonged survival, less toxicity, and greater convenience when compared to conventional systemic chemotherapy.2 Although there are some circumstances in which immunotherapy can be deployed without biomarker testing, testing is a prerequisite for targeted therapies and for the use of immunotherapy in certain clinical scenarios. Biomarker-driven therapies are also increasingly being used in the adjuvant setting after curative-intent surgery and ongoing clinical trials are assessing their use in the neoadjuvant setting.3, 4 The focus of this review, case scenarios, and discussions is to aid in performance of timely comprehensive biomarker testing for patients with advanced NSCLC.
The need to systematically perform comprehensive biomarker testing for eligible patients is highlighted by the potential for improved patient outcomes. Eligible patients with advanced NSCLC who are treated with targeted agents or immunotherapy can have response rates of 60% or more and median survival rates in excess of 5 years, considerably better outcomes than are generally attained with conventional chemotherapy.5, 6 Although different subtypes of NSCLC may have higher or lower likelihood of harboring an actionable mutation, and some oncogenic driver mutations are more common in certain patient subpopulations, (e.g., in younger patients, women, people who never smoked, and Asian populations), these factors (except squamous versus non-squamous histology) should not be used to select patients who should or should not undergo biomarker testing.7–9 Current guidelines from the National Comprehensive Cancer Network and the joint statement by the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology recommend routine genomic biomarker testing in all patients with advanced NSCLC who have adenocarcinoma, large cell carcinoma, and NSCLC otherwise not specified histology, and in selected patients with squamous histology (e.g. young age or a history of non-use or light use of cigarette tobacco).10, 11 These guidelines support Program Death-Ligand 1 (PD-L1) expression evaluation by immunohistochemistry for all patients with NSCLC, regardless of histology.
Despite guidelines and evidence for the clinical benefit, biomarker testing and use of related therapies has been accompanied by disparities in patient access and variable implementation at the provider and institution levels. The indigent, racial minorities, and the under-insured are less likely to receive testing.12, 13 In one analysis of Medicare claims data, black race and residence in impoverished areas were independently associated with nearly 50% and 20% reduction in the odds of testing, respectively.14 The continuing development of biomarkers, associated therapies, as well as indications for their use over the last decade create a challenge in analyzing quality metrics using secondary databases, which often lack specific details on biomarkers. Provider and health care system factors associated with lower rates of biomarker testing include patient evaluation by generalist proceduralists and oncologists rather those who specialize in thoracic oncology, oncologists earlier in their clinical experience, and those practicing in non-academic institutions.13–15
Beyond the presence or absence of testing, additional care-delivery challenges involve the quality of testing: which biomarkers should be assessed and what are the best methods for their evaluation? The potential benefits of personalized therapies depend on timely diagnosis and the determination of predictive biomarkers, which includes genomic changes and protein expression profiles, that divide NSCLC into subsets. Highly effective treatments are available to subsets of patients eligible for biomarker-driven therapies, but depend entirely on matching therapies with the correct profile. The list of specific clinically actionable biomarkers is rapidly evolving, necessitating changes to the approach for biomarker testing from sequential single-gene tests to the idea of ‘comprehensive biomarker testing.’ At a minimum, comprehensive testing should include all “actionable” predictive markers, meaning those with current US Food and Drug Administration (FDA)-approved drugs, but may also include predictive markers for promising drugs in development. For example, therapies that have received FDA ‘breakthrough therapy’ designation or those that may be available as part of a clinical trial also offer potential benefits for patients in the present or at a future time. For example, the FDA recently granted a fast-track status designation for seribantumab for solid tumors containing NRG1 fusions. Testing for NRG1 fusions prior to full approval could help patients receive targeted therapy as part of a clinical trial or after the drug gains FDA approval.
As of October of 2022, comprehensive testing for advanced NSCLC includes assessing ten genetic alteration or “driver mutations”: EGFR mutations, BRAF V600E, MET exon 14 skip mutations, KRAS mutations, ERBB2 (HER2) mutations, rearrangements in ALK, ROS1, RET, and NTRK, as well as immunohistochemical testing for PD-L1 Tumor Proportion Score (Table 1).10, 11 PD-L1 testing directs the use of immune checkpoint inhibitor monotherapy for patients with metastatic squamous or non-squamous NSCLC with a PD-L1 Tumor Proportion Score ≥50%. As previously mentioned, the list of actionable biomarkers and their indications continue to evolve. For example, Trastuzumab Deruxtecan recently gained FDA approval in August of 2022 for the treatment of patients with ERBB2 (HER2) mutations. Given the growth of biomarker-driven therapies over time, the definition of what constitutes comprehensive biomarker testing for patients with advanced NSCLC continues to evolve and we advocate for adherence to societal guidelines which are continually updated to reflect advances in the field.10, 11
Table 1.
List of biomarkers, drug names, and date each drug gained its first FDA approval in NSCLC
| Biomarker | Drug Name | Date of FDA Approval in NSCLC67 |
|---|---|---|
| EGFR exon 19 deletions or exon 21 (L858R) substitution mutations | Erlotinib* | 5/14/2013 |
| Afatinib | 7/12/13 | |
| Gefitinib | 7/13/15 | |
| Dacomitinib | 9/27/18 | |
| Osimertinib | 4/18/18 | |
| EGFR T790M mutation | Osimertinib | 11/13/15 |
| EGFR exon 20 insertion mutations | Amivantamab | 5/21/21 |
| Mobocertinib | 9/15/21 | |
| ALK fusion | Crizotinib | 8/26/11 |
| Ceritinib | 4/29/14 | |
| Alectinib | 12/11/15 | |
| Brigatinib | 4/28/17 | |
| Lorlatinib | 11/2/18 | |
| ROS1 fusion | Crizotinib | 3/11/16 |
| Entrectinib | 8/15/19 | |
| NTRK fusion | Larotrectinib | 11/26/18 |
| Entrectinib | 8/15/19 | |
| BRAF V600 | Dabrafenib + Trametinib | 6/22/17 |
| RET fusion | Selpercatinib | 5/8/20 |
| Pralsetinib | 12/1/20 | |
| MET exon 14 skipping alterations | Capmatinib | 5/6/20 |
| Tepotinib | 2/3/21 | |
| KRAS G12C | Sotorasib | 5/28/21 |
| ERBB2 (HER2) | Trastuzumab Deruxtecan | 8/11/22 |
| PD-L1 (immunotherapy) | Nivolumab | 3/4/15 |
| Pembrolizumab | 10/2/15 | |
| Atezolizumab | 4/17/17 | |
| Cemiplimab | 2/22/21 |
Erlotinib was first approved in 2004, the date listed was its time of first approval with a requirement for EGFR biomarker testing
Comprehensive biomarker testing should ideally be performed using ‘next-generation sequencing’ (NGS) panels. While other methods are available and acceptable, NGS often allows for simultaneous evaluation of all actionable oncogenic driver mutations with less expenditures of time and tissue than other assays and strategies.16 It is worth noting that not all NGS testing is the same. Some NGS tests may provide additional genomic data beyond actionable biomarkers, examples would include whole exome and whole transcriptome sequencing. Other NGS testing may not provide all the data needed to assess all actionable mutations. NGS testing should ideally include an assessment of RNA, as DNA assessment alone does not adequately evaluate for all relevant genetic alterations (e.g., gene fusions).17 Laboratories should provide the option of including RNA-based analyses or other complementary techniques, such as fluorescent in situ hybridization (FISH) and/or well-validated immunohistochemistry. Assay development, evaluation, and comparisons of strengths and weaknesses are also rapidly changing, and specific guidelines are available to assist in proper assay choices for biomarkers.11 PD-L1 expression, which is not assessed by NGS testing, should be assessed by immunohistochemical staining in all patients with advanced NSCLC, regardless of histology. Assessing PD-L1 expression has its own issues including heterogeneity in expression across a tumor and over time, selecting predictive cut-off values, concordance of different assays and interpretations between pathologists.18–20 Laboratories should adhere to sample requirements including evaluation of a minimum of 100 tumor cells; given the impact of cold ischemia time, fixation duration and type, and age of the specimen on antigenicity, adherence to strict protocols on the part of proceduralists and laboratorians is essential to reduce the risk of false negative results.21–23 Decisions on how to achieve biomarker testing must balance potentially competing factors including local resources, expertise, costs, reimbursement, and quality of results.24 Notably, in 2018, the Center for Medicare and Medicaid Services enacted a policy covering FDA-approved NGS tests for patients with recurrent, relapsed, refractory, metastatic, or advanced stage III or IV cancer, eliminating a significant barrier to testing for patients.25
Suboptimal utilization of testing has stimulated discussion of ‘reflex testing’ (meaning standardized test ordering at the point of initial tissue procurement) at the institutional level. This has the potential advantage of eliminating inter-provider variation in testing and increasing the likelihood that results will be available by the time of treatment decision-making. Reflex testing requires institutional planning and agreement, including decisions on workflow and testing facility or vendor. In most cases, reflex testing implies that a pathologist triggers or orders biomarker testing at the time of diagnosis.26–28 Institutions and practices must create workflows that are compliant with relevant regulations that apply to reflex and standing orders, meant to prevent self-referral and unnecessary testing.29 If protocols and arrangements are not appropriately made, pathologists will have significant barriers assisting with test ordering. Alternatives to pathology-driven ordering include leveraging prompts in the electronic medical record and designating test ordering to a specific team member such as a nurse navigator, physician, or clinic staff member.
