Abstract
Studies were conducted to evaluate the effects of s.c. injected recombinant human growth hormone (GH) on the expression of the gene transcript of N-methyl-D-aspartate receptor subunits type 1 (NR1), type 2A (NR2A), and type 2B (NR2B) in the male rat hippocampus. The GH-induced effects on the expression of hippocampal gene transcripts of GH receptor (GHR) and GH-binding protein were also examined. Male Sprague–Dawley rats, kept in four groups of two different ages, was treated with the hormone or saline during 10 days before decapitation and tissue dissection. Brain tissues collected were analyzed for mRNA content by using the Northern blot technique. The results indicated that in adult young rats (11 weeks of age) the hormone elicited a decrease in the mRNA expression of NR1 but an increase in that of the NR2B subunit. In elderly adult rats (57–67 weeks of age) GH induced an increase in the expression of the hippocampal message for NR1 and NR2A. Meanwhile, the hormone induced a significant up-regulation of the GHR transcript in hippocampus of adult young rats but not in elderly adult rats. It was further found that a significant positive correlation exists between the level of GHR mRNA and the expression of the NR2B subunit transcript in adult young rats. The GH-induced increase in the expression of hippocampal mRNA for the NR2B subunit is compatible with a previously observed memory promoting effect seen for the hormone, because overexpression of this N-methyl-D-aspartate receptor subunit is shown to enhance cognitive capabilities.
Recent studies have shown that growth hormone (GH) may affect many functions related to the central nervous system. GH replacement therapy has improved psychological capabilities in young as well as adult GH-deficient (GHD) patients (1, 2). Young GHD patients suffer from sleep disturbances and are psychologically immature (3). Untreated adult GHD patients exhibit general fatigue, lack of concentration, memory disabilities, and overall, a diminished subjective well being (4–6). Treatment of adult GHD patients with recombinant human GH is reported to induce increased psychological well being (1, 2, 5, 7, 8) and to improve cognitive efficiency and memory function (7–9). GH is also shown to affect learning processes and memory function in rats. The hormone was found to facilitate the long-term memory in young but not in aged rats, whereas the extinction response as recorded in a behavioral assay was affected in both groups of animal (10). Recently, we observed dose-dependent improvements in memory and learning processes in hypophysectomized male rats after 1 week of daily injections of recombinant human GH (11).
The physiological and molecular mechanisms behind the improvements seen in cognitive functions after GH treatment are not known, but they could be due to direct effects by the hormone on cells or by GH-induced release of secondary mediators, such as insulin-like growth factor-1 (IGF-1), that can cross the blood–brain barrier. The possibility that the hormone itself may pass the blood–brain barrier is supported by studies showing increased GH concentrations in cerebrospinal fluid in patients subjected to s.c. injections of GH (12, 13). In one of these studies a dose-dependent increase in cerebrospinal fluid GH concentrations was confirmed (13). Further evidence for brain cells being targets for GH actions has emerged from the identification of specific receptors for the hormone in the brain (14, 15). Because hippocampus is a brain region associated with the functional anatomy of memory and learning processes, it is of particular interest that a hippocampal GH-binding receptor has been identified and characterized in both humans (16) and rats (17). In a recent study we also described the nucleotide sequence of the cDNA for the GH receptor (GHR) in rat hippocampus (18).
Regarding the molecular mechanism underlying a possible action mediated through GHR on memory function, the present knowledge is poor. Studies have suggested a potential role of the N-methyl-D-aspartate (NMDA) receptor in learning and memory processes (19, 20). The NMDA receptor complex consists of several types of subunits, including type 1 (NR1), type 2A (NR2A), and type 2B (NR2B) (21, 22). A recent study reported that overexpression of the NR2B subunit in the forebrain (including hippocampus) of transgenic mice elicits an enhanced activation of the NMDA receptor, coinciding with improved learning and memory capabilities (23)
In the present study we therefore have examined whether GH, by daily s.c. injection for 10 days, may affect the expression of the gene transcripts for the NMDA receptor subunits NR1, NR2A, and NR2B in hippocampus of male rats in two groups of different ages. The effect on the transcript for GHR and GH-binding protein (GHBP) hormone was analyzed as well.
