Abstract
Although fast and slow gating mechanisms have been described in gap junctions (GJs), their relative contributions to dependence on transjunctional voltage, Vj, is still unclear. We used cell lines expressing wild-type connexin 45 (Cx45) and connexin 43 fused with enhanced green fluorescent protein (Cx43-EGFP) to examine mechanisms of gating in homo- and heterotypic GJs formed of these connexins. Macroscopically Cx45/Cx45 channels show high sensitivity to Vj. Cx45 channels demonstrate two types of gating: fast transitions between open and residual states and slow transitions between open and completely closed states. Single-channel conductance of the Cx45 channel is ≈32 pS for the open state and ≈4 pS for the residual state. Cx45/Cx43-EGFP heterotypic junctions exhibit very asymmetrical Vj gating with the maximum junctional conductance shifted to Vj positive on the Cx45 side. Conductance of single Cx45/Cx43-EGFP channels is ≈55 pS for the open state and ≈4 pS for the residual state, values consistent with the simple-series connection of Cx45 and Cx43-EGFP hemichannels. At Vj = 0, the slow gate of many Cx45 hemichannels is closed in both homotypic Cx45/Cx45 and heterotypic Cx45/Cx43-EGFP junctions. Fast and slow Vj gates of both Cx45 and Cx43 hemichannels close for relative negativity at their cytoplasmic end. Coupling mediated by Cx45/Cx43-EGFP junctions can exhibit asymmetry that can be strongly modulated by small changes in difference of holding potentials. Cx45/Cx43 junctions are likely to be found in brain and heart and may mediate rectifying electrical transmission or modulatable chemical communication.
Keywords: intercellular communication‖channels‖voltage gating‖rectifaction‖ electrical coupling
The connexins are a large family of homologous integral membrane proteins that form gap junction (GJ) channels which provide a pathway for electrical signaling and metabolic communication (1, 2). Each GJ channel is composed of two hemichannels, each formed of six connexin (Cx) monomers. GJ channels generally respond to transjunctional voltage, Vj, with decay of junctional conductance (gj) to some steady state, gj∞ (3). Although sensitivity and kinetics depend on the connexin type, most homotypic junctions show a maximum gj at Vj = 0 and symmetric gj–Vj dependence, which has been ascribed to the presence of a gate in each hemichannel and their opposite orientation with respect to Vj. In general, as Vj is increased, gj seems to decline to a voltage-insensitive or residual conductance, gjmin, that ranges from ≈5 to 30% of gj at Vj = 0 mV, depending on the connexin. This property has been explained by single-channel studies showing that GJ channels close from the main open state with conductance, γopen, to a subconducting or residual conductance state, γres (4–7). Now, apparently an additional gating mechanism exists in each GJ hemichannel characterized by slower kinetics and full open/closed transitions which themselves are slow, often taking tens of milliseconds to complete (8, 9). We termed the two Vj-sensitive gating mechanisms “fast” and “slow”, with the fast gating mechanism referring to that which closes channels to the residual state and the slow gating mechanism that which closes channels completely. Two distinct gating mechanisms have also been reported in Cx40 (10), Cx30 (11), and Cx57 (12) channels, as well as in unapposed Cx46 and chimeric Cx32*Cx43E1 hemichannels (8, 13). Fast and slow gates have been supported by molecular studies, which show that fast gating can be abolished with little effect on slow gating (9, 14).
Here we report on the properties of Cx45 GJ channels and heterotypic channels formed by pairing of Cx45 and Cx43-enhanced green fluorescent protein (EGFP) expressing cells. Cx45 channels have both fast and slow gating mechanisms, and both gates are closed by the same polarity of Vj, relative negativity at the cytoplasmic end of the channel. The two gates per hemichannel operate as if they are in series where the state of one gate affects the voltage across the other gate (“contingent gating”). In Cx45/Cx45 junctions at Vj = 0, a significant fraction of slow gates are closed, and the slow gate rather than the fast gate seems to be responsible for the strong Vj dependence. In heterotypic Cx43/Cx43-EGFP junctions, the gj–Vj relation is very asymmetric, and this property can result in very marked directional asymmetry in electrotonic transmission across the junction. Moreover, small differences in resting potential between the coupled cells strongly modulate the degree of asymmetry, and electrical transmission across the junctions can be switched from bidirectional to unidirectional. Modulation of electrical and chemical communication across these junctions may have important functional consequences.
Materials and Methods
cDNAs, Cell Lines, Culture Conditions, and Transfections.
Experiments were performed on HeLa cells (ATCC no. CCL-2) stably transfected with cDNAs encoding rat Cx45 and rat Cx43-EGFP. Cx43-EGFP has the EGFP attached to its cytoplasmic carboxyl terminus, and its trafficking can be visualized in living cells (15). HeLa cells were grown in Dulbecco's medium supplemented with 10% FBS. To study homotypic junctions, cells of one type were seeded onto coverslips. To study Cx45/Cx43-EGFP heterotypic junctions, HeLaCx45 and HeLaCx43-EGFP cells were mixed together in equal quantities and seeded on coverslips.
Electrophysiological and Fluorescence Measurements.