Outside of advanced NSCLC, guidelines differ on recommendations for biomarker testing, in part because they are updated in different years and have to incorporate emerging evidence for benefits of biomarker-driven therapies in early-stage disease including the ADAURA, IMpower010, and Checkmate-816 trials.3, 4, 30 Given these trials are already changing widespread practices in lung cancer care, we expect future iterations of societal guidelines will more strongly support and define the role of biomarker testing across earlier stages of NSCLC. While issues surrounding biomarker testing and precision medicine care delivery is important, the focus of this manuscript surrounds issues for patients with advanced NSCLC.
Obtaining a Diagnosis and Securing Adequate Amounts of Tumor for Biomarker Testing
The ability to identify and treat patients with the appropriate targeted agents or immunotherapy regimens greatly depends on timely diagnosis and minimizing delays in securing adequate amounts of tumor tissue for comprehensive biomarker testing. This process begins before a diagnosis is established. Physicians performing diagnostic procedures in patients suspected of having new or recurrent lung cancer must procure sufficient tissue for both histologic diagnosis, staging, and comprehensive biomarker testing within their practice setting. Local variables, including differences in tissue processing, the selection of specific biomarker assays and platforms, and the performing laboratory influence the amount of tumor tissue required. Depending on these variables, the same tissue sample could be adequate or inadequate for comprehensive testing. Technologic advancements in testing, including the availability of NGS, as well as the continuing development of number of FDA approved therapies and associated biomarkers that require testing may continue to influence the amount of tissue required to accomplish comprehensive testing as well. Thus, understanding the amount of tissue needed for testing requires close and ongoingf communication between proceduralists, pathologists, oncologists, and the performing laboratory. When insufficient tissue is available, strong consideration should be given to procuring additional tissue, or using alternative pathways to biomarker testing, such as circulating tumor DNA assays (‘liquid biopsy’), which are increasingly available.31, 32
The common diagnostic pathway (Figure 1) for patients suspected to have NSCLC based on symptoms or imaging begins with: 1) history and physical examination; 2) radiologic detection; 3) tissue biopsy from a site selected to yield material sufficient for histologic diagnosis, staging, and comprehensive biomarker testing; 4) histologic or cytologic diagnosis; and 5) biomarker testing to direct treatment. For patients with advanced NSCLC, there is an inclination to initiate therapy as soon as possible. However, biomarker characterization should precede targeted therapy and monotherapy with immune checkpoint inhibitors, given the lack of benefit and possible harm (e.g. interstitial lung disease) when the latter are used in ineligible patients (those with EGFR mutations and ALK rearrangements).33–35 Patients with significant delays in diagnosis and biomarker testing may begin treatment with potentially inferior systemic chemotherapy regimens rather than waiting on biomarker test results.36 Although there is no consensus for the time it should take to evaluate a patient newly suspected to have advanced lung cancer, there should be a sense of urgency and teams should regularly monitor for deficiencies along the diagnostic process. Figure 1 provides a proposed timeline for this process. Depending on local resources, this timeline may be readily accomplished, otherwise teams should strive to achieve it through collaboration and modifications in organizational workflows. Future research should focus on improving the diagnostic process for patients with advanced lung cancer and its impacts on treatment and outcomes, including patient reported outcomes and patient satisfaction.
Figure 1.

Proposed timeline for diagnosing advanced NSCLC. *2018 CAP/IASLC/AMP/ guidelines suggest that ideal turnaround time from receipt of specimens to reporting of all results should be < 10 business days.11
Efficient comprehensive biomarker testing requires integration of testing into the initial diagnostic process for lung cancer. The goal of this guide is to aid multidisciplinary teams and health care providers involved in the initial diagnosis of lung cancer in achieving successful biomarker testing with a focus on timely decision-making around the time of diagnosis. The initial site selected for biopsy is critical to the diagnostic process and should be chosen with care. Fundamental to this discussion is an understanding of methods to establish lung cancer diagnosis and appropriate staging tests. Numerous references provide complementary information in these areas for those needing or interested in further reading.37–39
A final concept that will aid the use of this guide is a basic understanding of specimen types derived from small biopsies commonly obtained in the diagnostic evaluation of lung cancer (Figure 2). A histopathologic sample refers to one that has a section of intact tissue, and in general, are collected from resections, excisions, and larger gauge needle biopsies (also referred to as “core biopsies”), such as those commonly performed during CT-guided biopsies. Histopathologic samples can be paraffin-embedded and fixed in formalin creating a “block” of tissue which can be cut into thin sheets for diagnostic stains, molecular analyses, and protein expression assays. A cytologic sample refers to the examination of single cells or small clusters detached from surrounding tissue and are, in general, collected from samples of body fluids (e.g., pleural, peritoneal) or from small gauge needle biopsies (also referred to as fine needle aspirates), such as those performed during bronchoscopic transbronchial needle aspirations (TBNA) of mediastinal lymph nodes. Cytologic preparations (smears, touch preps, thin preps) are amenable to immunohistochemistry, cytogenetic, and molecular analyses, but fall outside of the routine histology workflow and thus require special handling and dedicated validation. As a result, many labs will not be able to perform lung cancer biomarker testing on these samples. Finally, a “cell block” is a special preparation of a cytology specimen in which the cytology specimen placed in a centrifuge to produce a pellet of cells, which is then embedded in paraffin and fixed in formalin to create a “block of tissue.” Creation of a cell block and is generally advisable for procedures that only collect cytology specimens, as more laboratories are able to use these samples for diagnostic and biomarker testing for NSCLC. There are several factors, including available resources of the performing molecular laboratory, that will influence the ability of each of these samples to be utilized for biomarker testing. Practitioners directing the diagnostic work-up and performing tissue acquisition should be aware of the individual tissue requirements for the labs used by their practice or institution. Many of the case scenarios highlight procedures and samples that can be used to achieve diagnosis, stage, and comprehensive biomarker testing from a single procedure. Additional and more detailed information are available for specific practices for optimal tissue handling by proceduralists and pathology labs to facilitate biomarker testing.40
Figure 2.
Summary of common biopsy types and preparations. A) Image of a cytologic smear preparation at scanning power. B) High power magnification (400x) of a cytologic smear preparation. C) Image of a cell block preparation at scanning power from a malignant pleural fluid. D) High power magnification (200x) of cell block preparation from a malignant pleural fluid. E) Image of an endobronchial biopsy sample at scanning power F) High power magnification (200x) of tumor cells in the airway wall in an endobronchial biopsy sample.
We derived the following clinical scenarios from real patients with newly diagnosed advanced NSCLC. We provide commentaries to highlight considerations and practices to optimize site selection for tissue sampling to safely facilitate timely diagnosis, efficient staging, and procurement of adequate tissue for comprehensive biomarker testing. Selecting the proper site may allow for all these goals to be accomplished with a single procedure. We summarize the key tenets for optimal biopsy site selection in Table 2. Also, in Figure 3, we provide a summary of the site selection considerations covered in the scenarios.
Table 2.
Goals for biopsy site selection in suspected lung cancer:
| 1. Confirm the diagnosis and subtype of lung cancer (squamous, adenocarcinoma, small cell, etc.). |
| 2. Histologically confirm the most advanced stage (within the limits of safety and yield). |
| 3. Secure sufficient quality and quantity of tissue for comprehensive biomarker testing. |
| 4. Minimize patient risk and cost exposure. |
Figure 3.
Summary of case scenarios and key takeaways for biopsy site selection for undiagnosed advanced lung cancer.