Materials and Methods
Animal Experiments.
Male Sprague–Dawley rats (Alab AB, Sollentuna, Sweden) were housed in four groups in air-conditioned rooms (lights on 0800–2000 h) at a temperature of 21°C and a humidity of 50–60%. Two of the groups consisted of animals of 11 weeks and the other two were of 57–67 weeks of age. They had free access to food and water, and each group consisted of seven animals. Before experiment the rats were allowed to adapt to the laboratory environment for 1 week. One group of each age received daily s.c. injections of human GH (Genotropin, Amersham Pharmacia) (1 mg/kg) or saline for 10 days. The dose was based from previous experience ensuring an effect on bone and body growth. In this study both groups of rats receiving GH exhibited a significant increase in body weight compared with controls. After completed hormone treatment animals were decapitated and the hippocampus was dissected out on ice, frozen on dry ice, and stored at −70°C until further analyzed.
Northern Blot Analysis.
The Northern blot procedure has been described elsewhere (18, 24). In brief, total RNA was extracted by using the single-step acid guanidium thiocyanate-phenol-chloroform method and separated on agarose 3-(N-morpholino)- propanesulfonic acid/formaldehyde gels. The RNA was transferred to nylon filters and hybridized against cDNA probes for GHR and the three NMDA receptor subunits, NR1, NR2A, and NR2B. After stringent washing the filters were subjected to autoradiography and the hybridization signals were measured by use of an image-analyzing system and normalized to that of glyceraldehyde-3-phosphate dehydrogenase mRNA, detected with a Pst fragment of a human glyceraldehyde-3-phosphate dehydrogenase cDNA.
Statistics.
The data are expressed as mean ± SE of seven rats at each point. Unpaired Student's t test was used for the statistical analysis of differences between groups. The correlation was tested by simple regression analysis. Significance was set at P < 0.05.
Results
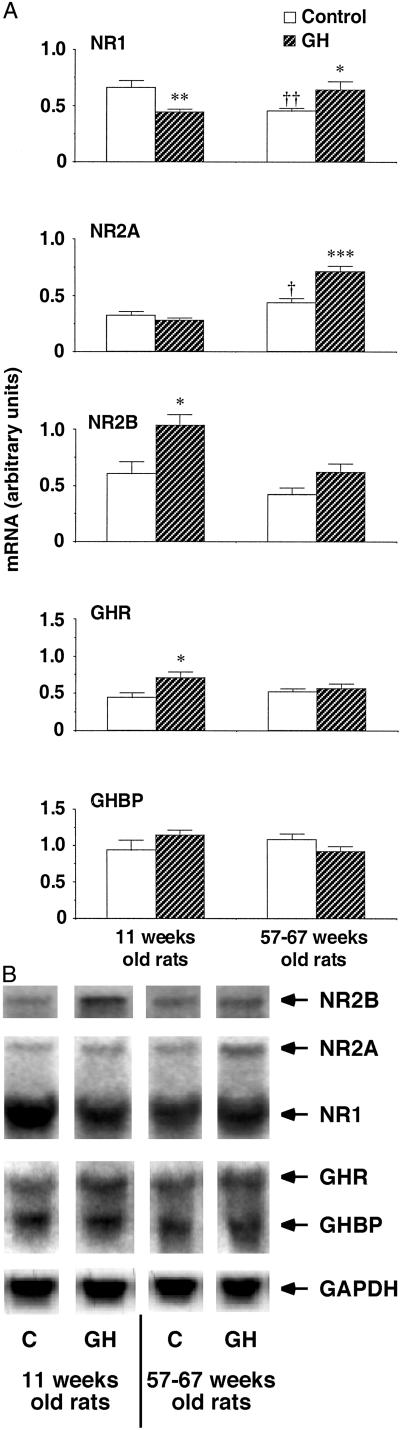
Northern blot analysis of material extracted from hippocampus (Fig. 1) revealed that the NR1 transcript was significantly lower in elderly adult rats (57–67 weeks) compared with the group of younger adult animals (11 weeks) (P < 0.05). Also NR2B mRNA tended to decrease with age, although this difference never reached significance (P = 0.22). On the contrary, NR2A gene expression was increased with age (P < 0.05).