For simultaneous electrophysiological and fluorescence recording, cells grown on coverslips were transferred to an experimental chamber mounted on the stage of an inverted Olympus IX-70 microscope equipped with OlymPix 2000 digital camera (Olympus, New Hyde Park, NY). Fluorescence of Cx43-EGFP was monitored by using UltraVIEW software (Perkin–Elmer Life Sciences). The chamber was perfused with a modified Krebs–Ringer solution [140 mM NaCl/4 mM KCl/2 mM CaCl2/1 mM MgCl2/5 mM Hepes/5 mM glucose/2 mM pyruvate (pH = 7.4)]. Patch pipettes were filled with a solution containing (in mM) NaCl (140), KCl (0.2), CaCl2 (1), MgCl2 (3), MgATP (5), Hepes (3, pH 7.2) EGTA (2, [Ca2+]i = 5 × 10−8 M). Junctional conductance was measured by using a dual whole-cell patch clamp as described (9). For data acquisition and analysis we used a MIO-16X A/D converter (National Instruments, Austin TX) and our own software.
Results
Macroscopic Voltage Gating of Cx45 Channels.
Fig. 1A shows a record from a Cx45 cell pair in response to a long Vj step of −100 mV (see Vj trace; negative Vj is defined for negative voltage step). Small, brief Vj ramps (V1 was changed linearly from −18 to 18 mV in 0.8 s with 0.2 s between ramps) were applied to measure gj before the long Vj step and after gj had reached steady state during the step. During the Vj step, gj (top trace) decreased from ≈8 to 1.2 nS. After the −100-mV step was terminated, gj increased, slowly reaching a maximum of ≈9 nS, i.e., exceeding the control value, and then subsided back to the initial level. The “overshoot” of gj, gjover, during recovery was greater for larger Vj and somewhat greater for positive than for negative Vj (Fig. 1C).
Figure 1.
Macroscopic measurement of Vj gating of homotypic Cx45 junctions. (A) Vj gating in response to a long Vj step of −100 mV applied to cell 1. Repeated (1 Hz) Vj ramps (from −18 to +18 mV in 0.8 s) were applied before, during, and after the Vj step to monitor gj. The gj time plot determined from Ij and Vj records shows that during recovery from the step, gj transiently exceeded the initial value; the maximum was termed gjover. (B) Pooled data of normalized steady state gj (Gj) vs. Vj measured in 15 Cx45 cell pairs. Gj was measured at the end of Vj steps ≈30 s in duration. The solid line is a fit of all of the data points by a four-state contingent gating model containing one gate per hemichannel (see below) with sensitivity parameters A = 0.3 mV−1 and V0 = 8.9 mV for negative Vj and A = 0.17 mV−1 and V0 = 7.5 mV. (C) Pooled data of normalized gjover vs. Vj.
The relation between normalized gj at steady state (Gj∞) and Vj was determined in 15 Cx45 cell pairs (summarized in Fig. 1B). The value of gj declined markedly for Vj of either sign, with slight asymmetry around Vj = 0 mV ascribable to dependence on Vm (16). gj reached a minimum at ±60 mV and then increased at higher Vj values. The solid line is a fit to the data with a model in which each hemichannel is assumed to contain a single gate whose state is dependent on the state of the other gate (contingent gating) (3, 17). Details of this model and the secondary increase in gj are discussed with respect to Figs. 3 and 6.
Figure 3.
Vj dependence of Cx45/Cx43-EGFP heterotypic junctions. (A) Phase contrast (Left) and fluorescence (Right) images of a Cx45/Cx43-EGFP cell pair. The Cx43-EGFP cell is identified by its fluorescence. The arrow indicates a junctional plaque. (B) Changes in Ij in response to positive and negative 60-mV Vj steps (positive Vj is defined as relatively positive on the Cx43-EGFP side). Ij increased slowly from an initial level of ≈13 pA during the negative Vj step and decreased rapidly during the positive Vj step. gj decreased by about 30% between the steps. (C) Normalized gj–V relation for Cx45/Cx43-EGFP heterotypic junctions in 14 cell pairs. The thin black line is a fit of a 4-state contingent gating model with one gate in each hemichannel to all data points (open and center-dotted circles). The parameters are A= 0.18 mV−1 and V0 = 1 mV for the Cx45 hemichannel and A = 0.03 mV−1 and V0 = 26 mV for the Cx43-EGFP hemichannel. The thick gray line is a fit of the data points indicated by dotted circles with a four-state contingent gating model of the channel containing fast and slow gates only in the Cx45 hemichannel. The parameters are A = 0.27 mV−1 and V0 = 3 mV for the slow gate and A = 0.46 mV−1 and V0 = 10 mV for the fast gate. Normalized gj decreased rapidly with voltage for relative positivity on the Cx43-EGFP side; the relatively high sensitivity indicates that this decrease is mediated by the Cx45 hemichannel and that its gating polarity is negative. An expanded view (Inset) demonstrates that the contingent model can explain the secondary increase in gj at large Vj (see Discussion and Fig. 6).
Figure 6.