These scenarios and commentaries are guides; care should always be individualized for patients. Further, this guide is not inclusive of all difficult scenarios. For example, all patient scenarios involve patients with relative clinical stability. Strategies that can be employed in effort to urgently treat rapidly declining patients (e.g., immunohistochemical staining that may require later confirmation, liquid biopsy, and other rapid testing strategies such as integrated multiplex PCR or NGS platforms) are not included. Additionally, we recognize the opportunity that liquid biopsies (i.e., assessment for mutations in circulating tumor DNA) offer to patients and physicians as a less invasive way to perform biomarker testing.31, 41 There are also potential pitfalls (e.g., false negative results, inability to detect PD-L1 expression or other tumor protein expression that may drive eligibility for drugs in the future).31 For these reasons, we have focused this guide, case scenarios, and learning points around issues of performing biomarker testing on patient’s tumor tissue and integration of biomarker testing into the initial evaluation of a patient efficiently.
Case Scenarios 1–4. Identifying Accessible Soft-Tissue Sites for Biopsy: Soft-tissue sites suspicious for involvement outside of the apparent primary lung tumor can yield material for diagnosis, staging, and biomarker testing with a single procedure.
Scenario # 1: Patient with a lung lesion suspicious for lung cancer and an accessible subcutaneous site.
A 57-year-old man with greater than 100 pack-year history of smoking presented with a two-month history of cough and increasing pain in his left buttock. Computed tomography (CT) of the chest demonstrated a 5 cm right upper lobe mass and mediastinal lymphadenopathy (Figure 4 A–B). The 5 cm right upper lobe mass, mediastinal lymph nodes, and gluteal mass were FDG avid (Figure 4 C). He underwent a mediastinoscopy and a fiberoptic bronchoscopy with endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA). The biopsy of the mass from the right upper lobe showed bronchial wall and lung parenchyma but was negative for tumor. Pathology from mediastinal lymph node stations 4R, 4L, and 7 showed lymph node tissue with no evidence of malignancy. The transbronchial needle aspiration yielded lymphocytes consistent with sampling of a lymph node but no malignant cells. A percutaneous 18 gauge needle biopsy of the gluteal soft tissue mass nearly 3 months after initial presentation confirmed squamous cell lung cancer. Immunohistochemistry studies showed PD-L1 expression in 2% of his tumor cells.
Figure 4.
A-B) Axial CT images of the chest demonstrates a lobulated right upper lobe mass measuring 5 cm (arrows, A, B) and mediastinal lymphadenopathy (asterisk, B). C) Fused axial image of PET/CT shows an FDG-avid soft tissue mass in the left gluteal muscle (arrow, C) with central photopenia.
Case Discussion # 1
The patient’s chest CT scan showed a lung mass which, when taken in consideration with his heavy smoking history and gluteal pain, was highly suspicious for metastatic lung cancer. Early on in his evaluation, the patient could have had a less invasive, safer, quicker, and less expensive outpatient procedure to obtain tissue from his left gluteal mass if attention had been paid to his presenting symptom of left gluteal pain. Percutaneous core needle biopsies typically yield adequate amounts of tumor tissue for comprehensive biomarker testing, with a recent meta-analysis of CT-guided biopsies showing a pooled adequacy rate of 95%.42 This patient could have been spared the bronchoscopy with EBUS-TBNA and cervical mediastinoscopy which did not yield a diagnosis and delayed his care by about three months. It is important to note that biopsy with mediastinoscopy and bronchoscopy with EBUS-TBNA do often have a high yield of diagnosis and both procedures are safe with a < 1% chance of severe procedure-related adverse events (e.g. pneumothorax, hemothorax, great vessel or airway injury, or death).43 In this case the mediastinal lymph nodes were not involved. While there are changes in both assays and the number of biomarkers that need to be assessed, a systematic review including studies until 2018 showed a pooled probability of adequacy of around 94% for EGFR mutations and ALK rearrangements.44 One single center’s experience with performing biomarker testing from fine needle aspiration and core biopsies from both CT-guided and bronchoscopy samples further highlights the success of these different samples.45 Large series and meta-analyses for testing on surgically resected specimens are lacking, though there is no reason to suspect a lower yield so long as the surgeon actually resects or biopsies the radiographically suspicious target. This case demonstrates an example of one potential pitfall of PET/CT, i.e., false-positive sites for cancer involvement. Although there was FDG uptake in the mediastinal lymph nodes, they were likely not involved given both a bronchoscopy with EBUS and mediastinoscopy showed no evidence of malignancy. Common causes of false-positive PET/CT results for thoracic lymph nodes include reactive lymphadenopathy due to active infection or granulomatous inflammation such as sarcoidosis, which can cause increased FDG avidity.
This case illustrates the importance of site selection and timely biopsy, as a non-diagnostic biopsy sets back the entire diagnosis-to-treatment pathway (Figure 1). Biopsies can be non-diagnostic for a variety of reasons including non-involvement with malignancy of the target site (as in this case), trouble sampling the target site during the procedure, low tumor cellularity in the lesion or in the sample collected, and extensive necrosis in the lesion. If a sample provides a diagnosis but there is not enough tissue for biomarker testing, it is generally advisable to repeat a biopsy for testing. However, the risk from delaying treatment, the likelihood of benefit from testing, patient preference, and the risk of the biopsy itself should be considered when pursuing repeat biopsy. This elderly patient had a squamous cell lung cancer, so genomic testing for oncogenic driver mutations is not mandatory given the lower yield of identifying targetable genomic changes.11 However, PD-L1 testing is advisable to guide the use of immunotherapy. This paradigm may change as driver mutations are investigated and discovered in squamous cell lung cancer.
Key Take-Aways for Case 1:
Biopsy the most accessible site that also confirms the stage. Suspicious painful soft-tissue lesions and enlarged peripheral lymph nodes (especially supraclavicular lymph nodes), if present, can often be safely sampled and provide staging information. A biopsy of the suspected primary lung tumor provides the least amount of staging information.
As illustrated in this case, initial site selection for biopsy has implications for the entire timeline for diagnosis and completion of biomarker testing, both of which are critical to initiating a treatment plan. Not all sites are equally informative, and care should be taken when selecting the initial biopsy site.
Squamous cell and non-squamous lung cancers are biologically different. Currently, PD-L1 testing is recommended for all metastatic NSCLC, regardless of histology, to direct the use of immunotherapy.10 For those with non-squamous NSCLC, testing for oncogenic driver mutations is indicated. Guidelines do support targeted therapy assessment in squamous cell lung cancer, especially for young patients and persons with a history of light or no smoking.10, 11 Furthermore, poorly differentiated cancers may be difficult to classify, and small biopsies may miss an adenocarcinoma component.
PET/CT is imperfect for detecting cancer and sites of potential metastasis; false positive sites may be caused by infectious or inflammatory disorders. Confirming stage histologically, rather than solely relying on imaging, is warranted.
Scenario # 2: Patient with a new left-sided lung lesion and FDG avid thoracic lymph nodes.
A 78-year-old woman presented 10 years after a right middle lobectomy of a T1bN0M0 squamous cell carcinoma with a chest CT scan showing a 1.8 cm left lower lobe pulmonary nodule and mildly enlarged mediastinal lymph nodes (Figure 5 A). A PET/CT scan revealed FDG avidity in the left lower lobe nodule as well as the left hilar, mediastinal, and supraclavicular lymph nodes (Figure 5 B–C). There were no other sites of FDG-avidity. She underwent a supraclavicular lymph node excision which showed metastatic adenocarcinoma. The NGS panel did not identify any actionable oncogenic driver mutations and the PD-L1 testing showed 95% of the tumor cells stained positive.
Figure 5.
A) Axial CT image of the chest demonstrates a 1.8 cm nodule in the left lower lobe (arrow, A). B) Fused axial image of PET/CT shows FDG-avid subcarinal lymph node (arrow, B) as well as other avid nodes in the left supraclavicular, right paratracheal, and left hilar regions (not shown).
Case Discussion # 2
The chest CT scan identified a 1.8 cm left lower lobe nodule suspicious for malignancy 10 years after resection of a right middle lobe lung cancer. This presentation suggests the new nodule represents a second primary tumor because the prior lung cancer was early stage, located in the contralateral lung, and 10 years had passed since its resection, which was confirmed later confirmed given the difference in histology between the two malignancies.46 The left lower nodule is easily accessible for a percutaneous biopsy, however, one still needs to assess potential spread to the regional lymph nodes, especially given lymphadenopathy evident on both the CT and the PET scans. In this case, the supraclavicular lymph node was excised, sparing the patient a more invasive procedure, such as bronchoscopy with EBUS-TBNA, CT-guided lung biopsy, or mediastinoscopy. The excision of the stage-defining supraclavicular lymph node confirmed adenocarcinoma and provided adequate tumor tissue for comprehensive biomarker testing. Given her otherwise good health, she was treated with pemetrexed, cisplatin, and chest radiotherapy with a good tumor response followed by durvalumab for her stage IIIB disease.47 Clinically, she is well two years after the lymph node biopsy and subsequent treatments.