Figure 1.
(A) Effects of daily s.c. injections of saline (n = 7 for each control group) or human GH (hGH) (1 mg/kg, n = 7 for each hGH-treated group) on NMDA receptor subunit, GHR and GHBP mRNAs in the adult rat hippocampus. *, P < 0 05; **, P <0.01; ***, P < 0.001 compared with the age-corresponding control group; †, P < 0.05, ‡‡, P < 0.01 compared with the 11-week-old control animals (unpaired Student's t test). (B) Representative Northern blot analysis of NMDA subunits, GHR, and GHBP mRNAs. Each well was loaded with 20 μg of total RNA. For correction of interlane variability in the amount of total RNA, the hybridization signals were normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA.
Daily treatment of GH for 10 days induces effects on the gene transcripts of NMDA receptor subunits in both young and elderly adult rats (Fig. 1). In young rats the hormone elicited a decrease in the NR1 subunit mRNA (P < 0.01) in contrast to an increase seen for the transcript of the NR2B subunit (P < 0.05). The expression of the NR2A mRNA was not affected in young adult rats. In elderly adult rats the hormone induced a significant enhancement in the expression of the gene transcript for NR1 and NR2A (P < 0.05 and P < 0.001, respectively), whereas the NR2B mRNA showed a tendency of an increase, close to significant level (P = 0.068).
The results obtained from analysis of the effects of GH on the expression of the gene transcripts for GHR and GHBP in hippocampus are also shown in Fig. 1. The levels of these two transcripts were not altered by the difference in age of the rats used in this study. In young adult rats the hormone induced a significant up-regulation of GHR mRNA (P < 0.05), whereas no effect was found for GHBP. In elderly adult rats no effect of GH was observed on the expression of mRNA for neither GHR nor GHBP.
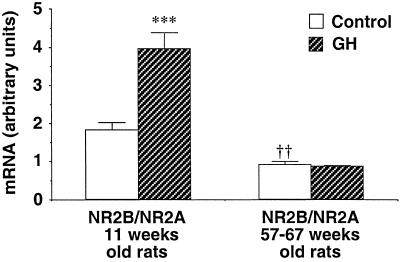
In young rats the hormone also affected the ratio of NR2B to NR2A mRNA. A significant increase appeared in the ratio in young GH-treated animals compared with that in controls (P < 0.001), whereas no difference occurred in the ratio between the two groups of elderly rats (Fig. 2). Furthermore, this ratio was clearly decreased in the control group of elderly adult rats compared with that of the younger animals (P < 0.01).
Figure 2.
The ratio of NR2B to NR2A mRNA in hippocampus of adult rats of two different ages. The animals received daily injections of saline (n = 7 for each control group) or hGH (1 mg/kg, n = 7 for each hGH-treated group). ***, P < 0.001 and †††, P < 0.001 compared with the control group of the 11-week-old rats (unpaired Student's t test).
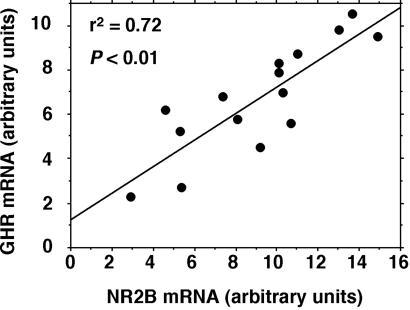
By comparing the expression of the gene transcripts for GHR and GHBP with those of the NMDA receptor subunits, a significant positive correlation was observed in young rats only, between the level of GHR mRNA and the level of the transcript for the NR2B subunit (r2 = 0.61, P < 0.01) (Fig. 3). No significant correlation could be seen between the expression level of mRNA for GHR, GHBP, and any other NMDA receptor subunits at any age of the animals.