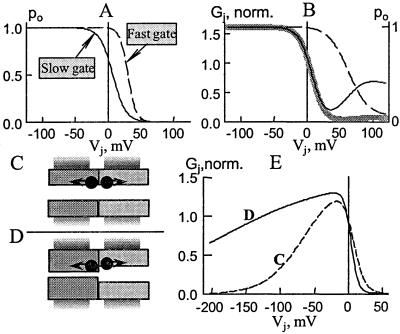
Summary of the fit to the experimental data obtained in heterotypic Cx45/Cx43-EGFP junctions by a four-state contingent gating model. (A) Vj sensitivity of open probability of fast (dashed line) and slow (solid line) gates that operate only in the Cx45 hemichannel. (B) At Vj = 0 ≈70% of Cx45 hemichannels are closed solely by the slow gate (Gj normalized to value at Vj = 0 is shown by the thick gray line and Po of the slow gate is shown by the solid line) and that the fast gate operates only at Vj values > ≈30 mV. (C and D) Heterotypic channels with identical (C) or different (D) conductances of hemichannels containing one slow gate (arrows) per hemichannel. (E) Gj–Vj dependence calculated for a four-state contingent gating model in which the two hemichannels have Vj sensitivity like that of Cx45 and Cx43 but are identical in unitary conductance that is equal to 230 pS (dashed line, C) or that they differ in both Vj sensitivity and unitary conductance (64 and 230 pS for the Cx45 and Cx43 hemichannels, respectively; solid line, D).
Fig. 1C shows the gjover vs. Vj plot. gjover was determined at the peak of the transient increase and normalized to gj at Vj = 0 mV. At Vj = ±150, gjover increased to ≈1.3. These results can be explained for the channels in which a substantial fraction of the hemichannels are closed at Vj = 0. Vj of one polarity tends to close gates in one hemichannel and open them in its partner hemichannel. Because at Vj = 0 no voltage occurs across either hemichannel, they might be expected to relax to their final open probability, Po, independently. gjover will be greater if the open hemichannels close more slowly than the closed hemichannels open.
Single Cx45 Channels Show Fast and Slow Vj Gating Mechanisms.
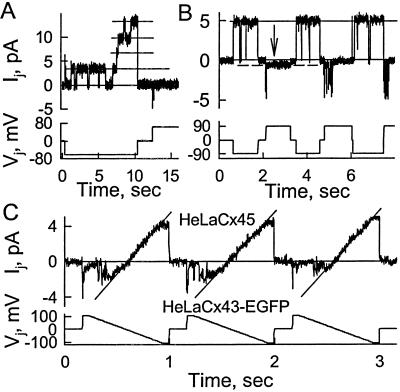
We examined Vj gating of single Cx45 channels in weakly coupled cell pairs with only one or two operational channels. Fig. 2A shows single-channel gating in response to Vj steps of −65 and +65 mV. At the onset of the voltage step one channel was open and underwent frequent unresolved closures until it closed to γres (dashed line); then several gating transitions of ≈1.7 pA (≈28 nS) occurred between the open (γopen) and residual states. The expanded record (Inset; Ij record was low-pass filtered at 200 Hz and sampled with 2-ms intervals) shows that 28-pS transitions between open and residual states were fast (no more than one point was recorded during each transition), and transitions from the residual to the closed state and back were slow (see arrows). The open-state conductance was ≈32 pS, and the residual state conductance was ≈4 pS. When Vj was reversed, the channel was still closed and after about 0.2 s opened slowly to the residual state. It then opened fully, closed to a level lower than the residual state, opened to the residual state, and then had a burst of poorly resolved transitions near the fully open state. In other cell pairs Ij–Vj relations of single channels were examined by applying voltage ramps (from +70 to −70 mV for the first two ramps and from +100 to −100 mV for the second two ramps; Fig. 2B). The channel closed after application of the positive shoulder of ramps and then opened again as Vj decreased, and then closed again for large negative Vj. Closures occurred to the residual state during the first two ramps and to the fully closed state in the second two, as indicated by arrows. Open-channel current increased linearly with voltage yielding a slope conductance of ≈32 pS. The residual state also seemed ohmic (see second ramp), although the resolution was compromised by the low value of the conductance.
Figure 2.
Vj gating of single Cx45 channels. (A) A negative and then a positive step of 60 mV were applied. At the beginning of the negative step a single channel was open (solid line) that showed closure to a substate of ≈4 pS (dashed lines), as well as a burst of apparently partial closings that were not well resolved. The closures were fast compared with the sampling interval of 2 ms. (B) Successive ramps repeated at 1/s showed fast gating to the residual state (first two ramps, dashed lines) and slow gating to the fully closed state (second two ramps, arrows). The channel was typically open at the onset of the ramp, closed at short latency, and then reopened (solid lines) at Vj approaching near zero.
Macroscopic Vj Gating of Heterotypic Cx45/Cx43-EGFP Channels.
These experiments utilized Cx45/Cx43-EGFP cell pairs, which exhibited EGFP fluorescence only in one cell, and had at least one fluorescent plaque present at the apposition between the cells (Fig. 3A; the arrow indicates a junctional plaque). Fig. 3B illustrates Ij changes in response to Vj steps of ±60 mV. For relative negativity on the Cx43-EGFP side, Ij increased over several seconds from about 13 pA to 28 pA (from ≈6 to ≈15 channels). During a brief stop at 0 mV, gj decreased by about 30%. During the following positive Vj step, Ij rapidly decreased to near zero; the residual current corresponded to gj = ≈16 pS, indicating that most of the channels had closed completely and ≈4 channels had closed to γres. In simple series placement, γres in homotypic Cx45 and heterotypic Cx45/Cx43-EGFP junctions would be given by the series sum of the hemichannel conductance of the residual state, γhem,res, and that of the open state, γhem,open. Thus, γres = γhem,res n/(n + 1), where n is the ratio, γhem,open/γhem,res. Because n is substantially greater than unity for homotypic Cx45 and even greater for Cx45/Cx43-EGFP heterotypic channels closed to the residual state on the Cx45 side, then γres ≈ γhem,res, i.e., γhem,resCx45 should be ≈4 pS.