Key Take-Aways for Scenario 2:
Target the most advanced stage-defining lesion for tissue sampling. Thoracic lymph nodes are commonly involved in advanced NSCLC when extra-thoracic sites are not available, and sampling is indicated to confirm the stage of lung cancer histologically.
Differentiating recurrence from a new primary lung cancer has important treatment implications. The clinical characteristics (including stage and time interval), histology, and biomarker profile can aid in this determination.
Although biomarker-driven therapies do not have an established indication for first-line therapy in unresected stage III NSCLC, a recent retrospective analysis of the PACIFIC trial suggested that the benefit of consolidative immunotherapy with Durvalumab was mostly in patients with PDL1 expression >1%.48 Furthermore, testing at the time of diagnosis can prepare for subsequent lines of therapy at the time of disease progression.
Scenario # 3: Patient with diffuse and extensive pulmonary nodules.
A 58-year-old woman with no smoking history presented with dyspnea on exertion and was found to have a 4.5 cm spiculated left upper lobe mass with innumerable diffuse bilateral nodules measuring up to 2.5 cm on a chest CT scan (Figure 6 A–B). There was no evidence of intra-thoracic lymph node involvement or metastatic disease on PET/CT or physical exam. She underwent bronchoscopy with transbronchial biopsies of the suspected primary tumor (left upper lobe mass) and bronchoalveolar lavage one month after presentation. The biopsy showed rare atypical cells with dysplasia and cytology examination of fluid from the bronchoalveolar lavage showed no malignant cells, failing to confirm a diagnosis of cancer. Nearly two months after bronchoscopy, she underwent a percutaneous biopsy of one of the smaller peripheral nodules. Five core biopsies were obtained which revealed an adenocarcinoma with 1% of the tumor cells staining positive for PD-L1 and an EGFR exon 20 insertion mutation on NGS. She was treated with empiric pemetrexed and carboplatin with a decrease in her dyspnea on exertion and stable disease radiographically.
Figure 6.
A-B) Axial CT images of the chest demonstrate a dominant mass in the left upper lobe (A, asterisk), measuring 4.5 cm, with innumerable bilateral pulmonary nodules up to 2.5 cm in size with somewhat confluent appearance (A and B).
Case Discussion # 3
This patient had a primary left upper lobe mass, which has an unfavorable location for transthoracic needle biopsy because it abuts the aorta and has a central location (Figure 6 A). In addition to the unfavorable location of the primary tumor, many of the pulmonary nodules suspicious for metastases present a better option for biopsy because they provide staging information that biopsy of the primary tumor does not. These sites are more easily accessed by transthoracic needle biopsy due to the proximity of many of the nodules to the lung periphery or even by EBUS-TBNA given proximity of nodules to the central airways. When intra-parenchymal metastases represent the best or only site to confirm diagnosis, stage, and collect tissue for comprehensive biomarker testing, CT-guided biopsy is often employed. One meta-analysis of CT-guided biopsies demonstrates a pooled adequacy for molecular testing of 95%.42 The complications of highest concern for trans-thoracic needle biopsies include pneumothorax and hemorrhage. One secondary database analysis of nearly 16,000 patients undergoing trans-thoracic needle biopsy found 15% of biopsies were complicated by pneumothorax, around half of which required a chest tube for management, and 1% experiencing hemorrhage.49
The patient had no history of smoking and pathology was consistent with adenocarcinoma, increasing the likelihood of having an actionable oncogenic driver mutation. An exon 20 EGFR insertion mutation was detected in her tumor tissue. There was no FDA approved targeted agent for patients with this mutation at the time she started therapy. Amivantanab, a bispecific antibody that targets EGFR and MET receptors, and mobocertinib, a tyrosine kinase inhibitor, were approved by the FDA in 2021 for the treatment of patients with EGFR exon 20 insertion mutations who have progressed during or after treatment with platinum-based chemotherapy and are now available to the patient.
Key Take-Aways for Scenario 3:
Current guidelines recommend that all patients with non-squamous histology should be assessed for targetable mutations and to test certain patients with squamous histology: e.g. young persons, those with light or no smoking history, and those with poorly differentiated tumors.10, 11 Although these same clinical factors may increase the likelihood of targetable mutations in patients with non-squamous NSCLC, guidelines recommend a systematic approach to testing all eligible patients with advanced non-squamous NSCLC. All patients with advanced NSCLC are recommended for PD-L1 testing, regardless of histology.
Delays in biopsy or re-biopsy and initiation of biomarker testing once tissue is collected is an inter-disciplinary problem. Communication between providers is essential to prevent and minimize delays in the diagnostic process for patients.
Biopsy of the suspected primary lung cancer is not recommended in cases when accessible metastatic lesions are present. In comparison to the primary cancer site, biopsy of intra-pulmonary metastasis would provide histologic evidence of a higher clinical stage. Multi-disciplinary discussion often helps determine the best site and technique for biopsy (e.g., bronchoscopy, ultrasound-guided biopsy, CT-guided biopsy, surgery, etc.).
Case Scenarios 4–7: Special Considerations: Pleural Effusions, Brain, and Bone Metastases. Biopsy of these sites can document metastatic disease and provide tissue for biomarker testing.
Scenario # 4: Patient with a lung mass and a pleural effusion.
A 61-year-old woman with no history of smoking presented with two months of increasing shortness of breath. A chest radiograph showed a large right-sided pleural effusion (Figure 7 A). A chest CT scan confirmed the effusion and revealed a 5 cm right upper lobe mass with collapse of the right middle and lower lobes, but no other sites of metastatic disease (Figure 7 B–C). A thoracentesis yielded one liter of pleural fluid and cytological examination showed an adenocarcinoma, consistent with metastatic spread from a primary lung cancer. Further immunohistochemical evaluation showed that the adenocarcinoma cells stained positive for ALK, which was confirmed by fluorescence in situ hybridization showing the ALK rearrangement in 82% of malignant cells. The findings from the formalin-fixed paraffin-embedded cell block were confirmed by an NGS panel showing an inversion at chromosome 2p2 fusing intron 6 of EML4 to intron 19 of ALK. She has remained on ALK inhibitor therapy for 5 years after initial diagnosis.
Figure 7.
A) The frontal chest radiograph shows a large right-sided pleural effusion (asterisk). B) Coronal CT image of the chest demonstrates a large pleural effusion with collapsed lung, with a mass in the right upper lobe (arrow). C) Axial CT image of the chest after drainage of the pleural effusion demonstrates a 5 cm lobulated mass in the right upper lobe (arrow).
Case Discussion # 4
The clinical presentation of a lung mass and pleural effusion is most consistent with a primary lung cancer with pleural metastasis. Thoracentesis can yield adequate numbers of tumors cells for a cytological diagnosis and concurrently confirm the clinical stage as IVA (M1a). Subsequent thoracentesis, after an initial non-diagnostic thoracentesis, has a higher diagnostic yield in cases with high suspicion for malignant pleural effusion (e.g. pleural involvement on imaging, lymphocyte-predominant exudate on first thoracentesis).37 Diagnostic yield for malignancy from an initial thoracentesis is approximately 40–60% and is influenced clinical factors, such as level of suspicion for malignant etiology, degree of pleural involvement by tumor, and type of malignancy.50–52 Patients do not require sedation for thoracentesis and complications such as pneumothorax and hemothorax are infrequent, occurring in fewer than ~5% of patients.53 Additionally, many laboratories can perform comprehensive biomarker testing on cytology specimens obtained at thoracentesis.54
Key Take-Away for Scenario 4:
Thoracentesis of suspected malignant pleural effusions represents a safe, inexpensive and convenient opportunity to confirm the diagnosis, establish stage IVA (M1a), and obtains cytologic tissue for biomarker testing. Thoracentesis can usually be performed in the outpatient setting.
Some laboratories may not be able to perform comprehensive biomarker testing on certain cytology samples. Cell block preparations of cytologic samples are widely accepted. Therefore, prior discussion with the biomarker testing laboratory is recommended.
Scenario # 5: Patient with a lung mass and brain lesions.