Figure 3.
Simple-regression analysis of the correlation between the NR2B and GHR mRNA in the hippocampus of young (11-week-old) adult rats (n = 14) treated with saline or hGH (1 mg/kg).
Discussion
The results of the present study indicate that chronic treatment of adult male rats with recombinant human GH affects the expression of the NMDA receptor subunit gene transcripts in hippocampus. Further, in young adult rats the hormone up-regulates the expression of the gene transcript for GHR. Moreover, a significant positive correlation between the GHR gene transcript and the message for the NR2B receptor subunit was confirmed. Accordingly, this study indicates a significant correlation between GH action and excitatory circuits in hippocampus. This result may have several implications connecting previously observed effects of both GH and the NMDA receptor complex to memory function.
It is well established that a decline of cognitive functioning with aging is paralleled with decreased circulating levels of GH. It is further suggested that GH and its secondary mediator IGF-1 contribute to the function of hippocampus (25). A pronounced and specific syndrome in GHD patients is memory deficit, which include both short- and long-term memory (26–28). Also, an age-related decrease in spatial memory have been observed in animals (29). However, as mentioned in the introductory statements, GH is shown to induce promoting effects on memory and learning processes, both in humans and rats. Thus, several studies (not all) suggest that GH replacement therapy improves cognitive performance (8, 9). By use of animal models, GH was shown also to modulate memory function in the rat (10). Moreover, a GH-releasing agent was found to prevent impairments in reference memory, as recorded in the Morris water maze (29).
The present study, demonstrating effects of GH on the expression of hippocampal NMDA receptor subunits mRNAs, suggests that the hormone may mediate its memory-promoting effects through an interaction with the NMDA receptor complex. This receptor has been shown to be important for synaptic plasticity and is suggested to be a common switch for many forms of learning and memory (19, 30). It is shown that the NMDA receptor function is enhanced with overexpression of the NR2B receptor subunit in the hippocampus which was demonstrated by improved learning and memory (23).
We here observed that in young adult male rats GH induced a significant enhancement in the expression of the hippocampal NR2B subunit mRNA. We also detected a significant increase in the ratio of NR2B to NR2A after hormone injection into these rats (Fig. 2). The NR2B/NR2A ratio is seen to decline with aging but was not affected by GH treatment of elderly adult rats. Accordingly, it seems that the hormone elicits an enhancement of the hippocampal NR2B/NR2A ratio in young but not in elderly adult rats, and this observation would be compatible with a GH-induced improvement of cognitive functions in young rats. In fact, GH treatment was shown to facilitate long-term memory in young (3 months) but not in aged (24 months) adult rats (10).
It has been suggested that the regulation of the NR2B/NR2A ratio can be involved in a mechanism modulating the induction of synaptic plasticity (31, 32). Thus, a high ratio of NR2B/NR2A, as shown for young adult rats in the present study, is suggested to correlate with enhanced long-term potentiation (LTP) and reduced long-term depression, whereas a low NR2B/NR2A ratio is considered to correlate with diminished LTP and increased long-term depression. We observed an increase in the expression of the NR2A mRNA in adult elderly control rats. This increase was potentiated by GH injection (Fig. 1), but the NR2B/NR2A ratio remained unaffected. The significance of the observed enhancement in NR2A in elderly rats is not clear; however, an age-related increase in the NR2A subunit protein, at least from 10 to 21 months of age, has been reported by others (33). In the same study, the authors demonstrated that intracerebroventricular infusion of IGF-1, a target for GHR-mediated action, can increase NR2A and NR2B subunit proteins in hippocampus of 30-month-old rats. The effects of GH on the NMDA receptor subunit transcripts seen in our study may also be mediated by IGF-1.