We studied gj–Vj dependence in 14 Cx45/Cx43-EGFP cell pairs in which gj ranged between 0.7 and 23 nS. The gj vs. Vj plot normalized to gj at Vj = 0 shows marked asymmetry (Fig. 3C). Gj decreases sharply with Vj values relatively positive on the Cx43-EGFP side followed by a small secondary increase at Vj values >40 mV. For the opposite polarity of Vj, Gj increases and decreases again at larger Vj values. Considering that Cx43-EGFP hemichannels only possess the slow Vj gate, the reduction in Gj on application of large negative Vj values, Vj positive to −40 mV (open circles in Fig. 3C) is attributable to closure of the slow gate in Cx43-EGFP hemichannels. The remaining conductance changes (indicated by dotted circles in Fig. 3C) are thus attributable to fast and slow gating of Cx45 hemichannels. We modeled the conductance changes by considering two series gates in Cx45 hemichannels that are in series with open Cx43-EGFP hemichannels. Assuming that γhem,open = ≈2γopen, Cx45 and Cx43-EGFP hemichannels were assigned conductances of 64 pS and 220 pS, respectively. A conductance of 4 pS was assigned to a channel with a closed Cx45 fast gate, and zero conductance was assigned to a channel with a closed Cx45 slow gate. The resulting state diagram consists of a circular reaction scheme containing four states: both fast and slow Cx45 gates open, only the Cx45 fast gate closed, only the Cx45 slow gate closed, and both fast and slow Cx45 gates closed; the slow gate of the Cx43-EGFP hemichannel was assumed to always remain open. In contingent gating, the gates interact such that the state of one gate alters the open probability of the other gate by changing the Vj it senses.
A fit of the data from Cx45/Cx43-EGFP junctions by the contingent gating model suggests that the slow gate tends to close at smaller Vj values than the fast gate; that a substantial fraction of slow gates are closed at Vj = 0; and that most fast gates are open at Vj = 0 (see Fig. 6 in Discussion). As Vj is increased, Gj decreases to near zero as slow gates tend to close channels completely. When Vj is in a range that closes fast gates, their closure reduces Vj at the slow gates, which tends to open them, giving rise to the secondary increase in Gj. On the basis of the asymmetry of gj–Vj dependence shown in Fig. 3C and gating asymmetry at the single channel level (see Fig. 4), we conclude that both fast and slow gates of Cx45 have negative gating polarities, i.e., close in response to Vj negative at their cytoplasmic ends. Our studies indicate that the slow gates in Cx45 hemichannels are largely responsible for closure of Cx45/Cx43-EGFP channels at Vj = near zero. Thus, we conclude that increase of Gj at negative Vj values is due to the slow gates opening in Cx45 hemichannels and the Gj decrease at higher negative Vj values is due to the slow gates closing in Cx43-EGFP hemichannels.
Figure 4.
Asymmetric Vj gating in heterotypic Cx43/Cx43-EGFP junctions at the single-channel level. (A) Example of opening of Cx45/Cx43-EGFP channels during a Vj step of −60 mV applied to the Cx43-EGFP cell. Opening and closing transitions were between open and closed states. During a subsequent Vj step of +60-mV channels closed completely at short latency. (B) During negative Vj steps, the single channel gated between the open state with a conductance of ≈55 pS and the fully closed state. During positive pulses, the channel gated to the residual state (first pulse) or to the closed state (second and third pulses). (C) Ramps at 1 Hz from +100 to −100 mV show closure from the open state (solid lines) to the residual or to the closed state. As Vj became less positive, the channel opened and remained open during the negative part of the ramp.
Vj Gating of Single Cx45/Cx43-EGFP Channels.
We studied single-channel conductance of Cx45/Cx43-EGFP heterotypic junctions in five weakly coupled cell pairs and in seven more strongly coupled cell pairs during recovery from uncoupling induced by CO2. Fig. 4A shows Ij and Vj records in a cell pair with at least four functional channels. A Vj step negative with respect to the Cx43-EGFP cell showed single-channel activity at the start of the pulse with gating transitions of ≈55 pS between open and fully closed states and then full opening of three additional channels. The amplitude of the gating transitions was consistent with the prediction that γopen of heterotypic channels results from the two hemichannels in series, i.e., γCx45/Cx43-EGFP,open = 1/(1/230 + 1/64) = ≈50 pS. An oppositely oriented Vj step closed the channels so fast that it was difficult to distinguish their numbers and closing times. These data demonstrate that at Vj = 0, a substantial fraction (three fourths in this case) of Cx45/Cx43-EGFP channels were closed. We conclude that it is the slow gate of Cx45 that closes the channels, because gating transitions were between open and fully closed states, and Cx43-EGFP hemichannels have a high open probability at Vj = 0 (9). In the record in Fig. 4B, apparently one functional channel was open at Vj = 0 when the negative Vj steps were applied on the Cx43-EGFP side, and gating transitions were between open and closed states. In response to positive Vj steps, the channel closed to a residual state (first pulse, dashed line) or flickered with unresolved degrees of closure and then closed completely (second and third pulses, solid line). Similar results were obtained by using a voltage ramp protocol (Fig. 4C). For a ramp beginning at Vj = 100 mV, positive on the Cx43-EGFP side, the single active channel quickly closed to the residual and then to the closed state. As Vj became less positive and then negative the channel opened and typically remained open for the remainder of the ramp.