A 44-year-old man with a 30 pack-year history of smoking presented with two months of headaches. A brain MRI showed multiple supra and infratentorial masses at the grey/white matter junction with surrounding vasogenic edema (Figure 8 A–C). The chest CT scan showed a 6 cm right apical mass with no suspicious lymphadenopathy or additional parenchymal lung nodules (Figure 8 D). The patient underwent a percutaneous core needle biopsy of the right upper lobe mass, which revealed squamous cell carcinoma. The NGS panel did not reveal an actionable oncogenic driver and the immunohistochemical staining showed 10% of the tumor cells stained positive for PD-L1. The patient was treated with paclitaxel, carboplatin, and an immune checkpoint inhibitor as well as stereotactic radiosurgery for the brain lesions. Following a year of chemotherapy and immunotherapy, the patient currently has no evidence of progressive disease.
Figure 8.
A-C) Contrast-enhanced T1-weighted MRI of the brain shows multiple enhancing supra- and infratentorial lesions (arrows, A, B, and C) with surrounding vasogenic edema, representing brain metastasis. D) The chest CT scan shows a 6 cm lobulated right apical mass (arrow, D).
Case Discussion # 5
This patient’s presenting symptom of persistent headaches prompted an MRI of the brain which showed multiple central nervous system lesions. This clinical scenario is worrisome for an extracranial malignancy with brain metastasis, the most common cause of which is lung cancer.55 Therefore, based on the patient’s smoking history, he underwent chest CT scanning. Given the potential morbidity of a brain biopsy and the relative certainty that these radiographic findings represent brain metastasis, biopsy of the brain lesions is typically unnecessary. Rather, it is more advisable to search for the primary tumor and extra-cranial sites of metastasis, which are safer and more accessible sites for biopsy. When the known sites of metastasis are relatively inaccessible, biopsy of the presumed primary lung tumor may represent the best option. The right upper lobe mass is accessible via a percutaneous core needle biopsy which in this case revealed a histologic diagnosis of squamous cell lung cancer and provided adequate amounts of tumor tissue for comprehensive biomarker testing (especially relevant in this case because of the patient’s relatively young age).
Key Take-Away for Scenario 5:
When there is overwhelming radiographic evidence of metastasis, a stage-confirmatory biopsy may not be necessary. In this case, the risk from biopsy of intracranial metastasis is high and the likelihood of gaining valuable information, not available from a safer, more accessible biopsy target, is low.
If the evidence for brain metastasis is equivocal, a multi-disciplinary discussion of the risks and benefits of diagnostic and management options, including biopsy or CSF analysis for suspected leptomeningeal disease, should guide decision-making.56, 57
Scenario # 6: Patient with a left upper lobe mass and bone lesions.
A 74-year-old man with a 20 pack-year history of smoking, who quit 35 years ago, presented with a 4-month history of left hip pain. An MRI of his lumbar spine demonstrated the presence of bone metastases, subsequently a chest CT scan showed a 5 cm left upper lobe lesion (Figure 9 A–B). A PET/CT scan revealed extensive FDG uptake in his axial skeleton including a prominent lytic lesion in the left iliac bone (Figure 9 C–D). He underwent bronchoscopy with EBUS-TBNA which yielded NSCLC with adenocarcinoma features. NGS failed to show any actionable mutations and PD-L1 immunohistochemical staining was positive in 20% of tumor cells.
Figure 9.
A-B) Axial CT images of the chest demonstrate an elongated and lobulated lesion in the left upper lobe (arrow, A, B), with adjacent enlarged aorto-pulmonary lymph node (asterisks, A, B).
C-D) Fused axial images of PET/CT scan demonstrated intense FDG uptake in the lung mass and in the aorto-pulmonary lymph node (arrow and asterisk, C). A lytic destructive lesion involving the left iliac bone associated with FDG-avid soft tissue extending into the associated musculature, as well as FDG-avid metastasis in the lumbar vertebrae (arrows, D).
Case Discussion # 6
Patients with lung cancer commonly present with bone metastases.58 Multiple lytic bone lesions assuredly suggest metastatic disease, so histologic or cytologic evidence of bone metastases is not needed in this case. The patient had an accessible lesion so that a bronchoscopy with EBUS-TBNA provided a cytologic diagnosis. It is challenging to perform comprehensive biomarker testing on lung cancer tissue from bone biopsies because they typically require decalcification, a process which often renders the DNA unsuitable for genomic testing.59 While some techniques, such as use of gentle chelating agents, may allow molecular testing after decalcification, it is critical to communicate with the laboratory performing biomarker analysis to discuss whether any bone specimens are accepted and to confirm what sample preparation is necessary to allow for successful testing if bone specimens can be used.60, 61 One important caveat: the presence of a significant soft-tissue component to a metastatic bone lesion, by obviating the need for decalcification, may permit biomarker testing and therefore make that lesion a preferable biopsy site. In this case, biopsy of the soft-tissue component of the iliac lesion would have been less invasive and histologically document a higher clinical stage.
Key Take-Aways for Scenario 6:
Similar to lesions in the brain, imaging is highly suggestive of metastatic disease to the bone and does not typically require histologic confirmation.
If the evidence for metastatic disease is NOT overwhelming (e.g., solitary suspicious boney site on PET scan), it may require sampling to confirm disease, especially if the patient would otherwise be excluded from a curative intent treatment. For instance, false positive PET signals can occur at sites of infection, arthritis, or osteonecrosis. An alternative approach to biopsy in such a case might be to perform a different radiologic evaluation of the suspicious site (e.g., MRI scan) to corroborate or refute the suspicion of metastasis.
Although boney sites of metastasis may be peripheral and easily biopsied, they are often a poor choice for biopsy because the decalcification process can render the sample unsuitable for biomarker testing and some laboratories may not accept any bone biopsies for biomarker testing. However, sites of bone metastasis with a significant soft-tissue component are unlikely to require decalcification.
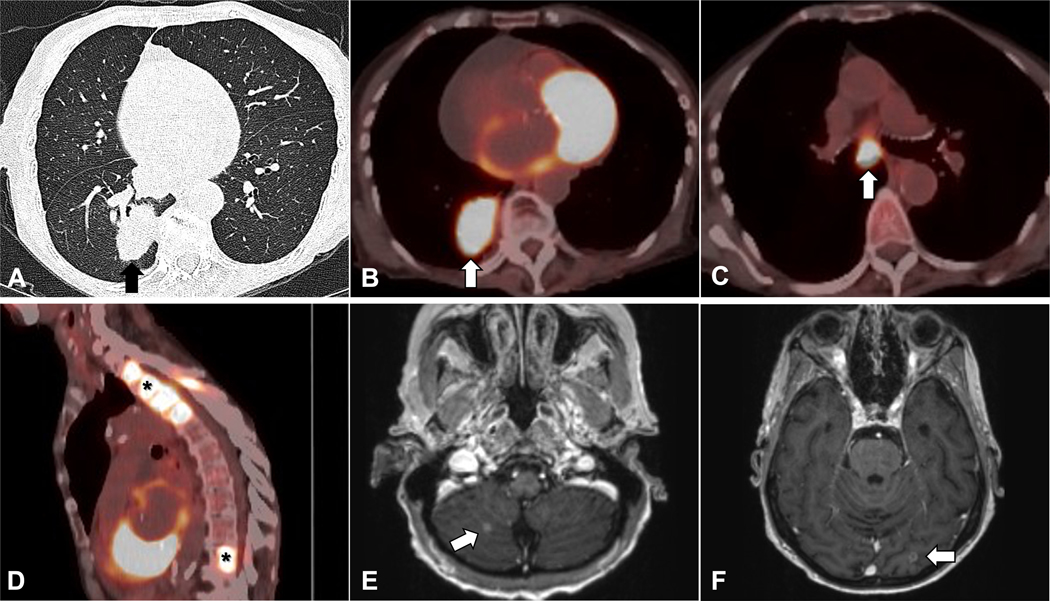
Scenario # 7: Patient with a lung mass and bone and brain lesions:
A 70-year-old woman with no history of smoking presented with dyspnea and was found to have a 4 cm lung mass and suspicious thoracic spine lesions with no mediastinal lymphadenopathy (defined as size > 1 cm in the short axis) on a non-contrast CT scan of the chest (Figure 10 A). A request was made for transthoracic needle biopsy of the suspected primary lung tumor rather than the vertebral lesions out of concern that they may fail to provide tissue for biomarker testing. However, because of the size of the mass and high likelihood it represented a primary lung malignancy, a multi-disciplinary tumor board recommended a PET/CT scan to assess for other metastatic disease. The patient’s PET scan demonstrated intense FDG avidity in mediastinal lymph nodes and the bone lesion (Figure 10 B–D). Notably, these lymph nodes were suspicious due to high PET avidity rather than their size. An MRI of the brain with and without contrast demonstrated multiple small enhancing lesions consistent with metastatic disease (Figure 10 E–F). The patient underwent bronchoscopy with EBUS-TBNA, which established a diagnosis of adenocarcinoma. Biomarker testing revealed an EGFR exon 20 insertion mutation. The patient had a rapidly declining performance status and cognitive issues, making her ineligible for chemotherapy or immunotherapy. Treatment with radiotherapy for the brain lesions and mobocertinib were offered and after discussion with the patient and their family, their decision was to forgo further treatment.