The age-related decrease in the expression of the NR1 gene transcript (Fig. 1) is in agreement with a recent observation based on quantitative Western blot analysis (34). However, only a slight, nonsignificant, age-related decrease was observed for the NR1 message, as assessed by kinetic reverse transcription–PCR technique. The loss in the expression of the NR1 subunit seen in aged rats was suggested to be associated with age-related deficits in LTP. Furthermore, the authors showed that life-long caloric restriction could prevent a decrease in the expression of NR1 and a deficit in LTP. It is well established that moderate caloric restriction increases GH levels in the rat (for review, see ref. 35). In the present work we found that in aged animals GH treatment mimicked the effect of caloric restriction and counteracted or prevented the decrease in NR1 mRNA expression, providing evidence for the hormone being able to ameliorate deficits in LTP in elderly rats. On the contrary, GH induced a significant decrease in the NR1 message in adult young rats. This decrease should be regarded in view of the potential increase seen in the ratio of NR2B/NR2A (Fig. 2), which may be of higher importance for the function of the NMDA receptor complex.
Of particular interest from the present study is the significant positive correlation found between the level of the GHR mRNA and the NR2B gene transcript (Fig. 3). This finding is indicative of a GHR-mediated effect on the synaptic function of the NMDA receptor. Moreover, it is compatible with a GH-induced memory promoting effect by a mechanism involving an up-regulation of the NR2B receptor subunit.
Regarding the mechanism by which GH affects the various gene transcripts of the NMDA receptor, its clear that an agonistic action of the hormone on hippocampal GHR may result in direct effects on brain circuits controlling glutamate transmission and the expression of its receptor complex. However, GH responses in the brain may also be mediated by a coordinated effect of both GH and IGF-1. As mentioned above IGF-1, given by intracerebroventricular infusion, is shown to increase the NR2A and NR2B receptor protein in the hippocampus (33). Also, some defects seen with aging are caused by a coordinated loss of the GH/IGF-1 function (7, 8, 12). The molecular mechanism by which GH signaling through GHR directly or by receptors of IGF-1 indirectly influences the regulation of the mRNAs of the NMDA receptor subunits is still poorly understood.
The GH-induced increase in the expression of the gene transcript for GHR seen in the adult young rats (Fig. 1) reflects an ability of the hormone to reach the brain and stimulate hippocampal cells. Agonist action of GH has shown differential effects on hepatic GHR transcription and GH binding, indicating a complex relationship that seems to depend on several parameters such as age, sex, and hormonal status (36). This item is poorly studied in the central nervous system. Our result shows a GH-induced stimulation of hippocampal GHR transcription in the group of younger animals. The lack of such an effect in the elderly rats indicates a partial resistance to GH in this group. Such age-related resistance to GH could decrease the response to the hormone and further contribute to the poor GH signaling often seen with age. Moreover, GH resistance has been described by a decrease of GH-stimulated IGF-1 gene expression in the liver of aging mice (37) and a decline of the hepatic IGF-1 secretion in humans (38). Few studies have been performed on autoregulation of central GHR transcripts in adult rats. Bennett et al. showed, by using in situ hybridization technique, that 6 days of continuous intracerebroventricular administration of the hormone to intact animals did not affect GHR transcripts in the hippocampus (39). On the other hand, acute peripheral GH treatment (2 h) has been shown to up-regulate GHR and GHBP mRNA in the brainstem, hypothalamus, and cortex of the rat (40).
Studies have described a decreased density of GH-binding sites with aging in the male rat brain (17). In certain human brain tissues both the GHR protein, as assessed by receptor binding, and the GHR mRNA decrease by aging (15, 16). In the present study, we did not observe any significant difference in hippocampal GHR or GHBP mRNA expression between untreated young and elderly adult male Sprague–Dawley rats. This relation on a transcriptional level has to our knowledge not been studied in the central nervous system, although it is in line with that found in the liver and other peripheral tissues of the Wistar rat (41).
As mentioned above, GH-replacement therapy in adult elderly GHD patients improves cognitive capability and memory function (7–9). In this study, focused on male rats, we did not observe any neurochemical evidence for a GH-induced enhancement of cognitive functions in elderly adult individuals. However, in contrast to GHD patients, these animals represent a normal healthy population of their particular age. In GH-deficient elderly male rats (hypophysectomized rats) GH treatment was recently demonstrated to improve memory and learning processes in a dose-dependent fashion (11). In this context it should also be emphasized that the GH effect on the expression of the NMDA receptor subunit gene transcripts may also depend on the given dose.