Asymmetry of Signal Transfer in Heterotypic Cx45/Cx43-EGFP Junctions.
In the next series of experiments, we examined if asymmetry of the gj–Vj relation of Cx45/Cx43-EGFP junctions could give rise to directional asymmetry in cell–cell coupling. In seven Cx45/Cx43-EGFP cell pairs, we examined electrical cell–cell coupling by voltage clamping one cell and current clamping the other. Fig. 5 A and B illustrate the experiment in which the potential in the Cx43-EGFP cell (V1) was clamped to repeated (3 Hz) positive or negative electrical pulses 30 ms in duration and 100 mV in amplitude from a holding potential (Vh1) of 0 mV. The amplitude of the electrotonic response in the Cx45 cell (V2) was much greater for negative than for positive pulses. Because gj is sensitive only to Vj, negative potentials in the Cx43-EGFP cell are equivalent to positive potentials in the Cx45 cell, and transmission of depolarization would be greater from the Cx45 cell to the Cx43-EGFP cell than in the opposite direction. Before the record in Fig. 5A, the holding current in the Cx45 cell was set to keep its holding potential, Vh2, at about +5 mV. During application of −100 mV pulses to cell 1, the potential in cell 2, V2, reached about −60 mV and decayed back to near that between pulses; the coupling coefficient for the pulses, k1–2,− = V2/V1, was ≈0.6. Because k1-2 is given by gj/(gj + g2), where g2 is the conductance of cell 2, gj was about twice g2. When positive pulses were applied in cell 1, the initial response in cell 2 was about 20 mV, and later responses became smaller over several seconds to reach a steady-state peak-to-trough value of ≈10 mV superimposed on a steady potential of ≈8 mV resulting in k1–2,+ = ≈0.1 for peak to trough and ≈0.18 from peak to baseline. The slower decay from the peak to a nonzero level of depolarization was caused by an increase in the coupling time constant, because relative negativity on the Cx45 side decreased gj. The ratio of coupling coefficients for positive and negative Vj, k1–2,+/k1–2,−, which we defined as the electrical coupling asymmetry coefficient (Kasym), for peak to trough in V2 was ≈0.15. When the holding current in cell 2 was reversed (after ≈20 s; in Fig. 5A), so that Vh2 in the steady state was about −8 mV, the first response in cell 2 was ≈20 mV and the following responses increased over the next several seconds so that the peak was ≈50 mV superimposed on the steady voltage. When the V1 pulses were reversed to +100 mV, the peak-to-trough value of V2 was rapidly decreased to about ≈3 mV, so that Kasym = 0.05. These data demonstrate that the difference between holding potentials of two cells, ΔVh = Vh1 − Vh2, strongly affects asymmetry of electrical cell–cell coupling (coupling asymmetry is lower for smaller V1 pulses). When the Cx45 cell was voltage clamped and pulsed and the Cx43-EGFP cell was current clamped, coupling was greater for positive pulses than for negative (Kasym was defined as k1–2,−/k1–2,+; not illustrated). Fig. 5C shows summarized data illustrating Kasym dependence on ΔVh measured in seven Cx45/Cx43-EGFP cell pairs when either the Cx43-EGFP cell (solid circles) or the Cx45 cell (open circles) was pulsed. ΔVh strongly affected electrical coupling asymmetry. Kasym increased to near 1 (almost no asymmetry) when the Cx45 cell was made more positive and decreased to near 0 (maximal asymmetry) when the Cx45 cell was made more negative.
Figure 5.
Asymmetry of electrical coupling mediated by heterotypic Cx45/Cx43-EGFP junctions. (A) Asymmetry of electrical coupling in a Cx45/Cx43-EGFP cell pair. The Cx43-EGFP cell (V1) was voltage clamped to positive or negative 100 mV, 30-ms rectangular pulses at 3 Hz from a holding potential of 0 mV. The Cx45 cell (V2) was current clamped, and the steady-state holding potential was varied from ≈5 mV (from 0 to 18 s) to about −8 mV (from 18 to 62 s). Coupling was much greater for the negative pulses, and coupling asymmetry increased by making the Cx45 side more negative. (B) Schematics of cell pair; pulses were applied to HelaCx43-EGFP cell. (C) Pooled data of the electrical coupling asymmetry coefficient, Kasym, vs. ΔVjh measured in seven Cx45/Cx43-EGFP cell pairs. Filled and open circles correspond to the experiments in which the Cx43-EGFP or Cx45 cell was voltage clamped and pulsed, respectively. Solid line shows data fit to sigmoid function, Kasym = A/{1 + exp[b(ΔVjh − ΔVjho)]}, with parameters A = 0.9338, b = 0.24 mV−1, ΔVjho = −6.4 mV.