Figure 10.
A) Axial images on CT demonstrate a 4 cm lung mass (arrow, A). B-D) PET/CT scan shows FDG uptake in the lung mass (arrow, B), mediastinal lymphadenopathy (arrow, C) and FDG-avid thoracic and lumbar spine lesions (asterisks, D). E-F) Brain MRI scan shows small enhancing lesions suspicious for metastatic disease (arrows, E, F).
Case Discussion # 7
Inter-disciplinary collaboration and communication improve cancer care quality and outcomes.62, 63 Inter-disciplinary communication, in the form of tumor board discussion, led to an improved choice for site of biopsy in this case. For patients lacking a confirmed diagnosis, those who have suspicious lesions that appear difficult or risky to biopsy, have already undergone a non-diagnostic procedure, or are being evaluated by physicians with little experience directing the work-up for suspected lung cancer anecdotally benefit from multi-disciplinary discussions. When initial imaging does not reveal a biopsy site with a favorable risk-to-benefit ratio, but advanced or metastatic disease is likely, PET/CT scanning is helpful in identifying the optimal biopsy site. For a lung mass > 3 cm, the likelihood of hilar/mediastinal involvement is high, and invasive mediastinal staging by bronchoscopy or mediastinoscopy would be indicated even with a negative PET scan if a curative surgical approach is contemplated.38 In this case, mediastinal lymph nodes involved with cancer were not detected to be abnormal by CT size criteria but by intense FDG avidity suggestive of disease involvement. Without identification of a more peripheral site, such as cervical lymphadenopathy or distant soft-tissue lesions, bronchoscopy with EBUS-TBNA provides the highest yield for biopsy to establish diagnosis, clinical stage, and provide material for biomarker testing at low risk to the patient.
A transthoracic needle biopsy for this patient would likely provide tissue for diagnosis and biomarker testing, however, it has a higher rate of complications, including an estimated ~15% risk of pneumothorax, compared to the low complication rate offered by bronchoscopy and EBUS-TBNA with ~1% risk of major complications.37, 49, 64–66 Bronchoscopy with EBUS-TBNA has demonstrated success for completing biomarker testing and confirms clinical nodal stage, as in this case. Most laboratories accept formalin-fixed paraffin-embedded biopsy specimens, but not all laboratories are capable of performing comprehensive biomarker testing on certain cytopathologic samples (e.g., touch preparations, direct smears).10 In this case, histologic confirmation of distant metastasis was not deemed necessary because of the overwhelming radiologic evidence of metastatic disease in brain and bone (see Scenarios 5 and 6 for a more detailed discussion of brain and bone sites of metastasis).
Despite recent advances, NSCLC remains a disease commonly diagnosed at advanced stages and associated with high morbidity and mortality. This case exemplifies the difficult decisions that still face physicians and patients and the need to further advance early detection and treatment for NSCLC. When comprehensive biomarker testing is not performed or delayed, it places pressure of patients and physicians to make treatment decisions that are not fully informed. While the patient ultimately pursued no treatment for their advanced lung cancer, this patient and their family did so in an ideal setting, with an informed discussion between the patient, family, and an oncologist and with comprehensive biomarker testing in-hand.
Key Take-Aways for Scenario 7:
A collaborative, multidisciplinary approach often improves care, as illustrated in this case. It is the preferred approach for managing thoracic malignancies.
PET/CT scanning, often done to evaluate the clinical stage of a newly suspected lung cancer, is also useful for identifying unsuspected sites of metastatic disease and alternative biopsy sites. For instance, PET/CT scans may reveal sites not detected by CT scanning. Such sites may be stage-defining and more easily accessible for biopsy.
Bronchoscopy with EBUS is safe and effective for lung cancer diagnosis, lymph node staging, and tissue collection for biomarker testing.
Conclusion
Biomarker-directed lung cancer treatments, including targeted therapy and immunotherapy, offer the potential for striking improvement in NSCLC outcomes, including convenience of treatment and prolonged survival. However, successful deployment of these therapies requires that tissue procurers adapt their practice to ensure safe and timely retrieval of sufficient quantities of tissue for diagnosis, staging, and comprehensive biomarker testing. Robust interdisciplinary communication optimizes biopsy site selection to achieve these three aims while reducing potential harm and costs to patients. Issues surrounding biomarker testing in NSCLC including access, equity, quality, and cost are chief concerns for the American Cancer Society National Lung Cancer Roundtable and research, dissemination, collaboration, and advocacy are all part of its strategic plan of ensuring optimal therapy for persons diagnosed with lung cancer.
Funding Statement:
AHF was supported by NIH grant: K12-CA157688. BEJ was supported by the Satherlie Family Research Fund and the Cyker Charitable Foundation.
Conflict of Interest Statement:
AHF owns stock in Merck. MN is a consultant to Daiichi Sankyo, AstraZeneca; Research grant to the institution from Merck, Canon Medical Systems, AstraZeneca, Daiichi Sankyo. RUO owns patents for a lymph node specimen collection kit; owns stocks in Eli Lilly, Gilead Sciences, and Pfizer; has worked as a paid research consultant for the American Cancer Society, the Association of Community Cancer Centers, Astra Zeneca, Biodesix, Eli Lilly, Triptych Healthcare Partners, and Genentech/Roche and the National Cancer Institute; and is founder of Oncobox Device, Inc; funding from NIH UG1CA189873, R01CA172253, and UM1CA233080. MPR reports funding from NIH/NCI. LSR reports that her salary is supported by unrestricted educational grants to the American Cancer Society from private and corporate foundations, and companies in the health sector related to the submitted work. RAS reports that the American Cancer Society receives unrestricted educational grants from private and corporate foundations, including foundations associated with companies in the health sector related to the submitted work. His salary is solely funded through American Cancer Society funds. FF received funds from the National Institutes of Health (NIH) (R01CA258352, U01HL162966) during this project. The NIH was not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. LMS reports consulting income to my institution from Genentech, Lilly; research funds to my institution from Genetech and Bristol Myers Squibb; personal consulting fees from AstraZeneca and GV20 Therapeutics. GAS reports consultant and research support Biodesix, research support Exact Sciences, consultant and research support Nucleix, consultant and research support Seer, research support Amgen. BEJ receives post-marketing royalties for a patent on epidermal growth factor receptor testing, research support from Cannon Medical Systems, paid Consultant to Novartis, Boston Pharmaceuticals, Checkpoint Therapeutics, Chugai, Daichi Sankyo, Foundation Medicine, G1 Therapeutics, Genentech, GSK, Hengrui Therapeutics, Janssen, Jazz Pharma, Lilly, Bluedot Bio, Unpaid Member of a Steering Committee for Pfizer.