The human variant of GH, which was used in this study, interacts with lactogenic or prolactin receptors in the rat. However, prolactin binding is found mainly in the female rat brain (42). Therefore, we believe that the hormone-induced effect on the NMDA receptor subunits seen here represents effects mediated through pure GHR sites, which are abundant in male rat hippocampus (17).
Even though the present work aimed to investigate the level of the gene transcripts for the NMDA receptor subunits as well as GHR and GHBP, the obtained result is in good agreement with what has been shown in earlier studies focused on the corresponding receptor proteins. Moreover, the observed GH-induced effects at transcriptional level agree with known function of the NMDA receptor complex regarding age and cognitive capabilities. Thus, the recorded alterations in the various transcripts seem to link previous observations made on the hormonal and transmitter system above in relation to age and memory function in a comprehensive fashion.
In conclusion, this study demonstrates that GH may elicit an increase in the hippocampal gene transcript for GHR and the NMDA receptor subunit NR2B in young but not in elderly adult rats. The ratio of the NR2B over the NR2A message is significantly enhanced in young, but not affected in aged rats. A distinct interaction between GH and the NMDA receptor is confirmed by the positive correlation found to exist between the level of GHR and NR2B mRNA. Because overexpression of the NR2B subunit is shown to be connected with enhanced cognitive performance, it is suggested from the present study that GH may induce its previously demonstrated memory-promoting effects by a mechanism including an enhanced synaptic function of the NMDA receptor.
Acknowledgments
This study was supported by Grant 9459 from the Swedish Medical Research Council.
Abbreviations
- GH
growth hormone
- GHR
growth hormone receptor
- GHBP
growth hormone-binding protein
- GHD
growth hormone deficient
- hGH
human growth hormone
- NMDA
N-methyl-d-aspartate
- NR1
NR2A, and NR2B, NMDA receptor subunit types 1, 2A, and 2B
- IGF-1
insulin-like growth factor-1
- LTP
long-term potentiation
References
- 1.Bengtsson B A, Eden S, Lonn L, Kvist H, Stokland A, Lindstedt G, Bosaeus I, Tolli J, Sjostrom L, Isaksson O G. J Clin Endocrinol Metab. 1993;76:309–317. doi: 10.1210/jcem.76.2.8432773. [DOI] [PubMed] [Google Scholar]
- 2.Burman P, Broman J E, Hetta J, Wiklund I, Erfurth E M, Hagg E, Karlsson F A. J Clin Endocrinol Metab. 1995;80:3585–3590. doi: 10.1210/jcem.80.12.8530603. [DOI] [PubMed] [Google Scholar]
- 3.Hayashi M, Shimohira M, Saisho S, Shimozawa K, Iwakawa Y. Brain Dev. 1992;14:170–174. doi: 10.1016/s0387-7604(12)80259-2. [DOI] [PubMed] [Google Scholar]
- 4.Bjork S, Jonsson B, Westphal O, Levin J E. Acta Paediatr Scand Suppl. 1989;356:55–56. doi: 10.1111/j.1651-2227.1989.tb11242.x. [DOI] [PubMed] [Google Scholar]
- 5.McGauley G A, Cuneo R C, Salomon F, Sonksen P H. Horm Res. 1990;33:52–54. doi: 10.1159/000181584. [DOI] [PubMed] [Google Scholar]