Discussion
Voltage-gating properties of Cx45 junctions have been examined in transfected cell lines and Xenopus oocytes, and these junctions have been shown to be one of the more sensitive to Vj and also to have sensitivity to Vm (16, 18, 19). Until recently, Vj sensitivity in all connexin channels was assumed to occur by a single mechanism. Complex kinetics of changes in gj in response to Vj and the observation that Vj closes some GJ channels completely suggested that there is more than one Vj-dependent gating mechanism (20–22). In correlating macroscopic and single-channel studies of Cx43 channels, we demonstrated that the distinct fast and slow kinetic components evident in macroscopic recordings segregated with transitions to γres and γclosed (9). Mutational studies demonstrated that the fast mechanism is governed by charged residues in the N-terminal domain (13, 23, 24). Moreover, C-terminal modifications of Cx43 including deletion (19) or attachment of aequorin or EGFP (25, 26), selectively abolish the fast gating component and transitions to γres (26). In summary, the existing data indicate that fast and slow gates are molecularly distinguishable and may well be common to all connexins.
Here we demonstrated that Cx45 channels possess both gating mechanisms and that the slow Vj gating mechanism is more sensitive to Vj than in Cx43 channels. In Cx43 junctions neither mechanism of Vj gating would have much influence on cell–cell coupling, except at large Vj values (> ≈60 mV). In Cx45 junctions, small Vj values can cause substantial changes in cell–cell coupling, and slow Vj gating makes a major contribution to these changes. Heterotypic Cx45/Cx43-EGFP junctions exhibit asymmetric Vj gating similar to that reported for Cx45/Cx43 junctions (18, 19). Only complete closures underlie the weak Vj dependence at large negative Vj values on the Cx43-EGFP side in Cx45/Cx43-EGFP junctions, consistent with closure of the slow Vj gate in Cx43-EGFP hemichannels on relative negativity. Thus, we could deduce that both the fast and slow Vj gates of Cx45 close on relative negativity on the Cx45 side. Complete opening and closings underlie the Vj dependence about Vj = 0 mV indicating that closure of the slow gate of Cx45 is responsible for the submaximal conductance at Vj = 0 mV. Opening of the slow gate of Cx45 at modest Vj values negative on the Cx43-EGFP side gives an increase in gj that peaks and declines at larger Vj values as the slow gate of Cx43-EGFP begins to close.
Because of the substantial fraction of channels closed by the slow gate of Cx45 at Vj = 0 mV, one might have predicted that in Cx45/Cx45 junctions, a still higher fraction of channels would be closed by this gate in response to Vj leaving little or no residual conductance (gmin). Nonetheless, we and others observed a residual conductance at large Vj values. At the single-channel level, we found γopen and γres of homotypic Cx45 channels to be ≈32 pS and ≈4 pS, respectively, in agreement with ref. 19, and in the absence of a slow gate, gmin should approximate the ratio γres/γopen = 4/32 = 0.13. However, at Vj = ≈60 mV, gj decreased to a value lower than predicted and then increased with increasing Vj up to about ±120 mV. The conductance increase at high Vj can be explained by having in Cx45 hemichannels fast and slow gates in series as described for Cx43 hemichannels (9). Macroscopic and single-channel data indicate that the slow gates of Cx45 hemichannels dominate at small Vj values. Higher Vj values should activate the fast gates, which have faster kinetics. This mechanism would tend to close preferentially channels to γres resulting in a large fraction of the Vj gradient to drop across the fast gate and near collapse of the Vj gradient at the sensor for the slow Vj gate. Because Cx43-EGFP hemichannels only contain the slow gate, which is weakly sensitive to Vj, we reasoned that Cx45/Cx43-EGFP channels provide a Vj range in which gating of Cx45 hemichannels can be examined in the absence of gating in the partner hemichannel. With data from Cx45/Cx43-EGFP junctions throughout the Vj range of −30 to −120 mV, a fit of the data by a contingent gating model showed that the presence of a residual conductance can be explained by interacting fast and slow gates (gray line in Fig. 3C). The fitted Boltzmann relations for each of the gates, if they acted alone, are plotted in Fig. 6A and show that the slow gate is more sensitive to Vj than the fast gate with a Po at Vj = 0 of ≈0.7. However, the fast gate has a steeper dependence on Vj. In contingent gating, Po of the slow gate decreases with Vj and then increases, because Vj is large enough to close the fast gate (Fig. 6B). This interaction produces a substantial fraction of channels residing in γres at larger Vj values. A plot of the predicted conductance normalized to that at Vj = 0 mV shows that the conductance changes at smaller Vj values closely follow Po of the slow gate. At higher Vj, conductance deviates from Po of the slow gate as fast gates close. Although the data can be explained by having two distinct gates that interact through their effects on the electric field inside the channel, we cannot rule out interactions that may occur through an allosteric mechanism or through sharing some molecular components.
A series arrangement of gap junction hemichannels, which can give rise to contingent gating, can also lead to differences in Vj sensitivity of the hemichannels in heterotypic channels as compared to that in homotypic channels. Consider a heterotypic channel formed of two series hemichannels with different open state conductances but with the same sensitivity to the voltage across them as they have in the homotypic channels. For Vj values where the open probability of the higher conductance hemichannels is large, the Vj sensitivity of the lower conductance hemichannel will be increased, because a larger fraction of Vj will appear across it. Conversely, when the lower conductance hemichannel is open, the Vj sensitivity of the higher conductance channel will be decreased. These effects are illustrated in Fig. 6E for a hypothetical heterotypic junction. The solid and dashed lines are Gj vs. Vj curves generated by a contingent gating model in which a single gate is assigned to each hemichannel. The gates on either side differ in Vj sensitivity giving rise to asymmetry in the Gj–Vj relation; the parameters used were those describing Vj dependence of Cx45 and Cx43-EGFP homotypic junctions. The dashed line (Fig. 6E) is generated when both hemichannels have identical conductances, illustrated schematically as hemichannels having the same pore diameter (Fig. 6C). With hemichannel conductances differing in the same ratio as those of Cx45 and Cx43-EGFP, but with the same sensitivites to the voltage developed across each hemichannel (Fig. 6D), Vj dependence in the hemichannel with the larger conductance is reduced, because a greater fraction of Vj drops across the smaller conductance hemichannel (solid line, Fig. 6E). Conversely, Vj dependence in the hemichannel with the smaller conductance is increased. At Vj = 0, series conductance has no effect on gating, and the open probabilities are the same in each case.