Abbreviation list:
- CT
Computed tomography
- EBUS-TBNA
Endobronchial ultrasound-guided transbronchial needle aspiration
- FDG
18F-fluoro-2-deoxy-D-glucose
- FDA
Federal Drug Administration
- NSG
Next-Generation Sequencing
- NSCLC
Non-small cell lung cancer
- PET
Positron Emission Tomography
- PD-L1
Programmed death-ligand 1
- TMB
Tumor mutation burden
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA: A Cancer Journal for Clinicians. 2022;72(1):7–33. doi: 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 2.Hanna NH, Robinson AG, Temin S, et al. Therapy for Stage IV Non–Small-Cell Lung Cancer With Driver Alterations: ASCO and OH (CCO) Joint Guideline Update. Journal of Clinical Oncology. 0(0):JCO.20.03570. doi: 10.1200/jco.20.03570 [DOI] [PubMed] [Google Scholar]
- 3.Wu Y-L, Tsuboi M, He J, et al. Osimertinib in Resected EGFR-Mutated Non–Small-Cell Lung Cancer. New England Journal of Medicine. 2020;383(18):1711–1723. doi: 10.1056/NEJMoa2027071 [DOI] [PubMed] [Google Scholar]
- 4.Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. Oct 9 2021;398(10308):1344–1357. doi: 10.1016/s0140-6736(21)02098-5 [DOI] [PubMed] [Google Scholar]
- 5.Mok T, Camidge DR, Gadgeel SM, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol. Aug 2020;31(8):1056–1064. doi: 10.1016/j.annonc.2020.04.478 [DOI] [PubMed] [Google Scholar]
- 6.Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med. Jan 11 2018;378(2):113–125. doi: 10.1056/NEJMoa1713137 [DOI] [PubMed] [Google Scholar]
- 7.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. May 20 2004;350(21):2129–39. doi: 10.1056/NEJMoa040938 [DOI] [PubMed] [Google Scholar]
- 8.Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. Jun 4 2004;304(5676):1497–500. doi: 10.1126/science.1099314 [DOI] [PubMed] [Google Scholar]
- 9.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. Sep 7 2004;101(36):13306–11. doi: 10.1073/pnas.0405220101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer. version 1.2022. 2022. Feb 11. [Google Scholar]
- 11.Lindeman NI, Cagle PT, Aisner DL, et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch Pathol Lab Med. Mar 2018;142(3):321–346. doi: 10.5858/arpa.2017-0388-CP [DOI] [PubMed] [Google Scholar]
- 12.Mileham KF, Schenkel C, Bruinooge SS, et al. Defining comprehensive biomarker-related testing and treatment practices for advanced non-small-cell lung cancer: Results of a survey of U.S. oncologists. Cancer Med. Jan 2022;11(2):530–538. doi: 10.1002/cam4.4459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox AH, Jett JR, Roy UB, et al. Knowledge and Practice Patterns Among Pulmonologists for Molecular Biomarker Testing in Advanced Non-small Cell Lung Cancer. Chest. Dec 2021;160(6):2293–2303. doi: 10.1016/j.chest.2021.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kehl KL, Lathan CS, Johnson BE, Schrag D. Race, Poverty, and Initial Implementation of Precision Medicine for Lung Cancer. J Natl Cancer Inst. Apr 1 2019;111(4):431–434. doi: 10.1093/jnci/djy202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smeltzer MP, Wynes MW, Lantuejoul S, et al. The International Association for the Study of Lung Cancer Global Survey on Molecular Testing in Lung Cancer. J Thorac Oncol. Sep 2020;15(9):1434–1448. doi: 10.1016/j.jtho.2020.05.002 [DOI] [PubMed] [Google Scholar]
- 16.Pennell NA, Mutebi A, Zhou Z-Y, et al. Economic Impact of Next-Generation Sequencing Versus Single-Gene Testing to Detect Genomic Alterations in Metastatic Non–Small-Cell Lung Cancer Using a Decision Analytic Model. JCO Precision Oncology. 2019;(3):1–9. doi: 10.1200/po.18.00356 [DOI] [PubMed] [Google Scholar]
- 17.Benayed R, Offin M, Mullaney K, et al. High Yield of RNA Sequencing for Targetable Kinase Fusions in Lung Adenocarcinomas with No Mitogenic Driver Alteration Detected by DNA Sequencing and Low Tumor Mutation Burden. Clinical Cancer Research. 2019;25(15):4712–4722. doi: 10.1158/1078-0432.Ccr-19-0225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lantuejoul S, Sound-Tsao M, Cooper WA, et al. PD-L1 Testing for Lung Cancer in 2019: Perspective From the IASLC Pathology Committee. J Thorac Oncol. Apr 2020;15(4):499–519. doi: 10.1016/j.jtho.2019.12.107 [DOI] [PubMed] [Google Scholar]
- 19.Mino-Kenudson M, Le Stang N, Daigneault JB, et al. The International Association for the Study of Lung Cancer Global Survey on Programmed Death-Ligand 1 Testing for NSCLC. J Thorac Oncol. Apr 2021;16(4):686–696. doi: 10.1016/j.jtho.2020.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rimm DL, Han G, Taube JM, et al. A Prospective, Multi-institutional, Pathologist-Based Assessment of 4 Immunohistochemistry Assays for PD-L1 Expression in Non-Small Cell Lung Cancer. JAMA Oncol. Aug 1 2017;3(8):1051–1058. doi: 10.1001/jamaoncol.2017.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Compton CC, Robb JA, Anderson MW, et al. Preanalytics and Precision Pathology: Pathology Practices to Ensure Molecular Integrity of Cancer Patient Biospecimens for Precision Medicine. Arch Pathol Lab Med. Nov 2019;143(11):1346–1363. doi: 10.5858/arpa.2019-0009-SA [DOI] [PubMed] [Google Scholar]
- 22.Forest F, Cote G, Laville D, et al. Impact of delayed fixation and decalcification on PD-L1 expression: a comparison of two clones. Virchows Arch. Dec 2019;475(6):693–699. doi: 10.1007/s00428-019-02613-w [DOI] [PubMed] [Google Scholar]
- 23.Gagné A, Wang E, Bastien N, et al. Impact of Specimen Characteristics on PD-L1 Testing in Non-Small Cell Lung Cancer: Validation of the IASLC PD-L1 Testing Recommendations. J Thorac Oncol. Dec 2019;14(12):2062–2070. doi: 10.1016/j.jtho.2019.08.2503 [DOI] [PubMed] [Google Scholar]
- 24.Phillips KA, Deverka PA, Marshall DA, et al. Methodological Issues in Assessing the Economic Value of Next-Generation Sequencing Tests: Many Challenges and Not Enough Solutions. Value Health. Sep 2018;21(9):1033–1042. doi: 10.1016/j.jval.2018.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Decision Memo for Next Generation Sequencing (NGS) for Medicare Beneficiaries with Advanced Cancer (CAG-00450N) (2018). [Google Scholar]
- 26.Anand K, Phung TL, Bernicker EH, Cagle PT, Olsen RJ, Thomas JS. Clinical Utility of Reflex Ordered Testing for Molecular Biomarkers in Lung Adenocarcinoma. Clin Lung Cancer. Sep 2020;21(5):437–442. doi: 10.1016/j.cllc.2020.05.007 [DOI] [PubMed] [Google Scholar]
- 27.Miller TE, Yang M, Bajor D, et al. Clinical utility of reflex testing using focused next-generation sequencing for management of patients with advanced lung adenocarcinoma. J Clin Pathol. Dec 2018;71(12):1108–1115. doi: 10.1136/jclinpath-2018-205396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zacharias M, Absenger G, Kashofer K, et al. Reflex testing in non-small cell lung carcinoma using DNA- and RNA-based next-generation sequencing-a single-center experience. Transl Lung Cancer Res. Nov 2021;10(11):4221–4234. doi: 10.21037/tlcr-21-570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazer R Clinical Laboratory Compliance Issues. J Helthcare Cimpliance. 2018;20(5):21–32, 59–60. [Google Scholar]
- 30.Forde PM, Spicer J, Lu S, et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. New England Journal of Medicine. 2022;386(21):1973–1985. doi: 10.1056/NEJMoa2202170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rolfo C, Mack P, Scagliotti GV, et al. Liquid Biopsy for Advanced NSCLC: A Consensus Statement From the International Association for the Study of Lung Cancer. J Thorac Oncol. Oct 2021;16(10):1647–1662. doi: 10.1016/j.jtho.2021.06.017 [DOI] [PubMed] [Google Scholar]
- 32.Merker JD, Oxnard GR, Compton C, et al. Circulating Tumor DNA Analysis in Patients With Cancer: American Society of Clinical Oncology and College of American Pathologists Joint Review. J Clin Oncol. Jun 1 2018;36(16):1631–1641. doi: 10.1200/jco.2017.76.8671 [DOI] [PubMed] [Google Scholar]
- 33.Mok TS, Wu Y-L, Thongprasert S, et al. Gefitinib or Carboplatin–Paclitaxel in Pulmonary Adenocarcinoma. New England Journal of Medicine. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699 [DOI] [PubMed] [Google Scholar]
- 34.Mazieres J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol. Aug 1 2019;30(8):1321–1328. doi: 10.1093/annonc/mdz167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee CK, Man J, Lord S, et al. Checkpoint Inhibitors in Metastatic EGFR-Mutated Non-Small Cell Lung Cancer-A Meta-Analysis. J Thorac Oncol. Feb 2017;12(2):403–407. doi: 10.1016/j.jtho.2016.10.007 [DOI] [PubMed] [Google Scholar]