- 6.Rosen T, Wiren L, Wilhelmsen L, Wiklund I, Bengtsson B A. Clin Endocrinol (Oxford) 1994;40:111–116. doi: 10.1111/j.1365-2265.1994.tb02452.x. [DOI] [PubMed] [Google Scholar]
- 7.Burman P, Deijen J B. Psychother Psychosom. 1998;67:154–167. doi: 10.1159/000012276. [DOI] [PubMed] [Google Scholar]
- 8.Gibney J, Wallace J D, Spinks T, Schnorr L, Ranicar A, Cuneo R C, Lockhart S, Burnand K G, Salomon F, Sonksen P H, Russell-Jones D. J Clin Endocrinol Metab. 1999;84:2596–2602. doi: 10.1210/jcem.84.8.5916. [DOI] [PubMed] [Google Scholar]
- 9.Deijen J B, de Boer H, van der Veen E A. Psychoneuroendocrinology. 1998;23:45–55. doi: 10.1016/s0306-4530(97)00092-9. [DOI] [PubMed] [Google Scholar]
- 10.Schneider-Rivas S, Rivas-Arancibia S, Vazquez-Pereyra F, Vazquez-Sandoval R, Borgonio-Perez G. Life Sci. 1995;56:L433–L441. doi: 10.1016/0024-3205(95)00171-2. [DOI] [PubMed] [Google Scholar]
- 11.Le Grevès M, Fhölenhag K, Zhou Q, Berg M, Meyerson B, Nyberg F. Growth Horm IGF Res. 2000;10:130. [Google Scholar]
- 12.Johansson J O, Larson G, Andersson M, Elmgren A, Hynsjo L, Lindahl A, Lundberg P A, Isaksson O G, Lindstedt S, Bengtsson B A. Neuroendocrinology. 1995;61:57–66. doi: 10.1159/000126813. [DOI] [PubMed] [Google Scholar]
- 13.Burman P, Hetta J, Wide L, Mansson J E, Ekman R, Karlsson F A. Clin Endocrinol (Oxford) 1996;44:319–324. doi: 10.1046/j.1365-2265.1996.617439.x. [DOI] [PubMed] [Google Scholar]
- 14.Nyberg F, Burman P. Horm Res. 1996;45:18–22. doi: 10.1159/000184753. [DOI] [PubMed] [Google Scholar]
- 15.Nyberg F. Front Neuroendocrinol. 2000;21:330–348. doi: 10.1006/frne.2000.0200. [DOI] [PubMed] [Google Scholar]
- 16.Lai Z, Roos P, Zhai O, Olsson Y, Fholenhag K, Larsson C, Nyberg F. Brain Res. 1993;621:260–266. doi: 10.1016/0006-8993(93)90114-3. [DOI] [PubMed] [Google Scholar]
- 17.Zhai Q, Lai Z, Roos P, Nyberg F. Acta Paediatr Suppl. 1994;406:92–95. doi: 10.1111/j.1651-2227.1994.tb13433.x. [DOI] [PubMed] [Google Scholar]
- 18.Thornwall-Le Grevès M, Zhou Q, Lagerholm S, Huang W, Le Greves P, Nyberg F. Neurosci Lett. 2001;304:69–72. doi: 10.1016/s0304-3940(01)01757-8. [DOI] [PubMed] [Google Scholar]
- 19.Tsien J Z, Huerta P T, Tonegawa S. Cell. 1996;87:1147–1148. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- 20.Castellano C, Cestari V, Ciamei A. Curr Drug Targets. 2001;2:273–283. doi: 10.2174/1389450013348515. [DOI] [PubMed] [Google Scholar]
- 21.Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg P. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- 22.Nakanishi S. Science. 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- 23.Tang Y P, Shimizu E, Dube G R, Rampon C, Kerchner G A, Zhuo M, Liu G, Tsien J Z. Nature (London) 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- 24.Le Grevès P, Huang W, Johansson P, Thornwall M, Zhou Q, Nyberg F. Neurosci Lett. 1997;226:61–64. doi: 10.1016/s0304-3940(97)00244-9. [DOI] [PubMed] [Google Scholar]