Elenes et al. (19) did not observe fast gating of the Cx43 hemichannel in Cx43/Cx45 junctions and concluded that docking of Cx43 with Cx45 blocks fast Vj gating in the Cx43 hemichannels. However, because the conductance of the Cx45 hemichannel is considerably smaller than that of the Cx43 hemichannel, ≈75% of Vj should act on the Cx45 hemichannel and ≈25% on the Cx43 hemichannel. The Vj sensitivity of the Cx43 hemichannel would be reduced relative to that in Cx43/Cx43 junctions, which could be interpreted as loss of fast Vj gating on the Cx43 side.
Cx45/Cx43-EGFP heterotypic junctions demonstrate marked asymmetry of electrical coupling for large values of Vj, largely because of the slow gating mechanism of Cx45 (Fig. 5). Moreover, the slow gate can operate relatively rapidly in response to Vj values comparable to amplitudes of action potentials (<0.1 s; see Figs. 3B and 4 A and B). Small variations of ΔVh of ±10 mV around zero very effectively modulate the degree of asymmetry, and at ΔVjh = ≈5–15 mV positive on the Cx43-EGFP side, electrical coupling is almost unidirectional for repeated signals comparable in amplitude to action potentials. Thus, a synapse with Cx45 on the presynaptic side and Cx43 on the postsynaptic side could be highly rectifying. We also observed markedly asymmetric electrical signaling in Cx45/Cx47 heterotypic junctions (data not shown) and in Cx32/37 junctions (F.F.B. and R. Weingart, unpublished data). Transmission at synapses using this mechanism would differ from known electrical synapses that are involved in escape systems, where the rectification is very rapid (<0.1 ms; refs. 27–29), and presumably is caused by single-channel rectification as seen in Cx26/Cx32 junctions (30, 31) and Cx46 hemichannels (8). It is interesting that the postsynaptic neuron may be at a more positive potential resulting in ΔVjh of ≈10 mV (32, 33).
In summary, asymmetric electrical signaling can arise in heterotypic junctions that demonstrate marked asymmetry in Vj gating, which can be highly increased if the hemichannel with higher Vj sensitivity has a smaller unitary conductance than the partner hemichannel. Cx45/Cx43 junctions are possible between oligodendrocytes and astrocytes (34), between Schwann cells and fibroblasts (which show asymmetric coupling like that in Fig. 3) (35), and in the conductive system of the heart and atrial and ventricular myocardium (36). Cardiac action potentials are long enough to alter coupling at the asymmetric junctions and may play a role in coupling asymmetry between cells of conductive and working myocardium. Astrocytes can be markedly depolarized by increases in extracellular potassium during electrical activity in the central nervous system. If oligodendrocytes were not depolarized to the same extent, their junctions with astrocytes would become lower in conductance and permeability. It appears that small changes in ΔVjh can be highly effective in regulation of electrical and metabolic communication between different cells and in different organ systems.
Acknowledgments
We thank Dr. D. W. Laird for kindly providing us with the Cx43-EGFP construct. This study was supported by National Institutes of Health Grants NS36706 (to F.F.B.) and GM54179 (to V.K.V.). M.V.L.B is the Sylvia and Robert S. Olnick Professor of Neuroscience and is supported in part by the F. M. Kirby Foundation.