- 36.Barlesi F, Mazieres J, Merlio JP, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet. Apr 2 2016;387(10026):1415–1426. doi: 10.1016/s0140-6736(16)00004-0 [DOI] [PubMed] [Google Scholar]
- 37.Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. May 2013;143(5 Suppl):e142S–e165S. doi: 10.1378/chest.12-2353 [DOI] [PubMed] [Google Scholar]
- 38.Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for Staging Non-small Cell Lung Cancer: Diagnosis and Management of Lung Cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. CHEST. 2013;143(5):e211S–e250S. doi: 10.1378/chest.12-2355 [DOI] [PubMed] [Google Scholar]
- 39.Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The Eighth Edition Lung Cancer Stage Classification. Chest. Jan 2017;151(1):193–203. doi: 10.1016/j.chest.2016.10.010 [DOI] [PubMed] [Google Scholar]
- 40.Roy-Chowdhuri S, Dacic S, Ghofrani M, et al. Collection and Handling of Thoracic Small Biopsy and Cytology Specimens for Ancillary Studies: Guideline From the College of American Pathologists in Collaboration With the American College of Chest Physicians, Association for Molecular Pathology, American Society of Cytopathology, American Thoracic Society, Pulmonary Pathology Society, Papanicolaou Society of Cytopathology, Society of Interventional Radiology, and Society of Thoracic Radiology. Arch Pathol Lab Med. May 13 2020;doi: 10.5858/arpa.2020-0119-CP [DOI] [PubMed] [Google Scholar]
- 41.Aggarwal C, Rolfo CD, Oxnard GR, Gray JE, Sholl LM, Gandara DR. Strategies for the successful implementation of plasma-based NSCLC genotyping in clinical practice. [DOI] [PubMed] [Google Scholar]
- 42.Zhang JH, Xia FF, Yang XS, Li Y. Computed tomography-guided lung biopsy for molecular tests: a meta-analysis. Kardiochir Torakochirurgia Pol. Jun 2022;19(2):96–101. doi: 10.5114/kitp.2022.117498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verdial FC, Berfield KS, Wood DE, et al. Safety and Costs of Endobronchial Ultrasound-Guided Nodal Aspiration and Mediastinoscopy. Chest. Mar 2020;157(3):686–693. doi: 10.1016/j.chest.2019.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Labarca G, Folch E, Jantz M, Mehta HJ, Majid A, Fernandez-Bussy S. Adequacy of Samples Obtained by Endobronchial Ultrasound with Transbronchial Needle Aspiration for Molecular Analysis in Patients with Non-Small Cell Lung Cancer. Systematic Review and Meta-Analysis. Ann Am Thorac Soc. Oct 2018;15(10):1205–1216. doi: 10.1513/AnnalsATS.201801-045OC [DOI] [PubMed] [Google Scholar]
- 45.Faber E, Grosu H, Sabir S, et al. Adequacy of small biopsy and cytology specimens for comprehensive genomic profiling of patients with non-small-cell lung cancer to determine eligibility for immune checkpoint inhibitor and targeted therapy. Journal of Clinical Pathology. 2022;75(9):612–619. doi: 10.1136/jclinpath-2021-207597 [DOI] [PubMed] [Google Scholar]
- 46.Johnson BE. Second lung cancers in patients after treatment for an initial lung cancer. J Natl Cancer Inst. Sep 16 1998;90(18):1335–45. doi: 10.1093/jnci/90.18.1335 [DOI] [PubMed] [Google Scholar]
- 47.Faivre-Finn C, Vicente D, Kurata T, et al. Four-Year Survival With Durvalumab After Chemoradiotherapy in Stage III NSCLC-an Update From the PACIFIC Trial. J Thorac Oncol. May 2021;16(5):860–867. doi: 10.1016/j.jtho.2020.12.015 [DOI] [PubMed] [Google Scholar]
- 48.Paz-Ares L, Spira A, Raben D, et al. Outcomes with durvalumab by tumour PD-L1 expression in unresectable, stage III non-small-cell lung cancer in the PACIFIC trial. Ann Oncol. Jun 2020;31(6):798–806. doi: 10.1016/j.annonc.2020.03.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiener RS, Schwartz LM, Woloshin S, Welch HG. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Ann Intern Med. Aug 2 2011;155(3):137–44. doi: 10.7326/0003-4819-155-3-201108020-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grosu HB, Kazzaz F, Vakil E, Molina S, Ost D. Sensitivity of Initial Thoracentesis for Malignant Pleural Effusion Stratified by Tumor Type in Patients with Strong Evidence of Metastatic Disease. Respiration. 2018;96(4):363–369. doi: 10.1159/000490732 [DOI] [PubMed] [Google Scholar]
- 51.Arnold DT, De Fonseka D, Perry S, et al. Investigating unilateral pleural effusions: the role of cytology. Eur Respir J. Nov 2018;52(5)doi: 10.1183/13993003.01254-2018 [DOI] [PubMed] [Google Scholar]
- 52.Porcel JM, Esquerda A, Vives M, Bielsa S. Etiology of pleural effusions: analysis of more than 3,000 consecutive thoracenteses. Arch Bronconeumol. May 2014;50(5):161–5. doi: 10.1016/j.arbres.2013.11.007 [DOI] [PubMed] [Google Scholar]
- 53.Cantey EP, Walter JM, Corbridge T, Barsuk JH. Complications of thoracentesis: incidence, risk factors, and strategies for prevention. Curr Opin Pulm Med. Jul 2016;22(4):378–85. doi: 10.1097/mcp.0000000000000285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roy-Chowdhuri S, Pisapia P, Salto-Tellez M, et al. Invited review-next-generation sequencing: a modern tool in cytopathology. Virchows Arch. Jul 2019;475(1):3–11. doi: 10.1007/s00428-019-02559-z [DOI] [PubMed] [Google Scholar]
- 55.Sacks P, Rahman M. Epidemiology of Brain Metastases. Neurosurg Clin N Am. Oct 2020;31(4):481–488. doi: 10.1016/j.nec.2020.06.001 [DOI] [PubMed] [Google Scholar]
- 56.Vogelbaum MA, Brown PD, Messersmith H, et al. Treatment for Brain Metastases: ASCO-SNO-ASTRO Guideline. Journal of Clinical Oncology. 2022;40(5):492–516. doi: 10.1200/jco.21.02314 [DOI] [PubMed] [Google Scholar]
- 57.Bönig L, Möhn N, Ahlbrecht J, et al. Leptomeningeal Metastasis: The Role of Cerebrospinal Fluid Diagnostics. Front Neurol. 2019;10:839. doi: 10.3389/fneur.2019.00839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grant SR, Smith BD, Colbert LE, et al. National Quality Measure Compliance for Palliative Bone Radiation Among Patients With Metastatic Non-Small Cell Lung Cancer. J Natl Compr Canc Netw. May 26 2021:1–6. doi: 10.6004/jnccn.2020.7688 [DOI] [PubMed] [Google Scholar]
- 59.Aisner DL, Marshall CB. Molecular pathology of non-small cell lung cancer: a practical guide. Am J Clin Pathol. Sep 2012;138(3):332–46. doi: 10.1309/ajcpfr12wjkceezz [DOI] [PubMed] [Google Scholar]
- 60.Confavreux CB, Girard N, Pialat JB, et al. Mutational profiling of bone metastases from lung adenocarcinoma: results of a prospective study (POUMOS-TEC). Bonekey Rep. 2014;3:580. doi: 10.1038/bonekey.2014.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schrijver WA, van der Groep P, Hoefnagel LD, et al. Influence of decalcification procedures on immunohistochemistry and molecular pathology in breast cancer. Mod Pathol. Dec 2016;29(12):1460–1470. doi: 10.1038/modpathol.2016.116 [DOI] [PubMed] [Google Scholar]
- 62.Ray MA, Faris NR, Fehnel C, et al. Survival Impact of an Enhanced Multidisciplinary Thoracic Oncology Conference in a Regional Community Health Care System. JTO Clinical and Research Reports. 2021/08/01/ 2021;2(8):100203. doi: 10.1016/j.jtocrr.2021.100203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Denton E, Conron M. Improving outcomes in lung cancer: the value of the multidisciplinary health care team. J Multidiscip Healthc. 2016;9:137–44. doi: 10.2147/jmdh.S76762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Asano F, Aoe M, Ohsaki Y, et al. Complications associated with endobronchial ultrasound-guided transbronchial needle aspiration: a nationwide survey by the Japan Society for Respiratory Endoscopy. Respir Res. May 10 2013;14(1):50. doi: 10.1186/1465-9921-14-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vaidya PJ, Munavvar M, Leuppi JD, Mehta AC, Chhajed PN. Endobronchial ultrasound-guided transbronchial needle aspiration: Safe as it sounds. Respirology. Aug 2017;22(6):1093–1101. doi: 10.1111/resp.13094 [DOI] [PubMed] [Google Scholar]
- 66.Heerink WJ, de Bock GH, de Jonge GJ, Groen HJ, Vliegenthart R, Oudkerk M. Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol. Jan 2017;27(1):138–148. doi: 10.1007/s00330-016-4357-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.U.S. Food and Drug Administration: Drug Databases: FDA-Approved Drugs. Accessed 2/2/2022. www.fda.gov/drugsatfda