- 25.van Dam P S, Aleman A, de Vries W R, Deijen J B, van der Veen E A, de Haan E H, Koppeschaar H P. Growth Horm IGF Res. 2000;10, Suppl. B:S69–S73. doi: 10.1016/s1096-6374(00)80013-1. [DOI] [PubMed] [Google Scholar]
- 26.Deijen J B, de Boer H, Blok B J, van der Veen E A. Psychoneuroendocrinology. 1996;21:313–322. doi: 10.1016/0306-4530(95)00050-x. [DOI] [PubMed] [Google Scholar]
- 27.Chrisoulidou A, Kousta E, Beshyah S A, Robinson S, Johnston D G. Bailliere's Clin Endocrinol Metab. 1998;12:261–279. doi: 10.1016/s0950-351x(98)80022-0. [DOI] [PubMed] [Google Scholar]
- 28.Aleman A, de Vries W R, de Haan E H, Verhaar H J, Samson M M, Koppeschaar H P. Neuropsychobiology. 2000;41:73–78. doi: 10.1159/000026636. [DOI] [PubMed] [Google Scholar]
- 29.Thornton P L, Ingram R L, Sonntag W E. J Gerontol A Biol Sci Med Sci. 2000;55:B106–B112. doi: 10.1093/gerona/55.2.b106. [DOI] [PubMed] [Google Scholar]
- 30.Tang Y, Wang H, Feng R, Kyin M, Tsien J Z. Neuropharmacology. 2001;41:779–790. doi: 10.1016/s0028-3908(01)00122-8. [DOI] [PubMed] [Google Scholar]
- 31.Quinlan E M, Olstein D H, Bear M F. Proc Natl Acad Sci USA. 1999;96:12876–12880. doi: 10.1073/pnas.96.22.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Philpot B D, Weisberg M P, Ramos M S, Sawtell N B, Tang Y P, Tsien J Z, Bear M F. Neuropharmacology. 2001;41:762–770. doi: 10.1016/s0028-3908(01)00136-8. [DOI] [PubMed] [Google Scholar]
- 33.Sonntag W E, Bennett S A, Khan A S, Thornton P L, Xu X, Ingram R L, Brunso-Bechtold J K. Brain Res Bull. 2000;51:331–338. doi: 10.1016/s0361-9230(99)00259-2. [DOI] [PubMed] [Google Scholar]
- 34.Eckles-Smith K, Clayton D, Bickford P, Browning M D. Brain Res Mol Brain Res. 2000;78:154–162. doi: 10.1016/s0169-328x(00)00088-7. [DOI] [PubMed] [Google Scholar]
- 35.Sonntag W E, Lynch C D, Cefalu W T, Ingram R, L, Bennett S A, Thornton P L, Khan A S. J Gerontol A Biol Sci Med Sci. 1999;54:B521–B538. doi: 10.1093/gerona/54.12.b521. [DOI] [PubMed] [Google Scholar]
- 36.Harvey S, Hull K L. In: Growth Hormone. Harvey S, Scanes C G, Daughaday W H, editors. Boca Raton, FL: CRC; 1995. pp. 303–337. [Google Scholar]
- 37.Xu X, Bennet S A, Ingram R L, Sonntag W E. Endocrinology. 1995;136:4551–4557. doi: 10.1210/endo.136.10.7664676. [DOI] [PubMed] [Google Scholar]
- 38.Lieberman S A, Mitchell A M, Marcus R, Hintz R L, Hoffman A R. Horm Metab Res. 1994;26:229–233. doi: 10.1055/s-2007-1001671. [DOI] [PubMed] [Google Scholar]
- 39.Bennett P A, Levy A, Sophokleous S, Robinson I C, Lightman S L. J Endocrinol. 1995;147:225–234. doi: 10.1677/joe.0.1470225. [DOI] [PubMed] [Google Scholar]
- 40.Hull K L, Harvey S. Growth Horm IGF Res. 1998;8:167–173. doi: 10.1016/s1096-6374(98)80107-x. [DOI] [PubMed] [Google Scholar]
- 41.Velasco B, Cacicedo L, Melian E, Fernandez-Vazquez G, Sanchez-Franco F. Eur J Endocrinol. 2001;145:73–85. doi: 10.1530/eje.0.1450073. [DOI] [PubMed] [Google Scholar]
- 42.Mustafa A, Adem A, Roos P, Nyberg F. Neurosci Res. 1994;19:93–99. doi: 10.1016/0168-0102(94)90012-4. [DOI] [PubMed] [Google Scholar]