Abbreviations
- Cx
connexin
- EGFP
enhanced green fluorescent protein
- GJ
gap junction
References
- 1.Bennett M V L, Barrio L C, Bargiello T A, Spray D C, Hertzberg E, Saez J C. Neuron. 1991;6:305–320. doi: 10.1016/0896-6273(91)90241-q. [DOI] [PubMed] [Google Scholar]
- 2.Elfgang C, Eckert R, Lichtenberg-Frate H, Butterweck A, Traub O, Klein R A, Hulser D F, Willecke K. J Cell Biol. 1995;129:805–817. doi: 10.1083/jcb.129.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris A L, Spray D C, Bennett M V L. J Gen Physiol. 1981;77:95–117. doi: 10.1085/jgp.77.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bukauskas F F, Weingart R. Pflügers Arch. 1993;423:152–154. doi: 10.1007/BF00374973. [DOI] [PubMed] [Google Scholar]
- 5.Weingart R, Bukauskas F F. Pflügers Arch. 1993;424:192–194. doi: 10.1007/BF00374611. [DOI] [PubMed] [Google Scholar]
- 6.Bukauskas F F, Weingart R. Biophys J. 1994;67:613–625. doi: 10.1016/S0006-3495(94)80521-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moreno A P, Rook M B, Fishman G I, Spray D C. Biophys J. 1994;67:113–119. doi: 10.1016/S0006-3495(94)80460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trexler E B, Bennett M V L, Bargiello T A, Verselis V K. Proc Natl Acad Sci USA. 1996;93:5836–5841. doi: 10.1073/pnas.93.12.5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bukauskas F F, Bukauskiene A, Bennett M V L, Verselis V K. Biophys J. 2001;81:137–152. doi: 10.1016/S0006-3495(01)75687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bukauskas F F, Elfgang C, Willecke K, Weingart R. Biophys J. 1995;68:2289–2298. doi: 10.1016/S0006-3495(95)80411-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valiunas V, Manthey D, Vogel R, Willecke K, Weingart R. J Physiol (London) 1999;519:631–644. doi: 10.1111/j.1469-7793.1999.0631n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manthey D, Bukauskas F, Lee C G, Kozak C A, Willecke K. J Biol Chem. 1999;274:14716–14723. doi: 10.1074/jbc.274.21.14716. [DOI] [PubMed] [Google Scholar]
- 13.Oh S, Abrams C K, Verselis V K, Bargiello T A. J Gen Physiol. 2000;116:13–31. doi: 10.1085/jgp.116.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anumonwo J M, Taffet S M, Gu H, Chanson M, Moreno A P, Delmar M. Circ Res. 2001;88:666–673. doi: 10.1161/hh0701.088833. [DOI] [PubMed] [Google Scholar]
- 15.Jordan K, Solan J L, Dominguez M, Sia M, Hand A, Lampe P D, Laird D W. Mol Biol Cell. 1999;10:2033–2050. doi: 10.1091/mbc.10.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barrio L C, Capel J, Jarillo J A, Castro C, Revilla A. Biophys J. 1997;73:757–769. doi: 10.1016/S0006-3495(97)78108-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vogel R, Weingart R. J Physiol. 1998;510:177–189. doi: 10.1111/j.1469-7793.1998.177bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steiner E, Ebihara L. J Membr Biol. 1996;150:153–161. doi: 10.1007/s002329900040. [DOI] [PubMed] [Google Scholar]
- 19.Elenes S, Martinez A D, Delmar M, Beyer E C, Moreno A P. Biophys J. 2001;81:1406–1418. doi: 10.1016/S0006-3495(01)75796-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bukauskas F F, Peracchia C. Biophys J. 1997;72:2137–2142. doi: 10.1016/S0006-3495(97)78856-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Revilla A, Castro C, Barrio L C. Biophys J. 1999;77:1374–1383. doi: 10.1016/S0006-3495(99)76986-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banach K, Weingart R. Pflügers Arch. 2000;439:248–250. doi: 10.1007/s004249900182. [DOI] [PubMed] [Google Scholar]
- 23.Verselis V K, Ginter C S, Bargiello T A. Nature (London) 1994;368:348–351. doi: 10.1038/368348a0. [DOI] [PubMed] [Google Scholar]
- 24.Ri Y, Ballesteros J A, Abrams C K, Oh S, Verselis V K, Weinstein H, Bargiello T A. Biophys J. 1999;76:2887–2898. doi: 10.1016/S0006-3495(99)77444-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin P E, George C H, Castro C, Kendall J M, Capel J, Campbell A K, Revilla A, Barrio L C, Evans W H. J Biol Chem. 1998;273:1719–1726. doi: 10.1074/jbc.273.3.1719. [DOI] [PubMed] [Google Scholar]
- 26.Bukauskas F F, Jordan K, Bukauskiene A, Bennett M V L, Lampe P D, Laird D W, Verselis V K. Proc Natl Acad Sci USA. 2000;97:2556–2561. doi: 10.1073/pnas.050588497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furshpan E J, Potter D D. J Physiol (London) 1959;145:289–325. doi: 10.1113/jphysiol.1959.sp006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Auerbach A A, Bennett M V L. J Gen Physiol. 1969;53:211–237. doi: 10.1085/jgp.53.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaslove S W, Brink P R. Nature (London) 1986;323:63–65. doi: 10.1038/323063a0. [DOI] [PubMed] [Google Scholar]
- 30.Bukauskas F F, Elfgang C, Willecke K, Weingart R. Pflügers Arch. 1995;429:870–872. doi: 10.1007/BF00374812. [DOI] [PubMed] [Google Scholar]
- 31.Oh S, Rubin J B, Bennett M V L, Verselis V K, Bargiello T A. J Gen Physiol. 1999;114:339–364. doi: 10.1085/jgp.114.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giaume C, Kado R T, Korn H. J Physiol (London) 1987;386:91–112. doi: 10.1113/jphysiol.1987.sp016524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pereda A E, Bell T D, Faber D S. J Neurosci. 1995;15:5943–5955. doi: 10.1523/JNEUROSCI.15-09-05943.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rash J E, Yasumura T, Dudek F E, Nagy J I. J Neurosci. 2001;21:1983–2000. doi: 10.1523/JNEUROSCI.21-06-01983.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bukauskas F, Shrager P, Peracchia C. Gating Properties of Gap Junction Channels in Schwann Cells and Fibroblasts Isolated from the Sciatic Nerve of Neonatal Rats. Amsterdam: IOS; 1998. [Google Scholar]
- 36.van Rijen H V, van Veen T A, van Kempen M J, Wilms-Schopman F J, Potse M, Krueger O, Willecke K, Opthof T, Jongsma H J, de Bakker J M. Circulation. 2001;103:1591–1598. doi: 10.1161/01.cir.103.11.1591. [DOI] [PubMed] [Google Scholar]